Abstract
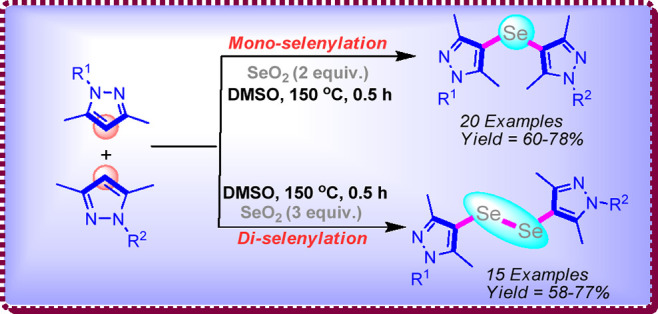
The insertion of selenium was achieved in the form of mono-selenides and di-selenides for the preparation of novel bis-heterocyclic compounds. This method is more general and provides scaffold diversity with high yields of products. The concentration-dependent mono- and di-selenylation reaction selectivity was achieved using SeO2 as an efficient selenylating reagent.
Introduction
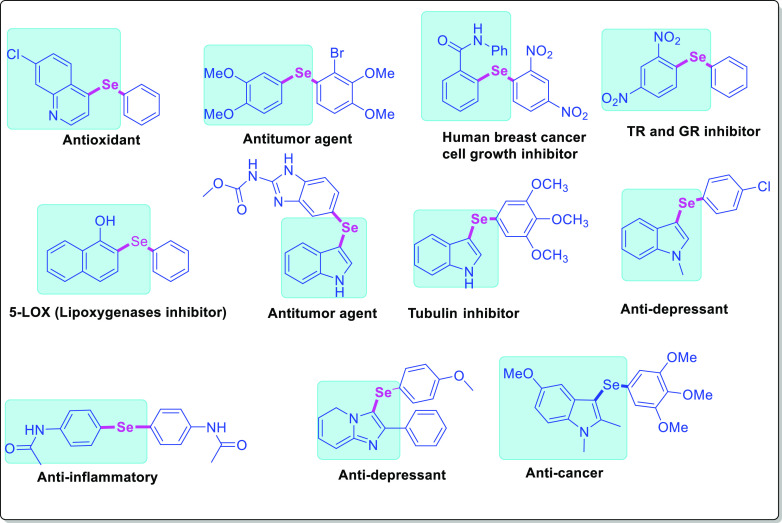
Organoselenium chemistry is a well-established field due to the applicability of selenium compounds as versatile tools in organic synthesis,1 catalysis,2 as well as privileged scaffolds in medicinal chemistry for bioactive molecules (Figure 1)3 such as ebselen used for ischemic stroke,4 hearing loss,5 and recently used for bipolar disorder.6 Elemental selenium has an important role in physiological functions and several dangerous viral infections (H1N1 influenza, SARS, HIV/AIDS, Ebola, etc.) are associated with selenium deficiency and outbreaks of them have originated either in bio-geo-chemically selenium-poor regions of China or selenium nutrient-depleted sub-Saharan Africa.7
Figure 1.
Biologically important aryl/heteroaryl selenide-based compounds with a wide range of activities.
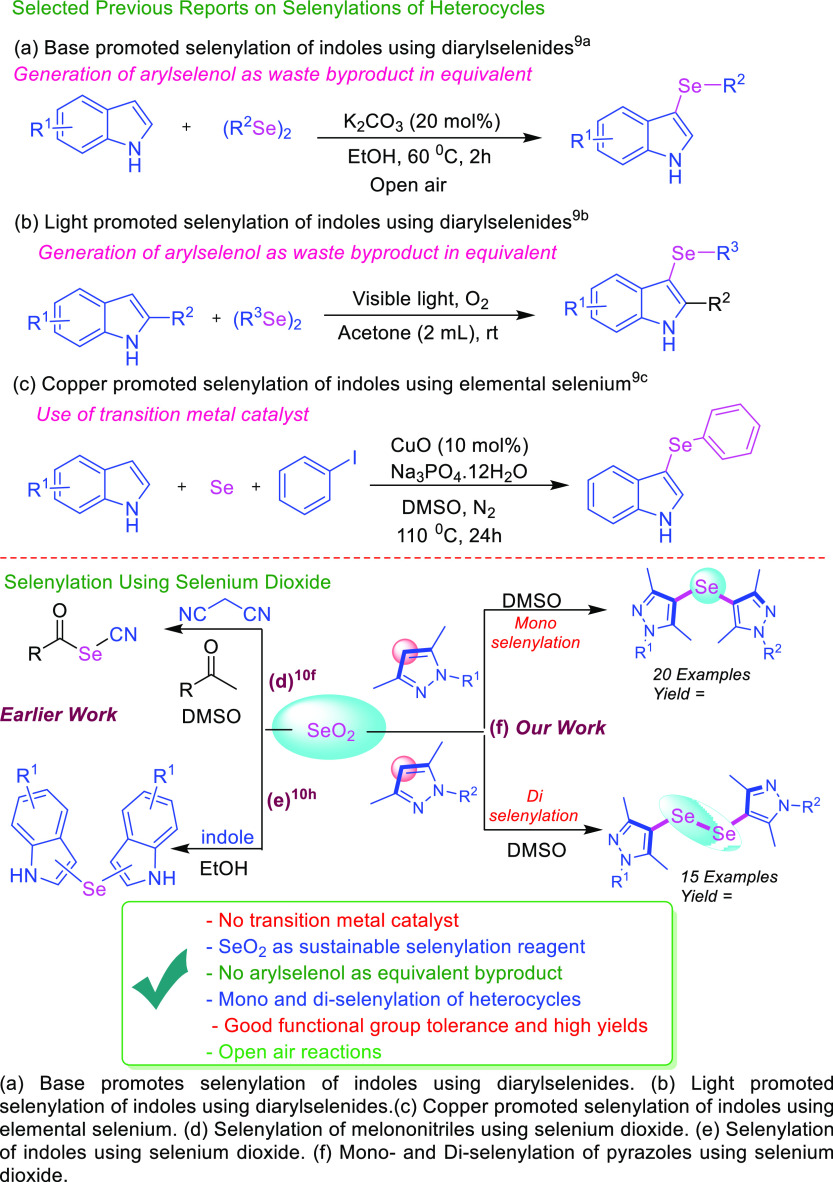
Organoselenium compounds are effective precursors in organic chemistry avoiding the protection/deprotection protocols.8 The construction of C–Se–C or C–Se–Se–C is mostly achieved by cross coupling of aryl boronic acids/aryl halides using selenium sources with transition metal catalysts, however, mostly limited to aryl substrates.9 The literature review about the selenylating reagents revealed the drawbacks of specified reaction protocols due to the instability in air and moisture, multiple preparation steps, various reactive side products, toxic waste in equivalent amount and many others. To overcome some of these serious drawbacks of earlier used selenylating reagents, SeO2 has been emerged as effective and stable alternative for various selenylation reactions.10 The installation of selenium11 in the heterocyclic scaffolds is an interesting and challenging task and further provides the opportunity for constructing bis-type of heterocyclic mono-selenide and di-selenide motifs.
Di-selenides resemble with the organic peroxides, although slightly more stable than peroxides, however, reactive enough to participate in electrophilic, nucleophilic, and radical processes. Pyrazoles12 are the privileged biorelevant scaffolds in organic synthesis due to their potential in the drug discovery process. Bis-pyrazoles13 incorporated with selenium offer more advantageous features to the molecules in terms of their biological applicability. Hence, developments of sustainable approaches for the construction of selenylated heterocyclic scaffolds are of high interest for chemists. In our recent work for the construction of bis-pyrazoles, the methylene (−CH2−) moiety was incorporated between two pyrazoles using the oxone/dimethyl sulfoxide (DMSO) system.14 Now, in continuation to this work on pyrazole scaffolds14,15 and understanding the biological importance of selenium,3−7 we directed our thought to incorporate the selenium between two pyrazole molecules in order to construct the selenylated bis-heterocycles (pyrazoles). Herein, we disclose a very simple protocol for the mono-selenylation and di-selenylation of pyrazoles for the construction of selenylated bis-pyrazoles using SeO2 as the selenylating reagent (Scheme 1).
Scheme 1. State of the Art on Selenylation Reactions.
Results and Discussion
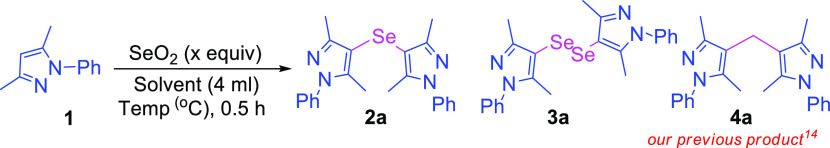
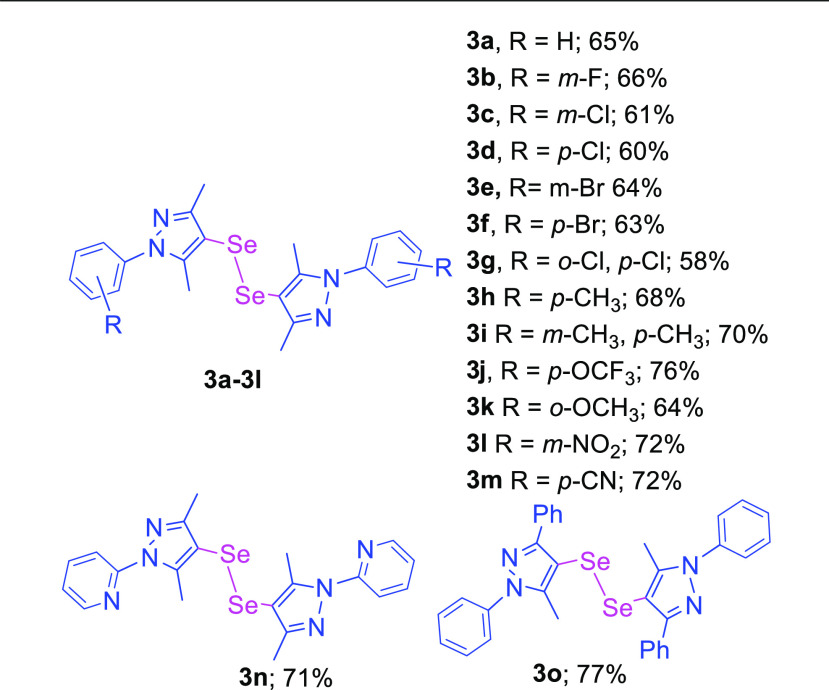
The optimization studies were initiated for the activation of pyrazoles for the synthesis of bis-pyrazole compounds using 3,5-dimethyl-1-phenyl-1H-pyrazole as the model substrate and SeO2 as the source of selenium, DMSO as the reaction solvent at 150 °C temperature, and systematically tested the required quantity of SeO2, reaction temperature, and solvents (Table 1). Initially, the quantity of SeO2 was optimized with the model reaction. While optimizing the quantities, 0.5 and 1.0 equiv of SeO2 afforded the mono-selenylated bis-pyrazole product 2a in 62 and 41 % yields, respectively (entries 1 and 2). On increasing the quantity of SeO2 to 1.5 equiv gave mono-selenylated bis-pyrazole (2a) as well as di-selenylated bis-pyrazole (3a) products with almost equal yields (entry 3). Furthermore, increasing the quantity of SeO2 to 2.0 equiv afforded the product 2a with major yield (71%) as compared to the 3a (9%) as minor (entry 4). On further increasing the quantity of SeO2 to 2.5 and 3.0 equiv provided di-selenylated product 3a as the major product and mono-selenylated product 2a as the minor product (entries 5 and 6). Hence, 2.0 equiv quantity of SeO2 for mono-selenylated product 2a and 3.0 equiv for di-selenylated product 3a were taken for the further optimization studies. The screening of different solvents was also carried out with model substrate 1. dimethylformamide (DMF), MeOH, 1,4-dioxane, and HCHO were found to be ineffective for the current reaction and afforded the desired products (2a or 3a) with low yields (entries 7–10). However, moderate yields of 2a were observed with ACN and DCM (entries 11 and 12). We also optimized the reaction temperature for the current model reaction. The reaction did not give the desired product at room temperature and at 50 °C (entries 13–16) as well. On increasing the temperature to 80 and 100 °C gave mono-selenylated product 2a only in low yields (entries 17–20). Raising the reaction temperature to 120 °C gave the mono-selenylated product 2a as the major (66%) product and di-selenylated product 3a as the minor (28%) product with 2 equiv of SeO2; however, with 3.0 equiv of SeO2, 2a was observed as the minor (37%) product and 3a as the major (57%) product (entries 21 and 22).
Table 1. Optimization Studiesa.
| entry | equiv SeO2 | solvent | temp. (°C) | yield 2ab (%) | yield 3ab (%) | yield 4ab (%) |
|---|---|---|---|---|---|---|
| 1 | 0.5 | DMSO | 150 | 62 | 8 | 0 |
| 2 | 1.0 | DMSO | 150 | 41 | 15 | 0 |
| 3 | 1.5 | DMSO | 150 | 51 | 48 | 0 |
| 4 | 2.0 | DMSO | 150 | 85 (71)c | 9 | 5 |
| 5 | 2.5 | DMSO | 150 | 15 | 35 | 12 |
| 6 | 3.0 | DMSO | 150 | 30 | 65 | 5 |
| 7 | 2.0 | DMF | 150 | 3 | 0 | 0 |
| 8 | 2.0 | MeOH | reflux | 1 | 0 | 0 |
| 9 | 2.0 | 1,4-dioxane | 100 | 19 | 1 | 0 |
| 10 | 2.0 | formalin | 150 | 0 | 0 | 40 |
| 11 | 2.0 | ACN | reflux | 67 | 0 | 0 |
| 12 | 2.0 | DCM | reflux | 50 | 0 | 0 |
| 13 | 2.0 | DMSO | rt | 0 | 0 | 0 |
| 14 | 3.0 | DMSO | rt | 0 | 0 | 0 |
| 15 | 2.0 | DMSO | 50 | 0 | 0 | 0 |
| 16 | 3.0 | DMSO | 50 | 0 | 0 | 0 |
| 17 | 2.0 | DMSO | 80 | 26 | 0 | 0 |
| 18 | 3.0 | DMSO | 80 | 26 | 0 | 0 |
| 19 | 2.0 | DMSO | 100 | 28 | <1 | <1 |
| 20 | 3.0 | DMSO | 100 | 33 | <1 | <1 |
| 21 | 2.0 | DMSO | 120 | 67 | 28 | 4 |
| 22 | 3.0 | DMSO | 120 | 37 | 57 | 5 |
Reaction conditions: 1 (1 mmol) and SeO2 (x equiv) in solvent (4 mL) were stirred upto 0.5 h.
LC–MS-based concentrations.
Isolated yield.
Hence, 2 equiv SeO2 with DMSO solvent at 150 °C for desired product 2a and 3.0 equiv SeO2 with DMSO solvent at 150 °C for product 3a were taken as optimized conditions for further study.
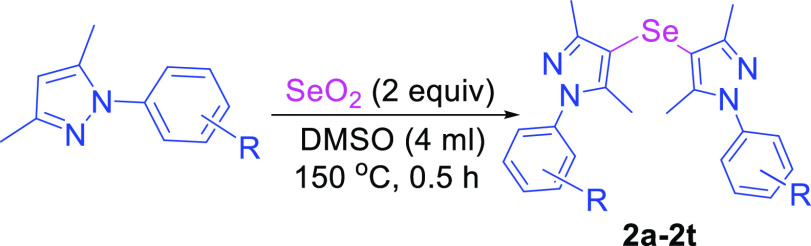
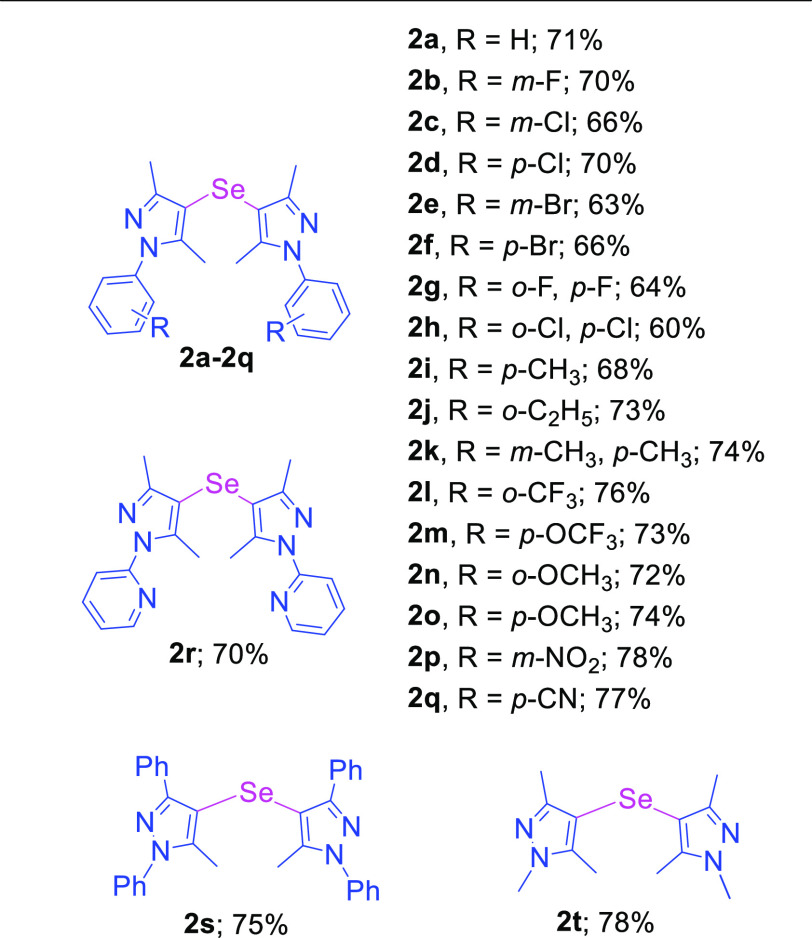
With the optimized reaction conditions in hand, we explored the generality of the developed method using various pyrazole substrates. In order to synthesize mono-selenylated bis-pyrazoles, various phenyl ring-substituted pyrazole compounds were investigated with SeO2 under the optimized conditions (Table 2). Simple phenyl ring derivative of pyrazole along with mono- and di-halogen atom bearing pyrazoles were tolerated under the developed method without affecting the different positions (o-, m-, and p-) of halogens on the phenyl ring to provide the corresponding mono-selenylated bis-pyrazoles in good yields (2a–2h).
Table 2. Synthesis of Mono-Selenylated Bis-Pyrazolesa,b.
Reaction conditions: 1 (1 mmol), SeO2 (2 equiv) were stirred in DMSO (4 mL) at 150 °C for 0.5 h.
Isolated yields.
The reaction of methyl-, ethyl-, and dimethyl-substituted pyrazoles provides the corresponding mono-selenylated bis-pyrazole products in high yields (2i–2k). The reaction of pyrazoles bearing pharmaceutically important16 trifluoromethyl and trifluoromethoxy groups also underwent the formation of corresponding mono-selenylated bis-pyrazoles in good yields (2l and 2m). The reaction of o- and p-methoxy-substituted pyrazoles proceeded in good yields (2n and 2o). The reaction of m-nitro- and p-cyano-substituted compounds afforded the corresponding selenylated products in good yields (2p and 2q). The reaction of pyrazole containing 2-pyridinyl ring also provided the targeted product in high yield (2r). The reaction of the 5-methyl-1,3-diphenyl-1H-pyrazole substrate also provided the corresponding mono-selenylated bis-pyrazole in high yield (2s). The reaction of the 1,3,5-trimethyl-1H-pyrazole moiety also proceeded well and provide the desired product in good yield (2t).
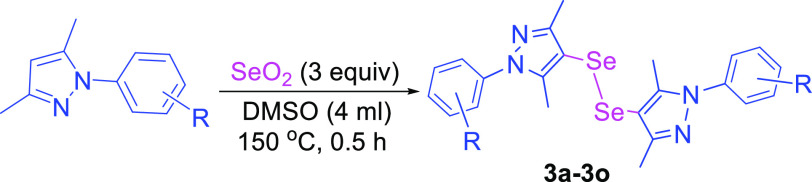
In order to further expand the scope of the developed method for the synthesis of di-selenylated bis-pyrazoles, reactions were performed with 3.0 equiv of SeO2 (Table 3). The reactions of the unsubstituted phenyl ring bearing pyrazole and other mono- and di-halogen-substituted (-F, -Cl, and -Br) pyrazoles provided the corresponding di-selenylated bis-pyrazoles in high yields (3a–3g). Methyl and dimethyl group substitution in the phenyl ring of pyrazole compounds did not affect the reaction yield and also provide the corresponding di-selenylated bis-pyrazoles products in a major quantity (3h and 3i). Reaction of the trifluoromethoxy-substituted pyrazole substrate also gave the desired products in high yields (3j). Reaction of o-methoxy-, m-nitro-, and p-cyano-substituted pyrazoles also afforded the corresponding di-selenylated bis-pyrazoles as the major products in high yields (3k–3m). The reaction of pyrazole having the 2-pyridinyl group instead of the phenyl ring also afforded the corresponding di-selenylated product in high yield (3n). The reaction of 5-methyl-1,3-diphenyl-1H-pyrazole substrate provides the corresponding di-selenylated product in high yield (3o).
Table 3. Synthesis of Di-Selenylated Bis-Pyrazolesa,b.
Reaction conditions: 1 (1 mmol) and SeO2 (3 equiv) were stirred in DMSO (4 mL) at 150 °C for 0.5 h.
Isolated yields.
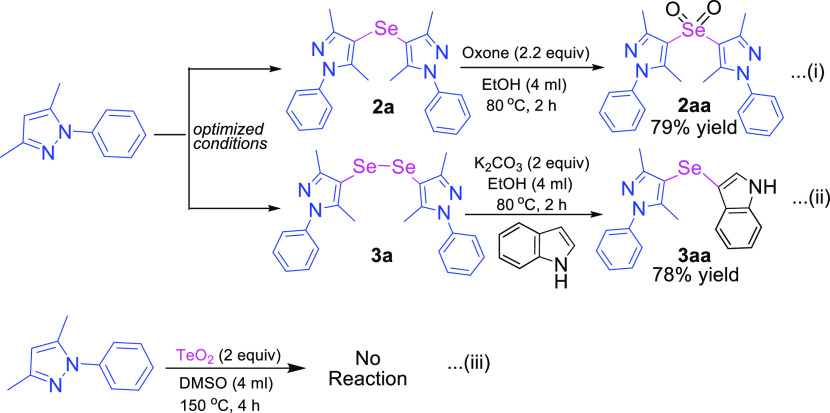
To further expand the scope and applicability of these products, mono- and di-selenylated bis-pyrazoles, we carried out some extension reactions using products 2a and 3a (Scheme 2). The oxidation of 2a afforded the corresponding dioxide product 2aa with oxone (2.2 equiv) in EtOH at 80 °C [Scheme 2, eq (i)]. As di-selenides are the important precursors in organic transformation, herein, we carried out the reaction of di-selenide bis-pyrazole (3a) with indole as the cross coupling substrate in the presence of a base in EtOH and successfully afforded the unsymmetric di-selenylated bis-pyrazole (3aa) in good yield [Scheme 2, eq (ii)]. Furthermore, the incorporation of tellurium (on reacting with TeO2) was not possible under the current developed method [Scheme 2, eq (iii)]. The current methods were not applicable for the direct synthesis of unsymmetrical heterocycles as very low conversions were observed for the reaction of the equimolar mixture of two different heterocycles (Schemes S1 and S2).
Scheme 2. Further Exploration of Products 2a and 3a.
The mechanism of the reaction is still unclear in the literature. Wilshire’s assumption17 suggested the possibilities for the formation of triselenide intermediate A during the formation of diaryl selenides 2a. Based on the existing reports for selenium incorporation,10,17 we proposed a hypothesis (Scheme 3) that SeO2 on reaction with pyrazole 1 forms the mono-selenylated bis-pyrazole product 2a via the triselenide intermediate A. We also proposed the possibility for the formation of intermediate B from the reaction of pyrazole 1 with SeO2 in the presence of DMSO. The intermediate B on further reaction with either mono-selenylated pyrazole 2a or with self-reacting provided the desired di-selenylated bis-pyrazole product 3a. However, further study is continuing in our laboratory for the confirmation of the proposed pathway.
Scheme 3. Plausible Reaction Pathway.
Conclusions
The current developed method is more general and successfully inserts the selenium in the form of mono-selenides and di-selenides using SeO2 as the selenylating reagent in DMSO as the solvent for the construction of mono- and di-selenylated bis-pyrazoles. Reaction conditions tolerated the various functionalities at the aromatic ring of the arylated pyrazole substrates and afforded the desired products in high yields. We also extended the current method for further adding the applicability to the obtained mono- and di-selenylated bis-pyrazole products.
Experimental Section
General
All reaction solvents were purified using the standard laboratory protocols. All reagents used in the synthesis were purchased from commercially available sources and used without further purification. Brucker AVANCE DPX FT-NMR 400 and 500 MHz instruments were used to record the 1H and 13C NMR spectra. 1H and 13C positive chemical shifts (δ, given in ppm) are downfield from the standard tetramethylsilane signal. Multiplicity [s = singlet, d = doublet, t = triplet, m = multiplet, br = broad singlet, coupling constant(s) are given in Hz, integration]. Agilent 6540 ultra-high-definition accurate-mass quadrupole time-of-flight liquid chromatography/mass spectrometry (LC/MS) system was used in order to record the high-resolution mass spectrometry (HRMS) spectra.
Synthesis of Aryl-Substituted Pyrazole Starting Compounds
The phenyl group (bearing various substitutions) containing pyrazole compounds, used as starting materials, were prepared using our earlier developed report.14,15
Typical Procedure for the Synthesis of Mono-Selenylated Bis-Pyrazoles
Pyrazole (1 mmol) and SeO2 (2 mmol) in DMSO (4 mL) at 150 °C were added in a dry round-bottom flask (25 mL) and heated at oil-bath for 0.5 h. The reaction was monitored by thin-layer chromatography (TLC), and after completion, the reaction mixture was transferred in ethyl acetate (20 mL) and washed with H2O (50 mL × three times). The combined organic phases were concentrated on a rotary evaporator and the crude obtained was purified over silica gel (ethyl acetate: n-hexane) to give mono-selenylated bis-pyrazole products.
Bis(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)selane (2a)
Pale yellow solid, mp 139–140 °C, yield = 71% (173 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.38–7.27 (m, 10H), 2.36 (s, 6H), 2.29 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 151.9, 142.3, 139.9, 129.0, 127.6, 124.8, 104.5, 13.2, 12.6 ppm. HRMS (ESI) calcd for C22H23N4Se [M + H]+, 423.1082; found, 423.1088.
Bis[1-(3-fluorophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2b)
Dark brown semisolid, yield = 70% (168 mg). Rf 0.3 (EtOAc/n-hexane = 1:9).1H NMR (400 MHz, CDCl3): δ 7.42 (dd, J = 14.5, 7.5 Hz, 2H), 7.24–7.15 (m, 4H), 7.08 (t, J = 8.0 Hz, 2H), 2.48 (s, 6H), 2.36 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 162.7 (d, J = 202.0 Hz), 152.41 (s), 141.8 (d, J = 131.3 Hz), 130.2 (d, J = 10.0 Hz), 120.0 (d, J = 3.0 Hz), 114.5 (d, J = 20.2 Hz), 112.1 (d, J = 20.2 Hz), 105.0, 13.3, 12.8 ppm. 19F NMR (400 MHz, CDCl3): δ −111.08 (dd, J = 15.2, 8.8 Hz). HRMS (ESI) calcd for C22H21F2N4Se [M + H]+, 459.0987; found, 459.0900.
Bis[1-(3-chlorophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2c)
Light yellow viscous, yield = 66% (157 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.38 (s, 2H), 7.32–7.22 (m, 6H), 2.38 (s, 6H), 2.28 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 152.4, 142.3, 140.9, 134.8, 130.0, 127.7, 124.9, 122.6, 105.0, 13.2, 12.7 ppm. HRMS (ESI) calcd for C22H21Cl2N4Se [M + H]+, 491.0303; found, 491.0308.
Bis[1-(4-chlorophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2d)
White solid, mp 160 °C, yield = 70% (166 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.34 (d, J = 8.7 Hz, 4H), 7.27 (d, J = 8.7 Hz, 4H), 2.35 (s, 6H), 2.27 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 152.2, 142.3, 138.4, 133.4, 129.2, 125.8, 104.8, 13.2, 12.6 ppm. HRMS (ESI) calcd for C22H21Cl2N4Se [M + H]+, 491.0303; found, 491.0308.
Bis[1-(3-bromophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2e)
Yellow semisolid, yield = 63% (145 mg). Rf 0.3 (EtOAc/n-Hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.54 (s, 2H), 7.42 (dd, J = 7.4, 1.5 Hz, 2H), 7.34–7.19 (m, 4H), 2.39 (s, 6H), 2.28 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 152.4, 142.4, 140.9, 130.6, 130.3, 127.7, 123.0, 122.6, 105.0, 13.3, 12.8 ppm. HRMS (ESI) calcd for C22H21Br2N4Se [M + H]+, 578.9293; found, 578.9298.
Bis[1-(4-bromophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2f)
Light yellow solid, mp 153–154 °C, yield = 66% (152 mg). Rf 0.3(EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.50 (d, J = 8.3 Hz, 4H), 7.21 (d, J = 8.3 Hz, 4H), 2.37 (s, 6H), 2.28 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 152.3, 142.3, 138.8, 132.2, 126.1, 121.3, 105.0, 13.3, 12.8 ppm. HRMS (ESI) calcd for C22H21Br2N4Se [M + H]+, 578.9293; found, 578.9298.
Bis[1-(2,4-difluorophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2g)
White solid, mp 101 °C, yield = 64% (152 mg). Rf 0.3 (EtOAc/n-hexane = 1:9).1H NMR (400 MHz, CDCl3): δ 7.35–7.29 (m, 2H), 6.92–6.86 (m, 4H), 2.23 (s, 6H), 2.19 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ162.6 (dd, J = 252.5, 11.1 Hz), 157.0 (dd, J = 255.5, 13.3 Hz), 152.8, 144.3, 130.0 (dd, J = 10.1, 1.0 Hz), 124.1 (dd, J = 13.1, 4.0 Hz), 111.9 (dd, J = 22.2, 3.0 Hz), 105.1, 104.9 (d, J = 2.0 Hz), 104.6, 103.6, 13.1, 11.3 (d, J = 4.0 Hz) ppm. 19F NMR (400 MHz, CDCl3): δ −107.65 (dd, J = 14.1, 7.8 Hz), −116.71 (t, J = 8.6 Hz). HRMS (ESI) calcd for C22H19F4N4Se [M + H]+, 495.0706; found, 495.0711.
Bis[1-(2,4-dichlorophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2h)
Light yellow solid, mp 173 °C, yield = 60% (139 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.45 (d, J = 1.3 Hz, 2H), 7.28 (dd, J = 8.7, 1.7 Hz, 2H), 7.23 (d, J = 8.4 Hz, 2H), 2.22 (s, 6H), 2.13 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 152.5, 144.0, 136.1, 135.8, 133.3, 130.5, 130.1, 127.9, 103.2, 13.1, 11.3 ppm. HRMS (ESI) calcd for C22H19Cl4N4Se [M + H]+, 558.9524; found, 558.9529.
Bis[3,5-dimethyl-1-(p-tolyl)-1H-pyrazol-4-yl]selane (2i)
White solid, mp 141–142 °C, yield = 68% (164 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.24 (d, J = 8.5 Hz, 4H), 7.20 (d, J = 8.5 Hz, 4H), 2.38 (s,6H), 2.36 (s, 6H), 2.33 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 151.6, 142.3, 137.6, 137.5, 129.6, 124.7, 104.2, 21.0, 13.2, 12.5 ppm. HRMS (ESI) calcd for C24H27N4Se [M + H]+, 451.1395; found, 451.1401.
Bis[1-(2-ethylphenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2j)
Light yellow semisolid, yield = 73% (173 mg). Rf 0.3 (EtOAc/n-hexane = 1:9).1H NMR (400 MHz, CDCl3): δ 7.39–7.34 (m, 4H), 7.30–7.24 (m, 2H), 7.16 (dd, J = 7.8, 1.2 Hz, 2H), 2.37–2.30 (m, 10H), 2.17 (s, 6H), 1.04 (t, J = 7.6 Hz, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 151.2, 143.1, 141.9, 138.2, 129.4, 129.3, 127.9, 126.5, 102.4, 23.8, 14.3, 13.1, 11.5 ppm. HRMS (ESI) calcd for C26H31N4Se [M + H]+, 479.1708; found, 479.1714.
Bis[1-(3,4-dimethylphenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2k)
White solid, mp 111–112 °C, yield = 74% (177 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.10 (d, J = 7.0 Hz, 4H), 7.00 (d, J = 8.1 Hz, 2H), 2.32 (s, 6H), 2.28 (s, 6H), 2.21 (s, 12H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 151.5, 142.2, 137.7, 137.5, 136.2, 129.9, 126.1, 122.1, 104.1, 19.7, 19.3, 13.2, 12.5 ppm. HRMS (ESI) calcd for C26H31N4Se [M + H]+, 479.1708; found, 479.1714.
Bis{3,5-dimethyl-1-[2-(trifluoromethyl)phenyl]-1H-pyrazol-4-yl}selane (2l)
Light brown solid, mp 132 °C, yield = 76% (181 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.70 (d, J = 7.7 Hz, 2H), 7.56 (t, J = 7.5 Hz, 2H), 7.49 (t, J = 7.6 Hz, 2H), 7.23 (d, J = 7.7 Hz, 2H), 2.20 (s, 6H), 2.03 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 151.7, 144.0, 137.5, 132.7, 130.4, 129.7, 128.5 (q, J = 30.3 Hz), 127.3 (q, J = 5.0 Hz), 124.1, 121.4, 102.8, 12.9, 11.2 ppm. 19F NMR (400 MHz, CDCl3): δ −60.53 (s). HRMS (ESI) calcd for C24H21F6N4Se [M + H]+, 559.0830; found, 559.0836.
Bis{3,5-dimethyl-1-[4-(trifluoromethoxy)phenyl]-1H-pyrazol-4-yl}selane (2m)
White solid, mp 110–112 °C, yield = 73% (167 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.36 (d, J = 8.9 Hz, 4H), 7.22 (d, J = 8.6 Hz, 4H), 2.37 (s, 6H), 2.27 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 152.4, 148.2, 142.4, 138.3, 126.0, 121.6 (d, J = 8.0 Hz), 119.1, 104.9, 13.2, 12.6 ppm. 19F NMR (400 MHz, CDCl3): δ −58.01 (s). HRMS (ESI) calcd for C24H21F6N4O2Se [M + H]+, 591.0728; found, 591.0734.
Bis[1-(2-methoxyphenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2n)
Light brown solid, mp 130 °C, yield = 72% (172 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.32 (dd, J = 11.3, 4.5 Hz, 2H), 7.21 (t, J = 9.3 Hz, 2H), 6.95 (dd, J = 15.9, 8.0 Hz, 4H), 3.69 (s, 6H), 2.24 (s, 6H), 2.15 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 154.6, 151.8, 144.4, 130.1, 129.0, 128.8, 120.9, 112.0, 102.6, 55.7, 13.2, 11.4 ppm. HRMS (ESI) calcd for C24H27N4O2Se [M + H]+, 483.1294; found, 483.1299.
Bis[1-(4-methoxyphenyl)-3,5-dimethyl-1H-pyrazol-4-yl]selane (2o)
Dark brown solid, mp 143 °C, yield = 74% (176 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.21 (d, J = 8.8 Hz, 4H), 6.87 (d, J = 8.8 Hz, 4H), 3.76 (s, 6H), 2.30 (s, 6H), 2.28 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 159.1, 151.5, 142.5, 133.1, 126.4, 114.2, 103.9, 55.5, 13.2, 12.4 ppm. HRMS (ESI) calcd for C24H27N4O2Se [M + H]+, 483.1294; found, 483.1299.
Bis[3,5-dimethyl-1-(3-nitrophenyl)-1H-pyrazol-4-yl]selane (2p)
Light yellow solid, mp 164 °C, yield = 78% (184 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 8.25 (t, J = 2.1 Hz, 2H), 8.16–8.13 (m, 2H), 7.77–7.74 (m, 2H), 7.59 (t, J = 8.1 Hz, 2H), 2.48 (s, 6H), 2.31 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.1, 148.5, 142.5, 140.7, 130.1, 129.9, 122.0, 119.1, 105.9, 13.3,13.0 ppm. HRMS (ESI) calcd for C22H21N6O4Se [M + H]+, 513.0784; found, 513.0790.
4,4′-[Selenobis(3,5-dimethyl-1H-pyrazole-4,1-diyl)]dibenzonitrile (2q)
White solid, mp 202 °C, yield = 77% (184 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.67 (d, J = 8.5 Hz, 4H), 7.51 (d, J = 8.5 Hz, 4H), 2.45 (s, 6H), 2.26 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.3, 143.2, 142.4, 133.1, 124.2, 118.0, 110.9, 106.2, 13.2 (2C) ppm. HRMS (ESI) calcd for C24H21N6Se [M + H]+, 473.0987; found, 473.0993.
Bis[3,5-dimethyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl]selane (2r)
White solid, mp 151 °C, yield = 70% (172 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 8.35 (d, J = 4.7 Hz, 2H), 7.73–7.68 (m, 4H), 7.11–7.07 (m, 2H), 2.72 (s, 6H), 2.27 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ153.3, 152.7, 147.5, 143.9, 138.2, 121.1, 116.2, 106.5, 14.4, 13.4 ppm. HRMS (ESI) calcd for C20H21N6Se [M + H]+, 425.0987; found, 425.0993.
Bis(5-methyl-1,3-diphenyl-1H-pyrazol-4-yl)selane (2s)
Dark yellow semisolid, yield = 75% (174 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.80–7.78 (m, 4H), 7.44–7.39 (m, 4H), 7.38–7.31 (m, 12H), 2.00 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.6, 143.7, 139.7, 133.4, 129.0, 128.8, 127.9, 127.9, 125.0, 103.8, 12.3 ppm. HRMS (ESI) calcd for C32H27N4Se [M + H]+, 547.1395; found, 547.1401.
Bis(1-methyl-1H-pyrazol-3-yl)selane (2t)
Dark yellow semisolid, yield = 78% (231 mg). Rf 0.3 (EtOAc/n-hexane = 4:6). 1H NMR (400 MHz, CDCl3): δ 7.47 (d, J = 9.0 Hz, 2H), 7.39 (d, J = 19.3 Hz, 2H), 3.84 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 144.5, 143.4, 135.1, 133.7, 104.2, 39.0 (d, J = 10.1 Hz) ppm. HRMS (ESI) calcd for C8H11N4Se [M + H]+, 243.0143; found, 243.0149.
Typical Procedure for the Synthesis of Di-Selenylated Bis-Pyrazoles
Pyrazole (1 mmol) and SeO2 (3 mmol) in DMSO (4 mL) were added to the dry round-bottom flask (25 mL) at 150 °C and heated on an oil-bath for 0.5 h. The reaction was monitored by TLC, and after completion, the reaction mixture was transferred in ethyl acetate (20 mL) and washed with H2O (50 mL × three times). The combined organic phases were concentrated on a rotary evaporator and crude was purified over silica gel (ethyl acetate: n-hexane) to give di-selenylated bis-pyrazole products.
1,2-Bis(3,5-dimethyl-1-phenyl-1H-pyrazol-4-yl)diselane (3a)
Light yellow semisolid, yield = 65% (190 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.37–7.33 (m, 5H), 7.30–7.26 (m, 5H), 2.17 (s, 6H), 2.02 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.2, 144.4, 139.7, 129.1, 127.8, 124.7, 104.7, 12.6, 11.8 ppm. HRMS (ESI) calcd for C22H23N4Se2 [M + H]+, 503.0248; found, 503.0253.
1,2-Bis[1-(3-fluorophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]diselane (3b)
Dark brown viscous, yield = 66% (187 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.34–7.28 (m, 2H), 7.06 (t, J = 7.7 Hz, 4H), 6.99 (t, J = 8.2 Hz, 2H), 2.15 (s, 6H), 2.05 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 162.8 (d, J = 248.4 Hz), 153.6 (s), 142.7 (d, J = 348.4 Hz), 130.3 (d, J = 9.0 Hz), 119.9 (d, J = 3.0 Hz), 114.7 (d, J = 20.2 Hz), 112.0 (d, J = 30.3 Hz), 105.4, 12.6, 12.0 ppm. 19F NMR (400 MHz, CDCl3): δ −110.72 (dd, J = 15.4, 8.3 Hz). HRMS (ESI) calcd for C22H21F2N4Se2 [M + H]+, 539.0059; found, 539.0065.
1,2-Bis[1-(3-chlorophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]diselane (3c)
Dark yellow semisolid, yield = 61% (169 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.35 (s, 2H), 7.28 (d, J = 6.5 Hz, 4H), 7.19–7.15 (m, 2H), 2.15 (s, 6H), 2.05 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.7, 144.5, 140.6, 135.0, 130.1, 127.9, 124.7, 122.4, 105.4, 12.6, 12.0 ppm. HRMS (ESI) calcd for C22H21Cl2N4Se2 [M + H]+, 570.9468; found, 570.9474.
1,2-Bis[1-(4-chlorophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]diselane (3d)
Dark yellow semisolid, yield = 60% (166 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.34 (d, J = 8.7 Hz, 4H), 7.22 (d, J = 8.7 Hz, 4H), 2.14 (s, 6H), 2.03 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.5, 144.4, 138.2, 133.7, 129.3, 125.7, 105.2, 12.5, 11.9 ppm. HRMS (ESI) calcd for C22H21Cl2N4Se2 [M + H]+, 570.9468; found, 570.9474.
1,2-Bis[1-(3-bromophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]diselane (3e)
White viscous, yield = 64% (168 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.51 (d, J = 1.9 Hz, 2H), 7.45–7.39 (m, 2H), 7.26–7.16 (m, 4H), 2.15 (s, 6H), 2.05 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.7, 144.5, 140.6, 130.9, 130.4, 127.6, 122.8, 122.8, 105.4, 12.6, 12.0 ppm. HRMS (ESI) calcd for C22H21Br2N4Se2 [M + H]+, 658.8458; found, 658.8463.
1,2-Bis[1-(4-bromophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]diselane (3f)
Dark yellow semisolid, yield = 63% (165 mg). Rf 0.3(EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.49 (d, J = 8.3 Hz, 4H), 7.16 (d, J = 8.3 Hz, 4H), 2.13 (s, 6H), 2.02 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 152.5, 143.3, 137.4, 131.2, 125.0, 124.8, 120.4, 104.2, 11.5, 10.8 ppm. HRMS (ESI) calcd for C22H21Br2N4Se2 [M + H]+, 657.8458; found, 658.8463.
1,2-Bis[1-(2,4-dichlorophenyl)-3,5-dimethyl-1H-pyrazol-4-yl]diselane (3g)
Dark yellow semisolid, yield = 58% (154 mg). Rf 0.4 (EtOAc/n-hexane = 1:9).1H NMR (400 MHz, CDCl3): δ 7.47 (s, 2H), 7.29 (s, 1H), 7.20–7.16 (m, 3H), 2.16 (s, 6H), 2.00 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.2, 145.7, 138.9, 133.4, 130.7, 129.8, 128.2, 122.1, 104.0, 12.8, 11.4 ppm. HRMS (ESI) calcd for C22H18Cl4N4Se2 [M + Na]+, 660.8514; found, 660.8514.
1,2-Bis[3,5-dimethyl-1-(p-tolyl)-1H-pyrazol-4-yl]diselane (3h)
Dark yellow liquid, yield = 68% (194 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.14 (s, 8H), 2.32 (s, 6H), 2.16 (s, 6H), 1.99 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.0, 144.4, 137.8, 137.2, 129.6, 124.6, 104.4, 21.0, 12.6, 11.7 ppm. HRMS (ESI) calcd for C24H27N4Se2 [M + H]+, 531.0561; found, 531.0566.
1,2-Bis[1-(3,4-dimethylphenyl)-3,5-dimethyl-1H-pyrazol-4-yl]diselane (3i)
Dark yellow semisolid, yield = 70% (190 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.07 (d, J = 8.1 Hz, 4H), 7.21 (t, J = 9.3 Hz, 2H), 6.95–6.93 (m, 2H), 2.21 (s, 6H), 2.18 (s, 6H), 2.16 (s, 6H), 1.98 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 152.8, 144.4, 137.6, 137.5, 136.4, 130.0, 125.9, 122.0, 104.2, 19.7, 19.3, 12.6, 11.8 ppm. HRMS (ESI) calcd for C26H31N4Se2 [M + H]+, 559.0874; found, 559.0879.
1,2-Bis{3,5-dimethyl-1-[4-(trifluoromethoxy)phenyl]-1H-pyrazol-4-yl}diselane (3j)
Dark yellow semisolid, yield = 76% (199 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.31 (d, J = 8.8 Hz, 4H), 7.20 (d, J = 8.6 Hz, 4H), 2.13 (s, 6H), 2.05 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.6, 148.3, 144.4, 138.1, 126.0 (d, J = 21.2 Hz), 121.7, 105.3, 12.5, 11.9 ppm. 19F NMR (400 MHz, CDCl3): δ −58.04 (s). HRMS (ESI) calcd for C24H21F6N4O2Se2 [M + H]+, 670.9894; found, 670.9899.
1,2-Bis[1-(2-methoxyphenyl)-3,5-dimethyl-1H-pyrazol-4-yl]diselane (3k)
Dark yellow semisolid, yield = 64% (178 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.31 (t, J = 7.7 Hz, 2H), 7.18–7.13 (m, 2H), 6.93 (t, J = 7.8 Hz, 4H), 3.65 (s, 6H), 2.18 (s, 6H), 1.92 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 154.5, 152.8, 146.4, 130.3, 129.0, 128.4, 120.8, 112.0, 103.2, 55.7, 12.6, 10.8 ppm. HRMS (ESI) calcd for C24H27N4O2Se2 [M + H]+, 563.0459; found, 563.0464.
1,2-Bis[3,5-dimethyl-1-(3-nitrophenyl)-1H-pyrazol-4-yl]diselane (3l)
Light yellow semisolid, yield = 72% (196 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 8.21 (t, J = 2.0 Hz, 2H), 8.15 (d, J = 8.2 Hz, 2H), 7.72 (d, J = 8.0 Hz, 2H), 7.58 (t, J = 8.1 Hz, 2H), 2.18 (s, 6H), 2.16 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 154.3, 148.6, 144.5, 140.5, 130.2, 129.6, 122.2, 118.9, 106.5, 12.6, 12.3 ppm. HRMS (ESI) calcd for C22H21N6O4Se2 [M + H]+, 592.9949; found, 592.9955.
4,4′-[Diselanediylbis(3,5-dimethyl-1H-pyrazole-4,1-diyl)]dibenzonitrile (3m)
Light yellow semisolid, yield = 72% (202 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.68 (d, J = 8.4 Hz, 4H), 7.48 (d, J = 8.4 Hz, 4H), 2.18 (s, 6H), 2.11 (s, 6H)ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 154.4, 144.4, 142.9, 133.2, 124.2, 117.9, 111.2, 106.9, 12.6, 12.5 ppm. HRMS (ESI) calcd for C24H21N6Se2 [M + H]+, 553.0153; found, 553.0158.
1,2-Bis[3,5-dimethyl-1-(pyridin-2-yl)-1H-pyrazol-4-yl]diselane (3n)
Dark yellow semisolid, yield = 71% (207 mg). Rf 0.4 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 8.25 (d, J = 4.6 Hz, 2H), 7.73–7.65 (m, 4H), 7.07 (t, J = 5.4 Hz, 2H), 2.33 (s, 6H), 2.18 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 153.7, 153.1, 147.6, 146.0, 138.2, 121.3, 116.3, 106.9, 13.6, 12.9 ppm. HRMS (ESI) calcd for C20H21N6Se2 [M + H]+, 505.0153; found, 505.0158.
1,2-Bis(5-methyl-1,3-diphenyl-1H-pyrazol-4-yl)diselane (3o)
Dark yellow viscous, yield = 77% (205 mg). Rf 0.3 (EtOAc/n-hexane = 1:9). 1H NMR (400 MHz, CDCl3): δ 7.87 (d, J = 1.7 Hz, 2H), 7.85 (t, J = 1.5 Hz, 2H), 7.37–7.31 (m, 7H), 7.30–7.26 (m, 7H), 7.24 (dd, J = 3.6, 1.9 Hz, 2H), 2.09 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 152.2, 144.3, 137.9, 131.2, 127.5, 126.7, 126.5, 126.4, 123.3, 100.7, 10.5 ppm. HRMS (ESI) calcd for C32H27N4Se2 [M + H]+, 627.0561; found, 627.0566.
Typical Procedure for the Oxidation of Mono-Selenylated Bis-Pyrazole (2a)
Pyrazole, 2a (1 mmol) and oxone (2.2 mmol) in EtOH (4 mL) were added to a dry round-bottom flask (25 mL) and heated at 80 °C in an oil-bath for 2 h. The reaction was monitored by TLC, and after completion, the reaction mixture was transferred in ethyl acetate (20 mL) and washed with H2O (50 mL × three times). The combined organic phases were concentrated on a rotary evaporator and crude was purified over silica gel (ethyl acetate/n-hexane) to give dioxide mono-selenylated bis-pyrazole product (2aa).
4,4′-Selenonylbis(3,5-dimethyl-1-phenyl-1H-pyrazole) (2aa)
White solid, mp 201–202 °C, yield = 79% (84 mg). Rf 0.3 (EtOAc/n-hexane = 4:6). 1H NMR (400 MHz, CDCl3): δ 7.44 (t, J = 8.8 Hz, 6H), 7.32 (d, J = 7.3 Hz, 4H), 2.57 (s, 6H), 2.42 (s, 6H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 148.3, 142.9, 138.0, 129.5, 129.2, 125.6, 118.4, 12.8, 11.7 ppm. HRMS (ESI) calcd for C22H23N4O2Se [M + H]+, 455.0981; found, 455.0986.
Typical Procedure for the Coupling of Di-Selenylated Bis-Pyrazole (3a) with Indole
Pyrazole, 3a (1 mmol), indole (1 mmol), and K2CO3 (2.2 mmol) in EtOH (4 mL) were added to a dry round-bottom flask (25 mL) and heated at 80 °C in an oil-bath for 2 h. The reaction was monitored by TLC, and after completion, the reaction mixture was transferred in ethyl acetate (20 mL) and washed with H2O (50 mL × three times). The combined organic phases were concentrated on a rotary evaporator and crude was purified over silica gel (ethyl acetate: n-hexane) to give unsymmetric di-selenylated bis-pyrazole product (3aa).
3-[(3,5-Dimethyl-1-phenyl-1H-pyrazol-4-yl)selanyl]-1H-indole (3aa)
Dark brown semisolid, yield = 78% (57 mg). Rf 0.3 (EtOAc/n-hexane = 3:7).1H NMR (400 MHz, CDCl3): δ 8.40 (s, 1H), 7.74 (d, J = 7.4 Hz, 1H), 7.45–7.39 (m, 2H), 7.36 (d, J = 7.4 Hz, 5H), 7.24–7.16 (m, 2H), 2.50 (s, 3H), 2.45 (s, 3H) ppm. 13C{1H} NMR (101 MHz, CDCl3): δ 152.2, 142.6, 139.9, 136.1, 129.5, 129.1,129.0, 127.5, 124.8, 122.6, 120.4, 120.1, 111.2, 105.1, 101.1, 13.2, 12.6 ppm. HRMS (ESI) calcd for C19H18N3Se [M + H]+, 368.0660; found, 368.0666.
Acknowledgments
The funding support from CSIR-India funded project HCP23 is gratefully acknowledged. P.K.V. and S.D.S. are thankful to DST-SERB for the Grant (GAP2139 and GAP2185). IIIM communication no. CSIR-IIIM/IPR/00363.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00323.
Characterization data of all compounds and 1H and 13C NMR and HRMS spectra for all compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Singh F. V.; Wirth T. Selenium and tellurium electrophiles in organic synthesis. Phy. Sci. Rev. 2019, 4, 20170131. 10.1515/psr-2017-0131. [DOI] [Google Scholar]; b Wirth T. Chiral selenium compounds in organic synthesis. Tetrahedron 1999, 55, 1–28. 10.1016/s0040-4020(98)00946-6. [DOI] [Google Scholar]; c Wirth T. Organoselenium chemistry in stereoselective reactions. Angew. Chem., Int. Ed. 2000, 39, 3740–3749. . [DOI] [PubMed] [Google Scholar]; d Curran S. P.; Connon S. J. Selenide ions as catalysts for homo- and crossed tishchenko reactions of expanded scope. Org. Lett. 2012, 14, 1074–1077. 10.1021/ol203439g. [DOI] [PubMed] [Google Scholar]; e Reich H. J. Functional group manipulation using organoselenium reagents. Acc. Chem. Res. 1979, 12, 22–30. 10.1021/ar50133a004. [DOI] [Google Scholar]; f Mugesh G.; du Mont W.-W.; Sies H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2180. 10.1021/cr000426w. [DOI] [PubMed] [Google Scholar]; g Mukherjee A. J.; Zade S. S.; Singh H. B.; Sunoj R. B. Organoselenium chemistry: role of intramolecular interactions. Chem. Rev. 2010, 110, 4357–4416. 10.1021/cr900352j. [DOI] [PubMed] [Google Scholar]; h Nicolaou K. C.; Claremon D. A.; Barnette W. E.; Seitz S. P. N-Phenylselenophthalimide (N-PSP) and N-phenylselenosuccinimide (N-PSS). Two versatile carriers of the phenylseleno group. Oxyselenation of olefins and a selenium-based macrolide synthesis. J. Am. Chem. Soc. 1979, 101, 3704–3706. 10.1021/ja00507a069. [DOI] [Google Scholar]
- a Freudendahl D. M.; Santoro S.; Shahzad S. A.; Santi C.; Wirth T. Green chemistry with selenium reagents: Development of efficient catalytic reactions. Angew. Chem., Int. Ed. 2009, 48, 8409–8411. 10.1002/anie.200903893. [DOI] [PubMed] [Google Scholar]; b Singh F. V.; Wirth T. Selenium-catalyzed regioselective cyclization of unsaturated carboxylic acids using hypervalent iodine oxidants. Org. Lett. 2011, 13, 6504–6507. 10.1021/ol202800k. [DOI] [PubMed] [Google Scholar]; c Alberto E. E.; Braga A. L.; Detty M. R. Imidazolium-containing diselenides for catalytic oxidations with hydrogen peroxide and sodium bromide in aqueous solutions. Tetrahedron 2012, 68, 10476–10481. 10.1016/j.tet.2012.08.004. [DOI] [Google Scholar]; d Perin G.; Lenardão E. J.; Jacob R. G.; Panatieri R. B. Synthesis of vinyl selenides. Chem. Rev. 2009, 109, 1277–1301. 10.1021/cr8004394. [DOI] [PubMed] [Google Scholar]; e Braga A.; Ludtke D.; Vargas F. Enantioselective synthesis mediated by catalytic chiral organoselenium compounds. Curr. Org. Chem. 2006, 10, 1921–1938. 10.2174/138527206778521204. [DOI] [Google Scholar]
- a Wincent E.; Shirani H.; Bergman J.; Rannug U.; Janosik T. Synthesis and biological evaluation of fused thio- and selenopyrans as new indolocarbazole analogues with aryl hydrocarbon receptor affinity. Bioorg. Med. Chem. 2009, 17, 1648–1653. 10.1016/j.bmc.2008.12.072. [DOI] [PubMed] [Google Scholar]; b Wen Z.; Xu J.; Wang Z.; Qi H.; Xu Q.; Bai Z.; Zhang Q.; Bao K.; Wu Y.; Zhang W. 3-(3,4,5-Trimethoxyphenylselenyl)-1H-indoles and their selenoxides as combretastatin A-4 analogs: Microwave-assisted synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 90, 184–194. 10.1016/j.ejmech.2014.11.024. [DOI] [PubMed] [Google Scholar]; c Nogueira C. W.; Zeni G.; Rocha J. B. T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. Chem. Rev. 2004, 104, 6255–6286. 10.1021/cr0406559. [DOI] [PubMed] [Google Scholar]; d Back T. G.; Moussa Z. Diselenides and allyl selenides as glutathione peroxidase mimetics. Remarkable activity of cyclic seleninates produced in situ by the oxidation of allyl omega-hydroxyalkyl selenides. J. Am. Chem. Soc. 2003, 125, 13455–13460. 10.1021/ja0357588. [DOI] [PubMed] [Google Scholar]; e Chen Z.; Lai H.; Hou L.; Chen T. Rational design and action mechanisms of chemically innovative organoselenium in cancer therapy. Chem. Commun. 2020, 56, 179–196. 10.1039/c9cc07683b. [DOI] [PubMed] [Google Scholar]; f Chen X.; Wu X.; He Z.; Zhang J.; Cao Y.; Mao D.; Feng C.; Tian B.; Chen G. Molecular docking-assisted design and synthesis of an anti-tumor quercetin–Se(IV) complex. New J. Chem. 2020, 44, 8434–8441. 10.1039/c9nj06136c. [DOI] [Google Scholar]
- Yamaguchi T.; Sano K.; Takakura K.; Saito I.; Shinohara Y.; Asano T.; Yasuhara H. Ebselen in acute ischemic stroke. Stroke 1998, 29, 12–17. 10.1161/01.str.29.1.12. [DOI] [PubMed] [Google Scholar]
- Kil J.; Pierce C.; Tran H.; Gu R.; Lynch E. D. Ebselen treatment reduces noise induced hearing loss via the mimicry and induction of glutathione peroxidase. Hear. Res. 2007, 226, 44–51. 10.1016/j.heares.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Singh N.; Halliday A. C.; Thomas J. M.; Kuznetsova O. V.; Baldwin R.; Woon E. C. Y.; Aley P. K.; Antoniadou I.; Sharp T.; Vasudevan S. R.; Churchill G. C. A safe lithium mimetic for bipolar disorder. Nat. Commun. 2013, 4, 1332. 10.1038/ncomms2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Arnér E. S. J.; Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]; b Li S.; Zhao Q.; Zhang K.; Sun W.; Jia X.; Yang Y.; Yin J.; Tang C.; Zhang J. Se deficiency induces renal pathological changes by regulating selenoprotein expression, disrupting redox balance, and activating inflammation. Metallomics 2020, 12, 1576–1584. 10.1039/d0mt00165a. [DOI] [PubMed] [Google Scholar]; c Harthill M. Review: Micronutrient selenium deficiency influences evolution of some viral infectious diseases. Biol. Trace Elem. Res. 2011, 143, 1325–1336. 10.1007/s12011-011-8977-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Nicolau K. C.; Petasi N. A.. Selenium in natural products synthesis; CIS: Philadelphia, PA, 1984. [Google Scholar]; b Paulmier C.Selenium reagents and intermediates in organic synthesis; Pergamon: Oxford, U.K.., 1986. [Google Scholar]; c Patai S.; Rappoport Z.. The chemistry of organic selenium and tellurium compounds; Wiley: New York, 1986; Vol. 1. [Google Scholar]; d Liotta D.Organoselenium chemistry; Wiley: New York, 1987. [Google Scholar]; e Krief A.; Hevesi L.. Organoselenium chemistry I; Springer: Berlin, 1988. [Google Scholar]; f Back T. G.Organoselenium chemistry: A pratical approach; Oxford University Press: Oxford, U.K.., 1999. [Google Scholar]; g Reich H. J. Functional group manipulation using organoselenium reagents. Acc. Chem. Res. 1979, 12, 22–30. 10.1021/ar50133a004. [DOI] [Google Scholar]; h Wang Q.; Li P.; Li T.; Liu M.; Zuo S.; Liu J.; Xu L.; Zhang X.; Yu L. Methylselenized glucose: Improvement of the stability of glucose-supported selenium via the end-capping strategy. Ind. Eng. Chem. Res. 2021, 60, 8659–8663. 10.1021/acs.iecr.1c01437. [DOI] [Google Scholar]
- a Li Y.; Wang H.; Li X.; Chen T.; Zhao D. CuS/Fe: a novel and highly efficient catalyst system for coupling reaction of aryl halides with diaryl diselenides. Tetrahedron 2010, 66, 8583–8586. 10.1016/j.tet.2010.09.061. [DOI] [Google Scholar]; b Singh D.; Alberto E. E.; Rodrigues O. E. D.; Braga A. L. Eco-friendly cross-coupling of diaryl diselenides with aryl and alkyl bromides catalyzed by CuO nanopowder in ionic liquid. Green Chem. 2009, 11, 1521–1524. 10.1039/b916266f. [DOI] [Google Scholar]; c Chatterjee T.; Ranu B. C. Solvent-controlled halo-selective selenylation of aryl halides catalyzed by Cu(II) supported on Al2O3. A general protocol for the synthesis of unsymmetrical organo mono- and bis-selenides. J. Org. Chem. 2013, 78, 7145–7153. 10.1021/jo401062k. [DOI] [PubMed] [Google Scholar]
- a Laloo B. M.; Mecadon H.; Rohman M. R.; Kharbangar I.; Kharkongor I.; Rajbangshi M.; Nongkhlaw R.; Myrboh B. Reaction of selenium dioxide with aromatic ketones in the presence of boron trifluoride etherate: A protocol for the synthesis of triarylethanones. J. Org. Chem. 2012, 77, 707–712. 10.1021/jo201985n. [DOI] [PubMed] [Google Scholar]; b Rohman M. R.; Kharkongor I.; Rajbangshi M.; Mecadon H.; Laloo B. M.; Sahu P. R.; Kharbangar I.; Myrboh B. One-pot synthesis of unsymmetrical benzils by oxidative coupling using selenium dioxide and p-toluenesulfonic acid monohydrate. Eur. J. Org. Chem. 2012, 2012, 320–328. 10.1002/ejoc.201101012. [DOI] [Google Scholar]; c Shangpliang O. R.; Kshiar B.; Wanniang K.; Marpna I. D.; Lipon T. M.; Laloo B. M.; Myrboh B. Selenium dioxide as an alternative reagent for the direct α-selenoamidation of aryl methyl ketones. J. Org. Chem. 2018, 83, 5829–5835. 10.1021/acs.joc.8b00558. [DOI] [PubMed] [Google Scholar]; d Shangpliang O. R.; Wanniang K.; Kshiar B.; Marpna I. D.; Lipon T. M.; Mizar P.; Myrboh B. PTSA-catalyzed reaction of alkyl/aryl methyl ketones with aliphatic alcohols in the presence of selenium dioxide: A protocol for the generation of an α-ketoacetals library. ACS Omega 2019, 4, 6035–6043. 10.1021/acsomega.9b00361. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Wanniang K.; Shangpliang O. R.; Marpna I. D.; Lipon T. M.; Laloo B. M.; Myrboh B. Metal-free three-component coupling reaction of ketones with electron-rich arenes and selenium dioxide for the synthesis of α-arylselanyl ketones. J. Org. Chem. 2020, 85, 15652–15659. 10.1021/acs.joc.0c02028. [DOI] [PubMed] [Google Scholar]; f Marpna I. D.; Wanniang K.; Lipon T. M.; Shangpliang O. R.; Myrboh B. Selenocyanation of aryl and styryl methyl ketones in the presence of selenium dioxide and malononitrile: An approach for the synthesis of α-carbonyl selenocyanates. J. Org. Chem. 2021, 86, 1980–1986. 10.1021/acs.joc.0c02630. [DOI] [PubMed] [Google Scholar]; g Marpna I. D.; Shangpliang O. R.; Wanniang K.; Kshiar B.; Lipon T. M.; Laloo B. M.; Myrboh B. Trifluoroacetic acid-mediated oxidative self-condensation of acetophenones in the presence of SeO2: A serendipitous approach for the synthesis of fused [1,3]dioxolo[4,5-d][1,3]dioxoles. ACS Omega 2021, 6, 14518–14524. 10.1021/acsomega.1c01466. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Talukdar R. A study on the reactions of SeO2 with pyrroles and N-substituted indoles in non-anhydrous ethanol under non-inert atmosphere. Asian J. Org. Chem. 2019, 8, 88–92. 10.1002/ajoc.201800584. [DOI] [Google Scholar]; i Quell T.; Mirion M.; Schollmeyer D.; Dyballa K. M.; Franke R.; Waldvogel S. R. Solvent-dependent facile synthesis of diaryl selenides and biphenols employing selenium dioxide. ChemistryOpen 2016, 5, 115–119. 10.1002/open.201500206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Ferreira N. L.; Azeredo J. B.; Fiorentin B. L.; Braga A. L. Synthesis of 3-selenylindoles under ecofriendly conditions. Eur. J. Chem. 2015, 2015, 5070–5074. 10.1002/ejoc.201500514. [DOI] [Google Scholar]; b Rathore V.; Kumar S. Visible-light-induced metal and reagent-free oxidative coupling of sp2 C–H bond with organo-dichalcogenides: Synthesis of 3-organochalcogenyl indoles. Green Chem. 2019, 21, 2670–2676. 10.1039/c9gc00007k. [DOI] [Google Scholar]; c Luo D.; Wu G.; Yang H.; Liu M.; Gao W.; Huang X.; Chen J.; Wu H. Copper-catalyzed three-component reaction for regioselective aryl- and heteroarylselenation of indoles using selenium powder. J. Org. Chem. 2016, 81, 4485–4493. 10.1021/acs.joc.6b00229. [DOI] [PubMed] [Google Scholar]
- a Pinto D. C. G. A.; Silva A. M. S.; Cavaleiro J. A. S.; Elguero J. New Bis(chalcones) and Their Transformation into Bis(pyrazoline) and Bis(pyrazole) Derivatives. Eur. J. Org. Chem. 2003, 2003, 747–755. 10.1002/ejoc.200390117. [DOI] [Google Scholar]; b Youssef A. M.; Neeland E. G.; Villanueva E. B.; White M. S.; El-Ashmawy I. M.; Patrick B.; Klegeris A.; Abd-El-Aziz A. S. Synthesis and Biological Evaluation of Novel Pyrazole Compounds. Bioorg. Med. Chem. 2010, 18, 5685–5696. 10.1016/j.bmc.2010.06.018. [DOI] [PubMed] [Google Scholar]
- a Parekh K. D.; Dash R. P.; Pandya A. N.; Vasu K. K.; Nivsarkar M. Implication of Novel Bis-Imidazopyridines for Management of Alzheimer’s Disease and Establishment of its Role on Protein Phosphatase 2A Activity in Brain. J. Pharm. Pharmacol. 2013, 65, 1785–1795. 10.1111/jphp.12149. [DOI] [PubMed] [Google Scholar]; b Reddy K. I.; Aruna C.; Manisha M.; Srihari K.; Sudhakar Babu K.; Vijayakumar V.; Sarveswari S.; Priya R.; Amrita A.; Siva R. Synthesis, DNA binding and In-vitro Cytotoxicity Studies on Novel Bis-pyrazoles. J. Photochem. Photobiol. B: Biol. 2017, 168, 89–97. 10.1016/j.jphotobiol.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Kour J.; Venkateswarlu V.; Verma P. K.; Hussain Y.; Dubey G.; Bharatam P. V.; Sahoo S. C.; Sawant S. D. Oxone-DMSO triggered methylene insertion and C(sp2)–C(sp3)-H–C(sp2) bond formation to access functional bis-heterocycles. J. Org. Chem. 2020, 85, 4951–4962. 10.1021/acs.joc.9b03477. [DOI] [PubMed] [Google Scholar]
- Venkateswarlu V.; Kour J.; Kumar K. A. A.; Verma P. K.; Reddy G. L.; Hussain Y.; Tabassum A.; Balgotra S.; Gupta S.; Hudwekar A. D.; Vishwakarma R. A.; Sawant S. D. Direct N-Heterocyclization of Hydrazines to Access Styrylated Pyrazoles: Synthesis of 1,3,5-Trisubstituted Pyrazoles and Dihydropyrazoles. RSC Adv. 2018, 8, 26523–26527. 10.1039/c8ra04550j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Wang J.; Sánchez-Roselló M.; Aceña J. L.; del Pozo C.; Sorochinsky A. E.; Fustero S.; Soloshonok V. A.; Liu H. Fluorine in Pharmaceutical Industry: Fluorine-Containing Drugs Introduced to the Market in the Last Decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. 10.1021/cr4002879. [DOI] [PubMed] [Google Scholar]; b Smits R.; Cadicamo C. D.; Burger K.; Koksch B. Synthetic Strategies to α-Trifluoromethyl and α-Difluoromethyl Substituted α-Amino Acids. Chem. Soc. Rev. 2008, 37, 1727–1739. 10.1039/b800310f. [DOI] [PubMed] [Google Scholar]; c Berger R.; Resnati G.; Metrangolo P.; Weber E.; Hulliger J. Organic Fluorine Compounds: A Great Opportunity for Enhanced Materials Properties. Chem. Soc. Rev. 2011, 40, 3496–3508. 10.1039/c0cs00221f. [DOI] [PubMed] [Google Scholar]; d Bégué J.-P.; Bonnet-Delpon D.; Crousse B.; Legros J. The Chemistry of Trifluoromethyl Imines and Related Acetals Derived from Fluoral. Chem. Soc. Rev. 2005, 34, 562–572. 10.1039/b401707m. [DOI] [PubMed] [Google Scholar]; e Purser S.; Moore P. R.; Swallow S.; Gouverneur V. Fluorine in Medicinal Chemistry. Chem. Soc. Rev. 2008, 37, 320–330. 10.1039/b610213c. [DOI] [PubMed] [Google Scholar]
- Wilshire J. The reaction of some indoles with selenium dioxide. Aust. J. Chem. 1967, 20, 359–364. 10.1071/ch9670359. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.