Abstract

A strong correlation between brain metabolite accumulation and oxidative stress has been observed in Alzheimer’s disease (AD) patients. There are two central hypotheses for this correlation: (i) coaccumulation of toxic amyloid-β and Myo-inositol (MI), a significant brain metabolite, during presymptomatic stages of AD, and (ii) enhanced expression of MI transporter in brain cells during oxidative stress-induced volume changes in the brain. Identifying specific interactive effects of MI with cellular antioxidant enzymes would represent an essential step in understanding the oxidative stress-induced AD pathogenicity. This study demonstrated that MI inhibits catalase, an essential antioxidant enzyme primarily inefficient in AD, by decreasing its kcat (turnover number) and increasing Km (Michaelis–Menten constant) values. This inhibition of catalase by MI under in vivo studies increased cellular H2O2 levels, leading to decreased cell viability. Furthermore, MI induces distortion of the active heme center with an overall loss of structure and stability of catalase. MI also alters distances of the vital active site and substrate channel residues of catalase. The present study provides evidence for the involvement of MI in the inactivation of the antioxidant defense system during oxidative stress-induced pathogenesis of AD. Regulation of MI levels, during early presymptomatic stages of AD, might serve as a potential early-on therapeutic strategy for this disease.
Introduction
The leading cause of Alzheimer’s disease, the most common neurodegenerative disease in older people, has not been completely understood but is generally characterized by the deposition of extracellular β-amyloid (Aβ) aggregates and intracellular neurofibrillary tangles of hyper-phosphorylated tau protein.1−4 The amyloid cascade hypothesis claims that Aβ deposition drives the remaining AD pathology. Therefore, AD is detected more commonly by analyzing Aβ levels in cerebrospinal fluid at the biochemical level.5,6 Moreover, the accumulation of such toxic protein species results in increased oxidative stress, leading to synaptic dysfunction and eventual cell death.7,8 Molecular-level understanding of the increase in oxidative stress is currently fairly low. It is well-known that despite an increase in the expression of the antioxidant enzyme system (as an attempt to suppress the increased oxidative stress) during AD pathology, the activity of the main enzyme, catalase, is drastically reduced, resulting in increased oxidative stress.9 One central mechanism for the reduced catalase activity is believed to be the direct interaction of Aβ with antioxidant enzymes, which inhibits antioxidant functions.10 Thus, post-translational events (rather than the transcriptional processes) are believed to result in catalase inactivation, leading to impairment in the intracellular hydrogen peroxide (H2O2) degradation. Furthermore, AD pathophysiological conditions also involve specific alterations in the neuronal metabolites, including Myo-inositol (MI), choline, glutamate, N-acetyl-aspartate, and sorbitol.11,12 Nevertheless, the molecular mechanisms underlying the altered metabolite levels and the inactivation of the antioxidant mechanism remain largely unexplored.
Among metabolites, MI is the most relevant molecule in AD, as its elevated level is strongly correlated with the Aβ-induced pathology and the subsequent decline in cognitive performance.13−15 Interestingly, abnormal brain levels of MI have been detected even in the presymptomatic stages of AD, before any detectable Aβ deposits are observed.15 Clinical studies have also demonstrated that part of the increased oxidative stress is taken care of via upregulating MI uptake up to several millimolar concentrations. Thus, MI appears to be one of the critical metabolites associated with the decline of the antioxidant potential in AD pathophysiology. In this study, we attempted to investigate the functional consequences of MI on the main cytoplasmic antioxidant enzyme, catalase. We discovered that MI inhibits catalase function by increasing Km and altering the enzyme’s overall structure and active site. The study highlights a novel role of MI in AD pathophysiology and mediating cross-talk between the cellular antioxidant enzyme systems and metabolites.
Results
Effect of MI on Catalase Activity
The activity of catalase was measured by monitoring the decrease in absorbance of H2O2 at 240 nm in the presence and absence of the polyol osmolytes. Kinetic curves, obtained by plotting initial velocity (V0) versus substrate concentration, were analyzed for Michaelis–Menten constant (Km) and catalytic constant (kcat) (Table 1). It was observed that, in the presence of increasing concentrations of MI, Km increases and kcat decreases. Furthermore, with increasing concentration of MI, kcat/Km , a measure of catalytic efficiency, decreases from 4.38 × 107 to 1.63 × 107 M–1 s–1. In addition to this, activity of catalase was further carried out in other polyol compounds like mannitol, glycerol, and sorbitol. It was observed that Km decreases while kcat increases at all polyol concentrations which signify increased kcat/km in the presence of the polyols. Altogether, activity results imply that unlike other polyols MI reduces the activity of catalase substantially (Table 1).
Table 1. Kinetic Parameters of Catalase in the Presence and Absence of the Polyols.
| concentration (M) | Km (mM) | kcat (s–1) (× 106) | kcat/Km (s–1 M–1) (× 107) |
|---|---|---|---|
| Myo-inositol | |||
| 0.00 | 31.22a | 1.37× | 4.38 ± 0.13 |
| 0.05 | 32.11 | 1.29 | 4.01 ± 0.14 |
| 0.15 | 34.71 | 1.10 | 3.17 ± 0.09 |
| 0.30 | 37.28 | 0.81 | 2.18 ± 0.08 |
| 0.60 | 38.30 | 0.62 | 1.63 ± 0.05 |
| Mannitol | |||
| 0.00 | 31.22 | 1.37 | 4.38 ± 0.13 |
| 0.25 | 27.74 | 1.74 | 6.29 ± 0.23 |
| 0.50 | 25.90 | 2.04 | 7.87 ± 0.31 |
| 0.70 | 24.53 | 2.74 | 11.17 ± 0.49 |
| Glycerol | |||
| 0% | 31.22 | 1.37 | 4.38 ± 0.13 |
| 10% | 30.33 | 1.34 | 4.43 ± 0.14 |
| 20% | 27.92 | 1.40 | 5.02 ± 0.20 |
| 30% | 26.39 | 1.54 | 5.86 ± 0.22 |
| 40% | 20.78 | 12.1 | 58.1 ± 0.37 |
| Sorbitol | |||
| 0.00 | 31.22 | 1.37 | 4.38 ± 0.13 |
| 0.25 | 30.54 | 1.38 | 4.53 ± 0.21 |
| 0.50 | 28.53 | 1.55 | 5.42 ± 0.22 |
| 0.75 | 25.11 | 1.80 | 7.16 ± 0.24 |
| 1.00 | 22.40 | 2.02 | 9.01 ± 0.36 |
A p-value <0.0001 was observed for all the measurements.
Effect of MI on Catalase Structure
Secondary Structure
For this, far-UV CD spectral measurements of catalase were carried out in the presence of different concentrations of MI (0–0.6 M). As can be seen in Figure 1A, no significant change in the secondary structure of catalase was observed in the presence of MI (Figure 1A).
Figure 1.
Measurement of catalase structure in the presence of MI. Panel A represents far-UV CD spectra of catalase in the absence and presence of different concentrations of MI. Panel B is representative of Heme absorption spectra of catalase at a wavelength range of 350–450 nm in the presence of MI. Inset shows the variation of λmax versus [MI]. Panels C and D represent intrinsic and extrinsic ANS fluorescence spectra in the presence of MI, respectively. Symbols at the right corner of the respective panel represent different concentrations of MI used. Panel E represents acrylamide fluorescence quenching measurements of catalase: Stern–Volmer plots for the acrylamide quenching of fluorescence of catalase in the absence (■) and presence of highest concentrations of MI (*) used. Spectra and results shown are representative of at least three independent measurements.
Tertiary Structure
Soret Absorption of Catalase Heme
Absorption spectra of catalase, exhibiting maximum soret absorption peak around 403 nm, was obtained in the presence of different concentrations of MI. Upon addition of MI, a concentration-dependent change in heme absorption intensity with a significant red shift of about 6 nm (from 403 to 409 nm) was observed (Figure 1B).
Intrinsic Fluorescence
Here, changes in the microenvironment of the tryptophan residues were monitored in the presence of MI. Upon excitation at 280 nm, fluorescence emission spectra were recorded with an absorption maximum at 336 nm. In presence of MI, a concentration-dependent decrease in tryptophan fluorescence intensity with a red shift of around 8 nm was observed (Figure 1C).
Solvent-Accessible Hydrophobic Patches in the Presence of MI
To monitor for any change in exposure of hydrophobic patches, ANS binding experiments were carried out. With an increase in MI concentration, an increase in ANS fluorescence intensity (enhanced binding) with a prominent red shift of about 11 nm was observed, which indicates increased exposure of hydrophobic residues to the solvent (Figure 1D).
Effect of MI on Compactness of Catalase
Acrylamide quenching of fluorescence of catalase provides information about the accessibility of the quencher to the intrinsic fluorophore (trp) as well as any alteration in the microenvironment of the fluorophore. For this, acrylamide-induced quenching of trp fluorescence in the presence of MI was carried out and KD values were calculated using eq 2 (Figure 1E). For control, the value for KD was found to be 1.28 ± 0.04, while it was found to be 1.51 ± 0.06 in the presence of MI. Increased KD values in the presence of MI indicate enhanced exposure of the trp residues to the surrounding aqueous environment.
Effect of MI on the Conformational Stability of Catalase
To monitor for any MI-induced change in the overall stability of catalase, thermal denaturation studies of catalase were carried out in the presence of MI. Thermal denaturation profiles, observed in the presence and absence of MI, were analyzed by using eq 3, and the observed fD values were plotted as a function of temperature (Figure S1). These plots were further analyzed for Tm by nonlinear fitting using eq 4. At 0.6 M MI, a decrease of around 3.7 °C was observed in Tm of catalase, which suggests an overall decreased stability of catalase in the presence of MI.
In Silico Studies of Catalase
Docking Studies
To elucidate any possible interaction between MI and catalase, molecular docking studies were carried out. Twenty docking conformations were obtained with MI, and the docked conformations were found to occupy and cluster at different sites around the catalase (Figure S2). The binding affinity of MI for catalase was found to be in the range of −4.7 to −4.2 kcal/mol and the lowest energy conformation was located near the opening of the heme binding active site channel of catalase, indicating favorable binding of MI at this position.
Molecular Dynamics Simulation Studies
To understand the effect of MI on the overall conformation/structure of catalase, MDS studies were carried out in the presence of an MI cosolvent system, where the concentrations for MI ranged up to 0.6 M with simulation in water alone serving as a control (Table 2). Simulation analysis was performed wherein MDS parameters like root mean square deviation (RMSD), radius of gyration (Rg), and hydrogen bonds were estimated (Table 3). In the MI cosolvent system, catalase was found to be highly dynamic and fluctuating with longer times of equilibration (Figure 2A, Table 3). With respect to RMSD of peptide backbone atoms, the values were found to decrease insignificantly for MI-bound catalase (5.13 Å) when compared to catalase alone (5.42 Å). On the other hand, Rg values showed almost no change in the presence of MI. However, significant changes in RMSD values were observed for the protein–heme region with an almost two-fold increase in RMSD value in the presence of MI (0.63 Å) compared to catalase alone (0.35 Å). Results obtained for the heme region indicate that the heme group of catalase in the MI cosolvent system was found to undergo pronounced structural fluctuations (Figure 2B). Additionally, the RMSF plot indicated that fluctuations of the amino acids residues from 5–70 and 380–480 are quite enhanced (Figure 2C).
Table 2. Summary of the Cosolvent Simulation Systems Used in the Study.
| system | [MI], M | No. of water molecules | No. of MI molecules | total No. of atoms | simulation length (ns) |
|---|---|---|---|---|---|
| water | 0.00 | 20765 | 0 | 70240 | 20 |
| Myo-inositol | 0.60 | 17789 | 192 | 65992 | 20 |
Table 3. Summary Statistics from the Trajectory Analysisa.
| RMSD |
No.
of hydrogen bonds |
|||||||
|---|---|---|---|---|---|---|---|---|
| system | protein Cα (Å) | heme group (Å) | Rg (Å) | intraprotein | protein–water | protein–heme | protein–MI | MI–MI |
| water | 5.42 ± 0.32 | 0.35 ± 0.04 | 24.77 ± 0.16 | 129 ± 13 | 526 ± 69 | 4 ± 1 | – | – |
| myoinositol | 5.13 ± 0.64 | 0.63 ± 0.07 | 24.82 ± 0.20 | 130 ± 12 | 389 ± 15 | 4 ± 1 | 32 ± 5 | 10 ± 4 |
The last 10 ns of the trajectory were considered for the analysis.
Figure 2.
Simulation studies of catalase in the presence of MI. Representative plots of RMSD of Protein Cα atoms (A) and Heme group (B), RMSF of Cα atoms (C), and Rg of Cα atoms (D). Profiles observed from the water and MI simulations are represented in black and red color, respectively.
Effect of MI on the Hydrogen Bonding Pattern of Catalase
Presence of hydroxyl groups in MI may allow it to interact with either water or surface protein groups. To explore the effect of MI on the interaction network of catalase, hydrogen bond interactions within the protein, and between the protein, MI, water, and the heme group were analyzed (Table 3 and Figure S3). The number of intraprotein hydrogen bonds in the presence of MI was found to be same as that of water alone (Table 3). However, in the MI system a profound decrease in the number of hydrogen bonds between protein and water was observed with a decrease from 526 (in water) to 389 (in MI) (Table 3, Figure S3). This loss of hydration sphere around catalase might be responsible for the decreased hydrogen bonding pattern of the catalase with water. As expected, a very small number of protein–MI hydrogen bonds were observed (Table 3 and Figure S3).
Effect on Heme Binding Active Site of Catalase
Altered catalytic activity of catalase in the presence of MI encouraged us to investigate its effect on the heme binding active site cavity of catalase (Figure 3, Figure S4 and S5). Here, the representative structure of the catalase (Figure 3A) was subjected to active site cavity measurements using CASTp program, and the identified active site cavities were visualized (Figures 3B,C). As can be seen from Table S1, an expansion of the active site cavity near the entrance of the channel with a volume of 577 Å3 was observed in the presence of MI. In addition to this, noticeable fluctuations in the threading arm (residues 1–66) and wrapping loop (residues 376–439) of catalase (Figures 3A,C) were observed. In the superposed conformation (Figure 3A), we could see that the threading arm and the wrapping loop, in the case of the MI system (gray and orange), were getting rearranged and coming close to the β-barrel part, that is, central core of the catalase fold.
Figure 3.
Binding of MI to heme active site of catalase. Panel A is a representative structure of catalase in water (gray) and MI cosolvent system (orange). Panels B and C represent an active site cavity identified in water (B) and MI (C) cosolvent system using CASTp program.
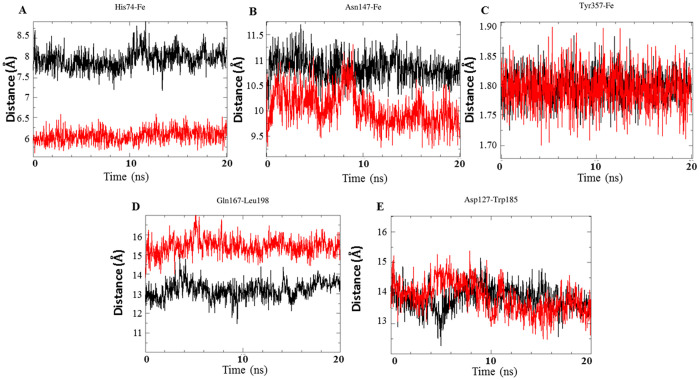
To further elucidate the observed effects of MI on the heme binding active center (Figure S4 and S5), the distance of some of the catalytically important residues, such as His74 and Asn147, with respect to the iron in the heme center, was monitored. As can be seen in Figure 4, Asn147 and Tyr357 are maintained at a larger distance from the heme iron in the presence of MI (Figure 4A–C). Results suggest that the altered distance between the residues and the heme iron might have contributed towards the MI-induced modulation of catalase activity. Additionally, the distance between other important channel residues, including Gln167-Leu198 and Asp127-Trp185, was also analyzed (Figure 4D,E). In the presence of MI, a respective increase and decrease in the distance of Cα atoms of the Gln167-Leu198 and Asp127-Trp185, with respect to the heme center, was observed. Furthermore, MI shows binding interaction with various hydrophobic residues lining the heme active site channel, including Val125, Arg126, Trp185, Val181, His465, Trp185, and Val181 (Figures S5 and S6).
Figure 4.
Representative plots of distance between catalytically important residues and iron of heme group in catalase. Panels represent distances of His74 (A), Asn147 (B), and Tyr357 (C) and regions Gln167-Leu198 (D) and Asp127-Trp185 (E) in the absence and presence of MI. Profiles from the water and MI simulations are represented in black and red color, respectively.
Effect of MI on the Redox State of Heme Iron
To verify whether MI has any effect on the oxidation state of Fe-center of heme, H2O2 decomposition by hemin chloride was monitored in the presence and absence of MI (Figure 5A). As can be seen in Figure 5A, no significant change was observed in the rate of H2O2 degradation by hemin chloride in the presence of MI.
Figure 5.
Effect of MI on redox status of heme. Panel A represents H2O2 degradation profile of hemin chloride and panel B represents EPR spectra of catalase in the presence and absence of MI. All the experiments were done in triplicate, and results presented are derived from three independent measurements. For all measurements, the standard mean error (SEM) ranged from 0.01% to 0.05% across all the wavelengths monitored.
Furthermore, EPR measurements of catalase were carried out in the presence of MI to confirm any perturbation in the redox state of catalase heme. As can be seen in Figure 5B, no alteration in the redox status of the catalase heme moiety was observed in the presence of MI. Altogether, results infer that the MI-induced loss of catalase activity is not due to any change in the redox status of heme center of catalase.
Effect of MI on Cell Viability
Using MTT assay, the effect of MI on viability of HeLa cell lines was investigated. On treatment of cells with MI, a percentage decrease of about 20% was observed in the concentration of viable HeLa cells, suggesting a cytotoxic effect of MI on HeLa cells (Figure 6A).
Figure 6.
Effect of MI on cell viability. Panel A represents cell viability in the presence of MI with right bar showing the percent viability of Hela cell lines in the presence of MI. Panels B and C represent flow cytometric analysis of MI-induced cell cycle arrest and ROS generation in Hela cell lines, respectively. Experiments were done in triplicate. A p-value <0.0001 was considered statistically significant.
Effect of MI on Cell Cycle Progression
For this, quantification of cell cycle phases of HeLa cells after 24 h of MI treatment was performed using propidium iodide dye, which stains cellular DNA. Flow cytometry analysis showed that in the presence of MI, the cell cycle progression stopped and the cells were arrested at the G1 phase, as indicated by the increased population of cells (about 51%) in this phase (Figure 6B).
Effect of MI on ROS generation
Using fluorescence microscopy, the effect of MI on ROS generation in cells was monitored with the help of a fluorescent dye, DCFH-DA (Figure 6C). Intracellular esterases cleave DCFH-DA, producing a relatively polar and cell membrane impermeable nonfluorescence product (H2DCF). This nonfluorescent molecule accumulates intracellularly, and subsequent oxidation yields the highly fluorescent product DCF. The redox state of the sample was then monitored by measuring fluorescent intensity. In our case, HeLa cells were treated with MI (0.2 M) and then stained with DCHF-DA, a ROS scavenger. It was observed that upon treatment with MI, the fluorescence intensity was found to increase 5-fold (Figure 6C), indicating oxidation of H2DCF to DCF, which is possible due to increased ROS levels in HeLa cells.
Discussion
Our results on activity measurements revealed inhibition of catalase function by MI with a Km increase of 7.08 mM (Table 1). Consequently, the overall catalytic efficiency (kcat/Km) decreased to 63% in the presence of the highest concentration of MI (0.6M). Interestingly, the inhibitory behavior was found to be confined to MI only as all other polyols (the class of compounds to which MI belongs) increased functional activity of the catalase (Table 1). Inhibition of catalase activity indicates that MI may perhaps be a ligand for catalase. For this, we have intentionally performed blind docking of MI to the native catalase using Autodock Vina. As evident in Figure S2, MI binds at the vicinity of the heme binding site (comprising Val125, Arg126, Trp185, and Val181) with a free energy change of −4.7 to −4.2 kcal/mol (Figure S2). Thus, inactivation of catalase by MI might be due to binding of MI in the vicinity of the active site of catalase which in turn should affect the substrate accessibility.
The peroxidase activity of catalase is due to the presence of iron (Fe2+) in a specialized porphyrin ring present in the protein.16−18 The Fe2+ form of iron could donate one electron to H2O2 and cleave it into O2 and H2O, thereby converting the enzyme into its inactive oxidized state (Fe3+). Therefore, a change in the oxidation state from Fe2+ to Fe3+ should be a signature for the nonfunctionality of catalase. To verify this possibility, we performed two key experiments. First, we analyzed the oxidation state of the MI-treated catalase by measuring EPR spectra. Second, the peroxidase activity of the hemin (non-protein part of catalase) was also examined in the presence of MI. As evident in Figure 5B, there is no alteration in the oxidation state and consequently no significant change in the peroxidase activity of hemin. Taken together, the results indicate that MI-mediated enzyme inactivation is not related to the non-protein part (porphyrin ring) and might thus involve the protein part of catalase. It is possible that MI-induced conformational changes in catalase might result in its inability to pick a substrate due to the decreased accessibility of the substrate to the active site or hampering its catalytic mechanism in some other way.
Conformational analysis revealed no change in the secondary structure (Figure 1A), but the tertiary contacts appeared to be distorted (Figure 1C). In terms of the tryptophan microenvironment, significant changes were observed in λmax and hyperchromicity (Figure 1C,D). Concomitantly, we also observed a slight increase in acrylamide quenching behavior in the MI-treated catalase as compared to the control (Figure 1E) which must have resulted in the blue shift in trp microenvironment. The observed red shift in Figure 1C might be due to unusual reshuffling of many of the trp residues in the nonpolar environment while few might be in the polar environment exposed to the solvent. Enhanced binding of ANS in MI-treated catalase further indicates that some of the hydrophobic groups are also exposed to the solvent (Figure 1D). Furthermore, the overall tertiary changes force the exposition of the catalytic heme center to the solvent (Figure 1B). In agreement, there is an overall decrease in the thermodynamic stability of the protein (Figure S1), which may be due to the distortion of the tertiary contacts. Taken together, the results suggest that MI binding induces a functionally deficient non-native state in catalase, characterized by an intact secondary structure with altered tertiary contacts and distorted heme microenvironment.
Next, we investigated the molecular level structural alterations in catalase due to MI binding by performing MD simulations in the presence of the MI cosolvent system. Generally, a quantitative description of the global effect of ligands on the overall conformation of proteins is described in terms of RMSD and Rg profiles, followed by measuring dynamics of inter/intramolecular hydrogen bonding interactions. Similar to the results obtained in secondary structure measurements (Figure 1A), there is no significant change in RMSD and Rg parameters in catalase-MI system when compared to catalase alone (Table 3 and Figure 2). Furthermore, the significant decrease in catalase–water hydrogen bonding interaction in the presence of MI and the consequent increase in MI–protein hydrogen bonding interaction indicates that MI binds to catalase by replacing water molecules from the protein hydration sphere (Table 3).
We also observed a large increase in RMSD values of heme moiety in the presence of MI (almost two-fold compared to the control), implying major structural fluctuations in the microenvironment of the heme. Catalase contains four heme groups buried deep within the individual subunits of catalase. It has a long amino-terminal threading arm, an antiparallel eight-stranded β-barrel, a wrapping loop, four α-helical domains, and a C-terminal helical domain. The extended threading arm (residues 5–70) stabilizes the quaternary structure by interconnecting two subunits and hooking through a long wrapping loop (residues 380–480) around another subunit. The heme is positioned in the middle of each subunit, with the distance between the iron and the center of the tetramer being around 23 Å and around 20 Å between the iron and the external surface. In addition to the changes in the heme binding site, noticeable fluctuations in the threading arm and wrapping loop of catalase (Figure 3A,C) were observed. In fact, in the superposed conformation (Figure 3A), we could see that the threading arm and wrapping loop of catalase (in the presence of MI) (gray and orange) come close to the β-barrel part of catalase (Figure 3B,C). RMSF measurements also revealed higher fluctuations in residues related to the threading arm and wrapping loop regions of the protein (Figure 2C), indicating minute tertiary/quaternary level conformational changes. Thus, the results on MDS studies are in excellent agreement with those of the spectroscopic studies.
In catalase, substrate accessibility towards the heme iron (or proper affinity) is regulated with the help of a 20 Å funnel-shaped substrate channel, that is, the part of the enzyme responsible for connecting the deeply buried heme with the surface. At the neck of this channel are four residues including Asp 127, Trp185, Glu167, and Leu198, which play a key role in catalytic activity of the enzyme. It is also known that distances between Glu167–Leu198 and Asp127–Trp185 play a major role in determining the channel diameter. Therefore, we intentionally studied the channel size and the distances of these two residues (Figure 4). Interestingly, an overall decrease in the channel diameter with a consequent increase in distance between Asp127–Trp185 (but not Glu167–Leu198) was observed. The distances between His74 and Asn147 with the iron center, aiding in electron transfer reaction during catalysis, were also altered and significantly decreased due to reduction in channel diameter (Figure 4A,B). Furthermore, MI also forms binding contacts with various hydrophobic residues associated with the narrow heme part of active site channel, including Ala116, Phe152, Phe153, Trp185, Val181, Val 185, Val125, and Arg126 (Table S1, Figures S5 and S6). Thus, binding to these residues and the longer distance between Asp127–Trp185 might force a decrease in channel diameter, thereby limiting the substrate entry to the catalytic site. In support of this argument, it has been demonstrated that modulation of catalase activity by various compounds has been attributed to the altered channel size by inducing overall conformational changes in the enzyme.19,20
To further support our results on MI-mediated catalase inhibition, we performed cellular level studies by treating HeLa cells with MI and measured cell viability and oxidative stress. As evident in Figure 6, a decrease in cell viability was observed in the presence of MI (Figure 6A) with around a 5-fold increase in ROS level (Figure 6C) leading to inhibition of the cell cycle at the G1 phase (Figure 6B). Intracellular esterases cleave DCFH-DA at the two ester bonds, producing a relatively polar and cell membrane impermeable product, H2DCF. This nonfluorescent molecule accumulates intracellularly, and subsequent oxidation yields the highly fluorescent product DCF. The redox state of the sample was then monitored by detecting the fluorescent intensity. In our case, HeLa cells after treatment with MI (0.2 M), followed by staining with DCHF-DA, a ROS scavenger, observed a 5-fold increase in the fluorescence intensity (Figure 6C), indicating oxidation of H2DCF to DCF and thus resulting in increased ROS level generation in HeLa cells. Altogether, the results conclude that decreased cell viability, observed in MTT assay, is due to the arrest of cells in G1 phase probably due to the inefficient antioxidant system as enhanced production of ROS was observed in the presence of MI. The inefficient antioxidant system in turn results in oxidative stress in the cells. Since H2O2 is a signaling molecule involved in cell proliferation, autophagy, and apoptosis,21−24 the results indicate that regulation of MI levels during oxidative stress disorders could serve as a potential therapeutic strategy for neurodegenerative diseases like Alzheimer’s.
Significance
Our results indicate that MI might act as a specific inhibitory ligand for catalase and could be responsible for the inefficient, otherwise overexpressed, antioxidant enzyme system during presymptomatic stages of AD. In other words, one of the primary causes for the compromised antioxidant defense system during oxidative stress stages of AD could be attributed to the increased MI concentrations in the brain. Furthermore, the observed results suggest that caution should be taken with prolonged use of MI and its derivatives as promising supplements for preventing female infertility, restoring polycystic ovary syndrome, type-II diabetes, bronchial dysplasia, and cardiovascular disorder.25−31
Materials and Methods
Bovine liver catalase, myo-inositol, and 8-anilino-1-naphthalene sulfate (ANS) were obtained from Sigma Chemical Co. U.S.A. Disodium hydrogen orthophosphate and sodium dihydrogen orthophosphate were purchased from Himedia laboratories while H2O2 was obtained from Merck, Darmstadt, Germany. All other reagents used in the study were of analytical grade. HeLa cells were purchased from National Centre for Cell Science, Pune, India.
Preparation of Stock Solutions
The stock solution of catalase was prepared in 0.05 M degassed sodium phosphate buffer of pH 7.0. Prior to its use, the protein solution was dialyzed overnight in 0.1 M KCl at 4 °C, and its concentration was determined spectrophotometrically using molar extinction coefficient (ε405) value of 3.24 × 105 M–1cm–1. H2O2 solution, prepared in 50 mM phosphate buffer of pH 7.0, was always fresh and its concentration was determined using a molar extinction coefficient (ε240) value of 40 M–1cm–131. The stock solution of MI was prepared in 50 mM sodium phosphate buffer, pH 7.0. All the reaction mixtures were checked for any pH change upon addition of MI, which in most of the cases was found to be insignificant.
Measurement of Catalase Activity
Activity measurement of catalase was carried out by spectrophotometric method, using Agilent Cary 100 UV/vis spectrophotometer, wherein a decrease in the absorbance of the substrate, H2O2, was monitored in the presence of catalase.32,33 For monitoring the effect of MI on catalase activity, catalase at a concentration of 10 nM was preincubated in different concentrations of MI. A reaction was initiated upon addition of H2O2 to the reaction mixture containing preincubated catalase, and the catalase-mediated degradation of the substrate was followed by measuring the change in the absorbance of H2O2 at 240 nm for 20 min. From each progress curve and at a given substrate concentration, the initial velocity (V0) was determined from the linear portion of the kinetic curve. Catalase-mediated H2O2 degradation reaction observed first order rate kinetics, wherein the rate of reaction was found to depend on H2O2 concentration.32 Plots of V0 versus substrate concentrations were analyzed for the kinetic parameters (Km and kcat) using eq 1
| 1 |
where V0 is the initial velocity of the enzyme, Km is Michaelis–Menten constant, S is substrate concentration, and Vmax is the maximum velocity of the enzyme.
Absorption Measurements
Absorption spectra of catalase (0.3 μM) in the presence and absence of MI (0.05, 0.15, 0.3, and 0.6 M) at 25 ± 0.1 °C were obtained in the wavelength range of 350–450 nm by using Agilent Cary 100 UV–vis spectrophotometer. The soret region of catalase showed a maximum absorbance around 403–405 nm, a typical region for the presence of a heme group.34,35 The effect of MI on the absorption spectra of catalase heme was monitored by following change in absorption intensity and maximum absorption wavelength (λmax) shift.
Fluorescence Measurements
Intrinsic Fluorescence
Intrinsic fluorescence measurements of catalase in the presence and absence of MI were carried out at 25 °C, pH 7.0 by using Cary Eclipse fluorescence spectrofluorometer. Fluorescence spectral measurements were recorded in the wavelength range of 300–500 nm with excitation wavelength of 295 nm and bandwidth of 10 nm.
Extrinsic (ANS) Fluorescence
Extrinsic fluorescence measurements, through ANS binding assays, were carried out to detect any change in the exposure of surface hydrophobic patches of catalase in the presence of different concentrations of the MI. For this, the excitation wavelength was set at 360 nm, and the emission spectra were recorded in the wavelength range of 400–600 nm with a catalase concentration of 0.3 μM. All the samples were run in triplicates. ANS concentration was kept 16-fold higher than that of the protein concentration, and all the samples were prepared in dark to avoid photodecomposition of ANS.
Acrylamide Quenching
Acrylamide quenching of intrinsic fluorescence of catalase at 295 nm was carried out by titrating catalase with acrylamide in the presence of MI. The data obtained was analyzed by Stern–Volmer model using eq 2
| 2 |
where F0 and Fi represent protein fluorescence intensity in absence and presence of varying concentrations of quencher, respectively. Q is the concentration of quencher used and KD is the Stern–Volmer constant related to the fluorophore lifetime and the bimolecular quenching constant.
Circular Dichroism Measurement
Far-UV circular dichroism (CD) spectral measurements in the presence and absence of MI were carried out at a wavelength range of 200–240 nm with a catalase concentration of 0.5 μM. The spectra were recorded on Jasco J-810 spectropolarimeter equipped with a temperature-controlled Peltier system under constant nitrogen flow at a scan speed of 50 nm/min in a cuvette of path length 0.1 cm. Necessary corrections for reference samples were also carried out.
Thermal Stability Measurement
In this, thermal denaturation of catalase in the presence of MI was monitored by using Cary 100 UV–vis spectrophotometer equipped with a temperature-controlled Peltier system. All the solutions were prepared in 0.05 M phosphate buffer, pH 7.0. Each sample with a catalase concentration of 0.2 μM was heated from 25 to 85 °C at a rate of 1 °C/min and the change in absorbance was followed at 280 nm. Heat-induced denaturation curves were analyzed for fD (fraction unfolded) using eq 3
| 3 |
where y is the optical property at a certain temperature and MI concentration, and yN and yD are the optical properties of native and denatured states of catalase, respectively.
The heat-induced transition curves were further analyzed for melting temperature, Tm, using a nonlinear least-squares analysis method according to eq 4
| 4 |
where y(T) is the optical property at temperature T (Kelvin), YN(T) and YD(T) are the optical properties of the native and denatured protein at T (K), respectively, R is the gas constant, and ΔHm is the enthalpy change.
Molecular Docking Measurements
The crystal structure of the catalase with PDB ID 1TGU was selected for the docking studies. The chain A of the crystal structure with heme moiety in the binding site was optimized using the protein preparation wizard in Schrodinger Release 2016-1.36 The structure of MI (Pubchem ID: 892) was obtained from Pubchem Database and optimized using the ligprep module of Schrodinger. The docking studies were performed using Autodock Vina with a grid box covering the whole protein structure and the number of conformations at 100.37
Molecular Dynamic Simulation (MDS) Measurements
Simulation System
An optimized structure of the catalase chain A with PDB ID 1TGU was used for the simulation studies. Two separate simulation systems were prepared, one without MI (in water alone) and one with MI as a cosolvent. Details of the simulation systems are summarized in Table 2. The simulation system was prepared using Chimera38 and simulation setup option in Desmond. A cubic box with a 10 Å buffer between the system and the edge of the box was used including periodic boundary conditions. In case of the cosolvent systems, the MI molecules, as per their molarity, were placed inside the box. The system was solvated using the TIP3P water model and ions were added to neutralize the system. The system was relaxed using the default protocol in Desmond. Subsequently, MDS studies were carried out for 20 ns for each system in the NPT ensemble using a Nose–Hoover chain thermostat (300 K) and a Martyna–Tobias–Klein barostat (1 Atm) with OPLS 2005 force field parameters.
MDS Analysis
The analysis of the three simulated trajectories were carried out using the simulation quality analysis in Maestro in the Schrodinger Release 2016-139 and VMD1.9.4.40 Trajectories were analyzed for total energy, root mean square deviation (RMSD), root mean square fluctuation (RMSF), radius of gyration (Rg), number of hydrogen bonds, and radial distribution function (RDF). The plots obtained were generated using Qtgrace.44 A representative structure from the three simulations was selected based on the clustering approach using the clustering plugin in VMD 1.9.4.40 On the basis of CA atoms with an RMSD cutoff of 3 Å, the last 10 ns of the trajectory from each simulation was considered for clustering. The protein conformations were visualized using Chimera,38 and the heme interaction diagram was prepared using Ligplus.41 The cavity volumes were measured using the CASTp program,42 and the cartoon representations of the protein structures were generated using Chimera.
Measurement of H2O2 Decomposition by Hemin Chloride
Absorption spectra of the MI treated and untreated hemin chloride were recorded with Agilent Cary 100 UV/vis spectrophotometer equipped with a Peltier-type temperature controller at 37 °C. The concentrations of hemin and H2O2 were 5 and 300 μM, respectively, and the decomposition progress was followed at 240 nm for 30 min. A sample cell of 1.0 cm path length was used for all measurements.
Electron Paramagnetic Resonance (EPR) Measurements
EPR measurements were carried out in a Bruker EMX MicroX spectrometer. Conditions used for the measurements included gain, 1 × 105; modulation amplitude, 7G; microwave power, 0.677 mW; temperature, 298 K and conversion time, 40 ms. Samples with catalase concentration of 20 μM were loaded in sealed quartz capillary tubes and transferred to the EPR cavity to obtain spectra.
Cell Viability Assay
For this, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was conducted as outlined by Mosmann with slight modifications.43 Briefly, cells with a density of 5 × 104 cells/ml were seeded in a 96-well plate and incubated in a humidified CO2 incubator for 24 h to allow for cell attachment. After 24 h of MI (200 mM) treatment, the cell viability was measured. Following this, 20 μL of MTT reagent (5 mg/mL) was added to each well and incubated for 4 h. Formazan crystals were dissolved by adding DMSO and absorbance at 570 nm was monitored by using an ELISA microplate reader.
Measurement of Reactive Oxygen Species (ROS) Generation
HeLa Cells (1 × 106 cell/ml) under standard conditions were seeded in a 12-well plate containing media and allowed to attach for 24 h. Following this, the cells were treated with MI (200 mM). After 24 h of incubation, the media was removed and 25 μM of DCFDA dye was added to each well and kept in the dark for 45 min before fluorescence imaging by using Flow cytometry.
Cell Cycle Analysis
Cell cycle progression of HeLa cells was monitored by using propidium iodide (PI) dye. Analysis of cell cycle progression was performed on the basis of DNA content of cells treated with MI. After 24 h of incubation, cells were treated with RNase A and PI dye and were kept in the dark. After centrifugation, the cells obtained were analyzed for cell cycle phase distribution by using flow cytometry.
Statistical Analysis
Results are expressed as mean ± SEM; n is indicated in the figures and/or legends. Results were analyzed by two-tailed student’s test or 1- or 2- way ANOVA, wherever appropriate, using GraphPad prism software. A Bonferri posthoc test was used to test for significant differences revealed by ANOVA. A p-value of <0.05 was considered statistically significant.
The t test was also performed on the MD simulation data for RMSD (protein Cα and heme group), Rg, and no. of hydrogen bonds (intraprotein, protein–water, protein–heme) between water and MI simulations using R 4.1.2.45 The data from the last 10 ns of the trajectory from each simulation were used for the analysis. A significant difference (p-values <0.001) was observed between simulation data (RMSD, Rg, and no. of H-bonds) from water and MI simulations.
Acknowledgments
Financial support provided by CSIR, New Delhi India (Grant 37(1653)/15/EMR-11/2015) to T.A.D. is acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.1c06990.
(Figure S1) stability measurements of catalase; (Figure S2) docking measurements of catalase in the presence of MI; (Figure S3) hydrogen bonding network profile of catalase in the presence of MI; (Figure S4) interaction of heme group with catalase in the two simulation systems, water (A) and MI (B); (Figure S5) interactions of MI near the entrance of the heme binding site; (Figure S6) ligand Interaction of MI with hydrophobic surface of protein; (Table S1) details of the heme binding site amino acid residues and the volume changes in the two simulation systems identified using CASTp program (PDF)
Author Contributions
∇ F.A. and U.M. contributed equally.
Author Contributions
Conceptualization, F.A., T.A.D., and L.R.S.; Methodology, F.A., U.M., R.B., A.K.B., and S.M.S.; Formal Analysis, T.A.D., L.R.S., S.M.S.; Investigation, F.A., T A D., S M S.; Writing, original draft, F.A., T.A.D., L.R.S., S.M.S.; Writing, review and editing, T.A.D., L.R.S., V.U., K.S.C.; Visualization, F.A., T.A.D., L.R.S.; Supervision, T.A.D., L.R.S.; Funding Acquisition, T.A.D.
The authors declare no competing financial interest.
Supplementary Material
References
- Tcw J.; Goate A. M. Genetics of β-amyloid precursor protein in Alzheimer’s Disease. Cold Spring Harb. Perspect. Med. 2017, 7, a024539 10.1101/cshperspect.a024539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villemagne V. L.; Doré V.; Burnham S. C.; Masters C. L.; Rowe C. C. Imaging tau and amyloid-β proteinopathies in Alzheimer disease and other conditions. Nat. Rev. Neurol. 2018, 14, 225–36. 10.1038/nrneurol.2018.9. [DOI] [PubMed] [Google Scholar]
- LaFerla F. M.; Oddo S. Alzheimer’s disease: Aβ, tau and synaptic dysfunction. Trends Mol. Med. 2005, 11, 170–6. 10.1016/j.molmed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Parihar M. S.; Hemnani T. Alzheimer’s disease pathogenesis and therapeutic interventions. J. Clin. Neurosci. 2004, 11, 456–67. 10.1016/j.jocn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Ritchie C.; Smailagic N.; Noel-Storr A. H.; Takwoingi Y.; Flicker L.; Mason S. E.; McShane R. Plasma and cerebrospinal fluid amyloid beta for the diagnosis of Alzheimer’s disease dementia and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst. Rev. 2014, (6), CD008782. 10.1002/14651858.CD008782.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoonsari P. E.; Haggmark A.; Lonnberg M.; Mikus M.; Kilander L.; Lannfelt L.; Bergquist J.; Ingelsson M.; Nilsson P.; Kultima K.; Shevchenko G. Analysis of the cerebrospinal fluid proteome in Alzheimer’s disease. PloS One 2016, 11, e0150672 10.1371/journal.pone.0150672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S.; Okamoto S.; Lipton S. A.; Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol. Neurodegener. 2014, 9, 48. 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. K.; Kashyap M. P.; Tripathi V. K.; Singh S.; Garg G.; Rizvi S. I. Neuroprotection through rapamycin-induced activation of autophagy and PI3K/Akt1/mTOR/CREB signaling against amyloid-β-induced oxidative stress, synaptic/neurotransmission dysfunction, and neurodegeneration in adult rats. Mol. Neurobiol. 2017, 54, 5815–28. 10.1007/s12035-016-0129-3. [DOI] [PubMed] [Google Scholar]
- Omar R. A.; Chyan Y. J.; Andorn A. C.; Poeggeler B.; Robakis N. K.; Pappolla M. A. Increased expression but reduced activity of antioxidant enzymes in Alzheimer’s disease. J. Alzheimers Dis. 1999, 1 (3), 139–145. 10.3233/JAD-1999-1301. [DOI] [PubMed] [Google Scholar]
- Habib L. K.; Lee M. T.; Yang J. Inhibitors of catalase-amyloid interactions protect cells from β-amyloid-induced oxidative stress and toxicity. J. Biol. Chem. 2010, 285, 38933–43. 10.1074/jbc.M110.132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson N. J.; Lewis R. H.; Cowan F. M.; Allsop J. M.; Counsell S. J.; Edwards A. D.; Cox I. J. Early increases in brain myo-inositol measured by proton magnetic resonance spectroscopy in term infants with neonatal encephalopathy. Pediatr. Res. 2001, 50 (6), 692–700. 10.1203/00006450-200112000-00011. [DOI] [PubMed] [Google Scholar]
- Ashwal S.; Holshouser B.; Tong K.; Serna T.; Osterdock R.; Gross M.; Kido D. Proton spectroscopy detected myoinositol in children with traumatic brain injury. Pediatr. Res. 2004, 56, 630–8. 10.1203/01.PDR.0000139928.60530.7D. [DOI] [PubMed] [Google Scholar]
- Miller B. L.; Moats R. A.; Shonk T.; Ernst T.; Woolley S.; Ross B. D. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology 1993, 187 (2), 433–437. 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]
- Haris M.; Cai K.; Singh A.; Hariharan H.; Reddy R. In vivo mapping of brain myo-inositol. Neuroimage 2011, 54, 2079–85. 10.1016/j.neuroimage.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang W.; Alexander G. E.; Daly E. M.; Shetty H. U.; Krasuski J. S.; Rapoport S. I.; Schapiro M. B. High brain myo-inositol levels in the predementia phase of Alzheimer’s disease in adults with Down’s syndrome: A 1H MRS Study. Am. J. Psychiatry 1999, 156 (12), 1879–1886. 10.1176/ajp.156.12.1879. [DOI] [PubMed] [Google Scholar]
- Vetrano A. M.; Heck D. E.; Mariano T. M.; Mishin V.; Laskin D. L.; Laskin J. D. Characterization of the oxidase activity in mammalian catalase. J. Biol. Chem. 2005, 280 (42), 35372–35381. 10.1074/jbc.M503991200. [DOI] [PubMed] [Google Scholar]
- Poulos T. L. Heme enzyme structure and function. Chem. Rev. 2014, 114 (7), 3919–3962. 10.1021/cr400415k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck D. E.; Shakarjian M.; Kim H. D.; Laskin J. D.; Vetrano A. M. Mechanisms of oxidant generation by catalase. Ann. N.Y. Acad. Sci. 2010, 1203, 120–125. 10.1111/j.1749-6632.2010.05603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najjar F. M.; Ghadari R.; Yousefi R.; Safari N.; Sheikhhasani V.; Sheibani N.; Moosavi-Movahedi A. A. Studies to reveal the nature of interactions between catalase and curcumin Using computational methods and optical techniques. Int. J. Biol. Macromol. 2017, 95, 550–556. 10.1016/j.ijbiomac.2016.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam C. D.; Arvai A. S.; Bourne Y.; Tainer J. A. Active and inhibited human catalase structures: ligand and NADPH binding and catalytic mechanism. J. Mol. Biol. 2000, 296, 295–309. 10.1006/jmbi.1999.3458. [DOI] [PubMed] [Google Scholar]
- Lennicke C.; Rahn J.; Lichtenfels R.; Wessjohann L. A.; Seliger B. Hydrogen Peroxide - production, fate and role in redox signaling of tumor cells. Cell Commun. Signal. 2015, 13, 39. 10.1186/s12964-015-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Marzo N.; Chisci E.; Giovannoni R. The role of hydrogen peroxide in redox-dependent signaling: homeostatic and pathological responses in mammalian cells. Cells 2018, 7 (10), 156. 10.3390/cells7100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal E.; Day A. Hydrogen peroxide as a signaling molecule. Antioxid. Redox signalling 2011, 15 (1), 147–151. 10.1089/ars.2011.3968. [DOI] [PubMed] [Google Scholar]
- Redza-Dutordoir M.; Averill-Bates D. A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim Biophys Acta, - Mol. Cell Res. 2016, 1863 (12), 2977–2992. 10.1016/j.bbamcr.2016.09.012. [DOI] [PubMed] [Google Scholar]
- Facchinetti F.; Bizzarri M.; Benvenga S.; D’Anna R.; Lanzone A.; Soulage C.; Di Renzo G. C.; Hod M.; Cavalli P.; Chiu T. T.; Kamenov Z. A.; Bevilacqua A.; Carlomagno G.; Gerli S.; Oliva M. M.; Devroey P. Results from the international consensus conference on myo-inositol and d-chiro-inositol in obstetrics and gynecology: The Link between Metabolic Syndrome and PCOS. Eur. J. Obstet. & Gynecol. 2015, 195, 72–76. 10.1016/j.ejogrb.2015.09.024. [DOI] [PubMed] [Google Scholar]
- Laganà A. S.; Garzon S.; Casarin J.; Franchi M.; Ghezzi F. Inositol in polycystic ovary syndrome: Restoring fertility through a pathophysiology-based approach. Trends Endocrinol Metab.m 2018, 29 (11), 768–780. 10.1016/j.tem.2018.09.001. [DOI] [PubMed] [Google Scholar]
- Ciotta L.; Stracquadanio M.; Pagano I.; Carbonaro A.; Palumbo M.; Gulino F. Effects of myo-inositol supplementation on oocyte’s quality in PCOS patients: A Double Blind Trial. Eur. Rev. Med. Pharmacol. Sci. 2011, 15 (5), 509–514. [PubMed] [Google Scholar]
- Khandelwal M.; Reece E. A.; Wu Y. K.; Borenstein M. Dietary myo-inositol therapy in hyperglycemia-induced embryopathy. Teratology 1998, 57 (2), 79–84. . [DOI] [PubMed] [Google Scholar]
- D’Anna R.; Santamaria A.; Alibrandi A.; Corrado F.; DI Benedetto A.; Facchinetti F. Myo-Inositol for the prevention of gestational diabetes mellitus. A Brief Review. J. Nutr. Sci. Vitaminol. 2019, 65, S59–S61. 10.3177/jnsv.65.S59. [DOI] [PubMed] [Google Scholar]
- Pintaudi B.; Di Vieste G.; Bonomo M. The effectiveness of myo-Inositol and D-chiro inositol treatment in type 2 Diabetes. Int. J.Endocrinol. 2016, 2016, 9132052. 10.1155/2016/9132052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolshakov I. A.; Vygodina T. V.; Gennis R.; Karyakin A. A.; Konstantinov A. A. Catalase activity of cytochrome c oxidase assayed with hydrogen peroxide-sensitive electrode microsensor. Biochemistry (Moscow) 2010, 75, 1352–60. 10.1134/S0006297910110064. [DOI] [PubMed] [Google Scholar]
- Iwase T.; Tajima A.; Sugimoto S.; Okuda K. I.; Hironaka I.; Kamata Y.; Mizunoe Y. A simple assay for measuring catalase activity: a visual approach. Sci. Rep. 2013, 3 (1), 1–4. 10.1038/srep03081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwan M. H. Simple spectrophotometric assay for measuring catalase activity in biological tissues. BMC Biochem. 2018, 19 (1), 7. 10.1186/s12858-018-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J.; Maśalakiewicz P.; Rodakiewicz-Nowak J.; Walde P. Activity and spectroscopic properties of bovine liver catalase in sodium bis(2-ethylhexyl) sulfosuccinate/isooctane reverse micelles. Eur. J. Biochem. 1993, 217 (2), 567–573. 10.1111/j.1432-1033.1993.tb18278.x. [DOI] [PubMed] [Google Scholar]
- Andersson L. A.; Johnson A. K.; Simms M. D.; Willingham T. R. Comparative analysis of catalases: spectral evidence against heme-bound water for the solution enzymes. FEBS letters 1995, 370 (1–2), 97–100. 10.1016/0014-5793(95)00651-O. [DOI] [PubMed] [Google Scholar]
- Sastry G. M.; Adzhigirey M.; Day T.; Annabhimoju R.; Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided. Mol. Des. 2013, 27 (3), 221–234. 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- Trott O.; Olson A. J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2009, 31 (2), 455–461. 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Couch G. S.; Greenblatt D. M.; Meng E. C.; Ferrin T. E. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25 (13), 1605–1612. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Release, S. 2016-1: Maestro; Schrödinger, LLC: New York, 2016.
- Humphrey W.; Dalke A.; Schulten K. VMD: Visual Molecular Dynamics. J. Molecular Graphics Modell. 1996, 14 (1), 33–38. 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- Qtgrace. http://plasma-gate.weizmann.ac.il/Grace.
- Laskowski R. A.; Swindells M. B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J.Chem.Inf. Model. 2011, 51 (10), 2778–2786. 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Tian W.; Chen C.; Lei X.; Zhao J.; Liang J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46 (W1), W363–W367. 10.1093/nar/gky473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol Methods. 1983, 65 (1–2), 55–63. 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- R 4.1.2. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria, 2013. http://www.R-project.org/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.