Abstract
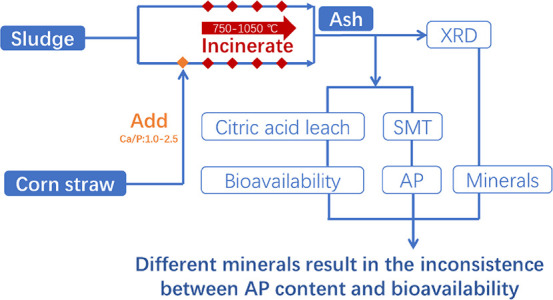
Phosphorus is a depletable resource, and the consumption of phosphorus fertilizer increases with the growing population size. Phosphorus recycled from incinerated sludge ash can be a complement to phosphatic fertilizers in districts suffering from phosphorus resource shortages (e.g., Germany, Japan, and Sweden). The apatite inorganic phosphorus (AP) content in incinerated sludge ash is a key factor influencing the recoverability and bioavailability. Biomass straw is rich in calcium and magnesium minerals and can be used as an additive to be blended with sludge to increase the AP content. However, most of the current studies added excessive amounts of calcium-based or biomass additives, and the bioavailability of various Ca–Mg–P minerals generated after the addition of biomass has not been systematically discussed. In this study, the changes of the phosphorus form in the mixed sludge and biomass with Ca/P in the range of 1.0–2.5 are studied, and the influence of temperature and additives on the phosphorus form and the bioavailability of phosphorus in the ash samples are discussed by combining X-ray diffraction and citric acid (CA) leaching experiments. The AP content is very low in the residue of the sludge or corn straw (CS) that has been burned individually. The sludge and the blended sludge and CS were incinerated at various temperatures. As the incineration temperature increased, the conversion of non-apatite inorganic phosphorus (NAIP) to AP was promoted, but the bioavailability did not change until 1050 °C for samples with a Ca/P of 2.5. In the range from 750 to 950 °C, higher temperature promotes the formation of Ca2P2O7 and CaP2O6. CaP2O6 is insoluble in CA; thus, the bioavailability changes little from 750 to 950 °C, although the AP content increases. With the increase of Ca/P, the conversion of NAIP to AP and the bioavailability of phosphorus were promoted. For the blended sludge and CS ash, Ca7Mg2P6O24 appears at 950 and 1050 °C and the bioavailability also increases.
1. Introduction
Phosphorus is one of the essential elements in the cell structure and metabolism of plants and animals. In the terrestrial circulation system, as part of the phosphorus that is washed and transported by water precipitates into the bottom mud of rivers, lakes, and seas, phosphorus resources are less available on the land.1 Phosphorus is a nonrenewable resource, but human demand for phosphorus resources increases with a larger population.2 It is predicted that the global consumption of phosphate fertilizer will increase to 200 million tons per year in 2030,3 and geological phosphate rock, the main raw material for phosphate fertilizer production, will be exhausted in 90–130 years.4−6 China is rich in phosphate rock resources, but high-grade phosphate rock resources only account for 10% of the total reserves, while the phosphorus content in sewage sludge (SS) is close to the poor phosphate rock (10–20 wt % of P2O5), and the annual SS discharge is as high as 50 million tons.7 We can calculate that if the phosphorus in the sludge can be reused, it is equivalent to supplementing 10% of China’s annual phosphate fertilizer demand.8 Germany, Sweden, and Japan began the industrialization practice of phosphorus resource recovery from sludge as early as 2010.9,10 The phosphorus in the incinerated sludge ash can be leached and crystallized into struvite or calcium hydroxyapatite to recover phosphorus, or the sludge ash after incineration can be directly used as citric fertilizer.11,12 Incineration of sludge has significant advantages such as the complete destruction of organic pollutants, volume reduction, and heat recovery.13−16 The form of phosphorus in the incinerated sludge ash is a key factor that affects the recovery and reuse of phosphorus and determines the recyclable percentage and bioavailability of phosphorus.17 In sludge ash, only apatite inorganic phosphorus (mainly calcium magnesium phosphate) can be directly absorbed by plants,18 so how to increase the apatite inorganic phosphorus content in the incineration sludge ash is one of the keys to realizing the efficient utilization of phosphorus resources in sludge.
In order to facilitate and unify the procedure to measure the bioavailability/mobility of phosphorus in sludge, the Commission of the European Communities has formulated a standard measurement protocol [standards, measurements, and testing (SMT)] that is easy to implement under routine experimental conditions and separately stipulates determination procedures for total phosphorus (TP), inorganic phosphorus (IP), and organic phosphorus (OP), where TP = IP + OP. IP is divided into apatite inorganic phosphorus (AP, Ca/Mg–P) and non-apatite inorganic phosphorus (NAIP, Fe/Al/Mn–P).19−21 One of the drawbacks of the method is that the differentiation between the apatite and non-apatite fractions is not so accurate due to the reagents employed that determine the releasing mechanism from the sludge. Despite the limitations inherent to the method, the SMT protocol is a good approach for phosphorus fractionation in sludge.20 Fe/Al/Mn–phosphate exhibits low bioavailability and cannot be used by plants directly. Ca/Mg–phosphate could be utilized as a slow-release P fertilizer in the long term. Many of the research focuses on how to promote the conversion of NAIP to AP in order to improve the bioavailability of the phosphorus in sludge ash.18,22−24 The bioavailability is usually evaluated by the soluble phosphorus content in the citric acid (CA) solution. However, the AP content is not equal to the content of biologically available phosphorus. The comparison of the AP content and the soluble phosphorus content in some research shows that the soluble phosphorus content in the CA solution is less than the AP content.25,26
Factors affecting the phosphorus form in sludge ash include reaction time, atmosphere, temperature, and additives. Li et al. found that iron–phosphorus compounds, aluminum–phosphorus compounds, and amorphous calcium–phosphorus compounds in sludge would be converted to AP during incineration at 750–950 °C.27 Qian et al.’s research shows that low-temperature combustion (below 600 °C) is conducive to the conversion of phosphorus to an alkali-soluble state, and high temperatures (600–800 °C) are conducive to the conversion of phosphorus to an acid-soluble state with high bioavailability, such as calcium phosphate and hydroxyapatite.28 Additives containing Ca or Mg were introduced to promote the formation of apatite inorganic phosphorus. Li et al. added CaO to the sludge to react with AlPO4 to form Ca2P2O7 and Ca3(PO4)2.27 Xu et al. found that the concentration of IP and AP in the sludge hydrothermal carbonization product reaches the maximum at the same time when 20 wt % CaCl2 was added.29 The equation Ca10–x(PO4)6–x(HPO4)x(OH)2–x → (1 – x)Ca10(PO4)6(OH)2 + 3xCa3(PO4)2 in Joris and Amberg’s research shows that Ca/P of 1.67–1.5 is beneficial to the formation of apatite inorganic phosphorus theoretically.30 However, most of the research studies have added far more additives than the value according to this ratio, which is not economic if the method is conducted in industrial practice. The biomass straw was also blended with sludge to act as additives as the calcium and magnesium content of the ash is high. Zhao et al. compared the effects of corn, wheat, cotton straws, and wood chips and found that cotton straws were the most effective in promoting the conversion of NAIP to AP.31 Ca and Mg compounds provide chemical reaction sites for phosphorus, forming new phosphate mineral phases such as Ca18Mg2H2(PO4)14, Ca2P2O7, and so forth.31,32 Ren et al. found that Ca9MgK(PO4)7 and KAlSi3O8 in the mixed ash of wheat straw and sludge inhibit the reaction of alkaline compounds with silica, and the reaction of alkali metals with phosphorus-containing compounds promotes the formation of K–Ca–P compounds.33 However, the bioavailability of these compounds was not discussed.
In summary, biomass straws can be blended with sludge as an additive to promote the conversion of NAIP to AP as the abundant calcium and magnesium minerals in biomass straws. However, the AP content is not equal to the content of biologically available phosphorus. AP refers to many kinds of calcium or magnesium phosphates, of which the bioavailability is different. Most of the current studies add excessive amounts of calcium-based or biomass additives, and the bioavailability of various Ca–Mg–P minerals generated has not been discussed. This study aims to explore the changes of the phosphorus species in the sludge and blended corn straws (CSs) and sludge within the range of Ca/P of 1.0–2.5. The relationship between phosphorus species and the bioavailability of the mixed ash is discussed by combining with X-ray diffraction (XRD) and CA leaching testing results.
2. Experimental Procedures
2.1. Materials
The SS in this experiment is from a water purification plant in Kunming, Yunnan Province, China. The SS (moisture 80%) was dried for 48 h under natural ventilation, and then crushed to below 2 mm. The crushed SS powder sample was dried at 105 °C for 48 h to remove the external moisture. The dried SS was milled to less than 0.15 mm using a planetary mill, and the obtained SS was stored in a dryer.
The CS used in this study is from the Guanshan Lake District, Guiyang City, Guizhou Province, China. The collected CS was naturally air dried and chopped to less than 20 mm, then it was dried at 105 °C for 48 h and crushed to a powder that was less than 0.425 mm using a multifunctional crusher and stored in a dryer storage.
The SS ash and CS ash were prepared according to the procedures in the proximate analysis of coal (GB/T212-2001,China) and proximate analysis of solid biofuels (GB/T28731-2012,China). About 5 g of the SS powder in a crucible was burned in a temperature-controlled muffle furnace. During the process, the furnace temperature was first raised to 500 °C and then kept constant for 1 h. The temperature was raised to 815 °C and held for 2 h subsequently. The residua were burned for 30 min again after the mass of the residua was determined. Repeat burning for 30 min and determine the mass of sample until the difference in the mass value two times is less than 0.2 mg. For the CS, a similar operation was conducted to prepare the corn ash, but the final temperature of the procedure was 550 °C.
2.2. Physical and Chemical Properties of Raw Materials
With reference to the proximate analysis of coal (GB/T 212-2001) and ultimate analysis of coal (GB/T 476residua2001), proximate analysis and elemental analysis of the samples were carried out. The results are listed in Table 1. SS has a higher ash content, while CS has a higher volatile content, an extremely low ash content, and a higher fixed carbon content. The chemical composition of SS ash and CS ash was analyzed by PANalytical Axios X-ray fluorescence, and the corresponding results are listed in Table 2.
Table 1. Proximate and Ultimate Analysis of Raw Materials.
| proximate analysis/% |
ultimate analysis/% |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| sample | M | V | A | FC | C | H | O | N | S |
| SS | 5.80 | 31.76 | 62.22 | 0.22 | 12.68 | 3.37 | 49.78 | 1.97 | 0.44 |
| CS | 10.34 | 69.16 | 4.67 | 15.83 | 42.93 | 5.80 | 46.00 | 0.60 | |
Table 2. Chemical Composition of Raw Ash.
| SiO2 | Al2O3 | Fe2O3 | P2O5 | CaO | SO3 | K2O | MgO | TiO2 | Na2O | Cl | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SS | 27.54 | 23.69 | 19.51 | 10.00 | 8.67 | 1.84 | 2.38 | 3.02 | 0.79 | 0.20 | 0.07 |
| CS | 43.74 | 0.43 | 0.30 | 2.29 | 10.29 | 2.68 | 27.72 | 11.67 | 0.03 | 0.14 | 0.56 |
2.3. Incineration Experiments
The molar ratio of calcium to phosphorus (Ca/P) of the original SS ash is about 1:1, and the Ca/P of CS ash is 6:1. CS was added to the sludge to increase the overall calcium to phosphorus ratio. According to the equation mentioned in Joris and Amberg’s research,30 Ca10–x(PO4)6–x(HPO4)x(OH)2–x → (1 – x)Ca10(PO4)6(OH)2 + 3xCa3(PO4)2, a Ca/P of 1.67–1.5 is beneficial to the formation of apatite inorganic phosphorus. The calcium–phosphorus molar ratios of this experimental sample are in the range from 1.0 to 2.5 in order to cover this ideal range of the Ca/P. The corresponding range of (Ca + Mg)/P is 1.64–6.53. The SS and CS were blended according to the abovementioned calcium–phosphorus ratio and pulverized in a ball mill for 10 min to mix well. About 5 g of the sample in a crucible was placed in the constant temperature zone of a tubular furnace. The sample was heated to the target temperatures (750, 850, 950, or 1050 °C) at 10 °C/min and held for 1 h. Air at a rate of 100 mL·min–1 was injected during the heating process. The obtained ash sample was marked as SCx–y based on its Ca/P and incinerated temperature, where x represents the ratio of Ca/P in the blended sample and y represents the temperature. For example, the ash sample with a Ca/P of 1.5, incinerated at 750 °C, is denoted as SC1.5–750.
2.4. Standards, Measurements, and Testing (SMT)
Different forms of phosphorus in ash samples were separated by the SMT method. The detailed analysis and extraction process are shown in Figure 1. According to SMT protocols, different forms of phosphorus were extracted from samples, including TP, IP, OP, NAIP (Al/Fe/Mg–P), and apatite inorganic phosphate (AP, Ca/Mg–P).19−21
Figure 1.
SMT method for the fractional extraction of various forms of phosphorus in sludge ash.
2.5. Molybdenum Blue Spectrophotometry
The supernatant obtained in the SMT extraction process was filtrated and then diluted to an appropriate concentration to facilitate detection. The phosphorus concentration of the diluent was measured by molybdenum blue colorimetry. The standard curve of phosphorus concentration–absorbance established according to the molybdenum blue colorimetric method is shown in Figure 2. It can be found from Figure 2 that the standard curve determined by the molybdenum blue colorimetric method has good regression and the phosphorus concentration has a significant positive correlation with the absorbance (R2 = 0.9999), which can be used for the detection of the phosphorus content in sludge ash. According to the absorbance of the diluted solution, the measured phosphorus concentration of the diluted solution was obtained from the standard curve shown in Figure 2, and the phosphorus content Pi (mg/g) of various forms in the sludge ash was calculated by the following formula.
Pi—the phosphorus content of different forms in the sample, the subscript i stands for TP, IP, OP, NAIP, and AP, mg/g; v—the diluent volume in a colorimetric tube with a plug, mL; c—the phosphorus concentration in a diluent, μg/mL; β—dilution times; and m—sample mass, g
Figure 2.
Standard working curve of phosphorus.
Generally, the dry weight of the sample to be tested is 0.2 g. In order to ensure the reliability of the results, three parallel tests were carried out during the experiment, and the results listed in the article were average values.
2.6. X-Ray Diffraction
The incinerated ash sample was tested in XRD analysis using the X’Pert PRO MPD X-ray diffractometer from PANalytical Company. The residual ash samples after incineration were milled to less than 0.0075 mm using an agate mortar for powder tablet pressing. The scanning angle was 5–80° and the scanning speed was 5°/min. The radiation was Cu Kα (1.5406 nm) under the conditions of 40 kV and 40 mA.
2.7. Phosphorus Bioavailability Test
The solubility of phosphorus-containing compounds in a 2% CA solution is an important indicator to measure the biological effectiveness of phosphorus in the compound.23 About 0.2 g of the sample was leached by 20 mL of a 2% CA solution at room temperature for 16 h. The lixivium was separated by centrifugal filtration. The total amount of soluble phosphorus in the lixivium was measured by molybdenum blue spectrophotometry as mentioned in Section 2.5.
3. Results and Discussion
3.1. Distribution of Phosphorus Forms in Sludge and CS Ashes
The phosphorus speciation in dry sludge, sludge ash, and CS ash is shown in Figure 3. The TP and IP contents in the dry sludge are 27.95 and 23.74 mg/g, respectively, and the OP content is relatively high. The TP content in the SS ash is 37.59 mg/g, and the OP content is only 0.79 mg/g. The proportion of OP decreases, which may be attributed to the decomposition of organic matter during combustion, causing phosphorus in the organics to be released and further react with other minerals in sludge.34 The content of AP in dry sludge is 9.9 mg/g, which is lower than the content of NAIP. The proportion of AP in the sludge ash is still less than 50%. The AP content in CS ash is much lower than that of NAIP. This result indicates that the proportion of AP is relatively low in the residual ash of SS or CS incinerated individually.
Figure 3.
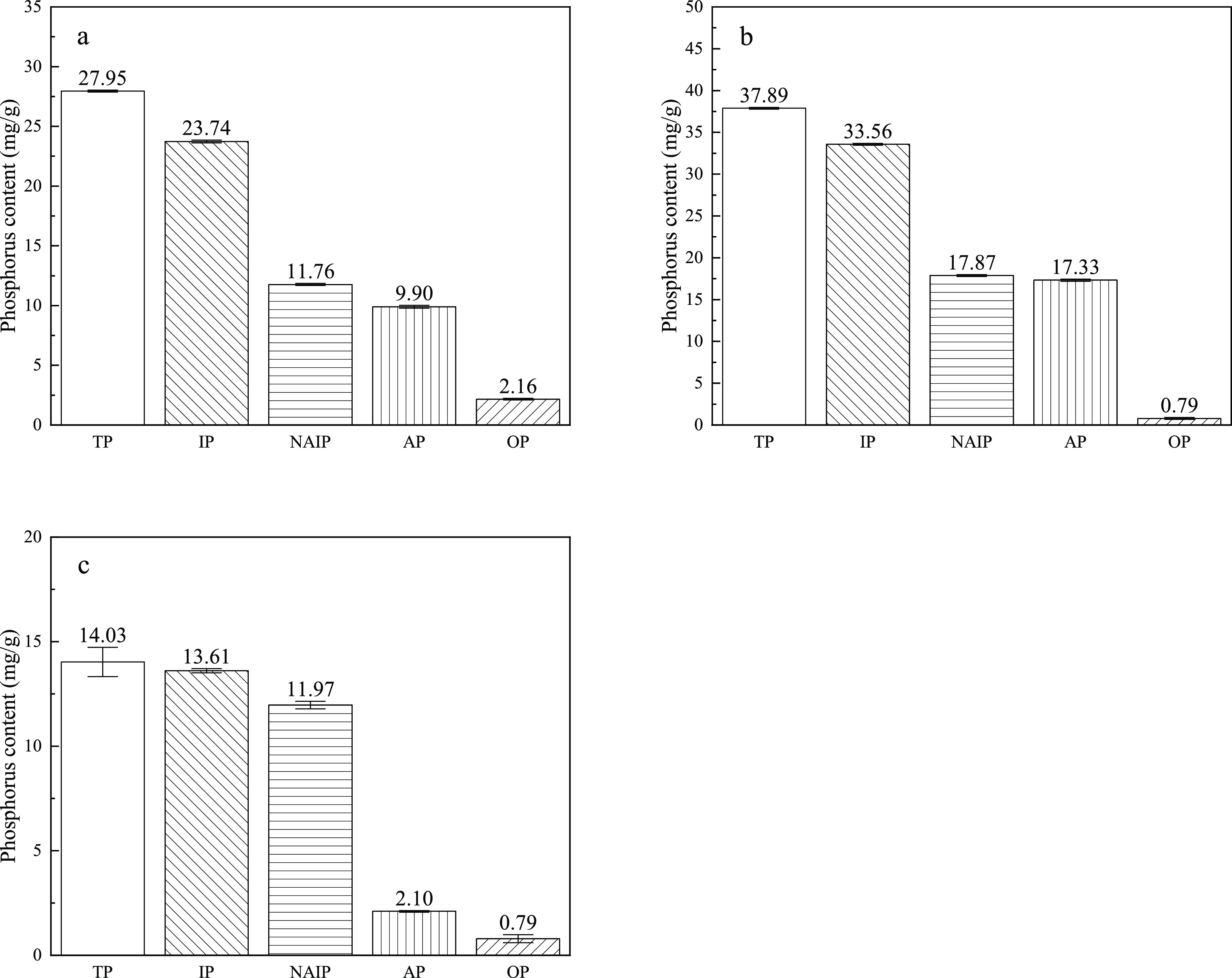
Phosphorus content of different forms in the dry sludge and the samples burned separately at 750 °C (a) dry sludge, (b) sludge ash, and (c) CS ash.
3.2. Influence of Temperature on Phosphorus Specification in Incinerated Ash
Figure 4 shows the effect of temperature on phosphorus speciation in the sludge ash and the mixed sludge and CS ash. The Ca/P of the original sludge ash is about 1:1, and the TP content in the sludge ash decreases from 37.59 mg/g at 750 °C to 27.96 mg/g at 1050 °C. At higher temperatures, the phosphorus transfer into the fly ash is more and the measured phosphorus in the residual ash is less.31,35 With the increase in temperature, the content of NAIP gradually decreases and the content of AP increases, indicating that the increase in temperature is conducive to the conversion of phosphorus in the sludge into AP. Figure 4b–d shows the phosphorus speciation in mixed sludge and CS samples with a Ca/P of 1.5–2.5. As the temperature increases, the NAIP content decreases and AP content increases gradually. We can conclude that for samples with different Ca/P, the increase in temperature is conducive to the conversion of NAIP to AP. In addition, the TP content in the mixed sludge and CS samples changed slightly with the increase in temperature, which indicates that the addition of CS is beneficial to the fixation of phosphorus.
Figure 4.
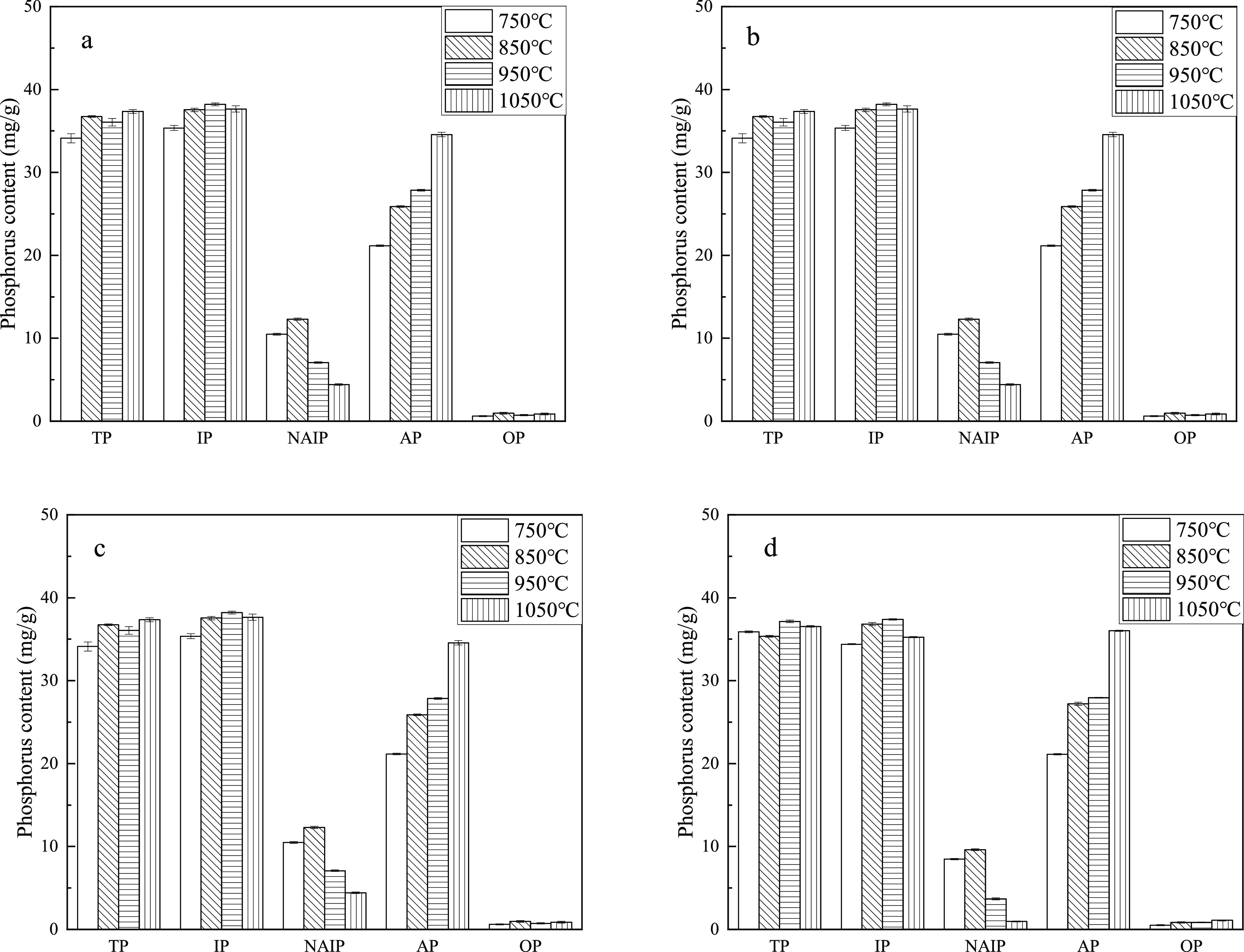
Distribution of various phosphorus forms in combustion ash at different temperatures, (a) Ca/P 1.0, (b) Ca/P 1.5, (c) Ca/P 2.0, and (d) Ca/P 2.5.
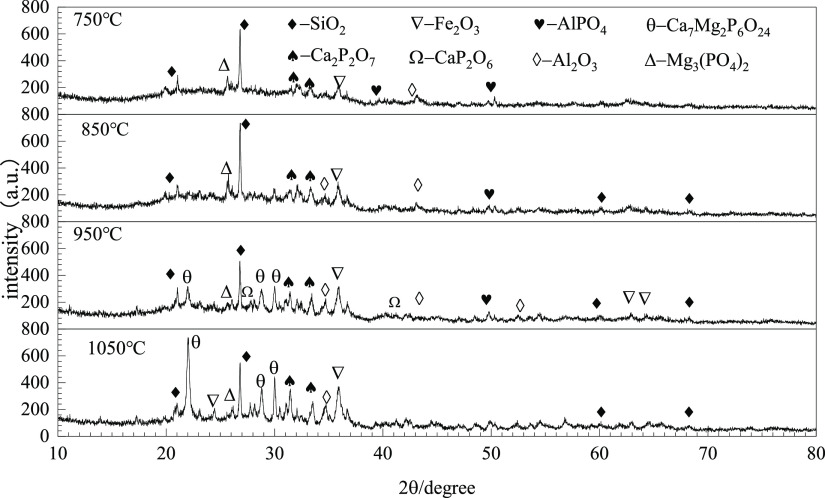
Figure 5 shows the crystal diffraction pattern of sludge ash burned at 750 and 1050 °C. The main diffraction peaks detected in the sludge at 750 °C are FePO4, AlPO4, and SiO2. It is consistent with the high proportion of NAIP in Figure 4. Ca2P2O7 and Mg3(PO4)2 are also detected, but the peaks are weak. From the XRD pattern of ash prepared at 1050 °C, the diffraction peaks of Ca7Mg2P6O24, CaP2O6, Fe2O3, and Al2O3 appeared in the diffraction pattern of sludge ash. Meanwhile, the intensity of the diffraction peaks of FePO4 and AlPO4 decreased, and the intensity of the diffraction peaks of Ca2P2O7 increased. As the (Ca + Mg)/P in Ca7Mg2P6O24 is 1.5 and the content of P2O5, CaO, and MgO in the sludge ash is 10.00, 8.67, and 3.02, respectively, meaning a (Ca + Mg)/P of 1.62 in the sludge ash, the content of Ca and Mg mineral is enough for the formation of Ca–P or Ca–Mg–P. However, the AP content in sludge ash at 1050 °C indicates that the conversion is not complete under the conditions. The comparison of crystal components in ash that was prepared at 750 and 1050 °C shows that the phosphates that originally existed mainly as Fe– and Al–phosphate can react with the inner Ca- and Mg-containing minerals in sludge ash to generate Ca- and Ca/Mg-containing phosphate at higher temperatures, which agrees with the upward trend of the AP content with temperatures in sludge ash.
Figure 5.
XRD pattern of sludge ash after incineration at 750 and 1050 °C.
Figure 6 shows the crystal diffraction pattern of ash samples with a Ca/P of 2.5 at 750–1050 °C. The diffraction peaks of Mg3(PO4)2, Ca2P2O7, AlPO4, Fe2O3, and Al2O3 exist in SC2.5-750. The diffraction peaks of Mg3(PO4)2, Fe2O3, and Ca2P2O7 increased at 850 °C. In SC2.5–950, the diffraction peaks of Ca7Mg2P6O24 and CaP2O6 appeared, and in SC2.5–1050. The intensity of peaks of Al2O3 and Fe2O3 gradually increase with temperature, which can be attributed to the transformation of Fe–P and Al–P to Ca–P as shown in the eqs 1 and 2.27 The diffraction peaks of Ca7Mg2P6O24 did not exist in SC2.5–850 and increase significantly at 1050 °C. Ca7Mg2P6O24 was reported to utilize as a fertilizer.36 As the intensity of diffraction peaks of Ca7Mg2P6O24 and Ca2P2O7 increases with temperature, it can be speculated that both Ca7Mg2P6O24 and Ca2P2O7 both have contributed to the increase of the AP content measured in the SMT result.
| 1 |
| 2 |
Figure 6.
XRD pattern of burning ash at a Ca/P of 2.5.
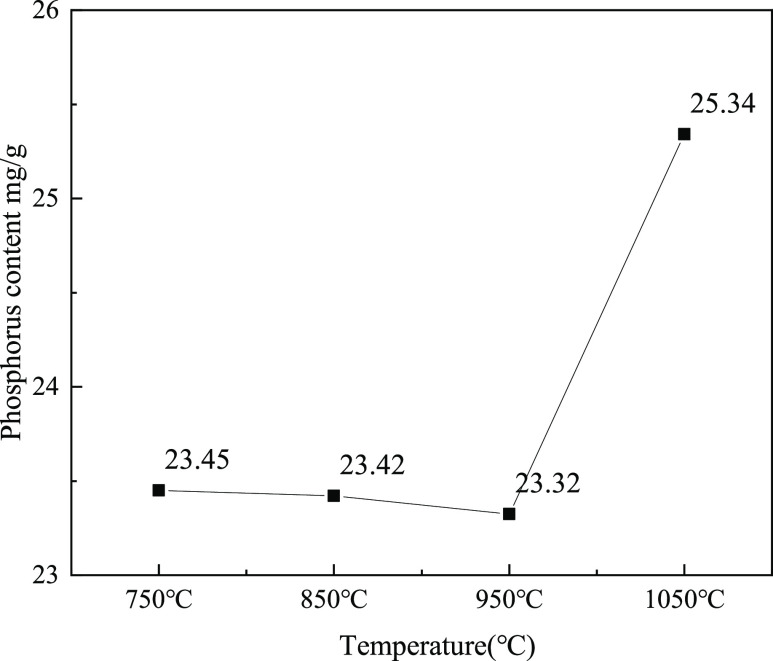
However, the AP content in SMT is not always equal to the biologically available phosphorus.25,26 The bioavailability of samples was tested by the leaching experiment of CA, and the results are shown in Figure 7. For the individual sludge ash, the content of dissolved phosphorus in 2% CA is 20.37 mg/g at 1050 °C and 23.55 mg/g at 750 °C, which is inconsistent with the change of the AP content in the two samples. This phenomenon illustrates that the mineral type of AP is also important. For the mixed samples, the content of dissolved phosphorus in 2% CA remains unchanged from 750 to 950 °C and increases to 25.34 mg/g at 1050 °C. It is worth noting that the intensity of the diffraction peak of Ca7Mg2P6O24 also becomes stronger, and CaP2O6 disappears in the process of rising from 950 to 1050 °C. Ca2P2O7, CaP2O6, and Ca7Mg2P6O24 are all present as AP in SMT, but CaP2O6 is insoluble in the CA.37 Ca7Mg2P6O24 appears above 850 °C and only abounds in the ash samples that burned at a higher temperature (1050 °C in this study). Ca7Mg2P6O24 may play the most important role in raising the bioavailability of phosphorus in sludge ash.
Figure 7.
Phosphorus content dissolved in 2% CA of ash with a Ca/P of 2.5 prepared at different temperatures.
3.3. Effect of Ca/P on the Phosphorus Specification in Ash Samples
Figure 8 shows the distribution of NAIP, AP, and OP in the combustion ash with a Ca/P of 1.0–2.5. At 750 °C, the proportion of NAIP dropped from 50 to 28%, while the proportion of AP rose from 48 to 70% with the increase of Ca/P. The comparison of the AP content in samples with a Ca/P of 1.0 and 1.5 shows that the change from individual sludge to blended ash of CS and sludge is substantial. It implies that the external calcium resource from CS can react with the phosphorus in sludge at 750 °C. For samples at higher temperature, the AP content increases with the Ca/P and NAIP content decreases, which is similar to the trend of samples prepared at 750 °C. For samples prepared at 1050 °C, the AP proportion did not change much with Ca/P as Ca/P is higher than 1.5, but NAIP still exist even in the sample with Ca/P of 2.5. In the SC2.5–1050, the AP content reaches 95% and NAIP is 3%. It can be concluded that for samples incinerated at different temperatures, the increase of Ca/P can promote the conversion of NAIP to AP, but the conversion of NAIP to AP is still not complete in SC2.5–1050. The Ca/P of 2.5 may be a proper ratio with regard to providing enough calcium or magnesium resources for the reaction that forms AP mineral.
Figure 8.
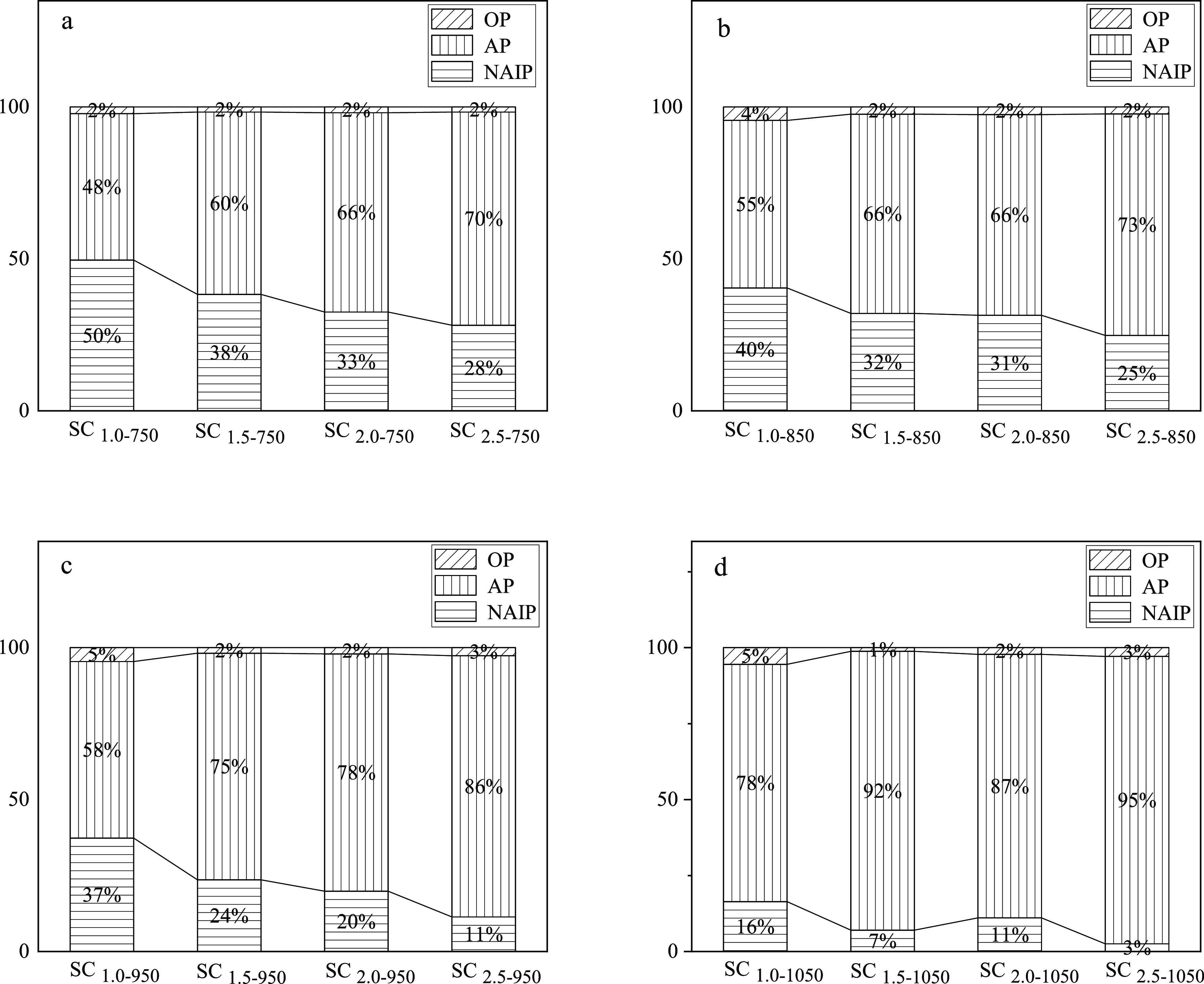
Distribution of NAIP, AP, and OP in different Ca/P combustion ash, (a) 750, (b) 850, (c) 950, and (d) 1050 °C.
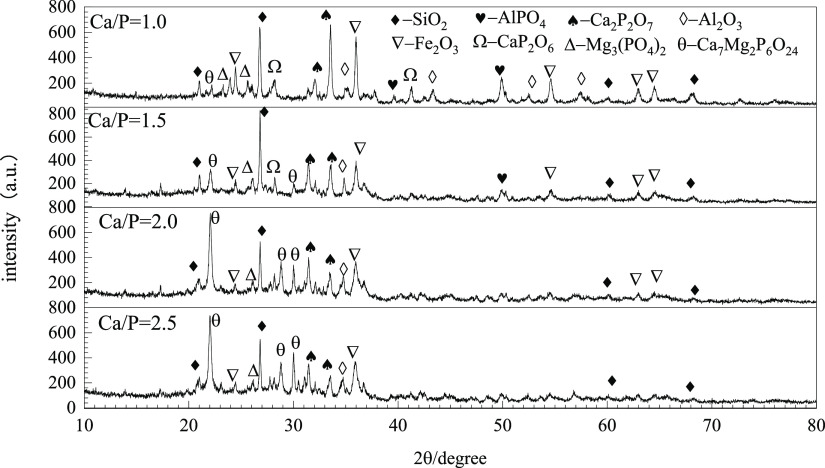
Figure 9 shows the XRD pattern of the combustion ash with a Ca/P ratio of 1.0–2.5 at 1050 °C. The diffraction peaks of Ca2P2O7, SiO2, Fe2O3, CaP2O6, Ca7Mg2P6O24, AlPO4, and Mg3(PO4)2 exist in SC1.0–1050. The intensity of the diffraction peaks of Ca2P2O7 is weaker and the intensity of the diffraction peaks of Ca7Mg2P6O24 becomes stronger as Ca/P increase. The diffraction peak of CaP2O6 disappeared in SC2.0–1050. The Ca/P in CaP2O6 and Ca2P2O7 is 0.5 and 1.0, respectively. Enough calcium resources are conducive for the formation of Ca2P2O7, which has a better bioavailability than CaP2O6 although they are both Ca-containing phosphate. The diffraction peak of Ca7Mg2P6O24 is more prominent in SC2.0–1050 and SC2.5–1050. It is speculated that the change of species is related to Ca/P, but the AP (mainly refers to Ca–P and Mg–P) content did not change much as they are all Ca-containing phosphates. However, the AP species are different in their solubility in CA. The effect of Ca/P on the AP content at 1050 °C is weak, but the AP species still change with Ca/P.
Figure 9.
XRD pattern of ash with different Ca/P prepared at 1050 °C.
Figure 10 shows the dissolved phosphorus content in 2% CA for samples prepared at 1050 °C with Ca/P from 1.0 to 2.5. As Ca/P increased from 1.0 to 2.5, the bioavailability of phosphorus increased, and the dissolved phosphorus content in 2% CA rose from 20.37 to 25.34 mg/g. The AP content in the samples burned at 1050 °C did not change much with Ca/P, but the variation of AP species results in the dissolved content of phosphorus. Higher Ca/P ratios in the blended ash are conducive for the formation of Ca7Mg2P6O24, which may have better bioavailability than Ca2P2O7.
Figure 10.
Phosphorus content dissolved in 2% CA of samples prepared at 1050 °C.
4. Conclusions
In this study, the changes of phosphorus species in the sludge and the blended samples with Ca/P in the range of 1.0–2.5 were studied, and the influence of temperature and additives on the phosphorus form and bioavailability of the sludge ash and the blended sludge and CS was discussed by combining XRD and CA leaching experiments.
The apatite inorganic phosphorus content is low in the residual ash of the sludge or CSs that were burned individually. The results of phosphorus species in samples incinerated at different temperatures show that the conversion of NAIP to AP was promoted as the incineration temperature increased. Higher temperatures promote the production of Ca2P2O7 and CaP2O6, but the bioavailability changes little. Because CaP2O6 is insoluble in CA, the bioavailability changes little with temperatures, although the AP content increases. The increase in Ca/P promotes the conversion of NAIP to AP and also the bioavailability of phosphorus in the mixed ash samples. For the blended sludge and CS, Ca7Mg2P6O24 appear at 950 °C and 1050 °C and the bioavailability also increases. Ca7Mg2P6O24 is easier to form at high temperatures (>950 °C) with enough calcium and magnesium resources.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (nos. 21808045 and 42063008), the Guizhou Provincial Science and Technology Foundation ([2019]1066 and [2019]1230), the Guizhou Science and Technology Platform Talents ([2018]5781).
The authors declare no competing financial interest.
References
- Acelas N. Y.; López D. P.; Brilman D. W. F.; Kersten S. R. A.; Kootstra A. M. J. Supercritical water gasification of sewage sludge: gas production and phosphorus recovery. Bioresour. Technol. 2014, 174, 167–175. 10.1016/j.biortech.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Desmidt E.; Karel G.; Yang Z.; Luc P.; Bart V. D. B.; Willy V.; Korneel R.; Boudewijn M. Global Phosphorus Scarcity and Full-Scale P-Recovery Techniques: A Review. Crit. Rev. Environ. Sci. Technol. 2014, 45, 336–384. 10.1080/10643389.2013.866531. [DOI] [Google Scholar]
- Tilman D.; Fargione J.; Wolff B.; D’Antonio C.; Dobson A.; Howarth R.; Schindler D.; Schlesinger W. H.; Simberloff D.; Swackhamer D. Forecasting Agriculturally Driven Global Environmental Change. Science 2001, 292, 281–284. 10.1126/science.1057544. [DOI] [PubMed] [Google Scholar]
- Li R.; Jing Y.; Weiyun W.; Yanlong L.; Ziheng Z. Transformation of phosphorus during drying and roasting of sewage sludge. Waste Manag. 2014, 34, 1211–1216. 10.1016/j.wasman.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Shepherd J. G.; Rosanna K.; Jaleh B.; Lee H.; Lalith S.; Elke V.; Kimo C. V. D. The future of phosphorus in our hands. Nutr. Cycling Agroecosyst. 2015, 104, 281–287. 10.1007/s10705-015-9742-1. [DOI] [Google Scholar]
- Nakakubo T.; Tokai A.; Ohno K. Comparative assessment of technological systems for recycling sludge and food waste aimed at greenhouse gas emissions reduction and phosphorus recovery. J. Clean. Prod. 2012, 32, 157–172. 10.1016/j.jclepro.2012.03.026. [DOI] [Google Scholar]
- GEP-Research . Global and China Sludge Treatment and Disposal Industry Development Research Report, 2019. http://www.gepresearch.com/77/view-767588-1.html.
- Havukainen J..; Mai T. N.; Ludwig H.; Mika H.; Mirja M.; Ivan D.; Lassi L. Potential of phosphorus recovery from sewage sludge and manure ash by thermochemical treatment. Waste Manag. 2016, 49, 221–229. 10.1016/j.wasman.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Donatello S.; Christopher R. C. Recycling and recovery routes for incinerated sewage sludge ash (ISSA): A review. Waste Manag. 2013, 33, 2328–2340. 10.1016/j.wasman.2013.05.024. [DOI] [PubMed] [Google Scholar]
- Herzel H.; Oliver K.; Ludwig H.; Christian A. Sewage sludge ash--A promising secondary phosphorus source for fertilizer production. Sci. Total Environ. 2015, 542, 1136–1143. 10.1016/j.scitotenv.2015.08.059. [DOI] [PubMed] [Google Scholar]
- Tarayre C.; Lies D. C.; Raphaëlle C.; Evi M.; Erik M.; Miller C.; Frank D. New perspectives for the design of sustainable bioprocesses for phosphorus recovery from waste. Bioresour. Technol. 2016, 206, 264–274. 10.1016/j.biortech.2016.01.091. [DOI] [PubMed] [Google Scholar]
- Ohtake H.; Tsuneda S.. Phosphorus Recovery and Recycling Full-Scale plants, 1st ed.; Springer: Singapore, 2019; pp 8–13. [Google Scholar]
- Wang J.-Y.; DiSong Z.; Olena S.; JooHwa T. Evaluation of electrokinetic removal of heavy metals from sewage sludge. J. Hazard. Mater. 2005, 124, 139–146. 10.1016/j.jhazmat.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Yao Y.; WenBing L.; QingNa K.; YuYong W.; Ruo H.; DongSheng S. Content, mobility and transfer behavior of heavy metals in MSWI bottom ash in Zhejiang province, China. Fuel 2010, 89, 616–622. 10.1016/j.fuel.2009.06.016. [DOI] [Google Scholar]
- Carbonell G.; Pro J.; Gómez N.; Babín M. M.; Fernández C.; Alonso E.; Tarazona J. V. Sewage sludge applied to agricultural soil: Ecotoxicological effects on representative soil organisms. Ecotoxicol. Environ. Saf. 2009, 72, 1309–1319. 10.1016/j.ecoenv.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Wang T.; Marta C.; Mike H.; Peter B. Predicting phosphorus bioavailability from high-ash biochars. Plant Soil 2012, 357, 173–187. 10.1007/s11104-012-1131-9. [DOI] [Google Scholar]
- Huang R.; Ci F.; Xiaowei L.; Rongfeng J.; Yuanzhi T. Transformation of Phosphorus during (Hydro)thermal Treatments of Solid Biowastes: Reaction Mechanisms and Implications for P Reclamation and Recycling. Environ. Sci. Technol. 2017, 51, 10284–10298. 10.1021/acs.est.7b02011. [DOI] [PubMed] [Google Scholar]
- Xiaodi H.; Jinglun Y.; Ranbin L.; Yuan L.; Fusheng L. Phosphorus recovery from excess sludge incineration ash and its technical progress. Acta Sci. Circumstantiae 2020, 40, 1149–1159. [Google Scholar]
- Ruban V.; López-Sánchez J. F.; Pardo P.; Rauret G.; Muntau H.; Quevauviller P. Development of a harmonised phosphorus extraction procedure and certification of a sediment reference material. Journal of Environmental Monitoring 2001, 3, 121–125. 10.1039/b005672n. [DOI] [PubMed] [Google Scholar]
- Pardo P.; López-Sánchez J. F.; Rauret G. Relationships between phosphorus fractionation and major components in sediments using the SMT harmonised extraction procedure. Anal. Bioanal. Chem. 2003, 376, 248–254. 10.1007/s00216-003-1897-y. [DOI] [PubMed] [Google Scholar]
- Thin M. M.; Sacchi E.; Setti M.; Re V. A Dual Source of Phosphorus to Lake Sediments Indicated by Distribution, Content, and Speciation: Inle Lake (Southern Shan State, Myanmar). Water 2020, 12, 1993. 10.3390/w12071993. [DOI] [Google Scholar]
- Tan Z.; Lagerkvist A. Phosphorus recovery from the biomass ash: A review. Renew. Sustain. Energy Rev. 2011, 15, 3588–3602. 10.1016/j.rser.2011.05.016. [DOI] [Google Scholar]
- Adam C.; Peplinski B.; Michaelis M.; Kley G.; Simon F. G. Thermochemical treatment of sewage sludge ashes for phosphorus recovery. Waste Manag. 2009, 29, 1122–1128. 10.1016/j.wasman.2008.09.011. [DOI] [PubMed] [Google Scholar]
- Li R.; Teng W.; Li Y.; Wang W.; Cui R.; Yang T. Potential recovery of phosphorus during the fluidized bed incineration of sewage sludge. J. Clean. Prod. 2017, 140, 964–970. 10.1016/j.jclepro.2016.06.177. [DOI] [Google Scholar]
- Jie X.; Qunxing H.; Xiangdong M.; Huaping G. Effects of calcium-based additives on phosphorus speciation and bioavailability of sewage sludge during hydrothermal carbonization. Chem. Ind. Eng. Prog. 2021, 40, 3507–3514. [Google Scholar]
- Meng X.; Qunxing H.; Huaping G.; Kangrou T.; Jianhua Y. Improved utilization of phosphorous from sewage sludge (as Fertilizer) after treatment by Low-Temperature combustion. Waste Manag. 2018, 80, 349–358. 10.1016/j.wasman.2018.09.034. [DOI] [PubMed] [Google Scholar]
- Li R.; Ziheng Z.; Yanlong L.; Wenchao T.; Weiyun W.; Tianhua Y. Transformation of apatite phosphorus and non-apatite inorganic phosphorus during incineration of sewage sludge. Chemosphere 2015, 141, 57–61. 10.1016/j.chemosphere.2015.05.094. [DOI] [PubMed] [Google Scholar]
- Qian T.-T.; Hong J. Migration of Phosphorus in Sewage Sludge during Different Thermal Treatment Processes. ACS Sustain. Chem. Eng. 2014, 2, 1411–1419. 10.1021/sc400476j. [DOI] [Google Scholar]
- Xu Y.; Fei Y.; Liang Z.; Xin W.; Ying S.; Qiang L.; Guangren Q. Migration and transformation of phosphorus in municipal sludge by the hydrothermal treatment and its directional adjustment. Waste Manag. 2018, 81, 196–201. 10.1016/j.wasman.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Joris S. J.; Amberg C. H. Nature of deficiency in nonstoichiometric hydroxyapatites. I. Catalytic activity of calcium and strontium hydroxyapatites. J. Phys. Chem. 1971, 75, 3167–3171. 10.1021/j100689a024. [DOI] [Google Scholar]
- Zhao Y.; Qiangqiang R.; Yongjie N. Phosphorus Transformation from Municipal Sewage Sludge Incineration with Biomass: Formation of Apatite Phosphorus with High Bioavailability. Energy Fuels 2018, 32, 10951–10955. 10.1016/j.fuel.2017.11.044. [DOI] [Google Scholar]
- Zhao Y.; Qiangqiang R.; Yongjie N. Promotion of cotton stalk on bioavailability of phosphorus in municipal sewage sludge incineration ash. Fuel 2018, 214, 351–355. [Google Scholar]
- Ren Q.; Li L. Co-combustion of Agricultural Straw with Municipal Sewage Sludge in a Fluidized Bed: Role of Phosphorus in Potassium Behavior. Energy Fuels 2015, 29, 4321–4327. 10.1021/acs.energyfuels.5b00790. [DOI] [Google Scholar]
- Andrieux F.; Aminot A. A two-year survey of phosphorus speciation in the sediments of the Bay of Seine (France). Cont. Shelf Res. 1997, 17, 1229–1245. 10.1016/S0278-4343(97)00008-3. [DOI] [Google Scholar]
- Zhao Y.; Qiangqiang R.; Yongjie N. Potential utilization of phosphorus in fly ash from industrial sewage sludge incineration with biomass. Fuel Process. Technol. 2019, 188, 16–21. 10.1016/j.fuproc.2019.02.005. [DOI] [Google Scholar]
- Myojin S.; Kuroki T.; Manabe W.; Yamasaki C.; Yamasaki N. Hydrothermal Detoxization of Slate Containing Asbestos and the Possibility of Application for Fertilizer of its Products. AIP Conf. Proc. 2010, 1251, 348–351. 10.1063/1.3529320. [DOI] [Google Scholar]
- Rongsheng L. Production of calcium metaphosphate fertilizer from yellow phosphorus and phosphate rock. Yunnan Chem. Technol. 1989, 3, 44–46. [Google Scholar]