Abstract
Competitive inhibitors of dihydrofolate reductase (DHFR) are used in chemotherapy or prophylaxis of many microbial pathogens, including the eukaryotic parasites Plasmodium falciparum and Toxoplasma gondii. Unfortunately, point mutations in the DHFR gene can confer resistance to inhibitors specific to these pathogens. We have developed a rapid system for testing inhibitors of DHFRs from a variety of parasites. We replaced the DHFR gene from the budding yeast Saccharomyces cerevisiae with the DHFR-coding region from humans, P. falciparum, T. gondii, Pneumocystis carinii, and bovine or human-derived Cryptosporidium parvum. We studied 84 dicyclic and tricyclic 2,4-diaminopyrimidine derivatives in this heterologous system and identified those most effective against the DHFR enzymes from each of the pathogens. Among these compounds, six tetrahydroquinazolines were effective inhibitors of every strain tested, but they also inhibited the human DHFR and were not selective for the parasites. However, two quinazolines and four tetrahydroquinazolines were both potent and selective inhibitors of the P. falciparum DHFR. These compounds show promise for development as antimalarial drugs.
The treatment of diseases caused by eukaryotic pathogens is particularly difficult because of the similarity between their cell biology and that of their human host. The selection for pathogens resistant to currently effective drugs and the increase in immunocompromised individuals have added urgency to the search for new therapies directed specifically against these pathogens. One fruitful avenue for identification of chemotherapeutic drugs is to screen compounds that have already been synthesized in order to identify those that might be active against these increasingly important pathogens. We have adopted this strategy and screened a large library of compounds that are directed against the enzyme dihydrofolate reductase (DHFR) (EC 1.5.1.3). DHFR is a central enzyme in nucleic acid and amino acid synthesis in all cells, but the active sites of enzymes from different organisms show subtle differences that allow the identification of inhibitors specific for a particular species (3, 16–18, 24). For example, pyrimethamine is a selective inhibitor that is effective in the nanomolar range against the DHFRs from Plasmodium falciparum and Toxoplasma gondii, but the human enzyme is relatively insensitive to the drug (8, 14, 24). Thus, pyrimethamine has been used in malaria and toxoplasmosis therapy for many years (9, 49).
We have designed an easy and inexpensive system to test in budding yeast (Saccharomyces cerevisiae) potential DHFR inhibitors against the enzymes from a variety of parasites. Function of the endogenous dfr1 gene was eliminated from the yeast (15), and the defect was complemented by expression of a heterologous DHFR gene from P. falciparum, T. gondii, Pneumocystis carinii, Cryptosporidium parvum, or humans (4). DHFR inhibitors function principally as competitive inhibitors of the enzyme. We have shown that the sensitivity of our engineered yeast strains to DHFR inhibitors depends on the interaction of the drug and the heterologous DHFR enzyme (4, 44, 48). For example, point mutations within the coding region of the P. falciparum DHFR gene can render the enzyme resistant to pyrimethamine. As one would expect, yeast that depends on a pyrimethamine-sensitive (Pyrs) allele of the P. falciparum DHFR gene are killed by treatment with nanomolar concentrations of pyrimethamine, but the same yeast strain dependent upon a mutant pyrimethamine-resistant (Pyrr) allele of DHFR is resistant to the drug. We have expanded this approach to design a rapid screen to identify DHFR inhibitors that are effective against yeast strains that depend upon a series of Pyrr alleles of P. falciparum and against DHFR enzymes from other parasites.
In this paper, we report the analysis of 84 compounds to determine their efficacy against the P. falciparum, T. gondii, C. parvum, P. carinii, and human DHFR enzymes. We have identified six compounds that are potent inhibitors of all of the enzymes. Several of the compounds show selective inhibition of one or several of the parasite enzymes compared with the human DHFR. Among these selective inhibitors, a large number were effective against pyrimethamine-resistant alleles of P. falciparum. These data will allow further refinement of the structure-resistance profiles of these parasite enzymes and the design of more effective, selective inhibitors.
MATERIALS AND METHODS
Yeast strains.
The S. cerevisiae strain used as a recipient of all of the plasmids was the dfr1 mutant TH5 (MATa leu2-3,112 trp1 ura3-52 dfr1::URA3 tup1), generously provided by Tun Huang (15). Yeast was cultured for all experiments at 30°C on minimal, dropout, or rich (yeast extract-peptone-dextrose medium, using standard yeast genetics techniques (1, 13). Growth of the dfr1 mutant was supported by supplementation of the medium with 100 μg of dTMP (Sigma, St. Louis, Mo.) per ml.
The TH5 yeast strain was transfected with a set of vectors that each expressed a heterologous DHFR enzyme. The parent expression vector, pEH2, is derived from pRS314 (45, 48). The DHFR-coding region from P. carinii (Pc-yeast) (11), S. cerevisiae (Sc-yeast) (12), or humans (Hu-yeast) (26) was cloned into the vector flanked at its 5′ end by a portion of the yeast DHFR promoter region and at its 3′ end by a portion of the yeast DHFR terminator, as described in detail by Brophy et al. (4). In Apicomplexan parasites, the DHFR enzyme is one domain of a bifunctional protein that also contains the thymidylate synthase (TS) activity (5, 18, 46). The same plasmid that contains the DHFR and TS domains from T. gondii was a gift from David Roos and Mary Reynolds (27). The DHFR domains from two strains of C. parvum were used, one derived from an infected human (hCp-yeast) and the other from a bovine infection (bCp-yeast) (46). Although the two differ at nine positions, none of these differences occur in amino acids that would be expected to cause changes in drug sensitivity; we have detected no differences in this study or in a previous one (4, 46). A set of yeast strains that expressed the DHFR domain from P. falciparum was also constructed (48). Each strain expressed an allele of P. falciparum DHFR whose sensitivity to the DHFR inhibitor pyrimethamine was known (18). The P. falciparum-derived strains are designated by their amino acid differences compared with the pyrimethamine-sensitive allele (S108). The S108N, N51I + S108N, C59R + S108N, N51I + C59R + S108N, and N51I + C59R + S108N + I164L alleles exhibit progressively higher levels of resistance to pyrimethamine (18). Two novel DHFR alleles (Y57H and I164M) were also tested; both show somewhat higher pyrimethamine resistance than the wild type (22). All of these heterologous DHFR enzymes complemented the dfr1 mutation in the TH5 yeast strain.
Synthesis of the test compounds.
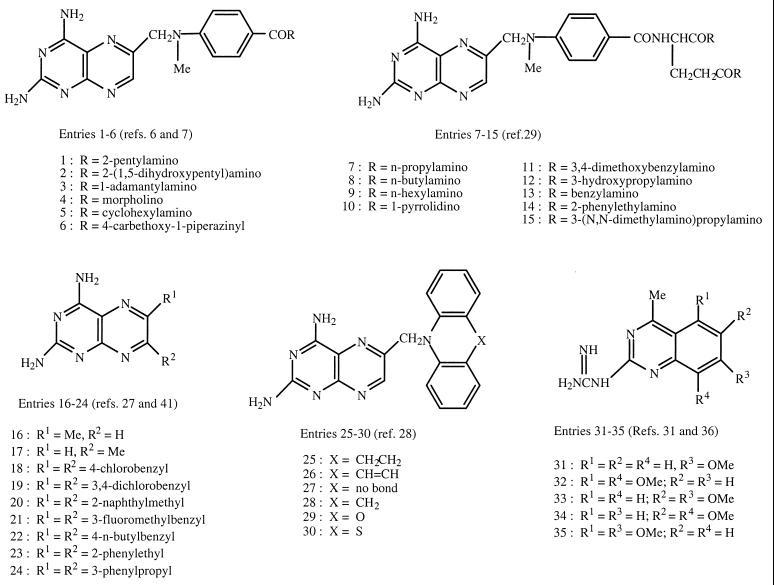
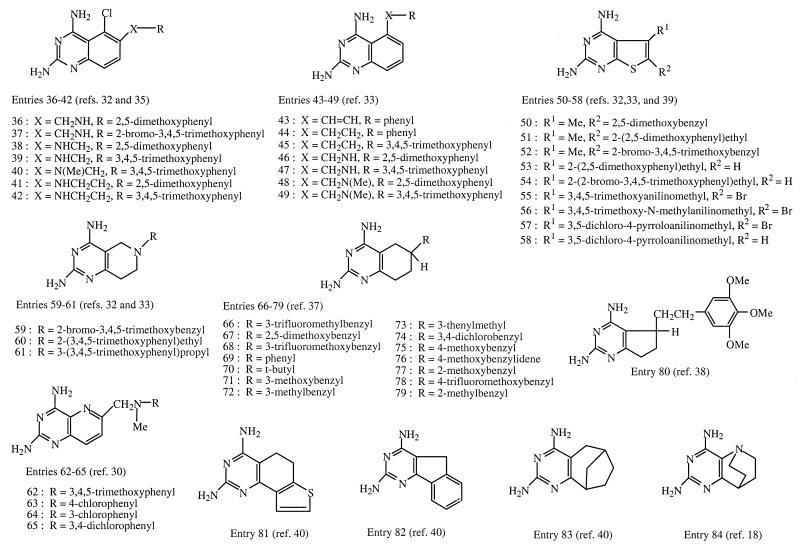
The 84 compounds tested in this work are listed by structure in Fig. 1 and 2. These were archival samples with a purity of ≥90% as determined by thin-layer chromatography. The compounds in entries 1 to 6 were made from 4-[N-(2,4-diaminopteridin-6-yl)methyl-N-methyl]aminobenzoic acid and amines by the mixed anhydride method using isobutyl chloroformate and triethylamine (6, 7), whereas those in entries 7 to 15 were made by reaction of amines with diethyl N-[4-(2,4-diaminopteridin-6-yl)methyl-N-methyl]aminobenzoyl-l-glutamate (methotrexate diethyl ester) (30). 2,4-Diamino-6-methylpteridine (entry 16) and its 7-isomer (entry 17) were synthesized from 2,4,5,6-tetraaminopyrimidine and 1,3-dihydroxyacetone as described previously (43). The 6,7-disubstituted pteridines (entries 18 to 24) were made from 2,4,5,6-tetraaminopyrimidine and 1,2-diketones (28). The pteridines with a diarylamine side chain (entries 25 to 30) were obtained from 2,4-diamino-6-bromomethylpteridine and the appropriate diarylamine (29). The guanidinoquinazolines (entries 31 to 35) were prepared from arylamines, cyanoguanidine, and acetone (32, 38). The 2,4-diamino-5-chloroquinazolines were obtained by reductive coupling of 2,4-diamino-5-chloroquinazoline-6-carbonitrile with an arylamine (entries 36 and 37), by reductive coupling of 2,4,6-triamino-5-chloroquinazoline with an aromatic aldehyde (entries 38, 41, and 42), or by N methylation of a preformed 6-arylmethylaminoquinazoline with formaldehyde and sodium cyanoborohydride (entry 40) (37). The 2,4-diaminoquinazolines with an aromatic substituent at the position 5 were obtained from 2,4-diamino-5-iodoquinazoline and an arylalkene or arylalkyne via a palladium-catalyzed coupling reaction followed by catalytic hydrogenation (entries 43 to 45), by reductive coupling of 2,4-diaminoquinazoline-5-carbonitrile with an arylamine (entries 46 and 47), or by N methylation of a preformed 5-anilinomethylquinazoline (entries 48 and 49) (35). The 5- and 6-substituted 2,4-diaminothieno[2,3-d]pyrimidines (entries 50 to 54) were made from the corresponding 2-aminothiophene-3-carbonitriles and chloroformamidine hydrochoride (33, 36), whereas the 2,4-diamino-6-anilinomethylthieno[2,3-d]pyrimidines (entries 55 to 58) were made via a four-step sequence from 2,4-diamino-5-methylthieno[2,3-d]pyrimidine (41). One member of the latter group (entry 58) was obtained by reductive dehalogenation of the corresponding 6-bromo compound (41). The pyrido[4,3-d]pyrimidines (entries 59 to 61) were made by alkylation of the unsubstituted amine (33, 41), whereas the compounds in entries 62 to 65 were made from 2,4-diamino-6-bromomethylpyrido[3,2-d]pyrimidine and anilines or N-methylanilines (31). The tetrahydroquinazolines (entries 66 to 79) and other several other di- and tricyclic pyrimidines (entries 80 to 84) were made from cyclic ketones and cyanoguanidine (39, 40, 42).
FIG. 1.
Structures of test compounds (entries 1 to 35).
FIG. 2.
Structures of test compounds (entries 36 to 84).
Drug sensitivity assays.
The radial assay used here has been described in detail by Sibley et al. (44) and Brophy et al. (4). Briefly, drugs were dissolved in dimethyl sulfoxide (DMSO) at 10−2 M and stored at −70°C until use. Drug dilutions in DMSO were made just before use and added to the growth medium or plate at a maximum final concentration of 1% DMSO in any solution. Addition of sulfanilamide increased the sensitivity of the yeast strains to the test drugs on plates. Therefore, for drug sensitivity experiments on solid medium, sulfanilamide was spread on the surface of the plate at a final concentration of 1 mM for tests of the complete set of heterologous strains. For some experiments testing only the P. falciparum set, 0.4 mM sulfanilamide was used on the plates. Drug sensitivity tests were made using a double replica plating procedure because this improved the discrimination of growth. A 10-μl volume of the test drug was added directly to the center of the plate. After 3 days of growth, each strain was scored for sensitivity by comparison with growth on the control plate without drug. Each drug was tested in triplicate.
The quantitative drug sensitivity assays were also conducted as previously described (44). Log-phase yeast cells were diluted uniformly into wells of a 96-well plate to generate the final concentrations required. Control wells lacked drug but contained a concentration of DMSO equal to that used in drug treatment; these were scored as 100% growth. The DMSO concentration was always <1%. The optical densities at 650 nm of the various drug dilutions were divided by this control value to determine percentage growth at each drug concentration. The 50% inhibitory concentration (IC50) was calculated using the two values that flanked the 50% mark and the formula y = mx + b, where m and b were the slope and y intercept, respectively, calculated using the two flanking drug concentrations. The solution for x at y = 50% yielded the IC50.
RESULTS AND DISCUSSION
The overall goal of this study was to rapidly screen a set of lipophilic compounds that were designed as inhibitors of DHFR in order to identify those effective against several human pathogens. We were especially interested in identifying inhibitors that are effective against the alleles of DHFR from Pyrr P. falciparum, but the DHFR enzymes from C. parvum, P. carinii, and T. gondii were included as well. The gene that encoded the enzyme from each pathogen was expressed in the same strain of DHFR-deficient S. cerevisiae; the growth of each yeast strain was dependent upon the activity of the heterologous enzyme (4). Two additional specificity controls were included: the same yeast strain dependent on the human DHFR or the S. cerevisiae enzyme expressed from the same single-copy plasmid as the heterologous DHFR genes.
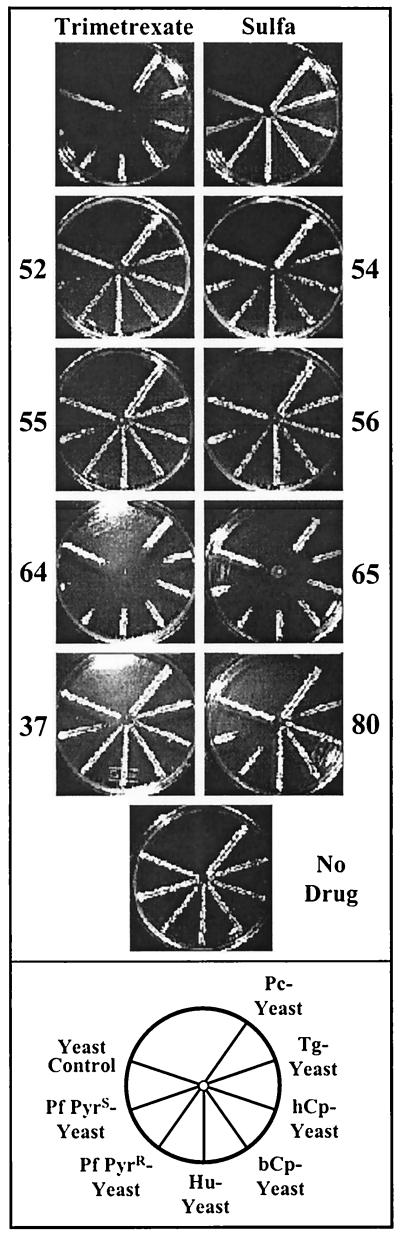
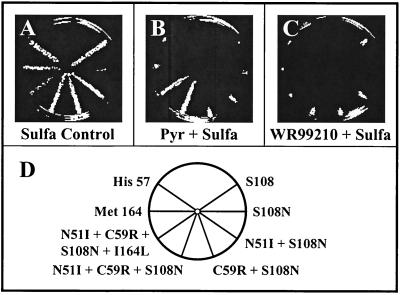
The compounds are listed in Fig. 1 and 2. Each compound was tested in a simple radial assay; the results from a typical experiment are shown in Fig. 3 to illustrate the strategy for screening. The yeast strains were streaked on master plates in a radial pattern as depicted at the bottom of Fig. 3. Eight compounds were tested in a set with two kinds of controls. Trimetrexate is a potent inhibitor of all DHFR enzymes (20) and was included in each series as a positive control. In order to increase sensitivity to the DHFR inhibitor, all plates contained 1 mM sulfanilamide. As a result, the yeasts were also replica plated from a master plate to a plate with sulfanilamide alone to ensure that growth was not inhibited by that addition. Each plate was then made from the master plate and spotted with 10 μl of a 10 mM solution of a test drug dissolved in DMSO. To ensure that the transfer was complete, a final plate with no drug was also included, as shown at the bottom of Fig. 3. In each case, the relative growth of the yeast strain reflected the relative inhibition of the DHFR enzyme expressed by the test strain.
FIG. 3.
Outline of basic screening protocol. Plates shown at the top are those normally included in each set. All plates except the no-drug control contain 1 mM sulfanilamide. The sulfa control shows growth of each yeast strain with no additional drug, and each plate with a designated compound was spotted with 10 μl of a 10-mg/ml solution of the compound dissolved in DMSO as described in Materials and Methods. Each set also contained a test plate with trimetrexate, a drug known to efficiently inhibit all test strains. At the bottom, a map of the location of each strain on the plates is shown. Pc-yeast, dfr1 yeast dependent upon the DHFR enzyme from P. carinii; Tg-yeast, DHFR-TS from T. gondii; hCp-yeast, DHFR-TS from human-derived C. parvum; bCp-yeast, DHFR-TS from bovine-derived C. parvum; Hu-yeast, DHFR from human; Pf PyrR-yeast, DHFR domain from the pyrimethamine-resistant (N51I + S108N) allele of P. falciparum; Pf PyrS-yeast, DHFR domain from the pyrimethamine-sensitive (S108) allele of P. falciparum; yeast control, DHFR gene from S. cerevisiae.
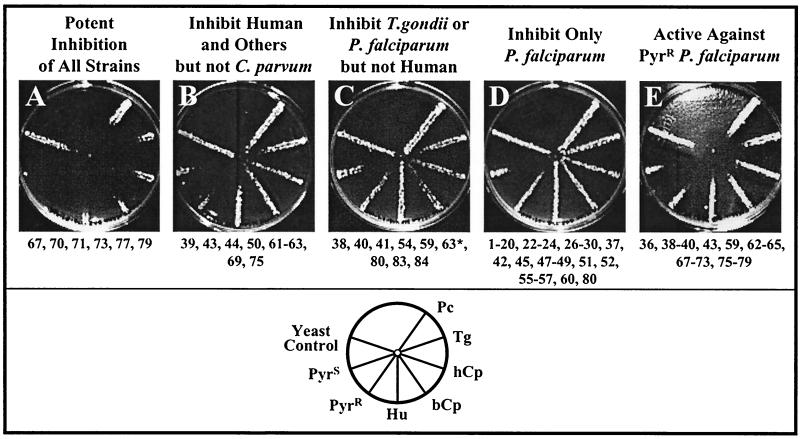
Figure 3 also illustrates that different drugs showed different patterns of inhibition against the set of yeast strains. For example, the two pyrido[3,2-d]pyrimidines, compounds 64 and 65, were potent inhibitors of all of the strains, similar to the pattern displayed by trimetrexate. In contrast, the quinazoline, compound 37, and the thieno[2,3-d]pyrimidines, compounds 55 and 56, inhibited only the pyrimethamine-sensitive enzyme from P. falciparum, while 2,4-diamino-5-[3-(3,4,5-trimethoxyphenyl)ethyl]-6,7-dihydro-5H-cyclopenta[d]pyrimidine, compound 80, was effective against both the Pyrs and Pyrr alleles of the P. falciparum enzyme. Differences of this sort in the patterns of inhibition reflect variations in drug potency (4) and were the basis for our classification of the 84 compounds into five categories. These are summarized in Fig. 4. A table with data for all of the compounds is located on our website (http://depts.washington.edu/genetics/spokeassay.htm).
FIG. 4.
Summary of patterns of drug activity observed on radial assay. The diagram at the bottom shows a typical pattern used to define each category. All drugs were tested three times, and if minor differences were observed, the category was determined by two of three patterns. The protocol was as described in Fig. 3 and in Materials and Methods. ∗, the Hu-yeast was more sensitive to compound 63 than the two Cp-yeast strains but was more resistant than the Pf- or Tg-yeasts, and thus the drug appears in categories panels B, C, and E. A complete table of the data for all of the compounds is available on our website (http://depts.washington.edu/genetics/spokeassay.htm).
There are presently no effective drugs for treatment of C. parvum infections, so we specifically searched for compounds that would inhibit yeast expressing the C. parvum DHFR. Figure 4A shows the typical pattern for the six tetrahydroquinazolines that were extremely potent inhibitors of all of the yeast strains, including those dependent upon C. parvum. The nine compounds shown in Fig. 4B were effective against the human enzyme and at least some others but showed no inhibition of the C. parvum-dependent strains. This group contained three quinazolines, two tetrahydroquinazolines, and four pyrimidine derivatives. The nine compounds listed in Fig. 4C, comprised of pyrimidine derivatives and quinazolines, inhibited P. falciparum and the related pathogen T. gondii. Figure 4D lists a large number of compounds that were specific inhibitors of the P. falciparum enzyme alone. Most of the pteridines tested fell into this category, and a large number of the quinazolines did so as well.
It was of particular interest that a number of compounds were effective inhibitors of the yeast strain that expressed a pyrimethamine-resistant enzyme from P. falciparum; these are displayed in Fig. 4E. Some compounds in this category are also listed in Fig. 4B because they were ineffective against the C. parvum-dependent strains, but these represent an important subset of lead compounds to be studied as potential antimalarial drugs. Some of the pyrido[3,2-d]pyrimidines and most of the tetrahydroquinazolines fell into this category. Some of these compounds had been tested in vitro against the purified DHFR enzymes of P. carinii, T. gondii, or rat liver (39). We assumed that the inhibition of the rat and human enzymes would be similar, and this allowed us to compare the radial assay with the in vitro assay. The correspondence was excellent. For example, the tetrahydroquinazolines were generally potent inhibitors of all of the yeast strains tested and were scored as positive in the radial assay. We noted that neither compound 69 [(6R, 6S)-2,4-diamino-6-phenyl-5,6,7,8-tetrahydroquinazoline] nor compound 74 [(6R, 6S)-2,4-diamino-6-(3,4-dichlorobenzyl)-5,6,7,8-tetrahydroquinazoline] fell into this group. Both of these compounds had shown little or no inhibition in vitro of the purified DHFR enzymes of P. carinii, T. gondii, or rat liver (39), demonstrating the congruence of this yeast-based screening system with the results observed from direct assay of the purified enzyme.
Due to their highly lipophilic nature, some of the compounds were only sparingly soluble in the aqueous agar and precipitated as they were spotted on the plate. For this reason, six compounds (compounds 20, 47, 49, 53, and 55) were not amenable to testing by this assay method, which requires a very high initial concentration of the drug to be spotted in the center of the plate. However, any of these drugs might be effective if tested in vitro, where a lower initial concentration could be used.
These experiments also illustrate an interesting advantage of testing the compounds against living cells. Each assay included the same yeast strain dependent upon expression of the S. cerevisiae DHFR from the same plasmid used to express the heterologous enzymes. Even trimetrexate showed an extremely modest inhibition of this strain (Fig. 3B). If no inhibition of the yeast control was observed, this demonstrated that nonspecific toxicity of that compound was not a problem, at least for yeast cells. Compounds 36, 63 to 68, 70 to 76, 77, and 79 showed this kind of pattern (see Fig. 4B and D for examples). Whenever a compound did inhibit the yeast control, that compound was retested to determine whether addition of dTMP and the drug would restore the yeast growth. This reversal of inhibition was the case for these compounds, and we concluded that the growth deficit resulted from specific inhibition of the folate pathway by the drug. Most of the drugs in this category were in the tetrahydroquinazoline group. The tetrahydroquinazoline compound 78 showed incomplete reversal of growth inhibition in the presence of dTMP; this likely reflects at least some nonspecific inhibition of the yeast growth. Although the inhibition of the yeast enzyme was generally modest, compounds in this group may show promise as leads for development of antifungal agents.
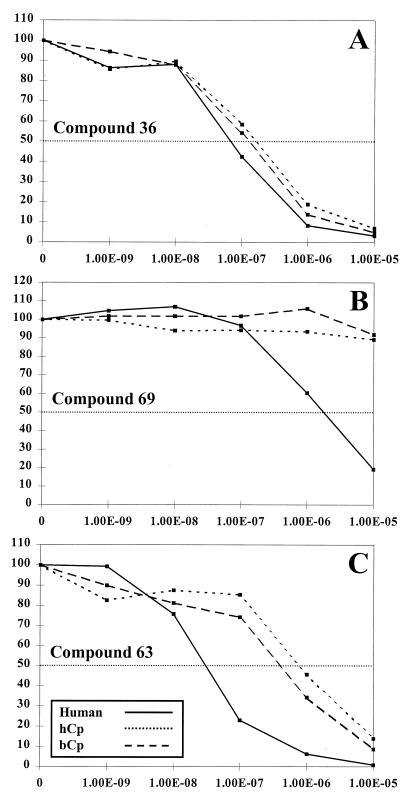
The radial assay is only semiquantitative; to more precisely test the potency and specificity of promising compounds, we grew the yeast strains in a range of drug concentrations in liquid and measured their growth relative to that of the same strain without drug. We first tested the relative effectiveness of the compounds against C. parvum and human DHFR. An example of these data is shown in Fig. 5. Compound 36 (Fig. 5A) had been shown to be a reasonable inhibitor of the P. carinii, T. gondii, and rat liver enzymes in vitro (39), and it was a potent inhibitor of all three strains tested in this assay, showing IC50s in the 10−7 M range. Both compounds 63 and 69 were in the category shown in Fig. 4B and more effectively inhibited the Hu-yeast than the two C. parvum strains. In this liquid growth assay, compound 69 inhibited the Hu-yeast in the micromolar range but was ineffective against the two Cp-yeasts (Fig. 5B). Compound 63 inhibited all three strains, but the IC50 was about 10-fold lower against the Hu-yeast (Fig. 5C). We next focused on correlating the qualitative categories defined by the radial assay with the IC50s for the same compounds. We concentrated on comparing the human- and C. parvum-dependent yeast strains, and the data for 14 compounds are summarized in Table 1. The three compounds (compounds 36, 63, and 69) displayed in Fig. 5 are in boldface in Table 1. In addition, Table 1 lists the published IC50s for the same compounds tested in vitro against the purified enzyme from rat liver. Several conclusions can be drawn. First, compounds that were ineffective in vitro were equally ineffective in the yeast assay (compounds 43, 50, 52, 54, 69, and 81). However, there was one compound, compound 28, that apparently did not penetrate the yeast, since it was without activity against any of the yeast strains, but showed good potency in vitro. Second, the relative effectiveness of the compounds against the rat liver enzyme and the Hu-yeast was similar, as one would expect for two mammalian enzymes. The most effective against rat liver were compounds 36 and 39, and these were also the most potent against the Hu-yeast. This direct comparison allows assessment of the relative sensitivity of the human enzyme and the DHFR from bovine-derived and human-derived C. parvum. Compounds 36 and 39 were also the most effective in this group against the two Cp-yeast strains, but the efficiency against the parasite DHFR was three- to fourfold lower than that against the Hu-yeast. No differences in sensitivity of the two forms of the C. parvum enzyme were observed. The correlation between the qualitative radial assay and the IC50 determinations, along with their correspondence with earlier assays against the purified enzyme, support the utility of the rapid screen as a first step in identification of drugs that are effective against these pathogens.
FIG. 5.
Quantitative determination of efficacy of drugs against Hu-yeast, hCp-yeast, and bCp-yeast. Each strain was grown to log phase and then resuspended in growth medium containing 1 mM sulfanilamide and one of the indicated drugs at a concentration range of 0 to 10−5 M. The growth at each concentration relative to that of the no-drug control was calculated and graphed. Each determination was done in triplicate, and the mean value was used to calculate the growth. The IC50 was calculated as described in Materials and Methods.
TABLE 1.
Comparison of IC50s of 14 compoundsa
| Compound | IC50 (μM) for:
|
Radial assay (Fig. 4)b | |||
|---|---|---|---|---|---|
| In vitro rat enzyme | Hu-yeast | hCp-yeast | bCp-yeast | ||
| 4 | NDc | 10.0 | >10 | >10 | D |
| 28 | 0.003 | >10 | >10 | >10 | D |
| 36 | 0.004 | 0.08 | 0.29 | 0.2 | E |
| 39 | 0.006 | 0.07 | 4.61 | 3.75 | B, E |
| 40 | 0.038 | 0.5 | 0.81 | 0.78 | C, E |
| 43 | 0.51 | 3.0 | >10 | >10 | B, E |
| 50 | 0.4 | 3.8 | >10 | >10 | B |
| 52 | 0.93 | 10.0 | 10.2 | 10.0 | D |
| 54 | 2.8 | >10 | >10 | >10 | C |
| 63 | 0.022 | 0.05 | 0.91 | 0.65 | B, C, E |
| 66 | 0.19 | 0.7 | 5.75 | 3.84 | —d |
| 69 | 1.9 | 3.0 | >10 | >10 | B, E |
| 75 | 0.077 | 0.09 | 0.53 | 0.36 | B, E |
| 81 | 30.0 | >10 | >10 | >10 | — |
The most promising lead that emerged from the qualitative screen was the effectiveness of several categories of compounds against the Pyrr allele of P. falciparum. To examine this lead in more detail, we tested the compounds that were effective against the Pyrr allele further against a set of yeast strains that carry eight different alleles of P. falciparum DHFR with known sensitivity to pyrimethamine. These were arrayed in a radial pattern with the pyrimethamine-sensitive allele (S108) at the upper right and five progressively more pyrimethamine-resistant forms in a clockwise pattern, as shown in Fig. 6D. In addition, two novel alleles with a fivefold elevation in pyrimethamine resistance were included (Met164 and His57) (22). This set of P. falciparum-dependent strains was classified in comparison to their sensitivity to pyrimethamine and to the potent experimental DHFR inhibitor 4,6-diamino-1,2-dihydro-2,2-dimethyl-1-[3′-(2,4,5-trichlorophenoxy)-propyloxy]-1,3,5- triazine hydrochloride (WR99210) (21), as shown in Fig. 6B and C. The yeast that carried the human DHFR allele was not included in this part of the screen because the human enzyme is much more resistant to the test compounds, and we could not have assayed all of the P. falciparum alleles on the same plate had it been included. For these experiments, a lower level of sulfanilamide, 0.4 mM, was present in the plates for the first 20 of these compounds, and no sulfanilamide was used for the remaining 13 compounds. When sulfanilamide was added, a sulfa-only control was always included, and this is depicted in Fig. 6A. In each case, the effectiveness of the test compound was measured against WR99210 or pyrimethamine under the same conditions.
FIG. 6.
Protocol for testing DHFR alleles from P. falciparum. Yeast strains dependent upon the DHFRs from eight different P. falciparum alleles were radially arrayed as shown in panel D. The master plates were grown for 2 to 3 days at 30°C and replica plated to plates that contained 0.4 mM sulfanilamide. Each test plate was spotted with 10 μl of the drug to be tested, and the results were tabulated after an additional 3 days of growth. The control plate with sulfanilamide alone is shown for each set of test plates, since growth of two of the strains was slowed somewhat by the sulfanilamide. Each strain is designated by the genotype of the allele, with the amino acid change from the wild type indicated. The DHFR domains from the first six strains are derived from standard reference strains of P. falciparum whose sensitivity to pyrimethamine has been established. The Met 164 and His 57 alleles are novel mutations that confer a low level of pyrimethamine resistance (21).
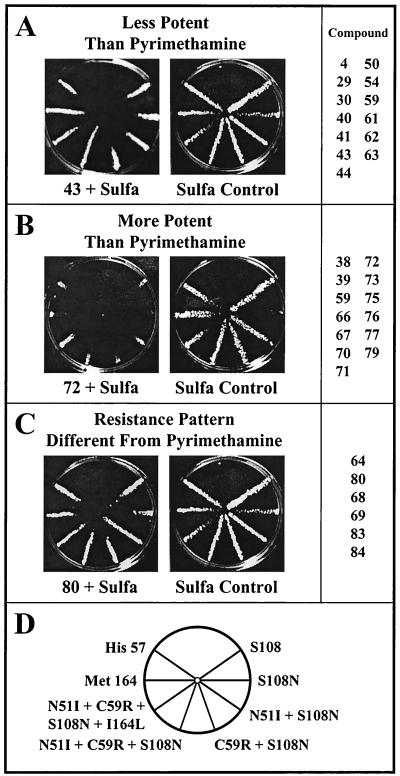
Figure 7A summarizes these data for the 33 compounds that showed activity against the Pyrr P. falciparum. We were interested both in the potency of the compounds and in whether the strains with more mutations showed higher levels of resistance to these drugs, as they do against pyrimethamine (18). Because of the chemical similarity of the compounds, we assumed that diffusion would be similar enough for all compounds to allow us to categorize the drugs qualitatively. Strains were placed in two categories: less or more potent than pyrimethamine (Fig. 7A and B), but with a pattern of resistance similar to that observed for pyrimethamine. The third group (Fig. 7C) included compounds that had a novel pattern of resistance. For example, on the plate shown, the most resistant strain carries the N51I + S108N allele, whereas for most compounds tested, the resistance was highest in strains that carried three or four mutations. Compounds 43, 72, and 80 were then tested in liquid culture to determine the IC50s against four of these DHFR alleles: the most pyrimethamine-sensitive (S108), two double mutant alleles that confer intermediate pyrimethamine resistance (N51I + S108N and C59R + S108N) and the highly resistant allele (N51I + C59R + S108N + I164L). Compounds 26, 70, and 77 were tested against only the highly resistant allele. Table 2 summarizes these data in comparison with both pyrimethamine and WR99210. As expected, pyrimethamine is extremely effective against the S108 allele but shows about a 10-fold higher IC50 against both double mutants and no effect on the quadruple mutant. In contrast, WR99210 was effective against all of the alleles tested, even the highly pyrimethamine-resistant mutant form (21). Among the test compounds, compound 72 showed excellent potency, in the same range as pyrimethamine and WR99210, against all of the alleles except the quadruple mutant. This compound, (6R, 6S)-2,4-diamino-6-(3-methylbenzyl)-5,6,7,8-tetrahydroquinazoline, showed about fourfold selectivity when the purified DHFR of T. gondii was compared in vitro with the human DHFR as well (39). The quinazolines, compounds 38 and 39, and the related tetrahydroquinazolines, compounds 67, 70, 71, and 75, all showed a similar excellent potency and reasonable selectivity, even against the triple (N51I + C59R + S108N) mutant P. falciparum DHFR allele.
FIG. 7.
Summary of efficacy of drugs tested against eight different P. falciparum alleles of DHFR. The drugs tested were categorized in comparison to the potency of pyrimethamine and the experimental DHFR inhibitor WR99210. (A and B) Compounds in which the effectiveness of the drug against the set of reference alleles was similar to the pattern for pyrimethamine. (C) Compounds in which the relative effectiveness of the drug against the reference alleles was different from the pattern for pyrimethamine.
TABLE 2.
Efficacy of selected compounds against four reference strains of P. falciparum DHFR
| DHFR allele | IC50 (μM) of compound:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Pyrimethamine | WR99210 | 26 | 43 | 70 | 72 | 77 | 80 | |
| S108 | 0.007 | 0.01 | NDa | 0.5 | ND | 0.017 | ND | 4.4 |
| N51I + S108N | 0.07 | 0.06 | ND | 3.0 | ND | 0.05 | ND | 7.2 |
| C59R + S108N | 0.07 | 0.06 | ND | 4.4 | ND | 0.08 | ND | 9.5 |
| N51I + C59R + S108N + I164L | >10 | 0.07 | >10 | >10 | 10.0 | 2.9 | 3.7 | >10 |
ND, not determined.
The pattern of resistance of P. falciparum DHFR to pyrimethamine is well studied. In that case, the loss of potency is gradual as one examines DHFR alleles with increasing numbers of mutations. The pattern for the tetrahydroquinazolines is different; the drugs efficiently inhibit all of the alleles with one to three mutations. The change from isoleucine to leucine at amino acid 164 has a profound effect, abrogating the effectiveness of all drugs in this class. Only WR99210 was effective against the quadruple mutant allele (N51I + C59R + S108N + I164L). This mutant has not yet been observed in African populations of P. falciparum (2, 19, 23, 25, 47), and thus a compound effective against the common double and triple mutant alleles is an extremely interesting lead for further development.
While DHFR inhibitors are extremely effective drugs, point mutations have been rapidly selected in P. falciparum populations whenever they have been used. The mutations selected by pyrimethamine have been clearly identified, and their pattern of sensitivity seems to be similar in the set of compounds studied here (10, 18). However, identification of inhibitors still effective against the most pyrimethamine-resistant alleles holds the potential for reversing this pattern. WR99210 is one example of such a situation; some mutations that confer resistance to WR99210 increase the sensitivity to pyrimethamine (48). If other compounds also show this “opposing selection,” it may be possible to slow the selection of parasites resistant to these newer DHFR inhibitors.
The large libraries of already-synthesized inhibitors of DHFR are a potentially fruitful source of lead compounds. Several of the tetrahydroquinazolines surveyed here show real promise both for more detailed analysis of the active site of P. falciparum and for further development as antimalarials or drugs effective against T. gondii. The ease with which this yeast-based assay is performed and the opportunity to screen compounds against DHFR enzymes from a variety of pathogens make it a reasonable first step in narrowing the search for drugs that are effective against these infectious diseases of humans or domestic animals.
ACKNOWLEDGMENTS
We thank Khang Le for his assistance with this study and the Sibley lab for their support.
This work received financial support from National Institutes of Health grants AI 42321 (to C.H.S.) and AI 29904 (to A.R.).
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. 2. J. New York, N.Y: Wiley; 1991. [Google Scholar]
- 2.Basco L K, Ringwald P. Molecular epidemiology of malaria in Yaounde, Cameroon I. Analysis of point mutations in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Am J Trop Med Hyg. 1998;58:369–373. doi: 10.4269/ajtmh.1998.58.369. [DOI] [PubMed] [Google Scholar]
- 3.Blakley R L. Eukaryotic dihydrofolate reductase. Adv Enzymol Mol Biol. 1995;70:23–102. doi: 10.1002/9780470123164.ch2. [DOI] [PubMed] [Google Scholar]
- 4.Brophy V H, Vasquez J, Nelson R G, Forney J R, Rosowsky A, Sibley C H. Identification of Cryptosporidium parvum dihydrofolate reductase inhibitors by complementation in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 2000;44:1019–1028. doi: 10.1128/aac.44.4.1019-1028.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bzik D J, Li W B, Horii T, Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci USA. 1987;84:8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaykovsky M, Brown B L, Modest E J. Methotrexate analogs. 6. Replacement of glutamic acid by various amino acid esters and amines. J Med Chem. 1975;18:909–912. doi: 10.1021/jm00243a010. [DOI] [PubMed] [Google Scholar]
- 7.Chaykovsky M, Rosowsky A, Papathanasopoulos N, Chen K K, Modest E J, Kisliuk R L, Gaumont Y. Methotrexate analogs. 3. Synthesis and biological properties of some side-chain altered analogs. J Med Chem. 1974;17:1212–1216. doi: 10.1021/jm00257a015. [DOI] [PubMed] [Google Scholar]
- 8.Chio L C, Queener S F. Identification of highly potent and selective inhibitors of Toxoplasma gondii dihydrofolate reductase. Antimicrob Agents Chemother. 1993;37:1914–1923. doi: 10.1128/aac.37.9.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clyde D F. Observations on monthly pyrimethamine prophylaxis in an East African village. East Afr Med J. 1953;31:41–46. [PubMed] [Google Scholar]
- 10.Cowman A F. The mechanisms of drug action and resistance in malaria. Mol Genet Drug Resist. 1997;3:221–246. [Google Scholar]
- 11.Edman J C, Edman U, Cao M, Lundgren B, Kovacs J A, Santi D V. Isolation and expression of the Pneumocystis carinii dihydrofolate reductase gene. Proc Natl Acad Sci USA. 1989;86:8625–8629. doi: 10.1073/pnas.86.22.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fling M E, Kopf J, Richards C A. Nucleotide sequence of the dihydrofolate reductase gene of Saccharomyces cerevisiae. Gene. 1988;63:165–174. doi: 10.1016/0378-1119(88)90522-7. [DOI] [PubMed] [Google Scholar]
- 13.Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. Vol. 194. San Diego, Calif: Academic Press; 1991. [Google Scholar]
- 14.Hitchings G S, Burchall J J. Inhibition of folate biosynthesis and function as a basis for chemotherapy. Adv Enzymol. 1965;27:417–468. doi: 10.1002/9780470122723.ch9. [DOI] [PubMed] [Google Scholar]
- 15.Huang T, Barclay B J, Kalman T I, VonBorstel R C, Hastings P J. The phenotype of a dihydrofolate reductase mutant in Saccharomyces cerevisiae. Gene. 1992;121:167–171. doi: 10.1016/0378-1119(92)90177-q. [DOI] [PubMed] [Google Scholar]
- 16.Huennekens F M. In search of dihydrofolate reductase. Protein Sci. 1996;5:1201–1208. doi: 10.1002/pro.5560050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huennekens F M. The methotrexate story: a paradigm for development of cancer chemotherapeutic agents. Adv Enzyme Regul. 1994;34:397–419. doi: 10.1016/0065-2571(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 18.Hyde J E. The dihydrofolate reductase-thymidylate synthase gene in the drug resistance of malaria parasites. Pharmacol Ther. 1990;48:45–59. doi: 10.1016/0163-7258(90)90017-v. [DOI] [PubMed] [Google Scholar]
- 19.Jelinek T, Ronn A M, Lemnge M M, Curtis J, Mhina J, Duraisingh M T, Bygbjerg I C, Warhurst D C. Polymorphisms in the dihydrofolate reductase (DHFR) and dihydropteroate synthetase (DHPS) genes of Plasmodium falciparum and in vivo resistance to sulfadoxine/pyrimethamine in isolates from Tanzania. Trop Med Int Health. 1998;3:605–609. doi: 10.1046/j.1365-3156.1998.00280.x. [DOI] [PubMed] [Google Scholar]
- 20.Kuyper L F, Baccanari D P, Jones M L, Hunter R N, Tansik R L, Joyner S S, Boytos C M, Rudolph S K, Knick V, Wilson H R, Caddell J M, Friedman H S, Comley J C, Stables J N. High-affinity inhibitors of dihydrofolate reductase: antimicrobial and anticancer activities of 7,8-dialkyl-1,3-diaminopyrrolo[3,2-f]quinazolines with small molecular size. J Med Chem. 1996;39:892–903. doi: 10.1021/jm9505122. [DOI] [PubMed] [Google Scholar]
- 21.Milhous W K, Weatherly N F, Bowdre J H, Desjardins R E. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob Agents Chemother. 1985;27:525–530. doi: 10.1128/aac.27.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mookherjee S, Howard V, Nzila-Mouanda A, Watkins W, Sibley C H. Identification and analysis of dihydrofolate reductase alleles from Plasmodium falciparum present at low frequency in polyclonal patient samples. Am J Trop Med Hyg. 1999;61:131–140. doi: 10.4269/ajtmh.1999.61.131. [DOI] [PubMed] [Google Scholar]
- 23.Nzila-Mounda A, Mberu E K, Sibley C H, Plowe C V, Winstanley P A, Watkins W M. Kenyan Plasmodium falciparum field isolates: correlation between pyrimethamine and chlorcycloguanil activity in vitro and point mutations in the dihydrofolate reductase domain. Antimicrob Agents Chemother. 1998;42:164–169. doi: 10.1128/aac.42.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peters W. Chemotherapy and drug resistance in malaria. 2nd ed. London, United Kingdom: Academic Press; 1987. [Google Scholar]
- 25.Plowe C V, Djimde A, Wellems T E, Diop S, Kouriba B, Doumbo O K. Community pyrimethamine-sulfadoxine use and prevalence of resistant Plasmodium falciparum genotypes in Mali: a model for deterring resistance. Am J Trop Med Hyg. 1996;55:467–471. doi: 10.4269/ajtmh.1996.55.467. [DOI] [PubMed] [Google Scholar]
- 26.Prendergast N J, Delcamp T J, Smith P L, Freisheim J H. Expression and site-directed mutagenesis of human dihydrofolate reductase. Biochemistry (Moscow) 1988;27:3664–3671. doi: 10.1021/bi00410a022. [DOI] [PubMed] [Google Scholar]
- 27.Reynolds M G, Roos D S. A biochemical and genetic model for parasite resistance to antifolates. Toxoplasma gondii provides insights into pyrimethamine and cycloguanil resistance in Plasmodium falciparum. J Biol Chem. 1998;273:3461–3469. doi: 10.1074/jbc.273.6.3461. [DOI] [PubMed] [Google Scholar]
- 28.Rosowsky A, Chaykovsky M, Lin M, Modest E J. Pteridines. 2. New 6,7-disubstituted pteridines as potential antimalarial and antitumor agents. J Med Chem. 1973;16:869–875. doi: 10.1021/jm00266a001. [DOI] [PubMed] [Google Scholar]
- 29.Rosowsky A, Cody V, Galitsky N, Fu H, Papoulis A T, Queener S F. Structure-based design of selective inhibitors of dihydrofolate reductase: synthesis and antiparasitic activity of 2,4-diaminopteridine analogues with a bridged diarylamine side chain. J Med Chem. 1999;42:4853–4860. doi: 10.1021/jm990331q. [DOI] [PubMed] [Google Scholar]
- 30.Rosowsky A, Ensminger W D, Lazarus H, Yu C S. Methotrexate analogs. 8. Synthesis and biological evaluation of bisamide derivatives of potential prodrugs. J Med Chem. 1978;21:925–930. doi: 10.1021/jm00217a012. [DOI] [PubMed] [Google Scholar]
- 31.Rosowsky A, Forsch R A, Queener S F. 2,4-Diaminopyrido[3,2-d]pyrimidine inhibitors of dihydrofolate reductase from Pneumocystis carinii and Toxoplasma gondii. J Med Chem. 1995;38:2615–2620. doi: 10.1021/jm00014a014. [DOI] [PubMed] [Google Scholar]
- 32.Rosowsky A, Modest E J. Chemical and biological studies on dihydro-s-triazines. XVI. NMR evidence for the formation of 2-guanidino-4-methylquinazolines as anomalous byproducts in the three-component synthesis. J Heterocycl Chem. 1972;9:637–643. [Google Scholar]
- 33.Rosowsky A, Mota C E, Queener S F. Brominated trimetrexate analogues as inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Heterocycl Chem. 1996;33:1959–1966. [Google Scholar]
- 34.Rosowsky A, Mota C E, Queener S F. Synthesis and antifolate activity of 2,4-diamino-5,6,7,8-tetrahydropyrido[4,3-d]pyrimidine analogues of trimetrexate and piritrexim. J Heterocycl Chem. 1995;32:335–340. [Google Scholar]
- 35.Rosowsky A, Mota C E, Queener S F, Waltham M, Ercikan-Abali E, Bertino J R. 2,4-Diamino-5-substituted-quinazolines as inhibitors of a human dihydrofolate reductase with a site-directed mutation at position 22 and of the dihydrofolate reductases from Pneumocystis carinii and Toxoplasma gondii. J Med Chem. 1995;38:745–752. doi: 10.1021/jm00005a002. [DOI] [PubMed] [Google Scholar]
- 36.Rosowsky A, Mota C E, Wright J E, Freisheim J H, Heusner J J, McCormack J J, Queener S F. 2,4-Diaminothieno[2,3-d]pyrimidine analogues of trimetrexate and piritrexim as potential inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Med Chem. 1993;36:3103–3112. doi: 10.1021/jm00073a009. [DOI] [PubMed] [Google Scholar]
- 37.Rosowsky A, Mota C E, Wright J E, Queener S F. 2,4-Diamino-5-chloroquinazoline analogues of trimetrexate and piritrexim: synthesis and antifolate activity. J Med Chem. 1994;37:4522–4528. doi: 10.1021/jm00052a011. [DOI] [PubMed] [Google Scholar]
- 38.Rosowsky A, Nadel M E, Modest E J. Chemical and biological studies on dihydro-s-triazines. XVII. Modifications of the three-component synthesis favoring the formation of 2-guanidino-4-methylquinazoline byproducts. J Heterocycl Chem. 1972;9:645–650. [Google Scholar]
- 39.Rosowsky A, Papoulis A T, Forsch R A, Queener S F. Synthesis and antiparasitic and antitumor activity of 2,4-diamino-6-(arylmethyl)-5,6,7,8-tetrahydroquinazoline analogues of piritrexim. J Med Chem. 1999;42:1007–1017. doi: 10.1021/jm980572i. [DOI] [PubMed] [Google Scholar]
- 40.Rosowsky A, Papoulis A T, Queener S F. 2,4-Diamino-6,7-dihydro-5H-cyclopenta[d]pyrimidine analogues of trimethoprim as inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Med Chem. 1998;41:913–918. doi: 10.1021/jm970614n. [DOI] [PubMed] [Google Scholar]
- 41.Rosowsky A, Papoulis A T, Queener S F. 2,4-Diaminothieno[2,3-d]pyrimidine lipophilic antifolates as inhibitors of Pneumocystis carinii and Toxoplasma gondii dihydrofolate reductase. J Med Chem. 1997;40:3694–3699. doi: 10.1021/jm970399a. [DOI] [PubMed] [Google Scholar]
- 42.Rosowsky A, Papoulis A T, Queener S F. One-step synthesis of novel 2,4-diaminopyrimidine antifolates from bridged alicyclic ketones and cyanoguanidine. J Heterocycl Chem. 1999;36:723–728. [Google Scholar]
- 43.Seeger D R, Cosulich D B, Smith J M, Jr, Hultquist M E. Analogs of pteroylglutamic acid. III. 4-Amino derivatives. J Am Chem Soc. 1949;71:1753–1759. doi: 10.1021/ja01170a066. [DOI] [PubMed] [Google Scholar]
- 44.Sibley C H, Brophy V H, Cheesman S, Hamilton K L, Hankins E G, Wooden J M, Kilbey B. Yeast as a model system to study drugs effective against apicomplexan proteins. Methods. 1997;13:190–207. doi: 10.1006/meth.1997.0511. [DOI] [PubMed] [Google Scholar]
- 45.Sikorski R S, Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vasquez J R, Gooze L, Kim K, Gut J, Petersen C, Nelson R G. Potential antifolate resistance determinants and genotypic variation in the bifunctional dihydrofolate reductase-thymidylate synthase gene from human and bovine isolates of Cryptosporidium parvum. Mol Biochem Parasitol. 1996;79:153–165. doi: 10.1016/0166-6851(96)02647-3. [DOI] [PubMed] [Google Scholar]
- 47.Wang P, Lee C S, Bayoumi R, Djimde A, Doumbo O, Swedberg G, Dao L D, Mshinda H, Tanner M, Watkins W M, Sims P F G, Hyde J E. Resistance to antifolates in Plasmodium falciparum monitored by sequence analysis of dihydropteroate synthetase and dihydrofolate reductase alleles in a large number of field samples of diverse origins. Mol Biochem Parasitol. 1997;89:161–177. doi: 10.1016/s0166-6851(97)00114-x. [DOI] [PubMed] [Google Scholar]
- 48.Wooden J M, Hartwell L H, Vasquez B, Sibley C H. Analysis in yeast of antimalaria drugs that target the dihydrofolate reductase of Plasmodium falciparum. Mol Biochem Parasitol. 1997;85:25–40. doi: 10.1016/s0166-6851(96)02808-3. [DOI] [PubMed] [Google Scholar]
- 49.Zumla A, Croft S L. Chemotherapy and immunity in opportunistic parasitic infections in AIDS. Parasitology. 1992;105:S93–S101. doi: 10.1017/s0031182000075405. [DOI] [PubMed] [Google Scholar]