Abstract
A one-year prospective human intervention study was performed to examine the effects of fermented lingonberry juice (FLJ), used as a mouthwash for six months, on salivary parameters. A total of 25 adult participants used 10 mL of FLJ as mouthwash 30 s daily for 6 months in addition to their normal oral homecare routines. Standard oral examinations and gathering of samples were performed at the beginning of the study and after six months and one year. Resting and stimulated saliva secretion rates, resting saliva pH, and stimulated saliva buffering capacity were determined. A questionnaire of participants’ subjective sensations of mouth dryness was also recorded at each timepoint. Fermented lingonberry juice mouthwash had positive effect to all five salivary parameters and were, according to the omnibus test, statistically significant during the study period. Analysis of the subjective dry mouth sensation questionnaires revealed that symptoms of xerostomia decreased due to the use of FLJ. This study revealed that the once-a-day use of FLJ mouthwash had a beneficial, increasing effect on salivary flow rates, buffering capacity, and salivary pH. FLJ thus can be safely used as an adjunctive and beneficial therapy in oral homecare, protecting teeth and oral mucosa, including periodontium, and also relieving dry mouth symptoms.
Keywords: fermented lingonberry juice, resting saliva, stimulated saliva, salivary buffering capacity, saliva pH, dry mouth, xerostomia
1. Introduction
Saliva has multiple functions in maintaining and controlling oral health. It protects and lubricates the oral mucosa, exerts buffering action against acidity variations after meals, takes part in the mineralization and demineralization of tooth enamel and dentin, has antimicrobial activity with its immunological and non-immunological components, as well enhances taste and begins the digestive process. Whole saliva is a mixture of water secretion produced by major salivary glands (parotid, submandibular, and sublingual), minor salivary glands (lower lip, tongue, palate, cheeks, and pharynx), and gingival crevicular fluid secretions [1] containing minerals, proteins and mucins. Diseases, such as diabetes mellitus, the autoimmune disease Sjögren’s syndrome, and medications or radiotherapy cause salivary gland dysfunction and may lead to xerostomia, altered dysbiotic microbial balance and dental caries, periodontal diseases, or mucosal lesions, such as candidosis [2,3,4,5,6]. Normal pH of saliva is 6–7, unstimulated saliva flow rate is 0.3–0.4 mL/min, the stimulated saliva flow rate 1–3 mL/min, and the buffering capacity 10–12. In hyposalivation the resting saliva flow rate is <0.1 mL/min, and the stimulated saliva flow rate < 0.7 mL/min. Eating or drinking causes alterations to saliva pH and secretion rates, and depending on beverage acidity, sugar content, and consumption frequency, buffering occurs in 5 to 15 min [7,8]. The non-microbial pellicle layer on teeth surfaces is an important intermediate in protecting and balancing environmental pH changes. The consumption of fermentable carbohydrates, acidic liquids, or sugar in the presence of acidogenic microbes causes the initiation of enamel demineralization under the critical pH 5.5 described by R.M. Stephan [9]. Saliva acts to counteract this process, but low buffering capacity and saliva pH predispose to erosion. There are limited means to regulate salivary flow. Topical management with lubricating gels, toothpastes, sprays, or artificial saliva may relieve sensations of dry mouth and xerostomia [10], acting as salivary stimulants or substitutes. Salivary-flow-inducing xylitol chewing gum or acidic substances eventually give temporary relief. Medications inducing salivary flow, such as systemically acting parasympatomimetic pilocarpine and cevimeline, exert side effects or are even contraindicated in patients with iritis, narrow-angle glaucoma, gastric ulcer, uncontrolled asthma, or in patients using β-blockers [11,12]. Several natural compounds have been evaluated for oral use. Lingonberries contain bioactive phenolic compounds with antioxidative, anti-inflammatory, and antimicrobial properties [13]. Based on previous in vitro and in vivo clinical human oral studies [14,15], FLJ used as mouthwash has revealed antimicrobial, anti-inflammatory, and anti-proteolytic effects, but the effects of lingonberries on salivary parameters have not been studied.
The aims of this prospective human intervention study were to evaluate the effects of FLJ on saliva secretion rates, saliva pH, and buffering capacity with basic clinical saliva test methods in randomly selected adult patients from two private dental clinics in Finland. The hypothesis of our study was whether fermented lingonberry juice, used as a mouthwash once a day in addition to oral homecare, could increase salivary flow and buffering capacity without decreasing pH, thus relieving symptoms of hyposalivation safely.
2. Materials and Methods
Twenty-five adults (28–91 years, mean 65.29 +/− 16.23 years; M/F ratio 10/15) were recruited randomly from two separate private dental clinics in Helsinki and Joensuu (Finland). The study took place from June 2020 to June 2021. Healthy patients or with controlled systemic diseases, good cognitive ability, users of removable dental prostheses (one patient with upper whole denture and three patients with upper partial denture), with health adequate periodontal tissue, with or without xerostomia, and without mucosal inflammation were included to the study. Medical evaluations revealed underlying diseases such as cardiovascular diseases and diabetes mellitus but no autoimmune diseases. Exclusion criteria were the use of other mouthwash products than FLJ, such as chlorhexidine, or current use of antibiotics during the study.
Resting saliva flow rate and pH, stimulated saliva flow rate, and buffering capacity were measured and analyzed by Saliva-Check BUFFER test kit (GC America Inc., Illinois, USA) at the onset and after 6 and 12 months. Patients were instructed not to eat, drink, or brush teeth for one hour prior to the examination appointment. Saliva collections were carried out in the afternoon. The patient was guided to swallow all saliva in the mouth, followed by resting saliva drain in a face down position into a cup without spitting or facial movements for 5 min, and asked to spit all saliva from the mouth at the end of the gathering. Resting saliva flow rate > 0.3 mL/min was considered normal, 0.1–0.3 low, and <0.1 mL/min extremely low. The Saliva-Check BUFFER test kit includes pH test papers and the buffer capacity measuring strips. The pH-paper and all four pads on the plastic buffer capacity strip were wetted thoroughly with resting saliva using a pipette and turned sideways on a paper towel to absorb the excess saliva. The color changes were compared to the specific color chart within suggested time limit provided by the manufacturer to obtain numerical values. Buffering capacity values (scale 0–12) of 0–5 were considered extremely low, 6–9 low, and 10–12 normal/high. Values of saliva pH (scale 5.0–7.8, scale interval 0.2) of 5.0–5.8 were considered highly acidic, 6.0–6.6 moderately acidic, and 6.8–7.8 normal [16]. Stimulated saliva secretion was induced by chewing wax and collected for 5 min. Stimulated saliva secretion rate >1 mL/min was considered normal, 0.7–1 mL/min low, and <0.7 mL/min extremely low. Diseases and medications were recorded.
Medications causing xerostomia (all classes from weak to strong effect pooled) were recorded according to Wolff et al. [2]. Additionally, a questionnaire for subjective sensations of xerostomia was filled at each timepoint (Table 1). Answers (yes/no) with indications of xerostomia were recorded, counted, and the points summarized for each patient.
Table 1.
Xerostomia sensations questionnaire.
| 1. Does your mouth feel dry after eating? 2. Do you have difficulties in swallowing? 3. Are you able to eat dry bread or biscuit without drinking? 4. Do you feel your saliva secretion is low? 5. How often do you wake up at night because of dry mouth sensations? |
Ten milliliters of FLJ (Lingora®, Vantaa, Finland) [14] was used by the participants as a mouthwash for thirty seconds daily for six months in addition to their daily oral homecare, following a six-month washout period without the FLJ mouthwash regimen. Phenolic compound concentration of the mouthwash was 0.219 % (w/w) and the pH was 2.77. The patients used nonprofessional toothpaste in oral homecare throughout the study, and professional topical fluoride was applied at each timepoint. This study has received approval from the ethical committee of Stockholm Community, Sweden (2016-08-24/2016/1:8 and 2016-1-24) and the Helsinki University Central Hospital, Finland (360/13/03/00/13 and 51/13/02/2009). Informed consent was obtained from all subjects involved in the study.
Salivary parameters (resting saliva, resting saliva pH, stimulated saliva, buffering capacity) and subjective dry mouth sensations from 21 subjects were measured at 3 time points (0, 6, and 12 months). The overall differences between the time points in the levels of each salivary parameter were determined with non-parametric Friedman’s test and pairwise post hoc analysis with Bonferroni corrected Wilcoxon signed-rank tests. SPSS (version 27; IBM Corp., Armonk, NY, USA); the rmcorr package (version 0.4.1) in the R statistical software version 3.6.3 was used in the statistical analyses, and p < 0.05 was considered as statistically significant. For one-way repeated measures analysis of variance, effect size = 0.40, α = 0.05, and 95% power for each group was considered to be appropriate for 18 participants. Previous studies regarding FLJ to calculate effect size were not available.
3. Results
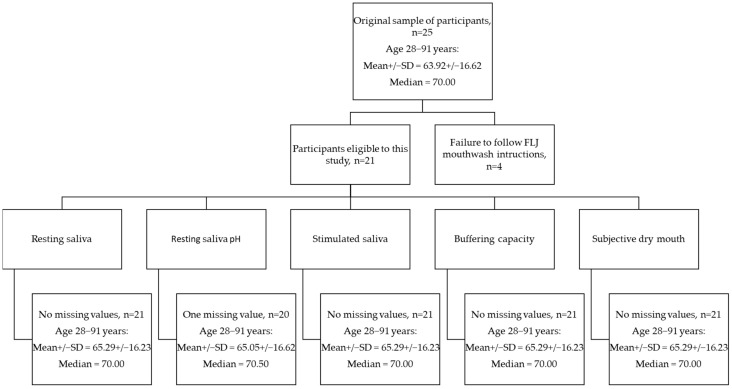
A total of 21 of the recruited participants used the mouthwash according to instructions (10 mL/once a day) and were included in the analyses. The number of the final sample and age of the participants may be seen in Figure 1. None of the participants discontinued the study. One patient had so low resting saliva secretion that the pH could not be measured. Four of the patients did not use FLJ as directed: One patient used FLJ mouthwash 5 mL/once a day, two used 10 mL irregularly, and one used 20 mL/once a day. In Table 2, patient characteristics, the specifications of the diseases, and the used medications with frequencies (%) among the participants are shown. The cohort selected for our study represents typical ambulatory middle-aged Scandinavian people visiting their dentists once yearly seemingly well.
Figure 1.
Flowchart of sample number and age of participants in the current study.
Table 2.
Patient characteristics.
| Age (mean ± standard deviation) Gender (female/male,%) Smoking (yes%) Diseases (mean, range) Medications (mean, range) Medications inducing xerostomia (mean, range) |
65.29 ± 16.23 years 61.9/38.1% 19.0% 1.76, 0–4 2.95, 0–9 1.33, 0–4 |
Number of diseases belonging to endocrine and metabolic, mental and behavioral, circulatory system, and respiratory system were classified according to the International Statistical Classification of Diseases and Related Health Problems [17]. The number of medications reported with higher or moderate level of evidence to induce xerostomia included medications for endocrine and metabolic (diabetes), the circulatory system (high blood pressure), and mental and behavioral diseases [2].
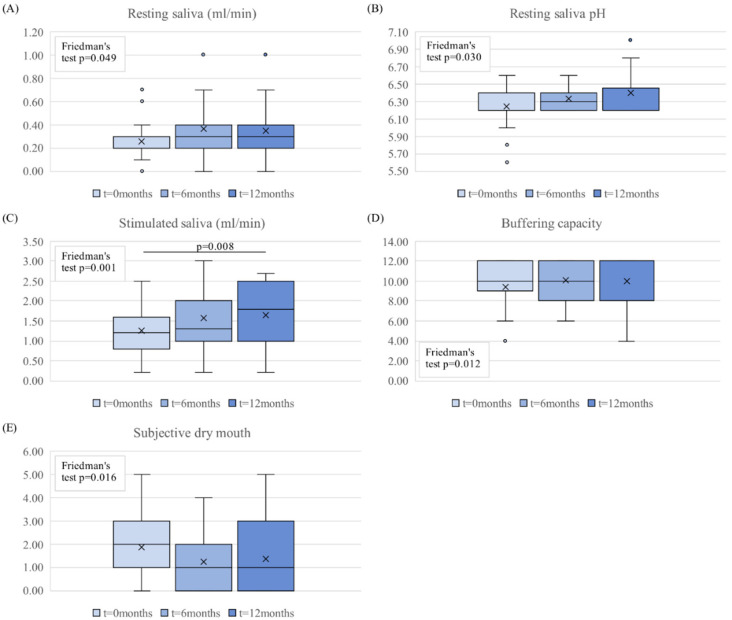
The results of the salivary parameters are shown in Figure 2. The FLJ mouthwash had positive effect on all five salivary parameters, and according to the omnibus test, all parameters were statistically significant during the study period (three measuring points).
Resting saliva flow rate increased from low to normal levels during the FLJ period and remained at this level during the washout period (Figure 2A);
Resting saliva pH increased progressively during the trial (Figure 2B);
Stimulated saliva flow rate increased during the FLJ period and continued to increase during the washout period (Figure 2C);
Buffering capacity increased from near-low values to normal values during the FLJ period and stayed at normal level during the washout period (Figure 2D);
In the beginning of the study, there was a small correlation between patient’s subjective dry mouth symptoms and resting and stimulated salivary flow, resting saliva pH, and buffering capacity (r = −0.432, p = 0.05; r = −0.482, p = 0.027; r = −0.357, p = 0.123; and r = −0.287, p = 0.207; respectively). The results from the questionnaire showed that subjective dry mouth symptoms decreased during the FLJ mouthwash period, kept to lower levels during the washout period compared to the beginning of the study, and had a negative correlation with resting and stimulated saliva flow rates and resting saliva pH. In other words, the increase in these parameters decreased the sensation of mouth dryness;
Frequencies (N) of participants in each classification of variables during the study are shown in Table 3.
Figure 2.
The boxplots of the salivary parameters (A) resting saliva, (B) resting saliva pH, (C) stimulated saliva, (D) buffering capacity, and (E) subjective dry mouth sensations for 3 time points of 0, 6, and 12 months (n = 21). The omnibus analysis of these parameters at three timepoints was conducted with Friedman’s test and pairwise post hoc analysis with Bonferroni corrected Wilcoxon signed-rank tests. The boxplots show the median, mean (x), quartiles, and extreme values (o).
Table 3.
Frequencies of participants in each classification of variables during the study.
| Saliva sampling (months) | 0 | 6 | 12 |
| Resting saliva flow (N) | |||
| Extremely low (<0.1 ml/min) | 4 | 2 | 3 |
| Low (0.1–0.7ml/min) | 13 | 15 | 14 |
| Normal (>0.3 ml/min) | 8 | 8 | 8 |
| Stimulated saliva (N) | |||
| Extremely low (<0.7 ml/min) | 2 | 1 | 2 |
| Low (0.7–1 ml/min) | 10 | 8 | 6 |
| Normal (>1 ml/min) | 13 | 16 | 17 |
| Resting saliva pH (N) | |||
| Highly acidic (5–5,8) | 2 | 0 | 0 |
| Moderately acidic (6–6.6) | 20 | 24 | 20 |
| Healthy (6.8–7.8) | 2 | 0 | 4 |
| Buffering capacity (N) | |||
| Very low (0–5) | 2 | 0 | 1 |
| Low (6–9) | 7 | 8 | 5 |
| Normal/high (10–12) | 16 | 17 | 19 |
Noteworthy, 4 respondents out of 25 did not obey the instructions provided, and their buffering capacities had no statistically significant changes. No erosive lesions or mucosal irritation were noticed during the intervention, and no adverse effects were reported from FLJ mouthwash by the participants.
4. Discussion
Sufficient salivary flow rates, buffering capacity, and pH are crucial factors in protecting the oral mucosa and the teeth. The sugar clearance time, buffering capacity, and pH of saliva decrease with flow rate [18]. Reduced salivary pH, flow rate, and buffering capacity have been found from patients suffering from diabetes mellitus or asthma and chronic obstructive pulmonary disease (COPD) [19,20]. Alteration in salivary parameters also from older adults, adolescents, and xerostomia have been associated with higher prevalence of caries, periodontal disease, and candidosis [5,21]. The autoimmune disease, Sjögren’s syndrome, causes oral symptoms related to decreased saliva secretion [22]. To our best understanding, our cohort represented a characteristic and representative ambulatory set of the Scandinavian middle-aged population in respect to age, habits, weights, sex, diseases, and their medications. Sensations of dry mouth were correlated with resting and stimulated saliva flow rates and resting saliva pH in the current study. Stimulating saliva secretion is one of the main treatment options in relieving these symptoms [23]. The results from the current study reveal significant increases in the stimulated saliva flow rate and resting saliva pH and flow rate and salivary buffering capacity clearly increased during the FLJ mouthwash period.
The current study shows that FLJ has a sustained beneficial effect in maintaining safe saliva pH, and this is a beneficial effect compared to CHX, which in fact has been shown in another study to decrease saliva pH and buffering capacity [24], which might lead to more acidic conditions for potential periodontopathogens and oral microbes to thrive. The remineralization of enamel and dentin occurs if the exposure time is short enough to overcome demineralization [25]. Liquids are buffered faster compared to solids. The role of MMPs in dental caries and erosion has also been proposed, and inactivation of MMP-8 with chlorhexidine, FeSO4 and green tea has been shown to inhibit dental erosion development [26,27]. Fermented lingonberry juice has anti-inflammatory [13,15] and remarkably similar antimicrobial and anti-proteolytic effects as CHX exerts [14,15,28,29], and indeed, our previous in vitro study extended and further confirmed these findings, revealing that FLJ can inhibit Candida glabrata cell-wall-induced activation of pro-MMP-8 [30].
Several in vitro studies have shown that lingonberry phenolic compounds exert antibacterial [31,32,33], antiviral [34], antioxidative [35,36,37,38,39,40,41,42], anti-inflammatory [30,35], and anticancerous effects [43,44,45,46]. In vivo mouse studies show that lingonberry juice has anti-atherothrombic and anti-inflammatory effects, and lingonberries reduce hyperglycaemia, high cholesterol, obesity, improve metabolic/brain functions, and reduce gut inflammation [47,48,49,50]. Lingonberries, lingonberry puree, and lingonberry nectar have shown in vivo effects on glucose, insulin, and free fatty acid release responses in humans [51,52]. Oral human in vivo effects with FLJ show reduced S. mutans and Candida counts, as well as anti-inflammatory effects [15]. Saliva composition and volume have an important role in lubricating the mucosa and protecting from infections as part of the innate immunity, and it has been proposed that the glycoprotein profile of mucins may have a role in the regulation of the infection spreading and could even be used as a biomarker to predict disease susceptibility, disease progression, and response to therapy of COVID-19 patients [53]. Microbial proteases may modify these glycoproteins and cause pathogen entry. Lingonberry polyphenols show several antiviral mechanisms in vitro [54].
Sufficient saliva secretion has a pivotal role in the oral environment, and keeping these beneficial effects of lingonberries in mind, FLJ is designed for safe oral use in addition to oral homecare and may be used safely for long periods of time. Because FLJ juice is all-natural and considered similar to lingonberry juice, general recommendations regarding daily consumption of berries and berry juices are considered safe: 10 mL of FLJ is equal to appr. 1 dl of lingonberries; the consumption of berries, fruits, and vegetables are recommended 250 g/day in a healthy diet [55]. It has no additives, may be swallowed, and has no known interactions with medications and no side-effects. Due to the fermentation process, it contains only a fraction of naturally occurring sugars as compared to unfermented lingonberry juice, and the recommended amount once daily has positive effects on the saliva parameters. In the current study, topical fluoride application at each sampling time point was controlled professionally, but no specific toothpaste product was provided to the participants by the researcher, and this might be a limitation of the study.
Attempts to develop products to alleviate dry mouth symptoms are based on several mechanisms, e.g., protection of the mucosa by replacing missing saliva by artificial saliva, moisture-binding surface agents, mechanically increasing saliva secretion by chewing gums or drug-related routes. They often have only short-term effects or side-effects and are not actually sialagogues. Natural products have gained interest in the treatment of xerostomia [56]. The downside with most of these products is that they are based on acids, which pose a risk for mucosal irritation and have adverse effects on dentition due to the decrease in saliva pH or are incapability of inducing salivary flow [57]. The precise mechanisms of the effects of FLJ on salivary parameters are not precisely known. Potential mechanisms may include the induction of salivary gland saliva secretion by acids and resulting increase also in saliva pH and buffer capacity. The complex FLJ polyphenol composition has additional mucous membrane protective antioxidant, anti-inflammatory, anti-proteolytic, and antimicrobial effects, which may contribute to saliva properties via the effects on oral microbiota composition and its metabolic products.
5. Conclusions
The results of this prospective human intervention study demonstrated for the first time, that a daily regimen of 10 mL of FLJ for 6 months improved saliva secretion rates, saliva buffering capacity, increased saliva pH, and decreased xerostomia These effects continued and were monitored even at the end of the six-month washout period. These positive effects of FLJ on saliva parameters also make it a potential natural aid in relieving dry mouth symptoms safely. Considering the importance of salivary parameters also on the risk for caries, periodontal diseases and candidosis, larger randomized placebo-controlled studies are warranted to confirm our results.
Acknowledgments
Open access funding provided by University of Helsinki.
Author Contributions
Investigation, P.P. and S.L.; data curation, I.T.R.; writing—review and editing, P.P., S.L., I.T.R., T.T., and T.S.; visualization, I.T.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Minerva Foundation’s Selma and Maja-Lisa Selander’s fund, Finland; Helsinki and Uusimaa Hospital District (HUS) (TYH2016251, TYH2017251, TYH2018229, TYH2019319, TYH2022225, Y1014SL017, Y1014SL018, Y1014SULE1), Finland; Finnish Dental Association Apollonia; Karolinska Institute, Sweden.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Stockholm Community, Sweden (2016-08-24/2016/1:8 and 2016-1-24) and the Helsinki University Central Hospital, Finland (360/13/03/00/13 and 51/13/02/2009).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting reported results can be obtained from the authors on request.
Conflicts of Interest
PP is the inventor of EP 2585087B1 (2017) and the trademark holder of Lingora®. The other authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Humphrey S.P., Williamson R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001;85:162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- 2.Wolff A., Joshi R.K., Ekström J., Aframian D., Pedersen A.M., Proctor G., Narayana N., Villa A., Sia Y.W., Aliko A., et al. A Guide to Medications Inducing Salivary Gland Dysfunction, Xerostomia and Subjective Sialorrhea: A Systematic Review Sponsored by the World Workshop on Oral Medicine VI. Drugs R&D. 2017;17:1–28. doi: 10.1007/s40268-016-0153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pedersen A.M.L., Sørensen C.E., Proctor G.B., Carpenter G.H., Ekström J. Salivary secretion in health and disease. J. Oral. Rehabil. 2018;45:730–746. doi: 10.1111/joor.12664. [DOI] [PubMed] [Google Scholar]
- 4.Rosier B.T., Marsh P.D., Mira A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J. Dent. Res. 2018;97:371–380. doi: 10.1177/0022034517742139. [DOI] [PubMed] [Google Scholar]
- 5.Chan A., Tamrakar M., Jiang C.M., Lo E., Leung K., Chu C.H. Common Medical and Dental Problems of Older Adults: A Narrative Review. Geriatrics. 2021;6:76. doi: 10.3390/geriatrics6030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proctor G.B., Shaalan A.M. Disease-Induced Changes in Salivary Gland Function and the Composition of Saliva. J. Dent. Res. 2021;100:1201–1209. doi: 10.1177/00220345211004842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Järvinen V.K., Rytömaa I.I., Heinonen O.P. Risk factors in dental erosion. J. Dent. Res. 1991;70:942–947. doi: 10.1177/00220345910700060601. [DOI] [PubMed] [Google Scholar]
- 8.Hans R., Thomas S., Garla B., Dagli R.J., Hans M.K. Effect of Various Sugary Beverages on Salivary pH, Flow Rate, and Oral Clearance Rate amongst Adults. Scientifica. 2016;2016:5027283. doi: 10.1155/2016/5027283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stephan R.M., Miller B.F. A quantitative method for evaluating physical and chemical agents which modify production of acids in bacterial plaques on human teeth. J. Dent. Res. 1943;22:45–51. doi: 10.1177/00220345430220010601. [DOI] [Google Scholar]
- 10.Turner M.D. Hyposalivation and Xerostomia: Etiology, Complications, and Medical Management. Dent. Clin. North Am. 2016;60:435–443. doi: 10.1016/j.cden.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Napeñas J.J., Brennan M.T., Fox P.C. Diagnosis and treatment of xerostomia (dry mouth) Odontology. 2009;97:76–83. doi: 10.1007/s10266-008-0099-7. [DOI] [PubMed] [Google Scholar]
- 12.Millsop J.W., Wang E.A., Fazel N. Etiology, evaluation, and management of xerostomia. Clin. Dermatol. 2017;35:468–476. doi: 10.1016/j.clindermatol.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 13.Pärnänen P., Lähteenmäki H., Tervahartiala T., Räisänen I.T., Sorsa T. Lingonberries—General and Oral Effects on the Microbiome and Inflammation. Nutrients. 2021;13:3738. doi: 10.3390/nu13113738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pärnänen P. A Preparation for Balancing the Composition of the Oral Microbial Flora. No. 2585087B1. European Patent. 2017 December 13;
- 15.Pärnänen P., Nikula-Ijäs P., Sorsa T. Antimicrobial and anti-inflammatory lingonberry mouthwash—A clinical pilot study in the oral cavity. Microorganisms. 2019;7:331. doi: 10.3390/microorganisms7090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Walsh L.J. Saliva Testing: Good Practice, Good Sense. GC Asia Dental Pte. Ltd.; Singapore: 2002. [Google Scholar]
- 17.WHO . International Statistical Classification of Diseases and Related Health Problems. 11th ed. World Health Organization; Geneva, Switzerland: 2019. [Google Scholar]
- 18.Almståhl A., Wikström M. Oral microflora in subjects with reduced salivary secretion. J. Dent. Res. 1999;78:1410–1416. doi: 10.1177/00220345990780080601. [DOI] [PubMed] [Google Scholar]
- 19.Seethalakshmi C., Reddy R.C., Asifa N., Prabhu S. Correlation of Salivary pH, Incidence of Dental Caries and Periodontal Status in Diabetes Mellitus Patients: A Cross-Sectional Study. J. Clin. Diagn. Res. 2016;10:ZC12. doi: 10.7860/JCDR/2016/16310.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bozejac B.V., Stojšin I., Ðuric M., Zvezdin B., Brkanić T., Budišin E., Vukoje K., Sečen N. Impact of inhalation therapy on the incidence of carious lesions in patients with asthma and COPD. J. Appl. Oral Sci. 2017;25:506–514. doi: 10.1590/1678-7757-2016-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González-Aragón Pineda A.E., García Pérez A., García-Godoy F. Salivary parameters and oral health status amongst adolescents in Mexico. BMC Oral Health. 2020;20:190. doi: 10.1186/s12903-020-01182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aframian D.J., Konttinen Y.T., Carrozzo M., Tzioufas A.G. Urban legends series: Sjögren’s syndrome. Oral Dis. 2013;19:46–58. doi: 10.1111/j.1601-0825.2012.01930.x. [DOI] [PubMed] [Google Scholar]
- 23.Ship J. Diagnosing, managing, and preventing salivary gland disorders. Oral Dis. 2002;8:77–89. doi: 10.1034/j.1601-0825.2002.2o837.x. [DOI] [PubMed] [Google Scholar]
- 24.Brookes Z., Belfield L.A., Ashworth A., Casas-Agustench P., Raja M., Pollard A.J., Bescos R. Effects of chlorhexidine mouthwash on the oral microbiome. J. Dent. 2021;113:103768. doi: 10.1016/j.jdent.2021.103768. [DOI] [PubMed] [Google Scholar]
- 25.Meurman J.H., ten Gate J.M. Pathogenesis and modifying factors of dental erosion. European J. Oral Sci. 1996;104:199–206. doi: 10.1111/j.1600-0722.1996.tb00068.x. [DOI] [PubMed] [Google Scholar]
- 26.Kato M.T., Leite A.L., Hannas A.R., Buzalaf M.A. Gels containing MMP inhibitors prevent dental erosion in situ. J. Dent. Res. 2010;89:468–472. doi: 10.1177/0022034510363248. [DOI] [PubMed] [Google Scholar]
- 27.Buzalaf M.A.R., Hannas A.R., Kato M.T. Saliva and dental erosion. J. Appl. Oral Sci. 2012;20:493–502. doi: 10.1590/S1678-77572012000500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sorsa T., Suomalainen K., Helenius J., Lindy S., Saari H., Konttinen Y.T., Uitto V.-J. Periodontal disease. (A letter) N. Engl. J. Med. 1990;323:133–134. [Google Scholar]
- 29.Sorsa T., Sahni V., Buduneli N., Gupta S., Räisänen I.T., Golub L.M., Lee H.M., Pätilä T., Bostanci N., Meurman J., et al. Active matrix metalloproteinase-8 (aMMP-8) point-of-care test (POCT) in the COVID-19 pandemic. Expert Rev. Proteom. 2021;18:707–717. doi: 10.1080/14789450.2021.1976151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pärnänen P., Sorsa T., Tervahartiala T., Nikula-Ijäs P. Isolation, characterization and regulation of moonlighting proteases from Candida glabrata cell wall. Microb. Pathog. 2020;149:104547. doi: 10.1016/j.micpath.2020.104547. [DOI] [PubMed] [Google Scholar]
- 31.Nohynek L.J., Alakomi H.-L., Kähkönen M.P., Heinonen M., Helander I.M., Oksman-Caldentey K.-M., Puupponen-Pimiä R.H. Berry phenolics: Antimicrobial properties and mechanisms of action against severe human pathogens. Nutr. Cancer. 2006;54:18–32. doi: 10.1207/s15327914nc5401_4. [DOI] [PubMed] [Google Scholar]
- 32.Heinonen M. Antioxidant activity and antimicrobial effect of berry phenolics—A Finnish perspective. Mol. Nutr. Food Res. 2007;51:684–691. doi: 10.1002/mnfr.200700006. [DOI] [PubMed] [Google Scholar]
- 33.Riihinen K.R., Ou Z.M., Gödecke T., Lankin D.C., Pauli G.F., Wu C.D. The antibiofilm activity of lingonberry flavonoids against oral pathogens is a case connected to residual complexity. Fitoterapia. 2014;97:78–86. doi: 10.1016/j.fitote.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Nikolaeva-Glomb L., Mukova L., Nikolova N., Badjakov I., Dincheva I., Kondakova V., Doumanova L., Galabov A.S. In vitro antiviral activity of a series of wild berry fruit extracts against representatives of Picorna-, Orthomyxo- and Paramyxoviridae. Nat. Prod. Commun. 2014;9:51–54. doi: 10.1177/1934578X1400900116. [DOI] [PubMed] [Google Scholar]
- 35.Kylli P., Nohynek L., Puupponen-Pimiä R., Westerlund-Wikström B., Leppänen T., Welling J., Moilanen E., Heinonen M. Lingonberry (Vaccinium vitis-idaea) and European Cranberry (Vaccinium microcarpon) Proanthocyanidins: Isolation, Identification and Bioactivities. J. Agric. Food Chem. 2011;59:3373–3384. doi: 10.1021/jf104621e. [DOI] [PubMed] [Google Scholar]
- 36.Ho K.Y., Huang J.S., Tsai C.C., Lin T.C., Hsu Y.F., Lin C.C. Antioxidant Activity of Tannin Components from Vaccinium vitis-idaea L. J. Pharm. Pharmacol. 1999;51:1075–1078. doi: 10.1211/0022357991773410. [DOI] [PubMed] [Google Scholar]
- 37.Kähkönen M.P., Hopia A.I., Heinonen M. Berry Phenolics and Their Antioxidant Activity. J. Agric. Food Chem. 2001;49:4076–4082. doi: 10.1021/jf010152t. [DOI] [PubMed] [Google Scholar]
- 38.Zheng W., Wang S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries, and lingonberries. J. Agric. Food Chem. 2003;51:502–509. doi: 10.1021/jf020728u. [DOI] [PubMed] [Google Scholar]
- 39.Viljanen K., Kylli P., Kivikari R., Heinonen M. Inhibition of Protein and Lipid Oxidation in Liposomes by Berry Phenolics. J. Agric. Food Chem. 2004;52:7419–7424. doi: 10.1021/jf049198n. [DOI] [PubMed] [Google Scholar]
- 40.Määttä-Riihinen K.R., Kähkönen M.P., Törrönen A.R., Heinonen M. Catechins and Procyanidins in Berries of Vaccinium Species and Their Antioxidant Activity. J. Agric. Food Chem. 2005;53:8485–8491. doi: 10.1021/jf050408l. [DOI] [PubMed] [Google Scholar]
- 41.Wu C.-H., Yen G.-C. Inhibitory Effect of Naturally Occurring Flavonoids on the Formation of Advanced Glycation End-Products. J. Agric. Food Chem. 2005;53:3167–3173. doi: 10.1021/jf048550u. [DOI] [PubMed] [Google Scholar]
- 42.Mane C., Loonis M., Juhel C., Dufour C., Malien-Aubert C. Food Grade Lingonberry Extract: Polyphenolic Composition and In Vivo Protective Effect against Oxidative Stress. J. Agric. Food Chem. 2011;59:3330–3339. doi: 10.1021/jf103965b. [DOI] [PubMed] [Google Scholar]
- 43.Hoornstra D., Vesterlin J., Parnanen P., Al-Samadi A., Zlotogorski-Hurvitz A., Vered M., Salo T. Fermented Lingonberry Juice Inhibits Oral Tongue Squamous Cell Carcinoma Invasion In Vitro Similarly to Curcumin. In Vivo. 2018;32:1089–1095. doi: 10.21873/invivo.11350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bomser J., Madhavi D.L., Singletary K., Smith M.A. In vitro anticancer activity of fruit extracts from Vaccinium species. Planta Med. 1996;62:212–216. doi: 10.1055/s-2006-957862. [DOI] [PubMed] [Google Scholar]
- 45.Olsson M.E., Gustavsson K.-E., Andersson S., Nilsson Å., Duan R.-D. Inhibition of Cancer Cell Proliferation in Vitro by Fruit and Berry Extracts and Correlations with Antioxidant Levels. J. Agric. Food Chem. 2004;52:7264–7271. doi: 10.1021/jf030479p. [DOI] [PubMed] [Google Scholar]
- 46.McDougall G.J., Ross H.A., Ikeji M., Stewart D. Berry Extracts Exert Different Antiproliferative Effects against Cervical and Colon Cancer Cells Grown in Vitro. J. Agric. Food Chem. 2008;56:3016–3023. doi: 10.1021/jf073469n. [DOI] [PubMed] [Google Scholar]
- 47.Kivimäki A.S., Ehlers P.I., Siltari A., Turpeinen A.M., Vapaatalo H., Korpela R. Lingonberry, cranberry and blackcurrant juices affect mRNA expressions of inflammatory and atherotrombotic markers of SHR in a long-term treatment. J. Funct. Foods. 2012;4:496–503. doi: 10.1016/j.jff.2012.02.010. [DOI] [Google Scholar]
- 48.Kivimäki A.S., Siltari A., Ehlers P.I., Korpela R., Vapaatalo H. Lingonberry juice negates the effects of a high salt diet on vascular function and low-grade inflammation. J. Funct. Foods. 2014;7:238–245. doi: 10.1016/j.jff.2014.02.005. [DOI] [Google Scholar]
- 49.Eid H.M., Ouchfoun M., Brault A., Vallerand D., Musallam L., Arnason J.T., Haddad P.S. Lingonberry (Vaccinium vitis-idaea L.) Exhibits Antidiabetic Activities in a Mouse Model of Diet-Induced Obesity. Evid. Based Complementary Altern. Med. 2014;2014:645812. doi: 10.1155/2014/645812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marungruang N., Kovalenko T., Osadchenko I., Voss U., Huang F., Burleigh S., Ushakova G., Skibo G., Nyman M., Prykhodko O., et al. Lingonberries and their two separated fractions differently alter the gut microbiota, improve metabolic functions, reduce gut inflammatory properties, and improve brain function in ApoE−/− mice fed high-fat diet. Nutr. Neurosci. 2020;23:600–612. doi: 10.1080/1028415X.2018.1536423. [DOI] [PubMed] [Google Scholar]
- 51.Törrönen R., Kolehmainen M., Sarkkinen E., Mykkänen H., Niskanen L. Postprandial glucose, insulin, and free fatty acid responses to sucrose consumed with blackcurrants and lingonberries in healthy women. Am. J. Clin. Nutr. 2012;96:527–533. doi: 10.3945/ajcn.112.042184. [DOI] [PubMed] [Google Scholar]
- 52.Törrönen R., Kolehmainen M., Sarkkinen E., Poutanen K., Mykkänen H., Niskanen L. Berries reduce postprandial insulin responses to wheat and rye breads in healthy women. J. Nutr. 2013;143:430–436. doi: 10.3945/jn.112.169771. [DOI] [PubMed] [Google Scholar]
- 53.Bose M., Mitra B., Mukherjee P. Mucin signature as a potential tool to predict susceptibility to COVID-19. Physiol. Rep. 2021;9:e14701. doi: 10.14814/phy2.14701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pärnänen P., Lähteenmäki H., Räisänen I., Tervahartiala T., Sorsa T. Lingonberry polyphenols: Potential SARS-CoV-2 inhibitors as nutraceutical tools? Physiol. Rep. 2021;9:e14741. doi: 10.14814/phy2.14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Finnish National Nutrition Council . Food Recommendations. 5th ed. Finnish National Nutrition Council; Helsinki, Finland: 2014. (In Finnish) [Google Scholar]
- 56.Burci L., Barbosa P., da Silva C.B., Zanin S., Miguel O., Dias J., Miguel M. Patentability potential of natural products for xerostomia treatment. Braz. Dent. Sci. 2016;19:4–12. doi: 10.14295/bds.2016.v19i1.1168. [DOI] [Google Scholar]
- 57.Navarro Morante A., Wolff A., Bautista Mendoza G.R., López-Jornet P. Natural products for the management of xerostomia: A randomized, double-blinded, placebo-controlled clinical trial. J. Oral Pathol. Med. 2017;46:154–160. doi: 10.1111/jop.12487. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting reported results can be obtained from the authors on request.