Abstract
This study aimed to deliver a cationic nanoemulsion carrying miconazole nitrate (MCN) to control fungal infections using excipients for synergism. Peceol (oil) and labrasol (surfactant) were selected based on maximum solubility and zone of inhibition values against Candida albicans and Aspergillus niger. Optimized MCNE11 was evaluated [size, zeta potential, % entrapment efficiency (%EE), % transmittance, viscosity, refractive index, extrudability, polydispersity (PDI), morphology, and pH]. An in vitro drug release study was conducted for comparison between DS (drug suspension) and MNE11. In vitro hemolysis was studied at two different concentrations (0.625 and 2.5 μg/mL). Permeation profiles were generated using rat skin. A Draize test was conducted using rabbit to negate irritability issues. Finally, a stability test of MCNE11 was conducted for 12 months. The results showed that MCNE11 (cationic) was the most optimized in term of size, %EE, and PDI. The drug release from MCNE11 was higher compared to DS but comparable to MNE11 (anionic), suggesting no impact of the imposed cationic charge on the release behavior. Moreover, permeation parameters of MCNE11 were significantly (p < 0.05) greater than MNE11, which may be attributed to the combined impact of size (low), surfactant (for reversible changes), and electrostatic interaction (nanoglobules–skin surface). Thus, stable MCN11 possessing high %EE (89.8%), low size (145 nm), maximum flux (5.7 ± 0.1 μg/cm2/h), high drug deposition (932.7 ± 41.6 μg/cm2), optimal viscosity (44.17 ± 0.8 cP), low PDI (0.21), optimal zeta potential (+28.1 mV), and low hemolysis can be promising alternatives to conventional cream to control resistant and recurring types of fungal infections.
1. Introduction
The prevalence of fungal infections is still increasing globally, and >35 million population are affected from topical fungal infections.1 Approximately 138 million women are globally infected with vulvovaginal candidiasis, and that number is expected to reach 158 million by 2030 including recurrent types.2,3 The progression of these infections can be rapid and serious because they compromise the immune system, particularly the deeper portion of the epidermis layer and andrigenic hairs.4 The recurrence of fungal infections, emergence of drug resistance, drug related toxicity, and challenged efficacy of current products are the prime clinical problems associated with conventional dosage forms.5 The resistance could be related to nontargeted drug administration to the infected location at suboptimal concentrations.6 Conventional topical application to a subtherapeutic level at the site of infection results in poor delivery of modalities and an active p-glycoprotein (P-gp) efflux system, leading to frequent recurrence and chronic infections.7
Miconazole nitrate (MCN) is a broad-spectrum synthetic imidazole derivative to control local fungal infections (vaginal, skin, and nail) caused by yeasts and dermatophytes. The drug is associated with limited water solubility (763 ng/mL), low oral bioavailability, optimal molecular weight (416.1 g/mol), and high lipophilicity (Log P = 6.25 in octanol–water system).8,9 The limited aqueous solubility resulted in poor dissolution properties and severe gastrointestinal disturbance (nausea, diarrhea, and rashes) after oral delivery, and marketed tablets exhibit drug interactions that subsequently cause hepatotoxicity.10−12 The drug exhibits elimination problems, and therefore, it is contraindicated in renal-impaired patients.13 Recently, oral delivery of the drug was reported in an NLC (nanostructured lipid carrier) despite the limited oral pharmacokinetics.14 Furthermore, localized infection with Candida albicans often penetrates to deeper viable epidermis, and miconazole nitrate has low bioavailability in skin strata causing drug resistance and recurrence of infections in the skin.7 Hence, there is a need to develop a new strategy for localizing the drug to the target site for efficient and effective therapy. The approach should be an alternative carrier to conventional dosage forms and use a topical route of delivery because of the physicochemical properties and intestinal constraints of miconazole. Considering the aforementioned issues, several attempts have been made to employ nanotechnology-based nanocarriers such as liposomes, ethosomes, and niosome.8,14,15 However, these formulations did not show the promised results to eradicate the complete infections from the skin (viable epidermis) due to physical, chemical, and storage instability of vesicular carrier systems.
A cationic nanocarrier-based drug delivery approach showed great potential in epidermal targeting to control cutaneous infections and diseases.16 To augment cellular internalization with pathogen and infected cells, several strategies have been implemented to achieve additive therapeutic efficacy with reduced dose and side effects. These strategies include the use of a submicron-sized carrier (nanoemulsion) for enhanced access intracellularly, compelled electrostatic interaction (cationic–anionic interaction between cationic nanoemulsion and pathogenic cell surface), and synergism-employing excipients (lipid or surfactant) possessing innate antimycotic effects.17−20 Nanoemulsions are basically related to their low interfacial tension, large surface area, small globular size, and low viscosity for maximized cellular access of various drugs (lipophilic, hydrophilic, and amphiphilic).21−23 Nanocarriers have the ability to enhance the penetration of both hydrophilic and lipophilic drugs via the subcutaneous (SC) layer of skin in comparison to other available systems.24 Despite the potential antimicrobial effects of several lipids, no commercial products containing an active ingredient or a lipid possessing an innate antimicrobial effect have been approved so far. Therefore, we aimed to develop a cationic nanoemulsion ferrying miconazole nitrate for enhanced efficacy to control topical fungal infections. In this plan, we screened lipid and surfactants based on the solubility of the drug and in vitro antifungal (C. albicans and A. niger) activities (maximum zone of inhibition). Then the selected excipients were employed as carriers for tailoring a cationic nanoemulsion laden with miconazole nitrate. The optimized formulation was then evaluated for in vitro characterizations, ex vivo permeation profiles (rat model), and in vivo data. The obtained findings were compared against the drug suspension and negatively charged nanoemulsion of the same composition.
2. Materials
A sample of miconazole nitrate (98% pure) was obtained as a gift from Velite Pharmaceuticals Ludhiana (Punjab, India). Castor oil (CO) and olive oil (OO) were purchased from Loba Chemical Pvt. Ltd. (Mumbai, India). Peceol was procured from Abitec Corp. (Janesville, Germany). Tween 80, Tween 20, span 80, propylene glycol (PG), and polyethylene glycol-400 (PEG400) were purchased from Avarice Laboratories (Ghaziabad, India). Methanol was purchased from SD Fine Chemicals (Mumbai, India). Labrasol (LAB) was generously gifted from Gattefosse (Saint-priest, Cedex, France). A. niger and C. albicans were procured and maintained as per IMTECH (Indian Institute of Microbial Technology), Punjab, India. All reagents and solvents were of analytical grade.
3. Methods
Solubility Analysis
The solubility analysis of MCN in different ingredients (excipients) was carried out according to the method reported earlier.17 Quantitative analysis of MCN was estimated in various oils such as peceol, castor oil, olive oil, surfactants, (labrasol, Tween 80, span 80), cosurfactants (PG and PEG400), and solvents (methanol and water). Briefly MCN was dissolved in excipient (5 mL) using stoppered glass vials (10 mL) and vortexed for 10 min. Each sample was kept at temperature (25 ± 2 °C) for 72 h, and a water shaker bath was used to keep the temperature constant. After a predetermined time (72 h), sample was taken out and centrifuged at 8000 rpm for 5 min and filtered with a syringe filter. After suitable dilution with methanol, absorbance was taken on UV–vis spectrophotometer (UV-1280, Shimadzu Corp, Japan) at a wavelength of 272 nm.
In Vitro Antifungal Activities
To investigate the antifungal potential of the explored excipients, it was necessary to assay against C. albicans and A. niger. Therefore, peceol, castor oil, olive oil, labrasol, Tween 80, PG, and PEG400 were subjected for in vitro antifungal activities against these strains. For this, lipophilic oil was first emulsified in water using transcutol-HP (no antifungal potential as assayed in our laboratory) to make it feasible for diffusion in culture media (10% v/v in final emulsion). Labrasol, PG, PEG400, and Tween 80 were aqueous solutions (10% v/v) for this study. Span 80 is not active against these strains, and therefore, the surfactant was dropped out of this study. In brief, the agar-well diffusion method was adopted as reported previously by us.17 Both cultures were first recultured and grown in sabouraud dextrose nutrient broth media (previously sterilized) at pH 7.0. Each culture (5.8 × 106 CFU (colony forming unit)/mL and 2.5 × 106 CFU/mL, for C. albicans and A. niger, respectively) was separately mixed with 25 mL of the potato dextrose agar media (PDA) at room temperature (30 °C) just before solidification. Then it was immediately transferred to labeled and previously sterilized Petri dishes. The plates were kept for solidification before wells were created under aseptic conditions. The test sample (10%v/v) was then transferred to the well, and the closed plates were subjected for incubation at 37 °C for 24 h. After completion of the incubation time, the plates were observed for the generated zone of inhibition (mm) around the well and reported as the mean (±standard deviation).
Construction of Phase Diagrams
On the basis of the results obtained from the solubility of MCN in various excipients and the in vitro antifungal activity assay (zone of inhibition), the selected lipid (peceol), surfactant (labrasol), and cosurfactant (PG) were utilized to fabricate various cationic nanoemulsions. In this, a constant amount (0.05%) of oleylamine (OA) was used as a positive charge inducer. Pseudoternary phase diagrams were constructed (Chemix School Ver.3.50 software USA) for the formulation of cationic CNE using different Smix ratios (surfactant to cosurfactant) (1:0, 1:1, 1:2, 2:1, 3:1). The aqueous phase comprising water, labrasol, and PG was used to titrate the organic phase (peceol, OA, and MCN) slowly following the method reported before.25 Titration was carried out slowly (dropwise) with intermittent shaking of the mixture to obtain a clear solution as an isotropic mixture. Formulations showing turbidity or any sign of instability (precipitation or phase separation) were discarded from further studies. Stable, clear, and transparent formulations were selected for further characterization and evaluation parameters. Each formulation contains 2%w/w of MCN.
Preparation of Drug-Loaded MCNE and MNE11 Formulations
Formulations were prepared by dissolved MCN (20 mg) and OA (0.05%) in peceol as the oil phase followed by slow titration with an aqueous phase containing an Smix ratio (labrasol and PG). The developed formulations were kept at room temperature (30 °C) followed for 24 h under constant physical observation for any signs of instability (precipitation, phase separation, and coloration). The final concentration of MCN in the formulations was 2% w/w for topical administration. A total of 14 formulations were prepared with different combinations (oil: Smix) (Table 2). The pH of the prepared formulations was measured in the range of 6.8–7.4 for biocompatibility with skin layer.
Table 2. Composition of CNE Formulations Containing a Constant Amount of MCN (20 mg) and OA (0.05%).
| code | Peceol (% w/w) | Smixa (% w/w) | Smix ratioa | water (% w/w) | size (nm) | PDIa | ZP (mV) | %EE |
|---|---|---|---|---|---|---|---|---|
| MCNE1c | 40.8 | 35.50 | 1:0 | 21.65 | 177.0 | 0.71 | + 21.1 | 41.5 |
| MCNE2c | 47.5 | 38.43 | 1:0 | 14.02 | 210.0 | 0.42 | + 18.8 | 46.9 |
| MCNE3 | 29.9 | 21.17 | 1:1 | 51.12 | 161.0 | 0.43 | + 37.1 | 50.1 |
| MCNE4c | 31.6 | 25.36 | 1:1 | 42.99 | 178.0 | 0.32 | +35.6 | 52.6 |
| MCNE5c | 31.0 | 29.00 | 1:1 | 39.95 | 198.0 | 0.31 | + 37.3 | 58.2 |
| MCNE6 | 24.7 | 22.10 | 1:2 | 53.15 | 188.0 | 0.32 | + 32.4 | 61.7 |
| MCNE7c | 31.7 | 22.65 | 1:2 | 45.60 | 178.0 | 0.32 | + 36.9 | 65.5 |
| MCNE8c | 32.1 | 24.98 | 1:2 | 42.87 | 162.0 | 0.33 | + 38.7 | 65.7 |
| MCNE9 | 26.9 | 18.50 | 2:1 | 54.55 | 178.0 | 0.30 | + 33.0 | 71.6 |
| MCNE10 | 25.3 | 22.01 | 2:1 | 52.64 | 169.0 | 0.29 | + 32.4 | 78.2 |
| MCNE11b | 18.4 | 25.00 | 2:1 | 56.55 | 145.0 | 0.21 | + 28.1 | 89.8 |
| MCNE12c | 31.9 | 26.17 | 3:1 | 41.88 | 192.0 | 0.29 | + 35.8 | 72.3 |
| MCNE13c | 28.7 | 24.92 | 3:1 | 46.33 | 219.0 | 0.29 | + 35.9 | 73.7 |
| MCNE14c | 30.2 | 47.00 | 3:2 | 22.75 | 173.0 | 0.32 | + 34.3 | 79.4 |
| MNE11b | 18.4 | 25.00 | 2:1 | 56.6 | 137.0 | 0.24 | - 30.2 | 85.9 |
Smix = Lab/PG ratio; PDI: polydispersity index.
The optimized formulation and MNE11 were free of OA for the comparison study.
Unstable formulations after 24 h of benchtop standing (room temperature). ZP = zeta potential (mV).
Globular Size, Polydispersity index (PDI), and Zeta Potential
The average globule size and PDI of the developed formulations were measured after 10× dilution (Milli-Q water) using a size analyzer (Beckman Coulter, Delsa Nano C, USA). Moreover, the zeta potential of formulations was determined without dilution at ambient temperature (Beckman Coulter, Delsa Nano C, USA). The study was replicated for mean and standard values (mean ± standard deviation).
Total Drug Content (TDC)
All formulations (MCNE1–MCNE14 and MNE11) were assayed for drug content after 24 h. For this, 2 mL of the test sample was dissolved in a chloroform/methanol (1:1) mixture. The mixture was stirred for 5 min at 25 °C in a beaker. Then, the mixture was filtered and the drug was estimated using UV–vis spectrophotometer (U-1800, Hitachi) at 272 nm.10 The study was performed in triplicate for mean values (n = 3).
Percent Entrapment Efficiency (%EE)
The %EE was determined for all formulation. For this, the sample (1.0 mL) was magnetically stirred 1 h by taking ethanol as a dialysis solvent. Whole unentrapped drug was release in ethanol and rest of the formulation was dissolve in mixture of chloroform and ethanol (1:1). The content of drug was estimated using a UV–vis spectrophotometer (U-1800, Hitachi) at 258 nm after suitable dilutions. %EE was calculated using the following formula:
| 1 |
The optimized formulations were used to evaluate other parameters such as % transmittance (spectrophotometer), conductance, refractive index (Fisherbrand refractometer, Fisher Scientific, USA), viscosity (Bohlin visco88, Viscometer, Malvern, USA), pH (calibrated digital pH meter), and extrudability (extrusion method) following the reported method.26
Morphological Assessment (Electron Microscopy)
Morphological analysis of the optimized MCNE11 was examined by electron microscopy (high-resolution transmission electron microscopy; HR-TEM and field emission scanning electron microscopy; FE-SEM). Prior to observation under HR-TEM and FE-SEM, the MCNE11 formulation was diluted (10×) with distilled water for clear visualization of nanoglobules and well-resolved images during scanning. The procedure for FE-SEM included placing the sample on a Nucleopore Track-Etch membrane and drying at room temperature (30 °C). The dried membrane was attached to the silicon wafer using double-sided carbon tape followed by sputter coating with gold and observing under FE-SEM (FE-SEM SU8000, Hitachi, Japan). For HR-TEM, the sample was stained with 0.2% w/v of phosphotungstic acid in phosphate buffer at pH 6.8, for 5 min. Then the excess phosphotungstic acid was removed using a filter paper. The stained sample was spread over a carbon-coated copper grid and was observed under HR-TEM (H-7500, Hitachi, Japan) at a voltage of 200 kV for morphology (shape and size).6
In Vitro Antifungal Activity of the Optimized Formulations
This study was performed for the final optimized formulations (MCNE11 and MNE11) against Candida albicans and Aspergillus niger at the reported MIC (minimum inhibitory concentration) values. The MIC values of miconazole were 0.5 and 4 μg/mL against Candida and Aspergillus, respectively.27,28 Therefore, MCNE11 and MNE11 were diluted to the same concentration using sterilized water for injection before pouring to the respective well. The adopted procedure and method was same as discussed before. Control groups for Candida and Aspergillus were not treated with formulation. Placebo MCNE11 and MNE11 were also taken as test sample. Finally, Petri dishes were incubated at 37 °C for 48 h and the zones of inhibition (ZOI) were measured.27 The results were reported as average and standard deviation (n = 3, SD).
In Vitro Hemolysis Study
Preliminary cellular toxicity of developed formulations (MCNE11, MNE11, placebo MCNE11, placebo MNE11, negative control PBS, and positive control triton-X-100) was determined after exposure to red blood cells (RBCs). Briefly, test samples (1 mL) with varying concentrations (0.625 and 2.5 μg/mL) were interacted with 1 mL of RBCs suspension (4% suspension of RBCs separated from plasma). A final volume (4 mL) was made with PBS. Furthermore, RBCs were also interacted with PBS 7.4, and Triton-X-100 separately, for controls (negative and positive). The sample was gently mixed with the RBCs suspension and allowed for incubation period (2 h) at 37 ± 1 °C. Then the tube was removed undisturbed and centrifuged at 8000 rpm for about 5 min, and the supernatant was taken out to estimate the released hemoglobin. The absorbance was taken using a UV–vis spectrophotometer (UV-1601, Shimadzu) at 540 nm (λmax).29
In Vitro Release Study
The in vitro release behavior of MCNE11, MNE11, and DS was studied using a dialysis membrane (12 K Dalton molecular weight cut-offs, HiMedia, Laboratories, Pvt., Mumbai, India) as per reported method.30 The test sample (equivalent to 10 mg of miconazole) was loaded in dialysis membrane and tied from both ends with an inert thread. The membrane was soaked in water for 24 h prior to commencement of the experiment. The tied membrane was suspended in a medium (PBS 7.4) containing 5.0% DMSO (dimethyl sulfoxide) to maintain sink condition. Sampling (1 mL) was withdrawn at various time points (0.5, 1, 2, 4, 8, 12, and 24 h). The sample taken was replaced with fresh medium (equal volume) after every time point. The sample was processed, filtered, and analyzed using UV–vis spectrophotometer (U-1800, Hitachi) at 272 nm after suitable dilutions.
Ex Vivo Release Study Across Rat Skin
A permeation study from rat skin was performed, and skin was obtained from male albino rats (200–220 g; 14–16 weeks) after they were euthanatized by the cervical dislocation method. The study protocol was approved (PCTE/LDH/1369/2013) by the Institute Animal Ethics Committee of Punjab PCTE Institute of Pharmacy, Punjab, India). All animal experiments comply with the ARRIVE guidelines and performed according to the EU Directive 2010/63/EU for animal. Abdominal rat skin was cleaned and hairs were removed without making any surgical cuts or injury. The hair-free skin was placed between the donor and receptor phase of the Franz diffusion cell in such a manner that the inner dermal skin layer is toward the receptor medium (PBS, pH 7.4) set at 37 ± 1 °C under continued stirring. MCNE11, MNE11, and DS placements were separately studied under same experimental conditions for 24 h. The sampled volume (1 mL) was replaced with fresh release medium to maintain the sink conditions. The withdrawn sample was assayed for the amount of drug permeated across the same skin. Permeation parameters were calculated and compared against control DS. The experiment was replicated for mean and standard deviation values (n = 6). The samples (1 mL) were collected from the receptor side at various time points (0.5, 1, 2, 4, 8, 12, and 24 h). The permeated drug was estimated using a UV–vis spectrophotometer.
Skin Retention Studies
The content of the drug deposited inside the skin was also estimated after completion of the permeation study. The exposed skin region (effective area of skin responsible for permeation) was carefully cleaned by washing the loaded sample using running water. Then the skin was sliced into fine pieces for extraction. These pieces of skin were placed in a beaker containing a methanol + chloroform mixture (1:2) under constant stirring 500 rpm for complete extraction (6 h). Finally, the mixture was filtered to remove tissue, and the filtrate was subjected to centrifugation. The process of centrifugation removed fine fibers and debris by settling at the bottom. The supernatant was used to estimate the amount of the drug deposited using a spectrophotometer.
Acute Dermal Irritation Study
White albino rabbits (1.2–1.8 kg) of either sex were selected for dermal irritation/toxicity study. Animals were placed properly in clean labeled cages 24 h before the start of the experiment. Three circles (2 cm2 each) were made by removing the hairs on the back side of rabbits. An appropriate volume (0.5 mL) of test samples (Placebo MCNE11, MCNE11, and MNE11) was applied topically on these different labeled sites. To ensure continuous contact of the formulation with the sites they were covered with soft net. The treatment sites were visually inspected frequently, and signs of toxicity, edema, inflammation, and swelling were recorded until 24 h.
Stability Studies
The formulation MCNE11 was selected as the most suitable formulation intended for topical application based on findings. Therefore, it was required to assess its physical (in term of globular size and PDI), and chemical stability over varied time points. In general, nanoemulsions are prone to be unstable after storage for a long period of time due to Ostwald ripening.31 We expected extended stability due to imposed electrostatic repulsion to avoid Ostwald ripening. For stability studies, developed MCNE11 was stored in a glass vial at 30 ± 2 °C/65 ± 5% RH (relative humidity) and cold temperature (2–8 °C) for 360 days. The formulation was evaluated for particle size, PDI, and %EE after 90, 180, and 360 days.32
Statistical Analysis
All of the statistical analysis was carried out using GraphPad prism software (version 5.01, GrapPad Software, Inc., La Jolla, CA). Data were statistically calculated one way ANOVA followed by a Tukey (Sigma Stat Software, 2.03).
4. Results and Discussion
Drug Solubility, Optimized Smix, and Microscopy Studies
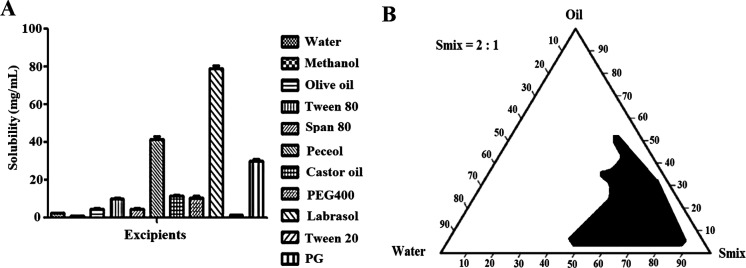
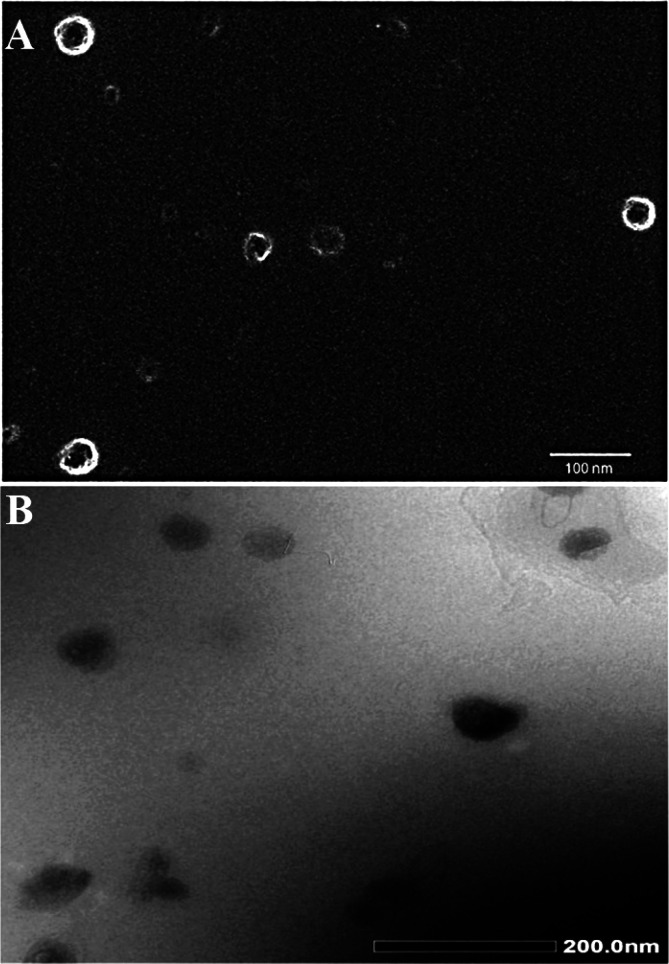
The results of the solubility analysis in different oils and surfactants and cosurfactants are shown in Figure 1A. Among the screened oils, peceol showed the greatest solubility (41.53 ± 1.36 mg/mL) of the drug, whereas labrasol and PG exhibited maximum solubility among surfactants. Furthermore, labrasol as the surfactant was found to have the maximum solubility (79.0 ± 1.4 mg/mL) of miconazole, while Tween 20 had the lowest solubility (1.37 ± 0.04 mg/mL). The optimized Smix ratio to construct a nanoemulsion with a maximized delineated zone of nanoemulsion was 2:1 (Figure 1B). Electron microscopy clearly demonstrated their spherical shape and small globular size, which in turn were used for maximum attachment to fungal cells to eradicate the infection and prevent recurrence of disease (Figure 2A-B).33
Figure 1.
Solubility of miconazole nitrate in various excipients (A) and pseudoternary phase diagram of the optimized ratio of Smix (2:1) for nanoemulsion preparation exhibiting maximally delineated zone of stable nanoemulsion (B).
Figure 2.
Morphological assessment of MCNE11 under: (A) FE-SEM scanning (magnification at 30000×) and (B) HR-TEM scanning (magnification at 40000×) using the freshly prepared sample.
In Vitro Antifungal Assay: Zone of Inhibition Assessment
Both species (Candida and Aspergillus) are responsible to cause acute and chronic cutaneous candidiasis. Therefore, it was required to investigate in vitro antifungal activities against these prime causative factors. The result of in vitro antifungal activities of explored excipients have been illustrated in Table 1. The control group showed apparent colonies of cultures (Candida and Aspergillus), whereas the test sample elicited a well-marked clear zone of inhibition depending upon the compound potential. However, few of them exhibited no clear zone of inhibition. Kinnunen and Koskela reported that 30% PG was about as effective as 10% hexylene glycol against C. albicans within 20 h.34 Moreover, PG was reported to have antimycotic potential (against C. albicans) at a concentration of 30–90 mg/mL (well tolerated concentration to skin) which may be attributed to keratolytic effect of PG.35
Table 1. Result of Zone of Inhibition (ZOI, mm) of Excipients against Candida and Aspergillus.
| zone
of inhibition (mm, ±sd) |
||
|---|---|---|
| excipients (10%) | Candida albicans | Aspergillus niger |
| labrasol | 25 ± 1.2 | 24 ± 1.0 |
| Tween 80 | 0 ± 0 | 0 ± 0 |
| PGa | 20 ± 0.8 | 16.0 ± 0.5 |
| PEG400 | 0 ± 0 | 0 ± 0 |
| olive oil | 11 ± 0.4 | 14 ± 0.2 |
| castor oil | 13 ± 0.5 | 18 ± 1.1 |
| Peceol | 28 ± 1.5 | 23 ± 1.6 |
| Transcutol-HPb | 0 ± 0 | 0 ± 0 |
PG = Propylene glycol, PEG400 = Polyethylene glycol 400.
Transcutol HP was 10% aqueous solution.
Castor oil showed antifungal activities against A. niger and the similar finding was obtained at 10%v/v concentration of the same oil in a reported literature.36 Olive oil enriched with oleic acid (65–85%) and various fatty acids, and ozononized olive oil are known to check microorganism growth.37,38 Therefore, olive oil exhibited zone of inhibition against both strains and these values were 11.0 ± 0.4 and 14.0 ± 0.2 mm against C. albicans and A. niger, respectively. The maximum value of zone of inhibition was achieved in peceol against both strains at explored concentration (10%) which may possibly be due to its composition (consists of mono-, di- and triglycerides of oleic (C18:1) acid). Moreover, this peceol was also reported to improve the efficacy of amphotericin b when formulated as a parenteral delivery system at a dose of 50 mg/kg/day.39
Preparation of MCNE and MNE Formulations and Evaluation Parameters
Formulations were prepared selecting peceol, labrasol, and PG to serve as as lipid, surfactant, and cosurfactant, respectively. The selection was rationalized on the basis of the maximum drug solubility and in vitro antifungal activity (zone of inhibition) of the excipient, which may establish synergism to control cutaneous fungal infections (acute or chronic) and to sensitize drug resistant fungal strain. Therefore, preliminary findings of ZOI and solubility dictated use of these three excipients in suitable proportions to control various dermal fungal infections using PG possessing an innate antifungal potential and keratolytic effect.35 Olive oil and peceol showed antifungal potential, which may be due to the use of oleic acid as a major component of oils producing a detrimental effect on fungal cells with or without antifungal drug as described earlier.39 OA is a positive charge inducer used at low concentration (0.05%) as the higher concentration of OA in the nanaoemulsion formulation could not improve the zeta potential and emulsification efficiency.40 Therefore, it was rationalized for use at low concentrations. However, it is potent stabilizer and reducing agent for nanoparticle synthesis and nanonization available at low cost than pure alkylamine. Chemically, OA is a long chain alkyl amine capable to interact with positively charge membrane on exposure and subsequently results in increased membrane fluidization for enhanced permeation for drug-loaded nanocarriers and stabilization of nanoparticles through repulsive electrostatic interactions.40,41 Therefore, several miconazole-loaded cationic (MCNE) and anionic nanoemulsion (MNE11) formulations were prepared as shown in Table 2. Details of the composition and characterized parameters (globular size, zeta potential, PDI, and %EE) have been illustrated in Table 2. Each formulation contained a constant amount of the drug and OA except MNE11. These were observed overnight at room temperature for any sign of physical instability. Seven formulations (MCNE1, MCNE2, MCNE4, MCNE7, MCNE8, MCNE12, and MCNE14) were found to be unstable due to phase separation, which may be due to the low content of the aqueous phase (14–21%) containing Smix (35–38%) lesser than the organic phase (∼40–48%). MNE11 was OA free formulation for comparative investigation in further studies. Notably, MCNE11 and MNE11 were approximately similar in composition except OA.
Characterization Parameters
Prepared formulations were immediately characterized for globular size, PDI, zeta potential, and %EE. The results are demonstrated in Table 2. The values for the globular size, PDI, zeta potential, and %EE of all cationic nanoemulsion formulations were found to be in the range of 145–219 nm, 0.21–0.71, 18.8–38.7 (+mV), and 41.5–89.8%, respectively, whereas these values of MNE11 (anionic NE) were observed as 137.0 nm, 0.24, −30 mV, and 85.9%. It is obvious that the negative zeta potential of MNE11 was due to the absence of OA (cationic charge inducer). Considering the impact of Smax and lipid content on globular size, size distribution, zeta potential, and %EE, it would be easy to explain based on the general concept of surfactant/cosurfactant mediated emulsification and subsequent consequences such as concentration dependent (Smix) emulsification efficiency, size reduction, homogeneous size distribution, and increased surface area and drug loading ability. In Table 2, MCNE1, MCNE2, MCNE4, MCNE5, MCNE7, MCNE8, MCNE12, MCNE13, and MCNE14 were unstable after 24 h which may be due to either relatively large globular size or insufficient Smix (relative to peceol content) responsible for phase separation. The presence of cosurfactant (PG) assisted in decreasing the globular size and increasing the %EE, whereas MCNE1 and MCNE2 were found to be formulations with large globular size and low %EE values (Table 2). MCNE11 exhibited the most desired globular size (145 nm), PDI (0.21), ZP (+28.1), and %EE (89.8%) as compared to other formulations. Reformulated MNE11 without OA was characterized, and the results revealed approximately similar evaluated values of the studied parameters except ZP (negative due to absence of OA and presence of lipid peceol). Chemically, labrasol is comprised of caprylocaproyl polyoxyl-8 glycerides and is associated with high HLB (hydrophilic–lipophilic balance) value (14.0). This is the most prominent surfactant to increase drug solubility, emulsification, and permeation across cell membrane by reducing P-gp efflux pump.42 Moreover, PG is a water-soluble cosolvent, biocompatible and efficiently effective on keratocyte cells, which further supported generation of an oil in water nanoemulsion (o/w) for dermal delivery.36 Moreover, the presence of two hydroxyl groups in PG conferred its dual functionalities such as (a) forming hydrogen bond with water for improved drug solubilization and (b) induced swelling of keratin for moisturizing effect.43 Upon increasing the relative content of labrasol in Smix (MCNE9, MCNE10, and MCNE11 with 2:1 ratio), a significant improvement in %EE (∼ 71–90%) was observed which may be attributed to the surfactant (labrasol) mediated efficient emulsification to reduced size and isotropic system among them. Notably, further increase in labrasol content (3:1) resulted in formulation instability as shown in MCNE12, MCNE13, and MCNE14. Therefore, MCNE11 was considered the most robust formulation with reduced size (for high surface are), high %EE, and optimal concentration of Smix. The lowest value of size (145 nm) and high %EE are optimal and suitable for topical delivery of miconazole to control superficial epidermal (tinea) and deep residing dermal fungal (Candida and Aspergillus) infections. Thus, MCNE11 and anionic MNE11 were selected for further evaluations to generate complete proof of concept for topical delivery of miconazole.6
Other Evaluation Parameters of the Optimized Formulations
Two formulations were selected from Table 2 for comparative study, and the results are presented in Table 3. To investigate the impact of a positive charge inducer, it was necessary to assess % transmittance, conductance, refractive index, change in pH, viscosity, and extrudability. It is clear that there was no substantial difference between MCNE11 and MNE11 in terms of % transmittance, conductance, refractive index, viscosity, and extrudability. However, there was a slight variation in pH values (Table 3) which may be attributed to free amine group of OA imposed on cationic nanoemulsion globules of MCNE11.40,41 The values of % transmittance and refractive index (optical property of clear solution) for both formulations were close to reference water as control suggesting homogeneous and isotropic nature of thermodynamically stable MCNE11 and MNE11.
Table 3. Other Physicochemical Characterization of MCNE11 and MNE11.
| S no. | parameters | MCNE11 | MNE11 |
|---|---|---|---|
| 1 | transmittance (%) | 92.4 ± 8.18 | 97.05 ± 1.27 |
| 2 | conductance (μs/cm) | 16.11 ± 1.6 | 17.92 ± 1.18 |
| 3 | refractive index | 1.3441 ± 0.021 | 1.341 ± 0.011 |
| 4 | pH | 7.4 | 6.9 |
| 5 | viscosity (cp) | 44.17 ± 0.85 | 48.82 ± 0.93 |
| 6 | extrudability (%) | 97.4 ± 3.8% | 94.6 ± 5.13% |
Morphological Assessment
Both formulations (MCNE11 and MNE11) were evaluated for globular size, PDI, zeta potential, and other parameters as shown in Tables 2 and 3. It was clear that they were not significantly different in terms of evaluated parameters except pH. Thus, imposed cationic charge could not interfere the formulation characteristics at explored concentration of OA. Therefore, we visualized MCNE11 under FE-SEM and HR-TEM. The representative images have been portrayed in Figure 2A,B. FE-SEM confirmed spherical shape and well dispersed nature of nanoemulsion globules as shown in Figure 2A. This may be correlated to OA mediated repulsive electrostatic repulsion existing among globules to confer improved stability by avoiding coalescence (sign of Ostwald ripening).41 HR-TEM report further supported this finding as shown in Figure 2B. The globular size obtained from DLS and HR-TEM were slightly different due to instrumental error (preferential adsorption of small globule by the grid over larger globules) observed in TEM.44 In general, this variation is reported in term of “fold error”.44 We found a fold error value of ∼1.4 (acceptable range <2.0).
Antifungal Efficacy
To confirm the antifungal potential of MCN-loaded MCNE11 and MNE11, we investigated the zone of inhibition against both strains of fungi. Moreover, this finding was also for comparative investigation between anionic and cationic nanoemulsion loaded with MCN. The purpose of this study was to understand the impact of the imposed charge on nanoglobules of nanoemulsion for detrimental effect against fungal strains in vitro. Results were obtained against both strains and compared for relative sensitivity to the formulations in term of zone of inhibition under experimental conditions. The MNE11-treated group revealed an apparent zone of inhibition around the well against both strains. Likewise, MCNE11-treated wells elicited a relatively larger zone of inhibition against both strains. The values of zone of inhibition by MNE11 against C. albicans and A. niger were found to be 12.6 ± 0.4 and 21.4 ± 1.0 mm, respectively. Moreover, these values of the MCNE11-treated groups against C. albicans and A. niger were found to be 18.7 ± 0.8 and 27.5 ± 1.5 mm, respectively. The results clearly confirmed the relatively high sensitivity of MCNE11 against both strains, which may be attributed to the imposed cationic charge and improved globular internalization with strain cells. Furthermore, these finding may be correlated with previous in vitro results where C. albicans was found to be more sensitive due to innate antifungal potential of labrasol and peceol standalone. Furthermore, higher sensitivity of both strains against cationic MCNE11 as compared to MNE11 can be attributed to OA mediated augmented cellular internalization of the drug-loaded nanoemulsion globules around fungal cell and maximized adherence to the surface. This may result in prolonged access of the drug to fungal cell for generating micropore in the cell and subsequent accumulation in the cell membrane to toxic level for cytoplasmic oozing and cell fragmentation.19
Hemolytic Toxicity
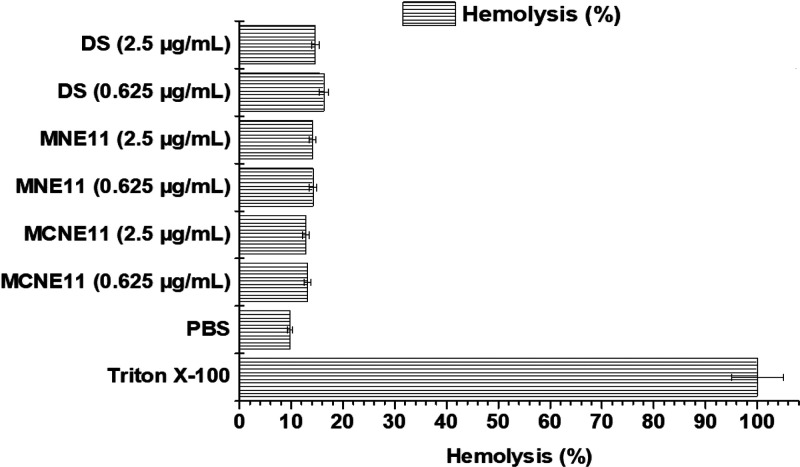
The developed formulation was intended for topical delivery and drug access to the deep dermal region to control dermal infection. The dermal region is enriched with a highly vascularized network of fine blood capillaries. Fungal infections reside in the deeper dermal region, which is a highly vascularized area.6 Therefore, it was required to negate the hemolysis potential of the developed formulation at an explored low concentration (0.05%) of OA as positive charge inducer. Erythrocytes are negatively charged blood cells that may interact with a cationic nanoemulsion if delivered to systemic system. This may alter morphological and physiological behavior of erythrocytes. The probable chance of hemolytic activity of MCNE11 and MNE11 was performed and compared against the negative and positive controls. The in vitro result of hemolysis is presented in Figure 3 wherein MCNE11 and MNE11 exhibited low % hemolysis (<14.0%) andare comparable to the negative control (13.2%) group (PBS 7.4). Positive control triton-X-100 caused significant lysis (p < 0.001) of erythrocytes and release of hemoglobin after incubation. This may be due to corrosive nature of this surfactant responsible to pore creation and membrane destabilization. Placebo formulations (MCNE11 and MNE11) were found to be safe and hemocompatible as evidenced with the in vitro hemolysis value comparable to control PBS. In order to investigate the probable chance of the concentration-dependent hemolysis potential of the optimized formulations, two different dilutions were made (0.625 and 2.5 μg/mL) and incubated. The results showed no significant difference in hemolysis over an explored period of incubation (Figure 3). Therefore, the ME-loaded MCNE11 formulation may be a suitable substitute to deliver drug at therapeutic concentration without causing any adverse reactions.45
Figure 3.
In vitro hemolysis study of various formulations at different concentrations using human RBCs. Formulations showed significantly (p < 0.001) low hemolysis as compared to positive control (n = 3; mean ± SD).
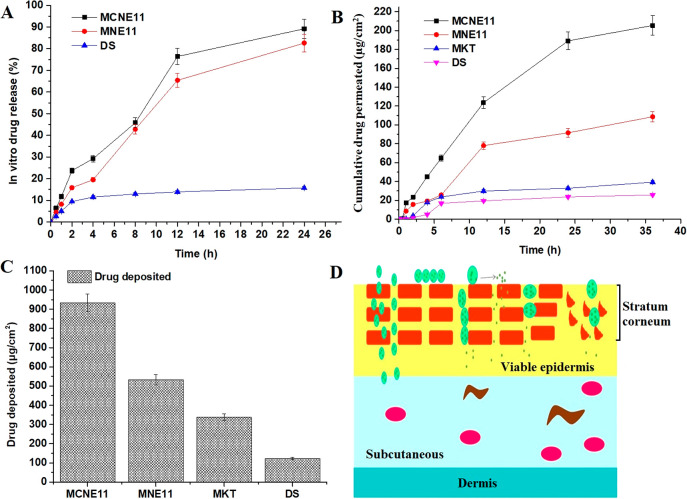
In Vitro Release Study
The results of the in vitro drug release behavior of the drug from MCNE11, MNE11, and DS (suspension) are illustrated in Figure 4A. The drug is lipophilic in nature, and therefore, its suspension showed limited drug release (15.7 ± 0.8%) in the studied medium over a period of 24 h. However, both formulations (MCNE11 and MNE11) exhibited substantial drug release following similar release behaviors over 24 h. MCNE11 and MNE11 showed total drug release as 98.2 ± 4.5% and 82.6 ± 4.0% at the end of 24 h, respectively. A similar pattern of drug release behavior indicated that there is no impact of the imposed cationic charge over the nanoglobules of the drug-loaded nanoemulsion on the drug release across the dialysis membrane. MCNE11 and MNE11 demonstrated 5.9- and 4.7-fold higher drug release as compared to DS, which may be good to correlate with the drug poor aqueous solubility in the suspension (DS). The absence of any burst effect in both formulations suggested the least free drug, and it is available in solubilized form of nanoemulsion for slow and sustained delivery.
Figure 4.
(A) In vitro release behavior of miconazole nitrate loaded formulations (MCNE11, MNE11, DS) through the dialysis membrane (n = 3). (B) Permeation study across rat skin placed for various formulations (MCNE11, MNE11, MKT, and DS) using Franz diffusion cell (% cumulative permeation). (C) Ex vivo drug retention behavior across the rat skin. (D) Various mechanisms via drug passes from organized skin layers to the deeper layers. MKT stands for the marketed product (conventional cream) miconazole.
Ex Vivo Skin Permeation and Drug Retention Studies
The results obtained from the drug permeation study of MCNE11, MNE11, MKT (marketed product), and DS are illustrated in Figure 4B. The cumulative amounts of the drug permeated across rat skin was found to be 205.4 ± 10.3, 108.6 ± 5.2, 39.3 ± 3.1, and 25.8 ± 1.1 μg/cm2 from MCNE11, MNE11, MKT, and DS, respectively, at the end of 36 h. The order of the permeation potential was observed as MCNE11 > MNE11 > MKT > DS, which may be envisaged on the basis of the combined impact of formulation related factors such as the nanocarrier and electrostatic interaction between the negatively charged cell membrane and globules of nanoemulsion and surfactant-mediated perturbation created after application to the surface. The least permeation from MKT and DS was due to semisolid cream base and insolubility of the drug, respectively. MCNE11 exhibited permeation higher than that of MNE11 despite their identical composition except for OA, which played a major role in enhanced permeation. MCNE11 elicited approximately 8 times higher permeation as compared to DS. Moreover, the higher permeation of MCNE11 is attributed to the presence of a labrasol, and PG which causes reversible occlusivity, temporary perturbation, and keratolysis.33 Estimated values of permeation flux were found to be 5.7 ± 0.1, 3.0 ± 0.05, 1.0 ± 0.01, and 0.72 ± 0.008 μg/cm2/h from MCNE11, MNE11, MKT, and DS, respectively. Figure 4C demonstrated the drug deposition from MCNE11, MNE11, MKT and DS and these were found to be as 932.7 ± 41.6, 331.8 ± 22.6, 338.1 ± 15.6, and 123.3 ± 6.2 μg/cm2, respectively. Thus, MCNE11 exhibited maximum cumulative permeation, permeation flux, and the drug deposition (retention) at the end of 36 h. This may be attributed to the composition of nanoemulsion, imposed cationic charge and lowest globular size loaded with soluble miconazole. Other factors also play a critical role for enhanced permeation which need to investigate by transepidermal water loss (TEWL) assessment, and visualization under electron microscopy for mechanistic perspective in the future. Therefore, a prospective mechanistic approach has been illustrated in Figure 4D wherein cationic nanoemulsions are capable to be permeated through intracellular spaces, surfactant mediated reversible changes in the protein layer, paracellular pathway, and follicular route (low percent).17,44
Acute Dermal Irritation Study
In general, cationic polymers or surfactants in the dermal product are known to cause toxicity/dermal irritation due to their ability to bind with hairs and keratin protein (strong ionic bonds with the epidermal protein) of the skin surface.46 Therefore, it was mandatory to investigate the impact of MCNE11, MNE11, and Placebo MCNE11 after topical application on rabbit skin. All rabbits were observed for possible toxicity/irritancy (erythema and edema) after topical application of these formulations. Treated animals were inspected for toxicity signs in terms of redness, erythema (inflammation), and edema (accumulation fluid) (Figure 5A–C). The visual observation for irritancy (toxicity) was graded on the basis of the appearance of toxicity signs. Fortunately, placebo MCNE11, MCNE11, and MNE11 illustrated no sign of any abnormalities such as redness, edema, and irritation. The untreated control group (image not included) and treated groups (Figure 5A–C) were found to be free from any signs of abnormality.
Figure 5.
Draize test to negate irritation behavior of the formulations. Various formulations were applied on the marked skin surface using rabbits: (A) placebo MCNE11, (B) MCNE11, and (C) MNE11.
Stability Study
There were no significant changes (p > 0.001) observed in particle size, size distribution (PDI), and %EE at 2–8 and 30 °C temperatures up to 360 days. This corroborated the stability of MCNE11 (Table 4) for prolonged times at 2–8 and 30 °C. The globular size values were found as 138, 141, and 143 nm at the end of 90, 180, and 360 days, respectively, at 2–8 °C. These values were quite close to those of the freshly prepared formulation at day zero. The globular size was found to be slightly increased (from 135 to 156 nm) after 360 days of storage at 30 ± 2 °C/65 ± 5% RH (relative humidity). Similarly, no coalescence or globular aggregation was observed as evidenced with no significant change o PDI values at both temperatures suggesting no significant changes in kinetics behavior.47,48 Chemical assessment for %EE estimation was carried out for 12 months, and it was found that % loss of the drug was <3% at both temperatures, suggesting stable product with a high shelf life at the explored temperature. Moreover, the stability of the product is going on at accelerated temperature to establish shelf life and possible impurities. These outcomes may be reported in ensuing publications.
Table 4. Stability Study of MCNE11 under Varying Temperatures and Relative Humidity.
| parameters | 0 days | 90 days | 180 days | 360 days |
|---|---|---|---|---|
| 2–8 °C (n = 3) | ||||
| globular size (nm) | 134 ± 7.0 | 138 ± 6 | 141.0 ± 8 | 144 ± 5 |
| size distribution (PDI) | 0.211 ± 0.03 | 0.215 ± 0.01 | 0.219 ± 0.04 | 0.222 ± 0.02 |
| %EE | 88.1 ± 1.2 | 85.2 ± 1.1 | 84.1 ± 1.4 | 83.1 ± 1.5 |
| 30 ± 2 °C/65 ± 5% RH (n = 3) | ||||
| globular size (nm) | 135 ± 11.0 | 144.0 ± 7.0 | 151.0 ± 8 | 156 ± 10 |
| size distribution (PDI) | 0.219 ± 0.01 | 0.217 ± 0.03 | 0.232 ± 0.06 | 0.254 ± 0.04 |
| %EE | 89.1 ± 4.3 | 88.8 ± 5.5 | 86.7 ± 9.1 | 86.5 ± 2.5 |
5. Conclusion
Miconazole nitrate is a potential antifungal drug to control topical and deep dermal infection. Its poor aqueous solubility challenged formulation scientists to find alternative options to improve the topical permeation and efficacy to control infections primarily caused by the Candida and Aspergillus strains. Considering the in vitro, ex vivo, and in vivo findings of the present study suggested a promising approach to control resistant and nonresistant fungal infections using excipients possessing innate antifungal potential. The in vitro zone of inhibition suggested labrasol and peceol possessed considerable inhibition at the explored concentration (10%). Therefore, the drug-laden MCNE11 caused significant inhibition and established synergism in inhibition. Furthermore, an imposed cationic charge on nanoglobules further increased the zone of inhibition against both strains. Thus, three strategies (nanonization, excipient with innate activity, and imposed cationic charge for electrostatic interaction) worked together for maximized detrimental effects against these strains. Ex vivo permeation and drug deposition results showed relatively high permeation parameters associated with MCNE11 as compared to MNE11 and DS, which further supported the impact of cationic charge for augmented electrostatic interaction between skin cells and nanoglobules and then maximized adherence to the fungal cells, possibly due to increased internalization with the negatively charged fungal cell surface. In vitro hemolysis negated the probable chance of hemolysis of stable MCNE11 at the explored concentrations. This stable formulation can be used as a safe product with maximum therapeutic benefits. Finally, the irritation study supported the hemolysis findings and confirmed no irritation after topical application. There were no signs of abnormalities such as edema, inflammation, irritation, or tissue damage. On the basis of the complete proof of concept, the present approach is promising to prevent resistance and recurrence of cutaneous mycoticl infections.
Author Contributions
Mudassar Shahid: Conceptualization, and validation, Afzal Hussain: Conceptualization, writing and drafting; Azmat Ali Khan: Funding and formal review, Amer M. Alanazi: Review and software; Ahmed L. Alaofi: Data curation and reviewing; Mahboob Alam: analysis and data curation; Mohhammad Ramzan: data analysis, software, and method validation. All authors have read and agreed to the published version of the manuscript.
This work was funded by the Researchers Supporting Project No. (RSP-2021/339) King Saud University, Riyadh, Saudi Arabia.
The authors declare no competing financial interest.
Notes
Institutional Review Board Statement. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Department, the Institutional Ethical Committee (PTU) (PCTE/LDH/1369/2013), and the Institute Animal Ethics Committee of Punjab PCTE Institute of Pharmacy, Punjab, India) and approved for the study as per ARRIVE guidelines. The protocol was followed as per ARRIVE guidelines.
References
- Ameen M. Epidemiology of superficial fungal infections. Clin Dermatol. 2010, 28, 197–201. 10.1016/j.clindermatol.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Denning D.; Kneale M.; Sobel J.; Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infectious Diseases. 2018, 18, e339. 10.1016/S1473-3099(18)30103-8. [DOI] [PubMed] [Google Scholar]
- Sobel J. D. Recurrent vulvovaginal candidiasis. American Journal of Obstetrics and Gynecology. 2016, 214, 15–21. 10.1016/j.ajog.2015.06.067. [DOI] [PubMed] [Google Scholar]
- Havlickova B.; Czaika V. A.; Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses 2008, 51, 2–15. 10.1111/j.1439-0507.2008.01606.x. [DOI] [PubMed] [Google Scholar]
- Pereira P. M.; He Q.; Valente F. M. A.; Xavier A. V.; Zhou J. Z.; Pereira I. A. C.; Louro R. O. Energy metabolism in Desulfovibrio vulgaris Hildenborough: insights from transcriptome analysis. Antonie Van Leeuwenhoek 2008, 93, 347–362. 10.1007/s10482-007-9212-0. [DOI] [PubMed] [Google Scholar]
- Ramzan M.; Kaur G.; Trehan S.; Agrewala J. N.; Michniak-Kohn B. B.; Hussain A.; Mahdi W. A.; Gulati J. S.; Kaur I. P. Mechanistic evaluations of ketoconazole lipidic nanoparticles for improved efficacy, enhanced topical penetration, cellular uptake (L929 and J774A.1), and safety assessment: In vitro and in vivo studies. Journal of Drug Delivery Science and Technology. 2021, 65, 102743. 10.1016/j.jddst.2021.102743. [DOI] [Google Scholar]
- Blanco D.; van Rossem K. A prospective two-year assessment of miconazole resistance in Candida spp. With repeated treatment with 0.25% miconazole nitrate ointment in neonates and infants with moderate to severe diaper dermatitis complicated by cutaneous candidiasis. Pediatr Dermatol. 2013, 30 (6), 717–24. 10.1111/pde.12107. [DOI] [PubMed] [Google Scholar]
- Bhalekar M. R.; Pokharkar V.; Madgulkar A.; Patil N.; Patil N. Preparation and Evaluation of Miconazole Nitrate-Loaded Solid Lipid Nanoparticles for Topical Delivery. AAPS PharmSciTechnol. 2009, 10, 289–296. 10.1208/s12249-009-9199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deresinski S. C.; Lilly R. B.; Levine H. B.; Galgiani J. N.; Stevens D. A. Treatment of fungal meningitis with miconazole. Archives of Internal Medicine. 1977, 137, 1180. 10.1001/archinte.137.9.1180. [DOI] [PubMed] [Google Scholar]
- Hosny K. M.; Aldawsari H. M.; Bahmdan R. H.; Sindi A. M.; Kurakula M.; Alrobaian M. M.; Aldryhim A. Y.; Alkhalidi H. M.; Bahmdan H. H.; Khallaf R. A.; El Sisi A. M. Preparation, Optimization, and Evaluation of Hyaluronic Acid-Based Hydrogel Loaded with Miconazole Self-Nanoemulsion for the Treatment of Oral Thrush. AAPS PharmSciTechnol. 2019, 20, 297. 10.1208/s12249-019-1496-7. [DOI] [PubMed] [Google Scholar]
- Prausnitz M. R.; Mitragotri S.; Langer R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discovery 2004, 3 (2), 115–24. 10.1038/nrd1304. [DOI] [PubMed] [Google Scholar]
- Tanner T.; Marks R. Delivering drugs by the transdermal route: review and comment. Skin Research and Technology 2008, 14 (3), 249–260. 10.1111/j.1600-0846.2008.00316.x. [DOI] [PubMed] [Google Scholar]
- Stevens D. A.; Levine H. B.; Deresinski S. C. Miconazole in coccidioidomycosis: II. Therapeutic and pharmacologic studies in man. American Journal of Medicine. 1976, 60, 191–202. 10.1016/0002-9343(76)90428-9. [DOI] [PubMed] [Google Scholar]
- Hosny K. M.; Sindi A. M.; Ali S.; Alharbi W. S.; Hajjaj M. S.; Bukhary H. A.; Badr M. Y.; Mushtaq R. Y.; Murshid S. S. A.; Almehmady A. M.; Bakhaidar R. B.; Alfayez E.; Kurakula M. Development, optimization, and evaluation of a nanostructured lipid carrier of sesame oil loaded with miconazole for the treatment of oral candidiasis. Drug Delivery 2022, 29 (1), 254–262. 10.1080/10717544.2021.2023703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljaeid B. M.; Hosny K. M. Miconazole-loaded solid lipid nanoparticles: formulation and evaluation of a novel formula with high bioavailability and antifungal activity. International Journal of Nanomedicine. 2016, 11, 441–447. 10.2147/IJN.S100625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa S.; Kasamaki M. Enhanced Delivery of Retinoic Acid to Skin by Cationic Liposomes. Chem. Pharm. Bull. 2006, 54, 242–244. 10.1248/cpb.54.242. [DOI] [PubMed] [Google Scholar]
- Hussain A.; Singh S.; Webster T. J.; Ahmad F. J. New perspectives in the topical delivery of optimized amphotericin B loaded nanoemulsions using excipients with innate anti-fungal activities: A mechanistic and histopathological investigation. Nanomedicine: Nanotechnology, Biology and Medicine. 2017, 13, 1117–1126. 10.1016/j.nano.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Khachane P. V.; Jain A. S.; Dhawan V. V.; Joshi G. V.; Date A. A.; Mulherkar R.; Nagarsenker M. S. Cationic nanoemulsions as potential carriers for intracellular delivery. Saudi Pharmaceutical Journal. 2015, 23, 188–194. 10.1016/j.jsps.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N.; Verma S. M.; Singh S. K.; Verma P. R. P.; Ahsan M. N. Antibacterial activity of cationised and non-cationised placebo lipidic nanoemulsion using transmission electron microscopy. Journal of Experimental Nanoscience. 2015, 10, 299–309. 10.1080/17458080.2013.830199. [DOI] [Google Scholar]
- Thormar H.; Hilmarsson H. The role of microbicidal lipids in host defense against pathogens and their potential as therapeutic agents. Chemistry and Physics of Lipids. 2007, 150, 1–11. 10.1016/j.chemphyslip.2007.06.220. [DOI] [PubMed] [Google Scholar]
- Lawrence M. J.; Rees G. D. Microemulsion-based media as novel drug delivery systems. Adv. Drug Delivery Rev. 2000, 45, 89–121. 10.1016/S0169-409X(00)00103-4. [DOI] [PubMed] [Google Scholar]
- Pavoni L.; Maggi F.; Mancianti F.; Nardoni S.; Ebani V. V.; Cespi M.; Bonacucina G.; Palmieri G. F. Microemulsions: An effective encapsulation tool to enhance the antimicrobial activity of selected EOs. Journal of Drug Delivery Science and Technology. 2019, 53, 101101. 10.1016/j.jddst.2019.05.050. [DOI] [Google Scholar]
- Pavoni L.; Perinelli D. R.; Bonacucina G.; Cespi M.; Palmieri G. F. An Overview of Micro- and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials (Basel). 2020, 10, 135. 10.3390/nano10010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastiti C. M. R. R.; Ponto T.; Abd E.; Grice J. E.; Benson H. A. E.; Roberts M. S. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics. 2017, 9, 37. 10.3390/pharmaceutics9040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabale V.; Vora S. Formulation and evaluation of microemulsion-based hydrogel for topical delivery. International Journal of Pharmaceutical Investigation. 2012, 2 (3), 140–149. 10.4103/2230-973X.104397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurana S.; Jain N. K.; Bedi P. M. S. Nanoemulsion based gel for transdermal delivery of meloxicam: Physico-chemical, mechanistic investigation. Life Sciences. 2013, 92, 383–392. 10.1016/j.lfs.2013.01.005. [DOI] [PubMed] [Google Scholar]
- Isham N.; Ghannoum M. A. Antifungal activity of miconazole against recent Candida strains. Mycoses. 2010, 53, 434–437. 10.1111/j.1439-0507.2009.01728.x. [DOI] [PubMed] [Google Scholar]
- Tokarzewski S.; Ziółkowska G.; Nowakiewicz A. Susceptibility testing of Aspergillus niger strains isolated from poultry to antifungal drugs - A comparative study of the disk diffusion, broth microdilution (M 38-A) and Etest methods. Polish journal of veterinary sciences. 2012, 15, 125–133. 10.2478/v10181-011-0123-7. [DOI] [PubMed] [Google Scholar]
- Hussain A.; Samad A.; Singh S. K.; Ahsan M. N.; Haque M. W.; Faruk A.; Ahmed F. J. Nanoemulsion gel-based topical delivery of an antifungal drug: in vitro activity and in vivo evaluation. Drug Delivery. 2016, 23, 642–657. 10.3109/10717544.2014.933284. [DOI] [PubMed] [Google Scholar]
- Alshehri S.; Hussain A.; Altamimi M. A.; Ramzan M. In vitro, ex vivo, and in vivo studies of binary ethosomes for transdermal delivery of acyclovir: A comparative assessment. Journal of Drug Delivery Science and Technology. 2021, 62, 102390. 10.1016/j.jddst.2021.102390. [DOI] [Google Scholar]
- Chebil A.; Desbrieres J.; Nouvel C.; Six J.-l.; Durand A. Ostwald ripening of nanoemulsions stopped by combined interfacial adsorptions of molecular and macromolecular nonionic stabilizers. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2013, 425, 24–30. 10.1016/j.colsurfa.2013.02.028. [DOI] [Google Scholar]
- Shukla T.; Pandey S. P.; Khare P.; Upmanyu N. Development of ketorolac tromethamine loaded microemulsion for topical delivery using D-optimal experimental approach: Characterization and evaluation of analgesic and anti-inflammatory efficacy. Journal of Drug Delivery Science and Technology. 2021, 64, 102632. 10.1016/j.jddst.2021.102632. [DOI] [Google Scholar]
- Damasceno B.; Dominici V.; Urbano I.; Silva J.; Araujo I.; Santos-Magalhaes N.; Silva A.; Medeiros A.; Oliveira A.; Egito S. Amphotericin B Microemulsion Reduces Toxicity and Maintains the Efficacy as an Antifungal Product. Journal of biomedical nanotechnology. 2012, 8, 290–300. 10.1166/jbn.2012.1374. [DOI] [PubMed] [Google Scholar]
- Kinnunen T.; Koskela M. Antibacterial and antifungal properties of propylene glycol, hexylene glycol, and 1,3-butylene glycol in vitro. Acta Derm Venereol. 1991, 71, 148–150. [PubMed] [Google Scholar]
- Faergemann J.; Fredriksson T. Antimycotic activity of propane-1,2-diol (propylene glycol). Sabouraudia: Journal of Medical and Veterinary Mycology 1980, 18, 163–166. 10.1080/00362178085380301. [DOI] [PubMed] [Google Scholar]
- Mahilrajan S.; Nandakumar J.; Kailayalingam R.; Manoharan N. A.; SriVijeindran S. Screening the antifungal activity of essential oils against decay fungi from palmyrah leaf handicrafts. Biological Research. 2014, 47, 35. 10.1186/0717-6287-47-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geweely N. S. Antifungal activity of ozonized olive oil (Oleozone). International Journal of Agriculture & Biology. 2002, 8, 670–675. [Google Scholar]
- Varol K.; Koc A. N.; Atalay M. A.; Keles I. Antifungal Activity of Olive Oil and Ozonated Olive Oil Against Candida Spp. and Saprochaete Spp. Ozone: Science & Engineering. 2017, 39, 462–470. 10.1080/01919512.2017.1322490. [DOI] [Google Scholar]
- Risovic V.; Rosland M.; Sivak O.; Wasan K. M.; Bartlett K. Assessing the Antifungal Activity of a New Oral Lipid-Based Amphotericin B Formulation Following Administration to Rats Infected with Aspergillus Fumigatus. Drug Dev. Ind. Pharm. 2007, 33, 703–707. 10.1080/03639040601077349. [DOI] [PubMed] [Google Scholar]
- Beg S.; Sharma G.; Thanki K.; Jain S.; Katare O. P.; Singh B. Positively charged self-nanoemulsifying oily formulations of olmesartan medoxomil: Systematic development, in vitro, ex vivo and in vivo evaluation. Int. J. Pharm. 2015, 493, 466–482. 10.1016/j.ijpharm.2015.07.048. [DOI] [PubMed] [Google Scholar]
- Mbewana-Ntshanka N. G.; Moloto M. J.; Mubiayi P. K. Role of the amine and phosphine groups in oleylamine and trioctylphosphine in the synthesis of copper chalcogenide nanoparticles. Heliyon. 2020, 6, e05130. 10.1016/j.heliyon.2020.e05130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong D. H.; Tran T. H.; Ramasamy T.; Choi J. Y.; Lee H. H.; Moon C.; Choi H.-G.; Yong C. S.; Kim J. O. Development of Solid Self-Emulsifying Formulation for Improving the Oral Bioavailability of Erlotinib. AAPS PharmSciTechnol. 2016, 17, 466–473. 10.1208/s12249-015-0370-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai R.; Ueda K.; Nishida T.; Toyohara M.; Mori O. Effects of Ionic and Surfactant Agents on the Antimicrobial Activity of Polyhexamethylene Biguanide. Eye & Contact Lens. 2011, 37, 85–89. 10.1097/ICL.0b013e31820cebc3. [DOI] [PubMed] [Google Scholar]
- Hussain A.; Altamimi M. A.; Alshehri S.; Imam S. S.; Shakeel F.; Singh S. K. Novel Approach for Transdermal Delivery of Rifampicin to Induce Synergistic Antimycobacterial Effects Against Cutaneous and Systemic Tuberculosis Using a Cationic Nanoemulsion Gel. Int. J. Nanomedicine. 2020, 15, 1073–1094. 10.2147/IJN.S236277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi J.; Suthar D.; Patel H.; Shelat P.; Parejiya P. Development and characterization of microemulsion based topical gel of essential oil of clove (Syzygium aromaticum) for superficial fungal infections. Advances in Traditional Medicine. 2021, 21, 519–534. 10.1007/s13596-020-00462-6. [DOI] [Google Scholar]
- Bujak T.; Nizioł-Łukaszewska Z.; Ziemlewska A. Amphiphilic cationic polymers as effective substances improving the safety of use of body wash gels. International Journal of Biological Macromolecules. 2020, 147, 973–979. 10.1016/j.ijbiomac.2019.10.064. [DOI] [PubMed] [Google Scholar]
- Verma D.; Thakur P. S.; Padhi S.; Khuroo T.; Talegaonkar S.; Iqbal Z. ″Design expert assisted nanoformulation design for co-delivery of topotecan and thymoquinone: Optimization, in vitro characterization and stability assessment.″. J. Mol. Liq. 2017, 242, 382–394. 10.1016/j.molliq.2017.07.002. [DOI] [Google Scholar]
- Khuroo T.; Dharani S.; Mohamed E. M.; Immadi S.; Wu Z.; Khan M. A.; Lu D.; Nehete P.; Rahman Z. ″Ultra-long acting prodrug of dolutegravir and delivery system-physicochemical, pharmacokinetic and formulation characterizations.″. Int. J. Pharm. 2021, 607, 120889. 10.1016/j.ijpharm.2021.120889. [DOI] [PubMed] [Google Scholar]