Abstract
Our aim was to investigate the distribution of acid-base disorders in patients with COVID-19 ARDS using both the Henderson–Hasselbalch and Stewart’s approach and to explore if hypoxemia can influence acid-base disorders. COVID-19 ARDS patients, within the first 48 h of the need for a non-invasive respiratory support, were retrospectively enrolled. Respiratory support was provided by helmet continuous positive airway pressure (CPAP) or by non-invasive ventilation. One hundred and four patients were enrolled, 84% treated with CPAP and 16% with non-invasive ventilation. Using the Henderson–Hasselbalch approach, 40% and 32% of patients presented respiratory and metabolic alkalosis, respectively; 13% did not present acid-base disorders. Using Stewart’s approach, 43% and 33% had a respiratory and metabolic alkalosis, respectively; 12% of patients had a mixed disorder characterized by normal pH with a lower SID. The severe hypoxemic and moderate hypoxemic group presented similar frequencies of respiratory and metabolic alkalosis. The most frequent acid-base disorders were respiratory and metabolic alkalosis using both the Henderson–Hasselbalch and Stewart’s approach. Stewart’s approach detected mixed disorders with a normal pH probably generated by the combined effect of strong ions and weak acids. The impairment of oxygenation did not affect acid-base disorders.
Keywords: COVID-19, ARDS, acid-base disorders, non-invasive ventilation
1. Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), due to the high tropism of the virus not only for the respiratory tract, but also for the bowel, heart, kidney and nervous system, can result in a high spectrum of disorders such as acute respiratory failure, acute heart failure, acute kidney injury, coagulopathy, extensive microvascular thrombosis, a dysregulated inflammatory response, sepsis and multiorgan failure [1,2,3,4]. As reported by Huang et al., patients with COVID-19 presented ARDS in 29%, acute kidney injury in 13% and cardiac failure in 12% of the cases [5].
In the presence of an acute respiratory failure, 81% of the patients required oxygen support and, based on the severity of acute respiratory failure (i.e., level of hypoxemia and dyspnea), most of these patients also required a respiratory support. The main applied respiratory supports included high flow oxygen therapy, continuous positive airway pressure (CPAP), non-invasive (NIV) and invasive mechanical ventilation. The proportion of patients treated with non-invasive respiratory supports varied from 62% in China to 20% and 11% in North America and Italy, respectively [1,6,7].
The presence of an altered organ function (lung, heart and kidney) influencing the patient homeostasis could induce an alteration in the acid-base balance according to the severity of the underlying disease [8]. However, limited data have been available to describe the acid-base characteristics of COVID-19 patients in the early phase of hospital admission. In more than one thousand critically ill patients, the alteration in pH at admission was significantly associated with the mortality [7]. Alfano et al., in a retrospective study of COVID-19 patients receiving oxygen therapy, found acid-base disturbances in 80% of the patients and the main alterations were the metabolic and respiratory alkalosis [9]. Patients with respiratory alkalosis had higher ratios of underlying disease and were more likely to die compared to patients without respiratory alkalosis [10].
The traditional classification of the acid-base disorders, in terms of presence of alkalemia or acidemia due to a respiratory versus metabolic disorder, has been based on the Henderson–Hasselbalch equation, which focuses on the plasma bicarbonate concentration ([HCO3−]), plasma carbon dioxide tension (PCO2) and the negative logarithm of the apparent dissociation constant (pK1′) for carbonic acid (H2CO3) in plasma [11]. Although this approach is the most widely used to identify an acid-base derangement, it is merely descriptive rather than mechanistic in nature and often unable to provide a diagnosis in critically ill patients [12]. Thus, in the late 1970s a mathematical model based on physicochemical principles was proposed by Peter Stewart to describe the alterations in acid-base balance according to three different variables: the strong ion difference (SID), carbon dioxide and weak acids [13,14]. In non-COVID-19 critically ill patients, Stewart’s approach showed, compared to the traditional evaluation, a greater identification of acid-base disorders [15,16,17].
Our aim was: (1) to investigate the distribution of acid-base disorders in a cohort of patients with acute respiratory distress syndrome due to COVID-19 using both the Henderson–Hasselbalch “physiological” approach and the Stewart “physicochemical” approach within the first 48 h of the need for a non-invasive respiratory support, and (2) to explore if the hypoxemia, as marker of the severity of the disease, can influence the acid-base disorders.
2. Materials and Methods
2.1. Study Population
Adults (>18 years) with acute respiratory failure caused by COVID-19 pneumonia, PaO2/FiO2 < 300, with ground glass bilateral opacities at chest X-ray or lung CT and requirement for non-invasive respiratory support, were retrospectively enrolled. They were admitted at the intermediate-high Dependency Unit of the ASST Santi Paolo e Carlo, San Paolo Hospital, Milan between September 2020 and March 2021 and treated with non-invasive respiratory support. Exclusion criteria were: the need for immediate endotracheal intubation (ETI) and Glasgow Coma Scale < 15.
The study was approved by the local ethical board (Comitato Etico Milano Area I; 17263/2020-2020/ST/095), and informed consent was acquired from each patient.
2.2. Study Protocol and Data Collection
Upon their emergency department admission demographics, comorbidities and chronic therapies were recorded at admission.
Respiratory support was provided by helmet continuous positive airway pressure (CPAP) or by mask delivered non-invasive ventilation (NIV) at the discretion of the attending physician to maintain the peripheral oxygen saturation (SpO2) > 92% and a respiratory rate < 25 bpm.
After the transfer to the High Dependency, vital signs, Borg scale dyspnea score and Work Of Breathing (WOB) score [18], laboratory parameters, non-invasive respiratory support settings, respiratory rate and arterial blood gas analysis within the first 48 hours from the onset of the respiratory support were collected.
The same blood sample using a Siemens RAPIDPoint 500 blood gas analyzer (Siemens HealthCare, Erlangen, Germany) was analyzed to investigate acid-base disorders using both the Henderson–Hasselbalch approach, based on bicarbonate-carbon dioxide, and the physicochemical approach (Stewart’s).
According to the Henderson–Hasselbalch approach, firstly, patients were classified as acidemic, alkalemic and with no pH disorder; secondly, the Primary Acid-Base Disturbance was identified and an eventual compensatory secondary response, assessed using the Boston Rules [11].
-
-
A pH of less than 7.38 was categorized as acidemia; a pH of more than 7.42 was categorized as alkalemia; a pH between 7.38 and 7.42, with PaCO2 between 38 and 42 mmHg and [HCO3-] between 22 and 26 mMol/L was categorized as no disorder;
-
-
Respiratory acidosis pH < 7.38 and PaCO2 > 42 mmHg; respiratory acidosis with the secondary acute metabolic response if [HCO3−] is increased by 1 mMol/liter for each PaCO2 increase of 10 mmHg above 40 mm Hg; respiratory acidosis with the secondary chronic metabolic response if [HCO3−] is increased by 4–5 mMol/liter for each PaCO2 increase of 10 mmHg above 40 mmHg; superimposed metabolic alkalosis or acidosis may be diagnosed if the calculated [HCO3−] is greater or less than predicted;
-
-
Metabolic acidosis pH < 7.38 and bicarbonate [HCO3−] < 22 mMol/L; metabolic acidosis with secondary respiratory response if PaCO2 = 1.5 × [HCO3−] + 8 ± 2 mmHg; superimposed respiratory acidosis or alkalosis may be diagnosed if the calculated PaCO2 is greater or less than predicted;
-
-
Respiratory alkalosis pH > 7.42 and PaCO2 < 38 mmHg; respiratory alkalosis with the secondary acute metabolic response if is decreased by 2 mMol/L for each PaCO2 decrease of 10 mmHg below 40 mmHg; respiratory alkalosis with the secondary chronic metabolic response if [HCO3−] is decreased by 4–5 mMol/L for each PaCO2 decrease of 10 mmHg below 40 mmHg; superimposed metabolic alkalosis or acidosis may be diagnosed if the calculated [HCO3−] is greater or less than predicted;
-
-
Metabolic alkalosis pH > 7.42 and [HCO3−] > 26 mMol/L; metabolic alkalosis with secondary respiratory response if PaCO2 = 0.7 × ([HCO3−] − 24) + 40 ± 2 mmHg; superimposed respiratory acidosis or alkalosis may be diagnosed if the calculated PaCO2 is greater or less than predicted.
We also calculated plasmatic Anion Gap as defined as: Anion Gap (mEq/L) = [Na+] – [Cl−] – [HCO3−].
According to the physicochemical approach (Stewart’s), we classified acidemia, alkalemia and no pH disorder based on the PCO2 and the electrolyte composition of blood (the apparent SID) [13]. The apparent SID was calculated as: [aSID] (mEq/L) = [Na+] + [K+] − [Cl−] − [Lactates−].
-
-
A pH of less than 7.38 was categorized as acidemia; a pH of more than 7.42 was categorized as alkalemia; a pH between 7.38 and 7.42, with PaCO2 between 38 and 42 mmHg and [aSID] between 38 and 42 mEq/L was categorized as no disorder;
-
-
Respiratory acidosis: pH < 7.38, PaCO2 > 42 mm Hg and [aSID] between 38 and 42 mEq/L;
-
-
Metabolic acidosis secondary to aSID: pH < 7.38, PaCO2 between 38 and 42 and [aSID] < 38 mEq/L;
-
-
Other metabolic acidosis: pH < 7.38, PaCO2 between 38 and 42 and [aSID] between 38 and 42 mEq/L;
-
-
Respiratory alkalosis: pH > 7.42, PaCO2 < 38 mmHg and [aSID] 38–42 mEq/L;
-
-
Metabolic alkalosis secondary to aSID: pH > 7.42, PaCO2 between 38 and 42 mmHg and [aSID] > 42 mEq/L;
-
-
Other metabolic alkalosis: pH > 7.42, PaCO2 between 38 and 42 mmHg and [aSID] between 38 and 42 mEq/L;
-
-
Mixed disorder pH 7.38–7.42 with PaCO2 > 42 and [aSID] > 42 mEq/L or PaCO2 < 38 and [aSID] < 38 mEq/L.
No advice was given to the physician regarding acid-base derangements treatment; however, sodium bicarbonate was not used in the patients.
Finally, we divided the whole population according to the severity of hypoxemia in terms of the median value of PaO2/FiO2.
2.3. Statistical Analysis
Categorical data are reported as % (number), while continuous variables are expressed as median [IQR]; normality of distribution was assessed by the Shapiro–Wilks tests. A One-Way Analysis of Variance (ANOVA) or Kruskal–Wallis test were used to assess differences among acid-base disturbance groups; Student’s t-test or Wilcoxon–Mann–Whitney test were used to assess differences between groups divided according to the median value of PaO2/FiO2. All analyses were performed with R Studio (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
A total of 104 patients were enrolled in the study. The baseline characteristics at emergency department were shown in Table 1. The median age was 58 (52–64) years, 77 (73%) were males with a body mass index of 28 (25–33) kg/m2. Patients presented a median period of 6 (4–8) days from onset of the symptoms to the emergency department admission. At the hospital admission, all patients received oxygen therapy with a PaO2/FiO2 of 264 (204–301) and presented respiratory alkalosis with PaCO2 31.8 (28.4–34.2) mmHg.
Table 1.
Baseline characteristics of the study population at emergency department admission.
| Number = 104 | |
|---|---|
| Age, years | 58 (52–64) |
| Male gender, % (n) | 73 (77) |
| Weight, kg | 83 (72–97) |
| BMI, kg/m2 | 28 (25–33) |
| Time from symptoms onset to hospital admission, days | 6 (4–8) |
| Time from hospital admission to respiratory support start, days | 1 (0–3) |
| Arterial pH | 7.45 (7.43–7.48) |
| PaCO2, mmHg | 31.8 (28.4–34.2) |
| PaO2, mmHg | 63.0 (55.7–74.0) |
| PaO2/FiO2 | 264 (204–301) |
| White blood cell count, cells/μL | 6600 (5200–8780) |
| Haemoglobin, g/dL | 14.4 (13.1–15.4) |
| Platelets, cells/μL | 198 (152–241) |
| INR | 1.14 (1.08–1.22) |
| GOT, U/L | 55 (41–76) |
| GPT, U/L | 46 (31–71) |
| Total bilirubin, mg/dL | 0.6 (0.4–1.0) |
| Creatinine, mg/dL | 0.8 (0.7–1.0) |
| LDH, mg/dL | 382 (292–472) |
| D-dimer, ng/mL | 300 (226–394) |
| SOFA score | 2 (2–3) |
Data are presented as median [IQR]. BMI: body mass index; PaCO2: arterial carbon dioxide partial pressure; PaO2: arterial oxygen partial pressure; INR: international normalized ratio; GOT: glutamic oxaloacetic transaminase; GTP: glutamic pyruvic transaminase; LDH: L-lactate dehydrogenase; SOFA: sequential organ failure assessment.
Within 48 h from the onset of the non-invasive respiratory support, 87 (84%) and 17 (16%) patients were treated with CPAP and non-invasive ventilation, respectively (Table 2). The median applied PEEP level was 8 (7.5–10) cmH2O, with a PaO2/FiO2 of 199 (139–246). The median pH was 7.44 (7.43–7.46), with a PaCO2 of 38 (35–41) mmHg and bicarbonate of 25.8 (24.1–27.4) mMol/L. The hospital mortality was 14%.
Table 2.
Comparison among groups classified according to Henderson–Hasselbalch approach.
| Study Population | No Acid-Base Disorder | Respiratory Alkalosis | Metabolic Alkalosis |
Respiratory Acidosis |
Mixed Alkalosis |
p | |
|---|---|---|---|---|---|---|---|
| Number (%) | 104 (100) | 14 (13) | 42 (40) | 34 (32) | 5 (5) | 9 (10) | - |
| Age, years | 60 (53–69) | 59 (52–71) | 61 (55–68) | 59 (52–66) | 58 (57–69) | 63 (59–70) | 0.824 |
| Female gender, n (%) | 28 (27) | 21 (3) | 17 (7) | 41 (14) | 100 (5) | 13 (1) | 0.845 |
| BMI, kg/m2 | 28 (25–33) | 25 (25–28) | 28 (25–31) | 28 (26–34) | 30 (30–30) | 30 (26–36) | 0.804 |
| Time from symptoms to ED, days | 6 (4–8) | 6 (4–7) | 5 (4–8) | 6 (3–8) | 7 (5–7) | 6 (5–8) | 0.989 |
| Respiratory rate, bpm | 19 (17–24) | 20 (17–24) | 20 (17–23) | 18 (16–22) | 18 (16–20) | 20 (18–24) | 0.791 |
| FiO2 | 70 (60–70) | 70 (60–80) | 70 (60–80) | 65 (60–70) | 70 (60–75) | 60 (60–70) | 0.286 |
| PEEP, cmH2O | 8 (7.5–10) | 10 (8–10) | 7.5 (7.5–10) | 8 (7.5–10) | 7.5 (7.5–10) | 7.5 (7.5–8) | 0.220 |
| Borg Score | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.203 |
| WOB Score | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–4) | 2 (1–3) | 0.684 |
| Arterial pH | 7.44 (7.43–7.46) | 7.40 (7.39–7.42) | 7.44 (7.44–7.46) | 7.45 (7.44–7.46) | 7.36 (7.36–7.36) | 7.48 (7.48–7.49) | <0.001 |
| PaCO2, mmHg | 38 (35–41) | 40 (39–42) | 35 (33–36) | 41 (40–44) | 48 (44–51) | 36 (35–37) | <0.001 |
| PaO2, mmHg | 123 (92–155) | 139 (101–180) | 125 (94–177) | 108 (92–150) | 98 (74–149) | 130 (108–146) | 0.762 |
| PaO2/FiO2 | 199 (139–246) | 212 (173–227) | 199 (139–261) | 167 (140–234) | 163 (124–212) | 221 (180–246) | 0.780 |
| HCO3−, mMol/L | 25.8 (24.1–27.4) | 25.2 (23.8–26.0) | 24.2 (22.9–25.0) | 28.0 (27.1–29.7) | 27 (24.5–28) | 27.0 (26.1–27.4) | <0.001 |
| BE, mMol/L | 1.6 (0.1–3.6) | 1.2 (−0.6–1.7) | 0.2 (−1.0–0.8) | 4.1 (2.9–6.1) | 1.3 (−0.8–3.6) | 3.8 (2.1–4.1) | <0.001 |
| Apparent SID, mEq/L | 36.6 (34.9–38.2) | 37.8 (36.8–38.8) | 35.8 (33.9–37.2) | 37.6 (36.6–38.9) | 35.6 (35.0–36.5) | 36.2 (34.9–37.4) | 0.004 |
| Sodium, mEq/L | 136 (134–138) | 138 (135–139) | 136 (134–139) | 136 (135–138) | 137 (133–137) | 135 (133–137) | 0.333 |
| Potassium, mEq/L | 4.1 (3.8–4.3) | 4.1 (3.8–4.4) | 4.0 (3.8–4.4) | 4.1 (3.8–4.3) | 4.1 (4.0–4.5) | 3.8 (3.6–4.0) | 0.270 |
| Cloride, mEq/L | 103 (100–105) | 102 (100–105) | 104 (101–105) | 102 (100–104) | 100 (99–102) | 102 (100–103) | 0.151 |
| Lactates, mMol/L | 1.3 (1.0–1.7) | 1.3 (1.0–1.8) | 1.4 (1.2–1.7) | 1.2 (1.0–1.4) | 1.5 (1.4–2.5) | 1.3 (1.0–1.4) | 0.075 |
| Anion Gap, mEq/L | 8.1 (6.3–10.0) | 9.9 (7.9–10.8) | 9.0 (8.0–10.7) | 6.6 (5.5–7.9) | 7.7 (6.0–8.8) | 6.9 (5.9–8.0) | <0.001 |
| Creatinine, mg/dL | 0.9 (0.7–1.1) | 0.8 (0.7–0.95) | 0.9 (0.8–1.1) | 0.7 (0.6–0.9) | 1.0 (0.9–1.2) | 1.0 (0.9–1.2) | 0.007 |
| Ventilation type, n (%) | 0.290 | ||||||
| CPAP | 84 (87) | 100 (14) | 81 (34) | 85 (29) | 80 (4) | 67 (6) | |
| NIV | 16 (17) | 0 (0) | 19 (8) | 15 (5) | 20 (1) | 33 (3) | |
| Endotracheal Intubation, n (%) | 19 (20) | 26 (4) | 19 (8) | 9 (3) | 2 (40) | 33 (3) | 0.223 |
| Mortality, n (%) | 14 (13) | 2 (14) | 5 (2) | 15 (5) | 40 (2) | 33 (3) | 0.065 |
Continuous variables are compared using One-Way ANOVA or Kruskal–Wallis Test, as appropriate; categorical variables are compared using χ2 test. Data are presented as median [IQR]. BMI: body mass index; FiO2: inspired oxygen fraction; ED: emergency department; PEEP: positive end-expiratory pressure; WOB: work of breathing; PaCO2: arterial carbon dioxide partial pressure; PaO2: arterial oxygen partial pressure; HCO3−: arterial bicarbonate concentration; BE: base excess; SID: strong ion difference; CPAP: continuous positive airway pressure; NIV: non-invasive ventilation.
3.1. Acid-Base Disturbance According to Henderson–Hasselbalch Approach
Considering the different acid-base disorders, respiratory rate, applied PEEP and PaO2/FiO2 were not different among groups (Table 2).
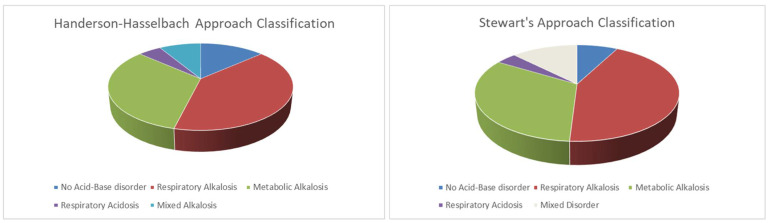
Forty-two (40%) and 34 (32%) patients presented respiratory alkalosis and metabolic alkalosis, respectively (Figure 1). Patients with respiratory alkalosis had a median pH of 7.44 (7.44–7.46), with a PaCO2 of 35 (33–36) mmHg and bicarbonate of 24.2 (22.9–25) mMol/L. In this group of patients, the PaCO2 was not related to the respiratory rate nor to the hypoxemia (p = 0.423, R2 = 0.01; p = 0.237, R2 = 0.01).
Figure 1.
Acid-base disturbances frequencies according to Henderson–Hasselbalch (on the left) and Stewart’s approach (on the right).
Patients with metabolic alkalosis presented values of median pH of 7.45 (7.44–7.46), PaCO2 of 41 (40–44) mmHg and bicarbonate of 28 (27.1–29.7) mMol/L.
Nine (10%) patients presented a mixed alkalosis due to a decreased PaCO2 (36 (35–37) mmHg) together with an increased bicarbonate concentration (27.0 (26.1–27.4) mMol/L).
Fourteen did not present any acid-base disorder.
The sodium, potassium and chloride concentrations were not different among groups and creatinine levels were within normal range. No patients had a metabolic acidosis according to our criteria.
3.2. Acid-Base Disturbance According to Stewart’s Method
Using Stewart’s approach forty-five (43%), twenty (19%) and fourteen (14%) had a respiratory alkalosis, a metabolic alkalosis secondary to aSID and other metabolic alkalosis, respectively (Table 3, Figure 1). Comparing these three groups, the pH was similar while the PaCO2 was, as expected, lower in the respiratory alkalosis, while the SID was slightly higher, even if within the normal range in the metabolic alkalosis group and slightly lower in the respiratory alkalosis group. The serum creatinine was not different between these groups.
Table 3.
Comparison among groups classified according to Stewart approach.
| Study Population |
No Acid-Base Disorder | Respiratory Alkalosis | Metabolic Alkalosis due to aSID | Other Alkalosis |
Respiratory Acidosis | Mixed Disorder |
p | |
|---|---|---|---|---|---|---|---|---|
| Number (%) | 104 (100) | 8 (8) | 45 (43) | 20 (19) | 14 (14) | 4 (4) | 13 (13) | - |
| Age, years | 60 (53–69) | 58 (53–64) | 60 (53–68) | 61 (50–70) | 60 (54–68) | 64 (58–69) | 62 (58–65) | 0.943 |
| Female gender, n (%) | 28 (27) | 1 (12) | 8 (18) | 11 (52) | 6 (43) | 25 (1) | 8 (1) | 0.654 |
| BMI, kg/m2 | 28 (25–33) | 25 (25–28) | 28 (25–31) | 28 (26–34) | 28 (26–33) | 30 (26–36) | 30 (30–30) | 0.734 |
| Time from symptoms to ED, days | 6 (4–8) | 6 (4–7) | 5 (4–8) | 4 (4–6) | 6 (4–8) | 6 (5–8) | 7 (5–7) | 0.783 |
| Respiratory rate, bpm | 19 (17–24) | 19 (17–26) | 19 (18–22) | 18 (16–22) | 18 (16–22) | 19 (18–22) | 19 (16–24) | 0.668 |
| FiO2 | 70 (60–70) | 70 (60–70) | 70 (60–70) | 70 (60–70) | 60 (60–70) | 75 (70–75) | 60 (60–70) | 0.532 |
| PEEP, cmH2O | 8 (7.5–10) | 10 (7.5–10) | 7.5 (7.5–10) | 8 (7.5–10) | 8 (7.5–10) | 10 (9–10) | 10 (7.5–10) | 0.141 |
| Borg Score | 0 (0–0) | 0 (0–1) | 0 (0–1) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–1) | 0.145 |
| WOB Score | 1 (1–2) | 1 (1–3) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 1 (1–2) | 0.971 |
| Arterial pH | 7.44 (7.43–7.46) | 7.41 (7.40–7.42) | 7.45 (7.44–7.48) | 7.45 (7.44–7.46) | 7.44 (7.45–7.46) | 7.36 (7.36–7.36) | 7.41 (7.40–7.42) | <0.001 |
| PaCO2, mmHg | 38 (35–41) | 41 (40–42) | 35 (33–36) | 40 (39–42) | 39 (38–42) | 48 (45–54) | 37 (34–43) | <0.001 |
| PaO2, mmHg | 123 (92–155) | 96 (81–190) | 120 (93–165) | 122 (90–152) | 126 (97–145) | 124 (92–195) | 138 (88–145) | 0.983 |
| PaO2/FiO2 | 199 (139–246) | 221 (118–298) | 199 (138–276) | 173 (150–230) | 168 (146–240) | 188 (148–278) | 198 (143–234) | 0.923 |
| HCO3−, mMol/L | 25.8 (24.1–27.4) | 25.6 (24.2–25.9) | 24.7 (23.6–25.6) | 27.4 (26.4–29.6) | 28.0 (26.9–29.4) | 26.2 (24.4–28.5) | 23.4 (22.0–27.0) | 0.001 |
| BE, mMol/L | 1.6 (0.1–3.6) | 1.6 (0.9–1.9) | 0.5 (−0.6–1.4) | 3.4 (2.4–6.0) | 3.6 (2.5–6.1) | 0.25 (−1.7–2.8) | 1.4 (−2–3.6) | 0.001 |
| Apparent SID, mEq/L | 36.6 (34.9–38.2) | 38.6 (38.0–39.1) | 38.9 (38.4–39.4) | 42.2 (42.1–43.0) | 40.0 (38.6–40.5) | 38.8 (38.0–39.6) | 35.6 (34.4–37.4) | 0.034 |
| Sodium, mEq/L | 136 (134–138) | 139 (136–139) | 136 (133–139) | 137 (135–138) | 137 (135–138) | 137 (136–138) | 137 (134–139) | 0.437 |
| Potassium, mEq/L | 4.1 (3.8–4.3) | 4.2 (4.0–4.4) | 4.0 (3.8–4.3) | 4.0 (3.7–4.2) | 4.1 (3.7–4.3) | 4.3 (4.1–4.5) | 4.3 (4.0–4.4) | 0.198 |
| Cloride, mEq/L | 103 (100–105) | 102 (99–103) | 103 (101–105) | 101 (100–104) | 101 (100–104) | 102 (100–104) | 103 (102–106) | 0.209 |
| Lactates, mMol/L | 1.3 (1.0–1.7) | 1.1 (0.9–1.3) | 1.3 (1.1–1.6) | 1.1 (1.0–1.4) | 1.1 (1.1–1.4) | 2.0 (1.4–2.6) | 1.7 (1.2–1.9) | 0.087 |
| Creatinine, mg/dL | 0.9 (0.7–1.1) | 0.8 (0.7–0.95) | 0.9 (0.8–1.1) | 1.0 (1.0–1.2) | 1.1 (0.9–1.1) | 1.0 (0.9–1.2) | 0.7 (0.6–0.9) | 0.004 |
| Ventilation type, n (%) | 0.489 | |||||||
| CPAP | 84 (87) | 8 (100) | 35 (78) | 10 (50) | 10 (71) | 3 (75) | 12 (92) | |
| NIV | 16 (17) | 0 (0) | 10 (22) | 10 (50) | 4 (29) | 1 (25) | 1 (8) | |
| Endotracheal Intubation, n (%) | 19 (20) | 4 (50) | 9 (20) | 2 (10) | 2 (14) | 2 (50) | 2 (15) | 0.132 |
| Mortality, n (%) | 13 (14) | 1 (12) | 5 (11) | 4 (20) | 1 (7) | 2 (50) | 1 (8) | 0.136 |
Continuous variables are compared using One-Way ANOVA or Kruskal–Wallis Test, as appropriate; categorical variables are compared using χ2 test. Data are presented as median [IQR]. AB: acid-base; BMI: body mass index; FiO2: inspired oxygen fraction; PEEP: positive end-expiratory pressure; ED: emergency department; WOB: work of breathing; PaCO2: arterial carbon dioxide partial pressure; PaO2: arterial oxygen partial pressure; HCO3−: arterial bicarbonate concentration; BE: base excess; SID: strong ion difference; CPAP: continuous positive airway pressure; NIV: non-invasive ventilation.
We did not find patients with superimposed respiratory and metabolic alkalosis considering the apparent SID according to Stewart’s approach.
Thirteen patients (12%) had a mixed disorder characterized by a normal pH 7.41 (7.40–7.42) with a lower SID and a lower PaCO2 compared with patients without any acid-base disorder.
Eight (8%) patients did not have any acid-base disorder.
The sodium, potassium and chloride concentrations were not different among groups and creatinine levels were within normal range.
3.3. Hypoxemia and Acid-Base Disturbance
The whole population was divided according to the median PaO2/FiO2 value of 199 (Table 4). The median PaO2/FiO2 were 140 (115–164) in the severe hypoxemic patients and 250 (222–300) in the moderate hypoxemic patients. The respiratory rate and applied PEEP were not different. No relationship between the oxygenation and the respiratory rate was found (p = 0.600, R2 = 0.02).
Table 4.
Comparison between groups classified according to the median value of PaO2/FiO2.
| Severe Hypoxemia |
Moderate Hypoxemia |
p | |
|---|---|---|---|
| Number, (%) | 51 (51) | 53 (49) | - |
| Age, years | 62 (54–68) | 59 (53–70) | 0.941 |
| BMI, kg/m2 | 28 (26–33) | 28 (25–33) | 0.366 |
| Time from symptoms to ED, days | 6 (4–7) | 5 (4–9) | 0.692 |
| Respiratory rate, bpm | 19 (17–24) | 19 (16–22) | 0.520 |
| FiO2 | 60 (60–70) | 70 (60–70) | 0.028 |
| PEEP, cmH2O | 8 (7.5–10) | 7.5 (7.5–10) | 0.380 |
| Borg Score | 0 (0–0) | 0 (0–0) | 0.410 |
| WOB Score | 1 (1–2) | 1 (1–2) | 0.126 |
| Arterial pH | 7.44 (7.43–7.46) | 7.44 (7.42–7.46) | 0.829 |
| PaCO2, mmHg | 38 (35–42) | 38 (34–41) | 0.185 |
| PaO2, mmHg | 92 (78–99) | 155 (140–204) | <0.001 |
| PaO2/FiO2 | 139 (115–162) | 246 (221–398) | <0.001 |
| HCO3−, mMol/L | 25.8 (24.4–27.4) | 25.8 (23.6–27.4) | 0.320 |
| BE, mMol/L | 1.6 (0.4–3.6) | 1.4 (−0.68–3.8) | 0.528 |
| Apparent SID, mEq/L | 36 (35–38) | 37 (35–38) | 0.761 |
| Sodium, mEq/L | 136 (135–139) | 137 (133–138) | 0.189 |
| Potassium, mEq/L | 4.1 (3.8–4.4) | 4.1 (3.8–4.2) | 0.199 |
| Cloride, mEq/L | 103 (101–105) | 102 (100–104) | 0.062 |
| Lactates, mMol/L | 1.3 (1.1–1.6) | 1.3 (1.0–1.7) | 0.780 |
| Creatinine, mg/dL | 0.9 (0.7–1.1) | 0.8 (0.7–1.0) | 0.448 |
| Endotracheal intubation, n (%) | 13 (25) | 7 (13) | 0.271 |
| Acid-base disorders, n (%) | 0.491 | ||
| No Acid-base disorder | 5 (10) | 9 (18) | |
| Respiratory Acidosis | 3 (5) | 2 (3) | |
| Respiratory Alkalosis | 20 (40) | 22 (41) | |
| Metabolic Alkalosis due to aSID | 10 (20) | 4 (9) | |
| Other Metabolic Alkalosis | 10 (20) | 10 (18) | |
| Mixed Alkalosis | 3 (5) | 6 (11) |
Continuous variables are compared using Student’s t-test or Wilcoxon–Mann–Whitney Test, as appropriate; categorical variables are compared using X2 test. Data are presented as median [IQR]. ED emergency Department; BMI: body mass index; FiO2: inspired oxygen fraction; PEEP: positive end-expiratory pressure; WOB: work of breathing; PaCO2: arterial carbon dioxide partial pressure; PaO2: arterial oxygen partial pressure; HCO3−: arterial bicarbonate concentration; BE: base excess; SID: strong ion difference.
The pH was similar between the groups (7.44 (7.43–7.46) vs. 7.44 (7.42–7.46)). Using the Henderson–Hasselbalch approach, severe hypoxemic and moderate hypoxemic group presented similar frequency of respiratory alkalosis, metabolic alkalosis due to aSID and other metabolic alkalosis: 22 (41%) versus 20 (40%), 4 (9%) versus 10 (20%) and 10 (18%) versus 10 (20%).
4. Discussion
The main findings of this observational study in COVID-19 patients with ARDS can be summarized as follows: (1) up to forty percent of the patients presented respiratory alkalosis, (2) up to thirty percent of the patients presented metabolic alkalosis, using both the Henderson–Hasselbalch and Stewart approach, (3) Stewart’s method allows us to classify 12% of the patients with a mixed disorder not detected by the traditional method and (4) the impairment of oxygenation did not affect the acid-base disorders.
A normal acid-base homeostasis is fundamental to guarantee normal physiology and cell function. The presence of any acid-base disorders is associated with higher risk for a worse outcome [19,20]. In non-COVID-19 critically ill patients the most frequently reported acid-base derangement is the acidemia, ranging between 14% and 42%. In a prospective observational study enrolling more than 2500 critically ill patients, 8% had acidemia with an associated intensive care mortality of 57% [8].
The acid-base alterations are generated by several conditions such as respiratory failure, shock, renal and hepatic failure [11]. The severe acid-base derangements are potentially life-threating conditions; thus, a precocious and accurate identification is necessary to improve the outcome [19].
According to the physiological approach the acid-base status depends on the proton concentration (i.e., the pH), on the bicarbonate concentrations [HCO3-] and on carbon dioxide (PCO2) [11,21]. Acidemia and alkalemia are defined as the accumulation/increase or loss of proton into or from the plasma resulting in a lower or higher pH in absence of any compensatory response. These conditions arise from a respiratory or metabolic alteration [11,12]. In addition, a combination of single derangements in the respiratory or metabolic function can generate a mixed acid-base disorder [11].
The clinical consequences of COVID-19 could range from an asymptomatic condition to a severe disease requiring hospital admission in up to 50% with an associated mortality between 40% and 80% [22,23]. Critically ill patients more often have pneumonia with hypoxemia but can also present other organ dysfunctions such as a cardiovascular, renal and liver failure [6,24]. Thus, these patients, due to several different clinical failures, can present a wide spectrum of acid-base disorders. However, at the present time, only a few studies are available regarding the early assessment of the acid-base disorders in COVID-19 patients [9,25]. The presence of respiratory alkalosis has been detected in between 29.0% and 30.3% [9,25].
In the present study, enrolling 105 COVID-19 patients, 40% of these presented a respiratory alkalosis. Eighty-four percent received a CPAP support, while 16% received non-invasive ventilation. In non-COVID-19 patients the respiratory alkalosis or hypocapnic alkalosis is related to the hyperventilation (increase in respiratory and or tidal volume) associated with the hypoxemia in presence of a pulmonary or central nervous system disease [26]. Wu et al. reported that COVID-19 patients with respiratory alkalosis within the first day from hospital admission had higher rates of underlying diseases and inflammatory markers but similar extension of the disease at lung CT compared to patients without respiratory alkalosis [25]. Similarly, Chen et al., in a cohort of 799 patients, found that the arterial CO2 was significantly lower in the patients who died compared to the survivors [27].
Unfortunately, in the present study the tidal volume during CPAP could not be measured. However, the respiratory rate was not different among the acid-base groups and was not related to the PaCO2 (p = 0.205, R2 = 0.01); thus, we hypothesized that the greater minute ventilation was due to the higher tidal volume. In addition, the respiratory rate was not related to the hypoxia and the oxygenation was similar among the groups confirming that, contrary to non-COVID-19 patients with acute respiratory failure, in COVID-19 patients the minute ventilation was not related to the amount of hypoxia [28]. This lower response to the hypoxemia in COVID-19 could be related to the several effects of the virus on the central nervous system and in the lung [28,29], but remains to be elucidated.
The second more frequent acid-base disorder was the metabolic alkalosis with a reported rate similar to those previously showed by Alfano et al. in COVID-19 patients analyzed within the first 48 hours from the hospital admission (31% and 33%, respectively) [9]. In non-COVID-19 the loss of gastric fluid and the use of diuretics account for the majority of cases of metabolic alkalosis [11]. In our population, we can exclude the presence of vomiting or diuretics and the main hypothesis could be the simultaneous presence of fever with previous dehydration due to the long stay at home before hospital admission and the possible activation of the Renin Angiotensin system due to SARS-CoV-2 infection [30].
A minority of the patients presented respiratory acidosis with just a slight increase in the arterial PCO2; this was related to the early application of the non-invasive ventilation which was able to guarantee an adequate minute ventilation [31].
No patients developed metabolic acidosis because all the patients presented an adequate hemodynamic, without any lactate accumulation or acute renal failure [32].
However, the physiological approach has been questioned because it ignores, according to the principles of physical chemistry the role of water dissociation as a determinant of the pH [13,33]. Taking into account this principle, Stewart suggested a mathematical approach which showed that pH is determined by only three independent variables: the SID, carbon dioxide and the concentration of the weak acids (protein and phosphate). Stewart’s method, by computing the components of the acid-base disorders individually, offers a better understanding of the pathogenesis; in fact, a respiratory acidosis/alkalosis is generated by an increase or decrease in the carbon dioxide, while a metabolic acidosis/alkalosis by a decrease or increase in the SID or in an increase or decrease in the weak acid concentration [13].
In the present study, the SID was computed considering the difference between only the most present anions and cations as sodium, potassium, chloride and lactate. According to this method, 43% and 33% of patients presented a respiratory and metabolic alkalosis, respectively, similar to those obtained by the physiological approach.
Patients with other metabolic alkalosis presented median SID values within normal range, suggesting the alkalemia was mainly generated by the effect of weak acid, in particular by the albumin and by phosphate. Thus, a decrease in albumin in COVID-19 patients, probably due to a not adequate nutrition or to an increased catabolism, caused a reduction in the weak acids with an alkalinizing effect.
Similarly, 13 patients (12%) had a mixed acid-base disorder characterized by a normal pH with a reduction in SID and a normal or decreased PaCO2, then we can hypothesize when PaCO2 is within normal range that the normal pH was generated by the combined effect of the strong ions and of the weak acids.
Moreover, in our population the mixed alkalosis, identified using the Henderson–Hasselbalch approach, can be detected by Stewart’s approach in a subgroup of patients with respiratory alkalosis and with an associated alkalinizing effect of weak acids.
Interestingly, regarding the possible contribution of the hypoxemia in the acid-base disorders, we did not find any effects on either respiratory or metabolic alterations. This could be explained by an adequate oxygen delivery to the organs guaranteed by the non-invasive respiratory support and by the absence of a hemodynamic failure.
Limitations
Possible limitations of the present study are the retrospective nature, the absence of any data on the patients at hospital admission in the emergency department and the absence of the albumin and phosphate values to calculate the effective SID.
5. Conclusions
In conclusion, COVID-19 patients with ARDS treated with non-invasive respiratory support within the first 48 h mainly presented respiratory or metabolic alkalosis. Thus, based on this data, strictly acid-base monitoring should be necessary during the first days of hospital admission in order both to prevent further clinical derangements and to provide an adequate assistance.
Author Contributions
Conceptualization, D.C. and S.C.; methodology, T.P. and S.C.; validation, D.C. and G.F.S.P.; formal analysis, T.P. and S.C.; investigation, I.F., L.M. and M.M.; data curation, T.P., I.F., L.M. and M.M.; writing—original draft preparation, D.C. and S.C.; writing—review and editing, T.P., G.F.S.P. and S.C.; supervision, D.C. and S.C.; funding acquisition, G.F.S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Regione Lombardia as part of the Registry for COVID-19 Emergency (RECOVER).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of MILANO AREA 1 (protocol code 17263/2020-2020/ST/095).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arentz M., Yim E., Klaff L., Lokhandwala S., Riedo F.X., Chong M., Lee M. Characteristics and Outcomes of 21 Critically Ill Patients with COVID-19 in Washington State. JAMA—J. Am. Med. Assoc. 2020;323:1612–1614. doi: 10.1001/jama.2020.4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu Y., Xu D., Fu S., Zhang J., Yang X., Xu L., Xu J., Wu Y., Huang C., Ouyang Y., et al. Patients with COVID-19 in 19 ICUs in Wuhan, China: A cross-sectional study. Crit. Care. 2020;24:219. doi: 10.1186/s13054-020-02939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ronco C., Reis T., Husain-syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Middeldorp S., Coppens M., van Haaps T.F., Foppen M., Vlaar A.P., Müller M.C.A., Bouman C.C.S., Beenen L.F.M., Kootte R.S., Heijmans J., et al. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J. Thromb. Haemost. 2020;18:1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang X., Yu Y., Xu J., Shu H., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., et al. Clinical Course and outcomes of critically ill patients with COVID19 in Wuhan China. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanella A., Florio G., Antonelli M., Bellani G., Berselli A., Bove T., Cabrini L., Carlesso E., Castelli G.P., Cecconi M., et al. Time course of risk factors associated with mortality of 1260 critically ill patients with COVID-19 admitted to 24 Italian intensive care units. Intensive Care Med. 2021;47:995–1008. doi: 10.1007/s00134-021-06495-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung B., Rimmele T., Le Goff C., Chanques G., Corne P., Jonquet O., Muller L., Lefrant J.Y., Guervilly C., Papazian L., et al. Severe metabolic or mixed acidemia on intensive care unit admission: Incidence, prognosis and administration of buffer therapy. A prospective, multiple-center study. Crit. Care. 2011;15:R238. doi: 10.1186/cc10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alfano G., Fontana F., Mori G., Giaroni F., Ferrari A., Giovanella S., Ligabue G., Ascione E., Cazzato S., Ballestri M., et al. Acid base disorders in patients with COVID-19. Int. Urol. Nephrol. 2021;54:405–410. doi: 10.1007/s11255-021-02855-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berend K., de Vries A.P.J., Gans R.O.B. Physiological Approach to Assessment of Acid–Base Disturbances. N. Engl. J. Med. 2014;371:1434–1445. doi: 10.1056/NEJMra1003327. [DOI] [PubMed] [Google Scholar]
- 12.Rastegar A. Clinical utility of Stewart’s method in diagnosis and management of acid-base disorders. Clin. J. Am. Soc. Nephrol. 2009;4:1267–1274. doi: 10.2215/CJN.01820309. [DOI] [PubMed] [Google Scholar]
- 13.Stewart P.A. Modern quantitative acid-base chemistry. Can. J. Physiol. Pharmacol. 1983;61:1444–1461. doi: 10.1139/y83-207. [DOI] [PubMed] [Google Scholar]
- 14.Stewart P.A. Independent and dependent variables of acid-base control. Respir. Physiol. 1978;33:9–26. doi: 10.1016/0034-5687(78)90079-8. [DOI] [PubMed] [Google Scholar]
- 15.Boniatti M.M., Cardoso P.R.C., Castilho R.K., Vieira S.R.R. Acid-base disorders evaluation in critically ill patients: We can improve our diagnostic ability. Intensive Care Med. 2009;35:1377–1382. doi: 10.1007/s00134-009-1496-2. [DOI] [PubMed] [Google Scholar]
- 16.Fencl V., Jabor A., Kazda A., Figge J. Diagnosis of metabolic acid-base disturbances in critically III patients. Am. J. Respir. Crit. Care Med. 2000;162:2246–2251. doi: 10.1164/ajrccm.162.6.9904099. [DOI] [PubMed] [Google Scholar]
- 17.Dubin A., Menises M.M., Masevicius F.D., Moseinco M.C., Kutscherauer D.O., Ventrice E., Laffaire E., Estenssoro E. Comparison of three different methods of evaluation of metabolic acid-base disorders. Crit. Care Med. 2007;35:1264–1270. doi: 10.1097/01.CCM.0000259536.11943.90. [DOI] [PubMed] [Google Scholar]
- 18.Apigo M., Schechtman J., Dhliwayo N., Al Tameemi M., Gazmuri R.J. Development of a work of breathing scale and monitoring need of intubation in COVID-19 pneumonia. Crit. Care. 2020;24:4–6. doi: 10.1186/s13054-020-03176-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raphael K.L., Zhang Y., Wei G., Greene T., Cheung A.K., Beddhu S. Serum bicarbonate and mortality in adults in NHANES III. Nephrol. Dial. Transplant. 2013;28:1207–1213. doi: 10.1093/ndt/gfs609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell J.H., Wildenthal K., Johnson R.L. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int. 1972;1:375–389. doi: 10.1038/ki.1972.48. [DOI] [PubMed] [Google Scholar]
- 21.Berend K. Diagnostic use of base excess in acid-base disorders. N. Engl. J. Med. 2018;378:1419–1428. doi: 10.1056/NEJMra1711860. [DOI] [PubMed] [Google Scholar]
- 22.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA-J. Am. Med. Assoc. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., Tobin K.A., Cerfolio R.J., Francois F., Horwitz L.I. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C., Wang G., Zhang Q., Yu B., Lv J., Zhang S., Wu G., Wu S., Zhong Y. Association Between Respiratory Alkalosis and the Prognosis of COVID-19 Patients. Front. Med. 2021;8:564635. doi: 10.3389/fmed.2021.564635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laffey J.G. Hypocapnia. N. Engl. J. Med. 2002;347:43–53. doi: 10.1056/NEJMra012457. [DOI] [PubMed] [Google Scholar]
- 27.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G., Ma K., Xu D., Yu H., Wang H., et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bulfamante G., Chiumello D., Canevini M.P., Priori A., Mazzanti M., Centanni S., Felisati G. First ultrastructural autoptic findings of SARS-CoV-2 in olfactory pathways and brainstem. Minerva Anestesiol. 2020;86:678–679. doi: 10.23736/S0375-9393.20.14772-2. [DOI] [PubMed] [Google Scholar]
- 30.Alfano G., Guaraldi G., Fontana F., Ferrari A., Magistroni R., Mussini C., Cappelli G. The Role of the Renin-Angiotensin System in Severe Acute Respiratory Syndrome-CoV-2 Infection. Blood Purif. 2021;50:263–267. doi: 10.1159/000507914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coppola S., Santus P., Sotgiu G., Mondoni M., Gandola A., Saad M., Sferrazza Papa G.F., Centanni S., Saderi L., Chiumello D.A., et al. Feasibility and clinical outcomes of a step up noninvasive respiratory support strategy in patients with severe COVID-19 pneumonia. J. Clin. Med. 2021;10:5444. doi: 10.3390/jcm10225444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nechipurenko Y.D., Semyonov D.A., Lavrinenko I.A., Lagutkin D.A., Generalov E.A., Zaitceva A.Y., Matveeva O.V., Yegorov Y.E. The role of acidosis in the pathogenesis of severe forms of COVID-19. Biology. 2021;10:852. doi: 10.3390/biology10090852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyle M., Baldwin I. Introduction to an alternate view of acid/base balance: The strong ion difference or Stewart approach. Aust. Crit. Care. 2002;15:14–20. doi: 10.1016/S1036-7314(02)80039-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.