Abstract
In Denmark surveillance of the in vitro susceptibility to ciprofloxacin of Neisseria gonorrhoeae was established in 1990. The proportion of N. gonorrhoeae strains with decreased susceptibility or resistance to ciprofloxacin (MIC ≥ 0.06 μg/ml) was low (0.3 to 2.3%) up to 1995. Between 1995 and 1998 the rate of less-susceptible and resistant strains rose from 6.9 to 13.2%. Among ciprofloxacin-resistant strains (MIC ≥ 1 μg/ml), 81% were highly resistant (MIC ≥ 4 μg/ml). Thirty-five N. gonorrhoeae strains (40 isolates) for which ciprofloxacin MICs were 4 to 32 μg/ml were investigated for the frequency and patterns of mutations within the gyrA and parC genes. The quinolone resistance-determining regions of the gyrA and parC genes were amplified by PCR, and the amplicons were directly sequenced. Alterations at Ser-91 and Asp-95 in GyrA and a single or double alteration in ParC were identified in 32 strains (91%). Ser-91-to-Phe and Asp-95-to-Gly alterations in GyrA were detected in 28 strains (80%). The most common ParC alteration, Asp-86 to Asn, was found in 19 strains (54%). The strains were analyzed for genetic relationship by pulsed-field gel electrophoresis (PFGE). The analysis showed that nine strains with the same mutation pattern in the gyrA and parC genes, originating from different geographical areas over 3 years, had the same PFGE patterns after SpeI as well as NheI digestion (only one strain with one band difference in the NheI pattern), suggesting that a resistant clone had spread worldwide. The results from this study strongly suggest that double gyrA mutations plus a parC mutation(s) play an important role in the development of high-level fluoroquinolone resistance in N. gonorrhoeae.
After the emergence and worldwide spread of penicillin- and tetracycline-resistant Neisseria gonorrhoeae strains, fluoroquinolones have been recommended as primary therapy for uncomplicated gonorrhea in many countries (31). Over the past 10 years, N. gonorrhoeae strains with decreased susceptibility or resistance to fluoroquinolones have been found in several countries, particularly in southeast Asia and western Pacific areas (7, 10, 12, 13, 25, 30). An N. gonorrhoeae strain with high-level ciprofloxacin resistance was reported for the first time in 1994 (2). During recent years the rate of gonorrhea has fallen dramatically in Denmark. Simultaneously, the proportion of multiresistant gonococci has risen sharply (15). Surveillance of susceptibility to ciprofloxacin in N. gonorrhoeae was initiated in 1990. The proportion of strains showing reduced susceptibility or resistance to ciprofloxacin (MIC ≥ 0.06 μg/ml) was low (0.3 to 2.3%) up to 1995. Two gonococcal isolates resistant to ciprofloxacin (0.3% of the isolates tested; MIC, 1 μg/ml) were found in 1992. Resistant strains were not detected between 1993 and 1994. However, since 1995 the numbers of N. gonorrhoeae strains with decreased susceptibility or resistance to ciprofloxacin have risen markedly.
To analyze quinolone resistance mechanisms in N. gonorrhoeae a number of studies of laboratory mutant strains (1, 4) and clinical isolates (22–24, 29) were performed. The resistance to fluoroquinolones was shown primarily to be associated with mutations in the gyrA gene, coding for DNA gyrase, and in the parC gene, coding for DNA topoisomerase IV (1, 4, 22, 23, 28), and less often with reduced uptake and accumulation of fluoroquinolone in the cells (21). The level of fluoroquinolone resistance appears to correlate to the location and number of mutations in gyrA and parC. Among the strains examined so far only a few strains exhibited high-level resistance to fluoroquinolone. In this paper, we report the occurrence of fluoroquinolone resistance among N. gonorrhoeae strains isolated in Denmark from 1995 to 1998 and the frequency and patterns of mutations involving gyrA and parC genes in 35 strains (40 isolates) highly resistant to ciprofloxacin. We also examined the genetic relationship of the 40 isolates by pulsed-field gel electrophoresis (PFGE).
MATERIALS AND METHODS
Bacterial strains.
From 1995 to 1998 a total of 809 culture-confirmed cases of gonorrhea occurred in Denmark. The methods used for primary isolation and identification of gonococci were the same as those described previously (20). All strains were sent to the National Reference Laboratory for antimicrobial susceptibility testing. In total 40 clinical isolates of N. gonorrhoeae for which ciprofloxacin MICs ranged from 4 to 32 μg/ml were available for the study. Information about the origin of the strains investigated was available from the request form sent to the reference laboratory or from the attending physician, who is obliged to obtain information about country of exposure according to the compulsory notification system for communicable diseases.
Antimicrobial susceptibility testing.
Penicillinase production was demonstrated by means of the chromogenic cephalosporin test (18). All strains were examined for susceptibility to ciprofloxacin, penicillin, tetracycline, and ceftriaxone by the agar plate dilution method using Danish chocolate agar medium (17). The antibiotic concentrations (twofold dilution steps) used were as follows: ciprofloxacin, 0.001 to 32 μg/ml; penicillin, 0.016 to 4.0 μg/ml; tetracycline, 0.125 to 64 μg/ml (32 μg/ml in 1995); ceftriaxone, 0.001 to 0.25 μg/ml. Strains for which the MICs of ciprofloxacin were 0.03 μg/ml or less, 0.06 to 0.5 μg/ml, and 1 μg/ml or more were designated susceptible, less susceptible, and resistant, respectively (25).
Plasmid analysis.
The plasmid profiles of penicillinase-producing N. gonorrhoeae (PPNG) strains were analyzed as described previously (20).
Determination of changes in gyrA and parC genes.
PCR and direct DNA sequencing of the amplicons were performed to identify mutations within the gyrA and parC genes. PCR primers specific for genes corresponding to the quinolone resistance-determining regions within GyrA and ParC were the same as those used by Tanaka et al. (22, 23) and described by Belland et al. (1). The DNA sequences were determined in both directions by using the ABI PRISM dye terminator cycle sequencing ready reaction kit (Perkin-Elmer, Applied Biosystems, Foster City, Calif.) and an ABI 377A automated DNA sequencer and analyzed by using Sequence Navigator software (Perkin-Elmer, Applied Biosystems).
PFGE analysis.
Genomic DNA was prepared as described previously (3). Slices of DNA-containing agarose blocks were digested with SpeI and NheI (New England Biolabs) overnight at 37°C in 100 μl of restriction endonuclease buffer containing 10 U of enzyme. Digested blocks were electrophoresed in a 1% agarose gel in a contour-clamped homogeneous electric field (CHEF DR III) system (Bio-Rad). Running conditions were 22 h at 12°C at a voltage of 6 V/cm with pulse times of 1 to 40 s.
The gels were stained with ethidium bromide, destained in distilled water, and photographed under UV transillumination. Subsequently, the patterns were scanned using a scientific imaging system (Digital Science ID; Eastman Kodak Company, Rochester, N.Y.). PFGE fingerprints were analyzed with GelCompar software (version 4.1; Applied Maths, Kortrijk, Belgium). The similarity of the PFGE banding patterns was estimated with the Dice coefficient and the unweighted pair group method using arithmetic averages. A tolerance in the band positions of 1.0% and an optimization setting of 0.5% were used. Bands smaller than 5 kb were not included in the analysis of the NheI pattern. A 90% similarity threshold was used to divide the outputs from the dendrograms into clusters.
RESULTS
Susceptibility to ciprofloxacin.
In Denmark the first strains displaying high-level quinolone resistance (MIC ≥ 4 μg/ml) appeared in 1995. From 1995 to 1998 the percentage of strains exhibiting decreased susceptibility to ciprofloxacin increased from 2.9 to 7.1% and the percentage of ciprofloxacin-resistant strains rose from 3.9 to 6.1% (Table 1). The MIC at which 90% of isolates were inhibited increased from 0.016 to 0.128 μg/ml. Among the 42 resistant strains isolated during the period, the MICs for 7 strains were 1 or 2 μg/ml and those for 35 (83%) were between 4 and 32 μg/ml.
TABLE 1.
Susceptibility to ciprofloxacin of N. gonorrhoeae strains isolated in Denmark from 1995 to 1998
| Yr | No. of strains testeda | No. (%) of strains with MICs (μg/ml) of CIPb:
|
MIC (μg/ml)c
|
||||
|---|---|---|---|---|---|---|---|
| ≤0.03 | 0.06–0.5 | ≥1 | 50% | 90% | Range | ||
| 1995 | 276 | 259 (93.1) | 8 (2.9) | 11 (3.9) | 0.004 | 0.016 | 0.002–16 |
| 1996 | 159 | 146 (91.8) | 4 (2.5) | 9 (5.7) | 0.004 | 0.016 | 0.002–8 |
| 1997 | 177 | 156 (88.1) | 11 (6.2) | 10 (5.6) | 0.004 | 0.128 | ≤0.001–32 |
| 1998 | 197 | 171 (86.8) | 14 (7.1) | 12 (6.1) | 0.004 | 0.128 | ≤0.001–16 |
One isolate per patient.
CIP, ciprofloxacin.
50% and 90%, MICs at which 50 and 90% of the isolates were inhibited, respectively.
Study population.
Table 2 summarizes data on the phenotypes and origin of 40 highly resistant N. gonorrhoeae isolates from 35 patients. From five patients pairs of isolates were available, of which four had been obtained before and after treatment with ciprofloxacin. For all five pairs the second isolate exhibited the same phenotypic features as the first one. Hence, only 35 strains were available for the comparative analyses. Among these strains, 23 were PPNG and 12 were non-PPNG. All the isolates were susceptible to ceftriaxone. Tetracycline-resistant strains were common, but none was suspected of carrying the tetM determinant (MIC ≤ 8 μg/ml). Of the 35 patients, 24 had been infected abroad, primarily in east Asia. Of the four who had acquired their infection in Denmark, three had contacts with patients infected abroad. For seven patients the country of acquisition was unknown. Among 27 strains isolated from 1995 to 1997 21 were PPNG (78%), while only 1 of 8 was PPNG in 1998 (13%).
TABLE 2.
Characterization and origin of 40 isolates of N. gonorrhoeae used in the study
| Strainc | β-Lactamase production | MIC (μg/ml)d of:
|
Size of β-lactamase coding plasmid (kb)e | Place of acquisition | |||
|---|---|---|---|---|---|---|---|
| CIP | PEN | CRO | TET | ||||
| 31/95 | + | 16 | >4 | 0.008 | ≤4 | 7.2 | Far East |
| 76/95 | + | 8 | >4 | 0.008 | ≤4 | 4.9 | Philippines |
| 81/95 | + | 8 | >4 | 0.004 | ≤4 | 4.9 | Thailand |
| 86/95 | − | 8 | 1 | 0.008 | ≤4 | Unknown | |
| 121/95* | + | 8 | >4 | 0.004 | ≤4 | 4.9 | Thailand |
| 128/95* | + | 8 | >4 | 0.004 | ≤4 | 4.9 | Thailand |
| 139/95 | + | 8 | >4 | 0.004 | ≤4 | 7.2 | Philippines |
| 152/95 | + | 8 | >4 | 0.064 | ≤4 | 5.1 | Thailand |
| 160/95† | − | 8 | 0.5 | 0.004 | ≤4 | PRCf | |
| 165/95† | − | 8 | 0.5 | 0.008 | ≤4 | PRC | |
| 169/95 | + | 8 | >4 | 0.002 | ≤4 | 4.9 | Unknown |
| 181/95 | + | 8 | >4 | 0.008 | ≤4 | 4.9 | Hong Kong |
| 253/95 | + | 16 | >4 | 0.002 | ≤4 | 4.9 | Thailand |
| 66/96 | + | 8 | >4 | 0.004 | 2 | 4.9 | Thailand |
| 72/96 | + | 8 | >4 | 0.008 | 4 | 4.9 | Philippines |
| 80/96‡ | + | 8 | >4 | 0.016 | 4 | 4.9 | Philippines |
| 88/96‡ | + | 8 | >4 | 0.008 | 4 | 4.9 | Philippines |
| 109/96 | + | 8 | >4 | 0.016 | 4 | 7.2 | Philippines |
| 110/96 | + | 8 | >4 | 0.008 | 4 | 4.9 | Philippines |
| 135/96 | − | 8 | 1 | 0.008 | 4 | Philippines | |
| 21/97 | + | 8 | >4 | 0.002 | 1 | 4.9 | Thailand |
| 24/97 | + | 8 | >4 | <0.001 | 1 | 5.1 | Thailand |
| 41/97 | − | 8 | 2 | 0.031 | 4 | Unknown | |
| 125/97 | + | 8 | >4 | 0.004 | 0.5 | 4.9 | Iraq |
| 134/97 | − | 32 | 0.5 | 0.004 | 4 | India | |
| 137/97 | + | 8 | >4 | 0.016 | 4 | 4.9 | Rumania |
| 163/97 | + | 8 | >4 | 0.016 | 4 | 4.9 | Denmark |
| 165/97 | + | 8 | >4 | 0.008 | 4 | 4.9 | New Guinea |
| 167/97a | + | 8 | >4 | 0.016 | 4 | 4.9 | Denmark |
| 176/97a | + | 8 | >4 | 0.016 | 8 | 4.9 | Denmark |
| 56/98 | − | 4 | 4 | 0.031 | 4 | Unknown | |
| 63/98 | − | 8 | 1 | 0.016 | 4 | PRC | |
| 88/98 | − | 4 | 0.5 | 0.004 | 1 | Unknown | |
| 105/98 | − | 16 | 2 | 0.016 | 4 | Unknown | |
| 182/98b§ | − | 4 | 1 | 0.016 | 2 | Denmark | |
| 193/98§ | − | 4 | 1 | 0.016 | 2 | Denmark | |
| 186/98 | − | 8 | 2 | 0.016 | 4 | Unknown | |
| 183/98∥ | − | 4 | 2 | 0.016 | 2 | Poland | |
| 194/98∥ | − | 4 | 2 | 0.016 | 2 | Poland | |
| 202/98 | + | 8 | >4 | 0.008 | 2 | 7.2 | Philippines |
Strain from a patient known to have contact with the source of strain 165/97.
Strain from a female patient known to have contact with the source of strain 183/98.
Pairs of isolates from patients are indicated by identical symbols.
CIP, ciprofloxacin; PEN, penicillin; CRO, ceftriaxone; TET, tetracycline.
7.2 kb, Asian type plasmid; 5.1 kb, African type plasmid; 4.9 kb, Toronto type plasmid.
PRC, People's Republic of China.
Plasmid analysis.
All the 23 PPNG strains included in the study contained the 4.2-kb cryptic plasmid as well as the 38.9-kb conjugative plasmid except one strain which did not contain the 38.9-kb plasmid; 17 out of 23 PPNG strains carried the 4.9-kb Toronto plasmid, four carried the 7.2-kb Asian plasmid, and two carried the 5.1-kb African plasmid. Thus four different plasmid profiles were represented among the strains: 17 strains contained the 4.2-, 4.9-, and 38.9-kb plasmids, 3 carried the 4.2-, 7.2-, and 38.9-kb plasmids, 2 carried the 4.2-, 5.1-, and 38.9-kb plasmids, and 1 carried only the 4.2- and 7.2-kb plasmids.
Mutation patterns in the gyrA and parC genes.
Table 3 shows the mutation patterns found in gyrA and parC genes in 35 high-level quinolone-resistant strains. We identified multiple mutations in gyrA and parC genes among these strains. All strains except one had double mutations at codons 91 and 95 in the gyrA gene with a single, double, or triple mutation(s) in the parC gene. One strain (24/97) contained only a single mutation at codon 91 in the gyrA gene.
TABLE 3.
Alterations in the GyrA and ParC proteins in 35 N. gonorrhoeae strains
| Pattern | Amino acid substitutions in:
|
No. of strains | CIPa MIC (μg/ml) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| GyrA
|
ParC
|
|||||||||
| Ser-91 (TCC) | Asp-95 (GAC) | Asp-86 (GAC) | Ser-87 (AGT) | Ser-88 (TCC) | Glu-91 (GAG) | Tyr-104 (TAT) | Leu-131 (CTC) | |||
| M1 | Phe (TTC) | 1 | 8 | |||||||
| M2 | Phe (TTC) | Gly (GGC) | Leu (CTG) | 2 | 4–8 | |||||
| M3 | Phe (TTC) | Ala (GCC) | Asn (AAT) | 1 | 4 | |||||
| M4 | Tyr (TAC) | Gly (GGC) | Asn (AAC) | 1 | 8 | |||||
| M5 | Phe (TTC) | Gly (GGC) | Asn (AAC) | 3 | 8 | |||||
| M6 | Phe (TTC) | Gly (GGC) | Pro (CCC) | 1 | 8 | |||||
| M7 | Phe (TTC) | Gly (GGC) | Lys (AAG) | 1 | 16 | |||||
| M8 | Phe (TTC) | Asn (AAC) | Asn (AAC) | Leu (CTG) | 3 | 4–8 | ||||
| M9 | Phe (TTC) | Gly (GGC) | Asn (AAC) | Leu (CTG) | 11 | 8 | ||||
| M10 | Phe (TTC) | Gly (GGC) | Lys (AAG) | Leu (CTG) | 1 | 32 | ||||
| M11 | Phe (TTC) | Gly (GGC) | Asn (AAT) | Lys (AAG) | 8 | 8–16 | ||||
| M12 | Phe (TTC) | Asn (AAC) | Asn (AAC) | Pro (CCC) | Leu (CTG) | 1 | 4 | |||
| M13 | Phe (TTC) | Gly (GGC) | Arg (CGT) | Tyr (TAC) | Leu (CTG) | 1 | 16 | |||
CIP, ciprofloxacin.
Among the GyrA alterations identified, Ser-91 to Phe (Ser-91 of N. gonorrhoeae GyrA corresponds to Ser-83 of Escherichia coli GyrA [1]) was the most common, having been identified in 34 of the 35 gonococcal strains (97%); a Ser-91-to-Tyr alteration was detected in one strain (41/97). An Asp-95-to-Gly alteration (Asp-95 of N. gonorrhoeae GyrA corresponds to Asp-87 of E. coli GyrA [1]) was found in 30 strains (86%). An Asp-95-to-Asn alteration was identified in four strains (11%). An Asp-95-to-Ala alteration which has not been described previously, was found in one strain (56/98). The double GyrA alteration of Ser-91 to Phe and Asp-95 to Gly was detected in 28 of the 35 strains (80%).
Sequence analysis of the parC gene demonstrated a variety of mutations at codons 86, 87, 88, 91, 104, and 131. The most common ParC alteration, Asp-86 to Asn (Asp-86 of N. gonorrhoeae ParC corresponds to Asp-79 of E. coli ParC [1]), was found in 19 strains (54%). A silent mutation in the DNA sequence from CTC to CTG at codon 131 was also identified in 19 strains (54%). A Glu-91-to-Lys alteration was found in 10 strains (29%), a Ser-87-to-Asn alteration was found in 9 strains (26%), a Ser-88-to-Pro alteration was found in 2 strains (6%), a Ser-87-to-Arg alteration was found in 1 strain (3%), and a silent mutation at codon 104 (Tyr) was found in one strain (3%).
Combination of gyrA mutations and parC mutations gave 13 different mutation patterns among the 35 strains (Table 3). The alterations of Ser-91 to Phe and Asp-95 to Gly in GyrA and Asp-86 to Asn in ParC were found in 14 strains (40%). Some mutation patterns have not been observed previously. There was no difference between the first and the second isolates within pairs or between isolates from sex partners either in antimicrobial susceptibility or in the mutation patterns of gyrA and parC genes.
PFGE analysis.
Genomic DNA from all 40 isolates of N. gonorrhoeae was examined by PFGE after treatment with endonucleases SpeI and NheI. The PFGE profiles obtained after SpeI digestion showed 12 to 17 DNA fragments ranging in size from approximately 23 to 540 kb. The PFGE profiles identified by NheI digestion showed 12 to 19 DNA fragments ranging from approximately 20 to 650 kb.
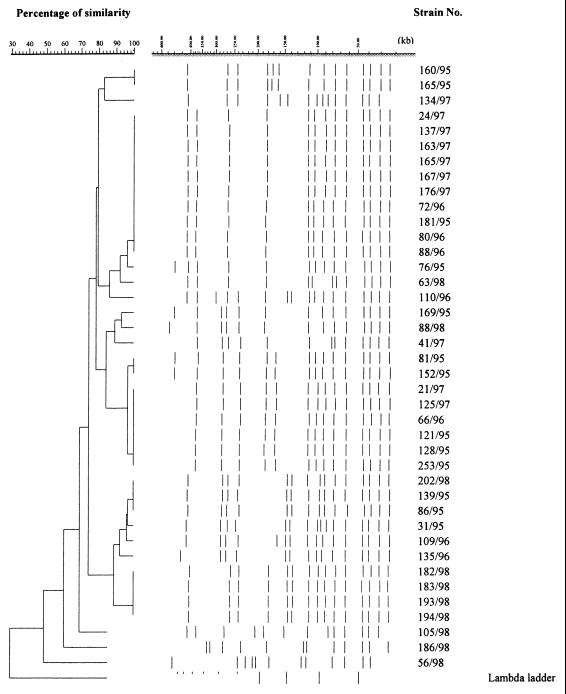
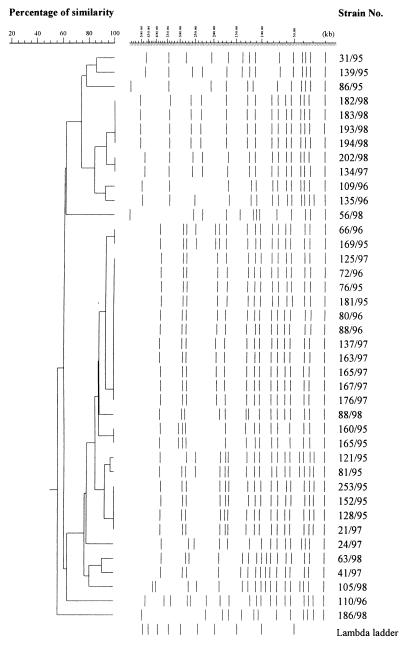
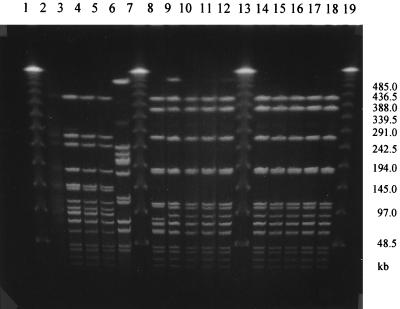
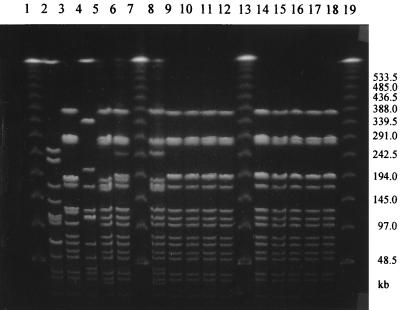
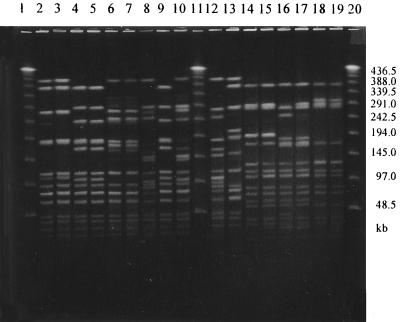
The Dice coefficient and a 90% similarity threshold gave 12 different SpeI profiles and 15 NheI profiles among the 40 isolates (Fig. 1 and 2). Out of eight strains, each of which had a unique pattern of mutations in the gyrA and parC genes, seven also had unique SpeI and NheI patterns, indicating that these strains were genetically unrelated. Among the 11 strains with the most common mutation pattern (M9), 9 (76/95, 181/95, 80/96, 72/96, 137/97, 163/97, 165/97, 167/97, 176/97) were classified into one cluster with identical SpeI patterns (Fig. 3) as well as NheI patterns (Fig. 4) except for one strain (76/95) that had one band difference from the other strains: the addition of a large DNA fragment of approximately 540 kb in the SpeI pattern. These nine strains were all PPNG carrying the 4.9-kb Toronto type plasmid but originated from various places over 3 years. Of the eight strains with the second-most-common mutation pattern (M11), six were classified into the same cluster on the basis of the SpeI patterns or NheI patterns at 96 and 90% similarity thresholds, respectively. Five strains contained the 4.9-kb Toronto type plasmid, and one harbored the 5.1-kb African type plasmid. Among these six strains, five originated from Thailand and one originated from Iraq.
FIG. 1.
Dendrogram based on SpeI PFGE patterns of 40 N. gonorrhoeae isolates.
FIG. 2.
Dendrogram based on NheI PFGE patterns of 40 N. gonorrhoeae isolates.
FIG. 3.
PFGE analysis of genomic DNAs from N. gonorrhoeae strains digested with SpeI. Lanes 1, 7, 13, and 19, lambda DNA ladder; lanes 2 to 6, 8 to 12, and 14 to 18, strains 182/98, 183/98, 193/98, 194/98, 56/98, 63/98, 76/95, 181/95, 72/96, 80/96, 137/97, 163/97, 165/97, 167/97, and 176/97, respectively. (See Table 2 for epidemiologic data.) The DNA preparation of strain 182/98 was tested before and gave the same pattern as that for 183/98, 193/98, and 194/98 (data not shown).
FIG. 4.
PFGE analysis of genomic DNAs from N. gonorrhoeae strains digested with NheI. Lanes 1, 7, 13, and 19, lambda DNA ladder; lanes 2 to 6, 8 to 12, and 14 to 18, strains 56/98, 88/98, 86/95, 21/97, 66/96, 81/95, 76/95, 181/95, 72/96, 80/96, 137/97, 163/97, 165/97, 167/97, and 176/97, respectively. (See Table 2 for epidemiologic data.)
Pairs of isolates recovered from the same patients (Fig. 5) and strains from sexual partners (Fig. 3 and 4) showed the same PFGE patterns after SpeI and NheI digestion. One pair of isolates (121/95 and 128/95) had the same SpeI pattern but differed in NheI pattern: that of the posttreatment isolate (128/95) lacked two small bands of approximately 250 and 40 kb found in that of 121/95 and had a large band of approximately 290 kb not found in that of 121/95 (Fig. 5).
FIG. 5.
PFGE analysis of genomic DNAs from N. gonorrhoeae strains digested with SpeI and NheI. Lanes 1, 11, and 20, lambda DNA ladder; lanes 2 to 10 and 12 to 13 (after SpeI digestion), strains 80/96, 88/96, 121/95, 128/95, 160/95, 165/95, 31/95, 253/95, 109/96, 134/97, and 105/98, respectively; lanes 14 to 19 (after NheI digestion), strains 80/96, 88/96, 121/95, 128/95, 160/95, and 165/95, respectively. (See Table 2 for epidemiologic data.)
DISCUSSION
Fluoroquinolones act by inhibiting the topoisomerase enzymes essential for DNA replication and recombination. Fluoroquinolone resistance in bacteria has been associated with alterations in the GyrA subunit of DNA gyrase (type II topoisomerase) and in the ParC subunit of DNA topoisomerase IV and with the reduction of drug accumulation in the cells. The development of high-level fluoroquinolone resistance occurs through stepwise mutations (6). Studies on laboratory mutants and clinical isolates of N. gonorrhoeae have revealed that gyrA mutation plays an important role in the development of fluoroquinolone resistance and that simultaneous parC and gyrA mutation plays a complementary role in increasing the level of resistance (1, 4, 28). The reduced fluoroquinolone accumulation in the bacteria is thought to have an additional but lesser effect (21). Previous studies have revealed that gonococcal isolates fully susceptible to ciprofloxacin had no alterations in GyrA and ParC or had a single alteration in GyrA alone (24); strains exhibiting decreased susceptibility (MICs, 0.125 to 0.5 μg/ml) generally contained alterations only in GyrA (4, 10). When a ParC alteration was detected in a less-susceptible strain, only a single GyrA alteration was present (29). Ciprofloxacin-resistant strains (MICs, ≥ 1 μg/ml), with few exceptions, had GyrA alterations at positions 91 and 95 and at least one ParC alteration (4, 28, 29).
In this study we demonstrated that 91% of strains for which ciprofloxacin MICs were 4 to 32 μg/ml and which were isolated between 1995 and 1998 contained the double GyrA alteration at the 91 and 95 positions and a single or double ParC alteration(s). Our results strongly suggest that the double gyrA mutation plus parC mutation play an important role in the development of high-level resistance to fluoroquinolone in N. gonorrhoeae. There was no significant correlation between the number of mutations in the parC gene and the susceptibility to ciprofloxacin. Strains with the same mutation pattern for parC but with different levels of ciprofloxacin resistance (MICs, 8 to 64 μg/ml) have been found previously (28).
In our study 63% of the high-level ciprofloxacin-resistant strains were PPNG. A high proportion of PPNG strains among quinolone-resistant strains was also reported by Tapsall et al. (26). Interestingly, the majority of the PPNG strains (83%) contained the 4.9-kb Toronto type plasmid. All the PPNG strains containing the 4.9-kb plasmid also contained the 38.9-kb conjugative plasmid, which is in agreement with previous studies (14, 20). A shift from predominantly PPNG strains to non-PPNG strains among the ciprofloxacin-resistant strains was observed in 1998.
A wide variety of phenotypes of quinolone-resistant N. gonorrhoeae strains with decreased susceptibilities to fluoroquinolones have been reported in Hong Kong and Japan (5, 11). A single strain or closely related strains of N. gonorrhoeae have been demonstrated to be associated with a focal area of endemic transmission in Cleveland, Ohio, (12) and Ontario, Canada (9). Among quinolone-resistant N. gonorrhoeae strains a shift from multiple subtypes being imported to a limited number of subtypes sustained domestically was documented in Australia (26), and the spread of quinolone-resistant strains has been assumed to be due in part at least to the presence of these phenotypes in international commercial sex workers and their clients (26). An increasing proportion of N. gonorrhoeae strains highly resistant to ciprofloxacin (MIC, ≥ 4 μg/ml) was observed among female sex workers in the Philippines from 1994 to 1997 (13). In Denmark infections with quinolone-resistant strains were most often acquired abroad or through contact with persons infected abroad (16). Although few in number, they made up a high proportion of all cases. According to the country-wide laboratory surveillance system, endemic spread of gonorrhea was infrequent from 1995 onwards (16).
PFGE is often considered the “gold standard” of molecular typing methods (19) and has been applied successfully to a wide range of bacterial species, both gram positive and gram negative (27). In this study the most interesting observation documented by PFGE is the identification of a cluster of nine strains originating from different places (Philippines, Hong Kong, Rumania, New Guinea, and Denmark) over 3 years, which suggests that a common epidemic clone may be widely spread. They also had identical patterns of mutations in the gyrA and parC genes, which also substantiated the assumption that they were genetically related. Close genetic relationships between strains of N. gonorrhoeae recovered from distinct locations separated by thousands of miles over a 2-year period have already been demonstrated by Xia et al. (32). No differences in the gyrA and parC mutation patterns or in susceptibility to ciprofloxacin between pretreatment and posttreatment isolates from the same patients or between sexual partners were observed. Pairs of isolates from sexual partners had indistinguishable SpeI and NheI patterns, confirming that they were isogenic and epidemiologically related. An interesting point was the one band difference in PFGE pattern between two isolates (121/95 and 128/95) from the same patient. On the basis of the criteria defined by Tenover et al. (27), a single genetic event such as a point mutation in the posttreatment isolate could be reflected as a difference of one to three bands. PFGE pattern variation between the posttreatment isolate and the original isolate was previously reported by Harnett et al. (8). In their report the interval between the recovery of the two isolates was 4 months. The interval was short in our patient, only 5 days.
In conclusion, the observations described in this study showed that the increase in the number of ciprofloxacin-resistant isolates of N. gonorrhoeae in Denmark was due to the importation of resistant strains from abroad, that a resistant bacterial clone which might have originated in east Asia seems to have spread worldwide, and that double alterations at Ser-91 and Asp-95 in GyrA plus a single or double ParC alteration(s) play an important role in the development of high-level fluoroquinolone resistance in N. gonorrhoeae.
ACKNOWLEDGMENT
We gratefully acknowledge the staff of the Clinical Biochemistry Department, Statens Serum Institut, for technical assistance with the sequencing.
REFERENCES
- 1.Belland R J, Morrison S G, Ison C A, Huang W M. Neisseria gonorrhoeae acquires mutations in analogous regions of gyrA and parC in fluoroquinolone resistant isolates. Mol Microbiol. 1994;14:371–380. doi: 10.1111/j.1365-2958.1994.tb01297.x. [DOI] [PubMed] [Google Scholar]
- 2.Birley H, McDonald P, Carey P. High level ciprofloxacin resistance in Neisseria gonorrhoeae. Genitourin Med. 1994;70:292–293. doi: 10.1136/sti.70.4.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bygraves J A, Maiden M C J. Analysis of the clonal relationships between strains of Neisseria meningitidis by pulsed field gel electrophoresis. J Gen Microbiol. 1992;138:523–531. doi: 10.1099/00221287-138-3-523. [DOI] [PubMed] [Google Scholar]
- 4.Deguchi T, Yasuda M, Nakano M, Ozeki S, Ezaki T, Saito I, Kawada Y. Quinolone-resistant Neisseria gonorrhoeae; correlation of alterations in the GyrA subunit of DNA gyrase and the ParC subunit of topoisomerase IV with antimicrobial susceptibility profiles. Antimicrob Agents Chemother. 1996;40:1020–1023. doi: 10.1128/aac.40.4.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deguchi T, Yasuda M, Maeda S I, Saito I, Kawada Y. Serotyping of quinolone-resistant Neisseria gonorrhoeae isolates with alterations in GyrA and ParC. J Antimicrob Chemother. 1998;41:418–420. doi: 10.1093/jac/41.3.418. [DOI] [PubMed] [Google Scholar]
- 6.Fisher L M. New insights into mechanisms of quinolone action and resistance. Mol Biol Genet. 1998;15(Suppl. E):55–60. [Google Scholar]
- 7.Fox K K, Knapp J S, Holmes K K, Hook III E W, Judson F N, Thompson S E, Washington J A, Whittington W L. Antimicrobial resistance in Neisseria gonorrhoeae in the United States, 1988–1994: the emergence of decreased susceptibility to the fluoroquinolones. J Infect Dis. 1997;175:1396–1403. doi: 10.1086/516472. [DOI] [PubMed] [Google Scholar]
- 8.Harnett N, Brown S, Terro R, Krishnan C, Pauze M, Yeung K-H. High-level tetracycline-resistant Neisseria gonorrhoeae in Ontario, Canada—investigation of a cluster of isolates, showing chromosomally mediated resistance to penicillin combined with plasmid-mediated resistance to tetracycline. J Infect Dis. 1997;176:1269–1276. doi: 10.1086/514122. [DOI] [PubMed] [Google Scholar]
- 9.Harnett N, Brown S, Riley G, Terro R, Krishnan C, Pauze M, Yeung K-H. Analysis of Neisseria gonorrhoeae in Ontario, Canada, with decreased susceptibility to quinolones by pulse-field gel electrophoresis, auxotyping, serotyping and plasmid content. J Med Microbiol. 1997;46:383–390. doi: 10.1099/00222615-46-5-383. [DOI] [PubMed] [Google Scholar]
- 10.Ison C A, Woodford P J, Madders H, Claydon E. Drift in susceptibility of Neisseria gonorrhoeae to ciprofloxacin and emergence of therapeutic failure. Antimicrob Agents Chemother. 1998;42:2919–2922. doi: 10.1128/aac.42.11.2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kam K M, Wong P W, Cheung M M, Ho N K Y, Lo K K. Quinolone-resistant Neisseria gonorrhoeae in Hong Kong. Sex Transm Dis. 1996;23:103–108. doi: 10.1097/00007435-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Kilmarx P H, Knapp J S, Xia M, St. Louis M E, Neal S W, Sayers D, Doyle L J, Roberts M C, Whittington W L. Intercity spread of gonococci with decreased susceptibility to fluoroquinolones: a unique focus in the United States. J Infect Dis. 1998;177:677–682. doi: 10.1086/514234. [DOI] [PubMed] [Google Scholar]
- 13.Klausner J D, Aplasca M-R, Mesola V P, Bolan G, Whittington W L, Holmes K K. Correlates of gonococcal infection and antimicrobial-resistant Neisseria gonorrhoeae among female sex workers, Republic of the Philippines, 1996–1997. J Infect Dis. 1999;179:729–733. doi: 10.1086/314625. [DOI] [PubMed] [Google Scholar]
- 14.Knapp J S, Mesola V P, Neal S W, Wi T E, Tuazon C, Manalastas R, Perine P L, Whittington W L. Molecular epidemiology, in 1994, of Neisseria gonorrhoeae in Manila and Cebu City, Republic of the Philippines. Sex Transm Dis. 1997;24:2–7. doi: 10.1097/00007435-199701000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Lind I. Antimicrobial resistance in Neisseria gonorrhoeae. Clin Infect Dis. 1997;24(Suppl. 1):S93–S97. doi: 10.1093/clinids/24.supplement_1.s93. [DOI] [PubMed] [Google Scholar]
- 16.Lind I, Smith E. Gonorrhoea 1997. EPI News Denmark. 1998;49:1. [Google Scholar]
- 17.Lind I. Gonorrhoea. Curr Probl Dermatol (Basel) 1996;24:12–19. doi: 10.1159/000424878. [DOI] [PubMed] [Google Scholar]
- 18.O'Callaghan C H, Morris A, Kirby S M, Shingler A H. Novel method for detection of β-lactamases by using a chromogenic cephalosporin substrate. Antimicrob Agents Chemother. 1972;1:283–288. doi: 10.1128/aac.1.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olive D M, Bean P. Principles and applications of methods for DNA-based typing of microbial organisms. J Clin Microbiol. 1999;37:1661–1669. doi: 10.1128/jcm.37.6.1661-1669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reimann K, Bollerup A C, Lind I. The emergence of penicillinase-producing Neisseria gonorrhoeae strains carrying the 4.9 kb (Toronto) plasmid in Denmark and of a novel large plasmid in two nonpenicillinase-producing Neisseria gonorrhoeae strains. Sex Transm Dis. 1992;19:206–212. doi: 10.1097/00007435-199207000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Tanaka M, Sakuma S, Takahashi K, Nagahuzi T, Saika T, Kobayashi I, Kumazawa J. Analysis of quinolone resistance mechanisms in Neisseria gonorrhoeae isolates in vitro. Sex Transm Infect. 1998;74:59–62. doi: 10.1136/sti.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka M, Otsuki M, Nishino T, Kobayashi I, Matsumoto T, Kumazawa J. Mutation in DNA gyrase of norfloxacin-resistant clinical isolates of Neisseria gonorrhoeae. Genitourin Med. 1996;72:295–297. doi: 10.1136/sti.72.4.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka M, Takahashi K, Saika T, Kobayashi I, Ueno T, Kumazawa J. Development of fluoroquinolone resistance and mutations involving GyrA and parC proteins among Neisseria gonorrhoeae isolates in Japan. J Urol. 1998;159:2215–2219. doi: 10.1016/S0022-5347(01)63308-1. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka M, Nakayama H, Haraoka M, Saika T, Kobayashi I, Naito S. Antimicrobial resistance of Neisseria gonorrhoeae and high prevalence of ciprofloxacin-resistant isolates in Japan, 1993 to 1998. J Clin Microbiol. 2000;38:521–525. doi: 10.1128/jcm.38.2.521-525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tapsall J W, Phillips E A, Shultz T R, Thacker C. Quinolone-resistant Neisseria gonorrhoeae isolated in Sydney, Australia, 1991 to 1995. Sex Transm Dis. 1996;23:425–428. doi: 10.1097/00007435-199609000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Tapsall J W, Limnios E A, Shultz T R. Continuing evolution of the pattern of quinolone resistance in Neisseria gonorhoeae isolated in Sydney, Australia. Sex Transm Dis. 1998;25:415–417. doi: 10.1097/00007435-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 27.Tenover F C, Arbeit R D, Goering R V. How to select and interpret molecular strain typing methods for epidemiological studies of bacterial infections: a review for healthcare epidemiologists. Infect Control Hosp Epidemiol. 1997;18:426–439. doi: 10.1086/647644. [DOI] [PubMed] [Google Scholar]
- 28.Trees D L, Sandul A L, Whittington W L, Knapp J S. Identification of novel mutation patterns in the parC gene of ciprofloxacin-resistant isolates of Neisseria gonorrhoeae. Antimicrob Agents Chemother. 1998;42:2103–2105. doi: 10.1128/aac.42.8.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trees D L, Sandul A L, Peto-Mesola V, Aplasca M, Leng H B, Whittington W L, Knapp J S. Alterations within the quinolone resistance-determining regions of GyrA and ParC of Neisseria gonorrhoeae isolated in the Far East and United States. Int J Antimicrob Agents. 1999;12:325–332. doi: 10.1016/s0924-8579(99)00081-3. [DOI] [PubMed] [Google Scholar]
- 30.WHO Western Pacific Gonococcal Antimicrobial Surveillance Programme. Resistance in gonococci isolated in the WHO Western Pacific region to various antimicrobials used in the treatment of gonorrhoea, 1997. Commun Dis Intell. 1998;22:288–291. doi: 10.33321/cdi.1998.22.62. [DOI] [PubMed] [Google Scholar]
- 31.World Health Organization. STD treatment strategies. WHO/VDT/89.447. Geneva, Switzerland: World Health Organization; 1989. [Google Scholar]
- 32.Xia M, Roberts M C, Whittington W L, Holmes K K, Knapp J S, Dillon J R, Wi T. Neisseria gonorrhoeae with decreased susceptibility to ciprofloxacin: pulsed-field gel electrophoresis typing of strains from North America, Hawaii, and the Philippines. Antimicrob Agents Chemother. 1996;40:2439–2440. doi: 10.1128/aac.40.10.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]