Abstract
Genetic defects in the SHANK2 gene, encoding for synaptic scaffolding protein, are associated with a variety of neurodevelopmental conditions, including autism spectrum disorders and mild to moderate intellectual disability. Until now, limited patient clinical descriptions have been published. Only 13 unrelated patients with SHANK2 pathogenic variations or microdeletions have been reported worldwide. By Exome Sequencing, we identified a de novo stop-gain variant, c.334C>T, p.(Gln112*), in an Italian patient with a neurodevelopmental disorder. The patient (9 years old) presented the following facial features: a flat profile, thick eyebrows, long eyelashes, a bulbous nasal tip and a prominent columella, retracted ears, dental anomalies. The patient showed speech delay and mild neuromotor delay but not autism spectrum disorder. In conclusion, this patient with a novel pathogenic variant in SHANK2 enlarges the phenotypic spectrum of SHANK2-mutated patients and demonstrates that the severity of SHANK2-associated disorders is highly variable.
Keywords: SHANK2, neurodevelopmental disorder, language impairment, exome sequencing (ES)
1. Introduction
Neurodevelopmental disorders (NDDs) are a group of heterogeneous conditions affecting 2–5% of children and include autism spectrum disorder (ASD), intellectual disability (ID), developmental delay (DD), and epilepsy [1]. Different phenotypes often coexist in the same patient, thus making classification difficult. The genetic etiology underlying NDDs is highly heterogeneous, with varying degrees of genetic overlap and penetrance or expressivity across the phenotypes [2,3,4,5,6,7,8,9,10].
Genes of the SHANK (SH3 and multiple ankyrin repeat domains protein) family (comprising SHANK1, SHANK2 and SHANK3) have been linked to a spectrum of neurodevelopmental disorders. The SHANK2 gene encodes for a postsynaptic scaffolding protein at glutamatergic synapses in the brain, essential for proper synapse formation, development and plasticity [11,12]. By genome-wide microarray analysis for copy number variants (CNVs) in a German cohort of 184 unrelated individuals with ID and a series of 396 Canadian individuals with ASD, Berkel et al. found one patient in each cohort who had a de novo deletion in the SHANK2 gene [13]. In the SFARI gene database, SHANK2 is categorized as a high-confidence autism risk gene. Shank2 mutant mice exhibit ASD-like behavioral deficits and synaptic dysfunctions [14]. To date, only 13 SHANK2 mutated patients have been described worldwide, and thus the phenotypic spectrum of the condition is only partially described [15,16,17,18,19,20,21,22,23,24]. Among these patients, all individuals carried de novo variants, six variants resulting in a premature stop codon, and five microdeletions encompassing the gene. All patients had mild to moderate ID, and autism was reported in nearly all of them. There are also reported cases with rare missense SHANK2 variants inherited from unaffected parents, suggesting the existence of low-penetrance alleles [13].
In the present study, we performed clinical exome sequencing in a patient with a neurodevelopmental disorder and found a de novo SHANK2 pathogenic variant, c.334C>T, p.(Gln112*). Interestingly, the patient showed mild intellectual disability and speech delay, without any signs of ASD, expanding the phenotypic spectrum associated with SHANK2 mutations.
2. Materials and Methods
2.1. Sample and DNA Extraction
Peripheral blood samples were collected in EDTA tubes from the proband and her parents at the Medical Genetics Unit of the Azienda Ospedaliera Universitaria Senese (A.O.U.S, Siena, Italy). Following written informed consent for both diagnostic and research purposes, genomic DNA samples were extracted using MagCore HF16 (Diatech Lab Line, Jesi, Ancona, Italy).
2.2. Exome Sequencing
Exome sequencing was performed on genomic DNA samples of the proband and both parents.
Library preparation and exome capture were obtained using the Illumina Nextera Flex for Enrichment KIT according to the manufacture’s protocol, and sequencing was performed using the Illumina NovaSeq 6000 platform (Illumina San Diego, CA, USA). Alignment of raw paired-end reads to the reference genome (version hg19) was performed with BWA Enrichment (v2.1.2). Variant calling was obtained using the Genome Analysis ToolKit (GATK).
2.3. Filtering and Variant Prioritization
Exome sequencing data were filtered using eVai software (enGenome v2.3). Variants’ prioritization was obtained by using increasingly enlarged filters: (i) phenotype, using the HPO terms Delayed speech and language developmental (HP: 0000750) and Intellectual Disability (HP: 0001249); (ii) classification (pathogenic, likely pathogenic and uncertain); (iii) effect (frameshift, stop-gain, splice site and missense variants).
All variants were screened according to their frequency, location, mutation category, literature, and mutation database data (ClinVar database, LOVD database, HGMD database). Polymorphisms (minor allele frequency, MAF < 0.01) were excluded, and synonymous variants were assumed to be benign or likely benign. Missense variants were predicted to be damaging by CADD-Phred prediction tools for functional effect prediction. Frameshift, stop-gain, and splice site variants were prioritized as pathogenic. The following public databases were used for the interpretation of the variants: ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/, accessed on 10 February 2022), LOVD (https://databases.lovd.nl/shared/genes, accessed on 10 February 2022), the Human Genome Mutation Database (HGMD, http://www.hgmd.cf.ac.uk/ac/index.php3, accessed on 10 February 2022), Varsome (https://varsome.com/ accessed on 10 February 2022).
Finally, for the interpretation of the variants, the American College of Medical Genetics and Genomics (ACMG) 2015 guidelines were used [25].
2.4. Sanger Sequencing
To confirm the SHANK2 variant, we performed Sanger sequencing of exon 3, including the flanking intron sequences of the gene (NM_133266.3) in the proband and her parents. DNA was amplified by PCR using specific primer pairs. Subsequently, the purified PCR products were applied to Sanger sequencing to affirm the mutation, using an ABI PRISM3130 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). The Sanger sequencing results were analyzed with Sequencher software V.4.9 (Gene Codes, Ann Arbor, MI, USA).
2.5. Mutation Nomenclature
The mutation is described according to Human Genome Variations Society (HGVS). Nucleotide numbers are derived from the cDNA sequence of SHANK2 (GenBank accession NM_133266.3).
3. Results
3.1. Clinical Description
The patient was a 9-year-old female, the first child of healthy parents.
She was born from an uncomplicated pregnancy at full term. Family history was negative for ASD or other neurodevelopmental disorders. The auxological parameters at birth were: length 51 cm (71° percentile) and weight 3450 g (25°–50° percentile).
The mother reported that the baby showed hypovalid suction. She described the onset of crawling at 12 months, autonomous walking at 15 months, production of single words at 2 years and 6 months and production of simple sentences at 6 years. Sphincter control was acquired at about 3 years. Eye contact with parents has always been good. At the age of about 2 years and 6 months, the girl began psychomotor therapy and speech therapy, with significant benefits. The girl has support at school and shows considerable difficulties in mathematical calculation. Good interaction with peers is reported. Her diet is varied and her sleep–wake rhythm is normal. She underwent audiometric, ophthalmological, brain MRI and EEG evaluation, all of which were normal.
Physical examination showed height of 127 cm (10° percentile), weight of 24 kg (5°–10° percentile) and head circumference of 51 cm (10°–25° percentile). Facial dysmorphisms included: a flat profile, thick eyebrows, long eyelashes, a nose with a bulbous tip and a prominent columella, large and spaced teeth, retracted ears (Figure 1).
Figure 1.
Photographs of the proband showing the main clinical features: thick eyebrows, long eyelashes, a nose with a bulbous tip and a prominent columella, flat profile, retracted ears, large and spaced teeth.
3.2. Genetic Analysis
Exome Sequencing analysis was performed on the patient and her parents. We obtained a mean depth of coverage of 80X. A total of 11.670 genetic variants on average for each sample was yielded.
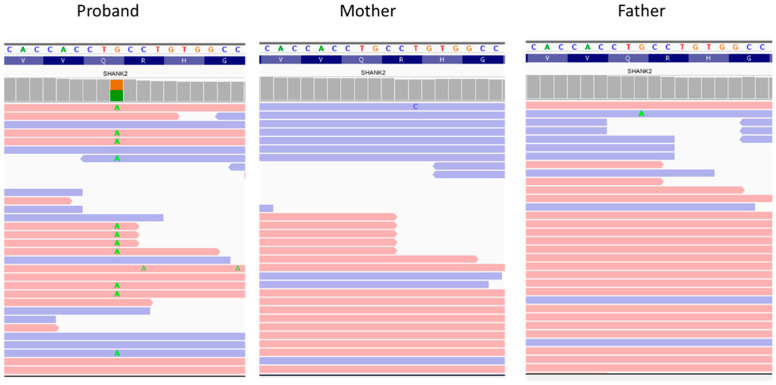
The analysis by classification filtering revealed a de novo stop-gain variant c.334C>T, p.(Gln112*) in exon 3 of the SHANK2 gene on chromosome 11 (Figure 2).
Figure 2.
Visualization of the variant in SHANK2 is show with an integrative genomics viewer. The variant c.334C>T was heterozygous in the proband and absent in the parents.
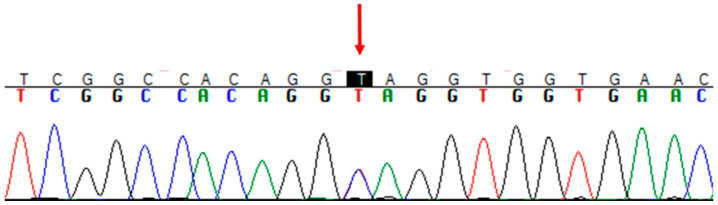
Sanger sequencing confirmed the heterozygous variant in the proband and not in parents (Figure 3). This frameshift deletion has not been previously reported and it is absent in the ExAC and gnomAD databases. The variant has been submitted to the LOVD database (https://www.LOVD.nl, accessed on 10 February 2022) with the following ID: 0000836786.
Figure 3.
Confirmation of the variant c.334C>T, conducted by Sanger sequencing.
4. Discussion
In the present study, we report the identification of one rare patient with a SHANK2 pathogenic variant, c.334C>T, p.(Gln112*), showing a neurodevelopmental disorder. To date, only 13 cases with de novo SHANK2 alterations have been reported worldwide, and the resulting phenotypes are poorly described (Table 1) [15,16,17,18,19,20,21,22,23,24].
Table 1.
Clinical features of the present case compared with the previously reported patients with SHANK2 variants.
| Present Study | Caumes et al., 2020 | Zhou et al., 2019 | Guo et al., 2018 | Bowling et al., 2017 | Marcou et al., 2016 | Leblond et al., 2014 | Leblond et al., 2012 | Wischmeijer et al., 2011 | Pinto et al., 2010 | Berkel et al., 2010 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pat.1 | Pat.1 | Pat.2 | Pat.1 | Pat.1 | Pat.1 | Pat.1 | Pat.1 | Pat.1 | Pat.1 | Pat.1 | Pat.1 | Pat.2 | Pat.3 | |
| AGE | 9 | 6 | 6 | 4 | NA | NA | 12 | NA | 11 | 8 | NA | NA | NA | NA |
| GENDER | F | M | F | M | M | NA | F | M | M | F | M | F | M | M |
| VARIANT | c.334C>T | c.1322del | c.132dup | c.2540_2541del | c.87C>G | c.1896dup | 11q13.2 to 11q13.4 | del_11q13.3q13.4 (all SHANK2 exons) | loss of exon 5-16 | del_11q13.3q13.4 (all SHANK2 exons) | del exon 5-16 | del exon 7 | del exon 7-6 | c.2521C>T |
| p.(Gln112*) | p.(Ile441Thsfs*8) | p.(Asp45Argfs*3) | p.(Ser847*) | p.(Tyr29*) | p.(Asp633Argfs*3) | p.(?) | p.(?) | p.(?) | p.(?) | p.(?) | p.(?) | p.(?) | p.(Arg841*) | |
| PATTERNS OF INHERITANCE | de novo | de novo | de novo | de novo | de novo | de novo | de novo | de novo | de novo | de novo | de novo | de novo | de novo | de novo |
| SPEECH DELAY | + | + | + | + | + | + | + | + | + | + | + | + | + | NA |
| AUTISM/ASD | − | ASD | AUTISM | ASD | ASD | NA | NA | AUTISM | AUTISM | AUTISM | AUTISM | AUTISM | AUTISM | AUTISM |
| ID | − | Mild | Moderate | NA | + | Moderate | Moderate/ Severe |
Severe | Moderate | Moderate/Severe | Mild | Moderate | Mild | Moderate |
| MOTOR DELAY | − | − | − | − | − | NA | + | + | + | + | NA | + | NA | NA |
| NEUROLOGICAL SIGNS | Difficulties in mathematical calculation | Sleep and attention disorders | Anxiety | Sleep disorder | Attentional problems Febrile seizures infancy repetitive behavior | Hyperactivity | − | Oral dyspraxia Signs of cerebellar dysfunction Slight hypotonia | − | NA | Slow reaction and limited mimicry | NA | NA | |
| CLINICAL EXAMINATION | Flat profile, thick eyebrows, long eyelashes, bulbous tip and prominent columella, large and spaced teeth, retracted ears | NA | NA | NA | NA | Microcephalic Mild malar hypoplasia, mild retrognathia. Fine hair. Prominent forehead Bilateral epicanthal folds, long palpebral fissures, deep-set eyes. Broad nasal tip, depressed nasal bridge. Small mouth, down-turned corners. Wide-spaced teeth. Long slender fingers, clinodactyly. Long, slender feet, mild pes planus, piezogenic papules |
Clinodactyly Deep-set eyes strabismus and ptosis. Large ears Retrognathia Wide nasal bridge Thin upper lip | Prominent chin, hypermetropia and astigmatism | Congenital hip dysplasia, downward slanting palpebral fissures, deep-set eyes, ptosis of the left eyelid, long and fine lashes, broad nasal bridge, tubular nose with round overhanging tip and hypoplastic nares, short philtrum, small and thin upper lip, preauricular tag and small low-set simple ears |
Hypermetropia, large and prominent ears, flat feet | NA | NA | NA | |
| MRI | Normal | Normal | Normal | NA | − | NA | Normal | NA | NA | Normal | Normal | NA | NA | NA |
| EEG | Normal | NA | NA | − | − | NA | Normal | NA | Normal | Normal | NA | NA | NA | NA |
* means change in a stop codon; NA, not available; M, male; F, female; +, present; −, absent.
All reported patients had ID, ranging from mild to moderate [15,16,17,18,19,20,21,22,23,24]. In accordance, the girl described here showed mild intellectual disability. Nearly all previous patients showed autism, while our case does not show any sign of ASD. Our patient manifested language delay, and this represents a key feature of the condition being present in all reported patients [15,16,17,18,19,20,21,22,23,24]. Additional neurologic features such as anxiety or repetitive behavior were frequently observed but were absent in the present case [13,15,16,21,22,23,24]. The delineation of a common facial phenotype is hampered by the lack of facial descriptions in previous papers. Here, the patient shows a flat profile, thick eyebrows, long eyelashes, a nose with a bulbous tip and a prominent columella, large and spaced teeth, retracted ears.
This variant, c.334C>T, p.(Gln112*), in SHANK2 is an early truncating variant with abolishment of the major domains of the protein (SH3, PDZ domain, Proline-rich region, SAM). In accordance with the present case, SHANK2 truncating variants are all de novo events in affected individuals. Only SHANK2 missense variants found in patients with NDD are inherited from healthy parents and probably represent low-penetrance alleles [13]. Dhaliwal et al. identified a missense variant in the SHANK2 gene in association with multiple inherited variants in other ASD-associated genes (RELN, SHANK2, DLG1, SCN10A, KMT2C and ASH1L) in a family with three affected children and hypothesized that additional genetic/epigenetic factors together might be necessary to develop ASD [26]. Moreover recent studies support that genetic defects in the SHANK2 gene are implicated in neuropsychiatric disorders [27,28,29]. The mutation type as well as the presence of modifiers in the genome can be fundamental. Leblond and colleagues recently identified CNVs as putative modifiers of the phenotype in accordance with a “multiple-hit model” [18]. Additional studies are necessary to delineate more robust genotype–phenotype correlations.
In Shank2 mutant mouse models, anxiety-like behavior, hyperactivity, abnormal social behavior and dysregulation of synaptic molecules have been observed in specific regions of the brain [30].
A possible pathogenic mechanism has been elucidated by the use of human induced pluripotent stem cells (hiPSCs). Lutz et al. generated hiPSCs from a patient carrying a heterozygous deletion of SHANK2 and from the unaffected parents. They observed a reduction in growth cone size and a transient increase in neuronal soma size during neuronal maturation in patients’ derived cells. Furthermore, the extracellular signal-regulated kinase (ERK) pathway resulted dysregulated [31]. Another hiPSC study showed that human neurons with ASD-associated haploinsufficient SHANK2 mutations had increased synaptic connectivity relative to controls [32]. These findings reinforce the hypothesis of SHANK2 involvement in neurodevelopmental disorders. In addition, the generation of animal models and hiPSC studies are important to discover and design therapeutic strategies.
Regarding the treatment of ASD, the supplementation of zinc (Zn) is a possible preventive strategy. Zinc is a metal involved in the developmental and functioning of the central nervous system (CNS) and is crucial for normal cognitive functions and emotional behaviors. Zn is also responsible for maintaining viable synapses by stabilizing SHANK2/3 postsynaptic scaffolding proteins [33,34]. Zn deficiency is a risk factor that may contribute to the pathophysiology of neurodevelopmental disorders such as ASD. Zinc supplements improved the behavioral deficits in animal models of ASD and in Shank3-mutated animals [35]. Clinical trials are still needed to validate the beneficial therapeutic effects of zinc supplements in ASD patients.
In conclusion, our findings underline that ASD is not necessarily a constant clinical feature in SHANK2-mutated patients and, in accordance with previous literature, indicate language impairment as a major feature in these patients. A peculiar facial phenotype may be observed, but additional studies with comprehensive clinical descriptions are required.
Acknowledgments
The authors thank the family for participating in the study.
Author Contributions
G.D. performed the experiments, made important contributions to the interpretation of the molecular results and drafted the manuscript. A.F. and M.A.M. conducted the genetic counseling to the family and drafted the manuscript. V.S. and R.C. performed the neuropsychiatric clinical evaluation. A.R. and F.A. made substantial contributions to conceptions and design and were involved in drafting the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were not required for the study on human participants in accordance with local legislation and institutional requirements.
Informed Consent Statement
The parents gave informed consent for diagnostic testing and research studies.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Boyle C.A., Boulet S., Schieve L.A., Cohen R.A., Blumberg S.J., Yeargin-Allsopp M., Visser S., Kogan M.D. Trends in the prevalence of developmental disabilities in US children, 1997–2008. Pediatrics. 2011;127:1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 2.The Deciphering Developmental Disorders Study Prevalence and architecture of de novo mutations in developmental disorders. Nature. 2017;542:433–438. doi: 10.1038/nature21062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilissen C., Hehir-Kwa J.Y., Thung D.T., van de Vorst M., van Bon B.W., Willemsen M.H., Kwint M., Janssen I.M., Hoischen A., Schenck A., et al. Genome sequencing identifies major causes of severe intellectual disability. Nature. 2014;511:344–347. doi: 10.1038/nature13394. [DOI] [PubMed] [Google Scholar]
- 4.Turner T.N., Coe B.P., Dickel D.E., Hoekzema K., Nelson B.J., Zody M.C., Kronenberg Z.N., Hormozdiari F., Raja A., Pennacchio L.A., et al. Genomic patterns of de novo mutation in simplex autism. Cell. 2017;171:710–722. doi: 10.1016/j.cell.2017.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samocha K.E., Robinson E.B., Sanders S.J., Stevens C., Sabo A., McGrath L.M., Kosmicki J.A., Rehnstrom K., Mallick S., Kirby A., et al. A framework for the interpretation of de novo mutation in human disease. Nat. Genet. 2014;46:944–950. doi: 10.1038/ng.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Iossifov I., O’Roak B.J., Sanders S.J., Ronemus M., Krumm N., Levy D., Stessman H.A., Witherspoon K.T., Vives L., Patterson K.E., et al. The contribution of de novo coding mutations to autism spectrum disorder. Nature. 2014;515:216–221. doi: 10.1038/nature13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krumm N., Turner T.N., Baker C., Vives L., Mohajeri K., Witherspoon K., Raja A., Coe B.P., Stessman H.A., He Z.X., et al. Excess of rare, inherited truncating mutations in autism. Nat. Genet. 2015;47:582–588. doi: 10.1038/ng.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Roak B.J., Vives L., Girirajan S., Karakoc E., Krumm N., Coe B.P., Levy R., Ko A., Lee C., Smith J.D., et al. Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature. 2012;485:246–250. doi: 10.1038/nature10989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders S.J., Murtha M.T., Gupta A.R., Murdoch J.D., Raubeson M.J., Willsey A.J., Ercan-Sencicek A.G., DiLullo N.M., Parikshak N.N., Stein J.L., et al. De novo mutations revealed by whole-exome sequencing are strongly associated with autism. Nature. 2012;485:237–241. doi: 10.1038/nature10945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The Deciphering Developmental Disorders Study Large-scale discovery of novel genetic causes of developmental disorders. Nature. 2015;519:223–228. doi: 10.1038/nature14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sheng M., Sala C. PDZ domains and the organization of supramolecular complexes. Annu. Rev. Neurosci. 2001;24:1–29. doi: 10.1146/annurev.neuro.24.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Boeckers T.M., Bockmann J., Kreutz M.R., Gundelfnger E.D. ProSAP/Shank proteins—A family of higher order organizing molecules of the postsynaptic density with an emerging role in human neurological disease. J. Neurochem. 2002;81:903–910. doi: 10.1046/j.1471-4159.2002.00931.x. [DOI] [PubMed] [Google Scholar]
- 13.Berkel S., Marshall C.R., Weiss B., Howe J., Roeth R., Moog U., Endris V., Roberts W., Szatmari P., Pinto D., et al. Mutations in the SHANK2 synaptic scaffolding gene in autism spectrum disorder and mental retardation. Nat. Genet. 2010;42:489–491. doi: 10.1038/ng.589. [DOI] [PubMed] [Google Scholar]
- 14.Jiang Y.H., Ehlers M.D. Modeling autism by SHANK gene mutations in mice. Neuron. 2013;78:8–27. doi: 10.1016/j.neuron.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berkel S., Tang W., Treviño M., Vogt M., Obenhaus H.A., Gass P., Scherer S.W., Sprengel R., Schratt G., Rappold G.A. Inherited and de novo SHANK2 variants associated with autism spectrum disorder impair neuronal morphogenesis and physiology. Hum. Mol. Genet. 2012;21:344–357. doi: 10.1093/hmg/ddr470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinto D., Pagnamenta A.T., Klei L., Anney R., Merico D., Regan R., Conroy J., Magalhaes T.R., Correia C., Abrahams B.S., et al. Functional impact of global rare copy number variation in autism spectrum disorders. Nature. 2010;466:368–372. doi: 10.1038/nature09146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wischmeijer A., Magini P., Giorda R., Gnoli M., Ciccone R., Cecconi L., Franzoni E., Mazzanti L., Romeo G., Zuffardi O., et al. Olfactory Receptor-Related Duplicons Mediate a Microdeletion at 11q13.2q13.4 Associated with a Syndromic Phenotype. Mol. Syndromol. 2011;1:176–184. doi: 10.1159/000322054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leblond C.S., Heinrich J., Delorme R., Proepper C., Betancur C., Huguet G., Konyukh M., Chaste P., Ey E., Rastam M., et al. Genetic and functional analyses of SHANK2 mutations suggest a multiple hit model of autism spectrum disorders. PLoS Genet. 2012;8:e1002521. doi: 10.1371/journal.pgen.1002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leblond C.S., Nava C., Polge A., Gauthier J., Huguet G., Lumbroso S., Giuliano F., Stordeur C., Depienne C., Mouzat K., et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A gradient of severity in cognitive impairments. PLoS Genet. 2014;10:e1004580. doi: 10.1371/journal.pgen.1004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcou C.A., Studinski Jones A.L., Murphree M.I., Kirmani S., Hoppman N.L. De novo 11q deletion including SHANK2 in a patient with global developmental delay. Am. J. Med. Genet. A. 2017;173:801–805. doi: 10.1002/ajmg.a.38075. [DOI] [PubMed] [Google Scholar]
- 21.Bowling K.M., Thompson M.L., Amaral M.D., Finnila C.R., Hiatt S.M., Engel K.L., Cochran J.N., Brothers K.B., East K.M., Gray D.E., et al. Genomic diagnosis for children with intellectual disability and/or developmental delay. Genome Med. 2017;9:43. doi: 10.1186/s13073-017-0433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo H., Wang T., Wu H., Long M., Coe B.P., Li H., Xun G., Ou J., Chen B., Duan G., et al. Inherited and multiple de novo mutations in autism/developmental delay risk genes suggest a multifactorial model. Mol. Autism. 2018;9:64. doi: 10.1186/s13229-018-0247-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou W.Z., Zhang J., Li Z., Lin X., Li J., Wang S., Yang C., Wu Q., Ye A.Y., Wang M., et al. Targeted resequencing of 358 candidate genes for autism spectrum disorder in a Chinese cohort reveals diagnostic potential and genotype-phenotype correlations. Hum. Mutat. 2019;40:801–815. doi: 10.1002/humu.23724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caumes R., Smol T., Thuillier C., Balerdi M., Lestienne-Roche C., Manouvrier-Hanu S., Ghoumid J. Phenotypic spectrum of SHANK2-related neurodevelopmental disorder. Eur. J. Med. Genet. 2020;63:104072. doi: 10.1016/j.ejmg.2020.104072. [DOI] [PubMed] [Google Scholar]
- 25.Richards S., Aziz N., Bale S., Bick D., Das S., Gastier-Foster J., Grody W.W., Hegde M., Lyon E., Spector E., et al. ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhaliwal J., Qiao Y., Calli K., Martell S., Race S., Chijiwa C., Glodjo A., Jones S., Rajcan-Separovic E., Scherer S., et al. Contribution of Multiple Inherited Variants to Autism Spectrum Disorder (ASD) in a Family with 3 Affected Siblings. Genes. 2021;12:1053. doi: 10.3390/genes12071053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eltokhi A., Gonzalez-Lozano M.A., Oettl L.L., Rozov A., Pitzer C., Röth R., Berkel S., Hüser M., Harten A., Kelsch W., et al. Imbalanced post- and extrasynaptic SHANK2A functions during development affect social behavior in SHANK2-mediated neuropsychiatric disorders. Mol. Psychiatry. 2021;26:6482–6504. doi: 10.1038/s41380-021-01140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han K.A., Yoon T.H., Shin J., Um J.W., Ko J. Differentially altered social dominance- and cooperative-like behaviors in Shank2- and Shank3-mutant mice. Mol. Autism. 2020;11:87. doi: 10.1186/s13229-020-00392-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang H., Wang D., Chen J., Li X., Yi Q., Shi Y. Identification of SHANK2 Pathogenic Variants in a Chinese Uygur Population with Schizophrenia. J. Mol. Neurosci. 2021;71:1–8. doi: 10.1007/s12031-020-01606-8. [DOI] [PubMed] [Google Scholar]
- 30.Lee Y.S., Yu N.K., Chun J., Yang J.E., Lim C.S., Kim H., Park G., Lee J.A., Lee K., Kaang B.K., et al. Identification of a novel Shank2 transcriptional variant in Shank2 knockout mouse model of autism spectrum disorder. Mol. Brain. 2020;13:54. doi: 10.1186/s13041-020-00595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lutz A.K., Pérez Arévalo A., Ioannidis V., Stirmlinger N., Demestre M., Delorme R., Bourgeron T., Boeckers T.M. SHANK2 Mutations Result in Dysregulation of the ERK1/2 Pathway in Human Induced Pluripotent Stem Cells-Derived Neurons and Shank2(−/−) Mice. Front. Mol. Neurosci. 2021;14:773571. doi: 10.3389/fnmol.2021.773571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaslavsky K., Zhang W.B., McCready F.P., Rodrigues D.C., Deneault E., Loo C., Zhao M., Ross P.J., El Hajjar J., Romm A., et al. SHANK2 mutations associated with autism spectrum disorder cause hyperconnectivity of human neurons. Nat. Neurosci. 2019;22:556–564. doi: 10.1038/s41593-019-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grabrucker A.M., Schmeisser M.J., Schoen M., Boeckers T.M. Postsynaptic ProSAP/Shank scaffolds in the cross-hair of synaptopathies. Trends Cell Biol. 2011;21:594–603. doi: 10.1016/j.tcb.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Alsufiani H.M., Alkhanbashi A.S., Laswad N.A.B., Bakhadher K.K., Alghamdi S.A., Tayeb H.O., Tarazi F.I. Zinc deficiency and supplementation in autism spectrum disorder and Phelan-McDermid syndrome. J. Neurosci. Res. 2022;100:970–978. doi: 10.1002/jnr.25019. [DOI] [PubMed] [Google Scholar]
- 35.Baj J., Flieger W., Flieger M., Forma A., Sitarz E., Skórzyńska-Dziduszko K., Grochowski C., Maciejewski R., Karakuła-Juchnowicz H. Autism spectrum disorder: Trace elements imbalances and the pathogenesis and severity of autistic symptoms. Neurosci. Biobehav. Rev. 2021;129:117–132. doi: 10.1016/j.neubiorev.2021.07.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.