Abstract
(1) Introduction: There is an increasing literature describing neonates born to mothers with SARS-CoV-2 infection (MIS-N) and infants infected with SARS-CoV-2 who presented with a severe disease (MIS-C). (2) Methods: To investigate clinical features of multisystem inflammatory syndrome in neonates and infants under six months of age, we used a systematic search to retrieve all relevant publications in the field. We screened in PubMed, EMBASE and Scopus for data published until 10 October 2021. (3) Results: Forty-eight articles were considered, including 29 case reports, six case series and 13 cohort studies. Regarding clinical features, only 18.2% of MIS-N neonates presented with fever; differently from older children with MIS-C, in which gastrointestinal symptoms were the most common manifestation, we displayed that cardiovascular dysfunction and respiratory distress are the prevalent findings both in neonates with MIS-N and in neonates/infants with MIS-C. (4) Conclusions: We suggest that all infants with suspected inflammatory disease should undergo echocardiography, due to the possibility of myocardial dysfunction and damage to the coronary arteries observed both in neonates with MIS-N and in neonates/infants with MIS-C. Moreover, we also summarize how they were treated and provide a therapeutic algorithm to suggest best management of these fragile infants.
Keywords: neonate, SARS-CoV-2, COVID-19, infant, children, infection, autoantibodies, heart, respiratory distress
1. Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) can infect all age groups. To date, children seem to have a more favourable clinical course of the related disease COVID-19, as observed during the last two years of pandemic [1]. Neonatal SARS-CoV-2 infections seem less frequent even now [2], probably due to the lower expression of SARS-CoV-2 entry receptors in nasal epithelium in both term and preterm neonates, compared with adults [3]. Neonatal cases are mostly linked to horizontal transmission due to familial clusters [4], although the rare possibility of a maternal fetal transmission has been demonstrated by Vivanti et al. [5].
However, there is an increasing literature describing SARS-CoV-2 infected children who become critically sick [6] because of the onset of a multisystem inflammatory syndrome named MIS-C. MIS-C has emerged as a significant COVID-19 related consequence, apparently not sparing neonates and infants, who can even require hospitalization and intensive care unit (ICU) support to survive [7,8]. This seems temporally associated with a SARS-CoV-2 infection.
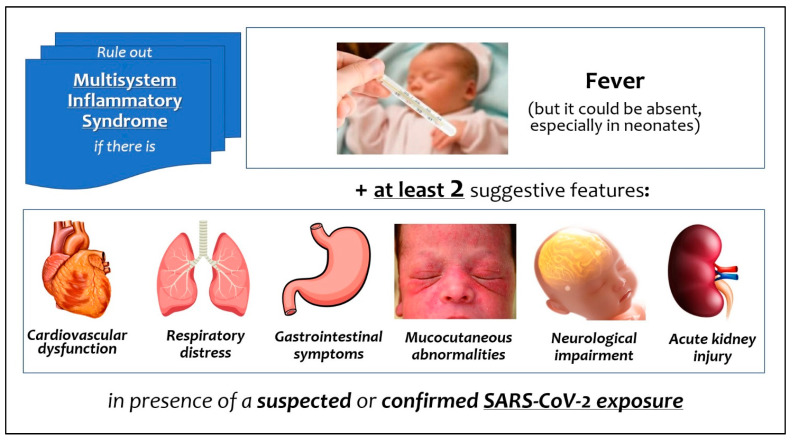
The most frequent mode of onset is fever and multi-organ involvement (Figure 1), associated with a rise in inflammatory biomarkers [9]. A similar multisystem inflammatory syndrome has been documented in adults (MIS-A) [10]. Patients with MIS-C are mostly children older than 7 years [7], but Pawar et al. [11] were the first to distinguish the early neonatal inflammatory syndrome (with onset within one week of life) in infants born to mothers with COVID-19 contracted in pregnancy (MIS-N), from that complicating the neonatal infection contracted after birth [11].
Figure 1.
Signs and symptoms of multisystem inflammatory syndrome related to SARS-CoV-2 in neonates and infants.
In this review we conducted a thorough analysis and synthesis of previously described cases of MIS-N and MIS-C in neonates and infants, aiming to describe clinical features of multisystem inflammatory syndrome in these delicate patients. Moreover, we also summarize how these infants were treated, in order to provide preliminary suggestions for management in this new clinical scenario.
2. Materials and Methods
We performed this systematic review following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines throughout the whole project. Prior to commencing the search, a detailed protocol was agreed to determine search modalities, eligibility criteria, and all methodological details. We searched for cohort, cross-sectional and case-control studies, as well as case series or case reports published as articles or letters to the editors describing neonates with multisystem inflammatory syndrome following maternal SARS-CoV-2 infection (MIS-N) or neonates and infants within first six months of life infected with SARS-CoV-2 and with MIS-C features.
We conducted an extensive search of the following databases (accessed on 10 October 2021): PubMed (https://pubmed.ncbi.nlm.nih.gov/), Scopus (https://www.scopus.com/search/form.uri?display=basic#basic) and Embase (https://www.embase.com/?phase=continueToApp#search). We used the following terms: ((“pediatric” AND “multisystem” AND “inflammatory” AND “syndrome”) OR (“multisystem” AND “inflammatory” AND “syndrome” AND “in” AND “children”)) AND “SARS-CoV-2”. Additional studies were identified by authors based on their knowledge in the field, if not already included by literature search. We excluded (1) all retrieved articles written in non-English language; (2) articles which did not clearly report the number of infants under six months of age.
Articles were assessed by independent researchers (DUDR, FP, MC, SR, SC, CM, LM and AS). Investigators evaluated abstracts and (where necessary) the full text of each article, excluding those not meeting the eligibility criteria, and removing duplicates. The CARE (Consensus-based Clinical Case Reporting Guideline Development) recommendations, specifically dedicated to case reports and series, were followed during the evaluation process. If an article was eligible but reported data on neonates and infants mixed with those on older children, eligible data were directly extracted. If there were uncertainties, they were resolved by discussion between the independent researchers and, if no agreement was reached, with the senior researcher (CA). We developed a dedicated online data extraction sheet (Excel 16: Microsoft Corporation, Redmond, WA, USA). Data from included records were independently extracted by each investigator using this data extraction sheet and then cross-verified.
Since we expected the majority of analyzed articles to be case reports or case series, we used the Mayo Evidence-Based Practice Centre tool that is specifically dedicated to the evaluation of case report/series quality [12]. Two investigators (DUDR and CA) independently summarized the results of this evaluation by aggregating the eight binary responses into a 0–8 score. Evaluation results were also qualitatively summarized (Low-Intermediate-Good), as recommended by the tool creators. If discrepancies or uncertainties persisted, they were resolved by discussion between the two researchers (DUDR and CA). We performed calculations and statistics with Excel 16 (Microsoft Corporation, Redmond, WA, USA), reporting continuous data as median (interquartile range, IQR), and categorical data as numbers and percentages.
3. Results
3.1. Workflow of Review and Synthesis
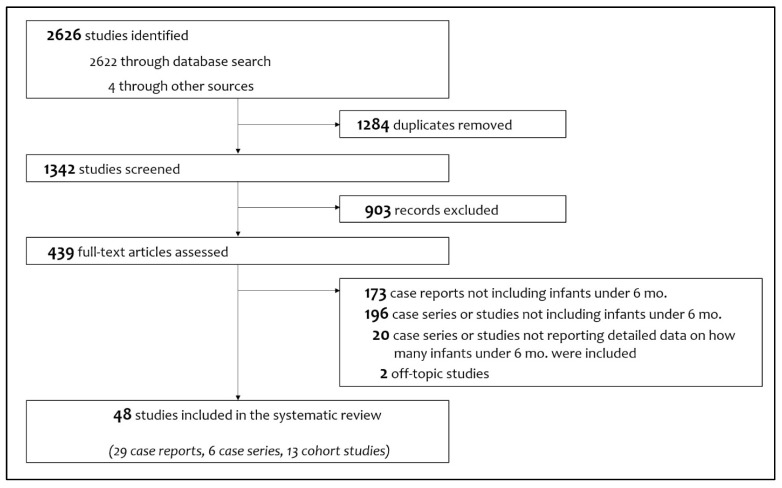
Figure 2 shows the study flow chart with included and excluded items (and the reasons for their exclusions). Finally, 48 articles were considered, consisting of 29 case reports [5,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40], six case series [11,41,42,43,44,45] and 13 cohort studies [34,46,47,48,49,50,51,52,53,54,55,56,57].
Figure 2.
Flowchart of study selection process.
We describe in Table 1 the characteristics of the included papers: we assessed as intermediate (median score 5 (5,6)) the methodological quality of case reports, case series and cohort studies which provided supplemental data with case descriptions. Ninety cases of neonates and infants under 6 months of age with multisystem inflammatory syndrome were described in the literature at the time of the search.
Table 1.
Characteristics of articles included in the systematic review. Methodological quality of case description was assessed only for case report and case series, or cohort studies which provided supplemental data with case descriptions.
| First Author | Article Type | Country | Quality Score | Overall Quality | Neonates and Infants < 6 Months with MIS (n) |
|---|---|---|---|---|---|
| Abdel-Haq [46] | Cohort study | United States | N.A. | N.A. | 1 |
| Acharyya [13] | Case report | India | 4 | Intermediate | 1 |
| Agrawal [14] | Case report | India | 6 | Good | 1 |
| Alharbi [47] | Cohort study | Saudi Arabia | 5 | Intermediate | 2 |
| Amonkar [15] | Case report | India | 5 | Intermediate | 1 |
| Antúnez-Montes [48] | Cohort study | Mexico | N.A. | N.A. | 3 |
| Caro-Dominguez [49] | Cohort study | Spain | N.A. | N.A. | 1 |
| Chandran [50] | Cohort study | India | N.A. | N.A. | 3 |
| Cui [16] | Case report | China | 6 | Good | 1 |
| Del Barba [17] | Case report | Italy | 5 | Intermediate | 1 |
| Diggikar [18] | Case report | India | 5 | Intermediate | 1 |
| Divekar [19] | Case report | United States | 5 | Intermediate | 1 |
| Diwakar [20] | Case report | India | 5 | Intermediate | 1 |
| Dugue [21] | Case report | United States | 3 | Intermediate | 1 |
| Dufort [51] | Cohort study | United States | N.A. | N.A. | 1 |
| Esteve-Sole [52] | Cohort study | Spain | 3 | Intermediate | 1 |
| Falah [53] | Cohort study | Pakistan | 3 | Intermediate | 2 |
| Frauenfelder [22] | Case report | United Kingdom | 6 | Good | 1 |
| García-Howard [23] | Case report | Spain | 5 | Intermediate | 1 |
| Giacomet [24] | Case report | Italy | 5 | Intermediate | 1 |
| Godfred-Cato [54] | Cohort study | United States | N.A. | N.A. | 1 |
| Grewal [55] | Cohort study | United States | N.A. | N.A. | 9 |
| Güllü [56] | Cohort study | Turkey | N.A. | N.A. | 3 |
| Kappanyil [25] | Case report | India | 6 | Good | 1 |
| Khaund Borkotoky [26] | Case report | India | 5 | Intermediate | 1 |
| Jones [27] | Case report | United States | 5 | Intermediate | 1 |
| Lad [28] | Case report | India | 2 | Low | 1 |
| Lima [29] | Case report | Brazil | 6 | Good | 1 |
| Lorenz [30] | Case report | Germany | 3 | Intermediate | 1 |
| Luna Santiago [31] | Case report | Mexico | 3 | Intermediate | 1 |
| Malle [41] | Case series | United States | 6 | Good | 1 |
| Mariani [32] | Case report | United States | 3 | Intermediate | 1 |
| Marino [42] | Case series | Italy | 4 | Intermediate | 1 |
| McCarty [33] | Case report | United States | 5 | Intermediate | 1 |
| Mehra [57] | Cohort study | India | N.A. | N.A. | 1 |
| Niño-Taravilla [58] | Cohort study | Chile | 2 | N.A. | 2 |
| Orlanski-Meyer [34] | Case report | Israel | 5 | Intermediate | 1 |
| Pawar [11] | Case series | India | 6 | Good | 20 |
| Rakha [43] | Case series | Egypt | 5 | Intermediate | 5 |
| Raut [35] | Case report | India | 5 | Intermediate | 1 |
| Richardson [40] | Case report | United Kingdom | 6 | Good | 1 |
| Rodriguez-Gonzalez [36] | Case report | Spain | 5 | Intermediate | 1 |
| Saha [37] | Case report | India | 6 | Good | 1 |
| Schoenmakers [38] | Case report | Netherlands | 6 | Good | 1 |
| Shaiba [39] | Case report | Saudi Arabia | 6 | Good | 2 |
| Shaiba [44] | Case series | Saudi Arabia | 5 | Intermediate | 2 |
| Villacis-Nunez [45] | Case series | United States | 6 | Good | 1 |
| Vivanti [5] | Case report | France | 6 | Good | 1 |
| Total of included infants | 90 | ||||
N.A. Not available.
3.2. Neonates with MIS-N
Thirteen included papers [5,11,14,15,19,20,25,26,29,30,33,38,39] fully described 33 cases of neonates with multisystem inflammatory syndrome in the first week after birth, born to mothers with SARS-CoV-2 infection (Table 2). Twenty-four (72.7%) of these neonates, were born preterm, with a median gestational age of 34 [33,34,35,36] weeks and a median birthweight of 2020 [1890–2620] grams. The diagnosis was obtained with a median age of 2 (0–3) days. All infants were admitted to Neonatal Intensive Care Unit (NICU) and 17 (51.5%) of them required mechanical ventilation.
Table 2.
Characteristics of neonates with MIS-N with fully described cases.
| Subject Number | First Author | Age/ Sex/ Birthweight/GA |
Neonatal RT-PCR and Serology for SARS-CoV-2 | Prior Maternal SARS-CoV-2 Exposure | Fever | Organ Involvement | Laboratory Work-Up | Imaging | Treatment | NICU Admission/Need for MV/Length of Hospital Stay | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Agrawal [14] |
44 h/M/3300 g/39 w | RT-PCR neg/IgG pos/IgM neg | YES—positive maternal IgG (without vaccination): she had a history of contact with COVID-19 4 weeks before delivery but remained asymptomatic. | Yes | Hypotension; respiratory distress requiring MV; indurated ulcer with erythema noted on the occiput; non-bilious vomiting and abdominal distension—surgical abdomen | Leukocytosis (23,940), metabolic acidosis (pH 7.18), PCT 10.76, NT-proBNP 4297, D-dimer 1331 | Normal inflation with minimal pulmonary infiltrates bilaterally (CXR); normal echocardiogram; dilated small and large intestines with absence of rectal gas and no radiological evidence of intestinal atresia or NEC (AXR) | IVIG, steroids, inotropes (dopamine), enoxaparin, aspirin | Yes/Yes/16 days | Favorable |
| 2 | Amonkar [15] |
6 days/M/2400 g/full-term | RT-PCR neg/IgG pos/IgM pos | YES—maternal asymptomatic infection with positive IgG and IgM on day 11 of life in both mother and neonate | No | Respiratory distress requiring ventilatory support (not specified); progressive blackish discoloration of the toes of the right lower limb; irritability | Leukocytosis (17,100), CRP 18.6, PCT 1.28, albumin 3, NT-proBNP 12,194, ferritin 515, D-dimer 4110, IL-6 20.29 | Acute thrombosis of abdominal aorta below renal arteries (80–90% occlusion at echocardiogram) | Steroids, r-TPA and surgical embolectomy, aspirin after limb amputation, unfractionated heparin | Yes/No/28 days | Favorable but he required amputation of the leg |
| 3 | Divekar [19] |
At birth/F/1300 g/30 w | RT-PCR neg/IgG pos/IgM neg | YES—positive maternal RT-PCR prior to delivery | No | Hypotension and inadequate urine output; hypoxic respiratory failure requiring surfactant and HFOV; generalized anasarca; oliguric renal failure | Tn 189, NT-proBNP >5000, IL-6 21.9 | Cardiomegaly and pulmonary edema (CXR); small pericardial effusion without tamponade, pathological coronary artery dilation and ventricular systolic dysfunction (echocardiogram) | IVIG | Yes/Yes/2 months | Favorable |
| 4 | Diwakar [20] |
19 days/M/3910 g/39 w | RT-PCR neg/Positive IgG | YES—maternal fever a week before delivery; she resulted positive when the baby was admitted | Yes | Rash over forehead and cheeks on day 2; diarrhea | Thrombocytopenia (97 × 109/L), transient neutropenia, CRP 12.13, hyponatremia (127), albumin 2.92, D-dimer 710, IL-6 1624 | Clear lung fields (CXR); normal structure and function with normal coronary arteries (echocardiogram) | IVIG, aspirin | Yes/No/6 days | Favorable |
| 5 | Kappanayil [25] |
24 days/F/3750 g/term | RT-PCR neg/IgG pos/IgM neg | YES—mother with a history of positive RT-PCR, with mild COVID-19 at 31 weeks GA, which was managed with symptomatic and supportive measures. | No | Hypotension and tachycardia; hypoxic respiratory failure; cool peripheries with erythema over the occiput and at bilateral gluteal regions; drowsiness | CRP 0.65, hyper-transamin-asemia (ALT 866 U/L and AST 2240 U/L), metabolic acidosis (pH 7.13), Tn 123, NT-proBNP 157,000, ferritin 56,400, D-dimer 20,000 | Cardiomegaly (CXR); severe biventricular dysfunction; coronary arteries with normal luminal dimensions, but appeared prominent and hyper-choic (echocardiogram) | IVIG, steroids, inotropes (epinephrine and milrinone), furosemide, vitamin C and D, zinc, heparin | Yes/Yes/29 days | Favorable |
| 6 | Khaund Borkotoky [26] |
4 h/M/4840 g/38 w | RT-PCR neg/IgG pos/IgM neg | YES—maternal fever and cough 3 weeks before delivery. On the day of delivery, her throat and nasal swab for COVID-19 PCR were negative. Anti-SARS-CoV-2 IgG were positive. | Yes | PPHN; hypoxic respiratory failure at 4 h of life and again on day 7; vasculitic rash on day 14; features suggestive of early NEC on day 14 | Leukocytosis (19,800), thrombocytopenia (70 × 109/L), CRP 3.91, albumin 2.2, Tn 171.2, NT-proBNP 6125, ferritin 1432, D-dimer >10,000, IL-6 43.49 | Bilateral haze (CXR) and bilateral ground opacities considered consistent with COVID-19 (chest CD); PPHN (echocardiogram) | Steroids, sildenafil, inotropes (dopamine), furosemide | Yes/Yes/34 days | Favorable |
| 7 | Lima [29] |
3 days/F/2400 g/33 w | RT-PCR pos/IgG pos/IgM neg | YES—flu-like symptoms at 29 weeks GA. At 32 weeks, the fetal echocardiogram revealed significant pericardial effusion with dilation of the vena cava and an overload in the cardiovascular system | No | Pericardial effusion; hemodynamic instability or bradycardia; respiratory distress and apnea requiring mechanical ventilation | CRP 1.1, metabolic acidosis (pH 7.12), Tn 38,000, ferritin 358, INR 1.4, D-dimer 2200, IL-6 56.4 in serum and 202.9 in pericardial fluid | Inflammatory ground glass pattern affecting less than 25% of the lung parenchyma (chest CT); pericardial effusion (echocardiogram) | Pericardiocentesis; red blood cell concentrate transfusion, inotropes (not specified amine) | Yes/Yes/23 days | Favorable |
| 8 | Lorenz [30] |
24 h/F/NA/40 w | RT-PCR pos/NA | YES—mother with mild respiratory infection and loss of smell and taste, and fever during delivery (38 °C) | No | Respiratory distress at about 80 h of life, requiring CPAP and oxygen therapy until day 6 of life; encephalitic symptoms (lethargic but severely hyperexcitable, high pitched crying) at 54 h of life | NA | Bilateral viral pneumonia (CXR) | Paracetamol and caffeine | Yes/No/NA | Favorable |
| 9 | McCarty [33] |
1st day/M/NA/34 w | RT-PCR neg/Serology NA | YES—mother with COVID-19 symptoms and positive RT-PCR | Yes | Severe pulmonary hypertension; respiratory distress requiring surfactant, mechanical ventilation and inhaled nitric oxide | Lymphopenia (230 at 48 h of life), thrombocytopenia (25 × 109/L), CRP 6.78, metabolic acidosis (pH 7.00) | Diffuse bilateral granular opacities (CXR); severe PPHN (echocardiogram) | None | Yes/Yes/22 days | Favorable |
| 10 | Pawar [11] |
1st day/F/4000 g/38 w | RT-PCR NA/IgG pos on day 1/IgM neg | YES—maternal RT-PCR positive 3 weeks before delivery | Yes | Hypotension | CRP 1.4, PCT 1.3, ferritin 1500, D-dimer 5088 | LV dysfunction (echocardiogram) | IVIG, steroids, inotropes (not specified) | Yes/No/13 days | Favorable |
| 11 | Pawar [11] |
1st day/M/2020 g/35 w | RT-PCR NA/IgG pos on day 2/IgM neg | YES—asymptomatic, COVID-19 contact 8 weeks before delivery | No | Shock | NT-proBNP >30,000, ferritin 393, D-dimer 5100 | Bilateral effusions (CXR); LV dysfunction (echocardiogram) | IVIG, steroids, LMWH | Yes/No/14 days | Favorable |
| 12 | Pawar [11] |
4 days/F/2000 g/33 w | RT-PCR NA/IgG pos on day 6/IgM neg | YES—asymptomatic, COVID-19 contact 6 weeks before delivery, with positive IgG | No | Severe bradycardia, prolonged QTc and 2:1 AVB; respiratory distress requiring surfactant and MV | ferritin 407, D-dimer 3020 | NA | IVIG, steroids | Yes/Yes/16 days | Favorable |
| 13 | Pawar [11] |
1st day/M/2000 g/36 w | RT-PCR NA/IgG pos on day 1/IgM neg | YES—asymptomatic, COVID-19 contact 6 weeks before delivery | No | Dilated RA/RV, pericardial effusion, RV dysfunction; shock; respiratory distress | CRP 1.2, NT-proBNP >25,000, D-dimer 6848 | Pleural effusions (CXR); dilated hypertrophied RV with dysfunction, moderate TR, large thrombus at LPA origin on day 3 | IVIG, steroids, inotropes (not specified), alteplase, LMWH | Yes/Yes/19 days | Favorable |
| 14 | Pawar [11] |
3 days/M/3500 g/38 w | RT-PCR NA/IgG pos on day 5/IgM neg | YES—Febrile illness at 7 months of gestation | No | Hypotension and intermittent bradycardia; respiratory distress requiring MV; feeding intolerance; lethargy | Leukocytosis (18,600), CRP 6.0, PCT 2.1, ferritin 878, D-dimer 6483 | NA | IVIG, steroids, inotropes (not specified) | Yes/Yes/13 days | Favorable |
| 15 | Pawar [11] |
2 days/M/2300 g/34 w | RT-PCR NA/IgG pos on day 12/IgM neg | YES—Febrile illness 2 weeks before delivery | No | Hypotension and intermittent bradycardia; respiratory distress requiring CPAP; rash, pedal edema, oral and skin lesions, skin peeling; feeding intolerance, brown gastric aspirates on day 4, treated like NEC, bleeding; decreased activity on day 2 | Thrombocytopenia (39 × 109/L), CRP 2.4, D-dimer 4200 | NA | IVIG, steroids, inotropes (not specified), LMWH | Yes/No/38 days | Favorable |
| 16 | Pawar [11] |
3 days/F/1400 g/34 w | RT-PCR NA/IgG pos on day 1/IgM neg | YES—Asymptomatic, positive RT-PCR at 5th month of gestation | No | Supraventricular tachycardia from day 8; brown gastric aspirates from day 3, frank melena from day 6 | CRP 5.0, D-dimer 5100 | Bilateral pleural effusions (CXR and echocardiogram) | IVIG, steroids, beta-blockers, LMWH | Yes/No/20 days | Favorable |
| 17 | Pawar [11] |
2 days/M/1900 g/32 w | RT-PCR NA/IgG pos on day 1/IgM neg | YES—Asymptomatic, positive RT-PCR at 3rd month of gestation | No | Bradycardia with prolonged QTc and 2:1 AVB on day 2; respiratory distress requiring MV | CRP 4.3, D-dimer 6600, IL-6 116 | NA | IVIG, steroids, inotropes (not specified), LMWH | Yes/Yes/23 days | Favorable |
| 18 | Pawar [11] |
2 days/F/1900 g/33 w | RT-PCR NA/IgG pos on day 4/IgM neg | YES—Asymptomatic, COVID-19 contact 8 weeks before delivery | No | Bradycardia with prolonged QTc and 2:1 AVB on day 2 | CRP 3.5, D-dimer 10,000 | NA | IVIG, steroids, LMWH | Yes/No/18 days | Favorable |
| 19 | Pawar [11] |
2 days/M/1600 g/33 w | RT-PCR NA/IgG pos on day 5/IgM neg | YES—Asymptomatic, COVID-19 contact 8 weeks before delivery | No | Bradycardia with prolonged QTc and 2:1 AVB on day 4 | D-dimer 10,000 | NA | IVIG, steroids, LMWH | Yes/No/18 days | Favorable |
| 20 | Pawar [11] |
4 days/F/2050 g/34 w | RT-PCR NA/IgG pos on day 4/IgM neg | YES—Febrile illness 3 weeks before delivery, with IgG level below cut-off | No | Bradycardia with prolonged QTc and 2:1 AVB on day 4 | PCT 1.8, NT-proBNP >25,000, D-dimer 4840 | NA | IVIG, steroids, inotropes (not specified), LMWH | Yes/No/11 days | Favorable |
| 21 | Pawar [11] |
4 days/M/2100 g/34 w | RT-PCR NA/IgG pos on day 4/IgM neg | YES—Febrile illness 3 weeks before delivery, with IgG level below cut-off | No | Bradycardia with prolonged QTc and 2:1 AVB on day 4 | PCT 1.4, D-dimer 5932 | NA | IVIG, steroids, inotropes (not specified), LMWH | Yes/No/11 days | Favorable |
| 22 | Pawar [11] |
3 days/F/1000 g/27 w | RT-PCR NA/IgG pos on day 4/IgM neg | YES—Asymptomatic, COVID-19 contact 8 weeks before delivery | No | Bradycardia with prolonged QTc and 2:1 AVB on day 4 | PCT 51, NT-proBNP 25,000, D-dimer 10,000 | NA | IVIG, steroids, LMWH | Yes/Yes/death on day 11 due to NEC | Death on day 11 (NEC) |
| 23 | Pawar [11] |
2 days/M/2400 g/36 w | RT-PCR NA/IgG pos on day 6/IgM neg | YES—Asymptomatic, COVID-19 contact 10 weeks before delivery, with positive IgG | No | Cardiogenic shock on day 5; echo-dilated coronaries severe TR, mild MR, ASD, PDA, Severe pulmonary hypertension; refusing feed on day 2 | CRP 1.1, D-dimer 4700 | Cardiomegaly (CXR); dilated coronaries, severe TR, mild MR, ASD, PDA, PPHN (echocardiogram) | IVIG, steroids, inotropes (not specified), aspirin, LMWH | Yes/Yes/14 days | Favorable |
| 24 | Pawar [11] |
4 days/M/2000 g/36 w | RT-PCR NA/IgG pos on day 4/IgM neg | YES—Asymptomatic, COVID-19 contact 4 weeks before delivery | No | Shock, bradycardia, mild LV dysfunction; acute renal failure, hyperkalemia; seizures | CRP 1.8, Cr 1.9, K+ 6.9, NT-proBNP 14,500, D-dimer 4700 | Small ASD, dilated all four chambers, mild LV dysfunction (echocardiogram) | IVIG, steroids, inotropes (not specified), peritoneal dialysis | Yes/Yes/death on day 8 due to MIS-C | Death on day 8 (multi-organ dysfunction) |
| 25 | Pawar [11] |
1st day/F/2000 g/36 w | RT-PCR NA/IgG pos on day 6/IgM neg | YES—Asymptomatic, COVID-19 contact 4 weeks before delivery, with positive IgG | Yes on day 1 | Tachypnea, desaturation; feeding intolerance, vomiting | CRP 6.2, PCT 2.4, NT-proBNP 7361, D-dimer 9734 | NA | IVIG, steroids | Yes/No/10 days | Favorable |
| 26 | Pawar [11] |
2 days/F/1500 g/32 w | RT-PCR NA/IgG neg/IgM neg | YES—Asymptomatic, COVID-19 contact 10 weeks before delivery | No | Bradycardia with prolonged QTc and 2:1 AVB on day 3; respiratory distress | CRP 1.8, D-dimer 12,000 | NA | IVIG, steroids, inotropes (not specified) | Yes/No/13 days | Favorable |
| 27 | Pawar [11] |
2 days/F/1500 g/32 w | RT-PCR NA/IgG below cut-off level/IgM negative | YES—Febrile illness 8 weeks before delivery | No | Bradycardia with prolonged QTc and 2:1 AVB on day 3; respiratory distress | CRP 2.5, NT-proBNP 23,700, D-dimer 10,000 | NA | IVIG, steroids | Yes/No/14 days | Favorable |
| 28 | Pawar [11] |
1st day/M/1900 g/34 w | IgG below cut-off level/IgM negative | YES—Febrile illness 6 weeks before delivery; IgG below cut-off levels, negative IgM | No | Dilated coronaries; not cried after birth; tachypnea, crepitations; pitting edema over chest wall; hepatomegaly | Thrombocytopenia (93 × 109/L), NT-proBNP >25,000, D-dimer 2820 | Pleural effusion (CXR); pericardial effusion, dilated coronaries, large PDA, mild TR, normal function on inotropes (echocardiogram); ascites and hepatomegaly (abdomen US) | IVIG, steroids, inotropes (not specified), aspirin, LMWH | Yes/Yes/NA | Favorable |
| 29 | Pawar [11] |
1st day/F/2700 g/38 w | RT-PCR NA/IgG pos on day 2/IgM neg | YES—Febrile illness 6 weeks before delivery; positive IgG | No | Poor peripheral pulsation and hypotension; not cried after birth; respiratory distress requiring surfactant; mottling | CRP 5.3, NT-proBNP 17,018, D-dimer 3942 | Intracardiac thrombus in RA on day 4, normal LV function (echocardiogram) | IVIG, steroids, inotropes (not specified), LMWH | Yes/Yes/NA | Favorable |
| 30 | Schoenmakers [38] |
At birth/F/1800 g/31 w | RT-PCR neg/Serology neg | YES—maternal SARS-CoV-2 infection during the third trimester associated with a placental inflammatory reaction and subsequent placental dysfunction | No | PPHN; hypoxic respiratory failure managed with surfactant, HFOV and inhaled nitric oxide; elevated creatinine; intraventricular hemorrhage | Leukopenia, thrombocytopenia, elevated liver function tests, elevated Cr, Tn and NT-proBNP, ferritin 14,272, elevated D-dimer | Bilateral opacities (CXR), significantly enlarged LMCA, flattened IVS, mild-to-moderate TR, small PDA with right-to-left shunt (echocardiogram) | IVIG, steroids, inotropes (not specified), aspirin | Yes/Yes/NA | Favorable |
| 31 | Shaiba [39] |
At birth/F/3004 g/36 w | RT-PCR neg/IgG pos/IgM not tested | YES—The mother tested positive for the SARS-CoV-2 virus in the second trimester, and then again positive 19 days prior to delivery | No | Poor systolic function and hypotension; respiratory distress requiring MV and inhaled nitric oxide; elevated creatinine | CRP 15, PCT 73 (day 1) and 1.22 (day 4), ALT 119 and AST 71, GGT 378, Cr 1, metabolic acidosis, Tn 130, NT-proBNP 3433, ferritin 384, INR 2.59 | Moderately dilated LV with poor systolic function, echogenic papillary muscles (could be secondary to ischemia vs. acidosis), wide PDA with a bidirectional shunt and myocarditis (echocardiogram) | IVIG, steroids, plasma transfusion, PGE1, inotropes (dobutamine) | Yes/Yes/12 days | Favorable |
| 32 | Shaiba [39] |
At birth/F/1700 g/32 w | RT-PCR pos/Serology neg | YES—Ten hours after delivery the mother’s swab result turned out to be positive for SARS-CoV-2 | No | Myocarditis, grunting and tachypnea, requiring CPAP; elevated creatinine | CRP 2.02, GGT 141, Tn 51.9, NT-proBNP 5610, ferritin 567, INR 1.06, D-dimer 1060 | Bilateral ground glass appearance with bilateral haziness and good lung volume (CXR); myocarditis (echocardiogram) | IVIG, steroids, | Yes/Yes/30 days | Favorable |
| 33 | Vivanti [5] |
3 days/M/2540 g/35 w | RT-PCR pos/Serology NA | YES—Severe cough since 2 days before hospitalization; positive RT-PCR in blood, amniotic fluid, nasopharyngeal and vaginal swabs | No | Respiratory distress requiring mechanical ventilation; poor feeding on day 3; irritability, axial hypertonia and opisthotonos on day 3 | GGT 290, Tn 43 | Brain MRI on day 11 showed bilateral gliosis on the deep white periventricular and subcortical matter, with slightly left predominance | None | Yes/Yes/18 days | Favorable |
Abbreviations: ALT = alanine transaminase, ASD = atrial septal defect; AST = aspartate transaminase, AVB = atrioventricular block; AXR = abdominal X-ray; CPAP = continuous positive airway pressure; Cr = serum creatinine; CRP = C-reactive protein; CT = computed tomography; CXR = chest X-ray; F = female; g = grams; GA = gestational age; GGT = gamma-glutamyl-transferase; HFOV = high frequency oscillator ventilation; IgG = immunoglobulin G; IgM = immunoglobulin M; IL-6 = interleukin-6; INR = International normalized ratio of prothrombin time; IVIG = intravenous immunoglobulin; IVS = interventricular septum; K+ = potassium; LAD = left anterior descending coronary artery; LMCA = left main coronary artery; LDH = lactate dehydrogenase; LMWH = low molecular weight heparin; LPA = left pulmonary artery; LV = left ventricle; M = male; MR = mitral regurgitation; MRI = magnetic resonance imaging; MV = mechanical ventilation; NA = not available; NEC = necrotizing enterocolitis; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PCT = procalcitonin; PDA = patent ductus arteriosus; PPHN = persistent pulmonary hypertension of the new-born; QTc = corrected QT interval; RA = right atrium; RCA = right coronary artery; r-TPA = recombinant tissue plasminogen activator; RT- PCR = reverse transcription-polymerase chain reaction; RV = right ventricle; SVT = supraventricular tachycardia; Tn = troponin; TR = tricuspid regurgitation; w = weeks. Measure units: Albumin = mg/dl; ALT and AST = U/L; Cr = mg/dl; CRP = mg/dl; D-dimer = mg/dl; Ferritin = ng/mL; GGT = U/L; IL-6 = pg/mL; K+ = mEq/L; LDH = U/L; NT-proBNP = pg/mL; PCT = ng/mL; Tn = ng/L.
Prior maternal SARS-CoV-2 exposure occurred in all infants. Neonatal RT-PCR for SARS-CoV-2 resulted positive only in four neonates (12.1%), whereas a positive serology yielded a maternal perinatal SARS-CoV-2 infection in 25 cases (75.8%).
Fever was observed only in six neonates (18.2%). Organ system involvement included cardiovascular dysfunction in 26 neonates (78.8%), respiratory distress in 22 (66.7%), gastrointestinal symptoms in nine (27.3%), mucocutaneous abnormalities in nine (27.3%), neurological impairment in eight (24.2%) and acute kidney injury in five (15.2%). None presented with musculoskeletal anomalies.
Laboratory tests showed mostly an increase in C-reactive protein (60.6%) and procalcitonin (27.3%), raised brain natriuretic peptide (NT-proBNP, 51.5%) and troponin (24.2%), increased D-dimer (84.4%) and interleukin-6 (18.2%), thrombocytopenia (18.2%), metabolic acidosis (15.1%), prolonged prothrombin time (6.1%), and hypoalbuminemia (9.1%).
Chest X-ray or chest CT discovered a pulmonary involvement in 12 neonates (36.4%), whereas echocardiography showed depressed ventricular functions and coronary anomalies in 17 (51.5%).
Twenty-seven neonates (81.8%) received intravenous immunoglobulins (IVIG: a total of 2 g/kg splitted in two infusions of 1 g/kg/day, without a further dose) and intravenous steroids (mostly methylprednisolone). Eighteen neonates (54.5%) needed inotropic support. None was treated with biologic medications or antivirals. The use of large-spectrum antibiotics was described in 10/13 cases (76.9%). Acetylsalicylic acid was given to 6 neonates (18.2%) while thromboprophylaxis was used in 13 cases (39.4%).
The median length of stay was 16 [13,14,15,16,17,18,19,20,21,22] days. The outcome was favourable in 30 neonates (90.9%); of these, a full-term male without comorbidities survived but required amputation of the right leg (because of an acute thrombosis of abdominal aorta) and a late preterm survived after peritoneal dialysis. Only two neonates died (the first on day 8 due to a multi-organ dysfunction and the second on day 11 because of necrotizing enterocolitis).
3.3. Neonates and Infants with MIS-C
Twenty-five included papers [13,16,17,18,21,22,23,24,27,28,31,32,34,35,36,37,40,41,42,43,44,45,47,52,53] describe 32 neonates and infants under the age of six months with multisystem inflammatory syndrome after the acquired SARS-CoV-2 infection (Table 3). Only six (18,8%) of these infants, had comorbidities. Thirteen infants (40.6%) required ICU admission, of whom 9/13 (69.2%) undergoing mechanical ventilation. Twenty-one infants (65.6%) had a positive RT-PCR for SARS-CoV-2, whereas in 12 (37.5%) previous exposure to SARS-CoV-2 was confirmed by a positive serology. In most cases, MIS-C was related to the positivity for SARS-CoV-2 of a family member in previous days or weeks (where reported): only one infant, hospitalized twice because of a late-onset sepsis, might have contracted the virus as a nosocomial infection.
Table 3.
Characteristics of neonates and infants under six months of age with MIS-C with fully described cases.
| Subject Number | First Author | Age/Sex/Comorbidities | RT-PCR and Serology for SARS-CoV-2 | Parental SARS-CoV-2 Exposure | Fever | Organ Involvement | Laboratory Work-Up | Imaging | Treatment | ICU Admission/Need for MV/Length of Hospital Stay | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Acharyya [13] |
4 months/M/NA | RT-PCR pos/NA | YES—his mother was also subsequently found positive for SARS-CoV-2. | Yes | Diffuse ectasia of coronary arteries; Erythematous macular rash over the trunk, palm and sole on day 2; Red lips, congested throat and small cervical lymphadenopathy; Irritability | CRP 11.56, Albumin 3, Anemia | Perivascular brightness and diffuse ectasia of coronary arteries (echocardiogram) | IVIG, aspirin | No/No/NA | Favorable |
| 2 | Alharbi [47] |
1 month/F/No | RT-PCR pos/NA | NA | No | Hypotension, increase in troponin; respiratory distress requiring MV for 12 days | Tn 2410, NT-proBNP 1127, ferritin 6130, IL-6 9.1 | Ejection fraction 60%, normal coronary arteries (echocardiogram) | IVIG, Anakinra, inotropes (not specified) | Yes/Yes/13 days | Death |
| 3 | Alharbi [47] |
3 months/M/No | RT-PCR pos/NA | NA | No | Hypotension, increase in troponin; respiratory distress requiring MV for 15 days | Tn 1294, NT-proBNP 2241, ferritin 813, IL-6 9.3 | Ejection fraction 80,5%, normal coronary arteries (echocardiogram) | IVIG, Anakinra | Yes/Yes/73 days | Favorable |
| 4 | Cui [16] |
55 days/F/No | RT-PCR pos/IgM pos | YES—a week earlier (parents) | No | Tachycardia; productive cough and hypoxic respiratory failure requiring oxygen supplementation through a nasal cannula (day 7 of illness) | CRP 5.6, Tn 25,000, D-dimer 54,000 | Ground-glass opacity (CXR); Patchy shadows and ground-glass opacity in the right lung (day 4 of illness), pneumonia (day 9) at chest CT | Inhaled interferon α-1b, reduced glutathione, urso-deoxycholic acid, and traditional Chinese medicine lotus qingwen | No/No/20 days | Favorable |
| 5 | Del Barba [17] |
38 days/M/No | RT-PCR pos/NA | YES—Both parents diagnosed with COVID-19 | Yes | Increase in troponin; rhinitis; modest hypo-reactivity | Thrombocytosis (525 × 109/L), PCT 3.28, Tn 82, NT-proBNP 208, D-dimer 133,000, fibrinogen 128 | Mild thickening of broncho-vascular markings, but no pulmonary parenchymal opacities (CXR); minimal pericardial effusion (cardiac MRI) | None | No/No/14 days | Favorable |
| 6 | Diggikar [18] | 7 days/NA/No | RT-PCR pos/IgG neg/IgM neg | YES—the mother tested positive for SARS-CoV-2 infection one day before the presentation | Yes | Small coronary artery aneurysm; apnoea and respiratory distress requiring MV; reduced tone, sluggish reflexes and seizures | CRP 60, D-dimer 5000 | Small coronary artery aneurysm (echocardiogram); diffuse changes involving the periventricular white matter, external capsule and internal capsule, while peripheral bilateral thalami show T2 fluid-attenuated inversion recovery hyperintensity with diffusion restriction (brain MRI) | Steroids, remdesivir, enoxaparin, levetiracetam and phenobarbitone | Yes/Yes/NA | Favorable |
| 7 | Dugue [21] | 6 weeks/M/No | RT-PCR positive for SARS-CoV-2 and Rhinovirus/NA | NA | Yes | Cough; mottled appearance; brief episodes of upward gaze associated with bilateral leg stiffening and altered responsiveness | Leukopenia (5070) | Excess of temporal sharp transients for age and intermittent vertex delta slowing with normal sleep-wake cycling (long-term EEG) | None | No/No/1 day | Favorable |
| 8 | Esteve-Sole [52] |
3 months/M/NA | RT-PCR neg/serology neg | YES | Yes | Peripheral extremity changes; gastrointestinal symptoms (not specified); irritability | Leukopenia (4000), CRP 12.8, Albumin 2.3, NT-proBNP 3628, D-dimer 2000 | Coronary abnormalities (not specified at echocardiogram) | IVIG, steroids, aspirin, clopidogrel | No/No/NA | Favorable |
| 9 | Falah [53] |
4 months/M/No | RT-PCR pos/NA | NA | Yes | Isolated coronary artery disease; Rash, conjunctival injection, lips and oral cavity changes; Irritability, neurological symptoms (not specified) | Leukocytosis, CRP 11.56, Albumin 3 | Coronary artery dilation (echocardiogram) | IVIG, aspirin | No/No/NA | Favorable |
| 10 | Falah [53] |
6 months/F/No | RT-PCR pos/NA | NA | Yes | Rash, conjunctival injection, lips and oral cavity changes; Not specified GI symptoms, poor feeding; Irritability, neurological symptoms (not specified) | Leukocytosis, CRP 13.3, Albumin 2.8 | Faint opacity in the left mid-lung zone (CXR) | IVIG, aspirin | No/No/NA | Favorable |
| 11 | Frauenfelder [22] |
32 days (corrected age 37 + 3 weeks)/M/Prematurity | RT-PCR pos/NA | YES—recent contact with family members and asymptomatic healthcare workers | No | Need for inotropes, mildly elevated troponin; significant glottic swelling and copious airway secretions; hypoxic respiratory failure requiring HFOV and inhaled nitric oxide | Lymphopenia (1.45 × 109/L), CRP 4.2, Albumin 2, Tn 138, Ferritin 138, D-dimer 1143 | Mild bilateral ground-glass opacities (CXR); Small patent foramen ovale with left-to-right shunt, mild dilation left side structures, and mild MR (echocardiogram) | Steroids, remdesivir, not specified anticoagulation (not specified, because of a line-associated femoral arterial thrombus), inotropes (noradrenaline and adrenaline) | Yes/Yes/18 days | Favorable |
| 12 | García-Howard [23] |
3 months/F/No | RT-PCR pos/NA | YES | Yes | Rhinorrhea and cough; Diarrhea; Mild hypotonia, staring gaze, clonic movements of the face and right extremities, and repeating sucking movements of the mouth, lasting <5 min | CRP 0.67, Ferritin 385, Increased D-dimer, negative CSF culture | Normal interictal EEG, Normal cerebral MRI | Levetiracetam, Hydroxychloroquine | No/No/9 days | Favorable |
| 13 | Giacomet [24] |
2 months/F/No | RT-PCR pos/NA | YES— Father and older brother tested positive |
Yes | Intermittent tachycardia; mottled skin; non-bloody diarrhea and vomiting | Anemia (Hb 7.9), Tn 103, NT-proBNP 12,507, D-dimer 1918, IL-6 236 | Hypokinesia of the inferior left ventricular wall and the inferior interventricular septum, with a mild decrease in the left ventricular ejection fraction | IVIG, Packed red blood cells transfusion | Yes/No/NA | NA |
| 14 | Jones [27] |
6 months/F/No | RT-PCR pos/NA | YES—her 9-year-old sibling had upper respiratory symptoms 3 weeks before | Yes | Sinus tachycardia during fever; mild congestion and subcostal retractions; Prominent tongue papilla; a blanching, polymorphous, maculopapular rash, limbic sparing conjunctivitis, and dry cracked lips; Irritability | Left-shifted white blood cell count with bandemia, normocytic anemia, CRP 13.3, Albumin 2.8 | Faint opacity in the left midlung zone (CXR) | IVIG, aspirin | No/No/NA | Favorable |
| 15 | Lad [28] |
4 months/F/NA | RT-PCR neg/IgG pos | YES—Father with COVID-19 a month before | Yes | Compensated shock; Vomiting and fresh blood in stool; Lethargy | Neutrophilic leukocytosis; Anemia (Hb 8) | Both coronaries dilated with high z-score > 2.5; Abdominal CT showed dilated jejunum and proximal ileum suggestive of obstruction. | IVIG, steroids, exploratory laparotomy (showing extensively congested ileum and tiny fibrous band about 2.5 cm crossing from antimesenteric border to mesentery in proximal ileum, without any obvious volvulus), packed red blood cells transfusion | No/No/NA | Favorable |
| 16 | Luna Santiago [31] |
2 months/M/Griscelli syndrome type 2 | RT-PCR pos/NA | NA | Yes | Shock; respiratory distress; Hepatosplenomegaly | Pancytopenia (in the context of a familial hemophagocytic lympho-histiocytosis triggered by SARS-CoV-2 at bone marrow aspiration), with increased CRP, D-dimer, triglycerides and IL-6 | NA | Steroids, anticoagulation therapy (not specified); inotropes (not specified), multiple transfusions, cyclosporine, cytarabine and inhibitor of JAK signaling | Yes/Yes/NA | Death |
| 17 | Malle [41] |
6 months/F/Down syndrome and CHD | RT-PCR pos/serology neg | YES—her father had contracted COVID-19 | Yes | Heart dysfunction and distributive shock; cough; Maculopapular erythematous rash with peripheral desquamation, edematous hands; Vomiting; Fatigue | Lymphopenia and neutrophilic leukocytosis, increased CRP and PCT, Hypoalbuminemia, Increased Tn, ferritin, D-dimer, fibrinogen and IL-6; Increased STAT3 phosphorylation and increased FcɣRI and ICAM1 on neutrophils and monocytes | Myocarditis and coronary dilatation (echocardiogram) | IVIG, steroids, lopinavir/ritonavir and hydroxychloroquine | Yes/No/4 months | Favorable |
| 18 | Mariani [32] |
5 months/F/NA | RT-PCR pos/IgG pos | YES—five weeks earlier, her father had tested positive | Yes | Intermittent tachycardia | Thrombocytopenia (36 × 109/L) in the context of a Severe transient pancytopenia with dys-erythropoiesis and dys-megakaryopoiesis; hypoalbuminemia, NT-proBNP 3617, D-dimer 8060 | Mild to moderate TR with small pericardial effusion (echocardiogram) | Steroids | No/No/NA | Favorable |
| 19 | Marino [42] | 5 months/F/No | RT-PCR neg/NA | No | Yes | Isolated coronary artery disease; Erythematous rash at the trunk; Sterile pyuria; Diarrhea; Irritability | CRP 6.4 and PCT 0.96, Tn 13, NT-proBNP 1019, Ferritin 259, D-dimer 1053 | Dilatation of both right coronary artery (RCA) and left main coronary artery (LMCA); dim opacity of the left lung base (CXR); gallbladder hydrops (abdominal US); | IVIG, steroids, aspirin | No/No/NA | Favorable |
| 20 | Orlanski-Meyer [34] | 8 weeks/F/No | RT-PCR neg/IgG pos | YES—both parents tested positive at 2 weeks of age. The positive serology was unlikely to represent the passive transfer of maternal antibodies. | No | Tachycardia; Cracked lips; Profuse watery diarrhea, transient bloody stool, vomiting | Thrombocytosis (958 × 109/L), ALT 173 and AST 140, GGT 274, Albumin 1.4, NT-proBNP 1011, Ferritin 385, Fibrinogen 393, IL-6 37.5 | Mild-moderate MR with normal coronary arteries and systolic function (echocardiogram); nonspecific intestinal wall changes and mucosal flattening and splenomegaly (abdominal US); patchy erythema and scattered pinpoint (colonoscopy) | IVIG, steroids, anakinra | No/No/NA | Favorable |
| 21 | Rakha [43] | 5 months/NA/No | NA/IgG and IgM pos | NA | Yes | Isolated coronary artery disease; not specified respiratory and GI symptoms | Leukocytosis, increased CRP and ferritin | Diffuse ectasia of RCA and LCMA; initial fractional shortening 35% (echocardiogram) | IVIG, aspirin | NA/NA/from 6 to 14 days | Favorable |
| 22 | Rakha [43] | 5 months/NA/No | NA/IgG and IgM pos | NA | Yes | Isolated coronary artery disease; not specified respiratory and GI symptoms | Leukocytosis, increased CRP and ferritin | Medium aneurysm LMCA, ectasia of LAD and diffuse ectasia of RCA; initial fractional shortening 29% (echocardiogram) | IVIG, aspirin | NA/NA/from 6 to 14 days | Favorable |
| 23 | Rakha [43] | 3 months/NA/No | NA/IgG and IgM pos | NA | Yes | Isolated coronary artery disease; not specified respiratory and GI symptoms | Leukocytosis, increased CRP and ferritin | Multiple medium and giant aneurysms in left circumflex, and LAD; initial fractional shortening 28% (echocardiogram) | IVIG, aspirin | NA/NA/2 days | Death on second day of admission |
| 24 | Rakha [43] | 6 months/NA/No | NA/IgG and IgM pos | NA | Yes | Coronary artery disease with myopericarditis; not specified respiratory and GI symptoms | Leukocytosis, increased CRP and ferritin | Ectasia of RCA, LCA, and LAD with decreased contractility; initial fractional shortening 22% (vomiting) | IVIG, aspirin | NA/NA/from 6 to 14 days | Favorable |
| 25 | Rakha [43] | 36 days/NA/No | NA/IgG and IgM pos | NA | Yes | Supraventricular tachycardia; not specified respiratory and GI symptoms | Leukocytosis, increased CRP and ferritin | Initial fractional shortening 28% (echocardiogram) | None | NA/NA/from 6 to 14 days | Favorable |
| 26 | Raut [35] | 5 months/M/No | RT-PCR pos/NA | NA | Yes | Isolated coronary artery disease; Skin rash and bilateral non-purulent conjunctivitis; Irritability | CRP 21.5, PCT 8.6, Albumin 2.4, NT-proBNP 2025, Ferritin 975 | Normal left ventricular function, with coronary dilatation in LMCA and LAD (echocardiogram) | IVIG, aspirin | No/No/22 days | Favorable |
| 27 | Richardson [40] | 5 months/F/No | RT-PCR neg/IgG pos a week after hospitalization | NA | Yes | Isolated coronary artery disease; respiratory distress requiring high-flow oxygen on day 5 of illness; Erythematous rash on trunk and extremities (at presentation) with peeling skin on her hands and feet and cracked lips (day 10) | CRP 50, Albumin 2.2, Ferritin 937, D-dimer 6692, Fibrinogen 4700 | Coronary artery aneurysm (echocardiogram) | IVIG, steroids, Anakinra then Infliximab, aspirin | Yes/No/NA | Favorable |
| 28 | Rodriguez-Gonzalez [36] |
6 months/M/short bowel syndrome (secondary to multiple intestinal resections during the neonatal period), antithrombotic prophylaxis due to previous local thrombotic obstructions of the central line | RT-PCR neg/IgG pos on day 21 of illness | NA | Yes | Cardiogenic shock secondary to severe pulmonary hypertension and new onset right ventricular failure; respiratory distress requiring MV | Thrombocytopenia (98 × 109/L), PCT 3.46, Tn 90, NT-proBNP 26,000, Ferritin 7634, Fibrinogen 179, IL-6 198 | Massive pulmonary thromboembolism, with a pattern of ground glass and numerous consolidations of predominance in the posterior-basal segments of both lungs (chest CT); Severely dilated right chambers, severe right ventricular systolic dysfunction, and supra-systemic pulmonary hypertension (echocardiogram); irregular pleural line, B-lines, some coalescent, with bilateral patchy distribution, and small peripheral consolidations, which were larger in posterior-basal areas (lung US) | Steroids, Tocilizumab, previous antithrombotic prophylaxis, hydroxychloroquine and inotropic support (milrinone and norepinephrine) | Yes/Yes/21 days | Favorable |
| 29 | Saha [37] | 25 days/F/Previous hospitalization due to bacterial late-onset sepsis | RT-PCR pos/NA | NO—No family members had signs and symptoms suggestive of SARS-CoV-2. She was in 2 different hospitals previously and may have contracted the virus there. | Yes | Cardiogenic shock; respiratory distress requiring MV; disseminated maculopapular rash; acute kidney injury post-resuscitation; hepatosplenomegaly and greenish watery stool; short-duration seizures | Thrombocytopenia (100 × 109/L), PCR 2.9, Metabolic acidosis, increased NT-proBNP, Ferritin 16,000, D-dimer 16,500 | Atelectasis of both lower lobes of lung (chest CT); Significant systolic dysfunction, with ejection fraction of 40% and mild pericardial infusion (echocardiogram) | IVIG, steroids, enoxaparin, inotropes (adrenaline and milrinone), phenobarbitone, furosemide, packed red blood cells transfusion | Yes/Yes/50 days | Favorable |
| 30 | Shaiba [44] | 30 days/F/NA | RT-PCR pos/NA | NA | Yes | Increased cardiac enzymes; respiratory distress requiring MV; impaired renal function; not specified GI symptoms | Thrombocytopenia (43 × 109/L), CRP 1.1 and PCT 1.7, Hyponatremia (123), Cr 2.44, Tn 684, NT-proBNP 971, Ferritin 2316, D-dimer 5500, Fibrinogen 225, | NA | IVIG, steroids, anakinra, heparin, hydralazine, amlodipine | Yes/Yes/15 days | Death |
| 31 | Shaiba [44] | 90 days/M/NA | RT-PCR pos/NA | NA | Yes | Increased cardiac enzymes; respiratory distress requiring MV | CRP 5.6, ALT 1070 and 1178, Cr 0.89, Tn 108, NT-proBNP 1370, Ferritin 813, INR 1.49, D-dimer 1320 | NA | IVIG, steroids, anakinra, aspirin, enoxaparin, hydralazine, amlodipine, sildenafil | Yes/Yes/75 days | Favorable |
| 32 | Villacis-Nunez [45] | 4 months/M/Prematurity, twin | RT-PCR pos/IgG pos on day 19 of illness | NA | Yes | Isolated coronary artery disease; respiratory distress requiring non-invasive positive-pressure ventilation; rash, hand and foot swelling, conjunctivitis; diarrhea | Increased CRP (>3) | Giant LAD and RCA aneurysms identified on day 21 of illness (echocardiogram); coronary involvement with possible LAD artery mural thrombus (cardiac CT) | IVIG, steroids, infliximab, remdesivir, aspirin, enoxaparin, clopidogrel | Yes/No/ 26 days | Favorable |
Abbreviations: ALT = alanine transaminase, ASD = atrial septal defect; AST = aspartate transaminase, AVB = atrioventricular block; AXR = abdominal X-ray; CPAP = continuous positive airway pressure; Cr = serum creatinine; CRP = C-reactive protein; CSF = cerebrospinal fluid; CT = computed tomography; CXR = chest X-ray; EEG = electroencephalogram; F = female; FCɣRI = FCɣ receptor I; g = grams; GGT = gamma-glutamyl-transferase; Hb = Hemoglobin; HFOV = high frequency oscillator ventilation; ICAM1 = Intercellular Adhesion Molecule 1; IgG = immunoglobulin G; IgM = immunoglobulin M; IL-6 = interleukin-6; INR = International normalized ratio of prothrombin time; IVIG = intravenous immunoglobulin; IVS = interventricular septum; K+ = potassium; LAD = left anterior descending coronary artery; LMCA = left main coronary artery; LDH = lactate dehydrogenase; LMWH = low molecular weight heparin; LPA = left pulmonary artery; LV = left ventricle; M = male; MR = mitral regurgitation; MRI = magnetic resonance imaging; MV = mechanical ventilation; NA = not available; NEC = necrotizing enterocolitis; NT-proBNP = N-terminal pro–B-type natriuretic peptide; PCT = procalcitonin; PDA = patent ductus arteriosus; PPHN = persistent pulmonary hypertension of the new-born; QTc = corrected QT interval; RA = right atrium; RCA = right coronary artery; r-TPA = recombinant tissue plasminogen activator; RT- PCR = reverse transcription-polymerase chain reaction; RV = right ventricle; STAT3 = Signal Transducer And Activator Of Transcription 3 gene; SVT = supraventricular tachycardia; Tn = troponin; TR = tricuspid regurgitation; US = ultrasound; w = weeks. Measure units: Albumin = mg/dl; ALT and AST = U/L; Cr = mg/dl; CRP = mg/dl; D-dimer = mg/dl; Ferritin = ng/mL; fibrinogen = mg/dl; GGT = U/L; Hb = g/dl; IL-6 = pg/mL; K+ = mEq/L; LDH = U/L; NT-proBNP = pg/mL; PCT = ng/mL; Tn = ng/L.
Twenty-seven infants (84.4%) presented with fever. Organ system involvement included cardiovascular dysfunctions in 26 infants (81.3%), respiratory distress in 23 (71.9%), gastrointestinal symptoms in 17 (53.1%), neurological impairment in 15 (46.9%), mucocutaneous abnormalities in 14 (43.8%) and acute kidney injury in three (9.4%). Only one (3.1%) presented with fatigue as musculoskeletal anomaly. Laboratory tests showed increase in C-reactive protein (68.8%) more than in procalcitonin (18.8%), elevated ferritin (56.2%), raised brain natriuretic peptide (NT-proBNP, 40.6%) and troponin (34.3%), increased D-dimer (46.9%) and interleukin-6 (21.8%), hypoalbuminemia (34.3%), and thrombocytopenia (15.7%).
Eight infants (25%) presented pulmonary involvement by chest X-ray and/or CT scan, whereas depressed ventricular function and coronaries anomalies were detected by echocardiography in 21 (65.6%).
Twenty-two infants (68.8%) received intravenous immunoglobulins (IVIG: a total of 2 g/kg splitted in two infusions of 1 g/kg/day, without a further dose) and fifteen (46.9%) intravenous steroids (mostly methylprednisolone). Five infants (15.6%) needed inotropic neonatal support. Nine infants (28.1%) received biologic medications (five anakinra, one anakinra and then infliximab, one infliximab, one tocilizumab, one inhaled interferon α-1b). Three infants (9.4%) were treated with remdesivir as antiviral.
The use of large-spectrum antibiotics was described in 17 cases (53.1%). Acetylsalicylic acid was given to 14 infants (43.8%); thromboprophylaxis was used in 14 cases (43.8%) and warfarin was given to one infant (3.1%).
The median length of stay was 15 (10–24) days. The outcome was favourable in 29 infants (90.6%), whereas four infants (12.5%) died: two neonate females within one month of life without comorbidities, a 2-months-old male with a familial hemophagocytic lymphohistiocytosis secondary to Griscelli syndrome type 2, and a 3-months-old baby with isolated coronary artery disease.
Analyzing data of neonates separately, among infants with MIS-C, only five (15.6%) had less than a month or before reaching term age if preterm born [18,22,37,39,46]. Three of five (60%) had fever. All had a cardiovascular and respiratory involvement, whereas two (40%) had gastrointestinal symptoms. Three (60%) received IVIG and four (80%) steroids. All required ICU admission and mechanical ventilation. Only two neonates (40%) died.
3.4. Differences between Neonates with MIS-N and Neonates with MIS-C
When SARS-CoV-2 infection was acquired postnatally, fever was a frequent feature (60%). rather than in neonates with MIS-N (18.2%). All neonates had a severe course, requiring NICU admission. Organ system involvement was similar for both neonate groups, with prevalent cardiovascular and respiratory signs and symptoms. We found no significant differences between the two groups for laboratory tests and treatments.
Two neonates died in both groups, with a slightly higher incidence in the MIS-C group although not significantly (2/33 MIS-N neonates versus 2/5 MIS-C neonates, p = 0.07).
3.5. Incidence of Multisystem Inflammatory Syndrome within Six Months of Age
Twelve cohort studies [44,46,47,48,49,50,51,54,55,56,57,58], described 1063 children with multisystem inflammatory syndrome, included 31 infants within six months of age (Table 4). These data allow calculation that 2.9% of reported cases are related to infants younger than six months of age: of these, 10 were neonates (32.3%) and 21 were infants (67.7%).
Table 4.
Characteristics of infants with MIS-N and MIS-C belonging to cohort studies and not fully described.
| First Author | Total Number of Children with MIS-C | Number of Infants under Six Months of Age | Available Description |
|---|---|---|---|
| Abdel-Haq [46] |
13 | 1 | A 3-months-old girl with a positive RT-PCR for SARS-CoV-2 and dilated coronary arteries, successfully treated with IVIG and infliximab |
| Alharbi [47] |
5 | 2 | Full description in Table 3 (Case 2 and Case 3) |
| Antúnez-Montes [48] | 95 | 3 | 3 infants under the age of a month, but their specific clinical characteristics were not described |
| Caro-Domínguez [49] | 37 | 1 | A 6-months-old boy with cardiac failure and positive RT-PCR for SARS-CoV-2 |
| Chandran [50] |
17 | 3 | Two infants survived (a 1-month-old infant who had prematurity as comorbidity and a 6-months-old infant who had previously undergone Kasai procedure at 2 months of age for biliary atresia). A 1-month-old infant with refractory thrombocytopenia and multiorgan involvement, treated with IVIG, methylprednisolone and cyclosporine, died. |
| Dufort [51] |
191 | 1 | A neonate with MIS-N, born to a positive mother (asymptomatic at delivery) who presented with fever and left breast cellulitis between 14 and 28 days of age. Laboratory work-up showed increasing troponin levels; echocardiogram showed good ventricular function and unremarkable coronary arteries. Two molecular tests for SARS-CoV-2 were negative. The discharge diagnoses were cellulitis, myocarditis, and shock. |
| Godfred-Cato [54] |
85 | 1 | The specific clinical characteristics of infants < 6 months of age are not described. |
| Grewal [55] |
92 | 9 | Nine infants under the age of 6 months, none with acute kidney injury. |
| Güllü [56] |
320 | 3 | A 1-month-old girl, followed up with aortic coarctation, resulted to be positive for SARS-CoV-2 and had fever and poor feeding. A 3-months-old boy, followed up with ventricular septal defect and pulmonary hypertension, had fever, diarrhoea (leading to a severe dehydration and lack of urine). A 6-days-old boy, who applied with fever, vomiting, decreased feeding and respiratory distress for 2 days. It was learned that his aunt and grandfather had been diagnosed with COVID-19 and they had loved and cared for the baby. |
| Mehra [57] |
120 | 1 | Among four deaths, one was a 3-month-old infant with acute COVID-19 related severe acute respiratory distress syndrome and shock |
| Niño-Taravilla [58] |
26 | 2 | Two neonates with MIS-C (the specific clinical characteristics are not described) |
| Shaiba LA [44] |
36 | 2 | Full description in Table 3 (Case 30 and Case 31) |
4. Discussion
This systematic review is the first to synthesize the current data regarding MIS-N in neonates and MIS-C in neonates and infants younger than six months of age. The literature relating to multisystem inflammatory syndrome in these patients is mainly centred on case reports or small case series: we summarized the most frequent clinical features to describe this syndrome in neonates and provide some therapeutic suggestions.
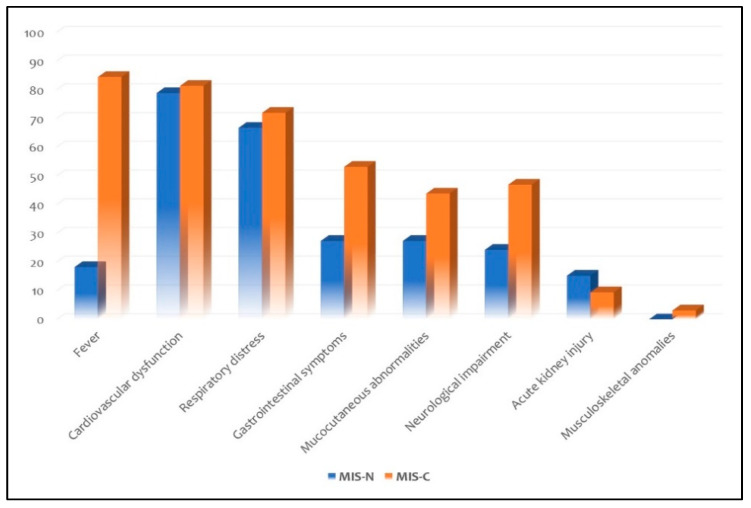
Regarding clinical features, fever is a milestone in older children with MIS-C (99.3%) [59]: we confirmed this trend in young infants with MIS-C (84.4%). Conversely, only the 18.2% of neonates with MIS-N presented with fever, but temperature changes are not a constant finding in the onset of infectious and even in the inflammatory febrile pathologies in preterm and term neonates [60,61]. Therefore, differences in age of MIS-N and MIS-C infants could explain this disparity in fever incidence. Differently from older children with MIS-C, in which gastrointestinal symptoms were the most common manifestation (87.3%) [62], we demonstrated that cardiovascular dysfunction and respiratory distress are the prevalent findings both in neonates with MIS-N and in neonates/infants with MIS-C (Figure 3).
Figure 3.
Distribution of clinical features in the subgroups of MIS-N and MIS-C.
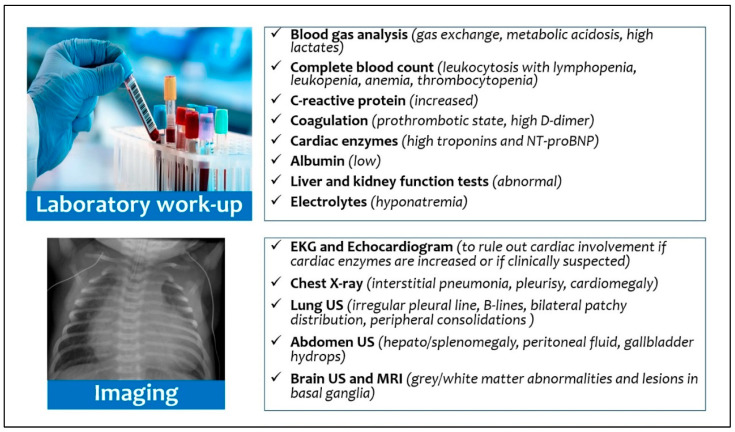
From the diagnostic point of view (Figure 4), although the current diagnostic reference standard for neonatal respiratory distress includes chest X-ray [63], only a third of patients with MIS-N or MIS-C showed pathologic chest X-ray. Recently, Musolino et al. suggested performing lung ultrasound (LUS) in patients with clinical suspicion of MIS-C, or without a certain diagnosis: the finding of many B-lines and pleural effusion would support the diagnosis of a systemic inflammatory disease [64]. Moreover, LUS can also reduce X-ray exposure, but its efficacy as diagnostic tool has been described only in a 6-months-old infant with MIS-C: the authors found an irregular pleural line, B-lines, with bilateral patchy distribution, and small peripheral consolidations [38].
Figure 4.
Diagnostic work-up for multisystem inflammatory syndrome in neonates and infants.
All infants with suspected inflammatory disease should undergo echocardiography, due to the possibility of myocardial dysfunction and damage to the coronary arteries observed both in neonates with MIS-N [11,19,25,26,29,33,38,39] and in neonates/infants with MIS-C [13,18,22,24,28,32,35,36,37,40,41,42,43,45,47,52,53]. Indeed, functional echocardiography can provide a direct bed-side assessment of cardiovascular anomalies and hemodynamics [65]. We suggest performing echocardiography in neonates with MIS-N after 24–48 h of life (or previously in case of symptoms), considering hemodynamic changes that physiologically occur during transition from fetal to neonatal life, while in MIS-C infants echocardiography should be carried on admission. Furthermore, neonates with MIS-N had a higher need of inotropic support (54.5%) than infants with MIS-C (15.6%); targeted echocardiography also offers the advantage of longitudinally assessing infants and their response to therapeutic intervention [66]. The increase in cardiac enzymes (troponin) and cardiac function-related proteins (NT-proBNP) must induce a strong suspicion of myocardial involvement and requires careful monitoring [67]. Additionally, the presence of microvascular dysfunction has recently been characterized in pediatric patients with SARS-CoV-2 pneumonia [68].
Inflammatory markers could be also raised, but we identified that neonates with MIS-N had lower levels than neonates/infants with MIS-C. Similarly, Zhao et al. assessed that younger children with MIS-C had lower levels of inflammatory markers when compared to middle-age children and adolescents with MIS-C [69].
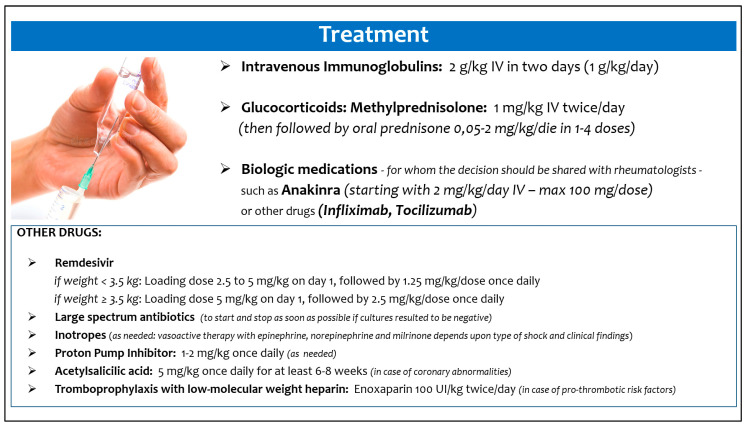
Moreover, we also tried to summarize how these infants were treated, in order to provide preliminary suggestions in management of this new clinical scenario (Figure 5), although data did not come from randomized studies [70]. The best treatment approach and the appropriate timing should be defined on an individual basis, as suggested by Cattalini et al. [71].
Figure 5.
Suggested treatment of multisystem inflammatory syndrome in neonates and infants.
MIS-C and MIS-N seem to originate from immune-mediated mechanisms, in the setting of a suspected or confirmed SARS-CoV-2 infection: however, in MIS-N the primary source is the maternal infection in pregnancy, with transplacental passage of maternal antibodies [62], whereas in MIS-C the infant contracts a postnatal infection and mounts an antibody response with intact neutralization capability [72]. The efficacy of intravenous immunoglobulins (IVIG) in MIS-C, a common approach to activating inhibitory Fc-receptors and preventing membrane-attack complexes by complement factors, thereby mitigating autoantibody-mediated pathology [73], lends support to the hypothesis that autoantibodies contribute to MIS-C pathogenesis [74].
The majority of infants described in the literature received IVIG [11,13,14,19,20,24,25,27,28,34,35,37,38,39,40,42,43,44,45,47,52,53] and steroids (mostly methylprednisolone) [11,14,15,18,22,25,26,28,31,32,34,36,37,38,39,40,41,42,44,45,52]. According to results of a French study, the treatment with IVIG and methylprednisolone vs IVIG alone was associated with a more favourable course among children with MIS-C [18,22,45]. However, data interpretation is limited by the observational design of the study [75].
Contrarily, antivirals (such as remdesivir) were poorly used (in none of the neonates with MIS-N and only 9.4% of infants with MIS-C) [18,22,45]. Similarly, biologic medications were not given to neonates with MIS-N, whereas about a third of infants with MIS-C required treatment with anakinra, infliximab, tocilizumab, and inhaled interferon α-1b [16,34,36,40,44,45,47].
Most neonates and infants were also treated with large-spectrum antibiotics, given the overlapping clinical signs and symptoms with those of sepsis. Recently, Yock-Corrales and colleagues set the alarm regarding the high rate of antibiotic prescriptions (24.5%) in children with COVID-19 (in particular in those with more severe forms) [76]. Considering the high need for ICU admission in neonates with MIS-N (100%) and infants with MIS-C (40.6%) described in the included studies of this review, and given that an antibiotic treatment is often guaranteed in younger infants, the length of antibiotic therapy should be carefully evaluated: reducing patient exposure to large-spectrum antibiotics can result in avoiding the spread of multi-drug resistant organisms [77]. The time to positivity of blood cultures performed on admission could guide decisions on antibiotics administration in neonates, because most bacterial pathogens grow within 48 h [78,79].
Although the outcome was favourable in the majority of cases, the observed mortality was 9.2% in neonates with MIS-N and infants younger than under six months with MIS-C. Conversely, the reported mortality rate was 1.9% when all pediatric MIS-C cases reported in the literature were considered [59]. This is an expected finding, considering the higher cardiovascular and respiratory involvement in younger infants.
This study had several limitations. First, the available studies were mostly case reports or case series, with possible selection biases. Second, data on some variables were not accessible in all papers or were not uniformly reported. Third, there is no consensus about diagnostic criteria in MIS-C, whereas recently Pawar et al. proposed modified criteria for MIS-N [11]. Fourth, most of these data have been obtained in the “pre-Omicron” period. Indeed, since late November 2021, with the emergence of the new SARS-CoV-2 variant B.1.1.529 (named Omicron), the number of COVID-19 cases increased substantially. Although the symptoms of the new cases are reported to be mild to moderate [80], we do not know what the actual impact will be in younger infants, for whom a vaccine is not yet available.
5. Conclusions
Multisystem inflammatory syndrome, named MIS-N or MIS-C, related to SARS-CoV-2 exposure can occur in a high percentage of neonates and infants. In affected neonates common findings are cardiac dysfunction and the coronary artery dilation or aneurysms; thus, a complete echocardiography is strongly recommended in the diagnostic approach.
The studies that we have consulted reported an overall good prognosis, despite the frequent need of NICU and ICU admissions.
Further epidemiological, clinical, immunological, and neurodevelopmental studies are needed to better clarify short- and long-term outcomes of neonates and infants with this inflammatory condition related to SARS-CoV-2 exposure.
Author Contributions
Conceptualization, D.U.D.R. and C.A.; Methodology, D.U.D.R. and C.A.; Formal analysis, D.U.D.R., F.P., M.C., S.R., S.C., C.M., L.M. and A.S.; Data curation, D.U.D.R., F.P., M.C., S.R., S.C., C.M., L.M. and A.S.; Writing—original draft preparation, D.U.D.R., F.P. and C.M.; Writing—review and editing, A.D. and C.A.; Supervision, C.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available in the included articles. No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Viner R.M., Mytton O.T., Bonell C., Melendez-Torres G.J., Ward J., Hudson L., Waddington C., Thomas J., Russell S., van der Klis F., et al. Susceptibility to SARS-CoV-2 Infection among Children and Adolescents Compared with Adults: A Systematic Review and Meta-Analysis. JAMA Pediatr. 2021;175:143–156. doi: 10.1001/jamapediatrics.2020.4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Auriti C., De Rose D.U., Mondì V., Stolfi I., Tzialla C. Neonatal SARS-CoV-2 Infection: Practical Tips. Pathogens. 2021;10:611. doi: 10.3390/pathogens10050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heinonen S., Helve O., Andersson S., Janér C., Süvari L., Kaskinen A. Nasal expression of SARS-CoV-2 entry receptors in newborns. Arch. Dis. Child Fetal Neonatal. Ed. 2022;107:F95–F97. doi: 10.1136/archdischild-2020-321334. [DOI] [PubMed] [Google Scholar]
- 4.Olivini N., Calò Carducci F.I., Santilli V., De Ioris M.A., Scarselli A., Alario D., Geremia C., Lombardi M.H., Marabotto C., Mariani R., et al. A neonatal cluster of novel coronavirus disease 2019: Clinical management and considerations. Ital. J. Ped. 2020;46:1–9. doi: 10.1186/s13052-020-00947-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivanti A.J., Vauloup-Fellous C., Prevot S., Zupan V., Suffee C., Do Cao J., Benachi A., De Luca D. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11:3572. doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams N., Radia T., Harman K., Agrawal P., Cook J., Gupta A. COVID-19 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: A systematic review of critically unwell children and the association with underlying comorbidities. Eur. J. Ped. 2021;180:689–697. doi: 10.1007/s00431-020-03801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiang L., Tang K., Levin M., Irfan O., Morris S.K., Wilson K., Klein J.D., Bhutta Z.A. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020;20:e276–e288. doi: 10.1016/S1473-3099(20)30651-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soma V.L., Shust G.F., Ratner A.J. Multisystem inflammatory syndrome in children. Curr. Opin. Ped. 2021;33:152–158. doi: 10.1097/MOP.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 9.Esposito S., Principi N. Multisystem Inflammatory Syndrome in Children Related to SARS CoV 2. Pediatr. Drugs. 2021;23:1–11. doi: 10.1007/s40272-020-00435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris S.B., Schwartz N.G., Patel P., Abbo L., Beauchamps L., Balan S., Lee E.H., Paneth-Pollak R., Geevarughese A., Lash M.K., et al. Case Series of Multisystem Inflammatory Syndrome in Adults Associated with SARS-CoV-2 Infection—United Kingdom and United States, March–August 2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:1450–1456. doi: 10.15585/mmwr.mm6940e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pawar R., Gavade V., Patil N., Mali V., Girwalkar A., Tarkasband V., Loya S., Chavan A., Nanivadekar N., Shinde R., et al. Neonatal multisystem inflammatory syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: A case series. Children. 2021;8:572. doi: 10.3390/children8070572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acharyya B.C., Acharyya S., Das D. Novel Coronavirus Mimicking Kawasaki Disease in an Infant. Indian Pediatr. 2020;57:753–754. doi: 10.1007/s13312-020-1924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agrawal G., Wazir S., Arora A., Sethi S.K. Multisystem inflammatory syndrome in a neonate masquerading as surgical abdomen. BMJ Case Rep. 2021;14:e246579. doi: 10.1136/bcr-2021-246579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amonkar P.S., Gavhane J.B., Kharche S.N., Kadam S.S., Bhusare D.B. Aortic thrombosis in a neonate with COVID-19-related fetal inflammatory response syndrome requiring amputation of the leg: A case report. Paediatr. Int. Child Health. 2021;41:211–216. doi: 10.1080/20469047.2021.1968596. [DOI] [PubMed] [Google Scholar]
- 16.Cui Y., Tian M., Huang D., Wang X., Huang Y., Fan L., Wang L., Chen Y., Liu W., Zhang K., et al. A 55-day-old female infant infected with 2019 novel coronavirus disease: Presenting with pneumonia, liver injury, and heart damage. J. Infect. Dis. 2020;221:1775–1780. doi: 10.1093/infdis/jiaa113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Barba P., Canarutto D., Sala E., Frontino G., Guarneri M.P., Camesasca C., Baldoli C., Esposito A., Barera G. COVID-19 cardiac involvement in a 38-day old infant. Pediatr. Pulmonol. 2020;55:1879–1881. doi: 10.1002/ppul.24895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diggikar S., Nanjegowda R., Kumar A., Kumar V., Kulkarni S., Venkatagiri P. Neonatal Multisystem Inflammatory Syndrome secondary to SARS-CoV-2 infection. J. Paediatr. Child Health. 2021:2–4. doi: 10.1111/jpc.15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Divekar A.A., Patamasucon P., Benjamin J.S. Presumptive Neonatal Multisystem Inflammatory Syndrome in Children Associated with Coronavirus Disease 2019. Am. J. Perinatol. 2021;38:632–636. doi: 10.1055/s-0041-1726318. [DOI] [PubMed] [Google Scholar]
- 20.Diwakar K., Gupta B.K., Uddin M.W., Sharma A., Jhajra S. Multisystem inflammatory syndrome with persistent neutropenia in neonate exposed to SARS-CoV-2 virus: A case report and review of literature. J. Neonatal. Perinatal. Med. 2021:1–5. doi: 10.3233/NPM-210839. [DOI] [PubMed] [Google Scholar]
- 21.Dugue R., Cay-Martínez K.C., Thakur K.T., Garcia J.A., Chauhan L.V., Williams S.H., Briese T., Jain K., Foca M., McBrian D.K., et al. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94:1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frauenfelder C., Brierley J., Whittaker E., Perucca G., Bamford A. Infant with SARS-CoV-2 infection causing severe lung disease treated with remdesivir. Pediatrics. 2020;146:e20201701. doi: 10.1542/peds.2020-1701. [DOI] [PubMed] [Google Scholar]
- 23.García-Howard M., Herranz-Aguirre M., Moreno-Galarraga L., Urretavizcaya-Martínez M., Alegría-Echauri J., Gorría-Redondo N., Planas-Serra L., Schlüter A., Gut M., Pujol A., et al. Case Report: Benign Infantile Seizures Temporally Associated With COVID-19. Front. Pediatr. 2020;8:507. doi: 10.3389/fped.2020.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giacomet V., Manfredini V.A., Meraviglia G., Peri C.F., Sala A., Longoni E., Gasperetti A., Stracuzzi M., Mannarino S., Zuccotti G.V. Acute inflammation and elevated cardiac markers in a two-month-old infant with severe acute respiratory syndrome coronavirus 2 infection presenting with cardiac symptoms. Pediatr. Infect. Dis. J. 2020;39:e149–e151. doi: 10.1097/INF.0000000000002750. [DOI] [PubMed] [Google Scholar]
- 25.Kappanayil M., Balan S., Alawani S., Mohanty S., Leeladharan S.P., Gangadharan S., Jayashankar J.P., Jagadeesan S., Kumar A., Gupta A., et al. Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to SARS-CoV-2: A case report. Lancet Child Adolesc. Health. 2021;5:304–308. doi: 10.1016/S2352-4642(21)00055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khaund Borkotoky R., Banerjee Barua P., Paul S.P., Heaton P.A. COVID-19-Related Potential Multisystem Inflammatory Syndrome in Childhood in a Neonate Presenting as Persistent Pulmonary Hypertension of the Newborn. Pediatr. Infect. Dis. J. 2021;40:e162–e164. doi: 10.1097/INF.0000000000003054. [DOI] [PubMed] [Google Scholar]
- 27.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Segal J.B., Nguyen E.L., Barsh G.R., Maskatia S., Mathew R. COVID-19 and Kawasaki Disease: Novel Virus and Novel Case. Hosp. Pediatr. 2020;10:537–540. doi: 10.1542/hpeds.2020-0123. [DOI] [PubMed] [Google Scholar]
- 28.Lad S.S., Suryawanshi P.B., Jadhav P., Kait S.P., Lad P., Mujawar J., Khetre R., Kataria P., Balte P., Neela A., et al. Fresh Per Rectal Bleeding in Pediatric Inflammatory Multisystem Syndrome Temporally Associated with SARS-CoV-2 (PIMS-TS) Indian J. Pediatr. 2021;88:607. doi: 10.1007/s12098-021-03728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lima A., Cardoso C.C., Bentim P., Voloch C.M., Rossi Á.D., da Costa R., da Paz J., Agostinho R.F., Figueiredo V., Júnior J., et al. Maternal SARS-CoV-2 Infection Associated to Systemic Inflammatory Response and Pericardial Effusion in the Newborn: A Case Report. J. Pediatr. Infect. Dis. Soc. 2021;10:536–539. doi: 10.1093/jpids/piaa133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenz N., Treptow A., Schmidt S., Hofmann R., Raumer-Engler M., Heubner G., Gröber K. Neonatal Early-Onset infection with SARS-CoV-2 in a newborn presenting with encephalitic symptoms. Pediatr. Infect. Dis. J. 2020;39:e212. doi: 10.1097/INF.0000000000002735. [DOI] [PubMed] [Google Scholar]
- 31.Luna Santiago L., Aguilar-Martinez N., Cabanas-Espinosa B., Ramirez-Machuca X. SARS-CoV-2-related multisystem inflammatory syndrome in familial refractory hemophagocytic lymphohistiocytosis: A case report. Pediatr. Crit. Care Med. 2021;22:352. doi: 10.1097/01.pcc.0000741260.86597.07. [DOI] [Google Scholar]
- 32.Mariani R., Liu H. Severe transient pancytopenia with dyserythropoiesis and dysmegakaryopoiesis in COVID-19–associated MIS-C. Blood. 2020;136:2964. doi: 10.1182/blood.2020009479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCarty K.L., Tucker M., Lee G., Pandey V. Fetal inflammatory response syndrome associated with maternal SARS-CoV-2 infection. Pediatrics. 2021;147:e2020010132. doi: 10.1542/peds.2020-010132. [DOI] [PubMed] [Google Scholar]
- 34.Orlanski-Meyer E., Yogev D., Auerbach A., Megged O., Glikman D., Hashkes P.J., Bar-Meir M. Multisystem Inflammatory syndrome in children associated with SARS-CoV-2 in an 8-week old infant. J. Pediatr. Infect. Dis. Soc. 2020;9:781–784. doi: 10.1093/jpids/piaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raut S., Roychowdhoury S., Bhakta S., Sarkar M., Nandi M. Incomplete Kawasaki Disease as Presentation of COVID-19 Infection in an Infant: A Case Report. J. Trop. Pediatr. 2021;67:1–4. doi: 10.1093/tropej/fmaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodriguez-Gonzalez M., Rodríguez-Campoy P., Sánchez-Códez M., Gutiérrez-Rosa I., Castellano-Martinez A., Rodríguez-Benítez A. New onset severe right ventricular failure associated with COVID-19 in a young infant without previous heart disease. Cardiol. Young. 2020;30:1346–1349. doi: 10.1017/S1047951120001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saha S., Pal P., Mukherjee D. Neonatal MIS-C: Managing the Cytokine Storm. Pediatrics. 2021;148:e2020042093. doi: 10.1542/peds.2020-042093. [DOI] [PubMed] [Google Scholar]
- 38.Schoenmakers S., Snijder P., Verdijk R.M., Kuiken T., Kamphuis S., Koopman L.P., Krasemann T.B., Rousian M., Broekhuizen M., Steegers E., et al. Severe acute respiratory syndrome coronavirus 2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. J. Pediatr. Infect. Dis. Soc. 2021;10:556–561. doi: 10.1093/jpids/piaa153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shaiba L.A., Hadid A., Altirkawi K.A., Bakheet H.M., Alherz A.M., Hussain S.A., Sobaih B.H., Alnemri A.M., Almaghrabi R., Ahmed M., et al. Case Report: Neonatal Multi-System Inflammatory Syndrome Associated With SARS-CoV-2 Exposure in Two Cases from Saudi Arabia. Front. Pediatr. 2021;6:652857. doi: 10.3389/fped.2021.652857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richardson K.L., Jain A., Evans J., Uzun O. Giant coronary artery aneurysm as a feature of coronavirus-related inflammatory syndrome. BMJ Case Rep. 2021;14:1–6. doi: 10.1136/bcr-2020-238740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Malle L., Bastard P., Martin-Nalda A., Carpenter T., Bush D., Patel R., Colobran R., Soler-Palacin P., Casanova J.L., Gans M., et al. Atypical Inflammatory Syndrome Triggered by SARS-CoV-2 in Infants with Down Syndrome. J. Clin. Immunol. 2021;41:1457–1462. doi: 10.1007/s10875-021-01078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marino A., Varisco T., Quattrocchi G., Amoroso A., Beltrami D., Venturiello S., Ripamonti A., Villa A., Andreotti M., Ciuffreda M., et al. Children with Kawasaki disease or Kawasaki-like syndrome (MIS-C/PIMS) at the time of COVID-19: Are they all the same? Case series and literature review. Reumatismo. 2021;73:48–53. doi: 10.4081/reumatismo.2021.1331. [DOI] [PubMed] [Google Scholar]
- 43.Rakha S., Sobh A., Hager A.H., Hafez M., Alsawah G.A., Abuelkheir M.M., Zeid M.S., Nahas M., Elmarsafawy H. Cardiac Implications of Multisystem Inflammatory Syndrome Associated with COVID-19 in Children under the age of Five Years. Cardiol. Young. 2021:1–6. doi: 10.1017/S1047951121003140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shaiba L.A., Altirkawi K., Hadid A., Alsubaie S., Alharbi O., Alkhalaf H., Alharbi M., Alruqaie N., Alzomor O., Almughaileth F., et al. COVID-19 Disease in Infants Less Than 90 Days: Case Series. Front. Pediatr. 2021;9:1–8. doi: 10.3389/fped.2021.674899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villacis-Nunez D.S., Hashemi S., Nelson M.C., Flanagan E., Thakral A., Rodriguez F., 3rd, Jaggi P., Oster M.E., Prahalad S., Rouster-Stevens K.A. Giant Coronary Aneurysms in Multisystem Inflammatory Syndrome in Children Associated with SARS-CoV-2 Infection. JACC Case Rep. 2021;3:1499–1508. doi: 10.1016/j.jaccas.2021.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdel-Haq N., Asmar B.I., Deza Leon M.P., McGrath E.J., Arora H.S., Cashen K., Tilford B., Charaf Eddine A., Sethuraman U., Ang J.Y. SARS-CoV-2-associated multisystem inflammatory syndrome in children: Clinical manifestations and the role of infliximab treatment. Eur. J. Pediatr. 2021;180:1581–1591. doi: 10.1007/s00431-021-03935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alharbi M., Kazzaz Y.M., Hameed T., Alqanatish J., Alkhalaf H., Alsadoon A., Alayed M., Hussien S.A., Shaalan M.A., Al Johani S.M. SARS-CoV-2 infection in children, clinical characteristics, diagnostic findings and therapeutic interventions at a tertiary care center in Riyadh, Saudi Arabia. J. Infect. Public Health. 2021;14:446–453. doi: 10.1016/j.jiph.2020.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Antúnez-Montes O.Y., Escamilla M.I., Figueroa-Uribe A.F., Arteaga-Menchaca E., Lavariega-Saráchaga M., Salcedo-Lozada P., Melchior P., de Oliveira R.B., Tirado Caballero J.C., Redondo H.P., et al. COVID-19 and Multisystem Inflammatory Syndrome in Latin American Children: A Multinational Study. Pediatr. Infect. Dis. J. 2020;40:e1–e6. doi: 10.1097/INF.0000000000002949. [DOI] [PubMed] [Google Scholar]
- 49.Caro-Domínguez P., Navallas M., Riaza-Martin L., Ghadimi Mahani M., Ugas Charcape C.F., Valverde I., D’Arco F., Toso S., Shelmerdine S.C., van Schuppen J., et al. Imaging findings of multisystem inflammatory syndrome in children associated with COVID-19. Pediatr. Radiol. 2021;51:1608–1620. doi: 10.1007/s00247-021-05065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chandran J., James E.J., Verghese V.P., Kumar T.S., Sundaravalli E., Vyasam S. Clinical Spectrum of Children with Multisystem Inflammatory Syndrome Associated With SARS-CoV-2 Infection. Indian Pediatr. 2021;58:955–958. doi: 10.1007/s13312-021-2330-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dufort E.M., Koumans E.H., Chow E.J., Rosenthal E.M., Muse A., Rowlands J., Barranco M.A., Maxted A.M., Rosenberg E.S., Easton D., et al. New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem Inflammatory Syndrome in Children in New York State. N. Engl. J. Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Esteve-Sole A., Anton J., Pino-Ramirez R.M., Sanchez-Manubens J., Fumadó V., Fortuny C., Rios-Barnes M., Sanchez-de-Toledo J., Girona-Alarcón M., Mosquera J.M., et al. Similarities and differences between the immunopathogenesis of COVID-19-related pediatric multisystem inflammatory syndrome and Kawasaki disease. J. Clin. Investig. 2021;131:e144554. doi: 10.1172/JCI144554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Falah N.U., Hashmi S., Ahmed Z., Jaan A., Akhtar A., Khalid F., Farooque U., Shera M.T., Ali S., Javed A. Kawasaki Disease-Like Features in 10 Pediatric COVID-19 Cases: A Retrospective Study. Cureus. 2020;12:e11035. doi: 10.7759/cureus.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Godfred-Cato S., Tsang C.A., Giovanni J., Abrams J., Oster M.E., Lee E.H., Lash M.K., Le Marchand C., Liu C.Y., Newhouse C.N., et al. Multisystem Inflammatory Syndrome in Infants <12 months of Age, United States, May 2020–January 2021. Pediatr. Infect. Dis. J. 2021;40:601–605. doi: 10.1097/INF.0000000000003149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grewal M.K., Gregory M.J., Jain A., Mohammad D., Cashen K., Ang J.Y., Thomas R.L., Valentini R.P. Acute Kidney Injury in Pediatric Acute SARS-CoV-2 Infection and Multisystem Inflammatory Syndrome in Children (MIS-C): Is There a Difference? Front. Pediatr. 2021;9:692256. doi: 10.3389/fped.2021.692256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Güllü U.U., Güngör Ş., İpek S., Yurttutan S., Dilber C. Predictive value of cardiac markers in the prognosis of COVID-19 in children. Am. J. Emerg. Med. 2021;48:307–311. doi: 10.1016/j.ajem.2021.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mehra B., Pandey M., Gupta D., Oberoi T., Jerath N., Sharma R., Lal N., Singha C., Malhotra B., Manocha V., et al. COVID-19 associated multisystem inflammatory syndrome in children: A multicentric retrospective cohort study. Indian J. Crit. Care Med. 2021;25:1174–1180. doi: 10.5005/jp-journals-10071-23996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Niño-Taravilla C., Otaola-Arca H., Lara-Aguilera N., Zuleta-Morales Y., Ortiz-Fritz P. Multisystem inflammatory syndrome in children, Chile, May-August 2020. Emerg. Infect. Dis. 2021;27:1457–1461. doi: 10.3201/eid2705.204591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yasuhara J., Watanabe K., Takagi H., Sumitomo N., Kuno T. COVID-19 and multisystem inflammatory syndrome in children: A systematic review and meta-analysis. Pediatr. Pulmonol. 2021;56:837–848. doi: 10.1002/ppul.25245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Rose D.U., Coppola M., Gallini F., Maggio L., Vento G., Rigante D. Overview of the rarest causes of fever in newborns: Handy hints for the neonatologist. J. Perinatol. 2021;41:372–382. doi: 10.1038/s41372-020-0744-8. [DOI] [PubMed] [Google Scholar]
- 61.Hofer N., Müller W., Resch B. Neonates presenting with temperature symptoms: Role in the diagnosis of early onset sepsis. Pediatr. Int. 2012;54:486–490. doi: 10.1111/j.1442-200X.2012.03570.x. [DOI] [PubMed] [Google Scholar]
- 62.Lakshminrusimha S., Hudak M.L., Dimitriades V.R., Higgins R.D. Multisystem Inflammatory Syndrome in Neonates following Maternal SARS-CoV-2 COVID-19 Infection. Am. J. Perinatol. 2021:1–6. doi: 10.1055/a-1682-3075. [DOI] [PubMed] [Google Scholar]
- 63.Hiles M., Culpan A.M., Watts C., Munyombwe T., Wolstenhulme S. Neonatal respiratory distress syndrome: Chest X-ray or lung ultrasound? A systematic review. Ultrasound. 2017;25:80–91. doi: 10.1177/1742271X16689374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Musolino A.M., Boccuzzi E., Buonsenso D., Supino M.C., Mesturino M.A., Pitaro E., Ferro V., Nacca R., Sinibaldi S., Palma P., et al. The Role of Lung Ultrasound in Diagnosing COVID-19-Related Multisystemic Inflammatory Disease: A Preliminary Experience. J. Clin. Med. 2022;11:234. doi: 10.3390/jcm11010234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tissot C., Singh Y. Neonatal functional echocardiography. Curr. Opin. Pediatr. 2020;32:235–244. doi: 10.1097/MOP.0000000000000887. [DOI] [PubMed] [Google Scholar]
- 66.Giesinger R.E., McNamara P.J. Hemodynamic instability in the critically ill neonate: An approach to cardiovascular support based on disease pathophysiology. Semin. Perinatol. 2016;40:174–188. doi: 10.1053/j.semperi.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 67.Haghighi Aski B., Manafi Anari A., Abolhasan Choobdar F., Zareh Mahmoudabadi R., Sakhaei M. Cardiac abnormalities due to multisystem inflammatory syndrome temporally associated with COVID-19 among children: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2021;33:100764. doi: 10.1016/j.ijcha.2021.100764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bottari G., Damiani E., Confalone V., Scorcella C., Casarotta E., Gandolfo C., Stoppa F., Cecchetti C., Donati A. Microvascular dysfunction in pediatric patients with SARS-COV-2 pneumonia: Report of three severe cases. Microvasc. Res. 2022;141:104312. doi: 10.1016/j.mvr.2022.104312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Y., Yin L., Patel J., Tang L., Huang Y. The inflammatory markers of multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19: A meta-analysis. J. Med. Virol. 2021;93:4358–4369. doi: 10.1002/jmv.26951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McArdle A.J., Vito O., Patel H., Seaby E.G., Shah P., Wilson C., Broderick C., Nijman R., Tremoulet A.H., Munblit D., et al. BATS Consortium. Treatment of Multisystem Inflammatory Syndrome in Children. N. Engl. J. Med. 2021;385:1–12. doi: 10.1056/NEJMoa2102968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cattalini M., Taddio A., Bracaglia C., Cimaz R., Paolera S.D., Filocamo G., La Torre F., Lattanzi B., Marchesi A., Simonini G., et al. Rheumatology Study Group of the Italian Society of Pediatrics. Childhood multisystem inflammatory syndrome associated with COVID-19 (MIS-C): A diagnostic and treatment guidance from the Rheumatology Study Group of the Italian Society of Pediatrics. Ital. J. Pediatr. 2021;47:1–6. doi: 10.1186/s13052-021-00980-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gruber C.N., Patel R.S., Trachtman R., Lepow L., Amanat F., Krammer F., Wilson K.M., Onel K., Geanon D., Tuballes K., et al. Mapping systemic inflammation and antibody responses in multisystem inflammatory syndrome in children (MIS-C) Cell. 2020;183:982–995. doi: 10.1016/j.cell.2020.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kazatchkine M.D., Kaveri S.V. Immunomodulation of autoimmune and inflammatory diseases with Intravenous Immune Globulin. N. Engl. J. Med. 2001;345:747–755. doi: 10.1056/NEJMra993360. [DOI] [PubMed] [Google Scholar]
- 74.Consiglio C.R., Cotugno N., Sardh F., Pou C., Amodio D., Rodriguez L., Tan Z., Zicari S., Ruggiero A., Pascucci G.R., et al. The immunology of multisystem inflammatory syndrome in children with COVID-19. Cell. 2020;183:968–981.e7. doi: 10.1016/j.cell.2020.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ouldali N., Toubiana J., Antona D., Javouhey E., Madhi F., Lorrot M., Léger P.L., Galeotti C., Claude C., Wiedemann A., et al. French COVID-19 Paediatric Inflammation Consortium. Association of Intravenous Immunoglobulins Plus Methylprednisolone vs Immunoglobulins Alone with course of fever in Multisystem Inflammatory Syndrome in Children. JAMA. 2021;325:855–864. doi: 10.1001/jama.2021.0694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yock-Corrales A., Lenzi J., Ulloa-Gutiérrez R., Gómez-Vargas J., Antúnez-Montes O.Y., Rios Aida J.A., Del Aguila O., Arteaga-Menchaca E., Campos F., Uribe F., et al. High rates of antibiotic prescriptions in children with COVID-19 or multisystem inflammatory syndrome: A multinational experience in 990 cases from Latin America. Acta. Paediatr. 2021;110:1902–1910. doi: 10.1111/apa.15847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Branstetter J.W., Barker L., Yarbrough A., Ross S., Stultz J.S. Challenges of antibiotic stewardship in the pediatric and neonatal intensive care units. J. Pediatr. Pharmacol. Ther. 2021;26:659–668. doi: 10.5863/1551-6776-26.7.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Rose D.U., Perri A., Auriti C., Gallini F., Maggio L., Fiori B., D’Inzeo T., Spans T., Vento G. Time to positivity of blood cultures could inform decisions on antibiotics administration in neonatal early-onset sepsis. Antibiotics. 2021;10:123. doi: 10.3390/antibiotics10020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kuzniewicz M.W., Mukhopadhyay S., Li S., Walsh E.M., Puopolo K.M. Time to Positivity of Neonatal Blood Cultures for Early-onset Sepsis. Pediatr. Infect. Dis. J. 2020;39:634–640. doi: 10.1097/INF.0000000000002632. [DOI] [PubMed] [Google Scholar]
- 80.Rahmani S., Rezaei N. Omicron (B.1.1.529) variant: Development, dissemination, and dominance. J. Med. Virol. 2022;94:1787–1788. doi: 10.1002/jmv.27563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the included articles. No new data were created or analyzed in this study.