Abstract

Fluid repellency of a hydrophobic surface has been typically demonstrated in terms of water sliding angle. A drop shape analysis method with a written computer algorithm monitoring the image brightness was proposed to precisely estimate the sliding angle. A hydrophobic surface coated with silanized silicon dioxide or polytetrafluoroethylene was selected as a known sample for the method validation. Average pixel brightness in an 8-bit grayscale unit rapidly increased after a water drop rolled off the surface, thus removing its black pixels. The resulting sliding angle was then determined as the tilt angle of the sample stage related to the sliding time at the brightness leap. The optimized angular speed of the rotor at 0.1 degrees per frame was chosen to avoid an overestimation of the sliding angle due to the deceleration. The proposed method yielded accurate sliding angles with an error of less than 0.2 degrees. It was then applied to study the fluid resistance of commercial face masks including disposable surgical masks and reusable fabric masks. It was found that the outermost layer of the single-use surgical masks can moderately repel a water drop with a sliding angle of 49.4 degrees. Meanwhile, the pre-coated fabric masks retained high protection efficiency at a sliding angle of less than 45 degrees after about 20 wash cycles. In addition, a raw muslin fabric coated with a commercial water-repellent spray could be a promising and affordable alternative to the surgical mask during the pandemic with high water repellency even after a few washes. The results suggested that, besides the hydrophobicity indicated by the typical contact angle, the precise sliding angle estimated by the proposed alternative method could additionally provide crucial information that might lead to a detailed discussion of the fluid repellency of rough materials.
1. Introduction
The wettability of a solid material surface has been widely studied to evaluate its wetting preference for specific liquids, particularly water and nonpolar oils. Different interactions between solid and liquid without a chemical reaction could lead to the drop transformations: the spreading of the liquid over a surface, the penetration or adsorption into a porous material, or the repellence of the fluid. Contact angle, defined as the angle between the liquid–air boundary and the contact solid surface, is one of the most important factors that was typically used to indicate the hydrophobicity of the surface.1−3 It is quantitatively estimated within the range from zero to 180 degrees related to the solid baseline, which typically depends on the surface characteristics such as morphological roughness and surface energy.4−6
The water contact angle could be very low or close to zero degrees in the case of a hydrophilic or perfect-wetting surface, for example, a superhydrophilic titanium dioxide coated on polycarbonate substrates.7 On the contrary, a superhydrophobic material with low surface energy could have a contact angle higher than 150 degrees such as a poly(tetrafluoroethylene)-coated material8 or a cotton fabric coated by metal nanoparticle-incorporated graphene oxide.9 An advanced material was recently developed with a tunable contact angle, for example, by fabricating micro-patterns on a stainless steel sheet10 or by infiltrating a hydrophobic polymer into a one-dimensional Al/Al2O3 nanostructure.11 However, the contact angle alone is inadequate and regularly inconsistent for the evaluation of the water repellency of a material surface. For example, various types of hydrophobic polymers were reported with large contact angle difference but with the same sliding angle.12 In contrast, the hydrophilic silica nanoparticle that was coated on a stainless steel mesh surface significantly increased the sliding angle due to the improved surface energy but the contact angle was still unchanged.13 Thus, a sliding angle or a roll-off angle, defined as the angle of inclination of a surface at which a water drop begins to roll off it,14 was introduced to additionally describe the water repellency or self-cleaning ability of a surface.
The water sliding angle has been widely reported along with the typical water contact angle to indicate the water repellency and surface hydrophobicity of materials. It has a direct relation to the contact angle hysteresis, which is related to the difference between the ascending and receding contact angles during the drop movement.15 The sliding angle mainly depends on the surface roughness, the water adhesion or friction, and the size of the droplet.16−18 Such unique characteristics of superhydrophobicity,19,20 marine antifouling,21,22 self-cleaning known as the lotus effect,23,24 and oil/water separation or chemical-selective permeability25,26 were previously discussed by using the criteria of both sliding and contact angles. Recently, critical sliding angles with a novel super-repellent surface, including a silanized silicon dioxide27 or silica with controlled multi-scaled roughness,28 were reported at lower than 2 degrees. To develop a hydrophobic and water-repellent surface, a measurement of the sliding angle should be used for a reliable and precise experiment.
The old-fashioned measurement of a sliding angle was done by placing a liquid drop with a certain weight on a sloped plate. The inclination was increasing with a higher surface angle until the drop began to roll off it.29,30 The sliding angle could be estimated by manually selecting the roll-off frame in the entire recorded video.31 Although this method was uncomplicated with a simple apparatus, the result could be more reliable and precise by using an advanced technique. A recent and the most common automated measurement of sliding angle was performed by using a commercial drop shape analyzer with specific image-analysis software.16 The detail of this optical-based measurement could be found in a user manual, for example, a technical note by Kruss, Germany.32 Briefly, a sequence of images was being recorded while the sample base with a water drop was being rotated. Three-phase points at the boundary of solid, liquid, and surrounding gas phases were located at both sides of the drop. The sliding angle was defined as the angle at which the three-phase points were displaced by 40 pixels or about one millimeter. Monitoring the baseline or the three-phase point movement, particularly on the surface with roughness or defects, could be complicated by using a difficult image-processing algorithm.33 Thus, it might consume a large computation time or yield high standard deviations of the sliding angle or even the contact angle. Besides the optical methods, force-based techniques were introduced to accurately determine the sliding angle by measuring the friction force between water drops and various surfaces.34,35 However, an experimental setup with a small drop holder and a nanotribometer with a highly sensitive force sensor could be somewhat complicated to install and analyze. Herein, to measure the sliding angle by using a typical optical-based experiment, a simple and precise automated method with custom computational algorithms written in MATLAB was introduced in this work described in the first part. Since the digital image of a water drop can be analyzed by using pixel-based image processing, an image brightness of a grayscale image calculated by averaging the intensity of all pixels was taken into account. The sliding angle could be estimated at the time that the dark shadow of the drop suddenly disappeared. After the proposed method was validated, a fluid repellency of face masks will be investigated to demonstrate their protection efficiency.
During the COVID-19 pandemic, wearing a face mask was recommended by the United States Centers for Disease Control and Prevention (CDC) and later by the World Health Organization (WHO) to effectively protect a wearer from respiratory infections.36−38 However, there was a shortage of mask supply in the community at the beginning of the outbreak, and thus surgical masks were strictly reserved for medical staff. These masks typically consist of three layers known as three-ply. The outermost layer of these masks was made of hydrophobic non-woven polypropylene, which was claimed to be somewhat repellent and protective against viruses or bacteria carried in fluid droplets.39 One or two dense layers made of melt-blown polymer were added in the middle in order to improve filtration capacity. The soft inner layer directly contacting to facial skin was designed to adsorb moisture and secretions. In addition, according to the increasing usage of a surgical mask, its non-reusability has raised public attention about the microplastic waste that could be harmful pollution in the environment.40 Therefore, a fabric mask made of different types of fabrics was used as an alternative for personal protective equipment with recent reports of its protection performance.41−44 Commercial fabric masks are reusable, durable, affordable, and can be made by oneself, and thus they are still popular among users until now. In the next section of this work, the proposed and validated method was then applied to study the fluid repellency mainly in terms of the sliding angle of commercial surgical and fabric masks available in a local store. The repellency improvement by using a hydrophobic coating on the fabric masks and raw fabrics will be investigated along with the change in their surface characteristics.
2. Materials and Methods
The hydrophobic silanization agent, dichloro(dimethyl)silane (DDS), and silicon dioxide porous nanoparticles with the size of 5–15 nm were purchased from Sigma-Aldrich, Singapore. A commercial hydrophobic micrometer-scale fine powder, polytetrafluoroethylene (PTFE), was purchased from Hubei Everflon Polymer Co., China. Adhesive spray number 75 was obtained from 3 M Co., Ltd. Ethanol was purchased from QReC, New Zealand, and utilized without any purification. The typical glass slides (CAT. no. 7105) were purchased from Sail Brand.
Hydrophobic surfaces were prepared by coating either DDS-SiO2 or PTFE on a glass slide. An amount of 0.5 g of silicon dioxide nanoparticle was added into 20 mL of 2.5% v/v of DDS in ethanol solution. Meanwhile, a suspension with 1% w/v of PTFE in ethanol was prepared by adding 0.5 g of PTFE powder into 50 mL of absolute ethanol and magnetically stirred for 10 min. The adhesive was sprayed on a glass slide for a few seconds with a spraying distance of about 20–30 cm. The glass slide was quickly dipped in the prepared solutions and was dried in air for a few minutes. These coating steps of glue and particles were defined as one cycle of preparation. The ferroconcrete-like structured coating was fabricated by 10 cycles of preparation. The silanized SiO2 nanoparticles were identified by using Fourier transform infrared spectroscopy (2000 FTIR, Perkin Elmer).
Typical disposable surgical masks regularly used by medical staff were collected from the Department of Nursing, Burapha University, and Burapha University Hospital, Thailand. They were made of a melt-blown polypropylene layer placed between an outermost colored water-repellent non-woven fabric and an innermost white non-woven fabric. Their protection performance has met the national standard TISI 2424-2562 as a single-use medical grade. Reusable fabric masks as an alternative during the shortage of the surgical mask were purchased from a local store. These can be made of different kinds of fabric textiles, for example, cotton, hemp, muslin, salu, synthetic, or linen. Salu and muslin fabrics were made of loosely woven cotton fabric to decrease the thickness and improve air permeability. Water-repellent sprays, which might be useful to improve the hydrophobicity of the fabric masks, were purchased from an online store and were applied to the fabric masks following the given instruction. The surface morphology of the raw fabric samples was observed by using scanning electron microscopy (1450 VP SEM, LEO).
Contact angle and sliding angle of the coated glass slide were measured by a video-imaging of a water droplet placed on the surface. Since the sliding angle depends on gravity, the volume of the drop was varied from 10 to 30 μL not only to verify the size effect but also to study the accuracy of the proposed method. A drop shape analyzer DSA-30 (Kruss, Germany) controlled by DSA-4 software was used in this work. A tilting stage PA4240 was installed at the sample base. The camera Stingray F046B IRF was operated at a camera framerate of 25 Hz and a camera angle of about 0.5 degrees related to the sample plane. It was not attached to the rotor so the image plane will not rotate along with the stage. The pixel size of the recorded image was 24.4 μm per pixel. The contact angle was calculated by correlating the tangent line of the drop circle and the surface baseline. To estimate the sliding angle, the sample was rotated from zero to 90 degrees with the rotor step sizes of 0.1 and 0.5 degrees per frame and total video recording times of about 36 and 7.2 s, respectively. The instrument rotor speed was validated at various units per second and different tilting angle ranges, resulting in the tilting speed of 1.59 degrees per unit.
To study the durability of the mask samples, selected fabric masks and coated raw fabrics will be washed by using a regular washing powder purchased from a local store. The solution was prepared by dissolving 1.27 g of the washing powder in 1.5 L of water as recommended by the manufacturer. The fabric was stirred in the solution for 20 min and dried in air. The sliding angle was measured after each wash cycle until the fabric cannot repel a water drop and adsorbed it into the surface. The sliding angle was reported by measuring water drop with a volume of 30 μL, which was in the limited range of a typical camera used in this work. The water repellency of the selected volume could provide a protection performance of the masks against expiratory droplets following recent studies using tens of microliters of a water drop on the facial masks coated with, for example, carbon nanotubes,33 graphene,45 or metal oxides.46
3. Results and Discussion
3.1. Proposed Method to Estimate the Sliding Angle of a Hydrophobic Surface
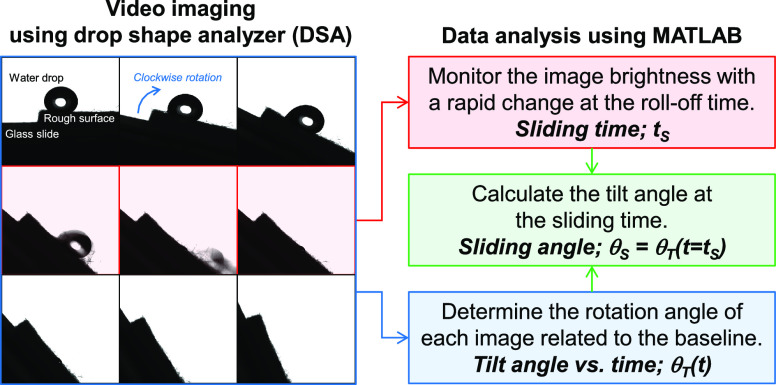
An experimental procedure with written computational algorithms was proposed in this work to precisely measure the sliding angle of a hydrophobic material with a rough surface. The schematic illustration with a series of video images taken by a drop shape analyzer and a computational calculation step is shown in Figure 1. First, the image brightness was monitored to indicate the sliding time that the water drop rapidly moved out of the surface. The roll-off frame will be pointed out with a rapid change of the image brightness due to the sudden depletion of the dark area that belonged to the water drop. The rotational angle of the sample surface tilted from zero to 90 degrees was then determined by the tangent method of the current surface baseline related to the original position. It was expressed by a linear relationship between the tilt angle and the acquisition time with an angular speed of the rotor as a slope. Finally, the sliding time was plugged into the linear expression, resulting in a precise sliding angle of the surface. These simple calculations could yield such a reliable numerical degree that shows the water repellency of the materials.
Figure 1.
Schematic illustration of the proposed sliding angle measurement by using the drop shape analyzer and computational calculations.
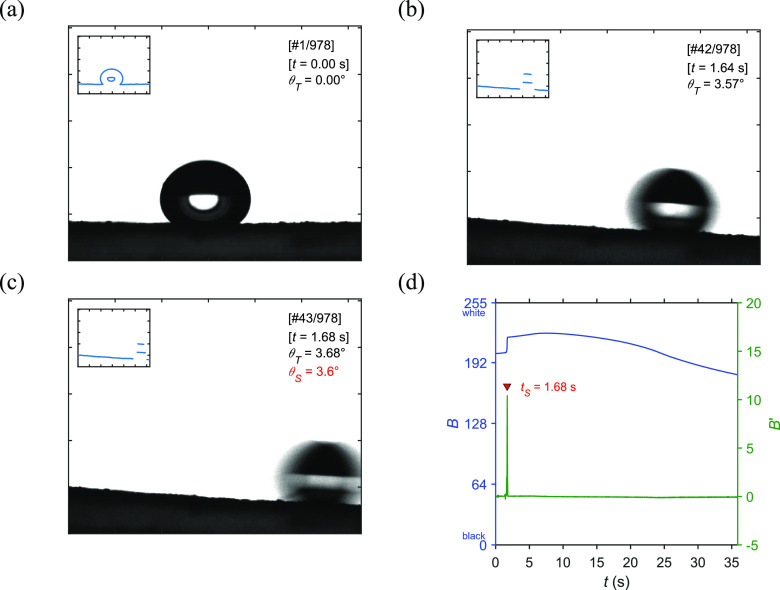
A water drop was placed on a glass slide coated by silanized silicon dioxide nanoparticles (DDS-SiO2), which appeared to be a non-transparent surface. The water drop was video imaged while the sample stage was being rotated clockwise from zero to 90 degrees. A drop shape video with a drop volume of 20 μL will be discussed as an example result (see the Movie S1 in the Supporting Information). An angular speed of 0.1 degrees per frame and a camera framerate of 25 Hz were adjusted. The first frame and the consecutive roll-off images are shown in Figure 2a–c. The initial contact angle was 131.2 degrees indicating the hydrophobicity of the surface. It was found that the water drop was sliding off the surface as seen in the movie from frame number 39 to frame number 44, suggesting a sliding time (tS) roughly determined between 1.52 and 1.72 s. The roll-off speed of the water drop was estimated at 43.8 mm/s. However, it could be impractical to manually search for the roll-off image in the entire video.
Figure 2.
(a–c) Video images of a water drop on a glass slide coated with DDS-SiO2 the corresponding boundary line shown in the inset. Each tick mark represents 100 pixels. (d) The average pixel brightness (B) is plotted against time with its differentiation (B′).
The image pixel was recorded in an 8-bit grayscale of which the complete black and white colors were assigned by 0 and 255, respectively, and the shade of gray was in between. An average of this color code over the entire pixels of an image could be considered as the average pixel brightness (B). The material surface and the water drop were imaged in black color with the white background from an illumination. While the sample stage was being rotated, a part of the black area of the surface shadow either appeared in the image or moved out of the frame. This caused a slight change in the average pixel brightness during the rotation as shown in Figure 2d.
After the water drop rolled off the surface, its dark area was suddenly removed and replaced by a white background. Thus, the average pixel brightness sharply increased by about 10 units in grayscale per pixel. The rapid change can be detected by calculating the differentiation of the brightness (B′) as shown in the solid green line. The corresponding peak is located at the brightness leap with the sliding time (tS) of 1.68 s. Thus, it was found that the written computational algorithms could be useful to automatically estimate the roll-off image by searching for the sudden brightness change in the entire video.
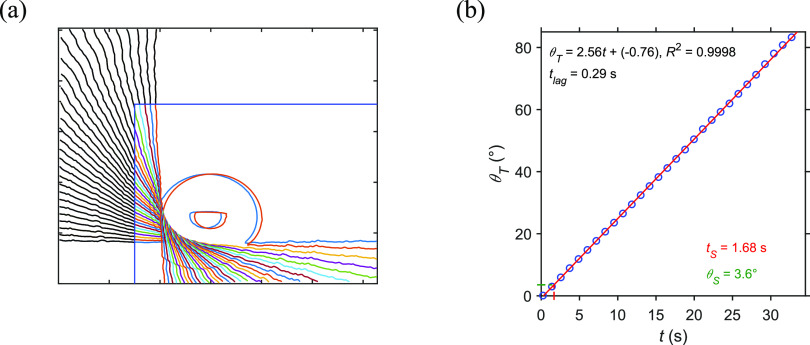
The pixel composition of the grayscale video image as seen above can be divided into two different colors. The water drop and the material surface were in black, while the illuminated background was in white. Thus, there was a boundary between these colors, which can be extracted by using a partial area effect technique, which is an image-processing function written in MATLAB available online.47 The image contrast was pre-adjusted in order to improve the color difference at the boundary. The resulting boundary lines are shown in Figure 3a in different colors with an interval of 30 frames to present a clear and distinguishable illustration. The surface was seemingly flat due to a somewhat uniform coating. The surface boundary was then cropped at 100 pixels along both axes as shown in the solid black line on the left. The angular speed of the rotor and the tilt angle will be calculated from these straight lines.
Figure 3.
(a) The boundary line between the white and black areas of each video image is shown with an interval of 30 frames. The solid black lines are the boundary segments within the first 100 pixels. (b) A linear relationship of the tilt angle (θT) with the acquisition timestep (t) yielded a sliding angle (θS) of 3.6 degrees.
Each set of data points of the single cropped boundary line was fitted to a linear equation, resulting the slope of the original baseline in the first frame (m0) and the slope of frame number i (mi). The tilt angle (θT) was calculated by using the tangent method following an equation, θT(i) = tan–1[(mi – m0)/(1 + mi·m0)]. To standardize the angular speed of the rotor used in this work, the resulting θT(i) and the successive timestep (ti) were fitted to a linear equation as shown in Figure 3b. The standard curve yielded a linear relationship, θT = 2.56·t – 0.76, with R2= 0.9998. The linearity is clearly shown the smooth and consistent rotation. An angular speed of the rotor of 2.56 degrees per second at a camera framerate of 25 Hz can be converted to a rotation resolution of 0.1 degrees per frame.
To decrease an error in the calculations due to the latency of the device, the camera will be synchronized with the rotor by determining the lagged time of the rotor at the beginning and the extended period of the imaging. The lagged time (tlag) of 0.29 s was calculated from the standard curve, indicating a slow start of the rotation after the camera imaging. The image brightness remained steady after the end time of 35.88 s as shown in Figure S1 in the Supporting Information. This was caused by the waiting period of the camera to ensure that the rotation was complete. Thus, the images taken before tlag and after tF will be neglected in the calculations to avoid latency, resulting in precise measurement and a corrected sliding angle.
The sliding time of 1.68 s calculated by monitoring the image brightness as described above was plugged into the linear equation of the standard curve. This yielded a sliding angle (θS) of 3.6 degrees. Although it can be compared to the tilt angle of 3.68 degrees at the roll-off frame, the calculated sliding angle was more reliable due to the correction by using the standard curve of the rotation explained above. These results showed that the proposed experimental method and the written computational scripts for a straightforward data analysis could be useful to precisely measure the sliding angle of a material surface.
3.2. Sliding Angle Measurements of a Superhydrophobic Surface, Effect of Water Drop Volume, and Angular Speed Optimization
The proposed measurement was used to study the effect of the water drop volume on the sliding angle. The drop volume was varied from 10 to 30 μL, in which a larger drop was expected to slide off the surface easier due to gravity. The resulting sliding angles of a glass slide coated with silanized silicon dioxide nanoparticles (DDS-SiO2) and polytetrafluoroethylene (PTFE) are shown in Table 1. It was found that the size of the water drop had a significant effect on the sliding angle as expected. The sliding angles were decreasing for a water drop with a larger volume obviously due to more weight from the gravity pushing the drop to slide off the surface. This result could suggest that a sliding angle should be reported along with the size of the water drop.
Table 1. Sliding Angle of a Glass Slide Coated with DDS-SiO2 or PTFE with Distinct Water Drop Volumes and Different Angular Speeds of the Rotor (Φ).
| θS (°) |
|||
|---|---|---|---|
| coating material | water drop volume (μL) | Φ = 0.1 °/s | Φ = 0.5 °/s |
| DDS-SiO2 | 10 | 6.2 ± 0.9 | 6.3 ± 1.0 |
| 15 | 5.9 ± 0.9 | 6.5 ± 0.7 | |
| 20 | 4.4 ± 0.6 | 4.6 ± 0.4 | |
| 25 | 4.3 ± 0.5 | 6.1 ± 1.0 | |
| 30 | 3.7 ± 0.5 | 5.4 ± 1.7 | |
| PTFE | 10 | 23.0 ± 1.9 | 24.2 ± 2.4 |
| 15 | 17.3 ± 0.9 | 18.9 ± 1.0 | |
| 20 | 12.1 ± 1.5 | 13.2 ± 1.3 | |
| 25 | 9.1 ± 0.7 | 11.2 ± 0.4 | |
| 30 | 9.0 ± 0.4 | 11.0 ± 0.4 | |
To optimize a rotation resolution, an angular speed (Φ) of 0.1 and 0.5 degrees per second was performed with a total video length of about 36 and 7.2 s, respectively. The slower speed could allow the camera to capture the roll-off events in detail. The results showed a significant difference with lower sliding angles by using the slower angular speed at the same drop volume. The slower stage rotation could finely detect the starting point of a water drop motion before rolling off the surface. In the meantime, the faster speed might add a significant centrifugal force to the drop that decelerates the roll-off movement, resulting in an overestimation of the sliding angle. In addition, inconsistency of the sliding angle measured by using the faster angular speed was observed at the increasing volumes. Therefore, the measurement with an angular speed of 0.1 degrees per second could yield a reliable sliding angle with a somewhat acceptable recording time. The latter sliding angle measurements in this work will be performed by using this angular speed to enhance the accuracy.
The water repellency and hydrophobicity of each layer of the superhydrophobic coating were studied by using the proposed method. The resulting sliding angle with a water drop volume of 30 μL and the corresponding contact angle at an early time before an expansion or adsorption of the water drop are shown in Table 2. A pristine glass slide hardly repelled a water drop with a sliding angle of 73.5 degrees, and its low hydrophobicity is shown with the starting contact angle of 67.6 degrees. A commercial adhesive spray that was coated on a slide could not repel a water drop in the observed rotation range up to 90 degrees, and thus the sliding angle was reported at higher than 90 degrees. Although the contact angle increased to 76.8 degrees indicating higher hydrophobicity, the water drop was still attached to the polymer glue by a strong adhesive force. The hydrophobic silanization agent alone could functionalize the silicate surface with a water-repellent silane group, which reduced the sliding angle to 15.5 degrees and increased the contact angle to 89.0 degrees. Without the silanization agent, a glass slide coated with the adhesive spray and the silicon dioxide nanoparticle could not repel a water drop. In addition, it quickly adsorbed the drop to the porous surface in a few seconds due to the strong hydrophilicity. Thus, the sliding angle could not be measured without the water droplets left on the surface and the contact angle was reasonably reported at zero degrees.
Table 2. Sliding Angle and Contact Angle of a Hydrophobic Glass Slide Coated with a Mixture of Silanized Silicon Dioxide and an Adhesive and Its Individual Compositions.
| coating material | θS (°) | θC (°) |
|---|---|---|
| DDS-SiO2 + adhesive | 3.7 | 140.0 |
| SiO2 + adhesive | - | 0.0 |
| DDS | 15.5 | 89.0 |
| adhesive | >90.0 | 76.8 |
| pristine glass slide | 73.5 | 67.6 |
The hydrophobicity of the silanized silicon dioxide nanoparticle (DDS-SiO2) mixed with the adhesive spray could be enhanced by the chemical and physical water repulsion of the silane groups and the roughness of the nanoparticle coating. The FTIR spectra of the pristine SiO2, hydrophobic DDS-SiO2, and the adhesive are shown in Figure S2. The main component of the adhesive spray is a polymer with long chains of hydrocarbons. The spectra of the SiO2 and DDS-SiO2 shown similar peaks at about 3360 and 1050 cm–1, which are attributed to the silanol groups and the Si–O–Si structure.48,49 The silanization of SiO2 nanoparticles by DDS yielded C–H stretching and C–H bending at 2958 and 1263 cm–1, respectively, which belonged to the methyl group of DDS. The functionalization with non-polar groups could increase the hydrophobicity of SiO2 nanoparticles. The sliding angle was estimated at 3.7 degrees, indicating the superhydrophobicity of the surface. Whereas the corresponding contact angle drastically increased to 140.0 degrees. The rough structures obtained from SiO2 nanoparticles and the low surface energy of DDS played an important role in the enhancement of the water repellency of the glass slide. The superhydrophobic results agreed well with a previous report that shown the ferroconcrete-like structure of the silanized SiO2 with adhesive.27 Thus, the proposed sliding angle measurement could be practical for a study of the water repellency in a broad range including superhydrophobic materials.
3.3. Determination of Fluid Repellency of Commercial Face Masks
The proposed sliding angle measurement was well-validated and thus applied to study the water repellency of commercial face masks including surgical and fabric masks. Since the virus outbreak worldwide, there was a shortage of surgical masks in the early days of the pandemic. Thus, a fabric mask made of different kinds of textiles was an alternative and is still popular as of yet. The face masks made of clothes are affordable, reusable, washable, and normally designed in an up-to-date style. The study of the water repellency mimicking the ability to resist a secretion droplet containing an infectious disease could be useful to indicate the efficiency of the face masks that are available in the market.
Surgical masks (SM), which are normally worn by medical staff, were collected from a local healthcare unit. Among six different SM samples (denoted by SM1 to SM5), the SM5 was selected to represent the sliding angle measurement of the outmost layer as shown in Figure 4. Since the mask was made of a synthetic fiber, an additional experimental setup was introduced to deal with a rough and hairy surface. The rough baseline could be hardly fitted to a linear equation, causing a deviation in the standard curve calculation described above. Thus, a bare flat glass slide was placed on the side of the image without being covered by the mask sample, which follows an experimental approach reported previously.50 This resulted in a straight rotated baseline as a part of the boundary lines as shown in Figure S3b. The corresponding tilt angle was perfectly fitted to a linear equation against the acquisition time, yielding an angular speed of 2.39 degrees per second. This additional method in the imaging could improve the accuracy of the sliding angle measurement of a rough surface.
Figure 4.
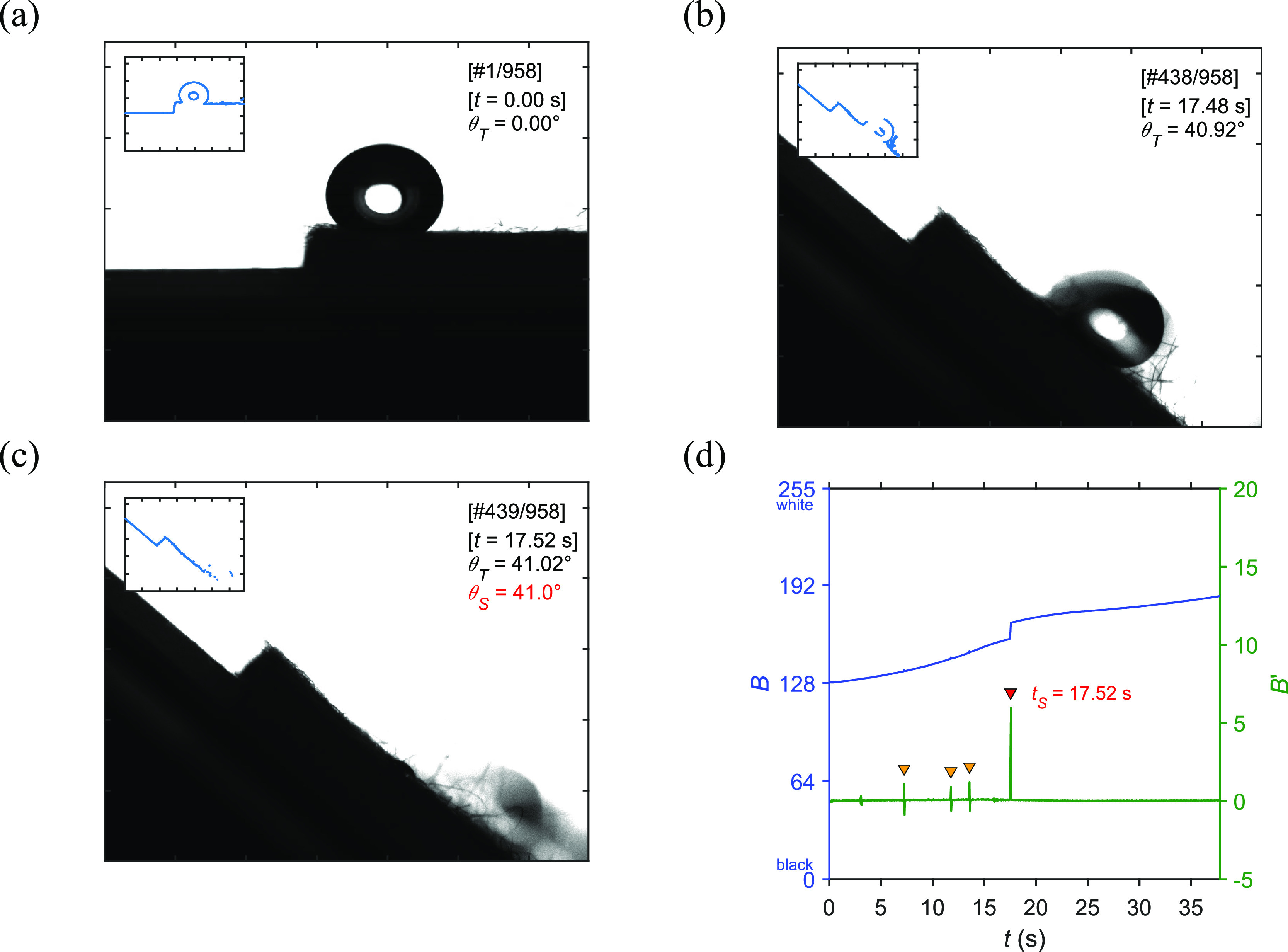
(a–c) Video image of a water drop on a surgical mask sample (SM5). (d) The average pixel brightness (B) plotted against time with its differentiation (B′).
The water drop with a volume of 30 μL, placed on the outermost layer of the SM5 sample, rapidly rolled off the surface as shown in frame numbers 438 and 439 in Figure 4b,c. The entire movie is shown in Movie S2 in the Supporting Information. The roll-off speed of the water drop was also estimated at about 109.8 mm/s, which is higher than that of the DDS-SiO2 due to the larger sliding angle affected by a higher amount of the angle sine multiplied by gravitational force. The tS of 17.52 s corresponding to frame number 439 was detected at the highest peak of the differentiation of the image brightness as shown in Figure 4d. This peak only appeared in a positive value, indicating a permanent removal of the black area of the rolling-off water drop. The resulting sliding angle of 41.0 degrees was then calculated by plugging the tS into the fitted linear equation of the stage rotation as described above. This showed a moderate water repellency of a surgical mask that could effectively protect the wearer from a respiratory droplet containing a contagious disease.
Additionally, three small peaks marked in orange color were observed in a shorter time in between approximately 6 to 14 s. In contrast to the highest peak, these waveform-like peaks showed such an equal value of both positive and negative sides. Their unique characteristics could refer to a slight shaking or a slide-and-stop movement of the water drop (see Figure S3) as shown in frame numbers 182, 295, and 340. This movement initially turned the black area of the drop to grayish or brighter and then changed it back to the original color or darker. This caused the shape of the peak that the B′ increased with a slightly brightened image area and then inversely decreased due to the dimmed image. The tiny movements were surprisingly noticed by a simple method monitoring the image brightness. It can be further studied on the distance of each jump and the hindrance that stopped the complete rolling-off movement.
The accuracy of the sliding angle measurements proposed in this work was then discussed. The rotor step size of 0.1 degrees per frame used in this work was used to determine an error that occurred in both roll-off speeds. For the slower speed of the water drop on the DDS-SiO2 surface, the drop movement started at frame number 41, whereas the image with the sudden change of the brightness was observed at frame number 43. This late estimation could yield an error of the measured sliding angle of 0.2 degrees. For the SM5 surface with a faster roll-off speed, the discrepancy of the sliding angle between the starting point and the estimated time was about 0.1 degrees. Thus, the proposed simple and rapid measurement of the sliding angle in this work was validated with an acceptable error of less than 0.2 degrees.
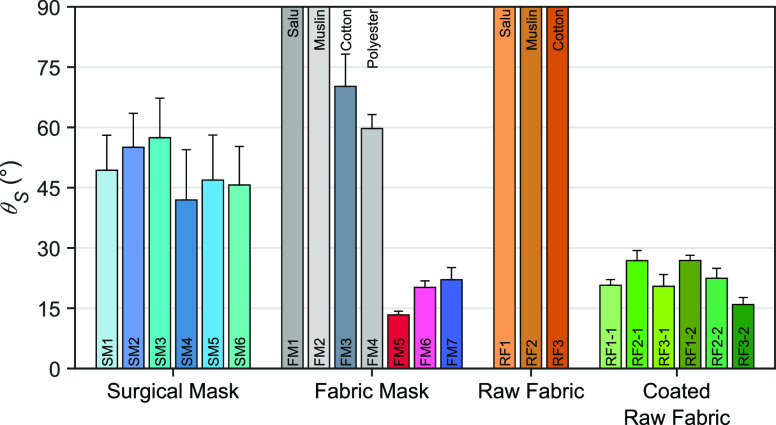
Face masks were purchased and collected including a surgical mask that was normally used by medical staff in a local healthcare unit and a fabric mask (FM) available in a local store. The sliding angle of each face mask sample with a standard deviation of the repeated measurements at 10 different sampled areas is shown in Figure 5. The corresponding contact angles are shown in Figure S4. Six different products of the surgical mask showed a moderate sliding angle of their outermost layer of 49.4 degrees on average. The SM4 was preferable with the lowest sliding angle although the contact angle of the group was indistinguishable at about 120 degrees on average. The results suggested that the SMs could effectively protect a wearer from infectious diseases via droplet transmission when the masks are worn properly.
Figure 5.
Sliding angle of face mask samples including surgical masks (SMs), fabric masks (FMs), raw fabrics (RFs), and the raw fabric coated by water-repellent sprays (−1 and −2).
Alternatively, regular face masks made of salu, muslin, cotton, and polyester fabrics were purchased from a local market as denoted by FM1 to FM4. The results showed that the FM1 and FM2 could not repel a water drop with no evaluated sliding angle. It was plotted at 90 degrees or higher with no water repellency to avoid confusion with the sliding angle of zero degrees, which indicates the highest water repellency. The water drop did not roll off the surface but was quickly adsorbed into the materials, yielding a contact angle of zero degrees. The superhydrophilicity might be due to the porosity of the fabrics. Meanwhile, the FM3 could hardly repel a water drop with a sliding angle of 70.2 degrees even though the contact angle of 120.7 degrees showed a robust hydrophobicity similar to those of the SMs. This might be caused by a strong water adhesion of the tightly woven cellulose fibers. The sliding angle of the FM4 was estimated at 59.7 degrees, showing the lowest in the group as expected. Unfortunately, the low air-permeability of the synthetic polyester likely made a wearer hard to breathe, and thus this type of fabric was not popular among users.
Water-repellent fabric masks (denoted by FM5 to FM7) were pre-coated with secret commercial repellent agents by the manufacturer. These were the most popular fabric mask due to the advertised protection efficiency even though they cost up to seven times more than the regulars. However, the average sliding angle of 18.5 degrees showed an outstanding water repellency as advertised. The sliding angle was significantly lower than those of surgical masks, but the contact angle was not different between these groups. Thus, the results suggested that the accurate sliding angle could provide such crucial information of the fluid repellency, which presents an efficiency of the face masks.
Raw and non-tailored fabrics including salu, muslin, and cotton (denoted by RF1 to RF3) had a strong water adhesion due to the hydrophilic cellulose fibers, and thus a water drop will not slide off the surface. The sliding angle was reported at 90 degrees showing no water repellent properties of these fabrics. Their contact angle was zero degrees due to the rapid adsorption indicating the perfect wetting, except the RF3 with a contact angle of 99.0 degrees. It can hold a water drop for a few seconds before the penetration likely due to the densely woven cotton fabric.
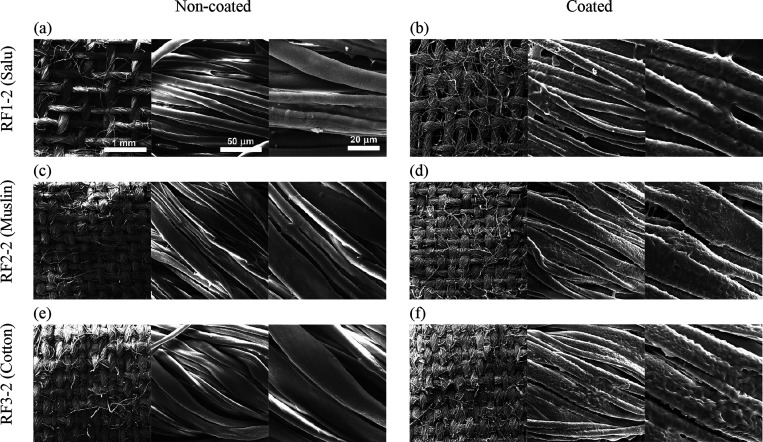
The raw fabrics were coated with a water-repellent spray to improve the water repellency of the materials. Two different water-repellent sprays were denoted by the suffixes −1 and −2 as shown in the plot. The sliding angle was drastically decreased to 22.2 degrees on average, which is similar to the pre-coated fabric masks. An average contact angle of 131.1 degrees was determined. Microscopic images by using a scanning electron microscope are shown in Figure 6. The pristine raw fabric showed a smooth surface of the fibers in a thread. The roughness of each single cellulose fiber was clearly observed after the spray coating, resulting in an improvement of the water repellency. The thickness and thread density of the fabrics analyzed from the SEM images are shown in Table S2. The RF3 was made of thick cotton fiber with an average diameter of 16.9 μm, which was woven into a thick and dense fabric with a thickness of 0.18 mm and a thread density of 100.0 percent with no visible cavity. On the other hand, the RF2 and RF1 were loosely woven with a thread density of 89.1 and 67.2 percent, respectively. The water-repellent spray tended to attach to a dense fabric with a lower cavity, and thus the RF3 yielded the highest water repellency. However, the sliding angle of the washable fabric masks and coated raw fabrics should be measured after each wash cycle to determine the durability of the masks.
Figure 6.
SEM images of non-coated and hydrophobic-coated raw fabrics: RF1-2 (a, b), RF2-2 (c, d), and RF3-2 (e, f). The same scale bar of each image set is presented in the first group.
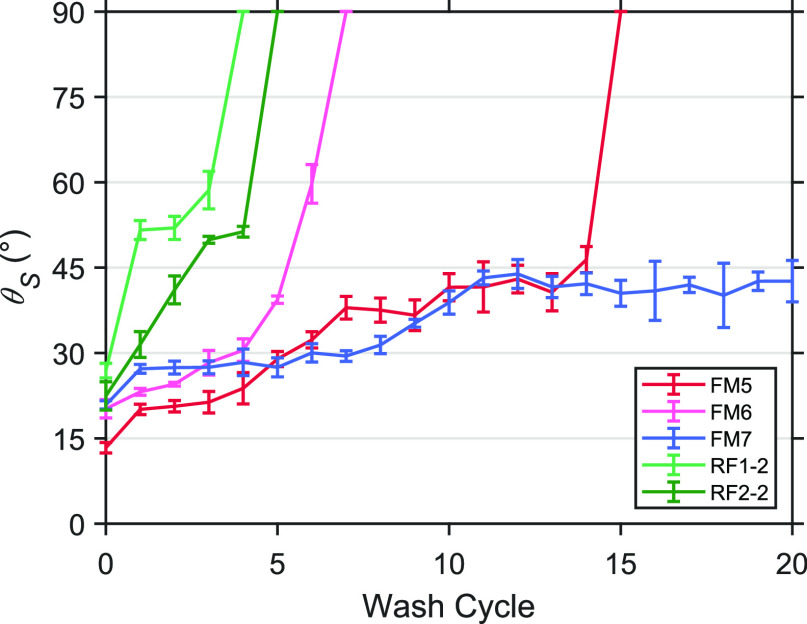
The sliding angle of the washable fabric masks and hydrophobic-coated raw fabrics measured after each wash cycle is shown in Figure 7. Only samples that maintained the water repellent characteristic after one wash cycle are shown in the plot. Among the reusable samples, the group of pre-coated fabric masks lasted the longest. The FM7 retained the sliding angle of less than 45 degrees even after 20 wash cycles, similar to those of the surgical masks. This robustness was superior as advertised due to their advanced coating agent and experienced finishing technique in the industry. The FM5 could be another promising candidate with high water repellency after about 15 wash cycles.
Figure 7.
Sliding angle measured after each wash cycle of the face masks and coated raw fabrics.
The raw fabrics made of salu and muslin coated with the water-repellent spray number 2 (RF1-2 and RF2-2) can hold a sliding angle lower than 60 degrees after about 3–4 wash cycles. The coating could be easily washed out of the densely woven cotton fabric because it accumulated only on the top layer of the surface. The washing detergent could easily penetrate into a loose salu fabric, washing the coating agent off the thread. This result suggested that the raw fabric made of muslin could be cut and sewed into a fabric face mask and coated by a water-repellent spray to improve the repellency. The coating can be repeatedly sprayed on the outermost layer of the fabric mask after a few uses. The coated fabric mask was significantly water-repellent, affordable, reusable, washable, durable, and eco-friendly, and thus it could be a feasible alternative to a surgical mask during the pandemic. More importantly, the precise sliding angle measured by a simple method with written image-analysis scripts has presented important information on the fluid repellency along with the typical contact angle of the materials. In addition, the method involving an image brightness could be further applied to study the sliding distance, the rolling-off speed, and the contact angle hysteresis to provide a better understanding of water repellent dynamics by using a simple technique.
4. Conclusions
The water sliding angle of a hydrophobic surface was measured by the proposed experimental drop shape analysis with custom computational algorithms. A consecutive video image was being recorded while the sample stage was rotated to 90 degrees. The image brightness averaging over an 8-bit grayscale of each pixel was monitored to locate a sliding time with a sudden increase of the brightness due to the depletion of the dark pixels of the drop. The tilt angle of the rotating stage was calculated by the tangent method related to the original baseline, which was in a linear relationship with the acquisition time. The water sliding angle defined by the tilt angle at the sliding time was then precisely estimated via the standard curve. It was found that an error of the resulting sliding angles was less than 0.2 degrees. Moreover, a slight drop movement including an immobilized shaking and a short-distance slipping was detected. It was then neglected in the calculations to avoid an underestimate of the sliding angle. In addition, an error caused by the latency of the operating devices was suppressed by neglecting an excessive recording at the lagged time of the rotor and the waiting period of the camera.
An effect of the water drop volume on the sliding angle was studied by using a superhydrophobic glass slide coated by either silanized silicon dioxide or polytetrafluoroethylene. The results showed that the sliding angle decreased with a larger drop due to the gravity as expected, and thus a volume of 30 μL was used in the following experiments. An angular speed of the rotor at 0.1 and 0.5 degrees per second was performed to optimize the rotation resolution. The slower speed allowed the camera to timely detect the starting point of a water drop motion before sliding off the surface, avoiding an overestimation of the sliding angle due to the retardation.
The fluid repellency of face masks including surgical masks, fabric masks, and raw fabric was determined by using the proposed method. It was found that the sampled surgical masks showed a moderate sliding angle of their outermost layer of 49.4 degrees on average, while the pre-coated fabric masks exhibited such outstanding efficiency with a sliding angle of 18.5 degrees. The pricey commercial hydrophobic fabric masks maintained high water repellency after about 20 wash cycles as advertised. However, a washable self-coated muslin fabric could be a promising alternative to the surgical mask during the pandemic due to its useful fluid repellency after a couple of wash cycles. It was affordable, reusable, durable, eco-friendly, and also breathable due to the loose weave. The resulting precise sliding angle measured in this work brought a thorough discussion on the fluid repellency of different materials, which could not be differentiated by solely considering the typical contact angle. The proposed straightforward analysis method could be an alternative measurement of the sliding angle with fast and reliable calculations compared to conventional optical-based methods. Thus, it could be used for image processing obtained by a simple imaging apparatus such as a typical digital camcorder or even a cellphone camera to have a better understanding of the water repellency of materials.
Acknowledgments
The authors gratefully acknowledged the use of services, facilities, and research grants from the Department of Chemistry, Faculty of Science, Burapha University. We also thank Ms. Prakaiwan Kotchapun for her assistance in our laboratory. We appreciated the help from the faculty of the Department of Nursing and the medical staff of the Burapha hospital. This research was supported by a basic research fund from the Thailand Science Research and Innovation (grant numbers 1499754/2021 and 2472757/2022).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00628.
Brightness analysis at a late time, sliding angle of a glass slide coated by DDS-SiO2 and PTFE with different angular speeds, FTIR, video images of a water drop on a surgical mask sample, fabric characteristics, and contact angle of the mask and fabric samples (PDF)
Video of a water drop sliding off a surface coated with DDS-SiO2 (MP4)
Video of a water drop sliding off the selected surgical mask (MP4)
The authors declare no competing financial interest.
Supplementary Material
References
- Good R. J. Contact Angle, Wetting and Adhesion: a critical review. J . Adhes. Sci. technol. 1992, 6, 1269–1302. 10.1163/156856192X00629. [DOI] [Google Scholar]
- Kwok D. Y.; Neumann A. W.. Contact Angle Measurement and Contact Angle Interpretation; 1999; Vol. 81, 10.1016/S0001-8686(98)00087-6. [DOI] [Google Scholar]
- Huhtamäki T.; Tian X.; Korhonen J. T.; Ras R. H. A. Surface-Wetting Characterization Using Contact-Angle Measurements. Nat. Protoc. 2018, 13, 1521–1538. 10.1038/s41596-018-0003-z. [DOI] [PubMed] [Google Scholar]
- Kozbial A.; Li Z.; Conaway C.; McGinley R.; Dhingra S.; Vahdat V.; Zhou F.; D’Urso B.; Liu H.; Li L. Study on the Surface Energy of Graphene by Contact Angle Measurements. Langmuir 2014, 30, 8598–8606. 10.1021/la5018328. [DOI] [PubMed] [Google Scholar]
- Busscher H. J.; van Pelt A. W. J.; de Boer P.; de Jong H. P.; Arends J. The Effect of Surface Roughening of Polymers on Measured Contact Angles of Liquids. Colloids Surf. 1984, 9, 319–331. 10.1016/0166-6622(84)80175-4. [DOI] [Google Scholar]
- Wang J.; Wu Y.; Cao Y.; Li G.; Liao Y. Influence of Surface Roughness on Contact Angle Hysteresis and Spreading Work. Colloid Polym. Sci. 2020, 298, 1107–1112. 10.1007/s00396-020-04680-x. [DOI] [Google Scholar]
- Adachi T.; Latthe S. S.; Gosavi S. W.; Roy N.; Suzuki N.; Ikari H.; Kato K.; Katsumata K. i.; Nakata K.; Furudate M.; Inoue T.; Kondo T.; Yuasa M.; Fujishima A.; Terashima C. Photocatalytic, Superhydrophilic, Self-Cleaning TiO 2 Coating on Cheap, Light-Weight, Flexible Polycarbonate Substrates. Appl. Surf. Sci. 2018, 458, 917–923. 10.1016/j.apsusc.2018.07.172. [DOI] [Google Scholar]
- Zhang J.; Li J.; Han Y. Superhydrophobic PTFE Surfaces by Extension. Macromol. Rapid Commun. 2004, 25, 1105–1108. 10.1002/marc.200400065. [DOI] [Google Scholar]
- Bhattacharjee S.; Macintyre C. R.; Wen X.; Bahl P.; Kumar U.; Chughtai A. A.; Joshi R. Nanoparticles Incorporated Graphene-Based Durable Cotton Fabrics. Carbon 2020, 166, 148–163. 10.1016/j.carbon.2020.05.029. [DOI] [Google Scholar]
- Jagdheesh R.; Diaz M.; Marimuthu S.; Ocana J. L. Robust Fabrication of μ-Patterns with Tunable and Durable Wetting Properties: Hydrophilic to Ultrahydrophobic via a Vacuum Process. J. Mater. Chem. A 2017, 5, 7125–7136. 10.1039/c7ta01385j. [DOI] [Google Scholar]
- Ali A. A.; Haidar A.; Polonskyi O.; Faupel F.; Abdul-Khaliq H.; Veith M.; Aktas O. C. Extreme Tuning of Wetting on 1D Nanostructures: From a Superhydrophilic to a Perfect Hydrophobic Surface. Nanoscale 2017, 9, 14814–14819. 10.1039/c7nr05336c. [DOI] [PubMed] [Google Scholar]
- Murase H.; Fujibayashi T. Characterization of Molecular Interfaces in Hydrophobic Systems. Prog. Org. Coat. 1997, 31, 97–104. 10.1016/S0300-9440(97)00023-4. [DOI] [Google Scholar]
- Zeng X.; Qian L.; Yuan X.; Zhou C.; Li Z.; Cheng J.; Xu S.; Wang S.; Pi P.; Wen X. Inspired by Stenocara Beetles: From Water Collection to High-Efficiency Water-in-Oil Emulsion Separation. ACS Nano 2017, 11, 760–769. 10.1021/acsnano.6b07182. [DOI] [PubMed] [Google Scholar]
- Yang X.; Liu X.; Lu Y.; Zhou S.; Gao M.; Song J.; Xu W. Controlling the Adhesion of Superhydrophobic Surfaces Using Electrolyte Jet Machining Techniques. Sci. Rep. 2016, 6, 1–9. 10.1038/srep23985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao L.; McCarthy T. J. Contact Angle Hysteresis Explained. Langmuir 2006, 22, 6234–6237. 10.1021/la060254j. [DOI] [PubMed] [Google Scholar]
- Miwa M.; Nakajima A.; Fujishima A.; Hashimoto K.; Watanabe T. Effects of the Surface Roughness on Sliding Angles of Water Droplets on Superhydrophobic Surfaces. Langmuir 2000, 16, 5754–5760. 10.1021/la991660o. [DOI] [Google Scholar]
- Yoshimitsu Z.; Nakajima A.; Watanabe T.; Hashimoto K. Effects of Surface Structure on the Hydrophobicity and Sliding Behavior of Water Droplets. Langmuir 2002, 18, 5818–5822. 10.1021/la020088p. [DOI] [Google Scholar]
- Ding Y.; Jia L.; Peng Q.; Guo J. Critical Sliding Angle of Water Droplet on Parallel Hydrophobic Grooved Surface. Colloids Surf., A 2020, 2019, 124083 10.1016/j.colsurfa.2019.124083. [DOI] [Google Scholar]
- Zhou C.; Chen Z.; Yang H.; Hou K.; Zeng X.; Zheng Y.; Cheng J. Nature-Inspired Strategy toward Superhydrophobic Fabrics for Versatile Oil/Water Separation. ACS Appl. Mater. Interfaces 2017, 9, 9184–9194. 10.1021/acsami.7b00412. [DOI] [PubMed] [Google Scholar]
- Zhu J.; Liu B.; Li L.; Zeng Z.; Zhao W.; Wang G.; Guan X. Simple and Green Fabrication of a Superhydrophobic Surface by One-Step Immersion for Continuous Oil/Water Separation. J. Phys. Chem. A 2016, 120, 5617–5623. 10.1021/acs.jpca.6b06146. [DOI] [PubMed] [Google Scholar]
- Hu P.; Xie Q.; Ma C.; Zhang G. Silicone-Based Fouling-Release Coatings for Marine Antifouling. Langmuir 2020, 36, 2170–2183. 10.1021/acs.langmuir.9b03926. [DOI] [PubMed] [Google Scholar]
- Banerjee I.; Pangule R. C.; Kane R. S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- Lu Y.; Sathasivam S.; Song J.; Crick C. R.; Carmalt C. J.; Parkin I. P. Robust Self-Cleaning Surfaces That Function When Exposed to Either Air or Oil. Science 2015, 347, 1132–1135. 10.1126/science.aaa0946. [DOI] [PubMed] [Google Scholar]
- Niu J. J.; Wang J. N. A Novel Self-Cleaning Coating with Silicon Carbide Nanowires. J. Phys. Chem. B 2009, 113, 2909–2912. 10.1021/jp808322e. [DOI] [PubMed] [Google Scholar]
- Chen B.; Qiu J.; Sakai E.; Kanazawa N.; Liang R.; Feng H. Robust and Superhydrophobic Surface Modification by a “Paint + Adhesive” Method: Applications in Self-Cleaning after Oil Contamination and Oil-Water Separation. ACS Appl. Mater. Interfaces 2016, 8, 17659–17667. 10.1021/acsami.6b04108. [DOI] [PubMed] [Google Scholar]
- Tong W.; Xiong D.; Wang N.; Yan C.; Tian T. Green and Timesaving Fabrication of a Superhydrophobic Surface and Its Application to Anti-Icing, Self-Cleaning and Oil-Water Separation. Surf. Coat. Technol. 2018, 352, 609–618. 10.1016/j.surfcoat.2018.08.035. [DOI] [Google Scholar]
- Chen C.; Weng D.; Chen S.; Mahmood A.; Wang J. Development of Durable, Fluorine-Free, and Transparent Superhydrophobic Surfaces for Oil/Water Separation. ACS Omega 2019, 4, 6947–6954. 10.1021/acsomega.9b00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D. H.; Jeong J.; Han S. W.; Kang D. P. Superhydrophobic Surfaces with Near-Zero Sliding Angles Realized from Solvent Relative Permittivity Mediated Silica Nanoparticle Aggregation. J. Mater. Chem. A 2014, 2, 17165–17173. 10.1039/c4ta03198a. [DOI] [Google Scholar]
- Murase H.; Nanishi K.; Kogure H.; Fujibayashi T.; Tamura K.; Haruta N. Interactions between Heterogeneous Surfaces of Polymers and Water. J. Appl. Polym. Sci. 1994, 54, 2051–2062. 10.1002/app.1994.070541307. [DOI] [Google Scholar]
- Pierce E.; Carmona F. J.; Amirfazli A. Understanding of Sliding and Contact Angle Results in Tilted Plate Experiments. Colloids Surf., A 2008, 323, 73–82. 10.1016/j.colsurfa.2007.09.032. [DOI] [Google Scholar]
- Morita K.; Sakaue H. Characterization Method of Hydrophobic Anti-Icing Coatings. Rev. Sci. Instrum. 2015, 86, 115108. 10.1063/1.4935585. [DOI] [PubMed] [Google Scholar]
- Thomsen F.; Winkler T.. Roll-Off of Sliding Angle and Dynamic Contact Angles. In Kruss Technical Note TN317e; 2012; pp. 1–4.
- Liu K.; Vuckovac M.; Latikka M.; Ras R. H. A. Improving surface-wetting characterization. Science 2019, 363, 1147–1148. 10.1126/science.aav5388. [DOI] [PubMed] [Google Scholar]
- Beitollahpoor M.; Farzam M.; Pesika N. S. Determination of the Sliding Angle of Water Drops on Surfaces from Friction Force Measurements. Langmuir 2022, 38, 2132–2136. 10.1021/acs.langmuir.1c03206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C. W.; Tang S.; Sebastian D.; Tadmor R. Sliding of Water Droplets on Micropillar-Structured Superhydrophobic Surfaces. Appl. Surf. Sci. 2020, 504, 144493 10.1016/j.apsusc.2019.144493. [DOI] [Google Scholar]
- Greenhalgh T.; Schmid M. B.; Czypionka T.; Bassler D.; Gruer L. Face Masks for the Public during the Covid-19 Crisis. BMJ 2020, 369, 1–4. 10.1136/bmj.m1435. [DOI] [PubMed] [Google Scholar]
- Cheng K. K.; Lam T. H.; Leung C. C. Wearing Face Masks in the Community during the COVID-19 Pandemic: Altruism and Solidarity. Lancet 2020, 2020, 2019–2020. 10.1016/S0140-6736(20)30918-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikenberry S. E.; Mancuso M.; Iboi E.; Phan T.; Eikenberry K.; Kuang Y.; Kostelich E.; Gumel A. B. To Mask or Not to Mask: Modeling the Potential for Face Mask Use by the General Public to Curtail the COVID-19 Pandemic. Infect. Dis. Model. 2020, 5, 293–308. 10.1016/j.idm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragaw T. A. Surgical Face Masks as a Potential Source for Microplastic Pollution in the COVID-19 Scenario. Mar. Pollut. Bull. 2020, 159, 111517. 10.1016/j.marpolbul.2020.111517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliu F.; Veronelli M.; Raguso C.; Barana D.; Galli P.; Lasagni M. The Release Process of Microfibers: From Surgical Face Masks into the Marine Environment. Environ. Adv. 2021, 4, 100042. 10.1016/j.envadv.2021.100042. [DOI] [Google Scholar]
- Wang D.; You Y.; Zhou X.; Zong Z.; Huang H.; Zhang H.; Yong X.; Cheng Y.; Yang L.; Guo Q.; Long Y.; Liu Y.; Huang J.; Du L. Selection of Homemade Mask Materials for Preventing Transmission of COVID-19: A Laboratory Study. PLoS One 2020, 15, 1–13. 10.1371/journal.pone.0240285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S.; Bahl P.; Chughtai A. A.; MacIntyre C. R. Last-Resort Strategies during Mask Shortages: Optimal Design Features of Cloth Masks and Decontamination of Disposable Masks during the COVID-19 Pandemic. BMJ Open Respir. Res. 2020, 7, 1–10. 10.1136/bmjresp-2020-000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee S.; Bahl P.; De Silva C.; Doolan C.; Chughtai A. A.; Heslop D.; Macintyre C. R. Experimental Evidence for the Optimal Design of a High-Performing Cloth Mask. ACS Biomater. Sci. Eng. 2021, 7, 2791–2802. 10.1021/acsbiomaterials.1c00368. [DOI] [PubMed] [Google Scholar]
- Talic S.; Shah S.; Wild H.; Gasevic D.; Maharaj A.; Ademi Z.; Li X.; Xu W.; Mesa-Eguiagaray I.; Rostron J.; Theodoratou E.; Zhang X.; Motee A.; Liew D.; Ilic D. Effectiveness of Public Health Measures in Reducing the Incidence of Covid-19, SARS-CoV-2 Transmission, and Covid-19 Mortality: Systematic Review and Meta-Analysis. BMJ. 2021, e068302. 10.1136/bmj-2021-068302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong H.; Zhu Z.; Lin J.; Cheung C. F.; Lu V. L.; Yan F.; Chan C. Y.; Li G. Reusable and Recyclable Graphene Masks with Outstanding Superhydrophobic and Photothermal Performances. ACS Nano 2020, 14, 6213–6221. 10.1021/acsnano.0c02250. [DOI] [PubMed] [Google Scholar]
- Li W.; Wang Y.; Tang X.; Yuen T. T. T.; Han X.; Li J.; Huang N.; Chan J. F. W.; Chu H.; Wang L. Liquid Repellency Enabled Antipathogen Coatings. Mater. Today Bio 2021, 12, 100145 10.1016/j.mtbio.2021.100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo-Pino A.; Krissian K.; Alemán-Flores M.; Santana-Cedrés D. Accurate Subpixel Edge Location Based on Partial Area Effect. Image Vis. Comput. 2013, 31, 72–90. 10.1016/j.imavis.2012.10.005. [DOI] [Google Scholar]
- Zulfiqar U.; Hussain S. Z.; Awais M.; Khan M. M. J.; Hussain I.; Husain S. W.; Subhani T. In-Situ Synthesis of Bi-Modal Hydrophobic Silica Nanoparticles for Oil-Water Separation. Colloids Surf. A 2016, 508, 301–308. 10.1016/j.colsurfa.2016.08.074. [DOI] [Google Scholar]
- Khan S. A.; Zulfiqar U.; Hussain S. Z.; Zaheer U.; Hussain I.; Husain S. W.; Subhani T. Fabrication of Superhydrophobic Filter Paper and Foam for Oil–Water Separation Based on Silica Nanoparticles from Sodium Silicate. J. Sol-Gel Sci. Technol. 2017, 81, 912–920. 10.1007/s10971-016-4250-6. [DOI] [Google Scholar]
- Nongnual T.; Damnong N.; Srimongkol S.; Phanrangsee S.; Benjalersyarnon T. An Experimental Approach to Image the Rotated Straight Baseline for the Water Sliding Angle Measurement of a Rough Surface. Thai J. Math. 2021, 19, 793–803. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.