Highlights
-
•
Repetitive transcranial magnetic stimulation (rTMS) is effective in treating depression; however, the effect on physical activity, sleep and recovery is unclear.
-
•
PHQ-9, GAD-7 and ReQol scores significantly improved with large effect sizes. Fitbit activity and sleep results werenon-significant.
-
•
Improvements on the ReQoL and aspects of sleep and activity indicate the positive impact of rTMS on functioning and quality of life.
Keywords: Depression, Fitbit, Exercise, Activity, Sleep, Recovery, rTMS
Abstract
Background
Repetitive transcranial magnetic stimulation (rTMS) is effective in treating depression; however, the effect on physical activity, sleep and recovery is unclear. This study investigated rTMS effect on physical activity and sleep through providing patients with a Fitbit and software apps; and reports the impact of rTMS on depression, anxiety and mental health recovery.
Methods
Study design was a pre and post data collection without a control, with twenty-four participants with treatment-resistant depression (TRD). Measures used were Fitbit activity and sleep data, and patient-rated Recovering Quality of Life (ReQoL-20), Patient Health Questionnaire (PHQ-9) and Generalised Anxiety Disorder (GAD-7).
Results
Response and remission rates were, respectively: 34.8% and 39% for PHQ-9; 34.8% and 47.8% for GAD-7. ReQoL-20 response and reliable improvement were 29.4% and 53%. PHQ-9, GAD-7 and ReQol-20 scores significantly improved, with large effect sizes. Analysis of Fitbit activity and sleep data yielded non-significant results. The Fitbit data machine learning model classified two levels of depression to 82% accuracy.
Limitations
rTMS treatment was open-label and adjunct to existing antidepressant medication. No control group. Female patients were overrepresented.
Conclusions
Improvements on the ReQoL-20 and aspects of sleep and activity indicate the positive impact of rTMS on the individual's real world functioning and quality of life. A wearable activity tracker can provide feedback to patients and clinicians on sleep, physical activity and depression levels. Further research could be undertaken through a sufficiently powered RCT comparing rTMS versus rTMS with use of a Fitbit, its software applications, and sleep and physical activity advice.
1. Introduction
Mental health and existence of mental illness are determined by a complex and individualised array of genetic, personal, social and environmental factors. Symptoms of depression, physical health, psychological wellbeing, sleep quality, physical activity levels, and mental health recovery are interlinked; each factor can have an effect on one another (Kaseva et al., 2019; WHO, 2020; Driver and Taylor, 2000; Faulkner and Taylor, 2005; Adam and Oswald, 1983). For example, the positive impact of engaging in physical activity on sleep quality is likely to be mediated by psychological functioning, as activity is associated with depression and anxiety symptom reduction as well as increased self-esteem and wellbeing (Biddle and Mutrie, 2007, Dunn et al., 2005; Faulkner and Taylor, 2005; Kaseva et al., 2019; ). Furthermore, treatments for depression can impact on the above factors; for example, antidepressant medication can change the levels of brain chemicals involved in generating normal effective sleep resulting in lower quality and less stable sleep (Steiger and Pawlowski, 2019). A relatively new treatment for depression is repetitive transcranial magnetic stimulation (rTMS). rTMS is a type of clinical neuromodulation: a non-invasive and non-convulsive technique in which an electromagnetic coil is placed against the scalp to deliver a short, powerful magnetic field pulse to induce electric currents in the cerebral cortex (Hardy et al., 2016). rTMS treatment for depression comprises single daily sessions lasting about 10 to 30 min, over a period typically around 5 weeks (Hardy et al., 2016). rTMS results in changes in brain activity, metabolism and connectivity that relate to emotional processing (Kito et al., 2008); however, the precise mechanism of treatment action is unknown (Hardy et al., 2016).
The Food and Drug Administration (FDA) approved TMS for treatment of depression in the USA in 2008 (Janicak and Dokucu, 2015). In the UK, the National Institute for Health and Care Excellence (NICE) recommended TMS for the treatment of depression, including TRD, in which it was specified that TMS does not require anaesthesia and can be performed on an outpatient basis (NICE, 2015). A systematic review of 45 RCTs found rTMS to be robustly effective versus sham rTMS on depression symptoms, response and remission (Health Quality Ontario, 2016).
In research trials, response and remission rates of rTMS for depression range between 25% - 50% and 12% - 35%, respectively (Allan et al., 2011; Berlim et al., 2014; Gross et al., 2007; Herrmann and Ebmeier, 2006; Kozel and George, 2002; Lam et al., 2008; Schutter, 2010; Slotema et al., 2010; Xie et al., 2013). Peer-reviewed published studies reported remission and therapeutic response in clinical service settings ranging between 29% - 51% and 6% - 37%, respectively (Carpenter et al., 2012; Connolly et al., 2012; Galletly et al., 2015; Taylor et al., 2017). In the rTMS service that was the recruitment centre for the present study, the reported response and remission rates, respectively, were 10% and 28.6% for depression (effect size = 0.27); and 24.6% and 28.8% for generalised anxiety disorder (effect size = 0.43) (GAD) (Griffiths et al. 2019a).
Symptoms of anxiety are common in people with a diagnosis of depression; frequent or constant anxiety symptoms have been reported by 55.6% of depressive disorder patients (Karpov et al., 2016; Young et al., 2013). Patients treated for depression with rTMS have recorded a reduction in anxiety symptoms (Diefenbach et al., 2013; LaSalle-Ricci et al., 2014; Caulfield et al., 2016; White and Tavakoli, 2015; Griffiths et al. 2019b). However, rTMS is not currently a FDA or NICE recommended treatment for GAD.
Physical activity can be an indicator of mental health, for example low levels of physical activity (bodily movement requiring energy expenditure caused by skeletal muscles) or sedentary behaviour are correlated with depression symptom severity (Schuch et al., 2017). Doing physical activity is correlated with lower levels of depressive symptoms in those who have a diagnosis of depression (Teychenne et al., 2008). In the general population, around 26% of adult activity levels can be labelled as sedentary (less than 5000 steps per day), 27% as low level activity (5000 to 7500 steps per day), 17% as somewhat active (7500–9999 steps per day), 8% as active (10,000–12,499 steps per day), and 7% as highly physically active (12,500 steps and over per day) (Tudor-Locke et al., 2009). People diagnosed with depression tend to have lower activity levels and more sedentary behaviour than the general population (Schuch et al., 2017).
Sleep quality can also be linked to mental and physical wellbeing; more than nine hours or less than seven hours night-time sleep has negative consequences on an individual's health (Watson et al., 2015). Around 35% of the general adult population get less than 7 h sleep (CDC, 2014), and this is associated with increased risk of adult depression (Zhai et al., 2015). Conversely, healthy levels of night-time sleep may reduce risk of stress and mental illness through, for example, healthy secretion of cortisol (Kumari et al., 2009, Meerlo et al., 2008).
Disturbed night-time sleep (insomnia symptoms, e.g. difficulty in initiating or maintaining sleep) is highly prevalent in depression (up to 40%), and compared with the general population, more people with depression report that sleep is less refreshing (Gupta et al., 2009; Nutt et al., 2008). A reason for this may be because symptoms of depression include changes to individuals’ sleep cycle patterns, which can be detrimental to the restorative value of sleep (WHO, 2020; Steiger and Pawlowski, 2019). Poor quality sleep can be distressing, is often unresolved by sleep treatment prescribed, and can lead to reduced quality of life (Nutt et al., 2008).
With the paramount role of physical activity and sleep in depression evidenced, there is a need to employ methods to assess the impact of rTMS on physical activity and sleep. One potential method is to use a wearable activity tracker, such as a Fitbit. A Fitbit is a wrist worn physical activity tracker measuring activity and sleep to an effective level of accuracy (Beattie et al., 2017). Fitbits are used by millions of people across the world to track and improve their physical activity, sleep, health and wellbeing; it is European Conformity (CE) marked as a wireless activity tracker.
Fitbit devices use a microelectronic triaxial accelerometer to capture body motion data, analysed using algorithms to identify patterns of motion to report daily steps, accuracy comparisons within ±10% error in free-living settings (real life daily activity); with a tendency to slightly overestimate steps in free-living settings (Feehan et al., 2018). Sleep latency, sleep duration, and sleep efficiency can be measured to a reasonable degree of accuracy using wrist-worn activity tracking devices (Haghayegh et al., 2019). Using a wearable physical activity tracker is useful to increase physical activity, and related self-awareness, as well as motivation and goal-setting (Chum et al., 2018). People with a diagnosis of serious mental illness have found using a Fitbit to be acceptable, motivating, and useful for enabling goal-setting and healthier lifestyles (Naslund et al., 2015).
It is important to measure the holistic impact of treatment for depression, which extends beyond the symptoms of depression. The recovering quality of life (ReQoL-20) measure is a patient-reported outcome measure (PROM) for individuals aged 16 and over experiencing mental health difficulties, co-produced with patients and clinicians (Keetharuth et al., 2020). It asks people how they feel about themselves, for example, their functioning, trust of others, sense of control, emotions, hopefulness, ability to cope, confidence, purpose in life, and motivation. It has not been previously used to assess the impact of rTMS. The ReQoL-20 is part of the International Consortium for Health Outcome Measurement (ICHOM) standard set of measures (ICHOM, 2021).
This project sought to determine whether treatment resistant depression (TRD) patients will wear a Fitbit and keep it charged. This project investigated the impact of rTMS on depression (PHQ-9), anxiety (GAD-9), mental health recovery related quality of life (ReQoL-20), as well as physical measures such as sleep and activity. Previously undertaken analysis of rTMS impact in the same service on depression (PHQ-9) and anxiety (GAD-9) were compared to results to when patients were given a Fitbit in addition to routine intervention. The ReQoL-20 is a relatively new measure, thus the current research aimed to contextualise its usage by provision of correlations with depression (PHQ-9) and anxiety (GAD-9) in the study population. Using machine learning techniques we sought to understand if depression severity could be predicted using Fitbit data.
2. Methods
2.1. Design
Pre and post intervention data collection, with no control group.
2.2. Recruitment and setting
The sample was recruited purposefully from those referred to an rTMS outpatient service within an NHS trust. Participants undergoing rTMS for depression were recruited between July 2019 and December 2020.
2.3. rTMS treatment
The site of stimulation was determined using EEG cap and treatment at F3 (Tsuzuki et al., 2016). Patients received FDA (FDA, 2011) depression protocol high frequency stimulation to the left dorsolateral prefrontal cortex. Participants received daily treatment for an average period of five weeks.
2.4. Fitbit provision
Participants were provided with a Fitbit and received verbal and written instructions on how to use the Fitbit and access Fitbit software applications. Support was provided to enable participants to wear and use the Fitbit and its apps.
2.5. Inclusion/exclusion criteria
Inclusion criteria: diagnosed with depression; 18 or over; and ability to read English. Exclusion criteria: have a heart condition that a doctor determined negates participation; taking any photosensitive medicine; have epilepsy; bruise easily that prevents wearing of a wrist device; carpal tunnel syndrome that prevents wearing of a wrist device; and lack of capacity to consent.
2.6. Procedure
Participants were required to download the Fitbit app to their smartphone, register with Fitbit, wear the device continually for the period of rTMS treatment (apart from when undertaking the rTMS) and charge it when required. At the outpatient visits participants were reminded by clinical staff to wear the Fitbit and to charge it. Measures of anxiety, depression and recovery were collected at baseline and at the end of treatment point.
2.7. Ethical approval
Ethical approval was gained from United Kingdom's (UK) Health Research Authority (HRA), Research Ethics Committee (REC) reference: 19/NW/0272. All participants provided informed consent.
2.8. Measures
PHQ-9 is a self-report measure of depression; it has good sensitivity and specificity for major depression as well as good internal consistency (Kroenke et al., 2001). Remission is defined as a score of 9 or less, reliable improvement is a drop in 6 points, and recovery is defined as the simultaneous achievement of both reliable improvement and remission (Richards and Borglin, 2011).
GAD-7 is a self-report measure of anxiety; it has good sensitivity and specificity for generalised anxiety disorder and good internal consistency (Kroenke et al., 2007; Spitzer et al., 2006). Remission is defined as a score of 7 or less, reliable improvement is defined as a reduction of 5 points, and clinical recovery is defined as the simultaneous achievement of both remission and reliable improvement (Kroenke et al., 2007; Spitzer et al., 2006).
ReQol-20 is a self-reported quality of life (measure of the impact of mental health problems on peoples’ lives) for people with mental health conditions. Items cover the following areas of quality of life: meaningful activity; belonging and relationships; choice; control and autonomy; hope; self-perception; well-being, and physical health. Test and retest reliability is acceptable, it has robust structure properties and good internal construct validity (Keetharuth et al., 2018, 2020). An increase in 10 points or more denotes a reliable improvement. Clinical range of mental illness is defined as a score between 0 and 49; a score above 50 is considered non-clinical (ReQoL, 2021). ReQoL-20 was specifically designed to measure mental health service users’ perspectives of recovery and quality of life (Keetharuth et al., 2020).
2.9. Fitbit data parameters
Step data was ranked within basal activity (<2500 steps), limited activity (2500–4999 steps), low activity (5000–7499 steps), somewhat active (7500–9999 steps), active (10,000–12,499 steps), and very active (>12,500 steps) categories (Tudor-Locke et al., 2009). Active minutes were ranked according to NHS guidelines (NHS, 2019) as either healthy (22 min moderate or 10 min intense activity per day) or unhealthy. Sleep duration was categorised as healthy (7 to 9 h sleep), fairly-healthy (6–7 or 9–10) or unhealthy (<6 or >10); and wake after sleep onset (WASO) was categorised as healthy (<21 min), fairly-healthy (21–41) or unhealthy (>41) (Watson et al., 2015).
2.10. Statistical analysis
Analysis of change from baseline to post first course treatment scores and correlational analysis were carried out using appropriate statistical tests.
As continuous variables were not normally distributed, Wilcoxon signed-rank tests (Z) were used to compare baseline with post-treatment measures, together with the calculated effect sizes. Using non-parametric analysis (Pearson's chi-squared test and Mann-Whitney U test), the differences in demographic variables and between responders and non-responders on a number of variables were explored. All tests were 1-sided, at 5% level of statistical significance. Spearman's rho was used to calculate correlations. Data were analysed using the statistical software package SPSS.
2.11. Machine learning analysis
Machine learning analysis was carried out to explore the feasibility of classifying the level of depression severity using Fitbit data. Twelve features were collected from Fitbits which include six physical activity and six sleep features as follows:
Activity features: steps, minutes sedentary, minutes lightly active, minutes moderately active, minutes very active, activity calories.
Sleep features: minutes asleep, minutes awake, number of awakenings, and minutes of REM, light and deep sleep.
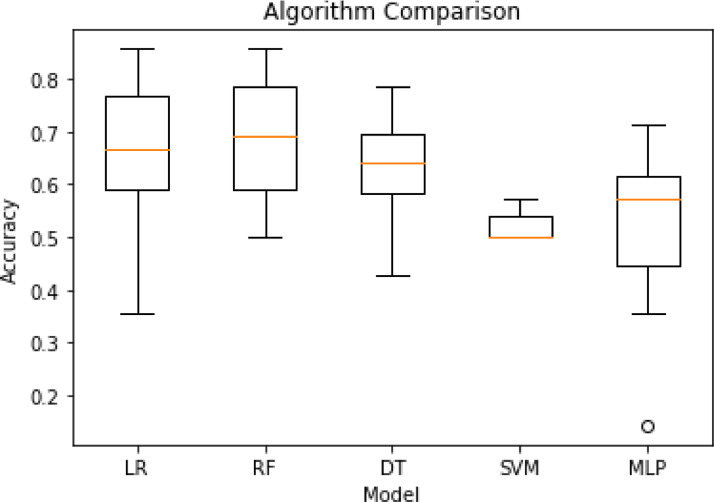
In addition, statistical features were extracted for our machine learning analysis. These were the difference and the second order of difference between consecutive days for all Fitbit features. In total, 36 features were used. The PHQ-9 scores collected were transformed into a binary variable with labels of ‘low to moderate’ (PHQ-9 < 20) and ‘high levels of depression’ (PHQ-9 ≥ 20). Due to missing data (missing Fitbit sensor data and/or PHQ-9), only 17 participants have been included in the machine learning analysis where a total of 160 days of data was used. An array of models including support vector machines (SVM), multi layered perceptron (MLP), logistic regression (LR) and random forest (RF) were tested in pilot training. Random forest performed the best in the pilot and hence was used for subsequent analysis (see Fig. 1). Scikit-learn (Pedregosa et al., 2011) implementation of randomised grid search was used to select the hyper-parameters of our random forest model. Ten-fold cross validation was performed on 90% of the data leaving 10% for unseen testing, stratified k-fold validation was used to ensure balanced classes.
Fig. 1.
Performance of different algorithms in pilot training.
3. Results
3.1. Participant characteristics
The sample included twenty-four TRD patients who were treated with rTMS. Cross tabulation indicated that female patients were overrepresented, χ2 (df = 1, n = 24) =6.31, p = 0.012. There were no differences in any of the measures between gender and age. All patients were diagnosed with TRD, with 11 patients diagnosed with comorbid GAD or other disorders (Table 1).
Table 1.
Baseline characteristics of participants (n = 24).
| Characteristic | |
|---|---|
| Age, Mean ± SD (Min-Max) | 46.83 ± 14.02 (21 - 69) |
| Sex, n (%) | |
| Male | 5 (21%) |
| Female | 19 (79%) |
| Diagnosis | |
| TRD | 13 (54.2%) |
| TRD and GAD | 5 (20.8%) |
| Other* | 6 (25%) |
*Other diagnosis includes PTSD, EUPD and bipolar disorder.
3.2. Treatment outcome
Baseline depression and anxiety scores were in the moderate to severe range, and 86.4% were in the clinical range for mental illness on the ReQol-20 (see Table 2). There was a statistically significant improvement on all measures following the rTMS treatment, with medium to large effect sizes.
Table 2.
Mean (SD) of pre-post treatment scores and associated Wilcoxon signed-rank test results.
| Rating scale | N | Mean ± SD [range] | Z | p | r |
|---|---|---|---|---|---|
| PHQ-9 | |||||
| Pre | 24 | 16.92 ± 5.48 [6 - 27] | −3.80 | < 0.001 * | 0.73 |
| Post | 23 | 10.75 ± 6.45 [0 - 25] | |||
| GAD-7 | |||||
| Pre | 24 | 14.67 ± 4.80 [5 - 21] | −3.34 | < 0.001 * | 0.84 |
| Post | 23 | 9.04 ± 4.68 [0 - 18] | |||
| ReQol-20 | |||||
| Pre | 22 | 29.32 ± 13.26 [10 - 61] | −1.99 | 0.046 ** | 0.46 |
| Post | 16 | 39.88 ± 18.56 [2 - 69] |
*Significant at <0.001.
**Significant at 0.05.
3.3. Measures of assessment
Statistically significance correlations were observed between mental health assessments at baseline and post intervention. Baseline ReQol-20 demonstrated a statistically significant positive correlation (p < 0.01) between baseline PHQ-9 and GAD-7 (rs = −0.55 and rs = 0.73 respectively). Post-treatment ReQol-20 correlations were stronger with Spearman's rho ranging from rs =−0.79 and 0.89 respectively. A statistically significant correlation between post intervention PHQ-9 and GAD-7 was observed (rs = 0.64) Table 3. illustrates correlations of change over time for each of the mental health assessments.
Table 3.
Correlations of improvement (change over time) from baseline to post intervention between each of the mental health measures.
| ReQol | GAD-7 | PHQ-9 | |||
|---|---|---|---|---|---|
| ReQol | ___ | ||||
| GAD-7 | .615* | ___ | |||
| PHQ-9 | .778* | .554* | ___ |
*Correlation is significant at the 0.01 level (1-tailed).
The effect sizes observed from Wilcoxon signed-rank significance tests on pre-post mental health assessments exceed those previously reported in the same the rTMS service (Griffiths et al., 2019a); see Table 2.
3.4. Reliable improvement, remission and recovery rates
PHQ-9 data analysis revealed 43.5% of participants made a reliable improvement, 39% achieved remission and 26.1% achieved a full clinical recovery. GAD-7 data analysis revealed 56.5% of participants made a reliable improvement, 47.8% achieved remission and 30.4% made a full clinical recovery. At baseline, 86.4% of participants fell within the clinical range ReQol-20 score, following the intervention this reduced to 62.5%; with 53% obtaining reliable improvement. Relatively few participants had deterioration in their mental health; see Table 4.
Table 4.
Percentage of participants that demonstrated mental health deterioration over time.
| Measure | Limit | Percentage |
|---|---|---|
| ReQol | Within 10 points | 25.0% |
| Exceeding 10 points | 6.3% | |
| GAD-7 | Within 5 points | 8.7% |
| Within 8 points | 4.3% | |
| PHQ-9 | Within 5 points | 8.7% |
3.5. Categorical response rates
Further analysis of change from baseline to post-intervention was carried out. Categorical response was defined as a 50% or greater drop as measured between the baseline and post-intervention assessments; partial response was defined as an improvement between 25% - 49% Table 5. illustrates response rates between baseline and post-intervention for PHQ-9, GAD-7, and ReQol-20.
Table 5.
Response rates following intervention for mental health assessments.
| Measure | No response | Partial response | Response |
|---|---|---|---|
| GAD-7 | 30.4% | 34.8% | 34.8% |
| PHQ-9 | 43.5% | 21.7% | 34.8% |
| ReQol-20 | 52.9% | 17.6% | 29.4% |
3.6. Sleep duration and WASO
While sleep duration and WASO measured by the Fitbit improved during the study, this was not statistically significant. For example, whilst 95.8% of participants had unhealthy WASO at baseline, this was reduced to 82.6% following the intervention. Similarly, 7.8% of participants moved from an unhealthy sleep range (< 6 or > 10 h sleep) at baseline to fairly-healthy sleep range (6 −7 or 9–10 h) post-intervention. Only one participant was sleeping 9–10 h at baseline and no-one at follow-up. Moreover, when participants with only a TRD diagnosis were analysed, it was found that following the intervention 11 of the 13 had reduced WASO, a statistically significant reduction (Mdn = −8.89 min) from baseline (Mdn = 70.29 min) to post-intervention (Mdn = 54.83 min), z = −2.900, p = 0.004 Table 6. illustrates pre and post differences of activity and sleep as measured by the Fitbit.
Table 6.
Participant Fitbit activity and sleep data according to defined categories.
| Measure | Category | Pre | Post |
|---|---|---|---|
| Steps | Basal activity (<2500 steps) | 4.20% | 4.20% |
| Limited activity (2500–4999 steps) | 29.20% | 29.20% | |
| Low activity (5000–7499 steps) | 37.50% | 25% | |
| Somewhat active (7500–9999 steps) | 16.70% | 25% | |
| Active (10,000–12,499 steps) | 12.50% | 12.50% | |
| Very active (>12,500 steps) | 0% | 4.20% | |
| NHS active minutes guideline | Unhealthy | 54.17% | 58.33% |
| Healthy | 45.83% | 41.67% | |
| Hours’ sleep | Unhealthy | 20.83% | 13.04% |
| Fairly healthy | 25% | 26.09% | |
| Healthy | 54.17% | 60.87% | |
| WASO | Unhealthy | 95.80% | 82.60% |
| Fairly healthy | 4.20% | 17.40% | |
| Healthy | 0.00% | 0.00% | |
3.7. Physical activity
Low physical activity levels were observed in patients, both pre and post-intervention, there was no significant change.
3.8. Classifying depression severity
To understand the importance of the different feature sets in the classification task, we analysed the activity and sleep feature sets individually before performing an analysis on the combined features sets (which include both activity and sleep, as well as statistical features). We used the following definitions for these performance metrics, where tp = true positive, fp = false positive, and fn = false negative:
| (1) |
| (2) |
| (3) |
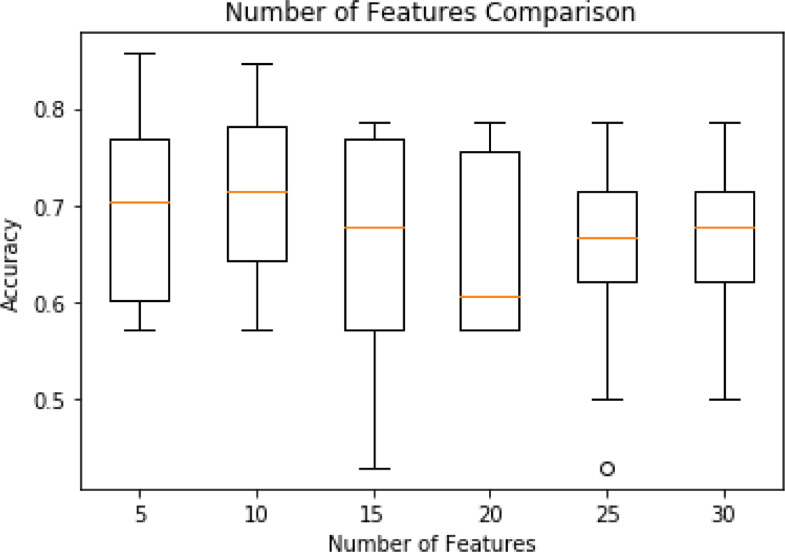
In addition, we performed univariate feature selection to examine if the model performance could be improved. Ten-fold cross validation was used on a tuned random forest model to find the optimal number of features. The top 5, 10, 15, 20, 25 and 30 features were tested, these results can be found in Fig. 2, showing that the model with 10 selected features achieved a reliably high performance.
Fig. 2.
Model (random forest) performance based on the number of selected features.
The results of our machine learning analysis are summarised in Table 7. Results on activity, sleep and combined feature sets provided some insight into the importance of each feature set with clear differences in performance. Specifically, our model is able to classify two levels of depression severity using the activity, sleep and combined features sets at 55%, 69% and 75% accuracy respectively. A model that utilises the 10 selected features outperformed all other models, achieving 82% accuracy.
Table 7.
Classification results of random forest (k-fold validation).
| Feature set | Precision | Recall | F1 | Accuracy |
|---|---|---|---|---|
| Activity features | 0.55 | 0.55 | 0.53 | 0.55 |
| Sleep features | 0.69 | 0.69 | 0.69 | 0.69 |
| Combined features (activity + sleep + statistical features) | 0.75 | 0.75 | 0.75 | 0.75 |
| 10 selected features | 0.82 | 0.81 | 0.81 | 0.81 |
In order to examine if our model could generalise to unseen patient, we assessed model performance with 10 selected features, using the leave-one-participation-out validation approach. For this analysis, only 9 participants had enough data points (minimum of 4 and maximum of 32) to be included in this model. The model achieved 76% accuracy which is comparable to the k-fold cross validation results (Table 8).
Table 8.
Classification results of random forest (leave-one-participant-out validation).
| Feature set | Precision | Recall | F1 | Accuracy |
|---|---|---|---|---|
| Leave-one-out validation on 10 selected features | 0.64 | 0.76 | 0.68 | 0.76 |
3.9. Feature importance analysis
Understanding the feature importance is valuable as it can provide useful insights into future work to improve classification performance, reduce model complexity, as well as to improve training and running speed. In addition, feature importance could provide some real-world insights into what seems to have the most impact on depression severity. The feature importance was estimated using 10-fold cross validation with scikit learns implementation of Gini importance using a random forest classifier (Pedregosa et al., 2011).
We ranked the importance of our 10 selected features based on the ‘importance score’ (see Table 9). We tested whether there was a statistical difference between high and low rated PHQ-9 levels for each feature, using Shapiro-Wilk test. Results of this statistical analysis revealed significant differences in six of these features. The majority of features selected were sleep related with the most important being number of awakenings during the night.
Table 9.
Analysis of feature importance of the selected 10 features.
| Feature | Importance | Median feature value | U test statistic | P value | |
|---|---|---|---|---|---|
| Low | High | ||||
| Number of Awakenings* | 0.21701 | 26.5 | 19.0 | 0.0 | 0.049 |
| Minutes Lightly Active | 0.19127 | 211.0 | 239.0 | 0.0 | 0.748 |
| Minutes Light Sleep 2nd Order of Difference | 0.11961 | 0.5 | 4.0 | 173.0 | 0.069 |
| Minutes Light Sleep* | 0.10999 | 292.0 | 269.0 | 0.0 | <0.001 |
| Minutes Very Active* | 0.10323 | 2.0 | 1.0 | 15,530.0 | <0.001 |
| Minutes Deep Sleep | 0.08600 | 58.0 |
72.0 | 173.0 | 0.001 |
| Minutes Awake* |
0.06130 | 58.0 | 53.0 | 18,071.5 | <0.001 |
| Minutes REM Sleep* | 0.05934 | 93.5 | 96.0 | 0.0 | 0.003 |
| Minutes Sedentary* | 0.03096 | 660.0 |
671.0 | 0.0 | <0.001 |
| Minutes Moderately Active | 0.02128 | 6.0 | 7.0 | 0.0 | 0.395 |
*Statistically significant at p<0.05.
4. Discussion
This study investigated rTMS effect on physical activity and sleep through providing patients with a Fitbit and software apps and reports the impact of rTMS on depression, anxiety and mental health recovery. The depression outcomes exceed or are similar to published RCT and service data response and remission rates (Allan et al., 2011; Berlim et al., 2014; Gross et al., 2007; Herrmann and Ebmeier, 2006; Kozel and George, 2002; Lam et al., 2008; Schutter, 2010; Slotema et al., 2010; Xie et al., 2013;Carpenter et al., 2012, Connolly et al., 2012, Galletly et al., 2015, Taylor et al., 2017). The recovery and reliable change results indicate that rTMS is an effective treatment for depression in patients with a TRD diagnosis.
The anxiety outcome results show that rTMS treatment in a clinical service can be effective in treating GAD symptoms in TRD patients. This supports the results of other published papers (Diefenbach et al., 2013; LaSalle-Ricci et al., 2014; Caulfield et al., 2016; White and Tavakoli, 2015; Griffiths et al., 2019a). However, there is currently a lack of RCT evidence on the effectiveness of rTMS on GAD in depression, and further well-designed and adequately powered RCTs are required to determine clinical recommendations (Kozel, 2018).
Patient improvements on the ReQoL-20 demonstrate a positive impact of rTMS on an individuals’ real world functioning and quality of life: on factors that are valued by people in their everyday lives. The ReQoL-20 demonstrated strong positive correlations between PHQ-9 and GAD-7 at baseline, follow-up, and change over time; indicating that it is an effective measure of the impact of symptoms on recovery-related quality of life. The ReQoL-20 was designed to enable a move from symptomology to broader recovery-based assessment, and its inclusion as a measure in clinical services can possibly facilitate a change in the focus of mental health services towards patient-defined mental health recovery (Franklin et al., 2021).
The Fitbit data confirms that people with a diagnosis of depression have lower levels of physical activity and are less highly active compared to the general population (Tudor-Locke et al., 2009; Schuch et al., 2017). Physical activity change was not statistically significant and NHS activity levels were lower. However, post-treatment levels of activity in the ‘somewhat active’ and ‘active’ categories exhibited an improvement above general population averages, indicating the potential impact of reduced depression and GAD symptoms on physical activity. Symptoms of depression can hinder efforts to increase physical activity, and reduced depression may improve motivation and self-confidence to facilitate an increase in physical activity (Searle et al., 2011). Compared to the general population (26%), our results (33.4%) confirm that people with a diagnosis of depression have high rates of sedentary behaviour (Tudor-Locke et al., 2009; Schuch et al., 2017). As physical activity can be beneficial, this highlights the need to provide support and interventions which support people with sedentary behaviour to become more physically active (Teychenne et al., 2008; Brand et al., 2010).
At 44.6% pre and 39.13% post-rTMS treatment, the percentage of hours of unhealthy sleep (less than 7 hrs) were greater than that of 35% in the general adult population (CDC, 2014); and no participants had what is considered healthy WASO either pre or post-treatment (Watson et al., 2015). This is an indication of sleep problems experienced by this population. Whilst there was a significant improvement in WASO for those with a TRD-only diagnosis and some improvement in healthy period of hours slept was seen, for the overall participant data set there were no statistically significant differences. A possible reason for why sleep quality did not show significant improvements is that sleep is affected by factors other than symptoms of depression that may be difficult to change, for example, levels of chronic pain, lifestyle factors, and environmental factors (Drake and Roehrs, 2003), which were not recorded in this study.
Some people moved from having ‘low activity’ to ‘somewhat active’ and ‘very active’, showing that rTMS can enable those who have a reasonable degree of activity to increase levels of activity. However, the results show that it is difficult to shift behaviour in those who are sedentary, who in the current study remained sedentary post-rTMS treatment. For a few participants recruited towards the end of the study, the impact of COVID-19 may have reduced physical activity due to restrictions imposed, as those with poor mental health had a higher risk of a reduction in physical activity than the general population (Herbec et al., 2021).
In comparison to patient depression and anxiety outcomes in the same service where a Fitbit was not provided (Griffiths et al., 2019a), there was greater response, remission and effect sizes on the GAD-7 and PHQ-9 in participants on the current study. This indicates the possible value of patients receiving and actively using a Fitbit and its app during their rTMS treatment. However, definitive reasons for differences cannot be ascertained as this was not a study with an RCT design whereby patients were randomised to either rTMS treatment or rTMS treatment with use of a Fitbit and its app. Results indicate the possible value of future research in this area employing an RCT design.
Our machine learning model is able to classify two levels of depression severity using the activity, sleep and combined features, utilising the ten selected features it achieved 82% accuracy. There are no direct comparisons to our machine learning results, as our dataset is unique due to the TRD participants. Other research mostly focuses on participants from the general population, using physiological, social media and smartphone data (Acharya et al., 2015; Intarasirisawat et al., 2020, 2020; Wang et al., 2013). A study classified depression using smartphone usage features, 138 university students were recruited to develop a model to classify if they had depressive symptoms post semester; an accuracy of 83.3% was achieved (Chikersal et al., 2021). Another study recruited adults from the general population and used GPS and smartphone usage to detect depression, using data of 28 participants the model achieved 86.6% accuracy on discriminating between participants below or equal to 5 and above 5 PHQ-9 (Wahle et al., 2016). A summary of the comparisons to other similar studies are presented in Table 10. In our analysis, the majority of features selected were sleep related with the most important being number of awakenings during the night. This shows that high depression level correlates with disturbances in sleep, which is consistent with literature (Zhai et al., 2015).
Table 10.
Comparison to other ML models developed to detect depression.
| Reference | Participants | Sensors | Classification | Accuracy |
|---|---|---|---|---|
| This Paper | 17 patients diagnosed with TRD | Fitness tracker |
Detection of depression severity daily |
Accuracy=81% |
| (Chikersal et al., 2021) | 138 university students | Smartphone sensors, usage statistics and fitness tracker | detection of depressive symptoms after a semester |
Accuracy=83.3% |
| (Saeb et al., 2015) | 28 adults | Location | Depression at the end of 2 weeks |
Accuracy=86.5% |
| (Wahle et al., 2016) | 36 adults | Smartphone sensors | Depression biweekly |
Accuracy=61.5% |
| (Farhan et al., 2016) | 79 university age people | Location | Clinical depression biweekly |
F1=0.82 |
| (Canzian and Musolesi, 2015) | 28 adults | Location | Detecting depression over different periods of time and in advance |
Sensitivity=0.71 Specificity=0.87 |
| (Wang et al., 2018) | 68 university students | Smartphone sensors | Depression weekly |
F1=0.75 |
| (Mehrotra et al., 2018) | 28 adults | location | Detecting depression over different periods of time | Sensitivity=0.77 Specificity=0.91 |
There were a number of limitations to the study. Due to COVID-19 restricting patient access, it was not possible to ensure full alignment of mental health assessments (at baseline and post-rTMS) with the Fitbit data. Therefore, correlations between the mental health data and Fitbit data did not always have perfectly aligned time points, which could have concealed the relationship between sleep patterns and mental health. Furthermore, COVID-19 restrictions on social activity and movement applied during the time of the study may have played a role in observing non-significant changes in the levels of activity. Trends of improved sleep patterns were noticeable in participants, but the power and sample size need to be larger to establish conclusive improvement. Female patients were overrepresented, reducing generalisation to males. rTMS treatment was open-label and adjunct to any existing antidepressant medication, with the absence of a control group. Data was collected from a single site in the UK limiting generalizability; however, partially negating this, patients come from across the UK to be treated at the site. The measure of depression used was the PHQ-9, a self-report measure of depression which is approved for and widely used in the UK's national health service: NHS; it is acknowledged that a limitation of the assessment of depression in this study was that it did not additionally employ a clinician assessment of depression. The focus of this study was on patient self-reported assessment of depression, their view of their depression. This study collected measures at baseline and at the end of treatment point, there was no later follow-up data collection – which is a limitation. It is recommended that future studies employ a later follow-up data collection point.
The results showing that Fitbit data analysed using machine learning techniques can predict depression severity indicates the potential value of wearable activity trackers in the monitoring of depression and providing feedback on depression change. However, further consideration needs to be given to factors such as inhibition in psychomotricity that might be linked to depressive features; for example, environmental stimuli or increase/decrease in anxiety and agitation may be potential mechanisms affecting physical activity. Fitbit data analysis could be combined with smart phone data analysis, which has been found to reliably classify five-level human emotions with up to 95% accuracy (Kanjo et al., 2019). In the future people with experience of depression could have ownership of an effective unobtrusive depression monitoring and feedback system, widely available at low-cost; to self-manage symptoms, find out what affects mood, take actions to improve mood, and seek help when needed. With dashboard visualisation, clinicians could observe depression changes over time, explore what works to improve symptoms and more effectively engage with, advise, and treat patients. Allowing effective assessment of the impact of prescribed treatment. The system could also be a measure of impact of new interventions in clinical research, further system development is required. However, our findings are preliminary and require further research in much larger numbers of patients, research on feasibility and acceptability of use is also required. Physical activity and sleep are just two aspects of complex range of symptoms associated with experience of depression.
The recorded Fitbit data showed that most patients with TRD undertaking rTMS treatment for depression will people wear a Fitbit consistently and charge it. The present research indicates the potential value of providing a wearable activity tracker and using the associated software applications. As people undergo rTMS treatment and have a period of regular contact with mental health services, there is potential value in providing a device such as the Fitbit building on the evidence of links between increased physical activity, improved sleep, enhanced wellbeing, better physical health, and lower depressive symptoms. Patients could benefit from the offer of a Fitbit and its apps alongside the provision of other depression treatments; for example, when patients are offered a course of medication or psychotherapy. Fitbits could be offered through primary care (GPs) or through psychotherapy services such as UK's Improving Access to Psychological Therapies (IAPT) services. There is potential value of the Fitbit and its software apps in other mental illness diagnoses where sleep and inactivity are known to be problematic, such as psychosis and bipolar disorder (McGlinchey et al., 2014; Soundy et al., 2013; Davies et al., 2017). Appropriately powered RCTs are warranted.
Authors' statement
All authors have materially participated in the research and/or article preparation
Chris Griffiths: conceptualization, investigation, methodolgy, supervision, writing - original draft, writing - review & editing
Dr Ksenija da Silva methodolgy, formal analysis, supervision, writing - original draft, writing - review & editing
Chloe Leathlean formal analysis, writing - original draft, writing - review & editing
Harmony Jiang writing - review & editing
Dr Chee Siang Ang methodolgy, supervision, formal analysis, writing - original draft, writing - review & editing
Ryan Searle formal analysis, writing - review & editing
All authors have inputted into writing of the paper and approved the final article
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We wish to thank the staff at the NHS Trust for their support in recruitment and the participants for their time.
References
- Acharya U.R., Sudarshan V.K., Adeli H., Santhosh J., Koh J.E.W., Puthankatti S.D., Adeli A. A novel depression diagnosis index using nonlinear features in EEG signals. Eur. Neurol. 2015;74(1–2):79–83. doi: 10.1159/000438457. [DOI] [PubMed] [Google Scholar]
- Adam K., Oswald I.A.N. Protein synthesis, bodily renewal and the sleep-wake cycle. Clin. Sci. 1983;65(6):561–567. doi: 10.1042/cs0650561. [DOI] [PubMed] [Google Scholar]
- Allan C.L., Herrmann L.L., Ebmeier K.P. Transcranial magnetic stimulation in the management of mood disorders. Neuropsychobiology. 2011;64(3):163–169. doi: 10.1159/000328951. [DOI] [PubMed] [Google Scholar]
- Beattie Z., Oyang Y., Statan A., Ghoreyshi A., Pantelopoulos A., Russell A., Heneghan C.J.P.M. Estimation of sleep stages in a healthy adult population from optical plethysmography and accelerometer signals. Physiological measurement. 2017;38(11):1968. doi: 10.1088/1361-6579/aa9047. [DOI] [PubMed] [Google Scholar]
- Berlim M.T., Van den Eynde F., Tovar-Perdomo S., Daskalakis Z.J. Response, remission and drop-out rates following high-frequency repetitive transcranial magnetic stimulation (rTMS) for treating major depression: a systematic review and meta-analysis of randomized, double-blind and sham-controlled trials. Psychological medicine. 2014;44(2):225–239. doi: 10.1017/S0033291713000512. [DOI] [PubMed] [Google Scholar]
- Biddle S., Mutrie N. Psychology of physical activity: Determinants, well-being and interventions. Routledge; 2007. [Google Scholar]
- Brand S., Gerber M., Beck J., Hatzinger M., Pühse U., Holsboer-Trachsler E. High exercise levels are related to favorable sleep patterns and psychological functioning in adolescents: a comparison of athletes and controls. J. Adolesc. Health. 2010;46(2):133–141. doi: 10.1016/j.jadohealth.2009.06.018. [DOI] [PubMed] [Google Scholar]
- Canzian L., Musolesi M. Proceedings of the 2015 ACM International Joint Conference on Pervasive and Ubiquitous Computing. 2015. Trajectories of depression: unobtrusive monitoring of depressive states by means of smartphone mobility traces analysis; pp. 1293–1304. Association for Computing Machinery. [Google Scholar]
- Carpenter L.L., Janicak P.G., Aaronson S.T., Boyadjis T., Brock D.G., Cook I.A., Demitrack M.A. Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depression and anxiety. 2012;29(7):587–596. doi: 10.1002/da.21969. [DOI] [PubMed] [Google Scholar]
- Caulfield K.A., Ketchabaw W.T., Pascual-Leone A., Press D.Z., Stern A.P. Reductions in depression and anxiety measures are correlated in patients receiving transcranial magnetic stimulation. Brain Stimul. 2016;9(5):e9. doi: 10.1016/j.brs.2016.06.028. [DOI] [Google Scholar]
- Centers for Disease Control and Prevention, 2014. Short Sleep duration among US adults. Retrieved from https://www.cdc.gov/sleep/data_statistics.html (accessed 04/04/2021).
- Chikersal P., Doryab A., Tumminia M., Villalba D.K., Dutcher J.M., Liu X., Cohen S., Creswell K.G., Mankoff J., Creswell J.D., Goel M., Dey A.K. Detecting depression and predicting its onset using longitudinal symptoms captured by passive sensing. ACM Trans. Comput.-Hum. Interact. 2021;28(1):1–41. doi: 10.1145/3422821. [DOI] [Google Scholar]
- Chum J., Kim M.S., Zielinski L. Acceptability of the Fitbit in behavioural activation therapy for depression: a qualitative study (vol 20, pg 128, 2017) Evidence-Based Mental Health. 2018;21(2):76. doi: 10.1136/eb-2017-102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly K.R., Helmer A., Cristancho M.A., Cristancho P., John P.O. Effectiveness of transcranial magnetic stimulation in clinical practice post-FDA approval in the United States: results observed with the first 100 consecutive cases of depression at an academic medical center. The Journal of clinical psychiatry. 2012;73(4):5611. doi: 10.4088/JCP.11m07413. [DOI] [PubMed] [Google Scholar]
- Davies G., Haddock G., Yung A.R., Mulligan L.D., Kyle S.D. A systematic review of the nature and correlates of sleep disturbance in early psychosis. Sleep Med. Rev. 2017;31:25–38. doi: 10.1016/j.smrv.2016.01.001. [DOI] [PubMed] [Google Scholar]
- Diefenbach G.J., Bragdon L., Goethe J.W. Treating anxious depression using repetitive transcranial magnetic stimulation. J. Affect. Disord. 2013;151(1):365–368. doi: 10.1016/j.jad.2013.05.094. [DOI] [PubMed] [Google Scholar]
- Drake C.L., Roehrs T., Roth T. Insomnia causes, consequences, and therapeutics: an overview. Depress. Anxiety. 2003;18(4):163–176. doi: 10.1002/da.10151. [DOI] [PubMed] [Google Scholar]
- Driver H.S., Taylor S.R. Exercise and sleep. Sleep Med. Rev. 2000;4(4):387–402. doi: 10.1053/smrv.2000.0110. [DOI] [PubMed] [Google Scholar]
- Dunn A.L., Trivedi M.H., Kampert J.B., Clark C.G., Chambliss H.O. Exercise treatment for depression: efficacy and dose response. American journal of preventive medicine. 2005;28(1):1–8. doi: 10.1016/j.amepre.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Farhan A., Yue C., Morillo R., Ware S., Lu J., Bi J., Kamath J., Russell A., Bamis A., Wang B. IEEE Wireless Health; 2016. Behavior vs. Introspection: Refining Prediction of Clinical Depression via Smartphone Sensing Data. 2016WH. [DOI] [Google Scholar]
- Faulkner G.E., Taylor A.H. first ed. Taylor and Francis; London: 2005. Exercise, Health and Mental Health: Emerging Relationships. [Google Scholar]
- Feehan L.M., Geldman J., Sayre E.C., Park C., Ezzat A.M., Yoo J.Y., Hamilton C.B., Li L.C. Accuracy of Fitbit devices: systematic review and narrative syntheses of quantitative data. JMIR mHealth uHealth. 2018;6(8):e10527. doi: 10.2196/10527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration, 2011. Class II special controls guidance document: repetitive transcranial magnetic stimulation (rTMS) systems—guidance for industry and FDA Staff. U.S. Department of Health and Human Service. Retrieved from https://www.fda.gov/media/81495/download (accessed 04/04/2021).
- Franklin M., Enrique A., Palacios J., Richards D. Psychometric assessment of EQ-5D-5L and ReQoL measures in patients with anxiety and depression: construct validity and responsiveness. Qual. Life. Res. 2021:1–15. doi: 10.1007/s11136-021-02833-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galletly C.A., Clarke P., Carnell B.L., Gill S. A clinical repetitive transcranial magnetic stimulation service in Australia: 6 years on. Australian & New Zealand Journal of Psychiatry. 2015;49(11):1040–1047. doi: 10.1177/0004867415607985. [DOI] [PubMed] [Google Scholar]
- Griffiths C., da Silva K., de Vai R., O'Neill-Kerr A. Repetitive transcranial magnetic stimulation (rTMS) in treatment resistant depression: retrospective data analysis from clinical practice. Open J. Depress. 2019;8(1):16–28. doi: 10.4236/ojd.2019.81003. [DOI] [Google Scholar]
- Griffiths C., O'Neill-Kerr A., De Vai R., Da Silva K. Impact of repetitive transcranial magnetic stimulation on generalized anxiety disorder in treatment-resistant depression. Ann. Clin. Psychiatry. 2019;31(4):236–241. [PubMed] [Google Scholar]
- Gross M., Nakamura L., Pascual-Leone A., Fregni F. Has repetitive transcranial magnetic stimulation (rTMS) treatment for depression improved? A systematic review and meta-analysis comparing the recent vs. the earlier rTMS studies. Acta Psychiatr. Scand. 2007;116(3):165–173. doi: 10.1111/j.1600-0447.2007.01049.x. [DOI] [PubMed] [Google Scholar]
- Gupta R., Dahiya S., Bhatia M.S. Effect of depression on sleep: qualitative or quantitative? Indian J. Soc. Psychiatry. 2009;51(2):117–121. doi: 10.4103/0019-5545.49451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghayegh S., Khoshnevis S., Smolensky M.H., Diller K.R., Castriotta R.J. Accuracy of wristband Fitbit models in assessing sleep: systematic review and meta-analysis. Journal of medical Internet research. 2019;21(11) doi: 10.2196/16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S., Bastick L., O'Neill-Kerr A., Sabesan P., Lankappa S., Palaniyappan L. Transcranial magnetic stimulation in clinical practice. BJPsych. Adv. 2016;22(6):373–379. doi: 10.1192/apt.bp.115.015206. [DOI] [Google Scholar]
- Health Quality Ontario Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis of randomized controlled trials. Ont. Health. Technol. Assess. Ser. 2016;16(5):1–66. [PMC free article] [PubMed] [Google Scholar]
- Herbec, A., Schneider, V., Fisher, A., Kale, D., Shahab, L., Lally, P., 2021. Correlates of and changes in aerobic physical activity and strength training before and after the onset of COVID-19 pandemic in the UK–findings from the HEBECO study. medRxiv. https://doi.org/10.1101/2021.01.16.21249925. [DOI] [PMC free article] [PubMed]
- Herrmann L.L., Ebmeier K.P. Factors modifying the efficacy of transcranial magnetic stimulation in the treatment of depression: a review. J. Clin. Psychiatry. 2006;67(12):1870–1876. doi: 10.4088/JCP.v67n1206. [DOI] [PubMed] [Google Scholar]
- ICHOM, 2021. International Consortium for Health Outcome Measurement: standard set reference guides. https://www.ichom.org/standard-sets/ (Accessed 04/04/2021).
- Intarasirisawat J., Ang C.S., Efstratiou C., Dickens L., Sriburapar N., Sharma D., Asawathaweeboon B. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies. Vol. 4. 2020. An automated mobile game-based screening tool for patients with alcohol dependence; pp. 1–23. [DOI] [Google Scholar]
- Intarasirisawat J., Ang C.S., Efstratiou C., Dickens L., Sriburapar N., Sharma D., Asawathaweeboon B. An Automated Mobile Game-based Screening Tool for Patients with Alcohol Dependence. Proceedings of the ACM on Interactive, Mobile, Wearable and Ubiquitous Technologies. 2020;4(3):1–23. [Google Scholar]
- Janicak P.G., Dokucu M.E. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr. Dis. Treat. 2015;11:1549–1560. doi: 10.2147/NDT.S67477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanjo E., Younis E.M., Ang C.S. Deep learning analysis of mobile physiological, environmental and location sensor data for emotion detection. Inf. Fus. 2019;49:46–56. doi: 10.1016/j.inffus.2018.09.001. [DOI] [Google Scholar]
- Karpov B., Joffe G., Aaltonen K., Suvisaari J., Baryshnikov I., Näätänen P., Koivisto M., Melartin T., Oksanen J., Suominen K., Heikknen M., Paunio T., Isometsä E. Anxiety symptoms in a major mood and schizophrenia spectrum disorders. Eur. Psychiatry. 2016;37:1–7. doi: 10.1016/j.eurpsy.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Kaseva K., Dobewall H., Yang X., Pulkki-Råback L., Lipsanen J., Hintsa T., Hintsanen M., Puttonen S., Hirvensalo M., Elovainio M., Raitakari O., Tammelin T. Physical activity, sleep, and symptoms of depression in adults-testing for mediation. Med. Sci. Sports Exerc. 2019;51(6):1162–1168. doi: 10.1249/MSS.0000000000001896. [DOI] [PubMed] [Google Scholar]
- Keetharuth A.D., Brazier J., Connell J., Bjorner J.B., Carlton J., Buck E.T., Ricketts T., McKendrick K., Browne J., Croudace T., Barkham M. Recovering quality of life (ReQoL): a new generic self-reported outcome measure for use with people experiencing mental health difficulties. Br. J. Psychiatry. 2018;212(1):42–49. doi: 10.1192/bjp.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keetharuth A.D., Bjorner J.B., Barkham M., Browne J., Croudace T., Brazier J. An item response theory analysis of an item pool for the recovering quality of life (ReQoL) measure. Qual. Life. Res. 2020;30(1):267–276. doi: 10.1007/s11136-020-02622-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kito S., Fujita K., Koga Y. Regional cerebral blood flow changes after low-frequency transcranial magnetic stimulation of the right dorsolateral prefrontal cortex in treatment-resistant depression. Neuropsychobiology. 2008;58(1):29–36. doi: 10.1159/000154477. [DOI] [PubMed] [Google Scholar]
- Kozel F.A. Clinical repetitive transcranial magnetic stimulation for posttraumatic stress disorder, generalized anxiety disorder, and bipolar disorder. Psychiatr. Clin. North. Am. 2018;41(3):433–446. doi: 10.1016/j.psc.2018.04.007. [DOI] [PubMed] [Google Scholar]
- Kozel F.A., George M.S. Meta-analysis of left prefrontal repetitive transcranial magnetic stimulation (rTMS) to treat depression. J. Psychiatr. Pract. 2002;8(5):270–275. doi: 10.1097/00131746-200209000-00003. [DOI] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med. 2001;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B., Monahan P.O., Löwe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann. Intern. Med. 2007;146(5):317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- Kumari M., Badrick E., Ferrie J., Perski A., Marmot M., Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. The Journal of Clinical Endocrinology & Metabolism. 2009;94(12):4801–4809. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam R.W., Chan P., Wilkins-Ho M., Yatham L.N. Repetitive transcranial magnetic stimulation for treatment-resistant depression: a systematic review and meta-analysis. J. Clin. Psychiatry. 2008;75(5):477–489. doi: 10.4088/JCP.13r08815. [DOI] [PubMed] [Google Scholar]
- LaSalle-Ricci V.H., Sisko E., DeBlasio K., Tendler S., Allsup H., DeLuca L., DeLuca M., Tendler A. Early improvement of depression and anxiety symptoms with repetitive transcranial magnetic stimulation (rTMS) augmentation of medication: case series. Brain Stimul. 2014;7(5):e20–e21. doi: 10.1016/j.brs.2014.07.013. [DOI] [Google Scholar]
- McGlinchey E.L., Gershon A., Eidelman P., Kaplan K.A., Harvey A.G. Physical activity and sleep: day-to-day associations among individuals with and without bipolar disorder. Ment. Health Phys. Act. 2014;7(3):183–190. doi: 10.1016/j.mhpa.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerlo P., Sgoifo A., Suchecki D. Restricted and disrupted sleep: effects on autonomic function, neuroendocrine stress systems and stress responsivity. Sleep medicine reviews. 2008;12(3):197–210. doi: 10.1016/j.smrv.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Mehrotra A., Musolesi M. Proc. ACM Interact. Mob. Wearable Ubiquitous Technol. Vol. 2. 2018. Using autoencoders to automatically extract mobility features for predicting depressive states. [Google Scholar]
- NHS, 2019. Physical activity guidelines for adults aged 19 to 64. Retrieved from https://www.nhs.uk/live-well/exercise/ (accessed 04/04/2021).
- Naslund J.A., Aschbrenner K.A., Barre L.K., Bartels S.J. Feasibility of popular m-health technologies for activity tracking among individuals with serious mental illness. Telemedicine and e-Health. 2015;21(3):213–216. doi: 10.1089/tmj.2014.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt D., Wilson S., Patterson L. Sleep disorders as core symptoms of depression. Dialogues Clin. Neurosci. 2008;10(3):329–336. doi: 10.31887/DCNS.2008.10.3/dnutt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedregosa F., Varoquaux G., Gramfort A., Michel V., Thirion B., Grisel O., Blondel M., Prettenhofer P., Weiss R., Dubourg V., Vanderplas J., Passos D., Brucher M., Perrot M., Duchesnay E. Scikit-learn: machine learning in python. J. Mach. Learn. Res. 2011;12:2825–2830. [Google Scholar]
- Richards D.A., Borglin G. Implementation of psychological therapies for anxiety and depression in routine practice: two year prospective cohort study. J. Affect. Disord. 2011;133(1–2):51–60. doi: 10.1016/j.jad.2011.03.024. [DOI] [PubMed] [Google Scholar]
- ReQoL, 2021. How to interpret ReQoL-10 scores. Retrieved from https://www.reqol.org.uk/p/scoring.html (accessed 04/04/2021).
- Saeb S., Zhang M., Karr C., Schueller S., Corden M., Kording K., Mohr D. Mobile phone sensor correlates of depressive symptom severity in daily-life behavior: an exploratory study. J. Med. Internet Res. 2015;17(7):e175. doi: 10.2196/jmir.4273. https://w w w.jmir.org/2015/7/e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuch F., Vancampfort D., Firth J., Rosenbaum S., Ward P., Reichert T., Bagatini N.C., Bgeginski R., Stubbs B. Physical activity and sedentary behavior in people with major depressive disorder: a systematic review and meta-analysis. J. Affect. Disord. 2017;210:139–150. doi: 10.1016/j.jad.2016.10.050. [DOI] [PubMed] [Google Scholar]
- Schutter D.J.L.G. Quantitative review of the efficacy of slow-frequency magnetic brain stimulation in major depressive disorder. Psychol. Med. 2010;40(11):1789–1795. doi: 10.1017/S003329171000005X. [DOI] [PubMed] [Google Scholar]
- Searle A., Calnan M., Lewis G., Campbell J., Taylor A., Turner K. Patients’ views of physical activity as treatment for depression: a qualitative study. British Journal of General Practice. 2011;61(585):e149–e156. doi: 10.3399/bjgp11X567054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotema C.W., Dirk Blom J., Hoek H.W., Sommer I.E. Should we expand the toolbox of psychiatric treatment methods to include repetitive transcranial magnetic stimulation (rTMS)? A meta-analysis of the efficacy of rTMS in psychiatric disorders. J. Clin. Psychiatry. 2010;71(7):873–884. doi: 10.4088/JCP.08m04872gre. [DOI] [PubMed] [Google Scholar]
- Soundy A., Wampers M., Probst M., De Hert M., Stubbs B., Vancampfort D., Attux C., Leutwyler H., Ströhle A. Physical activity and sedentary behaviour in outpatients with schizophrenia: a systematic review and meta-analysis. Int. J. Ther. Rehabil. 2013;20(12):588–595. doi: 10.12968/ijtr.2013.20.12.588. [DOI] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Steiger A., Pawlowski M. Depression and sleep. Int. J. Mol. Sci. 2019;20(3):607. doi: 10.3390/ijms20030607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tudor-Locke C., Johnson W.D., Katzmarzyk P.T. Accelerometer-determined steps per day in US adults. Med. Sci. Sports. Exerc. 2009;41(7):1384–1391. doi: 10.1249/MSS.0b013e318199885c. [DOI] [PubMed] [Google Scholar]
- Tsuzuki D., Watanabe H., Dan I., Taga G. MinR 10/20 system: quantitative and reproducible cranial landmark setting method for MRI based on minimum initial reference points. J. Neurosci. Methods. 2016;264:86–93. doi: 10.1016/j.jneumeth.2016.02.024. [DOI] [PubMed] [Google Scholar]
- Taylor S.F., Bhati M.T., Dubin M.J., Hawkins J.M., Lisanby S.H., Morales O., Wright J. A naturalistic, multi-site study of repetitive transcranial magnetic stimulation therapy for depression. Journal of affective disorders. 2017;208:284–290. doi: 10.1016/j.jad.2016.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teychenne M., Ball K., Salmon J. Physical activity and likelihood of depression in adults: a review. Prev. Med. 2008;46(5):397–411. doi: 10.1016/j.ypmed.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Watson N., Badr M.S., Belenky G., Bliwise D.L., Buxton O.M., Buysse D., Dinges D.F., Gangwisch J., Grandner M.A., Kushida C., Malhotra R.K., Martin J.L., Patel S.R., Quan S.F., Tasali E. Recommended amount of sleep for a healthy adult: a joint consensus statement of the American Academy of Sleep Medicine and Sleep Research Society. Sleep. 2015;38(6):591–592. doi: 10.5664/jcsm.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D., Tavakoli S. Repetitive transcranial magnetic stimulation for treatment of major depressive disorder with comorbid generalized anxiety disorder. Ann. Clin. Psychiatry. 2015;27(3):192–196. [PubMed] [Google Scholar]
- World Health Organization, 2020. Global recommendations on physical activity for health. https://www.who.int/standards/classifications/classification-of-diseases (accessed 27 March 2021).
- Xie J., Chen J., Wei Q. Repetitive transcranial magnetic stimulation versus electroconvulsive therapy for major depression: a meta-analysis of stimulus parameter effects. Neurol. Res. 2013;35(10):1084–1091. doi: 10.1179/1743132813Y.0000000245. [DOI] [PubMed] [Google Scholar]
- Wahle F., Kowatsch T., Fleisch E., Rufer M., Weidt S. Mobile sensing and support for people with depression: a pilot trial in the wild. JMIR mHealth uHealth. 2016;4(3):e111. doi: 10.2196/mhealth.5960. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Lei L., Wang X., Nie J., Chu X., Jin S. The exacerbating role of perceived social support and the “buffering” role of depression in the relation between sensation seeking and adolescent smartphone addiction. Personality and Individual Differences. 2018;130:129–134. [Google Scholar]
- Wang X., Zhang C., Ji Y., Sun L., Wu L., Bao Z. Vol. 7867. 2013. pp. 201–213. (A Depression Detection Model Based on Sentiment Analysis in Micro-blog Social Network. Revised Selected Papers of PAKDD 2013 International Workshops on Trends and Applications in Knowledge Discovery and Data Mining). VolumeApril 2013 Pages. [DOI] [Google Scholar]
- Young S., Pfaff D., Lewandowski K.E., Ravichandran C., Cohen B.M., Öngür D. Anxiety disorder comorbidity in bipolar disorder, schizophrenia and schizoaffective disorder. Psychopathology. 2013;46(3):176–185. doi: 10.1159/000339556. [DOI] [PubMed] [Google Scholar]
- Zhai L., Zhang H., Zhang D. Sleep duration and depression among adults: a meta-analysis of prospective studies. Depress. Anxiety. 2015;32(9):664–670. doi: 10.1002/da.22386. [DOI] [PubMed] [Google Scholar]