Abstract
The minimal fungicidal concentrations (MFCs) of voriconazole and itraconazole for five clinical isolates each of Aspergillus terreus, Aspergillus fumigatus, Aspergillus flavus, and Aspergillus niger were determined by a broth macrodilution method. Conidial suspensions as inocula were compared to hyphae as inocula since the invasive form of aspergillosis is manifested by the appearance of hyphal structures. In addition, cell viability staining with the dye FUN-1 was performed to assess time-dependent damage of hyphae exposed to various concentrations of the antifungal agents. With conidial inocula the MFC ranges of voriconazole were 0.5 to 4 μg/ml and those of itraconazole were 0.25 to 2 μg/ml, whereas the MFCs (2 to >16 μg/ml) with hyphal inocula were substantially higher (P < 0.01) for both itraconazole and voriconazole. Only minor differences between the tested antifungals were observed since 16 of 20 and 17 of 20 of the isolates of Aspergillus spp. tested appeared to be killed by voriconazole and itraconazole, respectively. The results of FUN-1 viability staining correlated closely to colony counts, but various time- and dose-dependent levels of viability of hyphae were also observed. In conclusion, our study demonstrates the importance of the type of inoculum used to test antifungals and the applicability of FUN-1 staining as a rapid and sensitive method for assaying the viability of hyphae.
Fungal pathogens are recognized as major and increasing sources of infection in immunocompromised hosts (7, 11, 21), with Aspergillus species, Fusarium species, zygomycetes, dematiaceous fungi, and other opportunistic fungi being the main pathogens (5, 6). The incidence of fungal infection may vary from 4% in patients with AIDS to 13% in patients undergoing heart transplantation and even 30% in bone marrow transplant recipients (8, 20). Infections with Aspergillus spp. are common causes of nosocomial pneumonia and are associated with an extremely high mortality rate of 40% (19). The most common species causing disease in patients are Aspergillus fumigatus (83%), Aspergillus flavus (9%), Aspergillus niger (5%), and Aspergillus terreus (3%) (6, 24).
Over the past 30 years, amphotericin B has remained the drug of choice for the treatment of invasive aspergillosis in immunosuppressed hosts, despite its toxicity and the low rates of response (30 to 55%) to the drug (6, 8, 13, 23). Recently, less toxic antifungal drugs have become available as treatment options, and antifungal susceptibility testing with the opportunistic pathogens mentioned above is important in the clinical laboratory. In the study described here we investigated the in vitro activities of voriconazole and itraconazole against clinical isolates of Aspergillus species because these substances are used for treatment of invasive aspergillosis (2, 22).
Since Aspergillus spp. adopt a hyphal form in tissue, we compared the minimal fungicidal concentrations (MFCs) of voriconazole and itraconazole for conidia versus hyphae. In addition, we developed a cell viability assay to assess the activities of both antifungals against fungal hyphae using the halogenated dye FUN-1 and evaluated the potential of this dye to examine time-dependent damage. This dye is converted to a red fluorescent agent in actively metabolizing fungal cells, which requires plasma membrane integrity and metabolic activity (4, 25).
MATERIALS AND METHODS
Strains.
The in vitro tests were performed with five clinical isolates each of A. fumigatus, A. flavus, A. niger, and A. terreus. The isolates were maintained as conidial suspensions in water at room temperature, and subcultures were grown on Sabouraud glucose agar (Merck, Darmstadt, Germany) and incubated at 35°C for 5 days. One in vitro and in vivo itraconazole-resistant isolate of A. fumigatus (isolate AF 72) was kindly provided by D. W. Denning and was included in the study.
Antifungal agents.
Voriconazole (Pfizer, Sandwich, United Kingdom) and itraconazole (Janssen Research Foundation, Beerse, Belgium) were kindly provided as standard powders by their respective manufacturers. All drugs were dissolved in dimethyl sulfoxide (Sigma, Vienna, Austria) to yield stock solutions containing 1,600 μg/ml. Serial dilutions of these agents to concentrations of 160 to 0.125 μg/ml were prepared in dimethyl sulfoxide and RPMI 1640 medium (BioWhittaker, Vienna, Austria) as described previously (9, 10).
Broth macrodilution test using conidia as inocula.
Fungi were tested by the broth macrodilution method for antifungal susceptibility testing of conidium-forming filamentous fungi as described previously (9). The conidial suspension was harvested by flooding each colony with 2 ml of sterile 0.85% saline. Turbidity was measured with a spectrophotometer at 530 nm (DU-64 spectrophotometer; Beckman, Foulerton, Minn.); the transmissions were adjusted with sterile 0.85% saline to 78 to 82% for A. flavus and A. niger and to 80 to 82% for A. fumigatus and A. terreus. The suspension was further diluted to 1:100 in RPMI 1640 medium to obtain 0.3 × 104 to 4 × 104 CFU/ml. A total of 100 μl of each of the drug dilutions was inoculated with 900 μl of the fungal suspensions, and the mixture was incubated at 35°C and evaluated after 24 and 48 h for visible growth. Final concentrations between 0.03 and 16 μg/ml for voriconazole or itraconazole were used. As a quality control an itraconazole-resistant A. fumigatus strain was used.
In order to obtain the MFCs, 100-μl volumes were taken from every well showing inhibition and were spread on Sabouraud dextrose agar. The numbers of CFU were counted after incubation of the plates at 35°C for 48 h until growth of subcultures from the growth control well was apparent (detection limit, 10 CFU/ml). The MFC was defined as the lowest drug concentration at which 99% of the inoculum was killed.
Broth macrodilution test using hyphae as inocula.
The conidial stock solutions were prepared as described above and were diluted 1:100 in RPMI 1640 medium containing 10 mM HEPES (Sigma, St. Louis, Mo.) to obtain the desired inoculum size of 0.1 × 104 to 5 × 104 CFU/ml. A total of 1 ml of each of these solutions was added to 24-well plates (Costar, Vienna, Austria), and the plates were incubated at 30°C for 16 to 22 h for the formation of hyphae. This allowed outgrowth of more than 95% of the conidia with hyphal lengths varying from 50 to 70 μm, as determined with an inverted microscope.
The wells were washed and refilled with 0.5 ml of RPMI 1640 medium. Voriconazole as well as itraconazole dilutions were added to final concentrations of 0.062 to 16 μg/ml, and the plates were incubated at 35°C. The MFCs of voriconazole and itraconazole were determined as described above.
FUN-1 viability staining of hyphae.
To assess the damage to the hypae, viability staining with FUN-1 (Molecular Probes, Eugene, The Netherlands) was performed at various time points. Samples from the various solutions containing hyphae as well as itraconazole and voriconazole at concentrations of 0.062 to 16 μg/ml were taken at time zero and after 30 min and 3, 9, 24, and 48 h of incubation at 35°C. For this purpose, supernatants were aspirated and fungi were washed with sterile water and resuspended in 0.7 ml of 10 mM HEPES. A total of 100 μl of the fungal culture suspension was added to 100 μl of the FUN-1 solution. Various concentrations of the FUN-1 solutions that ranged from 5 to 20 μmol were tested for each Aspergillus species. These cell suspensions were incubated for 30 min at 35°C and were centrifuged at 4,000 × g for 2 min.
As controls, Aspergillus isolates were incubated with medium without antifungals and were treated with 96% ethanol. Three samples from each well were assayed and were examined for staining with FUN-1. For comparison with the data obtained by FUN-1 staining, 100 μl was removed from every well, plated onto Sabouraud dextrose agar, and incubated at 35°C for 48 h.
Statistics.
The results of the in vitro tests with both antimycotics obtained with the conidia and hyphae of Aspergillus spp. were compared by the log-rank test.
RESULTS
Broth macrodilution test using conidia as inocula.
The MFC ranges of voriconazole and itraconazole for Aspergillus spp. were 0.5 to 4 and 0.25 to 2 μg/ml, respectively. Detailed results are given in Table 1. Voriconazole and itraconazole MFCs showed minor differences between and among the isolates of Aspergillus spp. tested.
TABLE 1.
Susceptibilities of Aspergillus species conidia to voriconazole and itraconazole
| Aspergillus sp.a (no. of isolates tested) | Antifungal agent | MFC (μg/ml)
|
|
|---|---|---|---|
| Range | 50% | ||
| A. flavus (5) | Voriconazole | 0.5–2 | 1 |
| Itraconazole | 0.5–1 | 0.5 | |
| A. terreus (5) | Voriconazole | 0.5–1 | 0.5 |
| Itraconazole | 0.5–1 | 1 | |
| A. niger (5) | Voriconazole | 1–4 | 1 |
| Itraconazole | 1–2 | 1 | |
| A. fumigatus (5) | Voriconazole | 0.5–1 | 0.5 |
| Itraconazole | 025–1 | 0.5 | |
| Itraconazole-resistant A. fumigatus (1) | Voriconazole | 0.5–1 | 0.5 |
| Itraconazole | >16 | NDb | |
Each isolate was tested three times.
ND, not determined.
Broth macrodilution test using hyphae as inocula.
According to the colony counts, three of five (60%) isolates of A. flavus, four of five (80%) isolates of A. terreus, five of five (100%) isolates of A. niger, and four of five (80%) isolates of A. fumigatus were killed by voriconazole at concentrations between 2 and 16 μg/ml, while five of five (100%) isolates of A. flavus, four of five (80%) isolates of A. terreus, three of five (60%) isolates of A. niger, and five of five isolates (100%) of A. fumigatus were killed by itraconazole at concentrations between 2 and 16 μg/ml. The MFC ranges are given in Table 2; the MFCs at which 50% of isolates are inhibited (MFC50s for both antifungals for Aspergillus spp. were 4 μg/ml.
TABLE 2.
Susceptibility of Aspergillus hyphae to voriconazole and itraconazole
| Aspergillus sp.a (no. of isolates tested) | Voriconazole MFC range (μg/ml) at 48 h | Voriconazole staining pattern(s)b | Itraconazole MFC range (μg/ml) at 48 h | Itraconazole staining pattern(s) |
|---|---|---|---|---|
| A. flavus (5) | 8–>16 | VID | 4–4 | ID |
| 2–2 | D | 2–2 | D | |
| 2–4 | D | 2–2 | D | |
| 8–>16 | VD | 4–4 | D | |
| 2–4 | D | 4–8 | D | |
| A. terreus (5) | 2–4 | D | 4–4 | D |
| 2–4 | ID | 4–4 | ID | |
| 16 | VD | 2–2 | D | |
| 2–4 | D | 8–>16 | VD | |
| 2–2 | D | 4–4 | D | |
| A. niger (5) | 8–16 | ID | 8–>16 | VD |
| 4–4 | D | 4–4 | D | |
| 2–2 | D | 8–>16 | VD | |
| 2–2 | D | 2–2 | D | |
| 2–2 | D | 2–2 | D | |
| A. fumigatus (5) | 2–2 | D | 2–2 | D |
| 2–4 | ID | 2–4 | ID | |
| 8–>16 | VID | 4–4 | ID | |
| 4–4 | D | 2–2 | D | |
| 2–2 | D | 2–4 | D | |
| Itraconazole-resistant A. fumigatus (1) | 2–2 | D | >16 | VID |
Each isolate was tested three times.
V, Viable hyphae represented by green fluorescent hyphae with red vacuolar structures; I, impaired hyphae represented by green fluorescent hyphae and lack of red fluorescent vacuolar structures; D, dead hyphae represented by green-yellow fluorescence without any vacuolar structures. The predominant patterns are underlined.
FUN-1 viability staining of hyphae.
Optimal FUN-1 staining of Aspergillus spp. was achieved at a final dye concentration of 5 μmol; the staining patterns of the hyphae were dependent on the voriconazole and itraconazole concentrations and the incubation time.
Various combinations of staining patterns were observed. Detailed results of tests of FUN-1 viability staining are given in Table 2.
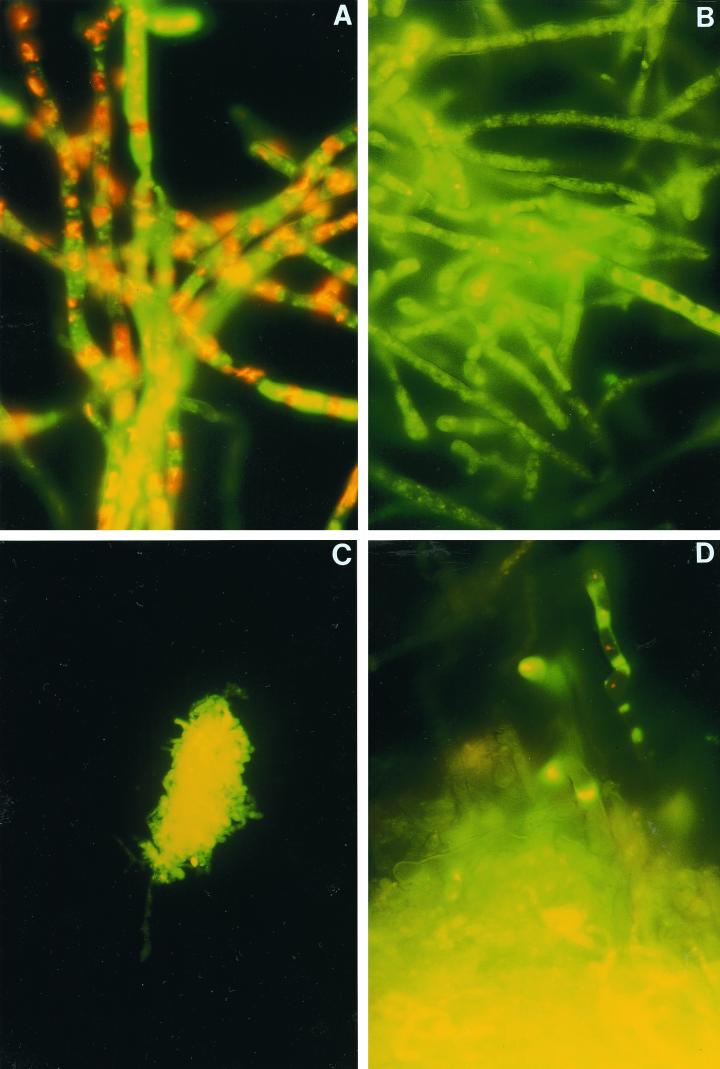
Untreated isolates of Aspergillus spp. showed green fluorescent hyphae with clearly red fluorescent vacuolar structures (Fig. 1A). Fungi were classified as viable, which was confirmed by the CFU counts. After 3 h of incubation with itraconazole and voriconazole at concentrations fungicidal for conidia, 11 of 20 and 15 of 20 isolates of Aspergillus spp., respectively, showed moderate reductions in red fluorescent vacuolar structures but no alteration in CFU counts. After 9 h of incubation at the same fungicidal concentrations, green fluorescent hyphae and a lack of red fluorescent vacuolar structures were shown by all aspergilli (Fig. 1B). Fungi were classified as impaired, and 13 isolates treated with voriconazole and 9 isolates treated with itraconazole showed a slightly delayed regrowth, but there was still no decrease in CFU counts. After 24 to 48 h of incubation, clusters of collapsed hyphae with extremely bright, diffuse, green-yellow fluorescence without vacuolar structures were observed for all Aspergillus spp. when antifungal concentrations between 2 and 16 μg/ml were used (Fig. 1C). These data correlated closely to the colony-count reductions; thus, these fungi were classified as dead. Similar patterns were obtained with Aspergillus isolates treated with 96% ethanol.
FIG. 1.
(A) Viable A. fumigatus showing green fluorescent hyphae with clearly red fluorescent vacuolar structures. (B) A. fumigatus hyphae treated with 2 μg of voriconazole per ml for 9 h. Impairment is shown by green fluorescent hyphae and a lack of red fluorescent vacuolar structures. (C) A. fumigatus hyphae treated with 8 μg of voriconazole per ml for 48 h. Clusters of collapsed hyphae with extremely bright, diffuse, green-yellow fluorescence without vacuolar structures are displayed by dead fungi. (D) A. fumigatus hyphae treated with 16 μg of voriconazole per ml for 48 h. Only one fragment of hyphae is apparently vital.
For isolates (n = 7) treated with concentrations of >16 μg/ml, clusters of dead fungi as well as small numbers of viable hyphae were found for both voriconazole and itraconazole (Fig. 1D and Table 2). For comparison, the itraconazole-resistant A.fumigatus strain showed masses of impaired structures combined with small numbers of viable and dead hyphae in medium containing 16 μg of itraconazole per ml.
Statistics.
Statistically significant differences in the MFCs obtained for conidia versus those obtained for hyphae (P < 0.01) of Aspergillus spp. were detected. The MFCs for hyphae were two- to fourfold higher than those for conidia.
DISCUSSION
The ranges of MFCs of voriconazole and itraconazole for conidial suspensions of Aspergillus spp. found in this study were comparable to those presented in previously published reports (12, 15, 18).
However, in order to kill hyphae of Aspergillus spp. in vitro, voriconazole and itraconazole must be applied at significantly higher concentrations (P < 0.01). Our findings are in agreement with results previously published for filamentous fungi other than Aspergillus spp. (12), showing that the MICs and MFCs for hyphae were always substantially higher than those for conidia. However, Manavathu et al. (14) reported that the MICs and MFCs of various antifungal agents for germinated conidia were identical to those for ungerminated conidia of A. fumigatus.
Aspergilli are respiratory pathogens, and pulmonary infections are usually acquired through inhalation of conidia (1), whereas the invasive form of infection is dominated by the appearance of hyphae. Therefore, the main target of antifungal agents should be the hyphal structure; consequently, impairment of hyphae under therapy must be guaranteed for therapy to be successful. This may be relevant for the prediction of in vivo efficacy and may explain therapeutic failures, despite low MICs and MFCs for conidia in the standard test.
Since the red fluorescence of FUN-1 is impaired by both drugs' interference with fungal metabolism and membrane damage (3), we evaluated the use of this dye to assess antifungal activities in a time- and dose-dependent manner. Direct microscopic visualization permitted identification of metabolically active fungi and differentiation from damaged, metabolically inactivated, and killed organisms, as described for Saccharomyces cerevisiae and Aspergillus nidulans (3, 16). An early drug-mediated impairment, as described for Candida spp. (26), was not detected. The 9-h incubation period for both antifungal agents when they were used at the MFCs determined for conidia resulted in a decrease in metabolic activity, expressed as a slightly delayed regrowth of a few isolates (Fig. 1B). Further investigations are necessary, however, to clarify this observation and to evaluate the clinical implications.
FUN-1 staining itself was seen to be applicable for susceptibility testing of Aspergillus spp., as deduced from the finding that the reduction in CFU counts closely corresponded to the occurrence of extremely bright, diffuse, green-yellow fluorescence and an absence of vacuolar structures. This was confirmed by using an itraconazole-resistant A. fumigatus strain. Masses of impaired fungal elements and small amounts of dead and viable cells were obtained, while for none of the other fungi did we find similar patterns with 16 μg of itraconazole per ml. No cross-resistance between itraconazole and voriconazole was observed. Voriconazole showed good activity against hyphae of the itraconazole-resistant A. fumigatus strain, with MFCs of 2 μg/ml.
For some isolates, however, clusters of dead hyphae accompanied by vital elements were detected in the presence of 16 μg of voriconazole or itraconazole per ml (Fig. 1D). The circumstances that accounted for this are not clear, nor is it known whether these results predict clinical resistance.
The results of our study, namely, that killing of hyphae required higher concentrations of antifungal drugs, may partly explain the difficulty in providing successful therapy. Both an exposure time of 24 to 48 h and high drug concentrations were necessary to cause a significant decrease in viable counts of hyphae. Concentrations in serum of 4.56 ± 0.68 μg/ml for voriconazole were achieved in a rat model of invasive pulmonary aspergillosis and delayed or prevented mortality (17). Therefore, the MFC50 of 4 μg of voriconazole per ml for Aspergillus spp. probably indicates clinically effective treatment.
Since the MFCs obtained for conidia had only a slight influence on the viability of the hyphae, the maximum tolerated dose for patients with diagnosed or probable fungal infections should be applied. The lack of an efficient host defense is dominant in patients with severe neutropenia. Fungicidal effects of the antifungal agents are therefore probably necessary to overcome invasive fungal infections.
In conclusion, our data show that both voriconazole and itraconazole have in vitro potencies against Aspergillus conidia and, at higher concentrations, against hyphae. Our data also demonstrate the importance of the type of inoculum used to test antifungal drugs. Furthermore, the use of FUN-1 is a simple and rapid way to assess the viability of Aspergillus spp. under antifungal treatment.
More studies are needed to implement a standard assay for the testing of fungal hyphae and to compare in vitro findings with in vivo outcome.
ACKNOWLEDGMENTS
We thank D. W. Denning for providing the itraconazole-resistant A. fumigatus strain. We also thank Pfizer Pharmaceutical and Janssen Research Foundation, for providing voriconazole and itraconazole, respectively.
REFERENCES
- 1.Beyer J, Schwartz S, Heineman V, Siegert W. Strategies in prevention of invasive pulmonary aspergillosis in immunosuppressed or neutropenic patients. Antimicrob Agents Chemother. 1994;38:314–319. doi: 10.1128/aac.38.5.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bradford C R, Prentice A G, Warnock D W, Copplestone J A. Comparison of the multiple dose pharmacokinetics of two formulations of itraconazole during remission induction of acute myeloblast leukaemia. J Antimicrob Chemother. 1991;28:555–560. doi: 10.1093/jac/28.4.555. [DOI] [PubMed] [Google Scholar]
- 3.Brul S, Nussbaum J, Dielbandhoesing S K. Fluorescent probes for wall porosity and membrane integrity in filamentous fungi. J Microbiol Methods. 1997;28:169–178. [Google Scholar]
- 4.Burgess S M, Delannoy M, Jensen R E. MMM1 encodes a mitochondrial outer membrane protein essential for establishing and maintaining the structure of yeast mitochondria. J Cell Biol. 1994;126:1375–1391. doi: 10.1083/jcb.126.6.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denning D W. Epidemiology and pathogenesis of systemic fungal infections in the immunocompromised host. J Antimicrob Chemother. 1991;28(Suppl. B):1–16. doi: 10.1093/jac/28.suppl_b.1. [DOI] [PubMed] [Google Scholar]
- 6.Denning D W. Invasive aspergillosis. Clin Infect Dis. 1998;26:781–805. doi: 10.1086/513943. [DOI] [PubMed] [Google Scholar]
- 7.Denning D W, Follansbee S E, Scolaro M, Norris S, Edelstein H, Stevens D. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:654–662. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- 8.Denning D W, Stevens D A. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1991;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 9.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Bresln B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinel-Ingroff A. In vitro activity of the new triazole voriconazole (UK-109,496) against opportunistic filamentous and dimorphic fungi and common and emerging yeast pathogens. J Clin Microbiol. 1998;36:198–202. doi: 10.1128/jcm.36.1.198-202.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerson L S, Talbot G H, Hurwitz S, Strom B L, Lusk E J, Cassileth P A. Prolonged granulocytopenia: the major risk factor for invasive pulmonary aspergillosis in patients with acute leukemia. Ann Intern Med. 1984;100:345–351. doi: 10.7326/0003-4819-100-3-345. [DOI] [PubMed] [Google Scholar]
- 12.Guarro J, Llop C, Aguilar C, Pujol I. Comparison of in vitro antifungal susceptibilities of conidia and hyphae of filamentous fungi. Antimicrob Agents Chemother. 1997;41:2760–2762. doi: 10.1128/aac.41.12.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hara K S, Ryu J H, Lie J T, Roberts G D. Disseminated Aspergillus terreus infection in immunocompromised hosts. Mayo Clin Proc. 1989;64:770–775. doi: 10.1016/s0025-6196(12)61749-2. [DOI] [PubMed] [Google Scholar]
- 14.Manavathu E K, Cutright J, Chandrasekar P. Comparative study of susceptibilities of germinated and ungerminated conidia of Aspergillus fumigatus to various antifungal agents. J Clin Microbiol. 1999;37:858–861. doi: 10.1128/jcm.37.3.858-861.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin M V, Yates J, Hitchcock C A. Comparison of voriconazole and itraconazole in prevention and treatment of Aspergillus fumigatus endocarditis in guinea pigs. Antimicrob Agents Chemother. 1997;41:13–16. doi: 10.1128/aac.41.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Millard P J, Roth B L, Thi H P, Yue S T, Haugland R P. Development of the FUN-1 family of fluorescent probes for vacuole labeling and viability testing of yeasts. Appl Environ Microbiol. 1997;63:2897–2905. doi: 10.1128/aem.63.7.2897-2905.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Murphy M, Bernard E M, Ishimaru T, Armstrong D. Activity of voriconazole against clinical isolates of Aspergillus species and its effectiveness in an experimental model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother. 1997;41:696–698. doi: 10.1128/aac.41.3.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oakley K L, Moore C B, Denning D W. In-vitro activity of voriconazole against Aspergillus spp. and comparison with itraconazole and amphotericin B. J Antimicrob Chemother. 1998;42:91–94. doi: 10.1093/jac/42.1.91. [DOI] [PubMed] [Google Scholar]
- 19.Offner F, Cordonnier C, Ljungman P, Prentice H G, Englhard D, DeBacqer D, Meunier F, Pauw B. Impact of previous aspergillosis on the outcome of bone marrow transplantation. Clin Infect Dis. 1998;26:1098–1103. doi: 10.1086/520274. [DOI] [PubMed] [Google Scholar]
- 20.Peterson P K, McGlave P, Ramseay N K. A prospective study of infectious diseases following bone marrow transplantation: emergence of Aspergillus and cytomegalovirus as the major causes of mortality. Am J Infect Control. 1998;4:81–89. doi: 10.1017/s0195941700057805. [DOI] [PubMed] [Google Scholar]
- 21.Richardson M D. Antifungal therapy in bone marrow failure. Br J Haematol. 1998;100:619–628. doi: 10.1046/j.1365-2141.1998.00600.x. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz S, Milatovic D, Thiel E. Successful treatment of cerebral aspergillosis with a novel triazole (voriconazole) in a patient with acute leukaemia. Br J Haematol. 1997;97:663–665. doi: 10.1046/j.1365-2141.1997.972911.x. [DOI] [PubMed] [Google Scholar]
- 23.Tritz D M, Woods G L. Fatal disseminated infection with Aspergillus terreus in immunocompromised hosts. Clin Infect Dis. 1993;16:118–122. doi: 10.1093/clinids/16.1.118. [DOI] [PubMed] [Google Scholar]
- 24.Walsh T J, Pizzo P A. Nosocomial fungal infections: a classification for hospital-acquired fungal infections and mycoses arising from endogenous flora or reactivation. Annu Rev Microbiol. 1988;42:517–545. doi: 10.1146/annurev.mi.42.100188.002505. [DOI] [PubMed] [Google Scholar]
- 25.Wenisch C, Linnau F, Parschalk B, Zedtwitz-Liebenstein K, Georgopoulos A. Rapid susceptibility testing of fungi by flow cytometry using vital staining. J Clin Microbiol. 1997;35:5–10. doi: 10.1128/jcm.35.1.5-10.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]