Abstract
Over the past decade, multiple sclerosis (MS), a chronic neuroinflammatory disease with severe personal and social consequences, has undergone a steady increase in incidence and prevalence rates worldwide. Despite ongoing research and the development of several novel therapies, MS pathology remains incompletely understood, and the prospect for a curative treatment continues to be unpromising in the near future. A sustained research effort, however, should contribute to a deeper understanding of underlying disease mechanisms, which will undoubtedly yield improved results in drug development. In recent years, the blood–brain barrier (BBB) has increasingly become the focus of many studies as it appears to be involved in both MS disease onset and progression. More specifically, neurovascular unit damage is believed to be involved in the critical process of CNS immune cell penetration, which subsequently favors the development of a CNS-specific immune response, leading to the classical pathological and clinical hallmarks of MS. The aim of the current narrative review is to merge the relevant evidence on the role of the BBB in MS pathology in a comprehensive and succinct manner. Firstly, the physiological structure and functions of the BBB as a component of the more complex neurovascular unit are presented. Subsequently, the authors review the specific alteration of the BBB encountered in different stages of MS, focusing on both the modifications of BBB cells in neuroinflammation and the CNS penetration of immune cells. Finally, the currently accepted theories on neurodegeneration in MS are summarized.
Keywords: blood–brain barrier, multiple sclerosis, neuroinflammation, neurodegeneration, microglia, immune cells
1. The Blood–Brain Barrier—Structure and Physiological Functions
Together with the blood-cerebrospinal fluid (CSF) barrier and the arachnoid barrier, the blood–brain barrier (BBB) plays an essential role in maintaining cerebral homeostasis by regulating, in a highly selective manner, the bidirectional exchanges between the circulatory system and the central nervous system (CNS) [1]. The BBB is a complex structure (Figure 1) comprising endothelial cells of the CNS microvessels and of additional structures that are fundamental for its proper functioning [2]. Furthermore, a myriad of circulatory molecules, including inflammatory factors and hormones, are responsible for ensuring the BBB’s physiological selective permeability [3].
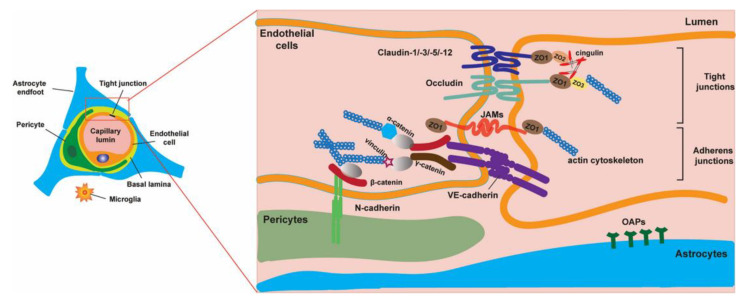
Figure 1.
The cellular and most significant molecular components of the blood–brain barrier as part of the neurovascular unit (Reprinted with permission from Kadry, H., Noorani, B. and Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17, 69 (2020). https://doi.org/10.1186/s12987-020-00230-3) [4].
1.1. Brain Microvascular Endothelial Cells
Several cellular components ensure the structural stability of the BBB, with endothelial cells (ECs) representing the central structural element of the barrier. This type of endothelial tissue is unique, consisting of flattened cells, closely linked together, lining the inside of the blood vessels. ECs are anchored to the basement membrane by cell adhesion molecules (CAMs), belonging to the classes of selectins, the immunoglobulin superfamily, and the integrin family. At the cerebral level, ECs have distinct characteristics, being more specialized compared to ECs located in other organs [5]. Brain microvascular ECs (BMECs) have an increased mitochondrial content [6], a lack of fenestrations [7], minimal pinocytotic activity [8], and the presence of an elaborate network of tight junctions (TJ) and adherens junctions (AJ) between cells [9].
TJs prevent paracellular diffusion and act as a physical barrier to regulate the movement of ions and other molecules between the peripheral blood flow and brain parenchyma. This complex consists of a set of several proteins, the most relevant being the transmembrane proteins (claudin, occludin, and junctional adhesion protein), which, although function independently of each other, work in unison to the effect of joining the plasma membranes of adjacent ECs to seal the paracellular space between them [10]. At the intracellular level, scaffolding proteins zona occludin-1 (ZO-1), ZO-2, and ZO-3 establish a link between the abovementioned transmembrane proteins and the actin cytoskeleton [11,12]. In addition, proteins are also associated with zonula adherens that form the adherens junctions formed by proteins such as the platelet-endothelial cell adhesion molecule (PECAM) and vascular endothelial-cadherin, also called cadherin-5. Forming a zipper-like structure, it regulates intercellular spaces and maintains BBB integrity [13].
Among the many claudin family members, claudin 5, 11, and 12 were reported to be found in the CNS [14]. Occludin, the first transmembrane protein identified at the TJ level, has a high molecular mass and complex structure, its intracellular domains interacting with zonula occludens (ZO) proteins in order to stabilize the TJ [15]. The presence of occludin in other areas of the body (gastric epithelium, testis, bone) and its changes in various pathologies suggest that occludin has other undiscovered roles, along with the simple structural role in the TJ component [16]. Finally, junctional adhesion molecule (JAM) proteins also enter the molecular structure of these structures, interacting with important intracellular elements, presumably zonula occludens (ZO), the structure that in turn interacts with actin, to maintain cytoskeletal integrity, and thus to ensure BBB impermeability [17].
While the TJs at the BBB greatly restrict the paracellular diffusion of many substances from the blood, small gaseous molecules such as O2 and CO2 can diffuse freely through lipid membranes [18]. The presence of specific transport systems on the luminal and abluminal membranes of the capillaries regulates the transcellular traffic of essential molecules between the bloodstream and brain; these include transporters and/or receptors for nutrients [19] and proteins such as insulin, leptin, and transferrin [20]. Restrictive transport across the BBB also results from reduced transcellular transport [21]. Transcytosis is the main process that ensures the transit of both smaller and larger molecules [21], being altered in physiological conditions such as aging [22] and during pathological states [23].
BBB endothelial cells also serve the role of transporting molecules against their concentration gradient. This is achieved via members of the well-known ATP-binding cassette (ABC) transporter superfamily in an energy-dependent process. Important subfamilies of transporters aiding the process of cellular efflux include the permeability glycoprotein (P-gp) and the multidrug resistance protein (MRP) transporters which are found on the luminal side of ECs, albeit there is recent research reporting the presence of transporters on the subendothelial side as well [24]. On the luminal side, MRP1 and MRP2 appear to carry out the translocation of organic non-polar negatively charged ions and HIV protease inhibitors, whereas MRP4 and MRP5 transport cyclic nucleotides alongside organic anions and weak acids [25,26]. Moreover, as part of the ABC transporter superfamily, breast cancer-related proteins (BCRPs) pump out both organic cations, and negatively charged drug molecules, as well as weak organic bases with non-polar regions [27].
Although BMECs with their morphological features represent the physical structure of the barrier, it should be noted that maintenance of the BBB is dependent upon the normal functioning of astrocytes (contacting the brain capillaries through numerous endfeet), pericytes (embedded within the basement membrane that they share with ECs), perivascular microglia, and the basal laminae, which are found in close proximity to the capillary and postcapillary venules of the CNS [28,29].
Therefore, for some years now, the term neurovascular unit (NVU) has been preferred, designating, on the one hand, the fact that several cells enter the BBB component, each having a special role in maintaining the quality of the barrier microenvironment, and, on the other hand, that any pathological change will involve alterations in the function and structure of these components [30,31].
1.2. Smooth Muscle Cells and Brain Pericytes
Smooth muscle cells (SMCs) make up the middle tunic of the blood vessel and, together with the brain pericytes, cover the vascular endothelium. SMCs are contractile cells present in arterioles and venules, whereas brain pericytes are perivascular cells adjacent to brain capillaries belonging to the lineage of SMCs [32]. Brain pericytes are an essential element in the induction and maintenance of the BBB properties [33], increasing the stability of the EC monolayer, and promoting the formation of TJ from the prenatal phase forwards [34]. Pericytes also appear to participate in the metabolic properties of the BBB by inducing the expression of enzymes in ECs [35] or by enhancing the expression of some efflux pumps (MRP6) [36]. Besides modulating vascular contractility, pericytes also synthesize type IV collagen, glycosaminoglycans, and laminin, important components of the basement membrane [37].
Pericytes contribute to the neuroimmune response and are potent modulators of BBB function due to their proximity to ECs. The close brain pericyte–EC association translates into maintaining viable TJ and a low level of transcytosis and CAM expression, thus including the very strict control of leukocyte transshipment at BBB level in physiological conditions [38]. Once the intercellular crosstalk relationships are established, both the pericytes and the EC will produce transforming growth factor beta (TGF-β) which, in turn, triggers the production of adhesion molecules such as laminin, while in the BMECs, it induces cadherin-2 (also known as N-cadherin) that promotes the adherence of pericytes [39]. Moreover, pericytes secrete cytokines and chemokines in cell culture and upregulate cytokines in response to lipopolysaccharide (LPS) [40,41]. They also present antigens in response to interferon (IFN)-γ, which may contribute to T-cell activation [42].
Pericytes serve to protect the endothelium of blood vessels and also ensure high BBB selectivity by sustaining vascular proliferation and development, as well as membrane hyperpolarization. This is thanks to the “intercellular crosstalk” with the other elements of the NVU [43]. New studies [44] question the contractile capacity of brain pericytes to the detriment of SMCs in the arteries and brain capillaries. To ensure neurovascular coupling, it is necessary to regulate blood flow according to the needs of the neuron, as there are variations according to location. The main cells that increase or decrease the vascular lumen remain the SMCs, which regulate blood flow in both healthy and pathological cases. Arteriolar SMCs are arranged on the circumference of the vessel, forming similar structures to bands [34]. Moreover, arteriolar SMCs have been found to express α-SMA in both human and mouse brains, explaining their contractile properties.
1.3. Astrocytes
The abundance of astrocytes in the brain parenchyma, as well as their location, suggest that glial cells have an important role in the NVU. Within the NVU, astrocytes are located in a position (between neurons, pericytes, and capillary ECs) that allows them to both receive neuronal input and communicate intercellularly with the endothelium. Thus, one of the roles is to provide support for the maintenance and repair of surrounding structures, as a great number of chemical mediators which promote the BBB phenotype, such as TGF-β, glial-derived neurotrophic factor (GDNF), basic fibroblast growth factor (bFGF), and angiopoetin-1 (Ang1), are also secreted by astrocytes [45]. Perivascular astrocyte endfeet wrap around endothelial cells and help regulate brain water transport primarily through the expression of aquaporin-4 [46]. Through this structure, the fluid flow at the CNS level is regulated, as is the excretion of toxic substances [47].
Additionally, astrocytes are also able to regulate the expression and localization of various transport proteins and enzyme systems on the endothelium [18]. Experiments using in vitro BBB models (endothelial cells cultured on filter in the presence of astrocytes) or conditioned medium have been shown to induce barrier properties by increasing trans-endothelial electrical resistance [48], or the expression of efflux pumps such as P-gp and some MRPs [36]. On the other hand, some studies suggest endothelial cells secrete factors involved in astrocyte growth and differentiation [49], further underscoring the importance of signaling between astrocytes and endothelial cells. ECs are thought to produce short peptides called endothelin that bind to specific receptors, promoting the production of key factors such as brain-derived neurotrophic factor (BDNF), GDNF, nerve growth factor, and neurotrophin-3 [50].
1.4. Oligodendrocytes
Although not directly involved in the structure of the BBB, the oligodendrocyte and the oligodendrocyte progenitor cell (OPC) seem to play an important role in maintaining BBB integrity. The main function of oligodendrocytes at the CNS level is to ensure correct myelin insulation of the axons. However, several recent studies have highlighted the additional capability of these cells to modulate BBB tightness via different mechanisms. For example, Seo et al. [51] revealed that OPCs are able to upregulate TJ proteins via TGF-β signaling, while Wang et al. [52] suggested Wnt/β-catenin to be the main regulatory pathway for claudin-5 expression in ECs. Extensive research related to the osmotic demyelinating syndrome revealed interesting data regarding the crosstalk between oligodendrocytes and the other cellular components of the BBB. Thus, the intercellular connections of oligodendrocytes with astrocytes situated in their proximity are essential for myelination activity [53], with studies suggesting that BBB leakage leads to damage in astrocytes as the first step [54], with oligodendrocyte alteration being a subsequent phenomenon. Multiple molecular mechanisms including the imbalance between protein synthesis and degradation [55], the impact of aquaporin-1 and aquaporin-4 [56], and finally the role of ionic equilibrium [57] have been proposed; however, the complete role of oligodendrocyte in the maintenance of BBB physiological function remains to be fully explained in the future.
1.5. Microglia
Microglia are considered to be the equivalent of immune cells in the CNS, being both an integral part of NVU structure, and serving the function of immune surveillance. From an embryological point of view, microglia are found in future CNS before EC migration, participating in the modulation of cerebrovascular development [58]. In the case of inflammation, microglia is the key player that will subsequently generate the cellular and humoral inflammatory response, with all the cascade of related reactions.
The link between microglial cells and BMECs and their effects in regulating BBB properties are incompletely explained. Microglia, the cells derived from hematopoietic precursors that migrate into the CNS parenchyma, have a primary immune and defense role in the BBB [59]. In this context, research thus far has focused on the role of microglia in CNS inflammation. Although representing only 10% of the cell population in the CNS, microglia are found in both white and gray matter, playing a central role in neuroinflammation. When these cells are activated, they lose their long processes, while expressing inflammatory cytokines (IL-1, tumor necrosis factor alpha (TNF)), chemokines, prostaglandins, and reactive oxygen species [60]. In addition, the transformation of microglial cells can theoretically follow two pathways, turning into the M1 (pro-inflammatory) or M2 phenotype (with anti-inflammatory valences by releasing vascular endothelial growth factor (VEGF) and chemokines that help repair tissues); however, newer research suggests a very fine border between the two states [61].
1.6. Neurons
In addition to the above-mentioned glial cells, neurons also exert an important direct influence at the BBB level. It is believed that neurons are at a maximum distance of 25 μm from capillaries, being in the vicinity of endothelial cells [62]. Thus, they are dependent on changes in the microenvironment, especially in terms of ionic composition [63]. Neurons regulate blood flow and BBB permeability by promoting tight junctions and downregulating efflux transport [64].
1.7. Extracellular Matrix
Within the NVU there are also relevant non-cellular elements, such as the extracellular matrix (ECM) or basement membrane of capillary cells, whose physiological role is not totally elucidated, but which undergoes changes in case of inflammation, for example, favoring increased BBB permeability and penetration of cells, and potentially toxic substances at the CNS level [65].
The cerebral capillary basement membrane remains the object of study of numerous papers in this area of research, the distinct feature being the increased presence of proteins, at least 30 different types, of which the most abundant are contractin-1, perlecan, agrin, and laminin [66]. Two structurally distinct basement membrane (BM) structures, the vascular BM and the parenchymal BM, secreted by pericytes and astrocytes, respectively, serve the function of a barrier against immune cell entry to the CNS [67]. Laminin α4 and α5 are major components of the vascular BM, whereas laminin α1 and α2 contribute to the structure of the parenchymal BM [68]. These are thought to play a role in controlling/regulating BBB function, including CD4 + lymphocytes acting differently on each membrane [69].
Another barrier to blood cells’ entry into brain parenchyma during inflammation and hemorrhage is the ECM [70]. ECM breakdown by metalloproteinases (MMPs) may contribute to BBB disruption, with consequent leukocyte trafficking, a mark of CNS inflammation [71]. Hyaluronan and its fragments bind Toll-like receptors to favor the neuro-immune environment [72].
1.8. The Physiological Roles of the BBB
Having a surface area between 12 and 18 m2 in adults, the BBB is essential for the bidirectional blood–brain exchange of substances [73].
As the name suggests, the BBB is a real barrier to many substances found in the blood, perhaps beneficial to other organs, but with potentially detrimental effects for sensitive brain tissue [74]. Among the substances that do not physiologically penetrate the BBB are macromolecules; their increased size, along with the polarity of the molecule, are some of the restrictive characteristics of the barrier passage [75]. In connection with this limitation of the entry of numerous substances at the CNS level, we also note the protective role of the BBB. Against both endogenous toxins, resulting from metabolism, and exogenous toxins that penetrate the human body, defense mechanisms enter the circulatory system and have potentially neurotoxic effects [76].
In neurophysiology, ion homeostasis is maintained by the BBB [77]. Specialized protein structures, alongside the Na-K pump, maintain the intra-/extracellular concentration of various electrolytes such as Na, K, Ca, Mg, and Cl—crucial for neuronal metabolism, electrophysiology, and overall adequate cell function [78].
Regarding metabolites and other substances essential for maintaining neuronal functions, these will cross the BBB via passive mechanisms such as diffusion down a concentration gradient, or via the active transport of liposoluble molecules [79].
Another function of the BBB is to adjust and maintain optimal levels of neurotransmitters within the CNS. If out of balance, some neurotransmitters can become neurotoxic. For example, glutamate in high concentrations can irreversibly destroy brain tissue by inducing neuronal death in the penumbra [80].
Finally, another important function of the BBB is that of eliminating toxic and residual end-products resulting from neuronal metabolism. Toxic end products cross the BBB to be released into the bloodstream, and subsequently for delivery to the kidneys or the digestive tract where excretion occurs [81].
Thus, the structurally intact BBB serves crucial roles in CNS physiology. Consequently, any local or systemic disorder that leads to inflammation directly or indirectly alters the structure of the BBB and potentially results in CNS damage (Table 1).
Table 1.
Physiological roles of the BBB and its dysfunctions in pathological conditions.
| Physiological Roles of the BBB | BBB Dysfunctions in Pathological Conditions (including MS) |
|---|---|
| Maintaining of ionic metastasis | Ionic imbalance (neuronal hyperpolarization) |
| Facilitating brain nutrition | Impaired brain nutrition |
| Regulating levels of neurotransmitters | Neurotransmitter imbalance (pathologic inhibition/stimulation) |
| Limiting plasma macromolecules penetrating the brain | Macromolecule leakage |
| Protecting the brain against neurotoxins | Penetration of neurotoxins in the brain |
| Facilitating molecules elimination from the brain | Impaired residual products elimination |
2. The Blood–Brain Barrier in Multiple Sclerosis—From Early Neuroinflammation to Neurodegeneration
BBB dysfunction is an early feature of several CNS pathologies such as autoimmune inflammatory diseases, CNS infections, and neurodegenerative diseases [82]. Multiple sclerosis (MS), a chronic neuroinflammatory CNS disorder, is currently affecting more and more people, with increasing incidence and prevalence observed worldwide [83]. Although many therapeutic options have emerged during the last two decades [84], no curative treatment is currently available. In this context, it is essential for researchers to better understand MS pathogenesis and the role of BBB breakdown in disease onset and evolution.
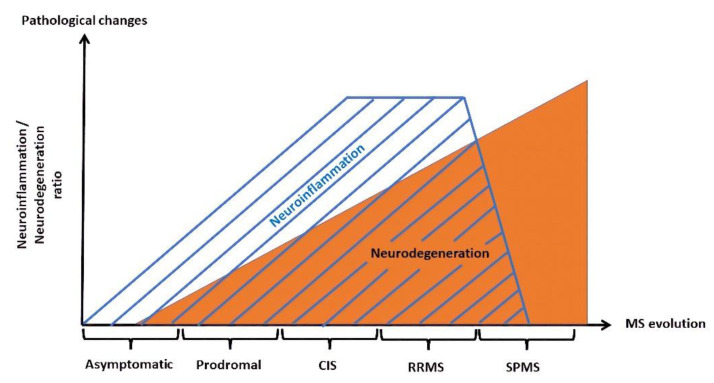
Two main pathological hallmarks are related to MS: early-stage chronic neuroinflammation, and neurodegeneration in the later stages and in particular subtypes of the disease, such as SPMS. Inflammation at the CNS level, also known as neuroinflammation, includes both positive and negative elements for the brain and spinal cord. Among the beneficial aspects, we mention immune surveillance, neuroinflammatory signaling by cytokines such as interleukin-1 (IL-1) and interleukin-4 (IL-4) that help memory and learning, remodeling by inflammation through the M2 microglia and, finally, eustress and “good inflammation” [85]. Another disorder, osmotic demyelinating syndrome, may offer relevant data related to the abovementioned concepts, as ionic rebalancing is the treatment approach for BBB disruption encountered within this syndrome, raising questions as to whether neuroinflammation has a real beneficial impact on pathological CNS conditions.
Neuroinflammation is more frequently associated with its negative aspects, such as those associated with early-stage neurological diseases, including early-stage MS (Figure 2).
Figure 2.
Neuroinflammation and neurodegeneration in multiple sclerosis.
2.1. Consequences of Neuroinflammation at the BBB Level
The BBB is the main structure altered in neuroinflammation, losing its permeability as a result of astrocyte activation, excessive production of cytokines and chemokines, and immune cell infiltration [86]. All cellular and non-cellular components of the BBB are damaged in inflammatory conditions [87] (Table 2).
Table 2.
Overview of alterations of the NVU components in the inflammatory state of multiple sclerosis.
| NVU Components and Other Related Cells Encountered in MS Pathogenesis |
Behavior in the Neuroinflammation Phase of MS | Most Relevant References | |
|---|---|---|---|
| Brain microvascular endothelial cells | Reduction in the expression of TJ proteins Upregulation of different membrane receptors (i.e., Toll-like receptors) Increased production of adhesion molecules Increased pinocytosis Upregulation of glucose transporters (GLUT1) Downregulation of amino acid transporters (LAT1) Alterations of P-glycoprotein expression Degradation of the glycocalyx |
[88,89,90,91,92,93,94,95,96] | |
| Pericytes | Increased production of proinflammatory cytokines and chemokines Differentiation into fibroblasts or phagocytes Secretion of endothelial-disrupting factors |
[97,98,99] | |
| Astrocytes | Increased glutamate production Decreased production of protective factors (angiopoietin-1, Shh, IGF-1) |
[100,101,102] | |
| Oligodendrocytes/oligodendrocyte progenitor cells (OPCs) | Increased expression of inflammatory genes Increased phagocytic capacity Inhibition of OPC recruitment and maturation Increased apoptosis of oligodendrocytes Regional CNS demyelination |
[53,103] | |
| Microglia | Activation of microglia (M1 and/or M2 phenotype) Increased production of inflammatory mediators (IL-1, IL-6, TNF-α), chemokines (CCL2, CX3CL1, MIP-1), matrix metalloproteinases (MMPs), and oxygen free radicals |
[104,105,106] | |
| Neurons | Dendritic transection Axonal transection Apoptosis |
[107] | |
| Immune cells | Th1 CD4 cells | Secretion of IFN-γ and TNF-α | [108] |
| Th17 CD4 cells | Production of IL-17, IL-21, IL-22 | ||
| CD8 T cells | Direct cytotoxic effect Production of IFN-γ and IL-17 |
[109] | |
| B cells | Antibody-dependent mechanisms (secretion of intrathecal IgG) Antibody-independent mechanisms (secretion of cytokines, neurotoxic factors, formation of tertiary lymphoid organs) |
[110] | |
| Neutrophils | Secrete pro-inflammatory cytokines (IL-1β, IL-6, IL-12, TNF-α, and IFN-γ) | [111] | |
| Monocytes | Change in phenotype (CD83 +, CD209 +) Promote the differentiation of Th1 and Th17 cells |
[112] | |
| Mast-cells | Production of TNF-α | [113] | |
2.1.1. Alteration of ECs during Inflammation
The alterations of ECs in inflammation are not limited to intracellular changes, and thus the endothelium must be seen as a whole and considered as a single component. Inflammatory mediators exert their effects on several levels, intervening with and affecting multiple processes. Firstly, they induce paracellular BBB leakage by modulating TJ and AJ proteins. While in physiological conditions ECs are impermeable because of the existence of TJ and AJ, in the setting of inflammation, these connections will be altered, resulting in unwanted BBB permeability [88]. Thus, by increasing the production of VEFG-A, certain inflammatory mediators (IL—1β, IL—6, IL—17, IFN—γ, TNF—α, and chemokine (C-C motif) ligand 2 (CCL2)) will reduce the expression of TJ proteins, such as occludin, claudin—5, ZO—1, and JAM—A, or change the location of these junctional molecules and therefore disrupt the integrity of the BBB [89]. Among the structural changes recorded in the affected TJ proteins, we mention hyperphosphorylation, which will cause alteration of the secondary and tertiary structure of the molecule, with the detachment of the protein chains [90].
Inflammatory mediators can also modulate transcytotic vesicular pathways, causing increased BBB permeability. The complex inflammatory process also alters other characteristics of BMECs, such as changing the concentration and function of different membrane receptors (mainly Toll-like receptors). In so doing, transcellular transporting mechanisms ensure the greater extravasation of immune cells (neutrophils, macrophages, lymphocytes). Regarding receptors and signaling pathways, Toll-like receptors (TLR) are one of the most intensively studied families, with research demonstrating their existence in the physiological conditions of TLR2, TLR3, TLR4, and TLR6 on both rat and human cerebral endothelial cells [91]. During inflammation, significant upregulation is observed, mediated by oxidative stress and TNF-α. More precisely, in the experiments conducted by Nagyoszi et al. [91], reactive oxygen species (ROS) equivalents induced a more than 5-fold increase in the expression of TLR2 and TLR3, an almost 4-fold increase in TLR4, and up to a 10-fold increase in TLR6. TLR2 and TLR3 were also strongly upregulated to TNF-α stimulation by about 10 and 8 times, respectively. The results are in line with other findings in ECs with different localization. For example, inflammatory stimuli also induce the expression of TLR2 receptors in human airway epithelial cells [114], TNF-α being the key player, activating TLR2, TLR4, and TLR6 by their specific ligands, which subsequently can increase the production of TNF, leading to a vicious circle [115]. There is increased vacuolar transendothelial transport as a result of increased pinocytosis [116]. Changes in the function of BMECs lead to the increased production of adhesion molecules and the production of VEGF, which subsequently increases permeability [117]. VEGF’s main function is to increase angiogenesis, increasing blood vessel permeability as part of its angiogenic properties. The increased expression of vascular adhesion molecules on the surface of BMECs means greater leukocyte infiltration into the brain, which maintains inflammation. The infiltration process requires interaction with adhesion proteins in BMECs (i.e., selectins) and anchoring and docking proteins (i.e., Intercellular Adhesion Molecule 1 (ICAM-1)) [92]. Furthermore, in severe cases, inflammation can irreversibly damage BMECs, leading to cell death. Apoptosis is believed to play a role, being reported in animal models of ischemia and in the deprivation of oxygen and glucose [118].
An increase in BBB permeability in the setting of inflammation is also believed to occur due to the modulation of several important transporters (such as the members of the solute carrier superfamily) by inflammatory mediators. In vitro studies have demonstrated a predominantly stimulatory effect of pro-inflammatory cytokines on glucose utilization. In this context, enhanced IL-1β expression and nitrous oxide (NO) production found in hypoxia stimulated the expression of the GLUT1 mRNA [93]. Other glucose transporters are also modulated, as another report has shown that LPS endotoxin enhanced GLUT3 [119]. Moreover, amino-acid transport at the BBB level suffers alteration in inflammatory states, as shown in a recent paper assessing the increase in tryptophan uptake by the transport mechanisms of tryptophan in brain capillary endothelial as an effect of neuroinflammation (LPS and TNF-α as main triggers) [120]. Another in vivo study [94] demonstrated the downregulation of L-type amino acid transporter 1 (LAT1) in inflammatory states, with the thyroid hormone passage being modulated similarly to other important biomolecules (adenosine, insulin) in neuroinflammation. Efflux transporters are also modulated, with P-gp expression and activity suffering changes in cases of neuroinflammation. In vitro, BBB P-gp activity was found to be downregulated after short-term exposure to inflammatory mediators, whereas its activity was upregulated following more prolonged exposure. Results from in vivo studies on human and animal subjects have suggested a link between CNS inflammation, peripheral inflammation, related clinical neuroinflammatory disorders, and alterations in the expression and activity of P-gp at the BBB level [95].
Lastly, subsequent to the degradation of the glycocalyx which occurs during inflammatory states, an increased passage of solutes across the endothelial barrier together with increased leukocyte adhesion to the endothelium were observed [121]. During prolonged inflammatory states, the glycocalyx suffers degradations caused by inflammatory factors, such as cytokines, metalloproteinases, heparinase, and hyaluronidase. Many pre-clinical and clinical studies have demonstrated the association between TNF-α, IL-1β, and IL-6 and an increasing trend in glycocalyx destruction markers (heparin sulfate, hyaluronan, syndecan-1). Nieuwdorp et al. [96] administered endotoxin followed by soluble TNF-α receptor etanercept in healthy male volunteers. By measuring endothelial glycocalyx thickness and other related parameters such as hyaluronan, the authors were able to quantify the amplitude of perturbation and destruction caused by TNF-α at the glycocalyx level. Results were in line with studies conducted on mice, where TNF-α alone, via heparanase activation, was sufficient to induce glycocalyx degradation in septic mice [122]. Elevated levels of IL-6, along with other inflammatory cytokines have also been reported in association with increased glycocalyx destruction, with an up to 2.5-fold increase according to a recently conducted observational trial on patients undergoing cardiac surgery with a cardiopulmonary bypass [123].
Other inflammatory markers that contribute to glycocalyx destruction include the proteolytic enzymes of the MMPs group. MMPs, in the setting of oxidative stress, exert their effects on glycocalyx to increase the expression and proteolytic activity of MMP-2 and MMP-9. This subsequently increases the shedding of syndecan-1 and limits extracellular superoxide dismutase (SOD3) activity [124]. Among glycocalyx destruction markers, glycosaminoglycans (GAGs) represented by heparan sulfate and hyaluronic acid are directly associated with inflammation and destruction intensity, respectively. Thus, because increased plasma levels are encountered in septic shock patients, with plasma levels being correlated to survival prognosis and inflammation severity, heparan sulfate and hyaluronic acid have been reported as having potential biomarker roles in the near future [125].
2.1.2. Pericytes Modification in Inflammatory Conditions
The pericyte to EC ratio of the cerebral capillary is increased compared to similar structures outside the CNS, where permeability is less regulated.
The pericyte also maintains and protects the integrity of the BBB through the secretion of various substances. Some of these include Ang-1, TGF-β1, and other factors involved in the maintenance of the basement membrane [126]. In the setting of inflammation, IL-1β and TNF-α will initiate the inflammatory cascade with downstream synthesis and the release of other proinflammatory cytokines that, in turn, exert their effects on the pericyte, as has been observed in HIV pathology studies [127] and in porcine brain pericytes [97].
In MS animal models, the administration of TNF-α resulted in the modification of pericyte α1/α2 integrin levels, with a subsequent downregulation of α-SMA and slowdown of cell differentiation [98].
Inflammation of the CNS leads to pericyte detachment from ECs with subsequent transformation into phagocytic or fibroblast cells. This pattern is associated with an increase in the number of EC vesicles, TJ disruption, and immune cell recruitment [128]. Furthermore, neuroinflammation is associated with fibrosis, the accumulation of pericyte-derived fibrin and scarring, and neurotoxic consequences that lead to cellular death [43]. Another indicator of pericyte differentiation into fibroblasts is fibroblast activity as measured by fibrin deposits.
The precise mechanisms whereby pericytes moderate inflammation in the CNS have yet to be understood, however. It remains unclear how pericytes interact with leukocytes to aid in their migration across the endothelial barrier. Pericyte detachment from the basement membrane and subsequent acquisition of phenotypical characteristics reminiscent of infiltrating macrophages may play a role [99]. In summary, pericytes appear to contribute to the neuroinflammatory response via: (1) favoring a leaky BBB by secretion of endothelial-disrupting factors or by physical detachment; (2) favoring transport of immune cells and pathogens into the brain; (3) by creating a pro-inflammatory environment locally and also through active recruitment of immune system cells to the site of inflammation.
2.1.3. Astrocyte Modification in Inflammatory Conditions
Through close contact with EC, under physiological conditions, the astrocyte maintains the functional integrity of the BBB and attenuates its damage. This function is possible by producing special substances (astrocyte-derived factors), whose release is inhibited or exacerbated in response to inflammation, with subsequent consequences at the level of BBB permeability [129].
Glutamate, produced mainly by neurons and to a lesser extent by astrocytes, despite being essential in the normal functioning of the CNS, when in excess (cerebral ischemia), will lead to excessive permeability of the BBB following NMDA receptor activation [100].
Protective factors produced by astrocytes have the role of counteracting the destructive effects of the astrocyte factors listed above. Thus, Ang-1 (even exogenously administered in excess) has a protective effect on BBB, causing the upregulation of ZO-1 and occludin in order to repair TJs after permanent ischemic damage in rat models [101]. Ang-1 also suppressed the VEGF-induced expression of ICAM-1 and vascular cell adhesion molecule 1 (VCAM-1), and reduced VEGF-induced leukocyte adhesion to human umbilical vein endothelial cells (HUVECs) [130]. Another protective factor is sonic hedgehog (Shh), which has an anti-apoptotic role in endothelial cells. There is also an effect at the TJ level, where Shh increases the expression of claudin-5, occludin, and ZO-1, promoting the restoration of BBB impermeability [102]. Glial-derived neurotrophic factor (GDNF) and retinoic acid act similarly to TJ proteins, stimulating the production of occludin and ZO-1. Insulin-like growth factor-1 (IGF-1), produced by astrocytes, but also found in other cells of the BBB, helps primarily to maintain the neuronal viability of endothelial cells, maintaining the intact structure of the BBB [131].
Through the released factors, the astrocyte also regulates the level of expression of the endothelial ICAM-1 and VCAM-1 molecules, which interact with integrin α4β1 (VLA-4) and lymphocyte function-associated antigen 1 (LFA-1) in leukocytes. In the context of inflammation, an increased expression of ICAM-1 and VCAM-1 will favor the penetration of leukocytes into the CNS.
2.1.4. Oligodendrocytes and OPCs in Neuroinflammation
Oligodendrocytes and their precursors (OPCs), known to be key factors in the early stages of MS (regional CNS demyelination), have recently gained attention in the context of chronic neuroinflammatory state related to BBB leakage. The intricate multidirectional cellular crosstalk among OPCs, astrocytes, and pericytes is of increasing importance, with cytokines and other inflammatory mediators being the main links within this microenvironment. During neuroinflammation, there is an increased expression of inflammatory genes together with increased phagocytosis in oligodendrocytes, mainly as a result of IFN-γ, IL-6, and IL-1 produced by other BBB cells such as astrocytes or microglia [53]. Another relevant aspect is the increased apoptosis of mature oligodendrocytes, while OPC maturation is heavily suppressed. Finally, newer hypotheses pointing towards oligodendrocyte dysfunction as the missing link between neuroinflammation and neurodegeneration (particularly in progressive MS), are incompletely understood and necessitate further scrutiny [103].
2.1.5. Microglia Activation in Inflammation
In the early phase of acute inflammation, the microglia are activated (within minutes) of the initiation of the CNS charge, the response being a long one, taking up to several weeks. This activation of the microglia means important changes both in terms of cellular phenotype and in terms of secreted substances. Known as the M1 proinflammatory phenotype, the cell will express Iba-1 on its surface and as exogenous products, reminiscent of the heterogeneous group of proinflammatory cytokines (IL-1, IL-6, TNF-α), chemokines (CCL2, C-X3-C motif chemokine ligand 1 (CX3CL1), and macrophage inflammatory protein 1 (MIP-1)), MMPs, and oxygen free radicals [104]. After overcoming the acute phase, the microglia phenotype changes into the anti-inflammatory, phagocytic M2-type, which will lead to angiogenesis and neuroprotection [132].
The BBB-microglia link becomes apparent when considering modifications in the structure and permeability of the BBB resulting from inflammation where microglia play a central role. Microglial activation occurs following neuronal damage, under the action of various damage-associated molecular patterns (DAMPS), a heterogeneous group of protein compounds such as S100 heat-shock proteins [133] or high-mobility group box 1 (HMGB-1) [134], nucleic acids, and ATP acids, and even cytokines such as neuron-derived fractalkines (CX3CL1) [105]. Even elements resulting from the destruction of ECs and the basement membrane of ECs could be DAMPS, leading to activation of microglia [135]. In addition, numerous studies have shown a direct link between microglial dysfunction, neuroinflammation, and the subsequent occurrence of neurodegenerative pathologies such as Alzheimer’s disease (AD) or Parkinson’s disease (PD) [136]. Thus, microglial activity is related to multiple AD modifications, from lipid transport to alterations in transmembrane proteins, cytoskeletal dynamics, and transcription factors. For example, ApoE microglial expression is encountered, explaining the deposition of amyloid beta (Aβ) in altered AD brain. ABCA7, an ATP-binding cassette transporter is presumed to play a role in membrane remodeling which, when altered, is correlated with impaired Aβ phagocytosis in mice [137]. Another example is the IL-1 receptor accessory protein (IL1RAP), a coreceptor with IL1R1 for IL-1 signaling, which enables proinflammatory signal transduction that may exacerbate Tau pathology [138]. Finally, research has also revealed the possible role of the myocyte enhancer factor 2C (MEF2C) transcription factor expressed by the microglia in downregulating the triggering receptor expressed on myeloid cells 2 (TREM2) [139].
The bidirectional relationship between microglia and the rest of the BBB components, both in physiological conditions and in acute or chronic inflammatory pathology, supports the central role of microglia in inflammation. One crosstalk pathway between microglia and the neuron is via fractalkine signaling, with the soluble CX3CL1 compound presumed to keep the microglial phenotype in a neuroprotective state. The disruption of CX3CL1–CX3CR1 signaling leads to microglial response dysregulation and neuronal damage. In AD, this pathway is supposed to be affected differently in the early versus later stages of the disease. In early AD, Aβ accumulation causes a mild decrease in neuron–microglia crosstalk via CX3CL1–CX3CR1 signaling, leading to the enhanced microglial phagocytosis of Aβ. As the disease progresses, the neuron–microglia intercommunication is further exacerbated, causing the dysregulation of microglia and abnormal excitation of the neuron, with subsequent neuron damage and loss [107].
Four theories have been postulated in an attempt to explain the link between BBB destruction and microglial activation: (1) the link factors result from the alteration of BBB cellular elements (EC, astroglia) to microglia [135]; (2) the link factors result from the destruction of pericytes and noncellular components of the BBB to microglia [128]; (3) systemic inflammatory markers pass the altered BBB and activate microglia [138]; and (4) chronic changes in the BBB stimulate the microglia (an effect known as microglia priming).
The effects being bidirectional mean that microglia also produce numerous inflammatory mediators, which influence BBB homeostasis. The most relevant cytokines produced by activated microglia appear to be IL-1 and TNFα. Microglia produce both IL-1α in the acute phase of inflammation (e.g., the first hours after stroke), and IL-1beta, the central element that increases BBB permeability [140]. A similar effect is TNFα produced by microglia, resulting in the downregulation of occludin with a subsequent increase in BBB permeability [141]. On the other hand, M2 microglia produce the same factors which, in this case, have a repairing, proangiogenic effect [106]. Important human M2 microglial markers are CD163 involved in the binding and internalization of the hemoglobin–haptoglobin complex [142], or TREM2, which is thought to be involved in debris clearance [143]. Other markers, such as arginase I implicated in tissue remodeling and wounding healing, or chitinase-like protein 3 (Ym1) likely involved in the degradation of extracellular matrix components [144], were detected in murine microglia, with studies in human microglia still in progress.
2.1.6. Immune Cells, BBB, and Inflammation
BBB structure and function also seem to be altered in the pathological processes which stimulate the migration of different populations of myeloid cells, neutrophils, monocytes, and mast cells [145]. Neutrophils, once in the perivascular space, begin to secrete pro-inflammatory cytokines (IL-1β, IL-6, IL-12, TNF-α, and IFN-γ), with different roles in maintaining inflammation. For example, IL-1β activates local antigen-presenting cells (APCs) which, in turn, reactivate encephalitogenic T cells [111]. T lymphocytes can also be stimulated by reactive oxygen species produced by activated neutrophils [146]. IL-1β also acts on the astrocyte, leading to increased VEGF-A production, resulting in altered TJ, resulting in increased permeability of immune cells and inflammatory mediators to the CNS [147].
Regarding monocytes, their transfer through the BBB is dependent on the homophilic interactions of activated leukocyte cell adhesion molecules (ALCAMs), JAM-A, PECAM-1, and CD99 which, in certain pathologies (e.g., HIV encephalitis), are upregulated [148]. Some monocytes (CD14 +), once migrated through the BBB, will change their phenotype to CD83 + CD209 + under the influence of TGF-β, promoting the differentiation of Th1 and Th17 cells [112]. Mast cells found physiologically in the meninges, choroid plexus, or hypothalamic region, when activated, secrete inflammatory mediators such as histamine, chymase, tryptase, TNF-α, IL-6, and IL-13, directly or indirectly affecting BBB permeability [113]. Histamine acts on H2 receptors at the BMEC level, increasing the permeability of the barrier. TNF-α produced by mast cells promotes neutrophil recruitment and focal ischemia in the CNS [149]. Compounds such as various environmental factors, oxidative stress and many yet to be studied, likely favor the initiation and maintenance of inflammation in the CNS; these will be detailed in another review.
Finally, the role T and B lymphocytes play in promoting neuronal damage in MS has been the focus of several studies, as MS was considered a T-cell mediated disease for a long time [150]. This hypothesis led to the development of one of the most used primary models that induce MS, the experimental autoimmune encephalomyelitis (EAE) model [151]. As members of both MHC class I and II molecules are risk factors for MS, there are also distinct populations of T cells involved in the demyelinating process. One important population consists of the CD4 myelin-reactive T cells which, compared to healthy controls, are more likely to be in an activated state and display a T helper (Th)1 phenotype in MS patients [108]. IFN-γ and TNF-α are the main proinflammatory cytokines secreted by Th1 CD4 T cells, being directly involved in the activation of local glial and antigen-presenting cells (APCs). Another relevant CD4 T cell fraction for MS includes the Th17 phenotype; however, this is found in a much lower proportion than Th1 cells in CSF and peripheral blood [152]. Th17 CD4 T cells produce other important inflammatory factors, including IL-17, IL-21, and IL-22, that subsequently can either promote the expression of other proinflammatory cytokines such as IL-6, which sustain the inflammatory cascade or stimulate the production of granzyme B that directly kills neurons through the glutamate receptor (GluR3) [153].
Another type of T cell that also plays a relevant role in MS pathogenesis is the CD8 T cell fraction. On one hand, CD8 lymphocytes act within MS patients via their well-known cytotoxic function by introducing granzymes into the cytosol of target cells [109]. Several studies in pathology have demonstrated the abundance of CD8 T cells within parenchymal lesions, with reports suggesting a positive association between their rate of detection in plaques, CSF, and peripheral blood and the intensity and destruction of MS lesions [154]. On the other hand, some studies suggest that particular subsets of CD8 cells are able to secrete cytokines, mainly IFN-γ and IL-17, which subsequently directly kill oligodendrocytes [155].
During the last decade, B cells have also been recognized as relevant participants within the immunological model of MS. Antibody-dependent (i.e., secreting intrathecal IgG) and -independent mechanisms are associated with B cell activity, both contributing to MS disease progression. Among antibody-dependent mechanisms, we mention the formation of autoantibodies that target specific CNS structures and the role of B cells in antigen presentation to T cells. Antibody-independent mechanisms consist of the secretion of cytokines and neurotoxic factors, and the formation of tertiary lymphoid organs and structures resembling germinal centers in the meninges of MS patients [110]. The role B cells play in BBB destruction in the context of MS has stimulated research in drug development; this has yielded potent disease-modifying drugs such as Rituximab [156], Ocrelizumab, and Ofatumumab [157], currently available on the market.
2.2. The BBB in Later Stages of MS—Focus on Neurodegeneration
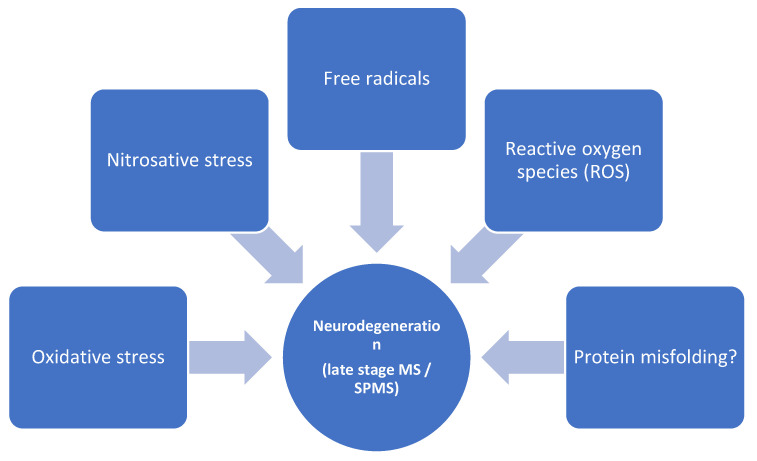
Although encountered in smaller proportions in earlier stages of MS (including clinically isolated syndromes), neurodegeneration gains importance as the disease progresses, characterizing mainly the secondary-progressive form of MS (SPMS). The continuous advancement of disability in the absence of relapses is caused by slow but steady dendrite, axonal, and finally neuronal loss [158]. Though inflammation is considered to be the main factor responsible for cortical degeneration during the early stages of the disease [159], in the advanced stages, other complementary mechanisms come into action. Similar to other neurodegenerative diseases (AD, PD), the impact of oxidative stress, as well as the pathological production of free radicals and ROS, are relevant steps in sustaining neuronal loss (Figure 3).
Figure 3.
Potential mechanisms involved in neurodegenerative phases of multiple sclerosis.
The BBB remains a central structure that is altered as a result of neurodegenerative processes, subsequently leading to cortical damage. Oxidative stress alters the BBB structure, with brain ECs being the primary targets. According to a recent review [160], there are at least four different mechanisms that explain BMECs, known as the “oxidative stress multifaceted association”. The brain is a highly metabolic organ; glucose transport is higher across the BBB than across homologous structures located elsewhere in the human body, creating an environment that favors physiologically high amounts of ROS. Moreover, the presence of endothelial nitric oxide synthase generates high levels of nitric oxide, while the transport of fatty acids as an alternative energy source explains the generation of lipid peroxidation. Finally, the abundance of mitochondria at this level favors the production of reactive species, including superoxide, during the final stages of MS, which supports an older theory known as the “Mitochondrial Free Radical Theory of Aging” [161].
Free radicals, also known as pro-oxidants, are highly reactive, unstable chemical species that contain one or more unpaired electrons in their external orbitals [162]. Although now demonstrated to play a dual role (destructive and protective) in living systems, free radicals are mainly toxic byproducts of the aerobic metabolism which generate and sustain oxidative and nitrosative stress, finally leading to tissue damage [163]. Active inflammation in the CNS is associated with increased oxygen and nitrogen free radicals; however, the effects of these compounds in later stages of the disease, in the absence of acute demyelination, remain to be elucidated. Other molecular changes such as lipid peroxidation or protein alteration may also be a source of pro-oxidants, providing another link to the vicious cycle of neurodegeneration encountered in progressive MS [164].
Lastly, the potential role misfolded proteins play in MS pathology is a scarcely studied topic, albeit a relevant one. Misfolded protein aggregates are a common hallmark in other neurodegenerative disorders such as AD or PD, where amyloid plaques or hyperphosphorylated Tau protein have already become valuable biomarkers for diagnosis and therapeutic monitoring [165]. Moreover, the bidirectional relationship between Aβ and the BBB is of relevance from different perspectives, including Aβ clearance, BBB structural damage, and increased brain toxicity [166]. From this point of view, researchers have wondered if the pathological Aβ or Tau could also be involved in MS, mainly during later stages of the disease. In this context, one of the first studies conducted by Valis et al. [167], showed a significant increase in Aβ42 in the CSF of MS patients; however, no difference related to total and phosphorylated Tau protein levels was observed. Similarly, the work of David and Tayebi [168] demonstrated the presence of soluble amyloid oligomers in the brain tissue and cerebral spinal fluid of MS patients, although their exact role within this pathology is still to be determined. On the other hand, inconsistent results from several clinical trials [169,170] suggest that misfolded proteins alone are not the cause for BBB leakage during the neurodegenerative stages of MS, and that there are other—still unknown—pathological events occurring.
3. Conclusions
The role of NVU structures in MS onset and evolution has received increasing attention over the past few years. The amounting evidence strongly suggests these structures are involved in neuroinflammation and neurodegeneration—both hallmarks of MS.
Future studies should aim to uncover how various components of NVUs interact with their environment in the setting of disease. For example, finding an answer to the role astrocytes and microglia play in MS pathology should constitute a priority, and receive increased attention in MS research.
Achieving a comprehensive understanding of all structures, functions, and molecular underpinnings involved in MS will stimulate drug development to yield improved treatment options, which is ultimately the desired end result of research, awaited by clinicians and patients alike.
Acknowledgments
The authors want to thank Kadry et al. [4] for the opportunity to use their figure in this article, copyright permission being received formally from Springer Nature. This paper is supported by European Union’s Horizon 2020 research and innovation programme under grant agreement No 952378, project BrainTwin (Development of a World-Class Neuroengineering Research Centre by European Twinning).
Author Contributions
T.G.S. and C.R. contributed to the study design and data collection; T.G.S. and B.O.P. contributed equally to data analysis and interpretation; T.G.S. and C.R. prepared the first draft; B.O.P. reviewed the manuscript and wrote its final version. All authors have read and agreed to the published version of the manuscript.
Funding
The publication fee was partially supported by the BrainTwin project, an European Union’s Horizon 2020 research and innovation programme under grant agreement No 952378.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data and materials supporting the results of the present study are available in the published article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Solár P., Zamani A., Kubíčková L., Dubový P., Joukal M. Choroid plexus and the blood-cerebrospinal fluid barrier in disease. Fluids Barriers CNS. 2020;17:35. doi: 10.1186/s12987-020-00196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbott N.J., Patabendige A.A.K., Dolman D.E.M., Yusof S.R., Begley D.J. Structure and function of the blood–brain barrier. Neurobiol. Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 3.Abbott N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 2000;20:131–147. doi: 10.1023/A:1007074420772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadry H., Noorani B., Cucullo L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020;17:69. doi: 10.1186/s12987-020-00230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daneman R., Prat A. The blood-brain barrier. Cold Spring Harb. Perspect. Biol. 2015;7:a020412. doi: 10.1101/cshperspect.a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oldendorf W.H., Cornford M.E., Brown W.J. The large apparent work capability of the blood-brain barrier: A study of the mitochondrial content of capillary endothelial cells in brain and other tissues of the rat. Ann. Neurol. 1977;1:409–417. doi: 10.1002/ana.410010502. [DOI] [PubMed] [Google Scholar]
- 7.Fenstermacher J., Gross P., Sposito N., Acuff V., Pettersen S., Gruber K. Structural and functional variations in capillary systems within the brain. Ann. N. Y. Acad. Sci. 1988;529:21–30. doi: 10.1111/j.1749-6632.1988.tb51416.x. [DOI] [PubMed] [Google Scholar]
- 8.Sedlakova R., Shivers R.R., Del Maestro R.F. Ultrastructure of the blood-brain barrier in the rabbit. J. Submicrosc. Cytol. Pathol. 1999;31:149–161. [PubMed] [Google Scholar]
- 9.Hawkins B.T., Davis T.P. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol. Rev. 2005;57:173–185. doi: 10.1124/pr.57.2.4. [DOI] [PubMed] [Google Scholar]
- 10.Wolburg H., Lippoldt A. Tight junctions of the blood-brain barrier: Development, composition and regulation. Vascul. Pharmacol. 2002;38:323–337. doi: 10.1016/S1537-1891(02)00200-8. [DOI] [PubMed] [Google Scholar]
- 11.Fanning A.S., Jameson B.J., Jesaitis L.A., Anderson J.M. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J. Biol. Chem. 1998;273:29745–29753. doi: 10.1074/jbc.273.45.29745. [DOI] [PubMed] [Google Scholar]
- 12.Tsukita S., Furuse M. Overcoming barriers in the study of tight junction functions: From occludin to claudin. Genes Cells. 1998;3:569–573. doi: 10.1046/j.1365-2443.1998.00212.x. [DOI] [PubMed] [Google Scholar]
- 13.Tietz S., Engelhardt B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell. Biol. 2015;209:493–506. doi: 10.1083/jcb.201412147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lv J., Hu W., Yang Z., Li T., Jiang S., Ma Z., Chen F., Yang Y. Focusing on claudin-5: A promising candidate in the regulation of BBB to treat ischemic stroke. Prog. Neurobiol. 2018;161:79–96. doi: 10.1016/j.pneurobio.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Yuan S., Liu K.J., Qi Z. Occludin regulation of blood-brain barrier and potential therapeutic target in ischemic stroke. Brain Circ. 2020;6:152–162. doi: 10.4103/bc.bc_29_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin T.A. The role of tight junctions in cancer metastasis. Semin. Cell Dev. Biol. 2014;36:224–231. doi: 10.1016/j.semcdb.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Jia W., Martin T.A., Zhang G., Jiang W.G. Junctional adhesion molecules in cerebral endothelial tight junction and brain metastasis. Anticancer Res. 2013;33:2353–2359. [PubMed] [Google Scholar]
- 18.Abbott N.J., Rönnbäck L., Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- 19.Abdullahi W., Tripathi D., Ronaldson P.T. Blood-brain barrier dysfunction in ischemic stroke: Targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 2018;315:C343–C356. doi: 10.1152/ajpcell.00095.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zlokovic B.V. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Villaseñor R., Lampe J., Schwaninger M., Collin L. Intracellular transport and regulation of transcytosis across the blood-brain barrier. Cell. Mol. Life Sci. 2019;76:1081–1092. doi: 10.1007/s00018-018-2982-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang A.C., Stevens M.Y., Chen M.B., Lee D.P., Stähli D., Gate D., Contrepois K., Chen W., Iram T., Zhang L., et al. Physiological blood-brain transport is impaired with age by a shift in transcytosis. Nature. 2020;583:425–430. doi: 10.1038/s41586-020-2453-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scalisi J., Balau B., Deneyer L., Bouchat J., Gilloteaux J., Nicaise C. Blood-brain barrier permeability towards small and large tracers in a mouse model of osmotic demyelination syndrome. Neurosci. Lett. 2021;746:135665. doi: 10.1016/j.neulet.2021.135665. [DOI] [PubMed] [Google Scholar]
- 24.Bendayan R., Ronaldson P.T., Gingras D., Bendayan M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J. Histochem. Cytochem. 2006;54:1159–1167. doi: 10.1369/jhc.5A6870.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronaldson P.T., Babakhanian K., Bendayan R. Drug transport in the brain. In: You G., Morris M.E., editors. Drug Transporters: Molecular Characterization and Role in Drug Disposition. Wiley; Hokoben, NJ, USA: 2007. pp. 411–461. [Google Scholar]
- 26.Miller D.S. Regulation of P-glycoprotein and other ABC drug transporters at the blood-brain barrier. Trends Pharmacol. Sci. 2010;31:246–254. doi: 10.1016/j.tips.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nicolazzo J.A., Katneni K. Drug transport across the blood-brain barrier and the impact of breast cancer resistance protein (ABCG2) Curr. Top. Med. Chem. 2009;9:130–147. doi: 10.2174/156802609787521580. [DOI] [PubMed] [Google Scholar]
- 28.Abbott N.J., Revest P.A., Romero I.A. Astrocyte-endothelial inter-action: Physiology and pathology. Neuropathol. Appl. Neurobiol. 1992;18:424–433. doi: 10.1111/j.1365-2990.1992.tb00808.x. [DOI] [PubMed] [Google Scholar]
- 29.Campisi M., Shin Y., Osaki T., Hajal C., Chiono V., Kamm R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 2018;180:117–129. doi: 10.1016/j.biomaterials.2018.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidari H., Taylor H. Review Article: Capturing the physiological complexity of the brain′s neuro-vascular unit in vitro. Biomicrofluidics. 2018;12:051502. doi: 10.1063/1.5045126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Villabona-Rueda A., Erice C., Pardo C.A., Stins M.F. The Evolving Concept of the Blood Brain Barrier (BBB): From a Single Static Barrier to a Heterogeneous and Dynamic Relay Center. Front. Cell. Neurosci. 2019;13:405. doi: 10.3389/fncel.2019.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Allt G., Lawrenson J.G. Pericytes: Cell biology and pathology. Cells Tissues Organs. 2001;169:1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 33.Lai C.H., Kuo K.H. The critical component to establish in vitro BBB model: Pericyte. Brain Res. Rev. 2005;50:258–265. doi: 10.1016/j.brainresrev.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Armulik A., Genove G., Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev. Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 35.Ramsauer M., Kunz J., Krause D., Dermietzel R. Regulation of a blood-brain barrier-specific enzyme expressed by cerebral pericytes (pericytic aminopeptidase N/pAPN) under cell culture conditions. J. Cereb. Blood Flow Metab. 1998;18:1270–1281. doi: 10.1097/00004647-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Berezowski V., Landry C., Dehouck M.P., Cecchelli R., Fenart L. Contribution of glial cells and pericytes to the mRNA profiles of P-glycoprotein and multidrug resistance-associated proteins in an in vitro model of the blood-brain barrier. Brain Res. 2004;1018:1–9. doi: 10.1016/j.brainres.2004.05.092. [DOI] [PubMed] [Google Scholar]
- 37.Fisher M. Pericyte signaling in the neurovascular unit. Stroke. 2009;40((Suppl. 1)):S13–S15. doi: 10.1161/STROKEAHA.108.533117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giannoni P., Badaut J., Dargazanli C., De Maudave A.F., Klement W., Costalat V., Marchi N. The pericyte-glia interface at the blood-brain barrier. Clin. Sci. 2018;132:361–374. doi: 10.1042/CS20171634. [DOI] [PubMed] [Google Scholar]
- 39.Chatterjee S., Naik U.P. Pericyte-endothelial cell interaction: A survival mechanism for the tumor vasculature. Cell Adhes. Migr. 2012;6:157–159. doi: 10.4161/cam.20252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabry Z., Fitzsimmons K.M., Herlein J.A., Moninger T.O., Dobbs M.B., Hart M.N. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J. Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- 41.Kovac A., Erickson M.A., Banks W.A. Brain microvascular pericytes are immunoactive in culture: Cytokine, chemokine, nitric oxide, and LRP-1 expression in response to lipopolysaccharide. J. Neuroinflamm. 2011;8:139. doi: 10.1186/1742-2094-8-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balabanov R., Beaumont T., Dore-Duffy P. Role of central nervous system microvascular pericytes in activation of antigen-primed splenic T-lymphocytes. J. Neurosci. Res. 1999;55:578–587. doi: 10.1002/(SICI)1097-4547(19990301)55:5<578::AID-JNR5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 43.Armulik A., Genové G., Mäe M., Nisancioglu M.H., Wallgard E., Niaudet C., He L., Norlin J., Lindblom P., Strittmatter K., et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–561. doi: 10.1038/nature09522. [DOI] [PubMed] [Google Scholar]
- 44.Alarcon-Martinez L., Yilmaz-Ozcan S., Yemisci M., Schallek J., Kılıç K., Villafranca-Baughman D., Can A., Di Polo A., Dalkara T. Retinal ischemia induces α-SMA-mediated capillary pericyte contraction coincident with perivascular glycogen depletion. Acta Neuropathol. Commun. 2019;7:134. doi: 10.1186/s40478-019-0761-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giovannoni F., Quintana F.J. The Role of Astrocytes in CNS Inflammation. Trends Immunol. 2020;41:805–819. doi: 10.1016/j.it.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tait M.J., Saadoun S., Bell B.A., Papadopoulos M.C. Water movements in the brain: Role of aquaporins. Trends Neurosci. 2008;31:37–43. doi: 10.1016/j.tins.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 47.Ikeshima-Kataoka H. Neuroimmunological Implications of AQP4 in Astrocytes. Int. J. Mol. Sci. 2016;17:1306. doi: 10.3390/ijms17081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raub T.J., Kuentzel S.L., Sawada G.A. Permeability of bovine brain microvessel endothelial cells in vitro: Barrier tightening by a factor released from astroglioma cells. Exp. Cell Res. 1992;199:330–340. doi: 10.1016/0014-4827(92)90442-B. [DOI] [PubMed] [Google Scholar]
- 49.Estrada C., Bready J.V., Berliner J.A., Pardridge W.M., Cancilla P.A. Astrocyte growth stimulation by a soluble factor produced by cerebral endothelial cells in vitro. J. Neuropathol. Exp. Neurol. 1990;49:539–549. doi: 10.1097/00005072-199011000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Berezowski V., Fukuda A.M., Cecchelli R., Badaut J. Endothelial Cells and Astrocytes: A Concerto en Duo in Ischemic Pathophysiology. Int. J. Cell Biol. 2012;2012:176287. doi: 10.1155/2012/176287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seo J.H., Maki T., Maeda M., Miyamoto N., Liang A.C., Hayakawa K., Pham L.D., Suwa F., Taguchi A., Matsuyama T., et al. Oligodendrocyte precursor cells support blood-brain barrier integrity via TGF-β signaling. PLoS ONE. 2014;9:e103174. doi: 10.1371/journal.pone.0103174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang L., Geng J., Qu M., Yuan F., Wang Y., Pan J., Li Y., Ma Y., Zhou P., Zhang Z., et al. Oligodendrocyte precursor cells transplantation protects blood-brain barrier in a mouse model of brain ischemia via Wnt/β-catenin signaling. Cell Death Dis. 2020;11:9. doi: 10.1038/s41419-019-2206-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nicaise C., Marneffe C., Bouchat J., Gilloteaux J. Osmotic Demyelination: From an Oligodendrocyte to an Astrocyte Perspective. Int. J. Mol. Sci. 2019;20:1124. doi: 10.3390/ijms20051124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gankam Kengne F., Nicaise C., Soupart A., Boom A., Schiettecatte J., Pochet R., Brion J.P., Decaux G. Astrocytes are an early target in osmotic demyelination syndrome. J. Am. Soc. Nephrol. 2011;22:1834–1845. doi: 10.1681/ASN.2010111127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gankam-Kengne F., Couturier B.S., Soupart A., Brion J.P., Decaux G. Osmotic Stress-Induced Defective Glial Proteostasis Contributes to Brain Demyelination after Hyponatremia Treatment. J. Am. Soc. Nephrol. 2017;28:1802–1813. doi: 10.1681/ASN.2016050509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Popescu B.F., Bunyan R.F., Guo Y., Parisi J.E., Lennon V.A., Lucchinetti C.F. Evidence of aquaporin involvement in human central pontine myelinolysis. Acta Neuropathol. Commun. 2013;1:40. doi: 10.1186/2051-5960-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gankam Kengne F., Soupart A., Pochet R., Brion J.P., Decaux G. Re-induction of hyponatremia after rapid overcorrection of hyponatremia reduces mortality in rats. Kidney Int. 2009;76:614–621. doi: 10.1038/ki.2009.254. [DOI] [PubMed] [Google Scholar]
- 58.Thion M.S., Ginhoux F., Garel S. Microglia and early brain development: An intimate journey. Science. 2018;362:185–189. doi: 10.1126/science.aat0474. [DOI] [PubMed] [Google Scholar]
- 59.Eyo U.B., Wu L.J. Microglia: Lifelong patrolling immune cells of the brain. Prog. Neurobiol. 2019;179:101614. doi: 10.1016/j.pneurobio.2019.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kettenmann H., Hanisch U.K., Noda M., Verkhratsky A. Physiology of microglia. Physiol. Rev. 2011;91:461–553. doi: 10.1152/physrev.00011.2010. [DOI] [PubMed] [Google Scholar]
- 61.Ransohoff R.M. A polarizing question: Do M1 and M2 microglia exist? Nat. Neurosci. 2016;19:987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 62.Andreone B.J., Lacoste B., Gu C. Neuronal and vascular interactions. Annu. Rev. Neurosci. 2015;38:25–46. doi: 10.1146/annurev-neuro-071714-033835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hübel N., Schöll E., Dahlem M.A. Bistable dynamics underlying excitability of ion homeostasis in neuron models. PLoS Comput. Biol. 2014;10:e1003551. doi: 10.1371/journal.pcbi.1003551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pulido R.S., Munji R.N., Chan T.C., Quirk C.R., Weiner G.A., Weger B.D., Rossi M.J., Elmsaouri S., Malfavon M., Deng A., et al. Neuronal Activity Regulates Blood-Brain Barrier Efflux Transport through Endothelial Circadian Genes. Neuron. 2020;108:937–952.e7. doi: 10.1016/j.neuron.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reed M.J., Damodarasamy M., Banks W.A. The extracellular matrix of the blood-brain barrier: Structural and functional roles in health, aging, and Alzheimer’s disease. Tissue Barriers. 2019;7:1651157. doi: 10.1080/21688370.2019.1651157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pozzi A., Yurchenco P.D., Iozzo R.V. The nature and biology of basement membranes. Matrix Biol. 2017;57–58:1–11. doi: 10.1016/j.matbio.2016.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu L., Nirwane A., Yao Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2018;4:78–82. doi: 10.1136/svn-2018-000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aumailley M. The laminin family. Cell Adhes. Migr. 2013;7:48–55. doi: 10.4161/cam.22826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Louboutin J.P., Strayer D.S. Blood-brain barrier abnormalities caused by HIV-1 gp120: Mechanistic and therapeutic implications. Sci. World J. 2012;2012:482575. doi: 10.1100/2012/482575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Del Zoppo G.J. Bleeding in the brain: Amyloid-beta may keep clots away. Nat. Med. 2009;15:1132–1133. doi: 10.1038/nm1009-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosenberg G.A. Matrix metalloproteinases in neuroinflammation. Glia. 2002;39:279–291. doi: 10.1002/glia.10108. Erratum in Glia 2002, 40, 130. [DOI] [PubMed] [Google Scholar]
- 72.Jiang D., Liang J., Noble P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zaragozá R. Transport of Amino Acids across the Blood-Brain Barrier. Front. Physiol. 2020;11:973. doi: 10.3389/fphys.2020.00973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brown G.C. The endotoxin hypothesis of neurodegeneration. J. Neuroinflamm. 2019;16:180. doi: 10.1186/s12974-019-1564-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tosi G., Duskey J.T., Kreuter J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin. Drug Deliv. 2020;17:23–32. doi: 10.1080/17425247.2020.1698544. [DOI] [PubMed] [Google Scholar]
- 76.McCall R.L., Cacaccio J., Wrabel E., Schwartz M.E., Coleman T.P., Sirianni R.W. Pathogen-inspired drug delivery to the central nervous system. Tissue Barriers. 2014;2:e944449. doi: 10.4161/21688362.2014.944449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yarlagadda A., Kaushik S., Clayton A.H. Blood brain barrier: The role of calcium homeostasis. Psychiatry. 2007;4:55–59. [PMC free article] [PubMed] [Google Scholar]
- 78.Jukkola P., Gu C. Regulation of neurovascular coupling in autoimmunity to water and ion channels. Autoimmun. Rev. 2015;14:258–267. doi: 10.1016/j.autrev.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tumani H., Huss A., Bachhuber F. The cerebrospinal fluid and barriers—Anatomic and physiologic considerations. Handb. Clin. Neurol. 2017;146:21–32. doi: 10.1016/B978-0-12-804279-3.00002-2. [DOI] [PubMed] [Google Scholar]
- 80.Belov Kirdajova D., Kriska J., Tureckova J., Anderova M. Ischemia-Triggered Glutamate Excitotoxicity from the Perspective of Glial Cells. Front. Cell. Neurosci. 2020;14:51. doi: 10.3389/fncel.2020.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Huang L., Li B., Li X., Liu G., Liu R., Guo J., Xu B., Li Y., Fang W. Significance and Mechanisms of P-glycoprotein in Central Nervous System Diseases. Curr. Drug Targets. 2019;20:1141–1155. doi: 10.2174/1389450120666190308144448. [DOI] [PubMed] [Google Scholar]
- 82.Subhramanyam C.S., Wang C., Hu Q., Dheen S.T. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin. Cell Dev. Biol. 2019;94:112–120. doi: 10.1016/j.semcdb.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 83.Lo J., Chan L., Flynn S. A Systematic Review of the Incidence, Prevalence, Costs, and Activity and Work Limitations of Amputation, Osteoarthritis, Rheumatoid Arthritis, Back Pain, Multiple Sclerosis, Spinal Cord Injury, Stroke, and Traumatic Brain Injury in the United States: A 2019 Update. Arch. Phys. Med. Rehabil. 2021;102:115–131. doi: 10.1016/j.apmr.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Piehl F. Current and emerging disease-modulatory therapies and treatment targets for multiple sclerosis. J. Intern. Med. 2021;289:771–791. doi: 10.1111/joim.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Goldstein E.Z., Church J.S., Hesp Z.C., Popovich P.G., McTigue D.M. A silver lining of neuroinflammation: Beneficial effects on myelination. Exp. Neurol. 2016;283 Pt B:550–559. doi: 10.1016/j.expneurol.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 86.Yang C., Hawkins K.E., Doré S., Candelario-Jalil E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019;316:C135–C153. doi: 10.1152/ajpcell.00136.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Šimić G., Španić E., Langer Horvat L., Hof P.R. Blood-brain barrier and innate immunity in the pathogenesis of Alzheimer′s disease. Prog. Mol. Biol. Transl. Sci. 2019;168:99–145. doi: 10.1016/bs.pmbts.2019.06.003. [DOI] [PubMed] [Google Scholar]
- 88.Jiang X., Andjelkovic A.V., Zhu L., Yang T., Bennett M.V.L., Chen J., Keep R.F., Shi Y. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog. Neurobiol. 2018;163–164:144–171. doi: 10.1016/j.pneurobio.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gavard J. Endothelial permeability and VE-cadherin: A wacky comradeship. Cell Adhes. Migr. 2014;8:158–164. doi: 10.4161/cam.29026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Van Itallie C.M., Anderson J.M. Phosphorylation of tight junction transmembrane proteins: Many sites, much to do. Tissue Barriers. 2018;6:e1382671. doi: 10.1080/21688370.2017.1382671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nagyoszi P., Wilhelm I., Farkas A.E., Fazakas C., Dung N.T., Haskó J., Krizbai I.A. Expression and regulation of toll-like receptors in cerebral endothelial cells. Neurochem. Int. 2010;57:556–564. doi: 10.1016/j.neuint.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Chodobski A., Chung I., Koz´niewska E., Koz´niewska K., Ivanenko T., Chang W., Harrington J.F., Duncan J.A., Szmydynger-Chodobska J. Early Neutrophilic Expression of Vascular Endothelial Growth Factor after Traumatic Brain Injury. Neuroscience. 2003;122:853–867. doi: 10.1016/j.neuroscience.2003.08.055. [DOI] [PubMed] [Google Scholar]
- 93.Yamagata K., Tagami M., Takenaga F., Yamori Y., Itoh S. Hypoxia-induced changes in tight junction permeability of brain capillary endothelial cells are associated with IL-1beta and nitric oxide. Neurobiol. Dis. 2004;17:491–499. doi: 10.1016/j.nbd.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 94.Wittmann G., Mohácsik P., Balkhi M.Y., Gereben B., Lechan R.M. Endotoxin-induced inflammation down-regulates L-type amino acid transporter 1 (LAT1) expression at the blood-brain barrier of male rats and mice. Fluids Barriers CNS. 2015;12:21. doi: 10.1186/s12987-015-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roberts D.J., Goralski K.B. A critical overview of the influence of inflammation and infection on P-glycoprotein expression and activity in the brain. Expert Opin. Drug Metab. Toxicol. 2008;4:1245–1264. doi: 10.1517/17425255.4.10.1245. [DOI] [PubMed] [Google Scholar]
- 96.Nieuwdorp M., Meuwese M.C., Mooij H.L., van Lieshout M.H., Hayden A., Levi M., Meijers J.C., Ince C., Kastelein J.J., Vink H., et al. Tumor necrosis factor-alpha inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis. 2009;202:296–303. doi: 10.1016/j.atherosclerosis.2008.03.024. [DOI] [PubMed] [Google Scholar]
- 97.Pieper C., Marek J.J., Unterberg M., Schwerdtle T., Galla H.J. Brain capillary pericytes contribute to the immune defense in response to cytokines or LPS in vitro. Brain Res. 2014;1550:1–8. doi: 10.1016/j.brainres.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 98.Nakamura K., Ikeuchi T., Nara K., Rhodes C.S., Zhang P., Chiba Y., Kazuno S., Miura Y., Ago T., Arikawa-Hirasawa E., et al. Perlecan regulates pericyte dynamics in the maintenance and repair of the blood-brain barrier. J. Cell Biol. 2019;218:3506–3525. doi: 10.1083/jcb.201807178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guillemin G.J., Brew B.J. Microglia, macrophages, perivascular macrophages, and pericytes: A review of function and identification. J. Leukoc. Biol. 2004;75:388–397. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 100.Hawkins R.A., Viña J.R. How Glutamate Is Managed by the Blood-Brain Barrier. Biology. 2016;5:37. doi: 10.3390/biology5040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu H., Wang P., An P., Xue Y. Recombinant human angiopoietin-1 ameliorates the expressions of ZO-1, occludin, VE-cadherin, and PKCα signaling after focal cerebral ischemia/reperfusion in rats. J. Mol. Neurosci. 2012;46:236–247. doi: 10.1007/s12031-011-9584-5. [DOI] [PubMed] [Google Scholar]
- 102.Alvarez J.I., Dodelet-Devillers A., Kebir H., Ifergan I., Fabre P.J., Terouz S., Sabbagh M., Wosik K., Bourbonnière L., Bernard M., et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334:1727–1731. doi: 10.1126/science.1206936. [DOI] [PubMed] [Google Scholar]
- 103.Psenicka M.W., Smith B.C., Tinkey R.A., Williams J.L. Connecting Neuroinflammation and Neurodegeneration in Multiple Sclerosis: Are Oligodendrocyte Precursor Cells a Nexus of Disease? Front. Cell. Neurosci. 2021;15:654284. doi: 10.3389/fncel.2021.654284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tang Y., Le W. Differential Roles of M1 and M2 Microglia in Neurodegenerative Diseases. Mol. Neurobiol. 2016;53:1181–1194. doi: 10.1007/s12035-014-9070-5. [DOI] [PubMed] [Google Scholar]
- 105.Limatola C., Ransohoff R.M. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front. Cell. Neurosci. 2014;8:229. doi: 10.3389/fncel.2014.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cherry J.D., Olschowka J.A., O’Banion M.K. Neuroinflammation and M2 microglia: The good, the bad, and the inflamed. J. Neuroinflamm. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Szepesi Z., Manouchehrian O., Bachiller S., Deierborg T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018;12:323. doi: 10.3389/fncel.2018.00323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Prajeeth C.K., Kronisch J., Khorooshi R., Knier B., Toft-Hansen H., Gudi V., Floess S., Huehn J., Owens T., Korn T. Effectors of Th1 and Th17 cells act on astrocytes and augment their neuroinflammatory properties. J. Neuroinflamm. 2017;14:204. doi: 10.1186/s12974-017-0978-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Salou M., Nicol B., Garcia A., Laplaud D.A. Involvement of CD8(+) T Cells in Multiple Sclerosis. Front. Immunol. 2015;6:604. doi: 10.3389/fimmu.2015.00604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wanleenuwat P., Iwanowski P. Role of B cells and antibodies in multiple sclerosis. Mult. Scler. Relat. Disord. 2019;36:101416. doi: 10.1016/j.msard.2019.101416. [DOI] [PubMed] [Google Scholar]
- 111.Wooff Y., Man S.M., Aggio-Bruce R., Natoli R., Fernando N. IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases. Front. Immunol. 2019;10:1618. doi: 10.3389/fimmu.2019.01618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gałuszka A., Stec M., Węglarczyk K., Kluczewska A., Siedlar M., Baran J. Transition Metal Containing Particulate Matter Promotes Th1 and Th17 Inflammatory Response by Monocyte Activation in Organic and Inorganic Compounds Dependent Manner. Int. J. Environ. Res. Public Health. 2020;17:1227. doi: 10.3390/ijerph17041227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Theoharides T.C., Tsilioni I., Ren H. Recent advances in our understanding of mast cell activation—or should it be mast cell mediator disorders? Expert Rev. Clin. Immunol. 2019;15:639–656. doi: 10.1080/1744666X.2019.1596800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Winder A.A., Wohlford-Lenane C., Scheetz T.E., Nardy B.N., Manzel L.J., Look D.C., McCray P.B., Jr. Differential effects of cytokines and corticosteroids on toll-like receptor 2 expression and activity in human airway epithelia. Respir. Res. 2009;10:96. doi: 10.1186/1465-9921-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jung Y.O., Cho M.L., Lee S.Y., Oh H.J., Park J.S., Park M.K., Park M.J., Ju J.H., Kim S.I., Park S.H., et al. Synergism of toll-like receptor 2 (TLR2), TLR4, and TLR6 ligation on the production of tumor necrosis factor (TNFα) in a spontaneous arthritis animal model of interleukin (IL)-1 receptor antagonist-deficient mice. Immunol. Lett. 2009;123:138–143. doi: 10.1016/j.imlet.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 116.Balabanov R., Goldman H., Murphy S., Pellizon G., Owen C., Rafols J., Dore-Duffy P. Endothelial Cell Activation Following Moderate Traumatic Brain Injury. Neurol. Res. 2001;23:175–182. doi: 10.1179/016164101101198514. [DOI] [PubMed] [Google Scholar]
- 117.Morgan R., Kreipke C.W., Roberts G., Bagchi M., Rafols J.A. Neovascularization Following Traumatic Brain Injury: Possible Evidence for Both Angiogenesis and Vasculogenesis. Neurol. Res. 2013;29:375–381. doi: 10.1179/016164107X204693. [DOI] [PubMed] [Google Scholar]
- 118.Winn R.K., Harlan J.M. The role of endothelial cell apoptosis in inflammatory and immune diseases. J. Thromb. Haemost. 2005;3:1815–1824. doi: 10.1111/j.1538-7836.2005.01378.x. [DOI] [PubMed] [Google Scholar]
- 119.Cidad P., Garcia-Nogales P., Almeida A., Bolaños J.P. Expression of glucose transporter GLUT3 by endotoxin in cultured rat astrocytes: The role of nitric oxide. J. Neurochem. 2001;79:17–24. doi: 10.1046/j.1471-4159.2001.00523.x. [DOI] [PubMed] [Google Scholar]
- 120.Gyawali A., Kang Y.S. Pretreatment Effect of Inflammatory Stimuli and Characteristics of Tryptophan Transport on Brain Capillary Endothelial (TR-BBB) and Motor Neuron Like (NSC-34) Cell Lines. Biomedicines. 2020;9:9. doi: 10.3390/biomedicines9010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Varatharaj A., Galea I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017;60:1–12. doi: 10.1016/j.bbi.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 122.Schmidt E.P., Yang Y., Janssen W.J., Gandjeva A., Perez M.J., Barthel L., Zemans R.L., Bowman J.C., Koyanagi D.E., Yunt Z.X., et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pesonen E., Passov A., Andersson S., Suojaranta R., Niemi T., Raivio P., Salmenperä M., Schramko A. Glycocalyx Degradation and Inflammation in Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2019;33:341–345. doi: 10.1053/j.jvca.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 124.Ali M.M., Mahmoud A.M., Le Master E., Levitan I., Phillips S.A. Role of matrix metalloproteinases and histone deacetylase in oxidative stress-induced degradation of the endothelial glycocalyx. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H647–H663. doi: 10.1152/ajpheart.00090.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nelson A., Berkestedt I., Bodelsson M. Circulating glycosaminoglycan species in septic shock. Acta Anaesthesiol. Scand. 2014;58:36–43. doi: 10.1111/aas.12223. [DOI] [PubMed] [Google Scholar]
- 126.Geranmayeh M.H., Rahbarghazi R., Farhoudi M. Targeting pericytes for neurovascular regeneration. Cell Commun. Signal. 2019;17:26. doi: 10.1186/s12964-019-0340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bertrand L., Cho H.J., Toborek M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain. 2019;142:502–511. doi: 10.1093/brain/awy339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ozen I., Boix J., Paul G. Perivascular mesenchymal stem cells in the adult human brain: A future target for neuroregeneration? Clin. Transl. Med. 2012;1:30. doi: 10.1186/2001-1326-1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Michinaga S., Koyama Y. Dual Roles of Astrocyte-Derived Factors in Regulation of Blood-Brain Barrier Function after Brain Damage. Int. J. Mol. Sci. 2019;20:571. doi: 10.3390/ijms20030571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chidiac R., Zhang Y., Tessier S., Faubert D., Delisle C., Gratton J.P. Comparative Phosphoproteomics Analysis of VEGF and Angiopoietin-1 Signaling Reveals ZO-1 as a Critical Regulator of Endothelial Cell Proliferation. Mol. Cell. Proteom. 2016;15:1511–1525. doi: 10.1074/mcp.M115.053298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zemva J., Schubert M. The role of neuronal insulin/insulin-like growth factor-1 signaling for the pathogenesis of Alzheimer′s disease: Possible therapeutic implications. CNS Neurol. Disord. Drug Targets. 2014;13:322–337. doi: 10.2174/18715273113126660141. [DOI] [PubMed] [Google Scholar]
- 132.Song Y., Li Z., He T., Qu M., Jiang L., Li W., Shi X., Pan J., Zhang L., Wang Y., et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics. 2019;9:2910–2923. doi: 10.7150/thno.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Panayi G.S., Corrigall V.M., Henderson B. Stress cytokines: Pivotal proteins in immune regulatory networks. Curr. Opin. Immunol. 2004;16:531–534. doi: 10.1016/j.coi.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 134.Scaffidi P., Misteli T., Bianchi M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418:191–195. doi: 10.1038/nature00858. Erratum in Nature 2010, 467, 622. [DOI] [PubMed] [Google Scholar]
- 135.Gülke E., Gelderblom M., Magnus T. Danger signals in stroke and their role on microglia activation after ischemia. Ther. Adv. Neurol. Disord. 2018;11:1756286418774254. doi: 10.1177/1756286418774254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hansen D.V., Hanson J.E., Sheng M. Microglia in Alzheimer’s disease. J. Cell Biol. 2018;217:459–472. doi: 10.1083/jcb.201709069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fu Y., Hsiao J.H., Paxinos G., Halliday G.M., Kim W.S. ABCA7 Mediates Phagocytic Clearance of Amyloid-β in the Brain. J. Alzheimers Dis. 2016;54:569–584. doi: 10.3233/JAD-160456. [DOI] [PubMed] [Google Scholar]
- 138.Zettergren A., Höglund K., Kern S., Thorvaldsson V., Johan Skoog M., Hansson O., Andreasen N., Bogdanovic N., Blennow K., Skoog I., et al. Association of IL1RAP-related genetic variation with cerebrospinal fluid concentration of Alzheimer-associated tau protein. Sci. Rep. 2019;9:2460. doi: 10.1038/s41598-018-36650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Casali B.T., Reed-Geaghan E.G. Microglial Function and Regulation during Development, Homeostasis and Alzheimer’s Disease. Cells. 2021;10:957. doi: 10.3390/cells10040957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Liu X., Quan N. Microglia and CNS Interleukin-1: Beyond Immunological Concepts. Front. Neurol. 2018;9:8. doi: 10.3389/fneur.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Raffaele S., Lombardi M., Verderio C., Fumagalli M. TNF Production and Release from Microglia via Extracellular Vesicles: Impact on Brain Functions. Cells. 2020;9:2145. doi: 10.3390/cells9102145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kowal K., Silver R., Slawinska E., Bielecki M., Chyczewski L., Kowal-Bielecka O. CD163 and its role in inflammation. Folia Histochem. Cytobiol. 2011;49:365–374. doi: 10.5603/FHC.2011.0052. [DOI] [PubMed] [Google Scholar]
- 143.Neumann H., Takahashi K. Essential role of the microglial triggering receptor expressed on myeloid cells-2 (TREM2) for central nervous tissue immune homeostasis. J. Neuroimmunol. 2007;184:92–99. doi: 10.1016/j.jneuroim.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 144.Raes G., Van den Bergh R., De Baetselier P., Ghassabeh G.H., Scotton C., Locati M., Mantovani A., Sozzani S. Arginase-1 and Ym1 are markers for murine, but not human, alternatively activated myeloid cells. J. Immunol. 2005;174:6561. doi: 10.4049/jimmunol.174.11.6561. [DOI] [PubMed] [Google Scholar]
- 145.Sayed A., Bahbah E.I., Kamel S., Barreto G.E., Ashraf G.M., Elfil M. The neutrophil-to-lymphocyte ratio in Alzheimer′s disease: Current understanding and potential applications. J. Neuroimmunol. 2020;349:577398. doi: 10.1016/j.jneuroim.2020.577398. [DOI] [PubMed] [Google Scholar]
- 146.Al-Yassin G.A., Bretscher P.A. Does T Cell Activation Require a Quorum of Lymphocytes? J. Immunol. 2018;201:2855–2861. doi: 10.4049/jimmunol.1800805. [DOI] [PubMed] [Google Scholar]
- 147.Saghazadeh A., Ferrari C.C., Rezaei N. Deciphering variability in the role of interleukin-1β in Parkinson’s disease. Rev. Neurosci. 2016;27:635–650. doi: 10.1515/revneuro-2015-0059. [DOI] [PubMed] [Google Scholar]
- 148.Morgello S. HIV neuropathology. Handb. Clin. Neurol. 2018;152:3–19. doi: 10.1016/B978-0-444-63849-6.00002-5. [DOI] [PubMed] [Google Scholar]
- 149.Kumar P., Misra S., Kumar A., Pandit A.K., Chakravarty K., Prasad K. Association between Tumor Necrosis Factor-α (−238G/A and −308G/A) Gene Polymorphisms and Risk of Ischemic Stroke: A Meta-Analysis. Pulse. 2016;3:217–228. doi: 10.1159/000443770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Weiner H. A 21 Point Unifying Hypothesis on the Etiology and Treatment of Multiple Sclerosis. Can. J. Neurol. Sci. J. Can. Des Sci. Neurol. 1988;25:93–101. doi: 10.1017/S0317167100033680. [DOI] [PubMed] [Google Scholar]
- 151.Constantinescu C.S., Farooqi N., O’Brien K., Gran B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS) Br. J. Pharmacol. 2011;164:1079–1106. doi: 10.1111/j.1476-5381.2011.01302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kalra S., Lowndes C., Durant L., Strange R.C., Al-Araji A., Hawkins C.P., Curnow S.J. Th17 cells increase in RRMS as well as in SPMS, whereas various other phenotypes of Th17 increase in RRMS only. Mult. Scler. J. Exp. Transl. Clin. 2020;6:2055217319899695. doi: 10.1177/2055217319899695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dos Passos G.R., Sato D.K., Becker J., Fujihara K. Th17 Cells Pathways in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorders: Pathophysiological and Therapeutic Implications. Mediat. Inflamm. 2016;2016:5314541. doi: 10.1155/2016/5314541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mockus T.E., Munie A., Atkinson J.R., Segal B.M. Encephalitogenic and Regulatory CD8 T Cells in Multiple Sclerosis and Its Animal Models. J. Immunol. 2021;206:3–10. doi: 10.4049/jimmunol.2000797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Denic A., Wootla B., Rodriguez M. CD8(+) T cells in multiple sclerosis. Expert Opin. Ther. Targets. 2013;17:1053–1066. doi: 10.1517/14728222.2013.815726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chisari C.G., Sgarlata E., Arena S., Toscano S., Luca M., Patti F. Rituximab for the treatment of multiple sclerosis: A review. J. Neurol. 2022;269:159–183. doi: 10.1007/s00415-020-10362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Florou D., Katsara M., Feehan J., Dardiotis E., Apostolopoulos V. Anti-CD20 Agents for Multiple Sclerosis: Spotlight on Ocrelizumab and Ofatumumab. Brain Sci. 2020;10:758. doi: 10.3390/brainsci10100758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Petrova N., Nutma E., Carassiti D., Rs Newman J., Amor S., Altmann D.R., Baker D., Schmierer K. Synaptic Loss in Multiple Sclerosis Spinal Cord. Ann. Neurol. 2020;88:619–625. doi: 10.1002/ana.25835. [DOI] [PubMed] [Google Scholar]
- 159.Dutta R., Trapp B.D. Mechanisms of neuronal dysfunction and degeneration in multiple sclerosis. Prog. Neurobiol. 2011;93:1–12. doi: 10.1016/j.pneurobio.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Banks W.A., Rhea E.M. The Blood–Brain Barrier, Oxidative Stress, and Insulin Resistance. Antioxidants. 2021;10:1695. doi: 10.3390/antiox10111695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sanz A., Stefanatos R.K. The mitochondrial free radical theory of aging: A critical view. Curr. Aging. Sci. 2008;1:10–21. doi: 10.2174/1874609810801010010. [DOI] [PubMed] [Google Scholar]
- 162.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Di Meo S., Venditti P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxidative Med. Cell. Longev. 2020;2020:9829176. doi: 10.1155/2020/9829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Angelova P.R. Sources and triggers of oxidative damage in neurodegeneration. Free Radic. Biol. Med. 2021;173:52–63. doi: 10.1016/j.freeradbiomed.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 165.Adiutori R., Puentes F., Bremang M., Lombardi V., Zubiri I., Leoni E., Aarum J., Sheer D., McArthur S., Pike I., et al. Analysis of circulating protein aggregates as a route of investigation into neurodegenerative disorders. Brain Commun. 2021;3:fcab148. doi: 10.1093/braincomms/fcab148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang D., Chen F., Han Z., Yin Z., Ge X., Lei P. Relationship Between Amyloid-β Deposition and Blood-Brain Barrier Dysfunction in Alzheimer’s Disease. Front. Cell. Neurosci. 2021;15:695479. doi: 10.3389/fncel.2021.695479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Valis M., Talab R., Stourac P., Andrys C., Masopust J. Tau protein, phosphorylated tau protein and beta-amyloid42 in the cerebrospinal fluid of multiple sclerosis patients. Neuroendocrinol. Lett. 2008;29:971–976. [PubMed] [Google Scholar]
- 168.David M.A., Tayebi M. Detection of protein aggregates in brain and cerebrospinal fluid derived from multiple sclerosis patients. Front. Neurol. 2014;5:251. doi: 10.3389/fneur.2014.00251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Szalardy L., Zadori D., Simu M., Bencsik K., Vecsei L., Klivenyi P. Evaluating biomarkers of neuronal degeneration and neuroinflammation in CSF of patients with multiple sclerosis-osteopontin as a potential marker of clinical severity. J. Neurol. Sci. 2013;331:38–42. doi: 10.1016/j.jns.2013.04.024. [DOI] [PubMed] [Google Scholar]
- 170.Zeydan B., Lowe V.J., Reichard R.R., Przybelski S.A., Lesnick T.G., Schwarz C.G., Senjem M.L., Gunter J.L., Parisi J.E., Machulda M.M., et al. Multiple sclerosis is associated with lower amyloid but normal tau burden on PET. Alzheimer’s Dement. 2020;16:e039179. doi: 10.1002/alz.039179. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials supporting the results of the present study are available in the published article.