Abstract
The poor oral bioavailability, rapid biotransformation to less active metabolites, and fast elimination from systemic circulation have been identified as the major limitations responsible for the clinical insignificance of many drug candidates and phytonutrients. Despite the technological advancements in the nanoformulations of synthetic drugs, there exist many challenges for nutritional therapy, due to the regulatory issues, use of high levels of synthetic emulsifiers and polymers, low stability, low loading levels, mainly liquid state, etc. Herein, we report the characterization and human pharmacokinetics of a natural self-emulsifying hybrid-hydrogel formulation of trans-resveratrol prepared by uniformly impregnating resveratrol micelles into the fenugreek galactomannan hydrogel scaffold to form a water-soluble micelle/hydrogel composite in powder form (RF-20). Fourier transform infrared spectroscopy (FTIR), powder X-ray diffraction (PXRD), scanning electron microscopy (SEM), particle size analysis by dynamic light scattering (DLS), and transmission electron microscopy (TEM) demonstrated the uniform impregnation of resveratrol micelles within the galactomannan hydrogel matrix to form a soluble (average particle size of 172.0 ± 10.4 nm and −21.0 ± 2.5 mV zeta potential) and amorphous powder form with smooth and translucent surface morphology for RF-20, with no chemical alterations. Upon pharmacokinetic studies on healthy human subjects (n = 16) following a randomized, double-blinded, placebo-controlled, 2-arm, 4-sequence crossover design and tandem mass spectrometry (UPLC-ESI-MS/MS), 80 mg of trans-resveratrol from RF-20 provided enhanced free resveratrol bioavailability and pharmacokinetic properties compared to the unformulated resveratrol with 98% purity. The enhancement in bioavailability was more when supplemented in sachet (12.98-fold) form than the capsule (10.48-fold) with improved absorption (Cmax = 50.97 ± 15.82 ng/mL), circulation half-life (t1/2 = 7.01 ± 1.44 h), and sustained delivery (Tmax = 4.71 ± 0.73 h), as compared to the unformulated form (Cmax = 15.07 ± 5.10 ng/mL; t1/2 = 1.58 ± 0.65 h; Tmax = 1.21 ± 0.42 h).
1. Introduction
The poor oral bioavailability of the bioactive forms has been shown to limit the therapeutic applications of many lipophilic drug candidates.1 In the case of phytonutrients, the poor bioavailability resulting from the low solubility, rapid intestinal/hepatic biotransformation to inactive or weak metabolites, and/or fast elimination from the systemic circulation have been identified as the main reasons for the observed gap between the interesting pharmacodynamics and clinical efficacy.2,3 Though nanodelivery forms such as liposomes, micelles, self-emulsifying systems, and solid–lipid nanoparticles could offer significant enhancement in the oral bioavailability, they very often suffer from several limitations, especially due to their liquid state, low loading level, and poor stability (both storage and in vivo stability), in addition to the possibility of fast clearance from systemic circulation and opsonization.3−5 The low thermodynamic stability and high free energy of the nanoformulations may also lead to aggregation/flocculation or fusion/coalescence, leading to the alterations of the structural integrity of the vesicles and hence the active molecule seepage.6 Extensive use of non-food-grade synthetic polymers and emulsifiers is another drawback, which is limiting their applications to nutrients (Nutraceuticals and Functional food). Surface modification of the nanostructures by chemical reactions has been reported to improve their stability, permeability, and hence in vivo delivery.7 We hypothesized that the hybrid-hydrogel formation using nanostructures and suitable natural biopolymers would be able to act as a surface modification or hydrogel trap to provide a “capping effect” to the nanostructures to overcome their limitations and hence to produce stable nanopowders with enhanced water solubility and stability. Hybrid-hydrogel, an emerging approach in drug delivery, may be defined as a composite formed by functionally, morphologically, and chemically/physically different building blocks with an effective hybridization at the molecular level.4 They are characterized by high water holding capacity, swelling/deswelling index, permeability, thermodynamic stability, enhanced solubility, low surface tension, biocompatibility, and biodegradability, in addition to their similarity with the extracellular matrix.4,8
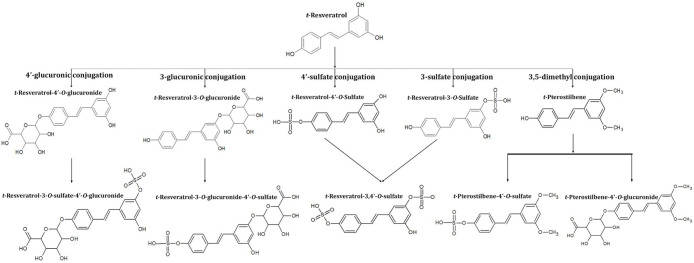
Resveratrol or 3,5,4′-trihydroxystilbene, [5-[(E)-2-(4-hydroxyphenyl) ethenyl benzene −1,3-diol], a nonflavonoid polyphenol found in various plants, including grapes, berries, cocoa, peanuts, tea, and certain herbs like Japanese knotweed (Polygonum cuspidatum), as a phytoalexin, is of great recent interest in Nutraceuticals and Functional food because of its beneficial health pharmacological effects, including antioxidant, anti-inflammatory, immunomodulatory, hepatoprotective, cardioprotective, neuroprotective, antiaging, and anticarcinogenic effects, through a pleiotropic mechanism of action involving the modulation of various gene/protein expressions and transcription factors.9−14 It has also been established as safe and tolerated even at a repeated dose as high as 5 g/day, without major side effects.11,15 However, this Class II BCS molecule (Biopharmaceutics Classification System) with poor solubility (<20 μg/mL) but good permeability undergoes rapid biotransformation to soluble glucuronides and sulfates in the intestine/liver, leading to the fast clearance and poor oral bioavailability of the native “free” (unconjugated) form (Figure 1).10,16−18 The free form in circulating plasma is rare with less than 1% bioavailability even upon repeated dosage as high as 5 g/day for 28 days.15,17,19 The free form is considered as the major bioactive form of resveratrol since the in vitro studies on the conjugated glucuronide and sulfate metabolites have shown mixed effects and in certain cases either weak or “no” activity as compared to the free resveratrol.8,20,21 High water solubility, relatively high molecular weight, low membrane permeability, and rapid renal elimination have been identified as the major limitations of the conjugated metabolites, albeit the hypothesis is that they may undergo deconjugation inside the cell by ubiquitously present sulfatase/glucuronidase to generate the free from in the cell.22−24 Thus, the therapeutic potential or functional benefit of resveratrol has been correlated to the poor bioavailability of the free form in the circulation and target tissues, despite the high intestinal absorption (∼75%) and micromolar plasma levels of glucuronides/sulfates.17,19
Figure 1.
Biotransformation of trans-resveratrol.
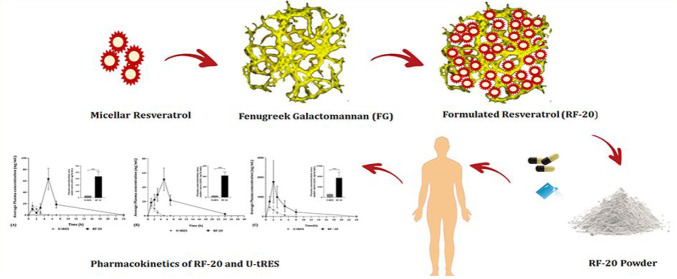
A number of attempts especially based on liposomes, micelles, solid–lipid nanoparticles, micronized particles, nanocrystals, and chemically modified forms have been reported to enhance the oral bioavailability of resveratrol in animals.19,25,26 However, none of the reports have provided significant improvement in the bioavailability of “free” form, though extensively conjugated forms were found in the plasma.11,26,27 Earlier, we had reported a green method to improve the bioavailability of lipophilic substances using the soluble dietary fiber (galactomannan) in fenugreek (Trigonella foenum graecum) seeds as a soft hydrogel scaffold (FENUMAT).28,29 When applied to curcumin, the bioactive molecule in turmeric, the technology could significantly enhance free curcumin bioavailability and blood–brain-barrier permeability.24,30 Herein we report the development of a modified FENUMAT technology, referred to as Hybrid-FENUMAT, as a “natural self-emulsifying reversible hybrid-hydrogel” delivery system (N’SERH) following the concept of hybrid-hydrogel. In this technology, resveratrol micelles were uniformly impregnated into the fenugreek hydrogel matrix to form a micelle/hydrogel composite (RF-20) in powder form. The present study investigated the solubility, particle size, and morphology of RF-20 and its influence on the bioavailability and pharmacokinetic properties of free resveratrol in human volunteers.
2. Materials and Methods
2.1. Preparation of the Hybrid-Hydrogel Form of Resveratrol (RF-20)
Hybrid-hydrogel was prepared by incorporating precasted resveratrol micelles into a fenugreek galactomannan hydrogel matrix by a gel-phase thin-film dispersion method followed by dehydration under vacuum. Briefly, 10 g of resveratrol was heated with 5 g of sunflower oil to form a solution and then slowly added to 15 g of lecithin dissolved in ethanol/water (25/75, v/v). The mixture was homogenized and kept stirring for 8 h. The solution was evaporated at 50 ± 5 °C to remove ethanol and to form the micelles. The micelles were then uniformly dispersed into fenugreek galactomannan hydrogel by homogenization and dehydrated to the powder form (RF-20). Reproducibility of the process was confirmed by repeating the process three times on three different occasions followed by the particle size and polydispersity index (PDI) analysis.
2.2. Characterization of RF-20
Resveratrol content in the formulation was determined by a validated high-performance liquid chromatography (HPLC) method,31 employing a Shimadzu LC 20AT instrument fitted with a M20A photodiode array detector (PDA) (Shimadzu Analytical Private Limited., Mumbai, India) and a reverse-phase C18 Phenomenex column (250 × 4.6 mm, 3 μm).
The surface morphology of the powder was analyzed by scanning electron microscopy (SEM) using a ZEISS Sigma 500 VP, ZEISS Microscopy, Oberkochen, Germany. Crystallinity was analyzed by powder X-ray diffraction (PXRD) employing a Bruker D8 Advance instrument: target Cu, k = 1.54 Å, Ni filter, voltage of 40 kV, a time constant of 5 min/s, and a scanning rate of 1°/min (Bruker AXS GmbH, Karlsruhe, Germany). Particle size analysis was conducted by a dynamic light-scattering (DLS) method, employing the Horiba SZ-100 particle size analyzer, Horiba India Private Limited, Bengaluru, India. Transmission electron microscopy (TEM) analysis was performed for the structural characterization of particles in solution (JEOL JEM-2100 LaB6, Jeol Co Limited, Japan). The entrapment of resveratrol in fenugreek and its structural integrity were confirmed by Fourier-transform infrared spectrometry (FTIR) (Nicolet iS50 FTIR Spectrometer, Thermo Fisher Scientific, Massachusetts, USA) in the wavelength range 400–4000 cm–1.
2.3. In Vitro Release Kinetics
In vitro release of resveratrol was evaluated at pH 6.5 and 2.0 using phosphate buffer solution and 0.1 M HCl, respectively, for 24 h, employing a USP dissolution apparatus (Electro lab, Mumbai, India). Briefly, a known amount of RF-20 (50 mg) was dispersed in a 10 mL solution of appropriate pH and kept under constant stirring at 37.0 ± 0.5 °C. About 500 μL of clear solution was carefully withdrawn from the mixture at various time intervals (1, 3, 5, 8, and 24 h) and made up to 50 mL with methanol. The amount of resveratrol content in the solution was determined by HPLC, with the help of a calibration curve. The experiment was performed in triplicate, and the mean cumulative percentage of released resveratrol was calculated and plotted against time to follow the release kinetics.
2.4. Pharmacokinetics Study
A randomized, double-blinded, placebo-controlled, 2-arm, 4-sequence crossover design was followed for the pharmacokinetic study. The study was conducted as per the clinical research guidelines of the Government of India and the Declaration of Helsinki. The protocol was evaluated and approved by an independent ethical committee and was registered in the clinical trial registry of India [CTRI/2018/03/012753, dated 22/03/2018]. The study was conducted at the clinical pharmacology unit of Sri Rama Hospital, Bangalore, India. Healthy volunteers (aged 20 to 55 years; n = 25), who provided written informed consent, were selected based on a standard clinical assessment comprising a diagnostic interview, medical history assessment, and hematology/biochemical analysis. Pregnant and breast-feeding women were excluded. The participants were not allowed to drink alcohol or wine or consume fruits and juices or peanuts for 2 days before the study day.
The pharmacokinetic study was carried out in two phases. In the first phase, identical hard shell gelatin capsules of either the formulated or unformulated resveratrol (RF-20 or U-tRES) containing 80 mg of trans-resveratrol were provided. Each of the formulated capsules contained 400 ± 10 mg of RF-20, having 20.2% (w/w) of trans-resveratrol content along with another 50 mg of microcrystalline cellulose as an excipient. The unformulated capsule contained 80 ± 5 mg of trans-resveratrol with 98.2% purity isolated from Japanese Knotweed (U-tRES) by an ethanol/water extraction process and about 300 mg of microcrystalline cellulose as an excipient. In the second phase, the same dose of either the formulated or the unformulated resveratrol was administered in identical stick packs, each weighing about 3 g. Maltodextrin was used as an additive to make up the sachet packs to 3 g, and the participants were asked to drink one sachet with about 240 mL of water. Both the dosage forms (sachets and capsules) were received from Akay Natural Ingredients, Cochin, India, in sealed bottles, along with a detailed certificate of analysis and declaration about its safety and suitability for human consumption.
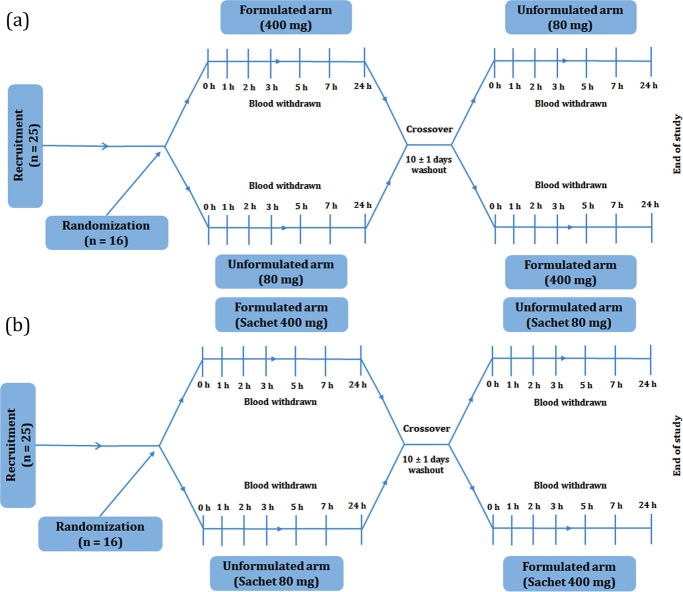
A schematic representation of the study procedure is shown in Figure 2. The selected participants were initially randomized into two groups and identified by a three-digit code. Each participant was then asked to report to the study center by 7–8 am in a fasting stage. After the withdrawal of the zero-time blood sample, either the RF-20 or U-tRES capsule was given for the first phase of the study. About 4 mL each of blood samples was withdrawn at regular postadministration time intervals (1, 2, 3, 5, 8, and 24 h), employing an indwelling venous cannula. Plasma was separated by centrifugation at 11 950g for 10 min at 4 °C and stored for a maximum of 2 days at −20 °C for analysis.
Figure 2.
Schematic representation of the pharmacokinetic study protocol. (a) Capsules and (b) sachets.
The same procedure was employed in the second phase of the study using sachets. All participants were provided with a standardized south Indian food comprising rice, vegetables, fish, and chicken curry for breakfast, lunch, and dinner. Breakfast was provided after the withdrawal of the blood sample at a 1 h time point, lunch after a 5 h time point, and dinner after an 8 h time point. A minimum of 10 days washout period was provided between the treatments.
2.5. UPLC-ESI-QTRAP-MS/MS Analyses of Resveratrol Content in Plasma
Plasma samples were extracted and subjected to mass spectrometric measurements, employing ultra performance liquid chromatography coupled with an electrospray ionization triple quadrupole ion trap tandem mass spectrometer (UPLC-ESI-QTRAP-MS/MS; 4500 QTRA, AB Sciex Private Limited, Singapore) for the detection, confirmation, and quantification of free form resveratrol, using their Multiple Reaction Monitoring (MRM). Separation of resveratrol was achieved with a Phenomenex Synergi 4 μm Fusion-RP 80 Å, LC column (50 × 2 mm, 1.8 μ), kept at 28 °C and using the mobile phase system consisting of (A) 5 mm of ammonium formate with 0.1% formic acid in water and (B) acetonitrile containing 0.1% formic acid in acetonitrile, set at a linear gradient of 20–100% B within 7 min at a 0.2 mL/min flow rate. A negative ion mode MRM was employed for MS/MS analysis: m/z (226.9 → 184.9; 226.9 → 142.9). Analyst workstation software version 1.7 with hotfix 3 was employed for data acquisition. The range and linearity of the extraction efficiency was determined by spiking 10 ng/mL of resveratrol in plasma along with the internal standard, salbutamol (10 ng/mL), followed by LC/MS/MS analysis. The accuracy and precision of the method were within the acceptable limits of 15%, as specified in ICH guidelines.
Free resveratrol from plasma was extracted with acetonitrile as previously mentioned.32−34 In a typical protocol, 1 mL of plasma was extracted with 4 × 1 mL of ice cold acetonitrile by vortex mixing for 1 min and centrifuged (9000g) at 4 °C for 15 min, and the top layer was collected. The extraction was repeated three times and evaporated at 40 ± 2 °C under a nitrogen atmosphere. The residue was reconstituted with 1 mL of acidified (0.1% formic acid) acetonitrile/water [90:10 (v/v)] and filtered through a 0.45 μm syringe filter; 3 μL was injected. The plasma concentration versus time plot was then constructed for both capsules and sachets, and the pharmacokinetics parameters were further deduced. The analytical standard of resveratrol (CAS No: 501-36-0) and the internal standard salbutamol (18559-94-9) were purchased from Sigma-Aldrich, Bangalore, India. All solvents used for analysis were of LC-MS grade and purchased from Merck, Mumbai, India.
2.6. Statistical Analysis
Statistical analysis was performed using SPSS software version 27, and all data points were expressed as mean ± SD. All intergroup comparisons of pharmacokinetic parameters and their mean and percentage changes from the baseline were performed using analysis of variance (ANOVA) followed by Dunnett’s test to estimate the differences between the groups. P < 0.05 was considered statistically significant. *P < 0.05; ***P < 0.001; GraphPad Prism Version 5.0 was used to plot the graph.
3. Results
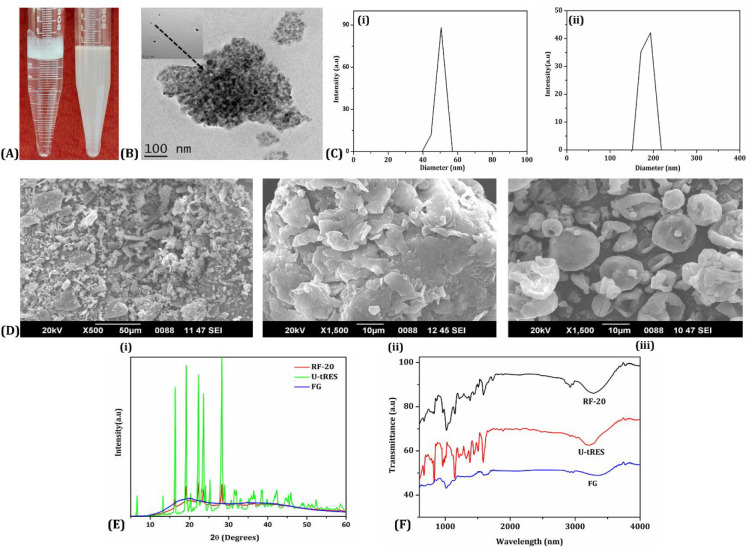
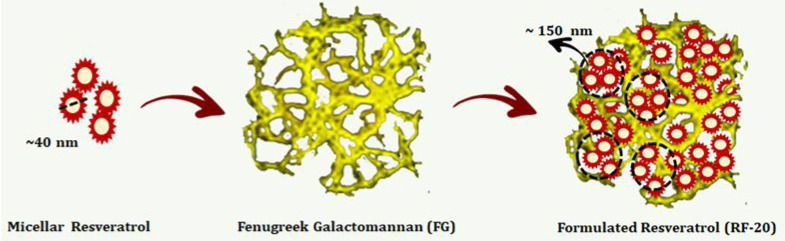
Unformulated trans-resveratrol (U-tRES) was isolated from Japanese Knotweed as a white powder with 98.2% purity, and the formulation RF-20 was a creamy white powder with 20.2% of resveratrol content, as per HPLC analysis. RF-20 exhibited enhanced solubility (Figure 3A), and TEM analysis revealed monodispersed and spherical micelles of resveratrol, with <40 nm uniformly entrapped and tightly packed within the galactomannan network as aggregated particles of around 150 nm (Figure 3B). Dynamic light scattering (DLS) particle size analysis indicated 47.2 ± 2.9 nm size for the precasted micelles before incorporation into the network [Figure 3C (i)] and the average size of 172.0 ± 10.4 nm for RF-20, due to the aggregation of micelles in the galactomannan hydrogel network, as evident from TEM [Figure 3C (ii)]. The SEM image of FG, U-tRES, and RF-20 is shown in Figure 3D. The crystalline nature of resveratrol in Figure 3D (i), amorphous FG matrix in Figure 3D (ii), and spherical amorphous particles with a smooth, translucent surface morphology due to the impregnation of crystalline resveratrol into the amorphous FG matrix in Figure 3D (iii) were clear from SEM images. This was further supported by the powder X-ray diffraction (PXRD) analysis in the 2θ range 6–60° (Figure 3E). While resveratrol provided sharp and intense peaks at 2θ values (6.6, 16.4, 19.2, 22.4, 23.6, 25.3, and 28.4°),35 the diffractogram for FG was characteristic of an amorphous substance. RF-20 on the other hand exhibited an amorphous nature, as evident from the less intense nature and efficiency of 92.10 ± 1.16%. The formation of micelle/hydrogel composite was confirmed by FTIR spectroscopy. Figure 3F shows the FTIR spectra of FG, U-tRES, and RF-20. The FTIR spectrum of U-tRES showed characteristic peaks corresponding to the key structural features of resveratrol. The peak observed at 3209 cm–1 corresponded to the O–H stretching of the phenolic hydroxyl groups. The stretching related to C=C bonds of the aromatic rings were visible at 1611–1500 cm–1. The peak observed around 1147 cm–1 can be attributed to the C–O stretching vibrations of phenolic groups, and the phenolic O–H stretching vibration was observed at 1381 cm–1. The characteristic peaks of alkene (=C–H) were observed at 962 cm–1, which confirmed the trans-configuration of the resveratrol. The stretching at 860–770 cm–1 was characteristic of =C–H vibration bands of arene conjugated to the olefinic group. All these peaks were found to be present in RF-20 as well, along with the characteristic peaks of FG (3200, 2914, 104, 1000–1200, 1653, 872, and 800–820 cm–1). This confirmed the encapsulation of resveratrol in the fenugreek galactomannan matrix without any chemical modification. Thus, the molecular arrangements of RF-20 from resveratrol, lecithin, and galactomannan as a hybrid-hydrogel (micelle/hydrogel composite) are schematically represented in Figure 4.
Figure 3.
Characterization of the hybrid-hydrogel formulation of trans-resveratrol, RF-20*. (A) Photograph of the aqueous solutions of U-tRES (left) and RF-20 (right) indicating the enhanced solubility and colloidal nature. (B) TEM image of RF-20. (C) DLS analysis of hydrodynamic size distribution of (i) precasted micelles before incorporation into the network (ii) RF-20. (D) SEM images of (i) U-tRES, (ii) FG, (iii) and RF-20. (E) Powder XRD diffractogram U-tRES, FG, and RF-20. (F) FTIR spectra of FG, U-tRES, and RF-20. *U-tRES – unformulated trans-resveratrol, FG – fenugreek galactomannan, and RF-20 – hybrid-hydrogel formulation of trans-resveratrol.
Figure 4.
Schematic representation of the molecular arrangements of resveratrol micelles in the fenugreek galactomannan network to form the hybrid-hydrogel (micelle/hydrogel composite) structure.
The reproducibility of the process of the formulation was confirmed by producing three different batches and analyzed for the particle size and polydispersity index. The observed particle size in the three batches was 172.0 ± 10.4, 152.0 ± 17.4, and 188.0 ± 24.8 nm, and their polydispersity indexes (PDI) were 0.221, 0.315, and 0.353.
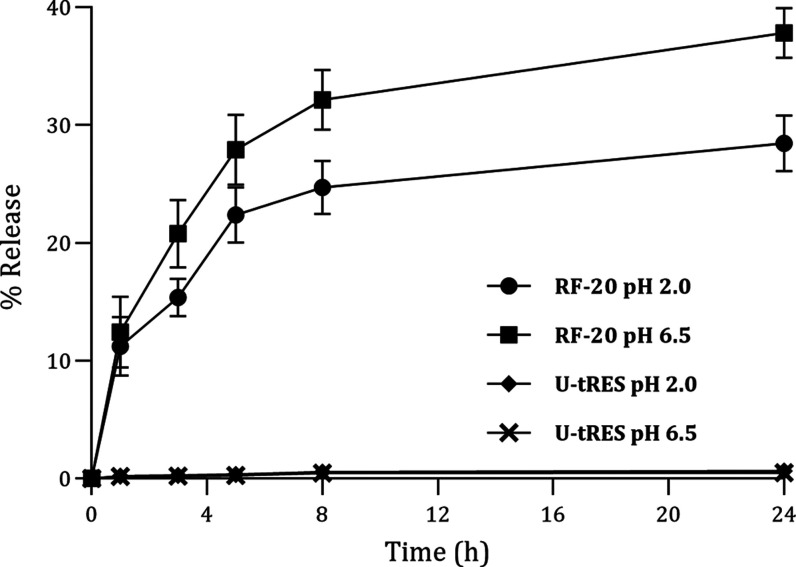
In vitro release of resveratrol from RF-20 is shown in Figure 5, which indicated a sustained release under both stomach and intestinal pH conditions. The powder form of RF-20 released almost 37.9% of resveratrol at pH 6.5 and 28.5% at pH 2.0, in 24 h. The unformulated resveratrol was insoluble.
Figure 5.
In vitro release of resveratrol from RF-20 and U-tRES at pH 6.5 and 2.0.
The double-blinded, placebo-controlled, randomized pharmacokinetic study was conducted in two phases, using both capsules and sachets, to investigate the influence of delivery form on the relative bioavailability of resveratrol from RF-20 (Figure 2). Each dose of either the formulated or the unformulated resveratrol delivered 80 ± 5 mg of trans-resveratrol. All the selected participants (n = 16; 10 males and 6 females) completed both the phases of the study without any significant side effects or adverse events, indicating the tolerability at the tested dosage. The demographic details of the participants and their blood routine analysis comprising the hematological and biochemical parameters are given in Supporting Information Table S1.
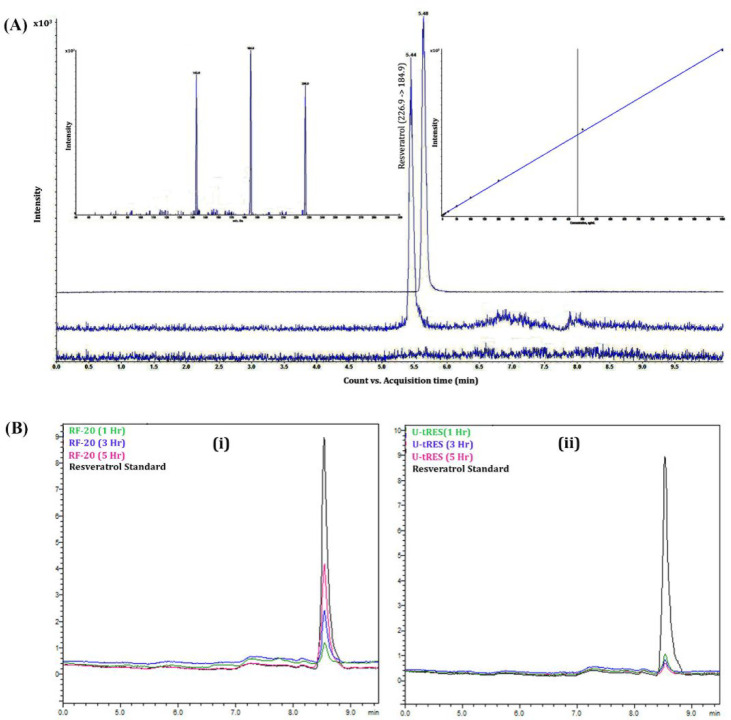
Tandem mass spectrometry using QTRAP technology has been established as a reliable method to detect, confirm, and quantify “free” resveratrol content in biomatrices. Acetonitrile-based sample preparation and further analysis by MRM transitions provided a limit of quantification of 1 ng/mL with a recovery of 82.16% (Figure 6A). The method showed linearity over a wide range (1 to 1000 ng/mL) of concentration with an r2 value of 0.9967 (Figure 6B). The assay was reproducible and showed an intra-assay precision of 4.1% and interassay precision of 7.8%. The assay also showed good accuracy, with intra-assay concentrations within 91.6 to 103.7% and interassay concentrations within 90.5 to 105.1% of the expected value. Matrix-matched calibration was performed in the present study. The blank plasma and resveratrol-spiked plasma samples did not show any interference at the respective retention times of each of the analytes. Significant improvement in the intensity of the peak corresponding to free trans-resveratrol in plasma followed by the ingestion of RF-20 is clear from Figure 6B.
Figure 6.
UPLC-PDA and UPLC-ESI-QTRAP-MS/MS analysis of trans-resveratrol in plasma. (A) Panel of MRM transitions that yielded MS/MS spectra with a signal/noise ratio >5.0 in blank plasma, standard trans-resveratrol, and plasma collected after 3 h of ingestion of RF-20, indicating the tandem mass spectrometric identification from a biomatrix. The MRM transitions for trans-resveratrol were m/z (226.9 → 184.9; 226.9 → 142.9) (inset). The linearity of a series of matrix-matched calibration solutions of trans-resveratrol in plasma is also given inset. (B) Panel (i) shows the UPLC-PDA chromatograms for blank plasma, standard trans-resveratrol, and the plasma samples collected from one of the volunteers at 1, 3, and 5 h after ingestion of the RF-20 capsule containing 80 mg of trans-resveratrol; (ii) shows the chromatograms obtained for blank plasma, standard trans-resveratrol, and the plasma samples collected from one of the volunteers at 1, 3, and 5 h after ingestion of the U-tRES capsule containing 80 mg of trans-resveratrol.
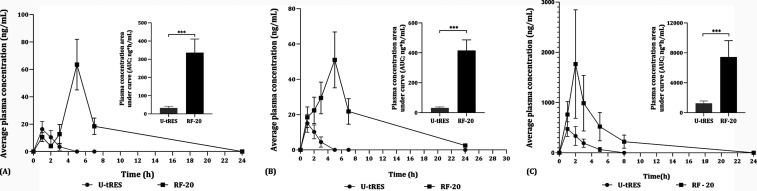
The pharmacokinetic parameters of the formulated and unformulated resveratrol (RF-20 and U-tRES) are given in Table 1. Our study was in agreement with the early reports that the bioavailability and circulation half-life of free resveratrol are very poor when administered as both a capsule and sachet. Upon the ingestion of RF-20, the plasma concentration of free resveratrol and its half-life was significantly increased (***P < 0.001) during 3 to 8 h of the postadministration period, irrespective of the delivery form (Figure 7). For capsules, the absorption maximum was at 4.86 ± 0.53 h (Tmax), with a maximum plasma concentration (Cmax) of 63.28 ± 16.87 ng/mL for RF-20, as compared to the Tmax of 1.07 ± 0.26 h and Cmax of 16.34 ± 5.67 ng/mL for the unformulated U-tRES (Table 1 and Figure 7A). The area under the curve of the plasma concentration versus time plot for capsules (AUC0–24h) for RF-20 (335.80 ± 75.41 ng/mL h) was 10.48-fold higher than that for the unformulated (32.05 ± 9.97 ng/mL h) (***P < 0.001) when delivered as a capsule (Table 1). Moreover, the absorbed resveratrol from RF-20 was found to stay in the circulation for a longer duration, as evidenced by the t1/2 (the time taken for 50% of absorbed resveratrol to degrade) values of 6.12 ± 1.31 h. In the case of sachets, the Cmax was 50.97 ± 15.82 ng/mL at a Tmax of 4.71 ± 0.73 h as compared to the Cmax of 15.07 ± 5.10 ng/mL and Tmax of 1.21 ± 0.42 h. The elimination half-life (t1/2) increased significantly (***P < 0.001) from 1.58 ± 0.65 h to 7.01 ± 1.44 h upon formulation (Table 1 and Figure 7B). It was noticed that the plasma concentration of free resveratrol remained higher than about 18 ng/mL for 1 to 8 h when delivered as a sachet, indicating the sustained release property of RF-20. On the other hand, the plasma levels were undetectable after 3 h for U-tRES, indicating the enhanced stability, absorption, and bioavailability of RF-20. The area under the curve calculation showed a 12.98-fold enhancement for the sachet. Therefore, the bioavailability of sachet delivery was higher than the capsule form (12.98-fold versus 10.48-fold) when the AUC0–24h was considered.
Table 1. Pharmacokinetic Parameters of the Unformulated trans-Resveratrol (U-tRES) and the Hybrid-Hydrogel Formulation RF-20, When Administered as a Capsule and Sachet Containing 80 mg of trans-Resveratrol per Dose.
| resveratrol:
capsule |
resveratrol:
sachet |
||||
|---|---|---|---|---|---|
| pharmacokinetic parameters | U-tRES | RF-20 | U-tRES | RF-20 | |
| free resveratrol | Cmax (ng/mL) | 16.34 ± 5.67 | 63.28 ± 16.87*** | 15.07 ± 5.10 | 50.97 ± 15.82*** |
| Tmax (h) | 1.07 ± 0.26 | 4.86 ± 0.53* | 1.21 ± 0.42 | 4.71 ± 0.73* | |
| t1/2 (h) | 1.58 ± 0.24 | 6.12 ± 1.31* | 1.58 ± 0.65 | 7.01 ± 1.44* | |
| AUC0–24 (ng h/mL) | 32.05 ± 9.97 | 335.80 ± 75.41*** | 31.93 ± 6.34 | 414.60 ± 72.31*** | |
| total resveratrola | Cmax (ng/mL) | 471.00 ± 145.96 | 1768.00 ± 1080.47*** | - | - |
| Tmax (h) | 1.17 ± 0.60 | 2.21 ± 0.89* | - | - | |
| t1/2 (h) | 1.79 ± 0.72 | 3.99 ± 1.12* | - | - | |
| AUC0–24 (ng h/mL) | 1254.00 ± 206.30 | 7422.00 ± 1552.00*** | - | - | |
Total resveratrol bioavailability and pharmacokinetic parameters of U-tRES and the RF-20 capsule measured by treating the plasma with a β-glucuronidase enzyme. U-tRES, unformulated resveratrol; RF-20, hybrid-FENUMAT-resveratrol formulation; Cmax, maximum plasma concentration; tmax, time taken to reach the maximum concentration in plasma; t1/2, time taken to reduce the plasma concentration to half of its maximum observed concentration; AUC, area under the curve. Mean values were significantly different from those of the U-tRES: *P < 0.05, ***P < 0.001
Figure 7.
Pharmacokinetics of RF-20 and U-tRES. (A) Plasma concentration versus time course for the free resveratrol upon ingestion of capsules. (B) Plasma concentration versus time course for the free resveratrol upon ingestion of sachets. (C) Total plasma resveratrol content measured upon enzymatic hydrolysis (β-glucosidase) versus time course upon ingestion of capsules (n = 16). Statistical analysis was performed using SPSS software version 27, and all data points were expressed as mean ± SD. P < 0.05 was considered statistically significant. *P < 0.05; ***P < 0.001; GraphPad Prism Version 5.0 was used to plot the graph.
The time course for the plasma resveratrol concentration, bioavailability, and elimination half-life for RF-20 was also significantly higher (6.0-fold, ***P < 0.001) when the total resveratrol content in plasma was estimated as the sum of free (unconjugated) and conjugated metabolites by treating the plasma with a glucuronidase enzyme (Table 1 and Figure 7C). The Cmax,Tmax, t1/2, and AUC0–24 h for the total resveratrol content when individuals are ingested with RF-20 were 1768.00 ± 1080.47 ng/mL, 2.21 ± 0.89 h, 3.99 ± 1.12 h, and 7422.00 ± 2215.41 ng h/mL, respectively, compared to the respective parameters for U-tRES as 471.00 ± 145.96 ng/mL, 1.17 ± 0.60 h, 1.79 ± 0.72 h, and 1254.00 ± 206.30 ng h/mL, indicating 6.0-fold enhancement in the bioavailability (7422.00 ± 1215.41 ng h/mL versus 1254.00 ± 206.30 ng h/mL) (Table 1).
4. Discussion
The free resveratrol concentrations in human plasma have turned out to be a marker for the plausible in vivo benefits of resveratrol since it possesses optimum hydrophobicity to interact with the lipid head groups and thiols for better membrane permeability and significant blood-brain-barrier permeability.3,16,27,34 The notion has been justified by the interesting pharmacodynamics of trans-resveratrol and the relatively weak activity of glucuronides/sulfates, despite the hypothesis that intracellular β-glucuronidase may deconjugate to generate the free form.8,13,21,23 Moreover, many clinical studies have also pointed out the importance of free resveratrol bioavailability, despite the micromolar range of plasma concentrations of glucuronides/sulfates.10,17,27 However, formulations capable of delivering significantly high levels of “free” form with longer circulation half-life remain a challenge.
The present study was aimed at the characterization and investigation of absorption, distribution, and bioavailability of “free” resveratrol, irrespective of its glucuronide and sulfate metabolites, following the ingestion of a novel formulation of trans-resveratrol as a natural self-emulsifying reversible hybrid-hydrogel (N’SERH) system. The concept of hybrid-hydrogels as an N’SERH delivery system was employed for the formulation since the previous reports on liquid state liposomal, micellar, and self-emulsifying nanodelivery forms using synthetic emulsifiers and polymers enhanced the resveratrol bioavailability despite their inherent limitations of low stability, low loading level, and excess usage of synthetic emulsifiers.19,25,36−38 We hypothesized that a surface modification of liposomes or micelles with hydrophilic polymers could be an effective strategy to enhance the in vivo stability and permeability, with significant reduction of presystemic conjugation and biotransformation. So, the present formulation (RF-20) was achieved using fenugreek galactomannans as a hydrogel trap in which precasted micelles of resveratrol were impregnated uniformly by a water-based gel-phase dispersion process followed by dehydration to provide a micelle/hydrogel composite in powder form. Due to the hydrophobic–hydrophilic balance and self-assembly to bilayer structures, sunflower oil containing phospholipids was employed for resveratrol micelle formation. The particle size and PDI analysis of the repeated batches confirmed the reproducibility of the formulation process.39
The powder (RF-20) was capable of absorbing water to swell extensively under gastrointestinal conditions to form a soft hydrogel and further to leach amphiphilic nanomicelles of resveratrol for better absorption (∼170 nm). The hybrid-hydrogel structure and its amorphous character were clear from TEM and PXRD analysis, which could reveal the entrapment of monodispersed micelles within the hydrogel matrix, as a “hydrogel trap” (Figure 3). Although such composite forms employing synthetic polymers and chemically modified biopolymers have already been reported as hybrid-hydrogels,4,8 this is the first human pharmacokinetic report of a hybrid-hydrogel to enhance the oral bioavailability by reducing the biotransformation. Since the highly mucoadhesive galactomannan biopolymer (soluble dietary fiber) from fenugreek was used without any chemical modification, the technology is natural and termed as hybrid-FENUMAT. RF-20 showed enhanced solubility and sustained release of soluble resveratrol. The poor aqueous solubility has been identified as the main reason for the low bioavailability of molecules, and decreasing particle size has been proposed as a method to circumvent the dissolution rate issues.40
The randomized, double-blinded, placebo-controlled, 2-arm, 4-sequence, crossover design in two phases to monitor the pharmacokinetic properties as capsules and sachets was employed to investigate the role of the delivery matrix in the bioavailability of RF-20. Considering the previous clinical studies, which have mainly used 75 to 150 mg/day either one time or two times a day12,34 and the regulatory guidelines for nutraceutical usage of resveratrol, the present study selected 80 ± 5 mg of trans-resveratrol (both as formulated (RF-20) and as unformulated (U-tRES)) as the dosage. The supplementation of trans-resveratrol at 75 mg/day was also shown to significantly improve the neurovascular coupling and cognitive performance in type 2 diabetes subjects.41 The study was carried out under fasting conditions since a high-fat diet prior to or immediately after the resveratrol dosage may affect the extent and time of absorption.42 The main objective of the study to follow the “free” resveratrol bioavailability was achieved by following the previous methods on “free” curcumin estimation in plasma without using β-glucuronidase/sulfatase enzyme assisted hydrolysis of glucuronide and sulfate metabolites to the free from.24,43
To our knowledge, this is the first pharmacokinetic investigation of resveratrol in Indian population. The study revealed very low bioavailability of unformulated resveratrol, as reported by previous studies. However, the plasma concentration versus time plot showed significantly high bioavailability for RF-20 when administered as both capsules and sachets. The plasma levels were undetectable after 3 h for the unformulated resveratrol, while the Cmax, Tmax, and t1/2 for RF-20 were significantly high, indicating its improved stability, absorption, and longer retention of free resveratrol. These results may be explained as the influence of surface modification of micelles by mucoadhesive galactomannans to stabilize them in the GI tract and further to affect their sustained release with improved paracellular and transcellular transport to the host cellular membrane bilayers. However, the observed bioavailability of sachets was higher than the capsules (12.98× versus 10.48×), indicating an effect of the food matrix, and the observed difference in bioavailability was statistically significant (*P < 0.05). The observation was in agreement with earlier reports that resveratrol absorption from the liquid form such as juice and wine was better than from capsules.44 The higher bioavailability of sachets may be attributed to the relatively fast swelling of RF-20 in the GI tract to immediately release the resveratrol micelles in the initial 1 to 3 h of the postadministration time itself.
The earliest pharmacokinetic studies by Goldberg et al. (2003) and Walle et al. (2004) could not find detectable levels of free resveratrol in plasma when supplemented at 25 mg dose, corresponding to a moderate wine consumption level, though around 300 ng/mL of conjugated metabolites was detected.17,45 Based on the urinary discretion and total metabolites, they concluded that the absorption of resveratrol is good (∼75%) but with poor bioavailability of free form (<1%).17,18 As an attempt to increase the bioavailability, dose-escalation and repeated dose studies were then tried at 25 to 5000 mg doses, with no significant enhancement in the bioavailability of free resveratrol.46,47 Almeida et al. also showed the possibility of saturation of metabolism upon repeated dosage at 4 h intervals. When supplemented at single and repeated doses (200 mg × 3/day), Nunes et al. observed around 23 ng/mL as the single-dose Cmax and 30 ng/mL as the repeated dose Cmax.48 Later, Brown et al. also reported a similar enhancement in Cmax upon the repeated dosage of 500, 1000, 2500, and 5000 mg for 28 days, with only 43.8 ng/mL Cmax for a 500 mg dose on the 28th day.15 Kennedy et al. also observed a very low Cmax of 5.65 and 14.4 ng/mL upon 250 and 500 mg single doses.49 Other previous studies have suggested good absorption but poor oral bioavailability of free form of resveratrol, irrespective of 8 to 20 times better absorption for the conjugated metabolites.18,42
Several formulations have also been tried for the oral bioavailability of resveratrol and their metabolites. A recent formulation as Veri-sperse failed to detect the free form in plasma when supplemented at 75 and 150 mg doses of trans-resveratrol, though it reported a 2-fold enhancement in sulfate/glucuronide metabolites.34 No dose-related enhancement was noted in this study, though Almeida et al. could establish a dose dependency at this dosage.47 Another liquid micellar formulation of grapevine shoot extract using polysorbate was reported to offer a 5-fold enhancement in total resveratrol metabolite bioavailability compared to the unformulated wine shoot extract. However, no free resveratrol was detected in this study.50 Yet another polysorbate-based soluble form of resveratrol reported an 8.8-fold enhancement in total resveratrol (sum of free and conjugated) bioavailability, with about 1.7-fold enhancement in free form.51 Wightman et al. reported no detection of free resveratrol and no significant enhancement in the oral bioavailability of resveratrol metabolites when 250 mg of resveratrol was supplemented along with 20 mg of piperine.52
Thus, it can be concluded that the significant absorption of free resveratrol distinguishes RF-20, irrespective of the number of folds of bioavailability enhancement previously reported by measuring the total resveratrol metabolites. The pharmacokinetic properties measured for the unformulated (U-tRES) resveratrol in the present study were in agreement with the early pharmacokinetic studies,15,47−49 indicating the reliability of the current tandem mass spectrometric plasma measurements. The pharmacokinetic properties of RF-20 are highly significant since it corresponds to better bioavailability, sustained release (Tmax of 4.7 h versus 1 h for unformulated), and longer circulation half-life (t1/2 of 7 h) compared to 0.5 to 1.5 h in early studies. The improved bioavailability and pharmacokinetic properties are expected to provide better health benefits, such as cardiovascular, neuroprotective, hepatoprotective, antioxidant, and anti-inflammatory effects at convenient dosages, which may be further evaluated clinically. The lack of side effects or adverse events with no significant variation in the biochemical and hematological parameters further indicates the safety and tolerability of RF-20 when consumed both as a capsule and as a sachet. The selection of fenugreek galactomannans as a mucoadhesive and self-emulsifying hydrogel matrix makes the present hybrid-hydrogel system attractive for nutritional applications since fenugreek galactomannans are cheap and possess biocompatibility, food-grade status, prebiotic potential, and functional benefits as a dietary fiber. Hybrid-hydrogel is an emerging approach in drug delivery with applications in regenerative medicine, tissue engineering, wound healing, and sustained drug/gene/nutrient delivery.4
5. Conclusion
In summary, the present study demonstrated the development of a prebiotic hybrid-hydrogel as a “natural self-emulsifying reversible hybrid-hydrogel system” (N’SERH) for the oral delivery of lipophilic phytonutrients like resveratrol for therapeutic/functional applications. The formulation of trans-resveratrol was achieved using a fenugreek galactomannan hydrogel, sunflower oil, and lecithin (RF-20) by incorporating the nanomicelles of resveratrol on a galactomannan hydrogel matrix using a gel-phase thin-film dispersion technique. RF-20 was amorphous with enhanced solubility and stability. The pharmacokinetic study on healthy subjects could establish sustained release and its potentiality to significantly enhance the “free” (unconjugated) resveratrol bioavailability and enhanced mean residence time in plasma when supplemented both as a single-dose capsule and as a sachet containing an average amount of 80 ± 5 mg of trans-resveratrol. The sachet form was found to offer slightly better bioavailability and more sustained plasma levels than the capsules (12.98× versus 10.48×), compared to the unformulated resveratrol. The amphiphilic micellar preparation of resveratrol with an oil/lecithin blend made it soluble and permeable, while the mucoadhesive galactomannan network acted as a soft “hydrogel trap” for the nanomicelles for better bioavailability with improved paracellular and transcellular transport. Thus, the hybrid-hydrogel form showed better bioavailability and reduced metabolic turnover brought about by the repel effect of the surface-bound hydrophilic galactomannan chains, leading to better in vivo stability and enhanced mean residence time in plasma.
Acknowledgments
The authors thank Leads Clinical Research & Bioservices Private Limited, Bangalore, India, for the registration and coordination of the human pharmacokinetic study.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c00116.
Demographic, biochemical, and hematological details of the participants are given in Table S1 (PDF)
Author Contributions
K.I.M. is the principal investigator who supervised the project and reviewed the manuscript. A.J. conducted the formulation development trials; A.B. validated the tandem mass spectrometry method for the analysis of the biomatrix samples and conducted the pharmacokinetic study; P.S. performed the characterization; and B.M. arranged the funding and reviewed the manuscript.
The authors declare the following competing financial interest(s): FENUMAT and Hybrid-FENUMAT, the technologies used in the present formulation of resveratrol are patented and registered by Akay Natural Ingredients, Cochin, India. Resverafen is the registered trademark of RF-20.
Supplementary Material
References
- Porter C.; Trevaskis N.; Charman W. Lipids and lipid-based formulations: optimizing the oral delivery of lipophilic drugs. Nat. Rev. Drug Discov 2007, 6 (3), 231–48. 10.1038/nrd2197. [DOI] [PubMed] [Google Scholar]
- Dima C.; Assadpour E.; Dima S.; Jafari S. Bioavailability of nutraceuticals: Role of the food matrix, processing conditions, the gastrointestinal tract, and nanodelivery systems. Compr. Rev. Food Sci. Food Saf. 2020, 19 (3), 954–94. 10.1111/1541-4337.12547. [DOI] [PubMed] [Google Scholar]
- Watkins R.; Wu L.; Zhang C.; Davis R.; Xu B. Natural product-based nanomedicine: recent advances and issues. Int. J. Nanomedicine 2015, 10, 6055–74. 10.2147/IJN.S92162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M.-H.; Chen X.-Y.; Fu L.-Q.; Du W.-L.; Yang X.; Mou X.-Z.; Hu P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anticancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol 2021, 9, 630943. 10.3389/fbioe.2021.630943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M.; Billingsley M.; Haley R.; Wechsler M.; Peppas N.; Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discovery 2021, 20 (2), 101–24. 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olusanya T.; Haj Ahmad R.; Ibegbu D.; Smith J.; Elkordy A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules. 2018, 23 (4), 907. 10.3390/molecules23040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siafaka P.; ÜstündagOkur N.; Karavas E.; Bikiaris D. Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses. Int. J. Mol. Sci. 2016, 17, 1440. 10.3390/ijms17091440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L.-X.; Heredia A.; Song H.; Zhang Z.; Yu B.; Davis C.; Redfield R. Resveratrol glucuronides as the metabolites of resveratrol in humans: characterization, synthesis, and anti-HIV activity. J. Pharm. Sci. 2004, 93 (10), 2448–57. 10.1002/jps.20156. [DOI] [PubMed] [Google Scholar]
- Griñán-Ferré C.; Bellver-Sanchis A.; Izquierdo V.; Corpas R.; Roig-Soriano J.; Chillón M.; et al. The pleiotropic neuroprotective effects of resveratrol in cognitive decline and Alzheimer’s disease pathology: From antioxidant to epigenetic therapy. Ageing Res. Rev. 2021, 67, 101271. 10.1016/j.arr.2021.101271. [DOI] [PubMed] [Google Scholar]
- Pezzuto J. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol. Ther. 2019, 27 (1), 1–14. 10.4062/biomolther.2018.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P.; Singh R.; Verma S. S.; Rai V.; Kaschula C. H.; Maiti P.; Gupta S. C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39 (5), 1851–91. 10.1002/med.21565. [DOI] [PubMed] [Google Scholar]
- Ramirez-Garza S.; Laveriano-Santos E.; Marhuenda-Munoz M.; Storniolo C.; Tresserra-Rimbau A.; Vallverdu-Queralt A.; Lamuela-Raventos R. Health Effects of Resveratrol: Results from Human Intervention Trials. Nutrients. 2018, 10 (12), 1892. 10.3390/nu10121892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambini J.; Inglés M.; Olaso G.; Lopez-Grueso R.; Bonet-Costa V.; Gimeno-Mallench L.; et al. Properties of Resveratrol: In Vitro and In Vivo Studies about Metabolism, Bioavailability, and Biological Effects in Animal Models and Humans. Oxid. Med. Cell. Longev. 2015, 2015, 837042. 10.1155/2015/837042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calamini B.; Ratia K.; Malkowski M. G.; Cuendet M.; Pezzuto J. M.; Santarsiero B. D.; Mesecar A. D. Pleiotropic mechanisms facilitated by resveratrol and its metabolites. Biochem. J. 2010, 429 (2), 273. 10.1042/BJ20091857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown V.; Patel K.; Viskaduraki M.; Crowell J.; Perloff M.; Booth T.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70 (22), 9003–11. 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amri A.; Chaumeil J.; Sfar S.; Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations?. J. Controlled Release 2012, 158 (2), 182–93. 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- Walle T. Bioavailability of resveratrol. Ann. N.Y. Acad. Sci. 2011, 1215 (1), 9–15. 10.1111/j.1749-6632.2010.05842.x. [DOI] [PubMed] [Google Scholar]
- Wenzel E.; Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49 (5), 472–81. 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- Chimento A.; de Amicis F.; Sirianni R.; Sinicropi M.; Puoci F.; Casaburi I. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019, 20 (6), 1381. 10.3390/ijms20061381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y.; Li C.; Li H.; Wu M.; Ren C.; Zhen Y.; et al. Differential sensitivities of bladder cancer cell lines to resveratrol are unrelated to its metabolic profile. Oncotarget 2017, 8 (25), 40289–304. 10.18632/oncotarget.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miksits M.; Wlcek K.; Svoboda M.; Kunert O.; Haslinger E.; Thalhammer T.; et al. Antitumor activity of resveratrol and its sulfated metabolites against human breast cancer cells. Planta Med. 2009, 75 (11), 1227–30. 10.1055/s-0029-1185533. [DOI] [PubMed] [Google Scholar]
- Ishii M.; Kanayama M.; Esumi H.; Ogawara K.; Kimura T.; Higaki K. Pharmacokinetic analysis of factors determining elimination pathways for sulfate and glucuronide metabolites of drugs. I: studies by in vivo constant infusion. Xenobiotica 2002, 32 (5), 441–50. 10.1080/00498250210123094. [DOI] [PubMed] [Google Scholar]
- Patel K.; Andreadi C.; Britton R.; Horner-Glister E.; Karmokar A.; Sale S.; et al. Sulfate metabolites provide an intracellular pool for resveratrol generation and induce autophagy with senescence. Sci. Transl. Med. 2013, 5 (205), 205ra133. 10.1126/scitranslmed.3005870. [DOI] [PubMed] [Google Scholar]
- Kumar D.; Jacob D.; Subash P.; Maliakkal A.; Johannah N. M.; Kuttan R.; et al. Enhanced bioavailability and relative distribution of free (unconjugated) curcuminoids following the oral administration of a food-grade formulation with fenugreek dietary fibre: A randomised double-blind crossover study. J. Funct. Foods. 2016, 22, 578–87. 10.1016/j.jff.2016.01.039. [DOI] [Google Scholar]
- de Vries K.; Strydom M.; Steenkamp V. Bioavailability of resveratrol: Possibilities for enhancement. J. Herb. Med. 2018, 11, 71–7. 10.1016/j.hermed.2017.09.002. [DOI] [Google Scholar]
- Smoliga J.; Blanchard O. Enhancing the delivery of resveratrol in humans: if low bioavailability is the problem, what is the solution?. Molecules 2014, 19 (11), 17154–72. 10.3390/molecules191117154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basavaraj S.; Betageri G. Improved oral delivery of resveratrol using proliposomal formulation: investigation of various factors contributing to prolonged absorption of unmetabolized resveratrol. Expert. Opin. Drug. Deliv 2014, 11 (4), 493–503. 10.1517/17425247.2014.878701. [DOI] [PubMed] [Google Scholar]
- Krishnakumar I.; Ravi A.; Kumar D.; Kuttan R.; Maliakel B. An enhanced bioavailable formulation of curcumin using fenugreek-derived soluble dietary fibre. J. Funct. Foods. 2012, 4 (1), 348–57. 10.1016/j.jff.2012.01.004. [DOI] [Google Scholar]
- Abhilash M. B.; Kumar D.; Deepti A.; Nair A.; Greet V.; An-Katrien V.; Mieke V. D. D.; Das Sivadasan S.; Maliakel B.; Chakrapani PS B.; Illathu Madhavamenon K. Enhanced absorption of curcuminoids and 3-Acetyl-11-keto-β-boswellic acid from fenugreek galactomannan hydrogel beadlets: A natural approach to the co-delivery of lipophilic phytonutrients. J. Funct. Foods. 2021, 79 (4), 104405. 10.1016/j.jff.2021.104405. [DOI] [Google Scholar]
- Krishnakumar I. M.; Maliakel A.; Gopakumar G.; Kumar D.; Maliakel B.; Kuttan R. Improved blood–brain-barrier permeability and tissue distribution following the oral administration of a food-grade formulation of curcumin with fenugreek fibre. J. Funct. Foods. 2015, 14, 215–25. 10.1016/j.jff.2015.01.049. [DOI] [Google Scholar]
- Jagwani S.; Jalalpure S.; Dhamecha D.; Hua G.; Jadhav K. A Stability Indicating Reversed Phase HPLC Method for Estimation of trans-Resveratrol in Oral Capsules and Nanoliposomes. Anal. Chem. Lett. 2019, 9 (5), 711–726. 10.1080/22297928.2019.1696227. [DOI] [Google Scholar]
- la Porte C.; Voduc N.; Zhang G.; Seguin I.; Tardiff D.; Singhal N.; Cameron D. W. Steady-State pharmacokinetics and tolerability of trans-resveratrol 2000 mg twice daily with food, quercetin and alcohol (ethanol) in healthy human subjects. Clin. Pharmacokinet. 2010, 49 (7), 449–54. 10.2165/11531820-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Bedada S.; Nearati P. Effect of resveratrol on the pharmacokinetics of carbamazepine in healthy human volunteers. Phytother. Res. 2015, 29 (5), 701–6. 10.1002/ptr.5302. [DOI] [PubMed] [Google Scholar]
- Briskey D.; Rao A. Trans-Resveratrol Oral Bioavailability in Humans Using LipiSperseTM Dispersion Technology. Pharmaceutics 2020, 12 (12), 1190. 10.3390/pharmaceutics12121190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkins S.; Kamath M.; Dhanasekaran; Ahmed S. Polycaprolactone scaffold engineered for sustained release of resveratrol: therapeutic enhancement in bone tissue engineering. Int. J. Nanomedicine 2013, 9 (1), 183. 10.2147/IJN.S49460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves A.; Lúcio M.; Martins S.; Lima J.; Reis S. Novel resveratrol nanodelivery systems based on lipid nanoparticles to enhance its oral bioavailability. Int. J. Nanomedicine 2013, 8, 177–87. 10.2147/IJN.S37840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen C.; Chang C.; Hsu M.; Wu Y. Self-Nanoemulsifying Drug Delivery System for Resveratrol: Enhanced Oral Bioavailability and Reduced Physical Fatigue in Rats. Int. J. Mol. Sci. 2017, 18 (9), 1853. 10.3390/ijms18091853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan S.; Zhang L.; Quan Y.; Wei K. Resveratrol-loaded PLGA nanoparticles: enhanced stability, solubility and bioactivity of resveratrol for non-alcoholic fatty liver disease therapy. R. Soc. open sci. 2018, 5, 181457. 10.1098/rsos.181457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo F.; Clogston J.; Calzolai L.; Rösslein M.; Prina-Mello A. Measuring particle size distribution of nanoparticle enabled medicinal products, the joint view of EUNCL and NCI-NCL. A step-by-step approach combining orthogonal measurements with increasing complexity. J. controlled release 2019, 299, 31–43. 10.1016/j.jconrel.2019.02.030. [DOI] [PubMed] [Google Scholar]
- Hintz R.; Johnson K. The effect of particle size distribution on dissolution rate and oral absorption. Int. J. Pharm. 1989, 51 (1), 9–17. 10.1016/0378-5173(89)90069-0. [DOI] [Google Scholar]
- Wong R.; Raederstorff D.; Howe P. Acute Resveratrol Consumption Improves Neurovascular Coupling Capacity in Adults with Type 2 Diabetes Mellitus. Nutrients 2016, 8 (7), 425. 10.3390/nu8070425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz-da-Silva M.; Loureiro A.; Falcao A.; Nunes T.; Rocha J. F.; Fernandes-Lopes C.; et al. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int. J. Clin. Pharmacol. Ther. 2008, 46 (11), 564–70. 10.5414/CPP46564. [DOI] [PubMed] [Google Scholar]
- Gatti G.; Perucca E. Plasma concentrations of free and conjugated silybin after oral intake of a silybin-phosphatidylcholine complex (silipide) in healthy volunteers. Int. J. Clin. Pharmacol. Ther. 1994, 32 (11), 614–7. [PubMed] [Google Scholar]
- Ortuño J.; Covas M.; Farre M.; Pujadas M.; Fito M.; Khymenets O.; et al. Matrix effects on the bioavailability of resveratrol in humans. Food Chem. 2010, 120 (4), 1123–30. 10.1016/j.foodchem.2009.11.032. [DOI] [Google Scholar]
- Goldberg D. M.; Yan J.; Soleas G. J. Absorption of three wine-related polyphenols in three different matrices by healthy subjects. ClinBiochem. 2003, 36 (1), 79–87. 10.1016/S0009-9120(02)00397-1. [DOI] [PubMed] [Google Scholar]
- Boocock D.; Faust G.; Patel K.; Schinas A.; Brown V.; Ducharme M.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007, 16 (6), 1246–52. 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- Almeida L.; Vaz-da-Silva M.; Falcão A.; Soares E.; Costa R.; Loureiro A.; et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53, S7–15. 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- Nunes T.; Almeida L.; Rocha J.; Falcão A.; Fernandes-Lopes C.; Loureiro A.; et al. Pharmacokinetics of trans-resveratrol following repeated administration in healthy elderly and young subjects. J. Clin. Pharmacol. 2009, 49 (12), 1477–82. 10.1177/0091270009339191. [DOI] [PubMed] [Google Scholar]
- Kennedy D. O; Wightman E. L; Reay J. L; Lietz G.; Okello E. J; Wilde A.; Haskell C. F Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. Am. J. Clin. Nutr. 2010, 91 (6), 1590–7. 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]
- Calvo-Castro L.; Schiborr C.; David F.; Ehrt H.; Voggel J.; Sus N.; et al. The Oral Bioavailability of trans-Resveratrol from a Grapevine-Shoot Extract in Healthy Humans is Significantly Increased by Micellar Solubilization. Mol. Nutr. Food Res. 2018, 62 (9), 1701057. 10.1002/mnfr.201701057. [DOI] [PubMed] [Google Scholar]
- Amiot M.; Romier B.; Anh Dao T.; Fanciullino R.; Ciccolini J.; Burcelin R.; et al. Optimization of trans-Resveratrol bioavailability for human therapy. Biochimie. 2013, 95 (6), 1233–8. 10.1016/j.biochi.2013.01.008. [DOI] [PubMed] [Google Scholar]
- Wightman E.; Reay J.; Haskell C.; Williamson G.; Dew T.; Kennedy D. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: a randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112 (2), 203–13. 10.1017/S0007114514000737. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.