Abstract
A growing amount of information about the different types, functions, and roles played by non-coding RNAs (ncRNAs) is becoming available, as more and more research is done. ncRNAs have been identified as potential therapeutic targets in the treatment of tuberculosis (TB), because they may be essential regulators of the gene network. ncRNA profiling and sequencing has recently revealed significant dysregulation in tuberculosis, primarily due to aberrant processes of ncRNA synthesis, including amplification, deletion, improper epigenetic regulation, or abnormal transcription. Despite the fact that ncRNAs may have a role in TB characteristics, the detailed mechanisms behind these occurrences are still unknown. The dark matter of the genome can only be explored through the development of cutting-edge bioinformatics and molecular technologies. In this review, ncRNAs’ synthesis and functions are discussed in detail, with an emphasis on the potential role of ncRNAs in tuberculosis. We also focus on current platforms, experimental strategies, and computational analyses to explore ncRNAs in TB. Finally, a viewpoint is presented on the key challenges and novel techniques for the future and for a wide-ranging therapeutic application of ncRNAs.
Keywords: noncoding RNAs, tuberculosis, epidemiology, RNA informatics, RNA structure-function relationships
1. Background
Tuberculosis (TB) is an infectious respiratory disease, caused by an intracellular pathogen referred to as Mycobacterium tuberculosis (Mtb), which is most commonly transmitted via inhalation and spreads to the alveolar space [1]. Since ancient times, tuberculosis has been a lethal disease that has posed a threat to public health, resulting in mortality [2]. A pandemic of tuberculosis in 1993 led the World Health Organization [3] to declare TB a global health emergency [3]. In accordance with the WHO, approximately ten million individuals were infected with TB, and approximately 6.4 million were newly diagnosed [4]. Furthermore, it was stated that about one million die from the disease every year [5].
As drug-resistant strains of Mtb have emerged, tuberculosis treatment has become more challenging [6]. Regardless of the fact that most of the research on tuberculosis has paid attention to the protein coding genes, the fact that approximately 97 percent of the human genetic material is composed of non-coding segments has prompted scientists to explore the genetic dark matter of tuberculosis [7]. The term untranslated RNAs refers to non-coding RNAs (ncRNAs) [8]. Micro-RNAs (miRNAs), which are short ncRNAs 22–25 nt; long non-coding RNAs (lncRNAs), which are the largest class of ncRNAs, with approximately 55,000 genes in human genetic material [7]; and circular RNAs (circRNAs) are the most extensively studied [9]. As ncRNAs exhibit tissue-specific expression patterns that are significantly dysregulated in tuberculosis, they are attractive diagnostic, prognostic, and therapeutic targets. As a result, elucidating the role of noncoding RNAs in tuberculosis in modern biology is a difficult task [10]. In the current review paper, we explain in silico and in vitro approaches to explore the noncoding RNA transcriptome, with a complete overview of strategies and tools for characterizing ncRNA structure and examining their role in tuberculosis and progression. Additionally, we discuss the implications of these approaches for translation.
2. Classification and Biogenesis of Noncoding RNAs
Only about 20,000 protein-coding genes are found in human DNA, accounting for less than 2% of the total genetic material. It was discovered using cutting-edge technologies that approximately 90% of the genome is actively transcribed [11]. The transcriptome of humans contains antisense, overlapping, and noncoding RNA expression, which makes it more complex than a collection of coding genes and splice variants [12]. Around 97 percent of the human genome is non-coding, prompting scientists to explore this genetic dark matter in a variety of diseases, including tuberculosis [13]. Certain classes of noncoding RNAs, called housekeeping ncRNAs, are expressed ubiquitously in all types of cells [14]. Transfer RNAs (tRNAs) and ribosomal RNAs (rRNAs) are two distinct types of RNAs involved in messenger RNA (mRNA) [15].
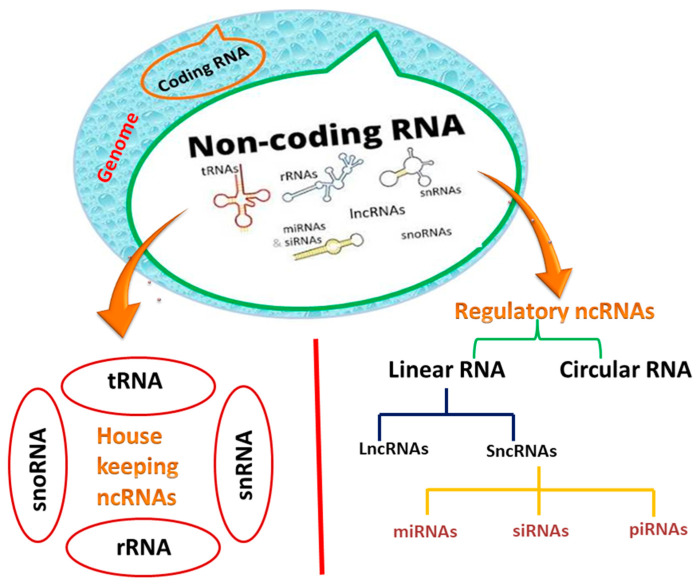
Another family of noncoding RNAs has recently sparked interest in the scientific community because of its regulatory roles [8]. Regulatory noncoding RNAs are classified into two types: (i) linear RNAs containing sncRNAs (small non-coding RNAs), which are further subdivided into microRNAs (miRNAs), small interfering RNAs (siRNAs), and long non-coding RNAs (lncRNAs); (ii) and circular RNAs (circRNAs) (Figure 1) [16]. Unlike linear RNAs, circRNAs can make a covalently closed loop in the absence of 5′ and 3′ polar regions. In the current review, we will concentrate on miRNAs and lncRNAs, including circular RNAs, and their biogenesis and roles in tuberculosis [17].
Figure 1.
Classification of non-coding RNAs (ncRNAs).
2.1. miRNA
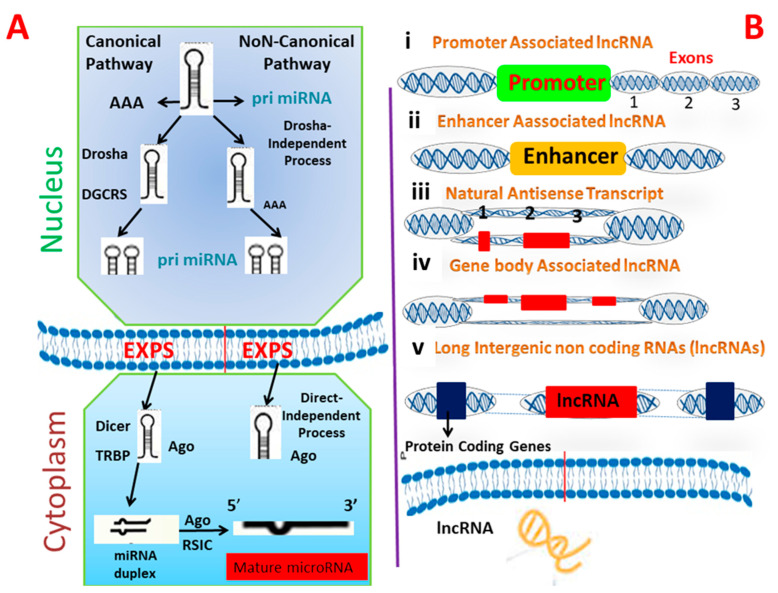
miRNAs are a subclass of noncoding short RNAs that range from 18 to 22 nucleotides (nt) [18]. MicroRNAs are crucial posttranscriptional regulators of gene expression and their roles in the pathogenesis of infectious diseases are well understood; they may be used as diagnostics. miRNAs are thought to be more stable biomarkers than mRNA, because of their small size and chemical structure [19]. Indeed, numerous recent publications suggest that changes in host’s miRNA expression are a common sign of bacterial infection at the cellular and organismal levels in humans, including those caused by Mtb [20]. All miRNAs undergo multistep maturation and processing steps that start in the nucleus and end in cytoplasm. RNA polymerase II synthesizes primary miRNA transcripts, in the form of a massive double-stranded primary transcript known as pri-miRNAs, which can be some 100 nt in length [21] and available in polyadenylated and capped hairpin forms. Drosha, an RNase III-like enzyme, converts this precursor into a double stranded miRNA precursor composed of 60–100 nt hairpins, referred to as pre-miRNAs. Pre-miRNAs have a stem-loop structure and are traded from nucleus to cytoplasm by exportin 5 [22]. Further processing of pre-miRNAs in the cytoplasm through RNase III Dicer enzyme results in the generation of an incomplete 22 nt double-stranded miRNA. The guide strand (miRNA) and the passenger strand (miRNA*) combine to form this unstable duplex [23]. The miRNA* is eliminated, while the guide strand miRNA matures. After maturation, mature miRNAs are integrated into the RNA-induced silencing complex (RISC), which distinguishes particular targets and promotes posttranscriptional gene silencing; thereby regulating gene expression. Recently, it was discovered that a novel biogenesis pathway exists in which microRNA enters RISC without being further processed by Dicer [24]. The strand enters RISC following Drosha processing (Figure 2A). The role of microRNA is well-defined by the genes that it targets and the effect of it on their expression. MiRNAs interact with bacteria in a variety of ways, depending on the bacteria’s characteristics [25].
Figure 2.
Schematic representation of biogenesis pathway of microRNA (A) and lncRNA (B). Number (i,ii,iii,iv) in (B) shows the number of biogenesis steps of lncRNAs while (1,2,3) shows the number of exons.
By regulating apoptosis in TB-infected cells and utilizing the host’s energy and metabolic processes, miRNAs contribute significantly to the pathogenesis of Mtb infection [26]. Hence, when Mtb is in the host, it changes the expression of miRNA genes.
Remarkably, anti-TB treatment restored the previously reduced expression of numerous miRNAs [27]. These findings imply that miRNAs provide the induction of inflammatory responses in human cells in response to bacterial pathogens. Future research on the miRNAs’ role and expression in tuberculosis may advance our understanding of Mtb pathogenesis and point the way toward improved diagnostics and/or novel treatment options [28].
2.2. lncRNAs
lncRNAs are a group of RNAs that do not undergo translation and are more than 200 nt long. Instead, they function as an RNA molecule. As with other mRNAs, long non-coding RNAs have a distinct 5′ cap structure and a 3′ polyadenylate structure, and were previously thought to lack an open reading frame [29]. Additionally, these RNAs are categorized according to their position relative to the coding gene, with the most common types of lncRNA such as forward long ncRNA, reverse lncRNAs, intragenic, bidirectional, and intergenic lncRNAs [30]. The presence of lncRNAs encoded short functional peptides shows that they can function simultaneously in RNA, as well as in peptides. Recognizing the biogenesis of lncRNAs is critical for differentiating them from the other types of RNA and elucidating their role and characteristics [31]. lncRNAs biogenesis is stage- and cell-type-specific, and is regulated by cell- and stage-specific stimuli. Numerous long noncoding RNAs (lncRNAs) are transcribed from diverse DNA genomic components, including promoters, and intergenic regions. Numerous mechanisms for the formation of lncRNAs have been proposed, including ribonuclease P cleavage of mature ends, the formation of small nucleolar protein (snoRNP) and snoRNA complex caps in ends and the formation of circular structures [32]. Additionally, distinct subnuclear structures known as paraspeckles were discovered recently surrounding specific lncRNAs during biogenesis (Figure 2B). The term “paraspeckles” refers to nuclear particles that are frequently observed near the nucleus in small foci. Paraspeckles may contribute to lncRNA-dependent gene expression, by transporting messenger RNA into the nucleoplasm in response to particular stimuli [33]. While the mechanisms underlying the biogenesis of various lncRNAs are unknown at the moment, additional information is expected to be obtained in the coming years as a result of the development of techniques such as RNA structure mapping, chromatin isolation by ribosome profiling, phylogenetic lineage tracing, RNA purification (ChIRP-Seq), and crosslinking immunoprecipitation (CLIP) [34,35]. Numerous long noncoding RNAs play a role in the regulation of biological processes such as cell lineage determination, cell organogenesis and differentiation, and tissue homeostasis in a wide range of diseases, including rheumatoid arthritis, tuberculosis, diabetes, and various types of cancer [36]. Most of the lncRNAs that are linked to diseases have a dysregulated expression in different cells. Dysregulation of long ncRNAs has been found in many infections, such as Mycobacterium tuberculosis, Salmonella typhimurium, and Escherichia coli [37].
2.3. CircRNAs
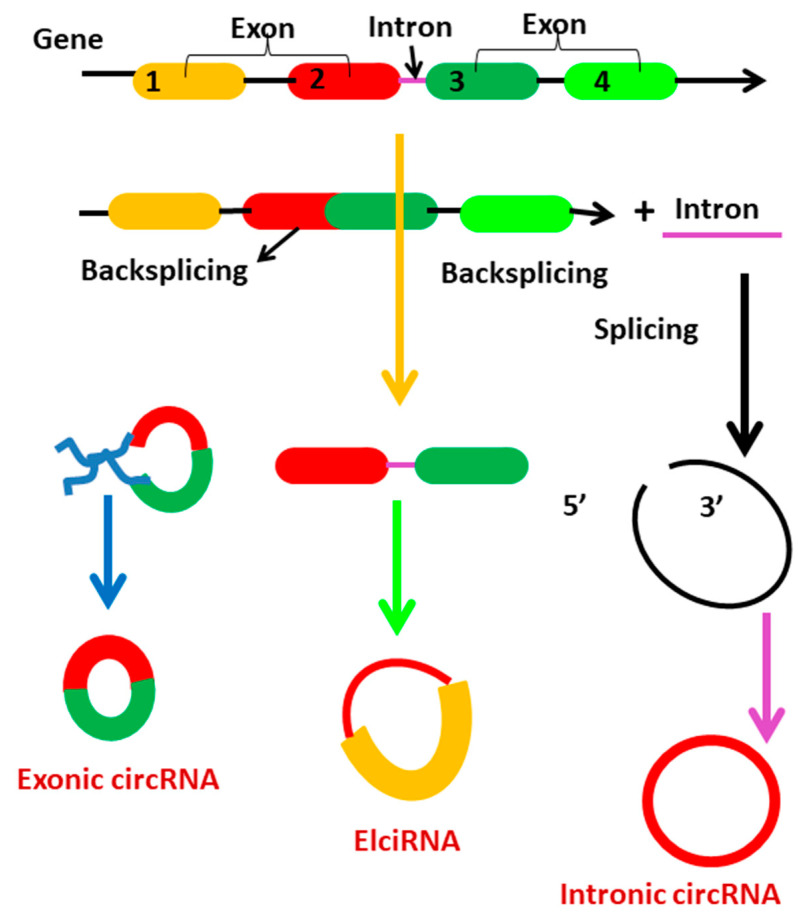
Circular RNAs are generated from primary transcripts via back-splicing and lariat-driven circularization, which compete with mRNA splicing [38]. Circularization of backsplicing occurs in response to cis- and trans-acting components and variables. Circularization occurs when cis-acting elements in upstream introns are base-paired with those in downstream introns [39]. TAFs are RNA-binding proteins (RBPs) that promote circularization through interacting with particular motifs within flanking introns. Circularization-driven circularization is another possibility for the biogenesis of circRNAs [40]. This occurs when pre-mRNA exons or introns are skipped or removed. Circular RNAs are classified according to their biogenesis into three types: exonic circRNAs (EcRNAs), exon-intron circRNAs (EIciRNAs), and circular intronic circRNAs (ciRNAs) (Figure 3). While both EIciRNAs and ciRNAs are circular RNAs with nuclear introns, most EcRNAs are synthesized in cytoplasm. While the majority of circular RNAs are synthesized from precursor mRNA, a minority are synthesized from precursor tRNA [41]. During tRNA maturation, the tRNA splicing endonuclease (TSEN) complex may cleave intron-containing pre-tRNA. RtcB ligase cyclizes intron termini, forming circular tRNA intronic RNAs (tricRNAs) [42].
Figure 3.
Representation of circRNA biogenesis. Numbers (1,2,3,4) in fig represents the exon 1, exon 2, exon 3 and exon 4.
3. Role of ncRNAs in the Molecular Epidemiologic Studies of Tuberculosis
3.1. The Role of miRNA
miRNA regulatory dysfunction is required for biological processes [43] and has been associated with a number of human diseases, including tuberculosis. Specifically, miRNA dysregulation could help us better understand human diseases [44]. Anomalies manifest in a variety of ways: (i) down regulation of microRNA expression due to mutation, or transcriptional downregulation; (ii) overexpression of microRNA due to gene amplification or transcriptional upregulation, which could result in decreased production of its target proteins; (iii) mutation in the 3UTR of mRNA can affect a microRNA binding site, rendering the microRNA inactive; and (iv) mutation in the miRNA itself may cause decreased production of its target proteins [45,46]. Numerous studies have established a link between miRNA dysregulation and tuberculosis. Recent research has established that the control of miRNA in tuberculosis can only be described by genetic or epigenetic abnormalities in the processing system [47]. This insight has been applied to a variety of infectious diseases, implying that miRNA could be used as clinical biomarkers [48]. Many studies have found that macrophages and natural killer cells from tuberculosis patients have different gene expression profiles when they are active and when latent [49].
This alteration in tuberculosis patients’ cellular makeup and gene expression is almost certainly caused by miRNAs. Numerous microRNAs have been identified to play a role in the regulation of T cell development and function. Additionally, microRNAs are involved in the regulation of macrophages and natural killer cells’ innate functions [50]. Next, we discuss the mechanism of action of a few miRNAs in the context of metabolic pathways.
3.1.1. miRNA-29
In vitro and clinical investigations have demonstrated that miR-29 is overexpressed in various human cell types, following infection with pathogenic Mycobacterium species [51]. By downregulating IFN-g, miR-29 reduces immunological responses to Mycobacterium TB [52]. Apart from targeting IFN-g mRNA at the 30UTR, miR-29a stimulates the interaction of IFN-g mRNA with Argonaute 2 (Ago2) protein, resulting in the formation of an RNA-induced silencing complex and subsequent suppression of IFN-g expression post-transcriptionally [53]. Additionally, several reports have indicated that miR-29 targets the antiapoptotic proteins B-cell lymphoma 2 (Bcl-2) and myeloid cell leukemia-1 (Mcl-1), as well as the kinase p85a and the GTP-binding protein Cdc42; implying that miR-29 plays a critical role in regulating the apoptotic pathway in immune cells [54]. Thus, overexpression of miR-29 in TB infection contributes one method by which Mycobacterium tuberculosis avoids macrophage digestion, by inhibiting IFN-g and boosting apoptosis of cells engaged in anti-tuberculosis responses [55]. Intriguingly, a contrary occurrence happened in a model of non-virulent Mycobacterium species infection [56]. In NK cells and T cells, Mycobacterium bovis Bacillus Calmette-Guerin (BCG) inhibited miR-29 expression and elevated IFN-g expression [57]. This result suggests that silencing miR-29 may have promoted the generation of IFN-g by these T cells and that miR-29 expression is controlled by Mycobacterium species-specific pathogenicity.
3.1.2. miRNA-147
A previous study demonstrated that miR-147 is increased in macrophages via the TLRs/NF-kB signaling pathway and inhibits the expression of proinflammatory cytokines such as TNF-a and IL-6 [58]. These findings suggest that miR-147 possesses significant anti-inflammatory activity. TNF-a and IL-6 levels in serum or peripheral blood mononuclear cells (PBMCs) were shown to be significantly higher in active tuberculosis compared to healthy controls [59]. A pivotal study discovered that miR-147 was significantly overexpressed in sputum taken from patients with active tuberculosis, as compared to controls [60]. Interestingly, this study discovered that the levels of TNF-a and IL-6 in sputum were not significantly different between active tuberculosis patients and controls, indicating that dysregulation of cytokines occurs mostly in the circulation, rather than the lungs [61]. Taken together, these findings suggest that miR-147 and miR-29 augment anti-tuberculosis immunity and operate as a negative regulator of the immune response to tuberculosis.
3.1.3. miRNA-21
Previous research indicates that miR-21 inhibits the expression of proinflammatory cytokines and promotes the synthesis of an anti-inflammatory cytokine, IL-10 [62]. A recent study discovered that miR-21 is increased in unsensitized DCs and macrophages following M. bovis BCG infection, both in vitro and in vivo, via the TLR/Erk/NF-kB pathway [63]. miR-21 expression is also increased when macrophages are challenged with the Mycobacterium TB early-secreted antigenic target 6 kDa protein (ESAT-6) antigen [64]. By directly targeting the 30UTR of IL-12 mRNA, miR-21 decreased host Th1 responses. Additionally, this study discovered that miR-21 decreased the expression of protective cytokines (TNF-a and IL-6) in M. tuberculosis infection, although these cytokines’ levels were not significantly altered in response to ESAT-6 exposure. Notably, miR-21 increased DC death by targeting Bcl-2; corroborating prior findings that M. tuberculosis can induce apoptosis in infected cells. However, the precise mechanism by which miR-21 influenced Bcl-2 expression remains unknown. Additionally, miR-21 inhibitors increased IL-12 production and elicited more robust antimycobacterial responses [65,66]. As a result, miR-21 may be a useful method for Mycobacteria to circumvent the host immune response and establish chronic infection.
3.1.4. miRNA-99b
Exposure to Mycobacterium TB but not to LPS increased miR-99b expression in murine DCs from MyD88-deficient mice. Further investigation revealed that inhibiting miR-99b (through antagomirs and knockdown) dramatically decreased M. tuberculosis growth and considerably increased proinflammatory cytokines such as TNF-a, IL-6, IL-12, and IL-1b. M. tuberculosis growth inhibition could be a result of an increase in the production of these proinflammatory cytokines [67,68,69]. Additionally, this study discovered that miR-99b directly targeted the tumor necrosis factor receptor superfamily, member 4 (TNFRSF-4) and TNF-a messenger RNA, to regulate the expression of a variety of cytokines and transcription factors involved in T cell differentiation pathways and tuberculosis clearance [70]. Additionally, treatment of DCs transfected with anti-miR-99b antibody resulted in an increase in bacterial mortality [71]. These findings demonstrate that miR-99b plays a critical role in M. tuberculosis development in DCs, by suppressing the production of TNF-a, which enables the bacteria to evade host protective immunological responses and survive within host phagocytes.
3.1.5. miRNA-125b
Rajaram et al. discovered that incubating human macrophages with Mycobacterium TB and its component, lipomannan, increases miR-125b expression, while decreasing TNF production [72,73]. miR-125b directly targets the 30UTR of the TNF mRNA transcript, inhibiting translation and, perhaps, accelerating its degradation; hence, inhibiting TNF production. Additionally, this study discovered that only virulent Mycobacterium species inhibit the activation of the mitogen-activated protein kinase (MAPK) p38 and Akt, two components that significantly contribute to TNF generation in mycobacterial-infected macrophages [74,75]. Additionally, miR-125b increases the stability of kB-Ras2, an NF-kB signaling inhibitor in human macrophages, thereby suppressing the inflammatory response. Taken together, these findings indicate that M. tuberculosis inhibits TNF production by increasing miR-125b expression. By contrast, infection with avirulent Mycobacterium species and exposure to lipomannan decreased miR-125b expression, which was associated with increased TNF production [76]. Further investigation revealed that silencing miR-125b increased the stability of TNF mRNA and promoted proinflammatory responses.
3.1.6. miRNA-155
miR-155 was discovered to be a multifunctional miRNA involved in a variety of biological processes, including infection, inflammation, and immunology. Previous research demonstrated that when miR-155 was knocked down in mice, more IL-4 and less IFN-g were produced, implying that miR-155 plays a critical role in regulating T cell-dependent responses. One study discovered contradictory data about miR-155 dysregulation in human macrophages infected with several Mycobacterium species [77,78]. The study discovered that M. tuberculosis infection and lipomannan exposure to human macrophages downregulated miR-155, resulting in decreased TNF production. This inhibition was mediated via the TLR-MAPK/Akt pathway. Further investigation demonstrated that M. tuberculosis inhibits the start and stability of TNF mRNA, resulting in decreased translation [79,80]. These findings, however, contradict a prior study, in which it was discovered that miR-155 targeted the 30UTR of the inositol phosphatase SH2-containing inositol 5-phosphatase (SHIP1) mRNA, a negative regulator of TNF production, resulting in its degradation and, thus, enhanced TNF production [81]. The other study discovered that overexpression of miR-155 increased TNF production by increasing the stability and half-life of mRNA.
3.1.7. miRNA-144*
miR-144* (present on complementary passenger strand) is overexpressed in patients with active TB. miR-144* targets genes involved in the Janus kinase/signal transducers and activators of transcription (Jak-STAT) signaling circuit, the MAPK signaling pathway, the Toll-like receptor signaling pathway, and interactions with cytokine receptors [82,83]. Further transfection of T cells with the miR-144* precursor revealed that miR-144* inhibits the production of TNF-a and IFN-g [84]. Given the critical function of TNF-a and IFN-g in protective immunity, it is possible that miR-144* has an effect on the development and outcome of tuberculosis. miR-144* may influence anti-tuberculosis immunity by modifying cytokine production and T cell proliferation.
3.1.8. miRNAs-223 and 424
Wang et al. [85] discovered that miR-223 and miR-424 were substantially expressed in the peripheral blood mononuclear cells (PBMCs) of patients with active tuberculosis [86,87]. A miR-223 silencing study in mice revealed an increased proportion of granulocytes, which are morphologically mature, hypersensitive to activating stimuli, and possess more fungicidal activity [87]. miR-424 stimulates monocyte development and then inhibits the transcription factor NFI-A78 expression. Wang et al. [85] discovered that several miRNAs target basic leucine zipper transcription factor 2/BTB and CNC homology 1 (BACH2) and B-cell CLL/lymphoma 7A (BCL7A) in active tuberculosis [88]. Wang et al. hypothesized that the decreased expression of these two genes, which are regulated by multiple miRNAs, such as miR-223 and miR-424, may result in an imbalance in the proportions of T cells and B cells in active tuberculosis patients, disrupting the delicate balance of immune control during Mycobacterium tuberculosis infection. Wang et al. [88] also discovered that miR-424 regulates the differentiation of B cells from plasma cells by targeting BACH2, BCL2, and BCL7A, which are important in cellular differentiation and development.
3.2. The Role of lncRNA
lncRNAs primarily regulate gene expression via chromatin remodeling, RNA–RNA interaction, transcriptional and translational activation or inhibition, and miRNA regulatory modulation [89]. mRNA splicing is one of the processes that lncRNAs can affect. For instance, it is unknown how the lncRNA MALAT1 regulates Ser/Arg splicing factors [90]. LncRNAs can affect the stability and trafficking of messenger RNA and proteins in the cytoplasm. Additionally, certain long noncoding RNAs are like a molecular sink or sponge for the proteins that interact with RNA (RBPs) [91]. RBPs function as chromatin modifiers, regulatory proteins for adjacent genes encoding proteins and transcription factors. MALAT1, snoRNAs, and NEAT1 are all instances of long ncRNAs that function as miRNA sponges, inhibiting/activating gene expression [92]. Certain lncRNAs can be synthesized from enhancer elements, which are frequently activated in a similar fashion to enhancers. Enhancer elements are DNA segments that have been covalently linked to specific proteins and function as transcriptional activators and enhancers for their target genes. Numerous enhancer regions produce RNAs called eRNAs or elncRNAs, which stand for enhancer-associated long noncoding RNAs [93,94]. Recent findings indicated that elncRNAs promote gene expression by increasing the accessibility of chromatin. Numerous functional lncRNAs, as mounting evidence indicates, exhibit variable expression patterns during microbial infections. These long noncoding RNAs have an essential function in regulating the interaction of host and pathogen. They originate in the host or are encoded by pathogen agents [95]. They can be activated as a result of pathogen growth and proliferation regulation or as a result of antimicrobial defense mechanisms. Here, we discuss the mechanism of action of a few lncRNAs in the context of their pathways.
3.2.1. lncRNA MEG3
Downregulation of lncRNA MEG3 in TB led in an increase in cell proliferation, prevention of apoptosis, and stimulation of autophagy [96]. To address this issue, a study using a variety of approaches to determine the involvement of lncRNA MEG3 in infected macrophage autophagy discovered that silencing lncRNA MEG3 expression increases autophagy in mycobacteria-infected macrophages [97]. On the other hand, interferon-induced autophagy in infected macrophages was associated with the persistence of MEG3 downregulation for more than 24 h.
3.2.2. lncRNA CD244
lncRNA CD244 and its signaling-related molecules are upregulated in tuberculosis infection, inhibiting T cell-dependent immunological responses and decreasing TNF- and IFN- production. Excessive production of lncRNA-BC050410, also known as lncRNA-CD244, has been shown to alter CD244 signaling and expression in the CD8+CD244+ T-cell subset [98]. CD244 lncRNA controls methylation events at the tnfa/infg loci and decreases the production of TNF-/IFN- in CD8+ T cells [99]. This inhibitory effect can be reversed by silencing lncRNA-CD244. Transfer of lncRNACD244–repressed CD8+ T cells reduced tuberculosis infection and pathology in Mtb-infected mice when compared to a control group with lncRNACD244–expressed CD8+ T cells [100]. It has been hypothesized that lncRNA-CD244 may be a significant target for tuberculosis infection treatment methods. CD4+ T cells play a critical role in the host defense against Mycobacterium TB, by modulating immunological homeostasis and regulating intracellular pathogen development.
3.2.3. lncRNA NEAT1
Recent research reveals that NEAT1 expression increases during Mtb infection and may be connected with TB prognosis. Reduced expression of NEAT1 may impair macrophage clearance of intracellular Mtb [101]. Additionally, TB infection dramatically boosted NEAT1 expression in T helper-1 cells. Using siRNA and NEAT1 knockout mice, we found that increased NEAT1 expression during tuberculosis infection was related with an increase in infection duration and that decreased NEAT1 expression may induce macrophage clearance of Mycobacterium tuberculosis from cells [102,103].
3.2.4. lncRNA PCED1B-AS1
lncRNA PCED1B-AS1 is expressed at a lower level in CD14+ monocytes from active tuberculosis patients than in healthy persons [104]. According to Li et al. [105] downregulation of the long noncoding RNA PCED1B-AS1 may decrease apoptosis and promote autophagy in infected macrophages [106]. Indeed, PCED1BAS1 as an endogenous sponge reduces miR-155 expression in macrophages via direct binding to miR-155 and abolishes miR-155’s influence on the target gene FOXO3/Rheb [107]. Thus, Mtb-induced PCED1B-AS1 downregulation lowers FOXO3 and Rheb expression, while increasing LC-3 expression and simultaneously decreasing caspase-3 expression and attenuating apoptosis [100,108]. Given this, PCED1B-AS1 may be used to augment autophagy in macrophages as a potential early diagnostic biomarker for active tuberculosis.
3.3. The Role of circRNA
CircRNAs perform several important roles, including modifying the expression of regulating gene transcription, functioning as microRNA sponges, binding to protein [109]. While the bulk of circRNAs are traded to the cytoplasm, a subclass of intron-carrying circRNAs are carried to the nucleus. Both EIciRNAs and ciRNAs regulate hereditary genes in a cis-regulatory manner [110]. EIciRNAs connect with U1 snRNP to create the EIciRNAs–U1 snRNP complex, which functions in conjunction with polymerase II (Pol II) to control host gene transcription in the promoter region. Similarly, circRNAs are accumulated at transcriptional sites to promote Pol II elongation, resulting in cis-regulatory effects on hereditary genes [111].
MiRNA sponges are widely regarded as one of circRNAs’ most critical functions. microRNAs are believed to operate as transcriptional regulators, connecting with particular mRNA locations to govern both pathological and normal gene expression. Numerous circRNAs have been identified as miRNA sponges [112]. CircRNAs have been demonstrated to influence gene expression by acting as protein sponges/decoys. For instance, circRNA100146 was shown to bind numerous types of the splicing factor family (SF3), which are highly associated with transcriptional regulation of genes among the upper hundred proteins, including SF3A1, SF3B2, and SF3B3 [113]. CircRNAs also act as scaffolds for proteins, stimulating chemical reactions or inhibiting the function of proteins. As exemplified by circ-Foxo3, which may operate as a protein scaffold for MDM2 and p53; hence, promoting p53 degradation. Numerous circular RNAs regulate gene expression and have a quick response to external stimuli via their association with particular proteins [114]. We next discuss the role of few circRNA in the context of metabolic pathways.
3.3.1. circRNA 051239
According to Liu et al. [105], circRNA 051239 was highly elevated in drug-resistant tuberculosis patients, suggesting that circRNA 051239 may operate as miR-320a sponges and play a critical role in the development of tuberculosis drug resistance [115]. The sponge of circRNA 051239 is involved in cytokine production regulation, and decreasing miR-320a expression may enhance tuberculosis progression, by reactivating cell migration and proliferation in lung tissue [116].
3.3.2. hsa-circRNA-100237
Previous research indicates that hsa-circRNA-100237 may play a role in tuberculosis pathogenesis by altering macrophage activities [108]. Due to the fact that the level of hsa-circRNA-100237 decreased during ATBI, it is possible that downregulated hsa-circRNA-100237 acted as a miR-33 sponge, promoting lipid storage by reducing mitochondrial fatty acid oxidation [117].
3.3.3. circAGFG1
circAGFG1 promotes autophagy and inhibits apoptosis in macrophages infected with Mycobacterium TB by targeting miRNA1257 to control Notch signaling. Additionally, the action of circAGFG1 may be mimicked in normal cells by suppressing miRNA-1257 [118]. Notch2 is a critical component of the Notch signaling pathway and was discovered to be increased in tuberculosis patients. Recent research suggests that by inhibiting the Notch signaling system, the Th1/Th2 imbalance in tuberculosis patients can be corrected [119].
3.3.4. CircTRAPPC6B
By targeting microRNA-874-3p, CircTRAPPC6B inhibits intracellular Mycobacterium tuberculosis growth, while promoting autophagy in macrophages. circ TRAPPC6B is formed from the TRAPPC6B gene’s exons 3 and 4. RNAse R digestion is ineffective in reducing circTRAPPC6B synthesis via PCR, implying a circular shape for circTRAPPC6B [120]. Along with the low level of expression of circTRAPPC6B in tuberculosis patients, it was discovered that anti-tuberculosis medication can restore its expression.
4. ncRNAs as a Diagnostic and Therapeutic Tool for Tuberculosis
4.1. The Potential of miRNAs as Diagnostic and Therapeutic Biomarkers for Tuberculosis
The diagnosis of tuberculosis is complicated by the low specificity and sensitivity of the currently available diagnostic assays. TB must be identified early, in order to prevent infection transmission and control the disease [114]. Thus, it would be extremely beneficial to develop novel tuberculosis biomarkers. However, examining microRNA profiles in tuberculosis patients prior to, and following, treatment may be beneficial. There is indication that changes in miRNA profiles are associated with therapeutic response [121]. Thus, the microRNA profile in serum can be used as a biomarker for tuberculosis and multidrug-resistant tuberculosis diagnosis and monitoring (Table 1). For instance, serum miR-125a-5p levels are increased in well-treated tuberculosis patients, but miR-148 b-3p miR-21-5p, and miR-92a-3p, levels are decreased. Additionally, following a directly observed treatment short-course (DOTS), miR-155 and miR-29a expression was increased, whereas miR-326 expression was decreased, and their expression was associated with decreased Th1 responses [93]. Furthermore, when compared with treated-patients, new TB-treatment patients, and healthy participants, patients with multidrug-resistant tuberculosis (MDR)-TB had the lowest serum level of miR-16. Intriguingly, regulatory miRNAs can be packaged in exosomes and transported to other cells, where they make changes in the target cell’s transcriptome and function, thereby serving as tuberculosis biomarkers [122]. For instance, it was discovered that the levels of exosomal miR-96, miR-484, and miR-425 in serum of tuberculosis patients correlate with infection grade and serve as a marker of diagnostic power [123]. Thus, combining miRNA measurements with established diagnostic markers may aid in tuberculosis detection and surveillance. However, these procedures are not without risk. The primary difficulty in this situation is that the detected miRNAs lack repeatability and diagnostic specificity. Despite numerous studies, the reported miRNA signatures are inconsistent [124]. These discrepancies may be explained by the source of the samples, which may be serum or plasma. Numerous findings have established that blood microRNAs instigate from a variety of tissues in healthy and sick individuals and that microRNAs in venous and arterial plasma exhibit distinct profiles of expression from those found in tissues [125]. Additionally, variations in results can be attributed to the sampling procedure, including sample preservation and processing, necessitating protocol standardization. The cohort size of a biomarker study should be representative of the population, and, thus, large enough to distinguish between healthy and diseased states [126]. Given the well-established role of miRNAs in tuberculosis infection outcome, interest in tuberculosis-specific miRNA targeting has increased. Thus, miRNAs administered to the lung may alter the lung’s resistance to microbial infections, providing an intriguing new approach to tuberculosis management and therapy [127]. One possible treatment technique is the administration of miRNAs associated with tuberculosis infection, such as miR-20, miR-27a, miR-155, mir-146a, or miR-33/33*. miR-155 is a frequently reported miRNA. MiRNA plays a complex role in tuberculosis and its biology, involving numerous pathways in the control of tuberculosis infection [128]. For instance, in the presence of Mtb, this miRNA enhances the activity of natural killer cells (NK cells). MiR-155, on the other hand, inhibits autophagy in infected macrophages by targeting a brain-expressed Ras homolog (Rheb). MiR-33/33* silencing promotes autophagy activation [129]. miR-23a specifically targets TLR2/MyD88/NF-B pathway-linked genes that influence the induction of autophagy and Mtb survival, implying that it may be used as a tuberculosis therapeutic target. Additionally, miRNAs playing roles in innate immunity or miRNAs targeting genes linked with cytokines may be reviewed for miRNA-based therapeutics. For instance, miR-99b stops the release of pro-inflammatory cytokines during Mycobacterium tuberculosis infection, whereas miR-20b inhibited the tuberculosis-induced inflammatory response in vivo by the NLRP3/caspase-1/IL-1 pathway. Anti-miR-mediated silencing of miRNAs is now hailed as a potentially game-changing tool used to treat infectious diseases. For example, miravirsen is a licensed anti-miR-122 oligonucleotide that has been modified with locked nucleic acid [130,131]. However, before the optimal settings for future miRNA-based tuberculosis therapy can be determined, these novel methods of miRNA delivery must be validated. Recent technological advances in gene delivery may facilitate the development of novel host-directed therapies (HDT) that specifically target miRNAs [132].
Table 1.
List of noncoding RNAs used in the etiology, diagnosis, and treatment of Tuberculosis.
| Noncoding RNA | Function in Tuberculosis | Action | References |
|---|---|---|---|
| miR-26-5p | Etiology | Inhibition of innate immunity | [145] |
| miR-132-3p | [146] | ||
| miR-155-5p | [147] | ||
| miR-29-3p | [148] | ||
| miR-21-5p | Suppression of inflammation | [149] | |
| miR-27b-3p | [150] | ||
| miR-99b-5p | [151] | ||
| miR-125-5p | [152] | ||
| miR-146a-5p | [153] | ||
| miR-223-3p | [154] | ||
| Let-7f | [155] | ||
| miR-20b-5p | [156] | ||
| miR-142-3p | [157] | ||
| miR-33 | Inhibition of phagosome maturation and autophagy | [158] | |
| miR-27a-5p | [159] | ||
| miR-125a-3p | [160] | ||
| miR-144-5p | [161] | ||
| miR-889-5p | [162] | ||
| miR-155-5p | Apoptosis Inhibition | [163] | |
| miR-582-5p | [20] | ||
| miR-769-5p | Diagnosis | Downregulation in TB patients | [164] |
| miR-320a | [165] | ||
| miR-22-3p | [166] | ||
| hsa_circ_0001380 | [167] | ||
| miR-423-5p | Upregulation in TB patients | [168] | |
| miR-17-5p | [169] | ||
| miR-20b-5p | [170] | ||
| lncRNA LOC152742 | [171] | ||
| hsa_circRNA_001937 | [172] | ||
| has_circRNA_051239 | [173] | ||
| hsa_circRNA_404022 | [174] | ||
| has_circRNA_029965 | [175] | ||
| lncRNAs NEAT1 | Treatment | Downregulation during drug treatment, linked with disease improvement | [176] |
| lncRNAs NEAT2 | [177] | ||
| lncRNA 152742 | [178] | ||
| circTRAPPC6B | [179] | ||
| lncRNAENST00000429730.1 | Downregulation during drug treatment, linked with entire inactivation of TB lesions from sputum negative patients | [180] | |
| lncRNA MSTRG.93125.4 | [181] |
4.2. LncRNAs as Diagnostic and Therapeutic Biomarkers for Tuberculosis
lncRNAs associated with infectious diseases are expressed abnormally in various tissues and cells [132]. Long ncRNAs are increasingly being implicated in regulatory processes and are being used as molecular markers for a variety of infections [133]. Disruption of long noncoding RNAs has been linked to many infections caused by S. typhimurium, M. tuberculosis, etc. A microarray analysis of plasma lncRNAs and mRNA expression levels in tuberculosis victim was performed in this regard. 348 long ncRNAs and 284 messenger RNAs were found to be downregulated, while 163 long non-coding RNAs were found to be upregulated [134]. The Kyoto encyclopedia of genes and genomes (KEGG), coding/noncoding co-expression (CNC), and gene ontology (GO) analyses revealed that differentially expressed mRNAs were associated with T-cell type selection, alpha-beta T cell activation, and the IFN-cellular response. KEGG identified a number of mRNAs as being involved in the T-cell receptor signaling pathway and the Jak-STAT signaling circuit [135]. Thus, it has been demonstrated that variable long ncRNA expression impairs T-cell and T helper cell activation, resulting in immunological deficits in tuberculosis patients [136]. Furthermore, microarray analysis revealed a greater role for non-coding RNAs in T cell regulation and related signaling networks. Furthermore, this aberrant expression may have an effect on the cAMP and calcium signaling pathways, as well as natural killer cell signaling pathways [137,138]. Differential expression of competitive endogenous RNAs (ceRNAs) was observed in ENST00000422183, ENST00000570366, NR 003142, and NR 038221 and was significantly linked with tuberculosis, implying that it may influence tuberculosis pathogenesis via its effect on the transduction of signals between immune cells and intracellular signaling pathways [139]. Ultimately, this analysis asserted that long ncRNAs serve as early detection biomarkers for tuberculosis. Another study in China examined 467 tuberculosis patients and 473 healthy controls, and lnc-AC145676 and lnc-TGS1–1 were analyzed. As determined by RT-qPCR analysis, both of these lncRNAs were significantly downregulated in tuberculosis patients. This could imply the involvement of lnc-AC145676. While, lnc-TGS1–1 significantly reduced infection risk with Mtb [140]. MiR-143, a miRNA that binds to lnc-TGS1–1, was found to be significantly upregulated during tuberculosis infection, resulting in decreased immune function via downstream gene downregulation. This could be because the expression of lnc-TGS1–1 had decreased, removing its sponge effect on miR-143. Furthermore, the absence of lnc-AC145676 increased miR-29a expression and interfered with the TLR signaling pathway and other immune response interactions in tuberculosis. Additionally, this study established a link between reduced lnc- TGS1–1 expression and the prevalence of thrombocytopenia in tuberculosis patients receiving anti-TB medication [141]. It was discovered that lnc-TGS1–1 and rs4737420 (a variant of lnc-TGS1–1) can function as prognostic markers for adverse drug reactions associated with anti-TB drugs (ATD-ADRs). Another study discovered that the patterns of RNA expression differ between patients with MDR-TB and drug-sensitive tuberculosis (DS-TB), as well as between the PBMCs of healthy people [142]. The majority of signaling pathways involved in the dysregulation of these lncRNAs have been identified, including those involved in Hippo signaling, NK cell-mediated cytotoxicity, and antigen presentation. Additionally, recently, single nucleotide polymorphisms (SNPs) in the lncRNA AC079767 were identified. Four genes have been linked with the growth of tuberculosis ADRs, and polymorphisms such as rs12477677 and rs1055229, as well as variants of the lncRNA AC079767, and may contribute to tuberculosis clinical presentation and may be potential biomarkers for the diagnosis and progression of tuberculosis [143]. Similarly, an analysis discovered that 2116 lncRNAs were differentially expressed in the plasma of tuberculosis infected persons, with 1102 lncRNAs being upregulated and 1014 being downregulated. The expression levels of ENST00000354432 and ENST00000427151 were confirmed in additional TB samples. It was proposed that the levels of lncRNA expression, particularly those of these two genuine long ncRNAs in plasma, could be used as potential biomarkers for early tuberculosis diagnosis [144].
4.3. CircRNAs as a Diagnostic and Therapeutic Biomarker for Tuberculosis
Delay in diagnosis and treatment of tuberculosis is a serious threat to public health. However, the sensitivity of the tuberculosis diagnostic methods presently available is limited [182]. As a result, novel biomarkers are now required to assist in the early detection and treatment of TB in the lungs. Zhang et al. identified and synthesized ceRNAs for 170 dysregulated circRNAs in lungs infected with tuberculosis [183]. Their findings indicated that circRNA-linked ceRNA-mediated regulation of gene is critical for pulmonary tuberculosis pathogenesis. Huang et al. determined that has-circ-001937 has a substantial diagnostic value for tuberculosis (under curve area = 0.873). It was associated with the severity of tuberculosis and served as a TB-specific signature circRNA, with notably higher levels in TB-infected people compared to pneumonia and lung cancer [184]. Zhuang et al. discovered that hsa-circ-0005836 and hsa-circ-0009128 were considerably downregulated in the PBMCs of patients with active TB and healthy people [185]. The key roles of differentially expressed (DE) circular RNAs were found to be associated with immune system activation, implying a connection between tuberculosis infection and immune system activity. Qian et al. identified DE circRNAs in tuberculosis patients’ PBMCs and used seven of them to create an individual tuberculosis index [186]. Tuberculosis patients and healthy controls had a significantly higher tuberculosis index (under curve area = 0.946) in validated groups. Additionally, Yi et al. found the plasma circular RNA expression profiles of patients with active tuberculosis and discovered that patients with tuberculosis had significantly lower levels of hsa-circRNA-103571 expression [187]. Additional computational biology analysis demonstrated that hsa-circRNA-103571 regulated T and B-cell receptor signaling pathways. Numerous circRNAs have been identified as diagnostic markers for the infection of tuberculosis and future investigations might focus on nontuberculous mycobacteria (NTB), which shares clinical symptoms with tuberculosis, resulting in frequent misdiagnosis and mistreatment [188,189]. Furthermore, the majority of NTBs are highly resistant to anti-tuberculosis medicines, posing a significant infection burden on society [190]. Circulating RNAs may one day serve as a novel biomarker to aid physicians in differentiating tuberculosis infection from non-tuberculosis infections.
5. Methods for Profiling ncRNAs Expression
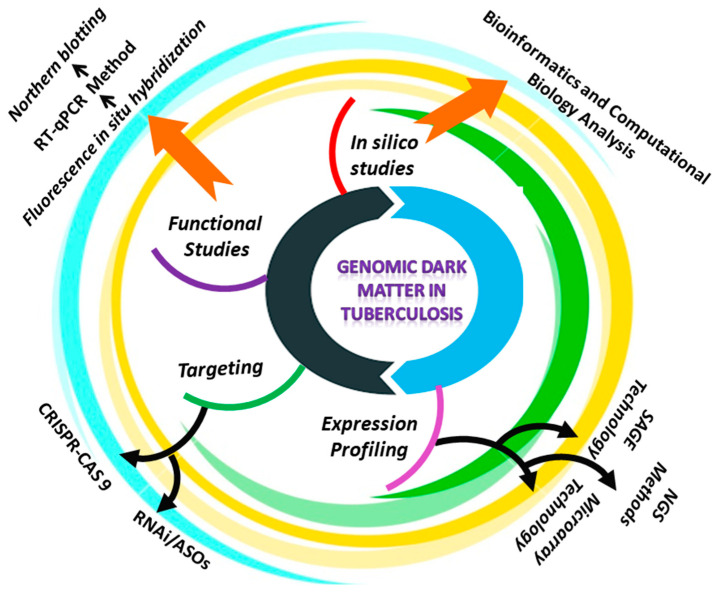
A common feature of tuberculosis in humans is aberrant ncRNA expression. Due to the specificity of ncRNAs for cell type, and tissue, RNA profiling has become a method for identifying valuable biomarkers of TB diagnosis, and metastasis [191]. When using both arrays and next generation sequencing (NGS) approaches for detecting and quantifying non-coding RNAs, several factors must be considered. In general, microRNAs and long noncoding RNAs are expressed at a lower level than messenger RNAs [192]. MiRNA profiling, necessitates the isolation of RNA to preserve the small fraction of RNA. Additionally, because microRNAs lack a conserved sequence, such as the poly (A) tail found in mRNAs, this class of non-coding RNAs must be detected selectively among various RNA species. Additionally, due to post-transcriptional modifications, miRNAs from the similar family can exhibit a high degree of divergence from the reference sequence [193]. When compared to mRNAs, lncRNAs share several characteristics with them, including size, transcription by RNA polymerase II, 5′-capping, and RNA splicing. Additionally, approximately 60% of the long ncRNAs contain a polyA tail. As a result, long ncRNAs may be profiled similarly to mRNAs, whereas microRNAs require a different strategy [194]. Despite this, the development of probes for the majority of lncRNAs is difficult to identify, because they are found at antisense transcripts of well-characterized protein-coding genes or intergenic regions with a high G-C content [195]. We describe several methods for comparing gene expression patterns in normal and TB-infected cells, in order to identify the noncoding RNAs that may be involved in tuberculosis (Figure 4).
Figure 4.
Schematic representation of the approaches reviewed in this study to investigate the role of ncRNAs in tuberculosis.
5.1. Microarray
Despite their origins in the profiling of protein-coding mRNAs, microarrays are a well-known technique for profiling miRNAs and long noncoding RNAs [196]. This technique is based on the hybridization of labelled RNA targets to their precise and complementary probes using nucleic acids. Microarrays have a number of advantages, including high parallelism, being inexpensive, and the power to identify extremely low abundance of RNA, without using PCR enrichment [197]. Numerous miRNA profiling platforms make use of a variety of non-amplification-based direct miRNA labelling techniques. Along with mRNAs, lncRNA microarray platforms facilitate the systematic profiling of lncRNAs. In general, lncRNA platforms employ in vitro transcription (IVT) for amplification and are slightly more technically sophisticated than miRNA platforms, primarily in terms of the number of long ncRNAs analyzed [198]. For instance, the Arraystar Long ncRNA microarray profiles 39,317 lncRNAs; and Thermo Fisher Scientific’s Clariom D human array profiles over 55,900 human long ncRNA NONCODE transcripts. Current techniques for non-coding RNA analysis have many limitations, including a limited linear quantification range, probe design constraints on known sequences, the requirement for continuous annotation updates, and a relative quantification that is restricted to comparing different grades (for example, control versus infected). Yang et al. profiled 31 TB-patients using an Arraystar Human LncRNA Microarray technology and identified two long ncRNAs (MIR3945HG V1 and MIR3945HG V2) as new candidate markers for the diagnosis of tuberculosis [199]. A Tiling array is another microarray-based technique distinguished by the use of probes that span distinct chromosomal sequences, or the entire genome. Bertone et al. discovered 10,595 previously unknown transcribed sequences in 2004, but this technology has been largely superseded in modern biology by next-generation sequencing approaches [200,201].
5.2. Serial Analysis of Gene Expression
SAGE is a breakthrough sequencing technique, designed specifically for the purpose of characterizing and quantifying the transcriptome, as well as noncoding RNAs [202]. It is based on the synthesis of small segments of unbiased cDNA sequences using restriction enzymes, followed by concatenation, cloning, and sequencing [203]. This technique has been employed in the “SuperSAGE” variant, which enables profiling of 26-basepair tags and in-tag-to-gene annotation at a lower cost/quality ratio. Gibb et al. used 24 million SAGE tags to determine the expression profile of long ncRNAs in 26 control and 19 tumor tissues [204].
5.3. RNA Sequencing (RNA-seq)
RNA-seq enables the identification and the quantification of all noncoding RNA groups by creating distinct cDNA libraries for each noncoding RNA type [205]. The desired transcripts are sequenced in massive parallel following the preparation of the cDNA library. While small RNA sequencing is appropriate for small noncoding RNAs, entire RNA sequencing is recommended for long noncoding RNAs, as many long noncoding RNAs are not poly-adenylated [206]. RNA-seq covers entire transcriptomes more thoroughly than a microarray. Notably, RNA-seq is a non-design-based technique that enables the finding of unknown/novel transcripts, as well as sequences with the difference of a single nucleotide, such as mutant or isoform-containing transcripts [207]. The primary drawbacks of RNA-seq are the complexity of the data analysis, and large number of deep reads needed to identify a small amount of target. Arnvig et al. published the first M. tuberculosis transcriptome investigation utilizing RNA-seq, examining whole-transcriptome expression throughout exponential growth and stationary phase [208]. Silva et al. used RNA-seq technology and identified three differentially expressed miRNAs (hsa-miR-486-3p, hsa-let-7g-5p, and hsa-miR-4732-5p) by differential expression analysis between tuberculosis patients and controls [209]. Luo et al. used RNA-seq technology to identified circRNA (hsa_circ_0001380) in peripheral blood, which can act as a biomarker for the diagnosis of tuberculosis [210]. Single cell transcriptomic sequencing is the cutting-edge application of RNA-seq. For instance, the designed primer-based RNA-seq approach (DP-seq) permits RNA amplification from 50 pg of sample, whereas Quartz-Seq is a single cell RNA-seq technique capable of revealing genetic differences amongst single cells of the same cell type, and cell cycle phase [211].
5.4. In Silico Investigation
Bioinformatics expertise is required to analyze the data generated by a variety of platforms, including those described above for microarray and NGS technologies [212]. NGS is increasingly being employed to decipher the genetic underpinnings of intra- and inter-individual variability, which is becoming more critical in the personalized medicine era [213]. The analysis of ncRNA can be used to accomplish a variety of objectives, including the detection and annotation of new non-coding, expression pattern profiling, authentication, and reconstruction of previously identified non-coding, as well as integrative analysis of their behavior and functions [214]. When dealing with array data, bioinformatics analysis entails the following steps: (i) identifying genes that are expressed differently between two groups (control versus TB-infected samples, pharmacologically treated vs. untreated cells); (ii) clustering, which classifies genes according to their level of expression; (iii) classification; and (iv) pathway and interaction network analysis. The processing of microarray raw data is divided into four stages: (1) pre-processing, which involves background adjustment, normalization, and summarization; (2) annotating pre-processed data to enrich it; and (3) data mining and statistical analysis [215]. For preprocessing microarray data, the algorithms GCRMA, RMA, MAS4.0, and PLIER are all well-known [216]. Background correction is required to eliminate the noise generated by non-specific hybridization in the optical detection system. Normalization is required within and between arrays, to eliminate the systemic technical artefacts caused by variations in reverse transcription, labelling, or hybridization efficiencies. Summarization is a technique that combines signals from multiple probes directed at the same transcript and distributed across the array [217]. Once the data has been summarized, it can be annotated with additional info such as gene symbols or functions. Data mining is a technique that involves comparing the sets of samples to determine which genes are expressed differentially, based on their expression values [218]. Numerous methods for visualizing and analyzing gene expression data are applicable to microarray and RNA-seq experiments, including gene set enrichment analysis, pathway analysis (WikiPathway, Reactome, KEGG, and Ingenuity), clustering analysis, and network analysis [219].
In RNA-seq technology, ncRNAs analysis begins with the un-processed NGS data. The first step is to eliminate low-quality reads from the unprocessed data. This is typically accomplished using raw files having FASTA-FASTQ-encoded short reads with tools that map the sequences to reference data. Blat, SHRiMP, MAQ, LastZ, and FASTX-Toolkit are just a few examples of such programs. The transcripts are assembled using BowTie or TopHat, following filtering. Following assembly, tools such as Bowtie are used to filter known DNA sequences. Following this phase, all DNA sequences that may denote ncRNAs must be evaluated and mapped using tools such as CPAT or Pfamscan [220]. This step is recommended with use of the NCBI NT and NR databases, because they contain sequences from all species. Sun et al. described lncRNAscan, a pipeline for identifying new lncRNAs from transcripts sequenced by RNA sequencing [216]. The pipeline explained above has been utilized in almost every bioinformatics study on noncoding RNAs that has corroborated the pattern of ncRNA expression with the clinical outcome of diseased-persons. Ultimately, the role of ncRNAs must be deduced through analysis of existing databases that contain a lot of ncRNA sequences and, when available, data from biological reports (Table 2) [221]. While these databases can be queried to find known noncoding RNAs within a given set of data, many known noncoding RNAs are valid only for well-characterized species, due to a lack of conservation. Jonathan et al. used the sRNAPredict2 program, applied for the identification of ncRNAs in the M. tuberculosis genome [222]. Reddy et al. published an integrated database named “The Tuberculosis Database (TBDB)”, which provides access to tuberculosis genetic resources, critical for the discovery and development of tuberculosis medicines, vaccines, and diagnostic biomarkers [223].
Table 2.
List of bioinformatics resources used in the prediction and analysis of ncRNAs.
| Tool Name | Description | References |
|---|---|---|
| miRanalyzer | miRNA detection tool for NGS experiments | [224] |
| miRTools | Toolbox for miRNA discovery and profiling | [225] |
| miRiadne | Tool for integrating the miRNA nomenclature | [226] |
| miRBase | Database of miRNA sequence and annotation | [227] |
| DIANA-mirGen | A tool to index promoters and regulator for miRNA | [228] |
| miRStart | miRNA’s Transcription Start Sites database | [229] |
| miRWalk 2.0 | Tool to predict miRNA-target interaction using Artificial intelligence algorithm | [229] |
| MatureBayes | miRNA-target prediction tool | [230] |
| miRanda | Tool to predict miRNA target using free energy feature | [231] |
| LNCipedia | lncRNA database | [232] |
| LNCBook | lncRNA database | [233] |
| LncDisease | lncRNA-disease associations predicting tool | [234] |
| NONCODE | Integrated knowledge database for ncRNA research | [235] |
| StarBase v2.0 | Tool to predict miRNA-ceRNA interaction | [236] |
| NRED | Database for expression information of lncRNAs | [237] |
| Circ2Traits | Toolbox for circularRNA discovery and analysis | [238] |
| CircRNABase | Tool to predict miRNA-circRNA interaction | [239] |
| circBase | circularRNA database | [240] |
| Circtools | Toolbox for circularRNA research | [241] |
| CircMarker | Tool for circularRNA detection | [242] |
6. Methods for Validating ncRNA Expression
It is necessary to validate microarray/RNA-seq data, and in-silico predictions. Noncoding RNAs can be quantified using a variety of techniques, including northern blot (NB), reverse transcription quantitative polymerase chain reaction (RT-qPCR), and fluorescence in situ hybridization (FISH) [243,244].
6.1. Northern Blotting (NB)
NB was the first method used to analyze the splicing variants of certain non-coding RNA’s gene expression [245]. The procedure begins with gel electrophoresis separation of RNA samples, then RNA transfer to nylon membrane, hybridization with an RNA probe, and lastly detection with an RNA probe. Wang et al. validated 28 novel small RNA-encoding regions in M. tuberculosis by northern blotting, which were identified from RNA-seq data [85]. Their study will help in the future to elucidate the complexity of gene expression regulation, mediated by sRNA in M. tuberculosis [246].
The primary disadvantage of this technique is its lower sensitivity, and prolonged execution time. Samples require a good amount of total RNA, which is extremely difficult to obtain when miRNAs are scarce or cell or tissue RNA sources are limited [247]. Additionally, the classical protocol’s use of isotope labelling is risky, and as a result, a large number of institutions prohibit its use. Numerous improvements to the classical method have been made in recent years, with the use of non-radioactive labelling agents, such as modified digoxigenin (DIG) probes [248].
6.2. RT-qPCR
RT-qPCR is a commonly used method for the identification and quantification of noncoding RNAs, due to its ease of integration into laboratory workflows [249]. As demonstrated by He et al. in their analysis of the differentially expressed profile of miRNAs in lung cancer patients’ peripheral blood, this technique is frequently used to validate microarray data. TaqMan and SYBR green assays are used in the RT-qPCR technique. The reverse transcription step is ncRNA-specific [250]. The TaqMan assay reverse transcripts miRNAs using a particular stem loop reverse transcription primer, whereas the SYBR green assay modifies a miRNA sequence by adding a poly-A tail, to facilitate primer binding. In the case of lncRNA, reverse transcription using random or particular primers is followed by qPCR employing TaqMan or SYBR green chemistry, to monitor the reaction product buildup in real time. Zhao et al. discovered the serum lncRNA n335659, which could be used as a biomarker for tuberculosis, and validated this biomarker using RT-qPCR technology [251].
6.3. FISH
Recent advancements in probe technology and identification methodologies have increased the imaging of ncRNAs via the FISH method, which is based on the employment of fluorescent probes with a high degree of complementarity to the nucleic acid sequence [252,253]. FISH provides novel information about the biological function of ncRNAs, by determining their spatial–temporal expression and subcellular localization. Confocal microscopy for FISH, for example, revealed that the long noncoding RNA NKILA is required in the cytoplasm, because it inhibits NF-B activation by stabilizing the NF-B/I-B complex; thereby, inhibiting cancer-associated inflammation; while, because of the small length and presence of repetitive sequences, using fluorophore-labeled DNA/RNA probes is extremely difficult. Branched-DNA probes, LNA probes, multiple oligo probe sets, are all examples of multiple oligo probe sets that have been fluorophore labeled [254]. In particular, it has been demonstrated that the usage of hapten-labeled LNA oligos is extremely advantageous for miRNA detection in clinical tissue samples, while there are few reports on lncRNA detection [255]. To circumvent these methods’ limitations in detecting low-abundance non-coding RNAs, scientists have established and applied single-molecule RNA FISH, a technique based on the hybridization of several small fluorescently-labelled oligonucleotides from a single cell. The risk of off-target hybridization is minimized by employing a single well-designed oligo probe, with low cross-reactivity to other RNAs [256].
7. Potentially Beneficial Therapeutic Interventions
The dysregulation of ncRNAs in TB-infected cells, in terms of expression profile and interactome, provides a rationale for considering them as a group of possible therapeutic markers [257]. Knowing the complexity of their promising mechanism of action, a variety of genomic and practical techniques have been designed to target ncRNAs directly/indirectly. We discuss the following: (1) post-transcriptional RNA degradation using synthetic antisense oligonucleotides (ASOs); (2) modulation of non-coding RNA genes via genome editing technologies. We discuss various preclinical studies that demonstrated the effectiveness of these strategies in functional investigations. Despite the fact that all of these approaches have the potential to be effective therapeutic interventions, numerous constraints, such as delivery systems, must be addressed before they can be translated into clinical practice.
7.1. Noncoding RNAs Targeting: ASOs
ASOs are sequences of synthetic nucleic acids which bind to complementary RNA substrates via Watson–Crick base pairing [258]. ASOs have been engineered to maintain drug-like characteristics through chemical variation of endogenous nucleotides. Phosphoro-thioate alteration of the linker protects ASOs against nuclease degradation and prolongs their half-life in blood, while maintaining RNaseH activity. These first-generation ASOs, which contained only deoxy residues, had a short clinical life. ASOs of the second generation have a core area of around ten phosphoro-thioate nucleotides bordered by nucleotides changed at the sugar (“gapmer” design). Third-generation ASOs are constructed of LNA-modified antisense oligonucleotides gapmers, with LNA-enriched flanking regions and a DNA-free middle gap [259,260]. LNAs are analogues of nucleotides, where the ribose ring is locked between the 2′ oxygen and the 4′ carbon through a methylene bridge. Gcanga et al. identified lincRNA-MIR99AHG, which is upregulated in macrophages but downregulated after Mtb infection and in patients with active tuberculosis [261]. However, they indicated for the first time that treatment with MIR99AHG ASOs significantly reduced the pathogenesis of Mtb infection.
7.2. Non-Coding RNAs Targeting: RNAi
RNA interference (RNAi) is an endogenous process of post-transcriptional regulation, which functions by pairing endogenous or exogenous double stranded RNA with a target mRNA [262]. To be precise, a dsRNA is first split into a 21-nucleotide RNA sequence known as siRNA by Dicer, and subsequently put into the cytosolic RISC. The promoter strand is removed, the guide strand is linked with the target mRNA, and silencing is produced by degradation, depending on complementarity. This physiological process has been extensively used experimentally in molecular oncology, for therapeutic purposes, and subsequently improved to execute high-throughput screening, employing the siRNAs’ pool [263]. Indeed, many libraries targeting microRNAs and long noncoding RNAs have been created, resulting in the discovery of non-coding RNAs involved in drug response. For instance, utilization of genome-wide microRNA libraries enabled the detection of miR 195’s synergic involvement in the response of microtubule-targeting agents to lung cancer [264]. On other hand, synthetic siRNAs have been employed to induce ncRNA degradation in a therapeutic setting. Additionally, siRNA-mediated inhibition of MALAT1 revealed that it plays a significant role in temozolomide resistance in glioblastoma multiforme, as its inhibition repaired drug sensitivity, while diminishing cancer stem cell stemness and proliferation [265].
7.3. ncRNAs Editing with CRISPR-Cas9
Clustered regularly interspaced short palindromic repeats (CRISPR)-associated nuclease 9 (CRISPR/Cas9) is a genome editing technique that functions like a molecular “scissor”. It was developed by manipulating the adaptive immune system of prokaryotes, so as to induce a distinct genetic alteration in eukaryotes using guide RNA (gRNA) and Cas9 protein [266]. gRNA has a length of 20-nt, and is homologous to a portion of the targeted DNA flanked by a three bp protospacer adjacent motif (PAM) sequence recognized by Cas9, an endonuclease capable of inducing a double stranded break (DSB). Cas9-mediated DSBs can repair using non-homologous end joining (NHEJ), by generating tiny indels disrupting the targeted locus (knock-out (KO) method) or via homology directed repair (HDR), in which donor DNA is used to insert a required sequence (knock-in). Numerous validation findings have been conducted using this technique, to determine the role of selected long noncoding RNAs [267,268]. For instance, CRISPR-Cas9 technology has been used to establish the functions of (1) LncRoR as an activator of the MAPK/ERK pathway, and (2) LncAK023948 as a positive regulator of the Akt pathway. By targeting the miRNA biogenesis region, the CRISPR-Cas9 method has also been utilized to significantly lower miRNA expression in vitro and in vivo. Diverse applications of the CRISPR-Cas9 method have enabled the identification of miR-210’s onco-suppressive involvement in renal cell cancer cell lines and miRNA182-onco-suppressive 5p’s activity in chronic myeloid leukemia [269]. Additionally, several research studies examined prospective delivery routes for miRNA therapies using the CRISPR-Cas9 system.
Given the enormous influence of this technique on molecular investigations, CRISPR-Cas9 has much improved the induction of progressively precise genetic modifications, up to the level of base editing [270]. Liu et al. conducted a illustrative investigation of CRISPR-interference-based screening in seven transfected cell lines, using 1600 lncRNAs as targets [271]. They discovered 499 lncRNA loci essential in cellular development and tissue-specific transcriptional control. Kurata et al. found miRNAs linked with cell fitness using a pooled CRISPR-Cas9 library based on 1600 annotated human miRNA stem-loops. For the first time, Liu et al. artificially synthesized a circRNA that was capable of inhibiting the proliferation of stomach cancer cells by miR-21 sponging [272]. This report generated a novel concept for the future clinical application of circular RNAs in tuberculosis. Additionally, disease-promoting circRNAs could be targeted using CRISPR/Cas9-based gene editing, to specifically target the back-splice junction of circRNAs [273].
The primary constraint on this technology is the off-target impacts, despite the fact that numerous measures are taken to address or at least mitigate this critical issue. In terms of clinical translation, CRISPR-Cas9-based techniques are still in their infancy, owing to the possibility of an unfavorable immune response to bacterial Cas9, which is often delivered via viral vectors, and due to ethical problems inherent in human genome editing applications [274].
8. Conclusions
In the current review, the research approaches used to explore the function of miRNAs, lncRNAs, and circular RNAs in tuberculosis were detailed. Progression of tuberculosis disease could be stopped by decreasing or increasing the expression of particular noncoding RNAs and then blocking/activating the genes they regulate. Indeed, they can have therapeutic potential too. The availability of new strong sequencing and molecular tools has enabled the overcoming of various constraints, including ncRNAs’ low quantity, subcellular spatial localization, and their instability. In terms of identification and functional validation, advances in wet laboratory techniques, combined with in-silico tools, have dramatically increased our understanding of the genome’s dark matter. Further developments in the coming years are likely to improve our understanding of the regulatory network behind ncRNA perturbations and, more importantly, facilitate the translation of experimental findings from bench to bedside. Given the existing data, translational research to test and include all examined markers into point-of-care tuberculosis testing is a worthwhile endeavor. This will enable research focused at overcoming the diagnostic problems associated with tuberculosis illness.
Acknowledgments
The researcher would like to thank the Deanship of Scientific Research, Qassim University for funding the publication of this project.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kiazyk S., Ball T. Tuberculosis (TB): Latent tuberculosis infection: An overview. Can. Commun. Dis. Rep. 2017;43:62. doi: 10.14745/ccdr.v43i34a01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rehm J., Samokhvalov A.V., Neuman M.G., Room R., Parry C., Lönnroth K., Patra J., Poznyak V., Popova S. The association between alcohol use, alcohol use disorders and tuberculosis (TB). A systematic review. BMC Public Health. 2009;9:450. doi: 10.1186/1471-2458-9-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Responding to Community Spread of COVID-19. Reference WHO/COVID-19/Community_Transmission/2020.1. 2020. [(accessed on 1 April 2022)]. Available online: https://www.who.int/publications/i/item/responding-to-community-spread-of-covid-19.
- 4.Zijenah L.S. The world health organization recommended TB diagnostic tools. Tuberculosis. 2018;2:71–90. [Google Scholar]
- 5.Ottenhoff T.H. Overcoming the global crisis: “Yes, we can”, but also for TB…? Eur. J. Immunol. 2009;39:2014–2020. doi: 10.1002/eji.200939518. [DOI] [PubMed] [Google Scholar]
- 6.Jain A., Mondal R. Extensively drug-resistant tuberculosis: Current challenges and threats. FEMS Immunol. Med. Microbiol. 2008;53:145–150. doi: 10.1111/j.1574-695X.2008.00400.x. [DOI] [PubMed] [Google Scholar]
- 7.Koch A., Cox H., Mizrahi V. Drug-resistant tuberculosis: Challenges and opportunities for diagnosis and treatment. Curr. Opin. Pharmacol. 2018;42:7–15. doi: 10.1016/j.coph.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tahir ul Qamar M., Zhu X., Khan M.S., Xing F., Chen L.L. Pan-genome: A promising resource for noncoding RNA discovery in plants. Plant Genome. 2020;13:e20046. doi: 10.1002/tpg2.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacques P.-É., Gervais A.L., Cantin M., Lucier J.-F.o., Dallaire G., Drouin G., Gaudreau L., Goulet J., Brzezinski R. MtbRegList, a database dedicated to the analysis of transcriptional regulation in Mycobacterium tuberculosis. Bioinformatics. 2005;21:2563–2565. doi: 10.1093/bioinformatics/bti321. [DOI] [PubMed] [Google Scholar]
- 10.Oliva G., Sahr T., Buchrieser C. Small RNAs, 5′ UTR elements and RNA-binding proteins in intracellular bacteria: Impact on metabolism and virulence. FEMS Microbiol. Rev. 2015;39:331–349. doi: 10.1093/femsre/fuv022. [DOI] [PubMed] [Google Scholar]
- 11.Dahariya S., Paddibhatla I., Kumar S., Raghuwanshi S., Pallepati A., Gutti R.K. Long non-coding RNA: Classification, biogenesis and functions in blood cells. Mol. Immunol. 2019;112:82–92. doi: 10.1016/j.molimm.2019.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Hombach S., Kretz M. Non-Coding RNAs Colorectal Cancer. Springer; Berlin, Germany: 2016. Non-coding RNAs: Classification, biology and functioning; pp. 3–17. [DOI] [PubMed] [Google Scholar]
- 13.Nelson W.C., Stegen J.C. The reduced genomes of Parcubacteria (OD1) contain signatures of a symbiotic lifestyle. Front. Microbiol. 2015;6:713. doi: 10.3389/fmicb.2015.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Memon D., Bi J., Miller C.J. In silico prediction of housekeeping long intergenic non-coding RNAs reveals HKlincR1 as an essential player in lung cancer cell survival. Sci. Rep. 2019;9:7372. doi: 10.1038/s41598-019-43758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun J., Lin Y., Wu J. Long non-coding RNA expression profiling of mouse testis during postnatal development. PLoS ONE. 2013;8:e75750. doi: 10.1371/journal.pone.0075750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guglas K., Bogaczyńska M., Kolenda T., Ryś M., Teresiak A., Bliźniak R., Łasińska I., Mackiewicz J., Lamperska K. lncRNA in HNSCC: Challenges and potential. Contemp. Oncol. 2017;21:259. doi: 10.5114/wo.2017.72382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B., Li W., Liu K., Jia Q., Zhang H., Bie Q. The roles of host noncoding RNAs in Mycobacterium tuberculosis infection. Front. Immunol. 2021;12:1689. doi: 10.3389/fimmu.2021.664787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn D.E., Martin M.M., Feldman D.S., Terry A.V., Jr., Nuovo G.J., Elton T.S. Experimental validation of miRNA targets. Methods. 2008;44:47–54. doi: 10.1016/j.ymeth.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bavelloni A., Ramazzotti G., Poli A., Piazzi M., Focaccia E., Blalock W., Faenza I. MiRNA-210: A current overview. Anticancer. Res. 2017;37:6511–6521. doi: 10.21873/anticanres.12107. [DOI] [PubMed] [Google Scholar]
- 20.Miotto P., Mwangoka G., Valente I.C., Norbis L., Sotgiu G., Bosu R., Ambrosi A., Codecasa L.R., Goletti D., Matteelli A. miRNA signatures in sera of patients with active pulmonary tuberculosis. PLoS ONE. 2013;8:e80149. doi: 10.1371/journal.pone.0080149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gan E.-S., Huang J., Ito T. Functional roles of histone modification, chromatin remodeling and microRNAs in Arabidopsis flower development. Int. Rev. Cell Mol. Biol. 2013;305:115–161. doi: 10.1016/B978-0-12-407695-2.00003-2. [DOI] [PubMed] [Google Scholar]
- 22.Beermann J., Piccoli M.-T., Viereck J., Thum T. Non-coding RNAs in development and disease: Background, mechanisms, and therapeutic approaches. Physiol. Rev. 2016;96:1297–1325. doi: 10.1152/physrev.00041.2015. [DOI] [PubMed] [Google Scholar]
- 23.Winter J., Jung S., Keller S., Gregory R.I., Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat. Cell Biol. 2009;11:228–234. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 24.Curtis H.J., Sibley C.R., Wood M.J. Mirtrons, an emerging class of atypical miRNA. Wiley Interdiscip. Rev. RNA. 2012;3:617–632. doi: 10.1002/wrna.1122. [DOI] [PubMed] [Google Scholar]
- 25.Li L., Li C., Lv M., Hu Q., Guo L., Xiong D. Correlation between alterations of gut microbiota and miR-122-5p expression in patients with type 2 diabetes mellitus. Ann. Transl. Med. 2020;8:1481. doi: 10.21037/atm-20-6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banada P.P., Sivasubramani S.K., Blakemore R., Boehme C., Perkins M.D., Fennelly K., Alland D. Containment of bioaerosol infection risk by the Xpert MTB/RIF assay and its applicability to point-of-care settings. J. Clin. Microbiol. 2010;48:3551–3557. doi: 10.1128/JCM.01053-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J.-Y., Lee M.-C., Shu C.-C., Lee C.-H., Lee L.-N., Chao K.-M., Chang F.-Y. Optimal duration of anti-TB treatment in patients with diabetes: Nine or six months? Chest. 2015;147:520–528. doi: 10.1378/chest.14-0918. [DOI] [PubMed] [Google Scholar]
- 28.Naing N.N., D’Este C., Isa A.R., Salleh R., Bakar N., Mahmod M.R. Factors contributing to poor compliance with anti-TB treatment among tuberculosis patients. Southeast Asian J. Trop. Med. Public Health. 2001;32:369–382. [PubMed] [Google Scholar]
- 29.Yang G., Lu X., Yuan L. LncRNA: A link between RNA and cancer. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2014;1839:1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Paraskevopoulou M.D., Hatzigeorgiou A.G. Long Non-Coding RNAs. Springer; Berlin, Germany: 2016. Analyzing miRNA–lncRNA interactions; pp. 271–286. [DOI] [PubMed] [Google Scholar]
- 31.Chen X., Yan G.-Y. Novel human lncRNA–disease association inference based on lncRNA expression profiles. Bioinformatics. 2013;29:2617–2624. doi: 10.1093/bioinformatics/btt426. [DOI] [PubMed] [Google Scholar]
- 32.Ferre F., Colantoni A., Helmer-Citterich M. Revealing protein–lncRNA interaction. Brief. Bioinform. 2016;17:106–116. doi: 10.1093/bib/bbv031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawaguchi T., Tanigawa A., Naganuma T., Ohkawa Y., Souquere S., Pierron G., Hirose T. SWI/SNF chromatin-remodeling complexes function in noncoding RNA-dependent assembly of nuclear bodies. Proc. Natl. Acad. Sci. USA. 2015;112:4304–4309. doi: 10.1073/pnas.1423819112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinodoz S., Guttman M. Long noncoding RNAs: An emerging link between gene regulation and nuclear organization. Trends Cell Biol. 2014;24:651–663. doi: 10.1016/j.tcb.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ansari M.A., Khan F.B., Safdari H.A., Almatroudi A., Alzohairy M.A., Safdari M., Amirizadeh M., Rehman S., Equbal M.J., Hoque M. Prospective therapeutic potential of Tanshinone IIA: An updated overview. Pharmacol. Res. 2021;164:105364. doi: 10.1016/j.phrs.2020.105364. [DOI] [PubMed] [Google Scholar]
- 36.Parasramka M.A., Maji S., Matsuda A., Yan I.K., Patel T. Long non-coding RNAs as novel targets for therapy in hepatocellular carcinoma. Pharmacol. Ther. 2016;161:67–78. doi: 10.1016/j.pharmthera.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koch L. Screening for lncRNA function. Nat. Rev. Genet. 2017;18:70. doi: 10.1038/nrg.2016.168. [DOI] [PubMed] [Google Scholar]
- 38.Pamudurti N.R., Bartok O., Jens M., Ashwal-Fluss R., Stottmeister C., Ruhe L., Hanan M., Wyler E., Perez-Hernandez D., Ramberger E. Translation of circRNAs. Mol. Cell. 2017;66:9–21.e27. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haque S., Harries L.W. Circular RNAs (circRNAs) in health and disease. Genes. 2017;8:353. doi: 10.3390/genes8120353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yi Z., Gao K., Li R., Fu Y. Dysregulated circRNAs in plasma from active tuberculosis patients. J. Cell. Mol. Med. 2018;22:4076–4084. doi: 10.1111/jcmm.13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fan X., Weng X., Zhao Y., Chen W., Gan T., Xu D. Circular RNAs in cardiovascular disease: An overview. BioMed Res. Int. 2017;2017:5135781. doi: 10.1155/2017/5135781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ebbesen K.K., Kjems J., Hansen T.B. Circular RNAs: Identification, biogenesis and function. Biochim. Biophys. Acta BBA-Gene Regul. Mech. 2016;1859:163–168. doi: 10.1016/j.bbagrm.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 43.Liu Y., Tahir ul Qamar M., Feng J.-W., Ding Y., Wang S., Wu G., Ke L., Xu Q., Chen L.-L. Comparative analysis of miniature inverted–repeat transposable elements (MITEs) and long terminal repeat (LTR) retrotransposons in six Citrus species. BMC Plant Biol. 2019;19:140. doi: 10.1186/s12870-019-1757-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumarswamy R., Volkmann I., Thum T. Regulation and function of miRNA-21 in health and disease. RNA Biol. 2011;8:706–713. doi: 10.4161/rna.8.5.16154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X.-C., Du L.-Q., Tian L.-L., Wu H.-L., Jiang X.-Y., Zhang H., Li D.-G., Wang Y.-Y., Wu H.-Y., She Y. Expression and function of miRNA in postoperative radiotherapy sensitive and resistant patients of non-small cell lung cancer. Lung Cancer. 2011;72:92–99. doi: 10.1016/j.lungcan.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Kadkhoda S., Ghafouri-Fard S. Function of miRNA-145-5p in the pathogenesis of human disorders. Pathol. Res. Pract. 2022;231:153780. doi: 10.1016/j.prp.2022.153780. [DOI] [PubMed] [Google Scholar]
- 47.Adams D.E., Shao W.-H. Epigenetic alterations in immune cells of systemic Lupus erythematosus and therapeutic implications. Cells. 2022;11:506. doi: 10.3390/cells11030506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang C., Huang M., Li T., Li L., Sussman H., Dai Y., Siemann D., Xie M., Tang X. Towards an integrative understanding of cancer mechanobiology: Calcium, YAP, and microRNA under biophysical forces. Soft Matter. 2022;18:1112–1148. doi: 10.1039/D1SM01618K. [DOI] [PubMed] [Google Scholar]
- 49.Doxakis E. Insights into the multifaceted role of circular RNAs: Implications for Parkinson’s disease pathogenesis and diagnosis. NPJ Parkinsons Dis. 2022;8:7. doi: 10.1038/s41531-021-00265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roy S., Ganguly N., Banerjee S. Exploring clinical implications and role of non-coding RNAs in lung carcinogenesis. Mol. Biol. Rep. 2022;49:1–13. doi: 10.1007/s11033-022-07159-w. [DOI] [PubMed] [Google Scholar]
- 51.Ma F., Xu S., Liu X., Zhang Q., Xu X., Liu M., Hua M., Li N., Yao H., Cao X. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat. Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 52.Laws M., Jin P., Rahman K.M. Efflux pumps in Mycobacterium tuberculosis and their inhibition to tackle antimicrobial resistance. Trends Microbiol. 2022;30:57–68. doi: 10.1016/j.tim.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Pauley K.M., Satoh M., Pauley B.A., Dominguez-Gutierrez P.R., Wallet S.M., Holliday L.S., Cha S., Reeves W.H., Chan E.K. Formation of GW/P bodies as marker for microRNA-mediated regulation of innate immune signaling in THP-1 cells. Immunol. Cell Biol. 2010;88:205–212. doi: 10.1038/icb.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Savan R. Post-transcriptional regulation of interferons and their signaling pathways. J. Interferon Cytokine Res. 2014;34:318–329. doi: 10.1089/jir.2013.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pai M., Joshi R., Bandyopadhyay M., Narang P., Dogra S., Taksande B., Kalantri S. Sensitivity of a whole-blood interferon-gamma assay among patients with pulmonary tuberculosis and variations in T-cell responses during anti-tuberculosis treatment. Infection. 2007;35:98–103. doi: 10.1007/s15010-007-6114-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ferrand R., Bothamley G., Whelan A., Dockrell H. Interferon-gamma responses to ESAT-6 in tuberculosis patients early into and after anti-tuberculosis treatment. Int. J. Tuberc. Lung Dis. 2005;9:1034–1039. [PubMed] [Google Scholar]
- 57.Cartwright N., Demaine A., Jahromi M., Sanders H., Kaminski E.R. A study of cytokine protein secretion, frequencies of cytokine expressing cells and IFN-G gene polymorphisms in normal individuals. Transplantation. 1999;68:1546–1552. doi: 10.1097/00007890-199911270-00019. [DOI] [PubMed] [Google Scholar]
- 58.Cavalla F., Biguetti C.C., Colavite P.M., Silveira E.V., Martins Jr W., Letra A., Trombone A.P.F., Silva R.M., Garlet G.P. TBX21-1993T/C (rs4794067) polymorphism is associated with increased risk of chronic periodontitis and increased T-bet expression in periodontal lesions, but does not significantly impact the IFN-g transcriptional level or the pattern of periodontophatic bacterial infection. Virulence. 2015;6:293–304. doi: 10.1080/21505594.2015.1029828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen S., Crabill G.A., Pritchard T.S., McMiller T.L., Wei P., Pardoll D.M., Pan F., Topalian S.L. Mechanisms regulating PD-L1 expression on tumor and immune cells. J. Immunother. Cancer. 2019;7:1–12. doi: 10.1186/s40425-019-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Galvin J., Tiberi S., Akkerman O., Kerstjens H., Kunst H., Kurhasani X., Ambrosino N., Migliori G. Pulmonary tuberculosis in intensive care setting, with a focus on the use of severity scores, a multinational collaborative systematic review. Pulmonology. 2022;28 doi: 10.1016/j.pulmoe.2022.01.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun X., Liu K., Zhao Y., Zhang T. High miRNA-378 expression has high diagnostic values for pulmonary tuberculosis and predicts adverse outcomes. BMC Mol. Cell Biol. 2022;23:14. doi: 10.1186/s12860-022-00413-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murdaca G., Paladin F., Tonacci A., Borro M., Greco M., Gerosa A., Isola S., Allegra A., Gangemi S. Involvement of Il-33 in the pathogenesis and prognosis of major respiratory viral infections: Future perspectives for personalized therapy. Biomedicines. 2022;10:715. doi: 10.3390/biomedicines10030715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sailliet N., Ullah M., Dupuy A., Silva A.K., Gazeau F., Le Mai H., Brouard S. Extracellular vesicles in transplantation. Front. Immunol. 2022;13:1521–1531. doi: 10.3389/fimmu.2022.800018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Adewumi A.T., Elrashedy A., Soremekun O.S., Ajadi M.B., Soliman M.E. Weak spots inhibition in the Mycobacterium tuberculosis antigen 85C target for antitubercular drug design through selective irreversible covalent inhibitor-SER124. J. Biomol. Struct. Dyn. 2022;40:2934–2954. doi: 10.1080/07391102.2020.1844061. [DOI] [PubMed] [Google Scholar]
- 65.Arish M., Naz F. Macrophage plasticity as a therapeutic target in tuberculosis. Eur. J. Immunol. 2022:1–9. doi: 10.1002/eji.202149624. [DOI] [PubMed] [Google Scholar]
- 66.Birhanu A.G., Gómez-Muńoz M., Kalayou S., Riaz T., Lutter T., Yimer S.A., Abebe M., Tønjum T. Proteome profiling of Mycobacterium tuberculosis cells exposed to nitrosative stress. ACS Omega. 2022;7:3470–3482. doi: 10.1021/acsomega.1c05923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lim Z.L., Drever K., Dhar N., Cole S.T., Chen J.M. Mycobacterium tuberculosis EspK has active but distinct roles in the secretion of EsxA and EspB. J. Bacteriol. 2022;10:e00060-22. doi: 10.1128/jb.00060-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Clarke C., Cooper D.V., Miller M.A., Goosen W.J. Detection of Mycobacterium tuberculosis complex DNA in oronasal swabs from infected African buffaloes (Syncerus caffer) Sci. Rep. 2022;12:1834. doi: 10.1038/s41598-022-05982-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pérez I., Campos-Pardos E., Díaz C., Uranga S., Sayes F., Vicente F., Aguiló N., Brosch R., Martín C., Gonzalo-Asensio J. The Mycobacterium tuberculosis PhoPR virulence system regulates expression of the universal second messenger c-di-AMP and impacts vaccine safety and efficacy. Mol. Ther. Nucleic Acids. 2022;27:1235–1248. doi: 10.1016/j.omtn.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lata S., Mahatha A.C., Mal S., Gupta U.D., Kundu M., Basu J. Unravelling novel roles of the Mycobacterium tuberculosis transcription factor Rv0081 in regulation of the nucleoid-associated proteins Lsr2 and EspR, cholesterol utilization and subversion of lysosomal trafficking in macrophages. Mol. Microbiol. 2022 doi: 10.1111/mmi.14895. [DOI] [PubMed] [Google Scholar]
- 71.Petruccioli E., Farroni C., Cuzzi G., Vanini V., Palmieri F., Vittozzi P., Goletti D. VIDAS® TB-IGRA reagents induce a CD4+ and CD8+ T-cell IFN-γ response for both TB infection and active TB. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Against Tuberc. Lung Dis. 2022;26:65–68. doi: 10.5588/ijtld.21.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khabibullina N.F., Kutuzova D.M., Burmistrova I.A., Lyadova I.V. The biological and clinical aspects of a latent tuberculosis infection. Trop. Med. Infect. Dis. 2022;7:48. doi: 10.3390/tropicalmed7030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al Tbeishat H. Novel in silico mRNA vaccine design exploiting proteins of M. tuberculosis that modulates host immune responses by inducing epigenetic modifications. Sci. Rep. 2022;12:4645. doi: 10.1038/s41598-022-08506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Junqueira-Kipnis A.P., de Castro Souza C., de Oliveira Carvalho A.C., de Oliveira F.M., Almeida V.P., de Paula A.R., Celes M.R., Kipnis A. Protease-based subunit vaccine in mice boosts BCG protection against Mycobacterium tuberculosis. Vaccines. 2022;10:306. doi: 10.3390/vaccines10020306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dockrell H.M., Ramachandran V., Bothamley G.H. Tuberculosis risk stratification of psoriatic patients before anti-TNF-a treatment. Immunol. Biomark. Tuberc. 2022;12:672894. doi: 10.3389/fimmu.2021.672894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mao L., Xu L., Wang X., Xing Y., Wang J., Zhang Y., Yuan W., Du J., Shi Z., Ma J. Enhanced immunogenicity of the tuberculosis subunit Rv0572c vaccine delivered in DMT liposome adjuvant as a BCG-booster. Tuberculosis. 2022;134:102186. doi: 10.1016/j.tube.2022.102186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Linnstaedt S.D., Gottwein E., Skalsky R.L., Luftig M.A., Cullen B.R. Virally induced cellular microRNA miR-155 plays a key role in B-cell immortalization by Epstein-Barr virus. J. Virol. 2010;84:11670–11678. doi: 10.1128/JVI.01248-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Faraoni I., Antonetti F.R., Cardone J., Bonmassar E. miR-155 gene: A typical multifunctional microRNA. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2009;1792:497–505. doi: 10.1016/j.bbadis.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 79.Hou L., Chen J., Zheng Y., Wu C. Critical role of miR-155/FoxO1/ROS axis in the regulation of non-small cell lung carcinomas. Tumor Biol. 2016;37:5185–5192. doi: 10.1007/s13277-015-4335-9. [DOI] [PubMed] [Google Scholar]
- 80.Zhuang G., Sun A., Teng M., Luo J. A tiny RNA that packs a big punch: The critical role of a viral miR-155 ortholog in lymphomagenesis in Marek’s disease. Front. Microbiol. 2017;8:1169. doi: 10.3389/fmicb.2017.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tili E., Croce C.M., Michaille J.-J. miR-155: On the crosstalk between inflammation and cancer. Int. Rev. Immunol. 2009;28:264–284. doi: 10.1080/08830180903093796. [DOI] [PubMed] [Google Scholar]
- 82.Xiao R., Li C., Chai B. miRNA-144 suppresses proliferation and migration of colorectal cancer cells through GSPT1. Biomed. Pharmacother. 2015;74:138–144. doi: 10.1016/j.biopha.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 83.Yu A., Zhang T., Zhong W., Duan H., Wang S., Ye P., Wang J., Zhong S., Yang Z. miRNA-144 induces microglial autophagy and inflammation following intracerebral hemorrhage. Immunol. Lett. 2017;182:18–23. doi: 10.1016/j.imlet.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 84.Chen C., Zhao C., Gu C., Cui X., Wu J. MiRNA-144-3p inhibits high glucose induced cell proliferation through suppressing FGF16. Biosci. Rep. 2019;39:BSR20181788. doi: 10.1042/BSR20181788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang W.-X., Rajeev B.W., Stromberg A.J., Ren N., Tang G., Huang Q., Rigoutsos I., Nelson P.T. The expression of microRNA miR-107 decreases early in Alzheimer’s disease and may accelerate disease progression through regulation of β-site amyloid precursor protein-cleaving enzyme 1. J. Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fix L.N., Shah M., Efferth T., Farwell M.A., Zhang B. MicroRNA expression profile of MCF-7 human breast cancer cells and the effect of green tea polyphenon-60. Cancer Genom. Proteom. 2010;7:261–277. [PubMed] [Google Scholar]
- 87.Carr T.J. Ph.D. Thesis. University of Delaware; Newark, DE, USA: 2017. Expanding the Avian Micro RNA Repertoire of Domesticated Poultry and Investigating the NLRP3 Inflammasome Messenger RNA and Micro RNA Transcriptomes in Anas Platyrhynchos (Ducks) [Google Scholar]
- 88.Wang C., Yang S., Sun G., Tang X., Lu S., Neyrolles O., Gao Q. Comparative miRNA expression profiles in individuals with latent and active tuberculosis. PLoS ONE. 2011;6:e25832. doi: 10.1371/journal.pone.0025832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang M., Lu H., Liu J., Wu S., Kim P., Zhou X. lncRNAfunc: A knowledgebase of lncRNA function in human cancer. Nucleic Acids Res. 2022;50:D1295–D1306. doi: 10.1093/nar/gkab1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie L., Huang G., Gao M., Huang J., Li H., Xia H., Xiang X., Wu S., Ruan Y. Identification of atrial fibrillation-related lncRNA based on bioinformatic analysis. Dis. Mark. 2022;2022:8307975. doi: 10.1155/2022/8307975. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 91.Chen Q., Lin G., Lin L., Huang J., Chen L., Lian N., Chen M., Zeng A., Lin Q. LncRNA XR_596701 protects H9c2 cells against intermittent hypoxia-induced injury through regulation of the miR-344b-5p/FAIM3 axis. Cell Death Discov. 2022;8:42. doi: 10.1038/s41420-022-00834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yan Q., Tian Y., Hao F. Downregulation of lncRNA UCA1 inhibits proliferation and invasion of cervical cancer cells through miR-206 expression. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2022;28:7–8. doi: 10.3727/096504018X15185714083446. [DOI] [PubMed] [Google Scholar]
- 93.Li X., Yang Y., Liang L., Fan M., Li X., Feng N., Pan Y., Tan Q., Xu Q., Xie Y. Effect of XBP1 deficiency in cartilage on the regulatory network Of LncRNA/circRNA-miRNA-mRNA. Int. J. Biol. Sci. 2022;18:315. doi: 10.7150/ijbs.64054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ansari M.A., Thiruvengadam M., Farooqui Z., Rajakumar G., Jamal Q.M.S., Alzohairy M.A., Almatroudi A., Alomary M.N., Chung I.-M., Al-Suhaimi E.A. Seminars in Cancer Biology. Volume 69. Academic Press; Cambridge, MA, USA: 2021. Nanotechnology, in silico and endocrine-based strategy for delivering paclitaxel and miRNA: Prospects for the therapeutic management of breast cancer; pp. 109–128. [DOI] [PubMed] [Google Scholar]
- 95.Tao S., Chen Y., Hu M., Xu L., Fu C.-B., Hao X.-B. LncRNA PVT1 facilitates DLBCL development via miR-34b-5p/Foxp1 pathway. Mol. Cell. Biochem. 2022;477:951–963. doi: 10.1007/s11010-021-04335-7. [DOI] [PubMed] [Google Scholar]
- 96.Zhu X., Wu Y.-B., Zhou J., Kang D.-M. Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via increasing FoxO1 expression. Biochem. Biophys. Res. Commun. 2016;469:319–325. doi: 10.1016/j.bbrc.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 97.Zhang H. Mechanism associated with aberrant lncRNA MEG3 expression in gestational diabetes mellitus. Exp. Ther. Med. 2019;18:3699–3706. doi: 10.3892/etm.2019.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Y., Zhong H., Xie X., Chen C.Y., Huang D., Shen L., Zhang H., Chen Z.W., Zeng G. Long noncoding RNA derived from CD244 signaling epigenetically controls CD8+ T-cell immune responses in tuberculosis infection. Proc. Natl. Acad. Sci. USA. 2015;112:E3883–E3892. doi: 10.1073/pnas.1501662112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liao K., Xu J., Yang W., You X., Zhong Q., Wang X. The research progress of LncRNA involved in the regulation of inflammatory diseases. Mol. Immunol. 2018;101:182–188. doi: 10.1016/j.molimm.2018.05.030. [DOI] [PubMed] [Google Scholar]
- 100.Fathizadeh H., Hayat S.M.G., Dao S., Ganbarov K., Tanomand A., Asgharzadeh M., Kafil H.S. Long non-coding RNA molecules in tuberculosis. Int. J. Biol. Macromol. 2020;156:340–346. doi: 10.1016/j.ijbiomac.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 101.Zhang M., Wu W., Wang Z., Wang X. lncRNA NEAT1 is closely related with progression of breast cancer via promoting proliferation and EMT. Eur. Rev. Med. Pharmacol. Sci. 2017;21:1020–1026. [PubMed] [Google Scholar]
- 102.Chakravarty D., Sboner A., Nair S.S., Giannopoulou E., Li R., Hennig S., Mosquera J.M., Pauwels J., Park K., Kossai M. The oestrogen receptor alpha-regulated lncRNA NEAT1 is a critical modulator of prostate cancer. Nat. Commun. 2014;5:5383. doi: 10.1038/ncomms6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yu H.M., Wang C., Yuan Z., Chen G.L., Ye T., Yang B.W. LncRNA NEAT1 promotes the tumorigenesis of colorectal cancer by sponging miR-193a-3p. Cell Prolif. 2019;52:e12526. doi: 10.1111/cpr.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yao Z., Zhang Q., Guo F., Guo S., Yang B., Liu B., Li P., Li J., Guan S., Liu X. Long noncoding RNA PCED1B-AS1 promotes the Warburg effect and tumorigenesis by upregulating HIF-1α in glioblastoma. Cell Transplant. 2020;29:0963689720906777. doi: 10.1177/0963689720906777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li Z., Yu X., Shen J. Long non-coding RNAs: Emerging players in osteosarcoma. Tumor Biol. 2016;37:2811–2816. doi: 10.1007/s13277-015-4749-4. [DOI] [PubMed] [Google Scholar]
- 106.Li C., Chen J., Zhang K., Feng B., Wang R., Chen L. Progress and prospects of long noncoding RNAs (lncRNAs) in hepatocellular carcinoma. Cell. Physiol. Biochem. 2015;36:423–434. doi: 10.1159/000430109. [DOI] [PubMed] [Google Scholar]
- 107.Su S., Gao J., Wang T., Wang J., Li H., Wang Z. Long non-coding RNA BANCR regulates growth and metastasis and is associated with poor prognosis in retinoblastoma. Tumor Biol. 2015;36:7205–7211. doi: 10.1007/s13277-015-3413-3. [DOI] [PubMed] [Google Scholar]
- 108.Wang Z., Xu H., Wei Z., Jia Y., Wu Y., Qi X., Li Y., Gao X. The role of non-coding RNA on macrophage modification in tuberculosis infection. Microb. Pathog. 2020;149:104592. doi: 10.1016/j.micpath.2020.104592. [DOI] [PubMed] [Google Scholar]
- 109.Zhao B., Zhou R., Ji C., Liu D., Wu T., Xu H., Lan D., Yao C., Xu Y., Fang L. The function of circRNA-0047604 in regulating the tumor suppressor gene DACH1 in breast cancer. BioMed Res. Int. 2022;2022:6589651. doi: 10.1155/2022/6589651. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 110.Wang H., Zhang Y., Zhang J., Du X., Li Q., Pan Z. circSLC41A1 resists porcine granulosa cell apoptosis and follicular atresia by promoting SRSF1 through miR-9820-5p sponging. Int. J. Mol. Sci. 2022;23:1509. doi: 10.3390/ijms23031509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Feng K., Jiang Z., Liu P., Liu J., Wen X., He L. Circular RNA, circ1-3p, is involved in cyflumetofen resistance by acting as a competitive RNA against miR-1-3p in Tetranychus cinnabarinus. J. Agric. Food Chem. 2022;70:1068–1078. doi: 10.1021/acs.jafc.1c07155. [DOI] [PubMed] [Google Scholar]
- 112.Zheng W., Su H., Lv X., Xin S., Xu T. Exon–intron circular RNA circRNF217 promotes innate immunity and antibacterial activity in teleost fish by reducing miR-130-3p function. J. Immunol. 2022;208:1099–1114. doi: 10.4049/jimmunol.2100890. [DOI] [PubMed] [Google Scholar]
- 113.Zhang J., Li H., Li J., Ke S. CircRNA CORO1C regulates miR-654-3p/USP7 axis to mediate laryngeal squamous cell carcinoma progression. Biochem. Genet. 2022;60:1–15. doi: 10.1007/s10528-021-10169-1. [DOI] [PubMed] [Google Scholar]
- 114.Xia R.-P., Zhao F., Ma T.-D., Zou C.-J., Xu G., Zhou C.-G. Circ-ITCH overexpression promoted cell proliferation and migration in Hirschsprung disease through miR-146b-5p/RET axis. Pediatric Res. 2022;91:1–9. doi: 10.1038/s41390-021-01860-5. [DOI] [PubMed] [Google Scholar]
- 115.Foruzandeh Z., Zeinali-Sehrig F., Nejati K., Rahmanpour D., Pashazadeh F., Seif F., Alivand M.R. CircRNAs as potent biomarkers in ovarian cancer: A systematic scoping review. Cell. Mol. Biol. Lett. 2021;26:41. doi: 10.1186/s11658-021-00284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang W., Bai C., Zhang L., Li Z., Tian Y., Yang Z., Wang L., Wu W. Correlation between serum circRNA and thyroid micropapillary carcinoma with cervical lymph node metastasis. Medicine. 2020;99:e23255. doi: 10.1097/MD.0000000000023255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Clerbaux L.A., Schultz H., Roman-Holba S., Ruan D.F., Yu R., Lamb A.M., Bommer G.T., Kennell J.A. The microRNA miR-33 is a pleiotropic regulator of metabolic and developmental processes in Drosophila melanogaster. Dev. Dyn. 2021;250:1634–1650. doi: 10.1002/dvdy.344. [DOI] [PubMed] [Google Scholar]
- 118.Yang R., Xing L., Zheng X., Sun Y., Wang X., Chen J. The circRNA circAGFG1 acts as a sponge of miR-195-5p to promote triple-negative breast cancer progression through regulating CCNE1 expression. Mol. Cancer. 2019;18:4. doi: 10.1186/s12943-018-0933-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 119.Wu F., Zhou J. CircAGFG1 promotes cervical cancer progression via miR-370-3p/RAF1 signaling. BMC Cancer. 2019;19:1067. doi: 10.1186/s12885-019-6269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Luo H.-L., Pi J., Zhang J.-A., Yang E.-Z., Xu H., Luo H., Shen L., Peng Y., Liu G.B., Song C.-M. Circular RNA TRAPPC6B inhibits intracellular Mycobacterium tuberculosis growth while inducing autophagy in macrophages by targeting microRNA-874-3p. Clin. Transl. Immunol. 2021;10:e1254. doi: 10.1002/cti2.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang W., Liu Y., Min Z., Liang G., Mo J., Ju Z., Zeng B., Guan W., Zhang Y., Chen J. circMine: A comprehensive database to integrate, analyze and visualize human disease–related circRNA transcriptome. Nucleic Acids Res. 2022;50:D83–D92. doi: 10.1093/nar/gkab809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Di Agostino S., Vahabi M., Turco C., Fontemaggi G. Secreted non-coding RNAs: Functional impact on the tumor microenvironment and clinical relevance in triple-negative breast cancer. Non-Coding RNA. 2022;8:5. doi: 10.3390/ncrna8010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Z., Lin W., Zhao S., Mo X., Wen Z., Cheung W.H., Fu D., Chen B. Identification of circRNA expression profiles in BMSCs from glucocorticoid-induced osteoporosis model. Stem Cells Int. 2022;2022:3249737. doi: 10.1155/2022/3249737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grabowska M., Misiorek J.O., Zarębska Ż., Rolle K. Clinical Applications of Non-Coding RNAs in Cancer. Elsevier; Amsterdam, The Netherlands: 2022. Applications of noncoding RNAs in brain cancer patients; pp. 17–64. [Google Scholar]
- 125.He S., Dou L., Li X., Zhang Y. Review of bioinformatics in Azheimer’s disease research. Comput. Biol. Med. 2022;143:105269. doi: 10.1016/j.compbiomed.2022.105269. [DOI] [PubMed] [Google Scholar]
- 126.Kumar S., Vishvakarma N.K., Kumar A. Clinical Applications of Non-Coding RNAs in Cancer. Elsevier; Amsterdam, The Netherlands: 2022. Clinical applications of noncoding RNAs in lung cancer patients; pp. 141–175. [Google Scholar]
- 127.Burzynski J., Mangan J.M., Lam C.K., Macaraig M., Salerno M.M., deCastro B.R., Goswami N.D., Lin C.Y., Schluger N.W., Vernon A. In-person vs electronic directly observed therapy for tuberculosis treatment adherence: A randomized noninferiority trial. JAMA Netw. Open. 2022;5:e2144210. doi: 10.1001/jamanetworkopen.2021.44210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ramakrishnan M., Ng W.L., Corbett M., Durcan L., Fitzpatrick F. Latent tuberculosis infection amongst patients with rheumatic diseases in an Irish tertiary referral centre–A five-year review. J. Infect. 2022;84:e18–e20. doi: 10.1016/j.jinf.2021.10.013. [DOI] [PubMed] [Google Scholar]
- 129.Wang R., Chopra N., Nho K., Maloney B., Obukhov A.G., Nelson P.T., Counts S.E., Lahiri D.K. Human microRNA (miR-20b-5p) modulates Alzheimer’s disease pathways and neuronal function, and a specific polymorphism close to the MIR20B gene influences Alzheimer’s biomarkers. Mol. Psychiatry. 2022;27:1–18. doi: 10.1038/s41380-021-01351-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kuskov A., Selina O., Kulikov P., Imatdinov I., Balysheva V., Kryukov A., Shtilman M., Markvicheva E. Amphiphilic poly (N-vinylpyrrolidone) nanoparticles loaded with DNA plasmids encoding Gn and Gc glycoproteins of the Rift valley fever virus: Preparation and in vivo evaluation. ACS Appl. Bio Mater. 2021;4:6084–6092. doi: 10.1021/acsabm.1c00426. [DOI] [PubMed] [Google Scholar]
- 131.Zhu T., Liu H., Su L., Dawood A., Hu C., Chen X., Chen H., Chen Y., Guo A. Identification of unique key miRNAs, TFs, and mRNAs in virulent MTB infection macrophages by network analysis. Int. J. Mol. Sci. 2022;23:382. doi: 10.3390/ijms23010382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chandra P., Coullon H., Agarwal M., Goss C.W., Philips J.A. Macrophage global metabolomics identifies cholestenone as host/pathogen cometabolite present in human Mycobacterium tuberculosis infection. J. Clin. Investig. 2022;132:e152509. doi: 10.1172/JCI152509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Di Marsico M., Paytuvi Gallart A., Sanseverino W., Aiese Cigliano R. GreeNC 2.0: A comprehensive database of plant long non-coding RNAs. Nucleic Acids Res. 2022;50:D1442–D1447. doi: 10.1093/nar/gkab1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Montico B., Giurato G., Pecoraro G., Salvati A., Covre A., Colizzi F., Steffan A., Weisz A., Maio M., Sigalotti L. The pleiotropic roles of circular and long noncoding RNAs in cutaneous melanoma. Mol. Oncol. 2022;16:565. doi: 10.1002/1878-0261.13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ross C.J., Ulitsky I. Discovering functional motifs in long noncoding RNAs. Wiley Interdiscip. Rev. RNA. 2022;13:e1708. doi: 10.1002/wrna.1708. [DOI] [PubMed] [Google Scholar]
- 136.Lan X., Han J., Wang B., Sun M. Integrated analysis of transcriptome profiling of lncRNAs and mRNAs in livers of type 2 diabetes mellitus. Physiol. Genom. 2022;54:86–97. doi: 10.1152/physiolgenomics.00105.2021. [DOI] [PubMed] [Google Scholar]
- 137.Valenzuela-Muñoz V., Gallardo-Escárate C., Benavente B.P., Valenzuela-Miranda D., Núñez-Acuña G., Escobar-Sepulveda H., Váldes J.A. Whole-genome transcript expression profiling reveals novel insights into transposon genes and non-coding RNAs during Atlantic salmon seawater adaptation. Biology. 2022;11:1. doi: 10.3390/biology11010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Anwar S., Almatroudi A., Alsahli M.A., Khan M.A., Khan A.A., Rahmani A.H. Natural products: Implication in cancer prevention and treatment through modulating various biological activities. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem. -Anti-Cancer Agents. 2020;20:2025–2040. doi: 10.2174/1871520620666200705220307. [DOI] [PubMed] [Google Scholar]
- 139.Sharma Y., Sharma A., Singh K., Upadhyay S.K. Long non-coding RNAs as emerging regulators of pathogen response in plants. Non-Coding RNA. 2022;8:4. doi: 10.3390/ncrna8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Li T., Wang L., Guo C., Zhang H., Xu P., Liu S., Hu X., Gao Q. Polymorphisms of SLC11A1 (NRAMP1) rs17235409 associated with and susceptibility to spinal tuberculosis in a southern Han Chinese population. Infect. Genet. Evol. 2022;98:105202. doi: 10.1016/j.meegid.2021.105202. [DOI] [PubMed] [Google Scholar]
- 141.Huang M., Tan Y., Zhang X., Wang Y., Su B., Xue Z., Wang J., Pang Y. Effect of mixed infections with Mycobacterium tuberculosis and nontuberculous Mycobacteria on diagnosis of multidrug-resistant tuberculosis: A retrospective multicentre study in China. Infect. Drug Resist. 2022;15:157. doi: 10.2147/IDR.S341817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kleinwaks G., Schmit V., Morrison J. Considering human challenge trials for tuberculosis vaccine development. Vaccine. 2022;40:173–174. doi: 10.1016/j.vaccine.2021.11.024. [DOI] [PubMed] [Google Scholar]
- 143.Wang Z., Posey D.L., Brostrom R.J., Morris S.B., Marano N., Phares C.R. Post-arrival evaluation of immigrant and refugee children in the USA with latent tuberculosis infection diagnosed overseas, 2007–2019. J. Pediatrics. 2022;S0022-3476(22)00080-4 doi: 10.1016/j.jpeds.2022.01.049. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Phuoc Long N., Heo D.Y., Park S., Thi Hai Yen N., Cho Y.-S., Shin J.-G., Oh J.Y., Kim D.-H. Molecular perturbations in pulmonary tuberculosis patients identified by pathway-level analysis of plasma metabolic features. PLoS ONE. 2022;17:e0262545. doi: 10.1371/journal.pone.0262545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Araujo L.S., Ribeiro-Alves M., Leal-Calvo T., Leung J., Durán V., Samir M., Talbot S., Tallam A., Mello F.C.d.Q., Geffers R. Reprogramming of small noncoding RNA populations in peripheral blood reveals host biomarkers for latent and active Mycobacterium tuberculosis infection. MBio. 2019;10:e01037-19. doi: 10.1128/mBio.01037-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lyu L., Zhang X., Li C., Yang T., Wang J., Pan L., Jia H., Li Z., Sun Q., Yue L. Small RNA profiles of serum exosomes derived from individuals with latent and active tuberculosis. Front. Microbiol. 2019;10:1174. doi: 10.3389/fmicb.2019.01174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ndzi E.N., Nkenfou C.N., Mekue L.M., Zentilin L., Tamgue O., Pefura E.W.Y., Kuiaté J.-R., Giacca M., Ndjolo A. MicroRNA hsa-miR-29a-3p is a plasma biomarker for the differential diagnosis and monitoring of tuberculosis. Tuberculosis. 2019;114:69–76. doi: 10.1016/j.tube.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 148.Chakrabarty S., Kumar A., Raviprasad K., Mallya S., Satyamoorthy K., Chawla K. Host and MTB genome encoded miRNA markers for diagnosis of tuberculosis. Tuberculosis. 2019;116:37–43. doi: 10.1016/j.tube.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 149.Alipoor S.D., Tabarsi P., Varahram M., Movassaghi M., Dizaji M.K., Folkerts G., Garssen J., Adcock I.M., Mortaz E. Serum exosomal miRNAs are associated with active pulmonary tuberculosis. Dis. Markers. 2019;2019:1907426. doi: 10.1155/2019/1907426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Barry S.E., Ellis M., Yang Y., Guan G., Wang X., Britton W.J., Saunders B.M. Identification of a plasma microRNA profile in untreated pulmonary tuberculosis patients that is modulated by anti-mycobacterial therapy. J. Infect. 2018;77:341–348. doi: 10.1016/j.jinf.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 151.Duffy F.J., Thompson E., Downing K., Suliman S., Mayanja-Kizza H., Boom W.H., Thiel B., Weiner Iii J., Kaufmann S.H., Dover D. A serum circulating miRNA signature for short-term risk of progression to active tuberculosis among household contacts. Front. Immunol. 2018;9:661. doi: 10.3389/fimmu.2018.00661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang C., Yang S., Liu C.-M., Jiang T.-T., Chen Z.-L., Tu H.-H., Mao L.-G., Li Z.-J., Li J.-C. Screening and identification of four serum miRNAs as novel potential biomarkers for cured pulmonary tuberculosis. Tuberculosis. 2018;108:26–34. doi: 10.1016/j.tube.2017.08.010. [DOI] [PubMed] [Google Scholar]
- 153.Cui J.-Y., Liang H.-W., Pan X.-L., Li D., Jiao N., Liu Y.-H., Fu J., He X.-Y., Sun G.-X., Zhang C.-L. Characterization of a novel panel of plasma microRNAs that discriminates between Mycobacterium tuberculosis infection and healthy individuals. PLoS ONE. 2017;12:e0184113. doi: 10.1371/journal.pone.0184113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang C., Liu C.-M., Wei L.-L., Shi L.-Y., Pan Z.-F., Mao L.-G., Wan X.-C., Ping Z.-P., Jiang T.-T., Chen Z.-L. A group of novel serum diagnostic biomarkers for multidrug-resistant tuberculosis by iTRAQ-2D LC-MS/MS and solexa sequencing. Int. J. Biol. Sci. 2016;12:246. doi: 10.7150/ijbs.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wagh V., Urhekar A., Modi D. Levels of microRNA miR-16 and miR-155 are altered in serum of patients with tuberculosis and associate with responses to therapy. Tuberculosis. 2017;102:24–30. doi: 10.1016/j.tube.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 156.Zheng M.-L., Zhou N.-K., Luo C.-H. MiRNA-155 and miRNA-132 as potential diagnostic biomarkers for pulmonary tuberculosis: A preliminary study. Microb. Pathog. 2016;100:78–83. doi: 10.1016/j.micpath.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 157.Xin H., Yang Y., Liu J., Li X., Li M., Feng B., Li Z., Zhang H., Li H., Shen F. Association between tuberculosis and circulating microRNA hsa-let-7b and hsa-miR-30b: A pilot study in a Chinese population. Tuberculosis. 2016;99:63–69. doi: 10.1016/j.tube.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 158.Xu Z., Zhou A., Ni J., Zhang Q., Wang Y., Lu J., Wu W., Karakousis P.C., Lu S., Yao Y. Differential expression of miRNAs and their relation to active tuberculosis. Tuberculosis. 2015;95:395–403. doi: 10.1016/j.tube.2015.02.043. [DOI] [PubMed] [Google Scholar]
- 159.Latorre I., Leidinger P., Backes C., Domínguez J., de Souza-Galvão M.L., Maldonado J., Prat C., Ruiz-Manzano J., Sánchez F., Casas I. A novel whole-blood miRNA signature for a rapid diagnosis of pulmonary tuberculosis. Eur. Respir. J. 2015;45:1173–1176. doi: 10.1183/09031936.00221514. [DOI] [PubMed] [Google Scholar]
- 160.Zhang C., Wang Q., Xi X., Jiao J., Xu W., Huang J., Lai Z. High serum miR-183 level is associated with the bioactivity of macrophage derived from tuberculosis patients. Int. J. Clin. Exp. Pathol. 2015;8:655. [PMC free article] [PubMed] [Google Scholar]
- 161.Zhang H., Sun Z., Wei W., Liu Z., Fleming J., Zhang S., Lin N., Wang M., Chen M., Xu Y. Identification of serum microRNA biomarkers for tuberculosis using RNA-seq. PLoS ONE. 2014;9:e88909. doi: 10.1371/journal.pone.0088909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Abd-El-Fattah A.A., Sadik N.A.H., Shaker O.G., Aboulftouh M.L. Differential microRNAs expression in serum of patients with lung cancer, pulmonary tuberculosis, and pneumonia. Cell Biochem. Biophys. 2013;67:875–884. doi: 10.1007/s12013-013-9575-y. [DOI] [PubMed] [Google Scholar]
- 163.Zhang X., Guo J., Fan S., Li Y., Wei L., Yang X., Jiang T., Chen Z., Wang C., Liu J. Screening and identification of six serum microRNAs as novel potential combination biomarkers for pulmonary tuberculosis diagnosis. PLoS ONE. 2013;8:e81076. doi: 10.1371/journal.pone.0081076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maertzdorf J., Weiner J., Mollenkopf H.-J., Network T., Bauer T., Prasse A., Müller-Quernheim J., Kaufmann S.H. Common patterns and disease-related signatures in tuberculosis and sarcoidosis. Proc. Natl. Acad. Sci. USA. 2012;109:7853–7858. doi: 10.1073/pnas.1121072109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Qi Y., Cui L., Ge Y., Shi Z., Zhao K., Guo X., Yang D., Yu H., Cui L., Shan Y. Altered serum microRNAs as biomarkers for the early diagnosis of pulmonary tuberculosis infection. BMC Infect. Dis. 2012;12:384. doi: 10.1186/1471-2334-12-384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fu Y., Yi Z., Wu X., Li J., Xu F. Circulating microRNAs in patients with active pulmonary tuberculosis. J. Clin. Microbiol. 2011;49:4246–4251. doi: 10.1128/JCM.05459-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lin Y., Zhang Y., Yu H., Tian R., Wang G., Li F. Identification of unique key genes and miRNAs in latent tuberculosis infection by network analysis. Mol. Immunol. 2019;112:103–114. doi: 10.1016/j.molimm.2019.04.032. [DOI] [PubMed] [Google Scholar]
- 168.Zhuang X., Li Z., Lin H., Gu L., Lin Q., Lu Z., Tzeng C.-M. Integrated miRNA and mRNA expression profiling to identify mRNA targets of dysregulated miRNAs in non-obstructive azoospermia. Sci. Rep. 2015;5:7922. doi: 10.1038/srep07922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Van Rensburg I., Du Toit L., Walzl G., Du Plessis N., Loxton A. Decreased neutrophil–associated miRNA and increased B-cell associated miRNA expression during tuberculosis. Gene. 2018;655:35–41. doi: 10.1016/j.gene.2018.02.052. [DOI] [PubMed] [Google Scholar]
- 170.Corral-Fernández N.E., Cortes-García J.D., Bruno R.-S., Romano-Moreno S., Medellín-Garibay S.E., Magaña-Aquino M., Salazar-González R.A., González-Amaro R., Portales-Pérez D.P. Analysis of transcription factors, microRNAs and cytokines involved in T lymphocyte differentiation in patients with tuberculosis after directly observed treatment short-course. Tuberculosis. 2017;105:1–8. doi: 10.1016/j.tube.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 171.Spinelli S.V., Fernández R.d.V., Zoff L., Bongiovanni B., Díaz A., D’Attilio L., Santucci N., Alvarez T., Marchesini M.M., Bogue C. miR-30c is specifically repressed in patients with active pulmonary tuberculosis. Tuberculosis. 2017;105:73–79. doi: 10.1016/j.tube.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 172.Pan D., Pan M., Xu Y.-M. Mir-29a expressions in peripheral blood mononuclear cell and cerebrospinal fluid: Diagnostic value in patients with pediatric tuberculous meningitis. Brain Res. Bull. 2017;130:231–235. doi: 10.1016/j.brainresbull.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 173.Zhou M., Yu G., Yang X., Zhu C., Zhang Z., Zhan X. Circulating microRNAs as biomarkers for the early diagnosis of childhood tuberculosis infection. Mol. Med. Rep. 2016;13:4620–4626. doi: 10.3892/mmr.2016.5097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Zheng L., Leung E., Lee N., Lui G., To K.-F., Chan R.C., Ip M. Differential microRNA expression in human macrophages with Mycobacterium tuberculosis infection of Beijing/W and non-Beijing/W strain types. PLoS ONE. 2015;10:e0126018. doi: 10.1371/journal.pone.0126018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wang J., Xu J., Han Y., Zhu Y., Zhang W. Diagnostic values of microRNA-31 in peripheral blood mononuclear cells for pediatric pulmonary tuberculosis in Chinese patients. Genet. Mol. Res. 2015;14:17235–17243. doi: 10.4238/2015.December.16.23. [DOI] [PubMed] [Google Scholar]
- 176.Fu Y., Yi Z., Li J., Li R. Deregulated micro RNA s in CD 4+ T cells from individuals with latent tuberculosis versus active tuberculosis. J. Cell. Mol. Med. 2014;18:503–513. doi: 10.1111/jcmm.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yi Z., Gao K., Li R., Fu Y. Changed immune and miRNA response in RAW264. 7 cells infected with cell wall deficient Mycobacterium tuberculosis. Int. J. Mol. Med. 2018;41:2885–2892. doi: 10.3892/ijmm.2018.3471. [DOI] [PubMed] [Google Scholar]
- 178.Spinelli S.V., Diaz A., D’Attilio L., Marchesini M.M., Bogue C., Bay M.L., Bottasso O.A. Altered microRNA expression levels in mononuclear cells of patients with pulmonary and pleural tuberculosis and their relation with components of the immune response. Mol. Immunol. 2013;53:265–269. doi: 10.1016/j.molimm.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 179.Tamgue O., Mezajou C.F., Ngongang N.N., Kameni C., Ngum J.A., Simo U.S.F., Tatang F.J., Akami M., Ngono A.N. Non-coding RNAs in the etiology and control of major and neglected human tropical diseases. Front. Immunol. 2021;12:703936. doi: 10.3389/fimmu.2021.703936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yao X., Yan C., Zhang L., Li Y., Wan Q. LncRNA ENST00113 promotes proliferation, survival, and migration by activating PI3K/Akt/mTOR signaling pathway in atherosclerosis. Medicine. 2018;97:e0473. doi: 10.1097/MD.0000000000010473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lungu P., Njelesani E., Sukwa T., Ngalamika O., Munsaka S., Kilembe W., Lakhi S., Mwaba P. Immune correlates of Mycobacterium tuberculosis patients in Zambia stratified by HIV serostatus and level of immunity-a cross-sectional analytical laboratory based study. PLoS ONE. 2022;17:e0262454. doi: 10.1371/journal.pone.0262454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Swain S.S., Pati S., Hussain T. Quinoline heterocyclic containing plant and marine candidates against drug-resistant Mycobacterium tuberculosis: A systematic drug-ability investigation. Eur. J. Med. Chem. 2022;232:114173. doi: 10.1016/j.ejmech.2022.114173. [DOI] [PubMed] [Google Scholar]
- 183.Zonghai C., Tao L., Pengjiao M., Liang G., Rongchuan Z., Xinyan W., Wenyi N., Wei L., Yi W., Lang B. Mycobacterium tuberculosis ESAT6 modulates host innate immunity by downregulating miR-222-3p target PTEN. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2022;1868:166292. doi: 10.1016/j.bbadis.2021.166292. [DOI] [PubMed] [Google Scholar]
- 184.Zhuang Z.-G., Zhang J.-A., Luo H.-L., Liu G.-B., Lu Y.-B., Ge N.-H., Zheng B.-Y., Li R.X., Chen C., Wang X. The circular RNA of peripheral blood mononuclear cells: Hsa_circ_0005836 as a new diagnostic biomarker and therapeutic target of active pulmonary tuberculosis. Mol. Immunol. 2017;90:264–272. doi: 10.1016/j.molimm.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 185.Qian Z., Liu H., Li M., Shi J., Li N., Zhang Y., Zhang X., Lv J., Xie X., Bai Y. Potential diagnostic power of blood circular RNA expression in active pulmonary tuberculosis. EBioMedicine. 2018;27:18–26. doi: 10.1016/j.ebiom.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sun Y., Jiang X., Lv Y., Liang X., Zhao B., Bian W., Zhang D., Jiang J., Zhang C. Circular RNA expression profiles in plasma from patients with heart failure related to platelet activity. Biomolecules. 2020;10:187. doi: 10.3390/biom10020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Micheni L.N., Kassaza K., Kinyi H., Ntulume I., Bazira J. Detection of Mycobacterium tuberculosis multiple strains in sputum samples from patients with pulmonary tuberculosis in south western Uganda using MIRU-VNTR. Sci. Rep. 2022;12:1656. doi: 10.1038/s41598-022-05591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Verma A.K., Bharti P.S., Rafat S., Bhatt D., Goyal Y., Pandey K.K., Ranjan S., Almatroodi S.A., Alsahli M.A., Rahmani A.H. Autophagy paradox of cancer: Role, regulation, and duality. Oxidative Med. Cell. Longev. 2021;2021:8832541. doi: 10.1155/2021/8832541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Sun W., Zhang L., Liang J., Li X., Yang Y., Sun W., Hou J. Comparison of clinical and imaging features between pulmonary tuberculosis complicated with lung cancer and simple pulmonary tuberculosis: A systematic review and meta-analysis. Epidemiol. Infect. 2022;150:1–25. doi: 10.1017/S0950268822000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Singer S.N., Ndumnego O.C., Kim R.S., Ndung’u T., Anastos K., French A., Churchyard G., Paramithiothis E., Kasprowicz V.O., Achkar J.M. Plasma host protein biomarkers correlating with increasing Mycobacterium tuberculosis infection activity prior to tuberculosis diagnosis in people living with HIV. EBioMedicine. 2022;75:103787. doi: 10.1016/j.ebiom.2021.103787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hosseini K., Ranjbar M., Pirpour Tazehkand A., Asgharian P., Montazersaheb S., Tarhriz V., Ghasemnejad T. Evaluation of exosomal non-coding RNAs in cancer using high-throughput sequencing. J. Transl. Med. 2022;20:30. doi: 10.1186/s12967-022-03231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Toden S., Goel A. Non-coding RNAs as liquid biopsy biomarkers in cancer. Br. J. Cancer. 2022;126:351–360. doi: 10.1038/s41416-021-01672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Diamantopoulos M.A., Georgoulia K.K., Scorilas A. Identification and expression analysis of ten novel small non-coding RNAs (sncRNAs) in cancer cells using a high-throughput sequencing approach. Gene. 2022;809:146025. doi: 10.1016/j.gene.2021.146025. [DOI] [PubMed] [Google Scholar]
- 194.Khamina K., Diendorfer A.B., Skalicky S., Weigl M., Pultar M., Krammer T.L., Fournier C.A., Schofield A.L., Otto C., Smith A.T. A MicroRNA next-generation-sequencing discovery assay (miND) for genome-scale analysis and absolute quantitation of circulating microRNA biomarkers. Int. J. Mol. Sci. 2022;23:1226. doi: 10.3390/ijms23031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lee J., Kang H. Role of microRNAs and long non-coding RNAs in sarcopenia. Cells. 2022;11:187. doi: 10.3390/cells11020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shah P., Chen C.-S. Systematic screening of penetratin’s protein targets by yeast proteome microarrays. Int. J. Mol. Sci. 2022;23:712. doi: 10.3390/ijms23020712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Han X., Cai L., Shi Y., Hua Z., Lu Y., Li D., Yang J. Integrated analysis of long non-coding RNA-mRNA profile and validation in diabetic cataract. Curr. Eye Res. 2022;47:382–390. doi: 10.1080/02713683.2021.1984536. [DOI] [PubMed] [Google Scholar]
- 198.Wang H., Gao Y., Vafaei S., Yu Q., Zhang J., Wang L. A chemoresistance lncRNA signature for recurrence risk stratification of colon cancer patients with chemotherapy. Mol. Ther. Nucleic Acids. 2022;27:427–438. doi: 10.1016/j.omtn.2021.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Baaklini I., Hraiky C., Rallu F., Tse-Dinh Y.C., Drolet M. RNase HI overproduction is required for efficient full-length RNA synthesis in the absence of topoisomerase I in Escherichia coli. Mol. Microbiol. 2004;54:198–211. doi: 10.1111/j.1365-2958.2004.04258.x. [DOI] [PubMed] [Google Scholar]
- 200.Piredda R., Mottola A., Cipriano G., Carlucci R., Ciccarese G., Di Pinto A. Next generation sequencing (NGS) approach applied to species identification in mixed processed seafood products. Food Control. 2022;133:108590. doi: 10.1016/j.foodcont.2021.108590. [DOI] [Google Scholar]
- 201.Harris C.M., Foley S.E., Goedken E.R., Michalak M., Murdock S., Wilson N.S. Merits and pitfalls in the characterization of covalent inhibitors of Bruton’s tyrosine kinase. Slas Discov. Adv. Life Sci. R&D. 2018;23:1040–1050. doi: 10.1177/2472555218787445. [DOI] [PubMed] [Google Scholar]
- 202.Gutiérrez-García C., Ahmed S.S., Ramalingam S., Selvaraj D., Srivastava A., Paul S., Sharma A. Identification of microRNAs from medicinal plant Murraya koenigii by high-throughput sequencing and their functional implications in secondary metabolite biosynthesis. Plants. 2022;11:46. doi: 10.3390/plants11010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Cheng S., Zhu B., Luo F., Lin X., Sun C., You Y., Yi C., Xu B., Wang J., Lu Y. Comparative transcriptome profiles of Schistosoma japonicum larval stages: Implications for parasite biology and host invasion. PLoS Negl. Trop. Dis. 2022;16:e0009889. doi: 10.1371/journal.pntd.0009889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gibb E.A., Vucic E.A., Enfield K.S., Stewart G.L., Lonergan K.M., Kennett J.Y., Becker-Santos D.D., MacAulay C.E., Lam S., Brown C.J. Human cancer long non-coding RNA transcriptomes. PLoS ONE. 2011;6:e25915. doi: 10.1371/journal.pone.0025915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Xu W., Yang W., Zhang Y., Chen Y., Zhang Q., Wang X., Song K., Jin W., Chen X. ISSAAC-seq enables sensitive and flexible multimodal profiling of chromatin accessibility and gene expression in single cells. bioRxiv. 2022 doi: 10.1101/2022.01.16.476488. [DOI] [PubMed] [Google Scholar]
- 206.Withanage M.H.H., Liang H., Zeng E. RNA-Seq experiment and data analysis. Methods Mol. Biol. 2022;2418:405–424. doi: 10.1007/978-1-0716-1920-9_22. [DOI] [PubMed] [Google Scholar]
- 207.De Paoli-Iseppi R., Joshi S., Wrzesinski T., Harrison P.J., Haerty W., Tunbridge E.M., Clark M.B. Using long-read RNA sequencing to decipher the role of RNA isoforms in disease. Pathology. 2022;54:S17. doi: 10.1016/j.pathol.2021.12.063. [DOI] [Google Scholar]
- 208.Arnvig K., Young D. Non-coding RNA and its potential role in Mycobacterium tuberculosis pathogenesis. RNA Biol. 2012;9:427–436. doi: 10.4161/rna.20105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Lee S.I., Park H., Kim S.J., Lee K.W., Son J.K., Hong J.H., Kim S.H., Cho H.J., Park J.B., Kim T.M. Transplantation Proceedings. Volume 53. Elsevier; Amsterdam, The Netherlands: 2021. Circulating RNA profiling in postreperfusion plasma from kidney transplant recipients; pp. 2853–2865. [DOI] [PubMed] [Google Scholar]
- 210.Cong L., Yang Q., Hu C., Yu Q., Hao S., Li D. Current status of functional studies on circular RNAs in bladder cancer and their potential role as diagnostic and prognostic biomarkers: A review. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019;25:3425. doi: 10.12659/MSM.916697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Feng H., Lin L., Chen J. scDIOR: Single cell RNA-seq data IO software. BMC Bioinform. 2022;23:16. doi: 10.1186/s12859-021-04528-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Dwight Z. Data Innovation Provides a Smooth Road to Production: Bioinformatics Needs to Accelerate. Volume 68. Oxford University Press; Oxford, UK: 2022. pp. 264–265. [DOI] [PubMed] [Google Scholar]
- 213.Yu C., Qi X., Yan W., Wu W., Shen B. Next-generation sequencing markup language (NGSML): A medium for the representation and exchange of NGS data. IEEE/ACM Trans. Comput. Biol. Bioinform. 2022;19:1. doi: 10.1109/TCBB.2022.3144170. [DOI] [PubMed] [Google Scholar]
- 214.Cheng J., Lin Y., Xu L., Chen K., Li Q., Xu K., Ning L., Kang J., Cui T., Huang Y. ViRBase v3. 0: A virus and host ncRNA-associated interaction repository with increased coverage and annotation. Nucleic Acids Res. 2022;50:D928–D933. doi: 10.1093/nar/gkab1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Federico A., Saarimäki L.A., Serra A., Giudice G.d., Kinaret P.A.S., Scala G., Greco D. Microarray Data Analysis. Springer; Berlin, Germany: 2022. Microarray data preprocessing: From experimental design to differential analysis; pp. 79–100. [DOI] [PubMed] [Google Scholar]
- 216.Kerachian M.A. Clinical Applications of Non-Coding RNAs in Cancer. Elsevier; Amsterdam, The Netherlands: 2022. Noncoding RNAs in patients with colorectal cancer; pp. 65–95. [Google Scholar]
- 217.Schon M.A., Lutzmayer S., Hofmann F., Nodine M.D. Precise transcript reconstruction with end-guided assembly. bioRxiv. 2022 doi: 10.1101/2022.01.12.476004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Bahonar E., Chahardowli M., Ghalenoei Y., Simjoo M. New correlations to predict oil viscosity using data mining techniques. J. Pet. Sci. Eng. 2022;208:109736. doi: 10.1016/j.petrol.2021.109736. [DOI] [Google Scholar]
- 219.Naphade S., Bhatnagar R., Hanson-Smith V., Choi I., Zhang A. Systematic comparative analysis of strand-specific RNA-seq library preparation methods for low input samples. Sci. Rep. 2022;12:1789. doi: 10.1038/s41598-021-04583-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Taban P.T.M.A.Q., Bhat B., Ahmad S.M., Dar M.A., Kashoo Z.A., Ganie N.A., Shah R.A. Expression of lncRNAs in response to bacterial infections of goat mammary epithelial cells reveals insights into mammary gland diseases. Microb. Pathog. 2022;162:105367. doi: 10.1016/j.micpath.2021.105367. [DOI] [PubMed] [Google Scholar]
- 221.Pucci P. Combination therapy and noncoding RNAs: A new era of cancer personalized medicine. Future Med. 2022;14:117–120. doi: 10.2217/epi-2021-0405. [DOI] [PubMed] [Google Scholar]
- 222.Sridhar J., Gunasekaran P. Computational small RNA prediction in bacteria. Bioinform. Biol. Insights. 2013;7:BBI-S11213. doi: 10.4137/BBI.S11213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ngabonziza J.C.S., Rigouts L., Torrea G., Decroo T., Kamanzi E., Lempens P., Rucogoza A., Habimana Y.M., Laenen L., Niyigena B.E. Multidrug-resistant tuberculosis control in Rwanda overcomes a successful clone that causes most disease over a quarter century. J. Clin. Tuberc. Other Mycobact. Dis. 2022;27:100299. doi: 10.1016/j.jctube.2022.100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hackenberg M., Sturm M., Langenberger D., Falcon-Perez J.M., Aransay A.M. miRanalyzer: A microRNA detection and analysis tool for next-generation sequencing experiments. Nucleic Acids Res. 2009;37:W68–W76. doi: 10.1093/nar/gkp347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wu J., Liu Q., Wang X., Zheng J., Wang T., You M., Sheng Sun Z., Shi Q. mirTools 2.0 for non-coding RNA discovery, profiling, and functional annotation based on high-throughput sequencing. RNA Biol. 2013;10:1087–1092. doi: 10.4161/rna.25193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Bonnal R.J., Rossi R.L., Carpi D., Ranzani V., Abrignani S., Pagani M. miRiadne: A web tool for consistent integration of miRNA nomenclature. Nucleic Acids Res. 2015;43:W487–W492. doi: 10.1093/nar/gkv381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kozomara A., Birgaoanu M., Griffiths-Jones S. miRBase: From microRNA sequences to function. Nucleic Acids Res. 2019;47:D155–D162. doi: 10.1093/nar/gky1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Georgakilas G., Vlachos I.S., Zagganas K., Vergoulis T., Paraskevopoulou M.D., Kanellos I., Tsanakas P., Dellis D., Fevgas A., Dalamagas T. DIANA-miRGen v3. 0: Accurate characterization of microRNA promoters and their regulators. Nucleic Acids Res. 2016;44:D190–D195. doi: 10.1093/nar/gkv1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Manual I. miRStar™ Human Cancer Focus miRNA & Target mRNA PCR Array. Arrastay, Inc.; Rockville, MD, USA: 2020. Instruction Manual Version 1.0. [Google Scholar]
- 230.Gkirtzou K., Tsamardinos I., Tsakalides P., Poirazi P. MatureBayes: A probabilistic algorithm for identifying the mature miRNA within novel precursors. PLoS ONE. 2010;5:e11843. doi: 10.1371/journal.pone.0011843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Schulhofer S.J. Reconsidering Miranda. Univ. Chic. Law Rev. 1987;54:435–461. doi: 10.2307/1599796. [DOI] [Google Scholar]
- 232.Volders P.-J., Helsens K., Wang X., Menten B., Martens L., Gevaert K., Vandesompele J., Mestdagh P. LNCipedia: A database for annotated human lncRNA transcript sequences and structures. Nucleic Acids Res. 2013;41:D246–D251. doi: 10.1093/nar/gks915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ma L., Cao J., Liu L., Du Q., Li Z., Zou D., Bajic V.B., Zhang Z. LncBook: A curated knowledgebase of human long non-coding RNAs. Nucleic Acids Res. 2019;47:D128–D134. doi: 10.1093/nar/gky960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wang J., Ma R., Ma W., Chen J., Yang J., Xi Y., Cui Q. LncDisease: A sequence based bioinformatics tool for predicting lncRNA-disease associations. Nucleic Acids Res. 2016;44:e90. doi: 10.1093/nar/gkw093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhao Y., Li H., Fang S., Kang Y., Wu W., Hao Y., Li Z., Bu D., Sun N., Zhang M.Q. NONCODE 2016: An informative and valuable data source of long non-coding RNAs. Nucleic Acids Res. 2016;44:D203–D208. doi: 10.1093/nar/gkv1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Li J.-H., Liu S., Zhou H., Qu L.-H., Yang J.-H. starBase v2. 0: Decoding miRNA-ceRNA, miRNA-ncRNA and protein–RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Dinger M.E., Pang K.C., Mercer T.R., Crowe M.L., Grimmond S.M., Mattick J.S. NRED: A database of long noncoding RNA expression. Nucleic Acids Res. 2009;37:D122–D126. doi: 10.1093/nar/gkn617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Ghosal S., Das S., Sen R., Basak P., Chakrabarti J. Circ2Traits: A comprehensive database for circular RNA potentially associated with disease and traits. Front. Genet. 2013;4:283. doi: 10.3389/fgene.2013.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chen X., Han P., Zhou T., Guo X., Song X., Li Y. circRNADb: A comprehensive database for human circular RNAs with protein-coding annotations. Sci. Rep. 2016;6:34985. doi: 10.1038/srep34985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Glažar P., Papavasileiou P., Rajewsky N. circBase: A database for circular RNAs. RNA. 2014;20:1666–1670. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Jakobi T., Uvarovskii A., Dieterich C. Circtools—A one-stop software solution for circular RNA research. Bioinformatics. 2019;35:2326–2328. doi: 10.1093/bioinformatics/bty948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Li X., Chu C., Pei J., Măndoiu I., Wu Y. CircMarker: A fast and accurate algorithm for circular RNA detection. BMC Genom. 2018;19:79–87. doi: 10.1186/s12864-018-4926-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Monteiro S., Rente D., Cunha M.V., Marques T.A., Cardoso E., Vilaça J., Coelho N., Brôco N., Carvalho M., Santos R. Discrimination and surveillance of infectious severe acute respiratory syndrome Coronavirus 2 in wastewater using cell culture and RT-qPCR. Sci. Total Environ. 2022;815:152914. doi: 10.1016/j.scitotenv.2022.152914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lee M.S., Hyun H., Park I., Kim S., Jang D.H., Kim S., Im J.K., Kim H., Lee J.H., Kwon T. Quantitative fluorescence in situ hybridization (FISH) of magnetically confined bacteria enables early detection of human bacteremia. Small Methods. 2022;6:2101239. doi: 10.1002/smtd.202101239. [DOI] [PubMed] [Google Scholar]
- 245.Green M.R., Sambrook J. Analysis of RNA by northern blotting. Cold Spring Harb. Protoc. 2022;2022:101741. doi: 10.1101/pdb.top101741. [DOI] [PubMed] [Google Scholar]
- 246.Stiens J., Arnvig K.B., Kendall S.L., Nobeli I. Challenges in defining the functional, non-coding, expressed genome of members of the Mycobacterium tuberculosis complex. Mol. Microbiol. 2022;117:20–31. doi: 10.1111/mmi.14862. [DOI] [PubMed] [Google Scholar]
- 247.Duarte I., Carraco G., de Azevedo N.T., Benes V., Andrade R.P. gga-miRNOME, a microRNA-sequencing dataset from chick embryonic tissues. Sci. Data. 2022;9:29. doi: 10.1038/s41597-022-01126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Green M.R., Sambrook J. Preparation of labeled DNA, RNA, and oligonucleotide probes. Cold Spring Harb. Protoc. 2022;2022:100578. doi: 10.1101/pdb.top100578. [DOI] [PubMed] [Google Scholar]
- 249.Torii S., Oishi W., Zhu Y., Thakali O., Malla B., Yu Z., Zhao B., Arakawa C., Kitajima M., Hata A. Comparison of five polyethylene glycol precipitation procedures for the RT-qPCR based recovery of murine hepatitis virus, bacteriophage phi6, and pepper mild mottle virus as a surrogate for SARS-CoV-2 from wastewater. Sci. Total Environ. 2022;807:150722. doi: 10.1016/j.scitotenv.2021.150722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Taylor S., Wakem M., Dijkman G., Alsarraj M., Nguyen M. A practical approach to RT-qPCR—Publishing data that conform to the MIQE guidelines. Methods. 2010;50:S1–S5. doi: 10.1016/j.ymeth.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 251.Mestdagh P., Van Vlierberghe P., De Weer A., Muth D., Westermann F., Speleman F., Vandesompele J. A novel and universal method for microRNA RT-qPCR data normalization. Genome Biol. 2009;10:R64. doi: 10.1186/gb-2009-10-6-r64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Levsky J.M., Singer R.H. Fluorescence in situ hybridization: Past, present and future. J. Cell Sci. 2003;116:2833–2838. doi: 10.1242/jcs.00633. [DOI] [PubMed] [Google Scholar]
- 253.Allemailem K.S., Almatroudi A., Alrumaihi F., Almansour N.M., Aldakheel F.M., Rather R.A., Afroze D., Rah B. Single nucleotide polymorphisms (SNPs) in prostate cancer: Its implications in diagnostics and therapeutics. Am. J. Transl. Res. 2021;13:3868. [PMC free article] [PubMed] [Google Scholar]
- 254.Nederlof P., Van der Flier S., Wiegant J., Raap A., Tanke H., Ploem J., Van der Ploeg M. Multiple fluorescence in situ hybridization. Cytom. J. Int. Soc. Anal. Cytol. 1990;11:126–131. doi: 10.1002/cyto.990110115. [DOI] [PubMed] [Google Scholar]
- 255.Liehr T. Fluorescence In Situ Hybridization (FISH) Springer; Berlin, Germany: 2017. [Google Scholar]
- 256.Trask B.J. Fluorescence in situ hybridization: Applications in cytogenetics and gene mapping. Trends Genet. 1991;7:149–154. doi: 10.1016/0168-9525(91)90103-W. [DOI] [PubMed] [Google Scholar]
- 257.Gonzalez Plaza J.J. Current roles of microRNAs in infectious diseases—Advancing into healthcare. Infektološki Glas. 2016;36:5–15. [Google Scholar]
- 258.Lalevée S., Feil R. Long noncoding RNAs in human disease: Emerging mechanisms and therapeutic strategies. Epigenomics. 2015;7:877–879. doi: 10.2217/epi.15.55. [DOI] [PubMed] [Google Scholar]
- 259.Grijalvo S., Alagia A., Jorge A.F., Eritja R. Covalent strategies for targeting messenger and non-coding RNAs: An updated review on siRNA, miRNA and antimiR conjugates. Genes. 2018;9:74. doi: 10.3390/genes9020074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Allemailem K.S., Almatroudi A., Alsahli M.A., Basfar G.T., Alrumaihi F., Rahmani A.H., Khan A.A. Recent advances in understanding oligonucleotide aptamers and their applications as therapeutic agents. 3Biotech. 2020;10:551. doi: 10.1007/s13205-020-02546-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Matsui M., Corey D.R. Non-coding RNAs as drug targets. Nat. Rev. Drug Discov. 2017;16:167–179. doi: 10.1038/nrd.2016.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Corrêa R.L., Steiner F.A., Berezikov E., Ketting R.F. MicroRNA–directed siRNA biogenesis in Caenorhabditis elegans. PLoS Genet. 2010;6:e1000903. doi: 10.1371/journal.pgen.1000903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Dykxhoorn D.M., Lieberman J. Running interference: Prospects and obstacles to using small interfering RNAs as small molecule drugs. Annu. Rev. Biomed. Eng. 2006;8:377–402. doi: 10.1146/annurev.bioeng.8.061505.095848. [DOI] [PubMed] [Google Scholar]
- 264.Castel S.E., Martienssen R.A. RNA interference in the nucleus: Roles for small RNAs in transcription, epigenetics and beyond. Nat. Rev. Genet. 2013;14:100–112. doi: 10.1038/nrg3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Fu S., Wang Y., Li H., Chen L., Liu Q. Regulatory networks of LncRNA MALAT-1 in cancer. Cancer Manag. Res. 2020;12:10181. doi: 10.2147/CMAR.S276022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Jiang F., Doudna J.A. CRISPR–Cas9 structures and mechanisms. Annu. Rev. Biophys. 2017;46:505–529. doi: 10.1146/annurev-biophys-062215-010822. [DOI] [PubMed] [Google Scholar]
- 267.Ran F., Hsu P.D., Wright J., Agarwala V., Scott D.A., Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Tang L., Zeng Y., Du H., Gong M., Peng J., Zhang B., Lei M., Zhao F., Wang W., Li X. CRISPR/Cas9-mediated gene editing in human zygotes using Cas9 protein. Mol. Genet. Genom. 2017;292:525–533. doi: 10.1007/s00438-017-1299-z. [DOI] [PubMed] [Google Scholar]
- 269.Doudna J.A., Charpentier E. The new frontier of genome engineering with CRISPR-Cas9. Science. 2014;346:1258096. doi: 10.1126/science.1258096. [DOI] [PubMed] [Google Scholar]
- 270.Peng R., Lin G., Li J. Potential pitfalls of CRISPR/Cas9-mediated genome editing. FEBS J. 2016;283:1218–1231. doi: 10.1111/febs.13586. [DOI] [PubMed] [Google Scholar]
- 271.Liu L., Zhan Y., Huang Y., Huang L. LncRNA FGD5-AS1 can be predicted as therapeutic target in oral cancer. J. Oral Pathol. Med. 2020;49:243–252. doi: 10.1111/jop.12989. [DOI] [PubMed] [Google Scholar]
- 272.Mei Y., Wang Y., Chen H., Sun Z.S., Ju X.-D. Recent progress in CRISPR/Cas9 technology. J. Genet. Genom. 2016;43:63–75. doi: 10.1016/j.jgg.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 273.Zhou J., Yuan M., Zhao Y., Quan Q., Yu D., Yang H., Tang X., Xin X., Cai G., Qian Q. Efficient deletion of multiple circle RNA loci by CRISPR-Cas9 reveals Os06circ02797 as a putative sponge for OsMIR408 in rice. Plant Biotechnol. J. 2021;19:1240–1252. doi: 10.1111/pbi.13544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhang Y., Nguyen T.M., Zhang X.-O., Wang L., Phan T., Clohessy J.G., Pandolfi P.P. Optimized RNA-targeting CRISPR/Cas13d technology outperforms shRNA in identifying functional circRNAs. Genome Biol. 2021;22:41. doi: 10.1186/s13059-021-02263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available within the article.