Abstract

Axially chiral 2-(2-(trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoic acid (TBBA) was used as a chiral derivatizing agent to evaluate the limits of absolute configuration assignment for β-chiral aminoalcohols. Seven Boc-aminoalcohols and eight variously N-substituted (S)-phenylglycinols were prepared, and their TBBA esters were analyzed by NMR spectroscopy. Diverse substitution at the β-position was employed to demonstrate the effect of structure on the general conformational model and reliability of the absolute configuration assignment. It was concluded that hydrogen bond formation and steric hindrance were the main factors affecting the correct assignment for Boc-aminoalcohols.
Introduction
The assignment of the absolute configuration of chiral compounds is an essential part of structure elucidation in chemistry. For this purpose, NMR spectroscopy is a valuable tool1 among other available analytical techniques, such as X-ray crystallography,2 circular dichroism (ECD, VCD),3 or other chiroptical methods to determine the absolute configuration. Most commonly, NMR methods designed for the assignment of absolute configurations use chemical derivatization with various chiral derivatization agents (CDAs) to convert the analyte into two diastereomers, and NMR spectra (commonly 1H or 13C) are compared.4−7 Then, the observed chemical shift differences are employed in a proposed conformational model to determine the spatial arrangement of substituents at the chiral center. Conformational models are generally based on NMR analyses of known chiral compounds and in silico calculations. The reliability of the most common CDAs has been assessed by multiple investigations, where diverse structural motifs have been evaluated to explore the scope and limits.5,8−11
Recently, we reported a benzimidazole-based axially CDA, 2-(2-(trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoic acid (TBBA), and its application toward α-chiral4 and β-chiral compounds.12 While the presented conformational model (Figure 1a–c) was reliable for various β-chiral analytes, the analysis of (S)-Boc-phenylglycinol assigned the opposite configuration (Figure 1d). We suspected that a hydrogen bond between the carbamate NH group and CF3 group locked the compound in a different conformation. This finding was unexpected since various aminoalcohols, which were converted with TBBA into amides with free hydroxyl groups, did not display any significant deviation from the proposed model due to the hydrogen bond(s).4 However, it was also reported that the presence of a polar group at the chiral center could change the conformational equilibrium.10 For these reasons, we decided to gain deeper insight into the limitations of TBBA as a CDA for β-chiral aminoalcohols. This study is focused on the relationship between the structure of aminoalcohols and the shielding effect of the TBBA benzimidazole ring.
Figure 1.
(a) Shielding effect of the (P)-TBBA benzimidazole group on the NHR2 substituent, (b) shielding effect of the (M)-TBBA benzimidazole group on the R1 substituent, (c) conformational model for analysis of primary β-chiral alcohols, and (d) inverted values of ΔδPM observed for (S)-Boc-phenylglycinol.
Results and Discussion
To explore whether anomalous ΔδPM values of (S)-Boc-phenylglycinol 1 are an exception or trend from the previously proposed model (Figure 1),12 we prepared more structurally diverse TBBA esters of Boc-aminoalcohols (Figure 2).
Figure 2.
Analyzed Boc-aminoalcohols 1–7 and their ΔδPM (ΔδPM = δR(P) – δR(M)) values in CDCl3 as TBBA esters. 9-Anthrylmethoxyacetic acid (9-AMA) values are shown in green. The values of 9-AMA esters for compounds 3 and 6 represent the inverse absolute configuration of Boc-aminoalcohols.
The Boc group showed a very low ΔδPM value (−0.002) in (S)-Boc-phenylglycinol 1 (Figure 2). However, based on the remaining ΔδPM differences at the ortho-H of the phenyl ring (−0.1) and NH group (+0.06), the opposite configuration would be deduced (Figure 1). The ΔδPM value for the Boc group (−0.002) could be considered anomalous and negligible in practical use to assign the absolute configuration of unknown compounds. Partial epimerization (approx. 10%) was observed during the preparation of 1; nevertheless, it did not hamper the assignment of the NMR signals and absolute configuration.
Other derivatives shown in Figure 2 also followed the anomalous trend in sign distribution in contrast to the previously proposed model. Boc-cyclohexylglycinol 2 showed a reliable distribution of ΔδPM: +0.53 for NH, +0.02 for Boc, and −0.1 ppm for the cyclohexyl CH proton. The substitution of the cyclohexyl ring for the less sterically demanding isopropyl group in valinol 3 achieved a similar distribution of ΔδPM values: −0.38 for NH; −0.01 for Boc; and +0.05, +0.06, and +0.15 for the isopropyl group (reversed values due to the opposite configuration). Further simplification of the structure by substituting the isopropyl group with a methyl group in alaninol 4 led to a slight change in the magnitudes of ΔδPM: the NH group now displayed a value of only +0.13 ppm, which is significantly smaller than that observed for compounds 2 and 3.
The substitution of methyl for benzyl in phenylalaninol 5 significantly increased the ΔδPM value at the benzylic position (−0.39 and −0.09 vs −0.11 in 4) and NH group (+0.47 vs +0.13 in 4). Further substitution of phenyl in 5 for isopropyl in leucinol 6 caused a reduction in the magnitude of ΔδPM. The amino group displayed a value of −0.3 ppm, while the methylene group showed a value of +0.19 ppm, which is significantly less than that of the similar methylene group in 5. The more remote isopropyl hydrogen atoms in 6 showed a difference of less than 0.1 ppm. Finally, the more polar benzyl-protected hydroxymethyl group of serine 7 caused a significant drop in ΔδPM values: −0.04 ppm for both methylene and benzyl protons and only +0.08 ppm for the NH unit in the carbamate group. The methyl groups in the Boc moiety showed no measurable difference among the diastereomers.
In addition, we also added reported values of analogous esters with 9-anthrylmethoxyacetic acid (9-AMA) for a comparison to Figure 2 since 9-AMA is also capable of projecting a strong shielding effect on remote positions.13 Chemical shift differences of analogous esters with Mosher’s acid were not reported in the literature.
Since the experimental results summarized in Figure 2 suggested a strong influence of the Boc group, we decided to continue with variously N-substituted (S)-phenylglycinols (Figure 3). First, we prepared N-methylated Boc-phenylglycinol 8 to remove any possible hydrogen bonding between the NH group and the hydrogen bond acceptor. The eliminated hydrogen bonding did not switch the sign distribution according to the previous model (Figure 1), but the observed ΔδPM value of ortho-Ar-H was smaller than that of nonmethylated derivative 1. A decreased ΔδPM value in 8 further indicates the presence of hydrogen bonds in conformational equilibrium.
Figure 3.
N-substituted (S)-phenylglycinols 8–15. ΔδPM values of minor rotamers are underlined.
Dimethylamino derivative 9 without a Boc group fully followed the previously proposed conformational model (−0.1 for methyl groups and +0.08 and +0.03 for the phenyl ring). The substitution of the Boc group for a smaller acetyl group in 10 showed significant differences compared to 1: +0.13 and +0.02 for acetyl and NH, respectively, and −0.2 and −0.11 for aromatic protons with the opposite sign distribution to the conformational model again.
To rule out NH as the hydrogen bond donor, N-methylated analogue 11 was synthesized and isolated as a mixture of rotamers, which complicated the structural assignment. Nevertheless, the sign distribution of the major isomer (+0.17, +0.09, and −0.01) did not follow the conformational model, as was previously observed for Boc analogue 8, and moreover, the minor rotamer complicated the configuration assignment with the irregular sign distribution: −0.17 for the acetyl group, +0.11 for the methyl group, and +0.03 for the phenyl ring. Further reduction of sterically bulkier acetyl to formyl did not offer an improvement. Compound 12 was isolated again as a mixture of two rotamers, showing an ambiguous sign distribution of ΔδPM for both rotamers.
Total N-deprotection of 1 with trifluoroacetic acid (TFA) yielded aminoester 13, which fully followed the conformational model, with ΔδPM values of −0.29 ppm for the amino group and +0.05 and +0.01 for the phenyl ring.
Dibenzyl derivative 14 and phthalimide 15 were prepared to evaluate the influence of synthetically interesting N-substituted groups that serve as ammonia equivalents.14,15
Derivative 14 displayed positive ΔδPM values at the phenyl ring for the ortho and meta protons (+0.18 and +0.02, respectively) and an anomalous value of −0.01 for the most remote para position. The benzyl groups complicated the assignment, with a zero difference at the aromatic hydrogens and opposing ΔδPM values at the benzylic methylene protons (+0.02 and −0.03). Uncertain evidence of the absolute configuration by 1H NMR spectra was arbitrated by the differences in 13C signals. The quaternary carbon atom in the phenyl ring displayed a ΔδPM value of +0.06 ppm, while a value of −0.07 ppm was observed for the benzylic methylene carbon atoms.
Ester 15 displayed a +0.01 ppm difference at the phthalimide hydrogen atoms, while the phenyl protons showed ΔδPM values of −0.04, +0.05, and +0.01 ppm for the ortho, meta, and para protons, respectively. Then, we analyzed the 13C NMR spectra to resolve the observed inconsistency in the sign distribution of the ΔδPM values. A positive difference (+0.19 ppm) was observed for the carbonyl carbon atoms, and a negative difference (−0.07 ppm) was observed for the quaternary carbon atom in the phenyl ring.
The chemical shift differences of TBBA esters in Figure 3 clearly revealed the significant role of the N-carbonyl moiety present as a carbamate 1–8, amide 10–12, or imide 15 functionality to change the equilibrium of conformers.
The supposed effect of the NHBoc group on conformational equilibrium in a nonpolar solvent was studied with software Spartan 18 (B3LYP-D3/6-31G*) to identify the theoretical lowest-energy conformers of 1 (Figure 4). It was revealed that the Boc group is always located out of the shielding zone of the benzimidazole cycle, which is in accordance with the observed small chemical shift differences of tert-butyl hydrogens. The position of the phenyl depends on the presence of intramolecular hydrogen bonds. If formed, the phenyl was positioned inside of the shielding zone of TBBA in (P)-1 (Figure 4a). Oppositely, the phenyl was located outside without an intramolecular hydrogen bond in (M)-1 (Figure 4b). These calculations were in agreement with the negative difference assigned for the ortho-positioned hydrogens of TBBA ester 1.
Figure 4.

Theoretical lowest-energy conformers of 1 in a nonpolar solvent (software Spartan 18). The conformer distribution was calculated with the MMFF model (≤100 kJ/mol), followed by the calculation of energy at the ground state using DFT in a nonpolar solvent (B3LYP-D3/6-31G*) to account for long-range nonbonded dispersion interactions. Hydrogens were omitted for clarity. (a) Most stable conformer of (P)-1 (Boltzmann weight: 0.901) with the hydrogen bond (light-blue dashed line) between the NHBoc and benzimidazole nitrogen. (b) Most stable conformer of (M)-1 (Boltzmann weight: 0.627) without hydrogen bonds. Please see the Supporting Information for more details.
The calculations showed the formation of intramolecular hydrogen bonds predominantly between the NHBoc and benzimidazole nitrogen at position 3. Since the NMR spectra did not show interactions between fluorine and hydrogen atoms (please see more details in the Supporting Information), we can exclude a strong hydrogen bonding between these atoms. The positive difference (+0.06) of NH hydrogen in phenylglycinol 1 (Figure 2) can be attributed to the higher ratio of conformers with intramolecular hydrogen bonds in diastereomer (P)-1 compared to (M)-1 (please see the Supporting Information for more details).
To evaluate the presence of the hydrogen bond, we conducted simple 1H NMR experiments in acetone-d6 as a possible hydrogen bond acceptor (Figure 5).
Figure 5.
Comparison of observed ΔδPM values in CDCl3 (black) and acetone-d6 (red) for compounds 1–8. Esters 1 and 4 were also measured in acetonitrile-d3 (blue).
The solvent change had a significant effect on the TBBA esters. Complete inversion of the ΔδPM sign was observed in two cases (Figure 5; compounds 1 and 4), and this effect was further confirmed in acetonitrile-d3. A partial inversion of ΔδPM was revealed in the case of derivatives 3, 5, and 7. Esters 2 and 6 did not show partial inversion; however, the magnitude of the difference approached the inverted value. Further evidence of intermolecular hydrogen bonds between the NH hydrogen and acetone carbonyl illustrates a drop of all NH differences in esters 1–7 since both diastereomers (P)- and (M)-TBBA participate more evenly in hydrogen bonding with acetone. A less significant decrease of ΔδPM for the NH hydrogens in 7 can originate from competitive formation of an intramolecular hydrogen bond between the NH and ether oxygen. As expected, no significant change was observed for ΔδPM in the case of N-methylated derivative 8. The change in chemical shift differences in Figure 5 supports the formation of hydrogen bonds, but the influence of steric hindrance (ester 2 vs 4) is also evident.
Conclusions
In summary, a small library of β-chiral N-Boc aminoalcohol TBBA esters was prepared. Their NMR spectra confirmed incongruity with the previously reported conformation model for β-chiral primary alcohols.12 Further expansion of the library with variously modified N-substituted phenylglycinols revealed similar nonconformity. The increased acidity of the hydrogen atom in the carbamate or amide functionality has a strong influence on the formation of hydrogen bonds capable of changing the conformational equilibrium. Moreover, the repulsion between the N-carbonyl moiety and trifluorobenzimidazole ring significantly impacts the conformer ratio. Both effects cause incorrect assignment since the resulting predominant conformers differ from the general conformational model.
To conclude, the presence of a carbonyl group at the N-substituent, where the nitrogen atom is not a constituent of a ring, is a limitation of the TBBA method to assign absolute configuration of β-chiral primary aminoalcohols. Analysis of such compounds with TBBA should be carried out with caution, and if possible, an alternative method including N-deprotection is much better for analyzing aminoalcohols such as TBBA amides with the chiral carbon at the α-position.4
Experimental Section
All reactions were carried out under normal conditions without any specific precautions to exclude moisture or air from the reaction unless otherwise stated. Reaction workup and column chromatography (CC) were performed with commercial-grade solvents without further purification. 1H NMR, 13C NMR, and 19F NMR spectra were measured on a Jeol ECA400II (400 MHz) or Jeol ECX-500SS (500 MHz) instrument in CDCl3, DMSO-d6, acetone-d6, or acetonitrile-d3 as a solvent. 1H and 13C spectra were calibrated using residual a nondeuterated solvent as an internal reference (7.26 and 77.16 ppm for CDCl3, 2.50 and 39.52 ppm for DMSO-d6, 2.05 and 29.84 ppm for acetone-d6, and 1.94 and 1.32 ppm for acetonitrile-d3). 19F spectra were calibrated by the addition of CFCl3 as an internal reference (δ = 0.0 ppm). All 13C NMR spectra were measured with broad-band 1H decoupling. 1H NMR data are reported as follows: δ, chemical shift; coupling constants (J are given in hertz, Hz), and integration. Abbreviations to denote the multiplicity of a particular signal were s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet), app (appears as), and br (broad).
Analytical thin-layer chromatography (TLC) was performed using Kieselgel 60 F254 plates (Merck). Compounds were detected by UV light (255 nm) and then by basic KMnO4 solution. Flash chromatography was performed using silica gel (35–70 μm particle size). HRMS analyses were carried out using an Exactive Plus Orbitrap high-resolution mass spectrometer with electrospray ionization (Thermo Fisher Scientific, MA, USA). Chromatographic pre-separation was performed using a HPLC system Dionex UltiMate 3000 (Thermo Fisher Scientific, MA, USA) equipped with a Phenomenex Gemini column (C18, 50 × 2 mm, 3.0 μm). The samples were dissolved in MeOH or acetonitrile and injected by an autosampler. Mobile phase compositions: isocratic elution of MeOH/water 95:5 + 0.1% (v/v) HCOOH with a flow rate of 0.3 mL/min.
The calculation of theoretical lowest-energy conformers was done using Spartan 18 (Wavefunction, USA). The conformer distribution was calculated with molecular mechanics applying the MMFF force field (≤100 kJ/mol), followed by the calculation of energy at the ground state using DFT in a nonpolar solvent with the B3LYP-D3 functional (6-31G* basis set) to account for long-range nonbonded (dispersion) interactions.
General Procedure for Esters 1–12, 14, and 15
TBBA (15 mg, 0.05 mmol, 1 equiv) was dissolved in dry DCM (1.5 mL). Then, alcohol (1 equiv, 0.05 mmol), DMAP (6 mg, 0.05 mmol, 1 equiv), and DCC (11 mg, 0.05 mmol, 1 equiv) were added. The mixture was stirred at room temperature for 16 h. After that, the precipitate was filtered off. The resulting filtrate was washed twice with 10% aq. HCl (2 mL) and 10% K2CO3 (2 mL) and once with brine and dried with MgSO4. After evaporation of DCM, the residue was purified by CC. The washing steps can be skipped if the analyte contains labile functional groups.
(P,S)-2-((tert-Butoxycarbonyl)amino)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-1
Purified by CC (hexane/EtOAc
5:1). Yield 15 mg (57%) as an amorphous solid. 1H NMR (400
MHz, CDCl3): δ 8.19 (dd, J = 7.8,
1.5 Hz, 1H), 7.95 (d, J = 7.9 Hz, 1H), 7.77 (td, J = 7.7, 1.7 Hz, 1H), 7.68 (td, J = 7.7,
1.3 Hz, 1H), 7.53–7.45 (m, 1H), 7.45–7.30 (m, 2H), 7.30–7.21
(m, 3H), 7.10 (dd, J = 7.9, 1.7 Hz, 2H), 6.95 (d, J = 8.9 Hz, 1H), 4.76 (br s, 2H), 4.30–4.01 (m, 2H),
1.39 (s, 9H). 13C NMR {1H} (101 MHz, CDCl3): δ 163.9, 155.2, 141.1 (q, J = 38.2
Hz), 140.8, 138.3, 137.6, 134.3, 133.9, 132.7, 130.7, 130.2, 128.8,
128.6, 128.0, 126.5, 126.1, 124.1, 121.6, 119.0 (q, J = 271.8 Hz), 110.8, 80.0, 67.3, 53.7, 28.4. 19F NMR (376
MHz, CDCl3): δ −62.01. HRMS (ESI) m/z: [M + H]+ calcd for C28H27N3O4F3, 526.1948;
found, 526.1951. 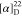 +52.86 (c 0.15, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.24 (ddd, J =
7.8, 1.6, 0.3 Hz,
1H), 7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.90–7.85
(m, 1H), 7.82 (td, J = 7.7, 1.3 Hz, 1H), 7.74 (dd, J = 7.8, 1.1 Hz, 1H), 7.44–7.37 (m, 2H), 7.37–7.28
(m, 4H), 7.28–7.22 (m, 1H), 7.07–6.99 (m, 1H), 6.46
(br s, 1H), 4.96 (br s, 1H), 4.31–4.10 (m, 2H), 1.38 (s, 9H). 1H NMR (400 MHz, acetonitrile-d3): δ 8.17 (dd, J = 7.8, 1.6 Hz, 1H), 7.92–7.83
(m, 2H), 7.77 (td, J = 7.7, 1.2 Hz, 1H), 7.63 (dd, J = 7.9, 0.5 Hz, 1H), 7.45–7.37 (m, 2H), 7.33–7.24
(m, 3H), 7.25–7.16 (m, 2H), 7.04–6.99 (m, 1H), 5.69
(br s, 1H), 4.79 (br s, 1H), 4.19 (dd, J = 11.2,
7.9 Hz, 1H), 4.10 (dd, J = 11.4, 4.9 Hz, 1H), 1.37
(s, 9H).
+52.86 (c 0.15, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.24 (ddd, J =
7.8, 1.6, 0.3 Hz,
1H), 7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.90–7.85
(m, 1H), 7.82 (td, J = 7.7, 1.3 Hz, 1H), 7.74 (dd, J = 7.8, 1.1 Hz, 1H), 7.44–7.37 (m, 2H), 7.37–7.28
(m, 4H), 7.28–7.22 (m, 1H), 7.07–6.99 (m, 1H), 6.46
(br s, 1H), 4.96 (br s, 1H), 4.31–4.10 (m, 2H), 1.38 (s, 9H). 1H NMR (400 MHz, acetonitrile-d3): δ 8.17 (dd, J = 7.8, 1.6 Hz, 1H), 7.92–7.83
(m, 2H), 7.77 (td, J = 7.7, 1.2 Hz, 1H), 7.63 (dd, J = 7.9, 0.5 Hz, 1H), 7.45–7.37 (m, 2H), 7.33–7.24
(m, 3H), 7.25–7.16 (m, 2H), 7.04–6.99 (m, 1H), 5.69
(br s, 1H), 4.79 (br s, 1H), 4.19 (dd, J = 11.2,
7.9 Hz, 1H), 4.10 (dd, J = 11.4, 4.9 Hz, 1H), 1.37
(s, 9H).
(M,S)-2-((tert-Butoxycarbonyl)amino)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-1
Purified by CC (hexane/EtOAc
5:1). Yield 21 mg (80%) as an amorphous solid. 1H NMR (400
MHz, CDCl3): δ 8.16 (dd, J = 7.8,
1.6 Hz, 1H), 7.97–7.91 (m, 1H), 7.76 (td, J = 7.7, 1.7 Hz, 1H), 7.68 (td, J = 7.7, 1.3 Hz,
1H), 7.47 (d, J = 7.2 Hz, 1H), 7.43–7.32 (m,
2H), 7.31–7.22 (m, 3H), 7.12 (d, J = 7.7 Hz,
2H), 6.95 (ddd, J = 7.9, 1.3, 0.8 Hz, 1H), 4.74 (m,
2H), 4.27 (br s, 1H), 4.08 (d, J = 10.2 Hz, 1H),
1.39 (s, 9H). 13C NMR {1H} (101 MHz, CDCl3): δ 163.8, 155.1, 141.0 (q, J = 38.5
Hz), 140.7, 138.2, 137.6, 134.2, 133.8, 132.5, 130.6, 130.1, 128.8,
128.6, 127.9, 126.4, 126.0, 124.0, 121.7, 118.9 (q, J = 272.0 Hz), 110.7, 80.0, 67.2, 53.6, 28.4. 19F NMR (376
MHz, CDCl3): δ −61.98. HRMS (ESI) m/z: [M + H]+ calcd for C28H27N3O4F3, 526.1948;
found, 526.1949. 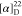 −34.0 (c 0.21,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.24 (dd, J = 7.9, 1.6 Hz,
1H), 7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.91–7.87
(m, 1H), 7.83 (td, J = 7.7, 1.2 Hz, 1H), 7.77–7.72
(m, 1H), 7.46–7.37 (m, 2H), 7.37–7.21 (m, 5H), 7.03–6.99
(m, 1H), 6.48 (br s, 1H), 4.91 (br s, 1H), 4.34–4.06 (m, 2H),
1.37 (s, 9H). 1H NMR (400 MHz, acetonitrile-d3): δ 8.17 (dd, J = 7.8, 1.7 Hz,
1H), 7.92–7.83 (m, 2H), 7.77 (td, J = 7.7,
1.3 Hz, 1H), 7.63 (dd, J = 7.8, 1.0 Hz, 1H), 7.45–7.36
(m, 2H), 7.34–7.24 (m, 3H), 7.21–7.14 (m, 2H), 7.01–6.97
(m, 1H), 5.73 (br s, 1H), 4.71 (br s, 1H), 4.15 (dd, J = 11.3, 7.8 Hz, 1H), 4.06 (dd, J = 11.3, 5.1 Hz,
1H), 1.37 (s, 9H).
−34.0 (c 0.21,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.24 (dd, J = 7.9, 1.6 Hz,
1H), 7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.91–7.87
(m, 1H), 7.83 (td, J = 7.7, 1.2 Hz, 1H), 7.77–7.72
(m, 1H), 7.46–7.37 (m, 2H), 7.37–7.21 (m, 5H), 7.03–6.99
(m, 1H), 6.48 (br s, 1H), 4.91 (br s, 1H), 4.34–4.06 (m, 2H),
1.37 (s, 9H). 1H NMR (400 MHz, acetonitrile-d3): δ 8.17 (dd, J = 7.8, 1.7 Hz,
1H), 7.92–7.83 (m, 2H), 7.77 (td, J = 7.7,
1.3 Hz, 1H), 7.63 (dd, J = 7.8, 1.0 Hz, 1H), 7.45–7.36
(m, 2H), 7.34–7.24 (m, 3H), 7.21–7.14 (m, 2H), 7.01–6.97
(m, 1H), 5.73 (br s, 1H), 4.71 (br s, 1H), 4.15 (dd, J = 11.3, 7.8 Hz, 1H), 4.06 (dd, J = 11.3, 5.1 Hz,
1H), 1.37 (s, 9H).
(P,S)-2-((tert-Butoxycarbonyl)amino)-2-cyclohexylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-2
Purified by CC (hexane/EtOAc
7:1). Yield: 12 mg (45%). 1H NMR (400 MHz, CDCl3): δ 8.29 (d, J = 7.6 Hz, 1H), 8.01–7.92
(m, 1H), 7.77 (td, J = 7.6, 1.7 Hz, 1H), 7.70 (td, J = 7.6, 1.4 Hz, 1H), 7.50–7.34 (m, 3H), 7.00 (dd, J = 7.2, 1.1 Hz, 1H), 4.20–4.03 (m, 2H), 3.97 (dd, J = 11.5, 3.5 Hz, 1H), 3.37 (tt, J = 9.5,
5.1 Hz, 1H), 1.60–1.48 (m, 3H), 1.42 (s, 9H), 1.39–1.30
(m, 1H), 1.09–0.91 (m, 3H), 0.85–0.72 (m, 3H), 0.64–0.52
(m, 1H). 13C NMR {1H} (101 MHz, CDCl3): δ 164.4, 155.8, 141.21 (q, J = 38.6 Hz),
140.8, 137.7, 133.9, 133.8, 133.1, 130.7, 130.1, 128.9, 126.2, 124.3,
121.7, 119.0 (q, J = 272.2 Hz), 111.0, 79.4, 66.0,
54.0, 37.9, 29.6, 29.1, 28.5, 26.2, 25.7, 25.7. 19F NMR
(376 MHz, CDCl3): δ −61.9. HRMS (ESI) m/z: [M + H]+ calcd for C28H33N3O4F3, 532.2418;
found, 532.2418. 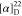 −60.83 (c 0.12,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.31 (dd, J = 7.9, 1.6 Hz,
1H), 7.94 (td, J = 7.7, 1.6 Hz, 1H), 7.92–7.86
(m, 1H), 7.83 (td, J = 7.7, 1.2 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.47–7.40 (m, 2H), 7.09 (ddt, J = 7.8, 5.3, 2.2 Hz, 1H), 5.45 (d, J =
10.1 Hz, 1H), 4.03 (d, J = 5.3 Hz, 2H), 3.47 (h, J = 5.4, 4.8 Hz, 1H), 1.69–1.49 (m, 5H), 1.39 (s,
9H), 1.18–1.05 (m, 4H), 1.01–0.85 (m, 2H).
−60.83 (c 0.12,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.31 (dd, J = 7.9, 1.6 Hz,
1H), 7.94 (td, J = 7.7, 1.6 Hz, 1H), 7.92–7.86
(m, 1H), 7.83 (td, J = 7.7, 1.2 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.47–7.40 (m, 2H), 7.09 (ddt, J = 7.8, 5.3, 2.2 Hz, 1H), 5.45 (d, J =
10.1 Hz, 1H), 4.03 (d, J = 5.3 Hz, 2H), 3.47 (h, J = 5.4, 4.8 Hz, 1H), 1.69–1.49 (m, 5H), 1.39 (s,
9H), 1.18–1.05 (m, 4H), 1.01–0.85 (m, 2H).
(M,S)-2-((tert-Butoxycarbonyl)amino)-2-cyclohexylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-2
Purified by CC (hexane/EtOAc
7:1). Yield: 6 mg (22%). 1H NMR (400 MHz, CDCl3): δ 8.34–8.22 (m, 1H), 7.97 (dd, J = 6.8, 1.9 Hz, 1H), 7.76 (td, J = 7.6, 1.8 Hz,
1H), 7.71 (td, J = 7.6, 1.5 Hz, 1H), 7.49–7.35
(m, 3H), 7.09–6.97 (m, 1H), 4.15 (dd, J =
11.5, 4.5 Hz, 1H), 3.90 (dd, J = 11.6, 3.4 Hz, 1H),
3.54 (d, J = 9.5 Hz, 1H), 3.40–3.24 (m, 1H),
1.70–1.58 (m, 2H), 1.51–1.42 (m, 2H), 1.40 (s, 9H),
1.21–0.99 (m, 3H), 0.88–0.67 (m, 3H). 13C
NMR {1H} (101 MHz, CDCl3): δ 164.6, 155.6,
141.12 (q, J = 39.3, 38.6 Hz), 140.7, 137.7, 137.7,
137.7, 133.8, 133.6, 133.2, 130.8, 130.3, 129.0, 126.4, 124.3, 121.8,
118.9 (q, J = 272.1 Hz), 110.9, 79.3, 66.2, 53.7,
37.8, 29.7, 29.2, 28.5, 26.2, 25.7, 25.6. 19F NMR (376
MHz, CDCl3): δ −61.9. HRMS (ESI) m/z: [M + H]+ calcd for C28H33N3O4F3, 532.2418;
found, 532.2420. 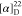 −63.08 (c 0.6,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.31 (dd, J = 7.9, 1.6 Hz,
1H), 7.94 (td, J = 7.7, 1.6 Hz, 1H), 7.92–7.87
(m, 1H), 7.84 (td, J = 7.7, 1.2 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.48–7.41 (m, 2H), 7.10–7.06
(m, 1H), 5.21 (d, J = 9.4 Hz, 1H), 4.08–3.99
(m, 2H), 3.47 (h, J = 5.1, 4.5 Hz, 1H), 1.71–1.50
(m, 5H), 1.38 (s, 9H), 1.23–1.06 (m, 4H), 0.92 (dddd, J = 24.9, 16.3, 12.5, 3.6 Hz, 2H).
−63.08 (c 0.6,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.31 (dd, J = 7.9, 1.6 Hz,
1H), 7.94 (td, J = 7.7, 1.6 Hz, 1H), 7.92–7.87
(m, 1H), 7.84 (td, J = 7.7, 1.2 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.48–7.41 (m, 2H), 7.10–7.06
(m, 1H), 5.21 (d, J = 9.4 Hz, 1H), 4.08–3.99
(m, 2H), 3.47 (h, J = 5.1, 4.5 Hz, 1H), 1.71–1.50
(m, 5H), 1.38 (s, 9H), 1.23–1.06 (m, 4H), 0.92 (dddd, J = 24.9, 16.3, 12.5, 3.6 Hz, 2H).
(P,R)-2-((tert-Butoxycarbonyl)amino)-3-methylbutyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-3
Purified by CC (hexane/EtOAc
4:1). Yield: 12 mg (50%). 1H NMR (400 MHz, CDCl3): δ 8.27 (dd, J = 7.6, 1.3 Hz, 1H), 7.97
(dd, J = 6.9, 1.4 Hz, 1H), 7.77 (td, J = 7.6, 1.8 Hz, 1H), 7.71 (td, J = 7.6, 1.4 Hz,
1H), 7.47–7.35 (m, 3H), 7.05–6.99 (m, 1H), 4.11 (dd, J = 11.6, 5.1 Hz, 1H), 3.92 (dd, J = 11.4,
4.0 Hz, 1H), 3.75 (d, J = 9.1 Hz, 1H), 3.41–3.28
(m, 1H), 1.40 (s, 9H), 0.74 (dd, J = 13.5, 6.7 Hz,
6H). 13C NMR {1H} (101 MHz, CDCl3): δ 164.4, 155.6, 141.04 (q, J = 38.4, 37.9
Hz), 140.8, 137.7, 133.8, 133.0, 130.7, 130.2, 129.0, 126.3, 124.2,
121.8, 118.9 (q, J = 272.1 Hz), 110.9, 79.4, 66.3,
54.6, 28.8, 28.5, 19.4, 18.6. 19F NMR (376 MHz, CDCl3): δ −62.0. δ HRMS (ESI) m/z: [M + H]+ calcd for C25H29N3O4F3, 492.2105;
found, 492.2107. 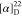 −19.13 (c 0.12,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.31 (dd, J = 7.8, 1.6 Hz,
1H), 7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.91–7.86
(m, 1H), 7.83 (td, J = 7.7, 1.1 Hz, 1H), 7.75–7.72
(m, 1H), 7.46–7.39 (m, 2H), 7.09–7.04 (m, 1H), 5.51
(d, J = 9.5 Hz, 1H), 4.07–3.97 (m, 2H), 3.53–3.43
(m, 1H), 1.51–1.44 (m, 1H), 1.38 (s, 9H), 0.79 (d, J = 6.8 Hz, 6H).
−19.13 (c 0.12,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.31 (dd, J = 7.8, 1.6 Hz,
1H), 7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.91–7.86
(m, 1H), 7.83 (td, J = 7.7, 1.1 Hz, 1H), 7.75–7.72
(m, 1H), 7.46–7.39 (m, 2H), 7.09–7.04 (m, 1H), 5.51
(d, J = 9.5 Hz, 1H), 4.07–3.97 (m, 2H), 3.53–3.43
(m, 1H), 1.51–1.44 (m, 1H), 1.38 (s, 9H), 0.79 (d, J = 6.8 Hz, 6H).
(M,R)-2-((tert-Butoxycarbonyl)amino)-3-methylbutyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-3
Purified by CC (hexane/EtOAc
4:1). Yield: 12 mg (50%). 1H NMR (400 MHz, CDCl3): δ 8.27 (d, J = 7.8 Hz, 1H), 7.96 (ddd, J = 8.1, 1.4, 0.8 Hz, 1H), 7.77 (td, J =
7.6, 1.7 Hz, 1H), 7.70 (td, J = 7.7, 1.4 Hz, 1H),
7.45 (d, J = 7.3 Hz, 1H), 7.41 (ddd, J = 8.0, 7.2, 1.4 Hz, 1H), 7.42–7.31 (m, 1H), 7.00 (ddd, J = 7.9, 1.5, 0.8 Hz, 1H), 4.14 (d, J =
9.6 Hz, 1H), 4.08 (dd, J = 11.5, 5.8 Hz, 1H), 3.95
(dd, J = 11.5, 3.9 Hz, 1H), 3.43–3.30 (m,
1H), 1.41 (s, 9H), 0.68 (dd, J = 19.0, 6.7 Hz, 6H). 13C NMR {1H} (101 MHz, CDCl3): δ
164.3, 155.8, 141.1 (q, J = 38.6 Hz), 140.8, 137.7,
133.9, 133.8, 133.0, 130.7, 130.2, 128.9, 126.1, 124.1, 121.7, 119.0
(q, J = 272.2 Hz), 110.9, 79.4, 66.2, 54.7, 28.6,
28.4, 19.3, 18.6. 19F NMR (376 MHz, CDCl3):
δ −62.0. HRMS (ESI) m/z: [M + H]+ calcd for C25H29N3O4F3, 492.2105; found, 492. 2104. 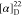 +84.35 (c 0.12, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.31 (dd, J = 7.9, 1.6 Hz, 1H),
7.94 (td, J = 7.7, 1.6 Hz, 1H), 7.91–7.86
(m, 1H), 7.83 (td, J = 7.7, 1.2 Hz, 1H), 7.74 (d, J = 7.8 Hz, 1H), 7.46–7.39 (m, 2H), 7.11–7.05
(m, 1H), 5.58 (d, J = 9.4 Hz, 1H), 4.03 (d, J = 5.3 Hz, 2H), 3.54–3.45 (m, 1H), 1.50–1.43
(m, 1H), 1.38 (s, 9H), 0.80 (dd, J = 6.8, 2.4 Hz,
6H).
+84.35 (c 0.12, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.31 (dd, J = 7.9, 1.6 Hz, 1H),
7.94 (td, J = 7.7, 1.6 Hz, 1H), 7.91–7.86
(m, 1H), 7.83 (td, J = 7.7, 1.2 Hz, 1H), 7.74 (d, J = 7.8 Hz, 1H), 7.46–7.39 (m, 2H), 7.11–7.05
(m, 1H), 5.58 (d, J = 9.4 Hz, 1H), 4.03 (d, J = 5.3 Hz, 2H), 3.54–3.45 (m, 1H), 1.50–1.43
(m, 1H), 1.38 (s, 9H), 0.80 (dd, J = 6.8, 2.4 Hz,
6H).
(P,S)-2-((tert-Butoxycarbonyl)amino)propyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-4
Purified by CC (hexane/EtOAc 4:1).
Yield: 10 mg (40%). 1H NMR (400 MHz, CDCl3):
δ 8.27 (dd, J = 7.8, 1.6 Hz, 1H), 7.97–7.93
(m, 1H), 7.77 (td, J = 7.6, 1.7 Hz, 1H), 7.70 (td, J = 7.7, 1.4 Hz, 1H), 7.47 (dd, J = 6.6,
1.0 Hz, 1H), 7.44–7.32 (m, 2H), 7.05–6.96 (m, 1H), 4.09
(br s, 1H), 4.00–3.84 (m, 2H), 3.66 (br s, 1H), 1.41 (s, 9H),
0.69 (d, J = 5.2 Hz, 3H). 13C NMR {1H} (101 MHz, CDCl3): δ 164.3, 155.1, 140.73
(q, J = 39.3 Hz), 140.70, 137.7, 133.9, 133.8, 132.9,
130.7, 130.2, 128.9, 126.2, 124.2, 121.7, 118.9 (q, J = 272.9 Hz), 110.9, 68.6, 68.5, 45.2, 45.2, 28.5, 16.8. 19F NMR (376 MHz, CDCl3): δ −62.0. HRMS (ESI) m/z: [M + H]+ calcd for C23H25N3O4F3, 464.1795;
found, 464.1795.  −56.0 (c 0.1, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.32 (dd, J = 7.9,
1.6 Hz, 1H),
7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.90–7.86
(m, 1H), 7.84 (td, J = 7.7, 1.2 Hz, 1H), 7.76–7.72
(m, 1H), 7.45–7.37 (m, 2H), 7.11–7.05 (m, 1H), 5.64
(d, J = 5.6 Hz, 1H), 3.97–3.86 (m, 2H), 3.77–3.68
(m, 1H), 1.37 (s, 9H), 0.93 (d, J = 6.8 Hz, 3H). 1H NMR (400 MHz, acetonitrile-d3): δ 8.25 (ddd, J = 7.8, 1.6, 0.3 Hz, 1H),
7.92–7.83 (m, 2H), 7.78 (td, J = 7.7, 1.4
Hz, 1H), 7.63 (dd, J = 7.8, 1.2 Hz, 1H), 7.45–7.37
(m, 2H), 7.08–7.03 (m, 1H), 4.93 (br s, 1H), 3.92–3.83
(m, 2H), 3.65 (br s, 1H), 1.37 (s, 9H), 0.86 (d, J = 6.9 Hz, 3H).
−56.0 (c 0.1, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.32 (dd, J = 7.9,
1.6 Hz, 1H),
7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.90–7.86
(m, 1H), 7.84 (td, J = 7.7, 1.2 Hz, 1H), 7.76–7.72
(m, 1H), 7.45–7.37 (m, 2H), 7.11–7.05 (m, 1H), 5.64
(d, J = 5.6 Hz, 1H), 3.97–3.86 (m, 2H), 3.77–3.68
(m, 1H), 1.37 (s, 9H), 0.93 (d, J = 6.8 Hz, 3H). 1H NMR (400 MHz, acetonitrile-d3): δ 8.25 (ddd, J = 7.8, 1.6, 0.3 Hz, 1H),
7.92–7.83 (m, 2H), 7.78 (td, J = 7.7, 1.4
Hz, 1H), 7.63 (dd, J = 7.8, 1.2 Hz, 1H), 7.45–7.37
(m, 2H), 7.08–7.03 (m, 1H), 4.93 (br s, 1H), 3.92–3.83
(m, 2H), 3.65 (br s, 1H), 1.37 (s, 9H), 0.86 (d, J = 6.9 Hz, 3H).
(M,S)-2-((tert-Butoxycarbonyl)amino)propyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-4
Purified by CC (hexane/EtOAc
4:1).
Yield: 14 mg (60%). 1H NMR (400 MHz, CDCl3):
δ 8.26 (dd, J = 7.8, 1.7 Hz, 1H), 7.98–7.93
(m, 1H), 7.77 (td, J = 7.6, 1.7 Hz, 1H), 7.71 (td, J = 7.6, 1.4 Hz, 1H), 7.46 (dd, J = 7.7,
1.1 Hz, 1H), 7.39 (pd, J = 7.2, 1.4 Hz, 2H), 7.02–6.98
(m, 1H), 4.07–3.82 (m, 3H), 3.67 (br s, 1H), 1.41 (s, 9H),
0.79 (br s, 3H). 13C NMR {1H} (101 MHz, CDCl3): δ 164.3, 155.0, 141.09 (q, J = 37.8
Hz), 140.8, 137.7, 133.9, 133.8, 132.9, 130.7, 130.2, 129.0, 126.2,
124.2, 121.7, 119.0 (q, J = 272.0 Hz), 110.8, 68.7,
28.5, 17.1. 19F NMR (376 MHz, CDCl3): δ
−61.95. HRMS (ESI) m/z: [M
+ H]+ calcd for C23H25N3O4F3, 464.1795; found, 464.1793. 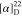 +13.57 (c 0.14, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.33 (dd, J = 7.8, 1.6 Hz, 1H),
7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.90–7.87
(m, 1H), 7.84 (td, J = 7.7, 1.2 Hz, 1H), 7.77–7.72
(m, 1H), 7.45–7.38 (m, 2H), 7.09–7.04 (m, 1H), 5.67
(d, J = 5.6 Hz, 1H), 3.92 (dd, J = 6.8, 3.9 Hz, 2H), 3.71 (dt, J = 9.5, 5.4 Hz,
1H), 1.38 (s, 9H), 0.90 (d, J = 6.4 Hz, 3H). 1H NMR (400 MHz, acetonitrile-d3): δ 8.26 (ddd, J = 7.8, 1.7, 0.4 Hz, 1H),
7.92–7.83 (m, 2H), 7.78 (td, J = 7.7, 1.4
Hz, 1H), 7.63 (dd, J = 7.8, 1.3 Hz, 1H), 7.46–7.37
(m, 2H), 7.10–7.00 (m, 1H), 4.96 (br s, 1H), 3.85 (d, J = 5.6 Hz, 2H), 3.59 (br s, 1H), 1.37 (s, 9H), 0.81 (d, J = 6.8 Hz, 3H).
+13.57 (c 0.14, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.33 (dd, J = 7.8, 1.6 Hz, 1H),
7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.90–7.87
(m, 1H), 7.84 (td, J = 7.7, 1.2 Hz, 1H), 7.77–7.72
(m, 1H), 7.45–7.38 (m, 2H), 7.09–7.04 (m, 1H), 5.67
(d, J = 5.6 Hz, 1H), 3.92 (dd, J = 6.8, 3.9 Hz, 2H), 3.71 (dt, J = 9.5, 5.4 Hz,
1H), 1.38 (s, 9H), 0.90 (d, J = 6.4 Hz, 3H). 1H NMR (400 MHz, acetonitrile-d3): δ 8.26 (ddd, J = 7.8, 1.7, 0.4 Hz, 1H),
7.92–7.83 (m, 2H), 7.78 (td, J = 7.7, 1.4
Hz, 1H), 7.63 (dd, J = 7.8, 1.3 Hz, 1H), 7.46–7.37
(m, 2H), 7.10–7.00 (m, 1H), 4.96 (br s, 1H), 3.85 (d, J = 5.6 Hz, 2H), 3.59 (br s, 1H), 1.37 (s, 9H), 0.81 (d, J = 6.8 Hz, 3H).
(P,S)-2-((tert-Butoxycarbonyl)amino)-3-phenylpropyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-5
Purified by CC (hexane/EtOAc
4:1). Yield: 21 mg (95%). 1H NMR (400 MHz, CDCl3): δ 8.28 (d, J = 7.5 Hz, 1H), 7.99–7.93
(m, 1H), 7.79 (td, J = 7.6, 1.7 Hz, 1H), 7.72 (td, J = 7.7, 1.4 Hz, 1H), 7.48 (d, J = 7.7
Hz, 1H), 7.43–7.36 (m, 2H), 7.24–7.13 (m, 3H), 7.08–7.03
(m, 1H), 6.90 (d, J = 6.9 Hz, 2H), 4.19 (d, J = 7.4 Hz, 1H), 3.94 (d, J = 4.5 Hz, 2H),
3.81 (br s, 1H), 2.37 (dd, J = 13.5, 6.3 Hz, 1H),
2.04 (dd, J = 13.0, 8.0 Hz, 1H), 1.40 (s, 9H). 13C NMR {1H} (101 MHz, CDCl3): δ
164.3, 155.2, 141.2 (q, J = 39.3 Hz), 140.8, 137.7,
137.1, 133.9, 133.9, 133.1, 130.8, 130.2, 129.2, 128.8, 128.6, 126.7,
126.3, 124.3, 123.0, 119.0 (q, J = 271.7 Hz), 110.9,
79.6, 66.5, 50.5, 37.0, 28.4. 19F NMR (376 MHz, CDCl3): δ −61.9. HRMS (ESI) m/z: [M + H]+ calcd for C29H29N3O4F3, 540.2105; found, 540.2108. 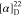 −26.19 (c 0.21,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.34 (dd, J = 7.9, 1.4 Hz,
1H), 7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.91–7.87
(m, 1H), 7.84 (td, J = 7.7, 1.2 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H), 7.44–7.39 (m, 2H), 7.24 (t, J = 7.2 Hz, 2H), 7.19–7.09 (m, 4H), 5.66 (d, J = 8.4 Hz, 1H), 4.00–3.97 (m, 2H), 3.93 (dd, J = 11.6, 6.0 Hz, 1H), 2.59 (q, J = 7.3,
6.8 Hz, 2H), 1.33 (s, 9H).
−26.19 (c 0.21,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.34 (dd, J = 7.9, 1.4 Hz,
1H), 7.95 (td, J = 7.7, 1.6 Hz, 1H), 7.91–7.87
(m, 1H), 7.84 (td, J = 7.7, 1.2 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H), 7.44–7.39 (m, 2H), 7.24 (t, J = 7.2 Hz, 2H), 7.19–7.09 (m, 4H), 5.66 (d, J = 8.4 Hz, 1H), 4.00–3.97 (m, 2H), 3.93 (dd, J = 11.6, 6.0 Hz, 1H), 2.59 (q, J = 7.3,
6.8 Hz, 2H), 1.33 (s, 9H).
(M,S)-2-((tert-Butoxycarbonyl)amino)-3-phenylpropyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-5
Purified by CC (hexane/EtOAc
4:1). Yield: 15 mg (40%). 1H NMR (400 MHz, CDCl3): δ 8.26 (dd, J = 7.8, 1.6 Hz, 1H), 7.98–7.92
(m, 1H), 7.79 (td, J = 7.6, 1.8 Hz, 1H), 7.73 (td, J = 7.7, 1.4 Hz, 1H), 7.48 (dd, J = 7.7,
1.0 Hz, 1H), 7.43–7.36 (m, 2H), 7.26–7.15 (m, 3H), 7.06–6.94
(m, 3H), 4.03–3.85 (m, 3H), 3.74 (br s, 1H), 2.55–2.41
(m, 1H), 2.40–2.31 (m, 1H), 1.38 (s, 9H). 13C NMR
{1H} (101 MHz, CDCl3): δ 164.3, 155.1,
141.13 (q, J = 38.5 Hz), 140.7, 137.7, 137.2, 133.9,
133.8, 132.9, 130.8, 130.2, 129.2, 128.9, 128.7, 126.7, 126.4, 124.3,
121.8, 119.0 (q, J = 272.1 Hz), 110.8, 79.6, 66.6,
50.5, 37.2, 28.4. 19F NMR (376 MHz, CDCl3):
δ −61.8. HRMS (ESI) m/z: [M + H]+ calcd for C29H29N3O4F3, 540.2105; found, 540.2109. 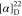 +21.0 (c 0.15, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.34 (dd, J = 7.8, 1.5 Hz, 1H),
7.96 (td, J = 7.7, 1.6 Hz, 1H), 7.89–7.83
(m, 2H), 7.75 (dd, J = 7.8, 1.1 Hz, 1H), 7.44–7.38
(m, 2H), 7.24 (tt, J = 8.1, 1.7 Hz, 2H), 7.20–7.16
(m, 1H), 7.16–7.11 (m, 2H), 7.10–7.07 (m, 1H), 5.68
(d, J = 8.1 Hz, 1H), 3.99 (qd, J = 11.0, 5.3 Hz, 2H), 3.93–3.86 (m, 1H), 2.62 (d, J = 7.1 Hz, 2H), 1.33 (s, 9H).
+21.0 (c 0.15, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.34 (dd, J = 7.8, 1.5 Hz, 1H),
7.96 (td, J = 7.7, 1.6 Hz, 1H), 7.89–7.83
(m, 2H), 7.75 (dd, J = 7.8, 1.1 Hz, 1H), 7.44–7.38
(m, 2H), 7.24 (tt, J = 8.1, 1.7 Hz, 2H), 7.20–7.16
(m, 1H), 7.16–7.11 (m, 2H), 7.10–7.07 (m, 1H), 5.68
(d, J = 8.1 Hz, 1H), 3.99 (qd, J = 11.0, 5.3 Hz, 2H), 3.93–3.86 (m, 1H), 2.62 (d, J = 7.1 Hz, 2H), 1.33 (s, 9H).
(P,R)-2-((tert-Butoxycarbonyl)amino)-4-methylpentyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-6
Purified by CC (hexane/EtOAc
5:1). Yield: 13 mg (50%). 1H NMR (500 MHz, CDCl3): δ 8.28 (d, J = 7.4 Hz, 1H), 7.96 (dd, J = 7.2, 1.1 Hz, 1H), 7.77 (td, J = 7.6,
1.7 Hz, 1H), 7.71 (td, J = 7.7, 1.3 Hz, 1H), 7.41
(ddd, J = 18.2, 16.2, 7.7 Hz, 3H), 7.01 (dd, J = 7.1, 1.1 Hz, 1H), 3.99 (dd, J = 11.0,
4.2 Hz, 1H), 3.90 (dd, J = 11.3, 3.6 Hz, 1H), 3.70
(d, J = 8.5 Hz, 1H), 3.60 (dd, J = 7.4, 3.5 Hz, 1H), 1.46–1.41 (m, 1H), 1.40 (s, 9H), 0.96–0.87
(m, 1H), 0.85 (d, J = 6.6 Hz, 3H), 0.81 (d, J = 7.0 Hz, 3H). 13C NMR {1H} (126
MHz, CDCl3): δ 164.5, 155.2, 141.1 (q, J = 38.3 Hz), 140.7, 137.7, 133.8, 133.0, 130.7, 130.2, 129.0, 126.3,
124.3, 121.8, 119.0 (q, J = 272.2 Hz), 115.7, 110.9,
79.4, 68.3, 47.5, 39.9, 28.5, 24.8, 22.8, 22.0. 19F NMR
(376 MHz, CDCl3): δ −61.88. HRMS (ESI) m/z: [M + H]+ calcd for C26H31N3O4F3, 506.2161;
found, 506.2162. 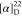 −10.77 (c 0.13,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.33 (dd, J = 7.8, 1.5 Hz,
1H), 7.94 (td, J = 7.7, 1.6 Hz, 1H), 7.91–7.86
(m, 1H), 7.86–7.81 (m, 1H), 7.73 (d, J = 7.8
Hz, 1H), 7.46–7.39 (m, 2H), 7.09–7.04 (m, 1H), 5.47
(d, J = 8.8 Hz, 1H), 3.93 (dt, J = 9.0, 4.2 Hz, 2H), 3.79 (dq, J = 8.5, 4.6, 3.8
Hz, 1H), 1.63–1.52 (m, 1H), 1.38 (s, 9H), 1.25–1.17
(m, 1H), 1.04 (ddd, J = 13.6, 8.9, 4.6 Hz, 1H), 0.85
(dd, J = 11.3, 6.6 Hz, 6H).
−10.77 (c 0.13,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.33 (dd, J = 7.8, 1.5 Hz,
1H), 7.94 (td, J = 7.7, 1.6 Hz, 1H), 7.91–7.86
(m, 1H), 7.86–7.81 (m, 1H), 7.73 (d, J = 7.8
Hz, 1H), 7.46–7.39 (m, 2H), 7.09–7.04 (m, 1H), 5.47
(d, J = 8.8 Hz, 1H), 3.93 (dt, J = 9.0, 4.2 Hz, 2H), 3.79 (dq, J = 8.5, 4.6, 3.8
Hz, 1H), 1.63–1.52 (m, 1H), 1.38 (s, 9H), 1.25–1.17
(m, 1H), 1.04 (ddd, J = 13.6, 8.9, 4.6 Hz, 1H), 0.85
(dd, J = 11.3, 6.6 Hz, 6H).
(M,R)-2-((tert-Butoxycarbonyl)amino)-4-methylpentyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-6
Purified by CC (hexane/EtOAc
5:1). Yield: 8 mg (30%). 1H NMR (500 MHz, CDCl3): δ 8.29 (d, J = 7.6 Hz, 1H), 7.98–7.95
(m, 1H), 7.77 (td, J = 7.6, 1.6 Hz, 1H), 7.71 (td, J = 7.7, 1.3 Hz, 1H), 7.45 (d, J = 7.7
Hz, 1H), 7.43–7.39 (m, 1H), 7.37 (ddd, J =
8.3, 7.2, 1.3 Hz, 1H), 7.02–7.00 (m, 1H), 3.96 (t, J = 5.9 Hz, 3H), 3.64 (dq, J = 8.8, 4.7
Hz, 1H), 1.42 (s, 9H), 1.39–1.34 (m, 1H), 0.77 (d, J = 7.0 Hz, 3H), 0.74 (d, J = 6.6 Hz, 3H),
0.72–0.64 (m, 1H). 13C NMR {1H} (126
MHz, CDCl3): δ 164.4, 155.4, 141.2 (q, J = 38.1 Hz), 140.8, 137.7, 133.9, 133.8, 133.1, 130.7, 130.1, 129.0,
126.2, 124.2, 121.8, 119.0 (q, J = 272.3 Hz), 111.0,
79.4, 68.2, 47.6, 39.6, 28.5, 24.7, 22.9, 22.0. 19F NMR
(376 MHz, CDCl3): δ −61.94. HRMS (ESI) m/z: [M + H]+ calcd for C26H31N3O4F3, 506.2161;
found, 506.2163. 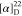 +85.00 (c 0.8, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.32 (dd, J = 7.8,
1.6 Hz, 1H),
7.94 (td, J = 7.7, 1.5 Hz, 1H), 7.91–7.86
(m, 1H), 7.86–7.81 (m, 1H), 7.74 (d, J = 7.8
Hz, 1H), 7.46–7.39 (m, 2H), 7.11–7.07 (m, 1H), 5.51
(d, J = 8.8 Hz, 1H), 3.93 (d, J =
5.4 Hz, 2H), 3.77 (dt, J = 10.4, 5.2 Hz, 1H), 1.67–1.52
(m, 1H), 1.38 (s, 9H), 1.24–1.13 (m, 1H), 1.01 (ddd, J = 13.7, 9.1, 4.6 Hz, 1H), 0.86–0.80 (m, 6H).
+85.00 (c 0.8, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.32 (dd, J = 7.8,
1.6 Hz, 1H),
7.94 (td, J = 7.7, 1.5 Hz, 1H), 7.91–7.86
(m, 1H), 7.86–7.81 (m, 1H), 7.74 (d, J = 7.8
Hz, 1H), 7.46–7.39 (m, 2H), 7.11–7.07 (m, 1H), 5.51
(d, J = 8.8 Hz, 1H), 3.93 (d, J =
5.4 Hz, 2H), 3.77 (dt, J = 10.4, 5.2 Hz, 1H), 1.67–1.52
(m, 1H), 1.38 (s, 9H), 1.24–1.13 (m, 1H), 1.01 (ddd, J = 13.7, 9.1, 4.6 Hz, 1H), 0.86–0.80 (m, 6H).
(P,S)-3-(Benzyloxy)-2-((tert-butoxycarbonyl)amino)propyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-7
Purified by CC (hexane/EtOAc
3:1). Yield: 23 mg (80%). 1H NMR (400 MHz, CDCl3): δ 8.23 (d, J = 7.6 Hz, 1H), 7.94 (dt, J = 8.6, 0.9 Hz, 1H), 7.76 (td, J = 7.7,
1.7 Hz, 1H), 7.68 (td, J = 7.7, 1.3 Hz, 1H), 7.45
(d, J = 7.4 Hz, 1H), 7.42–7.32 (m, 2H), 7.31–7.26
(m, 3H), 7.25–7.18 (m, 2H), 6.95 (d, J = 8.3
Hz, 1H), 4.55 (d, J = 8.0 Hz, 1H), 4.32 (s, 2H),
4.15 (dd, J = 11.6, 6.1 Hz, 1H), 4.02 (dd, J = 11.1, 5.3 Hz, 1H), 3.74 (br s, 1H), 3.14–2.97
(m, 2H), 1.41 (s, 9H). 13C NMR {1H} (101 MHz,
CDCl3): δ 164.0, 155.3, 141.09 (q, J = 37.8 Hz)140.7, 137.8, 137.6, 134.0, 133.8, 132.9, 130.7, 130.1,
128.9, 128.5, 127.9, 127.8, 126.1, 124.2, 121.7, 118.9 (q, J = 272.1 Hz), 110.9, 79.7, 73.3, 68.4, 65.0, 49.0, 28.4. 19F NMR (376 MHz, CDCl3): δ −62.0.
HRMS (ESI) m/z: [M + H]+ calcd for C30H31N3O5F3, 570.2210; found, 570.2214. 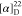 −29.13 (c 0.23,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.28 (ddd, J = 7.8, 1.6,
0.3 Hz, 1H), 7.94 (td, J = 7.7, 1.6 Hz, 1H), 7.90–7.86
(m, 1H), 7.82 (td, J = 7.7, 1.3 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.44–7.37 (m, 2H), 7.33–7.23
(m, 5H), 7.08–7.01 (m, 1H), 5.65 (d, J = 8.5
Hz, 1H), 4.42 (s, 2H), 4.14–4.05 (m, 2H), 3.93–3.83
(m, 1H), 3.36–3.25 (m, 2H), 1.38 (s, 9H).
−29.13 (c 0.23,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.28 (ddd, J = 7.8, 1.6,
0.3 Hz, 1H), 7.94 (td, J = 7.7, 1.6 Hz, 1H), 7.90–7.86
(m, 1H), 7.82 (td, J = 7.7, 1.3 Hz, 1H), 7.73 (d, J = 7.8 Hz, 1H), 7.44–7.37 (m, 2H), 7.33–7.23
(m, 5H), 7.08–7.01 (m, 1H), 5.65 (d, J = 8.5
Hz, 1H), 4.42 (s, 2H), 4.14–4.05 (m, 2H), 3.93–3.83
(m, 1H), 3.36–3.25 (m, 2H), 1.38 (s, 9H).
(M,S)-3-(Benzyloxy)-2-((tert-butoxycarbonyl)amino)propyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-7
Purified by CC (hexane/EtOAc
3:1). Yield: 28 mg (95%). 1H NMR (400 MHz, CDCl3): δ 8.21 (d, J = 7.5 Hz, 1H), 7.94 (d, J = 7.8 Hz, 1H), 7.81–7.73 (m, 1H), 7.68 (t, J = 7.7 Hz, 1H), 7.45 (dd, J = 7.8, 1.0
Hz, 1H), 7.41–7.27 (m, 4H), 7.26–7.16 (m, 3H), 6.95
(d, J = 7.7 Hz, 1H), 4.49 (d, J =
9.7 Hz, 1H), 4.41–4.26 (m, 2H), 4.11 (br s, 1H), 4.00 (dd, J = 10.9, 6.4 Hz, 1H), 3.78 (br s, 1H), 3.12 (m, 2H), 1.41
(s, 9H). 13C NMR {1H} (101 MHz, CDCl3): δ 164.0, 155.2, 141.0 (q, J = 38.6 Hz),
140.7, 137.9, 137.7, 133.9, 133.8, 132.9, 130.7, 130.2, 128.9, 128.5,
127.9, 127.7, 126.2, 124.2, 121.7, 118.9 (q, J =
272.1 Hz), 110.8, 79.7, 73.2, 68.3, 64.7, 48.9, 28.4. 19F NMR (376 MHz, CDCl3): δ −61.9. HRMS (ESI) m/z: [M + H]+ calcd for C30H31N3O5F3, 570.2210;
found, 570.2215. 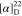 +9.29 (c 0.28, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.28–8.25 (m, 1H), 7.92 (td, J = 7.7, 1.6 Hz, 1H), 7.86–7.83 (m, 1H), 7.80 (td, J = 7.8, 1.2 Hz, 1H), 7.71 (dd, J = 7.9,
0.9 Hz, 1H), 7.41–7.34 (m, 2H), 7.29–7.19 (m, 5H), 6.99
(dd, J = 6.4, 2.3 Hz, 1H), 5.68 (d, J = 8.7 Hz, 1H), 4.41–4.27 (m, 2H), 4.05 (qd, J = 11.1, 6.3 Hz, 2H), 3.76 (p, J = 7.4, 6.3 Hz,
1H), 3.26–3.10 (m, 2H), 1.35 (s, 9H).
+9.29 (c 0.28, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.28–8.25 (m, 1H), 7.92 (td, J = 7.7, 1.6 Hz, 1H), 7.86–7.83 (m, 1H), 7.80 (td, J = 7.8, 1.2 Hz, 1H), 7.71 (dd, J = 7.9,
0.9 Hz, 1H), 7.41–7.34 (m, 2H), 7.29–7.19 (m, 5H), 6.99
(dd, J = 6.4, 2.3 Hz, 1H), 5.68 (d, J = 8.7 Hz, 1H), 4.41–4.27 (m, 2H), 4.05 (qd, J = 11.1, 6.3 Hz, 2H), 3.76 (p, J = 7.4, 6.3 Hz,
1H), 3.26–3.10 (m, 2H), 1.35 (s, 9H).
(P,S)-2-((tert-Butoxycarbonyl)(methyl)amino)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-8
Purified by CC (hexane/EtOAc
5:1). Yield: 19 mg (70%). 1H NMR (400 MHz, CDCl3): δ 8.21 (d, J = 6.3 Hz, 1H), 7.99–7.91
(m, 1H), 7.77 (t, J = 7.5 Hz, 1H), 7.67 (t, J = 7.5 Hz, 1H), 7.49 (d, J = 7.6 Hz, 1H),
7.42–7.33 (m, 2H), 7.32–7.23 (m, 3H), 7.10 (br s, 2H),
7.00–6.97 (m, 1H), 5.42 (d, J = 197.9 Hz,
1H), 4.46 (s, 2H), 2.51 (s, 3H), 1.41 (s, 9H). 13C NMR
{1H} (126 MHz, CDCl3): δ 163.5, 141.09
(q, J = 38.6 Hz), 140.8, 137.6, 136.8, 134.5, 133.8,
132.6, 130.6, 130.2, 128.8, 128.6, 127.9, 127.3, 127.0 126.0, 124.0,
121.7, 119.0 (q, J = 273.3 Hz), 110.8, 80.2, 62.8,
55.4, 29.8, 28.5. 19F NMR (471 MHz, CDCl3):
δ −61.4. HRMS (ESI) m/z: [M + H]+ calcd for C29H29N3O4F3, 540.2105; found, 540.2109. 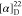 −10.53 (c 0.095,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.24 (dd, J = 7.8, 1.6 Hz,
1H), 7.99–7.92 (m, 1H), 7.90–7.85 (m, 1H), 7.83 (t, J = 7.4 Hz, 1H), 7.75 (d, J = 7.9 Hz, 1H),
7.45–7.38 (m, 2H), 7.34 (tt, J = 8.1, 1.9
Hz, 2H), 7.30–7.25 (m, 1H), 7.23 (d, J = 7.1
Hz, 2H), 7.08–7.03 (m, 1H), 5.47 (d, J = 109.5
Hz, 1H), 4.51 (s, 2H), 2.58 (s, 3H), 1.40 (s, 9H).
−10.53 (c 0.095,
CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.24 (dd, J = 7.8, 1.6 Hz,
1H), 7.99–7.92 (m, 1H), 7.90–7.85 (m, 1H), 7.83 (t, J = 7.4 Hz, 1H), 7.75 (d, J = 7.9 Hz, 1H),
7.45–7.38 (m, 2H), 7.34 (tt, J = 8.1, 1.9
Hz, 2H), 7.30–7.25 (m, 1H), 7.23 (d, J = 7.1
Hz, 2H), 7.08–7.03 (m, 1H), 5.47 (d, J = 109.5
Hz, 1H), 4.51 (s, 2H), 2.58 (s, 3H), 1.40 (s, 9H).
(M,S)-2-((tert-Butoxycarbonyl)(methyl)amino)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-8
Purified by CC (hexane/EtOAc
5:1). Yield: 22 mg (81%). 1H NMR (500 MHz, CDCl3): δ 8.21 (dd, J = 7.8, 1.5 Hz, 1H), 7.93
(d, J = 7.7 Hz, 1H), 7.77 (t, J =
7.5 Hz, 1H), 7.68 (s, 1H), 7.49 (d, J = 7.8 Hz, 1H),
7.40–7.33 (m, 2H), 7.31–7.22 (m, 3H), 7.09 (d, J = 30.8 Hz, 2H), 6.96 (d, J = 8.2 Hz,
1H), 5.44 (d, J = 182.5 Hz, 1H), 4.50 (dd, J = 11.4, 5.6 Hz, 1H), 4.34 (d, J = 60.2
Hz, 1H), 2.42 (s, 3H), 1.41 (s, 9H). 13C NMR {1H} (126 MHz, CDCl3): δ 163.5, 155.8, 140.92 (q, J = 39.1 Hz), 140.8, 137.7, 136.9, 134.5, 133.9, 132.5,
130.6, 130.2, 128.8, 128.7, 127.9, 127.3, 127.0, 126.1, 124.0, 121.6,
119.00 (app. d, J = 271.9 Hz), 110.8, 80.2, 63.3,
56.9, 29.3, 28.5. 19F NMR (471 MHz, CDCl3):
δ −61.4. HRMS (ESI) m/z: [M + H]+ calcd for C29H29N3O4F3, 540.2105; found, 540.2108. 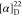 +12.27 (c 0.22, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.24 (dd, J = 7.8, 1.6 Hz, 1H),
7.98–7.91 (m, 1H), 7.90–7.79 (m, 2H), 7.74 (d, J = 7.8 Hz, 1H), 7.43–7.36 (m, 2H), 7.35–7.24
(m, 3H), 7.20 (d, J = 7.0 Hz, 2H), 7.01 (d, J = 8.2 Hz, 1H), 5.44 (d, J = 103.3 Hz,
1H), 4.52 (dd, J = 11.5, 5.4 Hz, 1H), 4.45 (d, J = 9.8 Hz, 1H), 2.45 (s, 3H), 1.41 (s, 9H).
+12.27 (c 0.22, CHCl3). 1H NMR (400 MHz, acetone-d6): δ 8.24 (dd, J = 7.8, 1.6 Hz, 1H),
7.98–7.91 (m, 1H), 7.90–7.79 (m, 2H), 7.74 (d, J = 7.8 Hz, 1H), 7.43–7.36 (m, 2H), 7.35–7.24
(m, 3H), 7.20 (d, J = 7.0 Hz, 2H), 7.01 (d, J = 8.2 Hz, 1H), 5.44 (d, J = 103.3 Hz,
1H), 4.52 (dd, J = 11.5, 5.4 Hz, 1H), 4.45 (d, J = 9.8 Hz, 1H), 2.45 (s, 3H), 1.41 (s, 9H).
(P,S)-2-(Dimethylamino)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-9
Purified by HPLC. Yield: 14 mg (50%). 1H NMR (500 MHz,
CDCl3): δ 8.06 (dd, J = 7.8, 1.6
Hz, 1H), 7.99–7.97 (m, 1H), 7.73 (td, J =
7.7, 1.6 Hz, 1H), 7.65 (td, J = 7.7, 1.3 Hz, 1H),
7.47–7.44 (m, 1H), 7.42 (ddd, J = 8.2, 7.2,
1.2 Hz, 1H), 7.36 (td, J = 7.7, 7.2, 1.1 Hz, 1H),
7.30–7.22 (m, 3H), 7.12–7.09 (m, 2H), 6.98 (dt, J = 8.1, 0.9 Hz, 1H), 4.29 (dd, J = 11.5,
6.5 Hz, 1H), 4.13 (dd, J = 11.5, 6.1 Hz, 1H), 2.98
(t, J = 6.3 Hz, 1H), 1.96 (s, 6H). 13C
NMR {1H} (126 MHz, CDCl3): δ 164.0, 141.1
(q, J = 38.5 Hz), 140.8, 137.8, 137.7, 134.0, 133.6,
132.5, 130.6, 130.0, 129.1, 128.4, 128.4, 127.8, 126.0, 124.0, 121.6,
118.9 (q, J = 272.1 Hz), 111.0, 68.3, 66.6, 42.7. 19F NMR (471 MHz, CDCl3): δ −61.3.
HRMS (ESI) m/z: [M + H]+ calcd for C25H23N3O2F3, 454.1737; found, 454.1735. 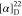 −53.57 (c 0.14,
CHCl3).
−53.57 (c 0.14,
CHCl3).
(M,S)-2-(Dimethylamino)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-9
Purified by HPLC. Yield: 14 mg (50%). 1H NMR (500 MHz,
CDCl3): δ 8.08 (dd, J = 7.9, 1.6
Hz, 1H), 7.96 (dt, J = 8.2, 0.9 Hz, 1H), 7.74 (td, J = 7.7, 1.6 Hz, 1H), 7.65 (td, J = 7.7,
1.3 Hz, 1H), 7.45 (dd, J = 7.8, 0.9 Hz, 1H), 7.40
(ddd, J = 8.3, 7.2, 1.1 Hz, 1H), 7.33 (ddd, J = 8.2, 7.2, 1.1 Hz, 1H), 7.27–7.21 (m, 3H), 7.04–7.00
(m, 2H), 6.94 (dt, J = 8.2, 1.0 Hz, 1H), 4.34 (dd, J = 11.5, 6.6 Hz, 1H), 4.14 (dd, J = 11.5,
5.9 Hz, 1H), 3.09 (t, J = 6.2 Hz, 1H), 2.06 (s, 6H). 13C NMR {1H} (126 MHz, CDCl3): δ
164.0, 141.10 (q, J = 38.5 Hz), 140.8, 137.7, 137.5,
134.1, 133.6, 132.5, 130.6, 130.0, 129.1, 128.4, 127.8, 126.0, 124.0,
121.6, 119.0 (q, J = 272.3 Hz), 110.9, 68.4, 66.5,
42.8. 19F NMR (471 MHz, CDCl3): δ −61.3.
HRMS (ESI) m/z: [M + H]+ calcd for C25H23N3O2F3, 454.1737; found, 454.1737. 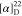 +44.29 (c 0.14, CHCl3).
+44.29 (c 0.14, CHCl3).
(P,S)-2-Acetamido-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-10
Purified by CC (hexane/EtOAc 1:1). Yield 5 mg (21%). 1H NMR (500 MHz, CDCl3): δ 8.23 (ddd, J = 7.8, 1.7, 0.3 Hz, 1H), 7.94 (dt, J = 8.2, 1.0
Hz, 1H), 7.78 (td, J = 7.7, 1.7 Hz, 1H), 7.71 (td, J = 7.7, 1.3 Hz, 1H), 7.47 (dd, J = 7.8,
1.2 Hz, 1H), 7.41 (ddd, J = 8.2, 7.2, 1.2 Hz, 1H),
7.35 (ddd, J = 8.3, 7.2, 1.1 Hz, 1H), 7.20–7.17
(m, 3H), 6.96–6.94 (m, 1H), 6.92–6.89 (m, 2H), 5.58
(d, J = 8.4 Hz, 1H), 5.10 (ddd, J = 8.2, 7.0, 4.0 Hz, 1H), 4.39 (dd, J = 11.7, 7.0
Hz, 1H), 4.12 (dd, J = 11.7, 4.0 Hz, 1H), 1.99 (s,
3H). 13C NMR {1H} (126 MHz, CDCl3): δ 169.8, 164.4, 141.25 (q, J = 38.8, 38.8,
38.4 Hz), 140.7, 134.1, 134.0, 133.1, 130.8, 130.1, 128.8, 128.4,
127.9, 126.4, 126.3, 124.4, 121.5, 118.9 (q, J =
273.0 Hz), 111.0, 67.4, 52.1, 23.3. 19F NMR (376 MHz, CDCl3): δ −61.67. HRMS (ESI) m/z: [M + H]+ calcd for C25H21N3O3F3, 468.1530; found, 468.1530. 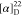 +12.73 (c 0.05, CHCl3).
+12.73 (c 0.05, CHCl3).
(M,S)-2-Acetamido-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-10
Purified by CC (hexane/EtOAc 1:1). Yield 10 mg (42%). 1H NMR (400 MHz, chloroform-d): δ 8.16 (dd, J = 7.8, 1.7 Hz, 1H), 7.97–7.92 (m, 1H), 7.78 (td, J = 7.7, 1.7 Hz, 1H), 7.70 (td, J = 7.7,
1.3 Hz, 1H), 7.48 (dd, J = 8.0, 0.9 Hz, 1H), 7.44–7.34
(m, 2H), 7.32–7.26 (m, 2H), 7.26–7.21 (m, 1H), 7.15–7.09
(m, 2H), 6.99–6.95 (m, 1H), 5.55 (d, J = 7.9
Hz, 1H), 5.07 (dt, J = 7.7, 3.7 Hz, 1H), 4.47 (dd, J = 11.7, 7.1 Hz, 1H), 3.99 (dd, J = 11.7,
4.2 Hz, 1H), 1.86 (s, 3H). 13C NMR {1H} (126
MHz, CDCl3): δ 169.7, 164.4, 141.2 (app. d, J = 38.6 Hz), 140.8, 137.7, 134.1, 134.1, 132.7, 130.8,
130.2, 128.9, 128.4, 128.1, 126.6, 126.2, 124.2, 121.8, 118.9 (q, J = 271.9 Hz), 110.8, 67.1, 52.4, 23.3. 19F NMR
(376 MHz, CDCl3): δ −61.89. HRMS (ESI) m/z: [M + H]+ calcd for C25H21N3O3F3, 468.1530;
found, 468.1532. 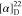 +60.0 (c 0.1, CHCl3).
+60.0 (c 0.1, CHCl3).
(P,S)-2-(N-Methylacetamido)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-11
Purified by CC (hexane/EtOAc
1:1).
Yield 17 mg (70%). Isolated as a mixture of rotamers. Peaks belonging
to the major rotamer are designated as M, and peaks belonging to the
minor rotamer are designated as m. 1H NMR (500 MHz, CDCl3): δ 8.19 (dd, J = 7.9, 1.5 Hz, 1H,
both rotamers), 7.94 (d, J = 8.2 Hz, 1H, both rotamers),
7.77 (td, J = 7.7, 1.6 Hz, 1H, both rotamers), 7.70
(td, J = 7.7, 1.3 Hz, 1H, both rotamers), 7.49 (d, J = 8.0 Hz, 1H, both rotamers), 7.43–7.39 (m, 1H,
both rotamers), 7.39–7.34 (m, 1H, both rotamers), 7.32–7.27
(m, 3H, both rotamers), 7.10 (dd, J = 7.3, 1.5 Hz,
1H, both rotamers), 7.05–7.01 (m, 1H, m), 7.00–6.97
(m, 1H, M), 6.09 (t, J = 7.5 Hz, 1H, M), 4.86 (dd, J = 8.8, 6.2 Hz, 1H, m), 4.55 (dd, J =
11.7, 6.0 Hz, 1H, m), 4.44 (d, J = 7.0 Hz, 2H, M),
4.29 (dd, J = 11.7, 8.9 Hz, 1H, m), 2.62 (s, 3H,
m), 2.59 (s, 1H, M), 2.06 (s, 3H, M), 1.96 (s, 3H, m). 13C NMR {1H} (126 MHz, CDCl3): δ 171.6,
171.2, 163.8, 163.6, 141.03 (q, J = 38.5 Hz), 140.8,
137.6, 136.3, 135.7, 134.4, 134.3, 133.9, 132.5, 132.5, 130.9, 130.9,
130.4, 130.2, 129.2, 128.9, 128.5, 128.5, 128.2, 128.1, 127.6, 126.6,
126.6, 126.2, 126.1, 124.2, 124.1, 121.8, 121.6, 119.0 (d, J = 272.1 Hz), 110.9, 110.7, 63.8, 62.5, 58.6, 53.4, 30.8,
28.2, 22.2, 21.8. 19F NMR (471 MHz, CDCl3):
δ −61.36, −61.44. HRMS (ESI) m/z: [M + H]+ calcd for C26H23N3O3F3, 482.1686;
found, 482.1685. 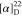 −392.36 (c 0.17,
CHCl3).
−392.36 (c 0.17,
CHCl3).
(M,S)-2-(N-Methylacetamido)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-11
Purified by CC (hexane/EtOAc
1:1).
Yield 12 mg (50%). Isolated as a mixture of rotamers. Peaks belonging
to the major rotamer are designated as M, and peaks belonging to the
minor rotamer are designated as m. 1H NMR (500 MHz, CDCl3): δ 8.19 (dd, J = 7.8, 1.6 Hz, 1H,
M), 8.16 (dd, J = 7.9, 1.6 Hz, 1H, m), 8.00–7.91
(m, 1H, M), 7.92 (dt, J = 8.2, 1.0 Hz, 1H, m), 7.81
(td, J = 7.7, 1.6 Hz, 1H, m), 7.77 (td, J = 7.7, 1.6 Hz, 1H, M), 7.73 (td, J = 7.7, 1.3 Hz,
1H, m), 7.69 (td, J = 7.7, 1.3 Hz, 1H, M), 7.52 (dd, J = 7.9, 1.2 Hz, 1H, m), 7.48 (dd, J =
7.7, 1.3 Hz, 1H, M), 7.43–7.24 (m, 10H, both rotamers), 7.15–7.06
(m, 2H, M), 7.00 (ddd, J = 7.9, 1.6, 0.8 Hz, 1H,
m), 6.99–6.97 (m, 1H, M), 6.95 (dt, J = 8.0,
1.1 Hz, 1H, m), 6.02 (dd, J = 9.2, 5.5 Hz, 1H, M),
4.97 (dd, J = 9.2, 5.7 Hz, 1H, m), 4.55 (dd, J = 11.5, 5.5 Hz, 1H, M), 4.49 (dd, J =
11.7, 5.7 Hz, 1H, m), 4.40 (dd, J = 11.5, 9.2 Hz,
1H, M), 4.28 (dd, J = 11.7, 9.3 Hz, 1H, m), 2.51
(s, 3H, m), 2.42 (s, 3H, M), 2.13 (s, 2H, m), 1.97 (s, 3H, M). 13C NMR {1H} (126 MHz, CDCl3): δ
171.3, 171.3, 163.8, 163.6, 141.20 (d, J = 38.3 Hz),
140.9, 140.7, 136.4, 135.7, 134.4, 134.3, 133.9, 132.7, 132.3, 130.9,
130.8, 130.1, 129.1, 128.9, 128.5, 128.4, 128.1, 127.6, 126.6, 126.3,
125.9, 124.2, 124.0, 121.7, 121.5, 118.97 (q, J =
272.5 Hz), 110.8, 63.7, 62.9, 58.6, 53.8, 31.0, 28.0, 22.3, 21.7. 19F NMR (471 MHz, CDCl3): δ −61.4,
−61.5. HRMS (ESI) m/z: [M
+ H]+ calcd for C26H23N3O3F3, 482.1686; found, 482.1685. 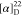 +127.5 (c 0.12, CHCl3).
+127.5 (c 0.12, CHCl3).
(P,S)-2-(N-Methylformamido)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-12
Purified by CC (hexane/EtOAc
2:1).
Yield 18 mg (78%). Isolated as a mixture of rotamers. Peaks belonging
to the major rotamer are designated as M, and peaks belonging to the
minor rotamer are designated as m. 1H NMR (500 MHz, CDCl3): δ 8.20 (dt, J = 7.8, 1.9 Hz, 1H,
both rotamers), 8.08 (s, 1H, m), 7.98–7.96 (m, 1H, M), 7.95
(dt, J = 8.2, 0.9 Hz, 1H, m), 7.81 (td, J = 7.7, 1.6 Hz, 1H, M), 7.77 (td, J = 7.6, 1.7 Hz,
1H, m), 7.75–7.72 (m, 1H, M), 7.72–7.69 (m, 1H, m),
7.54 (s, 1H, M), 7.52 (dd, J = 7.8, 1.2 Hz, 1H, M),
7.48 (dd, J = 7.9, 1.4 Hz, 1H, m), 7.47–7.44
(m, 2H, M), 7.43–7.36 (m, 2H, m), 7.35–7.27 (m, 5H,
both rotamers), 7.17–7.08 (m, 1H, M), 7.06–6.96 (m,
1H, both rotamers), 6.98 (dd, J = 1.4, 0.7 Hz, 1H,
m), 5.77 (dd, J = 10.0, 5.3 Hz, 1H, m), 4.60 (dd, J = 11.7, 10.0 Hz, 1H, M), 4.53–4.43 (m, 2H, m),
4.28 (dd, J = 11.7, 4.7 Hz, 1H, M), 4.15 (dd, J = 10.0, 4.6 Hz, 1H, M), 2.56 (s, 3H, m), 2.51 (s, 3H,
M). 13C NMR {1H} (126 MHz, CDCl3):
δ 163.8, 163.6, 163.5, 162.8, 141.26 (q, J =
34.0), 140.8, 140.7, 137.8, 137.6, 135.0, 134.6, 134.3, 134.2, 134.0,
132.8, 132.6, 130.9, 130.9, 130.2, 130.2, 129.2, 129.0, 128.8, 128.5,
128.4, 128.2, 127.7, 127.0, 126.4, 126.1, 124.3, 124.1, 121.6, 118.9
(q, J = 271.8 Hz), 111.0, 110.9, 62.4, 61.8, 59.4,
52.6, 30.1, 25.9. 19F NMR (471 MHz, CDCl3):
δ −61.3, −61.5. HRMS (ESI) m/z: [M + H]+ calcd for C25H21N3O3F3, 468.1530; found, 468.1531. 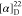 +23.33 (c 0.18, CHCl3).
+23.33 (c 0.18, CHCl3).
(M,S)-2-(N-Methylformamido)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-12
Purified by CC (hexane/EtOAc
2:1).
Yield 16 mg (70%). Isolated as a mixture of rotamers. Peaks belonging
to the major rotamer are designated as M, and peaks belonging to the
minor rotamer are designated as m. 1H NMR (500 MHz, CDCl3): δ 8.29–8.21 (m, 1H, m), 8.17 (s, 1H, m), 8.21–8.12
(m, 1H, m), 7.97–7.94 (m, 1H, m), 7.94–7.92 (m, 1H,
M), 7.81 (td, J = 7.7, 1.6 Hz, 1H, M), 7.77 (td, J = 7.7, 1.7 Hz, 1H, m), 7.73 (dd, J =
7.8, 1.3 Hz, 1H, M), 7.75–7.66 (m, 2H, m), 7.52 (dd, J = 7.8, 1.1 Hz, 1H, M), 7.47 (dd, J =
7.8, 1.3 Hz, 1H, m), 7.44–7.27 (m, 10H, both rotamers), 7.10
(m, 2H, m), 7.05 (dd, m, 2H, M), 7.01 (ddd, J = 7.8, 1.4, 0.7 Hz,
1H, m), 6.98 (d, m, 1H, M), 5.71 (dd, J = 10.4, 4.8
Hz, 1H, m), 4.68–4.55 (m, 1H, M), 4.61–4.58 (m, 1H,
m), 4.43 (dd, J = 9.8, 1.7 Hz, 1H, m), 4.43 (ddd, J = 11.6, 10.2, 8.2 Hz, 1H, m), 4.33 (dd, J = 7.1, 4.7 Hz, 1H, M), 2.43 (s, 3H, M), 2.25 (s, 3H, m). 13C NMR {1H} (126
MHz, CDCl3): δ 163.7, 163.7, 163.3, 162.8, 141.32
(app. d, J = 38.5 Hz), 140.9, 140.7, 137.7, 135.0,
134.7, 134.4, 134.3, 134.2, 134.0, 133.0, 132.4, 130.9, 130.9, 130.3,
130.1, 129.2, 129.0, 128.8, 128.5, 128.4, 128.2, 127.7, 127.0, 126.3,
126.0, 124.2, 124.0, 121.6, 121.5, 118.97 (q, J =
272.0 Hz), 111.1, 110.9, 62.5, 61.8, 59.4, 52.5, 29.8, 26.0. 19F NMR (471 MHz, CDCl3): δ −61.4,
−61.5. HRMS (ESI) m/z: [M
+ H]+ calcd for C25H21N3O3F3, 468.1530; found, 468.1531. 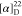 +67.50 (c 0.16, CHCl3).
+67.50 (c 0.16, CHCl3).
(P,S)-2-Amino-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-13
Compound (P)-1 (15 mg, 0.028 mmol)
was dissolved in dry DCM (2 mL), and TFA (250 μL) was added.
The reaction mixture was stirred at room temperature for 20 min. The
solvent was evaporated by a stream of nitrogen, and the resulting
solid residue was dissolved in EtOAc (2 mL) and extracted with sat.
NaHCO3. The organic layer was dried with MgSO4, and EtOAc was evaporated to yield 8.5 mg of a colorless oil (70%). 1H NMR (500 MHz, CDCl3): δ 8.28–8.25
(m, 1H), 8.01–7.98 (m, 1H), 7.79 (td, J =
7.6, 1.7 Hz, 1H), 7.73 (td, J = 7.7, 1.3 Hz, 1H),
7.51 (dd, J = 7.8, 1.2 Hz, 1H), 7.45–7.38
(m, 2H), 7.29–7.20 (m, 3H), 7.19–7.17 (m, 2H), 7.07–7.05
(m, 1H), 3.97–3.83 (m, 2H), 3.55 (dd, J =
9.0, 3.9 Hz, 1H), 1.07 (br s, 2H). 13C NMR {1H} (126 MHz, CDCl3): δ 164.3, 141.31 (app. d, J = 38.7 Hz), 140.8, 137.9, 133.9, 133.8, 133.1, 130.8,
130.1, 129.2, 128.7, 127.9, 126.8, 126.3, 124.3, 121.7, 119.0 (q, J = 272.0 Hz), 111.0, 71.6, 54.2. 19F NMR (471
MHz, CDCl3): δ −61.5. HRMS (ESI) m/z: [M + H]+ calcd for C23H19N3O2F3, 426.1424;
found, 426.1424. 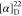 +70.59 (c 0.09, CHCl3).
+70.59 (c 0.09, CHCl3).
(M,S)-2-Amino-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-13
Compound (M)-1 (21 mg, 0.04 mmol) was
dissolved in dry DCM (2 mL), and TFA (250 μL) was added. The
reaction mixture was stirred at room temperature for 20 min. The solvent
was evaporated by a stream of nitrogen, and the resulting solid residue
was dissolved in EtOAc (2 mL) and extracted with sat. NaHCO3. The organic layer was dried with MgSO4, and EtOAc was
evaporated to yield 10 mg of a colorless oil (58%). 1H
NMR (500 MHz, CDCl3): δ 8.22 (dd, J = 7.8, 1.6 Hz, 1H), 8.00–7.98 (m, 1H), 7.79 (td, J = 7.7, 1.6 Hz, 1H), 7.72 (td, J = 7.7,
1.3 Hz, 1H), 7.51 (d, J = 7.7 Hz, 1H), 7.44 (ddd, J = 8.2, 7.3, 1.1 Hz, 1H), 7.38 (td, J =
7.8, 7.3, 1.1 Hz, 1H), 7.28–7.20 (m, 3H), 7.14–7.11
(m, 2H), 7.03–7.00 (m, 1H), 3.94–3.89 (m, 1H), 3.86
(dd, J = 10.9, 8.9 Hz, 1H), 3.57 (dd, J = 8.7, 4.0 Hz, 1H), 1.36 (br s, 2H). 13C NMR {1H} (126 MHz, CDCl3): δ 164.4, 141.21 (q, J = 38.1 Hz), 140.8, 137.8, 133.8, 132.8, 130.7, 130.1,
129.2, 128.7, 127.9, 126.7, 126.2, 124.3, 121.7, 119.00 (q, J = 272.1 Hz), 110.9, 71.6, 54.2. 19F NMR (471
MHz, CDCl3): δ −61.2. HRMS (ESI) m/z: [M + H]+ calcd for C23H19N3O2F3, 426.1424;
found, 426.1422.  +48.0 (c 0.1, CHCl3).
+48.0 (c 0.1, CHCl3).
(P,S)-2-(Dibenzylamino)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-14
Purified by CC (hexane/EtOAc 6:1). Yield 12 mg (40%). 1H NMR (500 MHz, CDCl3): δ 8.05 (dd, J = 7.8, 1.4 Hz, 1H), 7.86 (d, J = 8.1 Hz, 1H), 7.75
(td, J = 7.7, 1.6 Hz, 1H), 7.65 (td, J = 7.7, 1.3 Hz, 1H), 7.46–7.44 (m, 2H), 7.35–7.31 (m,
3H), 7.31–7.26 (m, 9H), 7.23 (ddd, J = 12.0,
7.3, 2.2 Hz, 3H), 7.13 (d, J = 6.9 Hz, 2H), 6.87
(dt, J = 8.2, 0.9 Hz, 1H), 4.53 (dd, J = 11.4, 6.7 Hz, 1H), 4.33 (dd, J = 11.4, 7.4 Hz,
1H), 3.78 (t, J = 7.0 Hz, 1H), 3.66 (d, J = 13.8 Hz, 2H), 3.23 (d, J = 13.8 Hz, 2H). 13C NMR {1H} (126 MHz, CDCl3): δ
163.8, 140.95 (q, J = 38.5 Hz), 140.7, 139.7, 137.6,
136.5, 134.3, 133.6, 132.3, 130.5, 130.2, 129.0, 128.9, 128.7, 128.4,
128.3, 127.7, 127.1, 125.9, 123.9, 121.6, 118.9 (q, J = 272.0 Hz), 110.7, 64.3, 60.4, 54.2. 19F NMR (471 MHz,
CDCl3): δ −61.3. HRMS (ESI) m/z: [M + H]+ calcd for C37H31N3O2F3, 606.2363;
found, 606.2364.  +11.67 (c 0.12, CHCl3).
+11.67 (c 0.12, CHCl3).
(M,S)-2-(Dibenzylamino)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-14
Purified by CC (hexane/EtOAc 6:1). Yield 14 mg (46%). 1H NMR (500 MHz, CDCl3): δ 8.04 (dd, J = 7.9, 1.6 Hz, 1H), 7.87 (d, J = 8.2 Hz, 1H), 7.74
(td, J = 7.7, 1.6 Hz, 1H), 7.65 (td, J = 7.7, 1.3 Hz, 1H), 7.47–7.44 (m, 2H), 7.37–7.31 (m,
2H), 7.31–7.26 (m, 10H), 7.23 (ddd, J = 13.0,
5.0, 2.8 Hz, 4H), 7.10 (d, J = 7.1 Hz, 2H), 6.89
(dt, J = 8.2, 0.9 Hz, 1H), 4.51 (dd, J = 11.4, 6.7 Hz, 1H), 4.42 (dd, J = 11.4, 7.5 Hz,
1H), 3.88 (t, J = 7.0 Hz, 1H), 3.65 (d, J = 13.8 Hz, 2H), 3.27 (d, J = 13.8 Hz, 2H). 13C NMR {1H} (126 MHz, CDCl3): δ
163.7, 141.03 (q, J = 38.3 Hz), 140.8, 139.7, 137.6,
136.5, 134.4, 133.6, 132.3, 130.5, 130.1, 128.9, 128.8, 128.7, 128.4,
128.3, 127.7, 127.1, 125.9, 123.9, 121.6, 119.0 (q, J = 272.0 Hz), 110.7, 64.1, 60.3, 54.2. 19F NMR (471 MHz,
CDCl3): δ −61.4. HRMS (ESI) m/z: [M + H]+ calcd for C37H31N3O2F3, 606.2363;
found, 606.2365.  +72.86 (c 0.14, CHCl3).
+72.86 (c 0.14, CHCl3).
(P,S)-2-(1,3-Dioxoisoindolin-2-yl)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (P)-15
Purified by CC (hexane/EtOAc 2:1). Yield 13 mg (46%). 1H NMR (500 MHz, CDCl3): δ 8.09 (dd, J = 7.9, 1.6 Hz, 1H), 7.87 (d, J = 8.1 Hz, 1H), 7.81
(dd, J = 5.5, 3.0 Hz, 2H), 7.74–7.72 (m, 1H),
7.77–7.67 (m, 2H), 7.63 (td, J = 7.7, 1.1
Hz, 1H), 7.42 (d, J = 7.7 Hz, 1H), 7.37–7.33
(m, 3H), 7.31–7.24 (m, 4H), 6.92 (d, J = 8.2
Hz, 1H), 5.32 (dd, J = 9.8, 5.5 Hz, 1H), 4.94 (dd, J = 11.4, 9.8 Hz, 1H), 4.72 (dd, J = 11.5,
5.5 Hz, 1H). 13C NMR {1H} (126 MHz, CDCl3): δ 167.9, 163.7, 140.85 (q, J = 38.4
Hz), 140.7, 134.2, 134.2, 133.8, 132.5, 131.9, 130.6, 130.2, 128.6,
128.1, 126.0, 124.0, 123.5, 121.7, 118.8 (q, J =
273.0 Hz), 110.6, 63.6, 53.6. 19F NMR (471 MHz, CDCl3): δ −61.5. HRMS (ESI) m/z: [M + H]+ calcd for C31H21N3O4F3, 566.1479; found, 566.1481.  −49.26 (c 0.13,
CHCl3).
−49.26 (c 0.13,
CHCl3).
(M,S)-2-(1,3-Dioxoisoindolin-2-yl)-2-phenylethyl 2-(2-(Trifluoromethyl)-1H-benzo[d]imidazole-1-yl)benzoate (M)-15
Purified by CC (hexane/EtOAc 2:1). Yield 14 mg (49%). 1H NMR (400 MHz, CDCl3): δ 8.11 (dd, J = 7.8, 1.6 Hz, 1H), 7.91 (dt, J = 8.2, 0.8 Hz,
1H), 7.82–7.77 (m, 2H), 7.73–7.68 (m, 3H), 7.63 (td, J = 7.7, 1.3 Hz, 1H), 7.44–7.41 (m, 1H), 7.34–7.24
(m, 6H), 7.20 (ddd, J = 8.2, 7.2, 1.1 Hz, 1H), 6.87
(dt, J = 8.2, 0.9 Hz, 2H), 5.18 (dd, J = 9.8, 5.4 Hz, 1H), 5.02 (dd, J = 11.4, 9.8 Hz,
1H), 4.62 (dd, J = 11.4, 5.5 Hz, 1H). 13C NMR {1H} (126 MHz, CDCl3): δ 167.9,
163.7, 140.95 (q, J = 38.8 Hz), 140.7, 137.6, 136.0,
134.2, 134.2, 133.9, 132.6, 131.9, 130.6, 130.2, 128.9, 128.6, 128.6,
128.0, 125.9, 123.9, 123.5, 121.8, 118.88 (q, J =
271.5 Hz), 110.5, 63.8, 53.6. 19F NMR (471 MHz, CDCl3): δ −61.4. HRMS (ESI) m/z: [M + H]+ calcd for C31H21N3O4F3, 566.1479; found, 566.1481. 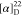 +21.43 (c 0.14, CHCl3).
+21.43 (c 0.14, CHCl3).
Methyl (S)-2-Formamido-2-phenylacetate
 Following the literature procedure16
Following the literature procedure16
(S)-Phenylglycine methylester hydrochloride
(603 mg, 3 mmol, 1 equiv) was dissolved in DI water (10 mL), and aq.
K2CO3 solution was added (10 mL, 10 wt %). The
solution was extracted with diethylether (3 × 20 mL), dried with
MgSO4, and evaporated to yield freebase (S)-phenylglycine methylester [360 mg of a clear oil (70%)]. This oil
was dissolved in formic acid (30 mL) and cooled in an ice bath. Acetic
anhydride (8.3 mL) was added dropwise while cooling. After the addition
was complete, the reaction mixture was stirred for 16 h. After 16
h, DI water was added (20 mL) and the solution was stirred for 20
min and evaporated. The oily residue was dissolved in EtOAc (50 mL)
and extracted with 10% aq. HCl (3 × 50 mL) and 10% aq. K2CO3 (3 × 50 mL), dried with MgSO4, and evaporated to yield a clear oil, which solidified upon standing
on room temperature or under high vacuum. Yield: 371 mg of a white
solid (75%). The reaction was reproduced on a 10 mmol scale, yielding
1.2 g (65%) of a white solid. 1H NMR (400 MHz, CDCl3): δ 8.25 (s, 1H), 7.38–7.33 (m, 5H), 6.60 (s,
1H), 5.67 (d, J = 7.4 Hz, 1H), 3.75 (s, 3H). 13C NMR {1H} (101 MHz, CDCl3): δ
171.1, 160.2, 136.2, 129.2, 128.9, 127.3, 55.2, 53.1. HRMS (ESI) m/z: [M + H]+ calcd for C10H12N3O1, 194.0812; found,
194.0813. 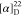 +87.62 (c 0.42, CHCl3).
+87.62 (c 0.42, CHCl3).
(S)-2-(Methylamino)-2-phenylethan-1-ol
 Modified literature procedure16
Modified literature procedure16
Methyl (S)-2-formamido-2-phenylacetate (400
mg, 2 mmol, 1 equiv) was added portionwise to a suspension of LiAlH4 (380 mg, 10 mmol, 5 equiv) in dry THF (15 mL) at 5 °C
(ice/water bath). After addition was completed, the mixture was refluxed
for 16 h. After reaction completion (TLC, EtOAc/MeOH 2:1), the reaction
mixture was cooled to room temperature and further cooled in an ice
bath, and aq. NaOH solution (15% by weight, 0.75 mL/mmol LiALH4) was added dropwise. The resulting suspension was filtered
through Celite and washed thoroughly with EtOAc, dried with MgSO4, and evaporated. The residual oil was purified by CC (EtOAc/MeOH
2:1), yielding 242 mg of a white solid (80%). The reaction was reproduced
on a 6.2 mmol scale, yielding a white solid, which was suspended in
chloroform and filtered, and after evaporation, 800 mg (85%) of a
white solid was obtained. 1H NMR (400 MHz, CDCl3): δ 7.39–7.34 (m, 2H), 7.32–7.27 (m, 3H), 3.75
(dd, J = 10.1, 4.1 Hz, 1H), 3.71–3.66 (m,
1H), 3.61 (dd, J = 10.0, 8.0 Hz, 1H), 2.69 (s, 2H),
2.36 (s, 3H). 13C NMR {1H} (101 MHz, CDCl3): δ 179.1, 129.4, 129.2, 128.2, 66.2, 64.5, 31.5, 23.8.
HRMS (ESI) m/z: [M + H]+ calcd for C9H14NO, 152.1070; found, 152.1070. 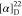 +39.89 (c 0.88, CHCl3).
+39.89 (c 0.88, CHCl3).
tert-Butyl (S)-(2-Hydroxy-1-phenylethyl)(methyl)carbamate
 Following
the literature procedure17
Following
the literature procedure17
(S)-2-(Methylamino)-2-phenylethan-1-ol (40
mg, 0.25 mmol, 1 equiv) was dissolved in EtOAc (10 mL). Boc2O was added at once, and the mixture was refluxed for 16 h. After
16 h, the reaction mixture was cooled to room temperature, washed
twice with water and once with brine, dried with MgSO4,
and evaporated to provide 53 mg of an oily product (85%). 1H NMR (400 MHz, CDCl3): δ 7.36–7.31 (m, 2H),
7.30–7.26 (m, 1H), 7.24–7.21 (m, 2H), 5.32–5.24
(m, 1H), 4.11–4.01 (m, 2H), 2.69 (s, 2H). 13C NMR
{1H} (101 MHz, CDCl3): δ 146.9, 128.8,
127.8, 127.5, 85.3, 80.4, 60.6, 28.6, 27.6. HRMS (ESI) m/z: [M + H]+ calcd for C14H22NO3, 252.1594; found, 252.1595. 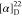 +55.17 (c 0.6, CHCl3).
+55.17 (c 0.6, CHCl3).
(S)-2-(Dibenzylamino)-2-phenylethan-1-ol
 Following the literature procedure18
Following the literature procedure18
(S)-Phenylglycinol (137 mg, 1 mmol, 1 equiv)
was dissolved in acetonitrile (7 mL). K2CO3 (280
mg, 2 mmol, 2 equiv) was added, followed by benzyl bromide (250 μL,
2.1 mmol, 2.1 equiv). The reaction mixture was stirred at 60 °C
for 24 h. After the reaction was complete (TLC, hexane/EtOAc 4:1),
the reaction mixture was filtered and the filtrate was evaporated
and purified by CC (hexane/EtOAc, gradient from 10:1 to 8:1). Isolated
as a colorless oil (199 mg, 62%). 1H NMR (400 MHz, CDCl3): δ 7.48–7.34 (m, 3H), 7.34 (d, J = 4.4 Hz, 8H), 7.27 (q, J = 3.9, 3.2 Hz, 4H), 4.14
(t, J = 10.6 Hz, 1H), 3.96–3.93 (m, 1H), 3.62
(dd, J = 10.8, 5.2 Hz, 1H), 3.16 (d, J = 13.4 Hz, 1H). 13C NMR {1H} (101 MHz, CDCl3): δ 139.3, 135.3, 129.4, 129.1, 128.7, 128.5, 128.2,
127.4, 63.2, 60.6, 53.7. HRMS (ESI) m/z: [M + H]+ calcd for C22H24NO, 318.1852;
found, 318.1853. 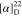 +122.33 (c 0.6, CHCl3).
+122.33 (c 0.6, CHCl3).
(S)-2-(2-Hydroxy-1-phenylethyl)isoindoline-1,3-dione
 Following the literature procedure19
Following the literature procedure19
(S)-Phenylglycinol (420 mg, 3 mmol, 1 equiv)
was suspended in toluene (10 mL). Phthalic anhydride (450 mg, 3 mmol,
1 equiv) was added, followed by triethylamine (50 μL, 0.3 mmol,
0.1 equiv). The reaction mixture was refluxed for 16 h and then cooled
to room temperature and evaporated, and the residue was dissolved
in EtOAc (25 mL) and extracted with 10% aq. HCl (3 × 25 mL) and
10% aq. K2CO3 (3 × 25 mL). The combined
organic layers were washed with brine, dried with MgSO4, and evaporated. The residue was purified by CC (hexane/EtOAc 2:1).
Yield 390 mg (50%). 1H NMR (400 MHz, CDCl3):
δ 7.83 (dd, J = 5.6, 3.2 Hz, 2H), 7.71 (td, J = 5.3, 2.1 Hz, 2H), 7.46 (dd, J = 6.9,
1.5 Hz, 2H), 7.39–7.23 (m, 3H), 5.47 (dd, J = 9.0, 5.0 Hz, 1H), 4.65 (dd, J = 11.7, 8.9 Hz,
1H), 4.24 (dd, J = 11.7, 5.0 Hz, 1H). 13C NMR {1H} (101 MHz, CDCl3): δ 169.0,
137.0, 134.3, 132.0, 128.9, 128.3, 128.0, 123.6, 62.5, 57.7. HRMS
(ESI) m/z: [M + H]+ calcd
for C16H14NO3, 268.0968; found, 268.
0967. 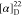 −45.17 (c 0.29,
CHCl3).
−45.17 (c 0.29,
CHCl3).
(S)-2-Acetamido-2-phenylethyl Acetate
 (S)-Phenylglycinol (670
mg, 5 mmol, 1 equiv)
and DMAP (70 mg, 0.5 mmol, 0.1 equiv) were dissolved in Ac2O (7 mL) and stirred at room temperature for 2.5 h. After 2.5 h,
the solution was added dropwise into an aq. solution of K2CO3 (10%, 15 mL). The solution was further neutralized
with solid K2CO3 until pH = 7 and then extracted
into DCM (3 × 30 mL). Organic layers were combined and dried
with MgSO4 and evaporated to yield a white solid (573 mg,
50%). 1H NMR (500 MHz, CDCl3): δ 7.37–7.33
(m, 2H), 7.31–7.27 (m, 3H), 6.09 (d, J = 6.4
Hz, 1H), 5.29 (td, J = 7.6, 4.7 Hz, 1H), 4.43 (dd, J = 11.5, 7.2 Hz, 1H), 4.26 (dd, J = 11.5,
4.7 Hz, 1H), 2.05 (s, 3H), 2.02 (s, 3H). 13C NMR {1H} (126 MHz, CDCl3): δ 171.4, 169.7, 138.5,
129.0, 128.1, 126.8, 66.2, 52.7, 23.5, 21.0. HRMS (ESI) m/z: [M + H]+ calcd for: C12H16NO3, 222.1130; found, 222.1125.
(S)-Phenylglycinol (670
mg, 5 mmol, 1 equiv)
and DMAP (70 mg, 0.5 mmol, 0.1 equiv) were dissolved in Ac2O (7 mL) and stirred at room temperature for 2.5 h. After 2.5 h,
the solution was added dropwise into an aq. solution of K2CO3 (10%, 15 mL). The solution was further neutralized
with solid K2CO3 until pH = 7 and then extracted
into DCM (3 × 30 mL). Organic layers were combined and dried
with MgSO4 and evaporated to yield a white solid (573 mg,
50%). 1H NMR (500 MHz, CDCl3): δ 7.37–7.33
(m, 2H), 7.31–7.27 (m, 3H), 6.09 (d, J = 6.4
Hz, 1H), 5.29 (td, J = 7.6, 4.7 Hz, 1H), 4.43 (dd, J = 11.5, 7.2 Hz, 1H), 4.26 (dd, J = 11.5,
4.7 Hz, 1H), 2.05 (s, 3H), 2.02 (s, 3H). 13C NMR {1H} (126 MHz, CDCl3): δ 171.4, 169.7, 138.5,
129.0, 128.1, 126.8, 66.2, 52.7, 23.5, 21.0. HRMS (ESI) m/z: [M + H]+ calcd for: C12H16NO3, 222.1130; found, 222.1125. 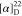 +80.77 (c 0.13, CHCl3).
+80.77 (c 0.13, CHCl3).
(S)-N-(2-Hydroxy-1-phenylethyl)acetamide
 (S)-2-Acetamido-2-phenylethyl acetate (300
mg, 1.35 mmol, 1 equiv) was dissolved in MeOH (15 mL). Then, a solution
of NaOH (270 mg, 6.75 mmol, 5 equiv dissolved in 5 mL of DI water)
was added and stirred at room temperature for 12 h. After 12 h, the
mixture was filtered through a pad of Celite and washed with 30 mL
of EtOAc/MeOH (1:1), and the filtrate was dried with MgSO4 and evaporated to yield a white solid (228 mg, 93%). 1H NMR (500 MHz, CDCl3): δ 7.37–7.33 (m, 2H),
7.31–7.27 (m, 3H), 6.38 (d, J = 3.5 Hz, 1H),
5.04 (dt, J = 7.1, 5.1 Hz, 1H), 3.85 (d, J = 5.1 Hz, 2H), 3.12 (s, 1H), 2.02 (s, 3H). 13C NMR {1H} (126 MHz, CDCl3): δ 171.0,
139.1, 129.0, 128.0, 126.9, 66.6, 56.1, 23.4 HRMS (ESI) m/z: [M + H]+ calcd for: C10H14NO2, 180.1019; found, 180.1019.
(S)-2-Acetamido-2-phenylethyl acetate (300
mg, 1.35 mmol, 1 equiv) was dissolved in MeOH (15 mL). Then, a solution
of NaOH (270 mg, 6.75 mmol, 5 equiv dissolved in 5 mL of DI water)
was added and stirred at room temperature for 12 h. After 12 h, the
mixture was filtered through a pad of Celite and washed with 30 mL
of EtOAc/MeOH (1:1), and the filtrate was dried with MgSO4 and evaporated to yield a white solid (228 mg, 93%). 1H NMR (500 MHz, CDCl3): δ 7.37–7.33 (m, 2H),
7.31–7.27 (m, 3H), 6.38 (d, J = 3.5 Hz, 1H),
5.04 (dt, J = 7.1, 5.1 Hz, 1H), 3.85 (d, J = 5.1 Hz, 2H), 3.12 (s, 1H), 2.02 (s, 3H). 13C NMR {1H} (126 MHz, CDCl3): δ 171.0,
139.1, 129.0, 128.0, 126.9, 66.6, 56.1, 23.4 HRMS (ESI) m/z: [M + H]+ calcd for: C10H14NO2, 180.1019; found, 180.1019. 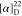 +45.26 (c 0.19, CHCl3).
+45.26 (c 0.19, CHCl3).
(S)-N-(2-Hydroxy-1-phenylethyl)-N-methylacetamide
 Following the literature procedure20
Following the literature procedure20
(S)-N-Methyl-phenylglycinol
(50 mg, 0.33 mmol, 1 equiv) was dissolved in DCM (1.5 mL), and acetylchloride
(30 μL, 0.4 mmol, 1.2 equiv) was added, followed by a dropwise
addition of 0.5 M NaOH (840 μL, 0.4 mmol, 1.2 equiv). The biphasic
system was stirred vigorously for 1 h. Then, the mixture was diluted
with water (10 mL) and extracted with DCM (3 × 10 mL). Organic
layers were combined, dried with MgSO4, and purified by
CC (EtOAc/MeOH 20:1) to yield 50 mg of a white solid (78%) as a mixture
of rotamers in a 10:4 ratio. Peaks belonging to the major rotamer
are designated as M, and peaks belonging to a minor rotamer are designated
as m. 1H NMR (400 MHz, CDCl3): δ 7.41–7.19
(m, 10H, both rotamers), 5.83 (dd, J = 9.3, 4.9 Hz,
1H, M), 5.09 (dd, J = 9.2, 4.9 Hz, 1H, m), 4.23–4.02
(m, 4H, both rotamers), 2.78 (s, 4H, both rotamers), 2.42 (dd, J = 7.2, 4.7 Hz, 1H, M), 2.28 (s, 3H, m), 2.19 (s, 3H, M),
2.15–2.05 (m, 1H, m). 13C NMR {1H} (126
MHz, CDCl3): δ 172.8 m, 172.4 m, 137.2 m, 137.0 m,
129.1 m, 128.8 m, 128.2 m, 127.93 m, 127.89 m, 127.0 m, 62.6 m, 61.9
m, 61.5 m, 58.4 m, 32.0 m, 28.1 m, 22.5 m, 22.2 m. HRMS (ESI) m/z: [M + H]+ calcd for: C11H16NO2, 194.1176; found, 194.1176.  −440.0 (c 0.13,
CHCl3).
−440.0 (c 0.13,
CHCl3).
(S)-2-(N-Methylformamido)-2-phenylethyl Formate
 Following the literature procedure21
Following the literature procedure21
HCOOH (150 μL, 4 mmol, 4 equiv)
dissolved in CHCl3 (2 mL) was added dropwise under cooling
into a solution of
DCC (412 mg, 2 mmol, 2 equiv) in CHCl3 (3 mL). After 5
min, a white suspension was added dropwise into the solution of (S)-N-methyl-phenylglycinol (151 mg, 1 mmol,
1 equiv) in a mixture of CHCl3 (3 mL) and pyridine (1.5
mL) and stirred in an ice bath for 16 h. After 16 h, the reaction
mixture was evaporated, suspended in diethylether (10 mL), and filtered,
and the filtrate was evaporated. The residue was then dissolved in
ethylacetate and extracted twice with 10% HCl, 10% K2CO3, and brine; dried with MgSO4; and purified by
CC (hexane/EtOAc 1:1) to yield 100 mg of an oily product (50%) as
a mixture of two rotamers in a 10:6 ratio. Peaks belonging to a major
rotamer are designated as M, and peaks belonging to a minor rotamer
are designated as m. 1H NMR (500 MHz, CDCl3):
δ 8.31 (s, 1H, M), 8.18 (s, 1H, m), 8.10 (s, 1H, M), 8.08 (s,
1H, m), 7.43–7.31 (m, 5H, both rotamers), 7.29–7.22
(m, 5H, both rotamers), 5.91 (dd, J = 9.5, 5.3 Hz,
1H, m), 4.91 (dd, J = 10.0, 4.6 Hz, 1H, M), 4.79–4.72
(m, 1H, both rotamers), 4.67–4.62 (m, 1H, both rotamers), 2.76
(s, 3H, m), 2.69 (s, 3H, M). 13C NMR {1H} (126
MHz, CDCl3): δ 163.5 m, 163.1 m, 160.6 m, 160.4 m,
135.2 m, 134.8 m, 129.3, 129.1, 128.9, 128.6, 127.9, 127.2, 60.8 m,
59.6 m, 52.7 m, 49.3 m, 34.1, 30.6 HRMS (ESI) m/z: [M + H]+ calcd for: C11H14NO3, 208.0968; found, 208.0967.  +89.47 (c 0.19, CHCl3).
+89.47 (c 0.19, CHCl3).
(S)-N-(2-Hydroxy-1-phenylethyl)-N-methylformamide
 (S)-2-(N-Methylformamido)-2-phenylethyl
formate (80 mg, 0.38 mmol, 1 equiv) was dissolved in MeOH (8 mL),
and NH3 was added (25% aq. solution, 90 μL, 1.15
mmol, 3 equiv). The reaction mixture was stirred at room temperature
for 2 h. Then, the solvent was evaporated. The resulting residue was
dissolved in EtOAc and extracted with brine three times. The organic
layer was separated, dried with MgSO4, and evaporated.
The residue was purified by CC (EtOAc) to yield 21 mg (30%) of a colorless
oil as a mixture of two rotamers in an aprox. 10:6 ratio. Peaks belonging
to the major rotamer are designated as M, and peaks belonging to the
minor rotamer are designated as m. 1H NMR (500 MHz, CDCl3): δ 8.33 (s, 1H, M), 8.21 (s, 1H, m), 7.40–7.22
(m, 10H, both rotamers), 5.41 (dd, J = 8.4, 5.4 Hz,
1H, m), 4.68 (dd, J = 8.7, 5.3 Hz, 1H, M), 4.17–4.08
(m, 4H, both rotamers), 2.80 (s, 3H, m), 2.70 (s, 3H, M). 13C NMR {1H} (126 MHz, CDCl3): δ 164.3,
163.9, 136.2, 136.1, 129.1, 129.0, 128.5, 128.3, 127.9, 127.4, 63.5,
61.6, 60.7, 58.7, 32.1, 26.6. HRMS (ESI) m/z: [M + H]+ calcd for: C10H14O2N1, 180.1019; found, 180.1019.
(S)-2-(N-Methylformamido)-2-phenylethyl
formate (80 mg, 0.38 mmol, 1 equiv) was dissolved in MeOH (8 mL),
and NH3 was added (25% aq. solution, 90 μL, 1.15
mmol, 3 equiv). The reaction mixture was stirred at room temperature
for 2 h. Then, the solvent was evaporated. The resulting residue was
dissolved in EtOAc and extracted with brine three times. The organic
layer was separated, dried with MgSO4, and evaporated.
The residue was purified by CC (EtOAc) to yield 21 mg (30%) of a colorless
oil as a mixture of two rotamers in an aprox. 10:6 ratio. Peaks belonging
to the major rotamer are designated as M, and peaks belonging to the
minor rotamer are designated as m. 1H NMR (500 MHz, CDCl3): δ 8.33 (s, 1H, M), 8.21 (s, 1H, m), 7.40–7.22
(m, 10H, both rotamers), 5.41 (dd, J = 8.4, 5.4 Hz,
1H, m), 4.68 (dd, J = 8.7, 5.3 Hz, 1H, M), 4.17–4.08
(m, 4H, both rotamers), 2.80 (s, 3H, m), 2.70 (s, 3H, M). 13C NMR {1H} (126 MHz, CDCl3): δ 164.3,
163.9, 136.2, 136.1, 129.1, 129.0, 128.5, 128.3, 127.9, 127.4, 63.5,
61.6, 60.7, 58.7, 32.1, 26.6. HRMS (ESI) m/z: [M + H]+ calcd for: C10H14O2N1, 180.1019; found, 180.1019.  +41.51 (c 0.21, CHCl3).
+41.51 (c 0.21, CHCl3).
(S)-2-(Dimethylamino)-2-phenylethan-1-ol
 Following the literature procedure22
Following the literature procedure22
(S)-Phenylglycinol (550 mg, 4 mmol, 1 equiv)
was dissolved in HCOOH (0.6 mL), and formaldehyde was added (38% aq.
solution, 0.6 mL). The reaction mixture was heated at 90 °C for
16 h. After 16 h, the solution was cooled to room temperature, neutralized
with the ammonia solution (25% aq. solution, 0.5 mL), and extracted
three times with DCM. The organic phases were combined and dried with
MgSO4, evaporated, and purified by CC (EtOAc/MeOH 20:1)
to yield 400 mg (60%) of a brown oil, which solidified after standing
at room temperature. 1H NMR (400 MHz, CDCl3):
δ 7.41–7.28 (m, 3H), 7.25–7.16 (m, 2H), 3.93 (dd, J = 10.7, 9.0 Hz, 1H), 3.68 (dd, J = 10.6,
5.3 Hz, 1H), 3.57 (dd, J = 9.0, 5.3 Hz, 1H), 2.21
(s, 3H). 13C NMR {1H} (101 MHz, CDCl3): δ 135.9, 129.1, 128.3, 128.0, 70.3, 61.4, 41.5. HRMS (ESI) m/z: [M + H]+ calcd for: C10H16NO, 166.1266; found, 166.1266.  +32.5 (c 0.36, CHCl3).
+32.5 (c 0.36, CHCl3).
Acknowledgments
The authors are grateful to the Internal Grant Agency of Palacký University (IGA_PrF_2021_024).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c07234.
1H and 13C NMR spectra of all prepared compounds in CDCl3, 1H NMR spectra of compounds 1–8 in acetone-d6, and 1H NMR spectra of compounds 1 and 4 in acetonitrile-d3 (PDF)
Author Present Address
§ Institute of Organic Chemistry and Biochemistry, The Czech Academy of Sciences, Flemingovo nám. 2, Prague 166 10, Czech Republic
Author Contributions
M.K. and D.P. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- Sullivan G. R.; Dale J. A.; Mosher H. S. Correlation of Configuration and Fluorine-19 Chemical Shifts of.Alpha.-Methoxy-.Alpha.-Trifluoromethylphenyl Acetate Derivatives. J. Org. Chem. 1973, 38, 2143–2147. 10.1021/jo00952a006. [DOI] [Google Scholar]
- Brummel B. R.; Lee K. G.; McMillen C. D.; Kolis J. W.; Whitehead D. C. One-Pot Absolute Stereochemical Identification of Alcohols via Guanidinium Sulfate Crystallization. Org. Lett. 2019, 21, 9622–9627. 10.1021/acs.orglett.9b03792. [DOI] [PubMed] [Google Scholar]
- Stephens P. J.; Devlin F. J.; Pan J.-J. The Determination of the Absolute Configurations of Chiral Molecules Using Vibrational Circular Dichroism (VCD) Spectroscopy. Chirality 2008, 20, 643–663. 10.1002/chir.20477. [DOI] [PubMed] [Google Scholar]
- Kriegelstein M.; Profous D.; Lyčka A.; Trávníček Z.; Přibylka A.; Volná T.; Benická S.; Cankař P. Axially Chiral Trifluoromethylbenzimidazolylbenzoic Acid: A Chiral Derivatizing Agent for α-Chiral Primary Amines and Secondary Alcohols To Determine the Absolute Configuration. J. Org. Chem. 2019, 84, 11911–11921. 10.1021/acs.joc.9b01770. [DOI] [PubMed] [Google Scholar]
- Seco J. M.; Quiñoá E.; Riguera R. Boc-Phenylglycine : The Reagent of Choice for the Assignment of the Absolute Configuration of α -Chiral Primary Amines by 1 H NMR Spectroscopy. J. Org. Chem. 1999, 64, 4669–4675. 10.1021/jo982305q. [DOI] [PubMed] [Google Scholar]
- Latypov S. K.; Seco J. M.; Quiñoá E.; Riguera R. Conformational Structure and Dynamics of Arylmethoxyacetates: DNMR Spectroscopy and Aromatic Shielding Effect. J. Org. Chem. 1995, 60, 504–515. 10.1021/jo00108a008. [DOI] [Google Scholar]
- Louzao I.; Seco M.; Quin E.; Riguera R. 13C NMR as a General Tool for the Assignment of Absolute Configuration. Chem. Commun. 2010, 46, 7903–7905. 10.1039/c0cc02774j. [DOI] [PubMed] [Google Scholar]
- Seco J. M.; Latypov S. K.; Quiñoá E.; Riguera R. Choosing the Right Reagent for the Determination of the Absolute Configuration of Amines by NMR: MTPA or MPA?. J. Org. Chem. 1997, 62, 7569–7574. 10.1021/jo970427x. [DOI] [Google Scholar]
- Seco J. M.; Quiñoá E.; Riguera R. The Assignment of Absolute Configurations by NMR of Arylmethoxyacetate Derivatives: Is This Methodology Being Correctly Used?. Tetrahedron Asymmetry 2000, 11, 2781–2791. 10.1016/s0957-4166(00)00228-7. [DOI] [Google Scholar]
- Freire F.; Seco J. M.; Quiñoá E.; Riguera R. Challenging the Absence of Observable Hydrogens in the Assignment of Absolute Configurations by NMR: Application to Chiral Primary Alcohols. Chem. Commun. 2007, 1, 1456–1458. 10.1039/b617184b. [DOI] [PubMed] [Google Scholar]
- Latypov S. K.; Seco J. M.; Quiñoá E.; Riguera R. MTPA vs MPA in the Determination of the Absolute Configuration of Chiral Alcohols by 1H NMR. J. Org. Chem. 1996, 61, 8569–8577. 10.1021/jo960719i. [DOI] [Google Scholar]
- Kriegelstein M.; Profous D.; Přibylka A.; Cankař P. The Assignment of Absolute Configuration of β-Chiral Primary Alcohols with Axially Chiral Trifluoromethylbenzimidazolylbenzoic Acid. J. Org. Chem. 2020, 85, 12912–12921. 10.1021/acs.joc.0c01510. [DOI] [PubMed] [Google Scholar]
- Latypov S. K.; Ferreiro M. J.; Quiñoá E.; Riguera R. Assignment of the Absolute Configuration of β-Chiral Primary Alcohols by NMR: Scope and Limitations. J. Am. Chem. Soc. 1998, 120, 4741–4751. 10.1021/ja972550b. [DOI] [Google Scholar]
- Erhardt P. W. Benzylamine and Dibenzylamine Revisited Syntheses of N-Substituted Aryloxypropanol Amines Exemplifying a General Route to Secondary Aliphatic Amines. Synth. Commun. 1983, 13, 103–114. 10.1080/00397918308061967. [DOI] [Google Scholar]
- Chen Z.-C.; Le Z. G.; Hu Y.; Zheng Q. G. Organic Reactions in Ionic Liquids: N-Alkylation of Phthalimide and Several Nitrogen Heterocycles. Synthesis 2004, 208–212. 10.1002/chin.200423058. [DOI] [Google Scholar]
- Boyle G. A.; Govender T.; Kruger H. G.; Maguire G. E. M. Synthesis of Chiral Pentacyclo-Undecane Ligands and Their Use in the Enantioselective Alkylation of Benzaldehyde with Diethylzinc. Tetrahedron Asymmetry 2004, 15, 2661–2666. 10.1016/j.tetasy.2004.07.038. [DOI] [Google Scholar]
- Agami C.; Couty F.; Hamon L.; Venier O. Chiral Oxazolidinones from N-Boc Derivatives of β-Amino Alcohols. Effect of a N-Methyl Substituent on Reactivity and Stereoselectivity. Tetrahedron Lett. 1993, 34, 4509–4512. 10.1016/0040-4039(93)88071-p. [DOI] [Google Scholar]
- Andrés J. M.; Barrio R.; Martínez M. A.; Pedrosa R.; Pérez-Encabo A. Synthesis of Enantiopure Syn-β-Amino Alcohols. A Simple Case of Chelation-Controlled Additions of Diethylzinc to α-(Dibenzylamino) Aldehydes. J. Org. Chem. 1996, 61, 4210–4213. 10.1021/jo960017t. [DOI] [PubMed] [Google Scholar]
- Zheng M.; Xue W.; Xue T.; Gong H. Ester Formation via Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Chloroformates. Org. Lett. 2016, 18, 6152–6155. 10.1021/acs.orglett.6b03158. [DOI] [PubMed] [Google Scholar]
- Micouin L.; Jullian V.; Quirion J.-C.; Husson H.-P. Origins of Diastereoselectivity in the Alkylation of N-Substituted Lactams and Amides Derived from Optically Active Aminoalcohols. Tetrahedron Asymmetry 1996, 7, 2839–2846. 10.1016/0957-4166(96)00374-6. [DOI] [Google Scholar]
- Waki M.; Meienhofer J. Efficient Preparation of N-Formylamino Acid Tert-Butyl Esters. J. Org. Chem. 1977, 42, 2019–2020. 10.1021/jo00431a046. [DOI] [PubMed] [Google Scholar]
- Paolucci C.; Rosini G. Approach to a Better Understanding and Modeling of (S)-Dihydrofuran-2-Yl, (S)-Tetrahydrofuran-2-Yl-, and Furan-2-Yl-β-Dialkylaminoethanol Ligands for Enantioselective Alkylation. Tetrahedron Asymmetry 2007, 18, 2923–2946. 10.1016/j.tetasy.2007.11.034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






