Abstract
Currently, there exist few satisfactory alternatives to vancomycin for therapy of serious methicillin-resistant Staphylococcus aureus (MRSA) infections. We employed a rat model of aortic valve endocarditis to assess the potential efficacy of evernimicin (SCH 27899) compared with vancomycin against infection with a strain susceptible to both agents (MICs of 0.25 and 0.50 μg/ml, respectively). Infected animals were assigned to one of three groups: controls (no treatment), evernimicin at 60 mg/kg of body weight by intravenous (i.v.) infusion once daily, or vancomycin at 150 mg/kg of body weight per day by continuous i.v. infusion. Therapy was administered for 5.5 days. At the start of therapy, colony counts in vegetations were 6.63 ± 0.44 log10 CFU/g. In both treatment groups, bacterial density within vegetations was significantly reduced in comparison with control animals that had not been treated. Final colony counts were as follows (mean ± standard deviation): controls, 10.12 ± 1.51 log10 CFU/g of vegetation; evernimicin, 7.22 ± 2.91 log10 CFU/g of vegetation; vancomycin, 5.65 ± 1.76 log10 CFU/g of vegetation. The difference between the evernimicin and vancomycin groups was not significant. These results confirmed the bacteriostatic activity of evernimicin in vivo in an experimental model of severe MRSA infection.
Infections with resistant gram-positive organisms have become an increasingly large problem in the United States in the 1990s (8). Methicillin-resistant Staphylococcus aureus (MRSA) strains are particularly important causes of nosocomial infections, such as endocarditis and other endovascular infections, including those associated with indwelling catheters or prosthetic devices (3–5, 8). This has prompted efforts to develop new agents with activity against these organisms. Evernimicin (SCH 27899) is an inhibitor of bacterial protein synthesis (P. V. Adrian and K. P. Klugman, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-110, p. 100, 1998; T. A. Black, W. Zhao, K. J. Shaw, and R. S. Hare, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C-106, p. 99, 1998) that demonstrates activity against a broad range of gram-positive organisms, including MRSA (9; Adrian and Klugman, 38th ICAAC; Black et al., 38th ICAAC). This everninomicin antibiotic was developed to provide greater potency and less nephrotoxicity than an earlier agent of this class, everninomicin D (9). Published reports indicate that this agent inhibits 90% of isolates of MRSA at concentrations of 0.5 to 0.78 μg/ml or less (9, 15) and is two- to fourfold more potent than vancomycin and teicoplanin against these strains. Evernimicin is primarily bacteriostatic against Staphylococcus aureus (15), with an in vitro postantibiotic effect of approximately 2 h (9).
Evernimicin has been studied in phase II and III clinical trials; a preliminary report suggested that the agent might be useful for treatment of pneumococcal pneumonia (J. M. L. Tsitsi, A. D. Calver, B. Luke, J. J. Garaud, P. Grint, J. Gupte, and J. C. Wherry, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-109, p. 580, 1998). The current study was undertaken to evaluate the potential effectiveness in vivo of evernimicin for the treatment of serious infection due to MRSA. For this purpose, we used a rat model of infective endocarditis and compared the activity of the new antimicrobial with that of vancomycin, the standard therapeutic agent used to treat human MRSA infections.
MATERIALS AND METHODS
Test organism.
MRSA 32 is a clinical isolate of S. aureus resistant to oxacillin, but susceptible to vancomycin and evernimicin (SCH 27899). Using an inoculum of approximately 8 × 105 CFU/ml, MICs determined in Mueller-Hinton broth were 0.25 μg/ml for evernimicin and 0.5 μg/ml for vancomycin (10). Neither drug was bactericidal, with minimal bactericidal concentrations (MBCs) of >8 and 16 μg/ml, respectively.
Antimicrobial agents.
Schering-Plough Research Institute, Kenilworth, N.J., kindly provided evernimicin (SCH 27899). A solution containing only excipients, termed placebo SCH 27899, was used as a diluent and for line-flushes. Vancomycin for intravenous (i.v.) use (Eli Lilly & Co., Indianapolis, Ind.) was obtained through our hospital pharmacy.
Creation of infective endocarditis.
Aortic valve endocarditis was produced in male Sprague-Dawley rats by slight modification of the method of Santoro and Levison (12), as described previously (14). A polyethylene catheter (PE 10; Becton-Dickinson, Sparks, Md.) was inserted via the right carotid artery and passed across the aortic valve. Twenty minutes following catherization, 0.5 ml of normal saline containing approximately 3 × 105 CFU of the test strain per ml was injected through the catheter, which was then sealed and left in place. Treatment was started 6 h after bacterial challenge, through an indwelling central venous catheter (silicone tubing; Baxter Healthcare Corp., Deerfield, Ill.) placed in the superior vena cava.
Treatment groups.
Infected animals were assigned to treatment with evernimicin or vancomycin, each administered for 5.5 days, or served as untreated controls. Evernimicin was given at a dose of 60 mg/kg of body weight by i.v. infusion over 3 to 5 min every 24 h, followed by infusion of 0.6 ml of SCH 27899 placebo to flush the catheter in order to prevent precipitation of the antibiotic. Following each dose, lines were kept open with 0.3 ml of 5% dextrose in water (D5W) per h. Vancomycin-treated animals received 150 mg/kg of body weight 24 h via continuous infusion in D5W. This dosage of vancomycin results in mean concentrations in serum of approximately 15 μg/ml (16). These doses were selected in order to achieve serum drug concentrations in rats comparable to those achievable in humans. Serum pharmacokinetics of evernimicin were studied in uninfected rats; concentrations were measured by an agar diffusion assay with Bacillus subtilis ATCC 6633.
Monitoring of therapy and outcome.
Animals were sacrificed 3 h after discontinuation of vancomycin or 24 h after the last dose of evernimicin (elimination half-life in rats of approximately 2 h) (P. Krieter, M. Thonoor, M. Wirth, S. Gupta, J. Patrick, and M. N. Cayen, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-112, p. 23, 1997) to permit elimination of antibiotics from the serum. At sacrifice, correct placement of the carotid catheter across the aortic valve was documented, and only animals with correct placement were included in the study. Cardiac vegetations were removed, homogenized in saline, and serially diluted. Twenty-five-microliter volumes of each dilution were plated on sheep blood agar plates in duplicate and on mannitol salt agar, again in duplicate. Results from all four plates were averaged to determine colony counts. Colonies were counted after 24 h of incubation and expressed as log10 CFU per gram of vegetation. This technique allows detection of approximately 2 log10 CFU/g of vegetation. Treated animals that did not survive for the duration of the experiment were included in statistical analysis of bacterial vegetation density only if they had received at least 4 days of antibiotic treatment.
Statistical analysis.
The significance of mortality differences between the groups was evaluated with the chi-square test with the Yates' correction. The Mann-Whitney rank sum test was used to assess the significance of differences in bacterial counts in cardiac vegetations.
RESULTS
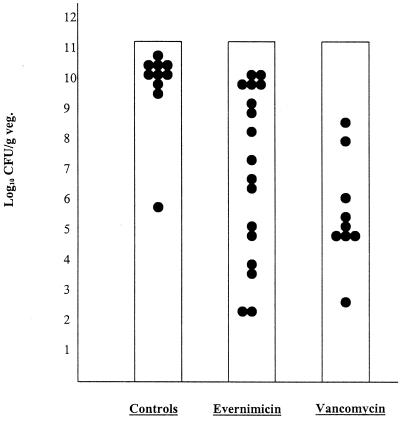
The results of treatment of aortic valve endocarditis in animals infected with MRSA 32 are shown in Fig. 1. At the time treatment was initiated, 6 h after inoculation, the mean (± standard deviation [SD]) colony count in vegetations from four rats was 6.63 ± 0.44 log10 CFU/g. Mean (± SD) counts of residual bacteria from cardiac vegetations at the conclusion of therapy were as follows: untreated controls, 10.12 ± 1.51 log10 CFU/g (n = 10); evernimicin, 7.22 ± 2.91 log10 CFU/g (n = 17); and vancomycin, 5.65 ± 1.76 log10 CFU/g (n = 9). Treatment with either evernimicin or vancomycin resulted in significantly lower bacterial density within vegetations compared with that in untreated controls (P = 0.001 and P < 0.001, respectively). However, the mean bacterial densities at the end of treatment were not statistically different from that observed at the start of therapy (P ≥ 0.19); hence, the effects of both antibiotics were primarily bacteriostatic. The difference in final colony counts between evernimicin- and vancomycin-treated animals was not statistically significant (P = 0.22). One rat in the evernimicin treatment group (5.9%) had bacterial counts below the limit of detection; all vancomycin-treated rats had a countable number of colonies in vegetations.
FIG. 1.
Viable bacteria recovered from cardiac vegetations (veg.) after treatment with evernimicin or vancomycin and from control rats. Each dot represents colony counts from a single animal.
As reflected in Fig. 1, the residual bacterial density for the group of animals treated with evernimicin was broadly distributed, from undetectable (≤2.45 log10 CFU/g) to 10.40 log10 CFU/g. Although the range of values obtained with vancomycin was also broad, values tended to cluster more closely about the mean. It is for this reason that more animals were entered into the evernimicin group in order to increase the precision of the measured response and thus to optimize statistical comparison of the two treatment groups (11).
Both treatments also provided a mortality benefit compared with controls. In the latter group, a 70% mortality rate was observed. All but one of the deaths among control animals occurred on day 3 or 4. Mortality was 12% in evernimicin-treated animals (P = 0.004) and 0% in vancomycin-treated rats (P = 0.007). There was no significant difference in mortality between the two groups receiving active antibiotic. In the evernimicin group, one death occurred after just one dose of the antibiotic, and the other occurred on the day of scheduled sacrifice. The bacterial count from this animal was 6.63 log10 CFU/g.
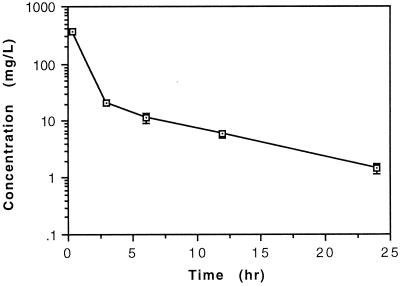
The concentrations of evernimicin in serum, determined over a 24-h interval following infusion of a 60-mg/kg dose of this compound, are shown in Fig. 2. The mean concentrations of evernimicin in serum remained above the MIC for the entire 24-h dose interval.
FIG. 2.
Concentrations of evernimicin in serum measured over time following administration of a 60-mg/kg dose infused i.v. over 3 to 5 min. Data points represent the mean concentration determined in two to four animals. Error bars depict the standard deviation.
DISCUSSION
MRSA strains are frequently resistant to multiple classes of antibiotics in addition to β-lactams, including tetracyclines, macrolides, and lincosamides; chloramphenicol; and trimethoprim-sulfamethoxazole (4), leaving vancomycin as the cornerstone of therapy. Nevertheless, treatment with vancomycin is not always successful, and some patients are intolerant of the drug. In addition, there is concern that continued heavy use of vancomycin may promote patient colonization with vancomycin-resistant enterococci or the further emergence of vancomycin-intermediate strains of S. aureus. Thus, new treatment options for gram-positive infections have been aggressively sought (3).
We employed a model of aortic valve infective endocarditis to study the efficacy of evernimicin against MRSA infection because of the prominent role of these organisms in clinical endovascular infections. We have previously shown that bactericidal activity in vitro is not a strict requirement for demonstration of activity in our animal model (16). This is because even though this is a model of endocarditis, it is not structured to assess rates of ultimate cure. Nevertheless, the aggressive nature of the infection created and the continued presence of the plastic intravascular catheter do make this model a rigorous means of testing antimicrobial activity in vivo. In this model, both evernimicin and the comparison agent, vancomycin, resulted in significant reductions in the density of viable bacteria on cardiac vegetations and the mortality rate compared with controls. It is important to note, however, that the final bacterial densities in animals from both treatment groups were similar to those at the start of therapy; hence, effects observed with either evernimicin or vancomycin should be described as primarily bacteriostatic. This is consistent with our in vitro data showing an MBC/MIC ratio of ≥32 for both antibiotics.
The dose of evernimicin chosen for these experiments, 60 mg/kg once daily, fortuitously approximated the 24-h bacteriostatic dose for this compound determined in the murine neutropenic thigh infection model, which was 45 to 61 mg/kg for infection due to MRSA (O. Vesga, and W. A. Craig, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-32, p. 6, 1997). The resulting area under the concentration-time curve for 24 h (AUC24), which was estimated to be approximately 400 μg-h/ml, was only modestly higher than the AUC (283 ± 23 μg-h/ml) calculated in humans receiving a 9-mg/kg dose of evernimicin over 1 h (T. Uematsu, O. Kozawa, S. Nagashima, M. Kanamaru, and Y. Ochi, Abstr. 38th Intersci. Conf. Antimicrob. Agents. Chemother., abstr. A-48, p. 15, 1998). By Monte Carlo pharmacodynamic simulation, this dose in humans is predicted to reach target goals for a bacteriostatic effect against S. aureus 96% of the time and to reach a maximal bactericidal effect approximately 50% of the time (G. L. Drusano, S. L. Preston, C. J. Hardalo, R. S. Hare, C. Banfield, and W. A. Craig, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1207, p. 37, 1999).
We do not have an explanation for the broad range of final bacterial densities in cardiac vegetations observed with evernimicin therapy. Although such broad distributions are not infrequent in staphylococcal endocarditis models, even when vancomycin therapy is employed (1, 2, 6), the range of values observed in evernimicin-treated animals was particularly large. We have seen a similarly broad range of bacterial densities within vegetations of animals treated with evernimicin in our studies of experimental enterococcal endocarditis (13). Our pharmacokinetic data suggested that differences in clearance of the compound in individual rats were not a likely explanation, given the small SDs about the mean serum drug concentrations shown in Fig. 2. Even at the 24-h trough, concentrations of evernimicin in serum exceeded the MIC for the test organism. However, evernimicin is highly protein bound (Vesga and Craig, 37th ICAAC), and its activity in vitro is substantially reduced in the presence of even 50% rat serum (13). Nevertheless, even assuming 96% protein binding (Vesga and Craig, 37th ICAAC), free drug concentrations would have remained above the MIC for the infecting organism for approximately 50% of the dosing interval. Although it might be argued that higher or more frequent doses would have resulted in better results, it is not clear that this would be the case. In our studies of evernimicin in an endocarditis model utilizing a vancomycin-resistant strain of Enterococcus faecium (evernimicin MIC of 0.25 μg/ml, identical to that for MRSA 32), doubling the dose from 60 mg/kg once a day to 120 mg/kg daily, either divided into twice-daily brief infusions or given by continuous 24-h infusions, did not appear to enhance the effectiveness of therapy in any obvious way (13). It is also conceivable that high protein binding may result in limited and nonhomogenous penetration of evernimicin into the vegetation mass.
Another potential explanation for the broad range of bacterial counts remaining at the end of therapy would be the emergence of evernimicin-resistant mutants on treatment. Although we did not test isolates recovered after therapy for susceptibility to the compound, we believe that this is not a likely explanation. In our enterococcal model, we found no evidence of increased resistance to evernimicin among colonies recovered from vegetations of treated animals. Furthermore, while colonies of S. aureus with reduced susceptibility to evernimicin can be selected following chemical mutagenesis (P. M. McNicholas, P. A. Mann, D. J. Najarian, L. Miesel, T. A. Black, R. S. Hare, and K. J. Shaw, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 846, p. 117, 1999), MICs for these strains are still below concentrations of the drug in rat serum. Indeed, difficulty in selection of resistant strains by antibiotic pressure alone has until recently complicated efforts to elucidate the mechanisms of action of this compound.
The results from this animal model of MRSA endocarditis have shown that evernimicin can have activity in vivo against serious staphylococcal infections. The effectiveness of the new agent appeared to be comparable to that of vancomycin, although with broader variability in final colony counts for individual animals; for both antibiotics, the effect observed at 5.5 days of therapy was bacteriostatic. The immediate relevance of these findings is limited by the recent decision to halt further clinical work with this compound. Clinical development of evernimicin was stopped after completion of phase II and III trials, based on data which failed to show a sufficient advantage of this agent in the treatment of infections due to vancomycin-susceptible or -resistant gram-positive bacteria, when compared with the clinical safety and efficacy profiles of approved agents. Nevertheless, we believe that these observations may prove useful points of comparison in the future, when other bacteriostatic inhibitors of protein synthesis are tested in similar experimental models.
ACKNOWLEDGMENT
This study was supported by a grant from Schering Plough Research Institute, Kenilworth, N.J.
REFERENCES
- 1.Chambers H F. In vitro and in vivo antistaphylococcal activities of l-695,256, a carbapenem with high affinity for the penicillin-binding protein PBP 2a. Antimicrob Agents Chemother. 1995;39:462–466. doi: 10.1128/aac.39.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Entenza J M, Drugeon H, Glauser M P, Moreillon P. Treatment of experimental endocarditis due to erythromycin-susceptible or -resistant methicillin-resistant Staphylococcus aureus with RP 59500. Antimicrob Agents Chemother. 1995;39:1419–1424. doi: 10.1128/aac.39.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fridkin S K, Edwards J R, Pichette S C, Pryor E R, McGowan J E, Jr, Tenover F C, Culver D H, Gaynes R P. Determinants of vancomycin use in adult intensive care units in 41 United States Hospitals. Clin Infect Dis. 1999;28:1119–1125. doi: 10.1086/514752. [DOI] [PubMed] [Google Scholar]
- 4.Gold H S, Moellering R C., Jr Antimicrobial-drug resistance. N Engl J Med. 1996;335:1445–1453. doi: 10.1056/NEJM199611073351907. [DOI] [PubMed] [Google Scholar]
- 5.Jensen A G, Wachmann C H, Poulsen K B, Espersen F, Scheibel J, Pkinhoj P, Frimodt-Moeller N. Risk factors for hospital-acquired Staphylococcus aureus bacteremia. Arch Intern Med. 1999;159:1437–1444. doi: 10.1001/archinte.159.13.1437. [DOI] [PubMed] [Google Scholar]
- 6.Kim Y S, Liu Q, Chow L L, Chambers H F, Täuber M G. Comparative efficacy of trovafloxacin in experimental endocarditis caused by ciprofloxacin-sensitive, methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1998;42:3325–3327. doi: 10.1128/aac.42.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landman D, Chockalingam M, Quale J M. Reduction in the incidence of methicillin-resistant Staphylococcus aureus and ceftazidime-resistant Klebsiella pneumoniae following changes in a hospital antibiotic formulary. Clin Infect Dis. 1999;28:1062–1066. doi: 10.1086/514743. [DOI] [PubMed] [Google Scholar]
- 8.Moellering R C., Jr Problems with antimicrobial resistance in gram-positive cocci. Clin Infect Dis. 1998;26:1177–1178. doi: 10.1086/520288. [DOI] [PubMed] [Google Scholar]
- 9.Nakashio S, Iwasawa H, Dun F Y, Kanemitsu K, Shimada J. Everninomicin, a new oligosaccharide antibiotic: its antimicrobial activity, postantibiotic effect and synergistic bactericidal activity. Drugs Exp Clin Res. 1995;XXI:7–16. [PubMed] [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7–A4. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 11.Piantadosi S. Clinical trials: a methodologic perspective. New York, N.Y: Wiley-Interscience; 1997. [Google Scholar]
- 12.Santoro J, Levison M E. Rat model of experimental endocarditis. Infect Immun. 1978;19:915–918. doi: 10.1128/iai.19.3.915-918.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Souli M, Thauvin-Eliopoulos C, Eliopoulos G M. In vivo activities of evernimicin (SCH 27899) against vancomycin-susceptible and vancomycin-resistant enterococci in experimental endocarditis. Antimicrob Agents Chemother. 2000;44:2733–2739. doi: 10.1128/aac.44.10.2733-2739.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thauvin C, Eliopoulos G M, Willey S, Wennersten C, Moellering R C., Jr Continuous-infusion ampicillin therapy of enterococcal endocarditis in rats. Antimicrob Agents Chemother. 1987;31:139–143. doi: 10.1128/aac.31.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urban C, Mariano N, Mosinka-Snipas K, Wadee C, Chahrour T, Rahal J J. Comparative in-vitro activity of SCH 27899, a novel everninomicin, and vancomycin. J Antimicrob Chemother. 1996;37:361–364. doi: 10.1093/jac/37.2.361. [DOI] [PubMed] [Google Scholar]
- 16.Yao J D C, Thauvin-Eliopoulos C, Eliopoulos G M, Moellering R C., Jr Efficacy of teicoplanin in two dosage regimens for experimental endocarditis caused by a β-lactamase-producing strain of Enterococcus faecalis with high-level resistance to gentamicin. Antimicrob Agents Chemother. 1990;34:827–830. doi: 10.1128/aac.34.5.827. [DOI] [PMC free article] [PubMed] [Google Scholar]