Abstract
Whole-cell assays were implemented to search for efflux pump inhibitors (EPIs) of the three multidrug resistance efflux pumps (MexAB-OprM, MexCD-OprJ, MexEF-OprN) that contribute to fluoroquinolone resistance in clinical isolates of Pseudomonas aeruginosa. Secondary assays were developed to identify lead compounds with exquisite activities as inhibitors. A broad-spectrum EPI which is active against all three known Mex efflux pumps from P. aeruginosa and their close Escherichia coli efflux pump homolog (AcrAB-TolC) was discovered. When this compound, MC-207,110, was used, the intrinsic resistance of P. aeruginosa to fluoroquinolones was decreased significantly (eightfold for levofloxacin). Acquired resistance due to the overexpression of efflux pumps was also decreased (32- to 64-fold reduction in the MIC of levofloxacin). Similarly, 32- to 64-fold reductions in MICs in the presence of MC-207,110 were observed for strains with overexpressed efflux pumps and various target mutations that confer resistance to levofloxacin (e.g., gyrA and parC). We also compared the frequencies of emergence of levofloxacin-resistant variants in the wild-type strain at four times the MIC of levofloxacin (1 μg/ml) when it was used either alone or in combination with EPI. In the case of levofloxacin alone, the frequency was ∼10−7 CFU/ml. In contrast, with an EPI, the frequency was below the level of detection (<10−11). In summary, we have demonstrated that inhibition of efflux pumps (i) decreased the level of intrinsic resistance significantly, (ii) reversed acquired resistance, and (iii) resulted in a decreased frequency of emergence of P. aeruginosa strains that are highly resistant to fluoroquinolones.
Active efflux of toxic compounds out of cells is a general mechanism that bacteria have developed to protect themselves against the adverse effects of their environments. Antibiotics that are used in clinical settings are among these toxic compounds, and extrusion of antibiotics from bacterial cells significantly decreases their clinical utility. Antibiotics are expelled from the cells by membrane transporter proteins, the so-called drug-efflux pumps. Of particular interest are efflux pumps capable of extruding out of the cell a large variety of structurally unrelated compounds with different antibacterial modes of action (13, 15, 30–32). Most of the genes encoding these multidrug resistance (MDR) pumps are normal constituents of bacterial chromosomes. Some of these genes have a relatively high level of constitutive expression and confer so-called intrinsic resistance to antibiotics. Expression of other genes that confer an efflux capability is not detected in wild-type cells, but such genes can become expressed after the acquisition of regulatory mutations.
In gram-negative bacteria, most of the efflux pumps that contribute to both intrinsic and acquired resistance to clinically useful antibiotics are three-component structures that traverse both inner membranes and outer membranes. Each tripartite pump contains a transporter located in the cytoplasmic membrane, an outer membrane channel, and a periplasmic linker protein, which is thought to bring the other two components into contact (54, 55). This structural organization allows extrusion of substrates directly into the external medium, bypassing the periplasm. Direct efflux as a mechanism of drug extrusion is required since these rather slow tripartite MDR pumps (46) rely heavily on the help of the outer membrane, which serves as a permeability barrier for both hydrophobic and hydrophilic antibiotics (33).
Several classes of MDR pumps have been identified on the basis of sequence comparisons (42). Most of the inner membrane components of clinically relevant tripartite efflux pumps from gram-negative bacteria belong to a single class of transporters called resistance-nodulation-division (RND) efflux pumps (5).
Pseudomonas aeruginosa is an important opportunistic pathogen which is highly resistant to antibiotic therapy. Fluoroquinolones, β-lactams, and aminoglycosides are among the primary agents available for treatment of infections caused by this pathogen. Four multicomponent MDR RND efflux pumps have been identified in P. aeruginosa, namely, MexAB-OprM (39), MexCD-OprJ (38), MexEF-OprN (12), and MexXY-OprM (1). These pumps have somewhat overlapping spectra of antibiotic substrates. For example, all four pumps confer various degrees of resistance to fluoroquinolones, and mutants that overexpress three of these pumps have been isolated among fluoroquinolone-resistant bacteria in clinical settings: nalB mutants that overproduce the MexAB-OprM pump (3), nfxB mutants that overproduce MexCD-OprJ (53), and nfxC mutants that overproduce the MexEF-OprN efflux pump (6). So far, overexpression of MexXY-OprM has not been reported as a cause of fluoroquinolone resistance in P. aeruginosa; however, this pump has recently been implicated in low-level resistance to aminoglycosides (1, 50). Of these four pumps, only MexAB-OprM is expressed at a level sufficient to confer intrinsic MDR in wild-type cells. Deletion of the mexA, mexB, or oprM gene renders P. aeruginosa more susceptible to multiple antibiotics, including fluoroquinolones (8, 39, 51). Importantly, overexpression of the MexCD-OprJ or the MexEF-OprN efflux pumps restores resistance to fluoroquinolones in strains lacking the MexAB-OprM efflux pump (9, 14, 18).
Besides efflux, resistance to fluoroquinolones is also conferred by target mutations. These mutations mainly occur in quinolone resistance-determining regions (QRDRs) (11, 36, 52) in DNA gyrase (encoded by gyrA and gyrB) and topoisomerase IV (encoded by parC and parE) in many organisms including P. aeruginosa (26). Importantly, it has been demonstrated recently in P. aeruginosa (18) and Escherichia coli (35) that when both target-based and efflux-mediated resistance mechanisms are present in the same cell, they contribute independently to fluoroquinolone resistance. As a result, deletion of the MexAB-OprM efflux pump from a strain in which this pump is overexpressed resulted in a 64-fold reduction in the MIC of levofloxacin, regardless of the presence of additional resistance mechanisms. It was also demonstrated that deletion of all described pumps significantly reduces the frequency of emergence of fluoroquinolone-resistant mutant strains (18).
On the basis of these genetic data, it appears that inhibition of efflux pumps in P. aeruginosa may significantly improve the clinical performance of fluoroquinolones. Inhibition of efflux pumps is expected to (i) decrease the level of intrinsic resistance, (ii) significantly reverse acquired resistance, and (iii) decrease the frequency of emergence of P. aeruginosa mutants highly resistant to fluoroquinolones.
The same considerations can also be applied to gram-positive bacteria. Indeed, when reserpine (which was long ago identified as an inhibitor of the mammalian MDR pump, P-glycoprotein, and which was later found to inhibit MDRs from gram-positive bacteria) was added to the selection media, it suppressed the emergence of Staphylococcus aureus and Streptococcus pneumonia mutants resistant to ciprofloxacin (19, 20). Recently, several new inhibitors of the NorA pump from S. aureus which potentiated the activity of ciprofloxacin against both wild-type and NorA-overexpressing strains have been reported (21). These compounds decreased the MICs of ciprofloxacin up to fourfold (at concentrations ranging from 0.2 to 1.5 μg/ml) for strains of S. aureus overexpressing the NorA pump. Some of these compounds also inhibited efflux-mediated resistance in S. pneumoniae. Importantly, all compounds dramatically suppressed the frequency of emergence of ciprofloxacin-resistant strains when selection was performed with 1 μg of ciprofloxacin per ml (21).
The potential benefits of broad-spectrum efflux pump inhibitors (EPIs) prompted us to screen our synthetic compound and natural product libraries to search for inhibitors of the Mex pumps from P. aeruginosa. Recently, we have described one such inhibitor, MC-207,110, and presented a description of a portion of our efforts to optimize the biological and physiochemical properties of this lead compound (41). Here we present an additional characterization of MC-207,110: evidence of its mode of action as an inhibitor of efflux pump activity, its potentiating effect of the activity of levofloxacin against both laboratory strains with multiple target mutations and recent clinical isolates of P. aeruginosa, and its ability to decrease the emergence of P. aeruginosa strains with clinically relevant resistance to levofloxacin.
MATERIALS AND METHODS
Bacterial strains and media.
The laboratory bacterial strains used in this study are listed in Table 1. Bacterial cells were grown in L broth (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, 0.5% [wt/vol] NaCl) or L agar (L broth plus 1.5% agar) at 37°C. Clinical isolates of P. aeruginosa were collected from the United States, Canada, England, and France between 1995 and 1998. Levofloxacin was synthesized at Daiichi Pharmaceutical Co., Ltd. (Tokyo, Japan). Nitrocefin was purchased from Calbiochem-Behring (La Jolla, Calif.). All other antibiotics as well as N-phenylnaphthylamine (NPN) and polymyxin B nonapeptide (PMBN) were purchased from Sigma Chemical Co. (St. Louis, Mo). MC-207,110 was from the synthetic compound library of Microcide Pharmaceuticals, Inc. MC-002,595, which is a close analog of MC-207,110, and MC-005,556 (alanine β-naphthylamide [Ala-Nap]) were synthesized at Microcide Pharmaceuticals, Inc.
TABLE 1.
Laboratory strains and plasmid used in this study
| Strain or plasmid | Genotype or descriptiona | Source or reference |
|---|---|---|
| P. aeruginosa | ||
| PAM1020 | PAO1 prototroph | 18 |
| PAM1032 | nalB (mexAB-oprM is overexpressed) | 18 |
| PAM1033 | nfxB (mexCD-oprJ is overexpressed) | 18 |
| PAM1034 | nfxC (mexEF-oprN is overexpressed) | 18 |
| PAM2381 | PAM1020/pAGH97 (mexXY) | This study |
| PAM1154 | oprM::ΩHg | 18 |
| PAM1561 | ΔmexAB-oprM::Cm ΔmexCD-oprJ::Gm | 18 |
| PAM1753 | nfxC ΔmexAB-oprM::Cm ΔmexCD-oprJ::Gm | This study |
| PAM1624 | ΔmexCD-oprJ::Gm ΔmexEF-oprN::ΩHg | 18 |
| PAM1723 | nalB ΔmexCD-oprJ::Gm ΔmexEF-oprN::ΩHg | This study |
| PAM1625 | ΔmexAB-oprM::Cm ΔmexEF-oprN::ΩHg | 18 |
| PAM1738 | nfxB ΔmexAB-oprM::Cm ΔmexEF-oprN::ΩHg | This study |
| PAM1626 | ΔmexAB-oprM::Cm ΔmexEF-oprN::ΩHg ΔmexCD-oprJ::Gm | 18 |
| PAM1548 | gyrA (Thr83→Ile) | 18 |
| PAM1573 | nalB gyrA (Thr83→Ile) | 18 |
| PAM1491 | nfxC gyrA (Asp87→Tyr) | 18 |
| PAM1582 | nalB gyrA (Thr83→Ile) parC (Ser87→Leu) | 18 |
| PAM1609 | nalB gyrA (Thr83→Ile) parC (Ser87→Leu) gyrA (Asp87→Tyr) | 18 |
| PAM1667 | gyrA (Thr83→Ile) parC (Ser87→Leu) mexA-phoA::Tc | 18 |
| PAM1669 | gyrA (Thr83→Ile) parC (Ser87→Leu) gyrA (Asp87→Tyr) mexA-phoA::Tc | 18 |
| PAM1619 | nalB gyrA (Thr83→Ile) parC (Ser87→Leu) Xb | 18 |
| PAM2005 | nalB (β-lactamase is fully expressed) | This study |
| PAM2035 | mexA::Tc (β-lactamase is fully expressed) | This study |
| E. coli | ||
| ATCC 25922 | K12 prototroph | Microcide collection |
| ECM1642 | marR1642 | 14 |
| ECM1668 | marR1642 ΔacrAB::Km | 14 |
| Plasmid pAGH97 | Ap; pAK1900 containing the mexXY operon | 1 |
ΩHg, Hg resistance-determining derivative of interposon Ω; Cm, chloramphenicol resistance; Gm, gentamicin resistance; Tc, tetracycline resistance; Km, kanamycin resistance; Ap, ampicillin resistance.
Mutation is located outside of QRDRs of gyrA and parC.
Construction of P. aeruginosa strains overexpressing a single Mex pump.
The mutants overexpressing each of the three Mex pumps individually (MexAB-OprM, MexCD-OprJ, or MexEF-OprN) were selected on L-agar plates containing levofloxacin at 1 μg/ml from strains lacking the two other pumps. The frequency of resistance was determined as the ratio of the number of CFU per milliliter appearing after overnight incubation on antibiotic-containing L-broth or L-agar plates versus the number of CFU per milliliter appearing after overnight incubation on antibiotic-free L-broth or L-agar plates.
MICs.
MIC determinations were carried out in 96-well microtiter plates by a twofold standard broth microdilution method (27) in Muller-Hinton broth (Difco) either with or without fixed concentrations of MC-207,110. The inocula in all experiments were 104 to 105 cells/ml.
Checkerboard titration assays.
Interactions between levofloxacin and MC-207,110 were assessed by a checkerboard titration assay. Levofloxacin was tested at 11 concentrations (0.008 to 8 μg/ml), while MC-207,110 was tested at 7 concentrations (0.06 to 40 μg/ml). Levofloxacin was dispensed alone in the first row and was combined with MC-207,110 in the remaining rows. MC-207,110 was also dispensed alone in the first column. Drug interactions were classified as synergistic if the fractional inhibitory concentration (FIC) index was <0.5. The FIC index is the sum of the FIC of each of the drugs, which in turn is defined as the MIC of each drug when used in combination divided by the MIC of the drug when used alone.
MC-005,556 (Ala-Nap) uptake assay.
Ala-Nap, which is not fluorescent in solution, is cleaved enzymatically inside the cells to produce highly fluorescent β-naphthylamine. The more Ala-Nap that enters the cells, the more fluorescence that is produced. Importantly, Ala-Nap is a substrate of the Mex pumps from P. aeruginosa as well as the AcrAB-TolC pump from E. coli (see Results). To assess the uptake of Ala-Nap, cultures of P. aeruginosa were grown to an optical density at 600 nm (OD600) of ∼1, washed, and resuspended in buffer at pH 7.0 containing K2HPO4 (50 mM), MgSO4 (1 mM), and glucose (0.4%) (buffer A). For treatment with carbonyl cyanide m-chlorophenylhydrazone (CCCP), the cells were resuspended in buffer A without glucose and mixed with 0.5 mM CCCP, and the mixture was incubated for 15 min. After treatment, the cells were washed with buffer A without glucose at least three times. Assays were performed in 96-well flat-bottom black plates (Applied Scientific or Costar) in a final volume of 200 μl and were initiated by the addition of Ala-Nap to suspensions of intact cells to a final concentration of 64 μg/ml. Fluorescence was measured on an fMAX spectrofluorometer (Molecular Devices) with excitation of 320 nm and emission of 460 nm. To measure the effects of the EPI or PMBN on the rate of Ala-Nap uptake, the cells were preincubated with different concentrations of these compounds prior to Ala-Nap addition.
NPN efflux assays.
NPN is an uncharged lipophilic molecule which fluoresces weakly in aqueous environments but which becomes strongly fluorescent in nonpolar environments such as the lipid bilayers of biological membranes. NPN is also a substrate of RND efflux pumps (34). Cultures of E. coli were grown in L broth to an OD600 of ∼1, washed, and resuspended in buffer A without glucose. Cells were treated with CCCP (100 μM), washed, and then loaded with NPN at 9 μM for 10 min. NPN efflux was initiated by the addition of glucose and was recorded as the decay in fluorescence. Assays were performed in 96-well spectrofluorometer cuvettes in a final volume of 200 μl. When stated, the EPI was added to the cells together with glucose.
Nitrocefin uptake assay.
Cells of P. aeruginosa (PAM2005 or PAM2035) with constitutive expression of chromosomal β-lactamase were grown overnight in L broth, harvested, washed in Mg2+-free buffer A, and resuspended in the same buffer at an OD600 of ∼0.5. To 100 μl of the cell suspension, 50 μl of either PMBN or MC-207,110 was added to give a final concentration of 1 to 128 μg/ml. Next, 50 μl of nitrocefin was added to give a final concentration of 32 μg/ml. Hydrolysis of nitrocefin was monitored spectrophotometrically by measurement of the increase in absorbance at 490 nm. Assays were performed in 96-well plates in a SpectraMAX Plus spectrophotometer (Molecular Devices).
31P NMR assay.
31P nuclear magnetic resonance (NMR) measurements were conducted to probe the intracellular pH of E. coli cells under different conditions. Assignments of resonance were based upon previously published in vivo NMR studies of E. coli (2, 48). A total of 1,000 ml of E. coli ATCC 25922 cells was grown to the mid-logarithmic phase (OD600, ∼1) in L broth. The cells were harvested (centrifugation at 3,000 × g for 10 min at 4°C) and washed twice with ice-cold phosphate-buffered saline buffer (pH 6.5). The cell pellet (7.5 ml) was resuspended in an equal volume of the following buffer at pH 6.5: KH2PO4 (2.5 mM), K2HPO4 (2.5 mM), morpholineethanesulfonic acid (100 mM), and NaCl (85 mM). The cells were kept on ice until needed (less than 2 h). Before use, the cells were allowed to reach room temperature. One milliliter of cell suspension was placed in a 5-mm NMR tube. The field was shimmed by using proton free induction decays. A total of 20 μl of 50% glucose was added to the cell suspension. When stated, 20 μl of either 20 mM MC-207,110 or CCCP was added to give a final concentration of 0.4 mM. 31P NMR spectra were obtained at 161.88 MHz. Free induction decays were accumulated at 5-min intervals for 30 min.
RESULTS
Screening for EPIs and criteria for efflux pump inhibitory mode of action of screening hits.
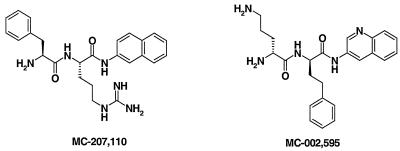
To identify potential efflux pump inhibitors, the growth inhibitory activity of synthetic compounds and natural products extracts was assayed using strains of P. aeruginosa overexpressing each of the three efflux pumps: MexAB-OprM (PAM1032), MexCD-OprJ (PAM1033), and MexEF-OprN (PAM1034) that were grown with and without subinhibitory concentrations of levofloxacin (47). Compounds or extracts that inhibited the growth of all three strains only in the presence of levofloxacin were chosen for follow-up assays to discriminate between compounds with false-positive results and true broad-spectrum EPIs. To qualify as EPIs, hit compounds had to satisfy several criteria: (i) must enhance the activities of levofloxacin and other antibiotics that are effluxed in strains containing functioning pumps, (ii) must not significantly potentiate the activities of antibiotics in a strain that lacks efflux pumps, (iii) must not potentiate the activities of antibiotics that are not effluxed, (iv) must increase the level of accumulation and decrease the level of extrusion of efflux pump substrates, and (iv) must not affect the proton gradient across the inner membrane. MC-207,110 (Fig. 1) was among the first efflux pump inhibitors identified in our screening efforts.
FIG. 1.
Efflux pump inhibitors used in this study.
MC-207,110 series satisfies microbiological criteria for EPIs.
To assess the interaction between levofloxacin and MC-207,110, we performed standard checkerboard assays in which the MICs of levofloxacin were determined in the presence of different concentrations of MC-207,110 (Table 2). While MC-207,110 itself was devoid of significant antibacterial activity (MICs, 64 or >512 μg/ml for the strains lacking or overexpressing efflux pumps, respectively), it increased the susceptibilities of the three pump-overexpressing mutants of P. aeruginosa to levofloxacin 64-fold. MC-207,110 also potentiated the activity of levofloxacin against strain PAM2391 containing plasmid pAGH97 (a gift from P. Plesiat) with the mexXY genes and against wild-type strain PAM1020. The latter result was expected since constitutive expression of the mexAB-oprM operon contributes to the intrinsic resistance to levofloxacin in this strain. Interactions between MC-207,110 and levofloxacin were clearly synergistic for all the strains expressing efflux pumps, with FIC indices ranging from 0.05 to 0.14. No synergy was observed against strain PAM1626, which lacks all known Mex pumps.
TABLE 2.
Synergistic in vitro activity of MC-207,110 combined with levofloxacin against the wild-type strain of P. aeruginosa and the strains overexpressing known efflux pumps
| Strain | Genotype | MC-207,110 MIC (μg/ml) | Levofloxacin MIC (μg/ml) in the presence of MC-207,110 at concn (μg/ml) of:
|
Emaxa | EC50b | FICindexc | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.625 | 1.25 | 2.5 | 5 | 10 | 20 | 40 | ||||||
| PAM1020 | Wild type | >512 | 0.125 | 0.125 | 0.125 | 0.125 | 0.03 | 0.015 | 0.008 | 0.008 | 16 | 5 | 0.140 |
| PAM1032 | nalB | >512 | 2 | 2 | 2 | 1 | 1 | 0.125 | 0.03 | 0.03 | 64 | 10 | 0.082 |
| PAM1033 | nfxB | >512 | 2 | 2 | 2 | 2 | 0.5 | 0.125 | 0.06 | 0.03 | 64 | 10 | 0.082 |
| PAM1034 | nfxC | >512 | 4 | 4 | 4 | 4 | 1 | 0.25 | 0.06 | 0.06 | 64 | 10 | 0.082 |
| PAM2391 | pMexXY | >512 | 0.25 | 0.25 | 0.25 | 0.25 | 0.03 | 0.008 | 0.008 | 0.008 | 32 | 5 | 0.052 |
| PAM1626 | ΔmexAB-oprM ΔmexCD-oprJ ΔmexEF-oprN | 64 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.015 | 0.008 | 0.008 | 2 | NDd | 1.156 |
Emax, ratio between the MIC without EPI and the MIC in the presence of a maximal potentiating concentration of EPI.
EC50, concentration of EPI at which a half potentiating effect is achieved.
To calculate the FIC index for the wild type and the pump overexpressing strains, 513 μg/ml was used as the MIC of MC-207,110.
ND, not determined.
MC-207,110 also had an effect on susceptibilities to other antibiotics that are substrates of efflux pumps (Table 3). In fact, the effect of MC-207,110 on the susceptibility of MexAB-OprM-overproducing strain PAM1032 to several antibiotics was comparable to the effect of inactivation of this pump by deleting oprM (PAM1154). Surprisingly, however, MC-207,110 did not potentiate the activities of all antibiotic substrates of the MexAB-OprM efflux pump to the same extent: at the concentration at which it completely reversed pump-mediated resistance to levofloxacin or erythromycin, it had only a very weak effect on susceptibility to carbenicillin and did not have any effect on susceptibility to ethidium bromide. The MICs of imipenem and gentamicin, which are not substrates of MexAB-OprM, were not affected by MC-207,110, as would be expected for an efflux pump inhibitor. Finally, this compound had little to no potentiating activity against the strains that lack efflux pumps (Tables 2 and 3). Thus, MC-207,110 generally satisfied the microbiological criteria for the EPI mode of action.
TABLE 3.
Comparison of effect of pump deletion with effect of the EPI MC-207,110 on susceptibility to various antibiotics
| Antibiotic | MIC ratio
|
||
|---|---|---|---|
| With or without MexAB-OprM for PAM1032 and PAM1154 | With or without MC-207,110 (20 μg/ml) for
|
||
| PAM1032 | PAM1154 | ||
| Levofloxacin | 64 | 32 | 2 |
| Chloramphenicol | 512 | 128 | 2 |
| Erythromycin | 32 | 32 | 4 |
| Sparfloxacin | 32 | 128 | 4 |
| Carbenicillin | 512 | 4 | 2 |
| Tetracycline | 256 | 8 | 2 |
| Ethidium bromide | 32 | 1 | 2 |
| Gentamicin | 1 | 1 | 1 |
| Imipenem | 1 | 1 | 1 |
MC-207,110 series increases levels of accumulation of efflux pump substrates inside the cell.
If MC-207,110 is an efflux pump inhibitor it should increase the level of accumulation of efflux pump substrates inside the cell. We (as well as others) have not been able to demonstrate directly, using several hydrophobic fluorescent dyes, efflux by Mex pumps from P. aeruginosa in membrane vesicles, probably due to the low levels of activity of pumps that rely on the outer membrane. Because of this, accumulation and efflux experiments had to be performed with intact cells. The interpretations of these assays could be complicated by the fact that both efflux pump inhibitors and permeabilizers of the outer membrane of P. aeruginosa may have similar effects on the accumulation of at least some test compounds (7, 16). To avoid such complications, we sought to identify a compound with the following features: (i) it is a substrate of efflux pumps, (ii) it is easily detected inside the cell, and (iii) its permeation is not restricted by the outer membrane in P. aeruginosa. If MC-207,110 increased the level of accumulation of such a compound, it could be interpreted to be a consequence only of inhibition of efflux.
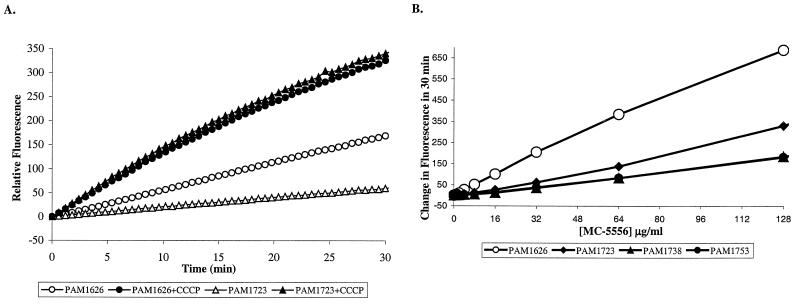
MC-005,556 (Ala-Nap), synthesized at Microcide, satisfied all criteria for such a test compound. Ala-Nap, which is not fluorescent in solution, is cleaved enzymatically inside the cells to produce the highly fluorescent compound β-naphthylamine. The fluorescence of β-naphthylamine itself is not quenched by the cells and is not affected by the presence or absence of efflux pumps (data not shown). The rate of production of β-naphthylamine (recorded as an increase in fluorescence) is limited by the rate of entry of Ala-Nap into the cell: cell extracts prepared from PAM1723 (overexpressing the MexAB-OprM) produced β-naphthylamine at an approximately fivefold higher rate than intact cells of the same strain (data not shown). The rate of cleavage of Ala-Nap was much higher in PAM1626 than in strains overexpressing an efflux pump, such as PAM1723. Importantly, the cells of both strains (when treated with CCCP to inhibit active efflux) cleaved Ala-Nap at the same rate (Fig. 2A). These data indicate that Ala-Nap is a substrate of the MexAB-OprM efflux pump. (The observation of an increase in the level of accumulation of Ala-Nap in the presence of CCCP in the strain from which the pump was deleted does not necessarily imply the presence of an additional pump in this strain. Since Ala-Nap is a weak base with a pKa of 8.2, the abolishment of the pH gradient across the membrane with CCCP leads to the spontaneous influx of Ala-Nap into the cell.) In analogous experiments it was demonstrated that Ala-Nap is also a substrate of the MexCD-OprJ and the MexEF-OprN pumps from P. aeruginosa (Fig. 2B) as well as the close homolog from E. coli, the AcrAB-TolC pump (data not shown). We then studied the uptake of Ala-Nap by PAM1723 cells in the presence of various concentrations (1 to 128 μg/ml) of PMBN, which is a polymyxin B derivative lacking the fatty acid moiety of the parent compound. PMBN is a potent permeabilizer of the outer membrane in P. aeruginosa (49). Assays were performed both in the presence and in the absence of Mg2+, which inhibits the activity of PMBN (37). In PAM1723 cells, PMBN did not increase the rate of conversion (or uptake) of Ala-Nap, even in Mg2+-free buffer (data not shown). The same result was obtained with cells of PAM1626 lacking efflux pumps (data not shown). Thus, Ala-Nap was an appropriate substrate for use in the assessment of the broad-spectrum efflux pump inhibitory activities of our lead compounds. It is also noteworthy that this particular compound apparently is a very good substrate for the efflux pumps since no help from the outer membrane was needed to demonstrate an effect of the efflux pump on its accumulation kinetics.
FIG. 2.
(A) A linear increase in fluorescence with time due to intracellular hydrolysis of MC-005,556 (Ala-Nap) is seen when Ala-Nap (32 μg/ml) is added to intact cells of P. aeruginosa. Open and filled triangles, cells of PAM1723 that overexpress MexAB-OprM and that were not treated or treated with CCCP, respectively; open and filled circles, cells of PAM1626 (the pump deletion mutant) that were not treated or treated with CCCP, respectively. Much less fluorescence is produced in the case of PAM1723 compared to the amount produced in the case of PAM1626, apparently due to MexAB-OprM-mediated efflux of Ala-Nap. As a result of CCCP treatment, efflux is abolished and both cell types produce the same amount of fluorescence. (B) The relationship between the change in fluorescence and the concentration of Ala-Nap for different cell types was determined. The amount of fluorescence produced by intracellular Ala-Nap hydrolysis in 30 min versus the concentration of Ala-Nap used is shown. Differential rates of uptake of Ala-Nap between cells overexpressing each of the three efflux pumps (MexAB-OprM [filled diamonds], MexCD-OprJ [filled triangles], MexEF-OprN [filled circles]) and the cells of PAM1626 (open circles) are seen over a wide range of Ala-Nap concentrations. The linear increase in fluorescence in PAM1626 in the presence of up to 128 μg of externally added Ala-Nap per ml indicates that the protease hydrolyzing Ala-Nap is not yet saturated at this concentration.
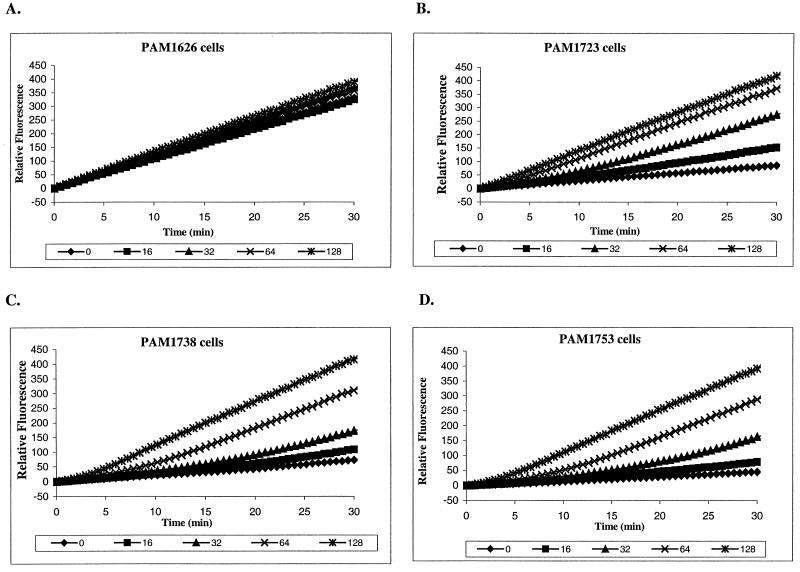
For the Ala-Nap uptake experiments we used MC-002,595 (Fig. 1), a close analog of MC-207,110 with microbiological activity similar to that of MC-207,110. MC-207,110 itself was not used since its own fluorescence interfered with the assay. Unlike PMBN, MC-002,595 increased the level of uptake of Ala-Nap in strains overexpressing each of the three efflux pumps in a dose-dependent manner (Fig. 3B to D). MC-002,595 also increased the level of uptake of Ala-Nap into cells of E. coli strain ECM1642, which overproduces AcrAB-TolC (data not shown). MC-002,595 showed no such effect for PAM1626 (Fig. 3A) or ECM1668 (ΔacrAB::Km, data not shown) or when the pump-overexpressing strains of P. aeruginosa or E. coli were treated with CCCP (data not shown).
FIG. 3.
An increase in the rates of fluorescence production due to hydrolysis of MC-055,556 (64 μg/ml) by intact cells of P. aeruginosa overexpressing Mex pumps is seen in the presence of different concentrations (in micrograms per milliliter, as indicated below each panel) of MC-002,595, a close analog of MC-207,110. No effect of compound was observed for cells of PAM1626. (A) PAM1626 lacking three Mex pumps; (B) PAM1723 overexpressing MexAB-OprM; (C) PAM1738 overexpressing MexCD-OprJ; (D) PAM1753 overexpressing MexEF-OprN.
MC-207,110 series inhibits efflux of NPN.
To evaluate further the modes of action of the MC-207,110 series of compounds, we studied the efflux of NPN by E. coli in the presence of MC-002,595 (to demonstrate an effect with a pump substrate that is structurally very dissimilar). Cells of E. coli either overexpressing (ECM1642) or lacking (ECM1668) the AcrAB-TolC efflux pump were treated with CCCP and loaded with NPN. When bound to the membranes, NPN becomes intensely fluorescent. Its efflux can be followed in real time as a decay of fluorescence after energization of the cells. Indeed, after the addition of glucose, efflux of NPN was observed in the case of ECM1642. Importantly, when MC-002,595 was added to the cells prior to the addition of glucose, it inhibited efflux in a dose-dependent manner (Fig. 4A). In fact, efflux was completely abolished with the EPI at 128 μg/ml. Apparent noncomplete inhibition is due to the quenching of NPN fluorescence by MC-002,595 (Fig. 4B). PMBN, which strongly increases the level of accumulation of NPN even in the presence of 1 mM Mg2+ (data not shown), did not have any effect on its efflux (data not shown).
FIG. 4.
(A) Cells of E. coli ECM1642 overexpressing AcrAB-TolC were treated with CCCP at 100 μM and preloaded with 9 μM NPN for 10 min. Extrusion was initiated by the addition of either glucose alone (filled squares) or by the addition of glucose mixed with different concentrations of MC-002,595 to give final concentrations of 16 to 128 μg/ml. Extrusion is visualized as the decay of NPN fluorescence in the presence of glucose versus that for the control (no glucose added) (open boxes). MC-002,595 inhibits extrusion of NPN in a dose-dependent manner. (B) Glucose alone or in combination with 128 μg of MC-002,595 per ml is added to CCCP-treated, NPN-loaded cells of ECM1668 (in which AcrAB-TolC is nonfunctional) or ECM1642. The similar level of NPN fluorescence in both cell types after MC-002,595 addition indicates complete inhibition of the AcrAB-TolC-mediated efflux of NPN. a.u., arbitrary units.
MC-207,110 does not affect the proton gradient across the inner membrane.
RND efflux pumps use the energy of the proton gradient across the inner membrane for drug extrusion: drug efflux is accompanied by the influx of protons (55). Compounds that disrupt the proton gradient may appear as efflux pump inhibitors. Therefore, it was important to demonstrate that the mode of action of MC-207,110 was not based on such disruption. To assess the effects of MC-207,110 on the proton gradient we used the method of determining the change in pH (ΔpH) based on measurement of the NMR spectrum of inorganic phosphate (31P), which is a pH-sensitive NMR probe. This particular method was chosen because many procedures of measuring ΔpH rely on the accumulation of pH-sensitive fluorescent probes [for example, various acridines or 2′,7′-bis-(2-carboxyethyl)-5 (and -6-)-carboxyfluorescein]. Many if not all of these compounds are substrates of efflux pumps. Consequently, it would be difficult to discriminate between the efflux pump inhibitory activity of the compound and its effect on the proton gradient.
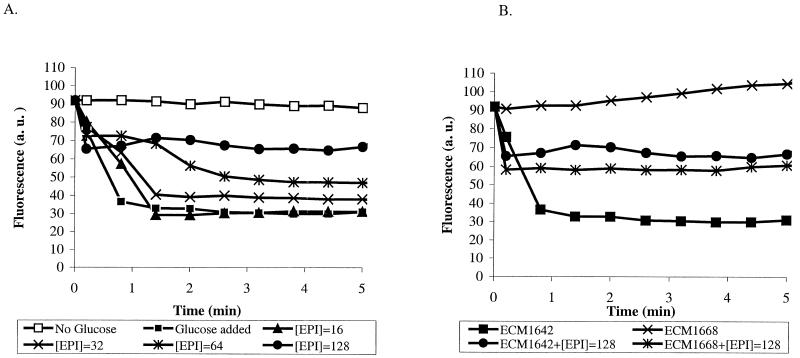
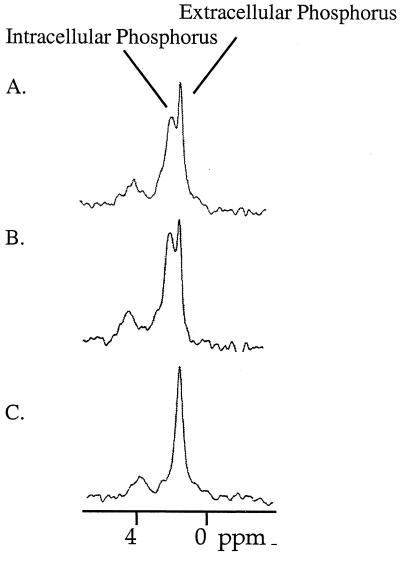
The results of the NMR experiment are presented in Fig. 5. When cells were resuspended in glucose-containing buffer, two peaks of inorganic phosphate (one corresponding to the internal pH and another corresponding to the external pH) were observed, indicating that the membrane was energized and able to maintain a pH gradient. When CCCP (0.4 mM) was added to the cell suspension, only one peak was observed throughout the experiment (even after 30 min), indicating that the cells were no longer able to generate a proton gradient across the inner membrane. At the same time, addition of MC-207,110 (0.4 mM, a concentration that is 10-fold higher than the EPI's potentiating concentration) neither abolished nor altered the magnitude of the pH gradient, as was evident by the detection of two 31P NMR peaks. The same results were obtained for MC-002,595 (data not shown). Therefore, the mode of action of the MC-207,110 series was not based on deenergization of an efflux pump.
FIG. 5.
The pH gradient is visualized as two peaks of the 31P NMR spectrum corresponding to intracellular and extracellular phosphorus, respectively. (A) Glucose alone; (B) glucose and MC-207,110 (0.4 mM); (C) glucose and CCCP (0.4 mM). The addition of MC-207,110 did not affect the transmembrane proton gradient.
On the basis of the results of the experiments described above, we concluded that MC-207,110 is indeed an inhibitor of the Mex pumps from P. aeruginosa and the AcrAB-TolC pump from E. coli.
MC-207,110 series has an additional mode of action.
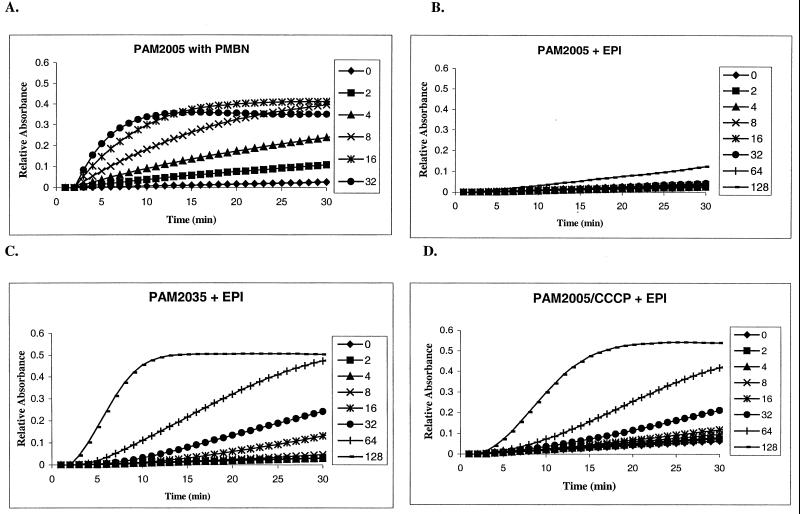
We wanted to know whether MC-207,110, besides efflux pump inhibition, might have an additional mode of action, namely, the ability to permeabilize the outer membrane. This possibility appeared to be feasible on the basis of the structure of MC-207,110, which is a dipeptide amide (41), with two positive charges at physiological pH (pKa1, ∼10.5; pKa2, ∼8.0). The outer membrane-permeabilizing effect of MC-207,110 at a wide range of concentrations was assessed by examining the rates of hydrolysis of a chromogenic β-lactam, nitrocefin, by intact cells of P. aeruginosa. An increased rate of hydrolysis in intact cells is indicative of increased permeation of nitrocefin to the periplasmic β-lactamase, since the rate of hydrolysis is limited by the rate of diffusion across the outer membrane (10, 56). For these experiments we used two strains of P. aeruginosa, PAM2005 and PAM2035. Both strains constitutively produce the β-lactamase AmpC, encoded by the corresponding gene normally present in the genome of this bacterium. PAM2005 also overproduces the MexAB-OprM efflux pump, while in PAM2035 this pump is nonfunctional due to insertional inactivation of the gene mexA. PMBN was used as a positive control, having a strong effect on the outer membrane permeability of PAM2005 (50% inhibitory concentration, ∼8 μg/ml), (Fig. 6A), as well as on that of PAM2035 (data not shown). In contrast, MC-207,110 did not have a significant effect on the outer membrane permeability of PAM2005 when it was tested over a wide concentration range (1 to 128 μg/ml) (Fig. 6B). However, it increased the permeability of the outer membrane of PAM2035, which lacks the functional MexAB-OprM efflux pump (albeit with less potency than PMBN [50% inhibitory concentration, ∼70 μg/ml]), and of that of PAM2005 when the strain was treated with CCCP (Fig. 6C and D). The permeabilizing effects of both PMBN and MC-207,110 were completely abolished by the addition of 1 mM Mg2+ (data not shown). Results that were similar both qualitatively and quantitatively were obtained for MC-002,595 (data not shown). In conclusion, it appears that the MC-207,110 series is capable of altering the permeability of the outer membrane in P. aeruginosa, but only in the absence of a functional MexAB-OprM efflux pump.
FIG. 6.
The outer membrane-permeabilizing activity of either PMBN or EPI is visualized as an increase in initial rates of nitrocefin hydrolysis by intact cells of P. aeruginosa in the presence of increasing concentrations (in micrograms per milliliter, as indicated beside each panel) of these compounds. PMBN was used at 2 to 32 μg/ml, and MC-207,110 was used at 2 to 128 μg/ml. Nitrocefin hydrolysis was monitored over time by monitoring the increase in absorbance at 490 nm. (A) The effect of PMBN was studied with PAM2005; (B) the effect of MC-207,110 was studied with PAM2005; (C) the effect of MC-207,110 on intact PAM2035 was studied; (D) the effect of MC-207,110 on CCCP-treated PAM2005 was studied.
MC-207,110 enhances the activity of levofloxacin against laboratory strains with multiple target mutations.
We demonstrated previously that the deletion of efflux pump genes increased susceptibility to fluoroquinolones even in strains containing mutations in genes encoding the fluoroquinolone targets gyrAB and parCE (18). Efflux pump inhibitors were expected to have a similar effect. To test whether this was indeed the case, the MICs of levofloxacin were determined for a series of isogenic laboratory mutants in the presence or absence of MC-207,110 at 20 μg/ml. The results are presented in Table 4. MC-207,110 enhanced the activity of levofloxacin against all strains containing a functional efflux pump (in this case, MexAB-OprM), regardless of the presence of one or even multiple target mutations. The magnitude of the potentiating effect was determined exclusively by the level of expression of the MexAB-OprM pump: an 8-fold decrease in the levofloxacin MIC for all strains expressing the mexAB-oprM operon at the wild-type level and a 32-fold decrease in the levofloxacin MIC for those strains overexpressing this operon.
TABLE 4.
Effect of MC-207,110 on susceptibility to levofloxacin in the strains containing the target-based mutations
| Strain | Genotype | MexAB-OprM status | Levofloxacin MIC (μg/ml) with:
|
|
|---|---|---|---|---|
| No addition | MC-207110 at 20 μg/ml | |||
| PAM1020 | Wild type | Wild type | 0.125 | 0.008 |
| PAM1548 | gyrA (Thr83→Ile) | Wild type | 1 | 0.125 |
| PAM1032 | nalB | Overexpressed | 2 | 0.03 |
| PAM1573 | nalB gyrA (Thr83→Ile) | Overexpressed | 8 | 0.25 |
| PAM1582 | nalB gyrA (Thr83→Ile) parC (Ser87→Leu) | Overexpressed | 32 | 1 |
| PAM1667 | mexA-phoA::Tc gyrA (Thr83→Ile) parC (Ser87→Leu) | Wild type | 4 | 0.25 |
| PAM1609 | nalB gyrA (Thr83→Ile) parC (Ser87→Leu) gyrA (Asp87→Tyr) | Overexpressed | 128 | 4 |
| PAM1669 | mexA-phoA::Tc gyrA (Thr83→Ile) parC (Ser87→Leu) gyrA (Asp87→Tyr) | Wild type | 8 | 1 |
| PAM1619 | nalB gyrA (Thr83→Ile) parC (Ser87→Leu) X | Overexpressed | 1,024 | 32 |
MC-207,110 enhances the activity of levofloxacin against clinical isolates of P. aeruginosa.
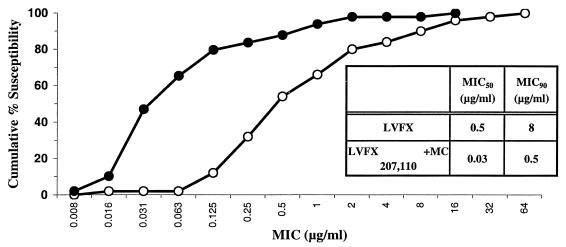
Fifty recent strains of P. aeruginosa for which the levofloxacin MIC range was wide (0.008 to >128 μg/ml) and that were collected from the United States, Canada, England, and France were used to determine whether MC-207,110 enhances the activity of levofloxacin. MC-207,110 was tested at a fixed concentration of 10 μg/ml. MC-207,110 decreased both the MIC at which 50% of strains are inhibited (MIC50) and the MIC90 of levofloxacin by ca. 16-fold (Fig. 7). The nearly parallel character of the curves constituting the cumulative susceptibility to levofloxacin with or without MC-207,110 indicates that this compound enhanced the activity of the antibiotic irrespective of the level of resistance of individual isolates. These results are in good accordance with the data reported above for laboratory mutants with multiple mechanisms of levofloxacin resistance.
FIG. 7.
The activity of the combination of MC-207,110 with levofloxacin against 50 clinical isolates of P. aeruginosa was determined with a fixed concentration of MC-207,110 (10 μg/ml). Open circles, levofloxacin alone; filled circles, levofloxacin in combination with EPI.
MC-207,110 decreases the emergence of P. aeruginosa strains with clinically relevant resistance to levofloxacin.
We have previously demonstrated (18) that deletion of known efflux pumps in P. aeruginosa significantly decreased the frequency of emergence of resistant variants when selection with the deletion mutant was performed with 1 μg of levofloxacin per ml (strains of P. aeruginosa for which the levofloxacin MIC is 2 μg/ml or higher are considered to be clinically resistant). On the basis of these data we have predicted that an efflux pump inhibitor should also affect the frequency of emergence of resistant bacteria. To test this prediction we performed selection experiments by plating cells of wild-type strain PAM1020 (MIC, 0.125 μg/ml) onto L-agar plates containing either levofloxacin at 1 μg/ml (eight times the MIC) or levofloxacin at 1 μg/ml in combination with MC-207,110 at 20 μg/ml. When the selection was performed with levofloxacin alone, the frequency of resistant variants was as high as 10−7 CFU/ml. However, when MC-207,110 was added to the selective medium, the frequency of emergence of strains with a clinically relevant level of resistance was less than 10−11 CFU/ml.
DISCUSSION
Inhibition of efflux pumps appears to be an attractive approach to improvement of the clinical efficacies of antibiotics that are substrates of such pumps. However, it is important to properly identify the antibiotics and target bacteria for which this approach would be the most productive. One should consider the prevalence of efflux-mediated resistance, other mechanisms besides efflux that contribute to resistance to a particular antibiotic, and interactions between different mechanisms of resistance. For example, inhibition of the transposon-encoded macrolide-specific efflux pumps MefA and MefE (4, 45) from gram-positive bacteria will enhance the efficacies of macrolides, but only against a population of resistant organisms containing the mef genes. Inhibitors of the predominant transporters TetA and TetB in gram-negative bacteria would reverse one type of tetracycline resistance. Such inhibitors have been identified among certain semisynthetic tetracycline analogs (28, 29). On the other hand, inhibition of a single RND MDR pump in Haemophilus influenzae that is encoded by a constitutively expressed housekeeping gene (43) should make the entire population of bacteria more susceptible to macrolides such as azithromycin or clarithromycin.
In P. aeruginosa, inhibitors of the MexAB-OprM efflux pump will enhance the activities of a variety of β-lactams (17, 24, 51), but only in strains that do not express high levels of β-lactamase (22, 25). In this important and clinically refractory organism, the efflux pump inhibitor approach appears to be particularly compelling when it is applied to fluoroquinolones.
Constitutively expressed efflux pumps contribute to intrinsic resistance to fluoroquinolones. The high-level acquired resistance is achieved due to independent contributions by both increased efflux and target modification. Therefore, efflux pump inhibitors are expected to enhance the activities of fluoroquinolones even against strains with target-based mutations (18). All of the Mex pumps from P. aeruginosa that confer resistance to fluoroquinolones belong to the RND family and are similar at the protein level. Fluoroquinolone pumps from other gram-negative bacteria also belong to the same family of transporters. This increases the possibility that a single inhibitor might be active against multiple efflux pumps.
In the study described in this report we expanded the characterization of the first inhibitor of multiple RND transporters from P. aeruginosa, MC-207,110, which was discovered as such by empiric screening of a small molecule library (41). The mode of action of efflux pump inhibition of MC-207,110 was confirmed in a series of microbiological assays. The activity of MC-207,110 was shown to be synergistic with that of levofloxacin when they were tested either against the wild-type strain or against strains overexpressing each of the known efflux pumps. MC-207,110 also potentiated the activities of other antibiotics that are substrates of the MexAB-OprM pump but did not potentiate the activities of antibiotics that are not. MC-207,110 did not have significant activity against strains lacking known efflux pumps. Interestingly, the magnitude of the effect of MC-207,110 was strongly dependent on the nature of a particular substrate, perhaps indicating that different antibiotics may have different binding sites on the pump and that inhibition by MC-207,110 is binding site specific. The possibility of multiple substrate binding sites was recently demonstrated for several MDR pumps (23, 40). According to this interpretation, when the pump is exposed to MC-207,110, it can still extrude some, but not all, substrates. Furthermore, MC-207,110 itself may be efficiently extruded by efflux pumps. Indeed, the Mex pumps conferred resistance to the weak antibacterial effect of MC-207,110: 64 or >512 μg of MC-207,110 per ml was needed to inhibit the growth of triple-pump-deletion strain PAM1626 or Mex pump-overexpressing strains, respectively (Table 2). It is noteworthy that many compounds that enhance the activities of anticancer agents against mammalian cells overexpressing the MDR pump P-glycoprotein have been shown to be efficiently extruded by this pump (44).
Additional confirmation that the mode of action of the MC-207,110 series is as an EPI was obtained in accumulation and efflux assays. The EPI increased the level of accumulation of the efflux pump substrate Ala-Nap inside cells of P. aeruginosa and decreased the level of efflux of another substrate, NPN, out of NPN-loaded cells of E. coli. In both assays, the EPI had activity only against cells expressing functional efflux pumps.
We have also demonstrated that the MC-207,110 series of EPIs may have an additional mode of action, namely, permeabilization of the outer membrane of P. aeruginosa. However, this permeabilizing effect of MC-207,110 was seen only for the strain of P. aeruginosa that lacked efflux pumps. This result is consistent with our hypothesis that MC-207,110 is an efflux pump substrate, whereupon the compound would be extruded by multicomponent Mex pumps directly into the external medium. Therefore, the active Mex pumps should decrease the concentration of MC-207,110 in all intracellular compartments, including the cytoplasm, the periplasm, and the outer membrane. By decreasing the MC-207,110 concentration in the outer membrane, the pump is expected to protect the cells from the outer membrane-permeabilizing effect of MC-207,110. We are exploring techniques that can be used to measure the efflux of MC-207,110 more directly.
In this report we have also demonstrated the numerous potential benefits of using MC-207,110 in combination with levofloxacin against P. aeruginosa. MC-207,110 decreased the intrinsic level of resistance to levofloxacin ca. 8-fold in the wild-type strain of P. aeruginosa, while in strains overexpressing efflux pumps, susceptibility was increased up to 64-fold. As expected, MC-207,110 potentiated the activity of levofloxacin irrespective of the presence of target-based mutations.
Recent clinical isolates of P. aeruginosa with a wide range of resistance phenotypes also showed increased susceptibilities to levofloxacin in the presence of MC-207,110. Remarkably, both the MIC50 and the MIC90 were decreased to the same extent (16-fold) by MC-207,110, providing additional evidence that the effect of MC-207,110 is not dependent on the absolute level of resistance but, rather, that the magnitude of this effect is determined only by the level of the efflux pump expression.
A major beneficial consequence of inhibition of multiple efflux pumps demonstrated in this report was a dramatic decrease in the frequency of emergence of P. aeruginosa strains with clinically relevant levels of resistance to fluoroquinolones. Resistant mutants for which the levofloxacin MIC was 1 μg/ml emerged at a frequency less than 10−11 (versus a resistance frequency of 10−7 in the absence of efflux pump inhibitors). This result was expected, since MC-207,110 decreases the MIC of levofloxacin for wild-type strain PAM1020 from 0.125 to 0.015 μg/ml, i.e., to the same level as that for the strain lacking efflux pumps. As we have demonstrated previously (18), for bacteria that lack efflux pumps (or in which such pumps are completely inhibited) to emerge under the selection conditions (levofloxacin at 1 μg/ml), they must acquire multiple target-based mutations: a strain that acquired a single target-based mutation that conferred only a four- to eightfold increase in resistance could not emerge under the selection conditions. Therefore, the frequency of emergence of mutants is decreased since the simultaneous acquisition of multiple mutations is an extremely rare event. Similar effects have been demonstrated with reserpine, which is an inhibitor of the efflux pumps NorA and PmrA from S. aureus and S. pneumoniae, respectively, that are implicated in intrinsic and acquired resistance to ciprofloxacin (19, 20). Recently, several novel inhibitors of NorA have been described and their in vitro activities have been characterized (21).
From our experience with clinical isolates of P. aeruginosa, it is evident that besides the utility of EPIs in combination with antibacterial agents for antibacterial therapy, these compounds may prove to be very useful for study of the contribution and prevalence of efflux in acquired and intrinsic resistance to multiple antibiotics in gram-negative bacteria.
While initial reports have demonstrated the multifactorial benefits of the EPI approach in combating drug resistance, substantial efforts are needed before inhibitors can be used clinically. Our efforts are focused on the optimization of both the activities and the pharmacological properties of the MC-207,110 series of compounds.
ACKNOWLEDGMENTS
We are grateful to G. Miller, Pat Martin, Paul Nakane, and Mike Dudley for critical reading of the manuscript. We are indebted to Hiroshi Nikaido for scientific advice and insightful comments.
This work was supported by Daiichi Pharmaceutical Co., Ltd., and Microcide Pharmaceuticals, Inc.
REFERENCES
- 1.Aires J R, Kohler T, Nikaido H, Plesiat P. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother. 1999;43:2624–2628. doi: 10.1128/aac.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Axe D, Bailey J. Application of 31-P nuclear magnetic resonance spectroscopy to investigate plasmid effects on Escherichia coli metabolism. Biotechnol Lett. 1987;9:83–88. [Google Scholar]
- 3.Chen H Y, Yuan M, Livermore D M. Mechanisms of resistance to beta-lactam antibiotics amongst Pseudomonas aeruginosa isolates collected in the UK in 1993. J Med Microbiol. 1995;43:300–309. doi: 10.1099/00222615-43-4-300. [DOI] [PubMed] [Google Scholar]
- 4.Clancy J, Petitpas J, Dib-Hajj F, Yuan W, Cronan M, Kamath A, Bergeron J, Retsema J. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol. 1996;22:867–879. doi: 10.1046/j.1365-2958.1996.01521.x. [DOI] [PubMed] [Google Scholar]
- 5.Dinh T, Paulsen I T, Saier M H., Jr A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of gram-negative bacteria. J Bacteriol. 1994;176:3825–3831. doi: 10.1128/jb.176.13.3825-3831.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fukuda H, Hosaka M, Iyobe S, Gotoh N, Nishino T, Hirai K. nfxC-type quinolone resistance in a clinical isolate of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1995;39:790–792. doi: 10.1128/AAC.39.3.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Germ M, Yoshihara E, Yoneyama H, Nakae T. Interplay between the efflux pump and the outer membrane permeability barrier in fluorescent dye accumulation in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1999;261:452–455. doi: 10.1006/bbrc.1999.1045. [DOI] [PubMed] [Google Scholar]
- 8.Gotoh N, Itoh N, Tsujimoto H, Yamagishi J, Oyamada Y, Nishino T. Isolation of OprM-deficient mutants of Pseudomonas aeruginosa by transposon insertion mutagenesis: evidence of involvement in multiple antibiotic resistance. FEMS Microbiol Lett. 1994;122:267–273. doi: 10.1111/j.1574-6968.1994.tb07179.x. [DOI] [PubMed] [Google Scholar]
- 9.Gotoh N, Tsujimoto H, Tsuda M, Okamoto K, Nomura A, Wada T, Nakahashi M, Nishino T. Characterization of the MexC-MexD-OprJ multidrug efflux system in ΔmexA-mexB-oprM mutants of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1998;42:1938–1943. doi: 10.1128/aac.42.8.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hancock R, Wong P. Compounds which increase the permeability of the Pseudomonas aeruginosa outer membrane. Antimicrob Agents Chemother. 1984;26:48–52. doi: 10.1128/aac.26.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper D C. Quinolone mode of action. Drugs. 1995;49:10–15. doi: 10.2165/00003495-199500492-00004. [DOI] [PubMed] [Google Scholar]
- 12.Kohler T, Michea-Hamzehpour M, Henze U, Gotoh N, Curty L K, Pechere J C. Characterization of MexE-MexF-OprN, a positively regulated multidrug efflux system of Pseudomonas aeruginosa. Mol Microbiol. 1997;23:345–354. doi: 10.1046/j.1365-2958.1997.2281594.x. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence L E, Barrett J F. Efflux pumps in bacteria: overview, clinical relevance, and potential pharmaceutical target. Exp Opin Invest Drugs. 1998;7:199–217. doi: 10.1517/13543784.7.2.199. [DOI] [PubMed] [Google Scholar]
- 14.Lee A, Mao W, Warren M, Mistry A, Hoshino K, Okumura R, Ishida H, Lomovskaya O. Interplay between efflux pumps may provide either additive or multiplicative effects on drug resistance. J Bacteriol. 2000;182:3142–3150. doi: 10.1128/jb.182.11.3142-3150.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lewis K. Multidrug resistance pumps in bacteria: variations on a theme. Trends Biochem Sci. 1994;19:119–123. doi: 10.1016/0968-0004(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 16.Li X, Zhang L, Poole K. Interplay between the MexA-MexB-OprM multidrug efflux system and the outer membrane barrier in the multiple antibiotic resistance of Pseudomonas aeruginosa. J Antimicrob Chemother. 2000;45:433–436. doi: 10.1093/jac/45.4.433. [DOI] [PubMed] [Google Scholar]
- 17.Li X Z, Ma D, Livermore D M, Nikaido H. Role of efflux pump(s) in intrinsic resistance of Pseudomonas aeruginosa: active efflux as a contributing factor to beta-lactam resistance. Antimicrob Agents Chemother. 1994;38:1742–1752. doi: 10.1128/aac.38.8.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lomovskaya O, Lee A, Hoshino K, Ishida H, Mistry A, Warren M S, Boyer E, Chamberland S, Lee V J. Use of a genetic approach to evaluate the consequences of inhibition of efflux pumps in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:1340–1346. doi: 10.1128/aac.43.6.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Markham P N. Inhibition of the emergence of ciprofloxacin resistance in Streptococcus pneumoniae by the multidrug efflux inhibitor reserpine. Antimicrob Agents Chemother. 1999;43:988–989. doi: 10.1128/aac.43.4.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markham P N, Neyfakh A A. Inhibition of the multidrug transporter NorA prevents emergence of norfloxacin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:2673–2674. doi: 10.1128/aac.40.11.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markham P N, Westhaus E, Klyachko K, Johnson M E, Neyfakh A A. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:2404–2408. doi: 10.1128/aac.43.10.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masuda N, Gotoh N, Ishii C, Sakagawa E, Ohya S, Nishino T. Interplay between chromosomal beta-lactamase and the MexAB-OprM efflux system in intrinsic resistance to beta-lactams in Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1999;43:400–402. doi: 10.1128/aac.43.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchell B, Paulsen I, Brown M, Skurray R. Bioenergetics of the staphylococcal multidrug export protein QacA. Identification of distinct binding sites for monovalent and divalent cations. J Biol Chem. 1999;274:3541–3548. doi: 10.1074/jbc.274.6.3541. [DOI] [PubMed] [Google Scholar]
- 24.Morshed S R, Lei Y, Yoneyama H, Nakae T. Expression of genes associated with antibiotic extrusion in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1995;210:356–362. doi: 10.1006/bbrc.1995.1669. [DOI] [PubMed] [Google Scholar]
- 25.Nakae T, Nakajima A, Ono T, Saito K, Yoneyama H. Resistance to beta-lactam antibiotics in Pseudomonas aeruginosa due to interplay between the MexAB-OprM efflux pump and beta-lactamase. Antimicrob Agents Chemother. 1999;43:1301–1303. doi: 10.1128/aac.43.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakano M, Deguchi T, Kawamura T, Yasuda M, Kimura M, Okano Y, Kawada Y. Mutations in the gyrA and parC genes in fluoroquinolone-resistant clinical isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1997;41:2289–2291. doi: 10.1128/aac.41.10.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standards NCCLS document M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 28.Nelson M L, Levy S B. Reversal of tetracycline resistance mediated by different bacterial tetracycline resistance determinants by an inhibitor of the Tet(B) antiport protein. Antimicrob Agents Chemother. 1999;43:1719–1724. doi: 10.1128/aac.43.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nelson M L, Park B H, Andrews J S, Georgian V A, Thomas R C, Levy S B. Inhibition of the tetracycline efflux antiport protein by 13-thio-substituted 5-hydroxy-6-deoxytetracyclines. J Med Chem. 1993;36:370–377. doi: 10.1021/jm00055a008. [DOI] [PubMed] [Google Scholar]
- 30.Nikaido H. Antibiotic resistance caused by gram-negative multidrug efflux pumps. Clin Infect Dis. 1998;27(Suppl. 1):32–41. doi: 10.1086/514920. [DOI] [PubMed] [Google Scholar]
- 31.Nikaido H. Multiple antibiotic resistance and efflux. Curr Opin Microbiol. 1998;1:516–523. doi: 10.1016/s1369-5274(98)80083-0. [DOI] [PubMed] [Google Scholar]
- 32.Nikaido H. Prevention of drug access to bacterial targets: permeability barriers and active efflux. Science. 1994;264:382–388. doi: 10.1126/science.8153625. [DOI] [PubMed] [Google Scholar]
- 33.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ocaktan A, Yoneyama H, Nakae T. Use of fluorescence probes to monitor function of the subunit proteins of the MexA-MexB-OprM drug extrusion machinery in Pseudomonas aeruginosa. J Biol Chem. 1997;272:21964–21969. doi: 10.1074/jbc.272.35.21964. [DOI] [PubMed] [Google Scholar]
- 35.Oethinger M, Kern W V, Jellen-Ritter A S, McMurry L M, Levy S B. Ineffectiveness of topoisomerase mutations in mediating clinically significant fluoroquinolone resistance in Escherichia coli in the absence of the AcrAB efflux pump. Antimicrob Agents Chemother. 2000;44:10–13. doi: 10.1128/aac.44.1.10-13.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piddock L J. Mechanisms of resistance to fluoroquinolones: state-of-the-art 1992–1994. Drugs. 1995;49:29–35. doi: 10.2165/00003495-199500492-00006. [DOI] [PubMed] [Google Scholar]
- 37.Plesiat P, Aires J, Godard C, Kohler T. Use of steroids to monitor alterations in the outer membrane of Pseudomonas aeruginosa. J Bacteriol. 1977;179:7004–7010. doi: 10.1128/jb.179.22.7004-7010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poole K, Gotoh N, Tsujimoto H, Zhao Q, Wada A, Yamasaki T, Neshat S, Yamagishi J, Li X Z, Nishino T. Overexpression of the mexC-mexD-oprJ efflux operon in nfxB-type multidrug-resistant strains of Pseudomonas aeruginosa. Mol Microbiol. 1996;21:713–724. doi: 10.1046/j.1365-2958.1996.281397.x. [DOI] [PubMed] [Google Scholar]
- 39.Poole K, Krebes K, McNally C, Neshat S. Multiple antibiotic resistance in Pseudomonas aeruginosa: evidence for involvement of an efflux operon. J Bacteriol. 1993;175:7363–7372. doi: 10.1128/jb.175.22.7363-7372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Putman M, Koole L, van Veen H, Konings W. The secondary multidrug transporter LmrP contains multiple drug interaction sites. Biochemistry. 1999;38:13900–13905. doi: 10.1021/bi991262k. [DOI] [PubMed] [Google Scholar]
- 41.Renau T, Leger R, Flamme E, Sangalang J, She M, Yen R, Gannon C, Mathias K, Lee A, Lomovskaya O, Chamberland S, Lee V J, Hecher S, Otha T, Nakayama K. Inhibitors of efflux pumps in Pseudomonas aeruginosa potentiate the activity of the fluoroquinolone antibacterial levofloxacin. J Med Chem. 1999;42:4928–4931. doi: 10.1021/jm9904598. [DOI] [PubMed] [Google Scholar]
- 42.Saier M H., Jr Molecular phylogeny as a basis for the classification of transport proteins from bacteria, archaea and eukarya. Adv Microb Physiol. 1998;40:81–136. doi: 10.1016/s0065-2911(08)60130-7. [DOI] [PubMed] [Google Scholar]
- 43.Sanchez L, Pan W, Vinas M, Nikaido H. The acrAB homolog of Haemophilus influenzae codes for a functional multidrug efflux pump. J Bacteriol. 1997;179:6855–6857. doi: 10.1128/jb.179.21.6855-6857.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stein W. Kinetics of the multidrug transporter (P-glycoprotein) and its reversal. Physiol Rev. 1997;77:545–590. doi: 10.1152/physrev.1997.77.2.545. [DOI] [PubMed] [Google Scholar]
- 45.Tait-Kamradt A, Clancy J, Cronan M, Dib-Hajj F, Wondrack L, Yuan W, Sutcliffe J. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2251–2255. doi: 10.1128/aac.41.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thanassi D G, Suh G S, Nikaido H. Role of outer membrane barrier in efflux-mediated tetracycline resistance of Escherichia coli. J Bacteriol. 1995;177:998–1007. doi: 10.1128/jb.177.4.998-1007.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trias, J., S. Chamberland, S. Hecker, and V. J. Lee. Nov. 1999. Efflux pump inhibitors. U. S. patent 5,989,832.
- 48.Urgubil K, Rotternberg H, Glynn P, Shulman R. 31-P nuclear magnetic resonance studies of bioenergetics and glycolisis in anaerobic Escherichia coli cells. Proc Natl Acad Sci USA. 1978;75:2244–2248. doi: 10.1073/pnas.75.5.2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaara M. Agents that increase the permeability of the outer membrane. Microbiol Rev. 1992;56:395–411. doi: 10.1128/mr.56.3.395-411.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Westbrock-Wadman S, Sherman D R, Hickey M J, Coulter S N, Zhu Y Q, Warrener P, Nguyen L Y, Shawar R M, Folger K R, Stover C K. Characterization of a Pseudomonas aeruginosa efflux pump contributing to aminoglycoside impermeability. Antimicrob Agents Chemother. 1999;43:2975–2983. doi: 10.1128/aac.43.12.2975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoneyama H, Ocaktan A, Tsuda M, Nakae T. The role of mex-gene products in antibiotic extrusion in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1997;233:611–618. doi: 10.1006/bbrc.1997.6506. [DOI] [PubMed] [Google Scholar]
- 52.Yoshida H, Bogaki M, Nakamura M, Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990;34:1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoshida T, Muratani T, Iyobe S, Mitsuhashi S. Mechanisms of high-level resistance to quinolones in urinary tract isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1994;38:1466–1469. doi: 10.1128/aac.38.7.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zgurskaya H I, Nikaido H. AcrA is a highly asymmetric protein capable of spanning the periplasm. J Mol Biol. 1999;285:409–420. doi: 10.1006/jmbi.1998.2313. [DOI] [PubMed] [Google Scholar]
- 55.Zgurskaya H I, Nikaido H. Bypassing the periplasm: reconstitution of the AcrAB multidrug efflux pump of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:7190–7195. doi: 10.1073/pnas.96.13.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimmermann W, Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977;12:368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]