Abstract
The purpose of this study was to compare vascular calcification (VC), serum osteoprotegerin (OPG) levels, and other biochemical markers to determine their value as available predictors of all-cause and cardiovascular (CV) mortality in patients on peritoneal dialysis (PD). A total of 197 patients were recruited from seven dialysis centers in Mexico City. VC was assessed with multi-slice computed tomography, measured using the calcification score (CaSc). OPG, albumin, calcium, hsC-reactive protein, phosphorous, osteocalcin, total alkaline phosphatase, and intact parathormone were also analyzed. Follow-up and mortality analyses were assessed using the Cox regression model. The mean age was 43.9 ± 12.9 years, 64% were males, and 53% were diabetics. The median OPG was 11.28 (IQR: 7.6–17.4 pmol/L), and 42% of cases had cardiovascular calcifications. The median VC was 424 (IQR:101–886). During follow-up (23 ± 7 months), there were 34 deaths, and 44% were cardiovascular in origin. In multivariable analysis, OPG was a significant predictor for all-cause (HR 1.08; p < 0.002) and CV mortality (HR 1.09; p < 0.013), and performed better than VC (HR 1.00; p < 0.62 for all-cause mortality and HR 1.00; p < 0.16 for CV mortality). For each mg/dL of albumin-corrected calcium, there was an increased risk for CV mortality, and each g/dL of albumin decreased the risk factor for all-cause mortality. OPG levels above 14.37 and 13.57 pmol/L showed the highest predictive value for all-cause and CV mortality in incident PD patients and performed better than VC.
Keywords: vascular calcification, diabetes mellitus, osteoprotegerin, cardiovascular mortality, risk factor, peritoneal dialysis
1. Introduction
Cardiovascular diseases (CVD) are the main cause of comorbidity and mortality in the population with chronic kidney disease (CKD) [1], but the incidence has not been fully explained by traditional risk factors. Cardiovascular calcifications have been included among non-traditional risk factors; they may involve cardiac valves and the intima layer (atherosclerosis) or the middle layer (arteriosclerosis) of coronary arteries and peripheral vessels [2].
Arterial calcifications are a frequent finding in patients with CKD, even in non-dialysis patients, and they are directly associated with the extent of renal damage [3]. Moreover, vascular calcifications (VC) are an important marker of cardiovascular risk [4].
Various studies have shown that their presence is associated with greater all-cause and CV mortality in hemodialysis patients [5]. On the basis of this association, coronary artery calcification, quantified by means of multi-detector spiral computed tomography (CT), has been suggested as a screening test to assess cardiovascular risk in patients undergoing renal replacement therapy. However, the limited availability of the technique and its operator dependency complicate its routine use [6] and have prompted the need for other calcification biomarkers. Moreover, one of the more controversial points comes from controlled clinical trials, in which various interventions, aimed at modifying the evolution of coronary artery calcifications, showed little or no effect on mortality [7].
In recent years, various studies have highlighted the value of some bone metabolism-related proteins as biomarkers of vascular wall calcification. Among them, osteoprotegerin (OPG) seems to be particularly promising. OPG is a soluble glycoprotein, belonging to the soluble proteins of the tumor necrosis factor (TNF) receptor superfamily, and is classified as an osteoclastogenesis inhibition factor [8] because it is a decoy of the receptor activator of nuclear factor kappa-β ligand (RANKL) and TNF-related apoptosis-inducing ligand (TRAIL) [9]. OPG is expressed in most human tissues, including bone and vasculature (endothelial and vascular smooth muscle cells (VSMC)). It is induced by inflammatory cytokines, such as pro-inflammatory mediators, such as TNFα [10].
Although studies in vitro and in animal models suggest that OPG inhibits vascular calcification, clinical studies suggest that elevated serum OPG levels are directly associated with vascular calcifications, coronary artery disease, stroke, and future cardiovascular events [11], and common carotid artery intima-media thickness (CCA-IMT). Moreover, it has been shown to be a prognostic marker of cardiovascular risk in dialyzed patients [12] and of mortality in hemodialysis (HD) patients [13]. Furthermore, in diabetic patients without CKD, elevated circulating OPG levels are associated with acute myocardial infarction and chronic heart failure of ischemic etiology [14], and mortality in patients with angina pectoris [15]. Additionally, OPG is a predictor of mortality in patients with other diseases, such as cancer or amyloidosis [16,17].
These findings may suggest that OPG could serve to evaluate cardiovascular risk in dialysis patients. This may be of greater importance in younger patients without evident comorbidities, as, in this context, it may lead to identifying subjects that need a cardiovascular evaluation and specific interventions, and may support treatment modulation.
The present study aimed to evaluate the role of osteoprotegerin compared to vascular calcification, and some mineral metabolism markers with all-cause and cardiovascular mortality in a multi-center cohort of patients on peritoneal dialysis (PD).
2. Materials and Methods
2.1. Study Design
A prospective observational cohort of incident patients in PD programs was recruited from 7 hospitals belonging to the National Network of the lnstituto Mexicano of Seguro Social (IMSS) in Mexico City and was followed up for at least 16 months. All-cause and CV mortality were the primary end points of the study.
2.2. Patient Selection
Participation was offered to all the patients who began peritoneal dialysis (>3 months and <4 months), either continuous ambulatory peritoneal dialysis (CAPD) or automated PD (APD) treatment, at the PD centers of each hospital. Written informed consent was obtained from the patients.
The inclusion criteria were: adult (>18 years), free of acute complications, including peritonitis and hospitalizations during the month prior to enrolment. Patients with previously known CVD (defined as heart failure, ischemic disease, arrhythmia, myocardial infarction), chronic infections, malignancies, chronic obstructive pulmonary disease, ongoing steroid therapy, positivity for hepatitis B or C and HIV were excluded. Patients with incomplete data were also excluded.
2.3. Dialysis Schedule
Patients received 2 L, four times a day, for CAPD and four or five exchanges per night and a wet day for APD. Only dextrose solutions were available and the concentration was prescribed by the attending nephrologist according to the patient’s needs. Dialysis adequacy was calculated by total Kt/V: renal Kt/V+ peritoneal Kt/V and peritoneal ultrafiltration. Kt/V is a number used to quantify the adequacy of peritoneal dialysis and hemodialysis, and represents the clearance of Urea by the peritoneum and/or by the kidney, normalized by total body water. K is the clearance of urea by the peritoneum or the kidney, in ml/min, t is the time on dialysis (min), and V is the volume of distribution of urea, approximately equal to the volume of the patient’s total body water. The minimal recommended values were a weekly urea Kt/V of 1.7 and a daily ultrafiltration of 750 mL [18]. Residual renal function was calculated as the mean of urea and creatinine clearance.
2.4. Data Collection
Demographic and relevant clinical data were collected from medical records by trained nurses. A CT was performed at the baseline stage. Patients were censored at the end of the follow-up: kidney transplantation, or in the shift to hemodialysis or transfer to other hospitals, in case of voluntary withdrawal or death. Causes of death were obtained from the death certificate and were reviewed according to a caregiver.
2.5. Biochemical Assessments
Blood samples were drawn from an antecubital vein without stasis, after overnight fasting. The samples were centrifuged and the plasma and serum were separated and stored at –70 °C until assayed. Osteoprotegerin (OPG) was determined using ELISA (MicroVue Eia Kit. Quidel Corp. Specialty Products, San Diego, CA, USA). The intra-assay precision was 3% and the inter-assay precision was 4.5%, with a limit of detection of 1.16 to 60 pmol/L. N-MID osteocalcin and intact parathormone (iPTH) were analyzed by electrochemiluminescence immunoassay (Elecsys Modular Analytics 2010 Roche, Mannheim, Germany) The intra and inter coefficients of variation (% CoV) were 2.5% and 2.0%, respectively. Serum phosphorous (P), serum albumin (Alb), and albumin-corrected calcium (cCa) were calculated with the formula: cCa = (Ca (mg/dL) + 0.8(4-Alb g/dL)); total cholesterol (Chol), glucose (Glu), creatinine (Cr), total alkaline phosphatase (tALP), and high-sensitivity C-reactive protein (hsCRP) were measured using standard techniques (Hitachi 902 autoanalyser, Tokyo, Japan). Twenty-four-hour urine and dialysate collection was performed for both CAPD and APD patients.
2.6. Measurement of Arterial Calcifications
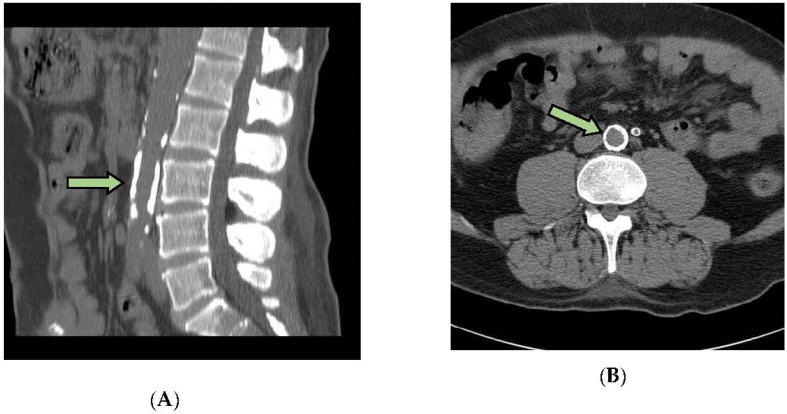
A 16-cut multi-slice computed tomography (MSCT) using Bright Speed, (GE, Beijing, China), was used to quantify the vascular calcifications (VC) in a standardized section of the abdominal aorta and pelvic vessels. The acquired images were reviewed using Advantage Workstation Swart Core software, v4.5, (GE, Waukesha, Wisconsin, WI, USA), and a calcification score (CaSc) was generated. The calcification score indicates the amount of calcified plaque in the arteries. The CaSc (AJ130 score) refers to the detection of densities of more than 130 Hounsfield units (HU) in areas of at least 1mm2. It is obtained from the product between the area of calcified plaque and its maximum density in Hounsfield units and is expressed in Agatston units (AU). A score of 0 implies the absence of calcified plaques, 1–10 AU shows minimal calcified plaques, 11–100 AU shows mild calcification, 101–300 AU shows moderate calcification, and >300 AU shows severe arterial calcification, as in other studies [19]. An example of a patient with calcifications is shown in Scheme 1.
Scheme 1.
Vascular calcification of a patient with high levels of OPG. (A) Sagittal view of calcification (CaSc) at the level of L3. (B) Axial view of calcification (CaSc) at the level of L3.
No contrast-enhancing agent was used; scanning was performed on sequential 2 mm thick layers. Two independent investigators, blind to the patient’s clinical history, evaluated the MSCT scans. Inter-observer reproducibility between the investigator and radiologist was assessed for all patients, with a coefficient of variation of 3.5%, the intra-observer coefficient of variation was 2.8% during three days, and the correlation between observers was 0.95 (CI%; 0.92 to 0.98).
2.7. Statistical Analysis
Data are expressed as mean ± standard deviation (SD) in the case of continuous variables with normal distribution, or median and interquartile range (IQR) in case of non-normal distribution, or as frequencies in the case of categorical variables. Differences between groups were analyzed using a Student’s t-test, Mann–Whitney U test, or Chi-square test, as appropriate. Cox proportional hazards regression, using the Enter method, was used to estimate the all-cause and cardiovascular mortality hazard ratios, unadjusted and adjusted by: serum OPG, cCa, sAlb, P, iPTH, and hsCRP as predicting variables, and hsCRP was converted to a logarithm. The Cox models were determined with baseline co-variables. We additionally performed a Cox regression analysis using the forward conditional method to determine the score statistics for each variable.
Receiver operating characteristic (ROC) curves were made to determine the diagnostic OPG value. The area under curve (AUC) was evaluated, and we determined the concentration of OPG as the cut-off point for all-cause mortality and CV death, according to the Youden index method, with a significance of p < 0.05. Analysis was performed using SPSS Statistics for Windows, version 21.0. (IBM Corp, Armonk, NY, USA).
3. Results
Figure 1 shows the study flow chart: 328 patients were assessed for eligibility at the dialysis centers, and 230 of them were eligible. Thirty-three were excluded because of several causes: 18 patients did not meet the selection criteria, mainly because of age, catheter dysfunction, peritonitis, and hospitalizations during the previous month; 7 patients refused to participate; 4 patients moved to another city; 4 patients lost social security coverage. Thus, 197 patients were included in the final analysis.
Figure 1.
Study flow chart.
3.1. Baseline Biochemical Data
Demographic and relevant clinical and biochemical data of the 197 included patients are shown inTable 1. The mean age was 43.9 ± 12.9 years old, 64% were males, and 53% were diabetics. The most frequent etiology was diabetic nephropathy, found in 52.8% of the patients. The median OPG was 11.28 pmol/L, with an interquartile range of 25–75; (7.6–17.1) comparable with other studies; 10.9 (IQR 8–10.3 pmol/L) [20].
Table 1.
Patient´s demographic, clinical and biochemical data.
| Variables | Mean ± SD |
|---|---|
| Number of patients. | 197 |
| Age (year) | 43.97 ± 12.92 |
| Sex: male (%) | 64% |
| Diabetes (%) | 53% |
| CAPD/APA (%) | 73/27% |
| Systolic blood pressure (mmHg) | 136.41 ± 26.44 |
| Diastolic blood pressure (mmHg) | 84.20 ± 15.71 |
| Total Kt/V, median (IQR) | 1.46(1.07–2.3) |
| Patients prescribed with Ca CO3 n (%) | 125(63.5) |
| Patients prescribed with Calcitriol n (%) | 105 (53.3) |
| Body mass index (kg/m2) | 24.83 ± 4.28 |
| Hemoglobin Hg (g/dL) | 10.15 ± 2.44 |
| Glucose (mg/dL) | 97.9 (87.6–138.1) |
| Creatinine (mg/dL) | 8.66 ± 3.22 |
| Total cholesterol (mg/dL) | 192.12 ± 44.26 |
| Triglycerides, (mg/dL), median(IQR) | 155 (119.1–226.3) |
| Albumin (g/dL) | 3.41 ± 0.51 |
| cCa(mg/dL) | 9.1 ± 1.38 |
| Phosphorus, mg/dL, median (IQR) | 4.5 (1.6–9.8) |
| Intact parathormone pg/mL, median(IQR) | 104.8 (50.4–199.7) |
| Osteocalcin, ng/mL, median (IQR) | 186.2 (105.4–300) |
| Ln C-Reactive protein, mg/L, median (IQR) | 1.8 (0.6–5.0) |
| Alkaline phosphatase, U/L, median (IQR) | 99.4 (77.2–138.2) |
| Osteoprotegerin, pmol/L median (IQR) | 11.28 (7.6–17.1) |
| Vascular calcification, CaSc, median (IQR) | 424 (101–886) |
Data are expressed as mean ± SD, or Median (IQR), interquartile range; (25–75). CAPD /APA: continuous ambulatory peritoneal dialysis/automated peritoneal dialysis. Total Kt/V = renal Kt/V+peritoneal Kt/V. cCa = albumin-corrected calcium.
A significant difference was noted between diabetic patients and non-diabetic ones in terms of OPG (16.78 ± 3.37 vs. 8.50 ± 3.88 pmol/L, respectively, p < 0.001), as well as in CaSc (424 (IQR, 144 to 928) vs. 118 (IQR 41 to 533), respectively, p < 0.001). Multiple correlations between variables adjusted by diabetes are shown in Table 2; OPG correlated positively with age, cCa, and VC, and negatively with albumin. With respect to treatments, we did not find differences between dialysis modalities, CAPD, or APD, in regard to OPG levels.
Table 2.
Multiple correlations between variables adjusted by diabetes.
| Variables | Age | SBP | cCa | PO4 | iPTH | OPG | Alb | CRP | |
|---|---|---|---|---|---|---|---|---|---|
| SBP | r | 0.142 0.055 |
10.000 | ||||||
| p | |||||||||
| cCa | r | 0.091 0.222 |
−0.034 0.643 |
10.000 | |||||
| p | |||||||||
| P | r | −0.197 0.008 b |
−0.055 0.459 |
0.094 0.206 |
10.000 | ||||
| p | |||||||||
| iPTH | r | −0.212 0.004 b |
0.123 0.098 |
−0.323 0.001a |
0.092 0.215 |
10.000 | |||
| p | |||||||||
| OPG | r | 0.333 0.001 a |
0.134 0.070 |
0.161 0.030 c |
−0.023 0.754 |
−0.087 0.242 |
10.000 | ||
| p | |||||||||
| Alb | r | −0.045 0.545 |
−0.108 0.147 |
−0.090 0.227 |
0.149 0.044 c |
0.078 0.292 |
−0.180 0.015 c |
10.000 | |
| p | |||||||||
| CRP | r | −0.029 0.694 |
−0.069 0.351 |
−0.044 0.552 |
0.041 0.585 |
0.001 0.986 |
−0.029 0.694 |
−0.153 0.039 c |
10.000 |
| p | |||||||||
| VC | r | 0.113 0.127 |
0.064 0.393 |
0.066 0.376 |
0.038 0.606 |
0.002 0.982 |
0.200 0.007 b |
0.015 0.836 |
0.015 0.839 |
| P |
SBP: systolic blood pressure, (mmHg), cCa: albumin-corrected calcium, (mg/dL), P: phosphorus, (mg/dL), iPTH: intact parathormone, (pg/mL), OPG: osteoprotegerin, (pmol/L), Alb: albumin, (g/dL), CRP: C reactive protein, (mg/L), VC: vascular calcification. (CaSc). Multivariate analysis adjusted by diabetes, a p < 0.001, b p < 0.01, c p < 0.05.
3.2. Follow-up
During a two-year follow-up, 34 patients died. Causes of death were: CVD in 15 patients (44%), including acute myocardial infarction, sudden death, arrhythmia, and heart failure, PD-related peritonitis in 2 patients (5.9%), non-peritoneal-related infections in 2 patients (5.9%), uremia/hyperkalemia in 5 patients (14.7%), cancer/stroke in 2 patients (5.9%), hypovolemic shock in 2 patients (5.9%), and hyperglycemia/acidosis in 6 patients (17.7%).
Table 3 shows the differences in patient demographics, as well as clinical and biochemical baseline data between the survivors and non-survivors. Non-survivors were older, more frequently diabetic, and had higher values of systolic blood pressure, OPG, and a higher incidence and severity of vascular calcifications. They also had lower levels of serum albumin.
Table 3.
Patient´s demographic, clinical and biochemical data according to survival.
| Variables | Survivors (n = 163) |
Non-Survivors (n = 34) |
p-Value |
|---|---|---|---|
| Age (year) | 42.25 ± 12.98 | 51.06 ± 9.91 | 0.001 a |
| Sex, male, (%) | 50.3% | 40% | 0.840 |
| Diabetes n (%) | 76 (46.6%) | 29 (85.3%) | 0.001 a |
| CAPD/APD (%) | (51.5%)/(48.5%) | (43.2%)/(56.8%) | 0.400 |
| Urine volume (mL/24 h) | 573.78 ± 516.71 | 585.7 ± 547.97 | 0.904 |
| Systolic blood pressure (mmHg) | 134.22 ± 26.34 | 146.79 ± 24.72 | 0.011 b |
| Dyastolic blood pressure (mmHg) | 84.61 ± 16.02 | 82.26 ± 14.25 | 0.431 |
| total Kt/V | 1.81 ± 1.1 | 1.85 ± 1.2 | 0.809 |
| Body mass index (kg/m2) | 24.57 ± 4.32 | 26.05 ± 3.94 | 0.067 |
| Hemoglobin (g/dL) | 10.03 ± 2.42 | 10.69 ± 3.01 | 0.171 |
| Glucose (mg/dL) | 95.7 (86.7–129.4) | 115.2 (95.9–185.9) | 0.004 a |
| Creatinine (mg/dL) | 8.81 ± 3.28 | 7.98 ± 2.89 | 0.173 |
| BUN (mg/dL) | 56.23 ± 19.79 | 57.07 ± 21.19 | 0.824 |
| Total Cholesterol (mg/dL) | 193.16 ± 44.04 | 187.14 ± 45.61 | 0.472 |
| Triglycerides (mg/dL) | 163.2 (112.6–232.1) | 140.6 (104.7–209.9) | 0.175 |
| Albumin (g/dL) | 3.48 ± 0.51 | 3.10 ± 0.44 | 0.001 a |
| Calcium (mg/dL) | 8.69 ± 1.17 | 8.76 ± 2.07 | 0.798 |
| cCa (mg/dL) | 9.09 ± 1.21 | 9.47 ± 2.07 | 0.168 |
| Phosphorus (mg/dL) | 4.45 (3.7–5.8) | 4.7 (3.5–6.0) | 0.663 |
| iPTH (pg/mL) | 107.15 (55.3–199.5) | 97.8 (22.2–216.7) | 0.922 |
| Osteocalcin (ng/mL) | 195.8 (104–300) | 111.5 (109–285) | 0.606 |
| Ln C-reactive protein (mg/L) | 0.53 (−0.5–1.6) | 0.69 (−0.5–1.9) | 0.519 |
| Alkaline phosphatase (U/L) | 95.5 (76.8–130.7) | 111.5 (86.4–151.3) | 0.249 |
| Osteoprotegerin (pmol/L) | 10.33 (7.23–15.34) | 17.61 (10.48–23) | 0.005 |
| Vascular calcification (Ca Sc) | 306.83 ± 898.91 | 960.16 ± 1888.08 | 0.014 a |
Data are expressed as mean ± SD or median (IQR). Total Kt/V = renal Kt/V+ DL Kt/V. cCa = albumin-corrected calcium. CaSc = calcium score. Student´s t-test and median test with significance of: a p < 0.005, b p < 0.05.
Table 4 presents the results of the unadjusted Cox model analysis (univariate analysis) of factors associated with all-cause and CV mortality. The presence of diabetes mellitus increases by 82% and 85%, respectively, the possibility of having the risk of all-cause and CV mortality. Diabetes mellitus, age (by year), systolic blood pressure (for each mmHg), OPG (pmol/L), VC (for each CaSc), and low albumin (for each g/dL) were predictors for all-cause mortality while, diabetes, age, OPG, cCa, VC, and decreased Alb were predictors for CV mortality.
Table 4.
Factors associated with all-cause and cardiovascular mortality in peritoneal dialysis patients.
| Univariate Cox Regression Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| All-Cause Mortality | Cardiovascular Mortality | |||||||
| Variable | p-Value | HR | CI 95% | p-Value | HR | 95% CI | ||
| Low | Upper | p | Low | Upper | ||||
| Diabetes (non) | 0.001 a | 0.182 | 0.070 | 0.472 | 0.013 b | 0.15 | 0.03 | 0.673 |
| Age (y) | 0.001 a | 1.018 | 1.004 | 1.031 | 0.014 b | 1.08 | 1.02 | 1.15 |
| SBP (mmHg) | 0.009 b | 1.02 | 1.00 | 1.03 | 0.107 | 1.02 | 0.99 | 1.04 |
| OPG(pmol/L) | 0.013 b | 1.09 | 1.01 | 1.16 | 0.001 a | 1.10 | 1.04 | 1.17 |
| sAlb (g/dL) | 0.001 a | 0.12 | 0.06 | 0.23 | 0.016 b | 0.30 | 0.11 | 0.79 |
| cCa (mg/dL) | 0.119 | 1.29 | 0.88 | 1.91 | 0.001 a | 1.41 | 1.15 | 1.73 |
| VC (CaSc) | 0.019 b | 1.00 | 1.00 | 1.00 | 0.003 a | 1.00 | 1.00 | 1.00 |
| P (mg/dL) | 0.723 | 0.96 | 0.76 | 1.21 | 0.885 | 0.90 | 0.69 | 1.37 |
| iPTH (pg/mL) | 0.530 | 1.00 | 1.00 | 1.01 | 0.965 | 1.00 | 0.99 | 1.01 |
| LnCRP (mg/L) | 0.057 | 1.28 | 0.99 | 1.65 | 0.147 | 1.32 | 0.91 | 1.92 |
Baseline variable Cox enter model analysis. a p < 0.005, b p < 0.05. SBP: systolic blood pressure, OPG: osteoprotegerin, sAlb: serum albumin, cCa: albumin-corrected calcium, VC: vascular calcification, P: phosphorus, iPTH: intact parathormone, lnCRP: C reactive protein.
Table 5 shows the multivariable analysis (adjusted Cox analysis) of mortality with baseline data of calcium metabolism biomarkers (Model 1); high OPG and low albumin levels were associated with all-cause mortality. OPG, cCa, and iPTH were associated with CV mortality. OPG was significant in both mortalities. Model 2 adds DM to Model 1. OPG was significant only in all-cause mortality; cCa and PTH were significant in cardiovascular mortality. Model 3 adds age to Model 2. Albumin was significant for all-cause mortality. cCa, PTH, and age were significant for CV mortality. OPG was not significant. This was due to the high association and collinearity that OPG had with age, as was shown in multiple correlations.
Table 5.
Factors associated with all-cause and cardiovascular mortality in peritoneal dialysis patients.
| Multivariate Cox Regression Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|
| All-Cause Mortality | Cardiovascular Mortality | |||||||
| Variable: | p | HR | 95% CI | p | HR | 95% CI | ||
| Model 1: OPG (pmol/L) | 0.002 a | 1.08 | 1.03 | 1.14 | 0.013 b | 1.09 | 1.02 | 1.66 |
| Alb (g/dL) | 0.012 b | 0.35 | 0.15 | 0.79 | 0.194 | 0.45 | 0.13 | 1.50 |
| cCa (mg/dL) | 0.353 | 1.13 | 0.87 | 1.46 | 0.001 a | 1.53 | 1.19 | 1.98 |
| VC (CaSc) | 0.616 | 1.00 | 1.00 | 1.00 | 0.163 | 1.00 | 1.00 | 1.00 |
| P (mg/dL) | 0.445 | 1.11 | 0.85 | 1.44 | 0.691 | 0.91 | 0.59 | 1.40 |
| PTH (pg/mL) | 0.297 | 1.00 | 0.99 | 1.01 | 0.030 b | 1.00 | 1.00 | 1.01 |
| LnCRP(mg/L) | 0.090 | 1.29 | 0.96 | 1.74 | 0.092 | 1.45 | 0.94 | 2.25 |
| Model 2: Model 1 + DM | ||||||||
| OPG (pmol/L) | 0.040 | 1.06 | 1.00 | 1.12 | 0.279 | 1.05 | 0.96 | 1.13 |
| Alb (g/dL) | 0.054 | 0.43 | 0.182 | 1.05 | 0.385 | 0.58 | 0.17 | 1.98 |
| cCa (mg/dL) | 0.231 | 1.17 | 0.90 | 1.53 | 0.001 a | 1.65 | 1.25 | 2.17 |
| VC (CaSc) | 0.791 | 1.00 | 1.00 | 1.00 | 2.242 | 1.00 | 1.00 | 1.00 |
| P (mg/dL) | 0.353 | 1.41 | 0.86 | 1.50 | 0.843 | 0.96 | 0.62 | 1.48 |
| iPTH (pg/mL) | 0.154 | 1.002 | 0.99 | 1.00 | 0.008 b | 1.00 | 1.00 | 1.01 |
| Ln CRP (mg/L) | 0.069 | 1.32 | 0.97 | 1.79 | 0.060 | 1.54 | 0.982 | 2.415 |
| DM (yes) | 0.087 | 0.343 | 0.101 | 1.16 | 0.054 | 7.14 | 0.966 | 52.786 |
| Model 3: Model 2 + Age | ||||||||
| OPG (pmol/L) | 0.127 | 1.048 | 0.987 | 1.112 | 0.409 | 1.03 | 0.954 | 1.124 |
| Alb (g/dL) | 0.042 b | 0.411 | 0.174 | 1.53 | 0.155 | 0.420 | 0.127 | 1.389 |
| cCa (mg/dL) | 0.225 | 1.181 | 0.903 | 1.544 | 0.001 a | 1.67 | 1.25 | 2.219 |
| VC (CaSc) | 0.819 | 1.00 | 1.00 | 1.00 | 0.190 | 1.00 | 1.00 | 1.00 |
| P (mg/dL) | 0.252 | 1.18 | 0.89 | 1.56 | 0.891 | 0.970 | 0.624 | 1.508 |
| iPTH (pg/mL) | 0.225 | 1.18 | 0.90 | 1.54 | 0.012 b | 1.01 | 1.00 | 1.009 |
| lnCRP (mg/L) | 0.074 | 1.32 | 0.97 | 1.79 | 0.079 | 1.51 | 0.954 | 2.393 |
| DM (yes) | 0.321 | 0.51 | 0.13 | 1.93 | 0.300 | 3.14 | 0.361 | 27.21 |
| Age (y) | 0.227 | 1.03 | 0.98 | 1.08 | 0.030 b | 1.08 | 1.007 | 1.159 |
Model 1 shows multivariable analysis with calcium metabolism biomarkers. Model 2 shows and adds DM to Model 1 plus DM. Model 3 shows and adds age to Model 1 plus DM and age2. Significance = a p < 0.005, b p < 0.05.
Vascular calcification was not a risk factor in multivariate analysis of either all-cause or CV mortality.
To know the categorical value of each variable in combination with all other variables, we performed Cox regression analysis using the forward conditional method (Table 6). The highest score, according to all-cause mortality, was obtained by OPG (18.77, p < 0.001, followed by sAlb (13.84, p < 0.001), DM (13.67, p < 0.001), Age (10.56, p > 0.006), VC (5.59, p > 0.018), and SBP (5.24, p < 0.022). For CV mortality; OPG had the highest score (11.90, p < 0.001), followed by cCa (11.07, p <0.001), VC (10.38, p < 0.001), DM (8.70, p < 0.003), Age (7.55, p < 0.006), and Alb (5.99, p < 0.014). With this analysis, it was possible to confirm the highest value of OPG as a predictor of death in combination with markers of calcium metabolism, inflammation, DM, and age.
Table 6.
Scores of variables associated with all-cause and cardiovascular mortality in incident peritoneal dialysis patients.
| Variable | All-Cause Mortality | Cardiovascular Mortality | ||
|---|---|---|---|---|
| Score | p-Value | Score | p-Value | |
| OPG (pmol/L) | 18.77 | 0.001 a | 11.90 | 0.001 a |
| sAlb (g/dL) | 13.84 | 0.001 a | 5.99 | 0.014 b |
| cCa (mg/dL) | 2.24 | 0.134 b | 11.07 | 0.001 a |
| VC (CaSc) | 5.59 | 0.018 | 10.38 | 0.001 a |
| P (mg/dL) | 0.03 | 0.865 | 0.08 | 0.775 |
| iPTH (pg/mL) | 0.16 | 0.690 | 0.04 | 0.838 |
| lnCRP (mg/L) | 3.09 | 0.078 | 2.38 | 0.122 |
| DM | 13.67 | 0.001 a | 8.70 | 0.003 a |
| Age (y) | 10.56 | 0.006 b | 7.55 | 0.006 b |
| SBP (mmHg) | 5.24 | 0.022 b | 2.54 | 0.111 |
Cox regression using forward conditional model analysis, a p < 0.005, b p < 0.05. OPG: osteoprotegerin, sAlb: serum albumin, cCa: albumin-corrected calcium, VC: vascular calcification, P: phosphorus, iPTH: intact parathormone, ln CRP: C reactive, protein, SBP: systolic blood pressure.
Figure 2a shows the diagnostic value of OPG for all-cause mortality in a ROC curve, where the area under the curve (AUC) was 0.72, p < 0.001 (95% CI: 0.627–0.821). The Youden index was identified as 14.37 pmol/L as the OPG cut-off value, with a sensitivity of 72.4% and a specificity of 62.5%. Figure 2b shows the diagnostic value of OPG for CV mortality in a ROC curve, where AUC was 0.70, p < 0.011 (95% CI: 0.552–0.845). The Youden index was identified as 13.57 pmol/L as the OPG cut-off value with a sensitivity of 77.3% and a specificity of 64.8%.
Figure 2.
(a) ROC curve of OPG for all-cause mortality (AUC 0.72, CI 95%: 0.627–0.821), cut-off point for OPG = 14.37 pmol/L, p < 0.001; with 72.4% sensitivity and a specificity of 62.5%. (b) ROC curve of OPG for CV mortality (AUC: 0.70; CI 95%: 0.552–0.845), cut-off point for OPG = 13.57 pmol/L, p < 0.011, with a sensitivity of 77.3% and a specificity of 64.8%.
Figure 3a shows the association of OPG concentration with all-cause mortality (Cox analysis), classified as 14.37 pmol/L. It is important to note that the highest OPG concentration (>14.37 pmol/L) had the lowest survival compared to those of the lowest OPG levels (<14.37 pmol/L), with an HR of 0.203 (95% CI: 0.096–0.43, p < 0.001). Figure 3b shows the association of OPG concentration with cardiovascular mortality (Cox analysis), classified as 13.57 pmol/L. It is important to note that the highest OPG concentration (>13.57 pmol/L) had the lowest survival compared to those of the lowest OPG levels (<13.57 pmol/L), HR of 0.198 (95%CI 0.006–0.432, p < 0.006).
Figure 3.
(a) All-cause survival analysis (Cox regression) according to the cut-off point for osteoprotegerin (14.37 pmol/L), p < 0.001. (b) Cardiovascular survival analysis (Cox regression) according to the cut-off point for osteoprotegerin (13.57 pmol/L), p < 0.006.
4. Discussion
Data from this study suggest that high OPG concentrations, a molecule related to mineral metabolism, inflammation, and vascular calcification, have a high predictive value for both all-cause (>14.37 pmol/L) and cardiovascular mortality (>13.57 pmol/L) in incident PD patients. Its predictive value is greater than those of other commonly used biomarkers, such as VC. Low albumin and high cCa levels were risk factors for only all-cause and cardiovascular death.
The novelty of this study resides in the fact that the PD cohort was composed of relatively young patients and that the selection criteria excluded those with higher mortality risk. The adopted inclusion/exclusion criteria allowed us to verify whether OPG, already associated with mortality in older cohorts of dialysis patients, was a mortality predictor in younger ones, a population in which focusing on cardiovascular risk may be of practical relevance.
The cut-off point values of OPG >14.37 pmol/L and >13.57 pmol/L were defined as a higher risk factor for all-cause and CV death using ROC curves. This was one of the main findings of the present study and is not surprising because of its relevance as a risk factor for the development and progression of heart valve calcification in PD patients [21], thoracic and femoral arterial calcification [22], as well as atherosclerosis, all-cause mortality, and cardiovascular dysfunction, which have already been demonstrated in other studies in hemodialysis and 3–5 CKD patients [23,24,25,26]. A recent meta-analysis showed that elevated circulating OPG levels independently predicted an increased risk for CV mortality in patients with CKD [27].
In the in vitro studies, OPG inhibits vascular calcification and protects endothelial cells from apoptosis, and it also promotes neovascularization in vivo as it is a soluble decoy receptor for TRAIL and RANKL [28,29,30,31]. In kidney patients, serum OPG levels increase, which is associated with vascular calcification and cardiovascular disease [11]. This apparent paradox can be understood as a compensatory mechanism to counteract calcification, endothelial damage, and ongoing inflammation. In this way, the positive association between OPG and diabetes, age, systolic blood pressure, calcium, vascular calcifications, as well as the negative correlation with serum albumin, found in the present study, can be explained.
Mechanisms other than mineral metabolism may be involved in increasing OPG, including the expansion of extracellular volume. In healthy humans, high sodium intake significantly elevates OPG in the blood. On the other hand, chronic inflammation through the increase in pro-inflammatory cytokines can also be a stimulus for an increase in circulating OPG [32]. Both conditions, extracellular expansion, and chronic inflammation, are frequent findings in PD patients [33]. Some studies have suggested that high levels of circulating OPG and inflammation have independent and additive values as predictors of death in patients with CKD and end-stage renal disease (ESRD) [34]. The lack of association of OPG with hsCRP in this and other studies does not invalidate the association, since hsCRP is not the only marker of inflammation [26]. As a member of the TNF superfamily, OPG may be involved in several inflammatory pathways. It is also important to mention that the OPG–inflammation relationship is bidirectional, and OPG can regulate the expression of interleukins in response to inflammatory stimuli, while its expression and production are regulated by several cytokines [35].
The ability of pro-inflammatory mediators, such as TNFα, interleukin-1, and platelet-derived growth factor, to enhance OPG expression and production in vascular cells, may explain the association between OPG concentrations and cardiovascular diseases [36].
Another important finding of our results was that OPG was a better predictor of both cardiovascular and all-cause mortality than VC or other biomarkers of calcium metabolism and inflammation. This suggests that OPG is associated with vascular damage and mortality through mechanisms independent of VC.
In keeping with our results, the authors of a published review concluded that circulating OPG levels could be used as an independent biomarker of cardiovascular disease in patients with acute or chronic cardio-metabolic diseases to improve the prognosis [37].
The useful prognostic value of calcification in patients with CKD is doubtful. It has been shown, in previous studies, that the quantification of coronary artery calcifications (CAC) with CT is valid for patients without CKD; however, VC association with cardiovascular death is not significant in CKD patients when adjusted for cardiovascular risk factor markers [38]. Our study confirms the findings that vascular calcifications in multivariate analysis (in combination with biomarkers of calcium metabolism and inflammation) were not associated with the risk of all-cause and cardiovascular death.
Although cardiovascular risk stratification with vascular calcification is used in the clinical setting, it is rather impractical for routine use. On the contrary, OPG, albumin, and cCa level determinations are widely available and can be repeatedly tested during patient follow-up by means of routine laboratory assessments.
Previous studies in PD patients showed that cCa was significantly associated with cardiovascular mortality and the results of our study are in line with these findings [39,40].
Serum albumin (sAlb) was the other circulating marker found to be associated with all-cause death. Previously, in several clinical studies, sAlb has been shown to be associated with CV death. It is speculated that hypoalbuminemia is a surrogate marker of proteinuria or inflammation [41].
The practical implications of the present study in the care of PD patients are that OPG and cCa levels could be an alternative or a complementary evaluation in the assessment of cardiovascular risk in PD patients. VC assessment, by means of a CT scan, could be reserved for specific cases due to its limited availability, high cost (150 USD compared to 13 USD for an OPG test) and its major operator dependency.
This study has some limitations; most importantly, the sample size is possibly considered to be too small. However, selection criteria allowed us to analyze a representative sample of a common population of PD patients in Mexico. Moreover, our study is the first, to date, to include only incident patients on PD and determine the risk of cardiovascular death, taking VC, OPG, and biochemical markers of mineral metabolism into account.
5. Conclusions
The data presented here show that OPG concentrations above 14.37 and 13.57 pmol/L have the highest all-cause and cardiovascular mortality predictive values in incident PD patients. The OPG predictive value overcomes other biomarkers, such as vascular calcification. Its systematic use could help in identifying patients with higher mortality risk, with the aim of providing a more intensive follow-up and adapting treatment. Future research could be conducted in intervention studies.
Acknowledgments
The authors thank Monica Ericsson (Division of Renal Medicine and Baxter Novum, Karolinska Institute) and Santa A. Prado (Hospital de Especialidades, CMN SSXI) for OPG and iPTH measurements. Susan Drier, Massimo Torreggiani and Giorgina Piccoli for reviewing the manuscript.
Author Contributions
(1). Conceptualization, M.Á. and R.P.; methodology, M.d.C.P., R.R., M.d.C.R., C.M. and M.T; tomography studies, validation, R.C., Mexican Nephrology Collaborative Study Group; recruited and maintained vigilance of patients, E.G.H., N.L. (2). Formal analysis, M.Á.; writing—original draft preparation, M.Á.; writing—review and editing, R.P. and M.T. (3). Providing intellectual content of critical importance to the work described, M.Á. and R.P. (4). Final Approval of the version to be published, M.Á. and R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by: Consejo Nacional de Ciencia y Tecnología, Mexico (CONACYT), Grant No. 111941, Sanofi Genzyme Corp, Grant No GZ-2010-10330. The APC was funded by both. Project administration: Foundation IMSS, A.C.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the National Ethics and Research Committees of the Instituto Mexicano del Seguro Social with approval number: 2009-785-087.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
The database used in the current study is not available in a public repository, but they are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Foley R.N., Murray A.M., Li S., Herzog C.A., McBean A.M., Eggers P.W., Collins A.J. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J. Am. Soc. Nephrol. JASN. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 2.London G.M. Arteriosclerosis and arterial calcifications in chronic kidney insufficiency. Nephrol. Ther. 2005;1((Suppl. 4)):S351–S354. [PubMed] [Google Scholar]
- 3.Gorriz J.L., Molina P., Cerveron M.J., Vila R., Bover J., Nieto J., Barril G., Martinez-Castelao A., Fernandez E., Escudero V., et al. Vascular calcification in patients with nondialysis CKD over 3 years. Clin. J. Am. Soc. Nephrol. CJASN. 2015;10:654–666. doi: 10.2215/CJN.07450714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raggi P., Boulay A., Chasan-Taber S., Amin N., Dillon M., Burke S.K., Chertow G.M. Cardiac calcification in adult hemodialysis patients. A link between end-stage renal disease and cardiovascular disease? J. Am. Coll. Cardiol. 2002;39:695–701. doi: 10.1016/S0735-1097(01)01781-8. [DOI] [PubMed] [Google Scholar]
- 5.Okuno S., Ishimura E., Kitatani K., Fujino Y., Kohno K., Maeno Y., Maekawa K., Yamakawa T., Imanishi Y., Inaba M., et al. Presence of abdominal aortic calcification is significantly associated with all-cause and cardiovascular mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 2007;49:417–425. doi: 10.1053/j.ajkd.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Zoccali C., London G. Con: Vascular calcification is a surrogate marker, but not the cause of ongoing vascular disease, and it is not a treatment target in chronic kidney disease. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2015;30:352–357. doi: 10.1093/ndt/gfv021. [DOI] [PubMed] [Google Scholar]
- 7.Raggi P., Chertow G.M., Torres P.U., Csiky B., Naso A., Nossuli K., Moustafa M., Goodman W.G., Lopez N., Downey G., et al. The ADVANCE study: A randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2011;26:1327–1339. doi: 10.1093/ndt/gfq725. [DOI] [PubMed] [Google Scholar]
- 8.Simonet W.S., Lacey D.L., Dunstan C.R., Kelley M., Chang M.S., Luthy R., Nguyen H.Q., Wooden S., Bennett L., Boone T., et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 9.Bernardi S., Voltan R., Rimondi E., Melloni E., Milani D., Cervellati C., Gemmati D., Celeghini C., Secchiero P., Zauli G., et al. TRAIL, OPG, and TWEAK in kidney disease: Biomarkers or therapeutic targets? Clin. Sci. 2019;133:1145–1166. doi: 10.1042/CS20181116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurz K., Herold M., Russe E., Klotz W., Weiss G., Fuchs D. Effects of Antitumor Necrosis Factor Therapy on Osteoprotegerin, Neopterin, and sRANKL Concentrations in Patients with Rheumatoid Arthritis. Dis. Markers. 2015;2015:276969. doi: 10.1155/2015/276969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Campenhout A., Golledge J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis. 2009;204:321–329. doi: 10.1016/j.atherosclerosis.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janda K., Krzanowski M., Chowaniec E., Kusnierz-Cabala B., Dumnicka P., Krasniak A., Podolec P., Sulowicz W. Osteoprotegerin as a marker of cardiovascular risk in patients on peritoneal dialysis. Pol. Arch. Med. Wewn. 2013;123:149–155. doi: 10.20452/pamw.1678. [DOI] [PubMed] [Google Scholar]
- 13.Winther S., Christensen J.H., Flyvbjerg A., Schmidt E.B., Jorgensen K.A., Skou-Jorgensen H., Svensson M. Osteoprotegerin and mortality in hemodialysis patients with cardiovascular disease. Clin. Nephrol. 2013;80:161–167. doi: 10.5414/CN107803. [DOI] [PubMed] [Google Scholar]
- 14.Avignon A., Sultan A., Piot C., Mariano-Goulart D., Thuan Dit Dieudonne J.F., Cristol J.P., Dupuy A.M. Osteoprotegerin: A novel independent marker for silent myocardial ischemia in asymptomatic diabetic patients. Diabetes Care. 2007;30:2934–2939. doi: 10.2337/dc07-0992. [DOI] [PubMed] [Google Scholar]
- 15.Pedersen E.R., Ueland T., Seifert R., Aukrust P., Schartum-Hansen H., Ebbing M., Bleie O., Igland J., Svingen G., Nordrehaug J.E., et al. Serum osteoprotegerin levels and long-term prognosis in patients with stable angina pectoris. Atherosclerosis. 2010;212:644–649. doi: 10.1016/j.atherosclerosis.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 16.Kastritis E., Gavriatopoulou M., Dimopoulos M.A., Eleutherakis-Papaiakovou E., Kanellias N., Roussou M., Pamboucas C., Toumanidis S.T., Terpos E. Osteoprotegerin is a significant prognostic factor for overall survival in patients with primary systemic amyloidosis independent of the Mayo staging. Blood Cancer J. 2015;5:e319. doi: 10.1038/bcj.2015.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vik A., Mathiesen E.B., Brox J., Wilsgaard T., Njolstad I., Jorgensen L., Hansen J.B. Serum osteoprotegerin is a predictor for incident cardiovascular disease and mortality in a general population: The Tromso Study. J. Thromb. Haemost. JTH. 2011;9:638–644. doi: 10.1111/j.1538-7836.2011.04222.x. [DOI] [PubMed] [Google Scholar]
- 18.Lo W.K., Bargman J.M., Burkart J., Krediet R.T., Pollock C., Kawanishi H., Blake P.G., ISPD Adequacy of Peritoneal Dialysis Working Group Guideline on targets for solute and fluid removal in adult patients on chronic peritoneal dialysis. Perit. Dial. Int. 2006;26:520–522. [PubMed] [Google Scholar]
- 19.Agatston A.S., Janowitz W.R., Hildner F.J., Zusmer N.R., Viamonte M., Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-T. [DOI] [PubMed] [Google Scholar]
- 20.Scialla J.J., Kao W.H., Crainiceanu C., Sozio S.M., Oberai P.C., Shafi T., Coresh J., Powe N.R., Plantinga L.C., Jaar B.G., et al. Biomarkers of vascular calcification and mortality in patients with ESRD. Clin. J. Am. Soc. Nephrol. 2014;9:745–755. doi: 10.2215/CJN.05450513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Avila-Diaz M., Mora-Villalpando C., Prado-Uribe Mdel C., Orihuela-Rodriguez O., Villegas-Antelo E., Gomez-Noriega A.M., Villanueva-Noches D., Hinojosa-Heredia H., Serrato-Avila J., Ilabaca B., et al. De novo development of heart valve calcification in incident peritoneal dialysis patients. Arch. Med. Res. 2013;44:638–644. doi: 10.1016/j.arcmed.2013.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Avila M., Mora C., Prado M.D.C., Zavala M., Paniagua R., Mexican Collaborative G. Osteoprotegerin Is the Strongest Predictor for Progression of Arterial Calcification in Peritoneal Dialysis Patients. Am. J. Nephrol. 2017;46:39–46. doi: 10.1159/000477380. [DOI] [PubMed] [Google Scholar]
- 23.Collado S., Coll E., Nicolau C., Azqueta M., Pons M., Cruzado J.M., de la Torre B., Deulofeu R., Mojal S., Pascual J., et al. Serum osteoprotegerin in prevalent hemodialysis patients: Associations with mortality, atherosclerosis and cardiac function. BMC Nephrol. 2017;18:290. doi: 10.1186/s12882-017-0701-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuzniewski M., Fedak D., Dumnicka P., Stepien E., Kusnierz-Cabala B., Cwynar M., Sulowicz W. Osteoprotegerin and osteoprotegerin/TRAIL ratio are associated with cardiovascular dysfunction and mortality among patients with renal failure. Adv. Med. Sci. 2016;61:269–275. doi: 10.1016/j.advms.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Sigrist M.K., Levin A., Er L., McIntyre C.W. Elevated osteoprotegerin is associated with all-cause mortality in CKD stage 4 and 5 patients in addition to vascular calcification. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2009;24:3157–3162. doi: 10.1093/ndt/gfp253. [DOI] [PubMed] [Google Scholar]
- 26.Marques G.L., Hayashi S., Bjallmark A., Larsson M., Riella M., Olandoski M., Lindholm B., Nascimento M.M. Osteoprotegerin is a marker of cardiovascular mortality in patients with chronic kidney disease stages 3–5. Sci. Rep. 2021;11:2473. doi: 10.1038/s41598-021-82072-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Q.X., Li J.B., Huang N., Huang X.W., Li Y.L., Huang F.X. Elevated Osteoprotegerin Concentration Predicts Increased Risk of Cardiovascular Mortality in Patients with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Kidney Blood Press. Res. 2020;45:565–575. doi: 10.1159/000508978. [DOI] [PubMed] [Google Scholar]
- 28.Emery J.G., McDonnell P., Burke M.B., Deen K.C., Lyn S., Silverman C., Dul E., Appelbaum E.R., Eichman C., DiPrinzio R., et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 29.Sato K., Niessner A., Kopecky S.L., Frye R.L., Goronzy J.J., Weyand C.M. TRAIL-expressing T cells induce apoptosis of vascular smooth muscle cells in the atherosclerotic plaque. J. Exp. Med. 2006;203:239–250. doi: 10.1084/jem.20051062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGonigle J.S., Giachelli C.M., Scatena M. Osteoprotegerin and RANKL differentially regulate angiogenesis and endothelial cell function. Angiogenesis. 2009;12:35–46. doi: 10.1007/s10456-008-9127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rochette L., Meloux A., Rigal E., Zeller M., Cottin Y., Vergely C. The Role of Osteoprotegerin and Its Ligands in Vascular Function. Int. J. Mol. Sci. 2019;20:705. doi: 10.3390/ijms20030705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu F.Q., Liu S.Q., Zhang Y., Wang Y., Chu C., Wang D., Pan S., Wang J.K., Yu Q., Mu J.J. Effects of Salt Loading on Plasma Osteoprotegerin Levels and Protective Role of Potassium Supplement in Normotensive Subjects. Circ. J. 2016;81:77–81. doi: 10.1253/circj.CJ-16-0756. [DOI] [PubMed] [Google Scholar]
- 33.Avila-Diaz M., Ventura M.D., Valle D., Vicente-Martinez M., Garcia-Gonzalez Z., Cisneros A., Furlong M.D., Gomez A.M., Prado-Uribe M.D., Amato D., et al. Inflammation and extracellular volume expansion are related to sodium and water removal in patients on peritoneal dialysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2006;26:574–580. doi: 10.1177/089686080602600510. [DOI] [PubMed] [Google Scholar]
- 34.Matsubara K., Stenvinkel P., Qureshi A.R., Carrero J.J., Axelsson J., Heimburger O., Barany P., Alvestrand A., Lindholm B., Suliman M.E. Inflammation modifies the association of osteoprotegerin with mortality in chronic kidney disease. J. Nephrol. 2009;22:774–782. [PubMed] [Google Scholar]
- 35.Baud’huin M., Duplomb L., Teletchea S., Lamoureux F., Ruiz-Velasco C., Maillasson M., Redini F., Heymann M.F., Heymann D. Osteoprotegerin: Multiple partners for multiple functions. Cytokine Growth Factor Rev. 2013;24:401–409. doi: 10.1016/j.cytogfr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Collin-Osdoby P. Regulation of vascular calcification by osteoclast regulatory factors RANKL and osteoprotegerin. Circ. Res. 2004;95:1046–1057. doi: 10.1161/01.RES.0000149165.99974.12. [DOI] [PubMed] [Google Scholar]
- 37.Rochette L., Meloux A., Rigal E., Zeller M., Cottin Y., Vergely C. The role of osteoprotegerin in the crosstalk between vessels and bone: Its potential utility as a marker of cardiometabolic diseases. Pharmacol. Ther. 2018;182:115–132. doi: 10.1016/j.pharmthera.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 38.Gregg L.P., Adams-Huet B., Li X., Colbert G., Jain N., de Lemos J.A., Hedayati S.S. Effect Modification of Chronic Kidney Disease on the Association of Circulating and Imaging Cardiac Biomarkers With Outcomes. J. Am. Heart Assoc. 2017;6:e005235. doi: 10.1161/JAHA.116.005235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Noordzij M., Korevaar J.C., Bos W.J., Boeschoten E.W., Dekker F.W., Bossuyt P.M., Krediet R.T. Mineral metabolism and cardiovascular morbidity and mortality risk: Peritoneal dialysis patients compared with haemodialysis patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2006;21:2513–2520. doi: 10.1093/ndt/gfl257. [DOI] [PubMed] [Google Scholar]
- 40.Paniagua R., Ventura M.D., Avila-Diaz M., Hinojosa-Heredia H., Mendez-Duran A., Cisneros A., Gomez A.M., Cueto-Manzano A., Trinidad P., Obrador G.T., et al. Reaching targets for mineral metabolism clinical practice guidelines and its impact on outcomes among Mexican chronic dialysis patients. Arch. Med. Res. 2013;44:229–234. doi: 10.1016/j.arcmed.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 41.Don B.R., Kaysen G. Serum albumin: Relationship to inflammation and nutrition. Semin. Dial. 2004;17:432–437. doi: 10.1111/j.0894-0959.2004.17603.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The database used in the current study is not available in a public repository, but they are available from the corresponding author on reasonable request.