Abstract
Intestinal diseases, such as inflammatory bowel diseases (IBDs) and colorectal cancer (CRC), are a significant source of morbidity and mortality worldwide. Epidemiological data have shown that IBD patients are at an increased risk for the development of CRC. IBD-associated cancer develops against a background of chronic inflammation and oxidative stress, and their products contribute to cancer development and progression. Therefore, the discovery of novel drugs for the treatment of intestinal diseases is urgently needed. Licorice (Glycyrrhiza glabra) has been largely used for thousands of years in traditional Chinese medicine. Licorice and its derived compounds possess antiallergic, antibacterial, antiviral, anti-inflammatory, and antitumor effects. These pharmacological properties aid in the treatment of inflammatory diseases. In this review, we discuss the pharmacological potential of bioactive compounds derived from Licorice and addresses their anti-inflammatory and antioxidant properties. We also discuss how the mechanisms of action in these compounds can influence their effectiveness and lead to therapeutic effects on intestinal disorders.
Keywords: Glycyrrhiza glabra-derived compounds, glycyrrhizin (G), glycyrrhetinic acid (GA), dipotassium glycyrrhizinate (DPG), inflammation, oxidative stress, intestinal disorders
1. Introduction
Licorice (Glycyrrhiza glabra) has been used in traditional Chinese medicine for thousands of years. Clinically, it is used widely to treat immune systems, respiratory, and digestive diseases [1,2,3,4,5,6], and no severe side effects have been reported so far [7]. In addition, Licorice-derived compounds possesses antiallergic, antibacterial, antiviral, anti-inflammatory, and anticarcinogenic effects [8,9,10]. These pharmacological properties aid in inflammatory disease treatment [11,12,13] (Figure 1).
Figure 1.
Licorice pharmacological properties.
The main bioactive compounds isolated from Licorice are glycyrrhizin (G) and glycyrrhetinic acid (GA) [14]. G is a triterpene glycoside complex and has been shown to possess cytotoxic effects against several cancer cell lines such as colon, lung, leukemia, melanoma, and glioblastoma (GBM) [9,15,16,17,18,19,20,21]. Additionally, the incidence of liver carcinogenesis in patients with hepatitis C was clinically reduced after G administration [22]. GA, an aglycone of G, has been demonstrated to have pro-apoptotic effects on human hepatoma, promyelocytic leukemia, stomach cancer, Kaposi sarcoma-associated herpesvirus-infected cells, and prostate cancer cells in vitro by inducing DNA fragmentation and oxidative stress [23,24,25]. In addition, several genotoxic studies have indicated that G is neither teratogenic nor mutagenic and may possess anti-genotoxic properties under certain conditions [26,27]. As a result, there is a high level of use of Licorice and GZ in the US with an estimated consumption of 0.027–3.6 mg/kg/day [27].
However, GA oral efficacy is impaired due to its low solubility and permeability through the gastrointestinal mucosa [28]. It has been shown that GA administered through nanocarriers (GA-F127/TPGS-MMs) [29], micellar carrier based on polyethylene glycol-derivatized GA (PEG-Fmoc-GA) [30], and microparticles [31] increase absorption significantly [28,29,30,31]. Both G and GA have been prescribed for several therapeutic purposes, such as cancer and inflammation; however, side effects have pointed out the problem of their toxicity [32].
Dipotassium glycyrrhizinate (DPG), a dipotassium salt of GA, has been recently used as a flavoring and skin conditioning agent with demonstrated anti-allergic and anti-inflammatory properties [32]. It can inhibit leukotriene and reduce histamine levels with an apparent lack of adverse side effects [32,33,34]. In addition, it has been demonstrated that DPG has anti-inflammatory, antioxidant, immunomodulatory, anti-ulcerative, and antitumoral properties [11,13,35].
In this context, this review examines recent studies on the pharmacological properties of some bioactive compounds derived from Licorice and addresses their anti-inflammatory and antioxidant properties, as well as their therapeutic effects on gastrointestinal disorders.
2. G, GA, and DPG-Mediated Anti-Inflammation Regulation
As stated previously, Licorice compounds such as G, GA, and DPG have anti-inflammatory, antioxidant, antiviral, immunomodulatory, and antitumor properties [11,12,13]. Inflammation is an evolutionarily conserved, tightly regulated protective mechanism that comprehends immune, vascular, and cellular biochemical reactions. The normal inflammatory response is temporally restricted and, in general, beneficial to the host. Chronic inflammatory response, on the other hand, is a risk factor for the development of several diseases such as ischemic heart disease, stroke, cancer, and diabetes mellitus, among others [36,37]. Taking this into account, natural compounds have been widely used to treat all sorts of inflammatory conditions.
The anti-inflammatory effects of G and GA have long been reported. G has exerted anti-inflammatory actions by inhibiting the generation of reactive oxygen species (ROS) by neutrophils, the most potent inflammatory mediator at the site of inflammation [38]. Moreover, G has enhanced interleukin (IL)-10 production by liver dendritic cells in mice with hepatitis [39]. GA has presented anti-inflammatory and anticarcinogen effects on several tumor cell lines such as human hepatoma (HLE), promyelocytic leukemia (HL-60), stomach cancer (KATO III), and prostate cancer (LNCaP e DU-145) by both DNA fragmentation and gene deregulation required for oxidative stress control [23,24,25].
More recently, one study exposed the U251 GBM cell line to different concentrations of GA (1 mM, 2 mM, and 4 mM), and the authors observed that the inhibition of cell proliferation and colony formation, apoptosis stimulation, and significant decrease in p65 protein, which is responsible for the activation of the nuclear factor kappa B (NF-κB) pathway [40]. The NF-κB pathway is constantly activated in GBM and is responsible for the aggressiveness of the disease and regulation of the expression of anti-apoptotic genes, and cell adhesion and invasion factors [41]. Thus, some studies have suggested that the inhibition of the NF-κB pathway could decrease the resistance of tumor cells to chemotherapy and contribute to increasing the survival of patients with GBM [42,43,44,45].
Recently, a study has shown that DPG exposure has anti-tumoral effects on GBM cell lines (U87MG and T98G) through cell proliferation decrease and apoptosis stimulation. Furthermore, DPG anti-tumoral effect was related to NF-κB pathway suppression by IRAK2 and TRAF6 mediating miR-16 and miR-146a, respectively. Finally, the authors have also shown that DPG was able to inhibit the subpopulation of stem cells essential for tumor formation, survival, and recurrence [46].
3. G, GA, and DPG-Mediated Crosstalk between Inflammation and Oxidative Stress Pathways
Oxidative stress consists of an imbalance of endogenous pro-oxidant and antioxidant activities, characterized by excessive formation of high ROS and reactive nitrogen species (RNS) [47]. Small amounts of ROS are synthesized physiologically and act on cell homeostasis; however, in the disease context, the excessive synthesis of ROS disrupts the antioxidant defense system, causing cellular apoptosis [47]. This condition is commonly associated with oxidative changes such as lipid peroxidation, protein carbonylation, carbonyl adduct, nitration, and DNA impairment as well as the induction of inflammatory processes, leading to several diseases [48,49]. Cyclooxygenase type 2 (Cox-2) and inducible nitric oxide synthase (iNOS) enzymes, responsible for the release of pro-inflammatory mediators, prostaglandin E2 (Pge-2), and nitric oxide (NO), play relevant roles in oxidative and acute inflammatory processes [49].
The high mobility group box 1 (Hmgb1) cytokine plays an important role in the pathologic process of endothelial permeability under oxidative stress [49]. DPG and G have presented antioxidant effects due to their negative modulation of Hmgb1 in the DSS-induced colitis mice model [49]. It has been shown that G inhibits Hmgb1-cytokine secretion by blocking the Cytochrome C release and caspase-3 activity, consequently inhibiting apoptosis in inflammation-related stroke rat models [50,51]. In addition, the G compound decreases the iNOS, TNF-α, IL-1β, and IL-6 expression levels by the modulation of p38 mitogen-activated protein kinases (p38-MAPK) and c-Jun N-terminal kinase (p-JNK) signaling pathways in brain vascular cells [51] and by preventing oxidative stress and apoptosis through the inhibition of p38-MAPK, p-JNK, and NF-κB signaling pathways in lung cells [52].
Accordingly, the G compound can inhibit oxidative stress and inflammatory response by attenuating the activity of the Hmgb1 and NF-κB signaling pathways, with decreased levels of malondialdehyde (MDA) and cytokines (TNF-α, IL-1β and IL-6) in lung cells [53]. Moreover, G increases glutathione-S-transferase (GSTs) levels, decreases MDA, and negatively regulates the expression of TNF-α, IL-6, iNOS, and monocyte chemotactic protein-1 (MCP-1) in liver cells [54]. G compound has been shown to suppress NF-κB pathway through inhibiting the toll-like receptor 4 (TLR4) in renal cells [55] and reducing the formation of intracellular ROS. Moreover, an activation of the AMP/nuclear factor erythroid-2-related factor-2 (NRF2) pathways in vitro was observed, positively regulating the antioxidant enzymes, HO-1, NQO-1, and GCLC and negatively regulating TNF-α, IL-1β, and IL-6 [56].
According to descriptions, GA also suppresses oxidative stress and neuroinflammation induced by A1C13 through TLR4/NF-κB signaling pathway inhibition [57]. In accordance, one study has observed that GA was able to attenuate oxidative stress and neuroinflammation induced by rotenone reducing the activation of the ionized calcium-binding adapter molecule-1 (Iba-1), preventing glutathione depletion, lipid peroxidation inhibition, and attenuation of the induction of COX-2 and iNOS [58]. In addition, a restored mitochondrial complex I and IV, a reduction in the generation of ROS, the release of Cytochrome C, and ultimately cell apoptosis inhibition after exposure to GA in brain tissue of adult Sprague Dawley Rats were observed [59].
GA can suppresses lipopolysaccharide (LPS)-induced oxidative stress, inflammation, and apoptosis through activation of the extracellular signal-regulated kinase (ERK) pathway, and inhibition of the NF-κB in renal cells [60]. GA also suppresses oxidative stress and inflammation through activation of the NRF-2 and HO-1 pathways and IκB and NF-κB p65 signaling inhibition in cardiac cells [61].
In the liver tissue of rats, GA inhibits NTiO2-induced apoptosis by superoxide dismutase (SOD) and glutathione peroxidase (GPx) activation [61]. Moreover, it has been shown that GA can inhibit caspase-3 and -9 at mitochondria in HepG2 cells, positively and negatively regulating Bcl-2 and Bax proteins expression, respectively [62]. Table 1 summarizes the studies used in this review.
Table 1.
Summary of studies showing the autoinflammatory and anti-tumoral effects of G, GA, and DPG.
| Model | Compound (Dose) | Mechanism | Reference |
|---|---|---|---|
| In vitro (KATO III and HL-60) | G (1 to 10 mg/mL) | Antitumor activity ↑ apoptosis | [23] |
| In vitro (HLE, KATO III, and HL-60) | G (0.1 to 1 mg/mL) | Antitumor activity ↑ apoptosis | [24] |
| In vitro (DU-145 and LNCaP) |
G (1 to 20 mM) | Antitumor activity ↑ apoptosis | [25] |
| In vitro (Caco3, HT29, and RAW 264.7) In vivo (Acute lung injury mice model) |
DPG (300 µM) DPG (3 and 8 mg/kg/day) |
↓ TNF-α, IL-1β, and IL-6, as well as HMGB1 receptors, RAGE and TLR4 | [34] |
| In vitro (neutrophils) | G (0.05, 0.5, and 5.0 µg/mL) | ↓ ROS | [38] |
| In vivo (Con A-induced hepatitis) Ex vivo (liver dendritic cells) |
G (2 mg/mouse) G (0.1 mg/mL) |
↑ IL-10 and ↓ liver inflammation | [39] |
| In vitro (U251) | GA (1, 2, 4 mM) | Anticancer effect ↓ proliferation and ↑ apoptosis possibly related to the NF-κB mediated pathway | [40] |
| In vitro (U87MG and T98G) | DPG (0.1 to 2 mM) | Anticancer effect ↓ proliferation and ↑ apoptosis. ↓ NF-κB pathway | [46] |
| In vivo (DSS-induced colitis mice model) | DPG (8 mg/kg/day) | ↓ colitis, at the earlier stages, ↓ inflammation though AMPK-COX-2-PGE. At later times ↓ iNOS and COX-2 in HMGB1-dependent manner | [49] |
| In vivo (mechanical thrombectomy rat model) | G (2, 4 and 10 mg/kg/day) | ↓ HMGB1 and its downstream inflammatory factors, and ↓ oxidative stress |
[50] |
| In vivo (Focal cerebral I/R injury rat model) | G (4 mg/kg/day) | ↓ HMGB1 and ↑ apoptosis through the blockage of the JNK and p38 | [51] |
| In vivo (Sepsis-induced acute lung injury rat model) | G (25 and 50 mg/kg/day) | ↓ inflammatory responses, oxidative stress damage, and apoptosis though ↓ NF-κB, JNK, and p38 MAPK |
[52] |
| In vivo (Acute lung injury mice model) | G (20 and 40 mg/kg/day) | ↓ LPS-induced lung injury via blocking HMGB1/TLRs/NF-κB pathway | [53] |
| In vitro (RAW 264.7 and bone marrow monocytes) | G (25 to 100 µM) | ↓ RANKL-induced osteoclastogenesis and oxidative stress through ↑ AMPK/Nrf2 and ↓ NF-κB and MAPK | [56] |
| In vivo (Parkinson rat model) | GA (50 mg/kg/day) | ↓ dopamine neuron loss and ↓ Iba-1 and GFAP ↑ antioxidant enzyme activity, ↓ lipid peroxidation, ↓ pro-inflammatory cytokines |
[58] |
| In vivo (Vascular dementia rat model) | GA (20 mg/kg/day) | ↓ release of cytochrome-c and ↑ Bcl2, and ↑ the endogenous antioxidants |
[59] |
| In vitro (HBZY-1) In vivo (sepsis-induced acute kidney injury mice model) |
GA (50 and 100 µM) GA (25 and 50 mg/kg/day) |
↓ oxidative stress via ↑ ERK signaling pathway. ↓ NF-κB | [60] |
| In vivo (myocardial ischemic injury-rat model) | GA (10 and 20 mg/kg/day) | ↓ oxidative stress and inflammatory cytokines. ↑ Nrf2 antioxidant response ↓ NF-κB activation |
[61] |
| In vitro (HEPG2) | G (5, 25 and 125 µg/mL) | ↓ H2O2-induced oxidative stress, ↑ apoptosis | [62] |
| In vitro (HT29) | GA (1, 5 and 10 µM) | ↓ TNF-α-mediated IL-8 through ↓ MAPK and the IKB/NF-κB pathway | [63] |
| In vivo (DSS-induced colitis mice model) | GA (10 and 50 mg/kg/day) | ↓ colitis, ↓ inflammation by regulating COX-2 and NF-κB | [64] |
| In vivo (rat model of ulcerative colitis) | G (40 mg/kg/day) | ↓ colitis, ↓ inflammatory injury via suppression of NF-κB, TNF-α, and ICAM-1 |
[65] |
| In vivo (TNBS-induced experimental colitis mice model) | G (10, 30 and 90 mg/kg/day) | ↓ colitis, ↓ IFN-γ, IL-12, TNF-α, and IL-17 and ↑ IL-10 | [66] |
| In vivo (DSS-induced colitis rat model) | G (2 mg rectally) | ↓ colitis, ↓ IL-1β, IL-6, TNF-α, Cxcl-2, Mcp1, and MPO | [67] |
| In vivo (TNBS-induced experimental colitis rat model) | GA (2, 10 and 50 mg/kg, rectally and 10 mg/kg/day) | ↓ colitis, ↓ serum levels of TNF-α and IL-1β, ↓ colon MPO and MDA, and ↑ SOD | [68] |
| In vivo (rat model of ulcerative colitis) | G (100 mg/kg/day) | ↓ colitis, when combined with emu synergistically ↓ of PPARγ and TNF-α | [69] |
| In vivo (TNBS-induced experimental colitis mice model) | G (50 mg/kg/day) | ↓ colitis, ↓ HMGB1 on DC/macrophage mediated Th17 proliferation | [70] |
| In vivo (indomethacin-induced small intestinal injury mice model) | GA (100 mg/kg/day) | ↓ TNF-α, IL-1β, and IL-6, ↑ indomethacin-induced small intestinal damage | [71] |
| In vivo (DSS-induced colitis mice model) | G (100 mg/kg/day) | ↓ colitis, regulated the phosphorylation of transcription factors such as NF-κB p65 and IκB α | [72] |
| In vivo (DSS-induced colitis mice model) | DPG (8 mg/kg/day) | ↑ mucosal healing by ↓ CXCL1, CXCL3, CXCL5, PTGS2, IL-1β, IL-6, CCL12, CCL7; ↑ wound healing genes COL3A1, MMP9, VTN, PLAUR, SERPINE, CSF3, FGF2, FGF7, PLAT, TIMP1 and ↑ extracellular matrix remodeling genes, VTN, and PLAUR |
[73] |
4. Therapeutic Effect of G, GA, and DPG for Intestinal Disorders
Crohn’s Disease (CD) and Ulcerative Colitis (UC) are the main inflammatory bowel diseases (IBD) that affect the gastrointestinal tract. These diseases are characterized by chronic and progressive inflammation of the gastrointestinal tract, associated with extraintestinal manifestations such as arthritis, uveitis, erythema nodosum, gangrenous pyoderma, and cholangitis [74,75]. It has been shown that inflammatory reaction and the increased production of ROS are commonly associated with the pathogenesis of IBD [49,76]. ROS are toxic to cells and their overproduction causes breakage of the various lines of defense that make up the mucosal barrier [76]. The dysregulation of the immune response and the exaggerated release of pro-inflammatory/interleukin cytokines (IL-1, IL-6, IL-8, and TNF-α) culminate in the exacerbation of intestinal inflammation [63,64]. Thus, oxidative stress is considered an initial step to the colonic epithelium inflammation of patients with IBD.
Patients with IBD are at increased risk of developing colorectal cancer (CRC). Epidemiological data from patients with UC estimate that the risk of CRC is approximately 2- to 3-fold more than the general population, and patients with CD appear to have a similar increased risk [77]. Chronic inflammation is the most important aspect of neoplastic progression, resulting in dysplastic precursor lesions that may arise from different areas of the colon [78]. The overproduction of ROS can damage the DNA of the chronically inflamed colonic mucosa cells, increasing the mutation rate in genes related to development of CRC [76,79]. Oxidative reactions are an integral part of the inflammatory response and are generally associated with CRC development [80]. The potential mechanisms for the natural alkaloids in the treatment of UC has been recently described, showing that its positive effects are closely related to the modulation of oxidative stress, immune response, intestinal microbiota, and improvement of the gut barrier function [81]. Considering that oxidative stress is one of the main factors related to the development of IBD and IBD-related CRC, it is possible that the use of active principles found in Glycyrrhiza glabra extract may be effective for the treatment and prevention of both diseases [82] (Table 1).
Several studies have evaluated the effectiveness of G, GA, and GL in experimental models of induced colitis [65,66,67,68,83]. The oral administration or application of enemas containing these drugs, alone or associated with other substances with anti-inflammatory activity, can reduce the inflammatory process of the colonic mucosa, and oxidative tissue damage as well as improve epithelial healing of the colonic mucosa [68,83].
Yuan et al. were the first authors to evaluate the effects of Glycyrrhizinate extract in an experimental model of acetic acid-induced colitis [65]. The authors described that Glycyrrhizinate extract has a potent anti-inflammatory effect that is mediated by the suppression of NF-κB, TNF-α, and ICAM-1 in colonic mucosa. Three years later, Sun et al. investigated the therapeutic potential of G in trinitrobenzene sulfonic acid (TNBS)-induced experimental colitis in mice [66]. After colitis induction by TNBS, G was administered by gavage (10 mg/kg, 20 mg/kg, and 30 mg/kg) for 10 days. G significantly ameliorated TNBS-induced colitis and dose-dependently decreased macroscopic and microscopic inflammation scores, and MPO activity. Mechanistically, G downregulated the colonic levels of the pro-inflammatory cytokines IFN-α, IL-12, TNF-α, and IL-17 and increased the anti-inflammatory cytokine IL-10. The efficacy of G topical application in the treatment of a rat model of UC was also evaluated in experimental colitis models induced by dextran sodium sulfate (DSS). G significantly ameliorated the extent of colitis, which was associated with a decrease in the expression levels of pro-inflammatory cytokines and chemokines, including interleukin IL-1β, IL-6, TNF-α, CXCL2, and CCL2 in the inflamed mucosa. G also inhibited MPO activity in the inflamed mucosa and had a therapeutic effect on experimental colitis in rats [67]. A synergistic effect was observed when G was combined with emu oil in a colitis rat model induced by acid acetic. The authors observed that the treatment combination significantly improved their ability to reduce macroscopic and microscopic lesions as well as to decrease MPO levels and enhanced downmodulation on PPARγ and TNF-α expression [69]. More recently, Chen et al. reported that G ameliorated colitis and decreased the production of inflammatory mediators such as HMGB1, IFN-γ, IL-6, TNF-α, and IL-17. Furthermore, G suppressed the proliferation of Th17 cells in colitis and inhibited the ability of dendritic cells and macrophages to induce the differentiation of Th17 cells that was enhanced in the presence of HMGB1 [70].
To find the best route of administration, Liu et al. compared rectal and oral treatments with GA in TNBS-induced colitis in rats. Both rectally and orally administered treatment effectively attenuated colitis at different dosages. Furthermore, administration by both routes decreased serum levels of TNF-α and IL-1β, colon MPO activity and MDA concentration, and elevated SOD activity [68]. It has been demonstrated that GA is capable of blocking prostaglandin-E2 synthesis via blockade of COX-2 resulting in concurrent augmentation of nitric oxide production on indomethacin-induced small intestinal injury in mice [71]. In addition, using an ulcerative colitis mice model, GA reduced IL-6 and IL-1β, regulating the phosphorylation of NF-κB and IkB-α, and the expression of COX-2 and PGE2 in an ulcerative colitis model [72]. The anti-inflammatory mechanisms of both G and GA were described as mediated by IFN-y, TNF-alpha, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, and IL-17. Moreover, G and GA mediated intercellular adhesion molecules 1, P-selectin, iNOS, and the NF-κB pathway through the nuclear translocation of NF-κB and activation of STATs 3 and 6 [11]. In human colonic epithelial cell line HT-29, GA exhibits the inhibitory activity on TNF-α and IL-8 production and the blockade of the MAPK and the IKB/NF-κB pathways [63].
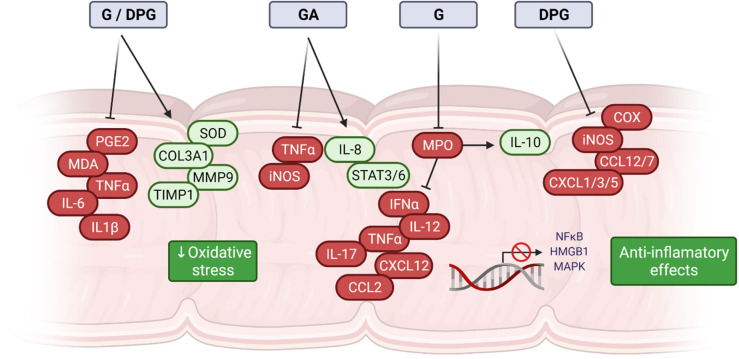
The use of DPG as a therapeutic strategy to overcome intestinal inflammation was also evaluated. Vitali et al. studied the DPG effects on HMGB1, an early pro-inflammatory cytokine that is released from injured cells during inflammation. In vitro assays show that DPG significantly reduces the release of HMGB1 as well as expression levels of pro-inflammatory cytokines, TNF-α, IL-1β and IL-6. In vivo, DPG decreases the severity of DSS-induced colitis as well as intestinal inflammation reduction mediated by a downregulation of the pro-inflammatory cytokines TNF-α, IL-1β, and IL-6, as well as HMGB1 receptors, RAGE, and TLR4 [34]. Posteriorly, the same research group showed that DPG has a protective effect on colitis and inflammation through the inhibition of oxidative tissue stress [49]. It was observed that DPG can decrease oxidative stress through the inhibition of iNOS and COX-2 ameliorating DSS-induced colitis in mice. It was demonstrated in vitro that DPG decreases inflammation-related oxidative stress, through (i) an earlier ability to promote AMP-activated kinase (AMPK)-phosphorylation and (ii) a later HMGB1-dependent mechanism. Moreover, DPG can also improve colonic inflammation in DSS-induced colitis mice model through downregulation of the pro-inflammatory genes (CXCL1, CXCL3, CXCL5, PTGS2, IL-1β, IL-6, CCL12, and CCL7), and upregulation of genes involved in healing (COL3A1, MMP9, VTN, PLAUR, SERPINE, CSF3, FGF2, FGF7, PLAT, and TIMP1), which contribute to accelerating intestinal mucosa repair in induced colitis [73]. It has recently been shown that DPG increases the expression of the receptors farnesoid-X-receptor (FXR), pregnane-X-receptor (PXR), and G-protein-coupled-receptor (GPCR; TGR5), decreasing the oxidative stress and consequently intestinal/hepatic inflammation in DSS colitis animal model, with a decrease in IL-8 [84]. Figure 2 summarizes the main mechanism of action of G, GA, and DPG.
Figure 2.
Molecular mechanisms of Glycyrrhiza glabra-derived compounds in intestinal disorders. Compounds derived from Glycyrrhiza glabra have anti-inflammatory potential. G, GA, and DPG act through the inhibition of HMGB1, TLR4, and RAGE receptors and significantly regulate important cytokines, interleukins, and genes involved in the inflammatory process. These effects are related to the capacity of regulating important inflammatory signaling pathways such as HMGB1, NF-κB, and MAPK. Oxidative stress is significantly reduced because of cellular and molecular changes, and consequently, the inflammatory process is attenuated as a result of treatment with these compounds.
The evidence from all these experimental studies suggests that the bioactive compounds from Licorice (Glycyrrhiza glabra) have anti-inflammatory and antioxidant effects in intestinal disorders through different mechanisms of action. Considering that these molecular features are also important in human intestinal disorders, it is reasonable to assume that Licorice might have similar activity in humans. Therefore, several clinical studies have focused on the pharmacological effects of Licorice on intestinal diseases (Table 2). However, to date, there is no clinical evidence showing the effect of Licorice in patients with IBD.
Table 2.
Clinical trials with Licorice in intestinal disorders.
| Drug | Clinical Trial | Phase | N of Pts | Status | Diseases | Results |
|---|---|---|---|---|---|---|
| Traditional Chinese Medication (containing 3 g of Licorice) | NCT03135821 | 2, 3 | 104 | Unknown | Irritable bowel syndrome | NA |
| Traditional Chinese Medicine (17 g herbal extract containing G) | NCT00676975 | 2 | 104 | Complete | Irritable bowel syndrome | NA |
| Modified Gegen Qinlian Decoction (containing 6 g of Licorice) | NCT04057547 | 1 | 60 | Recruiting | Ulcerative colitis | NA |
| Modified Gegen Qinlian Decoction (containing 6 g of Licorice) | NCT04312477 | 1 | 60 | Recruiting | Irritable bowel syndrome | NA |
| Traditional Chinese Medicine (17 g herbal extract containing 2 g of G) | NCT04368663 | NA | 100 | Recruiting | Pneumatosis cystoides intestinalis | NA |
Abbreviation: NA, not available.
It has also been shown that Licorice has potentially serious side effects for humans [27]. Clinical studies have shown that the most important side effects of Licorice and glycyrrhizin are hypertension and hypokalemic-induced secondary disorders [85]. Biochemical studies indicate that G inhibits 11beta-hydroxysteroid dehydrogenase, the enzyme responsible for inactivating cortisol. As a result, continuous, high-level exposure to GZ compounds can produce hypermineralocorticoid-like effects in both animals and humans [7,27]. Chronic use of Licorice can lead to hypokalemia and hypertension. Some people are more sensitive to licorice exposure [7]. Licorice side effects are increased by hypokalemia, prolonged gastrointestinal transient time, decreased 11-beta-hydroxysteroid dehydrogenase activities, hypertension, and anorexia nervosa [85]. These side-effects are reversible upon withdrawal of Licorice or Glycyrrhizin [27]. It can be assumed from these data that the consumption of Licorice extract products presents no concern for safe use as a supporting drug in patients with IBD. However, multicenter, randomized clinical studies that include a larger number of patients are still necessary to verify the benefits of using Licorice extracts in the treatment of IBD, and as a natural therapeutic strategy to prevent CRC in patients with extensive and active forms of long-term IBD.
5. Conclusions and Future Perspectives
The broad involvement of Licorice-derived compounds in intestinal disorders and their potential to overcome these disorders and the mechanism of action is presented in this review. In summary, the evidence from all these experimental studies suggests that the bioactive compounds obtained from Licorice have anti-inflammatory and antioxidant properties that affect anti-intestinal disorders through different mechanisms of action. This provides an interesting background for understanding how G, GA, and DPG compounds act and contributes to the development of natural therapeutic strategies and to the establishment of research models. In addition, more research is needed to determine the mechanism of action in different biological activities. Clinical trials on G, GA, and DPG are also required to validate these pharmacological effects, to establish these compounds as promising pharmaceuticals, and to fill some gaps regarding their safety and toxicological characteristics.
Acknowledgments
The financial support provided by the CAPES is gratefully acknowledged.
Author Contributions
C.d.S.L., G.A.B., J.C.S., C.A.R.M., M.M.O. and M.L.R. made a substantial contribution to all aspects of the preparation of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
M.L.R. acknowledges supports from CNPq (305402/2019-6). C.A.R.M. acknowledges supports from CNPq (303837/2018-7). Coordination of Superior Level Staff Improvement (CAPES) scholarship for C.d.S.L.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Deng Q.P., Wang M.J., Zeng X., Chen G.G., Huang R.Y. Effects of glycyrrhizin in a mouse model of lung adenocarcinoma. Cell. Physiol. Biochem. 2017;41:1383–1392. doi: 10.1159/000467897. [DOI] [PubMed] [Google Scholar]
- 2.Sun X., Zeng H., Wang Q., Yu Q., Wu J., Feng Y., Deng P., Zhang H. Glycyrrhizin ameliorates inflammatory pain by inhibiting microglial activation-mediated inflammatory response via blockage of the HMGB1-TLR4-NF-κB pathway. Exp. Cell Res. 2018;369:112–119. doi: 10.1016/j.yexcr.2018.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Yan T., Wang H., Cao L., Wang Q., Takahashi S., Yagai T., Li G., Krausz K.W., Wang G., Gonzalez F.J., et al. Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation. Drug Metab. Dispos. 2018;46:1310–1319. doi: 10.1124/dmd.118.082008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yao L., Sun T. Glycyrrhizin administration ameliorates Streptococcus aureus-induced acute lung injury. Int. Immunopharmacol. 2019;70:504–511. doi: 10.1016/j.intimp.2019.02.046. [DOI] [PubMed] [Google Scholar]
- 5.Paudel Y.N., Angelopoulou E., Semple B., Piperi C., Othman I., Shaikh M.F. Potential Neuroprotective Effect of the HMGB1 Inhibitor Glycyrrhizin in Neurological Disorders. ACS Chem. Neurosci. 2020;11:485–500. doi: 10.1021/acschemneuro.9b00640. [DOI] [PubMed] [Google Scholar]
- 6.Yang L., Jiang Y., Zhang Z., Hou J., Tian S., Liu Y. The anti-diabetic activity of licorice, a widely used Chinese herb. J. Ethnopharmacol. 2020;263:113216. doi: 10.1016/j.jep.2020.113216. [DOI] [PubMed] [Google Scholar]
- 7.Kwon Y.J., Son D.H., Chung T.H., Lee Y.J. A Review of the Pharmacological Efficacy and Safety of Licorice Root from Corroborative Clinical Trial Findings. J. Med. Food. 2020;23:12–20. doi: 10.1089/jmf.2019.4459. [DOI] [PubMed] [Google Scholar]
- 8.Shibata S. A drug over the millennia: Pharmacognosy, chemistry, and pharmacology of licorice. Yakugaku Zasshi. 2000;120:849–862. doi: 10.1248/yakushi1947.120.10_849. [DOI] [PubMed] [Google Scholar]
- 9.Menegazzi M., Di Paola R., Mazzon E., Genovese T., Crisafulli C., Dal Bosco M., Zou Z., Suzuki H., Cuzzocrea S. Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol. Res. 2008;58:22–31. doi: 10.1016/j.phrs.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 10.Gowda P., Patrick S., Joshi S.D., Kumawat R.K., Sen E. Glycyrrhizin prevents SARS-CoV-2 S1 and Orf3a induced high mobility group box 1 (HMGB1) release and inhibits viral replication. Cytokine. 2021;142:155496. doi: 10.1016/j.cyto.2021.155496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richard S.A. Exploring the Pivotal Immunomodulatory and Anti-Inflammatory Potentials of Glycyrrhizic and Glycyrrhetinic Acids. Mediat. Inflamm. 2021;2021:6699560. doi: 10.1155/2021/6699560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen K., Yang R., Shen F.-Q., Zhu H.-L. Advances in Pharmacological Activities and Mechanisms of Glycyrrhizic Acid. Curr. Med. Chem. 2019;27:6219–6243. doi: 10.2174/0929867325666191011115407. [DOI] [PubMed] [Google Scholar]
- 13.Rehman M.U., Farooq A., Ali R., Bashir S., Bashir N., Majeed S., Taifa S., Ahmad S.B., Arafah A., Sameer A.S., et al. Preclinical Evidence for the Pharmacological Actions of Glycyrrhizic Acid: A Comprehensive Review. Curr. Drug Metab. 2020;21:436–465. doi: 10.2174/1389200221666200620204914. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q., Ye M. Chemical analysis of the Chinese herbal medicine Gan-Cao (licorice) J. Chromatogr. A. 2009;1216:1954–1969. doi: 10.1016/j.chroma.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 15.Abe H., Ohya N., Yamamoto K.F., Shibuya T., Arichi S., Odashima S. Effects of glycyrrhizin and glycyrrhetinic acid on growth and melanogenesis in cultured B16 melanoma cells. Eur. J. Cancer Clin. Oncol. 1987;23:1549–1555. doi: 10.1016/0277-5379(87)90099-X. [DOI] [PubMed] [Google Scholar]
- 16.Chung J.G., Chang H.L., Lin W.C., Wang H.H., Yeh C.C., Hung C.F., Li Y.C. Inhibition of N-acetyltransferase activity and DNA-2-aminofluorene adducts by glycyrrhizic acid in human colon tumour cells. Food Chem. Toxicol. 2000;38:163–172. doi: 10.1016/S0278-6915(99)00151-9. [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi M., Fujita K., Katakura T., Utsunomiya T., Pollard R.B., Suzuki F. Inhibitory effect of glycyrrhizin on experimental pulmonary metastasis in mice inoculated with B16 melanoma. Anticancer Res. 2002;22:4053–4058. [PubMed] [Google Scholar]
- 18.Cassileth B.R., Deng G. Complementary and Alternative Therapies for Cancer. Oncologist. 2004;9:80–89. doi: 10.1634/theoncologist.9-1-80. [DOI] [PubMed] [Google Scholar]
- 19.Cragg G.M., Newman D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Khan R., Khan A.Q., Lateef A., Rehman M.U., Tahir M., Ali F., Hamiza O.O., Sultana S. Glycyrrhizic Acid Suppresses the Development of Precancerous Lesions via Regulating the Hyperproliferation, Inflammation, Angiogenesis and Apoptosis in the Colon of Wistar Rats. PLoS ONE. 2013;8:e56020. doi: 10.1371/journal.pone.0056020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang R.Y., Chu Y.L., Jiang Z.B., Chen X.M., Zhang X., Zeng X. Glycyrrhizin suppresses lung adenocarcinoma cell growth through inhibition of thromboxane synthase. Cell. Physiol. Biochem. 2014;33:375–388. doi: 10.1159/000356677. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda K., Arase Y., Kobayashi M., Saitoh S., Someya T., Hosaka T., Sezaki H., Akuta N., Suzuki Y., Suzuki F., et al. A long-term glycyrrhizin injection therapy reduces hepatocellular carcinogenesis rate in patients with interferon-resistant active chronic hepatitis C: A cohort study of 1249 patients. Dig. Dis. Sci. 2006;51:603–609. doi: 10.1007/s10620-006-3177-0. [DOI] [PubMed] [Google Scholar]
- 23.Hibasami H., Iwase H., Yoshioka K., Takahashi H. Glycyrrhizin induces apoptosis in human stomach cancer KATO III and human promyelotic leukemia HL-60 cells. Int. J. Mol. Med. 2005;16:233–236. doi: 10.3892/ijmm.16.2.233. [DOI] [PubMed] [Google Scholar]
- 24.Hibasami H., Iwase H., Yoshioka K., Takahashi H. Glycyrrhetic acid (a metabolic substance and aglycon of glycyrrhizin) induces apoptosis in human hepatoma, promyelotic leukemia and stomach cancer cells. Int. J. Mol. Med. 2006;17:215–219. doi: 10.3892/ijmm.17.2.215. [DOI] [PubMed] [Google Scholar]
- 25.Thiugnanam S., Xu L., Ramaswamy K., Gnanasekar M. Glycyrrhizin induces apoptosis in prostate cancer cell lines DU-145 and LNCaP. Oncol. Rep. 2008;20:1387–1392. doi: 10.3892/or_00000157. [DOI] [PubMed] [Google Scholar]
- 26.Sasaki Y.F., Kawaguchi S., Kamaya A., Ohshita M., Kabasawa K., Iwama K., Taniguchi K., Tsuda S. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res.—Genet. Toxicol. Environ. Mutagen. 2002;519:103–119. doi: 10.1016/S1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- 27.Isbrucker R.A., Burdock G.A. Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 2006;46:167–192. doi: 10.1016/j.yrtph.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 28.Yang J., Zhou L., Wang J., Wang C., Davey A.K. The disposition of diammonium glycyrrhizinate and glycyrrhetinic acid in the isolated perfused rat intestine and liver. Planta Med. 2008;74:1351–1356. doi: 10.1055/s-2008-1081328. [DOI] [PubMed] [Google Scholar]
- 29.Shen C., Zhu J., Song J., Wang J., Shen B., Yuan H., Li X. Formulation of pluronic F127/TPGS mixed micelles to improve the oral absorption of glycyrrhizic acid. Drug Dev. Ind. Pharm. 2020;46:1100–1107. doi: 10.1080/03639045.2020.1775634. [DOI] [PubMed] [Google Scholar]
- 30.Yang T., Lan Y., Cao M., Ma X., Cao A., Sun Y., Yang J., Li L., Liu Y. Glycyrrhetinic acid-conjugated polymeric prodrug micelles co-delivered with doxorubicin as combination therapy treatment for liver cancer. Colloids Surfaces B Biointerfaces. 2019;175:106–115. doi: 10.1016/j.colsurfb.2018.11.082. [DOI] [PubMed] [Google Scholar]
- 31.Sui X., Wei W., Yang L., Zu Y., Zhao C., Zhang L., Yang F., Zhang Z. Preparation, characterization and in vivo assessment of the bioavailability of glycyrrhizic acid microparticles by supercritical anti-solvent process. Int. J. Pharm. 2012;423:471–479. doi: 10.1016/j.ijpharm.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 32.Andersen F.A. Final report on the safety assessment of glycyrrhetinic acid, potassium glycyrrhetinate, disodium succinoyl glycyrrhetinate, glyceryl glycyrrhetinate, glycyrrhetinyl stearate, stearyl glycyrrhetinate, glycyrrhizic acid, ammonium glycyrrhizate, dipotassium glycyrrhizate, disodium glycyrrhizate, trisodium glycyrrhizate, methyl glycyrrhizate, and potassium glycyrrhizinate. Int. J. Toxicol. 2007;26:79–112. doi: 10.1080/10915810701351228. [DOI] [PubMed] [Google Scholar]
- 33.Shim J.Y., Bin Yim S., Chung J.H., Hong K.S. Antiplaque and antigingivitis effects of a mouthrinse containing cetylpyridinium chloride, triclosan and dipotassium glycyrrhizinate. J. Periodontal Implant. Sci. 2012;42:33–38. doi: 10.5051/jpis.2012.42.2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vitali R., Palone F., Cucchiara S., Negroni A., Cavone L., Costanzo M., Aloi M., Dilillo A., Stronati L. Dipotassium Glycyrrhizate Inhibits HMGB1-Dependent Inflammation and Ameliorates Colitis in Mice. PLoS ONE. 2013;8:e66527. doi: 10.1371/journal.pone.0066527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen J., Li L.F., Hu X.R., Wei F., Ma S. Network Pharmacology-Based Strategy for Elucidating the Molecular Basis Forthe Pharmacologic Effects of Licorice (Glycyrrhiza spp.) Front. Pharmacol. 2021;12:872. doi: 10.3389/fphar.2021.590477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kotas M.E., Medzhitov R. Homeostasis, Inflammation, and Disease Susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hunter P. The inflammation theory of disease. EMBO Rep. 2012;13:968–970. doi: 10.1038/embor.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akamatsu H., Komura J., Asada Y., Niwa Y. Mechanism of anti-inflammatory action of glycyrrhizin: Effect on neutrophil functions including reactive oxygen species generation. Planta Med. 1991;57:119–121. doi: 10.1055/s-2006-960045. [DOI] [PubMed] [Google Scholar]
- 39.Abe M., Akbar F., Hasebe A., Horiike N., Onji M. Glycyrrhizin enhances interleukin-10 production by liver dendritic cells in mice with hepatitis. J. Gastroenterol. 2003;38:962–967. doi: 10.1007/s00535-003-1179-7. [DOI] [PubMed] [Google Scholar]
- 40.Li S., Zhu J.H., Cao L.P., Sun Q., Liu H.D., De Li W., Li J.S., Hang C.H. Growth inhibitory in vitro effects of glycyrrhizic acid in U251 glioblastoma cell line. Neurol. Sci. 2014;35:1115–1120. doi: 10.1007/s10072-014-1661-4. [DOI] [PubMed] [Google Scholar]
- 41.Brassesco M.S., Roberto G.M., Morales A.G., Oliveira J.C., Delsin L.E.A., Pezuk J.A., Valera E.T., Carlotti C.G., Rego E.M., de Oliveira H.F., et al. Inhibition of NF-κB by Dehydroxymethylepoxyquinomicin Suppresses Invasion and Synergistically Potentiates Temozolomide and γ-Radiation Cytotoxicity in Glioblastoma Cells. Chemother. Res. Pract. 2013;2013:593020. doi: 10.1155/2013/593020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith D., Shimamura T., Barbera S., Bejcek B.E. NF-κB controls growth of glioblastomas/astrocytomas. Mol. Cell. Biochem. 2008;307:141–147. doi: 10.1007/s11010-007-9593-4. [DOI] [PubMed] [Google Scholar]
- 43.Galardi S., Mercatelli N., Farace M.G., Ciafrè S.A. NF-κB and c-Jun induce the expression of the oncogenic miR-221 and miR-222 in prostate carcinoma and glioblastoma cells. Nucleic Acids Res. 2011;39:3892–3902. doi: 10.1093/nar/gkr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanotto-Filho A., Braganhol E., Schröder R., De Souza L.H.T., Dalmolin R.J.S., Pasquali M.A.B., Gelain D.P., Battastini A.M.O., Moreira J.C.F. NFκB inhibitors induce cell death in glioblastomas. Biochem. Pharmacol. 2011;81:412–424. doi: 10.1016/j.bcp.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 45.Westhoff M.A., Zhou S., Nonnenmacher L., Karpel-Massler G., Jennewein C., Schneider M., Halatsch M.E., Carragher N.O., Baumann B., Krause A., et al. Inhibition of NF-κB signaling ablates the invasive phenotype of glioblastoma. Mol. Cancer Res. 2013;11:1611–1623. doi: 10.1158/1541-7786.MCR-13-0435-T. [DOI] [PubMed] [Google Scholar]
- 46.Bonafé G.A., dos Santos J.S., Ziegler J.V., Umezawa K., Ribeiro M.L., Rocha T., Ortega M.M. Growth inhibitory effects of dipotassium glycyrrhizinate in glioblastoma cell lines by targeting microRNAs through the NF-κB signaling pathway. Front. Cell. Neurosci. 2019;13:216. doi: 10.3389/fncel.2019.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Pol A., van Gilst W.H., Voors A.A., van der Meer P. Treating oxidative stress in heart failure: Past, present and future. Eur. J. Heart Fail. 2019;21:425–435. doi: 10.1002/ejhf.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pisoschi A.M., Pop A., Iordache F., Stanca L., Predoi G., Serban A.I. Oxidative stress mitigation by antioxidants-An overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021;209:112891. doi: 10.1016/j.ejmech.2020.112891. [DOI] [PubMed] [Google Scholar]
- 49.Vitali R., Palone F., Pierdomenico M., Negroni A., Cucchiara S., Aloi M., Oliva S., Stronati L. Dipotassium glycyrrhizate via HMGB1 or AMPK signaling suppresses oxidative stress during intestinal inflammation. Biochem. Pharmacol. 2015;97:292–299. doi: 10.1016/j.bcp.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 50.Mu S.W., Dang Y., Fan Y.C., Zhang H., Zhang J.H., Wang W., Sen Wang S., Gu J.J. Effect of HMGB1 and RAGE on brain injury and the protective mechanism of glycyrrhizin in intracranial-sinus occlusion followed by mechanical thrombectomy recanalization. Int. J. Mol. Med. 2019;44:813–822. doi: 10.3892/ijmm.2019.4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gong G., Xiang L., Yuan L., Hu L., Wu W., Cai L., Yin L., Dong H. Protective effect of glycyrrhizin, a direct HMGB1 inhibitor, on focal cerebral ischemia/reperfusion-induced inflammation, oxidative stress, and apoptosis in rats. PLoS ONE. 2014;9:e89450. doi: 10.1371/journal.pone.0089450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao H., Zhao M., Wang Y., Li F., Zhang Z. Glycyrrhizic Acid Prevents Sepsis-Induced Acute Lung Injury and Mortality in Rats. J. Histochem. Cytochem. 2016;64:125–137. doi: 10.1369/0022155415610168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ge X., Meng X., Fei D., Kang K., Wang Q., Zhao M. Lycorine attenuates lipopolysaccharide-induced acute lung injury through the HMGB1/TLRs/NF-κB pathway. 3 Biotech. 2020;10:369. doi: 10.1007/s13205-020-02364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao F.Q., Wang G.F., Xu D., Zhang H.Y., Cui Y.L., Wang Q.S. Glycyrrhizin mediated liver-targeted alginate nanogels delivers quercetin to relieve acute liver failure. Int. J. Biol. Macromol. 2021;168:93–104. doi: 10.1016/j.ijbiomac.2020.11.204. [DOI] [PubMed] [Google Scholar]
- 55.Emara N.A., Mahmoud M.F., El Fayoumi H.M., Mahmoud A.A.A. The renoprotective effect of glycyrrhizic acid in insulin-resistant rats exposed to aluminum involves the inhibition of TLR4/NF-κB signaling pathway. Naunyn Schmiedebergs Arch. Pharmacol. 2021;394:863–872. doi: 10.1007/s00210-020-02012-y. [DOI] [PubMed] [Google Scholar]
- 56.Li Z., Chen C., Zhu X., Li Y., Yu R., Xu W. Glycyrrhizin Suppresses RANKL-Induced Osteoclastogenesis and Oxidative Stress Through Inhibiting NF-κB and MAPK and Activating AMPK/Nrf2. Calcif. Tissue Int. 2018;103:324–337. doi: 10.1007/s00223-018-0425-1. [DOI] [PubMed] [Google Scholar]
- 57.Ali N.M., Mahmoud A.A.A., Mahmoud M.F., El Fayoumi H.M. Glycyrrhizic acid and silymarin alleviate the neurotoxic effects of aluminum in rats challenged with fructose-induced insulin resistance: Possible role of toll-like receptor 4 pathway. Drug Chem. Toxicol. 2019;42:210–219. doi: 10.1080/01480545.2018.1544984. [DOI] [PubMed] [Google Scholar]
- 58.Ojha S., Javed H., Azimullah S., Abul Khair S.B., Haque M.E. Glycyrrhizic acid Attenuates Neuroinflammation and Oxidative Stress in Rotenone Model of Parkinson’s Disease. Neurotox. Res. 2016;29:275–287. doi: 10.1007/s12640-015-9579-z. [DOI] [PubMed] [Google Scholar]
- 59.Sathyamoorthy Y., Kaliappan K., Nambi P., Radhakrishnan R. Glycyrrhizic acid renders robust neuroprotection in rodent model of vascular dementia by controlling oxidative stress and curtailing cytochrome-c release. Nutr. Neurosci. 2020;23:955–970. doi: 10.1080/1028415X.2019.1580935. [DOI] [PubMed] [Google Scholar]
- 60.Zhao H., Liu Z., Shen H., Jin S., Zhang S. Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress. Eur. J. Pharmacol. 2016;781:92–99. doi: 10.1016/j.ejphar.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 61.Xu C., Liang C., Sun W., Chen J., Chen X. Glycyrrhizic acid ameliorates myocardial ischemic injury by the regulation of inflammation and oxidative state. Drug Des. Devel. Ther. 2018;12:1311–1319. doi: 10.2147/DDDT.S165225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Su M., Yu T., Zhang H., Wu Y., Wang X., Li G. The antiapoptosis effect of glycyrrhizate on HepG2 cells induced by hydrogen peroxide. Oxid. Med. Cell. Longev. 2016;2016:6849758. doi: 10.1155/2016/6849758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang O.H., Kim J.A., Choi Y.A., Park H.J., Kim D.K., An Y.H., Choi S.C., Yun K.J., Nah Y.H., Cai X.F., et al. Inhibition of interleukin-8 production in the human colonic epithelial cell line HT-29 by 18 beta-glycyrrhetinic acid. Int. J. Mol. Med. 2005;15:981–985. doi: 10.3892/ijmm.15.6.981. [DOI] [PubMed] [Google Scholar]
- 64.Jeon Y.D., Kang S.H., Bang K.S., Chang Y.N., Lee J.H., Jin J.S. Glycyrrhetic acid ameliorates dextran sulfate sodium-induced ulcerative colitis in vivo. Molecules. 2016;21:523. doi: 10.3390/molecules21040523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yuan H., Ji W.S., Wu K.X., Jiao J.X., Sun L.H., Feng Y.T. Anti-inflammatory effect of Diammonium Glycyrrhizinate in a rat model of ulcerative colitis. World J. Gastroenterol. 2006;12:4578–4581. doi: 10.3748/wjg.v12.i28.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Y., Cai T.T., Shen Y., Bin Zhou X., Chen T., Xu Q. Si-Ni-San, a traditional Chinese prescription, and its active ingredient glycyrrhizin ameliorate experimental colitis through regulating cytokine balance. Int. Immunopharmacol. 2009;9:1437–1443. doi: 10.1016/j.intimp.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 67.Kudo T., Okamura S., Zhang Y., Masuo T., Mori M. Topical application of glycyrrhizin preparation ameliorates experimentally induced colitis in rats. World J. Gastroenterol. 2011;17:2223–2238. doi: 10.3748/wjg.v17.i17.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Y., Xiang J., Liu M., Wang S., Lee R.J., Ding H. Protective effects of glycyrrhizic acid by rectal treatment on a TNBS-induced rat colitis model. J. Pharm. Pharmacol. 2011;63:439–446. doi: 10.1111/j.2042-7158.2010.01185.x. [DOI] [PubMed] [Google Scholar]
- 69.Sethuraman S.N., Swaminathan S., Nelson S.B., Palaninathan P.S., Gopalan T.K., Velayudham P. Modulation of PPARγ and TNFα by emu oil and glycyrrhizin in ulcerative colitis. Inflammopharmacology. 2015;23:47–56. doi: 10.1007/s10787-014-0226-8. [DOI] [PubMed] [Google Scholar]
- 70.Chen X., Fang D., Li L., Chen L., Li Q., Gong F., Fang M. Glycyrrhizin ameliorates experimental colitis through attenuating interleukin-17-producing T cell responses via regulating antigen-presenting cells. Immunol. Res. 2017;65:666–680. doi: 10.1007/s12026-017-8894-2. [DOI] [PubMed] [Google Scholar]
- 71.Ishida T., Miki I., Tanahashi T., Yagi S., Kondo Y., Inoue J., Kawauchi S., Nishiumi S., Yoshida M., Maeda H., et al. Effect of 18β-glycyrrhetinic acid and hydroxypropyl γcyclodextrin complex on indomethacin-induced small intestinal injury in mice. Eur. J. Pharmacol. 2013;714:125–131. doi: 10.1016/j.ejphar.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 72.Jeon Y.D., Bang K.S., Shin M.K., Lee J.H., Chang Y.N., Jin J.S. Regulatory effects of glycyrrhizae radix extract on DSS-induced ulcerative colitis. BMC Complement. Altern. Med. 2016;16:459. doi: 10.1186/s12906-016-1390-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 73.Stronati L., Palone F., Negroni A., Colantoni E., Mancuso A.B., Cucchiara S., Cesi V., Isoldi S., Vitali R. Dipotassium glycyrrhizate improves intestinal mucosal healing by modulating extracellular matrix remodeling genes and restoring epithelial barrier functions. Front. Immunol. 2019;10:939. doi: 10.3389/fimmu.2019.00939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ramos G.P., Papadakis K.A. Mechanisms of Disease: Inflammatory Bowel Diseases. Mayo Clin. Proc. 2019;94:155–165. doi: 10.1016/j.mayocp.2018.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seyedian S.S., Nokhostin F., Malamir M.D. A review of the diagnosis, prevention, and treatment methods of inflammatory bowel disease. J. Med. Life. 2019;12:113–122. doi: 10.25122/jml-2018-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pravda J. Radical induction theory of ulcerative colitis. World J. Gastroenterol. 2005;11:2371–2384. doi: 10.3748/wjg.v11.i16.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fantini M.C., Guadagni I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: Pathogenesis and impact of current therapies. Dig. Liver Dis. 2021;53:558–565. doi: 10.1016/j.dld.2021.01.012. [DOI] [PubMed] [Google Scholar]
- 78.Shah S.C., Itzkowitz S.H. Colorectal Cancer in Inflammatory Bowel Disease: Mechanisms and Management. Gastroenterology. 2021;162:715–730.e3. doi: 10.1053/j.gastro.2021.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scalise J.R., Poças R.C.G., Caneloi T.P., Lopes C.O., Kanno D.T., Marques M.G., Valdivia J.C.M., Maximo F.R., Pereira J.A., Ribeiro M.L., et al. DNA Damage Is a Potential Marker for TP53 Mutation in Colorectal Carcinogenesis. J. Gastrointest. Cancer. 2016;47:409–416. doi: 10.1007/s12029-016-9846-0. [DOI] [PubMed] [Google Scholar]
- 80.Guina T., Biasi F., Calfapietra S., Nano M., Poli G. Inflammatory and redox reactions in colorectal carcinogenesis. Ann. N. Y. Acad. Sci. 2015;1340:95–103. doi: 10.1111/nyas.12734. [DOI] [PubMed] [Google Scholar]
- 81.Li C., Wang J., Ma R., Li L., Wu W., Cai D., Lu Q. Natural-derived alkaloids exhibit great potential in the treatment of ulcerative colitis. Pharmacol. Res. 2022;175:105972. doi: 10.1016/j.phrs.2021.105972. [DOI] [PubMed] [Google Scholar]
- 82.Cao S.Y., Ye S.J., Wang W.W., Wang B., Zhang T., Pu Y.Q. Progress in active compounds effective on ulcerative colitis from Chinese medicines. Chin. J. Nat. Med. 2019;17:81–102. doi: 10.1016/S1875-5364(19)30012-3. [DOI] [PubMed] [Google Scholar]
- 83.Lee J.Y., Kang H.S., Park B.E., Moon H.J., Sim S.S., Kim C.J. Inhibitory effects of Geijigajakyak-Tang on trinitrobenzene sulfonic acid-induced colitis. J. Ethnopharmacol. 2009;126:244–251. doi: 10.1016/j.jep.2009.08.035. [DOI] [PubMed] [Google Scholar]
- 84.Negroni A., Fiaschini N., Palone F., Vitali R., Colantoni E., Laudadio I., Oliva S., Aloi M., Cucchiara S., Stronati L. Intestinal Inflammation Alters the Expression of Hepatic Bile Acid Receptors Causing Liver Impairment. J. Pediatr. Gastroenterol. Nutr. 2020;71:189–196. doi: 10.1097/MPG.0000000000002759. [DOI] [PubMed] [Google Scholar]
- 85.Nazari S., Rameshrad M., Hosseinzadeh H. Toxicological Effects of Glycyrrhiza glabra (Licorice): A Review. Phytother. Res. 2017;31:1635–1650. doi: 10.1002/ptr.5893. [DOI] [PubMed] [Google Scholar]