Abstract
To provide novel data relating to the dispositions, effects, and toxicities of the artemisinin derivatives in severe malaria, we studied 30 Vietnamese adults with slide-positive falciparum malaria treated with intravenous artesunate. Twelve patients with complications (severe; group 1) and 8 patients without complications but requiring parenteral therapy (moderately severe; group 2) received 120 mg of artesunate by injection, and 10 patients with moderately severe complications (group 3) were given 240 mg by infusion. Serial concentrations of artesunate and its active metabolite dihydroartemisinin in plasma were measured by high-performance liquid chromatography. The time to 50% parasite clearance (PCT50) was determined from serial parasite densities. Full clinical (including neurological) assessments were performed at least daily. In noncompartmental pharmacokinetic analyses, group mean artesunate half-lives (t1/2) were short (range, 2.3 to 4.3 min). The dihydroartemisinin t1/2 (range, 40 to 64 min), clearance (range, 0.73 to 1.01 liters/h/kg), and volume of distribution (range, 0.77 to 1.01 liters/kg) were also similar both across the three patient groups (P > 0.1) and to previously reported values for patients with uncomplicated malaria. Parasite clearance was prompt (group median PCT50 range 6 to 9 h) and clinical recovery was complete under all three regimens. These data indicate that the pharmacokinetics of artesunate and dihydroartemisinin are not influenced by the severity of malaria. Since the pharmacokinetic parameters for both artesunate and dihydroartemisinin were similar regardless of whether injection or infusion was used, artesunate can be considered a prodrug that is converted stoichiometrically to dhydroartemisinin. Conventional doses of artesunate are safe and effective when given to patients with complications of falciparum malaria.
Artemisinin and its semisynthetic derivatives are potent, well-tolerated compounds that are used as first-line antimalarial therapy in many tropical countries (1, 16, 22, 38). The derivative with the widest potential application is artesunate (ARTS), a water-soluble prodrug that can be administered parenterally as well as by mouth and per rectum. A number of studies have demonstrated the efficacy of ARTS in uncomplicated (17, 19, 24, 26, 27, 28), severe (20, 21), and multidrug-resistant (11, 25, 34) falciparum malaria. Since ARTS clears parasites more rapidly than quinine and may improve survival (1, 16, 22, 24, 38), intravenous (i.v.) ARTS has become an alternative to i.v. quinine for the treatment of severe malaria in many countries.
The pharmacokinetics and pharmacodynamics of ARTS and other artemisinin derivatives have been assessed in patients with uncomplicated malaria (3, 6, 7, 9), but there have been no equivalent studies with patients with severe Plasmodium falciparum infections. The dosing regimens used for patients with complications remain empirical and do not differ significantly from those used for patients with milder malaria (16, 22, 38). Although neurotoxicity has yet to be demonstrated objectively in humans receiving conventional doses of artemisinin drugs, residual concerns remain (10, 12, 18, 23, 30, 35). Since neurological dysfunction leading to coma in its most severe form is a serious complication of falciparum malaria (37), there is an added need for data relating to the disposition and effects of ARTS in severe malaria.
We have studied the pharmacokinetics, pharmacodynamics, and toxicities of ARTS and its equipotent, longer-acting active metabolite dihydroartemisinin (DHA) in patients with severe malaria given a conventional dose of ARTS by i.v. injection. Since i.v. ARTS is converted rapidly to DHA and the half-life (t1/2) of DHA itself is short compared to the recommended interval between doses, administration of ARTS by i.v. infusion has been suggested as a way of increasing the duration of parasite exposure to therapeutic drug concentrations as well as limiting the potential toxicity of high peak levels postinjection (4). In a second study, therefore, we examined the disposition, antimalarial efficacy, and toxicity of ARTS given by injection or 4-h infusion to patients with moderately severe falciparum malaria.
MATERIALS AND METHODS
Patients.
We studied 30 Vietnamese farmers or rural laborers with falciparum malaria requiring parenteral therapy who were admitted to Cho Ray Hospital, Ho Chi Minh City, or to Bao Loc Hospital, Bao Loc, Lam Dong Province. After confirmation of the diagnosis by microscopy, a complete clinical assessment including drug history was performed. Patients were classified as having severe malaria (37) if they were (i) in a coma (Glasgow coma score [GCS], <11), (ii) jaundiced (serum bilirubin level, >50 μmol/liter; serum aspartate transaminase [AST] level, more than twice the upper limit of the reference range), (iii) in renal failure (serum creatinine level, >250 μmol/liter after rehydration), (iv) anemic (venous hematocrit level, <15%), or (v) hyperparasitemic (>250,000 asexual forms/μl of whole blood). Patients were considered as having moderately severe malaria if they did not have any of the features described above but could not be given oral therapy because of nausea, vomiting, or confusion.
Patients were excluded if they had been treated with ARTS or DHA in the previous 8 h, artemisinin in the previous 12 h, or artemether or arteether in the previous 24 h. If the patient's condition deteriorated significantly during the study, the attending physician was to withdraw the patient from the study and start appropriate resuscitation and supportive treatment. Informed consent was obtained from each patient or from a first-degree relative of those who presented in a coma. Patients were free to withdraw from the study at any stage without prejudice to their continuing care. The study was approved by the Ministry of Health of Vietnam and the University of Western Australia Human Rights Committee.
Study design and procedures.
The study was performed in two phases. In phase 1, 12 patients with severe falciparum malaria (group 1) were given 120 mg of ARTS (Guilin No. 2 Pharmaceutical Factory, Guangxi, China) diluted in 10 ml of 5% (wt/vol) dextrose and administered as an i.v. bolus over 2 min. Venous blood samples were obtained from the arm opposite that used for drug administration at 0 (predosing), 5, 7, 9, 12, 15, 20, 30, 45, 60, 90, 120, 180, and 240 min. Further 60-mg doses of i.v. ARTS were scheduled at 24 and 48 h, but additional doses could be given at the discretion of the attending physician. Mefloquine (15 mg/kg of body weight) was given at 72 h, or if this was precluded by the clinical state of the patient, daily ARTS administration was continued until oral mefloquine could be tolerated.
In phase 2, patients with moderately severe malaria were randomized to receive ARTS by either i.v. injection or infusion. Because infusions of ARTS had not been evaluated previously, we used a total initial dose of approximately 4 mg/kg/day, which is at the upper end of the range usually recommended for falciparum malaria (1, 16). Eight patients (group 2) were given 120 mg of ARTS i.v., and the same sampling protocol used for group 1 was followed. A second dose of 120 mg of ARTS was given i.v. immediately after the last (240 min) blood sample had been taken, but no other samples were drawn subsequently. Ten other patients (group 3) were given the same total dose of ARTS given to those in group 2 (240 mg), but the dose was diluted in 50 ml of 5% (wt/vol) dextrose and was given as an i.v. infusion over 4 h with a motor-driven infusion pump. For these patients, venous blood was drawn at 0, 5, 7, 9, 12, 15, 20, 30, 45, 60, 90, 120, 180, and 240 min during the infusion and then at 5, 7, 9, 12, 15, 20, 30, 45, 60, 90, and 120 min after the infusion had been stopped. Patients in phase 2 were scheduled to receive further doses of ARTS at 60 mg i.v. at 24 h and a single dose of mefloquine on the second or third day of hospitalization.
A complete physical examination, including full neurological assessment and determination of GCS, was performed at least daily for each patient, and the results were recorded on standard forms. All blood samples were collected in fluoride-oxalate tubes and were chilled immediately to prevent ARTS degradation by plasma esterases. Samples were centrifuged within 30 min to minimize hemolysis, and the separated plasma was stored below −20°C until analyzed. Thick and thin blood films were prepared at 0, 0.5, 1, 2, 3, 4, 6, 8, 12, 18, and 24 h and every 6 h thereafter until parasite clearance. Oral temperature was measured every 4 h. Patients were discharged when they were afebrile and aparasitemic.
Pharmacokinetic and pharmacodynamic analyses.
Plasma samples were assayed by a validated high-performance liquid chromatography (HPLC) method (2, 6). ARTS has previously been shown to be stable in 5% (wt/vol) dextrose at 30°C, with <10% being hydrolyzed in 7 h, while the stabilities of ARTS (780 and 4,560 nmol/liter) and DHA (1,060 and 6,160 nmol/liter) in plasma have been assessed for up to 12 months at −25°C and found to be within ±7.6% of those for replicate samples stored for equivalent lengths of time at −80°C (2, 4). As reported previously (3, 4, 6, 7), the batch purity of the i.v. ARTS used was checked by HPLC and was found to be equivalent to that stated by the manufacturer. Conventional pharmacokinetic parameters (area under the plasma concentration-time curve [AUC] from time zero to infinity [AUC0–∞i; with extrapolation to ∞ as Clast/λz, where Clast is the final measured concentration and λz is the terminal elimination rate constant], t1/2, clearance [CL], volume of distribution at pseudo-distribution equilibrium [Vz], maximum concentration in plasma [Cmax], and the time to Cmax [Tmax]) were determined from the plasma concentration-time data by noncompartmental analysis (36) and by assuming complete bioconversion of ARTS to DHA.
Thick blood films were Giemsa stained within 12 h of preparation. The number of asexual parasites per microliter of whole blood was determined by counting the white cells (WBCs) in high-power fields containing a total of 500 parasites in which the ratio of parasites/WBCs was more than 1 or the number of parasites per 1,000 WBCs in which the ratio of parasites/WBCs was less than 1. The parasitemia was calculated as the product of the parasite/WBC ratio and the WBC count. The time to a 50% reduction of the original parasite count (PCT50) was determined by linear interpolation of the parasite count-time data. Fever clearance time (FCT) was taken to be the time of the first of two oral temperature readings <37.5°C.
Statistical analysis.
Differences between means were analyzed by Student's t test or one-way analysis of variance (ANOVA), as appropriate, with the least-significant-difference post hoc test (SPSS for Windows; SPSS Inc., Chicago, Ill.). For variables that were not normally distributed by the Kolmogorov-Smirnov one-sample test, Wilcoxon-Mann-Whitney and Kruskall-Wallis tests were used. The Spearman rank correlation was used to evaluate relationships between pharmacokinetic and pharmacodynamic parameters. Data are reported as means ± standard deviations (SDs). Two-tailed significance levels were used.
RESULTS
Clinical course.
Details for the patients in the three groups at presentation are given in Table 1. Consistent with the criteria used for classification, the patients with severe malaria (group 1) had significantly greater serum creatinine, bilirubin, and AST concentrations than those in the other two groups (P < 0.01 by ANOVA in each case). Five group 1 patients had a GCS <11. All other demographic, clinical, and laboratory measurements for the three groups were similar (P > 0.1).
TABLE 1.
Presentation demographic, anthropometric, and laboratory data from patients with severe (group 1) and moderately severe (groups 2 and 3) malariaa
| Parameter | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| No. of patients | 12 | 8 | 10 |
| Age (yr) | 36.7 ± 16.2 | 34.8 ± 19.2 | 33.2 ± 12.3 |
| Sex (no. of men/no. of women) | 11/1 | 5/3 | 5/5 |
| Wt (kg) | 53.6 ± 4.8 | 55.4 ± 8.7 | 48.7 ± 6.2 |
| Oral temp (°C) | 38.3 ± 1.0 | 39.0 ± 1.3 | 38.4 ± 0.7 |
| Venous hematocrit level (%) | 28.0 ± 5.5 | 32.5 ± 7.5 | 28.0 ± 9.0 |
| Parasitemia (no. of parasites/μl [range]) | 14,990 (120–139,330) | 74,730 (520–176,810) | 74,710 (950–219,670) |
| Plasma glucose concn (mmol/liter) | 9.1 ± 5.0 | 8.6 ± 3.9 | 6.5 ± 1.9 |
| Plasma lactate concn (mmol/liter) | 4.1 ± 3.4 | 2.2 ± 1.0 | 2.0 ± 0.9 |
| Serum creatinine concn (μmol/liter) | 301 ± 248b | 89 ± 38 | 86 ± 66 |
| Serum bilirubin concn (μmol/liter) | 113 ± 77b | 28 ± 18 | 36 ± 39 |
| Serum AST concn (U/liter) | 183 ± 145b | 44 ± 29 | 61 ± 40 |
Unless indicated otherwise, data are means ± SDs.
P < 0.01 versus results for groups 2 and 3.
All 30 patients made a full recovery and were discharged from hospital between 3 and 21 days after admission. In the case of the patients admitted in a coma, three recovered consciousness fully (GCS, 15) within 72 h. The other two patients had a depressed GCS (GCS, <15) for 5 and 16 days after presentation, respectively. The latter patient had a normal cranial computed tomography scan and cerebrospinal fluid examination during the second week of his illness. As assessed from detailed clinical examinations, none of the 30 patients had any neurological deficit at discharge. One patient in group 2 developed features suggestive of a postmalaria neurological syndrome (33), including confusion and ataxia, after mefloquine had been administered and parasite and fever clearance had occurred. His symptoms were, however, self-limiting and did not require intervention including additional drug therapy.
Pharmacokinetic analysis. (i) ARTS.
Pharmacokinetic data for ARTS are summarized in Fig. 1 and Table 2. ARTS had a short mean t1/2 in each of the three groups, the shortest being in the severely ill patients (group 1). These patients also had a mean value of Vz that was significantly lower than those for the other two groups (Table 2). Mean CL values also tended to be lowest for patients in group 1 and highest for the patients with moderately severe malaria who received ARTS by infusion (group 3).
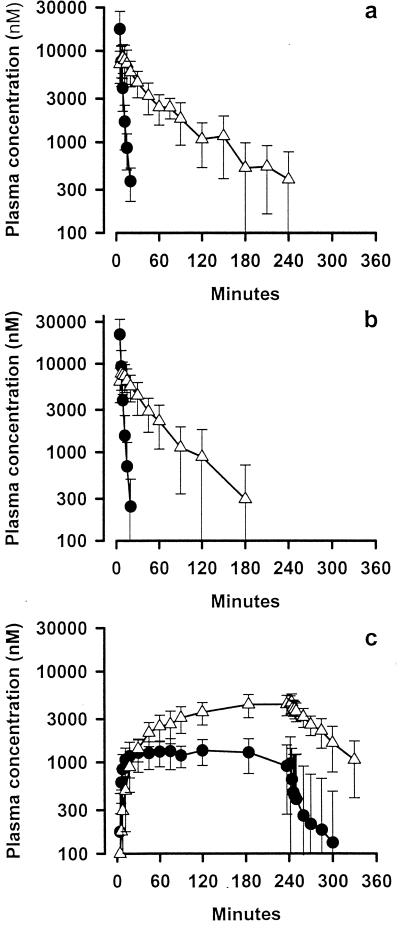
FIG. 1.
Mean ± SD concentrations of ARTS (closed circles) and DHA (open triangles) plasma in groups 1, 2, and 3 (panels a, b, and c, respectively).
TABLE 2.
Pharmacokinetic parameters for ARTS and DHA following i.v. injection of 120 mg (312.5 μmol) ARTS in groups 1 and 2 and a 4-h infusion of 240 mg (625 μmol) in groupa
| Drug and parameter | Group 1 | Group 2 | Group 3 |
|---|---|---|---|
| ARTS | |||
| t1/2 (min) | 2.3 ± 0.9 | 4.3 ± 2.6 | 3.2 ± 1.6 |
| CL (liters/h/kg) | 1.63 ± 0.96c | 2.49 ± 1.68 | 3.07 ± 0.90 |
| Vz (liter/kg) | 0.08 ± 0.04bc | 0.24 ± 0.17 | 0.23 ± 0.10 |
| DHA | |||
| t1/2 (min) | 40.0 ± 24.8 | 64.1 ± 28.8 | 46.2 ± 18.7 |
| CL (liters/h/kg) | 1.09 ± 0.53 | 0.73 ± 0.28 | 0.73 ± 0.28 |
| Vz (liters/kg) | 0.77 ± 0.28 | 1.01 ± 0.34 | 0.78 ± 0.31 |
| AUC (μmol · h/liter) | 7.3 ± 4.1 | 9.0 ± 3.9 | 19.6 ± 8.6 |
| Cmax (μmol/liter) | 8.5 ± 2.9 | 8.9 ± 3.8 | 3.2 ± 1.5d |
| Tmax (min) | 10.4 ± 7.5 | 9.9 ± 3.4 | 240d |
| Pharmacodynamicse | |||
| No. of patients | 8 | 6 | 10 |
| Initial parasitemia (no. of parasites/μl) | 9,260 (120–139,330) | 89,730 (520–176,810) | 74,710 (950–219,670) |
| PCT50 (h [range]) | 9 (2–20)cf | 6 (1–12) | 6 (1–12) |
| FCT (h [range]) | 34 (20–216) | 36 (20–95) | 32 (16–72) |
Data are given as means ± SDs. Pharmacodynamic parameters are also shown as group medians and (ranges).
P < 0.01 versus group 2.
P < 0.05 versus group 3.
End-of-infusion values.
Excludes those patients given unscheduled doses of ARTS during the first 24 h.
(ii) DHA.
Apart from AUC and Cmax, the pharmacokinetics of DHA after i.v. ARTS administration were similar in the three groups (Table 2). The mean AUC for group 3 was consistent with the dose of ARTS given, approximately double those for the other two groups, while the mean Cmax for group 3 (at the end of the infusion period) was significantly lower than those for the other two groups. Consistent with the t1/2 for each compound, steady-state concentrations of ARTS were achieved early on during the infusion in group 3 patients, whereas for DHA, steady state was reached late in the 240-min infusion period (Fig. 1).
Pharmacodynamic analysis.
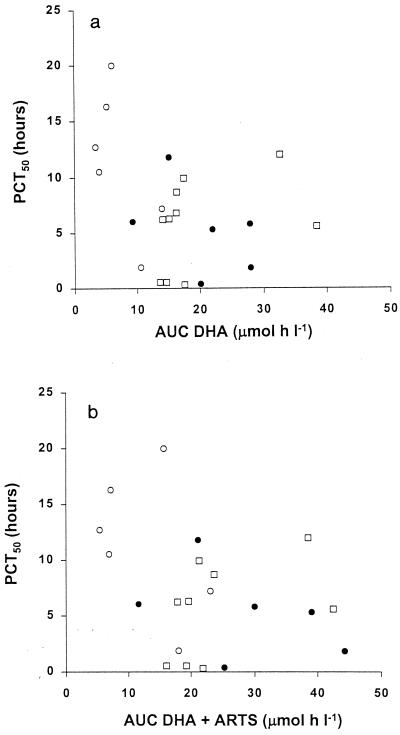
Four group 1 patients and two group 2 patients received additional unscheduled doses of i.v. ARTS within the first 24 h. Parasite and fever clearance data for these patients were therefore excluded from the pharmacodynamic analysis (Table 2). There were no between-group differences in FCT (P = 0.7). However, the six evaluable group 1 patients had the greatest PCT50s (P < 0.05 versus the values for groups 2 and 3). This was not a function of the initial parasitemia because there was no significant correlation with PCT50 (rs = −0.26; n = 24; P = 0.22). To determine whether the between-group differences in PCT50s resulted from the relatively greater severity of illness in group 1 patients or from the higher doses of i.v. ARTS given to patients in groups 2 and 3 during the first 24 h of treatment, we investigated the pharmacodynamics of ARTS and DHA by assessing correlations between drug AUC values and PCT50s. Because patients in group 2 had received two doses of 120 mg of ARTS 4 h apart, we doubled the AUC values for both DHA and DHA plus ARTS after the first injection for these subjects. There was a nonsignificant but borderline inverse correlation between PCT50 and AUC for DHA in the 24 patients (rs = −0.40; P = 0.06) (Fig. 2). Because ARTS and DHA both possess the endoperoxide moiety thought to be responsible for the antimalarial activities of the artemisinin drugs (22), ARTS may contribute to parasite clearance, despite its short t1/2. The correlation between PCT50 and total AUC for ARTS and DHA combined was similar to that between PCT50 and the AUC for DHA alone (rs = −0.40; P = 0.07) (Fig. 2). In both cases (AUC for DHA and AUC for DHA and ARTS combined), an AUC value of <10 μmol · h/liter was especially likely to be associated with a PCT50 >10 h.
FIG. 2.
PCT50 plotted against AUC for DHA (a) and against AUC for DHA plus ARTS (b). Open circles group 1 patients; closed circles, group 2 patients; open squares, group 3 patients.
DISCUSSION
The present studies were generated primarily by the concern that, analogous to quinine (15, 39), the complications of falciparum malaria could be associated with clinically significant changes in the pharmacokinetics of ARTS and DHA. Rational parenteral quinine regimens have been developed specifically for severely ill patients (37). By contrast, the relative paucity of pharmacokinetic data for ARTS, even in patients without complications, means that dosing regimens for severe malaria remain empirical. As well as providing novel pharmacokinetic data for ARTS and DHA in this situation, we wished to assess whether i.v. infusion of ARTS both achieved the predicted plasma DHA concentrations and cleared the parasitemia at least as effectively as ARTS given in equivalent doses by i.v. injection. Although the risk of toxicity associated with the artemisinin derivatives appears to be very low, the present study provided further opportunities to monitor the side effects of ARTS in a controlled situation.
Apart from a case study of a Caucasian patient who received i.v. ARTS by injection (13), our data are the first relating to the disposition and effects of an artemisinin derivative in severe falciparum malaria. The t1/2 of ARTS has been estimated to be between 2 and 5 min in a variety of previous studies in both healthy volunteers (40, 41) and patients with uncomplicated malaria (3, 6, 9). Mean values for our three groups of patients and that for our Caucasian patient (13) lie within this range. Although there was a significantly shorter t1/2 in our 12 patients with severe malaria compared with those in the 18 patients who had moderately severe malaria in the presence of a lower CL and Vz, these differences are unlikely to be of clinical significance since ARTS is rapidly metabolized and can thus be considered a prodrug. The pharmacokinetics of its longer-acting metabolite, DHA, were comparable across the three groups, apart from parameters relating to the double-dose and 4-h administration time for group 3 patients. Mean values for DHA elimination t1/2, CL, and Vz in the three groups were comparable to those after i.v. ARTS administration for healthy controls, patients with uncomplicated malaria, and the one patient with severe malaria reported previously (3, 6, 7, 8, 9, 13, 40, 41). We conclude, therefore, that the pharmacokinetics of ARTS and DHA are not influenced by the severity of the malaria.
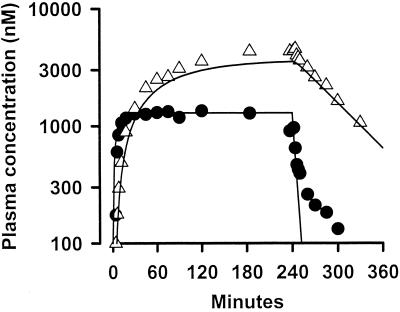
A further indication that ARTS and DHA pharmacokinetics are independent of infection severity is provided by Fig. 3. Mean concentration profiles for both ARTS and DHA in plasma for group 3 patients during and after ARTS infusion are shown in Fig. 3, together with theoretical plots derived from pharmacokinetic data for patients with uncomplicated falciparum and vivax malaria given ARTS by i.v. injection (4). The latter plots have been generated by using an open one-compartment model under the assumption that there is complete metabolism of ARTS to DHA. The theoretical and actual curves are similar, apart from some divergence at low plasma ARTS concentrations postinfusion. This divergence reflects the fact that the ARTS concentrations in some group 3 patients were below the assay detection limit (250 nmol/liter) at these times and were not used in the calculation of mean values.
FIG. 3.
Mean plasma ARTS (closed circles) and DHA (open triangles) concentration profiles for group 3 patients and predicted concentrations in plasma for patients with uncomplicated malaria (solid lines) derived from pharmacokinetic data describing levels after i.v. injection.
In a previous study with an isolated perfused rat liver model (5), we found that DHA had a high, concentration-dependent hepatic extraction ratio that was reduced by 20 to 30% in rodents with Plasmodium berghei malaria. Elimination of DHA-glucuronide, the principal biliary metabolite, was reduced by 40 to 50% in rats with malaria, probably as a result of decreased intrinsic metabolic clearance. By contrast, the present data suggest indirectly that hepatic dysfunction complicating falciparum malaria does not alter DHA clearance significantly. This may reflect the fact that glucuronidation is a high-capacity reaction occurring in many tissues (32) and may not be the only metabolic pathway for DHA in humans (31).
Parasite clearance was prompt in all three groups, with PCT50s that did not exceed 20 h. However, the PCT50 for the severely ill patients who received a lower dose of ARTS in the first 24 h was significantly longer than those for patients in the other two groups. When the data for all three groups were considered together, there was a trend to a shorter PCT50 for patients with greater AUC values for DHA both alone and in combination with ARTS. Although the PCT50s for the six group 1 and 2 patients given additional unscheduled doses of i.v. ARTS were close to the group medians (data not shown), our preliminary pharmacodynamic data suggest that it may be appropriate to give more than 120 mg of ARTS in the first 24 h of treatment to patients with at least moderately severe falciparum malaria. This might take the form of a second i.v. injection of 60 to 120 mg of ARTS at 4 to 8 h or the use of a high-dose ARTS infusion, as was done for group 3 patients. The precise mechanism of action of the artemisinin derivatives is not yet fully understood. Nevertheless, our data for the group 3 patients suggest that their rapidity of action does not depend on peak concentrations in plasma.
Other than those that are attributable to falciparum malaria per se (37) or that occur after parasite clearance, especially in the context of mefloquine administration (33), neurological symptoms and signs were not observed in any of our patients. Although there is evidence from in vitro and animal studies that artemisinin derivatives are neurotoxic (10, 12, 23, 35), this has not been documented in controlled, prospective studies with humans (1, 16, 22, 38). In recent single case reports of an association between treatment with an artemisinin derivative and cerebellar dysfunction (30) and tremor (18), it is difficult to know whether the neurological features were due to artemisinin, malaria itself, or other contemporaneous medications or disease processes (14). There have been no reports of similar features in severely ill patients treated with an artemisinin drug.
Severe malaria remains a condition still associated with unacceptably high rates of morbidity and mortality (37). Our results extend current knowledge of the disposition and effects of artemisinin derivatives to patients with complications of falciparum malaria and confirm that ARTS given at doses of 2 to 4 mg/kg/day is both safe and effective when given as an i.v. bolus or as an infusion, including to those presenting in a coma. Consistent with the results of previous in vitro and in vivo studies (1, 16, 22, 38), our results imply that rapid initial parasite clearance after ARTS administration can be achieved after a relatively brief exposure of parasitized erythrocytes to drug and/or active metabolite.
ACKNOWLEDGMENTS
We thank Trinh Kim Anh, Nguyen Phuc Tien, Truong Xuan Mai, and Vuong Van Chon, Cho Ray Hospital, and Vo Thanh Chien, Vu Nam Bien, Dang Thi Vinh Thuan, and the staff of the Malaria and Biochemistry & Hematology Departments, Bao Loc Hospital, for facilitating the conduct of this study.
This study was supported by a project grant from the National Health and Medical Research Council (NHMRC) of Australia (to T.M.E.D. and K.F.I.). K.T.B. was a recipient of an NHMRC Dora Lush (Biomedical) Scholarship.
REFERENCES
- 1.Barradell L B, Fitton A. Artesunate. A review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs. 1995;50:714–741. doi: 10.2165/00003495-199550040-00009. [DOI] [PubMed] [Google Scholar]
- 2.Batty K T. Pharmacokinetic studies of artesunate and dihydroartemisinin, p. 114–116. Ph.D. thesis. Nedlands, Australia: University of Western Australia; 1999. [Google Scholar]
- 3.Batty K T, Davis T M E, Thu L T A, Binh T Q, Anh T K, Ilett K F. Selective high-performance liquid chromatographic determination of artesunate and α- and β-dihydroartemisinin in patients with falciparum malaria. J Chromatogr B. 1996;677:345–350. doi: 10.1016/0378-4347(95)00428-9. [DOI] [PubMed] [Google Scholar]
- 4.Batty K T, Ilett K F, Davis T M E. Chemical stability of artesunate injection and proposal for its administration by intravenous infusion. J Pharm Pharmacol. 1996;48:22–26. doi: 10.1111/j.2042-7158.1996.tb05870.x. [DOI] [PubMed] [Google Scholar]
- 5.Batty K T, Ilett K F, Edwards G, Powell S M, Maggs J L, Park B K, Davis T M E. Assessment of the effect of malaria infection on hepatic clearance of dihydroartemisinin using rat liver perfusions and microsomes. Br J Pharmacol. 1998;125:159–167. doi: 10.1038/sj.bjp.0702023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batty K T, Thu L T A, Davis T M E, Ilett K F, Mai T X, Hung N C, Tien N P, Powell S M, Thien H V, Binh T Q, Kim N V. A pharmacokinetic and pharmacodynamic study of intravenous versus oral artesunate in uncomplicated falciparum malaria. Br J Clin Pharmacol. 1998;45:123–129. doi: 10.1046/j.1365-2125.1998.00655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batty K T, Thu L T A, Ilett K F, Tien N P, Powell S M, Hung N C, Mai T X, Chon V V, Thien H V, Binh T Q, Kim N V, Davis T M E. A pharmacokinetic and pharmacodynamic study of artesunate for vivax malaria. Am J Trop Med Hyg. 1999;59:823–827. doi: 10.4269/ajtmh.1998.59.823. [DOI] [PubMed] [Google Scholar]
- 8.Benakis A, Paris M, Loutan L, Plessas C T, Plessas S. Pharmacokinetics of artemisinin and artesunate after oral administration in healthy volunteers. Am J Trop Med Hyg. 1997;56:17–23. doi: 10.4269/ajtmh.1997.56.17. [DOI] [PubMed] [Google Scholar]
- 9.Bethell D B, Teja-Isavadharm P, Cao X T, Pham T T, Ta T T, Tran T N, Nguyen T T, Pham T P, Kyle D, Day N P, White N J. Pharmacokinetics of oral artesunate in children with moderately severe Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg. 1997;91:195–198. doi: 10.1016/s0035-9203(97)90222-4. [DOI] [PubMed] [Google Scholar]
- 10.Brewer T G, Grate S J, Peggins J O, Weina P J, Petras J M, Levine B S, Heiffer M H, Schuster B G. Fatal neurotoxicity of arteether and artemether. Am J Trop Med Hyg. 1994;51:251–259. doi: 10.4269/ajtmh.1994.51.251. [DOI] [PubMed] [Google Scholar]
- 11.Bunnag D, Kanda T, Karbwang J, Thimasarn K, Pungpak S, Harinasuta T. Artemether or artesunate followed by mefloquine as a possible treatment for multidrug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1996;90:415–417. doi: 10.1016/s0035-9203(96)90529-5. [DOI] [PubMed] [Google Scholar]
- 12.Classen W, Altmann B, Gretener P, Souppart C, Skelton-Stroud P, Krinke G. Differential effects of orally versus parenterally administered qinghaosu derivative artemether in dogs. Exp Toxic Pathol. 1999;51:507–516. doi: 10.1016/S0940-2993(99)80128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis T M E, Breheny F X, Kendall P A, Daly F, Batty K T, Singh A, Ilett K F. Severe falciparum malaria with hyperparasitaemia treated with intravenous artesunate. Med J Aust. 1997;166:416–418. doi: 10.5694/j.1326-5377.1997.tb123192.x. [DOI] [PubMed] [Google Scholar]
- 14.Davis T M E, Edwards G, McCarthy J S. Artesunate and cerebellar dysfunction in falciparum malaria. N Engl J Med. 1997;337:11. doi: 10.1056/NEJM199709113371116. [DOI] [PubMed] [Google Scholar]
- 15.Davis T M E, White N J, Looareesuwan S, Silamut K, Warrell D A. Quinine pharmacokinetics in cerebral malaria: predicted plasma concentrations after rapid intravenous loading using a two-compartment model. Trans R Soc Trop Med Hyg. 1988;82:542–547. doi: 10.1016/0035-9203(88)90498-1. [DOI] [PubMed] [Google Scholar]
- 16.de Vries P J, Dien T K. Clinical pharmacology and therapeutic potential of artemisinin and its derivatives in the treatment of malaria. Drugs. 1996;52:818–836. doi: 10.2165/00003495-199652060-00004. [DOI] [PubMed] [Google Scholar]
- 17.Duarte E C, Fontes C J, Gyorkos T W, Abrahamowicz M. Randomized controlled trial of artesunate plus tetracycline versus standard treatment (quinine plus tetracycline) for uncomplicated Plasmodium falciparum malaria in Brazil. Am J Trop Med Hyg. 1996;54:197–202. doi: 10.4269/ajtmh.1996.54.197. [DOI] [PubMed] [Google Scholar]
- 18.Elias Z, Bonnet E, Marchou B, Massip P. Neurotoxicity of artemisinin: possible counseling and treatment of side-effects. Clin Infect Dis. 1999;28:1330–1331. doi: 10.1086/517789. [DOI] [PubMed] [Google Scholar]
- 19.Hassan Alin M, Kihamia C M, Bjorkman A, Bwijo B A, Premji Z, Mtey G J, Ashton M. Efficacy of oral and intravenous artesunate in male Tanzanian adults with Plasmodium falciparum malaria and in vitro susceptibility to artemisinin, chloroquine, and mefloquine. Am J Trop Med Hyg. 1995;53:639–645. doi: 10.4269/ajtmh.1995.53.639. [DOI] [PubMed] [Google Scholar]
- 20.Hien T T, Arnold K, Vinh H, Cuong B M, Phu N H, Chau T T, Hoa N T, Chuong L V, Mai N T, Vinh N N. Comparison of artemisinin suppositories with intravenous artesunate and intravenous quinine in the treatment of cerebral malaria. Trans R Soc Trop Med Hyg. 1992;86:582–583. doi: 10.1016/0035-9203(92)90137-2. [DOI] [PubMed] [Google Scholar]
- 21.Hien T T, Phu N H, Mai N T H, Chau T T, Trang T T, Loc P P, Cuong B M, Dung N T, Vinh H, Waller D, White N J. An open randomized comparison of intravenous and intramuscular artesunate in severe falciparum malaria. Trans R Soc Trop Med Hyg. 1992;86:584–585. doi: 10.1016/0035-9203(92)90138-3. [DOI] [PubMed] [Google Scholar]
- 22.Hien T T, White N J. Qinghaosu. Lancet. 1993;341:603–608. doi: 10.1016/0140-6736(93)90362-k. [DOI] [PubMed] [Google Scholar]
- 23.Kamchonwongpaisan S, McKeever P, Hossler P, Ziffer H, Meshnick S R. Artemisinin neurotoxicity: neuropathology in rats and mechanistic studies in vitro. Am J Trop Med Hyg. 1997;56:7–12. doi: 10.4269/ajtmh.1997.56.7. [DOI] [PubMed] [Google Scholar]
- 24.Karbwang J, Na-Bangchang K, Thanavibul A, Bunnag D, Chongsuphajaisiddhi T, Harinasuta T. Comparison of oral artesunate and quinine plus tetracycline in acute uncomplicated falciparum malaria. Bull WHO. 1994;72:233–238. [PMC free article] [PubMed] [Google Scholar]
- 25.Karbwang J, Na-Bangchang K, Thanavibul A, Ditta-in M, Bunnag D, Harinasuta T. Comparative clinical trial of artesunate and the combination of artesunate-mefloquine in multidrug-resistant falciparum malaria. Clin Drug Investig. 1996;11:84–89. [Google Scholar]
- 26.Looareesuwan S, Viravan C, Vanijanonta S, Wilairatna P, Charoenlarp P, Andrial M. Randomised trial of artesunate and mefloquine alone and in sequence for acute uncomplicated falciparum malaria. Lancet. 1992;339:821–824. doi: 10.1016/0140-6736(92)90276-9. [DOI] [PubMed] [Google Scholar]
- 27.Looareesuwan S, Viravan C, Vanijanonta S, Wilairatana P, Pitisuttithum P, Andrial M. Comparative clinical trial of artesunate followed by mefloquine in the treatment of acute uncomplicated falciparum malaria: two- and three-day regimens. Am J Trop Med Hyg. 1996;54:210–213. doi: 10.4269/ajtmh.1996.54.210. [DOI] [PubMed] [Google Scholar]
- 28.Luxemburger C, Nosten F, Raimond S D, Chongsuphajaisiddhi T, White N J. Oral artesunate in the treatment of uncomplicated hyperparasitemic falciparum malaria. Am J Trop Med Hyg. 1995;53:522–525. doi: 10.4269/ajtmh.1995.53.522. [DOI] [PubMed] [Google Scholar]
- 29.Luxemburger C, ter Kuile F O, Nosten F, Dolan G, Bradol J H, Phaipun L, Chongsuphajaisiddhi T, White N J. Single day mefloquine-artesunate combination in the treatment of multi-drug resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1994;88:213–217. doi: 10.1016/0035-9203(94)90303-4. [DOI] [PubMed] [Google Scholar]
- 30.Miller L G, Panosian C B. Ataxia and slurred speech after artesunate treatment for falciparum malaria. N Engl J Med. 1997;336:1328. doi: 10.1056/NEJM199705013361818. [DOI] [PubMed] [Google Scholar]
- 31.Miners J O, Mackenzie P I. Drug glucuronidation in humans. Pharmacol Ther. 1991;51:347–369. doi: 10.1016/0163-7258(91)90065-t. [DOI] [PubMed] [Google Scholar]
- 32.Mulder G J. Glucuronidation and its role in regulation of biological activity of drugs. Ann Rev Pharmacol Toxicol. 1992;32:25–49. doi: 10.1146/annurev.pa.32.040192.000325. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen T H, Day N P, Ly V C, Waller D, Nguyen H P, Bethell D B, Tran T H, White N J. Post-malaria neurological syndrome. Lancet. 1996;348:917–921. doi: 10.1016/s0140-6736(96)01409-2. [DOI] [PubMed] [Google Scholar]
- 34.Price R N, Nosten F, Luxemburger C, Van Vugt M, Phaipun L, Chongsuphajaisiddhi T, White N J. Artesunate versus artemether in combination with mefloquine for the treatment of multidrug-resistant falciparum malaria. Trans R Soc Trop Med Hyg. 1995;89:523–527. doi: 10.1016/0035-9203(95)90094-2. [DOI] [PubMed] [Google Scholar]
- 35.Smith S L, Fishwick J, McLean W G, Edwards G, Ward S A. Enhanced in vitro neurotoxicity of artemisinin derivatives in the presence of haemin. Biochem Pharmacol. 1997;53:5–10. doi: 10.1016/s0006-2952(96)00591-6. [DOI] [PubMed] [Google Scholar]
- 36.Thomann P. Non-compartmental analysis methods. In: Heinzel G, Woloszczak R, Thomann P, editors. Topfit version 2.0; pharmacokinetic and pharmacodynamic data analysis system for the PC. Stuttgart, Germany: Gustav Fischer; 1993. [Google Scholar]
- 37.Warrell D A, Molyneux M E, Beales P F. Severe and complicated malaria (2nd edition) Trans R Soc Trop Med Hyg. 1990;84(Suppl. 2):1–65. [Google Scholar]
- 38.White N J. Clinical pharmacokinetics and pharmacodynamics of artemisinin and derivatives. Trans R Soc Trop Med Hyg. 1994;88(Suppl. 1):S41–S43. doi: 10.1016/0035-9203(94)90471-5. [DOI] [PubMed] [Google Scholar]
- 39.White N J, Looareesuwan S, Warrell D A, Warrell M J, Bunnag D, Harinasuta T. Quinine pharmacokinetics and toxicity in cerebral and uncomplicated falciparum malaria. Am J Med. 1982;73:564–572. doi: 10.1016/0002-9343(82)90337-0. [DOI] [PubMed] [Google Scholar]
- 40.Yang S D, Ma J M, Sun J H, Chen D X, Song Z Y. Clinical pharmacokinetics of a new effective antimalarial artesunate, a qinghaosu derivative. Chin J Clin Pharmacol. 1985;1:106–109. [Google Scholar]
- 41.Zhao K C, Chen Z X, Lin B L, Guo X B, Li G Q, Song Z Y. Studies on the phase 1 clinical pharmacokinetics of artesunate and artemether. Chin J Clin Pharmacol. 1988;4:76–81. [Google Scholar]