Abstract
This article shows that two extremely important families of fused heterocyclic assemblies, namely 6-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine and 5a-methyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine, can be synthesized from only two available building blocks (N-allenylpyrrole-2-carbaldehyde and o-phenylenediamine) by controlling only one reaction parameter (water content of the medium). It should be emphasized that the latter class of compounds (with an a/d arrangement) is previously unknown. If the allene group is introduced not into the starting compound, but during the reaction (in superbase media), a heterocyclic ensemble, 5-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazines, with a different position of the methyl group is formed.
Keywords: aldehydes, allenes, cyclization, heterocycles
1. Introduction
Fused heterocyclic compounds, namely, derivatives of imidazole, benzimidazole, and pyrazine, exhibit diverse biological activities. Imidazolopyrazines and imidazolopiperazines belong to the class of therapeutically efficient fused heterocycles. Imidazolopyrazine derivatives exert anti-inflammatory and antiviral actions, as well as inhibition MAPK-activated PK5 [1]. It was reported that imidazolopyrazines act as an effective CXCR3 antagonist (regulation of leukocyte transport) [2], and as a potent IGF-1R inhibitor [3]. The literature search reveals that the imidazolopiperazine motif is responsible for antimalarial action of known drugs [4], which can be enhanced via the introduction the pyrrole moiety in their structure.
Despite a wide applicability of such compounds, facile and straightforward methods for the synthesis of these biologically active scaffolds still remain limited. Currently, only a few examples of assembling such systems are documented in the literature. They can be divided into two groups: intramolecular and intermolecular reactions (Scheme 1). Polyheterocyclic systems are obtained via intramolecular cyclization in the presence of palladium [5] or copper [6,7] salts as catalysts at high temperatures. Intermolecular cyclizations are also promoted by catalysts: transition metals [8], salts [9] or acids [10]. All the above reactions require expensive toxic catalysts, long reaction times or high temperatures, which are disadvantages from a synthetic point of view.
Scheme 1.
Methods for the synthesis of polyheterocyclic systems: previous works.
2. Results
We have developed a strategy for the synthesis of benzimidazopyrrolopyrazines through the sequential addition of o-phenylenediamine (o-PDA) 2 to N-allenylpyrrole-2-carbaldehydes 1a–j under mild conditions. Our investigation commenced with the reaction of 5-(2-phenyl)-N-allenylpyrrole-2-carbaldehyde 1b and o-PDA 2, which proceeded under conditions developed by us earlier for N-vinylpyrrole-2-carbaldehydes [11] in ethyl alcohol at room temperature for 16 h (Scheme 2).
Scheme 2.
Synthesis of 6-methyl-3-phenylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine and 5a-methyl-3-phenyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine.
In contrast to the work with N-propargylindolecarbaldehyde [8], the allene fragment does not require activation with heavy metal salts (CuI), which opens up more possible directions for the reaction.
Experiments have shown that the reaction leads to a mixture of two products: the anticipated benzimidazopyrrolopyrazine 3b and the unexpected dihydrobenzimidazopyrrolopyrazine 4b. The process is influenced by the air humidity and hence by the presence of some amount of water in the reaction mixture. Thus, at a higher content of water in the air (wet season), the ratio of products 3b and 4b is 1:1 (NMR), while in a drier climate in the presence of commercial ethanol, this ratio is 64%:16% (19% of unreacted compound 1b). Consequently, we have optimized this reaction by the addition of water and varying the solvents in order to direct it selectively to one of the two products (Table 1).
Table 1.
Optimization of the reaction conditions a.
| Entry | Solvent | H2O (% from V Solvent) | T (°C) | t (h) | Ratio (1H NMR), % | ||
|---|---|---|---|---|---|---|---|
| 1b | 3b | 4b | |||||
| 1 b | EtOH | – | 20–25 | 12 | – | 50 | 50 |
| 2 c | DMSO | – | 20–25 | 12 | – | 50 | 50 |
| 3 c | DMSO | – | 65 | 1 | – | 50 | 50 |
| 4 d | EtOH | – | 20–25 | 12 | 19 | 64 | 16 |
| 5 | EtOH (dry) | – | 20–25 | 12 | – | 90 | 10 |
| 6 | EtOH (dry) | 10 | 20–25 | 12 | – | 60 | 40 |
| 7 | EtOH (dry) | 20 | 20–25 | 12 | – | 50 | 50 |
| 8 | EtOH (dry) | 50 | 20–25 | 12 | 100 | – | – |
| 9 | MeOH (dry) | – | 20–25 | 12 | – | 96 | traces |
| 10 | Benzene | – | 20–25 | 12 | – | 85 | 15 |
| 11 | Benzene (dry) | – | 20–25 | 12 | 48 | 48 | 4 |
a 1b (0.0011 mol), 2 (0.00121 mol), solvent (2.2 mL), CF3COOH (1%). b The reaction was carried out at high air humidity in unpressurized conditions (without drying agent). c Resinification. d Common ethanol.
Optimization of the reaction conditions has revealed that in commercial DMSO, which usually contains some water, the process occurs with similar efficiency to that observed in aqueous ethanol (Entry 2). When the reaction was carried out in DMSO at a higher temperature (65 °C) for a shorter time (1 h), the products were obtained in the same ratio (Entry 3). However, in this case, the reaction was accompanied by the formation of hardly identifiable side products and afforded the target products in lower yield. Therefore, we further used the initial temperature conditions. Subsequently, we carried out experiments with dried ethanol in an inert atmosphere to avoid the effect of air humidity. In dry ethanol, a mixture of two products 3b and 4b was formed in a 9:1 ratio. Evidently, the dried ethanol contained enough water to trigger a side reaction (Entry 5). In the presence of ethanol and 10% of water, a mixture of two compounds 3b and 4b was obtained in a 6:4 ratio (Entry 6). At a higher content of water (20%), the product ratio was again 1:1 (Entry 7), which evidenced the clear dependence of this ratio on the amount of water. However, when the process was implemented in a 1:1 mixture of ethanol-water (Entry 8), the starting compound 1b was completely recovered due to poor solubility of such a mixture. Replacement of ethanol with methanol, which usually contains much less water, led to almost selective formation of product 3b (Entry 9). The reaction in commercial (Entry 10) and dry benzene (Entry 11) also resulted in a higher selectivity with respect to compound 3b, the conversion in the latter being decreased.
Thus, to selectively synthesize compounds 3, it is most expedient to employ the reaction conditions shown in Entry 9. Under these conditions, a wide series of N-allenylpyrrole-2-carbaldehydes was involved in the process to selectively afford benzimidazopyrrolopyrazines 3a–j (Scheme 3).
Scheme 3.
Synthesis of 6-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazines.
We failed to selectively direct the reaction toward the formation of products 4. Therefore, aqueous ethanol (Scheme 2) was used, followed by the isolation of individual compounds by column chromatography. The structures and yields of the products obtained by both methods are shown in Table 2.
Table 2.
Structures of 6-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazines and 5a-methyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazines.
| 3 | Product | Yield (Aqueous EtOH) a, % | Yield (MeOH Dry) b, % | 4 | Product | Yield (Aqueous EtOH) a, % |
|---|---|---|---|---|---|---|
| a |

|
30 d | 42 | a c |

|
4 d |
| b |

|
34 | 87 | b |
|
31 |
| c |

|
32 | 85 | c |

|
30 |
| d |

|
40 | 90 | d |

|
35 |
| e |

|
38 | 89 | e |

|
33 |
| f |

|
34 | 82 | f |
|
32 |
| g |

|
37 | 84 | g |

|
33 |
| h |

|
28 | 79 | h |

|
19 |
| i |

|
34 | 76 | i |

|
28 |
| j |

|
41 | 88 | j |

|
37 |
a 1 (0.0011 mol), 2 (0.00121 mol), EtOH (2.2 mL), H2O (0.44 mL) CF3COOH (1%); b 1 (0.0011 mol), 2 (0.00121 mol), MeOH dry (2.2 mL), CF3COOH (1%); c Reaction time: 64 h; d Conversion of 1a was 74%.
As can be seen from Table 2, aromatic and heteroaromatic substituents in the α-position of the pyrrole ring have the same effect on the yield of the reaction products. This is also the case for donating or accepting substituents in the para position of the phenyl substituent. A slight decrease in yields for bulky compounds 4h and 4i is due to the steric hindrance. The decreased yield and incomplete conversion in the case of starting pyrrole 1a bearing donor alkyl substituents is expected for nucleophilic addition at the carbonyl group, since the donating substituents compensate the positive charge on the carbonyl carbon.
The structure of benzoimidazopyrrolopyrazines 3 was unambiguously proven by X-ray diffraction analysis using compound 3e as an example (Figure 1).
Figure 1.
X-ray structure of 3-(3-methoxyphenyl)-6-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]. pyrazine 3e.
The structure of 4 was established by NMR spectroscopy (1H, 13C, including 2D correlations, Figure 2a,b.
Figure 2.
(a) NOESY and (b) HMBC for 4i.
The formation of products 3 can be tentatively rationalized as follows [12,13]. The reaction starts with the addition of o-PDA to N-allenylpyrrole-2-carbaldehyde to furnish Schiff base A. The latter further undergoes intramolecular cyclization to give the benzimidazole skeleton B (5-exo-trig). This is a well-known reaction, in which ambient oxygen can act as an oxidizing agent for the intermediate imidazoline B. The NH-function of the benzimidazole attacks the central carbon atom (sp) of the allene moiety that leads to a 6-exo-dig cyclization to finally deliver benzimidazopyrrolopyrazines 3 (Scheme 4).
Scheme 4.
Possible mechanism of formation of 6-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazines.
It is more difficult to explain the formation of product 4. We have proposed two possible reaction pathways.
Possible pathway 1: The formation of by-product 4 likely also commences with the addition of o-PDA to N-allenylpyrrole-2-carbaldehyde to produce the Schiff base A. Afterward, the second amino group is not added to the C=N bond (as in Scheme 4), but at the central allenic carbon to form a nine-membered ring D (Baldwin’s rule [14,15,16]). Product 4 contains two more hydrogen atoms than compound A, i.e., some kind of reduction occurs. Apparently, the intermediate imidazoline B generated during the formation of compound 3 with the hydrogen transfer through the medium can act as a reducing agent. In the reduced intermediate E, the double bond is activated by the acid of the system to intramolecularly close the ring and to afford product 4 (Scheme 5).
Scheme 5.
Possible pathway 1 of formation of 5a-methyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazines.
As seen from the above mechanistic schemes, selectivity of the reaction depends on the behavior of the amino group in intermediate A, which attacks either the C=N or C=C bond. A possible reason for the effect of water on the reaction direction is assumed to be the presence of hydrogen bonding between water molecules and the amino group, which complicates the attack on the more sterically hindered C=N bond thus allowing an alternative addition at the C=C bond. This scheme is supported by the fact that the best ratio of products of 3:4 was 1:1 (Table 1, Entry 7); compound 4 cannot be formed without a reducing agent (intermediate B) delivering product 3. Besides, a further increase in the water content to direct the reaction selectively to product 4 had no success due to the reduction of solubility of the starting compound in the water–alcohol mixture (Table 1, Entry 8).
Possible pathway 2: It can also be assumed that the reaction is triggered by disproportionation of the starting pyrrolecarbaldehyde 1 (the Cannizzaro type reaction [17]) to furnish carboxylic acid F and alcohol G, which further interact with o-PDA. In the case of carboxylic acid, the process involves the formation of product 3 similar to the reaction with aldehyde (such reactions are well known [18,19,20]). In alcohol G, the substitution at the hydroxyl group occurs with participation the o-PDA amino group to deliver the intermediate H, followed by intramolecular addition of the second amino group at the allene producing a 9-membered ring I. The latter can undergo intramolecular cyclization due to the activation of the remaining double bond by the acid of the system to afford compound 4 (Scheme 6).
Scheme 6.
Possible pathway 2 of formation of 5a-methyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazines.
We indirectly proved such direction of the process by involving the separately obtained alcohol in the reaction. Among the reaction products, an intermediate compound was identified and characterized by NMR spectroscopy (see Supplementary Information). The obvious disadvantage of such an explanation of the possible mechanism is that upon disproportionation, the ratio of products should be close to 1:1, but in our case, compound 3 most often predominates. This can be rationalized by the fact that the starting pyrrolecarbaldehyde 1 is not only disproportionated, but also directly reacts with o-PDA (Scheme 2), thereby increasing the content of product 3 in the mixture. Probably, water here directly affects the direction of Cannizzaro reaction and somehow promotes the hydrogen transfer from the oxidizing site to the reducing one thus enhancing the contribution of Cannizzaro-type reaction to the overall process. This also explains the impossibility to shift the ratio of products 3 and 4 by more than 1:1. Similar to the case of pathway 1, a further increase in the concentration of water decreases solubility of the reactants and worsens the outcome of the reaction.
Non-conjugated dihydrobenzimidazopyrrolopyrazines 4 represent a rather rare class of compounds. To the best of our knowledge, chemical databases contain no such structures. We managed to find a structure with a similar 5/6/5 arrangement at the a/d position, containing only one nitrogen atom and synthesized by the Pauson–Khand reaction [21,22,23], all the obtained compounds being considered as promising alkaloids. Meanwhile, these compounds are expected to exhibit valuable biological properties and can be widely employed as synthons in organic synthesis.
Often, the relative position of substituents in a molecular backbone and the presence of free positions suitable for further functionalization are essential for biological applications. In all disclosed protocols for the assembly of benzimidazopyrrolopyrazines 3, the spacer between the pyrrole/indole nitrogen and the imidazole ring nitrogen either does not bear a methyl substituent at all, or contains a methyl moiety in the α-position relative to the imidazole ring. Synthesis of benzimidazopyrrolopyrazines with the methyl group located closer to the pyrrole/indole fragment will expand the synthetic potential, which possibly complements the existing backbones to afford new biological targets.
To address this challenge, we have employed the following synthetic approach based on our previous research (Scheme 7). At the first stage, a series of NH-pyrrolylbenzimidazoles 5 were prepared via the published procedure [11]. Further, the obtained compounds were involved in the reaction with propargyl chloride under the conditions reported earlier [24]. The superbase in this reaction has an advantage over the common bases: it catalyzes not only substitution of the NH-function with propargyl chloride, but also the complete (quantitative) acetylene-allene isomerization to furnish allene, which is almost always selectively attacked at the sp atom. Since in compound 5, the NH-function of imidazole (pKa = 14.4) is more mobile than that of pyrrole (pKa = 23.0), substitution occurs on the former. Product I undergoes isomerization into intermediate J, the sp atom of which is subjected to an intramolecular attack by the pyrrole NH-moiety and 6-exo-dig cyclization to deliver a new series of benzimidazopyrrolopyrazines 6, isomeric to compounds 3, but with a different disposition of the methyl group. We checked the generality of this strategy on a series of substrates, and the product yields are shown in Table 3.
Scheme 7.
Synthesis of 5-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazines.
Table 3.
Structures of 5-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazines a.
| 6 | Product | Yield, % |
|---|---|---|
| k |

|
82 |
| a |

|
61 |
| l |

|
51 |
| b |

|
81 |
| c |

|
85 |
| d |

|
90 |
| e |

|
88 |
| f |

|
83 |
| g |

|
96 |
| h |

|
79 |
| i |

|
84 |
a 5 (0.0011 mol), KOH (0.0044 mol), DMSO (2.2 mL), C3H3Cl (0.0022 mol), KOH (0.0132 mol).
We checked the generality of this strategy on a series of substrates, and the product yields are shown in Table 3.
The reaction was found to be of general character. It was shown that aromatic and heteroaromatic substituents did not affect yields of the products (6a–i, k–l). If the p-position of the phenyl ring contained donating (6d, 90%) or accepting substituents (6g, 96%), the yields were approximately the same. In the case of a donating substituent in the pyrrole ring (6l), the yield decreased to moderate.
The structure of the obtained compounds was unambiguously proven by X-ray single-crystal analysis using 6b as an example (Figure 3). For all the processes described above (Scheme 2 and Scheme 3; Table 3), at each stage of several possible (allowed) directions, according to Baldwin’s rule, only one is always realized, i.e., all reactions are regeoselective.
Figure 3.
X-ray structure of 3-(3-methoxyphenyl)-6-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]. pyrazine 6b.
3. Materials and Methods
3.1. General Information
All reagents were purchased from a commercial supplier, such as Sigma-Aldrich Co. LLC (St. Louis, MO, USA). N-Allenylpyrrole-2-carbaldehydes were obtained according to the procedure [25]. DMSO was used with a water content of 0.1–0.3%, ethyl alcohol with a water content of 5%. Methyl alcohol used 99.5%. Ethanol and benzene were dried according to the literature data [26]. Propargyl chloride was purified by simple distillation (58 °C). NMR spectra were recorded from solutions in CDCl3 and DMSO-d6 on Bruker DPX-400 and AV-400 spectrometers (400.1 MHz for 1H and 100.6 MHz for 13C). Chemical shifts (δ) are quoted in parts per million (ppm). The residual solvent peak, δH = 7.27 and δC = 77.16 for CDCl3, δH = 2.50 and δC = 39.52 for DMSO-d6, was used as a reference. Coupling constants (J) are reported in Hertz (Hz). The multiplicity abbreviations used are: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; br, broad signal.
3.2. General Procedures
General procedure for the synthesis products 3 and 4. A mixture of the N-allenylpyrrole-2-carbaldehyde 1 (0.0011 mol), o-PDA 2 (0.131 g, 0.00121 mol), EtOH (2.2 mL), H2O (0.44 mL) and CF3COOH (1%) was stirred at r.t. for 16 h. Solvent was evaporated. The resulting products were separated on a column with Al2O3 using flash chromatography (eluent hexane then hexan/diethyl ether 1:1).
General procedure for the synthesis products 3. A mixture of the N-allenylpyrrole-2-carbaldehyde 1 (0.0011 mol), o-PDA 2 (0.131 g, 0.00121 mol), MeOH (dry, 2.2 mL) and CF3COOH (1%) was stirred at r.t. for 16 h in an inert atmosphere. The resulting precipitate was collected.
General procedure for synthesis of 5. Compounds 5 were prepared according to procedure [11]. Compounds 5a, 5c–i, 5l were synthesized for the first time. Product yields depend on the complexity of the purification of the raw product. A mixture of NH-pyrrole-2-carbaldehyde (0.002 mol), o-PDA 2 (0.216 g, 0.002 mol) and CF3COOH (1% with respect to the combined mass of both reagents) in DMSO (2 mL) was stirred at 70–80 °C for 1 h with continuous air bubbling. The reaction mixture was diluted with aq 1% NaHCO3 soln (8 mL), extracted with Et2O (5 × 5 mL) and the extracts were dried (K2CO3). The solvent was evaporated, and the crude product was passed through a neutral alumina column (eluent hexane, then hexane/diethyl ether (1:1)) to give 5.
General procedure for synthesis of 6. A mixture of 5 (0.0011 mol), KOH pellets (0.286 g, 0.0044 mol) and DMSO (water content < 0.2%) (2.2 mL) was stirred at r.t. for 45 min. Subsequently, freshly distilled propargyl chloride (0.164 g, 0.0022 mol) was added over 10 min, while keeping the internal temperature between 28 and 30 °C (exothermic reaction). A further amount of KOH pellets (0.858 g, 0.0132 mol) was added, while heating between 35 and 40 °C for 20 min, and then the reaction mixture was poured into H2O under efficient stirring. The formed precipitate was filtered off.
3.3. Characterization Data of Products 3,4,5 and 6
3-Butyl-6-methyl-2-propylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (3a). White powder (0.078 g, 30% yield; 0.147 g, 42% yield). 1H NMR (400 MHz, CDCl3) δ 7.86–7.83 (m, 2H, Ph), 7.36 (t, J = 7.6 Hz, 1H, Ph), 7.21 (t, J = 7.7 Hz, 1H, Ph), 7.13 (s, 1H, CH), 6.92 (s, 1H, pyrrole), 2.83 (s, 3H, CH3), 2.79 (t, J = 7.6 Hz, 2H, CH2), 2.57 (t, J = 7.5 Hz, 2H, CH2), 1.71–1.66 (m, 2H, CH2), 1.61–1.56 (m, 2H, CH2), 1.44–1.39 (m, 2H, CH2), 1.01–0.95 (m, 6H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.8, 143.5, 131.2, 126.9, 125.6, 123.6, 121.3, 119.7, 119.3, 118.7, 112.5, 107.1, 105.6, 31.3, 28.3, 24.1, 23.8, 22.7, 18.1, 14.1, 14.0. Elemental analysis calcd (%) for C21H25N3: C 78.96, H 7.89, N 13.15; found: C 79.12; H 7.93; N 13.28.
6-Methyl-3-phenylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (3b). White powder (0.111 g, 34% yield; 0.284 g, 87% yield). 1H NMR (400 MHz, CDCl3) δ 7.87 (d, J = 7.8 Hz, 1H, Ph), 7.82 (d, J = 8.3 Hz, 1H, Ph), 7.54–7.47 (m, 4H, Ph), 7.42–7.38 (m, 1H, Ph), 7.37–7.35 (m, 1H, Ph), 7.33 (d, J = 4.0 Hz, 1H, pyrrole), 7.24 (s, 1H, CH), 7.22–7.18 (m, 1H, Ph), 6.72 (d, J = 4.0 Hz, 1H, pyrrole), 2.73 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.8, 143.5, 131.5, 131.2, 130.8, 129.1 (2C), 128.7 (2C), 128.1, 123.9, 121.9, 121.4, 120.9, 119.6, 112.9, 112.6, 107.5, 106.3, 18.0. Elemental analysis calcd (%) for C20H15N3: C 80.78, H 5.08, N 14.13; found: C 80.81, H 5.12, N 14.17.
6-Methyl-3-(p-tolyl)benzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (3c). White powder (0.109 g, 32% yield; 0.29 g, 85% yield). 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.1 Hz, 1H, Ph), 7.86 (d, J = 8.4 Hz, 1H, Ph), 7.46 (d, J = 8.0 Hz, 2H, Ph), 7.40–7.38 (m, 1H, Ph), 7.36–7.35 (m, 1H, Ph), 7.34–7.31 (m, 2H, Ph, pyrrole), 7.26–7.22 (m, 2H, Ph, CH), 6.71 (d, J = 3.9 Hz, 1H, pyrrole), 2.77 (s, 3H, CH3), 2.44 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.7, 143.5, 138.1, 131.2, 130.9, 129.8 (2C), 128.6 (2C), 128.5, 123.8, 121.8, 121.1, 120.7, 119.6, 112.6, 112.6, 107.6, 106.3, 21.4, 18.0. Elemental analysis calcd (%) for C21H17N3: C 81.00, H 5.50, N, 13.49; found: C 81.09, H 5.56, N 13.54.
3-(4-Methoxyphenyl)-6-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (3d). Pink powder (0.144 g, 40% yield; 0.324 g, 90% yield). 1H NMR (400 MHz, CDCl3) δ 7.90–7.87 (m, 2H, Ph), 7.49 (d, J = 8.7 Hz, 2H, Ph), 7.39 (t, J = 7.8 Hz, 1H, Ph), 7.35 (d, J = 3.9 Hz, 1H, pyrrole), 7.28–7.24 (m, 2H, CH, Ph), 7.06 (d, J = 8.7 Hz, 2H, Ph), 6.69 (d, J = 3.9 Hz, 1H, pyrrole), 3.89 (s, 3H, CH3), 2.81 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 159.4, 144.5, 143.3, 130.9, 130.4, 129.8 (2C), 123.6, 121.6, 120.6, 120.5, 119.2, 114.4 (2C), 112.4, 112.2, 107.3, 105.9, 55.3, 17.8. Elemental analysis calcd (%) for C21H17N3O: C 77.04, H 5.23, N 12.84; found: C 77.08, H 5.27, N 12.87.
3-(3-Methoxyphenyl)-6-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (3e). White powder (0.137 g, 38% yield; 0.32 g, 89% yield). 1H NMR (400 MHz, CDCl3) δ 7.90 (m, 2H, Ph), 7.45–7.40 (m, 2H, Ph), 7.38–7.33 (m, 2H, pyrrole, Ph), 7.28–7.25 (m, 1H, CH), 7.16–7.14 (m, 1H, Ph), 7.10 (s, 1H, Ph), 6.99–6.96 (m, 1H, Ph), 6.76 (d, J = 3.9 Hz, 1H, pyrrole), 3.88 (s, 3H, CH3), 2.81 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 160.1, 144.8, 143.4, 132.7, 131.2, 130.6, 130.1, 123.9, 121.9, 121.4, 120.99, 120.90, 119.6, 114.5, 113.4, 112.9, 112.6, 107.6, 106.3, 55.5, 18.0. Elemental analysis calcd (%) for C21H17N3O: C 77.04, H 5.23, N 12.84; found: C 77.06, H 5.25, N 12.86.
3-(4-Chlorophenyl)-6-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (3f). White powder (0.124 g, 34% yield; 0.299 g, 82% yield). 1H NMR (400 MHz, CDCl3) δ 7.90 (d, J = 8.1 Hz, 1H, Ph), 7.86 (d, J = 8.4 Hz, 1H, Ph), 7.49 (s, 4H, Ph), 7.39 (t, J = 7.6 Hz, 1H, Ph), 7.35 (d, J = 4.0 Hz, 1H, pyrrole), 7.27–7.25 (m, 1H, Ph), 7.20 (s, 1H, CH), 6.72 (d, J = 4.0 Hz, 1H, pyrrole), 2.79 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.8, 143.3, 134.1, 131.2, 129.98, 129.94 (2C), 129.5, 129.4 (2C), 124.0, 122.0, 121.7, 121.2, 119.7, 113.2, 112.6, 107.3, 106.4, 18.1. Elemental analysis calcd (%) for C20H14ClN3: C 72.40, H 4.25, N 12.66; found: C 72.47, H 4.29, N 12.67.
3-(4-Bromophenyl)-6-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (3g). White powder (0.153 g, 37% yield; 0.347 g; 84% yield). 1H NMR (400 MHz, CDCl3) δ 7.90 (d, J = 8.1 Hz, 1H, Ph), 7.87 (d, J = 8.3 Hz, 1H, Ph) 7.65 (d, J = 8.3 Hz, 2H, Ph), 7.43 (d, J = 8.4 Hz, 2H, Ph), 7.39–7.34 (m, 2H, pyrrole, Ph), 7.27–7.26 (m, 1H, Ph), 7.20 (s, 1H, CH), 6.73 (d, J = 3.9 Hz, 1H, pyrrole), 2.79 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.7, 143.3, 132.3 (2C), 131.2, 130.4, 130.1 (2C), 129.5, 124.0, 122.2, 122.0, 121.7, 121.2, 119.7, 113.1, 112.6, 107.2, 106.5, 18.0. Elemental analysis calcd (%) for C20H14BrN3: C 63.84, H 3.75, N 11.17; found, %: C 63.91, H 3.82, N 11.20.
6-Methyl-3-(naphthalen-2-yl)benzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (3h). White powder (0.107 g, 28% yield; 0.302 g, 79% yield). 1H NMR (400 MHz, CDCl3) δ 8.02–7.97 (m, 2H, Ph), 7.92–7.88 (m, 4H, Ph), 7.68–7.66 (m, 1H, Ph), 7.57–7.54 (m, 2H, Ph), 7.43–7.39 (m, 2H, pyrrole, Ph), 7.37 (s, 1H, CH), 7.29–7.25 (m, 1H, Ph), 6.85 (d, J = 4.0 Hz, 1H, pyrrole), 2.80 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.9, 143.5, 133.6, 132.9, 131.3, 130.8, 128.95, 128.91, 128.2, 127.9, 127.5, 126.8, 126.68, 126.61, 124.0, 122.0, 121.6, 121.0, 119.8, 113.4, 112.7, 107.7, 106.6, 18.1. Elemental analysis calcd (%) for C24H17N3: C 82.97, H 4.93, N 12.10; found: C 83.02, H 4.98, N 12.17.
6-Methyl-13,14-dihydrobenzo[g]benzo[4’,5’]imidazo[2’,1’:3,4]pyrazino[1,2-a]indole (3i). White powder (0.121 g, 34% yield; 0.270 g, 76% yield). 1H NMR (400 MHz, CDCl3) δ 7.90–7.88 (m, 2H, Ph), 7.70–7.65 (m, 2H, Ph), 7.42–7.33 (m, 3H, Ph), 7.28–7.24 (m, 1H, pyrrole), 7.22–7.19 (m, 2H, CH, Ph), 2.97–2.93 (m, 2H, indole), 2.90–2.88 (m, 5H, indole, CH3). 13C NMR (100 MHz, CDCl3): δ 144.8, 143.3, 137.2, 131.2, 129.0, 128.7, 126.8, 126.7, 126.2, 125.9, 123.9, 121.7, 121.3, 120.7, 120.2, 119.5, 112.6, 108.2, 104.6, 30.7, 22.6, 18.1. Elemental analysis calcd (%) for C22H17N3: C 81.71, H 5.30, N 12.99; found: C 81.75, H 5.37; N 13.34.
6-Methyl-3-(thiophen-2-yl)benzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (3j). White powder (0.137 g, 41% yield; 0.294 g, 88% yield). 1H NMR (400 MHz, CDCl3) δ7.78 (d, J = 8.1 Hz, 1H, Ph), 7.75 (d, J = 8.1 Hz, 1H, Ph), 7.32–7.31 (m, 1H, thienyl), 7.30–7.28 (m, 2H, thienyl), 7.22 (d, J = 4.0 Hz, 1H, pyrrole), 7.16–7.12 (m, 2H, CH, Ph), 7.09–7.07 (m, 1H, Ph), 6.70 (d, J = 4.0 Hz, 1H, pyrrole), 2.69 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.8, 143.1, 132.6, 131.1, 127.8, 126.3, 126.1, 124.0, 123.5, 122.0, 121.7, 121.2, 119.7, 114.1, 112.6, 107.6, 106.3, 18.1. Elemental analysis calcd (%) for C18H13N3S: C 71.26, H 4.32, N 13.85; found: C 71.29, H 4.37, N 13.90.
3-Butyl-5a-methyl-2-propyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine (4a). Orange oil (0.01 g, 4% yield). 1H NMR (400 MHz, CDCl3) δ 6.79 (m, 1H, Ph), 6.75–6.60 (m, 2H, Ph), 6.39 (d, J = 7.6 Hz, 1H, Ph), 5.81 (s, 1H, pyrrole), 4.47 (d, J = 14.8 Hz, 1H, CH2), 4.44 (d, J = 14.8 Hz, 1H, CH2), 3.92 (d, J = 12.0 Hz, 1H, CH2), 3.85 (s, 1H, NH), 3.70 (d, J = 12.0 Hz, 1H, CH2), 2.50–2.45 (m, 2H, CH2), 2.34 (t, J = 15.2 Hz, 2H, CH2), 1.56–1.50 (m, 2H, CH2), 1.43–1.39 (m, 2H, CH2), 1.37–1.31 (m, 2H, CH2), 1.26 (s, 3H, CH3), 0.95–0.90 (m, 6H, CH3).
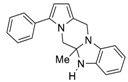
5a-Methyl-3-phenyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine (4b). Red oil (0.102 g, 31% yield). 1H NMR (400 MHz, CDCl3) δ 7.42–7.35 (m, 4H, Ph), 7.31–7.27 (m, 1H, Ph), 6.80 (t, J = 7.5 Hz, 1H, Ph), 6.64 (t, J = 7.5 Hz, 1H, Ph), 6.59–6.57 (m, 1H, Ph), 6.46 (d, J = 7.5 Hz, 1H, Ph), 6.27 (d, J = 3.5 Hz, 1H, pyrrole), 6.12 (d, J = 3.5 Hz, 1H, pyrrole), 4.65 (d, J = 14.6 Hz, 1H, CH2), 4.52 (d, J = 14.6 Hz, 1H, CH2), 4.16 (d, J = 12.1 Hz, 1H, CH2), 3.88 (d, J = 12.1 Hz, 1H, CH2), 3.68 (s, 1H, NH), 1.22 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 142.5, 138.8, 134.6, 132.5, 128.7 (2C), 128.2 (2C), 126.8, 126.1, 121.6, 119.3, 110.4, 108.2, 107.4, 105.2, 79.9, 52.1, 43.2, 24.7. Elemental analysis calcd (%) for C20H19N3: C 79.70, H 6.35, N 13.94; found: C 79.78, H 6.41, N 13.99.
5a-Methyl-3-(p-tolyl)-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine (4c). Red oil (0.104 g, 30% yield). 1H NMR (400 MHz, CDCl3) δ 7.31 (d, J = 8.0 Hz, 2H, Ph), 7.26 (d, J = 8.0 Hz, 2H, Ph), 6.84 (t, J = 7.5 Hz,1H, Ph), 6.68 (t, J = 7.5 Hz, 1H, Ph), 6.61 (d, J = 7.4 Hz, 1H, Ph), 6.49 (d, J = 7.5 Hz, 1H, Ph), 6.28 (d, J = 3.5 Hz, 1H, pyrrole), 6.15 (d, J = 3.5 Hz, 1H, pyrrole), 4.68 (d, J = 14.6 Hz, 1H, CH2), 4.55 (d, J = 14.6 Hz, 1H, CH2), 4.17 (d, J = 12.1 Hz, 1H, CH2), 3.90 (d, J = 12.1 Hz, 1H, CH2), 3.70 (s, 1H, NH), 2.43 (s, 3H, CH3), 1.24 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 142.4, 138.8, 136.5, 134.6, 129.6, 129.3 (2C), 128.2 (2C), 125.7, 121.6, 119.2, 110.3, 107.8, 107.3, 105.0, 79.9, 52.0, 43.2, 24.7, 21.2. Anal. Calcd for C21H21N3, %: C, 79.97; H, 6.71; N, 13.32. Found, %: C, 80.02; H, 6.74; N, 13.35.
3-(4-Methoxyphenyl)-5a-methyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine (4d). Red oil (0.127 g, 35% yield). 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 8.7 Hz, 2H, Ph), 6.95 (d, J = 8.6 Hz, 2H, Ph), 6.82–6.78 (m, 1H, Ph), 6.65–6.62 (m, 1H, Ph), 6.59 (d, J = 8.8 Hz, 1H, Ph), 6.45 (d, J = 7.4 Hz, 1H, Ph), 6.18 (d, J = 3.4 Hz, 1H, pyrrole), 6.10 (d, J = 3.4 Hz, 1H, pyrrole), 4.64 (d, J = 14.7 Hz, 1H, CH2), 4.51 (d, J = 14.7 Hz, 1H, CH2), 4.11 (d, J = 12.1 Hz, 1H, CH2), 3.84 (s, 3H, CH3), 3.80 (d, J = 12.1 Hz, 1H, CH2), 3.67 (s, 1H, NH), 1.22 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 158.7, 142.6, 138.8, 134.4, 129.6 (2C), 125.4, 125.1, 121.7, 119.2, 114.1 (2C), 110.4, 107.4, 104.9, 79.9, 77.3, 55.4, 52.0, 43.2, 24.8. Elemental analysis calcd (%) for C21H21N3O: C 76.11, H 6.39, N 12.68; found: C 76.18, H 6.47, N 12.74.
3-(3-Methoxyphenyl)-5a-methyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine (4e). Red oil (0.120 g, 33% yield). 1H NMR (400 MHz, CDCl3) δ 7.33–7.29 (m, 1H, Ph), 7.26–7.23 (m, 1H, Ph), 6.95–6.93 (m, 1H, Ph), 6.86–6.83 (m, 1H, Ph), 6.82–6.78 (m, 1H, Ph), 6.64 (t, J = 7.5 Hz, 1H, Ph), 6.59–5.57 (m, 1H, Ph), 6.46 (d, J = 7.5 Hz, 1H, Ph), 6.28 (d, J = 3.5 Hz, 1H, pyrrole), 6.12 (d, J = 3.5 Hz, 1H, pyrrole), 4.65 (d, J = 14.6 Hz, 1H, CH2), 4.51 (d, J = 14.6 Hz, 1H, CH2), 4.15 (d, J = 12.1 Hz, 1H, CH2), 3.91 (d, J = 12.1 Hz, 1H, CH2), 3.84 (s, 3H, CH3), 3.69 (s, 1H, NH), 1.22 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 159.8, 142.3, 138.9, 134.5, 133.8, 129.6, 126.2, 121.4, 120.6, 119.3, 113.8, 112.2, 110.1, 108.4, 107.3, 105.2, 79.9, 55.3, 52.1, 43.2, 24.7. Elemental analysis calcd (%) for C21H21N3O: C 76.11, H 6.39, N 12.68. found: C 76.20, H 6.45, N 12.78.
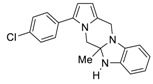
3-(4-Chlorophenyl)-5a-methyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine (4f). Red oil (0.118 g, 32% yield). 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 8.6 Hz, 2H, Ph), 7.30 (d, J = 8.6 Hz, 2H, Ph), 6.82 (t, J = 7.6 Hz, 1H, Ph), 6.66 (t, J = 7.5 Hz, 1H, Ph), 6.61 (d, J = 7.4 Hz, 1H, Ph), 6.47 (d, J = 7.5 Hz, 1H, Ph), 6.27 (d, J = 3.5 Hz, 1H, pyrrole), 6.13 (d, J = 3.5 Hz, 1H, pyrrole), 4.65 (d, J = 14.7 Hz, 1H, CH2), 4.52 (d, J = 14.7 Hz, 1H, CH2), 4.14 (d, J = 12.1 Hz, 1H, CH2), 3.82 (d, J = 12.1 Hz, 1H, CH2), 3.71 (s, 1H, NH), 1.23 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 142.5, 138.8, 133.4, 132.6, 130.9, 129.3 (2C), 128.9 (2C), 126.5, 121.7, 119.4, 110.5, 108.6, 107.4, 105.4, 79.8, 52.1, 43.1, 24.7. Elemental analysis calcd (%) for C20H18ClN3: C 71.53, H 5.40, N 12.51; found: C 71.59, H 5.48, N 12.55.
3-(4-Bromophenyl)-5a-methyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine (4g). Red oil (0.138 g, 33% yield). 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 8.4 Hz, 2H, Ph), 7.23 (d, J = 8.4 Hz, 2H, Ph), 6.83 (t, J = 7.4 Hz, 1H, Ph), 6.65 (t, J = 7.0 Hz, 1H, Ph), 6.60 (d, J = 7.5 Hz, 1H, Ph), 6.46 (d, J = 7.3 Hz, 1H, Ph), 6.27 (d, J = 3.5 Hz, 1H, pyrrole), 6.13 (d, J = 3.5 Hz, 1H, pyrrole), 4.64 (d, J = 14.7 Hz, 1H, CH2), 4.51 (d, J = 14.7 Hz, 1H, CH2), 4.13 (d, J = 12.1 Hz, 1H, CH2), 3.82 (d, J = 12.1 Hz, 1H, CH2), 3.69 (s, 1H, NH), 1.22 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 142.4, 138.7, 133.4, 131.8 (2C), 131.4, 129.6 (2C), 126.6, 121.7, 120.7, 119.4, 110.5, 108.7, 107.4, 105.4, 79.8, 52.1, 43.1, 24.7. Elemental analysis calcd (%) for C20H18BrN3: C 63.17, H 4.77, N 11.05; found: C 63.23, H 4.85, N 11.13.
5a-Methyl-3-(naphthalen-2-yl)-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine (4h). Red oil (0.073 g, 19% yield). 1H NMR (400 MHz, CDCl3) δ 7.89–7.83 (m, 3H, Ph), 7.79 (s, 1H, Ph), 7.55–7.46 (m, 3H, Ph), 6.83 (t, J = 7.5 Hz, 1H, Ph), 6.69 (t, J = 7.5 Hz, 1H, Ph), 6.60 (d, J = 7.4 Hz, 1H, Ph), 6.49 (d, J = 7.5 Hz, 1H, Ph), 6.41 (d, J = 3.5 Hz, 1H, pyrrole), 6.19 (d, J = 3.5 Hz, 1H, pyrrole), 4.69 (d, J = 14.6 Hz, 1H, CH2), 4.56 (d, J = 14.6 Hz, 1H, CH2), 4.26 (d, J = 12.2 Hz, 1H, CH2), 3.97 (d, J = 12.2 Hz, 1H, CH2), 3.67 (s, 1H, NH), 1.22 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 142.5, 138.8, 134.7, 133.6, 132.2, 129.9, 128.3, 127.9, 127.8, 126.7, 126.5, 126.4, 126.2, 125.9, 121.7, 119.3, 110.4, 108.8, 107.4, 105.5, 79.9, 52.4, 43.3, 24.8. Elemental analysis calcd (%) for C24H21N3: C 82.02, H 6.02, N 11.96; found: C 82.14, H 6.16, N 12.12.
14a-Methyl-5,8,14a,15-tetrahydro-6H,14H-benzo[g]benzo[4’,5’]imidazo[1’,2’:4,5]pyrazino[1,2-a]indole (4i). Red oil (0.1 g, 28% yield). 1H NMR (400 MHz, CDCl3) δ 7.29–7.24 (m, 2H, Ph), 7.21–7.18 (m, 1H, Ph), 7.06 (t, J = 7.4 Hz, 1H, Ph), 6.82 (t, J = 7.5 Hz, 1H, Ph), 6.68–6.62 (m, 1H, Ph), 6.44 (d, J = 7.7 Hz, 1H, Ph), 5.97 (s, 1H, pyrrole), 4.62 (d, J = 14.7 Hz, 1H, CH2), 4.55 (d, J = 14.7 Hz, 1H, CH2), 4.35 (d, J = 11.9 Hz, 1H, CH2), 4.20 (d, J = 11.9 Hz, 1H, CH2), 3.75 (s, 1H, NH), 2.95–2.82 (m, 2H, indole), 2.72–2.58 (m, 2H, indole), 1.29 (s, 1H, CH3). 13C NMR (100 MHz, CDCl3): δ 142.5, 138.7, 136.1, 129.5, 129.0, 128.6, 126.6, 126.1, 124.6, 122.7, 121.9, 120.3, 119.1, 110.8, 107.0, 103.8, 79.5, 77.3, 55.3, 42.8, 31.1, 24.4, 22.5. Elemental analysis calcd (%) for C22H21N3: C 80.70, H 6.46, N 12.83; found: C 80.78, H 6.51, N 12.90.
5a-Methyl-3-(thiophen-2-yl)-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d]pyrazine (4j). Red oil (0.125 g, 37% yield). 1H NMR (400 MHz, CDCl3) δ 7.29 (d, J = 3.6 Hz, 1H, thienyl), 7.10–7.08 (m, 1H, thienyl), 6.98 (d, J = 3.5 Hz, 1H, thienyl), 6.82 (t, J = 7.4 Hz, 1H, Ph), 6.67 (m, 1H, Ph), 6.62 (d, J = 7.2 Hz, 1H, Ph), 6.47 (d, J = 7.5 Hz, 1H, Ph), 6.35 (d, J = 3.5 Hz, 1H, pyrrole), 6.11 (d, J = 3.5 Hz, 1H, pyrrole), 4.63 (d, J = 15.1 Hz, 1H, CH2), 4.55 (d, J = 15.1 Hz, 1H, CH2), 4.11 (d, J = 12.2 Hz, 1H, CH2), 4.02 (d, J = 12.2 Hz, 1H, CH2), 3.74 (s, 1H, NH), 1.33 (s, 1H, CH3). 13C NMR (100 MHz, CDCl3): δ 142.1, 138.7, 134.3, 127.5, 126.6, 126.2, 124.9, 124.7, 121.6, 119.2, 110.5, 109.6, 107.1, 105.0, 79.6, 51.5, 42.5, 24.3. Elemental analysis calcd (%) for C18H17N3S: C 70.33, H 5.57, N, 13.67; found: C 70.45, H 5.67, N 13.76.
2-(5-Butyl-4-propyl-1H-pyrrol-2-yl)-1H-benzo[d]imidazole (5a). Yellow powder (0.157 g, 28% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.25 (s, 1H, NH), 11.18 (s, 1H, NH), 7.43 (s, 2H, Ph), 7.08–7.05 (m, 2H, Ph), 6.60 (s, 1H, pyrrole), 2.51–2.47 (m, 2H, CH2), 2.31 (t, J = 7.4 Hz, 2H, CH2), 1.54–1.45 (m, 4H, CH2), 1.26–1.19 (m, 2H, CH2), 0.89 (t, J = 7.4 Hz, 3H, CH3), 0.84 (t, J = 7.3 Hz, 3H, CH3). 13C NMR (100 MHz, DMSO-d6): δ 147.2, 132.7, 121.24, 121.4, 120.3, 119.9, 106.6, 32.2, 27.5, 24.8, 24.1, 21.9, 13.9, 13.8. Elemental analysis calcd (%) for C18H23N3: C 76.83, H 8.24, N 14.93; found: C 76.96, H 8.31, N 15.03.
2-(4,5,6,7-Tetrahydro-1H-indol-2-yl)-1H-benzo[d]imidazole (5l). Yellow powder (0.175 g, 37% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.27 (s, 1H, NH), 11.20 (s, 1H, NH), 7.85–7.38 (m, 2H, Ph), 7.10–7.08 (m, 2H, Ph), 6.55 (s, 1H, pyrrole), 2.57–2.55 (m, 2H, indole), 2.47–2.42 (m, 2H, indole), 1.74–1.67 (m, 2H, indole). 13C NMR (100 MHz, DMSO-d6): δ 177.6, 147.3, 130.8, 121.4, 121.3, 121.1, 120.5, 117.6, 110.4, 108.1, 30.75, 23.5, 22.9, 22.63, 22.62. Elemental analysis calcd (%) for C15H15N3: C 75.92, H 6.37, N 17.71; found: C 76.03, H 6.48, N 17.82.
2-(5-(p-Tolyl)-1H-pyrrol-2-yl)-1H-benzo[d]imidazole (5c). Beige powder (0.41 g, 75% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.48 (s, 1H, NH), 11.96 (s, 1H, NH), 7.76 (d, J = 8.0 Hz, 2H, Ph), 7.59–7.49 (m, 2H, Ph), 7.20–7.16 (m, 4H, Ph), 6.95 (d, J = 3.2 Hz, 1H, pyrrole), 6.65 (d, J = 3.2 Hz, 1H, pyrrole), 2.30 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6): δ 146.4, 143.9, 135.6, 134.8, 134.5, 129.2 (2C), 124.4 (2C), 123.8, 121.7, 121.3, 117.8, 111.1, 110.6, 107.1, 20.7. Elemental analysis calcd (%) for C18H15N3: C 79.10, H 5.53, N 15.37; found: C 79.15, H 5.58, N 15.41.
2-(5-(4-Methoxyphenyl)-1H-pyrrol-2-yl)-1H-benzo[d]imidazole (5d). Pink powder (0.121 g, 21% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.48 (s, 1H, NH), 11.90 (s, 1H, NH), 7.79 (d, J = 8.7 Hz, 2H, Ph), 7.57–7.47 (m, 2H, Ph), 7.16–7.14 (m, 2H, Ph), 6.97 (d, J = 8.7 Hz, 2H, Ph), 6.92 (d, J = 3.6 Hz, 1H, pyrrole), 6.57 (d, J = 3.6 Hz, 1H, pyrrole), 3.77 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6): δ 146.7, 144.2, 135.9, 135.2, 134.8, 129.6 (2C), 124.7 (2C), 124.2, 122.1, 121.6, 118.1, 111.4, 107.5, 21.1. Elemental analysis calcd (%) for C18H15N3O: C 74.72, H 5.23, N 14.52: found: C 74.77, H 5.29, N 14.58.
2-(5-(3-Methoxyphenyl)-1H-pyrrol-2-yl)-1H-benzo[d]imidazole (5e). Red powder (0.231 g, 40% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.53 (s, 1H, NH), 12.09 (s, 1H, NH), 7.59 (m, 1H, Ph), 7.51 (m, 1H, Ph), 7.48–7.47 (m, 1H, Ph), 6.40 –7.38 (m, 1H, Ph), 7.28 (t, J = 7.9 Hz, 1H, Ph), 7.17–7.16 (m, 2H, Ph), 6.94 (d, J = 3.0 Hz, 1H, pyrrole), 6.79–6.77 (m, 1H, Ph), 6.72 (d, J = 3.0 Hz, 1H, pyrrole), 3.83 (s, 3H, CH3). 13C NMR (100 MHz, DMSO-d6): δ 146.0, 133.4, 131.4, 131.2, 126.3 (2C), 124.6, 121.6 (2C), 119.1, 114.2, 111.2, 108.3. Elemental analysis calcd (%) for C18H15N3O: C 74.72, H 5.23, N 14.52; found: C 74.87, H 5.36, N 14.65.
2-(5-(4-Chlorophenyl)-1H-pyrrol-2-yl)-1H-benzo[d]imidazole (5f). Brown powder (0.487 g, 83% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.57 (s, 1H, NH), 12.10 (s, 1H, NH), 7.87 (d, J = 8.5 Hz, 2H, Ph), 7.59 (s, 1H, Ph), 7.47 (s, 1H, Ph), 7.44 (d, J = 8.5 Hz, 2H, Ph), 7.17–7.16 (m, 2H, Ph), 6.96 (d, J = 3.7 Hz, 1H, pyrrole), 6.73 (d, J = 3.7 Hz, 1H, pyrrole). 13C NMR (100 MHz, DMSO-d6): δ 146.2, 133.5, 130.9, 130.8, 128.8 (2C), 126.1 (2C), 124.6, 122.1, 121.6, 118.0, 111.3, 110.8, 108.4. Elemental analysis calcd (%) for C17H12ClN3: C 69.51, H 4.12, N 14.30; found: C 69.64, H 4.21, N 14.42.
2-(5-(4-Bromophenyl)-1H-pyrrol-2-yl)-1H-benzo[d]imidazole (5g). Light yellow powder (0.277 g, 41% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.66 (s, 1H, NH), 12.13 (s, 1H, NH), 7.84 (d, J = 8.4 Hz, 2H, Ph), 7.57–7.53 (m, 4H, Ph), 7.18–7.16 (m, 2H, Ph), 6.96 (d, J = 3.2 Hz, 1H, pyrrole), 6.74 (d, J = 3.2 Hz, 1H, pyrrole). 13C NMR (100 MHz, DMSO-d6): δ 146.0, 133.4, 131.4, 131.2, 126.3 (2C), 124.6, 121.6 (2C), 119.1, 114.2, 111.2, 108.3. Elemental analysis calcd (%) for C17H12BrN3: C 60.37, H 3.58, N 12.42; found: C 60.42, H 4.05, N 12.47.
2-(5-(Naphthalen-2-yl)-1H-pyrrol-2-yl)-1H-benzo[d]imidazole (5h). Beige powder (0.185 g, 30% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.54 (s, 1H, NH), 12.18 (s, 1H, NH), 8.48 (s, 1H, Ph), 8.02–7.99 (m, 1H, Ph), 7.92–7.87 (m, 3H, Ph), 7.63–7.61 (m, 1H, Ph), 7.53–7.44 (m, 3H, Ph), 7.19–7.17 (m, 2H, Ph), 7.00 (d, J = 3.2 Hz, 1H, pyrrole), 6.87 (d, J = 3.2 Hz, 1H, pyrrole). 13C NMR (100 MHz, DMSO-d6): δ 146.2, 143.9, 134.59, 134.52, 133.4, 131.7, 129.4, 128.0, 127.7, 127.5, 126.4, 125.5, 124.6, 123.5, 121.9, 121.8, 121.3, 117.8, 111.1, 110.7, 108.4. Elemental analysis calcd (%) for C21H15N3: C 81.53, H, 4.89, N 13.58; found: C 81.58, H 4.95, N 13.64.
2-(1H-Benzo[d]imidazol-2-yl)-4,5-dihydro-1H-benzo[g]indole (5i). Beige powder (0.313 g, 55% yield). 1H NMR (400 MHz, DMSO-d6) δ 12.43 (s, 1H, NH), 12.05 (s, 1H, NH), 7.85 (d, J = 8.5 Hz, 1H, Ph), 7.49 (s, 2H, Ph), 7.16–7.11 (m, 4H, Ph), 7.04–7.00 (m, 1H, Ph), 6.75 (s, 1H, pyrrole), 2.84 (t, J = 7.3 Hz, 2H, indole), 2.67 (t, J = 7.3 Hz, 2H, indole). 13C NMR (100 MHz, DMSO-d6): δ 146.5, 134.7, 130.4, 128.9, 127.9, 126.6, 125.4, 123.2, 121.4, 120.8, 120.7, 108.6, 29.4, 21.3. Elemental analysis calcd (%) for C19H15N3: C 79.98, H 5.30, N 14.73; found: C 80.02, H 5.36, N 14.77.
5-Methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (6k). Beige powder (0.199 g, 82% yield). 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.4 Hz, 1H, Ph), 7.61 (d, J = 7.9 Hz, 1H, Ph), 7.43–7.39 (m, 2H, Ph, pyrrole), 7.34–7.30 (m, 1H, pyrrole), 7.28–7.27 (m, 2H, CH, pyrrole), 6.79–6.78 (m, 1H, pyrrole), 2.53 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 143.8, 142.0, 129.6, 123.8, 121.6, 121.4, 120.1, 119.2, 115.7, 112.8, 109.1, 106.8, 105.4, 15.6. Elemental analysis calcd (%) for C14H11N3: C 76.00, H 5.01, N 18.99: found: C 76.12, H 5.17, N 19.13.
3-Butyl-5-methyl-2-propylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (6a). Yellow powder (0.214 g, 61% yield). 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 8.0 Hz, 1H, Ph), 7.43 (d, J = 8.0 Hz, 1H, Ph), 7.24 (t, J = 7.6 Hz, 1H, Ph), 7.15–7.13 (m, 2H, Ph, CH), 6.92 (s, 1H, pyrrole), 2.89–2.85 (m, 2H, CH2), 2.59 (s, 3H, CH3), 2.44 (t, J = 7.5 Hz, 2H, CH2), 1.62–1.56 (m, 2H, CH2), 1.49 –1.45 (m, 2H, CH2), 1.36–1.30 (m, 2H, CH2), 0.92–0.84 (m, 6H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.4, 142.7, 130.7, 129.7, 127.3, 123.8, 121.3, 121.1 (2C), 119.0, 108.9, 107.2, 106.2, 34.5, 28.4, 25.9, 23.8, 22.6, 18.6, 14.2, 13.9. Elemental analysis calcd (%) for C21H25N3: C 78.96, H 7.89, N 13.15; found: C 79.05, H 7.97, N 13.21.
6-Methyl-1,2,3,4-tetrahydrobenzo[4’,5’]imidazo[2’,1’:3,4]pyrazino[1,2-a]indole (6l). Beige powder (0.154 g, 51% yield). 1H NMR (400 MHz, CDCl3) δ 7.80 (d, J = 8.1 Hz, 1H, Ph), 7.44 (d, J = 8.0 Hz, 1H, Ph), 7.44 (t, J = 7.6 Hz, 1H, Ph), 7.33 (t, J = 7.6 Hz, 1H, Ph), 7.21 (t, J = 7.6 Hz, 1H, Ph), 7.06 (s, 1H, CH), 6.82 (s, 1H, pyrrole), 3.11 (t, J = 6.0 Hz, 2H, indole), 2.69 (t, J = 5.5 Hz, 2H, indole), 2.54 (s, 3H, CH3), 1.88–1.84 (m, 2H, indole), 1.77–1.74 (m, 2H, indole). 13C NMR (100 MHz, CDCl3): δ 144.3, 142.5, 129.7, 128.2, 124.1, 123.6, 121.7, 121.1, 119.0, 108.9, 106.0, 105.2, 77.36, 25.9, 24.0, 23.8, 22.7, 18.6. Elemental analysis calcd (%) for C18H17N3: C 78.52, H 6.22, N 15.26; found: C 78.65, H 6.35, N 15.36.
5-Methyl-3-phenylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (6b). Brown powder (0.264 g, 81% yield). 1H NMR (400 MHz, CDCl3) δ 88 (d, J = 8.1 Hz, 1H, Ph), 7.59 (d, J = 7.9 Hz, 1H, Ph), 7.48–7.46 (m, 2H, Ph), 7.45–7.38 (m, 5H, Ph, pyrrole), 7.30 (t, J = 7.5 Hz, 1H, Ph), 7.10 (s, 1H, CH), 6.65 (d, J = 3.9 Hz, 1H, pyrrole), 2.03 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.1, 142.7, 134.3, 133.1 131.1 (2C), 129.6, 128.6, 127.7 (2C), 124.0, 122.8, 122.0, 121.6, 119.2, 115.9, 109.1, 106.8, 106.7, 19.6. Elemental analysis calcd (%) for C20H15N3: C 80.78, H 5.08, N 14.13; found: C 80.83, H 5.14, N 14.19.
5-Methyl-3-(p-tolyl)benzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (6c). Yellow powder (0.291 g, 85% yield). 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 8.1 Hz, 1H, Ph), 7.51 (d, J = 7.9 Hz, 1H, Ph), 7.44 (d, J = 3.9 Hz, 1H, pyrrole), 7.40–7.36 (m, 3H, Ph), 7.28–7.24 (m, 3H, Ph), 6.95 (s, 1H, CH), 6.63 (d, J = 3.9 Hz, 1H, pyrrole), 2.48 (s, 3H, CH3), 2.00 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.1, 142.6, 138.4, 133.0, 131.1, 130.8 (2C), 129.5, 128.2 (2C), 123.7, 122.5, 121.8, 121.3, 119.0, 115.5, 109.0, 106.5, 106.3, 21.3, 19.4. Elemental analysis calcd (%) for C21H17N3: C 81.00, H 5.50, N 13.49; found: C 81.13, H 5.58, N 13.60.
3-(4-Methoxyphenyl)-5-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (6d). Brown powder (0.323 g, 90% yield). 1H NMR (400 MHz, CDCl3) δ 7.86 (d, J = 8.0 Hz, 1H, Ph), 7.55 (d, J = 8.1 Hz, 1H, Ph), 7.40 (d, J = 3.9 Hz, 1H, pyrrole), 7.38 (d, J = 8.5 Hz, 2H, Ph), 7.29–7.25 (m, 2H, Ph), 7.03 (s, 1H, CH), 6.94 (d, J = 8.7 Hz, 2H, Ph), 6.59 (d, J = 3.9 Hz, 1H, pyrrole), 3.87 (s, 3H, CH3), 2.00 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 159.9, 144.1, 142.7, 132.8, 132.2 (2C), 129.6, 126.3, 123.8, 122.5, 121.9, 121.4, 119.1, 115.6, 113.0 (2C), 109.0, 106.6, 106.4, 55.4, 19.4. Elemental analysis calcd (%) for C21H17N3O: C 77.04, H 5.23, N 12.84; found: C 77.10, H 5.28, N 12.91.
3-(3-Methoxyphenyl)-5-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (6e). Brown powder (0.317 g, 88% yield). 1H NMR (400 MHz, CDCl3) δ 7.76 (d, J = 8.1 Hz, 1H, Ph), 7.44 (d, J = 8.1 Hz, 1H, Ph), 7.30 (d, J = 3.9 Hz, 1H, pyrrole), 7.27–7.25 (m, 1H, Ph), 7.22–7.14 (m, 2H, Ph), 6.93–6.86 (m, 4H, CH, Ph), 6.52 (d, J = 3.9 Hz, 1H, pyrrole), 3.73 (s, 3H, CH3), 2.95 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 158.9, 144.2, 142.7, 135.5, 132.8, 129.7, 128.6, 123.9, 123.7, 122.9, 121.9, 121.6, 119.3, 116.8, 115.7, 114.2, 109.1, 106.8, 106.5, 55.4, 19.3. Elemental analysis calcd (%) for C21H17N3O: C 77.04, H 5.23, N 12.84; found: C 77.10; H 5.28, N 12.91.
3-(4-Chlorophenyl)-5-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (6f). Brown powder (0.303 g, 83% yield). 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.1 Hz, 1H, Ph), 7.61 (d, J = 8.0 Hz, 1H, Ph), 7.43–7.39 (m, 6H, Ph, pyrrole), 7.32 (t, J = 7.6 Hz, 1H, Ph), 7.13 (s, 1H, CH), 6.63 (d, J = 3.9 Hz, 1H, pyrrole), 2.05 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.2, 142.5, 134.7, 132.7, 132.2 (2C), 131.5, 129.6 (2C), 127.9, 124.1, 123.1, 121.7, 121.6, 119.4, 116.1, 109.1, 107.0, 106.7, 19.8. Elemental analysis calcd (%) for C20H14ClN3: C 72.40, H 4.25, N 12.66; found: C 72.49, H 4.31, N 12.74.
3-(4-Bromophenyl)-5-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (6g). Yellow powder (0.397 g, 96% yield). 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 8.1 Hz, 1H, Ph), 7.59–7.56 (m, 3H, Ph), 7.42–7.39 (m, 1H, pyrrole), 7.36 (d, J = 8.0 Hz, 2H, Ph), 7.33–7.27 (m, 2H, Ph), 7.10 (s, 1H, CH), 6.63 (d, J = 3.9 Hz, 1H, pyrrole), 2.04 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.1, 142.4, 133.1, 132.4 (2C), 131.4, 130.8 (2C), 129.5, 124.0, 123.1, 122.8, 121.6, 121.5, 119.2, 116.0, 109.1, 106.9, 106.6, 19.7. Elemental analysis calcd (%) for C20H14BrN3: C 63.84, H 3.75, N 11.17; found, %: C 63.95, H 3.81, N 11.28.
5-Methyl-3-(naphthalen-2-yl)benzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine (6h). Light brown powder (0.302 g, 79% yield). 1H NMR (400 MHz, CDCl3) δ 7.97 (s, 1H, Ph), 7.93–7.87 (m, 4H, Ph), 7.61–7.56 (m, 4H, Ph), 7.48 (d, J = 3.9 Hz, 1H, pyrrole), 7.41 (t, J = 7.3 Hz, 1H, Ph), 7.32 (t, J = 7.4 Hz, 1H, Ph), 7.13 (s, 1H, CH), 6.73 (d, J = 3.9 Hz, 1H, pyrrole), 2.06 (s, 3H, CH3). Elemental analysis calcd (%) for C24H17N3: C 82.97, H 4.93, N 12.10; found: C 83.11, H 5.07, N 12.19.
7-Methyl-13,14-dihydrobenzo[g]benzo[4’,5’]imidazo[2’,1’:3,4]pyrazino[1,2-a]indole (6i). Brown powder (0.299 g, 84% yield). 1H NMR (400 MHz, CDCl3) δ 7.89 (d, J = 7.9 Hz, 1H, Ph), 7.65 (d, J = 7.9 Hz, 1H, Ph), 7.42 (t, J = 7.4 Hz, 1H, Ph), 7.35–7.26 (m, 5H, Ph, pyrrole), 7.19–7.16 (m, 2H, Ph, CH), 2.95 (t, J = 6.7 Hz, 2H, indole), 2.79 (t, J = 6.7 Hz, 2H, indole), 2.58 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3): δ 144.4, 142.7, 136.8, 130.6, 129.9, 129.6, 129.3, 128.0, 125.9, 125.8, 125.1, 124.5, 124.1, 121.9, 121.7, 119.4, 109.3, 108.1, 105.9, 30.8, 23.4, 19.8. Elemental analysis calcd (%) for C22H17N3: C 81.71, H 5.30, N 12.99; found: C 81.78, H 5.39, N 13.07.
3.4. X-ray Crystallography
Crystal Data for 3e: Formula: C21H17N3O·CHCl3 (M = 327.38 g/mol): monoclinic, space group P21/c (No. 14), a = 10.3012(10) Å, b = 22.0073(19) Å, c = 7.2627(7) Å, β = 90.469(3)°, V = 1566.72(9) Å3, Z = 4, T = 296(2) K, µ(CuKα) = 1.076 mm−1, Dcalc = 1.261 g/cm3, 43,389 reflections measured (2.30° ≤ θ ≤ 30.06°), 4583 unique (Rint = 0.1327; Rsigma = 0.0865), which were used in all calculations. The final R1 was 0.0699 (I > 2σ(I)) and wR2 was 0.1920 (all data). CCDC 2102328 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk (accessed on 10 August 2021).
Crystal Data for 6b: Formula: C20H15N3·CHCl3 (M = 297.35 g/mol): monoclinic, space group P21/c (No. 14), a = 10.4033(3) Å, b = 20.1257(7) Å, c = 7.8873(3) Å, β = 108.427(2)°, V = 1646.4(3) Å3, Z = 4, T = 296(2) K, µ(CuKα) = 0.083 mm−1, Dcalc = 1.321 g/cm3, 42,575 reflections measured (3.36° ≤ θ ≤ 30.05°), 4804 unique (Rint = 0.0288; Rsigma = 0.0186), which were used in all calculations. The final R1 was 0.0555 (I > 2σ(I)) and wR2 was 0.0774 (all data). CCDC 2102327 contains the supplementary crystallographic data for this paper. The data can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk (accessed on 10 August 2021).
4. Conclusions
In conclusion, three different series of valuable and highly promising annulated heterocyclic assemblies have been synthesized from available building blocks, pyrrole/indolecarbaldehyde, o-phenylenediamine and propargyl chloride, by simply controlling the water content of the reaction. Moreover, 5a-methyl-5a,6-dihydro-5H,12H-benzo[4,5]imidazo[1,2-a]pyrrolo[1,2-d] 4 and 5-methylbenzo[4,5]imidazo[1,2-a]pyrrolo[2,1-c]pyrazine 6 with the methyl group in α-position to pyrrole were obtained for the first time. These compounds represent important scaffolds for pharmaceutical chemistry, given the wide biological activity of nitrogen-containing heterocycles and manifold possibilities of their further functionalization.
Acknowledgments
Experimental work was carried out using the equipment of the Baikal Analytical Centre for Collective Use of the Siberian Branch of the Russian Academy of Sciences. The authors would like to thank Rozentsveig I.B. (A.E. Favorskyay Irkutsk Institute of Chemistry SB RAS) for the helpful suggestions and fruitful discussion.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules27082460/s1. Synthesis S1: procedure for the synthesis intermediate H and 1H NMR spectra; Figures S1 and S2: 2D NOESY and HMBC of H; Figures S3–S80: 1H and 13C NMR spectra for 3, 4, 5 and 6; Figures S81–S88: selected 2D NMR spectra for 4i, 6e; Tables S1–S3: crystallographic data of 3e and 6d. References [27,28] are cited in the supplementary materials.
Author Contributions
Conceptualization, A.V.I.; investigation, S.V.M. and A.B.B.; visualization, A.V.I. and S.V.M.; formal analysis, T.N.B. and I.A.U. All authors have read and agreed to the published version of the manuscript.
Funding
The reported study was funded by RFBR, project number 19-33-90051.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds 1,3,4,5 and 6 are available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Andrews M.J.I., Edwards P., Brys R.C.X., Huxley P., Schmidt W., Birault V., Chambers M.S., Harris C.J., Macleod A., Hirst K.L., et al. Imidazolopyrazine compounds useful for the treatment of degenerative and inflammatory diseases. 817,365,6B2. US Patent. 2012 May 15;
- 2.Du X., Gustin D.J., Chen X., Duquette J., Mcgee L.R., Wang Z., Ebsworth K., Henne K., Lemon B., Ma J., et al. Imidazo-pyrazine derivatives as potent CXCR3 antagonists. Med. Chem. Lett. 2009;19:5200–8204. doi: 10.1016/j.bmcl.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 3.Jin M.A., Kleinberg A., Cooke A., Gokhale P.C., Foreman K., Dong H., Siu K.W., Bittner M.A., Mulvihill K.M., Yao Y., et al. Potent and Selective Cyclohexyl-Derived Imidazopyrazine Insulin-Like Growth Factor 1 Receptor Inhibitors with in vivo Efficacy. Bioorg. Med. Chem. Lett. 2011;21:1176–1180. doi: 10.1016/j.bmcl.2010.12.094. [DOI] [PubMed] [Google Scholar]
- 4.Wu T., Nagle A., Kuhen K., Gagaring K., Borboa R., Franeck C., Chen Z., Plouffe D., Goh A., Lakshminarayana S.B., et al. Imidazolopiperazines: Hit to Lead Optimization of New Antimalarial Agents. Med. Chem. 2011;54:5116–5130. doi: 10.1021/jm2003359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripathia K.N., Bansode A.H., Singh R.P. Palladium-Catalyzed Oxidative Annulation of Pyrrolylalkyl-1H-azoles: Towards the Synthesis of Polyheterocyclic Arenes. Synthesis. 2020;52:719–726. doi: 10.1055/s-0039-1691492. [DOI] [Google Scholar]
- 6.Tripathi K.N., Ray D., Singh R.P. Synthesis of Pyrrole-Annulated Heterocycles through Copper-Catalyzed Site-Selective Dehydrogenative Cross-Coupling. Eur. J. Org. Chem. 2017;2017:5809–5813. doi: 10.1002/ejoc.201700929. [DOI] [Google Scholar]
- 7.Ray D., Manikandan T., Roy A., Tripathia K.N., Singh R.P. Ligand–Promoted Intramolecular Dehydrogenative Cross-Coupling with Cu Catalyst: A Direct Access to Polycyclic Heteroarenes. Chem. Commun. 2015;51:7065–7068. doi: 10.1039/C5CC01817J. [DOI] [PubMed] [Google Scholar]
- 8.Ramesh S., Kr Ghosh S., Nagarajan R. Copper Catalyzed Synthesis of Fused Benzimidazolopyrazine Derivatives via Tandem Benzimidazole Formation/Annulation of δ-Alkynyl Aldehyde. Org. Biomol. Chem. 2013;11:7712–7720. doi: 10.1039/c3ob41044g. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasulu V., Shehadeh I., Khanfar M.A., Malik O.G., Tarazi H., Abu-Yousef I.A., Sebastian A., Baniowda N., O’Connor M.J., Al-Tel T.H. One-Pot Synthesis of Diverse Collections of Benzoxazepine and Indolopyrazine Fused to Heterocyclic Systems. J. Org. Chem. 2019;84:934–948. doi: 10.1021/acs.joc.8b02878. [DOI] [PubMed] [Google Scholar]
- 10.Dagar A., Joshi D.R., Kim I. Solvent-Controlled Divergent Syntheses of Polycyclic N-Fused Heteroaromatics. Synthesis. 2020;52:2841–2856. doi: 10.1055/s-0040-1707865. [DOI] [Google Scholar]
- 11.Trofimov B.A., Ivanov A.V., Skital’tseva E.V., Vasil’tsov A.M., Ushakov I.A., Petrushenko K.B., Mikhaleva A.I. A Straightforward Synthesis of 2-(1-Vinyl-1H-pyrrol-2-yl)-1H-benzimidazoles from 1-Vinyl-1H-pyrrole-2-carbaldehydes and o-Phenylenediamine. Synthesis. 2009;21:3603–3610. doi: 10.1055/s-0029-1216996. [DOI] [Google Scholar]
- 12.Eynde J.J.V., Delfosse F., Lor P., Haverbeke Y.V. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone, a mild catalyst for the formation of carbon-nitrogen bonds. Tetrahedron. 1995;51:5813–5818. doi: 10.1016/0040-4020(95)00252-4. [DOI] [Google Scholar]
- 13.Lin S., Yang L. A simple and efficient procedure for the synthesis of benzimidazoles using air as the oxidant. Tetrahedron Lett. 2005;46:4315–4319. doi: 10.1016/j.tetlet.2005.04.101. [DOI] [Google Scholar]
- 14.Gharpure S.J., Nanda S.K., Fartade D.J. Expeditious diastereoselective synthesis of medium ring heterocycle-fused chromenes via tandem 8/9-endo-dig and 8-exo-dig hydroalkoxylation-formal-[4 + 2] cycloaddition. Org. Biomol. Chem. 2019;17:8806–8810. doi: 10.1039/C9OB02030F. [DOI] [PubMed] [Google Scholar]
- 15.Wright N.E., Snyder S.A. 9-Membered Carbocycle Formation: Development of Distinct Friedel–Crafts Cyclizations and Application to a Scalable Total Synthesis of (±)-Caraphenol A. Angew. Chem. Int. Ed. 2014;53:3409–3413. doi: 10.1002/anie.201311299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilmore K., Mohamed R.K., Alabugin I.V. The Baldwin rules: Revised and extended. WIREs Comput. Mol. Sci. 2016;6:487–514. doi: 10.1002/wcms.1261. [DOI] [Google Scholar]
- 17.Smith M.B., March J. Advanced Organic Chemistry: Reactions, Mechanisms, and Structure. 6th ed. Wiley-Interscience; New York, NY, USA: 2007. [Google Scholar]
- 18.Huynh T.-K.-C., Nguyen T.-H.-A., Tran N.-H.-S., Nguyen T.-D., Hoang T.-K.-D. A facile and efficient synthesis of benzimidazole as potential anticancer agents. J. Chem. Sci. 2020;132:84–92. doi: 10.1007/s12039-020-01783-4. [DOI] [Google Scholar]
- 19.EL-Sayed T.H., Aboelnaga A., Hagar M. Ball Milling Assisted Solvent and Catalyst Free Synthesis of Benzimidazoles and Their Derivatives. Molecules. 2016;21:1111. doi: 10.3390/molecules21091111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malakooti R., Rostami-Nasab M., Mahmoudi H., Oskooie H.A., Heravi M., Karimi N., Amouchi A., Kohansal G. Synthesis of 2-substituted benzimidazoles and 2-aryl-1H-benzimidazoles using [Zn(bpdo)2·2H2O]2+/MCM-41 catalyst under solvent-free conditions. Reac. Kinet. Mech. Cat. 2014;111:663–677. doi: 10.1007/s11144-013-0672-0. [DOI] [Google Scholar]
- 21.Perez-Serrano L., Gonzalez-Perez P., Casarrubios L., Dominguez G., Perez-Castells J. Synthesis of Polycycloindoles by Stereoselective Pauson-Khand Reactions. Synlett. 2000;9:1303–1305. doi: 10.1055/s-2000-7145. [DOI] [Google Scholar]
- 22.Perez-Serrano L., Dominguez G., Perez-Castells J. New Approach to Indole Alkaloids Based on the Intramolecular Pauson-Khand Reaction. J. Org. Chem. 2004;69:5413–5418. doi: 10.1021/jo049309h. [DOI] [PubMed] [Google Scholar]
- 23.Tucker J.W., Narayanam J.M.R., Krabbe S.W., Stephenson C.R.J. Electron Transfer Phtoredox Catalysis: Intramolecular Radical Addition to Indoles and Pyrroles. Org. Lett. 2010;2:368–371. doi: 10.1021/ol902703k. [DOI] [PubMed] [Google Scholar]
- 24.Tarasova O.A., Brandsma L., Trofimov B.A. Facile One-Pot Syntheses of 1-Allenyipyrroles. Synthesis. 1993;6:571–572. doi: 10.1055/s-1993-25906. [DOI] [Google Scholar]
- 25.Martynovskaya S.V., Shcherbakova V.S., Ushakov I.A., Borodina T.N., Ivanov A.V. Expedient synthesis of a new class of organic building blocks: N-allenylpyrrole-2-carbaldehydes. Tetrahedron. Lett. 2020;61:152666. doi: 10.1016/j.tetlet.2020.152666. [DOI] [Google Scholar]
- 26.Ford R.A., Gordon A.J. The Chemist’s Companion. 1st ed. Wiley; Hoboken, NJ, USA: 1972. 560p [Google Scholar]
- 27.Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A.K., Puschmann H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009;42:339–341. doi: 10.1107/S0021889808042726. [DOI] [Google Scholar]
- 28.Sheldrick G.M. A short history of SHELX. Acta Cryst. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the Supplementary Material.












