Abstract
Background
Standardized diagnostic criteria for arrhythmogenic left ventricular cardiomyopathy (ALVC) have been recently proposed. The criteria emphasize structural left ventricle (LV) myocardial change on contrast-enhanced imaging and require the identification of gene variants associated with arrhythmogenic cardiomyopathy.
Case summary
A 21-year-old man presented for evaluation of exertional syncope and was found to have monomorphic ventricular tachycardia (VT) and an episode of polymorphic VT that degenerated to ventricular fibrillatory cardiac arrest. Documented premature ventricular contractions were of left bundle branch block, inferior axis morphology. Ventricular arrhythmias were successfully suppressed with β-blockade, amiodarone, and lidocaine, and a subcutaneous implantable cardioverter-defibrillator was implanted. Cardiac magnetic resonance imaging demonstrated normal-appearing right ventricle, reduced LV ejection fraction, and sub-epicardial scarring of basal-anterior and anterolateral LV segments. Endomyocardial biopsy showed lymphocytic myocarditis, and genetic testing revealed a pathogenic truncating mutation in the DSP gene, which encodes desmoplakin; this variant was also identified in the patient’s mother who carried a diagnosis of non-ischaemic cardiomyopathy. These findings are consistent with a diagnosis of ALVC.
Discussion
The clinical presentation of ALVC can be very dramatic. The differential for sub-epicardial LV myocardial fibrosis includes myocarditis, sarcoidosis, and in those with a suspicious family history or characteristic electrocardiogram findings, genetic cardiomyopathy. Prompt referral to a genetic counsellor can be lifesaving to patients and their family members.
Keywords: Arrhythmogenic left ventricular cardiomyopathy, Desmoplakin, Genetic testing, Ventricular tachycardia, Case report
For the podcast associated with this article, please visit https://academic.oup.com/ehjcr/pages/podcast
Learning points.
The 2020 Padua criteria emphasize contrast-enhanced cardiac magnetic resonance imaging findings and genetic testing in the diagnosis of arrhythmogenic left ventricular cardiomyopathy (ALVC).
Loss-of-function DSP mutations lead to failure of intercalated disc integrity and subsequent cardiomyocyte hypertrophy, inflammation, and often sub-epicardial fibrosis.
Ventricular arrhythmias and fibrosis in ALVC can be misattributed to viral myocarditis or sarcoidosis. A thorough cardiac family history that looks for heritable cardiomyopathy can be life-saving.
Introduction
The 2010 revision of the arrhythmogenic right ventricular cardiomyopathy (ARVC) Task Force diagnostic criteria included known pathogenic gene mutations as one of the major criteria for the diagnosis of ARVC.1 The 2019 Heart Rhythm Society guidelines for arrhythmogenic cardiomyopathy also incorporated genetic data into implantable cardioverter-defibrillator (ICD) recommendations.2 These changes reflected the work of the early 2000s, when familial mutations in genes encoding plakoglobin, desmoplakin, plakophilin, desmocollin, and desmoglein were isolated from various ARVC registries.3 Over the past decade, both biventricular and isolated arrhythmogenic left ventricular cardiomyopathy (ALVC) have also been recognized, though only recently have diagnostic criteria accounting for the left ventricular phenotype been proposed.4
Timeline
| Hour 0: Emergency room presentation | Administration of metoprolol, amiodarone, and lidocaine for ventricular tachycardia (VT). |
| Hour 3 | Sudden cardiac arrest from ventricular fibrillation with cardiopulmonary resuscitation (CPR) and return of spontaneous circulation. |
| Day 1 | Transthoracic echocardiogram (TTE) demonstrated both global and regional depressed left ventricular (LV) function with ejection fraction of 45%. Late gadolinium enhancement seen on cardiac magnetic resonance imaging in sub-epicardial regions of the LV. |
| Day 2 | No further VT. Lidocaine infusion stopped. |
| Day 3 | Endomyocardial biopsy showed lymphocytic myocarditis and scattered areas of myocyte hypertrophy and fibrosis. Totally subcutaneous implantable cardioverter-defibrillator (S-ICD) implanted for secondary prevention. |
| Day 4 | Discharge from hospital on oral amiodarone and metoprolol. |
| Month 3 | Repeat TTE showed recovery of LV function with ejection fraction of 54%. Cardiomyopathy gene panel revealed DSP truncation variant p. R1951X. This variant was also identified in the patient’s mother, who had a known cardiomyopathy. |
| Month 12 | No ventricular arrhythmias recorded on S-ICD; no new symptoms. Patient continues on metoprolol and amiodarone. |
Case presentation
A 21-year-old university student presented to the Emergency Department (ED) following an episode of syncope after exercise at home. He had no chest pain or shortness of breath. Two weeks prior, he had a self-limited diarrheal illness presumed to be viral gastroenteritis. In the ED, he was afebrile with a heart rate of 81 beats/min, blood pressure of 127/78 mmHg, and room air pulse oximetry of 98%. He was alert, appeared comfortable, and an initial cardiac examination showed a regular rate and rhythm. Jugular venous pulsation was not elevated at 30°, and lungs were clear to auscultation.
The patient had no significant past medical history. Social history was significant for occasional heavy alcohol use and complete abstinence from tobacco or illicit substances. His mother had an idiopathic cardiomyopathy and had undergone genetic testing several years prior with non-diagnostic results, and the details of the prior genetic panel were unavailable. His maternal grandmother and her brother had unspecified heart disease as well and were advised to have ICDs placed.
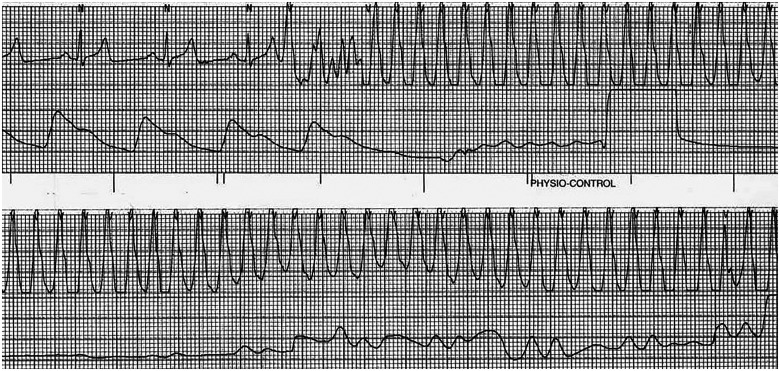
While awaiting testing in the ED, his telemetry monitoring showed repeated runs of sustained monomorphic ventricular tachycardia (VT) with a cycle length of 220 ms (Figure 1); he remained haemodynamically stable but felt lightheaded during those episodes. He was given intravenous metoprolol, amiodarone, and lidocaine and admitted to the coronary care unit. Upon arrival to the coronary care unit, the patient’s cardiac rhythm changed to polymorphic VT with degeneration to ventricular fibrillation, and he lost a pulse leading to a cardiac arrest. Advanced Cardiac Life Support was initiated, and he was defibrillated with 200 J of biphasic energy with return of spontaneous circulation and consciousness.
Figure 1.
Telemetry from the emergency department showing monomorphic ventricular tachycardia with cycle length 220 ms. The patient felt lightheaded but was not hypotensive. The episode self-terminated but recurred twice in the Emergency Department. The patient was started on intravenous metoprolol, amiodarone, and lidocaine.
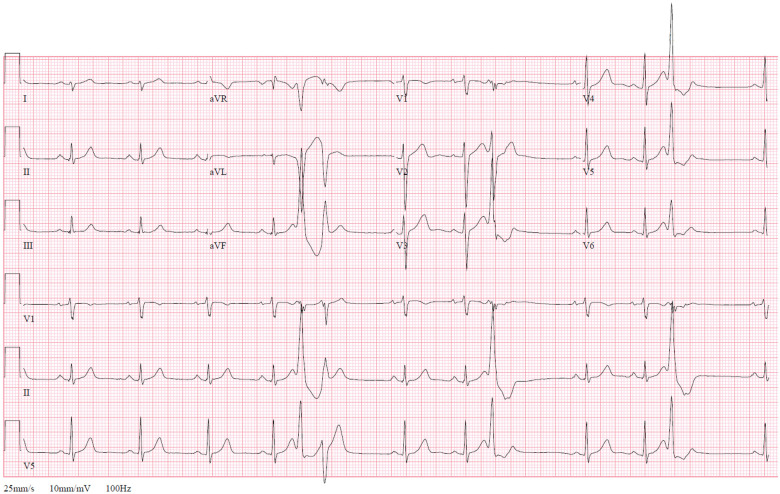
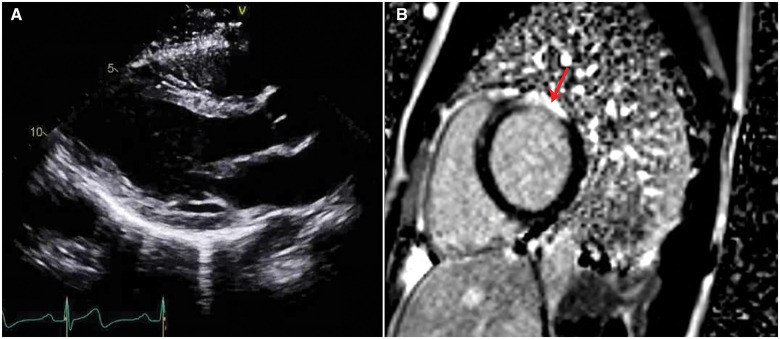
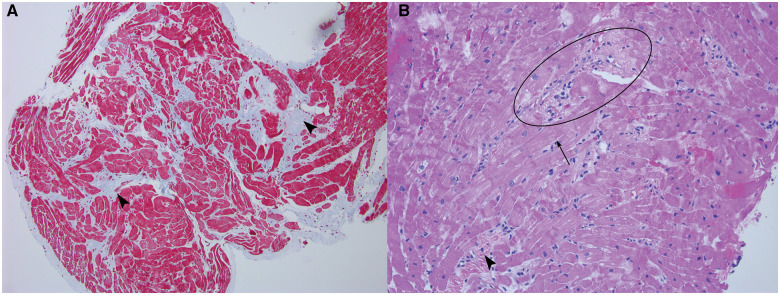
Baseline serum chemistry values were normal. Initial troponin I was 0.23 ng/mL (normal <0.04 ng/mL) and decreased thereafter. The pre-arrest electrocardiogram (ECG) demonstrated normal sinus rhythm with ventricular premature complexes of left bundle branch block and inferior axis morphology. There were no epsilon waves, precordial T wave inversions, intraventricular conduction delay, or QT prolongation (Figure 2). Post-arrest transthoracic echocardiogram showed a dilated left ventricle (LV) with an ejection fraction of 45%, severe hypokinesis of the basal-anterior segment and mild hypokinesis of the mid- and distal anterior, anterior septal, and apical lateral segments (Figure 3A, Video 1). Cardiac magnetic resonance (CMR) imaging showed sub-epicardial basal-anterior and basal anterolateral late gadolinium enhancement (LGE), and additional LGE in the mid-inferior and mid-anteroseptal segments (Figure 3B, Video 2). The right ventricle (RV) was of normal size and function and without LGE. An endomyocardial biopsy was performed to rule out giant cell myocarditis. Biopsy demonstrated mild lymphocytic myocarditis, interstitial fibrosis, and myocyte hypertrophy (Figure 4).
Figure 2.
Pre-arrest 12-lead surface electrocardiogram. Normal sinus rhythm with premature ventricular complexes of left bundle branch block, inferior axis morphology. Ventricular rate 82 beats/min, QRS 96 ms, and QTc 472 ms. Rightward axis and left posterior fascicular block. Upright T-waves in V2–V6. No epsilon waves.
Figure 3.
(A) Transthoracic echocardiogram, parasternal long-axis view. Left ventricular (LV) internal diameter in diastole was 5.5 cm. Left ventricular ejection fraction was 45%. (B) Cardiac magnetic resonance imaging, short-axis basal slice. Late gadolinium enhancement is demonstrated at the sub-epicardial left ventricular basal-anterior and basal anterolateral segments, involving >50% of wall thickness with some transmural foci (red arrow). The right ventricle had no late gadolinium enhancement.
Figure 4.
(A) Masson Trichrome stain of endomyocardial biopsy, 100× magnification. Pale blue areas demonstrate diffuse interstitial fibrosis (arrowheads). (B) Haematoxylin and eosin, 200× magnification. Areas of lymphocytic infiltrate and cardiomyocyte damage consistent with myocarditis (centre left oval). Variation in myocyte size, myocyte hypertrophy (arrow), and interstitial fibrosis (arrowhead) are unusual for a young patient and raise concern for an underlying cardiomyopathy. There was no evidence of iron deposition.
A diagnosis of polymorphic VT arrest due to lymphocytic myocarditis with likely underlying cardiomyopathy was given. The patient transitioned to oral amiodarone and metoprolol succinate and was provided a referral for cardiac genetic counselling. A subcutaneous ICD (S-ICD) was placed for secondary prevention of sudden cardiac death. This was chosen based on his young age and because anti-tachycardia pacing was unlikely to be efficacious for his very rapid VT, and at times polymorphic VT.
The patient was followed up for outpatient genetic counselling. Genetic testing identified a pathogenic heterozygous DSP gene truncation variant (p.R1951X) and the pathogenic heterozygous HFE variant (p.H63D). Three variants of uncertain significance were also reported (DSC2, p. M589T; LAMA4, p. S678F; and HFE, p. R224W). The HFE gene encodes the homeostatic iron regulator protein, DSC2 encodes desmocollin 2, and LAMA4 encodes the alpha 4 sub-unit of laminin. Only the DSP truncation variant, p. R1951X, segregated with disease in other family members and was therefore considered the primary responsible pathogenic variant (Figure 5).
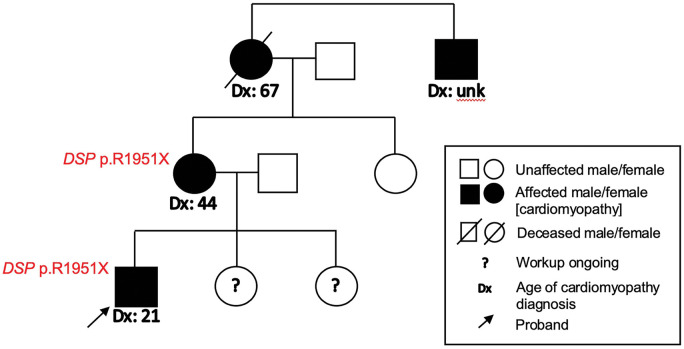
Figure 5.
Pedigree for patient. The DSP c.5851C>T (p.R1951X) truncation variant was present in the patient and his mother. The patient’s two siblings were asymptomatic and underwent non-invasive cardiac testing and genetic screening. The patient’s maternal grandmother was advised to have implantable cardioverter-defibrillator implantation but died of sudden cardiac arrest before this could occur.
At 1-year follow-up, the patient remained on amiodarone 200 mg daily and metoprolol 25 mg daily with plans to transition to sotalol. Repeat echocardiogram showed normalization of LV ejection fraction and no wall motion abnormalities. He has received no therapies from his S-ICD, and there have been no further episodes of VT on device interrogation.
Discussion
The DSP gene encodes desmoplakin, an intracellular protein that localizes to intercalated discs and associates with plakoglobin, plakophilin, desmocollin, and desmoglein near its N-terminus and with intermediate filaments near its C-terminus.2 Truncation variants lead to loss-of-function and failure of intercellular adhesion, and cardiomyocyte death leads to inflammation and fibrosis that can resemble infectious myocarditis on pathology.2,5
The young age at which our patient exhibited cardiomyopathic changes and cardiac arrest is notable. It is suspected that the fibrofatty LV replacement of ALVC seen in late disease reflects reparation after repeated inflammatory ‘hot phases’ in normally quiescent myocardium.5 Active myocardial inflammation predisposes younger patients to electrical instability and sudden cardiac death. Accordingly, our patient had mild troponin elevation and CMR demonstrating localized oedema on T2-weighted imaging but no obvious fatty infiltration. The triggers of these acute inflammatory phases are not fully elucidated. In our patient, no viral molecular testing was done on endomyocardial tissue, and the extent to which a viral myocarditis may have contributed to his dramatic presentation is unclear.
It is possible the genetic burden of additional DSC2 and HFE missense variants also contributed to his early presentation compared to other family members. The patient’s mother had congestive heart failure symptoms in her forties, and it was revealed that the patient’s maternal grandmother had declined a medical recommendation for an ICD and died in her sleep while in her sixties. While the recommendation for ICD implantation for secondary prevention was straightforward in our patient, implantation for primary prevention of sudden cardiac arrest in ALVC is less clear. Prospective studies on life-threatening ventricular arrhythmias in arrhythmogenic cardiomyopathy are primarily from ARVC registries. These studies have identified several risk factors for incident ventricular arrhythmias or delivery of ICD therapy when present: non-sustained VT, inducible VT in an electrophysiology study, LV ejection fraction ≤49%, male sex, high burden of premature ventricular contractions, RV dysfunction, multiple desmosomal variants, and the proband having sustained a life-threatening ventricular arrhythmia.6–9
The specific DSP truncation variant p. R1951X has been previously identified in a 41-year-old man with dilated cardiomyopathy and biventricular heart failure, and in one case of hypertrophic cardiomyopathy.10,11 However, DSP-related cardiomyopathy has a preference for scarring the LV and often leads to ALVC.12 The disease is under-recognized owing to an extensive differential for LV fibrosis; misattribution to myocarditis or sarcoidosis is common.5 The 2020 Padua criteria represent an effort to standardize the diagnosis of ALVC. Patients are diagnosed with ALVC if they exhibit structural LV myocardial abnormalities, manifesting as sub-epicardial or mid-myocardial LV in 1 segment(s) of the LV free wall or septum, are free of RV involvement, and harbour a gene mutation implicated in arrhythmogenic cardiomyopathy.4 The emphasis on CMR and genomics over ECG findings contrasts with the initial description of ALVC by Sen-Chowdhry et al. in 2008, where inclusion criteria required documented arrhythmia of LV origin or left precordial repolarization abnormalities.5 The patient in our case demonstrated neither ventricular arrhythmia of right bundle branch block morphology nor left precordial T wave changes; however, his malignant ventricular arrhythmias, localization of sub-epicardial LV fibrosis, and isolation of a pathogenic DSP variant warrant a diagnosis of ALVC under the 2020 Padua criteria.
Conclusion
Our patient with DSP-related arrhythmogenic cardiomyopathy featured LV predominant disease and met criteria for ALVC. Other than a suspicious family history, his clinical presentation could have been misattributed solely to a transient viral myocarditis. A thorough family history and prompt referral to genetic counselling helped identify the correct diagnosis, with important implications for immediate family members.

Lead author biography
Vincent Chen, MD, is a third-year resident in the Internal Medicine program at the McGaw Medical Center of Northwestern University. He is an alumnus of the Teach for America 2013 Milwaukee Corps and takes his passion for teaching onto rounds and into patients’ rooms. He is planning a career in cardiology. His interests include molecular mechanisms of cardiovascular disease and estimation of population disease burden.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The authors confirm that written consent for submission and publication of this case report including images and associated text has been obtained from the patient in line with COPE guidance.
Funding: The National Institutes of Health (HL128075); and the American Heart Association Arrhythmias and Sudden Cardiac Death Strategically Focused Research Network to E.M.
Conflict of interest: E.M.M. is a consultant to Amgen, Avidity, AstraZeneca, Cytokinetics, Janssen, Pfizer, Tenaya Therapeutics and is the founder of Ikaika Therapeutics. The content of this manuscript is unrelated to these activities. B.P.K. is a consultant to Boston Scientific, Inc., maker of the subcutaneous implantable defibrillator.
Supplementary Material
References
- 1.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Towbin JA, McKenna WJ, Abrams DJ, Ackerman MJ, Calkins H, Darrieux FCC. et al. 2019 HRS expert consensus statement on evaluation, risk stratification, and management of arrhythmogenic cardiomyopathy. Heart Rhythm 2019;16:e301–72. [DOI] [PubMed] [Google Scholar]
- 3.Syrris P, Ward D, Evans A, Asimaki A, Gandjbakhch E, Sen-Chowdhry S. et al. Arrhythmogenic right ventricular dysplasia/cardiomyopathy associated with mutations in the desmosomal gene desmocollin-2. Am J Hum Genet 2006;79:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corrado D, Perazzolo Marra M, Zorzi A, Beffagna G, Cipriani A, Lazzari MD. et al. Diagnosis of arrhythmogenic cardiomyopathy: the Padua criteria. Int J Cardiol 2020;319:106–114. [DOI] [PubMed] [Google Scholar]
- 5.Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D. et al. Left-dominant arrhythmogenic cardiomyopathy. An under-recognized clinical entity. J Am Coll Cardiol 2008;52:2175–2187. [DOI] [PubMed] [Google Scholar]
- 6.Mazzanti A, Ng K, Faragli A, Maragna R, Chiodaroli E, Orphanou N. et al. Arrhythmogenic right ventricular cardiomyopathy. J Am Coll Cardiol 2016;68:2540–2550. [DOI] [PubMed] [Google Scholar]
- 7.Pinamonti B, Dragos AM, Pyxaras SA, Merlo M, Pivetta A, Barbati G. et al. Prognostic predictors in arrhythmogenic right ventricular cardiomyopathy: results from a 10-year registry. Eur Heart J 2011;32:1105–1113. [DOI] [PubMed] [Google Scholar]
- 8.Orgeron GM, James CA, Te Riele A, Tichnell C, Murray B, Bhonsale A. et al. Implantable cardioverter‐defibrillator therapy in arrhythmogenic right ventricular dysplasia/cardiomyopathy: predictors of appropriate therapy, outcomes, and complications. JAHA 2017;6:e006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Link MS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear K. et al. Ventricular arrhythmias in the North American multidisciplinary study of ARVC. J Am Coll Cardiol 2014;64:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cuenca S, Ruiz-Cano MJ, Gimeno-Blanes JR, Jurado A, Salas C, Gomez-Diaz I, et al. ; Inherited Cardiac Diseases Program of the Spanish Cardiovascular Research Network (Red Investigación Cardiovascular). Genetic basis of familial dilated cardiomyopathy patients undergoing heart transplantation. J Heart Lung Transplant. J Heart Lung Transplant 2016;35:625–635. [DOI] [PubMed] [Google Scholar]
- 11.Bottillo I, D'Angelantonio D, Caputo V, Paiardini A, Lipari M, De Bernardo C. et al. Molecular analysis of sarcomeric and non-sarcomeric genes in patients with hypertrophic cardiomyopathy. Gene 2016;577:227–235. [DOI] [PubMed] [Google Scholar]
- 12.Smith ED, Lakdawala NK, Papoutsidakis N, Aubert G, Mazzanti A, McCanta AC. et al. Desmoplakin cardiomyopathy, a fibrotic and inflammatory form of cardiomyopathy distinct from typical dilated or arrhythmogenic right ventricular cardiomyopathy. Circulation 2020;141:1872–1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.