Abstract
The influences of dosing time and dosing schedule on the plasma alpha interferon (IFN-α) concentration and the production of anti-IFN-α neutralizing antibodies were investigated in ICR male mice adapted to cycles of 12 h of light and 12 h of dark. In mice pretreated with IFN-α for 21 days, the plasma IFN-α concentrations were significantly lower than those in control mice (P < 0.01). The clearance of IFN-α and its volume of distribution obtained at steady state were significantly higher in the animals with IFN-α pretreatment than in the mice without IFN-α pretreatment. The area under the concentration-time curve and the mean residence time of IFN-α were significantly smaller in IFN-α-pretreated animals than in control animals. The plasma IFN-α levels (measured 2 h after dosing) were significantly lower in mice treated daily with IFN-α, while the anti-IFN-α neutralizing antibody levels (measured 24 h after dosing) were significantly increased on days 15 and 21 of treatment. Plasma IFN-α levels were significantly decreased in association with the production of anti-IFN-α neutralizing antibodies in mice treated with IFN-α daily at either 0900 or 2100 h. By contrast, the plasma IFN-α levels (measured 2 h after dosing) remained stable in mice treated with IFN-α at 0900 h on alternate days, while they were significantly lower after 21 days of treatment in mice treated with IFN-α at 2100 h on alternate days. These changes were associated with a significant increase in the levels of anti-IFN-α neutralizing antibodies in the latter group. The present findings suggest that an appropriate dosing schedule and/or dosing time for IFN-α may reduce the level of production of anti-IFN-α neutralizing antibodies in experimental and clinical situations.
Interferons (IFNs), which belong to a group of cytokines, have been widely used as antiviral and antitumor agents in humans. However, therapy with alpha IFN (IFN-α) has been complicated by the production of neutralizing antibodies to IFNs (15, 16, 38, 48). Some reports suggest that antibodies appear to be of the immunoglobulin G class (11, 15, 42), and neutralizing antibodies have been found more frequently in patients treated with recombinant IFN-α2a (rIFN-α2a) than in those treated with rIFN-α2b or with natural IFN preparations such as human lymphoblastoid IFNs or leukocyte IFNs (3, 25, 26, 37, 43, 47). Generally, the response to the drug could be influenced by the sensitivities of living organisms to drugs and/or the pharmacokinetics of the drugs. Consequently, it is important to investigate the alterations of IFN pharmacokinetics associated with the production of anti-IFN neutralizing antibody.
One approach to increasing the efficiency of pharmacotherapy is administration of drugs at a time of day at which they are most effective and/or best tolerated. Certainly, the use of a chronopharmacological strategy can improve the effects of drugs and reduce toxicity (27, 28, 29, 30, 31, 32, 33, 34, 35). IFN-α is better tolerated by cancer patients when it is administered in the evening than when it is administered in the morning (1, 14). There are significant dosing time-dependent differences in the antitumor and myelosuppressive activities of IFN-α in mice (20, 21). Also, the rhythmic changes in IFN-induced fever and antiviral activity were examined in mice (22, 29). However, the influence of IFN-α dosing time on the production of anti-IFN-α neutralizing antibodies has not yet been investigated.
This study was designed to examine how the production of anti-IFN-α neutralizing antibodies in mice can modify the pharmacokinetics of IFN-α. Additionally, the effects of dosing time and dosing schedule on plasma IFN-α concentrations and the production of anti-IFN-α neutralizing antibodies were investigated.
MATERIALS AND METHODS
Experimental animals.
Male ICR mice (age, 5 weeks) were purchased from Charles River Japan Inc. (Kanagawa, Japan). Mice were housed at 10 mice per cage in a light-controlled room (lights on from 0700 to 1900 h) at a room temperature of 24 ± 1°C and a humidity of 60 ± 10%, with food and water provided ad libitum. All mice were adapted to their light-dark cycle for 2 weeks before the experiments.
Experimental design.
In experiment 1, the effects of IFN-α treatment on the time course of plasma IFN-α concentrations were evaluated in mice (n = 6) injected at 0900 h with a single daily dose of saline or IFN-α (106 IU [1 MIU]/kg of body weight subcutaneously [s.c.]) for 21 days. Control animals received an identical volume of normal saline. The mice in the two groups received injections of a single dose of IFN-α (1 MIU/kg intravenously [i.v.]) into the tail vein at 0900 h on day 22. Blood samples were taken continuously from the orbital sinus vein at 10 min and at 0.5, 1, 2, 3, and 4 h after IFN-α injection. Plasma samples were obtained after centrifugation and were stored at −20°C until assay. The relationship between the IFN-α concentration and the production of anti-IFN-α neutralizing antibody was investigated in experiment 2. A group of six mice received an injection of a single dose of IFN-α (1 MIU/kg s.c.) daily at 0900 h for 21 days. Blood samples were taken 2 and 24 h after dosing on days 1, 9, 15, and 21 for the determination of the plasma IFN-α concentration and for the detection of anti-IFN-α neutralizing antibody activity. Finally, the influence of dosing schedule and dosing time on the plasma IFN-α concentration and the production of anti-IFN-α neutralizing antibody were studied in experiment 3. Groups of six mice each received an injection of a single dose of IFN-α (1 MIU/kg s.c.) daily or on alternate days for 21 days at either 0900 or 2100 h. Blood samples were drawn 2 h after dosing on days 1, 9, 15, and 21 for the determination of the plasma IFN-α concentration and 24 h after administration of the last dose for the detection of anti-IFN-α neutralizing antibody activity.
Drug used.
IFN-α [Recombinant Human Interferon-α (2b); Pepro Tech EC Ltd., London, United Kingdom] was diluted with sterilize saline to a concentration of 2 MIU/ml. This drug solution was used within 30 min after preparation. The volume of injection was 0.05 ml/10 g of body weight.
Determination of IFN-α concentration in plasma.
Plasma IFN-α concentrations were determined with an enzyme-linked immunosorbent assay (ELISA) kit (IFN-α immunoassay kit; BioSource International Inc., Camarillo, Calif.). This kit quantitates IFN-α by a sandwich immunoassay. A total of 100 μl of a plasma sample was placed in a microtiter plate to which the capture antibody is bound, and the plate was then incubated for 1 h at room temperature. After washing, 100 μl of the antibody solution was added to each well, and the plate was then incubated for 1 h at room temperature. After the plate was washed, 100 μl of horseradish peroxidase conjugate solution was added to each well, and the plate was then incubated for 1 h at room temperature. After the plate was washed, 100 μl of tetramethylbenzidine solution was added to each well, and the plate was then incubated for 15 min at room temperature. After incubation, 100 μl of stop solution was added to each well, and then the contents of each well were mixed by swirling the plate gently. Within 5 min after the addition of the stop solution, the absorption at 450 nm was determined with a microplate reader. The standard curve for IFN-α is linear from 10 to 1,000 pg/ml. The lower limit of detectability is judged to be 10 pg/ml, at which the coefficient of variation is less than 10%. The titer was expressed in international units per milliliter.
Determination of anti-IFN-α neutralizing antibody in plasma.
The plasma samples were diluted 10-fold before assay. The mixture containing a plasma sample (100 μl) and a standard IFN-α solution (100 μl, 50 IU/ml) was incubated at 37°C for 1 h. The concentration of residual IFN-α was assayed by ELISA as described above, and the titer of anti-IFN-α neutralizing antibody was calculated.
Statistical analysis.
Statistical moment analysis was used to calculate the pharmacokinetic parameters such as area under the plasma concentration-time curve (AUC) and mean residence time (MRT). By using these parameters, clearance (CL) and the volume of distribution at steady state (VSS) were calculated. The statistical significance of differences between groups was validated by the Bonferroni method for multiple comparisons, Student's t test for comparison between two groups, and Spearman's rank test for the correlation coefficient between the IFN-α concentration and the production of anti-IFN-α neutralizing antibody. A probability level of <0.05 was considered significant.
RESULTS
Influence of IFN-α pretreatment on pharmacokinetic parameters after a single injection of IFN-α.
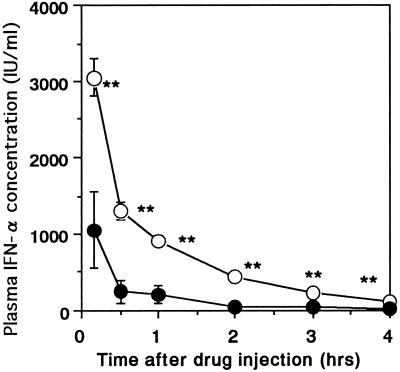
The time course of the plasma IFN-α concentrations was determined after administration of a single dose of IFN-α at 0900 h on day 22 (Fig. 1). The plasma IFN-α concentrations at 10 min and 0.5, 1, 2, 3, and 4 h after IFN-α injection were significantly lower in mice with pretreatment for 21 days with IFN-α than in mice without IFN-α pretreatment (P < 0.01). In fact, the results of the pharmacokinetic analysis presented in Table 1 show that CL (P < 0.01) and VSS (P < 0.05) were significantly higher and AUC (P < 0.01) and MRT (P < 0.01) were significantly lower in mice with IFN-α pretreatment for 21 days than in mice without IFN-α pretreatment.
FIG. 1.
Influence of IFN-α pretreatment on plasma IFN-α concentrations after administration of a single dose of IFN-α (1 MIU/kg i.v.) at 0900 h on day 22. ○, without IFN-α pretreatment; ●, pretreatment with a single dose of IFN-α (1 MIU/kg s.c.) daily for 21 days. Each point represents the mean ± standard error of six observations. ∗∗, P < 0.01 when the data for the two groups are compared.
TABLE 1.
Influence of IFN-α pretreatment on pharmacokinetic parameters of IFN-α after administration of a single dose of IFN-αa
| Pretreatment | CL (liters/h/kg) | VSS (liters/kg) | AUC (IU · h/ml) | Terminal t1/2 (h)b | MRT (h) |
|---|---|---|---|---|---|
| Without pretreatment | 0.353 ± 0.024 | 0.477 ± 0.041 | 2,896.0 ± 196.0 | 1.038 ± 0.034 | 1.343 ± 0.039 |
| With pretreatment | 2.882 ± 1.054 | 3.161 ± 1.206 | 661.0 ± 339.0 | 0.967 ± 0.110 | 1.055 ± 0.045 |
| Statistical significance | P < 0.01 | P < 0.05 | P < 0.01 | NS | P < 0.01 |
Mice were administered a dose of 1 MIU i.v. for pretreatment, and mice received injections of a single dose of IFN-α (1 MIU/kg s.c.) (with pretreatment) or saline (without pretreatment) daily for 21 days. Values are the means ± standard errors of six observations. Statistical significance is compared between the two groups. NS, not significant.
t1/2, half-life.
Time course of plasma IFN-α concentrations and anti-IFN-α neutralizing antibody titers during repetitive treatment with IFN-α.
The plasma IFN-α concentration and anti-IFN-α neutralizing antibody titers were determined in mice given a single dose of IFN-α at 0900 h daily for 21 days (Fig. 2). The plasma IFN-α concentration measured 2 h after dosing increased after IFN-α injection on day 9. However, it significantly decreased after IFN-α injection on days 15 and 21 (P < 0.05 and P < 0.01, respectively). The anti-IFN-α neutralizing antibody titers measured 24 h after the last injection significantly increased after IFN-α injection on day 15 or 21 (P < 0.01 for both days). The time course of antibody production showed an inverse correlation with the plasma IFN-α concentration (r = −0.920; P < 0.01).
FIG. 2.
Time course of plasma IFN-α concentration 2 h after dosing and plasma anti-IFN-α antibody levels 24 h after dosing in mice given a single dose of IFN-α (1 MIU/kg s.c.) at 0900 h daily for 21 days. ○, plasma IFN-α concentration; ●, anti-IFN-α antibody level. Each point represents the mean ± standard error of six observations. ∗, P < 0.05; ∗∗, P < 0.01 compared with the level on day 1.
Influence of dosing schedule and dosing time on IFN-α concentration and anti-IFN-α neutralizing antibody titer.
In mice given a single dose of IFN-α daily, the plasma IFN-α concentration measured 2 h after dosing increased after the IFN-α injection on day 9 (Fig. 3A). However, it decreased after the IFN-α injection on day 15 and then significantly decreased after the IFN-α injection on day 21 (P < 0.05). There was no significant dosing time-dependent difference in the IFN-α concentrations between mice injected with IFN-α at 0900 h and mice injected with the drug at 2100 h. The anti-IFN-α neutralizing antibody titers measured 24 h after administration of the last dose on day 21 also showed no time-dependent differences (Fig. 4A).
FIG. 3.
Influence of dosing time on plasma IFN-α concentration 2 h after dosing in mice given a single dose of IFN-α (1 MIU/kg s.c.) daily (A) or on alternate days (B) for 21 days. ○, dosing at 0900 h; ●, dosing at 2100 h. Each point represents the mean ± standard error of six observations. a, P < 0.05 when the levels on day 1 are compared; b, P < 0.05 when the data for the two groups are compared.
FIG. 4.
Influence of dosing time on plasma anti-IFN-α antibody levels 24 h after administration of the last dose in mice given a single dose of IFN-α (1 MIU/kg s.c.) daily (A) or on alternate days (B) for 21 days. □, dosing at 0900 h; ■, dosing at 2100 h. Each column represents the mean ± standard error of six observations. ∗, P < 0.05 when the data for the two groups are compared.
In mice injected with a single dose of IFN-α at 0900 h on alternate days, the plasma IFN-α concentrations measured 2 h after dosing remained the same over 21 days (Fig. 3B). By contrast, these plasma IFN-α concentrations decreased on day 21 of treatment and were significantly lower in mice treated at 2100 h (P < 0.05) compared with the concentrations in mice treated at 0900 h. The anti-IFN-α neutralizing antibody levels measured 24 h after the last injection on day 21 were significantly higher in mice treated on alternate days at 2100 h than in mice treated at 0900 h (P < 0.05) (Fig. 4B).
DISCUSSION
The plasma IFN-α concentrations after an i.v. injection of IFN-α were significantly lower in mice with IFN-α pretreatment for 21 days than in mice without IFN-α pretreatment. The decrease in plasma IFN-α concentrations was associated with increases in CL and VSS. IFN-α is quickly eliminated from the body via several pathways. The main route of excretion is the kidneys (4). Renal tubular cells extract and break down many plasma proteins (44). IFN-α is also internalized and catabolized intracellularly in the kidney via receptor-mediated endocytosis (5). Various immune cells express the receptors for IFNs and may contribute to the elimination of IFNs. The number of IFN receptors on mononuclear cells decreases by 42 to 80% during IFN treatment (23). IFNs have the inhibitory effect of P450 enzymes (36). Both factors may contribute not only to the decrease in plasma IFN concentrations but also to the increase. The time course of antibody production showed a significant inverse correlation with the plasma IFN-α concentration. Namely, the plasma IFN-α concentration was decreasing as the production of anti-IFN-α neutralizing antibodies was increasing. IFNs bind to specific antibodies, and their biological activities may rapidly disappear. Accordingly, the antibodies produced during IFN treatment may influence the clearance and disposition of a drug. However, the large change in the distribution of IFNs after IFN pretreatment may also be influenced by other factors such as third spacing, capillary leak, etc.
IFN-α has been used on a large scale to treat patients with hepatitis and tumors. The production of specific antibodies against IFNs has been demonstrated among patients who did not respond to treatment or who had relapses after an initial response (3, 6). For example, anti-IFN neutralizing antibody is detected in 75% of patients with no response to IFNs (6). Namely, the effective IFN concentration decreases because of the appearance of anti-IFN neutralizing antibodies, resulting in a reduced therapeutic effect. The production of anti-IFN neutralizing antibodies may be influenced by several factors, including dosing schedule (duration, dose, dosing time) and the use of an alternative IFN such as IFN-α, IFN-β, or IFN-γ (17, 39, 42, 46). One simple approach that can be used to prevent or overcome the production of anti-IFN antibodies is the use of a dosing schedule which reduces the level of production of anti-IFN antibodies. Plasma IFN-α levels significantly decreased in association with the production of anti-IFN-α neutralizing antibodies in mice treated daily with IFN-α at either 0900 or 2100 h. By contrast, the plasma IFN-α levels remained stable in mice treated with IFN-α at 0900 h on alternate days, while they were significantly lower after 21 days of treatment in mice treated with IFN-α at 2100 h on alternate days. These changes were associated with significant increases in the levels of anti-IFN-α neutralizing antibodies in the latter group. The present finding suggests that an appropriate IFN-α dosing schedule and/or an appropriate IFN-α dosing time may reduce the level of production of anti-IFN-α neutralizing antibodies.
The identification of a foreign substance and its subsequent destruction constitute the main tasks of the immune system (24). Often, macrophages first take up and analyze foreign substances. These cells bear major histocompatibility complex (class II) molecules on their surfaces and will either destroy the chemical or present it as an antigen to T lymphocytes. Subsequently, various cytokines will be secreted and several lymphocyte subsets will cooperate in the mounting of an immune response. In nocturnally active mice, macrophage reactivity to a phagocytic stimulus as gauged by zymosan-induced chemiluminescence is greatest in the late rest span (8, 19, 45) or early active span (8). Other phagocytic cells, such as polymorphonuclear cells, have increased migratory activities at these times (7). Also, the organism is more resistant to tumor cells in the late rest span, when increased numbers of natural killer lymphocytes are circulating. The circadian stage dependence of the immune system may be considered the mechanism underlying the dosing time-dependent production of anti-IFN neutralizing antibodies. Namely, in the present study the level of production of anti-IFN-α neutralizing antibodies as a result of drug administration increased at times at which macrophage activity and the number of natural killer lymphocytes were elevated.
The skin responses of subjects sensitized to tuberculin challenge (9) and the episodes of kidney allograft rejection in patients who have undergone renal transplantation (18) are significantly higher in the early active span. The immune data obtained for nocturnally active rodents usually correspond well to the data obtained for humans if the data for species-specific rest-activity cycle are compared. The present findings suggest that an appropriate IFN-α dosing schedule and/or IFN-α dosing time may reduce the level of production of anti-IFN-α neutralizing antibodies in certain experimental and clinical situations.
The antibody titers were higher in animals that had more exposure to the human protein (i.e., dosing daily versus dosing every other day). This is a typical response for an animal that develops antibodies to a foreign protein within 2 weeks of dosing. The response may be different from the phenomenon occurring in patients, although similar findings are observed for recombinant interleukins and IFNs (2, 10, 12, 40, 41). If administered protein bound to circulating antibodies is still biologically active, the use of a bioassay rather than an ELISA may be useful. The means of formulation and handling of IFN-α in the vial have also been changed, which may have corrected the immunogenicity problems associated with IFN-α administration (13). Therefore, these points should be considered in future experiments.
ACKNOWLEDGMENTS
This research was supported by a Grant-in-Aid for Scientific Research (c) from the Ministry of Education, Science, Sports and Culture of Japan (grant 00223884 to S.O.).
REFERENCES
- 1.Abrams P G, McClamrock E, Foon K A. Evening administration of alpha interferon. N Engl J Med. 1985;312:443–444. doi: 10.1056/NEJM198502143120714. [DOI] [PubMed] [Google Scholar]
- 2.Anderson T D, Arceo R, Hayes T J. Comparative toxicity and pathology associated with administration of recombinant HuIL-1α to animals. Int Rev Exp Pathol. 1993;34A:9–36. [PubMed] [Google Scholar]
- 3.Antonelli G, Currenti M, Turriziani O, Dianzani F. Neutralizing antibodies to interferon-alpha: relative frequency in patients treated with different interferon preparations. J Infect Dis. 1991;163:882–885. doi: 10.1093/infdis/163.4.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bino T, Edery H, Gertler A, Rosenberg H. Involvement of the kidney in catabolism of human leukocyte interferon. J Gen Virol. 1982;59:39–45. doi: 10.1099/0022-1317-59-1-39. [DOI] [PubMed] [Google Scholar]
- 5.Bocci V, Mogensen K E, Muscettola M, Pacini A, Paulesu L, Pessina G P, Skiftas S. Degradation of human 125I-interferon alpha by isolated perfused rabbit kidney and liver. J Lab Clin Med. 1983;101:857–863. [PubMed] [Google Scholar]
- 6.Bonetti P, Diodati G, Drago C, Casarin C, Scaccabarozzi S, Realdi G, Ruol A, Alberti A. Interferon antibodies in patients with chronic hepatitic C virus infection treated with recombinant interferon alpha-2α. J Hepatol. 1994;20:416–420. doi: 10.1016/s0168-8278(94)80018-9. [DOI] [PubMed] [Google Scholar]
- 7.Bureau J P, Garelly L, Coupe M, Labrecque G. Circadian rhythm studies on BCG-induced migration of PMN in normal and adrenolectomized mice. Annu Rev Chronopharmacol. 1985;1:333–336. [Google Scholar]
- 8.Carrere V, Dorfman P, Bastide M. Evaluation of various factors influencing the action of mouse αβ interferon on the chemiluminescence of mouse peritoneal macrophages. Annu Rev Chronopharmacol. 1988;5:9–12. [Google Scholar]
- 9.Cove-Smith J R, Kabler P, Pownall R, Knapp M S. Circadian variation in an immune response in man. Br Med J. 1978;2:253–254. doi: 10.1136/bmj.2.6132.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Green J D, Terrell T G. Utilization of homologous proteins to evaluate the safety of recombinant human proteins—case study: recombinant human interferon-gamma (rhIFN-γ) Toxicol Lett. 1992;64/65:321–327. doi: 10.1016/0378-4274(92)90204-w. [DOI] [PubMed] [Google Scholar]
- 11.Gutterman J U, Fine S, Quesada J, Horning S J, Levine J F, Alexanian R, Bernhardt L, Kramer M, Spiegel H, Colburn W, Trown P, Merigan T, Dziewanowski Z. Recombinant leukocyte A interferon: pharmacokinetics, single-dose tolerance, and biologic effects in cancer patients. Ann Intern Med. 1982;96:549–556. doi: 10.7326/0003-4819-96-5-549. [DOI] [PubMed] [Google Scholar]
- 12.Harada Y, Yahara I. Pathogenesis of toxicity with human-derived interleukin-2 in experimental animals. Int Rev Exp Pathol. 1993;34A:37–55. [PubMed] [Google Scholar]
- 13.Hochuli E. Interferon immunogenicity: technical evaluation of interferon-α2a. J Interferon Cytokine Res. 1997;17(Suppl. 1):S15–S21. [PubMed] [Google Scholar]
- 14.Iacobelli S, Garufi C, Irtelli L, Martino M T, Santobuono F, Vicario G, Tinari N, Fiorentino B, Innocenti P, Natoli C. A phase I study of recombinant interferon-α administered as a seven-day continuous venous infusion at circadian-rhythm modulated rate in patients with cancer. Am J Clin Oncol. 1995;18:27–31. doi: 10.1097/00000421-199502000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Itri L M, Campion M, Dennin R A, Palleroni A V, Gutterman J U, Groopman J E, Trown P W. Incident and clinical significance of neutralizing antibodies in patients receiving recombinant interferon alpha-2a by intramuscular injection. Cancer. 1987;59:668–674. doi: 10.1002/1097-0142(19870201)59:3+<668::aid-cncr2820591317>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs S J, Sullivan L M, Salfi M, Grossberg H, Spiegel R J, Leibowitz P J, Oden E M, Kelsey D K, Treuhaft M W. Minimal antigenicity of intron A in human recipients demonstrated by three analytical methods. J Biol Response Modifiers. 1988;7:447–456. [PubMed] [Google Scholar]
- 17.Janssen J T, De Pauw B E, Holdrinet R S. Treatment of hairy cell leukaemia with recombinant human α2-interferon. Lancet. 1984;i:1025–1026. doi: 10.1016/s0140-6736(84)92377-8. [DOI] [PubMed] [Google Scholar]
- 18.Knapp M S, Cove-Smith J R, Dugdale R, Mackenzie N, Pownall R. Possible effect of time on renal allograft rejection. Br Med J. 1979;1:75–77. doi: 10.1136/bmj.1.6156.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knyszynski A, Fischer H. Circadian fluctuations in the activity of phagocytic cells in blood, spleen and peritoneal cavity of mice as measured by zymosan-induced chemiluminescence. J Immunol. 1981;127:2508–2511. [PubMed] [Google Scholar]
- 20.Koren S, Fleischmann W R. Circadian variations in myelosuppressive activity of interferon-α in mice: identification of an optimal treatment time associated with reduced myelosuppressive activity. Exp Hematol. 1993;21:552–559. [PubMed] [Google Scholar]
- 21.Koren S, Whorton E B, Fleischmann W R. Circadian dependence of interferon antitumor activity in mice. J Natl Cancer Inst. 1993;85:1927–1932. doi: 10.1093/jnci/85.23.1927. [DOI] [PubMed] [Google Scholar]
- 22.Koyanagi S, Ohdo S, Yukawa E, Higuchi S. Chronopharmacological study of interferon-α in mice. J Pharmacol Exp Ther. 1997;283:259–264. [PubMed] [Google Scholar]
- 23.Lau J Y N, Sheron N, Morris A G, Bomford A B, Alexander G J, Williams R. Interferon-α receptor expression and regulation in chronic hepatitis B virus infection. Hepatology. 1991;13:332–338. [PubMed] [Google Scholar]
- 24.Levi F, Canon C, Dipalma M, Florentin I, Misset J L. When should the immune clock be reset? From circadian pharmacodynamics to temporally optimized drug delivery. Ann N Y Acad Sci. 1991;618:312–329. doi: 10.1111/j.1749-6632.1991.tb27251.x. [DOI] [PubMed] [Google Scholar]
- 25.Liao M J, Axelrod H R, Kuchler M, Yip Y K, Kirkbright E, Testa D. Absence of neutralizing antibodies to interferon in condyloma acuminata and cancer patients treated with natural human leukocyte interferon. J Infect Dis. 1992;165:757–760. doi: 10.1093/infdis/165.4.757. [DOI] [PubMed] [Google Scholar]
- 26.Lok S F, Lai C L. Incidence, neutralizing activity and clinical significance of interferon antibodies in chronic hepatitis B patients receiving recombinant α-interferons. In: Hollinger F B, Lemon S M, Margolis H S, editors. Viral hepatitis and liver disease. London, United Kingdom: The Williams & Wilkins Co.; 1991. pp. 643–645. [Google Scholar]
- 27.Ohdo S, Grass G M, Lee V H L. Improving the ocular to systemic ratio of topical timolol by varying the dosing time. Investig Ophthalmol Vis Sci. 1991;32:2790–2798. [PubMed] [Google Scholar]
- 28.Ohdo S, Inoue K, Yukawa E, Higuchi S, Nakano S, Ogawa N. Chronotoxicity of methotrexate in mice and its relation to circadian rhythm of DNA synthesis and pharmacokinetics. Jpn J Pharmacol. 1997;75:283–290. doi: 10.1254/jjp.75.283. [DOI] [PubMed] [Google Scholar]
- 29.Ohdo S, Koyanagi S, Yukawa E, Higuchi S. Circadian rhythm of fever induced by interferon-α in mice. Life Sci. 1997;61:PL95–PL100. doi: 10.1016/s0024-3205(97)00567-5. [DOI] [PubMed] [Google Scholar]
- 30.Ohdo S, Makinosumi T, Ishizaki T, Yukawa E, Higuchi S, Nakano S, Ogawa N. Cell cycle-dependent chronotoxicity of irinotecan hydrochloride in mice. J Pharmacol Exp Ther. 1997;283:1383–1388. [PubMed] [Google Scholar]
- 31.Ohdo S, Nakano S, Ogawa N. Chronopharmacological study of sodium valproate in mice: dose-concentration-response relationship. Jpn J Pharmacol. 1988;47:11–19. doi: 10.1254/jjp.47.11. [DOI] [PubMed] [Google Scholar]
- 32.Ohdo S, Nakano S, Ogawa N. An influencing factor to predict plasma valproate concentrations: circadian stage-dependent kinetics. Jpn J Clin Pharmacol Ther. 1990;21:747–754. [Google Scholar]
- 33.Ohdo S, Ogawa N, Nakano S, Higuchi S. Influence of feeding schedule on the chronopharmacological aspects of sodium valproate in mice. J Pharmacol Exp Ther. 1996;278:74–81. [PubMed] [Google Scholar]
- 34.Ohdo S, Ogawa N, Song J G. Chronopharmacological study of acetylsalicylic acid in mice. Eur J Pharmacol. 1995;293:151–157. doi: 10.1016/0926-6917(95)00011-9. [DOI] [PubMed] [Google Scholar]
- 35.Ohdo S, Watanabe H, Ogawa N, Yoshiyama Y, Sugiyama T. Circadian rhythm of embryotoxicity induced by sodium valproate in mice. Eur J Pharmacol. 1995;293:281–285. doi: 10.1016/s0922-4106(05)80056-2. [DOI] [PubMed] [Google Scholar]
- 36.Piscitelli S C, Reiss W G, Figg W D, Petros W P. Pharmacokinetic studies with recombinant cytokines: scientific issues and practical considerations. Clin Pharmacokinet. 1997;32:368–381. doi: 10.2165/00003088-199732050-00003. [DOI] [PubMed] [Google Scholar]
- 37.Porres J C, Carreno V, Ruiz M, Marron J A, Bartolome J. Interferon antibodies in patients with chronic HBV infection treated with recombinant interferon. J Hepatol. 1989;8:351–357. doi: 10.1016/0168-8278(89)90034-2. [DOI] [PubMed] [Google Scholar]
- 38.Quesada J R, Gutterman J U. Clinical study of recombinant DNA-produced leukocyte interferon (clone A) in an intermittent schedule in cancer patients. J Natl Cancer Inst. 1983;70:1041–1046. [PubMed] [Google Scholar]
- 39.Quesada J R, Rios A, Swanson D, Gutterman J U. Antitumor activity of recombinant-derived interferon alpha in metastatic renal cell carcinoma. J Clin Oncol. 1985;3:1522–1528. doi: 10.1200/JCO.1985.3.11.1522. [DOI] [PubMed] [Google Scholar]
- 40.Reiner G, Ronneberger H, Hintz-Obertreis P. Comparative toxicity of Escherichia coli and yeast rHuIL-3 in cynomolgus and rhesus monkeys. Int Rev Exp Pathol. 1993;34A:119–147. [PubMed] [Google Scholar]
- 41.Ryffel B, Mihatsch M J, Woerly G. Pathology induced by interleukin-6. Int Rev Exp Pathol. 1993;34A:79–89. [PubMed] [Google Scholar]
- 42.Spiegel R J, Spicehandler J R, Jacobs S L, Oden E M. Low incidence of serum neutralizing factors in patients receiving recombinant alpha-2b interferon (intron A) Am J Med. 1986;80:223–228. doi: 10.1016/0002-9343(86)90013-6. [DOI] [PubMed] [Google Scholar]
- 43.Steis R G, Smith II J W, Urba W J, Clark J W, Itri L M, Evans L M, Schoenberger C, Longo D L. Resistance to recombinant interferon alpha-2a in hairy-cell leukemia associated with neutralizing anti-interferon antibodies. N Engl J Med. 1988;318:1409–1413. doi: 10.1056/NEJM198806023182201. [DOI] [PubMed] [Google Scholar]
- 44.Strober W, Waldmann T A. The role of the kidney in the metabolism of plasma proteins. Nephron. 1974;13:35–66. doi: 10.1159/000180368. [DOI] [PubMed] [Google Scholar]
- 45.Szabo I, Kovats T, Halberg F. Circadian rhythms in murine reticulo-endothelial function. Chronobiologia. 1978;5:137–143. [PubMed] [Google Scholar]
- 46.Vallbracht A, Treuner T, Flehmig B, Joester K E, Niethammer D. Interferon neutralizing antibodies in a patient treated with human fibroblast interferon. Nature. 1981;287:496–498. doi: 10.1038/289496a0. [DOI] [PubMed] [Google Scholar]
- 47.Von Wussow P, Hartmann F, Freund M, Poliwoda H, Deicher H. Leucocyte-derived interferon-α in patients with antibodies to rIFN-α2b. Lancet. 1988;i:882–883. doi: 10.1016/s0140-6736(88)91628-5. [DOI] [PubMed] [Google Scholar]
- 48.Weck P K, Leventhal B G, Brand C, Finter N B. Detection and incidence of neutralizing antibodies to interferon-α-n1. J Interferon Res. 1989;9:S37–S43. [PubMed] [Google Scholar]