Abstract
Polyamines (PAs) are small, versatile molecules with two or more nitrogen-containing positively charged groups and provide widespread biological functions. Most of these aspects are well known and covered by quite a number of excellent surveys. Here, the present review includes novel aspects and questions: (1) It summarizes the role of most natural and some important synthetic PAs. (2) It depicts PA uptake from nutrition and bacterial production in the intestinal system following loss of PAs via defecation. (3) It highlights the discrepancy between the high concentrations of PAs in the gut lumen and their low concentration in the blood plasma and cerebrospinal fluid, while concentrations in cellular cytoplasm are much higher. (4) The present review provides a novel and complete scheme for the biosynthesis of Pas, including glycine, glutamate, proline and others as PA precursors, and provides a hypothesis that the agmatine pathway may rescue putrescine production when ODC knockout seems to be lethal (solving the apparent contradiction in the literature). (5) It summarizes novel data on PA transport in brain glial cells explaining why these cells but not neurons preferentially accumulate PAs. (6) Finally, it provides a novel and complete scheme for PA interconversion, including hypusine, putreanine, and GABA (unique gliotransmitter) as end-products. Altogether, this review can serve as an updated contribution to understanding the PA mystery.
Keywords: polyamines, CNS, astrocytes, neurons, glial cells, spermidine, spermine, agmatine, nutrition, transport
1. Introduction
The term polyamines (PAs) comprises small molecular compounds with two or more nitrogen-containing, positively charged groups, such as ammonium or guanidinium residues (Table 1). PAs may be derived from natural or artificial sources. PAs and their metabolites may be encountered throughout all kingdoms of life [1]. The important PAs in mammalian cells are putrescine (PUT), spermidine (SPD), and spermine (SPM), which also are present in all eukaryotic cells. Some prokaryotes may lack the ability to synthesize SPM [2], but thermophiles [3], especially, show a wide variety of other PAs (Table 1).
Table 1.
Designations, structures, and biological or pharmacological functions of important polyamines.
| Source | Substance | Sum Formula | Mass/g*mol−1 | Structure | Function/Purpose/Usage | References |
|---|---|---|---|---|---|---|
| plants/protozoa | Diaminopropane | C3H10N2 | 74,13 | NH2(CH2)3NH2 | proliferative agent | (1) |
| ubiquitous | Putrescine | C4H12N2 | 88,15 | NH2(CH2)4NH2 | precursor to spermidine | (2) |
| prokaryotes/E.coli | Cadaverine | C5H14N2 | 102,18 | NH2(CH2)5NH2 | decarboxylation product of L-Lysine | (3) |
| eukaryotes | Norspermidine | C6H17N3 | 131,22 | NH2(CH2)3NH(CH2)3NH2 | catabolic metabolite | (4) |
| ubiquitous | Spermidine | C7H19N3 | 145,25 | NH2(CH2)3NH(CH2)4NH2 | growth regulator in eukaryotic cells | (5) |
| ubiquitous | N1-Acetylspermidine | C9H21N3O | 187,28 | CH3CONH(CH2)3NH(CH2)4NH2 | catabolic metabolite | (6) |
| prokaryotes/E.coli | Aminopropylcadaverine | C8H21N3 | 159,27 | NH2(CH2)5NH(CH2)3NH2 | compensatory metabolite/growth regulator | (7) |
| plants/prokaryotes/algae | Homospermidine | C8H21N3 | 159,27 | NH2(CH2)4NH(CH2)4NH2 | essential precursor to pyrrolizidine alkaloids | (8) |
| plants/prokaryotes/algae | Norspermine | C9H24N4 | 188,31 | NH2(CH2)3NH(CH2)3NH(CH2)3NH2 | antiproliferative agent | (9) |
| plants/prokaryotes/algae | Thermospermine | C10H26N4 | 202,34 | NH2(CH2)3NH(CH2)3NH(CH2)4NH2 | growth regulator in plants | (10) |
| eukaryotes/prokaryotes | Spermine | C10H26N4 | 202,34 | NH2(CH2)3NH(CH2)4NH(CH2)3NH2 | growth regulator in eukaryotic cells | (5) |
| eukaryotes | N1-Acetylspermine | C12H28N4O | 244,38 | CH3CONH(CH2)3NH(CH2)4NH(CH2)3NH2 | catabolic metabolite | (6) |
| prokaryotes/E.coli | Bisaminopropylcadaverine | C11H28N4 | 216,37 | NH2(CH2)3NH(CH2)5NH(CH2)3NH2 | compensatory metabolite/growth regulator | (7) |
| plants/fungi | Canavalmine | C11H28N4 | 216,37 | NH2(CH2)4NH(CH2)3NH(CH2)4NH2 | growth inhibitor in murine leukemia cells | (11) |
| prokaryotes/E.coli | Homospermine | C12H30N4 | 230,39 | NH2(CH2)4NH(CH2)4NH(CH2)4NH2 | growth regulator in root nodule bacteria | (12) |
| thermophiles | Caldopentamine | C12H31N5 | 245,41 | NH2(CH2)3NH(CH2)3NH(CH2)3N(CH2)3NH2 | survival at extreme temperature | (13) |
| prokaryotes/E.coli | Aminopropylcanavalmine | C14H35N5 | 273,46 | NH2(CH2)3NH(CH2)4NH(CH2)3N(CH2)4NH2 | compensatory metabolite/growth regulator | (7) |
| plants | Homopentamine | C16H39N5 | 301,51 | NH2(CH2)4NH(CH2)4NH(CH2)4N(CH2)4NH2 | growth/differentiation | (14) |
| thermophiles | Caldohexamine | C15H38N6 | 302,5 | NH2(CH2)3NH(CH2)3NH(CH2)3NH(CH2)3NH(CH2)3NH2 | inhibition of PA-uptake | (9) |
| thermophiles | Homocaldohexamine | C16H40N6 | 316,53 | NH2(CH2)3NH(CH2)3NH(CH2)3NH(CH2)3NH(CH2)4NH2 | antiviral agent in plants | (15) |
| prokaryotes | Thermohexamine | C16H40N6 | 316,53 | NH2(CH2)3NH(CH2)3NH(CH2)4NH(CH2)3NH(CH2)3NH2 | inhibition of PA-uptake | (9) |
| plants/mammals | Agmatine | C5H14N4 | 130,19 | [(NH2)CNH]NH(CH2)4NH2 | neurotransmitter/precursor to putrescine | (16) |
| plants | N6-Methylagmatine | C6H16N4 | 144,22 | [(NH2)CN(CH3)]NH(CH2)4NH2 | nutrient | (17) |
| PA-analogue | Methylglyoxalbisguanylhydrazone (MGBG) | C5H12N8 | 184,2 | (NH2)(NH)CNHNCHC(CH3)NNHC(NH)(NH2) | antileucamic agent | (18) |
| PA-analogue | MDL 27695 | C27H44N4 | 424,7 | C6H5CH2NH2(CH2)3NH(CH2)7NH(CH2)3NH2CH2C6H5 | antimalaria agent | (19) |
| PA-analogue | N1,N11-Bisethylnorspermine | C13H32N4 | 244,42 | C2H5NH2(CH2)3NH(CH2)3NH(CH2)3NH2C2H5 | antiproliferative agent | (20) |
| PA-analogue | BE 4-4-4-4 | C20H47N5 | 357,6 | NH2(CH2)4NH(CH2)4NH(CH2)4N(CH2)4NH2 | antiproliferative agent | (21) |
| PA-analogue | trimer 44NMe | C33H69N9 | 592 | [1,3,4][(CH2)NH(CH2)4NH(CH2)4NH2]3(C6H6) | antiproliferative agent | (22) |
| Streptomyces spp. | Kanamycin A | C18H40N4O11 | 488,5 | 6-O-(3-Amino-3-desoxy-α-d-glucopyranosyl)-4-O- (6-amino-6-desoxy-α-d-glucopyranosyl)-2-desoxy- d-streptamin |
aminoglycoside antibiotic agent | (23) |
| Streptomyces spp. | Neomycin B | C23H46N6O13 | 614,6 | 4-O-2,6-Diamino-2,6-didesoxy-α-d-glucopyranosyl-5-O- [3-O-2,6-diamino-2,6-dideoxy-β-l-idopyranosyl- β-d-ribofuranosyl]-2-deosxy-d-streptamin |
aminoglycoside antibiotic agent | (23) |
| References | ||||||
| (1) Potter, M.J.; Gibson, M.K.; McCammon, J.A.; J. Am. Chem. Soc. 1994, 116, 10298–10299. | ||||||
| (2) Takao, K.; Sugita, Y.; Shirahata, A. Amino Acids 2010, 38, 533–539 | ||||||
| (3) Li, M.; Li, D; Huang, Y.; Liu, M.; Wang, H.; Tang, Q.; Lu, F. J. Ind. Microbiol. Biotechnol. 2014, 41, 701–709 | ||||||
| (4) Michael, A.J. J. Biol. Chem. 2016, 291, 14896–14903 | ||||||
| (5) Bergeron, R.J.;McManis, J.S.; Weimar, W.R.; Schreier, K.; Gao, F.; Wu, Q.; Ortiz-Ocasio, J.; Luchetta, G.R.; Porter, C.; Vinson, J.R.T. J. Med. Chem. 1995, 38, 2278–2285 | ||||||
| (6) Yu, C.; Liu, R.; Xie, C.; Zhang, Q.; Yin, Y.; Bi, K.; Li, Q. Anal. Bioanal. Chem. 2015, 407, 6891–6897 | ||||||
| (7) Igarashi, K.; Kashiwagi, K.; Hamasaki, H.; Miura, A.; Kakegawa, T.; Hirose, S.; Matsuzaki, S. J. Bacteriol. 1986, 166, 128–134 | ||||||
| (8) Ober, D.; Gibas, L.; Witte, L.; Hartmann, T. Phytochemistry 2003, 62, 339–344 | ||||||
| (9) Takao, K.; Sugita, Y.; Shirahata, A. Amino Acids 2010, 38, 533–539 | ||||||
| (10) Takano, A.; Kakehi, J.-I.; Takahashi, T. Plant Cell Physiol. 2012, 53, 606–616 | ||||||
| (11) Fujihara, S.; Nakashima, T.; Kurogochi, Y. Biochem. Biophys. Acta 1984, 805, 277–284 | ||||||
| (12) Fujihara, S.; Harada, Y. Biochem. Biophys. Res. Commun. 1989, 165, 659–666 | ||||||
| (13) Oshima T.; Moriya T.; Terui Y. Polyamines. Methods in Molecular Biology (Methods and Protocols); Pegg, A., Casero, R., Jr., Eds.; Humana Press, 2011; Volume 720, pp. 81–111 | ||||||
| (14) Bagni, N.; Tassoni, A. Amino Acids 2001, 20, 301–317 | ||||||
| (15) Sagor, G.H.M.; Liu, T.; Takahashi, H.; Niitsu, M.; Berberich, T.; Kusano, T. Plant Cell Rep. 2013, 32, 1477–1488 | ||||||
| (16) Weiss, T.; Bernard, R.; Bernstein, H.-G.; Veh, R.W.; Laube, G. Transl. Psychiatry 2018, 8, 201 | ||||||
| (17) Paik, W.K.; Kim, S. Amino Acids 1993, 4, 267–286 | ||||||
| (18) Von Hoff, D.D. Ann. Oncol. 1994, 5, 487–493 | ||||||
| (19) Edwards, M.L.; Stemerick, D.M.; Bitonti, A.J.; Dumont, J.A.; McCann, P.P.; Bey, P.; Sjoerdsma, A. J. Med. Chem. 1991, 34, 569–574 | ||||||
| (20) Thomas, T.J.; Thomas, T. Med. Sci. 2018, 6, 24 | ||||||
| (21) Basu, H.S.; Pellarin, M., Feuerstein, B.G.; Shirahata, A.; Samejima, K.; Deen, D.F.; Marton, L.J. Cancer Res. 1993, 53, 3948–3955 | ||||||
| (22) Muth, A.; Madan, M.; Archer, J.J.; Ocampo, N.; Rodriguez, L.; Otto Phanstiel, O. J. Med. Chem. 2014, 57, 348−363 | ||||||
| (23) Blagbrough I.S.; Metwally A.A.; Andrew J. Geall A.J. Polyamines. Methods in Molecular Biology (Methods and Protocols); Pegg A., Casero, R., Jr., Eds.; Humana Press, 2011; Volume 720, pp. 493–503 | ||||||
Biological functions are widespread, including simply buffering acidic compartments [4,5], stabilizing or condensing nucleic acids [6,7], promoting homology-directed DNA repair [8], protecting from oxidative damage [9,10], and increasing longevity [11,12,13], and are covered in a separate article of this special issue.
Historically, PAs had been discovered during the seventeenth century, when crystals appeared in samples of human semen left to cool [14]. A century later, these crystals were identified as an organic phosphate [15]. After another 100 years, the organic base was identified [16] and subsequently called “spermine” [16,17]. Later, PA biosynthesis, interconversions, and basic biological functions were established by E. Agostinelli, U. Bachrach, R. Casero, T. Eisenberg, G. Gilad, K. Igarashi, J. Jänne, F. Madeo, G. Park, M. Pegg, C. W. Porter, M. Rosenheim, H. Tabor, C. W. Tabor, H. Wallace, and other scientists (alphabetical order; for reviews see [18,19,20,21]). The interest in PAs has grown steadily, with 1 paper published on PA-research from 1951 to 1960, 2259 papers from 1981 to 1990, and 4113 papers from 2011 to 2020.
The existing literature on PAs is abundant but sometimes vague. The present review focuses on chemistry, nutritional uptake, and metabolism of PAs in the mammalian central nervous system (CNS) and may serve as a framework for an increased molecular understanding of PA homeostasis.
2. Chemistry of Polyamines
Most PAs consist of at least two amino or guanidinium groups that are positively charged and separated by a carbon backbone of varying length. In contrast to other cations, such as Mg2+ or Ca2+, PAs feature at least two charged groups connected by a flexible carbon chain. This adds hydrophobic effects [22,23] to the electrostatic interactions of the amino or guanidine groups. SPM and SPD are the most abundant PAs in mammals. PUT is the common precursor but is usually only present in low concentrations [3] or is even absent in some parasitic organisms [24]. Chemically, agmatine (AGM) also belongs to the PA family. This biogenic amine, however, exerts largely separate functions in mammalian tissues as compared to the other PAs [25,26], but may serve as a precursor for PUT [27,28,29].
3. Intake of Polyamines from Nutrition
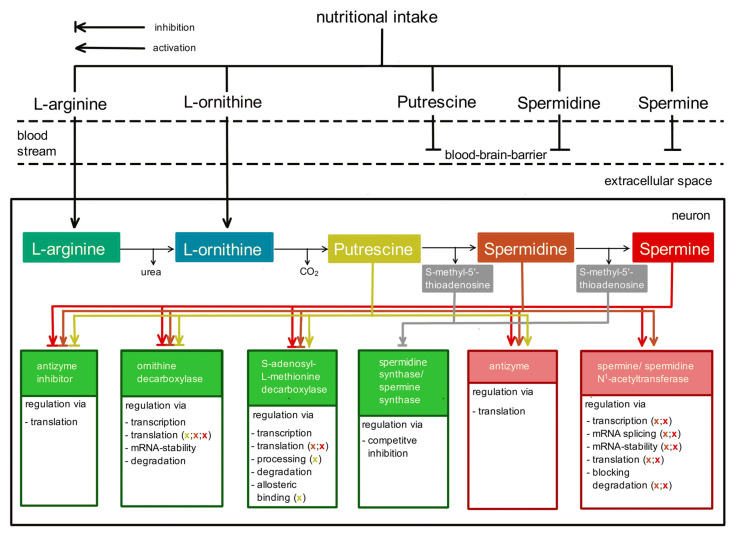
Polyamines (PAs) may be derived from alimentary sources or by biosynthesis. In the mammalian gut lumen, the predominant share of PAs stems from food intake [30], whereas a variable quantity may be produced by large intestine microbiota [31]. The intestinal flora in most mammals synthesizes mainly PUT, whereas its amount depends on the composition of the diet [31]. Food sources with relevant PA content include cheese, nuts, mushrooms, tea, fruit, vegetables, mollusks, and other meat products [32,33,34,35]. With respect to PA synthesis by gut microbiota, an arginine rich diet is favorable [31]. PAs in the gut lumen are absorbed predominantly by the duodenal and jejunal mucosa [36,37] and subsequently transferred [31] into the bloodstream (Figure 1). The estimated average of alimentary intake of PAs into the gut varies between 250 to 550 µmol/d, depending on the geographical region and associated food patterns [32]. In detail, PUT contributes 197 µmol/d, SPD with 74 µmol/d, and SPM with 46 µmol/d [34] to the daily PA intake.
Figure 1.
Schematic representation of nutritional intake and regulated biosynthesis to maintain polyamine homeostasis in the body. Nutritional intake into the blood stream includes arginine and orhithine in addition to the polyamines (PAs) putrescine, spermidine, and spermine. PAs are taken up mostly in the small intestine and delivered to the blood stream, but they cannot pass the blood–brain barrier. However, arginine and ornithine can and will represent starting materials of the regulated biosynthesis of PAs in the central nervous system. Inside the neuron, PA concentrations are tightly controlled by at least six proteins, represented as boxes in the bottom row: antizyme inhibitor, ornithine decarboxylase, S-adenosylmethionine decarboxylase, Spd/Spm-synthase, antizyme, and Spm/Spd-N1-acetyltransferase. Arrows or blocked arrows indicate which target proteins (green: increase PAs; red: decrease PAs) are modulated by a given PA. The colour of the (x) indicates which PA is involved.
PAs in the gut lumen may reach almost millimolar concentrations after a meal and disappear rapid and completely [37], whereby the luminal PA content returns to the fasting level in about 120 min [30]. PUT is metabolized almost completely inside the enterocytes. Plasma levels of SPD and SPM show only mild (up to 20 µM) increases after a meal [30]. Most likely, PA uptake in peripheral tissues keeps the plasma concentrations low (Table 2).
Table 2.
Polyamine concentrations in brain and body fluids.
| Arg | PUT | NAc-PUT | ref. | SPD | NAc-SPD | ref. | SPM | NAc-SPM | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| serum | 80 µM | 100 nM | (4) | 130 nM | (4) | 40 nM | (4) | ||||||
| 130 nM | (3) | 400 nM | (3) | 50 nM | (3) | ||||||||
| 320 nM | (1) | 35 nM | (5) | ||||||||||
| 60 nM | 2.5 nM | (8) | 4.0 nM | (8) | 43 nM | (8) | |||||||
| cerebrospinal fluid | 180 nM | (2) | 150 nM | (2) | 90 nM | (2) | |||||||
| 230 nM | (6) | 120 nM | (6) | 140 nM | (6) | ||||||||
| brain extracellular space | 750 nM | (5) | 420 nM | (5) | 480 nM | (5) | |||||||
| cytoplasm (fibroblasts) | 29 µM | (4) | 159 µM | (4) | 635 µM | (4) | |||||||
| cytoplasm (ascites cells) | 43 µM | (4) | 430 µM | (4) | 602 µM | (4) | |||||||
| hepatocytes | 1150 µM | (9) | 880 µM | (9) | |||||||||
| brain (Müller cells) | 800 µM | (7) | |||||||||||
| urine | 60.2 nM | 2.5 nM | (8) | 4.0 | 1.7 nM | (8) | 43.1 nM | 1.3 nM | (8) | ||||
| daily loss | 90.3 nmoles | 3.7 nmoles | (8) | 6.0 nmoles | 2.5 nmoles | (8) | 64.6 nmoles | 1.9 nmoles | (8) | ||||
| References | |||||||||||||
| (1) | Marton, L.J.; Russell, D.H.; Levy, C.C. Clin. Chem. 1973, 9, 923–926 | ||||||||||||
| (2) | Marton, L.J.; Heby, O.; Levin, V.A.; Lubich, W.P.; Crafts, D.C.; Wilson, C.B. Cancer Res. 1976, 36, 973–977 | ||||||||||||
| (3) | Bartos, F.; Bartos, D.; Grettie, D.P.; Campbell, R.A. Biochem. Biophys. Res. Commun. 1977, 75, 4 | ||||||||||||
| Seiler, N.; Atanassov, C.L. Progr. Drug Res. 1994, 43, 87–141 | |||||||||||||
| (4) | Morgan, D.M.L. Biochem. Soc. Trans. 1990, 18, 1080–1084 | ||||||||||||
| (5) | Dot, J.; Lluch, M.; Blanco, I.; Rodríguez-Alvarez, J. J. Neurochem. 2000, 75, 1917–1926 | ||||||||||||
| Dot, J.; Danchev, N.; Blanco, I.; Rodríguez-Alvarez, J. Neuroreport 2002, 13, 1083–108 | |||||||||||||
| (6) | Ekegrena, T.; Gomes-Trolin, C. Anal. Biochem. 2005, 338, 179–185 | ||||||||||||
| (7) | Kucheryavykh, Y.V.; Shuba, Y.M.; Antonov, S.M.; Inyushin, M.Y.; Cubano, L.; Pearson, W.L.; Kurata, H.; Reichenbach, A.; Veh, R.W.; Nichols, C.G.; Eaton, M.J.; Skatchkov, S.N. Glia 2008, 56, 775–790 | ||||||||||||
| (8) | Liu, R.; Li, Q.; Ma, R.; Lin, X.; Xu, H.; Bi, K. Anal. Chim. Acta 2013, 791, 36–45 | ||||||||||||
| (9) | Igarashi, K.; Kashiwagi, K. Int. J. Biochem. Cell Biol. 2019, 107, 104–115 | ||||||||||||
Loss of PAs from the body may be due to micturition and defecation. Concentrations in urine are very low (Table 2) and PA loss via diuresis is negligible. Estimated concentrations in feces are about 800 µM for PUT, about 40 µM for SPD, and 20 µM for SPM [36]. Considering fecal density of 1.09 kg/L and 155 g as an average daily amount of stool, loss of PAs in feces amounts to about 123 µmoles PUT, 5.9 µmoles SPD, and 2.9 µmoles SPM per day. Altogether, there is roughly a nutritional daily net intake into the body of 74 µmoles PUT, 68 µmoles SPD and 43 µmoles SPM. Actually, the uptake of PUT may be much higher, as gut microbiota can produce considerable amounts of PUT from arginine [31].
The brain apparently is excluded from taking up PAs from the plasma (Figure 1), as the blood–brain barrier (BBB) seems to be completely impermeable to PAs [38,39] (this special issue). Consequently, the brain depends on different sources to obtain the essential PAs. Most likely, arginine (Figure 1), which crosses the blood–brain-barrier via the CAT1 transporter [40], provides the necessary material for PA biosynthesis in brain.
4. Biosynthesis of Polyamines
When the brain is excluded from nutritional PA sources, homeostasis (Figure 1) depends on metabolism. With respect to the biological importance of PAs, it is plausible that their intracellular concentration is tightly controlled at several levels. This task is fulfilled by regulating biosynthesis (Figure 2) as well as degradation (Figure 3). The amino acids L-arginine, L-ornithine, glycine, L-proline, L-glutamate, and L-methionine [41,42,43] are effective sources. Biosynthesis predominantly follows two different pathways, both producing PUT as starting material for the following reactions (Figure 2).
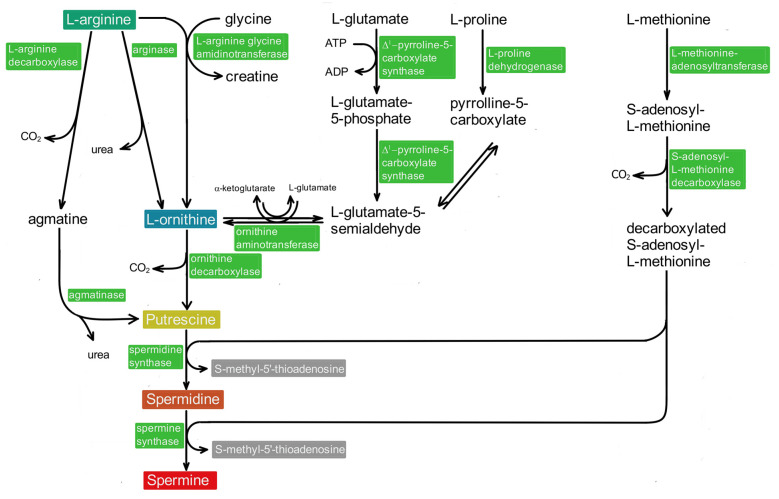
Figure 2.
Schematic representation of polyamine biosynthesis. The most important biosynthetic pathway begins with the action of arginase on arginine, forming ornithine, and its subsequent decarboxylation provides putrescine (PUT). PUT may also be obtained from arginine via decarboxylation to agmatine and subsequent action of agmatinase (left column). In addition, ornithine may be obtained from arginine and glycine via arginine-glycine-amidinotransferase. Glutamate and proline provide additional sources (middle columns). The additional carbon chains of spermidine and spermine are derived from methionine (right column).
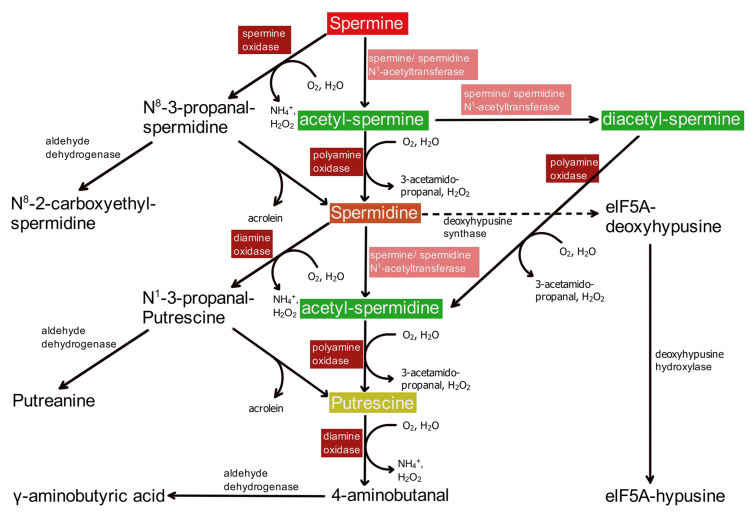
Figure 3.
Schematic representation of polyamine catabolism and conversions. In the first step, the classical degradation pathway for spermine and spermidine involves N-acetylation via the corresponding N-acetyl transferase. N-acetylated PAs are oxidized by peroxisomal polyamine oxidase, yielding spermidine or putrescine, respectively. Alternately, spermine may be directly oxidized by spermine oxidase to N8-3-propyl-spermidine (upper left side), which spontaneously splits off acrolein and thus is converted to spermidine. This molecule, instead of N-acetylation, may be oxidized by diamine oxidase to N1-3-propyl-putrescine (lower left side), which again under loss of acrolein forms putrescine. There are two side pathways. Spermidine may be attached to a lysine side chain of a nascent protein, which subsequently is hydroxylated to the functional elF5A-hypusine transcription factor (right side). In a separate pathway, N1-3-propyl-putrescine, the product of the oxidation of N-acetyl-spermidine by diamine oxidase, is reduced to putreanine.
4.1. The Ornithine Decarboxylase Pathway
In the “classical” ornithine decarboxylase (ODC) pathway (Figure 2), arginine is split by arginase, yielding ornithine and urea [42,44]. Alternately [42,45], ornithine may be obtained from arginine via a reaction with glycine, from glutamate, or from proline (Figure 2).
PUT, the starting product for the biosynthesis of SPD and SPM, is generated from ornithine via decarboxylation by ODC. This is the rate-limiting step and, consequently, mammalian PA synthesis is predominantly controlled here (Figure 1). The amount of ODC enzyme is increased by low levels, while its degradation is enhanced by high levels of PAs. ODC function is further subjected to regulation by ODC antizyme and, in addition, by an antizyme inhibitor protein [46,47,48]; for review see [3]), highlighting the intricate control mechanisms that guarantee intracellular PA homeostasis.
In the next biosynthesis step (Figure 2), spermidine synthase, an aminopropyl transferase, adds an aminopropyl group derived from decarboxylated S-adenosylmethionine (dcAdoMet) to PUT, thereby forming SPD. Subsequently, SPD is elongated the same way by another aminopropyl group, now via spermine synthase, to form SPM. Both aminopropyl transferases are subject to negative feedback control (Figure 1) via their reaction product S-methyladenosine [49]. Since a steady supply of dcAdoMet is crucial for de novo synthesis of SPD and SPM, its formation also represents a rate-limiting step in PA biosynthesis [3,44,46]. Consequently, the corresponding enzyme, S-adenosylmethionine decarboxylase (AdoMetDC), also is tightly regulated at several levels (Figure 1; [1,47]; for review see [3]).
4.2. The Agmatine Pathway
The second pathway starts with enzymatic cleavage of arginine (Figure 2) by arginine decarboxylase into agmatine (AGM). Subsequently, AGM is split into PUT and urea by agmatinase [50,51,52,53]. It still remains somewhat unclear whether the pathway for the biosynthesis of AGM, an important neurotransmitter, plays a major role for the production of PUT and other PAs.
The fact is that mouse knockouts of ODC, which are completely devoid of PA biosynthesis via the ODC pathway, do not survive early stages of embryonic development [54]. Blocking PA biosynthesis via difluoromethylornithine, an ODC inhibitor, stops glial cell proliferation [53] and causes developmental arrest of the embryo in mouse [55], rat [56,57], rabbit [58], hamster [59], and mink [60]. These data suggest that at least in early stages of embryonic development, the AGM pathway is not sufficient to overcome the absence of ODC for PA biosynthesis.
When, however, biosynthesis of PUT is blocked via antisense oligonucleotides against ODC, the AGM pathway may be sufficient to rescue PA biosynthesis [51]. In addition, the fact that the AGM pathway is widely distributed also in peripheral tissues supports a general role of AGM in PA biosynthesis [29,61]. Both apparently contradictory facts could be explained by assuming that the AGM pathway is fully developed only later in life.
5. Concentrations and Transport of Polyamines within the Brain
Tissue concentrations strongly differ among individual PAs (Table 2). When focusing on SPM, it appears rather surprising that the intracellular concentration is very high (about 1 mm), whereas it is very low in plasma/serum (50 nM or below) and in cerebrospinal fluid (CSF; about 100 nm). Such a steep gradient of PA concentrations between blood plasma and CSF, extracellular brain space, cytoplasm, and total PA content in cells requires explanation. Most likely, the systems use different transporters [62].
The fact that PAs in the brain cannot be acquired from the blood stream [38,39] (this special issue) and consequently must be synthesized in the CNS itself (Figure 1) leads to the question of where this biosynthesis takes place, in neurons or in glial cells.
Very likely arginine, which crosses the blood–brain-barrier via the CAT1 transporter [40], represents the starting material. Arginine may be converted to ornithine by arginase and subsequently to PUT by ODC. Alternately, PUT may be derived from AGM via agmatinase. Arginase, ODC, and agmatinase are found in neurons [63,64,65]. The presence of any protein inside a cell, however, contains no information on its activity. Thus, ODC in the adult human brain is predominantly associated with its antizyme protein [64], resulting in low or absent enzymatic activity.
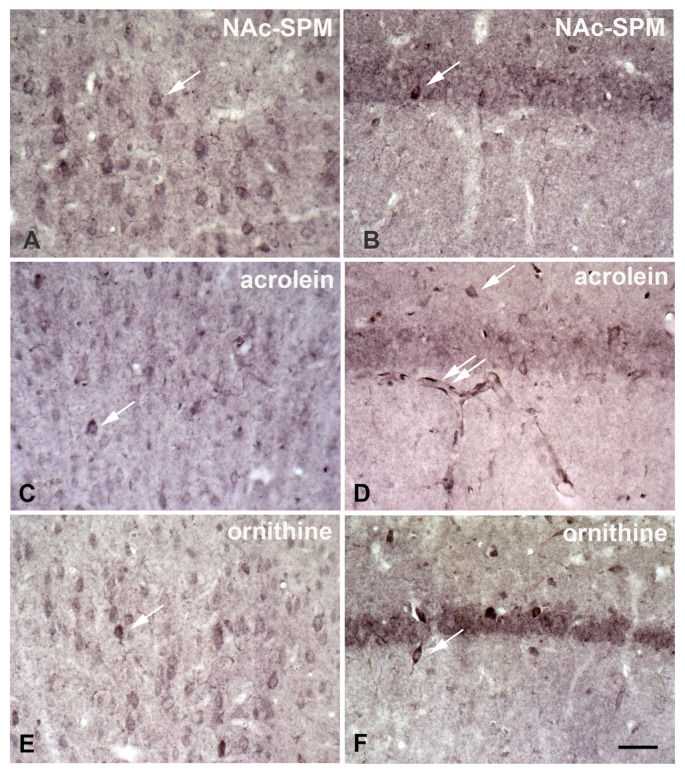
SPD/SPM immunoreactivity (Figure 4) is much more prominent in astrocytes as compared to neurons [66], which is surprising, because the synthesizing enzyme, ODC, appears to be restricted to neurons [64,67]. The data suggest that in the brain PAs may be primarily synthesized in neurons. This idea is strongly supported by the massive expression of SPD-synthase immunoreactivity exclusively in neurons [68]. PA degradation may occur in neurons and astrocytes, as SSAT [69], N-acetylspermine, and acrolein (Figure 5; from [70] this special issue and Figure S1) are localized in both cell types. PAs directly after N-acetylation can leave the cell [71] and are taken up by astrocytes. The capacity of this uptake system is enormous, allowing astrocytes to reach above 1 mM internal SPM after one hour (recalculation based on the data of [72]). Not all neurons, however, release all their intracellular Pas, as conspicuous neuronal SPD/SPM-like immunoreactivity is distributed in a regional- specific manner throughout the brain [73]. The biological meaning of separate synthesis and storage appears unclear at present. Novel data, however, suggest that there is an intense exchange of PAs during astrocytes and neurons. Thus, in the retina during daytime, PA-immunoreactivity is strongly enhanced in photoreceptor terminals, while at night, reactivity in Müller cells was predominant [74]. This indicates that the exchange of PAs between neurons and astrocytes may depend on respective activities.
Figure 4.
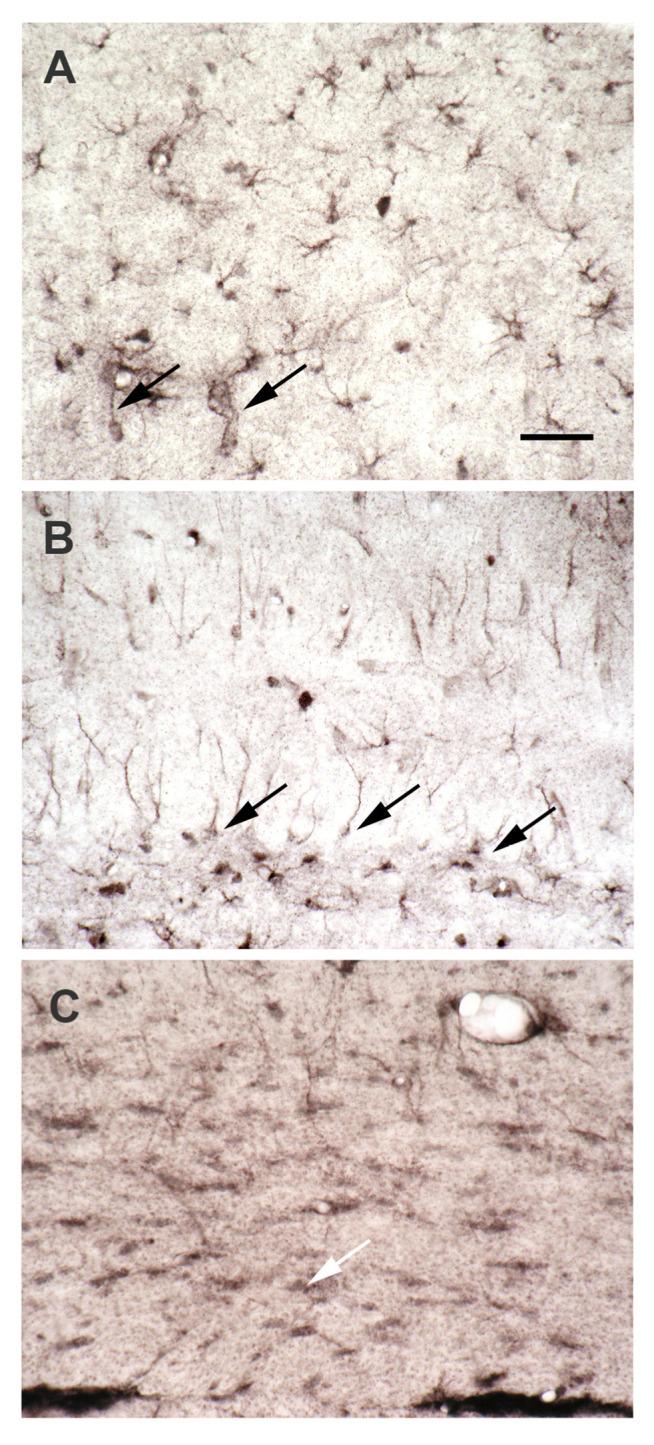
Spermidine/spermine immunoreactivity is predominantly localized in astrocytes, not in neurons. Coronal sections of rat hippocampus after immunocytochemical visualization of SPD/SPM. The antibody (raised in the author’s lab [66]) does not differentiate between tissue-bound spermidine and spermine. Thus, these two polyamines cannot be visualized separately. (A) Spd/spm-immunoreactivity in the CA1 region of the hippocampus is largely restricted to astrocytes. Some of their processes extend to capillaries (left arrow), forming endfeet there. Note the strong staining of the capillary walls (right arrow). Whether this staining is due to labeled astrocyte endfeet or to an immunopositive endothelium cannot be decided here. (B) In the dentate gyrus spd/spm-immunoreactivity of astrocytes displays a very different appearance. Many cell bodies are found at the lower border of the granule cell layer (arrows), with rather straight processes extending to the molecular layer. Other astrocytes with a similar morphology are found in more superficial regions of the dentate gyrus. In contrast, the bottom of the photograph presents the hilar area, where astrocytes show their usual appearance. (C) Among immunoreactive astrocytes, the corpus callosum also displays spd/spm-positive oligodendrocytes (white arrow). Taken from Höhlig et al., this special issue. Bar in (A) indicates 50 µm in (A–C).
Figure 5.
Immunocytochemical visualization of some components of polyamine metabolism. Coronal sections of rat cortex (A,C,E) and hippocampus (B,D,F) after immunocytochemical visualization of (1) N-acetylspermine, (2) acrolein, and (3) ornithine display staining predominantly in neurons. Antibodies had been raised in the author’s laboratory (1, 3) as described earlier [66] or were obtained from commercial sources (2, rabbit anti-acrolein; LS-C63521, MoBiTec, Göttingen, Germany). All control sections were negative. Surprisingly, immunoreactivity is more pronounced in interneurons (single arrows in all images) as compared to adjacent neurons in all sections. This indicates that there may be considerable differences between separate classes of neurons. Note the strong acrolein-immunoreactivity in capillary walls ((D), double arrow). Bar in (F) indicates 50 µm in all images. Taken from Höhlig et al., this special issue.
The total concentration of intracellular SPM in different cells (Table 2) is estimated (presumably to high, see Table 2) to about 3–10 mM [4,75] and most is bound to negatively charged molecules, such as DNA, RNA, phospholipids, acidic proteins, and others. In contrast, the concentration of free SPM as deduced from functional and biochemical tests is much smaller [74,76,77,78]. Unfortunately, this fact often is misunderstood. When SPM is largely bound to a number of macromolecules, this does not mean that it is unavailable for interactions with others. Binding between molecules always is an equilibrium process. Thus, availability of SPM does not depend on the amount, which is bound to other molecules, but only on the affinity of these interactions. When these are low (as they are) and affinities to ion channels or receptors are high (as they are), SPM moves very fast (below milliseconds) to such other binding sites. Therefore, the amount of free as compared to total PAs inside a cell is rather unimportant.
Transport of PAs in CNS uses neuronal and glial processes (Figure 6) showing multiple and bidirectional PA-fluxes. There is a high-affinity uptake of PAs in rodent cortex [79]. The authors, however, bathed slices of brain tissue in respective solutions and consequently could measure only the uptake from artificial CSF but not from the blood compartment. In living brains, PAs (Figure 4) are accumulated preferentially in glial cells [66,80] but not in neurons [66,73,81,82,83,84]. Unfortunately, the PA transport pathways from neurons to glia, from glia to neurons, and in the astrocytic network are still unknown.
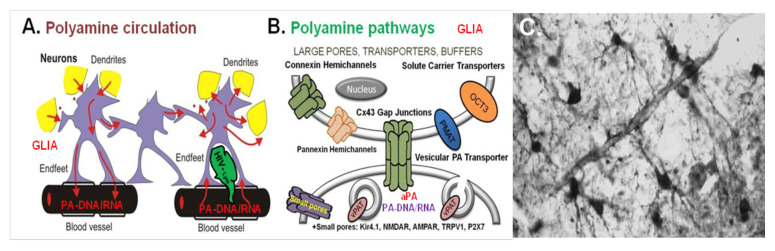
Figure 6.
Distinct mechanisms promote circulation of polyamines and acetylated polyamines in the brain. (A) Suggested interaction between astrocytes (violet), neuronal dendrites (yellow), synapses (yellow), lymphocytes (green), and blood vessels (black) based on bi-directional polyamine (PA) fluxes (red arrows). PAs and aPAs are taken up and released from glia to neurons as well as propagated distantly through the syncytium. (B) Suggested PA and acetylated PAs (aPAs) pathways (uptake and release) in glia via (i) connexin 43 (Cx43) hemichannels (green) or gap-junctions (green), (ii) transporters such as organic cation transporters (OCTs) SLC22A1-3 (orange), and (iii) vesicular PA transporter (vPAT) SLC18B1 with subsequent vesicular uptake/release (brown). Minor pathways (iv) are present in some channels (Kir4.1, NMDAR, AMPAR, TRPV1, P2X7). The scheme represents data from Laube and Veh, 1997; Masuko et al., 2003; Cui et al., 2009; Benedikt et al., 2012; Sala-Rabanal et al., 2013; Merali et al., 2014; Skatchkov et al., 2014; 2015; 2016; Kucheryavykh et al., 2017; Malpica-Nieves et al., 2020; 2021. (C) Astrocytes extend their endfeet to small vessels, as shown here after staining of rat hippocampus for spermine-like immunoreactivity.
PA transport in the brain has been identified as uptake/release via (i) organic cation transporters (OCT; [72,85,86,87,88,89], (ii) glial gap junctions [90], (iii) connexin-43 hemichannels (Cx43 HCs; [91]), and (iv) minor pathways through PA-permeable receptors and channels [92,93]. PA-transport through ion channels or receptors, however, is negligible when compared to the other fluxes.
5.1. Uptake of PAs in Astrocytes via Organic Cation Transporters
Adult astrocytes lack SPD synthesis [68] and use uptake to store PAs, to some degree even in vesicles [94]. Glial cells show several types of PA-uptake [53,72,84,85,86,91]. Recent experimental data [91] make the organic cation transporter 3 (OCT3, SLC22A3) a likely candidate to allow PA uptake in astrocytes. This hypothesis is supported by several lines of evidence. (i) It is present in astrocytes [86,91,95]. (ii) There is a high affinity uptake system in astrocytes with Km-values for SPD/SPM of about 2 µm [85]. As SPD/SPM concentrations in the extracellular space range about 0.5 µm (Table 2), uptake seems possible. Another, so far unidentified, high-affinity uptake system with a Km-value of about 0.5 µm had been described earlier [79] and also would allow PA uptake into astrocytes. (iii) PA uptake into astrocytes is inhibited by trimer 44 NMe, an inhibitor of organic cation transporters [91]. Only OCT3 is expressed in astrocytes in relevant amounts [96]. (iv) OCT3 is rather unspecific and transports quite a number of different compounds [97,98]. This is in good agreement with the fact that in addition to Pas, monoamines, the anesthetic ketamine [98], the anti-Parkinson drug L-Dopa [99], and the anti-diabetic drug metformin [100], as well as SPD can also be transported by a PA transporter into astrocytes [53,72,85,86,91,94,98,99]. PAs can interact with psychoactive substances during transport by OCT3 [98]. The data support a potential role for OCT3 in the mechanism by which astrocytes take up PAs. In strong contrast, however, is the fact that the K0.5-value for the uptake of SPM is about 1.0 mM for OCT heterologously expressed in Xenopus oocytes ([88]; see Table 1). At extracellular SPD/SPM concentration of about 0.5 µm (Table 2), which is about 2000-fold lower than the K0.5-value, OCT3 cannot provide a relevant contribution to the uptake of PAs by astrocytes. Consequently, the uptake mechanism(s) remain mysterious.
5.2. Distribution of PAs via Gap Junctions in the Astroglial Syncytium
Even conceding that the the astroglial network represents no real syncytium, electrical as well as molecular communication between individual astrocytes via gap junctions is intense (Figure 6). Gap junctions, also called electrical synapses, consist of arrays of connexons and macrochannels, which themselves are formed by members of the connexin family proteins. Most connexons are not permeable to PAs, except those formed from connexins Cx38 and Cx43 [20,101,102]. In astrocytes, gap junctions are composed mostly of connexin 43 (Cx43), but lower amounts of Cx26 and Cx30 also are present [103].
Exchange of information in the glial syncytium through these macrochannels is tightly regulated by a variety of extracellular and intracellular factors, including protein kinase C, calcium, and ATP [104]. PAs also belong to these regulators, and SPM at physiological intracellular concentrations (about 1 mM; [105]) efficiently keeps Cx43-containing gap channels open [90] by removing calcium and blocking hydrogen from Cx43 GJs [106,107]. Consequently, PAs may be distributed this way through the astrocytic network.
5.3. Release of PAs via Large Pore Connexin-43 (Cx43) Hemichannels
Gap junctions are formed when connexons of one cell dock with another connexon from a neighboring cell [103]. In contrast, when communication with the extracellular matrix is necessary, the cell may use hemi gap junctions (hemichannels, HCs) to exchange molecules with its intercellular environment (Figure 6).
At a resting state, external and internal calcium concentrations ([Ca+2]e: 1.2 to 1.8 mM; [Ca+2]i: <100 nM), Cx43 HCs are blocked [108,109,110,111,112,113]. Either strong decrease of ([Ca+2]e to about 0.2 mM [110] or mild increase of [Ca+2]i to below 500 nm) [113,114], however, relieves this block [115]. On the other hand, PA release may still occur via Cx43 HCs in high [Ca+2]e conditions [91] when intracellular calcium is still low or mildly increased.
In case of very strong neuronal activation, Cx43 HCs may be opened because extracellular calcium levels can drop dramatically [116]. During 5 s of spreading depression or 2 min of ischemia, [Ca+2]e may decrease to about 0.06 mM [116]. Because this value is below the K50 for opening, which is about 0.05–0.2 mM [109,110], Cx43 HCs will be opened. However, neither spreading depression nor ischemia represents physiological situations and need to be considered separately.
In conclusion, opening of HCs with the help of PAs provide pathways for the transfer of small ions, metabolites, and signaling molecules between the cytosol and the extracellular space around astrocytes. Release of PAs through HCs may modulate receptors in neurons such as NMDA and AMPA receptors via the delivery of these PA “gliotransmitters” from their astrocytic store to neurons.
6. Catabolism and Interconversion
The catabolism of PAs (Figure 3) in principle follows two pathways. One starts with the acetylation of SPM by cytosolic SPM/SPD-N1-acetyltransferase (SSAT), whereas in the second, SPM is directly oxidized via SPM oxidase (SMOX).
6.1. The Cetylation Pathway
Apparently, the most important pathway for catabolism and interconversion of PAs (Figure 3) starts with the acetylation of SPM by cytosolic SPM/SPD-N1-acetyltransferase (SSAT). N1-acetyl-SPM is converted into SPD via peroxisomal N1-PA oxidase (PAOX), which is highly specific for acetylated PAs as substrates [69,117,118]. Subsequently, SSAT also acetylates SPD, and the resulting N1-acetyl-SPD again is oxidized by PAOX, yielding PUT (Figure 3). SSAT may also further acetylate N1-acetyl-SPM to form N1,N12-diacetyl-SPM [119]. Peroxisomal PAOX then oxidizes N1,N12-diacetyl-SPM to N1-acetyl-SPD, which is converted to PUT, as stated above (Figure 3).
SSAT is the rate-limiting enzyme for the complete pathway [69,120,121]. Consequently, its activity is again intensely controlled to maintain PA homeostasis (Figure 1). This regulation comprises an increased expression of the SAT1 gene and an effective conversion to mRNA at high intracellular PA levels as well as an increased degradation of mRNA and SSAT protein at low intracellular PA levels (for details see [3]). Thus, intracellular PA-homeostasis is obtained by the combined regulation of biosynthetic and degradation pathways (Figure 1).
6.2. The Direct Oxidation Pathway
The second catabolic pathway starts with the direct oxidation of SPM via SPM oxidase (SMOX). The emerging aldehyde (N8-3-propanal-spermidine, Figure 3) either undergoes a beta-elimination step, yielding SPD and acrolein, or is further oxidized by aldehyde dehydrogenase to the corresponding acid, N8-2-carboxyethyl-spermidine [117,122]. SPD then is oxidized by the cytosolic diamine oxidase (DAOX) [117,123] to N1-3-propanal-putrescine (Figure 3), which subsequently may be either oxidized to putreanine by aldehyde dehydrogenase or undergo spontaneous beta-elimination to PUT and acrolein [117]. Finally, PUT may be oxidized further via diamine oxidase to form 4-aminobutanal, which is converted to GABA by the cytosolic aldehyde dehydrogenase [117,123]. This alternate pathway for GABA biosynthesis (Figure 3) is known to occur in midbrain dopamine neurons [124] and may be of special importance for the release of GABA by astrocytes [125,126].
Together with biosynthesis, the PA catabolism forms an effective interconversion cycle, enabling mammalian cells to quickly adapt their PA content to the actual demand [117,127]. It must be mentioned, however, that some catabolic reactions produce potentially cytotoxic metabolites like hydrogen peroxide, which might result in DNA damage [69,118]. The intracellular concentration of SPM can reach millimolar levels (Table 2), and thus the degradation of SPM via direct SMOX has been suspected to cause damage to the cell via cytotoxic metabolites [128,129]. Furthermore, a number of authors argue that products of PA oxidation might be involved in neurodegeneration based on the fact that overexpression of enzymes such as PAOX, SMOX and DAOX degrading PAs can result in severe damage of nervous tissue.
With respect to adult, healthy animals, these ideas, however, must be criticized. Most of the reports, which emphasize a high toxicity of PA degradation products, are based on experiments far away from physiological situations. Mostly they depend on the use of tissue culture, where peroxide or aldehydes often are added in grotesque amounts. Actually, experimental evidence for any damage produced by the degradation of PAs under physiological conditions is missing [130]. In addition, PAOX is located in peroxisomes [118,119,131], which allows the oxidation of N1-SPM/SPD in a closed environment. The resulting H2O2 on its own is neither toxic nor able to damage nucleic acids. In the presence of transition metals like copper, however, it may generate toxic hydroxyl radicals in a Fenton-like reaction [132,133]. But peroxisomes actually are free of nucleic acid-associated transition metals like copper. Consequently, the H2O2 generated within peroxisomes is not toxic and will be disposed of via catalase.
In animal models of injury, however, the situation may be different [134,135,136]. In a very precise report, it was shown that during ischemia, intracerebral levels of 3-aminopropanal are increased, preceding ischemic lesions [136]. Even considering that 3-aminopropanal is a very unstable compound, which spontaneously decays to acrolein and ammonium ions (Figure 3), it may not really be important whether ischemic damage is produced by 3-aminopropanal itself or by acrolein, its decomposition product. So, in pathological situations, PA catabolism actually may contribute to toxic effects in the animal brain.
Catabolism via oxidation may lead to the loss of cellular PAs, since the results of aldehyde dehydrogenase activity (Figure 3), N8-2-carboxyethyl-spermidine, putreanine, or GABA are end products of the PA system. The biological advantage of the cytosolic oxidation of SPM via SMOX for the interconversion cycle remains unclear. The two separate pathways, direct versus acetylation-dependent oxidation, may serve different purposes. By speculation, the SSAT pathway may interconvert PAs depending on the cellular demand. This idea is supported by its regulation via PA content (Figure 1). In contrast, the SMOX and the cytosolic pathway may be important to reduce the net PA content inside the cell, independent of fluxes of unmodified or acetylated PAs.
6.3. Additional Interconversion Products
In addition to the pathways described above, SPD serves as substrate for deoxyhypusine synthase (Figure 3). The transfer of an aminobutyl group of SPD onto a specific lysine residue is the first step of the posttranslational modification of the factor elF5A. The deoxyhypusine residue is subsequently converted to hypusine by a specific hydroxylase. The translation factor elF5A is essential for early embryonic development [137] and for the activation of the autophagy pathway, and the hypusine modification is crucial for its proper activity [44,138].
Putreanine (Figure 3) is yet another product of PA catabolism [122,139,140]. Initially, putreanine was thought to be a unique metabolite of mammalian central nervous PA catabolism [139] but was later also detected in rat liver and kidney [122]. At present, there is no known biological function for putreanine other than being the terminal product of cytoplasmatic SPD oxidation. Putreanine, however, accumulates in excretory organs, and one may speculate that it simply serves as a carrier for the extracorporal disposal of waste nitrogen. Certainly, to understand the biological role of putreanine more deeply, further investigation is needed.
7. Conclusions
In summary, we highlight discrepancies in the literature and add new data on the circulation of PAs in the brain and body, including important sources of PAs in gut lumen, blood plasma, and cerebrospinal fluid. We emphasize a large gap in concentrations between these compartments, resulting in surprisingly high accumulation of PAs in glial cytoplasm. We provide a novel and complete scheme for the biosynthesis/degradation of PAs, including glycine, glutamate, proline and others as PA precursors. We suggest a solution for the apparent contradiction in the literature, suggesting that when ODC knockout seems to be lethal, the AGM pathway is probably rescuing PUT production. We point to PA transport in glial cells, explaining how without synthesis these cells (but not neurons) preferentially accumulate PAs. We introduce a novel and complete scheme for PA interconversion, including hypusine, putreanine, and the unique gliotransmitter (GABA) as end-products. In addition, we highlight that S-adenosylmethionine decarboxylase promotes decreased PUT and increased SPM levels. This review can serve as an updated contribution to understanding the PA mystery in the CNS and its body.
Acknowledgments
We are grateful to Franziska Wagner, Priscila Sanabria, and Yuriy Kucheryavykh for excellent scientific help and discussions.
Abbreviations
| CNS | central nervous system |
| CSF | cerebrospinal fluid |
| PA | polyamine |
| PUT | putrescine |
| SPD | spermidine |
| SPM | spermine |
| AGM | agmatine |
| ODC | ornithine decarboxylase |
| dcAdoMet | decarboxylated S-adenosylmethionine |
| AdoMetDC | S-adenosylmethionine decarboxylase |
| SSAT | spermidine/spermine acetyltransferase |
| SMOX | spermine oxidase |
| PAOX | polyamine oxidase |
| DAOX | diamine oxidase |
| OCT3 | organic cation transporter 3 |
| SLC22A3 | solute carrier protein 22A3 (organic cation transporter 3) |
| Cx | connexin |
| HC | hemichannel |
| GABA | gamma aminobutyrate |
| ATP | adenosine triphosphate |
| CAT1 | cationic amino acid transporter 1 |
| PKC | protein kinase C |
| NMDAR | N-methyl-d-aspartate-receptor |
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/biom12040501/s1, Figure S1: Photographic documentation.
Author Contributions
R.W.V., M.J.E. and S.N.S. planned the research and the article. R.W.V., J.R., M.J.E., S.N.S. and C.D. performed the research, made the figures, and wrote the initial draft of the manuscript. R.W.V., J.R. and S.N.S. finalized the review. R.W.V. and S.N.S. provided resources and acquired funding. All authors reviewed and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institutes of Health grants: NIH NINDS RO1-NS-065201 (to S.N.S.), NIH NINDS R15-NS-116478 (to S.N.S.) and NIH NIMHD-PRCTRC-8U54MD007587-03 (to UCC), and NIH NIGMS G12MD007583 (UCC core facilities).
Institutional Review Board Statement
The animal study was reviewed and approved by the Animal Welfare Committees at the Charité—Universitätsmedizin Berlin, Germany, and at the Universidad Central del Caribe, Puerto Rico, USA.
Informed Consent Statement
Not applicable.
Data Availability Statement
Additional data put in the Supplement section and the raw data supporting the conclusions of this review will be made available without undue reservation.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Miller-Fleming L., Olin-Sandoval V., Campbell K., Ralser M. Remaining Mysteries of Molecular Biology: The Role of Polyamines in the Cell. J. Mol. Biol. 2015;427:3389–3406. doi: 10.1016/j.jmb.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Paulin L., Vehmaanperä J., Nykänen I., Pösö H. GTP-insensitive ornithine decarboxylase in acetobacteria able to synthesize spermine. Biochem. Biophys. Res. Commun. 1983;114:779–784. doi: 10.1016/0006-291X(83)90849-5. [DOI] [PubMed] [Google Scholar]
- 3.Pegg A.E. Mammalian polyamine metabolism and function. IUBMB Life. 2009;61:880–894. doi: 10.1002/iub.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe S.-I., Kusama-Eguchi K., Kobayashi H., Igarashi K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J. Biol. Chem. 1991;266:20803–20809. doi: 10.1016/S0021-9258(18)54780-3. [DOI] [PubMed] [Google Scholar]
- 5.Miska J., Rashidi A., Lee-Chang C., Gao P., Lopez-Rosas A., Zhang P., Burga R., Castro B., Xiao T., Han Y. Polyamines drive myeloid cell survival by buffering intracellular pH to promote immunosuppression in glioblastoma. Sci. Adv. 2021;7:eabc8929. doi: 10.1126/sciadv.abc8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Agostino L., Di Luccia A. Polyamines interact with DNA as molecular aggregates. Eur. J. Biochem. 2002;269:4317–4325. doi: 10.1046/j.1432-1033.2002.03128.x. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi K., Kashiwagi K. Modulation of protein synthesis by polyamines. IUBMB Life. 2015;67:160–169. doi: 10.1002/iub.1363. [DOI] [PubMed] [Google Scholar]
- 8.Lee C.-Y., Su G.-C., Huang W.-Y., Ko M.-Y., Yeh H.-Y., Chang G.-D., Lin S.-J., Chi P. Promotion of homology-directed DNA repair by polyamines. Nat. Commun. 2019;10:65. doi: 10.1038/s41467-018-08011-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rider J., Hacker A., Mackintosh C., Pegg A., Woster P., Casero R. Spermine and spermidine mediate protection against oxidative damage caused by hydrogen peroxide. Amino Acids. 2007;33:231–240. doi: 10.1007/s00726-007-0513-4. [DOI] [PubMed] [Google Scholar]
- 10.Tofalo R., Cocchi S., Suzzi G. Polyamines and gut microbiota. Front. Nutr. 2019;6:16. doi: 10.3389/fnut.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eisenberg T., Abdellatif M., Schroeder S., Primessnig U., Stekovic S., Pendl T., Harger A., Schipke J., Zimmermann A., Schmidt A., et al. Cardioprotection and lifespan extension by the natural polyamine spermidine. Nat. Med. 2016;22:1428–1438. doi: 10.1038/nm.4222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenberg T., Knauer H., Schauer A., Büttner S., Ruckenstuhl C., Carmona-Gutierrez D., Ring J., Schroeder S., Magnes C., Antonacci L., et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009;11:1305–1314. doi: 10.1038/ncb1975. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh I., Sankhe R., Mudgal J., Arora D., Nampoothiri M. Spermidine, an autophagy inducer, as a therapeutic strategy in neurological disorders. Neuropeptides. 2020;83:102083. doi: 10.1016/j.npep.2020.102083. [DOI] [PubMed] [Google Scholar]
- 14.Leeuwenhoek A.V. De natis e semine genitali animalculis. Roy. Soc. Lond. Philos. Trans. 1678;12:1040–1043. doi: 10.1098/rstl.1677.0068. [DOI] [Google Scholar]
- 15.Vauquelin L. Experiences sur le sperme humain. Ann. Chim. 1791;9:64–80. [Google Scholar]
- 16.Schreiner P. Ueber eine neue organische Basis in thierischen Organismen. Justus Liebigs Ann. Der Chem. 1878;194:68–84. doi: 10.1002/jlac.18781940107. [DOI] [Google Scholar]
- 17.Ladenburg A., Abel J. Ueber das aethylenimin (Spermin?) Ber. Der Dtsch. Chem. Ges. 1888;21:758–766. doi: 10.1002/cber.188802101139. [DOI] [Google Scholar]
- 18.Fraser A., Wallace H.M. Inhibitors of polyamine metabolism. Amino Acids. 2004;26:353–365. doi: 10.1007/s00726-004-0092-6. [DOI] [PubMed] [Google Scholar]
- 19.Pegg A.E. Introduction to the Thematic Minireview Series: Sixty plus years of polyamine research. J. Biol. Chem. 2018;293:18681–18692. doi: 10.1074/jbc.TM118.006291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skatchkov S., Antonov S., Eaton M. Glia and glial polyamines. Role in brain function in health and disease. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2016;10:73–98. doi: 10.1134/S1990747816010116. [DOI] [Google Scholar]
- 21.Wallace H.M., Fraser A.V., Hughes A. A perspective of polyamine metabolism. Biochem. J. 2003;376:1–14. doi: 10.1042/bj20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cu C., Bähring R., Mayer M.L. The role of hydrophobic interactions in binding of polyamines to non NMDA receptor ion channels. Neuropharmacology. 1998;37:1381–1391. doi: 10.1016/S0028-3908(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 23.Antony T., Hoyer W., Cherny D., Heim G., Jovin T.M., Subramaniam V. Cellular polyamines promote the aggregation of α-synuclein. J. Biol. Chem. 2003;278:3235–3240. doi: 10.1074/jbc.M208249200. [DOI] [PubMed] [Google Scholar]
- 24.Algranati I. Polyamine metabolism in Trypanosoma cruzi: Studies on the expression and regulation of heterologous genes involved in polyamine biosynthesis. Amino Acids. 2010;38:645–651. doi: 10.1007/s00726-009-0425-6. [DOI] [PubMed] [Google Scholar]
- 25.Li G., Regunathan S., Barrow C.J., Eshraghi J., Cooper R., Reis D.J. Agmatine: An endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- 26.Weiss T., Bernard R., Bernstein H.-G., Veh R.W., Laube G. Agmatine modulates spontaneous activity in neurons of the rat medial habenular complex—a relevant mechanism in the pathophysiology and treatment of depression? Transl. Psychiatry. 2018;8:201. doi: 10.1038/s41398-018-0254-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coleman C.S., Hu G., Pegg A.E. Putrescine biosynthesis in mammalian tissues. Biochem. J. 2004;379:849–855. doi: 10.1042/bj20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Iyo A.H., Zhu M.Y., Ordway G.A., Regunathan S. Expression of arginine decarboxylase in brain regions and neuronal cells. J. Neurochem. 2006;96:1042–1050. doi: 10.1111/j.1471-4159.2005.03544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu M.-Y., Iyo A., Piletz J.E., Regunathan S. Expression of human arginine decarboxylase, the biosynthetic enzyme for agmatine. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2004;1670:156–164. doi: 10.1016/j.bbagen.2003.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Milovic V. Polyamines in the gut lumen: Bioavailability and biodistribution. Eur. J. Gastroenterol. Hepatol. 2001;13:1021–1025. doi: 10.1097/00042737-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Kibe R., Kurihara S., Sakai Y., Suzuki H., Ooga T., Sawaki E., Muramatsu K., Nakamura A., Yamashita A., Kitada Y. Upregulation of colonic luminal polyamines produced by intestinal microbiota delays senescence in mice. Sci. Rep. 2014;4:4548. doi: 10.1038/srep04548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Atiya Ali M., Poortvliet E., Strömberg R., Yngve A. Polyamines in foods: Development of a food database. Food Nutr. Res. 2011;55:5572. doi: 10.3402/fnr.v55i0.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handa A.K., Fatima T., Mattoo A.K. Polyamines: Bio-molecules with diverse functions in plant and human health and disease. Front. Chem. 2018;6:10. doi: 10.3389/fchem.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muñoz-Esparza N.C., Latorre-Moratalla M.L., Comas-Basté O., Toro-Funes N., Veciana-Nogués M.T., Vidal-Carou M.C. Polyamines in food. Front. Nutr. 2019;6:108. doi: 10.3389/fnut.2019.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soda K. Spermine and gene methylation: A mechanism of lifespan extension induced by polyamine-rich diet. Amino Acids. 2020;52:213–224. doi: 10.1007/s00726-019-02733-2. [DOI] [PubMed] [Google Scholar]
- 36.Matsumoto M., Kakizoe K., Benno Y. Comparison of fecal microbiota and polyamine concentration in adult patients with intractable atopic dermatitis and healthy adults. Microbiol. Immunol. 2007;51:37–46. doi: 10.1111/j.1348-0421.2007.tb03888.x. [DOI] [PubMed] [Google Scholar]
- 37.Madeo F., Hofer S.J., Pendl T., Bauer M.A., Eisenberg T., Carmona-Gutierrez D., Kroemer G. Nutritional aspects of spermidine. Annu. Rev. Nutr. 2020;40:135–159. doi: 10.1146/annurev-nutr-120419-015419. [DOI] [PubMed] [Google Scholar]
- 38.Shin W.W., Fong W.F., Pang S.F., Wong P.C.L. Limited blood-brain barrier transport of polyamines. J. Neurochem. 1985;44:1056–1059. doi: 10.1111/j.1471-4159.1985.tb08724.x. [DOI] [PubMed] [Google Scholar]
- 39.Weiss T., Bernard R., Laube G.R.J., Eaton M., Skatchkov S.N., Veh R.W. Biotinylated Spermine as Valuable Tool to Analyze the Uptake of Polyamines from Blood Plasma and Cerebrospinal Fluid into the Brain. Biomolecules. 2022 submitted . [Google Scholar]
- 40.Watson C.P., Pazarentzos E., Fidanboylu M., Padilla B., Brown R., Thomas S.A. The transporter and permeability interactions of asymmetric dimethylarginine (ADMA) and L-arginine with the human blood–brain barrier in vitro. Brain Res. 2016;1648:232–242. doi: 10.1016/j.brainres.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Du J., Zhu S., Lim R.R., Chao J.R. Proline metabolism and transport in retinal health and disease. Amino Acids. 2021;53:1789–1806. doi: 10.1007/s00726-021-02981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenis Y.Y., Elmetwally M.A., Maldonado-Estrada J.G., Bazer F.W. Physiological importance of polyamines. Zygote. 2017;25:244–255. doi: 10.1017/S0967199417000120. [DOI] [PubMed] [Google Scholar]
- 43.Phang J.M. The proline regulatory axis and cancer. Front. Oncol. 2012;2:60. doi: 10.3389/fonc.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pegg A.E. Functions of polyamines in mammals. J. Biol. Chem. 2016;291:14904–14912. doi: 10.1074/jbc.R116.731661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomlinson C., Rafii M., Sgro M., Ball R.O., Pencharz P. Arginine is synthesized from proline, not glutamate, in enterally fed human preterm neonates. Pediatric Res. 2011;69:46–50. doi: 10.1203/PDR.0b013e3181fc6ab7. [DOI] [PubMed] [Google Scholar]
- 46.Ivanov I.P., Atkins J.F., Michael A.J. A profusion of upstream open reading frame mechanisms in polyamine-responsive translational regulation. Nucleic Acids Res. 2010;38:353–359. doi: 10.1093/nar/gkp1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perez-Leal O., Merali S. Regulation of polyamine metabolism by translational control. Amino Acids. 2012;42:611–617. doi: 10.1007/s00726-011-1036-6. [DOI] [PubMed] [Google Scholar]
- 48.Wu H.-Y., Chen S.-F., Hsieh J.-Y., Chou F., Wang Y.-H., Lin W.-T., Lee P.-Y., Yu Y.-J., Lin L.-Y., Lin T.-S. Structural basis of antizyme-mediated regulation of polyamine homeostasis. Proc. Natl. Acad. Sci. USA. 2015;112:11229–11234. doi: 10.1073/pnas.1508187112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pegg A.E., Poulin R., Coward J.K. Use of aminopropyltransferase inhibitors and of non-metabolizable analogs to study polyamine regulation and function. Int. J. Biochem. Cell Biol. 1995;27:425–442. doi: 10.1016/1357-2725(95)00007-C. [DOI] [PubMed] [Google Scholar]
- 50.Halaris A., Plietz J. Agmatine. CNS Drugs. 2007;21:885–900. doi: 10.2165/00023210-200721110-00002. [DOI] [PubMed] [Google Scholar]
- 51.Wang X., Ying W., Dunlap K.A., Lin G., Satterfield M.C., Burghardt R.C., Wu G., Bazer F.W. Arginine decarboxylase and agmatinase: An alternative pathway for de novo biosynthesis of polyamines for development of mammalian conceptuses. Biol. Reprod. 2014;90:1–15. doi: 10.1095/biolreprod.113.114637. [DOI] [PubMed] [Google Scholar]
- 52.Laube G., Bernstein H.-G. Agmatine: Multifunctional arginine metabolite and magic bullet in clinical neuroscience? Biochem. J. 2017;474:2619–2640. doi: 10.1042/BCJ20170007. [DOI] [PubMed] [Google Scholar]
- 53.Malpica-Nieves C.J., Rivera-Aponte D.E., Tejeda-Bayron F.A., Mayor A.M., Phanstiel O., Veh R.W., Eaton M.J., Skatchkov S.N. The involvement of polyamine uptake and synthesis pathways in the proliferation of neonatal astrocytes. Amino Acids. 2020;52:1169–1180. doi: 10.1007/s00726-020-02881-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pendeville H., Carpino N., Marine J.-C., Takahashi Y., Muller M., Martial J.A., Cleveland J.L. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell. Biol. 2001;21:6549–6558. doi: 10.1128/MCB.21.19.6549-6558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fozard J.R., Part M.-L., Prakash N.J., Grove J. Inhibition of murine embryonic development by α-difluoromethylornithine, an irreversible inhibitor of ornithine decarboxylase. Eur. J. Pharmacol. 1980;65:379–391. doi: 10.1016/0014-2999(80)90342-8. [DOI] [PubMed] [Google Scholar]
- 56.Reddy P., Rukmini V. α-Difluoromethylornithine as a postcoitally effective antifertility agent in female rats. Contraception. 1981;24:215–221. doi: 10.1016/0010-7824(81)90094-9. [DOI] [PubMed] [Google Scholar]
- 57.Méndez J., Diaz-Flones M., Duián G., Hicks J. Inhibition of rat embryonic development by the intrauterine administration of α-difluoromethylornithine. Contraception. 1983;28:93–98. doi: 10.1016/S0010-7824(83)80010-9. [DOI] [PubMed] [Google Scholar]
- 58.Fozard J.R., Part M.-L., Prakash N.J., Grove J., Schechter P.J., Sjoerdsma A., Koch-Weser J. L-Ornithine decarboxylase: An essential role in early mammalian embryogenesis. Science. 1980;208:505–508. doi: 10.1126/science.6768132. [DOI] [PubMed] [Google Scholar]
- 59.Galliani G., Colombo G., Luzzani F. Contragestational effects of DL-α-difluoro-methylornithine, an irreversible inhibitor of ornithine decarboxylase, in the hamster. Contraception. 1983;28:159–170. doi: 10.1016/0010-7824(83)90015-X. [DOI] [PubMed] [Google Scholar]
- 60.Lefèvre P.L., Palin M.-F., Murphy B.D. Polyamines on the reproductive landscape. Endocr. Rev. 2011;32:694–712. doi: 10.1210/er.2011-0012. [DOI] [PubMed] [Google Scholar]
- 61.Regunathan S., Reis D. Characterization of arginine decarboxylase in rat brain and liver: Distinction from ornithine decarboxylase. J. Neurochem. 2000;74:2201–2208. doi: 10.1046/j.1471-4159.2000.0742201.x. [DOI] [PubMed] [Google Scholar]
- 62.Akanuma S.-I., Shimada H., Kubo Y., Hosoya K.-I. Involvement of Carrier-Mediated Transport at the Blood–Cerebrospinal Fluid Barrier in Spermine Clearance from Rat Brain. Biol. Pharm. Bull. 2017;40:1599–1603. doi: 10.1248/bpb.b17-00394. [DOI] [PubMed] [Google Scholar]
- 63.Müller M., Cleef M., Röhn G., Bonnekoh P., Pajunen A., Bernstein H.-G., Paschen W. Ornithine decarboxylase in reversible cerebral ischemia: An immunohistochemical study. Acta Neuropathol. 1991;83:39–45. doi: 10.1007/BF00294428. [DOI] [PubMed] [Google Scholar]
- 64.Bernstein H.-G., Müller M. The cellular localization of the L-ornithine decarboxylase/polyamine system in normal and diseased central nervous systems. Prog. Neurobiol. 1999;57:485–505. doi: 10.1016/S0301-0082(98)00065-3. [DOI] [PubMed] [Google Scholar]
- 65.Peters D., Berger J., Langnaese K., Derst C., Madai V.I., Krauss M., Fischer K.-D., Veh R.W., Laube G. Arginase and arginine decarboxylase–where do the putative gate keepers of polyamine synthesis reside in rat brain? PLoS ONE. 2013;8:e66735. doi: 10.1371/journal.pone.0066735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Laube G., Veh R.W. Astrocytes, not neurons, show most prominent staining for spermidine/spermine-like immunoreactivity in adult rat brain. Glia. 1997;19:171–179. doi: 10.1002/(SICI)1098-1136(199702)19:2<171::AID-GLIA8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 67.Kilpeläinen P., Rybnikova E., Hietala O., Pelto-Huikko M. Expression of ODC and its regulatory protein antizyme in the adult rat brain. J. Neurosci. Res. 2000;62:675–685. doi: 10.1002/1097-4547(20001201)62:5<675::AID-JNR6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 68.Krauss M., Langnaese K., Richter K., Brunk I., Wieske M., Ahnert-Hilger G., Veh R., Laube G. Spermidine synthase is prominently expressed in the striatal patch compartment and in putative interneurones of the matrix compartment. J. Neurochem. 2006;97:174–189. doi: 10.1111/j.1471-4159.2006.03721.x. [DOI] [PubMed] [Google Scholar]
- 69.Zahedi K., Barone S., Soleimani M. Polyamine catabolism in acute kidney injury. Int. J. Mol. Sci. 2019;20:4790. doi: 10.3390/ijms20194790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Höhlig C.D.C., Rieck J., Beck H., Maslarova A., Veh R.W. Polyamine Degradation in Epilepsia. Biomolecules. 2022 submitted . [Google Scholar]
- 71.Kramer D.L., Diegelman P., Jell J., Vujcic S., Merali S., Porter C.W. Polyamine acetylation modulates polyamine metabolic flux, a prelude to broader metabolic consequences. J. Biol. Chem. 2008;283:4241–4251. doi: 10.1074/jbc.M706806200. [DOI] [PubMed] [Google Scholar]
- 72.Dot J., Danchev N., Blanco I., Rodríguez-Alvarez J. Polyamine uptake is necessary for a normal biochemical maturation of astrocytes in culture. NeuroReport. 2002;13:1083–1087. doi: 10.1097/00001756-200206120-00022. [DOI] [PubMed] [Google Scholar]
- 73.Laube G., Bernstein H.G., Wolf G., Veh R.W. Differential distribution of spermidine/spermine-like immunoreactivity in neurons of the adult rat brain. J. Comp. Neurol. 2002;444:369–386. doi: 10.1002/cne.10157. [DOI] [PubMed] [Google Scholar]
- 74.Vila A., Shihabeddin E., Zhang Z., Santhanam A., Ribelayga C.P., O’Brien J. Synaptic Scaffolds, Ion Channels and Polyamines in Mouse Photoreceptor Synapses: Anatomy of a Signaling Complex. Front. Cell. Neurosci. 2021;15:667046. doi: 10.3389/fncel.2021.667046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seiler N. The Neuropharmacology of Polyamines. Academic Press; London, UK: 1994. Formation, catabolism and properties of the natural polyamines; pp. 1–36. [Google Scholar]
- 76.Bowie D., Mayer M.L. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. doi: 10.1016/0896-6273(95)90049-7. [DOI] [PubMed] [Google Scholar]
- 77.Fakler B., Brändle U., Glowatzki E., Weidemann S., Zenner H.-P., Ruppersberg J. Strong voltage-dependent inward rectification of inward rectifier K+ channels is caused by intracellular spermine. Cell. 1995;80:149–154. doi: 10.1016/0092-8674(95)90459-X. [DOI] [PubMed] [Google Scholar]
- 78.Haghighi A.P., Cooper E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J. Neurosci. 1998;18:4050–4062. doi: 10.1523/JNEUROSCI.18-11-04050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harman R., Shaw G. High-affinity uptake of spermine by slices of rat cerebral cortex. J. Neurochem. 1981;36:1609–1615. doi: 10.1111/j.1471-4159.1981.tb00409.x. [DOI] [PubMed] [Google Scholar]
- 80.Skatchkov S.N., Eaton M.J., Krušek J., Veh R.W., Biedermann B., Bringmann A., Pannicke T., Orkand R.K., Reichenbach A. Spatial distribution of spermine/spermidine content and K+-current rectification in frog retinal glial (Müller) cells. Glia. 2000;31:84–90. doi: 10.1002/(SICI)1098-1136(200007)31:1<84::AID-GLIA80>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 81.Ingoglia N., Sharma S., Pilchman J., Baranowski K., Sturman J. Axonal transport and transcellular transfer of nucleosides and polyamines in intact and regenerating optic nerves of goldfish: Speculation on the axonal regulation of periaxonal cell metabolism. J. Neurosci. 1982;2:1412–1423. doi: 10.1523/JNEUROSCI.02-10-01412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Biedermann B., Skatchkov S.N., Brunk I., Bringmann A., Pannicke T., Bernstein H.G., Faude F., Germer A., Veh R., Reichenbach A. Spermine/spermidine is expressed by retinal glial (Müller) cells and controls distinct K+ channels of their membrane. Glia. 1998;23:209–220. doi: 10.1002/(SICI)1098-1136(199807)23:3<209::AID-GLIA4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 83.Gilad G.M., Balakrishnan K., Gilad V.H. The course of putrescine immunocytochemical appearance in neurons, astroglia and microglia in rat brain cultures. Neurosci. Lett. 1999;268:33–36. doi: 10.1016/S0304-3940(99)00375-4. [DOI] [PubMed] [Google Scholar]
- 84.Masuko T., Kusama-Eguchi K., Sakata K., Kusama T., Chaki S., Okuyama S., Williams K., Kashiwagi K., Igarashi K. Polyamine transport, accumulation, and release in brain. J. Neurochem. 2003;84:610–617. doi: 10.1046/j.1471-4159.2003.01558.x. [DOI] [PubMed] [Google Scholar]
- 85.Dot J., Lluch M., Blanco I., Rodríguez-Alvarez J. Polyamine uptake in cultured astrocytes: Characterization and modulation by protein kinases. J. Neurochem. 2000;75:1917–1926. doi: 10.1046/j.1471-4159.2000.0751917.x. [DOI] [PubMed] [Google Scholar]
- 86.Inazu M., Takeda H., Matsumiya T. The role of glial monoamine transporters in the central nervous system. Nihon Shinkei Seishin Yakurigaku Zasshi. 2003;23:171–178. [PubMed] [Google Scholar]
- 87.Inazu M., Takeda H., Maehara K., Miyashita K., Tomoda A., Matsumiya T. Functional expression of the organic cation/carnitine transporter 2 in rat astrocytes. J. Neurochem. 2006;97:424–434. doi: 10.1111/j.1471-4159.2006.03757.x. [DOI] [PubMed] [Google Scholar]
- 88.Sala-Rabanal M., Li D.C., Dake G.R., Kurata H.T., Inyushin M., Skatchkov S.N., Nichols C.G. Polyamine transport by the polyspecific organic cation transporters OCT1, OCT2, and OCT3. Mol. Pharm. 2013;10:1450–1458. doi: 10.1021/mp400024d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Makarov V., Kucheryavykh L., Kucheryavykh Y., Rivera A., Eaton M., Skatchkov S., Inyushin M. Transport reversal during heteroexchange: A kinetic study. J. Biophys. 2013;2013:683256. doi: 10.1155/2013/683256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Benedikt J., Inyushin M., Kucheryavykh Y.V., Rivera Y., Kucheryavykh L.Y., Nichols C.G., Eaton M.J., Skatchkov S.N. Intracellular polyamines enhance astrocytic coupling. Neuroreport. 2012;23:1021. doi: 10.1097/WNR.0b013e32835aa04b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Malpica-Nieves C.J., Rivera Y., Rivera-Aponte D.E., Phanstiel O., Veh R.W., Eaton M.J., Skatchkov S.N. Uptake of Biotinylated Spermine in Astrocytes: Effect of Cx43 siRNA, HIV-Tat Protein and Polyamine Transport Inhibitor on Polyamine Uptake. Biomolecules. 2021;11:1187. doi: 10.3390/biom11081187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bähring R., Bowie D., Benveniste M., Mayer M.L. Permeation and block of rat GluR6 glutamate receptor channels by internal and external polyamines. J. Physiol. 1997;502:575–589. doi: 10.1111/j.1469-7793.1997.575bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kucheryavykh Y.V., Pearson W.L., Kurata H.T., Eaton M.J., Skatchkov S.N., Nichols C.G. Polyamine permeation and rectification of Kir4.1 channels. Channels (Austin) 2007;1:172–178. doi: 10.4161/chan.4389. [DOI] [PubMed] [Google Scholar]
- 94.Hiasa M., Miyaji T., Haruna Y., Takeuchi T., Harada Y., Moriyama S., Yamamoto A., Omote H., Moriyama Y. Identification of a mammalian vesicular polyamine transporter. Sci. Rep. 2014;4:6836. doi: 10.1038/srep06836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vialou V., Balasse L., Callebert J., Launay J.M., Giros B., Gautron S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J. Neurochem. 2008;106:1471–1482. doi: 10.1111/j.1471-4159.2008.05506.x. [DOI] [PubMed] [Google Scholar]
- 96.Furihata T., Anzai N. Functional expression of organic ion transporters in astrocytes and their potential as a drug target in the treatment of central nervous system diseases. Biol. Pharm. Bull. 2017;40:1153–1160. doi: 10.1248/bpb.b17-00076. [DOI] [PubMed] [Google Scholar]
- 97.Amphoux A., Vialou V., Drescher E., Brüss M., La Cour C.M., Rochat C., Millan M.J., Giros B., Bönisch H., Gautron S. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 98.Maier J., Niello M., Rudin D., Daws L.C., Sitte H.H. The Interaction of Organic Cation Transporters 1-3 and PMAT with Psychoactive Substances. Handb. Exp. Pharmacol. 2021;266:199–214. doi: 10.1007/164_2021_469. [DOI] [PubMed] [Google Scholar]
- 99.Inyushin M., Huertas A., Kucheryavykh Y., Kucheryavykh L., Tsydzik V., Sanabria P., Eaton M., Skatchkov S., Rojas L., Wessinger W. L-DOPA uptake in astrocytic endfeet enwrapping blood vessels in rat brain. Parkinson’s Dis. 2012;2012:321406. doi: 10.1155/2012/321406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khan N.A., Wiernsperger N., Quemener V., Havouis R., Moulinoux J.P. Characterization of metformin transport system in NIH 3T3 cells. J. Cell. Physiol. 1992;152:310–316. doi: 10.1002/jcp.1041520212. [DOI] [PubMed] [Google Scholar]
- 101.Veenstra R.D. Control of Cell Proliferation by Polyamine Signaling through Gap Junctions, Feasible or Not? Bioessays. 2018;40:e1800043. doi: 10.1002/bies.201800043. [DOI] [PubMed] [Google Scholar]
- 102.Enkvetchakul D., Ebihara L., Nichols C. Polyamine flux in Xenopus oocytes through hemi-gap junctional channels. J. Physiol. 2003;553:95–100. doi: 10.1113/jphysiol.2003.047910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Giaume C., Koulakoff A., Roux L., Holcman D., Rouach N. Astroglial networks: A step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 104.Theis M., Söhl G., Eiberger J., Willecke K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005;28:188–195. doi: 10.1016/j.tins.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 105.Kucheryavykh Y.V., Shuba Y.M., Antonov S.M., Inyushin M.Y., Cubano L., Pearson W.L., Kurata H., Reichenbach A., Veh R.W., Nichols C.G. Complex rectification of Müller cell Kir currents. Glia. 2008;56:775–790. doi: 10.1002/glia.20652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skatchkov S.N., Bukauskas F.F., Benedikt J., Inyushin M., Kucheryavykh Y.V. Intracellular spermine prevents acid-induced uncoupling of Cx43 gap junction channels. Neuroreport. 2015;26:528. doi: 10.1097/WNR.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kucheryavykh L.Y., Benedikt J., Cubano L.A., Skatchkov S.N., Bukauskas F.F., Kucheryavykh Y.V. Polyamines preserve connexin43-mediated gap junctional communication during intracellular hypercalcemia and acidosis. Neuroreport. 2017;28:208. doi: 10.1097/WNR.0000000000000746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Contreras J.E., Sáez J.C., Bukauskas F.F., Bennett M.V. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc. Natl. Acad. Sci. USA. 2003;100:11388–11393. doi: 10.1073/pnas.1434298100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ye Z., Wyeth M.S., Baltan-Tekkok S., Ransom B.R. Functional hemichannels in astrocytes: A novel mechanism of glutamate release. J. Neurosci. 2003;23:3588–3596. doi: 10.1523/JNEUROSCI.23-09-03588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sáez J.C., Retamal M.A., Basilio D., Bukauskas F.F., Bennett M.V. Connexin-based gap junction hemichannels: Gating mechanisms. Biochim. Biophys. Acta (BBA)-Biomembr. 2005;1711:215–224. doi: 10.1016/j.bbamem.2005.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spray D.C., Ye Z.-C., Ransom B.R. Functionalconnexin “hemichannels”: A critical appraisal. Glia. 2006;54:758–773. doi: 10.1002/glia.20429. [DOI] [PubMed] [Google Scholar]
- 112.Bargiotas P., Monyer H., Schwaninger M. Hemichannels in cerebral ischemia. Curr. Mol. Med. 2009;9:186–194. doi: 10.2174/156652409787581646. [DOI] [PubMed] [Google Scholar]
- 113.De Vuyst E., Wang N., Decrock E., De Bock M., Vinken M., Van Moorhem M., Lai C., Culot M., Rogiers V., Cecchelli R. Ca2+ regulation of connexin 43 hemichannels in C6 glioma and glial cells. Cell Calcium. 2009;46:176–187. doi: 10.1016/j.ceca.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 114.Wang N., De Bock M., Antoons G., Gadicherla A.K., Bol M., Decrock E., Evans W.H., Sipido K.R., Bukauskas F.F., Leybaert L. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca 2+ elevation. Basic Res. Cardiol. 2012;107:304. doi: 10.1007/s00395-012-0304-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Giaume C., Naus C.C., Sáez J.C., Leybaert L. Glial connexins and pannexins in the healthy and diseased brain. Physiol. Rev. 2021;101:93–145. doi: 10.1152/physrev.00043.2018. [DOI] [PubMed] [Google Scholar]
- 116.Hansen A., Zeuthen T. Changes of brain extracellular ions during spreading depression and ischemia in rats. Acta Physiol. Scand. 1981;113:437–445. doi: 10.1111/j.1748-1716.1981.tb06920.x. [DOI] [PubMed] [Google Scholar]
- 117.Seiler N. Catabolism of polyamines. Amino Acids. 2004;26:217–233. doi: 10.1007/s00726-004-0070-z. [DOI] [PubMed] [Google Scholar]
- 118.Stewart T.M., Dunston T.T., Woster P.M., Casero R.A. Polyamine catabolism and oxidative damage. J. Biol. Chem. 2018;293:18736–18745. doi: 10.1074/jbc.TM118.003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Pegg A.E. Spermidine/spermine-N 1-acetyltransferase: A key metabolic regulator. Am. J. Physiol.-Endocrinol. Metab. 2008;294:E995–E1010. doi: 10.1152/ajpendo.90217.2008. [DOI] [PubMed] [Google Scholar]
- 120.Wang Y., Casero R.A., Jr. Mammalian polyamine catabolism: A therapeutic target, a pathological problem, or both? J. Biochem. 2006;139:17–25. doi: 10.1093/jb/mvj021. [DOI] [PubMed] [Google Scholar]
- 121.Minois N., Carmona-Gutierrez D., Madeo F. Polyamines in aging and disease. Aging (Albany N. Y.) 2011;3:716. doi: 10.18632/aging.100361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seiler N., Knödgen B., Haegele K. N-(3-aminopropyl) pyrrolidin-2-one, a product of spermidine catabolism in vivo. Biochem. J. 1982;208:189–197. doi: 10.1042/bj2080189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elmore B.O., Bollinger J.A., Dooley D.M. Human kidney diamine oxidase: Heterologous expression, purification, and characterization. JBIC J. Biol. Inorg. Chem. 2002;7:565–579. doi: 10.1007/s00775-001-0331-1. [DOI] [PubMed] [Google Scholar]
- 124.Kim J.-I., Ganesan S., Luo S.X., Wu Y.-W., Park E., Huang E.J., Chen L., Ding J.B. Aldehyde dehydrogenase 1a1 mediates a GABA synthesis pathway in midbrain dopaminergic neurons. Science. 2015;350:102–106. doi: 10.1126/science.aac4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yamasaki E.N., Barbosa V.D., De Mello F.G., Hokoç J.N. GABAergic system in the developing mammalian retina: Dual sources of GABA at early stages of postnatal development. Int. J. Dev. Neurosci. 1999;17:201–213. doi: 10.1016/S0736-5748(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 126.Szabó Z., Héja L., Szalay G., Kékesi O., Füredi A., Szebényi K., Dobolyi Á., Orbán T.I., Kolacsek O., Tompa T. Extensive astrocyte synchronization advances neuronal coupling in slow wave activity in vivo. Sci. Rep. 2017;7:6018. doi: 10.1038/s41598-017-06073-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Seiler N. Functions of polyamine acetylation. Can. J. Physiol. Pharmacol. 1987;65:2024–2035. doi: 10.1139/y87-317. [DOI] [PubMed] [Google Scholar]
- 128.Bonneau M.-J., Poulin R. Spermine oxidation leads to necrosis with plasma membrane phosphatidylserine redistribution in mouse leukemia cells. Exp. Cell Res. 2000;259:23–34. doi: 10.1006/excr.2000.4974. [DOI] [PubMed] [Google Scholar]
- 129.Saiki R., Park H., Ishii I., Yoshida M., Nishimura K., Toida T., Tatsukawa H., Kojima S., Ikeguchi Y., Pegg A.E. Brain infarction correlates more closely with acrolein than with reactive oxygen species. Biochem. Biophys. Res. Commun. 2011;404:1044–1049. doi: 10.1016/j.bbrc.2010.12.107. [DOI] [PubMed] [Google Scholar]
- 130.Seiler N. Oxidation of polyamines and brain injury. Neurochem. Res. 2000;25:471–490. doi: 10.1023/A:1007508008731. [DOI] [PubMed] [Google Scholar]
- 131.Holtta E. Oxidation of spermidine and spermine in rat liver: Purification and properties of polyamine oxidase. Biochemistry. 1977;16:91–100. doi: 10.1021/bi00620a015. [DOI] [PubMed] [Google Scholar]
- 132.Winterbourn C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995;82:969–974. doi: 10.1016/0378-4274(95)03532-X. [DOI] [PubMed] [Google Scholar]
- 133.Ha H.C., Sirisoma N.S., Kuppusamy P., Zweier J.L., Woster P.M., Casero R.A. The natural polyamine spermine functions directly as a free radical scavenger. Proc. Natl. Acad. Sci. USA. 1998;95:11140–11145. doi: 10.1073/pnas.95.19.11140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Doğan A., Rao A.M., Baskaya M.K., Hatcher J., Temiz C., Rao V.L., Dempsey R.J. Contribution of polyamine oxidase to brain injury after trauma. J. Neurosurg. 1999;90:1078–1082. doi: 10.3171/jns.1999.90.6.1078. [DOI] [PubMed] [Google Scholar]
- 135.Ivanova S., Batliwalla F., Mocco J., Kiss S., Huang J., Mack W., Coon A., Eaton J.W., Al-Abed Y., Gregersen P.K., et al. Neuroprotection in cerebral ischemia by neutralization of 3-aminopropanal. Proc. Natl. Acad. Sci. USA. 2002;99:5579–5584. doi: 10.1073/pnas.082609299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ivanova S., Botchkina G.I., Al-Abed Y., Meistrell M., Gregersen P.K., Eaton J.W., Tracey K.J. Cerebral Ischemia Enhances Polyamine Oxidation: Identification of Enzymatically Formed 3-Aminopropanal as an Endogenous Mediator of Neuronal and Glial Cell Death. J. Exp. Med. 1998;188:327–340. doi: 10.1084/jem.188.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nishimura K., Lee S.B., Park J.H., Park M.H. Essential role of eIF5A-1 and deoxyhypusine synthase in mouse embryonic development. Amino Acids. 2012;42:703–710. doi: 10.1007/s00726-011-0986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Park M.H. The post-translational synthesis of a polyamine-derived amino acid, hypusine, in the eukaryotic translation initiation factor 5A (eIF5A) J. Biochem. 2006;139:161–169. doi: 10.1093/jb/mvj034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kakimoto Y., Nakajima T., Kumon A., Matsuoka Y., Imaoka N., Sano I., Kanazawa A. Putreanine, N-(4-Aminobutyl)-3-aminopropionic Acid: An amino acid occurring uniquely in the mammalian central nervous system. J. Biol. Chem. 1969;244:6003–6006. doi: 10.1016/S0021-9258(18)63572-0. [DOI] [PubMed] [Google Scholar]
- 140.Konishi H., Nakajima T., Sano I. Metabolism of putrescine in the central nervous system. J. Biochem. 1977;81:355–360. doi: 10.1093/oxfordjournals.jbchem.a131466. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Additional data put in the Supplement section and the raw data supporting the conclusions of this review will be made available without undue reservation.