Key Points
Question
What is the durability of the antibody response to COVID-19 vaccines in patients with cancer undergoing treatment or who received a stem cell transplant?
Findings
In this cross-sectional study of 453 patients with cancer undergoing treatment or who received a stem cell transplant, the geometric mean titers for the anti–SARS-CoV-2 spike protein receptor binding domain were 470.38 U/mL 1 month after the second dose of the vaccine, 447.23 U/mL 6 months after the second dose, and 9224.85 U/mL 1 month after a third dose.
Meaning
This study suggests that for patients with cancer undergoing treatment or who received a stem cell transplant, antibody titers peak 1 month after the second dose of a messenger RNA vaccine and are sustained over 6 months; compared with the primary vaccine course, a 20-fold increase in geometric mean titers after a third suggests a robust B-cell response.
Abstract
Importance
The durability of the antibody response to COVID-19 vaccines in patients with cancer undergoing treatment or who received a stem cell transplant is unknown and may be associated with infection outcomes.
Objective
To evaluate anti–SARS-CoV-2 spike protein receptor binding domain (anti-RBD) and neutralizing antibody (nAb) responses to COVID-19 vaccines longitudinally over 6 months in patients with cancer undergoing treatment or who received a stem cell transplant (SCT).
Design, Setting, and Participants
In this prospective, observational, longitudinal cross-sectional study of 453 patients with cancer undergoing treatment or who received an SCT at the University of Kansas Cancer Center in Kansas City, blood samples were obtained before 433 patients received a messenger RNA (mRNA) vaccine (BNT162b2 or mRNA-1273), after the first dose of the mRNA vaccine, and 1 month, 3 months, and 6 months after the second dose. Blood samples were also obtained 2, 4, and 7 months after 17 patients received the JNJ-78436735 vaccine. For patients receiving a third dose of an mRNA vaccine, blood samples were obtained 30 days after the third dose.
Interventions
Blood samples and BNT162b2, mRNA-1273, or JNJ-78436735 vaccines.
Main Outcomes and Measures
Geometric mean titers (GMTs) of the anti-RBD; the ratio of GMTs for analysis of demographic, disease, and treatment variables; the percentage of neutralization of anti-RBD antibodies; and the correlation between anti-RBD and nAb responses to the COVID-19 vaccines.
Results
This study enrolled 453 patients (mean [SD] age, 60.4 [13,1] years; 253 [56%] were female). Of 450 patients, 273 (61%) received the BNT162b2 vaccine (Pfizer), 160 (36%) received the mRNA-1273 vaccine (Moderna), and 17 (4%) received the JNJ-7846735 vaccine (Johnson & Johnson). The GMTs of the anti-RBD for all patients were 1.70 (95% CI, 1.04-2.85) before vaccination, 18.65 (95% CI, 10.19-34.11) after the first dose, 470.38 (95% CI, 322.07-686.99) at 1 month after the second dose, 425.80 (95% CI, 322.24-562.64) at 3 months after the second dose, 447.23 (95% CI, 258.53-773.66) at 6 months after the second dose, and 9224.85 (95% CI, 2423.92-35107.55) after the third dose. The rate of threshold neutralization (≥30%) was observed in 203 of 252 patients (80%) 1 month after the second dose and in 135 of 166 patients (81%) 3 months after the second dose. Anti-RBD and nAb were highly correlated (Spearman correlation coefficient, 0.93 [0.92-0.94]; P < .001). Three months after the second dose, anti-RBD titers were lower in male vs female patients (ratio of GMTs, 0.52 [95% CI, 0.34-0.81]), patients older than 65 years vs patients 50 years or younger (ratio of GMTs, 0.38 [95% CI, 0.25-0.57]), and patients with hematologic malignant tumors vs solid tumors (ratio of GMTs, 0.40 [95% CI, 0.20-0.81]).
Conclusions and Relevance
In this cross-sectional study, after 2 doses of an mRNA vaccine, anti-RBD titers peaked at 1 month and remained stable over the next 6 months. Patients older than 65 years of age, male patients, and patients with a hematologic malignant tumor had low antibody titers. Compared with the primary vaccine course, a 20-fold increase in titers from a third dose suggests a brisk B-cell anamnestic response in patients with cancer.
This cross-sectional study evaluates anti–SARS-CoV-2 spike protein receptor binding domain and neutralizing antibody responses to COVID-19 vaccines longitudinally over 6 months in patients with cancer undergoing treatment or who received a stem cell transplant.
Introduction
Patients with cancer and recipients of stem cell transplants (SCTs) who acquire COVID-19 have high rates of mortality.1,2,3 Severely immunocompromised patients, such as recipients of solid organ transplants, have high rates of humoral seronegativity after vaccination.4,5 This prospective, longitudinal cross-sectional study evaluates the degree and durability of anti-SARS-CoV-2 spike protein receptor binding domain (anti-RBD) and neutralizing antibody (nAb) responses to COVID-19 vaccines over 6 months after the second dose of a messenger RNA (mRNA) vaccine (or 7 months after the JNJ-7846735 vaccine) in patients with cancer undergoing systemic treatment or who have received an SCT. This information is critical for physicians to make informed decisions about vaccinating individual patients undergoing systemic treatment and those who received an SCT.
Methods
Study Population and Outcome Measures
Eligible patients were 18 years or age or older with a cancer diagnosis, were planning to receive a COVID-19 vaccine, and were undergoing systemic anticancer treatment or planning to start treatment within 14 days, or had already received an autologous or allogeneic SCT or chimeric antigen receptor T-cell therapy (Table). Patients could receive any of the available vaccines (BNT162b2 [Pfizer], mRNA-1273 [Moderna], or JNJ-7846735 [Johnson & Johnson]). This study was approved by the institutional review board of the University of Kansas Medical Center. All patients provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Table. Characteristics of 453 Patients and Analysis of Demographic, Disease, and Treatment Variables Associated With Anti-RBD Antibody 1 Month and 3 Months After the Second Messenger RNA Vaccine Dose.
| Characteristic | No. (%) | 1 mo After second dose | 3 mo After second dose | ||||
|---|---|---|---|---|---|---|---|
| No. (n = 293) | GMT (95% CI) | GMT ratio | No. (n = 336) | GMT (95% CI) | GMT ratio | ||
| Age group, y | |||||||
| ≤50 | 89 (20) | 56 | 765.29 (306.94-1908.09) | 1 [Reference] | 60 | 716.35 (349.50-1468.25) | 1 [Reference] |
| 51-65 | 188 (41) | 131 | 557.35 (312.00-995.65) | 0.73 (0.41-1.30) | 133 | 551.42 (363.33-836.88) | 0.77 (0.51-1.17) |
| >65 | 176 (39) | 106 | 315.28 (176.68-562.60) | 0.41 (0.23-0.74) | 143 | 269.15 (176.15-411.26) | 0.38 (0.25-0.57) |
| Sex | |||||||
| Female | 253 (56) | 167 | 541.08 (325.79-898.62) | 1 [Reference] | 192 | 561.60 (393.30-801.92) | 1 [Reference] |
| Male | 200 (44) | 126 | 413.26 (235.09-726.46) | 0.76 (0.43-1.34) | 144 | 294.39 (189.92-456.31) | 0.52 (0.34-0.81) |
| Cancer type | |||||||
| Solid tumor | 319 (70) | 203 | 808.65 (544.11-1201.82) | 1 [Reference] | 244 | 547.44 (417.13-718.46) | 1 [Reference] |
| Hematologic malignant tumor | 134 (30) | 90 | 149.90 (67.77-331.58) | 0.19 (0.08-0.41) | 92 | 218.67 (108.22-441.85) | 0.40 (0.20-0.81) |
| Vaccine type | |||||||
| BNT162b2 | 273 (61) | 178 | 503.44 (318.84-794.91) | 1 [Reference] | 201 | 371.10 (267.31-515.19) | 1 [Reference] |
| mRNA-1273 | 160 (35) | 101 | 676.07 (337.90-1352.68) | 1.34 (0.67-2.69) | 122 | 553.54 (327.79-934.78) | 1.49 (0.88-2.52) |
| JNJ-7846735 | 17 (4) | 14 | 24.00 (8.31-69.34) | 0.05 (0.02-0.14) | 13 | 304.29 (74.57-1241.65) | 0.82 (0.20-3.35) |
| Treatment groupa | |||||||
| Chemotherapy | 180(40) | 114 | 609.48 (322.36-1152.31) | NA | 142 | 584.34 (392.75-869.38) | NA |
| Immunotherapy | 75 (17) | 51 | 1053.63 (537.35-2065.94) | NA | 57 | 442.74 (263.19-744.76) | NA |
| SCTb | 114 (25) | 77 | 325.35 (149.93-706.01) | NA | 78 | 454.36 (237.48-869.32) | NA |
| Chemoimmunotherapy | 21 (5) | 15 | 314.28 (71.67-1378.11) | NA | 16 | 262.98 (85.98-804.40) | NA |
| CDK4/6 inhibitors | 23 (5) | 12 | 1800.32 (580.83-5580.21) | NA | 15 | 1099.05 (459.09-2631.11) | NA |
| TKIsc | 32 (7) | 19 | 189.84 (54.86-656.91) | NA | 20 | 157.56 (56.51-439.36) | NA |
| Oral (other agents)d | 8 (2) | 5 | 1.73 (0.10-30.58) | NA | 8 | 3.31 (0.22-48.78) | NA |
Abbreviations: anti-RBD, anti–SARS-CoV-2 spike protein receptor binding domain; CDK, cyclin-dependent kinase; NA, not applicable; SCT, stem cell transplant; TKIs, tyrosine kinase inhibitors.
The geometric mean titers (GMTs) of individual treatment groups are included. Given the small numbers in some of the treatment categories, the GMT ratios for comparison are not shown.
Allogeneic SCT, Autologous SCT, and chimeric antigen receptor T-cell therapy.
Only 1 patient among the TKI treatment group in this analysis had a hematologic malignant tumor.
This category included 5 patients with chronic lymphocytic leukemia receving venetoclax and 2 patients receiving non-TKI, non-CDK4/6 inhibitors.
At the study start, only unvaccinated patients were eligible. Because of the rapidity of vaccine availability for cancer patients in our area, on April 3, 2021, the protocol was amended to include partially and fully vaccinated patients with known vaccination date(s). To get a clinical representation of the cancer population, patients with prior COVID-19 infection were not excluded. For the mRNA vaccines, blood samples were obtained before vaccination, 2 weeks after the first dose and 1 month, 3 months, and 6 months after the second dose; for the JNJ-7846735 vaccine, blood samples were obtained 1 month, 2 months, 4 months, and 7 months after the vaccine (baseline before vaccination [T0], 14 days after the first dose and before the second dose of a messenger mRNA vaccine [T1], 1 month [±1 week] after the second dose of an mRNA vaccine or 2 months after the JNJ-7846735 vaccine [T2], 3 months [±4 weeks] after the second dose of an mRNA vaccine or 4 months after the JNJ-7846735 vaccine [T3], 6 months [±4 weeks] after the second dose of an mRNA vaccine or 7 months after the JNJ-7846735 vaccine [T4]). Once a third vaccine dose was approved for patients with cancer, blood samples were obtained 1 month after this third dose. Patients who were enrolled after receiving 1 dose of the vaccine had missing blood samples at T0, and patients who were enrolled after receiving 2 doses of the vaccine had missing blood samples at T0 or T1. Measurement of anti-RBD was performed using the Roche Elecsys anti-SARS-CoV-2 S enzyme immunoassay. Neutralizing antibodies were measured using the SARS-CoV-2 Surrogate Virus Neutralization Test assay (GenScript). The primary end point was the geometric mean titer (GMT) of the anti-RBD.
Statistical Analysis
Anti-RBD antibody kinetics over time after vaccination were associated with demographic characteristics, cancer type, and vaccine type. Scatter plots of log-transformed anti-RBD antibody titers over time were created with the use of an in-house Python script. The covariates are presented as ratios of mean titers with 95% CI. Correlations between anti-RBD and neutralizing antibodies were assessed based on Spearman rank correlation. P < .05 was considered to indicate statistical significance.
Results
Between March 2 and September 30, 2021, we enrolled 453 patients. Of these 453 patients, 186 (41%) were receiving chemotherapy, 75 (16%) were receiving immunotherapy, 22 (5%) were receiving chemoimmunotherapy, 114 (25%) had received an SCT, and 63 (14%) were receiving a targeted oral agent; 23 (5%) were receiving a cyclin-dependent kinase 4/6 inhibitor, and 32 (7%) were receiving a tyrosine kinase inhibitor. A total of 1042 blood samples were obtained for antibody testing (112 were obtained at T0, 176 were obtained at T1, 293 were obtained at T2, 336 were obtained at T3, 98 were obtained at T4, and 27 were obtained after a third dose). Of 450 patients, 273 (61%) received the BNT162b2 vaccine, 160 (36%) received the mRNA-1273 vaccine, and 17 (4%) received the JNJ-7846735 vaccine.
Anti-RDB and nAb Titers by Time Points and Cancer Treatments
The GMTs (95% CI) of the anti-RBD antibody for all patients were 1.70 (95% CI, 1.04-2.85) at T0, 18.65 (95% CI, 10.19-34.11) at T1, 470.38 (95% CI, 322.07-686.99) at T2, 425.80 (95% CI, 322.24-562.64) at T3, 447.23 (95% CI, 258.53-773.66) at T4, and 9224.85 (95% CI, 2423.92-35107.55) after the third dose (median time from the second to the third dose, 165 days [range, 78-187 days]). The proportion of patients with an anti-RDB titer 100 U/mL or higher was 12% at T0, 29% at T1, 75% at T2, 80% at T3, and 79% at T4. The rate of seropositivity (anti-RBD titer ≥0.8 U/mL) was 26% at T0, 68% at T1, 93% at T2, 95% at T3, and 95% at T4.
The Table presents the GMTs with 95% CIs, the ratios of GMTs with 95% CIs for demographic, disease, and vaccine variables, and the GMTs of different treatment groups, 1 month and 3 months after the second dose of an mRNA vaccine (or 2 and 4 months after the JNJ-7846735 vaccine). Three months after the second dose, anti-RBD titers were lower in male patients compared with female patients (ratio of GMTs, 0.52 [95% CI, 0.34-0.81]), lower in patients older than 65 years compared with patients 50 years or younger (ratio of GMTs, 0.38 [95% CI, 0.25-0.57]), and lower in patients with a hematologic malignant tumor compared with patients with a solid tumor (ratio of GMTs, 0.40 [95% CI, 0.20-0.81]). Three months after the second dose, anti-RBD titers 100 U/mL or higher were seen in 50 of 60 of patients (83%) 50 years of age or younger vs 109 of 143 of patients (76%) older than 65 years, in 160 of 192 female patients (83%) vs 109 of 144 male patients (76%), and in 61 of 92 patients (6%) with a hematologic malignant tumor vs 208 of 244 patients (85%) with a solid tumor.
Results of nAb testing were available for 664 blood samples. The rate of threshold neutralization (≥30%) was observed in 15 of 102 patients (15%) at T0, 56 of 144 patients (39%) at T1, 203 of 252 patients (80%) at T2, and 135 of 166 patients (81%) at T3 (Figure 1).
Figure 1. Anti–SARS-CoV-2 Spike Protein Receptor Binding Domain (Anti-RBD) and Neutralizing Antibody Response to Vaccines.
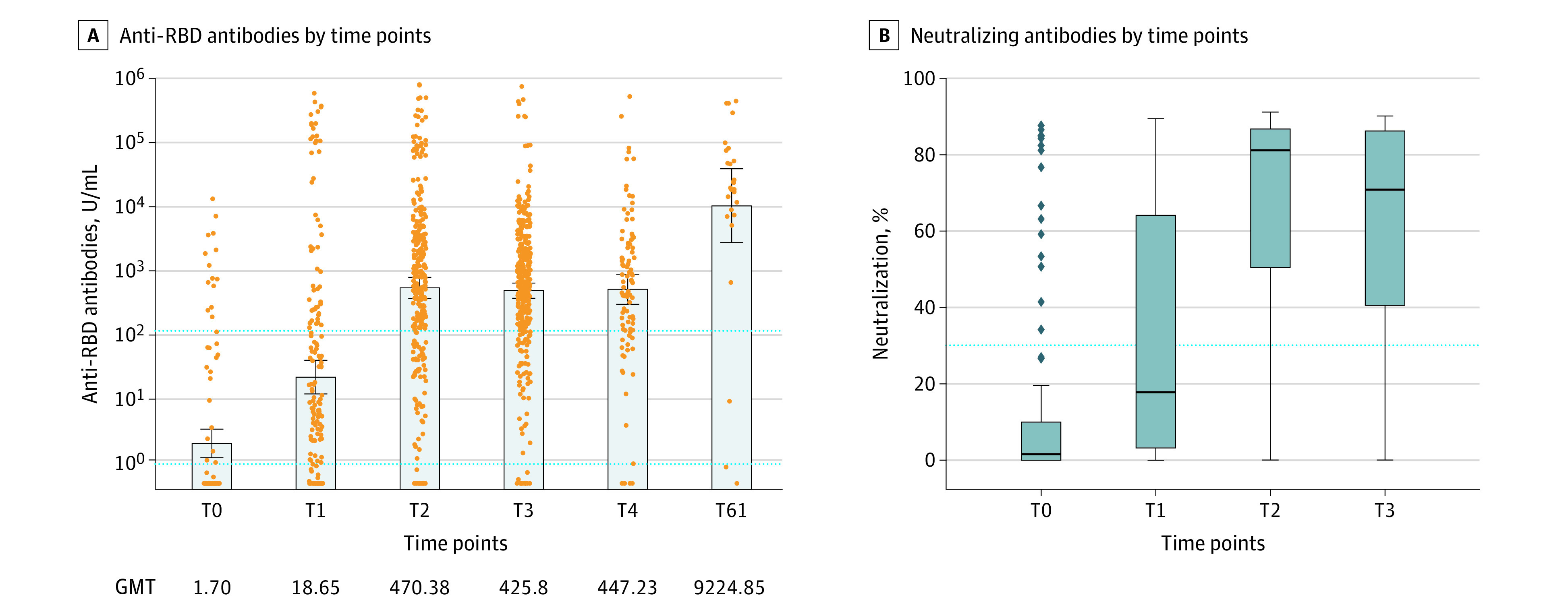
A, Anti-RBD titers for all 453 patients. A total of 1042 blood samples were obtained for antibody testing (112 at T0, 176 at T1, 293 at T2, 336 at T3, 98 at T4, and 27 after the third dose). Each dot represents a patient sample. The x-axis indicates time points for the collection of samples: baseline before vaccination (T0), 14 days after the first dose and before the second dose of a messenger RNA (mRNA) vaccine (T1), 1 month (±1 week) after the second dose of an mRNA vaccine or 2 months after the JNJ-7846735 vaccine (T2), 3 months (±4 weeks) after the second dose of an mRNA vaccine or 4 months after the JNJ-7846735 vaccine (T3), 6 months (±4 weeks) after the second dose of an mRNA vaccine or 7 months after the JNJ-7846735 vaccine (T4), and 4 weeks after the booster (third dose of an mRNA vaccine [T61]). The lower horizontal dotted line indicates seropositivity (0.8 U/mL), and the upper horizontal dotted line indicates the threshold value of 100 U/mL. The error bars indicate geometric mean titers (GMTs) with 95% CIs (whiskers). The GMTs are also displayed numerically. There was a 20-fold increase in titers 1 month after a booster compared with T2 (1 month after completion of the primary vaccination). Only 3 patients had titers lower than 100 U/mL after the booster, and all of them had a hematologic malignant tumor. B, Box-and-whisker plots of the percentage of neutralization using the Surrogate Viral Neutralization Test. The whiskers indicate the range, the boxes indicate the IQR, and the horizontal line within each box indicates the median. The dots indicate the outliers. The horizontal dotted line indicates threshold of neutralization (30%). The median percentage of neutralization was 1% at T0, 18% at T2, 81% at T2, and 71% at T3.
Correlation Between the 2 Antibodies
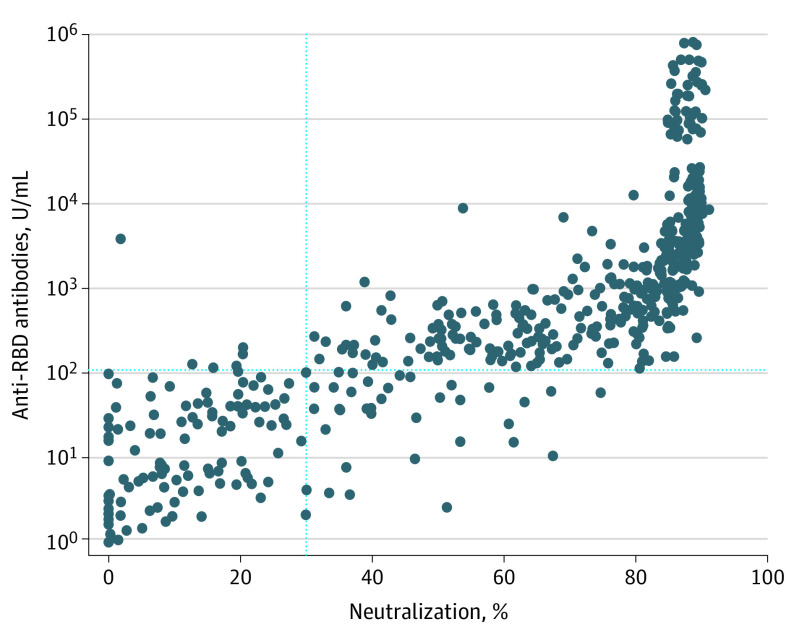
There was a strong correlation between anti-RBD titers and neutralization (Figure 2). The Spearman correlation coefficient between percentage of neutralization and anti-RBD was 0.93 and was statistically significant at P < .001.
Figure 2. Correlation Between Anti–SARS-CoV-2 Spike Protein Receptor Binding Domain (Anti-RBD) Antibody and Neutralizing Antibody Levels.
The correlation between anti-RBD antibody titers (y-axis) and percentage of neutralization by neutralizing antibody (x-axis) in 619 blood samples is shown. The vertical dotted line indicates the threshold of 30% neutralization; the horizontal dotted line indicates the threshold of 100 U/mL for the anti-RBD antibody. The Spearman correlation coefficient between the percentage of neutralization and the anti-RBD is 0.93, and it is statistically significant at P < .001.
Discussion
We have described the immune humoral responses to COVID-19 vaccines over 6 months after 2 doses of an mRNA vaccine (or 7 months after the JNJ-7846735 vaccine) in patients with cancer who were undergoing anticancer treatment or who had received an SCT. We measured an anti-RBD titer and an nAb titer and observed a strong linear association between the antibodies (Figure 2). A significant number of patients (32%) did not mount any antibody response after the first dose of a vaccine. After a sluggish response from the first dose, the anti-RBD response peaked 4 weeks after the second dose of an mRNA vaccine (2 months after the JNJ-7846735 vaccine) and was sustained at 6 months after the second dose of an mRNA vaccine. Despite antibody responses being durable over this time period, the peak antibody levels in these patients were well below the titers observed in healthy participants in published studies using the same assay.6,7 The 20-fold increase in titers from a booster dose compared with primary vaccination is indicative of a brisk anamnestic response from memory B cells.8
The association of higher antibody titers with younger age and female sex has been reported for patients without cancer, and we see a similar association among patients who were receiving anticancer therapy.7 Compared with patients with a hematologic malignant tumor, patients with a solid tumor had significantly higher titers, and more patients with a hematologic malignant tumor had anti-RBD titers less than 100 U/mL. This finding may explain the increased risk of severe COVID-19 among fully vaccinated patients with a hematologic malignant tumor.9
Higher levels of anti-RBD and neutralizing antibodies are associated with higher vaccine effectiveness.10,11 Although a threshold of humoral protection against breakthrough infections has not been established in humans, statistical modeling suggests that significantly lower antibody levels than those achieved by mRNA vaccines may be sufficient.12 While still an arbitrary cutoff, an anti-RBD level of 100 U/mL or higher has been associated with protection13 and has been used to evaluate the effectiveness of a third dose of an mRNA vaccine in a randomized clinical trial of patients who received a solid organ transplant.5
Limitations
Our study has some limitations. We did not include a control group of healthy participants. In addition, patients with a previous COVID-19 infection or a COVID-19 infection during the study were not excluded, and it is possible that they are not distributed evenly across subgroups, creating a potential bias.
Conclusions
The results of our cross-sectional study show that approximately 80% of the patients remained above the threshold of an anti-RBD level of 100 U/mL or higher at 6 months. Although more data are needed to confirm this level as protective, if established, anti-RBD can potentially be used to prioritize additional vaccine doses, especially in regions of the world with limited vaccine resources.
References
- 1.Bakouny Z, Hawley JE, Choueiri TK, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell. 2020;38(5):629-646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Al-Shamsi HO, Alhazzani W, Alhuraiji A, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an international collaborative group. Oncologist. 2020;25(6):e936-e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma A, Bhatt NS, St Martin A, et al. Clinical characteristics and outcomes of COVID-19 in haematopoietic stem-cell transplantation recipients: an observational cohort study. Lancet Haematol. 2021;8(3):e185-e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyarsky BJ, Werbel WA, Avery RK, et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021;325(17):1784-1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall VG, Ferreira VH, Ku T, et al. Randomized trial of a third dose of mRNA-1273 vaccine in transplant recipients. N Engl J Med. 2021;385(13):1244-1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller T. Antibodies against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) in individuals with and without COVID-19 vaccination: a method comparison of two different commercially available serological assays from the same manufacturer. Clin Chim Acta. 2021;518:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Terpos E, Trougakos IP, Apostolakou F, et al. Age-dependent and gender-dependent antibody responses against SARS-CoV-2 in health workers and octogenarians after vaccination with the BNT162b2 mRNA vaccine. Am J Hematol. 2021;96(7):E257-E259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turner JS, O’Halloran JA, Kalaidina E, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature. 2021;596(7870):109-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt AL, Labaki C, Hsu CY, et al. ; COVID-19 and Cancer Consortium . COVID-19 vaccination and breakthrough infections in patients with cancer. Ann Oncol. 2022;33(3):340-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bergwerk M, Gonen T, Lustig Y, et al. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021;385(16):1474-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gilbert PB, Montefiori DC, McDermott A, et al. Immune correlates analysis of the mRNA-1273 COVID-19 vaccine efficacy trial. medRxiv. Preprint posted online January 1, 2021.
- 12.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27(7):1205-1211. [DOI] [PubMed] [Google Scholar]
- 13.McMahan K, Yu J, Mercado NB, et al. Correlates of protection against SARS-CoV-2 in rhesus macaques. Nature. 2021;590(7847):630-634. [DOI] [PMC free article] [PubMed] [Google Scholar]