Abstract
Silver nanoparticles (AgNPs) biosynthesized using plant extracts as reducing and capping agents show multiple possibilities for solving various biological problems. The aim of this study was to expand the boundaries of AgNPs using a novel low toxicity and production cost phytochemical method for the biosynthesis of nanoparticles from Eucalyptus globulus and Salvia officinalis aqueous leaf extracts. Biosynthesized AgNPs were characterized by various methods (ultraviolet-visible spectroscopy (UV-vis), Fourier transform infrared (FTIR) spectroscopy with horizontal attenuated total reflectance (HART), transmission electron microscopy (TEM), energy-dispersive X-ray spectroscopy (EDS)). The determined antioxidative and antimicrobial activity of plant extracts was compared with the activity of the AgNPs. The UV-vis spectral analysis demonstrated the absorption peaks at 408 and 438 nm, which confirmed the synthesis of stable AgNPs from E. globulus and S. officinalis, respectively. FTIR-HART results suggested strong capping of phytochemicals on AgNPs. TEM results show mainly spherical-shaped AgNPs, whose size distribution depends on the plant leaf extract type; the smaller AgNPs were obtained with E. globulus extract (with size range of 17.5 ± 5.89 nm compared to 34.3 ± 7.76 nm from S. officinalis AgNPs). The in vitro antioxidant activity evaluated by radical scavenging assays and the reduction activity method clearly demonstrated that both the plant extracts and AgNPs showed prominent antioxidant properties. In addition, AgNPs show much stronger antimicrobial activity against broad spectrum of Gram-negative and Gram-positive bacteria strains than the plant extracts used for their synthesis.
Keywords: green synthesis, Eucalyptus globulus, Salvia officinalis, phytochemical analysis, silver nanoparticles, antioxidant activity, antibacterial activity
1. Introduction
In recent years, there has been an increasing focus on biomedicine and other sciences by studying the chemical diversity of plants and the raw materials they provide. The results of the research are important in the search for plant raw materials, in the development of medicinal products and food supplements of plant origin, and in finding new possibilities for the use plants and plant raw materials [1,2].
Green synthesis represents an environmentally benign, low production cost, relatively reproducible, simple method [3,4,5,6]. For obtaining plant-derived nanomaterials, the plant biodiversity is broadly regarded due to the selection of “green reagents” such as ketones, aldehydes, amides, phenols, flavonoids and terpenoids, carboxylic acids, and ascorbic acids, which are available in a huge class of plant extracts, particularly in plant leaves [2,7,8,9,10]. Eucalyptus and sage leaves are very valuable raw plant materials that possess many biological activities and are promising agents for the green synthesis of AgNPs [11].
It has been indicated in the scientific literature that extracts of sage have particularly strong antioxidant activity compared to other herbal plants [12]. This effect is primarily related to the complex of different groups of phenolic compounds (phenolic acids, luteolin, apigenin, quercetin, isorhamnetin glycosides) accumulated in sage leaves [13]. These extracts also show a strong anti-inflammatory effect due to the triterpene compounds accumulated in raw material, especially ursolic acid. Many studies have shown that extracts of sage have biological activity against various bacteria strains and yeasts [13]. Sage leaf extracts also have antispasmodic, hypoglycemic, and antimutagenic effects. Sage tea is used as a traditional herbal medicine to relieve the symptoms of inflammation of the mucous membranes of the mouth and throat, and to reduce heavy hyperhidrosis, dizziness, and mild symptoms of indigestion; recent studies indicate wider benefits [14,15,16,17].
Eucalyptus is one of the most widely utilized medicinal plants due to its wide spectrum of biological activities, which are mainly attributed to the diversity of phytochemical constituents in the plant parts. Eucalyptus leaf decoctions are used for the mouth cavity, rinsing wounds, compresses in trauma, upper respiratory tract pain, and rheumatism; it is suitable for inhalations in upper respiratory tract diseases, bronchitis. The biologically active compound complex found in eucalyptus leaves determines a particularly strong antioxidant effect of their extracts [18,19,20]. The most important phenolic compounds that largely determine the antiradical and reductive activity of eucalyptus leaves are phenolic acids, quercetin glycosides [21], and other groups of phenolic compounds of various structures [22]. Like sage leaf extract, eucalyptus leaf extract has extremely strong antibacterial and antifungal activity [23].
In general, the chemical, physical, mechanical, and antimicrobial properties of AgNPs depend on their chosen precursor and synthesis method [24]. Synthesis of nanoparticles can be achieved by many different methods, such as reduction in solutions (distilled water, ethanol, hexane, toluene, ethylene glycol, and others), electrochemical and sonochemical approaches [25,26], the microwave-assisted method (by choosing the right power, time, and medium) [27], the radiation-assisted method [8], and via biological routes and chemical reactions [28]. In general, nanoparticles are prepared by a variety of chemical and physical methods, but these approaches are expensive and harmful to the environment due to their use of perilous and toxic chemicals, which are responsible for biological risks and hazardous to human health [29]. However, AgNPs are mainly used in healthcare and medicine due to their strong antibacterial (inhibits DNA replication, enzyme functions, etc.), antiviral (blocks the attachment of viruses to the cell wall), antifungal (destroys the cell membrane), and other activities [30,31]. Therefore, it is imperative to develop non-hazardous, economical, and feasible methods for the synthesis of AgNPs to maximize their potential benefits to society.
The present paper reports the biological synthesis of AgNPs using the aqueous leaf extracts of Eucalyptus globulus Labill. (E. globulus) and Salvia officinalis L. (S. officinalis), and evaluation of the nanoparticles’ potential application as an antibacterial agent against various pathogenic bacteria strains, having an antioxidant potential.
2. Results and Discussion
2.1. Phytochemical Analysis and Morphology of Leaf Extracts
Silver nanoparticles were successfully synthesized by the reduction of AgNO3 with the leaf extracts of E. globulus (EuG-AgNPs) and S. officinalis (SaO-AgNPs). Various bioactive derivatives presented in leaf extracts are responsible for the bioreduction and capping/stabilization of AgNPs [32,33]. Among the most important biologically active compounds accumulated by plants are phenolic compounds, which are classified as natural antioxidants [34,35]. Oxidative stress causes changes in cellular metabolism associated with DNA and protein damage and lipid peroxidation [36,37]. Phenolic compounds neutralize reactive forms of oxygen and nitrogen [38,39], and have antimicrobial [40,41] anti-inflammatory effects [42,43]; they are therefore valuable in the prevention and treatment of many diseases. For this reason, studies on the qualitative and quantitative composition of plant raw materials that accumulate phenolic compounds are important and relevant.
To evaluate phytochemical composition, the UV-VIS spectrophotometric evaluation of the total composition of phenolic compounds, flavonoids, proanthocyanidins, and hydroxycinnamic acid derivatives in E. globulus and S. officinalis extracts, and AgNPs from these extracts, was performed. The obtained results are summarized in Table 1. The highest content of phenolic compounds was found in S. officinalis leaf extract (0.78 mg GAE/g), whereas a slightly lower amount (in 12%) was detected in the E. globulus extract. The results of phytochemical composition obtained for E. globulus and S. officinalis frequently differ from those obtained by other researchers [44,45]. This can be explained by the fact that other factors not studied may strongly affect the qualitative and quantitative content of the vegetative and generative organs of various plants; for example, the geochemical composition of the soil, the geographic region, the climate and meteorology, plant cultivation and raw material storage conditions, and foliar fertilization lead to increased phenol content.
Table 1.
Phytochemical analysis of plant extracts and biosynthesized AgNPs.
| Compound Name | E. globulus | S. officinalis | EuG-AgNPs | SaO-AgNPs |
|---|---|---|---|---|
| The total content of proanthocyanidins, mg EE/g | 0.13 ± 0.02 | 0.09 ± 0.00 | 0.09 ± 0.01 | 0.07 ± 0.04 |
| The total content of hydroxycinnamic acid derivatives, mg CAE/g | 1.38 ± 0.05 | 1.57 ± 0.02 | 1.24 ± 0.02 | 1.54 ± 0.01 |
| The total content of phenolic compounds, mg GAE/g | 0.69 ± 0.04 | 0.78 ± 0.00 | 0.58 ± 0.03 | 0.61 ± 0.02 |
| The total content of flavonoids, mg RE/g | 0.48 ± 0.04 | 0.44 ± 0.00 | 0.43 ± 0.01 | 0.41 ± 0.03 |
A similar dependence is characteristic for the content of hydroxycinnamic acid derivatives in S. officinalis and E. globulus extracts (1.57 and 1.38 mg CAE/g, respectively). Furthermore, E. globulus extract contains a larger amount of proanthocyanidin and flavonoid compounds compared to S. officinalis extract.
The total content of phenol, flavonoid, hydroxycinnamic acid, and proanthocyanidins after biosynthesis of EuG-AgNPs and SaO-AgNPs is lower compared to that of pure E. globulus and S. officinalis leaf extracts, but the character of changes is the same (Table 1). A higher content of total hydroxycinnamic acid and phenolic compounds was found in SaO-AgNPs, whereas slightly higher contents of proanthocyanidins and flavonoids were detected in the EuG-AgNPs. The content of phenolic compounds in the case of nanoparticles is 15–20% lower than that of plant extracts. It is supposed that the donating potential of polyphenols facilitates the formation of nanoparticles by bioreduction of Ag+ to Ag0 and further stabilization of nanoparticles [46].
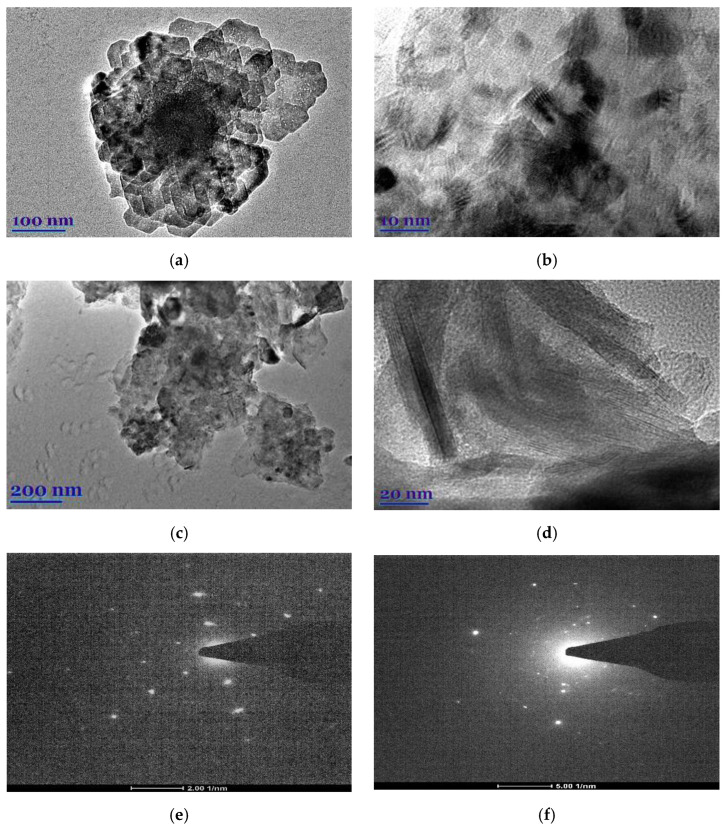
The morphological peculiarities of leaf extracts were examined by TEM and selected area electron diffraction (SAED). Figure 1a–d shows TEM images of E. globulus and S. officinalis leaf extracts. It was found that the natural compounds from extracts show highly ordered structures. In the case of E. globulus, nano-sized octagonal-shaped crystallites of 40 to 55 nm in size are closely aligned. In the case of S. officinalis, the needle-shaped crystallites are visible, whose longitudinal dimensions are considerably larger than those of the E. globulus derivatives. Thus, the obtained E. globulus and S. officinalis extracts are highly crystalline, as shown by clear lattice fringes and typical SAED (Figure 1e,f).
Figure 1.
TEM images (a–d) and SAED (e,f) of E. globulus (a,b,e) and S. officinalis (c,d,f) patterns.
2.2. Morphological Analysis of Biosynthesized AgNPs
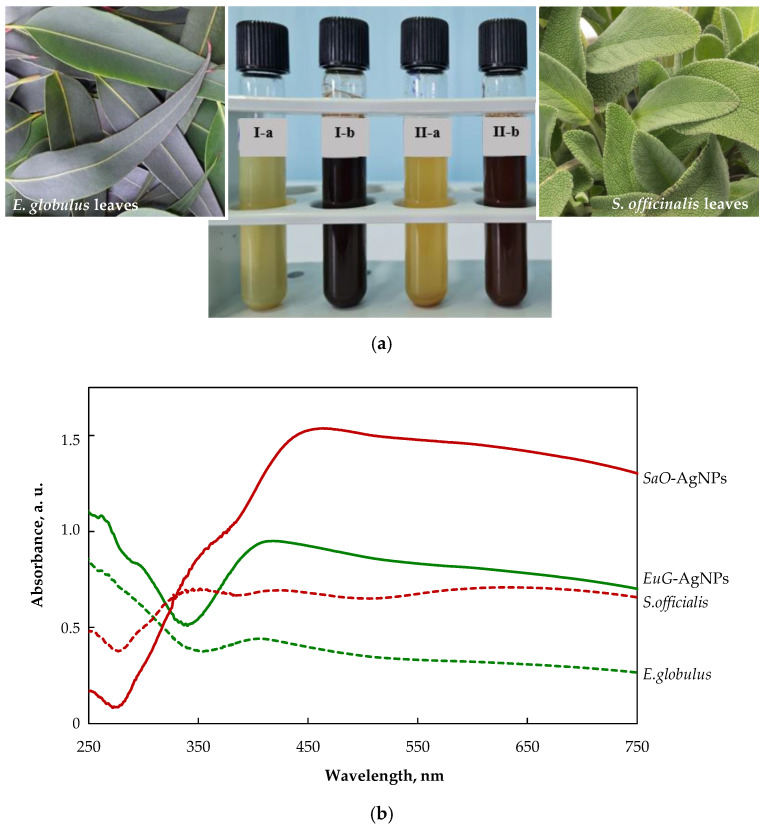
The color change of colloidal solutions from yellowish to reddish brown and dark brown visually evidences the formation of SaO-AgNPs and EuG-Ag NPs, respectively (Figure 2a). The color change of nanoparticles’ colloidal solutions happens due to localized surface plasmon resonance (LSPR) after the bioreduction of Ag+ ions to Ag0 by phytochemicals. It was confirmed by UV-visible spectroscopic analysis, as shown in Figure 2b. The biosynthesized SaO-AgNPs that were stabilized with S. officinalis extract show a strong LSPR peak at 438 nm, whereas for E. globulus extract, the stabilized EuG-AgNPs peak appears at 408 nm. Generally, the intensity and position of LSPR are dependent on the shape and size of nanoparticles, and the composition of the surrounding medium [47]. It is proposed that smaller nanoparticles primarily absorb light and have peaks near 400 nm, whereas larger spherical particles exhibit increased scattering and have peaks that broaden and shift towards longer wavelengths [48]. Thus, it is suggested that EuG-AgNPs should be smaller in size than SaO-AgNPs.
Figure 2.
(a) Changes in the color of the colloidal solutions indicating the formation of AgNPs (I-a—E. globulus + AgNO3; I-b—EuG-AgNPs; II-a—S. officinalis +AgNO3; II-b—SaO-AgNPs); (b) UV-vis absorption spectra of plant extracts and biosynthesized AgNPs.
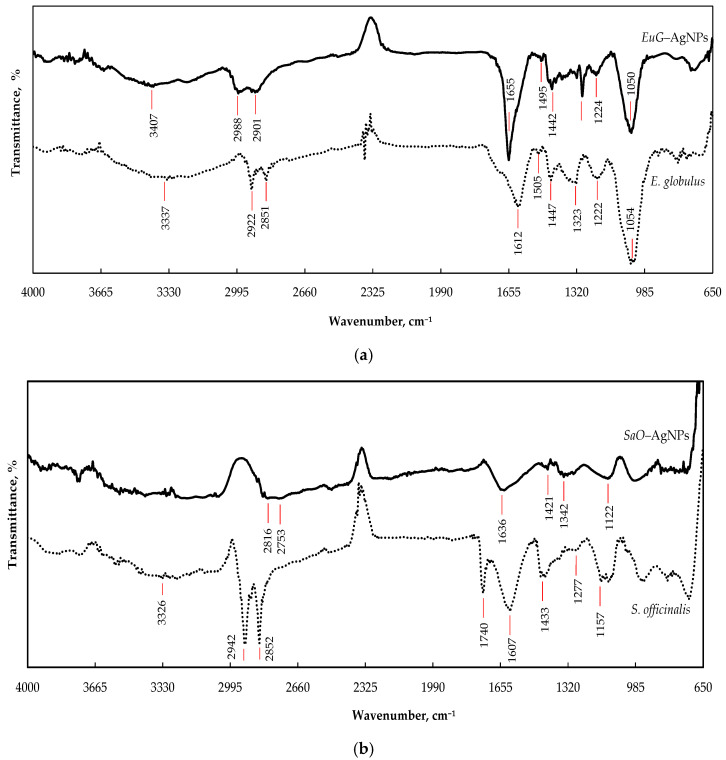
FTIR-HART analysis was used to identify the role of functional groups presented in E. globulus and S. officinalis extracts in the bioreduction, capping formation, and stabilization of AgNPs. Figure 3a demonstrates the possible functional groups of E. globulus extract. The wide absorbance band with a peak at 3337 cm−1 is attributed to the O–H stretching vibration of alkynes, alcohols, and phenols [49,50], whereas the overlapping peaks at 2922 and 2851 cm−1 can be assigned to the C–H stretching vibrations in CH/CH2/CH3 groups [51]. The absorbance bands in the region of 1600–1490 cm−1 correspond to C=C stretching vibrations of aromatic rings [51,52]. The peak observed at 1612 cm−1 can be attributed to N–H bending vibrations of alkenes, primary amines, and amides, and the peak at 1505 cm−1 can be assigned to N=O stretching of nitro groups (NO2) and N–H bending of secondary amines [49,51]. The peak at 1447 cm−1 is related to the C–H bending vibration of proteins. The peak at 1323 cm−1 may occur due to the bending vibration of nitro groups (N=O); the peak at 1222 cm−1 indicates the presence of the stretching vibration of the C–O bond from alcohols, esters, carboxylic acid, or ether [49,50]; and the prominent peak at 1054 cm−1 is typical for the C–N (amines) stretching vibration of proteins [52]. During formation of EuG-AgNPs, biomolecules of phytochemicals bind with the silver through various functional groups and form a coating on the surface of the nanoparticles. Therefore, in the FTIR spectrum of biosynthesized EuG-AgNPs, similar peaks as those of the spectrum of E. globulus leaf extract appear (Figure 3a). EuG-AgNPs’ spectrum shows the presence of O–H, C–H, C=O, N–H, N=O, C–O, and C–N functional groups, with peaks at 3407, 2988, 2901, 1655, 1495, 1442, 1293, 1224, and 1050 cm−1, respectively.
Figure 3.
FTIR spectra of AgNPs synthesized by the reduction of AgNO3 with the E. globulus (a) and S. officinalis (b) leaf extracts.
The spectral bands that reflect the chemical composition of S. officinalis extract can be seen from the FTIR spectrum shown in Figure 3b. The bands between 3000 and 3600 cm−1 are mainly due to OH stretching vibrations within phenols [53]. Two high intensity bands at 2924 and 2850 cm−1 can be attributed to methylene −CH2– and methyl −CH3 groups, respectively, whereas the carbonyl of the triglyceride linkage ester is observed at 1740 cm−1 [54]. The absorption peak appearing at 1607 cm−1 may be due to C=O bond stretching within the aromatic rings of different phenolic compounds present in S. officinalis. The region between 1500 and 1200 cm−1 includes mixed vibrations arising from the bending modes of the >CH2 and −CH3 groups in proteins, fatty acids, and phosphate-bearing compounds. The presence of carbohydrates and polysaccharides reveals the vibrations of the −C–O–C glycoside ring bond, C–O stretching in COOH, and O–H bending in the region from 1220 to 1000 cm−1 [54,55]. The spectral range between 900 and 650 cm−1 corresponds to the vibrations of bands arising from amino acids and nucleotides [54]. The FTIR spectrum of the SaO-AgNPs synthesized shows peaks at 3312, 2824, 2753, 1636, 1421, 1339, and 1121 cm−1, which are identical to the peaks that appear in the spectrum of S. officinalis leaf extract and indicate the presence of the organic compounds of the phytochemicals (Figure 3b).
Some changes in the FTIR spectra of the biosynthesized EuG-AgNPs and SaO-AgNPs, such as the decrease in the absorption bands’ intensity, and their shifting or disappearing comparing to the absorption bands of the pure plant extracts, confirm the involvement of biomolecules of phytochemicals in the bioreduction, capping, and stabilization of AgNPs. The organic compounds such as flavanones or terpenoids may be responsible for the bioreduction of nanoparticles, whereas proteins can be involved in the stabilization of AgNPs [49]. It is believed that proteins can bind to AgNPs through free amine groups or cysteine residues in the proteins [56], whereas flavanones and terpenoids are absorbed on the surface of AgNPs, possibly by interaction through carbonyl [51,57,58]. It was determined that terpenoids reduce metal ions by oxidation of aldehydic groups in the molecules to carboxylic acid [57].
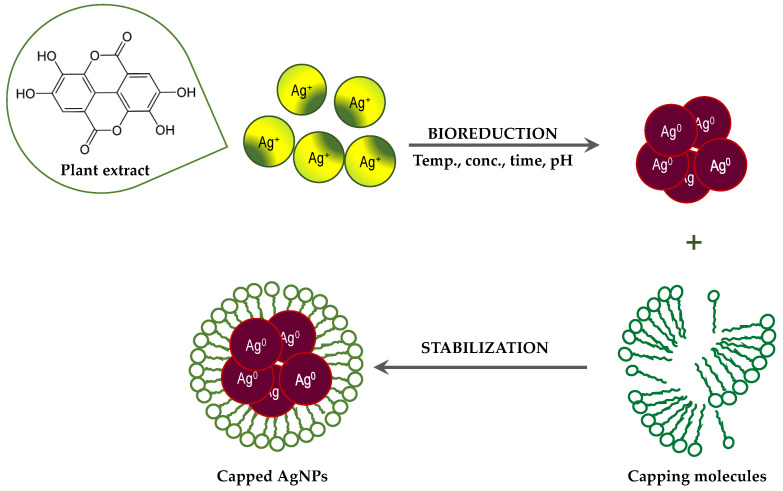
Although the process of biosynthesis of nanoparticles remains unclear, plausible mechanisms have been proposed to explain the formation of metallic AgNPs via bioreduction derived from plants [32,33]. Plants are rich in secondary metabolites, such as flavonoids, tannins, and phenolic acid. Many of these metabolites act as both reducing and stabilizing agents, and inhibit the aggregation of formed nanoparticles [32,59]. Generally, the Ag+ ions are inactivated via phytochelation, probably dye to the nucleophilic nature of the phenolic aromatic rings. It is supposed that ions are captured and immobilized by biological elements and subsequently undergo reduction, growth, and sintering processes, leading to the formation of AgNPs. Thus, the bioreduction of AgNPs by plant extracts can be divided into three phases [32]: (a) the activation phase, in which reduction and nucleation of Ag+ ions occurs; (b) the growth phase, when the small neighboring AgNPs combine to form larger particles, accompanied by an increase in the thermodynamic stability of the AgNPs; and (c) the termination phase, in which the final shape of the AgNPs is formed through (Figure 4).
Figure 4.
Suggested mechanisms for the formation of AgNPs via bioreduction derived from plant extracts.
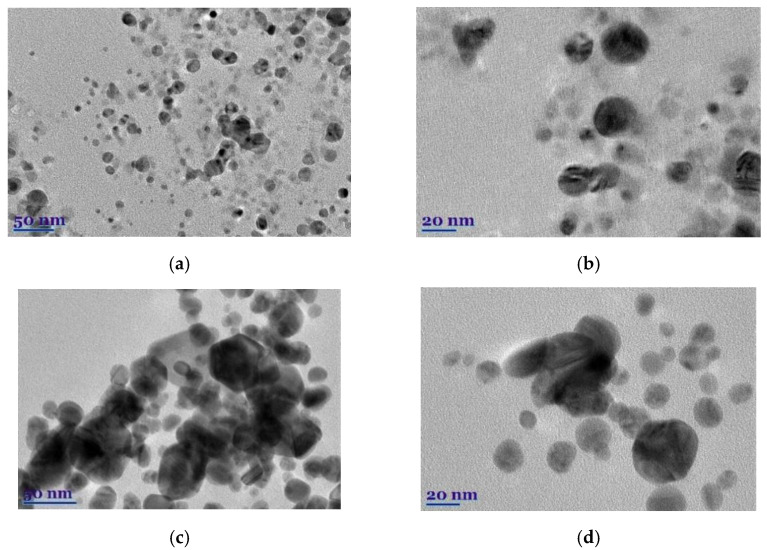
The morphology, shape, size, and chemical composition of biosynthesized EuG-AgNPs and SaO-AgNPs were examined by TEM and TEM-EDS. It is postulated that the type of plant extract and its concentration influence the morphology of the nanoparticles formed, whereas the temperature and pH of the extract medium controls the growth and size of the nanoparticles [32]. The shape, size, and morphology of green synthesized EuG-AgNPs and SaO-AgNPs are shown in Figure 5a–d. This shows that most of the nanoparticles are nearly spherical, whereas some of EuG-AgNPs and SaO-AgNPs have a triangular or hexagonal shape. A slight agglomeration of nanoparticles is also observed. After evaluating the particle distribution using the ImageJ application, it was found that the size of the nanoparticles depends on the plant extract used for bioreduction and stabilization. The diameter of the EuG-AgNPs was found to be in the range of 17.5 ± 5.89 nm (polydispersity approx. 4.5), whereas the SaO-AgNPs diameter is almost twice large and is in the range of 34.3 ± 7.76 nm (polydispersity approx. 6.0). The obtained results prove the suggestion that larger SaO-AgNPs show a UV-vis absorption peak at a longer wavelength (see Figure 2b).
Figure 5.
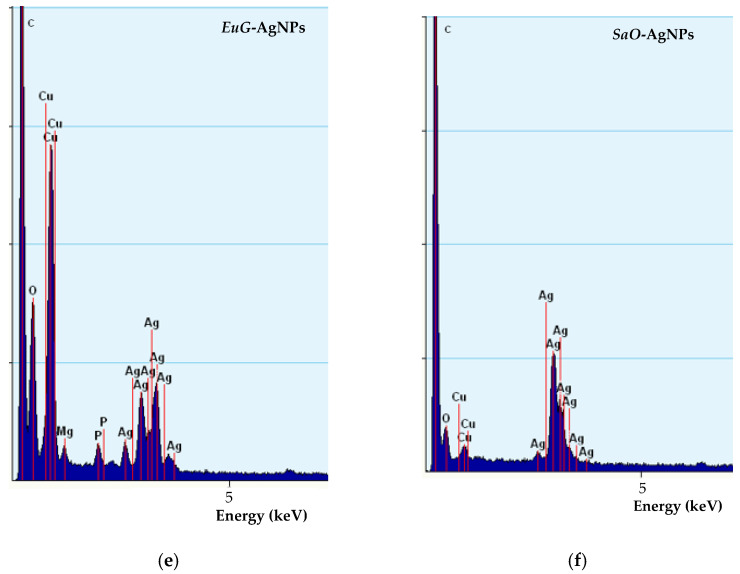
TEM images (a–d) and EDS spectra (e,f) of biosynthesized EuG-AgNPs (a,b,e) and SaO-AgNPs (c,d,f).
TEM-EDS spectra confirm the presence of biosynthesized nanoparticles (Figure 5e,f). EuG-AgNPs and SaO-AgNPs show a strong peak at approximately 3 keV, which is characteristic of metal silver due to LSPR [60]. In addition, other peaks for oxygen, carbon, and copper (from the TEM grid) were also observed.
2.3. Comparison of Bioactivity of the Plant Extracs and Biosynthesized AgNPs
2.3.1. Antioxidant Activity
Plant extracts have a variety of compounds that act as antioxidants through different reaction mechanisms. Therefore, antioxidant activity cannot be adequately tested using only one method. In the scientific literature, it is strongly recommended to use at least two different methods for the determination of antioxidant activity in plant extracts [61]. Therefore, in this study, four different antiradical and reduction activity determination methods were used for an in-depth evaluation of the antioxidant activity of the analyzed E. globulus and S. officinalis extracts. ABTS, DPPH, and TFPH assays are based on the ability of antioxidants to scavenge ABTS•+, DPPH•, and TFPH•+ free radicals [62,63], whereas the FRAP assay measures the reduction activity of antioxidants [64]. The ABTS•+ radical cation scavenging assay enables the determination of the antiradical activity of both lipophilic and hydrophilic antioxidants at a medium pH of 7.4 (medium pH is close to blood pH of 7.35–7.45) [63]. Furthermore, the DPPH• radical scavenging method is only suitable for evaluating the in vitro antiradical activity of compounds soluble in organic solvents. However, it limits the evaluation of antioxidant activity of hydrophilic antioxidants [65,66]. The TFPH•+ method is used to evaluate the activity of biologically active compounds in a strongly acidic medium [67].
The antioxidant activity of eucalyptus and salvia species has been reported by many researchers [17,18]. The antioxidant capacity of the studied E. globulus and S. officinalis leaf extracts is shown in Table 2. The E. globulus extract showed stronger in vitro antioxidant activity compared to the S. officinalis extract. This tendency is especially pronounced after the FRAP assay, when E. globulus showed more than 2 times stronger reducing activity than S. officinalis (9.23 and 4.23 mmol TE/g, respectively). The E. globulus extract also showed stronger antiradical activity evaluated by ABTS•+, DPPH•, and TFPH•+ radical scavenging methods. The antioxidant activity observed with the extracts of E. globulus and S. officinalis leaves is probably due to phenolic and proanthocyanidins compounds, which are well known for their antioxidant capacity [68,69].
Table 2.
Antioxidant activity of plant extracts and biosynthesized AgNPs (p > 0.05).
| Assay | E. globulus | S. officinalis | EuG-AgNPs | SaO-AgNPs |
|---|---|---|---|---|
| ABTS, mmol TE/g | 1.69 ± 0.07 c | 1.46 ± 0.04 d | 1.97 ± 0.01 b | 2.28 ± 0.04 a |
| DPPH, mmol TE/g | 0.96 ± 0.03 a | 0.37 ± 0.01 b | 0.98 ± 0.02 a | 0.39 ± 0.02 b |
| TFPH, mmol TE/g | 1.46 ± 0.64 a | 1.37 ± 0.43 a | 2.08 ± 0.12 a | 1.87 ± 0.01 a |
| FRAP, mmol TE/g | 9.23 ± 0.43 a | 4.23 ± 0.18 b | 9.11 ± 0.14 a | 4.02 ± 0.01 b |
The different superscript letters in the same line indicate statistically significant differences between the antioxidant activity of plant extracts (p < 0.05).
Further, the antioxidant activity of biosynthesized AgNPs was compared to the antioxidant activity of E. globulus and S. officinalis leaf extracts (Table 2). Data obtained by ABTS, DPPH, and TFPH in vitro assays show that biosynthesized AgNPs possess higher antioxidant potential due to the existence of silver in two oxidation states, Ag+ and Ag2+, and phytochemicals capped on the AgNPs’ surface [46]. EuG-AgNPs show stronger antioxidant capacity than SaO-AgNPs, with the exception of the antiradical activity determined by the ABTS method. According to the FRAP assay result, it is clear that both AgNPs indicate slightly lower reducing activity than the plant extracts.
2.3.2. Antibacterial Activity
It was determined that eucalyptus [70,71] and salvia [72,73] species exhibit antibacterial activity against various pathogens, and are promising alternatives to the use of hazardous chemicals, and may have potential applications in food, pharmaceutical products, etc. In this study, the antibacterial activity of E. globulus and S. officinalis leaf extracts and biosynthesized AgNPs was investigated against both Gram-positive (S. aureus, B. cereus, P. vulgaris, B. subtilis) and Gram-negative (E. coli, P. aeruginosa, K. pneumoniae, P. mirabilis) bacteria strains (Table 3). The results revealed that E. globulus and S. officinalis extracts are potentially effective in suppressing bacterial growth with a range of inhibition zone from 12.5 to 17.9 mm. Accordingly, E. globulus and S. officinalis extracts were found to show slightly higher antibacterial activity against Gram-positive bacteria than Gram-negative bacteria (p > 0.05) due to the variation in their cell wall structure [74,75]. E. globulus extract exhibits a stronger inhibitory effect against most pathogens, except S. aureus and B. cereus. Importantly, a high inhibition ability of E. globulus and S. officinalis was detected against K. pneumoniae and P. aeruginosa, which are biofilms that cause organisms’ resistance to multiple classes of antibiotics and can cause nosocomial infections [76]. Various mechanisms have been suggested to explain the mode of action of plant antimicrobials. Generally, these include damaging the bacterial cell membrane, inhibiting efflux pumps, and inhibiting DNA and protein biosynthesis [33].
Table 3.
Inhibition zones of the plant extracts and AgNPs against Gram-positive and Gram-negative bacteria strains (p > 0.05).
| Bacterial Strains | Inhibition Zone ± SD, mm: | ||||
|---|---|---|---|---|---|
| E. globulus | S. officinalis | EuG-AgNPs | SaO-AgNPs | ||
| Gram-positive | S. aureus | 14.4 ± 0.01 d | 17.9 ± 0.20 c | 20.0 ± 0.10 b | 24.4 ± 0.05 a |
| B. cereus | 13.2 ± 0.05 d | 15.7 ± 0.70 c | 20.4 ± 0.08 b | 24.0 ± 0.10 a | |
| P. vulgaris | 14.7 ± 0.10 c | 13.2 ± 0.55 d | 18.2 ± 0.09 b | 20.0 ± 0.22 a | |
| B. subtilis | 14.0 ± 0.01 c | 13.1 ± 0.01 d | 20.0 ± 0.20 a | 18.8 ± 0.18 b | |
| Gram-negative | E. coli | 14.0 ± 0.02 c | 13.9 ± 0.01 d | 19.0 ± 0.01 b | 22.4 ± 0.03 a |
| P. aeruginosa | 13.8 ± 0.01 c | 13.0 ± 0.10 d | 20.1 ± 0.03 b | 20.9 ± 0.27 a | |
| K. pneumoniae | 13.6 ± 0.04 c | 13.2 ± 0.25 d | 21.5 ± 0.01 b | 23.0 ± 0.14 a | |
| P. mirabilis | 12.5 ± 0.08 d | 12.8 ± 0.10 c | 19.7 ± 0.10 b | 20.7 ± 0.40 a | |
The different superscript letters in the same line indicate statistically significant differences between Gram-positive and Gram-negative bacteria strains of plant extracts (p < 0.05).
As can be seen from Table 3, biosynthesized EuG-AgNPs and SaO-AgNPs demonstrate 25–45% higher antibacterial activity against all tested pathogenic microorganisms compared to that of pure E. globulus and S. officinalis extracts. Comparing antibacterial properties between nanoparticles, SaO-AgNPs more effectively prevent the growth of bacteria tested, with a range of inhibition zone from 18.8 to 24.4 mm, whereas for EuG-AgNPs, the inhibition zone varies from 18.2 to 21.5 mm. It can be assumed that a higher content of hydroxycinnamic acid and phenolic compounds in the S. officinalis extract increases the antibacterial capacity of SaO-AgNPs. In addition, the antimicrobial activity of AgNPs also depends on the nanoparticle morphological characteristics, such as the shape and size [77,78].
The antibacterial mode of action of silver nanoparticles against bacteria is also not clearly understood. There are several hypotheses for the nanoparticles’ effect on the cell, such as their adhesion on the surface of the bacterial cell wall and membrane, penetration into the cell and disruption of intracellular organelles and biomolecules, induction of oxidative stress, and modulation of signal transduction [19,20,22,28,79].
Thus, AgNPs obtained by the reduction of AgNO3 with E. globulus and S. officinalis extracts have a broad spectrum of activity by inhibiting Gram-positive and Gram-negative bacteria strains.
3. Materials and Methods
3.1. Chemicals
Silver nitrate (AgNO3), ABTS•+ (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)), sodium acetate trihydrate (CH3COONa × 3H2O), iron(III) chloride hexahydrate (FeCl3 × 6H2O), TPTZ (2,4,6-Tris(2-pyridyl)-s-triazine), TFPH (trifluoperazine hydrochloride), and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid were purchased from Merck (Darmstadt, Germany); ethanol (96.3% v/v) was obtained from Stumbras, AB (Kaunas, Lithuania); potassium chloride (KCl) was obtained from Scharlau (Barcelona, Spain); potassium bisulfate (K2S2O8) and DPPH• (2,2-Diphenyl-1-(2,4,6-trinitrophenyl)hydrazin-1-yl) were obtained from Alfa Aesar GmbH & Co KG (Karlsruhe, Germany); sulfuric acid (H2SO4, 95% was purchased from Chempur (Piekary Śląskie, Poland). All chemicals used were of analytical grade.
3.2. Plant Materials
Finely cut Salvia officinalis (Švenčionių vaistažolės UAB, Švenčionys, Lithuania) and Eucalyptus globulus (Acorus Calamus UAB, Pakruojis, Lithuania) leaves were purchased from a public pharmacy operating in Kaunas (Lithuania). Plant material was ground to a powder using a mill (IKA® A11 basic, Staufen, Germany). Loss on drying before analysis was determined by drying about 1 g of powdered raw plant material in a moisture analyzer (Precisa HA 300, Precisa Instruments AG, Dietikon, Switzerland) to complete evaporation of water and volatile compounds at a drying temperature of 105 °C. The data were recalculated for absolute dry weight (DW).
3.3. Preparation of Plant Leaf Extracts
A quantity of 125 g of raw material (crushed E. globulus or S. officinalis leaves) was soaked for 3 h in 70% (v/v) ethanol. The soaked raw material was transferred to a percolator, covered with extractant, i.e., 70% (v/v) ethanol, and left to macerate for 48 h. Then, it was percolated at speed of 0.3 mL/min and high-concentration extract (85% of total extract amount) was obtained. Low-concentration extract was decanted until all biologically active substances were washed from the raw material. It was evaporated using an IKA® HB 10 rotary evaporator (IKA®-Werke GmbH & Co. KG, Breisgau, Germany) up to 15% of the total liquid extract amount. The remaining part of the low-concentration extract was transferred to a single container with the high-concentration extract, and liquid extract for antioxidant activity analysis was obtained. Ethanolic phase of E. globulus and S. officinalis leaf liquid extracts was evaporated at 70 °C for 4 h by a rotary evaporator (Buchi Rotavapor R-205, Buchi AG, Flawil, Switzerland) and aqueous phase extracts of yellowish color were obtained. These aqueous extracts were used as green reductants and capping agents for the biosynthesis of AgNPs. In addition, a portion of E. globulus and S. officinalis aqueous extracts was lyophilized at 0.01 mbar pressure and condenser temperature of −85 °C using a Zirbus lyophilizer (Zirbus technology GmbH, Bad Grund, Germany) for morphological studies.
3.4. Green Synthesis of Silver Nanoparticles
A quantity of 0.03 g of AgNO3 was dissolved in 2.5 mL distilled water and mixed with 30 mL of E. globulus or S. officinalis aqueous extract under vigorous stirring at room temperature and speed of 400 rpm for 2 h. The mixtures were incubated at room temperature for 24 h, and the change in color from yellowish to brown proved the formation of EuG-Ag NPs and SaO-AgNPs. After that, the material was stored in darkness at a 6 °C temperature.
3.5. Characterization of Leaf Extracts and AgNPs
The formation of EuG-AgNPs and SaO-AgNPs was checked using a Lambda 25 UV-vis spectrometer (PerkinElmer, Waltham, MA, USA). The analysis was performed in the wavelength range from 200 to 800 nm.
The functional characterization of materials to be tested was performed by Fourier transform infrared (FTIR) spectrometry. Spectra were recorded using the Spectrum GX FTIR spectrometer (PerkinElmer, Waltham, MA, USA), which was equipped with a horizontal attenuated total reflection (HATR) accessory. The FTIR-HATR spectra of samples were recorded at room temperature in the wave number range of 4000–600 cm−1 with a resolution of 1 cm−1. Collected spectra were processed with the Spectrum® v5.0.1 software from the PerkinElmer.
The microscopy analysis was applied for understanding morphological and structural features of the tested materials. A Tecnai G2 F20 X-TWIN transmission electron microscope (TEM) (FEI, Hillsboro, OR, USA) was used to examine the size and shape of the synthesized AgNPs. The diluted sample was deposited drop-wise onto carbon-coated copper TEM grids. The Schottky emission electron source was used with the accelerating voltage of 20–200 kV. The resolution of the microscope ranged from 0.8 to 1.0 nm. Elemental analysis was performed using an energy dispersive X-ray spectrometer (EDS) with an r-TEM detector and an 11 MPix ORIUS SC1000B (Gatan, Pleasanton, CA, USA) CCD camera. The spot/linear resolution was 0.25/0.102 nm.
3.6. Quantitative Phytochemical Analysis
Measurements were carried out using a double beam UV-Vis scanning spectrophotometer M550 (Spectronic CamSpec, Garforth, United Kingdom). The total phenolic content in the extracts of eucalyptus and sage leaves was determined by the Folin–Ciocalteu assay [80] and expressed as mg gallic acid equivalent (GAE) per gram of absolutely dry weight. The total content of flavonoids was determined using the described methodology [81] and expressed as mg rutin equivalent (RE) per gram of absolutely dry weight. The total content of proanthocyanidins was determined by the DMCA assay [82] and expressed as mg (-)-epicatechin equivalent (EE) per gram of absolutely dry weight. The total content of hydroxycinnamic acid derivatives was determined using the described methodology [83] and expressed as mg ChAE/g DW.
3.7. Evaluation of Antioxidant Activity
The ABTS•+ radical cation scavenging assay was applied according to the methodology described by Re et al. [63]. The DPPH• free radical scavenging activity was determined using the assay proposed by Brand-Williams et al. [62]. The TFPH•+ radical cation scavenging assay was applied using the methodology described by Asghar and Khan [67]. The reduction activity of extracts was determined using the FRAP assay proposed by Benzie and Strain [64]. The antioxidant activity of the extracts was expressed as mmol of the Trolox equivalent (TE) per one gram of absolutely DW.
3.8. Antibacterial Assay
The in vitro antibacterial activity was evaluated using the Agar diffusion test against Gram-positive Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, and Proteus vulgaris, and Gram-negative Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Proteus mirabilis bacteria strains. For this purpose, 0.5 McFarland unit density suspension (~108 CFU/mL) of bacterial strain was inoculated onto the cooled Mueller Hinton Agar (Oxoid, Basingstoke, UK), using sterile cotton swabs. Wells of 6 mm in diameter were punched in the agar and filled with 50 µL of extracts. Agar plates were incubated at 37 °C for 24 h and zones of the inhibition were measured and tabulated.
3.9. Statistical Analysis
All the experiments were carried out in triplicate. Means and standard deviations were calculated with STATISTICA 10 StatSoft, Inc., Tulsa, OK, USA) and Excel (Microsoft, Redmond, WA, USA) software. A one-way analysis of variance (ANOVA) with the post hoc Tukey’s HSD test was employed for statistical analysis. Differences were considered to be significant at p < 0.05.
4. Conclusions
The dispersed AgNPs were synthesized by an eco-friendly and cost-effective method using plant leaf extracts of E. globulus and S. officinalis as a reducing and capping agent. The results suggest that plant extract selection affected the morphology of nanoparticles. The size ranges of AgNPs mediated by E. globulus and S. officinalis extracts were found to be 17.5 ± 5.89 nm and 34.3 ± 7.76 nm, respectively. Flavonoids, phenolic compounds, hydroxycinnamic acid, and other phytochemicals found in plant leaf extracts and capped AgNPs are responsible for their biological activity. The biosynthesized AgNPs exerted prominent antioxidant properties and antibacterial potency against tested pathogenic bacteria strains.
Author Contributions
Conceptualization, A.B. and V.J. (Virginija Jankauskaitė); methodology, M.L., V.J. (Valdimaras Janulis) and E.G.; validation, M.L.; formal analysis, V.P., A.B. and V.J. (Virginija Jankauskaitė); investigation, A.B. and E.G.; resources, A.B.; data curation, M.L. and J.V.; writing—original draft preparation, A.B. and V.J. (Virginija Jankauskaitė); writing—review and editing, A.B. and V.J. (Virginija Jankauskaitė); visualization, V.P. and J.V.; supervision, V.J. (Virginija Jankauskaitė) and P.V.; project administration, A.B.; funding acquisition, P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This project has received funding from European Social Fund (project No 09.3.3-LMT-K-712-19-0120) under grant agreement with the Research Council of Lithuania (LMTLT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated during this study are included in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alabdallah N.M., Hasan M.M. Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci. 2021;28:5631–5639. doi: 10.1016/j.sjbs.2021.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chan Y.Y., Pang Y.L., Lim S., Chong W.C. Facile green synthesis of ZnO nanoparticles using natural-based materials: Properties, mechanism, surface modification and application. J. Environ. Chem. Eng. 2021;9:105417. doi: 10.1016/j.jece.2021.105417. [DOI] [Google Scholar]
- 3.Roy A., Bulut O., Some S., Mandal A.K., Yilmaz M.D. Green synthesis of silver nanoparticles: Biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv. 2019;9:2673–2702. doi: 10.1039/C8RA08982E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gour A., Jain N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019;47:844–851. doi: 10.1080/21691401.2019.1577878. [DOI] [PubMed] [Google Scholar]
- 5.Naikoo G.A., Mustaqeem M., Hassan I.U., Awan T., Arshad F., Salim H., Qurashi A. Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: A critical review. J. Saudi Chem. Soc. 2021;25:101304. doi: 10.1016/j.jscs.2021.101304. [DOI] [Google Scholar]
- 6.Balciunaitiene A., Viskelis P., Viskelis J., Streimikyte P., Liaudanskas M., Bartkiene E., Zavistanaviciute P., Zokaityte E., Starkute V., Ruzauskas M. Green synthesis of silver nanoparticles using extract of Artemisia absinthium L., Humulus lupulus L. and Thymus vulgaris L., physico-chemical characterization, antimicrobial and antioxidant activity. Processes. 2021;9:1304. doi: 10.3390/pr9081304. [DOI] [Google Scholar]
- 7.Dhand V., Soumya L., Bharadwaj S., Chakra S., Bhatt D., Sreedhar B. Green synthesis of silver nanoparticles using Coffea arabica seed extract and its antibacterial activity. Mater. Sci. Eng. C. 2016;58:36–43. doi: 10.1016/j.msec.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 8.Dzimitrowicz A., Jamroz P., Sergiel I., Kozlecki T., Pohl P. Preparation and characterization of gold nanoparticles prepared with aqueous extracts of Lamiaceae plants and the effect of follow-up treatment with atmospheric pressure glow microdischarge. Arab. J. Chem. 2019;12:4118–4130. doi: 10.1016/j.arabjc.2016.04.004. [DOI] [Google Scholar]
- 9.Kumar J.A., Krithiga T., Manigandan S., Sathish S., Renita A.A., Prakash P., Prasad B.N., Kumar T.P., Rajasimman M., Hosseini-Bandegharaei A. A focus to green synthesis of metal/metal based oxide nanoparticles: Various mechanisms and applications towards ecological approach. J. Clean Prod. 2021;324:129198. doi: 10.1016/j.jclepro.2021.129198. [DOI] [Google Scholar]
- 10.Iravani S., Korbekandi H., Mirmohammadi S.V., Zolfaghari B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014;9:385–406. [PMC free article] [PubMed] [Google Scholar]
- 11.Shivakumar M., Nagashree K., Yallappa S., Manjappa S., Manjunath K., Dharmaprakash M. Biosynthesis of silver nanoparticles using pre-hydrolysis liquor of Eucalyptus wood and its effective antimicrobial activity. Enzym. Microb. Technol. 2017;97:55–62. doi: 10.1016/j.enzmictec.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 12.Maliki I., Es-Safi I., El Moussaoui A., Mechchate H., El Majdoub Y.O., Bouymajane A., Cacciola F., Mondello L., Elbadaoui K. Salvia officinalis and Lippia triphylla: Chemical characterization and evaluation of antidepressant-like activity. J. Pharm. Biomed. Anal. 2021;203:114207. doi: 10.1016/j.jpba.2021.114207. [DOI] [PubMed] [Google Scholar]
- 13.Sabry M.M., Abdel-Rahman R.F., El-Shenawy S.M., Hassan A.M., El-Gayed S.H. Estrogenic activity of Sage (Salvia officinalis L.) aerial parts and its isolated ferulic acid in immature ovariectomized female rats. J. Ethnopharmacol. 2022;282:114579. doi: 10.1016/j.jep.2021.114579. [DOI] [PubMed] [Google Scholar]
- 14.Ehsani P., Farahpour M.R., Mohammadi M., Mahmazi S., Jafarirad S. Green fabrication of ZnO/magnetite-based nanocomposite-using Salvia officinalis extract with antibacterial properties enhanced infected full-thickness wound. Colloids Surf. Physicochem. Eng. Asp. 2021;628:127362. doi: 10.1016/j.colsurfa.2021.127362. [DOI] [Google Scholar]
- 15.Jedidi S., Sammari H., Selmi H., Hosni K., Rtibi K., Aloui F., Adouni O., Sebai H. Strong protective effects of Salvia officinalis L. leaves decoction extract against acetic acid-induced ulcerative colitis and metabolic disorders in rat. J. Funct. Foods. 2021;79:104406. doi: 10.1016/j.jff.2021.104406. [DOI] [Google Scholar]
- 16.Wilfried D., Nina C.D.G., Silvia B. Effectiveness of Menosan® Salvia officinalis in the treatment of a wide spectrum of menopausal complaints. A double-blind, randomized, placebo-controlled, clinical trial. Heliyon. 2021;7:e05910. doi: 10.1016/j.heliyon.2021.e05910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Francik S., Francik R., Sadowska U., Bystrowska B., Zawiślak A., Knapczyk A., Nzeyimana A. Identification of phenolic compounds and determination of antioxidant activity in extracts and infusions of salvia leaves. Materials. 2020;13:5811. doi: 10.3390/ma13245811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gullon B., Muniz-Mouro A., Lu-Chau T.A., Moreira M.T., Lema J.M., Eibes G. Green approaches for the extraction of antioxidants from eucalyptus leaves. Ind. Crops Prod. 2019;138:111473. doi: 10.1016/j.indcrop.2019.111473. [DOI] [Google Scholar]
- 19.Palma A., Díaz M.J., Ruiz-Montoya M., Morales E., Giráldez I. Ultrasound extraction optimization for bioactive molecules from Eucalyptus globulus leaves through antioxidant activity. Ultrason. Sonochem. 2021;76:105654. doi: 10.1016/j.ultsonch.2021.105654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonçalves S., Moreira E., Andrade P.B., Valentão P., Romano A. Effect of in vitro gastrointestinal digestion on the total phenolic contents and antioxidant activity of wild Mediterranean edible plant extracts. Eur. Food Res. Technol. 2019;245:753–762. doi: 10.1007/s00217-018-3197-y. [DOI] [Google Scholar]
- 21.Da Silva M.G., de Barros M.A.S.D., de Almeida R.T.R., Pilau E.J., Pinto E., Soares G., Santos J.G. Cleaner production of antimicrobial and anti-UV cotton materials through dyeing with eucalyptus leaves extract. J. Clean Prod. 2018;199:807–816. doi: 10.1016/j.jclepro.2018.07.221. [DOI] [Google Scholar]
- 22.Bello M., Jiddah-kazeem B., Fatoki T.H., Ibukun E.O., Akinmoladun A.C. Antioxidant property of Eucalyptus globulus Labill. Extracts and inhibitory activities on carbohydrate metabolizing enzymes related to type-2 diabetes. Biocatal. Agric. Biotechnol. 2021;36:10211. doi: 10.1016/j.bcab.2021.102111. [DOI] [Google Scholar]
- 23.González-Burgos E., Liaudanskas M., Viškelis J., Žvikas V., Janulis V., Gómez-Serranillos M.P. Antioxidant activity, neuroprotective properties and bioactive constituents analysis of varying polarity extracts from Eucalyptus globulus leaves. J. Food Drug Anal. 2018;26:1293–1302. doi: 10.1016/j.jfda.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aarthye P., Sureshkumar M. Green synthesis of nanomaterials: An overview. Mater. Today Proc. 2021;47:907–913. doi: 10.1016/j.matpr.2021.04.564. [DOI] [Google Scholar]
- 25.Hirsch T., Zharnikov M., Shaporenko A., Stahl J., Weiss D., Wolfbeis O.S., Mirsky V.M. Size-Controlled Electrochemical Synthesis of Metal Nanoparticles on Monomolecular Templates. Angew Chem. Int. Ed. 2005;44:6775–6778. doi: 10.1002/anie.200500912. [DOI] [PubMed] [Google Scholar]
- 26.Korotchenkov O., Cantarero A., Shpak A., Kunitskii Y.A., Senkevich A., Borovoy M., Nadtochii A. Doped ZnS: Mn nanoparticles obtained by sonochemical synthesis. Nanotechnology. 2005;16:2033. doi: 10.1088/0957-4484/16/10/008. [DOI] [PubMed] [Google Scholar]
- 27.Nadagouda M.N., Speth T.F., Varma R.S. Microwave-assisted green synthesis of silver nanostructures. Acc. Chem. Res. 2011;44:469–478. doi: 10.1021/ar1001457. [DOI] [PubMed] [Google Scholar]
- 28.Panda S., Sen S., Roy S., Moyez A. Synthesis of colloidal silver nanoparticles by reducing aqueous AgNO3 Using Green Reducing Agents. Mater. Today Proc. 2018;5:10054–10061. doi: 10.1016/j.matpr.2017.10.206. [DOI] [Google Scholar]
- 29.Corciova A., Ivanescu B. Biosynthesis, characterisation and therapeutic applications of plant-mediated silver nanoparticles. J. Serb. Chem. Soc. 2018;83:515–538. doi: 10.2298/JSC170731021C. [DOI] [Google Scholar]
- 30.Yin I.X., Zhang J., Zhao I.S., Mei M.L., Li Q., Chu C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020;15:2555–2562. doi: 10.2147/IJN.S246764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jankauskaitė V., Balčiūnaitienė A., Alexandrova R., Buškuvienė N., Žukienė K. Effect of cellulose microfiber silylation procedures on the properties and antibacterial activity of polydimethylsiloxane. Coatings. 2020;10:567. doi: 10.3390/coatings10060567. [DOI] [Google Scholar]
- 32.El-Seedi H.R., El-Shabasy R.M., Khalifa S.A., Saeed A., Shah A., Shah R., Iftikhar F.J., Abdel-Daim M.M., Omri A., Hajrahand N.H. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019;9:24539–24559. doi: 10.1039/C9RA02225B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khameneh B., Iranshahy M., Soheili V., Bazzaz B.S.F. Review on plant antimicrobials: A mechanistic viewpoint. Antimicrob. Resist. Infect. Control. 2019;8:1–28. doi: 10.1186/s13756-019-0559-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ndhlala A.R., Moyo M., Van Staden J. Natural antioxidants: Fascinating or mythical biomolecules? Molecules. 2010;15:6905–6930. doi: 10.3390/molecules15106905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pourreza N. Phenolic compounds as potential antioxidant. Jundishapur J. Nat. Pharm. Prod. 2013;8:149–150. doi: 10.17795/jjnpp-15380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meral A., Tuncel P., Sürmen-Gür E., Özbek R., Öztürk E., GÜnay Ü. Lipid peroxidation and antioxidant status in ß-thalassemia. Pediatr. Hematol. Oncol. 2000;17:687–693. doi: 10.1080/08880010050211402. [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B., Rafter J., Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: Direct or indirect effects? Antioxidant or not? Am. J. Clin. Nutr. 2005;81:268S–276S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 39.Almeida I.F., Fernandes E., Lima J.L., Costa P.C., Bahia M.F. Walnut (Juglans regia) leaf extracts are strong scavengers of pro-oxidant reactive species. Food Chem. 2008;106:1014–1020. doi: 10.1016/j.foodchem.2007.07.017. [DOI] [Google Scholar]
- 40.Alvesalo J., Vuorela H., Tammela P., Leinonen M., Saikku P., Vuorela P. Inhibitory effect of dietary phenolic compounds on Chlamydia pneumoniae in cell cultures. Biochem. Pharmacol. 2006;71:735–741. doi: 10.1016/j.bcp.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 41.Puupponen-Pimiä R., Nohynek L., Meier C., Kähkönen M., Heinonen M., Hopia A., Oksman-Caldentey K. Antimicrobial properties of phenolic compounds from berries. J. Appl. Microbiol. 2001;90:494–507. doi: 10.1046/j.1365-2672.2001.01271.x. [DOI] [PubMed] [Google Scholar]
- 42.Middleton E., Kandaswami C., Theoharides T.C. The effects of plant flavonoids on mammalian cells: Implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- 43.Subarnas A., Wagner H. Analgesic and anti-inflammatory activity of the proanthocyanidin shellegueain A from Polypodium feei METT. Phytomedicine. 2000;7:401–405. doi: 10.1016/S0944-7113(00)80061-6. [DOI] [PubMed] [Google Scholar]
- 44.Dos Santos Ferreira C.I., Pereyra A., Patriarca A.R., Mazzobre M.F., Polak T., Abram V., Buera M.d.P., Poklar Ulrih N. Phenolic compounds in extracts from Eucalyptus globulus leaves and Calendula officinalis flowers. J. Nat. Prod. Res. 2016;1:53–57. [Google Scholar]
- 45.Abdelkader M., Ahcen B., Rachid D., Hakim H. Phytochemical study and biological activity of sage (Salvia officinalis L.) Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014;8:1231–1235. [Google Scholar]
- 46.Afreen A., Ahmed R., Mehboob S., Tariq M., Alghamdi H.A., Zahid A.A., Ali I., Malik K., Hasan A. Phytochemical-assisted biosynthesis of silver nanoparticles from Ajuga bracteosa for biomedical applications. Mat. Res. Exp. 2020;7:075404. doi: 10.1088/2053-1591/aba5d0. [DOI] [Google Scholar]
- 47.Jeon H.B., Tsalu P.V., Ha J.W. Shape effect on the refractive index sensitivity at localized surface plasmon resonance inflection points of single gold nanocubes with vertices. Sci. Rep. 2019;9:13635. doi: 10.1038/s41598-019-50032-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silver Nanoparticles: Optical Properties. [(accessed on 20 March 2022)]. Available online: https://nanocomposix.com/pages/silver-nanoparticles-optical-properties.
- 49.Menon S., Agarwal H., Kumar S.R., Kumar S.V. Green synthesis of silver nanoparticles using medicinal plant Acalypha indica leaf extracts and its application as an antioxidant and antimicrobial agent against foodborne pathogens. Int. J. Appl. Pharm. 2017;9:42–50. doi: 10.22159/ijap.2017v9i5.19464. [DOI] [Google Scholar]
- 50.Senthil B., Devasena T., Prakash B., Rajasekar A. Non-cytotoxic effect of green synthesized silver nanoparticles and its antibacterial activity. J. Photochem. Photobiol. B Biol. 2017;177:1–7. doi: 10.1016/j.jphotobiol.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 51.Khoshraftar Z., Safekordi A.A., Shamel A., Zaefizadeh M. Synthesis of natural nanopesticides with the origin of Eucalyptus globulus extract for pest control. Green Chem. Lett. Rev. 2019;12:286–298. doi: 10.1080/17518253.2019.1643930. [DOI] [Google Scholar]
- 52.Jyoti K., Singh A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J. Genet. Eng. Biotechnol. 2016;14:311–317. doi: 10.1016/j.jgeb.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Malik M.A., Alshehri A.A., Patel R. Facile one-pot green synthesis of Ag–Fe bimetallic nanoparticles and their catalytic capability for 4-nitrophenol reduction. J. Mater. Res. Technol. 2021;12:455–470. doi: 10.1016/j.jmrt.2021.02.063. [DOI] [Google Scholar]
- 54.Tulukcu E., Cebi N., Sagdic O. Chemical fingerprinting of seeds of some Salvia species in Turkey by using GC-MS and FTIR. Foods. 2019;8:118. doi: 10.3390/foods8040118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albeladi S.S.R., Malik M.A., Al-thabaiti S.A. Facile biofabrication of silver nanoparticles using Salvia officinalis leaf extract and its catalytic activity towards Congo red dye degradation. J. Mater. Res. Technol. 2020;9:10031–10044. doi: 10.1016/j.jmrt.2020.06.074. [DOI] [Google Scholar]
- 56.Pandey S., De Klerk C., Kim J., Kang M., Fosso-Kankeu E. Eco friendly approach for synthesis, characterization and biological activities of milk protein stabilized silver nanoparticles. Polymers. 2020;12:1418. doi: 10.3390/polym12061418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Verma A., Mehata M.S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Rradiat. Res. Appl. Sci. 2016;9:109–115. doi: 10.1016/j.jrras.2015.11.001. [DOI] [Google Scholar]
- 58.Ahmad H., Venugopal K., Rajagopal K., De Britto S., Nandini B., Pushpalatha H.G., Konappa N., Udayashankar A.C., Geetha N., Jogaiah S. Green synthesis and characterization of zinc oxide nanoparticles using Eucalyptus globules and their fungicidal ability against pathogenic fungi of apple orchards. Biomolecules. 2020;10:425. doi: 10.3390/biom10030425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kharissova O., Dias H., Kharisov B., Pérez B., Pérez V. The greener synthesis of nanoparticles. Trends Biotechnol. 2013;31:240–248. doi: 10.1016/j.tibtech.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 60.Venil C., Malathi M., Velmurugan P., Renuka Devi P. Green synthesis of silver nanoparticles using canthaxanthin from Dietzia maris AURCCBT01 and their cytotoxic properties against human keratinocyte cell line. J. Appl. Microbiol. 2021;130:1730–1744. doi: 10.1111/jam.14889. [DOI] [PubMed] [Google Scholar]
- 61.Prior R.L., Wu X., Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 62.Brand-Williams W., Cuvelier M., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 63.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 64.Benzie I.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 65.Apak R., Güçlü K., Demirata B., Özyürek M., Çelik S.E., Bektaşoğlu B., Berker K.I., Özyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12:1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chao P., Lin S., Lin K., Liu Y., Hsu J., Yang C., Lai J. Antioxidant activity in extracts of 27 indigenous Taiwanese vegetables. Nutrients. 2014;6:2115–2130. doi: 10.3390/nu6052115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Asghar M., Khan I. Measurement of antioxidant activity with trifluoperazine dihydrochloride radical cation. Braz. J. Med. Biol. Res. 2008;41:455–461. doi: 10.1590/S0100-879X2008000600003. [DOI] [PubMed] [Google Scholar]
- 68.Biao L., Tan S., Meng Q., Gao J., Zhang X., Liu Z., Fu Y. Green synthesis, characterization and application of proanthocyanidins-functionalized gold nanoparticles. Nanomaterials. 2018;8:53. doi: 10.3390/nano8010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stagos D. Antioxidant activity of polyphenolic plant extracts. Antioxidants. 2020;9:19. doi: 10.3390/antiox9010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Clavijo-Romero A., Quintanilla-Carvajal M.X., Ruiz Y. Stability and antimicrobial activity of eucalyptus essential oil emulsions. Food Sci. Technol. Int. 2019;25:24–37. doi: 10.1177/1082013218794841. [DOI] [PubMed] [Google Scholar]
- 71.Sebei K., Sakouhi F., Herchi W., Khouja M.L., Boukhchina S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015;48:7. doi: 10.1186/0717-6287-48-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delamare A.P.L., Moschen-Pistorello I.T., Artico L., Atti-Serafini L., Echeverrigaray S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007;100:603–608. doi: 10.1016/j.foodchem.2005.09.078. [DOI] [Google Scholar]
- 73.Mendes F.S.F., Garcia L.M., da Silva Moraes T., Casemiro L.A., de Alcantara C.B., Ambrósio S.R., Veneziani R.C.S., Miranda M.L.D., Martins C.H.G. Antibacterial activity of salvia officinalis L. against periodontopathogens: An in vitro study. Anaerobe. 2020;63:102194. doi: 10.1016/j.anaerobe.2020.102194. [DOI] [PubMed] [Google Scholar]
- 74.Poh T.Y., Ali N., Mac Aogáin M., Kathawala M.H., Setyawati M.I., Ng K.W., Chotirmall S.H. Inhaled nanomaterials and the respiratory microbiome: Clinical, immunological and toxicological perspectives. Part Fibre Toxicol. 2018;15:46. doi: 10.1186/s12989-018-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fournomiti M., Kimbaris A., Mantzourani I., Plessas S., Theodoridou I., Papaemmanouil V., Kapsiotis I., Panopoulou M., Stavropoulou E., Bezirtzoglou E.E. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health Dis. 2015;26:23289. doi: 10.3402/mehd.v26.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Oliveira D.M., Forde B.M., Kidd T.J., Harris P.N., Schembri M.A., Beatson S.A., Paterson D.L., Walker M.J. Antimicrobial resistance in ESKAPE pathogens. Clin. Microbiol. Rev. 2020;33:e00181-e19. doi: 10.1128/CMR.00181-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liao C., Li Y., Tjong S.C. Bactericidal and cytotoxic properties of silver nanoparticles. Int. J. Mol. Sci. 2019;20:449. doi: 10.3390/ijms20020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ronavari A., Kovacs D., Igaz N., Vagvolgyi C., Boros I.M., Konya Z., Pfeiffer I., Kiricsi M. Biological activity of green-synthesized silver nanoparticles depends on the applied natural extracts: A comprehensive study. Int. J. Nanomed. 2017;12:871–883. doi: 10.2147/IJN.S122842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mikhailova E.O. Silver nanoparticles: Mechanism of action and probable bio-application. J. Funct. Biomater. 2020;11:84. doi: 10.3390/jfb11040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bobinaitė R., Viškelis P., Venskutonis P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012;132:1495–1501. doi: 10.1016/j.foodchem.2011.11.137. [DOI] [PubMed] [Google Scholar]
- 81.Urbonavičiūtė A., Jakštas V., Kornyšova O., Janulis V., Maruška A. Capillary electrophoretic analysis of flavonoids in single-styled hawthorn (Crataegus monogyna Jacq.) ethanolic extracts. J. Chromatogr. A. 2006;1112:339–344. doi: 10.1016/j.chroma.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 82.Heil M., Baumann B., Andary C., Linsenmair E.K., McKey D. Extraction and quantification of “condensed tannins” as a measure of plant anti-herbivore defence? Revisiting an old problem. Naturwissenschaften. 2002;89:519–524. doi: 10.1007/s00114-002-0366-3. [DOI] [PubMed] [Google Scholar]
- 83.Fraisse D., Felgines C., Texier O., Lamaison J.L. Caffeoyl derivatives: Major antioxidant compounds of some wild herbs of the Asteraceae family. Food Nutr. Sci. 2011;2:181–192. doi: 10.4236/fns.2011.23002. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated during this study are included in this article.