Abstract
Telithromycin (HMR 3647) is a novel ketolide antimicrobial with good activity against both common and atypical respiratory pathogens, including many resistant strains. This randomized, three-period crossover study determined the dose proportionality of telithromycin pharmacokinetics after single and multiple dosing in healthy subjects. In each treatment period, subjects received a single oral dose of 400, 800 or 1,600 mg of telithromycin followed 4 days later by the same dose once daily for 7 days. Blood and urine samples were taken throughout the study for determination of pharmacokinetic parameters for telithromycin and RU 76363, its main metabolite. Telithromycin and RU 76363 achieved steady state within 2 to 3 days of once-daily dosing. A slight accumulation of telithromycin was observed after 7 days of therapy, with values of the area under the concentration-time curve from 0 to 24 h approximately 1.5 times higher than those achieved with the single dose. The pharmacokinetics of telithromycin and RU 76363 deviated moderately from dose proportionality. At a dose of 800 mg/day, telithromycin attained mean maximal and trough plasma concentrations of 2.27 and 0.070 mg/liter respectively. Elimination was biphasic; initial and terminal half-lives were 2.87 and 9.81 h for the 800-mg dose. Study medication was well tolerated, although adverse events tended to be more frequent at the 1,600-mg dose. This study showed that telithromycin was generally well tolerated and suggests that a once-daily 800-mg oral dose of telithromycin maintains an effective concentration in plasma for the treatment of respiratory tract infections involving the key respiratory pathogens.
Telithromycin (HMR 3647) is the first of a novel family of antimicrobials, the ketolides, developed specifically for the treatment of community-acquired respiratory tract infections. The ketolides are a new addition to the macrolide-lincosamide-streptogramin B (MLS) group of antimicrobials. These agents inhibit bacterial protein synthesis via two mechanisms: first by directly blocking translation of mRNA and second by interfering with the assembly of new ribosomal units (7). Ketolides are characterized by a ketone group, which replaces the cladinose sugar at position 3 of the macrolactone ring. This ketone group not only confers excellent acid stability but also accounts for the fact that, unlike macrolides, telithromycin does not induce MLS resistance in vitro (4, 6, 14). Furthermore, the C11–12 carbamate side chain of telithromycin enhances binding to MLS-resistant ribosomes, and this may explain the activity of telithromycin against MLS-resistant organisms (11).
The global spread of resistance among respiratory tract pathogens is a matter of serious concern. Indeed, a U.S. analysis of Streptococcus pneumoniae isolates from the 1998–1999 respiratory season reported resistance rates of 14, 25, and 22% for penicillin (high-level resistance), cefuroxime, and clarithromycin-azithromycin, respectively (C. Thornsberry, I. A. Critchley, Y. Mauriz, J. Khan, G. Piazza, and D. F. Sahm, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother. abstr. 820, p. 109, 1999). Telithromycin has a spectrum of activity covering most common and atypical respiratory tract pathogens, irrespective of their susceptibilities to β-lactams or macrolides (1, 2, 3, 5, 10, 12, 13).
In vitro, telithromycin exhibits concentration-dependent killing and has a significant postantibiotic effect (D. Felmingham, S. Clark, M. J. Robbins, C. Dencer, and A. Bryskier, Abstr. 38th Intersci. Conf. Antimicrob. Agents, Chemother., abstr. E-133, p. 207, 1998). These properties are generally characteristic of antimicrobials for which, in relation to MIC, the amount of drug delivered rather than the time for which plasma levels are maintained above the MIC is a better predictor of outcome (9). This suggests that a once-daily dosage regimen of telithromycin may be suitable for further evaluation in humans. The present study was conducted to evaluate the single- and multiple-dose pharmacokinetics and dose proportionality of telithromycin given once daily over the dose range of 400 to 1,600 mg/day in healthy human subjects.
MATERIALS AND METHODS
This single-center, randomized, open-label, single- and multiple-dose, three-way crossover study was conducted between August 1998 and November 1998 in Bloemfontein, South Africa. The study was performed in accordance with the European Community Good Clinical Practice and International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals guidelines, and all subjects were required to provide written, informed consent.
Subjects.
This study recruited male subjects aged 18 to 45 years who were judged to be healthy by medical history, physical examination, routine laboratory tests, blood pressure, heart rate, and 12-lead electrocardiogram (ECG).
Subjects were excluded from the study if they were receiving concomitant medication or had received during the preceding 3 months an investigational drug or any drug with a well-defined potential for toxicity to a major organ or a potential to induce liver enzymes or any other medication within the last 2 weeks. Subjects were also excluded if they had any condition known to interfere with the absorption, distribution, metabolism, or excretion of drugs or if they had experienced symptoms of a clinically significant illness in the previous 3 months. Additional exclusion criteria included known long-QT syndrome or a history of allergic disease or of hypersensitivity to drugs with a structure similar to that of telithromycin. During the study, subjects were asked to refrain from smoking more than 10 cigarettes per day and to restrict their alcohol intake to no more than 0.5 liters of wine or equivalent per day.
Study design and procedures.
Each of the three treatment periods consisted of a single oral dose of telithromycin at either 400, 800, or 1,600 mg (day 1), followed 4 days later by the same oral dose of telithromycin given once daily for 7 days (days 5 to 11). Each treatment period was separated by a washout period of at least 7 days. The sequence in which subjects received the different dosages of telithromycin was determined by a Williams randomization plan (8). Study medication was administered with 240 ml of water after an overnight fast, and administration was followed by a light breakfast. Telithromycin (400-mg) tablets for oral administration were provided by Hoechst Marion Roussel (Romainville, France).
Subjects were housed for 24 h on the first day (day 1) and the last day (day 11) of each of the three treatment periods and reported to the clinic before breakfast to receive study medication on the other dosing days. Subjects were to abstain from strenuous physical exercise and consumption of tobacco, alcohol, and xanthine derivatives from 48 h before the first dose of telithromycin until 24 h after the last dosing of each treatment period. In addition, grapefruit juice was not permitted from 48 h before the first dose of telithromycin until 96 h after the last dosing of each treatment period.
Sample collection.
Blood samples (3.5 ml) for pharmacokinetic analysis were taken at the screening visit (2 weeks before study entry), before dosing on all days, and at the following times after dosing on days 1 and 11 of each treatment period: 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, 72, and 96 h. Urine for determination of telithromycin concentrations was collected at screening and for three consecutive 24-h periods, starting at the time of the last dose on days 1 and 11 of each treatment period. For safety evaluations, blood (30 ml at screening and 20 ml thereafter) was collected at screening, before the first dose of the first treatment period, 24 h after the last dose of each treatment period, and 96 h after the last dose of the third treatment period. Urine was collected at screening, before the first dose of the first treatment period, and 96 h after the last dose of the third treatment period. Plasma and urine samples were stored frozen at −20°C until analysis.
Analytical methodology. (i) Telithromycin.
Telithromycin was assayed in plasma using a validated liquid chromatography/mass spectrometry (LC/MS) method following precipitation of plasma proteins by acetonitrile in a 96-well format. The method has a standard curve range of 0.005 to 3 mg/liter using 100 μl of plasma and a limit of quantification of 0.005 mg/liter. Each run included calibration and quality controls over the standard range. The interbatch percent coefficient of variation (CV) for the quality control samples was between 2.8 and 8%. Telithromycin concentration in urine was assayed using a validated reverse-phase high-performance liquid chromatography method. The column eluent was monitored by fluorimetry (excitation at 263 nm and emission at 460 nm), and the detector signal was integrated to produce peak heights. The method has a standard curve range of 0.5 to 100 mg/liter using 50 μl of urine and a limit of quantification of 0.5 mg/liter. Each run included calibration (0.5 to 100 mg/liter) and quality controls (0.5 to 75 mg/liter). The interbatch percent CV for the quality control samples was between 0.8 and 2.9%.
(ii) RU 76363.
RU 76363, the main metabolite of telithromycin, was assayed in plasma by liquid chromatography-atmospheric pressure chemical ionization-mass spectrometry using the full-scan MS mode. Samples were deproteinated with acetonitrile, evaporated to dryness, and reconstituted in the analytical mobile phase prior to injection for reversed-phase chromatography. The validated method has a standard curve range of 0.0025 to 0.25 mg of RU 76363/liter (using 100 μl plasma) and a limit of quantification of 0.0025 mg/liter. Each run included calibration (0.0025 to 0.25 mg/liter) and quality controls (0.006 to 0.25 mg/liter). The interbatch percent CV for the quality control samples was between 4.2 and 9.6%.
Safety.
Safety was evaluated on the basis of ECG, blood pressure, heart rate, and laboratory variables (hematology, blood chemistry, and urinalysis) at screening, before dosing on day 1, 24 h after the last dose of each treatment period, and 96 h after the last dose of the third treatment period. Adverse events were recorded throughout the study. An adverse event, which could be nonserious or serious, was defined as any sign, symptom, syndrome, or illness that appeared or worsened in a subject during the observation period and that could impair the well-being of the subject.
Pharmacokinetic and statistical analysis.
A sample size of six subjects was calculated to provide 80% power to observe a difference of at least 1 mg · h/liter in the area under the concentration-time curve (AUC) standardized to the dose, with a risk of 5% and a mean square error of 0.5, based on observations from previous studies. However, since it is common to use 18 subjects for such a pivotal study, this was the number used.
Pharmacokinetic parameters were calculated using WinNonLin software (version 2.0), and descriptive statistics were performed using SAS software (version 6.12). Concentrations of telithromycin and its major metabolite, RU 76363, in plasma were determined for all sampling times throughout the study. The following pharmacokinetic parameters were calculated for telithromycin and RU 76363 after a single dose (day 1) and multiple doses (day 11): maximal plasma concentration (Cmax), time to reach maximal plasma concentration (tmax), trough plasma concentration 24 h after dosing (C24) AUC over 24 h (AUC0–24), AUC until the last quantifiable measurement (AUC0–z), AUC until infinity (AUC0–∞), (when the terminal half-life was available), amount of telithromycin excreted in urine over 24 h (Ae0–24), renal clearance of telithromycin [CLR(0–24)], accumulation ratio (Rac), Cmax and C24 standardized to the 800-mg dose (Cmax/dose and C24/dose, respectively), and the AUC standardized to the 800-mg dose (AUC0–24/dose and AUC0–z/dose). In addition, the ratio of AUC0–24 for RU 76363/AUC0–24 for telithromycin (R) was calculated for days 1 and 11, and C24 hs for telithromycin and RU 76363 were determined for each treatment period from days 6 to 11. The primary and terminal half-lives (t1/2λ1 and t1/2λz, respectively) were calculated using a one- or two-compartment model, depending on the profile. In most cases, t1/2λz could not be assessed for RU 76363, so this was not tabulated.
Dose proportionality for telithromycin and RU 76363 was tested for after oral administration of single and multiple doses of telithromycin using analyses of variance (ANOVA) of log-transformed data with subject, dose, and treatment period as the main effects for the following parameters: Cmax/dose, AUC0–24/dose, AUC0–z/dose; C24/dose; t1/2λ1, and, for telithromycin only, t1/2λz, CLR(0–24), and R. Comparisons between doses were performed using Tukey's test. The dose effect on tmax was assessed using the Kruskal-Wallis nonparametric test. The effect of dose on Rac was assessed using ANOVA with subject, dose, and treatment period as the main effects. Trough concentrations of telithromycin and RU 76363 in plasma determined for days 5 to 11 were compared for each dose to determine the day at which steady states were achieved. ANOVA were applied to the log-transformed data with subject and day as the main effects, followed by Tukey's test. Mean trough plasma concentrations on the last dosing day (day 11) were taken as the mean trough plasma concentrations at steady state.
RESULTS
Subjects.
Eighteen Caucasian, healthy male subjects of mean age 21.3 years (range, 18 to 29 years), mean height 183.2 cm (ranges, 170 to 195 cm) and mean weight 79.2 kg (ranges, 67.8 to 98.4 kg) were recruited. No subjects had concomitant illness, and none were receiving concomitant treatments at the time of inclusion. However, six subjects received concomitant medications for acute minor illnesses during the study, none of which are likely to interfere with the pharmacokinetics or safety of telithromycin. One subject withdrew because of an adverse event during dosing at 1,600 mg/day.
Telithromycin and RU 76363 assays.
The mean accuracy and precision of the plasma telithromycin assay were 95.7 to 100.7% (percent recovery relative to the theoretical concentration) and 7.5 to 11.7% (percent CV), respectively. For the urine telithromycin assay, the mean accuracy was −2.0 to +2.0% (percent relative error) and the precision was 5.5 to 8.8% (percent CV). The mean accuracy of the plasma RU 76363 assay was 98.8 to 103.3% (percent recovery relative to the theoretical concentration), and the precision was 4.6 to 7.1% (percent CV).
Pharmacokinetics.
Pharmacokinetic analysis was performed on data from 18 subjects for the 400- and 800-mg doses but from only 16 subjects for the 1,600-mg dose: one subject withdrew due to a severe adverse event, and in another subject the trough concentrations suddenly decreased by a factor of 2 between the third and fourth administrations. In the latter subject, the results could be due to a decrease in bioavailability, although no explanation for a potential decrease in bioavailability could be found, e.g. vomiting, concomitant medication, or compliance. Hence, the data from this subject were rejected from the 1,600-mg multiple-dose pharmacokinetic analysis of telithromycin and RU 76363.
(i) Telithromycin.
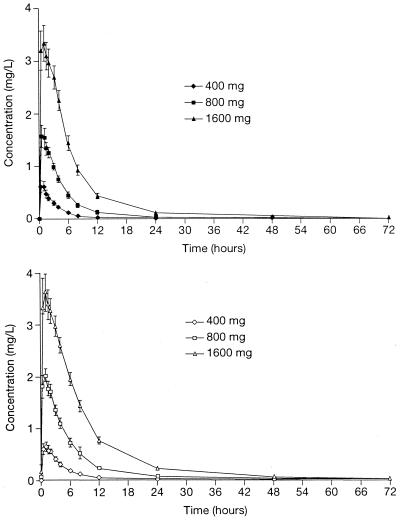
The pharmacokinetics and dose proportionality of single and multiple doses of telithromycin are shown in Tables 1 and 2, respectively. Following a single oral dose of 400, 800, or 1,600 mg, telithromycin was quantifiable in the plasma from the first time point (0.5 h) and reached tmax after a median of 1 h, irrespective of dose (Fig. 1). Telithromycin was no longer quantifiable in plasma 48, 72, and 96 h after dosing for the 400-, 800-, and 1,600-mg single doses, respectively, and 72 and 96 h after the final dose in the 7-day multiple-dose phase (400- and 800-mg groups). Telithromycin was still quantifiable in 14 out of 16 subjects after the final dose in the 7-day multiple-dose phase in the 1,600-mg group.
TABLE 1.
Pharmacokinetics and dose proportionality of telithromycin following a single oral dosea
| Dose (mg) | Cmax (mg/liter) | tmax (h)bc | C24 (mg/liter) | AUC0–24 (mg · h/liter) | AUC0–∞ (mg · h/liter) | CLR(0–24) (liters/h)c | t1/2λ1 (h)c | t1/2λz (h)d | Ae0–24 (%) | Cmax/dose (mg/liter) | AUC0–z/dose (mg · h/liter) | C24/dosec (mg/liter) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400 | 0.80 (57) | 1.0 (0.5–4.0) | 0.0069 (72) | 2.57 (40) | 3.09 (35) | 12.17 (26) | 2.13 (37) | 6.68 (24) | 7.42 (32)f | 1.59 (57) | 5.14 (40)f | 0.0141 (72) |
| 800 | 1.90 (42) | 1.0 (0.5–4.0) | 0.0296 (45) | 8.25 (31) | 8.96 (32) | 12.32 (17) | 2.43 (41) | 7.16 (19) | 12.66 (33)f | 1.90 (42) | 8.40 (32)f | 0.0296 (45) |
| 1,600 | 4.07 (30) | 1.0 (0.5–4.0 | 0.103 (53) | 23.1 (34) | 25.4 (35) | 13.28 (23) | 2.81 (31) | 10.13 (27)e | 18.4 (27)f | 2.04 (30)g | 12.4 (36)f | 0.0512 (53) |
Data are means, with percent CV in parentheses, except where indicated otherwise. Statistical significance was determined by ANOVA and Tukey's test. Elimination half-lives were calculated by a compartmental analysis.
Values are medians with ranges in parentheses.
None of the values are significantly different from each other.
For 400-, 800-, and 1,600-mg doses, n = 10, 12, and 15, respectively.
Significantly different from values at 400 and 800 mg (P < 0.001).
Significantly different from values for other doses (P < 0.001).
Significantly different from value for 400-mg dose (P < 0.01).
TABLE 2.
Pharmacokinetics and dose proportionality of telithromycin following 7 days of once-daily oral dosinga
| Dose (mg) | Cmax (mg/liter) | tmax (h)bc | C24 (mg/liter) | AUC0–24 (mg · h/liter) | AUC0–∞ (mg · h/liter)d | CLR(0–24) (liters/h)c | t1/2λ1 (h) | t1/2λz (h)d | Ae0–24 (%) | Cmax/dose (mg/liter) | AUC0–24/dose (mg · h/liter) | C24/dose (mg/liter) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 400 | 0.829 (42) | 1.0 (0.5–6.0) | 0.0172 (56) | 3.50 (31) | 4.01 (39) | 11.21 (28) | 2.62 (49) | 7.70 (21) | 9.57 (35)g | 1.66 (42)h | 7.00 (31)g | 0.0343 (56)g |
| 800 | 2.27 (31) | 1.0 (0.5–3.0) | 0.070 (72) | 12.5 (43) | 12.59 (22) | 12.5 (34) | 2.87 (50) | 9.81 (20) | 17.7 (27)g | 2.27 (31) | 12.5 (43)g | 0.070 (72)g |
| 1,600 | 4.48 (33) | 1.0 (0.5–3.0) | 0.217 (40) | 30.2 (22) | 35.9 (17) | 13.1 (31) | 3.76 (31)e | 18.7 (31)f | 24.4 (28)g | 2.24 (33) | 15.11 (22)g | 0.108 (40)g |
Data are means, with percent CV in parentheses, unless otherwise indicated. Statistical significance was determined by ANOVA and Tukey's test. Elimination half-lives were calculated by a compartmental analysis.
Values are medians with ranges in parentheses.
None of the values are significantly different from each other.
For 400-, 800-, and 1,600-mg doses, n = 10, 15, and 12, respectively.
Significantly different from the values at 400 and 800 mg (P < 0.01); n = 15.
Significantly different from the values at 400 and 800 mg (P < 0.001).
Significantly different from the values for other doses (P < 0.001).
Significantly different from the values at 800 and 1,600 mg (P < 0.001).
FIG. 1.
Mean concentration-time curves for telithromycin following oral administration of telithromycin at 400, 800, and 1,600 mg once daily to 18 healthy subjects for 1 day (top) or 7 days (bottom). Error bars, ±SEM.
Steady state was achieved on the second or third day of multiple dosing, as determined from the trough plasma concentrations. A slight accumulation of telithromycin was observed after 7 days of therapy, with AUC0–24 values 1.37 to 1.49 times higher than those achieved in the single-dose phase. Rac was unaffected by the dose.
Overall, there was a modest deviation from dose proportionality for Cmax, AUC, and C24 in both the single- and multiple-dose phases (Tables 1 and 2). After single dosing, Cmax was proportional to dose over the 400- to 800-mg and 800- to 1,600-mg intervals, although not over the entire dosage range. After multiple dosing, Cmax was proportional to dose only for the 800- to 1,600-mg interval. AUC deviated from dose proportionality in both the single- and multiple-dose phases; a doubling of dose resulted in a 2.6- to 3.6-fold increase in AUC. C24 deviated from dose proportionality only in the multiple-dose phase.
Telithromycin exhibited biphasic elimination, with a short (but predominant) t1/2λ1 followed by a longer t1/2λz. In the multiple-dose phase, t1/2λ1 increased significantly by 1.5-fold between the 800- and 1,600-mg doses, while t1/2λz increased by a factor of 1.8 over the 800- to 1,600-mg dose interval. CLR(0–24) for telithromycin was constant over the dose range after both single and multiple doses.
In the 72-h period following administration of a single dose of telithromycin of 400, 800, or 1,600 mg, 7.64, 13.0, and 19.0%, respectively, of the dose was eliminated unchanged in the urine. Corresponding values for the 72-h period following the final dose of the 7-day treatment period were 9.93, 18.4, and 25.8%. Approximately 96% of urinary excretion took place within 24 h of the final dose.
(ii) RU 76363.
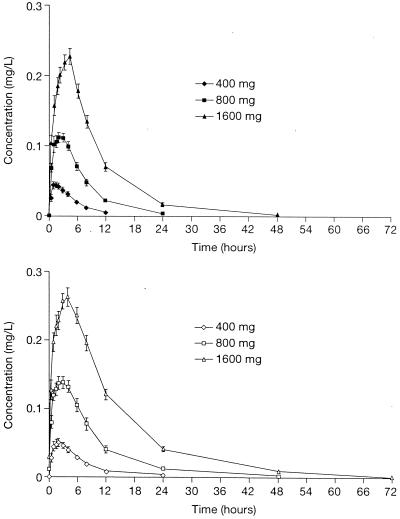
RU 76363, an alcohol resulting from loss of aryl rings, is the major hepatic metabolite of telithromycin. Following single doses of telithromycin at 400, 800, or 1,600 mg, RU 76363 was quantifiable in plasma at the same time points as telithromycin. The levels of RU 76363 peaked after those of the parent compound. The AUC0–24 of this metabolite was 10 to 12% that of the parent compound, a figure that was constant across the dose range.
The pharmacokinetics and dose proportionality for single and multiple doses of RU 76363 are shown in Tables 3 and 4. RU 76363 deviated moderately from dose proportionality in a manner similar to that for telithromycin, with a doubling of dose resulting in a 2.5- to 3.7-fold increase in AUC. For both Cmax and C24, RU 76363 deviated significantly from dose proportionality within the 400- to 800-mg subinterval, although dose proportionality was shown over the entire 400- to 1,600-mg dose range. In the single- and multiple-dose phases, t1/2λz increased by means of 17 and 26%, respectively, between 400 and 800 mg, and by means of 16 and 30%, respectively, between 800 and 1,600 mg.
TABLE 3.
Pharmacokinetics and dose proportionality of RU 76363 following a single oral dose of telithromycina
| Dose (mg) | Cmax (mg/liter) | tmax (h)b | C24 (mg/liter) | AUC0–24 (mg · h/liter) | AUC0–z (mg · h/liter) | t1/2λ1 (h)c | Cmax/dose (mg/liter) | AUC0–z/dose (mg · h/liter) | C24/dose (mg/liter) |
|---|---|---|---|---|---|---|---|---|---|
| 400 | 0.0512 (46) | 1.5 (1.0–4.0) | 0.00076 | 0.277 (47) | 0.277 (47) | 2.96 (27) | 0.102 (46)e | 0.554 (47)f | 0.0015g |
| 800 | 0.1286 (23) | 2.0 (1.0–4.0) | 0.00546 (48) | 0.974 (29) | 0.983 (31) | 3.45 (36) | 0.1286 (23) | 0.983 (31)f | 0.00546 (48) |
| 1,600 | 0.238 (19) | 3.0 (1.5–4.0) | 0.0181 (50) | 2.37 (24) | 2.61 (28) | 4.00 (23)d | 0.1190 (19) | 1.306 (28)f | 0.0090 (50) |
Values are means, with percent CV in parentheses, unless otherwise indicated. Statistical significance was determined by ANOVA and Tukey's test. Elimination half-lives were calculated by compartmental analysis.
Values are medians with ranges in parentheses. P < 0.001.
For values at 800 and 1,600 mg, n = 17.
Significantly different from the value for the 400-mg dose (P < 0.05).
Significantly different from the values for the 800- and 1,600-mg doses (P < 0.01).
Significantly different from the values for other doses (P < 0.001).
Significantly different from the values for the 800- and 1,600-mg doses (P < 0.001).
TABLE 4.
Pharmacokinetics and dose proportionality of RU 76363 following 7 days of once-daily oral dosing with telithromycina
| Dose (mg) | Cmax (mg/liter) | tmax (h) | C24 (mg/liter) | AUC0–24 (mg · h/liter) | AUC0–z (mg · h/liter) | t1/2λ1 (h)c | Cmax/dose (mg/liter) | AUC0–24/dose (mg · h/liter) | C24/dose (mg/liter) |
|---|---|---|---|---|---|---|---|---|---|
| 400 | 0.0601 (33) | 1.5 (1.0–6.0) | 0.00328 (95) | 0.430 (36) | 0.430 (36) | 3.70 (39)e | 0.1201 (33)f | 0.860 (36)g | 0.0066 (95)h |
| 800 | 0.1505 (26) | 2.0 (1.0–4.0) | 0.0137 (64) | 1.48 (38) | 1.60 (45) | 4.67 (15) | 0.1505 (26) | 1.48 (38)g | 0.0137 (64) |
| 1,600d | 0.271 (19) | 4.0 (1.5–6.0) | 0.0415 (29) | 3.38 (18) | 4.04 (21) | 6.06 (40) | 0.1355 (19) | 1.688 (19)g | 0.0208 (29) |
Data are means, with percent CV in parentheses, unless otherwise noted. Statistical significance was determined by ANOVA and Tukey's test. Elimination half-lives were calculated by compartmental analysis.
Values are medians with ranges in parentheses. P < 0.01.
For values at 800 and 1,600 mg, n = 17 and 14, respectively.
n = 16.
Significantly different from the value for 1,600 mg (P < 0.05).
Significantly different from the value for 800 mg (P < 0.01).
Significantly different from the values for the other doses (P < 0.001).
Significantly different from the value for 800 mg (P < 0.05).
After 7 days of dosing, there was a modest accumulation of RU 76363, with AUC0–24 values approximately 1.5 (1.43 to 1.61) times that attained with a single dose. Accumulation ratio was relatively constant over the dosage range. The concentration-time curve of RU 76363 is shown in Fig. 2.
FIG. 2.
Mean concentration-time curves for RU 76363 following oral administration of telithromycin at 400, 800, and 1,600 mg once daily to 18 healthy volunteers for 1 day (top) or 7 days (bottom). Error bars, ±SEM.
Safety.
No deaths or other serious adverse events were reported during the study. The incidence of possibly drug-related adverse events increased with the dose of telithromycin, being 5.6, 27.8, and 77.8% for 400, 800, and 1,600 mg, respectively. A total of 29 possibly drug-related adverse events were reported, of which most (24) occurred during multiple-dose administration. Adverse events most commonly affected the digestive tract and were manifest as diarrhea, nausea, and gastrointestinal pain and disorder. All were mild or moderate in intensity, except for one case of vomiting and diarrhea, which was of severe intensity and which occurred during the multiple-dose 1,600-mg phase. This patient was withdrawn from the study.
Telithromycin had no clinically significant effect on clinical laboratory assessments, vital signs (blood pressure, heart rate, and ECG), or physical examination. In addition, no QTc values above 450 ms were observed throughout the study.
DISCUSSION
Telithromycin (HMR 3647) is an innovative new ketolide antimicrobial, which has been specifically designed for the treatment of community-acquired respiratory tract infections. The pharmacokinetic profile and dose proportionality of telithromycin and its main circulating metabolite, RU 76363, have been established when the drugs are given as single and as multiple once-daily doses to healthy subjects. RU 76363, an alcohol formed from loss of aryl rings during hepatic metabolism, is 4- to 16-fold less active than telithromycin in vitro.
Following oral administration telithromycin was rapidly absorbed, reaching Cmax within 1 h of dosing. Steady state plasma concentrations of both telithromycin and its major metabolite, RU 76363, were reached within 2 to 3 days of multiple dosing, regardless of the dose. After 7 days of dosing, there was moderate accumulation of both telithromycin and RU 76363, with AUC values approximately 1.5-fold higher than those attained following a single dose. Rac was relatively constant over the dosage range. This moderate accumulation might be explained by a slight decrease in nonrenal clearance with multiple dosing, since the main (initial) elimination half-life increased by 20 to 30% while CLR(0–24) remained unchanged.
The pharmacokinetics of telithromycin deviated moderately from dose proportionality after single and multiple oral administration: a doubling of the dose resulted in an increase of approximately threefold in AUC. Cmax deviated only slightly from dose proportionality. t1/2λ1 and t1/2λz also increased significantly with dose in the multiple-dose phase: over the 800- to 1,600-mg dosage interval t1/2λ1 increased by a factor of 1.5, while t1/2λz increased 1.8-fold. CLR(0–24) remained constant over the 400- to 1,600-mg dose range. Consequently, the percentage of telithromycin eliminated unchanged in the urine increased with dose with a magnitude similar to that of the AUC.
The moderate deviation from dose proportionality observed in this study may reflect a decrease in the metabolic clearance of the drug and a slight increase in the bioavailability of telithromycin with increasing dose. As the amount of telithromycin eliminated in the initial phase represents the major fraction, it may be assumed that the 30% increase in the t1/2λ1 for a doubling of dose corresponds to a 30% decrease in plasma clearance. As CLR(0–24) was unchanged in this study, this suggests that nonrenal clearance (i.e., hepatic metabolic clearance) decreases with increasing dose.
RU 76363, the main circulating metabolite of telithromycin, is formed by hydrolysis of the aryl rings of the carbamate side chain of telithromycin; its AUC represents about 10 to 12% that of telithromycin. The decrease of telithromycin clearance cannot be attributed to a decrease in the formation of the RU 76363 metabolite, since the pharmacokinetics of this metabolite also deviated from dose proportionality in a manner similar to that observed for telithromycin. The dose proportionality of other circulating metabolites of telithromycin was not assessed since their AUC values represent only 1 to 2% of that of telithromycin (15).
In the present study, the Cmax and C24 values after 7 days of dosing with 800 mg telithromycin were 2.27 and 0.070 mg/liter, respectively. The telithromycin MICs at which 90% of the isolates are inhibited values for S. pneumoniae (including MLS-resistant strains) and Haemophilus influenzae are ≤0.06 and 2 mg/liter, respectively (1, 5). These data therefore suggest that telithromycin given at a daily dose of 800 mg will provide adequate plasma levels to maintain activity against respiratory pathogens, irrespective of macrolide susceptibility.
Telithromycin was generally well tolerated at all doses, though the incidence of adverse events tended to be higher at the 1,600-mg dose. These data, taken together with the pharmacokinetic profile of the compound, suggest that a once-daily 800-mg oral dose of telithromycin maintains an effective concentration in plasma and is suitable for evaluation in further pharmacokinetic and clinical trials for the treatment of community-acquired respiratory tract infections.
ACKNOWLEDGMENT
Funding for this study was provided by Hoechst Marion Roussel.
REFERENCES
- 1.Barry A L, Fuchs P C, Brown S D. Antipneumococcal activities of a ketolide (HMR 3647), a streptogramin (quinupristin-dalfopristin), a macrolide (erythromycin), and a lincosamide (clindamycin) Antimicrob Agents Chemother. 1998;42:945–946. doi: 10.1128/aac.42.4.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bébéar C M, Renaudin H, Aydin M D, Chantot J F, Bébéar C. In vitro activity of ketolides against mycoplasmas. J Antimicrob Chemother. 1997;39:669–670. doi: 10.1093/jac/39.5.669. [DOI] [PubMed] [Google Scholar]
- 3.Biedenbach D J, Barrett M S, Jones R N. Comparative antimicrobial activity and kill-curve investigations of novel ketolide antimicrobial agents (HMR 3004 and HMR 3647) tested against Haemophilus influenzae and Moraxella catarrhalis strains. Diagn Microbiol Infect Dis. 1998;31:349–353. doi: 10.1016/s0732-8893(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 4.Bonnefoy A, Agouridas C, Chantot J F. Proceedings of the 4th International Conference on Macrolides, Azalides, Streptogramins and Ketolides. New York, N.Y: Marcel Dekker, Inc.; 1998. HMR 3647: antibacterial activity of resistance; p. 25. [Google Scholar]
- 5.Boswell F J, Andrews J M, Ashby J P, Fogarty C, Brenwald N P, Wise R. The in-vitro activity of HMR 3647, a new ketolide antimicrobial agent. J Antimicrob Chemother. 1998;42:703–709. doi: 10.1093/jac/42.6.703. [DOI] [PubMed] [Google Scholar]
- 6.Bryskier A, Agouridas C, Chantot J F. Acid stability of new macrolides. J Chemother. 1993;5(Suppl. 1):158–159. [Google Scholar]
- 7.Champney W S, Tober C L. Inhibition of translation and 50S ribosomal subunit formation in S. aureus cells by 11 different ketolide antibiotics. Curr Microbiol. 1998;37:418–425. doi: 10.1007/s002849900403. [DOI] [PubMed] [Google Scholar]
- 8.Chow S G, Liu J P. Designs of bioavailability studies. In: Chow S G, Liu J P, editors. Design and analysis of bioavailability and bioequivalence studies. New York, N.Y: Marcel Decker Inc.; 1992. pp. 23–47. [Google Scholar]
- 9.Drusano G L, Craig W A. Relevance of pharmacokinetics and pharmacodynamics in the selection of antibiotics for respiratory tract infections. J Chemother. 1997;9(Suppl. 3):38–44. [PubMed] [Google Scholar]
- 10.Edelstein P H, Edelstein M A C. In vitro activity of the ketolide HMR 3647 (RU 6647) for Legionella spp., its pharmacokinetics in guinea pigs, and use of the drug to treat guinea pigs with Legionella pneumophila pneumonia. Antimicrob Agents Chemother. 1999;43:90–95. doi: 10.1128/aac.43.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen L H, Mauvais P, Douthwaite S. The macrolide-ketolide antibiotic binding site is formed by structures in domains II and V of 23S ribosomal RNA. Mol Microbiol. 1999;31:623–631. doi: 10.1046/j.1365-2958.1999.01202.x. [DOI] [PubMed] [Google Scholar]
- 13.Reinert R R, Bryskier A, Lutticken R. In vitro activities of the new ketolide antibiotics HMR 3004 and HMR 3647 against Streptococcus pneumoniae in Germany. Antimicrob Agents Chemother. 1998;42:1509–1511. doi: 10.1128/aac.42.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roblin R M, Kutlin A, Reznik T, Hammerschlag M R. Activity of grepafloxacin and other fluoroquinolones and newer macrolides against recent clinical isolates of Chlamydia pneumoniae. Int J Antimicrob Agents. 1999;12:181–184. doi: 10.1016/s0924-8579(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 15.Rosato A, Vicarini H H, Bonnefoy A, Chantot J F, Leclercq R. A new ketolide, HMR 3004, active against streptococci inducibly resistant to erythromycin. Antimicrob Agents Chemother. 1998;42:1392–1396. doi: 10.1128/aac.42.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sultan E, Namour F, Mauriac C, Lenfant B, Scholtz H. Proceedings of the 21st International Congress on Chemotherapy. Vol. 44. Oxford, United Kingdom: Oxford University Press; 1999. The ketolide antimicrobial, HMR 3647, is metabolised and eliminated predominantly in the faeces in man; p. 54. [Google Scholar]