Abstract
We have previously shown that the N-7 substituted acyclic nucleoside analog 2-amino-7-[1,3-dihydroxy-2-propoxy)methyl]purine (compound S2242) is, both in vitro and in animal models, a potent inhibitor of the replication of several herpesviruses (Neyts et al., Antimicrob. Agents Chemother. 39:56–60, 1995). Here we report on the potent and selective antiviral activity of S2242 against vaccinia virus (VV), an orthopoxvirus. The 50% effective concentrations for inhibition of VV-induced cytopathic effect and viral DNA synthesis in cell culture were 2.4 and 0.2 μg/ml, respectively. We next studied the efficacy of S2242 in VV-infected mice. Immunocompetent NMRI mice that had been inoculated intravenously with VV developed tail lesions. Mice that had been treated for 5 consecutive days via the subcutaneous (s.c.) route with 100 mg of the diacetate ester prodrug of S2242 (compound H961) per kg of body weight did not develop any lesions and demonstrated no adverse effects. Severe combined immunodeficient (SCID) mice that had been inoculated intraperitoneally with VV became sick and died within 1 month after infection. Following treatment with H961 at 100 mg/kg for 10 consecutive days (either via oral gavage or s.c. injection) VV-inoculated SCID mice were completely protected, for at least 3 months, against virus-induced morbidity and mortality. At that time, no virus could be recovered from the organs of these mice (as assessed by titration for infectious virus, a DNA hybridization assay, and a PCR for VV-specific sequences). Compound S2242 and its oral prodrug H961 could be useful in treatment of orthopoxvirus infections.
Until the introduction of effective vaccines in the early 1800s, poxviruses historically caused serious disease in humans. The last documented case occurred in 1977 in Somalia. The World Health Assembly declared that smallpox had been eradicated as of 1980. Before that time the virus had killed millions of people. Starting in 1977, the number of laboratories holding stocks of the variola virus was being reduced until all known stocks were consolidated at only two World Health Organization (WHO) collaborating centers: the Centers for Disease Control and Prevention in Atlanta, Ga., and the VECTOR labs in Kotsovo, Russia. Although outlawed by the Biological Weapons Convention in 1972, the potential existence of unregistered stocks of smallpox virus that can be used for bioterrorism or biological warfare is a source of concern. All vaccination programs against smallpox were discontinued after eradication of the disease. Thus, virtually all children and many adults are now fully susceptible to smallpox. There is only a limited stock of vaccine available, not all of which has been confirmed to be properly stored or monitored for potency (5). Currently, there is also no established effective treatment. A smallpox outbreak occurring today in a highly mobile and susceptible population would likely spread widely before effective measures could be taken. Therefore, if smallpox were used in an act of terrorism or warfare, it could result in a real catastrophe (12).
In 1994 destruction of all stocks of variola virus was recommended by a WHO expert committee on orthopoxvirus infections. In May of 1999, the WHO's 191 member states voted to delay destruction of the variola virus stocks until 2002 at the latest. A WHO advisory committee on variola virus research defined in December 1999 the following areas in which research should be conducted before the end of 2002: sequencing more completely the DNA of the smallpox virus, devising tests to detect the virus in humans, and developing drugs to treat human smallpox infections should they reappear (press release WHO/77, 10 December 1999).
Human monkeypox causes a systemic exanthem which resembles smallpox. It occurs sporadically in parts of western and central Africa. The disease is transmitted from animals (the probable reservoir being squirrels) to humans by physical contact. Also, secondary spread from human to human by aerosol is possible (11). Between February 1996 and October 1997 an outbreak of monkeypox occurred in the province Kasai Oriental in the Democratic Republic of Congo (1, 11, 15). It would also be important to have an effective treatment for monkeypox.
The use of recombinant vaccinia virus (rVV) expressing immunoreactive epitopes from the pathogen (or tumors) as immunoprophylaxis holds promise. However, generalized VV infection may occur in immuncompromised patients, particularly those infected with human immunodeficiency virus type 1 (10, 20). In such cases an effective antipoxvirus drug may be beneficial.
Several compounds have been shown to inhibit the in vitro replication of VV (9). Of these, some demonstrated activity in the mouse tail lesion model. These include 3-deazaneplanocin A, ribavirin, cytosine arabinoside, and 5-iodo-2′-deoxyuridine. Only one drug, methisazone (Marboran; 1-methylisatin-3-thiosemicarbazone), has ever been used and reported to show prophylactic efficacy against smallpox (2, 3). We have reported on the potent anti-VV activity of the acyclic nucleoside phosphonate analog cidofovir in both immunocompetent and immunodeficient mice (16). Recently, our findings were corroborated by the observation that cidofovir also protects mice from a lethal aerosolized or intranasal cowpoxvirus challenge (4).
Here we report the potent efficacy of the diacetyl ester prodrug (H961) of the N-7-substituted acyclic nucleoside analog 2-amino-7-[1,3-dihydroxy-2-propoxy)methyl]purine (S2242) in protecting mice against VV infections. Two infection models were used: (i) a model in which the virus is injected intravenously in immunocompetent mice and (ii) a model in which the virus is injected intraperitoneally in SCID mice. The NMRI mouse model allows the monitoring of pox lesion development on the tail in a nonlethal infection; the SCID mouse model allows the determination of protective effects on virus-induced mortality. Compound S2242 is a potent and selective inhibitor of the replication of herpesviruses (human cytomegalovirus, thymidine kinase-inducing and thymidine kinase-deficient strains of herpes simplex virus, and varicella-zoster virus) (17, 18). VV is, like variola virus, an orthopoxvirus; it has been used as a smallpox vaccine for the past 200 years, and there are no known natural hosts for this virus.
MATERIALS AND METHODS
Virus, cells, and compounds.
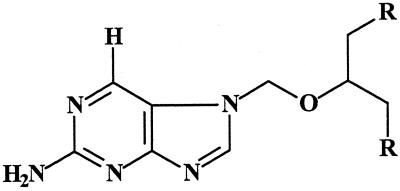
The VV strain used in this study was obtained from the Rijksentstofinrichting and was used in Belgium as a vaccine against smallpox. HEL (human embryonic lung) cells were obtained from the American Type Cell Culture Collection. Compound S2242 and its diacetate ester prodrug H961 (Fig. 1) were kindly provided by I. Winkler (Hoechst, Frankfurt am Main, Germany) and were synthesized as reported previously (13). Cidofovir was a kind gift from Gilead Sciences (Foster City, Calif.).
FIG. 1.
Chemical structure of S2242 (R = OH) and H961 [R = OC(O)CH3].
In vitro antiviral assays.
HEL cells were propagated in Eagle minimal essential medium (MEM) with 10% fetal calf serum (FCS) and 1 mM l-glutamine and were grown to confluency in 96-well microtiter plates. Cultures were infected for 2 h at 37°C with 100 PFU of VV. Following a 2-h adsorption period, the virus inoculum was removed and cultures were incubated at 37°C with different dilutions of the test compounds in MEM containing 2% FCS. At 5 days postinfection, cultures were fixed with 70% ethanol and stained with a 2% Giemsa solution and viral cytopathic effect (CPE) was determined microscopically. The 50% effective concentration (EC50) was defined as the concentration causing a 50% inhibition of virus-induced CPE.
Determination of cytostatic activity.
HEL cells were seeded in 96-well plates at 5,000/well. Cells were allowed to attach for 6 h, after which the test compounds were added. Following a 4-day incubation period, cells were trypsinized and counted with a Coulter Counter.
Determination of viral DNA levels.
Confluent cultures of HEL cells grown in 25-cm2 culture flasks were inoculated with 200 PFU of VV for 2 h. Following adsorption of the virus, the cultures were washed four times with 5 ml of medium to remove unattached virus, after which the cultures were incubated with the appropiate concentrations of the compounds diluted in medium containing 2% FCS. At day 5 postinfection, when mock-infected cultures showed 100% CPE, cells were collected by trypsinization and total cellular DNA was extracted (Qiagen Blood Kit). Ten micrograms of denaturated total cellular DNA was blotted onto a nylon membrane (Hybond-N; Amersham) and UV cross-linked, and then prehybridization was carried out for 1 h at 42°C. The probe was labeled with digoxigenin-11-dUTP in a PCR; the 5′-to-3′ primer sequences were TTG CAC CTT CAG ATG CCG AT and CAG AAT CCG CTG ATG GAA ACA, delineating a 386-bp sequence. The probe was gel purified, and hybridization was carried out for 18 h at 42°C with 30 ng of the digoxigenin-11-dUTP-labeled probe per ml. The membrane was washed at high stringency (2× SSC [1× SSC is 0.5 M NaCl plus 0.015 M sodium citrate]–0.1% sodium dodecyl sulfate [SDS]) for 10 min at room temperature followed by two washes of 15 min each in 0.1× SSC–0.1% SDS at 65°C. After incubation in blocking buffer, the filter was incubated with an antidigoxigenin antibody conjugated with alkaline phosphatase (Boehringer Mannheim), and chemiluminescence was detected by standard methods. Films were scanned densitometrically.
Inoculation, treatment, and evaluation.
NMRI mice weighing 15 to 20 g were infected intravenously with 4 × 103 PFU of VV per ml. SCID mice weighing about 15 g were inoculated intraperitoneally with 4 × 103 PFU/ml. H961 was administered either by oral gavage (in 0.5 ml of water) or by subcutaneous (s.c.) injection (in 0.2 ml of phosphate-buffered saline). (The compound was administered s.c. rather than intravenously or intraperitoneally because the s.c. route of administration would be most practical in case a large number of patients need to be treated, e.g., in the case of a terrorist attack.) Mortality and the number of pox lesions on the tail were recorded daily. Statistical significance of the mean day of death and the number of tail lesions was assessed using the two-tailed Student's t test, and statistical significance of the number of survivors was assessed by the χ2 test with Yates' correction.
RESULTS
In vitro anti-VV activity of S2242
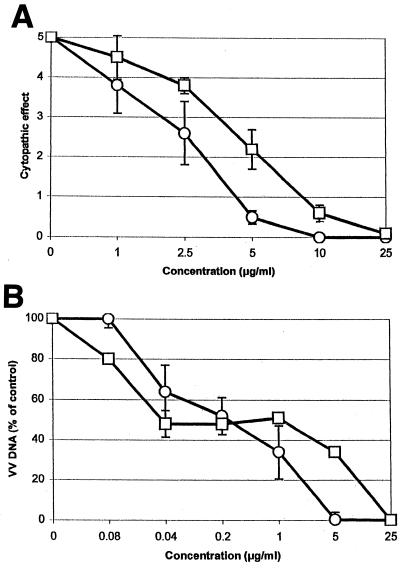
The in vitro anti-VV activities of S2242 and (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (HPMPC) were compared. As can be seen in Fig. 2A, S2242 efficiently inhibited the replication of VV in fibroblasts, with an EC50 of 2.6 μg/ml; the EC50 of HPMPC was 4.6 μg/ml. In parallel, viral DNA synthesis was monitored. A dose-dependent inhibition of VV DNA synthesis was observed, with EC50 of 0.2 μg/ml for both S2242 and HPMPC. S2242 and HPMPC had no effect on the morphology of HEL cells at concentrations of up to 200 μg/ml but inhibited the growth of exponentially growing uninfected HEL cells by 50% at concentrations of 32 ± 2 and 49 ± 8 μg/ml, respectively (data not shown).
FIG. 2.
Anti-VV activities of S2242 and HPMPC measured as virus-induced CPE (A) or viral DNA synthesis (B). ○, S2242; □, HPMPC.
Effect of H961 on VV-induced tail lesions in NMRI mice
NMRI mice (10 to 15 animals) were inoculated intravenously with VV. Tail lesions first appeared at day 4 and were counted at day 7 postinfection. On average, 20.8 ± 5.8 lesions were counted on the tails of untreated animals (Table 1). When the animals were treated with an s.c. injection of 100 mg of H961 per kg of body weight per day beginning at 2 h postinfection and continuing for 5 consecutive days, no lesions were detectable on the tail until the end of the experiment (P < 0.001). Also, treatment with H961 at 10 mg/kg/day reduced the number of lesions by 69%, to 5.7 ± 3.1 (P < 0.001). At a dose of 1 mg/kg no inhibitory effect on lesion development was observed (20.3 ± 8.2 lesions).
TABLE 1.
Effect of treatment with H961 on VV infection in NMRI and SCID micee
| Model | Mice | Treatment | Dose (mg/kg/day) | No. of tail lesionsb | No. of survivors/ total no. of mice | MDDd |
|---|---|---|---|---|---|---|
| Tail lesion | NMRI | Placebo | 20.8 ± 5.8 | |||
| H961a | 100 | 0** | ||||
| 10 | 5.7 ± 3.1** | |||||
| 1 | 20.3 ± 8.2 (NS) | |||||
| Lethal | SCID | Placebo | 0/10 | 28 ± 6.1 | ||
| H961c | 100 (orally) | 10/10** | ||||
| 100 (s.c.) | 10/10** |
For each condition, 10 to 15 mice were used. The compound was administered s.c. for five consecutive days starting at 2 h postinfection. Data are mean values from two independent experiments.
Numbers of tail lesions (± standard deviations) determined at day 7 postinfection.
H961 was administered s.c. or orally for two periods of five consecutive days with a 2-day interval.
MDD, mean day of death (± standard deviation).
∗∗, P < 0.001; NS, not significant.
Effect of H961 on VV-induced mortality in SCID mice.
The effect of H961 was studied in a lethal VV infection model (five animals per group). SCID mice that had been infected intraperitoneally became sick (ruffled fur, cachexia) at day 14 postinfection and died at 28 ± 6.1 days postinfection (Table 1). Compound H961 when administered orally or s.c. (starting at 2 h postinfection) at 100 mg/kg/day for two consecutive periods of 5 days each (separated by a 2-day interval) completely protected the animals against virus-induced morbidity and mortality for at least 3 months (second experiment) or 6 months (first experiment) (P < 0.001). At that time liver, lung, kidney, and brain tissue samples were collected from the surviving animals and analyzed for the presence of virus by (i) a plaque assay, (ii) a DNA-DNA hybridization dot blot assay, and (iii) a PCR specific for VV sequences. Neither infectious virus nor viral DNA could be detected by these methods, whereas infectious virus and viral DNA were readily detectable in organs of untreated animals (data not shown).
Next the efficacy of H961 was studied in VV-infected SCID mice, with the start of treatment being delayed for several days after infection (Table 2). H961 proved most effective when treatment was initiated at 2 h postinfection. When treatment with H961 was first initiated at 2 days postinfection, virus-induced mortality was delayed by 28 days. Even when treatment was delayed for 4 or 7 days postinfection, mice lived 11 to 17 days longer than the untreated controls, although all animals ultimately succumbed.
TABLE 2.
Effect of a delayed start of treatment with H961 on VV-induced mortality in SCID micea
| Treatment | No. of survivors | Mean day of death |
|---|---|---|
| Placebo | 0 | 29 ± 6 |
| H961 | ||
| Days 0–9 | 9** | 70 |
| Days 2–11 | 2 | 57 ± 20* |
| Days 4–13 | 0 | 40 ± 5*** |
| Days 7–16 | 0 | 46 ± 14** |
SCID mice that had been inoculated intraperitoneally with VV were treated with H961 at 100 mg/kg/day for 10 consecutive days starting at day 0 (2 h postinfection) or 2, 4, or 7 days postinfection. ∗∗∗, P < 0.0005; ∗∗, P < 0.005; ∗, P < 0.01.
DISCUSSION
Since there is no drug for the treatment or prevention of smallpox, the WHO has recently recommended that specific antiviral agents for the treatment of poxviruses should be developed before the destruction of the (officially remaining) stocks of variola.
We previously reported on the activity of some nucleoside phosphonate analogues against VV replication. The prototype compound of this family (S)-9-(3-hydroxy-2-phosphonylmethoxypropyl)adenine [(S)-HPMPA] was found to exhibit broad-spectrum activity against a wide variety of DNA viruses including VV (7). (S)-HPMPA also inhibited the development of tail lesions caused by VV in mice (8). Cidofovir [(S)-HPMPC], the cytosine congener of (S)-HPMPA, has an activity spectrum comparable to that of the parent compound but is less toxic in vitro and in vivo (i.e., in mice). We demonstrated that cidofovir possesses potent protective activity against lethal VV infection in SCID mice (16). Our observations were recently corroborated by the findings of Bray and colleagues, who demonstrated that cidofovir is also effective in the treatment of lethal aerosolized or intranasal cowpox virus infections (4). In the same report the authors revealed (their unpublished data) that cidofovir may rescue monkeys that received large quantities of aerosolized monkeypox (4). Cidofovir has also been shown to result in complete regression of lesions associated with poxviruses, i.e., severe molluscum contagiosum lesions (6, 14) and an ecthyma contagiosum (ORF) in immunodeficient patients (9a).
We have shown previously that S2242 selectively inhibits the replication of herpesviruses (17, 18). The compound is phosphorylated intracellularly first by deoxycytidine kinase and then further by cellular kinases to its 5′-triphosphorylated metabolite, which is expected to inhibit in a selective manner the viral DNA polymerase (19).
We have shown here that S2242 is at least as effective as cidofovir in inhibiting VV replication in vitro. Consequently, H961, the diacetylated oral prodrug form of S2242, was found to elicit potent activity against VV infections in both immunocompetent and SCID mice. Immunocompetent NMRI mice develop tail lesions but do not succumb to VV infection. Once the drug protects these mice against the acute phase of the infection, no recurrence of lesion formation is observed, because the animals mount an immune response. In contrast to immunocompetent mice, SCID mice that have been infected with VV die from the disease. In the SCID VV model, H961 was able to completely prevent virus-induced mortality and still afforded a marked protective effect (i.e., delay in virus-associated mortality) when the start of treatment was delayed.
At the doses used, no side effects were noted. In our study on the antiherpesvirus activity of S2242 (18) the body weight of 3- to 4-week-old NMRI mice was monitored over a 14-day period of treatment with S2242 at 100 mg/kg/day. No effect on body weight was observed compared to the control. Because ganciclovir, a structural analogue of S2242, is known to affect testicle histology (at least in rodents), the effect of S2242 on testicle histology was studied. Four weeks following a 14-day treatment period with S2242 at 100 mg/kg/day, all male mice displayed atrophy of the testicular germinal epithelium. Similar observations were reported for ganciclovir-treated animals. No other histological abnormalities were observed in the organs of S2242-treated animals. S2242 or analogues thereof may thus be useful in treatment of poxvirus infections.
ACKNOWLEDGMENTS
This work was supported by the Geconcerteerde Onderzoeksacties (GOA 00/12). J. Neyts is a postdoctoral research assistant supported by the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen.
We thank W. Zeegers and M. Stuyck for expert technical assistance and C. Callebaut and D. Brabants for dedicated editorial help.
REFERENCES
- 1.Anonymous. Human monkeypox—Kasai Oriental, Democratic Republic of Congo, February 1996–October 1997. Morb Mortal Wkly Rep. 1997;46:1168–1171. [PubMed] [Google Scholar]
- 2.Bauer D J, St. Vincent L, Kempe C H, Downie A W. Prophylactic treatment of smallpox contacts with N-methylisatin β-thiosemicarbazone. Lancet. 1963;ii:494–496. doi: 10.1016/s0140-6736(63)90230-7. [DOI] [PubMed] [Google Scholar]
- 3.Bauer D J. International encyclopedia of pharmacology and therapeutics. I. Chemotherapy of viral diseases. Oxford, United Kingdom: Pergamon Press Ltd.; 1972. Thiosemicarbazones; pp. 35–113. [Google Scholar]
- 4.Bray M, Martinez M, Smee D F, Kefauver D, Thompson E, Huggins J W. Cidofovir protects mice against lethal aerosol or intranasal cowpox virus challenge. J Infect Dis. 2000;181:10–19. doi: 10.1086/315190. [DOI] [PubMed] [Google Scholar]
- 5.Breman J G, Henderson D A. Poxvirus dilemmas—monkeypox, smallpox, and biologic terrorism. N Engl J Med. 1998;339:556–559. doi: 10.1056/NEJM199808203390811. [DOI] [PubMed] [Google Scholar]
- 6.Davies E G, Thrasher A, Lacey K, Harper J. Topical cidofovir for severe molluscum contagiosum. Lancet. 1999;353:2042. doi: 10.1016/s0140-6736(99)01782-1. [DOI] [PubMed] [Google Scholar]
- 7.De Clercq E, Holý A, Rosenberg I, Sakuma T, Balzarini J, Maudgal P C. A novel selective broad-spectrum anti-DNA virus agent. Nature. 1986;323:464–467. doi: 10.1038/323464a0. [DOI] [PubMed] [Google Scholar]
- 8.De Clercq E, Holý A, Rosenberg I. Efficacy of phosphonylmethoxyalkyl derivatives of adenine in experimental herpes simplex virus and vaccinia virus infections in vivo. Antimicrob Agents Chemother. 1989;33:185–191. doi: 10.1128/aac.33.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Clercq, E. Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin. Microbiol. Rev., in press. [DOI] [PMC free article] [PubMed]
- 9a.Geerinck, K., G. Lukito, R. Snoeck, R. De Vos, E. De Clercq, Y. Vanrenterghem, H. Degreef, and B. Maes. A case of human orf in an immunocompromised patient, successfully treated with cidofovir cream. J. Med. Virol., in press. [DOI] [PubMed]
- 10.Guillaume J C, Saiag P, Wechsler J, Lescs M C, Roujeau J C. Vaccinia from recombinant virus expressing HIV genes. Lancet. 1991;337:1034–1035. doi: 10.1016/0140-6736(91)92689-y. [DOI] [PubMed] [Google Scholar]
- 11.Heymann D L, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54:693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 12.Hooper C. Poxvirus dilemmas. N Engl J Med. 1998;339:2027–2028. doi: 10.1056/NEJM199812313392717. [DOI] [PubMed] [Google Scholar]
- 13.Jähne G, Kroha H, Müller A, Helsberg M, Winkler I, Gross G, Scholl T. Regioselective synthesis and antiviral activity of purine nucleoside analogues with acyclic substituents at N7. Angew Chem Int Ed Engl. 1994;33:562–563. [Google Scholar]
- 14.Meadows K P, Tyring S K, Pavia A T, Rallis T M. Resolution of recalcitrant molluscum contagiosum virus lesions in human immunodeficiency virus-infected patients treated with cidofovir. Arch Dermatol. 1997;133:987–990. [PubMed] [Google Scholar]
- 15.Mukinda V B, Mwema G, Kilundu M, Heymann D L, Khan A S, Esposito J J. Re-emergence of human monkeypox in Zaire in 1996. Monkeypox Epidemiologic Working Group. Lancet. 1997;349:1449–1455. doi: 10.1016/S0140-6736(05)63725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neyts J, De Clercq E. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)-cytosine for the treatment of lethal vaccinia virus infections in severe combined immune deficiency (SCID) mice. J Med Virol. 1993;41:242–246. doi: 10.1002/jmv.1890410312. [DOI] [PubMed] [Google Scholar]
- 17.Neyts J, Andrei G, Snoeck R, Jahne G, Winkler I, Helsberg M, Balzarini J, De Clercq E. The N-7-substituted acyclic nucleoside analog 2-amino-7-[(1,3-dihydroxy-2-propoxy)methyl]purine is a potent and selective inhibitor of herpesvirus replication. Antimicrob Agents Chemother. 1994;38:2710–2716. doi: 10.1128/aac.38.12.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neyts J, Jähne G, Andrei G, Snoeck R, Winkler I, De Clercq E. In vivo antiherpesvirus activity of N-7-substituted acyclic nucleoside analog 2-amino-7-[(1,3-dihydroxy-2-propoxy)methyl]purine. Antimicrob Agents Chemother. 1995;39:56–60. doi: 10.1128/aac.39.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neyts J, Balzarini J, Andrei G, Chaoyong Z, Snoeck R, Zimmermann A, Mertens T, Karlsson A, De Clercq E. Intracellular metabolism of the N7-substituted acyclic nucleoside analog 2-amino-7-(1,3-dihydroxy-2-propoxymethyl)purine, a potent inhibitor of herpesvirus replication. Mol Pharmacol. 1998;53:157–165. doi: 10.1124/mol.53.1.157. [DOI] [PubMed] [Google Scholar]
- 20.Redfield R R, Wright D C, James W D, Jones T S, Brown C, Burke D S. Disseminated vaccinia in a military recruit with human immunodeficiency virus (HIV) disease. N Engl J Med. 1987;316:673–676. doi: 10.1056/NEJM198703123161106. [DOI] [PubMed] [Google Scholar]