Abstract
Xylitol is effective in preventing acute otitis media by inhibiting the growth of Streptococcus pneumoniae. To clarify this inhibition we used fructose, which is known to block similar growth inhibition observed in Streptococcus mutans. In addition, we evaluated the efficacy of sorbitol in inhibiting the growth of pneumococci, as sorbitol is widely used for indications similar to those for which xylitol is used. The addition of 5% xylitol to the growth medium resulted in marked growth inhibition, an effect which was totally eliminated in the presence of 1, 2.5, or 5% fructose but not in the presence of 1 or 5% glucose, 1% galactose, or 1% sucrose. This finding implies that xylitol-induced inhibition of pneumococcal growth is mediated via the fructose phosphotransferase system in a way similar to that in which mutans group streptococcal growth is inhibited. The addition of sorbitol at concentrations of 1, 2.5, or 5% to the growth medium did not affect the growth of pneumococci and neither inhibited nor enhanced the xylitol-induced growth impairment. Thus, it seems that xylitol is the only commercially used sugar substitute proven to have an antimicrobial effect on pneumococci.
Xylitol is a five-carbon sugar alcohol that occurs naturally in certain fruits and that has been widely used as a sweetener, mainly in chewing gums (14). We have previously shown that xylitol at concentrations of 1 and 5% in the growth medium inhibits the growth of pneumococci (Streptococcus pneumoniae) (6) and that it also reduces the level of adherence of the otopathogens S. pneumoniae and Haemophilus influenzae to nasopharyngeal epithelial cells (7). Regular use of xylitol chewing gum or syrup prevented acute otitis media to the extent of 30 to 42% in day-care children (11, 12).
The mechanism of action of xylitol on the inhibition of growth of pneumococci may resemble that previously demonstrated for the xylitol-induced inhibition of growth of mutans group streptococci (Streptococcus mutans). Mutans group streptococci take xylitol into the cell via a fructose phosphotransferase system (10). Xylitol does not cause growth inhibition in mutans group streptococci in the presence of fructose (3), as the fructose phosphotransferase system prefers fructose. Our aim was to study whether fructose prevents xylitol-induced inhibition of growth of pneumococci as well. Other sugars were also tested to observe if the prevention of the xylitol-induced growth inhibition is a unique feature of fructose.
Another sugar alcohol, sorbitol, was included in this study because it is also commonly used as a sweetener in many chewing gums (14); in fact, it is used more frequently than xylitol because it is less expensive than xylitol. In addition to testing whether sorbitol inhibits the growth of pneumococci, we used a combination of sorbitol and xylitol to study whether sorbitol affects the xylitol-induced inhibition of pneumococcal growth.
MATERIALS AND METHODS
Twenty strains of pneumococci were isolated from consecutive routine patient samples taken from middle-ear effusions, and one pneumococcal strain was isolated from a sinus aspirate. Strain ATCC 49619 was used as a commercially available reference strain. The pneumococcal strains were identified from their colony morphologies and optochin sensitivities. The strains were kept frozen at −20°C in skim milk broth until they were used. They were serotyped at the National Health Institute Laboratory in Oulu, Finland. Strains of serotypes 6A, 6B (two strains), 7 (sinus aspirate), 9V (two strains), 14, 15 (four strains), 16, 19A (two strains), 23F (two strains), 38 (two strains), and pool G (serotype 29, 35, or 42) were used for the purposes of the xylitol and fructose studies (Table 1). One strain was not viable at the time of serotyping after storage. Ten of these strains (serotypes 6A, 6B [two strains], 9V, 14, 15 [three strains], 19A, and 38) were used in complementary studies and in the xylitol and sorbitol studies (Table 1). The serotype of strain ATCC 49619 was 19F (serotype 59 in the U.S. nomenclature according to the American Type Culture Collection [ATCC]).
TABLE 1.
Growth media used in the experimentsa
| Control medium | Test medium | No. of strainsb |
|---|---|---|
| BHI only | BHI + 2.5% xylitol | 11 |
| BHI only | BHI + 5% xylitol | 21 |
| BHI + 5% fructose | BHI + 5% fructose + 5% xylitol | 21 |
| BHI + 2.5% fructose | BHI + 2.5% fructose + 5% xylitol | 11 |
| BHI + 1% fructose | BHI + 1% fructose + 5% xylitol | 11 |
| BHI + 5% glucose | BHI + 5% glucose + 5% xylitol | 11 |
| BHI + 1% glucose | BHI + 1% glucose + 5% xylitol | 11 |
| BHI + 1% galactose | BHI + 1% galactose + 5% xylitol | 11 |
| BHI + 1% sucrose | BHI + 1% sucrose + 5% xylitol | 11 |
| BHI only | BHI + 1% sorbitol | 11 |
| BHI only | BHI + 2.5% sorbitol | 11 |
| BHI only | BHI + 5% sorbitol | 11 |
| BHI only | BHI + 2.5% xylitol + 2.5% sorbitol | 11 |
All concentrations are shown as weight/volume.
Including strain ATCC 49619; each test was carried out in triplicate for each strain.
Pneumococci were cultured in brain heart infusion broth (BHI) (Difco Laboratories, Detroit, Mich.) containing 0.2% glucose. To ensure optimal growth, 10% (vol/vol) heat-inactivated fetal calf serum (PAA Laboratories, Linz, Austria) was added. Xylitol (Sigma Chemical Co., St. Louis, Mo.), d-fructose (BDH Laboratory Supplies, Poole, England), d-(+)-glucose (anhydrous; Fluka Biochemika, Buchs, Switzerland), d-(+)-galactose (Merck, Darmstadt, Germany), sucrose (BDH Laboratory Supplies), or d-(−)-sorbitol (Merck) was added to the basic medium, and the mixture was sterilized by filtration (Ministart 0.2-μm-pore-size filter; Millipore Corp., Bedford, Mass.). The test media contained the sugar or sugar alcohol concentrations indicated in Table 1. Each strain was cultured aerobically in BHI at 35°C in a 5% CO2 atmosphere up to the exponential phase of growth (optical density [OD], 0.2 to 0.3). Three hundred microliters of this suspension was transferred into 3 ml of test medium containing various sugar alcohol or sugar concentrations (Table 1). All the test tubes with medium also contained 10% fetal calf serum at a volume of 3 ml. The test tubes were incubated at 35°C for 24 h. Each test was carried out in triplicate.
The OD of each tube was measured at a wavelength of 650 nm with an SFM 35 spectrophotometer (Perkin-Elmer Corp., Norwalk, Conn.) against the standard medium, with the measurements being performed every 1 to 2 h during the logarithmic phase of growth. The OD results were calculated as the means of three measurements. To confirm the relationship between the OD and the total number of viable bacterial cells, viability counts were made by the standard dilution method on sheep blood agar plates at the beginning, at the mid-phase of logarithmic growth, and at end of the logarithmic phase and also during the stationary phase. At the end of the experiment (24 h) the samples were cultured on sheep blood agar plates and in the basic medium to ensure the presence of viable pneumococci.
Statistical analyses were performed with the Arcus QuickStat biostatistical program. One-way analysis of variance was used to test the differences between the groups at each point in time. When there was a significant difference, the groups were further compared by the t test with the Bonferroni correction because of multiple comparisons.
RESULTS
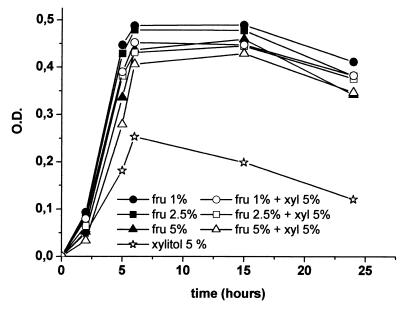
Marked growth inhibition was detected in the presence of 5% xylitol in the basic medium with 0.2% glucose, as in our previous studies (6). The addition of 1, 2.5, or 5% fructose eliminated the effect of the xylitol (Fig. 1). None of the differences was statistically significant. Xylitol-induced growth inhibition was prevented by all fructose concentrations used.
FIG. 1.
Growth of pneumococci (11 and 21 strains), measured in terms of OD counts, in media containing 1, 2.5, or 5% fructose (fru) with or without 5% xylitol (xyl) over 24 h and in BHI containing xylitol only.
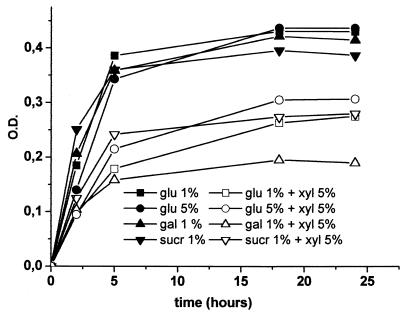
The addition of 1% glucose, 1% galactose, or 1% sucrose did not alter the effect of xylitol, and growth inhibition was detected (Fig. 2). The difference was greatest 5 h after the beginning of the observation when glucose or galactose was used, when the mean OD for the pneumococci was 0.38 (standard deviation [SD], 0.06) in 1% glucose, whereas it was 0.18 (SD, 0.07) in 5% xylitol combined with 1% glucose; i.e., it was 53% less in the xylitol-containing medium (95% confidence interval [CI], 45 to 63% [P < 0.0001]). The mean OD was 0.36 (SD, 0.07) in 1% galactose, whereas it was 0.16 (SD, 0.06) in 5% xylitol combined with 1% galactose; i.e., it was 53% less in the xylitol-containing medium (95% CI, 46 to 65% [P < 0.0001]). The difference was greatest 2 h after the beginning of the culture when sucrose was used. The mean OD for the pneumococci was 0.25 (SD, 0.09) in 1% sucrose, whereas it was 0.12 (SD, 0.07) in 5% xylitol combined with 1% sucrose; i.e., it was 50% less in the xylitol-containing medium (95% CI, 31 to 68% [P < 0.0001]) (Fig. 2). The mean OD for the pneumococci remained less in xylitol-containing medium than in the control medium throughout the observation period for all media tested (P < 0.0001). Xylitol-induced growth inhibition was also observed in the presence of 5% glucose. The difference was greatest 6 h after the beginning of the culture, when the mean OD for the pneumococci was 0.21 (SD, 0.07) in 5% xylitol, whereas it was 0.34 (SD, 0.07) in the control culture containing 5% glucose; i.e., it was 38% less in the xylitol-containing medium (95% CI, 26 to 47% [P < 0.0001]).
FIG. 2.
Growth of pneumococci (11 strains), measured in terms of OD counts, in media containing 1 or 5% glucose (glu), 1% galactose (gal), or 1% sucrose (sucr) with or without 5% xylitol (xyl) over 24 h.
The xylitol-induced inhibition of pneumococcal growth was systematically seen with strain ATCC 49619 and with all except one of the pneumococci isolated (n = 21). The serotype of this exceptional strain was 7, and it was the only strain isolated from a sinus aspirate.
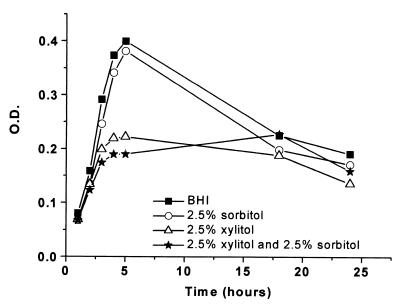
Sorbitol at concentrations of 1, 2.5, and 5% had no effect on the growth of pneumococci. Xylitol at a concentration of 2.5% was as effective alone as it was in combination with 2.5% sorbitol (Fig. 3). The mean OD for the pneumococci after 5 h of observation was 0.40 (SD, 0.07) in the control medium (BHI), whereas it was 0.19 (SD, 0.06) in the medium containing both 2.5% sorbitol and xylitol and 0.22 (SD, 0.07) in the medium containing 2.5% xylitol. The OD values for the cultures grown on the experimental media differed significantly from those for cultures grown on the control medium. Growth inhibition of 52% (95% CI, 35 to 68% [P < 0.0001]) and 46% (95% CI, 28 to 60% [P < 0.0001]), respectively, were observed.
FIG. 3.
Growth of pneumococci (11 strains), measured in terms of OD counts, in BHI, 2.5% sorbitol, 2.5% xylitol, and a medium containing both 2.5% xylitol and 2.5% sorbitol over 24 h.
The correlation between the OD values during the logarithmic phase and the growth of pneumococci was good and was independent of the medium used. An OD of 0.05 to 0.1 was equal to 1 × 106 to 2 × 106 CFU/ml, and a value of 0.4 corresponded to 1 × 109 CFU/ml. The pneumococci in the medium containing xylitol and sorbitol remained viable until the end of the test. The autolysis of pneumococci was seen in BHI and in the medium containing 1, 2.5, or 5% sorbitol, which is a typical phenomenon in pneumococcal cultures after rapid logarithmic growth and which is thought to be mediated by autolytic enzymes. In media containing xylitol the bacteria never achieved the highest point of logarithmic growth and autolysis was not seen. The bacteria in the media containing an extra carbon source for growth, i.e., 1, 2.5, or 5% fructose, 1 or 5% glucose, 1% galactose, or 1% sucrose, had high OD values at the end of the experiment, without autolysis. Growth of these bacteria was detected after transfer to fresh basic medium.
DISCUSSION
We were able to confirm our earlier finding that xylitol causes marked inhibition of pneumococcal growth (6). Extra glucose, galactose, or sucrose had no effect on this inhibition, but it was totally eliminated by fructose. This shows that the mechanism of growth inhibition in pneumococci is mediated by a system regulated by fructose. The phosphotransferase system of oral streptococci is a flexible mechanism capable of taking in different sugars depending on the current sugar environment (13). In mutans group streptococci the first step of xylitol metabolism is entry into the bacterial cell via the fructose phosphotransferase system, and xylitol does not cause growth inhibition in the presence of fructose (3, 10). Xylitol is then metabolized to xylitol-5-phosphate, which mutans group streptococci cannot utilize further and which may even be toxic to bacteria (10). It must therefore be expelled from the cell (9). This futile xylitol cycle consumes energy and results in growth inhibition. Our results show that inhibition of the growth of pneumococci exposed to xylitol is prevented by fructose, a finding that supports our hypothesis that this inhibition is attributable to the involvement of the fructose phosphotransferase system in a way similar to that of inhibition of growth of mutans group streptococci.
This finding that xylitol exposure restored the pneumococci to a viable state despite the impaired growth is in accordance with our observation that xylitol does not reduce the nasopharyngeal carriage of pneumococci (5, 11). Of the 14 serotypes that we tested, xylitol had no effect on the growth of strains of only 1 serotype, serotype 7. Although xylitol has been widely used as a sweetener in Finland for two decades, the results of our clinical trials indicate that most pneumococcal strains are sensitive to it (11, 12). Whether xylitol insensitivity results in a decrease in virulence in the case of pneumococci remains to be established.
Sorbitol, which has been shown to have minimal or no effects on the growth of mutans group streptococci in the presence of glucose, had no effect in the present study on the growth of pneumococci (2, 3). Sorbitol alone is also less effective than xylitol for the prevention of caries (4, 8). It may enhance the inhibitory potential of xylitol in mutans group streptococci by alternation of the intracellular metabolism of xylitol (1), but in pneumococci it neither enhanced nor inhibited the effect of xylitol on growth. It is unlikely that the combination of xylitol and sorbitol in chewing gums would provide any clinical benefit in the prevention of otitis media relative to the clinical benefits of chewing gums with pure xylitol. The dose of xylitol needed to prevent acute otitis media is in any case quite high, so that its partial replacement with sorbitol would be an illogical approach.
In conclusion, we found that the beneficial effect of xylitol in inhibiting the growth of pneumococci is totally eliminated by fructose. The underlying mechanism of xylitol-induced inhibition of growth of pneumococci may be mediated by a fructose phosphotransferase system in a manner similar to that of inhibition of growth of mutans group streptococci. In practice, this finding means that xylitol should not be combined with fructose in products intended to prevent acute otitis media.
ACKNOWLEDGMENT
This work was supported by a grant from the Maud Kuistila Foundation, Helsinki, Finland, and the Yrjö Johnsson Foundation, Helsinki, Finland.
REFERENCES
- 1.Assev S, Rølla G. Sorbitol increases the growth inhibition of xylitol on Strep. mutans OMZ 176. Acta Pathol Microbiol Immunol Scand Sect B. 1986;94:231–237. doi: 10.1111/j.1699-0463.1986.tb03046.x. [DOI] [PubMed] [Google Scholar]
- 2.Assev S, Vegarud G, Rølla G. Growth inhibition of Streptococcus mutans strain OMZ 176 by xylitol. Acta Pathol Microbiol Scand. 1980;88:61–63. doi: 10.1111/j.1699-0463.1980.tb02605.x. [DOI] [PubMed] [Google Scholar]
- 3.Assev S, Waler S M, Rølla G. Further studies on the growth inhibition of some oral bacteria by xylitol. Acta Pathol Immunol Scand. 1983;91:261–265. doi: 10.1111/j.1699-0463.1983.tb00044.x. [DOI] [PubMed] [Google Scholar]
- 4.Gales M A, Nguyen T M. Sorbitol compared with xylitol in prevention of dental caries. Ann Pharmacother. 2000;34:98–100. doi: 10.1345/aph.19020. [DOI] [PubMed] [Google Scholar]
- 5.Kontiokari T, Svanberg M, Mattila P, Leinonen M, Uhari M. Quantitative analysis of the effect of xylitol on pneumococcal nasal colonisation in rats. FEMS Microbiol Lett. 1999;178:313–317. doi: 10.1111/j.1574-6968.1999.tb08693.x. [DOI] [PubMed] [Google Scholar]
- 6.Kontiokari T, Uhari M, Koskela M. Effect of xylitol on growth of nasopharyngeal bacteria in vitro. Antimicrob Agents Chemother. 1995;39:1820–1823. doi: 10.1128/aac.39.8.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kontiokari T, Uhari M, Koskela M. Antiadhesive effects of xylitol on otopathogenic bacteria. J Antimicrob Chemother. 1998;41:563–565. doi: 10.1093/jac/41.5.563. [DOI] [PubMed] [Google Scholar]
- 8.Mäkinen K K, Bennett C A, Hujoel P P, Isokangas P J, Isotupa K P, Pape H R, Jr, Mäkinen K K. Xylitol chewing gums and caries rates: a 40-month cohort study. J Dent Res. 1995;74:1904–1913. doi: 10.1177/00220345950740121501. [DOI] [PubMed] [Google Scholar]
- 9.Söderling E, Pihlanto-Leppälä A. Uptake and expulsion of 14C-xylitol by xylitol-cultured Streptococcus mutans ATCC 25175 in vitro. Scand J Dent Res. 1989;97:511–519. doi: 10.1111/j.1600-0722.1989.tb00925.x. [DOI] [PubMed] [Google Scholar]
- 10.Trahan L, Bareil M, Gauthier L, Vadeboncoeur C. Transport and phosphorylation of xylitol by a fructose phosphotransferase system in Streptococcus mutans. Caries Res. 1985;19:53–63. doi: 10.1159/000260829. [DOI] [PubMed] [Google Scholar]
- 11.Uhari M, Kontiokari T, Koskela M, Niemelä M. Xylitol chewing gum in prevention of acute otitis media: double blind randomised trial. Br Med J. 1996;313:1180–1184. doi: 10.1136/bmj.313.7066.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Uhari M, Kontiokari T, Niemelä M. A novel use of xylitol sugar in preventing acute otitis media. Pediatrics. 1998;102:879–884. doi: 10.1542/peds.102.4.879. [DOI] [PubMed] [Google Scholar]
- 13.Vadeboncoeur C, Pelletier M. The phosphoenolpyruvate: sugar phosphotransferase system of oral streptococci and its role in the control of sugar metabolism. FEMS Microbiol Rev. 1997;19:187–207. doi: 10.1111/j.1574-6976.1997.tb00297.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y M, van Eys J. Nutritional significance of fructose and sugar alcohols. Annu Rev Nutr. 1981;1:437–475. doi: 10.1146/annurev.nu.01.070181.002253. [DOI] [PubMed] [Google Scholar]