Abstract
In Staphylococcus aureus infection hemolysis caused by the extracellular protein α-toxin encoded by hla is thought to contribute significantly to its multifactorial virulence. In vitro, subinhibitory concentrations of β-lactam antibiotics and fluoroquinolones increase the levels of hla and α-toxin expression, whereas aminoglycosides decrease the levels of hla and α-toxin expression. In the present study we investigated the effects of subinhibitory concentrations of amoxicillin, gentamicin, and moxifloxacin on hla and α-toxin expression and total hemolysis of S. aureus strain 8325-4, a high-level α-toxin producer, and its α-toxin-negative mutant, DU 1090, in vitro and in a rat model of chronic S. aureus infection. The levels of expression of hla and α-toxin and total hemolysis did not differ significantly when amoxicillin, gentamicin, or moxifloxacin was added to cultures of S. aureus strain 8325-4. In vivo, strain 8325-4 induced a significantly increased level of hemolysis in infected pouches compared to that in uninfected control pouches, but the hemolysis was reduced to control levels by treatment with doses of amoxicillin, gentamicin, or moxifloxacin that reduced bacterial numbers by 2 orders of magnitude. Additionally, the effects of subinhibitory concentrations of the three antibiotics on total hemolysis of four methicillin-resistant S. aureus and three methicillin-sensitive S. aureus (MSSA) clinical isolates were assessed in vitro. A significant increase in total hemolysis was observed for only one MSSA strain when it was treated with amoxicillin but not when it was treated with moxifloxacin or gentamicin. When purified α-toxin was incubated with purified human neutrophil elastase, α-toxin was cleaved nearly completely. The results suggest that the penicillin-induced increases in S. aureus α-toxin expression are strain dependent, that reduction of bacterial numbers in vivo counteracts this phenomenon effectively, and finally, that in localized S. aureus infections α-toxin activity is controlled by neutrophil elastase.
Staphylococcus aureus causes a broad range of life-threatening diseases in humans (19). Its virulence is multifactorial (1, 4), including the extracellularly released 33.2-kDa polypeptide α-toxin (α-hemolysin) (3, 31). α-Toxin is thought to be a major virulence factor of S. aureus since α-toxin-negative mutants of the wild-type strain 8325-4, such as DU 1090, were revealed to have significantly reduced levels of toxicity in animal models of human S. aureus infection (5, 25, 27, 28). In vitro data suggest that antibiotics modify the expression of α-toxin. For example, macrolides (21, 26), aminoglycosides (26), and clindamycin (26) reduced the level of α-toxin production, whereas β-lactam antibiotics strongly increased (18, 26) and fluoroquinolones slightly increased (26) its level of production. Sterile culture supernatants of S. aureus strains treated with nafcillin in vitro were significantly more toxic for rats than untreated culture supernatants (18).
On the basis of these results, it was hypothesized that β-lactam therapy may enhance the virulence of S. aureus strains and increase the symptoms of human S. aureus infections (18). At particular risk would be patients with the hereditary disease cystic fibrosis (CF), who often suffer from chronic S. aureus lung infections (13) and who therefore are repeatedly treated with β-lactam antibiotics and other classes of antibiotics.
The objectives of the present study were, first, to investigate the effects of subinhibitory concentrations of amoxicillin, gentamicin, or moxifloxacin on α-toxin expression of S. aureus in vitro and in a chronic S. aureus infection model of CF in rats. Second, we wanted to assess the effects of subinhibitory concentrations of these antibiotics in vitro on several clinical isolates of S. aureus that differed in their resistance to methicillin, and, finally, we investigated the hypothesis that α-toxin is cleaved by human neutrophil elastase. We provide evidence that hla and α-toxin expression and total hemolysis do not differ significantly when amoxicillin, gentamicin, or moxifloxacin is added to in vitro cultures of S. aureus strain 8325-4 and that these antibiotics are well-suited for the treatment of localized chronic S. aureus infections, when they are used at even subinhibitory concentrations, because they reduce the level of hemolysis by decreasing bacterial numbers. Furthermore, we show that the penicillin-induced increase in the level of α-toxin expression is largely strain dependent and, finally, that neutrophil elastase degrades α-toxin.
MATERIALS AND METHODS
Bacterial strains, antibiotics, and culture methods.
The following S. aureus strains were used: strain 8325-4, a high-level α-toxin-producing strain (24); its α-toxin-deficient mutant, DU 1090, prepared by insertion of a transposon in the hla gene (27); four methicillin-resistant clinical isolates of S. aureus (methicillin-resistant S. aureus [MRSA] isolates 1 to 4); and three methicillin-sensitive clinical isolates of S. aureus (methicillin-sensitive S. aureus [MSSA] isolates 1 to 3). Strains were cultured in vitro in tryptone soy broth (TSB; Oxoid, Basingstoke, England) with or without subinhibitory concentrations of antibiotics at 37°C with shaking (200 rpm). The antibiotics moxifloxacin (BAY 12-8039; Bayer AG) and amoxicillin, gentamicin, and nafcillin (Sigma-Aldrich, Deisenhofen, Germany) were used. Antibiotic resistance testing revealed that laboratory strains 8325-4 and DU 1090 were susceptible to the antibiotics tested, whereas the MRSA strains were resistant and the MSSA strains varied in their susceptibilities (Table 1). MICs for S. aureus strains were determined by the broth dilution method according to the NCCLS performance standards for antimicrobial testing (22). The subinhibitory concentrations are listed in Table 1. S. aureus strains were tested for β-lactamase activity by the nitrocefin assay (Oxoid). As positive controls, three Staphylococcus carnosus strains carrying an Escherichia coli plasmid encoding a β-lactamase were used (a kind gift of Fritz Götz). The mean β-lactamase activities for these strains were set equal to 100%. Besides strain MRSA 4 (42% β-lactamase activity), the other clinical isolates showed a mean ± standard deviation (SD) β-lactamase activity of 13.4% ± 5.6%, whereas laboratory strain 8325-4 and its α-toxin-deficient mutant, DU 1090, were totally negative for β-lactamase activity. Following overnight culturing, S. aureus strains were adjusted to an optical density at 600 nm (OD600) of 0.05 (Ultrospec III; Pharmacia Biotech, Freiburg, Germany). The suspension was again incubated as described above, and the bacteria were grown to an OD600 of 4.5, depending on the strain used and the antibiotic added, after 4.25 to 10.25 h. One milliliter of the samples was centrifuged (10,000 rpm [GR4-12; Jouan, Saint Nazaire, France]). Supernatants were subjected to Western blotting and to the hemolysis assay; the cells were subjected to Northern blotting.
TABLE 1.
MICs and subinhibitory concentrations
| Strain | Amoxicillin
|
Gentamicin
|
Moxifloxacin
|
|||
|---|---|---|---|---|---|---|
| MIC (mg/liter) | SICs (per liter)a | MIC (mg/liter) | SICs (per liter)a | MIC (mg/liter) | SICs (per liter)a | |
| 8325-4 | 0.25 | 1 μg, 10 μg | 2 | 10 μg, 100 μg | 0.125 | 1 μg, 10 μg |
| DU 1090 | 0.25 | 1 μg, 10 μg | 1 | 10 μg, 100 μg | 0.0625 | 1 μg, 10 μg |
| MRSA 1 | 32 | 1 mg, 10 mg | >128 | 10 mg, 100 mg | 8 | 100 μg, 1 mg |
| MRSA 2 | 64 | 1 mg, 10 mg | >128 | 10 mg, 100 mg | 8 | 100 μg, 1 mg |
| MRSA 3 | 32 | 1 mg, 10 mg | >128 | 10 mg, 100 mg | 8 | 100 μg, 1 mg |
| MRSA 4 | 8 | 100 μg, 1 mg | >128 | 10 mg, 100 mg | 4 | 100 μg, 1 mg |
| MSSA 1 | 4 | 100 μg, 1 mg | 8 | 100 μg, 1 mg | 0.0625 | 1 μg, 10 μg |
| MSSA 2 | 2 | 100 μg, 1 mg | 4 | 100 μg, 1 mg | 0.0625 | 1 μg, 10 μg |
| MSSA 3 | 0.25 | 1 μg, 10 μg | 8 | 100 μg, 1 mg | 0.0625 | 1 μg, 10 μg |
Two different subinhibitory concentrations (SICs) of antibiotics with variable relation to MICs were added to liquid cultures of selected S. aureus strains, which were grown as described in Materials and Methods.
Rat granuloma pouch model.
Pouches were prepared in 90 male Wistar rats (weight, 300 g) as described previously (10). Briefly, 1 ml of olive oil with 1% croton oil was injected together with 20 ml of air into the loose subcutaneous tissue between the shoulders of the rats. After 7 days, the pouches were filled with approximately 5 ml of an inflammatory exudate, characterized by a massive infiltration of neutrophils (10). The pouches were infected with 1 ml of a washed inoculum (108 CFU/ml) of S. aureus strain 8325-4 (40 rats) or DU 1090 (40 rats). Antibiotics were given to groups of 10 rats each (amoxicillin, two times at 100 mg/kg of body weight orally; gentamicin, two times at 30 mg/kg subcutaneously; moxifloxacin, one time at 50 mg/kg orally). Two more groups were infected with strain 8325-4 (10 rats) and DU 1090 (10 rats) without antibiotic treatment; 10 uninfected and untreated animals served as blanks. The antibiotic concentrations in the pouches were determined by a conventional cup-plate agar diffusion method with Bacillus subtilis as the indicator organism. One day after inoculation, the pouch exudate was collected and the bacteria were counted. The exudate (1 to 5 ml) was centrifuged, and the supernatant was frozen at −70°C until further investigation. The experiments were approved by the local ethics committee at Bayer AG, Wuppertal, Germany.
Hemolysis assay.
Total hemolysis was assessed as described previously (2) with rabbit erythrocytes, which are 100 times more sensitive to S. aureus α-toxin than human erythrocytes. Briefly, 100 μl of washed rabbit erythrocytes (5 × 106/ml; Froschek, Südbretten, Germany) was added to microtiter plates (Cellstar TC; Greiner, Frickenhausen, Germany), filled with 100 μl of serial dilutions of bacterial culture supernatants or pouch exudates, and incubated for 60 min at 37°C. As a positive control, saponin (Sigma) was used in each assay, whereas phosphate-buffered saline served as a negative control. Following centrifugation, the absorption at 450 nm (A450) of the resulting supernatants was determined with a computerized enzyme-linked immunosorbent assay reader (SLT Labinstruments, Crailsheim, Germany). One unit of hemolytic activity was defined as the amount of test solution able to liberate half of the total hemoglobin from the erythrocytes. All experiments were performed in at least three independent assays. In the case of SDs with P values between 0.1 and 0.05, experiments were repeated up to six times.
Effect of neutrophil elastase on α-toxin stability.
Neutrophil elastase was purified as described before (14). The specific enzymatic activity was demonstrated to be 3.4 mU by using the peptide methoxy-Suc–Ala–Ala–Pro–Val–p-nitroanilide (Bachem, Heidelberg, Germany) as the specific chromogenic substrate. One unit was defined as the release of 1 mol of p-nitroanilide/min/ml by using an extinction coefficient at 410 nm (ɛ410) of 8,800 M−1 cm−1. Serial elastase dilutions (0 ng, 1 ng, 10 ng, 100 ng, 1 μg) were incubated for 1 h at 37°C in phosphate-buffered saline (pH 7.2), together with aliquots of 100 ng of purified α-toxin (Sigma). In other experiments, a molar surplus of α1-antitrypsin was incubated with 1 μg of elastase for 30 min before α-toxin was added. Thereafter, the reaction products were subjected to Western blotting. Quantification of the remaining intact α-toxin was performed by densitometry, as described below.
Congo red proteolysis assay.
For investigation of the elastolytic activity in rat pouch exudates, an elastin-Congo red assay (Sigma) was used. This assay is based on the principle that elastin is cleaved from the Congo red, resulting in a change in color intensity at 495 nm. A 1-ml aliquot of the samples was mixed with 2 ml of an elastin-Congo red suspension (20 g/liter in 0.1 M Tris-maleate–1 mM CaCl2 [pH 8.4]), and the mixture was incubated with shaking for 6 to 8 h at 37°C. After centrifugation (5,000 rpm [Minifuge GL; Heraeus, Fellbach, Germany] for 10 min at 25°C), the A495 was measured in a spectrometer (Ultrospec III; Pharmacia). Calibration was performed with neutrophil elastase, purified as described by Goldstein and Döring (14), with minor changes.
Western blotting.
S. aureus strains 8325-4 and DU 1090 were incubated without and with the addition of subinhibitory concentrations of antibiotics as described above. Cultures were centrifuged and culture supernatants were subjected to sodium dodecyl sulfate (SDS)-polyacrylamide (12%) gel electrophoresis run at 200 V and 75 mA. Samples were blotted onto nitrocellulose (0.8 mA/cm2, 15 V) and stained with rabbit anti-α-toxin immunoglobulin G (IgG) (diluted 1:10,000) purified from a hyperimmune serum by protein A-Sepharose affinity chromatography (Pharmacia). Bound anti-α-toxin IgG was detected with a goat anti-rabbit IgG–horseradish peroxidase conjugate (Dako, Hamburg, Germany) that had been diluted 1:15,000. Detection was performed with the chemiluminescent reagent Lumigen PS-3 (Pharmacia), the ECL Detection System (Pharmacia), and X-ray film (Hyperfilm ECL; Pharmacia). Western blot bands were digitized (Video Copy Processor; Mitsubishi, Tokyo, Japan) and analyzed with the WinCam, version 2.2, software program (Cybertech, Berlin, Germany). The bands were compared to those for purified α-toxin standards added to the Western blots at concentrations of 2.5, 5, 10, 25, and 50 μg/liter.
Northern blotting.
hla mRNA quantification was performed as described previously (12). In short, following overnight growth strains 8325-4 and DU 1090 were adjusted to give an OD600 of 0.05 in a volume of 10 ml of TSB, and amoxicillin (10 μg/liter), gentamicin (100 μg/liter), or moxifloxacin (10 μg/liter) was added to strain 8325-4. All samples were grown to reach an OD600 of 4.5 to 5.0. After centrifugation the pellets were resuspended in 1 ml of ice-cold Trizol reagent (Gibco Life Technologies, Paisley, United Kingdom). Cell walls were disrupted with fastRNA tubes blue and Fast Prep FP 120 (both from Bio 101, Inc., Vista, Calif.). RNA was separated from cell proteins and was quantified spectroscopically (Ultrospec III; Pharmacia). A total of 2.5 μg of each RNA sample was loaded onto agarose gels (low-melting-point agarose [1%]; FMC Bioproducts, Rockland, Maine) supplemented with 10% formaldehyde for electrophoresis (65 V, 2.5 h). Alkaline capillary blotting was performed with positively charged nylon membranes (Roche, Mannheim, Germany) and a turboblotter (Schleicher & Schüll, Dassel, Germany) according to the instructions of the manufacturers. Blots were cross-linked with a GS Gene Linker UV Chamber (Bio-Rad, Hercules, Calif.). Prehybridization was performed for 30 min in Duran glass tubes (HB-OV-BS; Hybaid, Heidelberg, Germany) with a high-concentration SDS solution in a hybridization oven (Mini10; Hybaid) at 68°C, followed by an overnight hybridization after addition of a digoxigenin-labeled single-stranded PCR probe specific for hla (12). The amount of bound probe was detected with an antidigoxigenin antibody (Roche). After addition of CSPD (Roche), chemiluminescence was detected with Hyperfilm ECL (Pharmacia).
Statistical analysis.
Statistical analysis was performed with JMP software (fourth version, 1995; SAS Institute, Inc., Cary, N.C.). Raw data were checked for normality by the Shapiro-Wilks test; thereafter, significance was calculated by the Student t test or the Wilcoxon rank sum test. Probability values less than 0.05 were considered significant.
RESULTS
On the basis of previous reports that penicillins strongly increase the levels of S. aureus α-toxin production in vitro (18, 26), whereas aminoglycosides reduce it (26) and fluoroquinolones slightly increase it (26), we incubated high-level α-toxin-producing strain 8325-4 with subinhibitory concentrations of amoxicillin, gentamicin, or moxifloxacin and quantified hla expression by Northern blotting and α-toxin expression by Western blotting (Fig. 1).
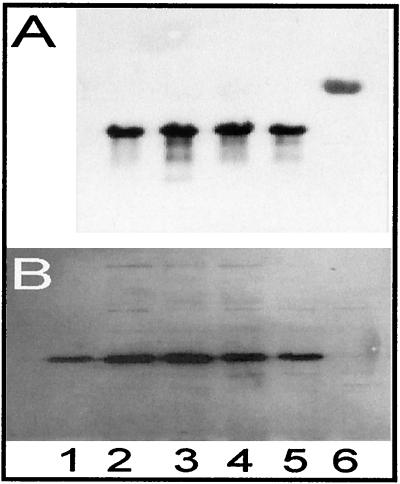
FIG. 1.
Effects of subinhibitory concentrations of amoxicillin, gentamicin, and moxifloxacin on the expression of hla and α-toxin of S. aureus strains 8325-4 and DU 1090 in vitro. (A) Total RNAs of S. aureus strains 8325-4 (lanes 2 to 5) and DU 1090 (lane 6) were electrophoresed, blotted, and hybridized with an hla-specific probe in the absence of drugs (lane 2) or in the presence of amoxicillin (lane 3), gentamicin (lane 4), or moxifloxacin (lane 5). (B) Culture supernatants of S. aureus strains 8325-4 (lanes 2–5) and DU 1090 (lane 6) grown in the absence of drugs (lane 2) or in the presence of amoxicillin (lane 3), gentamicin (lane 4), or moxifloxacin (lane 5) were electrophoresed and blotted onto nitrocellulose, and α-toxin was detected with specific rabbit antibodies against α-toxin. Lane 1, 25 ng of purified α-toxin.
Using a standard curve for purified α-toxin, we found that cultures of S. aureus strain 8325-4 (107 CFU/ml) produced 20.37 ± 9.76 μg of α-toxin per liter without the addition of antibiotics (Fig. 1B, lane 2). Addition of subinhibitory concentrations of amoxicillin (10 μg/liter) resulted in moderately increased levels of α-toxin production (Fig. 1B, lane 3). A similar, but also not significant, increase (1.59 times) in the level of hla mRNA expression was found after amoxicillin treatment (Fig. 1A, lane 3). Treatment of cultures of S. aureus strain 8325-4 with subinhibitory concentrations of moxifloxacin (10 μg/liter; Fig. 1B, lane 5) or gentamicin (100 μg/liter; Fig. 1B, lane 4) also failed to change the level of production of α-toxin significantly. Both Western blotting and Northern blotting yielded comparable results. As expected, the α-toxin-negative mutant of 8325-4, DU 1090, produced no hla transcript (Fig. 1A, lane 6) or α-toxin protein (Fig. 1B, lane 6). These results do not corroborate results from previous studies with other S. aureus strains, including strain 8325-4, which revealed significant increases in the level of α-toxin production in the presence of subinhibitory concentrations of penicillins (18, 26).
In addition to α-toxin, S. aureus may produce other extracellular toxins with hemolytic properties (11, 15, 23, 30) that may have altered transcription and translation patterns as a result of treatment with subinhibitory concentrations of antibiotics. Therefore, we studied the total hemolytic activity of culture supernatants of strains 8325-4 and DU 1090 and seven clinical isolates of S. aureus in a rabbit erythrocyte assay (Fig. 2).
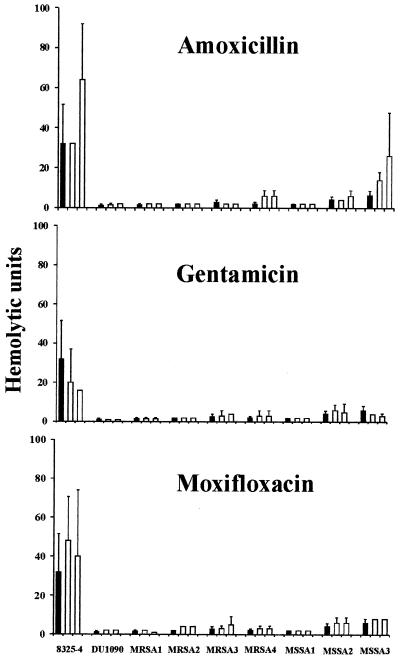
FIG. 2.
Effects of subinhibitory concentrations of amoxicillin, gentamicin, and moxifloxacin on the hemolytic activities of nine S. aureus strains. S. aureus cultures were grown in the absence (black bars) or presence (open bars) of two subinhibitory concentrations (Table 1) of amoxicillin, gentamicin, or moxifloxacin. Thereafter, culture supernatants were subjected to a rabbit erythrocyte hemolysis assay. Bars represent mean values of three or more independent experiments.
Addition of 10 μl of the culture supernatant of strain 8325-4 to rabbit erythrocytes yielded a hemolytic activity of 32.0 ± 19.6 hemolytic units (HUs), which corresponded to 20.37 ± 9.76 μg of α-toxin per liter, as determined by Western blotting (Fig. 1B, lane 2). In the presence of two different subinhibitory concentrations of amoxicillin, gentamicin, or moxifloxacin, strain 8325-4 did not show a significant increase in total hemolysis (Fig. 2A to C). To demonstrate the sensitivity of the assay, nafcillin, a β-lactam antibiotic which had previously been shown to induce a significant increase in the level of α-toxin in S. aureus strains (18), was used as a positive control. Indeed, the total hemolytic activity of strain 8325-4 was significantly increased from 32 to 80 HUs with 0.1 μg of nafcillin per liter (P < 0.05) and from 32 to 56 HUs with 0.01 μg of nafcillin per liter (P < 0.05). Next, we investigated the effect of amoxicillin, gentamicin, and moxifloxacin on seven clinical isolates of S. aureus which differed from each other with respect to their resistance to methicillin. With the exception of strain MSSA 3, for which a significant increase in the total level of hemolysis was revealed when it was treated with amoxicillin (1 μg/liter, P < 0.005; 10 μg/liter, P < 0.05) (Fig. 2A), all the other strains, regardless of whether they were sensitive or resistant to methicillin, had numbers of HUs in the range of that for α-toxin-deficient strain DU 1090. α-Toxin contributed the most significant portion of the total hemolytic activity of strain 8325-4, since its α-toxin-deficient mutant, DU 1090, caused hemolysis at a level of only 1.3 ± 0.5 HUs, corresponding to 4% of the hemolysis caused by strain 8325-4. On the basis of the calculation that 32 HUs corresponds to 20.37 μg of α-toxin per liter and that the hemolytic activity in this erythrocyte assay is largely due to α-toxin, the seven clinical isolates of S. aureus produced between 1.0 ng and 3.8 μg of α-toxin per liter.
These results show that in a biological cell assay subinhibitory concentrations of penicillins such as nafcillin or amoxicillin may increase the total level of hemolysis in a minority of S. aureus strains, whereas gentamicin and moxifloxacin do not affect the total level of hemolysis of S. aureus.
We then used strain 8325-4 and its α-toxin-negative mutant, DU 1090, in a localized S. aureus infection model of CF, the granuloma rat pouch, to assess the effects of amoxicillin, gentamicin, and moxifloxacin on the total hemolytic activity in the pouches (Fig. 3A and B). Analysis of the bacterial numbers in the pouch exudates yielded similar counts for strain 8325-4 [(1.1 ± 1.1) × 107 CFU/ml] and strain DU 1090 [(2.4 ± 1.2) × 107 CFU/ml) (Fig. 3A). When amoxicillin, gentamicin, and moxifloxacin were administered at high concentrations orally or subcutaneously to rats, the bacterial numbers in the rat pouches were reduced by about 2 orders of magnitude 24 h after inoculation. Quantitative determination of the antibiotics in the rat pouches showed that maximum concentrations of the antibiotics (amoxicillin, 5.2 mg/liter; gentamicin, 1.25 mg/liter; moxifloxacin, 1.2 mg/liter) were achieved between 120 and 140 min after application. The fact that approximately 105 CFU of S. aureus was still present in the rat pouches 24 h after inoculation, even though the half-lives of amoxicillin (2.15 h), gentamicin (0.87 h), and moxifloxacin (5.2 h) in the pouches were fairly long, may suggest that strains 8325-4 and DU 1090 have changed their in vitro phenotypes, rendering them more resistant to the antibiotics used. Thus, a significant proportion of the bacterial cells were confronted with subinhibitory antibiotic concentrations in the pouches.
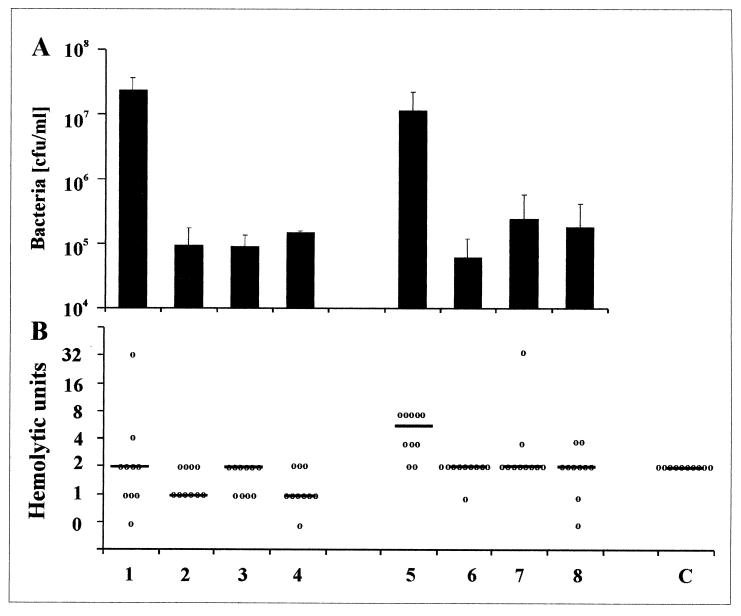
FIG. 3.
Bacterial numbers (A) and total hemolytic activity (B) in rat pouch exudates infected with S. aureus strains 8325-4 and DU 1090. Seven-day-old rat pouches were inoculated with S. aureus strains 8325-4 and DU 1090 and subinhibitory concentrations of amoxicillin, gentamicin, or moxifloxacin. After 24 h, pouch exudate was collected, bacterial numbers were counted, and the total hemolytic activity was determined in a rabbit erythrocyte hemolysis assay. Lanes 1 to 4, strain DU 1090 untreated (lane 1) or treated with amoxicillin (lane 2), gentamicin (lane 3), and moxifloxacin (lane 4). Lanes 5 to 8, strain 8325-4 untreated (5) or treated with amoxicillin (lane 6), gentamicin (lane 7), and moxifloxacin (lane 8). Lane C, sterile pouches. Bacterial numbers are given as means ± SDs (A); hemolysis is shown by separate values and medians (B).
The inflammatory exudate in sterile rat pouches was revealed to have hemolytic activity for rabbit erythrocytes of 2.0 ± 0.0 HUs (Fig. 3B). Infection of the pouches with S. aureus strain 8325-4 increased the total level of hemolytic activity significantly compared to that for the controls (P < 0.001) and compared to that for pouches infected with α-toxin-negative strain DU 1090 (P < 0.05), whereas the total levels of hemolysis in sterile pouches and those infected with strain DU 1090 did not differ significantly (P = 0.35) (Fig. 3B). These results suggest that the increase in hemolytic activity after infection with wild-type strain S. aureus 8325-4 is caused by α-toxin.
The treatment of rats with amoxicillin, gentamicin, or moxifloxacin reduced the total level of hemolytic activity in pouches infected with strain 8325-4 to levels observed in sterile pouch exudates (Fig. 3B). Thus, the 2-log reduction of bacterial numbers following antibiotic treatment is more important with respect to α-toxin levels in vivo than a potentially stimulatory effect of subinhibitory amoxicillin concentrations.
Culture supernatants of strain 8325-4, obtained after growth of 107 CFU/ml in vitro, contained 20.37 μg of α-toxin per liter, which corresponds to 32 HUs. Such hemolytic activity was not observed in rat pouch exudates infected with S. aureus 8325-4. Possibly, α-toxin is proteolytically degraded in vivo. Indeed, high levels of proteolytic activity toward elastin-Congo red were present in sterile rat pouch exudates and pouches infected with S. aureus 8325-4 or DU 1090 (Table 2). A comparison of the infected pouch exudate elastolytic activity with the elastolytic activity of human neutrophil elastase against elastin-Congo red revealed that approximately 100 to 220 mg of neutrophil elastase-like activity per liter was present in the pouches.
TABLE 2.
Proteolytic activities of rat pouch exudates and human neutrophil elastase against Congo red
| Samplea | S. aureus strain | A495 |
|---|---|---|
| Pouches | 8325-4 | 0.122 ± 0.021 |
| Pouches | DU 1090 | 0.171 ± 0.053 |
| Pouches | 0.199 ± 0.117 | |
| NE (100 μg/ml) | 0.100 ± 0.007 | |
| NE (1 mg/ml) | 1.005 ± 0.003 |
Ten rat pouches were infected with S. aureus strain 8325-4 or DU 1090, exudates were recovered after 24 h, and 1 ml of the exudates was added to 2 ml of a Congo red suspension. Additionally, 1 ml of purified human neutrophil elastase (NE; 100 μg/ml or 1 mg/ml) was added to 2 ml of the Congo red suspension. Values represent means ± SDs of three independent assays and are corrected for the value for the buffer.
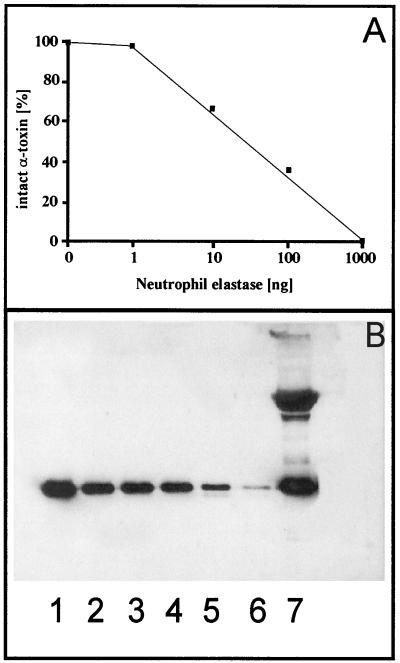
Since proteolytic degradation of α-toxin may also be relevant to the pathogenicity of S. aureus infections in the lungs of CF patients, we assessed the stability of α-toxin in the presence of human neutrophil elastase. Human neutrophil elastase is present in sputum samples of CF patients at concentrations of about 100 mg/liter of sputum (14). When 100 ng of purified α-toxin was incubated with 1 μg of neutrophil elastase, almost complete cleavage was observed by Western blotting (Fig. 4A and B).
FIG. 4.
Cleavage of purified α-toxin of S. aureus by human neutrophil elastase. (A) Quantitative determination of α-toxin cleavage by scanning of a Western blot. (B) Western blot of 100 ng of α-toxin (lane 1) pretreated with 0 ng (lane 2), 1 ng (lane 3), 10 ng (lane 4), 100 ng (lane 5), and 1,000 ng (lane 6) of neutrophil elastase or a mixture of 10 μg of α1-antitrypsin and 1 μg of neutrophil elastase (lane 7).
Taken together, our results confirm that penicillins may increase α-toxin-induced hemolysis in some S. aureus strains in vitro, whereas gentamicin and moxifloxacin do not stimulate hemolysis. The treatment of a localized S. aureus infection in rat pouches with concentrations of amoxicillin, gentamicin, or moxifloxacin low enough to allow bacterial persistence does not increase the levels of production of bacterial toxins with hemolytic activities. The virulence of S. aureus mediated by α-toxin may be further controlled by host-derived proteinases that inactivate α-toxin.
DISCUSSION
Previous in vitro data have shown that S. aureus α-toxin expression is enhanced by subinhibitory concentrations of penicillins (18, 26), suggesting that the symptoms of S. aureus infections may be aggravated by penicillin treatment. In the present study we have confirmed and extended these in vitro findings using nafcillin and amoxicillin. With six MSSA strains, Ohlsen et al. (26) showed a strong strain-dependent increase in the level of α-toxin production by methicillin that ranged from 1.5- to 12-fold. Increases were more dramatic for MRSA strains treated with subinhibitory methicillin concentrations (26). We did not observe such a difference between MSSA and MRSA strains in the present study. An increase in the level of α-toxin expression was seen for only one of seven clinical isolates including four MRSA strains. Methodological differences could account for the discrepancies in our findings. Interestingly, using nafcillin we observed a significant (twofold) increase in the level of α-toxin production only in high-level α-toxin-producing S. aureus strain 8325-4, corroborating similar data from Kernodle et al. (18), and in strain MSSA 3 treated with amoxicillin. The molecular basis for the stimulation of expression in these strains remains to be studied. In contrast to the observations of Ohlsen et al. (26), in our S. aureus strains we observed neither a decrease in the level of α-toxin expression with the aminoglycoside gentamicin nor an increase in the level of α-toxin expression with the fluoroquinolone moxifloxacin.
On the basis of the results of these in vitro experiments, the relevance of α-toxin expression for the clinical situation, which apparently depends on the S. aureus strain and on the antibiotics used, remains unclear. Therefore, high-level α-toxin producer 8325-4 and its α-toxin-negative mutant, DU 1090, were used in a localized S. aureus infection model for CF, the granuloma rat pouch. The effect of α-toxin on total hemolysis was evident in untreated control rats inoculated with strain 8325-4. The addition of either amoxicillin, gentamicin, or moxifloxacin at concentrations which allowed bacterial persistence at 105 CFU/ml of pouch exudate 24 h after challenge did not lead to increased α-toxin levels. The fact that the maximum concentrations of the three antibiotics in the pouches were much higher than the MICs determined for the strains in vitro may suggest that the bacteria have changed their phenotypes, rendering them more resistant to the antibiotics used. Support for a phenotypic switch stems from our previous characterization of S. aureus strains from CF patients (20) and from other rat pouch experiments (17). In both types of localized infections, S. aureus failed to express capsular polysaccharide type 5, which is a prominent surface component of the organism in vitro. Poly-N-succinyl glucosamine, which is involved in biofilm formation, is instead the predominant in vivo surface component of S. aureus in CF patients (20).
Our findings suggest that in clinical situations in which antibiotic treatment does not lead to a rapid and total eradication of the S. aureus load but causes only a partial reduction of bacterial numbers, increased α-toxin levels need not be feared. Attention must be paid, however, to situations in which antibiotic-resistant S. aureus strains such as MRSA or vancomycin-resistant strains cause infections in humans.
Other host factors, however, may control α-toxin levels. Using S. aureus strain 8325-4 and α-toxin-negative mutant DU 5725, Bramley and colleagues (5) reported that the presence of neutrophils at the site of infection, triggered by administration of endotoxin prior to S. aureus inoculation, led to the recovery of similar numbers of toxigenic and nontoxigenic staphylococci. The investigators concluded that under circumstances of chronic inflammation, in which the growth of bacteria and the consequent elaboration of α-toxin are controlled, toxigenicity may not contribute significantly to virulence. Interestingly, α-toxin itself triggers the recruitment of neutrophils to a site of infection (16). The importance of neutrophils in controlling S. aureus growth and toxin production is also evident from studies in which depletion of neutrophils resulted in a conversion of a subclinical infection with toxigenic staphylococci into acute mastitis (29). Neutrophils release serine proteases which may be capable of cleaving S. aureus α-toxin. Indeed, we showed here that neutrophil elastase readily cleaves α-toxin in vitro. Our rat pouch exudates contained high levels of elastolytic activity, corresponding to approximately 100 to 220 mg of human neutrophil elastase-like activity per liter. Even 1 μg of neutrophil elastase was able to completely cleave 100 ng of purified α-toxin in vitro, suggesting that in chronic inflammatory states with high levels of local proteolytic activity, as observed in patients with CF (14), the pathogenic effects of S. aureus α-toxin may be reduced or totally diminished. Inactivation of S. aureus α-toxin by neutrophil elastase may be a mechanism that prevents the hematogenous spread of the organism in patients with CF and that keeps the infection localized to the endobronchial site.
Besides host proteases, other factors may modulate α-toxin levels during infection. Previous studies with the rat pouch model have demonstrated a massively reduced partial O2 pressure (10, 17), which may reduce the levels of the staphylococcal accessory regulator Sar (6), first described by Cheung and colleagues (7). SarA, a regulatory DNA binding protein, binds to the P2 and P3 promoter regions of the accessory global regulator (agr) locus and thus increases the levels of both RNAII and RNAIII (9). This in turn positively regulates hla expression (9). In sar and agr double mutants the secretion of all hemolysins in vitro is absent (8). Thus, sar and/or agr expression may be reduced in S. aureus strains present in rat pouches. Direct transcript analysis of agr (RNAIII) and hla of S. aureus strains present in the respiratory tracts of CF patients, which also show massively reduced partial O2 pressures (32), revealed poor expression of RNAIII, although hla was irregularly expressed (12).
Taken together, antibiotic treatment with S. aureus-specific classes of antibiotics, including β-lactam antibiotics, fluoroquinolones, or aminoglycosides, which reduce the bacterial loads, together with host proteases such as neutrophil elastase, may control α-toxin production.
ACKNOWLEDGMENTS
We are indebted to S. Bhakdi, Mainz, Germany, for a hyperimmune serum of rabbit α-toxin and to D. Axmann-Krcmar, Tübingen, Germany, for statistical evaluation of the data.
REFERENCES
- 1.Arvidson S. Extracellular enzymes from Staphylococcus aureus. In: Easmon C S F, Adlam C, editors. Staphylococci and staphylococcal infections. London, United Kingdom: Academic Press; 1983. pp. 745–808. [Google Scholar]
- 2.Bernheimer A W. Assay of hemolytic toxins. Methods Enzymol. 1988;165:213–217. doi: 10.1016/s0076-6879(88)65033-6. [DOI] [PubMed] [Google Scholar]
- 3.Bhakdi S, Valeva A, Walev I, Zitzer A, Palmer M. Pore-forming bacterial cytolysins. Soc Appl Bacteriol Symp Ser. 1998;27:15S–25S. doi: 10.1046/j.1365-2672.1998.0840s115s.x. [DOI] [PubMed] [Google Scholar]
- 4.Bisognano C, Vaudaux P, Rohner P, Lew D P, Hooper D C. Induction of fibronectin-binding proteins and increased adhesion of quinolone-resistant Staphylococcus aureus by subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 2000;44:1428–1437. doi: 10.1128/aac.44.6.1428-1437.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bramley A J, Patel A H, O'Reilly M, Foster R, Foster T J. Roles of alpha-toxin and beta-toxin in virulence of Staphylococcus aureus for the mouse mammary gland. Infect Immun. 1989;57:2489–2494. doi: 10.1128/iai.57.8.2489-2494.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan P F, Foster S J. Role of SarA in virulence determinant production and environmental signal transduction in Staphylococcus aureus. J Bacteriol. 1998;180:6232–6241. doi: 10.1128/jb.180.23.6232-6241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung A L, Koomey J M, Butler C A, Projan S J, Fischetti V A. Regulation of exoprotein expression in Staphylococcus aureus by a locus (sar) distinct from agr. Proc Natl Acad Sci USA. 1992;89:6462–6466. doi: 10.1073/pnas.89.14.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheung A L, Ying P. Regulation of alpha- and beta-hemolysins by the sar locus of Staphylococcus aureus. J Bacteriol. 1994;176:580–585. doi: 10.1128/jb.176.3.580-585.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung A L, Bayer M G, Heinrichs J H. sar genetic determinants necessary for transcription of RNAII and RNAIII in the agr locus of Staphylococcus aureus. J Bacteriol. 1997;179:3963–3971. doi: 10.1128/jb.179.12.3963-3971.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dalhoff A, Frank G, Luckhaus G. The granuloma pouch: an in vivo model for pharmacokinetic and chemotherapeutic investigations. I. Biochemical and histological characterization. Infection. 1982;10:354–360. doi: 10.1007/BF01642299. [DOI] [PubMed] [Google Scholar]
- 11.Ferreras M, Hoper F, Dalla-Serra M, Colin D A, Prevost G, Menestrina G. The interaction of Staphylococcus aureus bi-component gamma-hemolysins and leucocidins with cells and lipid membranes. Biochim Biophys Acta. 1998;1414:108–126. doi: 10.1016/s0005-2736(98)00160-6. [DOI] [PubMed] [Google Scholar]
- 12.Goerke C, Campana S, Bayer M G, Döring G, Botzenhart K, Wolz C. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect Immun. 2000;68:1304–1311. doi: 10.1128/iai.68.3.1304-1311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goerke C, Kraning K, Stern M, Döring G, Botzenhart K, Wolz C. Molecular epidemiology of community-acquired Staphylococcus aureus in families with and without cystic fibrosis patients. J Infect Dis. 2000;181:984–989. doi: 10.1086/315331. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein W, Döring G. Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am Rev Respir Dis. 1986;134:49–56. doi: 10.1164/arrd.1986.134.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Gouaux E, Hobaugh M, Song L. Alpha-hemolysin, gamma-hemolysin, and leukocidin from Staphylococcus aureus: distant in sequence but similar in structure. Protein Sci. 1997;6:2631–2635. doi: 10.1002/pro.5560061216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbelin C, Poutrel B, Gilbert F B, Rainard P. Immune recruitment and bactericidal activity of neutrophils in milk of cows vaccinated with staphylococcal alpha-toxin. J Dairy Sci. 1997;80:2025–2034. doi: 10.3168/jds.S0022-0302(97)76147-2. [DOI] [PubMed] [Google Scholar]
- 17.Herbert S, Worlitzsch D, Dassy B, Boutonnier A, Fournier J-M, Bellon G, Dalhoff A, Döring G. Regulation of the Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J Infect Dis. 1997;176:431–438. doi: 10.1086/514061. [DOI] [PubMed] [Google Scholar]
- 18.Kernodle D S, McGraw P A, Barg N L, Menzies B E, Voladri R K, Harshman S. Growth of Staphylococcus aureus with nafcillin in vitro induces alpha-toxin production and increases the lethal activity of sterile broth filtrates in a murine model. J Infect Dis. 1995;172:410–419. doi: 10.1093/infdis/172.2.410. [DOI] [PubMed] [Google Scholar]
- 19.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 20.McKenney D, Tibbetts K L, Wang Y, Murthy V, Ulrich M, Döring G, Lee J C, Goldmann D A, Pier G B. Broadly-protective vaccine for Staphylococcus aureus based on an in vivo expressed antigen. Science. 1999;284:1523–1527. doi: 10.1126/science.284.5419.1523. [DOI] [PubMed] [Google Scholar]
- 21.Moneib N A, Shibl A M, el Said M A, el Masry E M. Macrolides induced suppression of virulence factors produced by Staphylococcus aureus. J Chemother. 1993;5:289–292. doi: 10.1080/1120009x.1993.11739246. [DOI] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7–A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1999. [Google Scholar]
- 23.Nilsson I M, Hartford O, Foster T, Tarkowski A. Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis. Infect Immun. 1999;67:1045–1049. doi: 10.1128/iai.67.3.1045-1049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Novick R P. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1963;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 25.O'Callaghan R J, Callegan M C, Moreau J M, Green L C, Foster T J, Hartford O M, Engel L S, Hill J M. Specific roles of alpha-toxin and beta-toxin during Staphylococcus aureus corneal infection. Infect Immun. 1997;65:1571–1578. doi: 10.1128/iai.65.5.1571-1578.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohlsen K, Ziebuhr W, Koller K-P, Hell W, Wichelhaus T A, Hacker J. Effects of subinhibitory concentrations of antibiotics on alpha-toxin (hla) gene expression of methicillin-sensitive and methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother. 1998;42:2817–2823. doi: 10.1128/aac.42.11.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Reilly M, de Azavedo J C, Kennedy S, Foster T J. Inactivation of the alpha-hemolysin gene of Staphylococcus aureus 8325-4 by site-directed mutagenesis and studies on the expression of its hemolysins. Microb Pathog. 1986;1:125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- 28.Patel A H, Nowlan P, Weavers E D, Foster T. Virulence of protein A-deficient and alpha-toxin-deficient mutants of Staphylococcus aureus isolated by allele replacement. Infect Immun. 1987;55:3103–3110. doi: 10.1128/iai.55.12.3103-3110.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schalm O W, Lasmanis J, Jain N C. Conversion of chronic staphylococcal mastitis to acute gangrenous mastitis after neutropenia in blood and bone marrow produced by an equine anti-bovine leukocyte serum. Am J Vet Res. 1976;37:885–890. [PubMed] [Google Scholar]
- 30.Sugawara N, Tomita T, Sato T, Kamio Y. Assembly of Staphylococcus aureus leukocidin into a pore-forming ring-shaped oligomer on human polymorphonuclear leukocytes and rabbit erythrocytes. Biosci Biotechnol Biochem. 1999;63:884–891. doi: 10.1271/bbb.63.884. [DOI] [PubMed] [Google Scholar]
- 31.Tomita T, Kamio Y. Molecular biology of the pore-forming cytolysins from Staphylococcus aureus, alpha- and gamma-hemolysins and leukocidin. Biosci Biotechnol Biochem. 1997;61:565–572. doi: 10.1271/bbb.61.565. [DOI] [PubMed] [Google Scholar]
- 32.Worlitzsch D, Meyer K C, Birrer P, Döring G. Assessing oxygen: a bronchoscopic view into sputum-plugged cystic fibrosis airways. Pediatr Pulmonol Suppl. 1998;17:333. [Google Scholar]