Abstract
This case series describes ipilimumab/nivolumab therapy in patients with metastatic pancreatic or biliary cancer with homologous recombination deficiency (HRD) pathogenic germline variants (PGVs).
Approximately 3% to 10% of patients with pancreatic ductal adenocarcinoma (PDAC) have pathogenic germline variants (PGVs) leading to homologous recombination deficiency (HRD), and approximately 15% to 17% have similar somatic alterations. These patients have increased sensitivity to platinum-containing chemotherapy and PARP inhibitors. There is increased genomic instability in this subgroup of patients; in a pan-cancer analysis, PDACs with biallelic loss of BRCA1 and BRCA2 (BRCA1/2) had a higher median tumor mutation burden (4.3 vs 1.7 mutations/Mb) and higher genomic loss of heterozygosity than wild-type tumors. Similar genomic instability leading to tumor-inflamed phenotype has been described in BRCA1/2-variant breast, ovarian, and prostate cancers. Immune checkpoint inhibitors (ICIs) have been ineffective in unselected patients with PDAC. Given the molecular rationale for increased susceptibility to ICIs in patients with PDAC associated with HRD PGVs, we investigated whether this subgroup may be sensitive to immunotherapy strategies.
Methods
This retrospective single-institution case series included patients with chemotherapy-refractory metastatic PDAC or biliary cancer who were treated between April 2018 and February 2021 with combination ipilimumab/nivolumab and had PGVs in HRD genes (detected by a Clinical Laboratory Improvement Amendments–approved assay). This study was approved by the University of Miami institutional review board, and a waiver of informed consent was given to collect deidentified data retrospectively. This study followed the reporting guideline for case series.
Patients received ipilimumab, 1 mg/kg, with nivolumab, 3 mg/kg, every 21 days for 4 doses, followed by nivolumab, 480 mg, every 28 days. Available pretreatment tumor specimens were analyzed by immunohistochemistry and RNA sequencing using the PanCancer IO 360 Panel. Data were analyzed using Prism, version 7, and 2-sided P < .05 was the level of significance.
Results
A total of 12 patients were included (7 [58%] men; median age, 66 [range, 47-73] years) (Table). Four patients achieved a complete response (CR) to therapy, 1 had a partial response, and 2 had stable disease. The objective response rate was 42%, with a disease control rate of 58%. The 4 patients who achieved CR remained without evidence of disease 11 to 41 months after starting therapy. Treatment-related adverse events were consistent with the known toxic effects of combination ipilimumab/nivolumab.
Table. Baseline Characteristics and Outcomes for 12 Patients With PDAC or Biliary Cancer Treated With Ipilimumab/Nivolumaba.
| Patient No./gender/age, y | Diagnosis | Germline variant | Best response | Duration of response, mo | PD-L1 combined positive score, % | TMB, mt/Mb | Somatic statusb | Previous therapies |
|---|---|---|---|---|---|---|---|---|
| 1/M/60s | PDAC | BRCA1 | CRc | 41.6 | NA | 4 | Biallelic | Resection and adjuvant gemcitabine/capecitabine |
| 2/M/70s | CCA | BRCA1 | CRc | 39.9 | 0 | 8 | Biallelic | Gemcitabine/cisplatin |
| 3/M/50s | PDAC | RAD51C | CRc | 26.4 | NA | 8 | Biallelic | FOLFIRINOX, olaparib, liposomal irinotecan/5-fluorouracil, and gemcitabine/nab-paclitaxel/cisplatin |
| 4/M/70s | AMP | BRCA2 | CRd | 11.5 | 2 | NA | Biallelic | Resection, adjuvant gemcitabine/cisplatin, and olaparib |
| 5/M/40s | PDAC | BRCA1 | PR | 7.3 | NA | NA | Biallelic | Gemcitabine/cisplatin/nab-paclitaxel and olaparib |
| 10/F/50s | PDAC | ATM | SD | 4.1 | NA | NA | Monoallelic | FOLFIRINOX, gemcitabine/nab-paclitaxel, and olaparib |
| 6/F/70s | PDAC | BRCA1 | SD | 3.5 | NA | 5 | Biallelic | Neoadjuvant gemcitabine/cisplatin and resection |
| 12/F/60s | PDAC | BRCA2 | PD | 2.6 | NA | 4 | NA | Neoadjuvant FOLFIRINOX, resection, and gemcitabine/nab-paclitaxel |
| 7/M/60s | PDAC | BRCA2 | PD | 2.3 | NA | 3 | NA | Resection, adjuvant gemcitabine/cisplatin, FOLFIRINOX, and olaparib |
| 9/F/50s | PDAC | RAD51D | PD | 2.0 | 0 | 6 | NA | Gemcitabine/nab-paclitaxel, FOLFIRINOX, and olaparib |
| 8/F/60s | PDAC | BRCA2 | PD | 1.8 | 0 | 3 | Monoallelic | Gemcitabine/cisplatin/nab-paclitaxel |
| 11/M/60s | PDAC | BRCA2 | PD | 1.3 | NA | 3 | Biallelic | FOLFIRINOX and olaparib |
Abbreviations: AMP, ampullary carcinoma; CCA, cholangiocarcinoma; CR, complete response; FOLFIRINOX, 5-fluorouracil, folinic acid, irinotecan, and oxaliplatin; mt, mutations; NA, not available; PD, progressive disease; PD-L1, programmed cell death 1 ligand 1; PDAC, pancreatic adenocarcinoma; PR, partial response; SD, stable disease; TMB, tumor mutation burden.
All patients had microsatellite-stable tumors.
Somatic status refers to the somatic zygosity of the gene with the pathogenic germline variant.
Ongoing response after discontinuing therapy.
Ongoing response while receiving therapy.
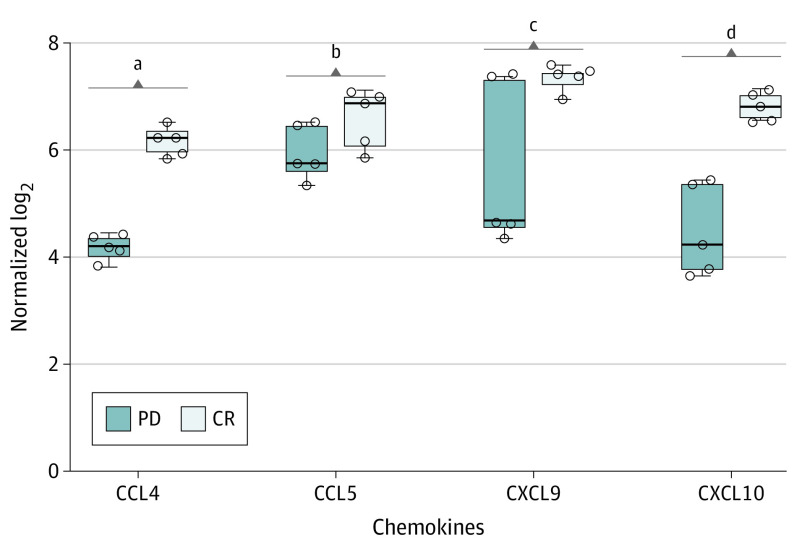
Adequate archival biopsy results were available for 4 patients (2 with CRs and 2 with progressive disease). Responders had higher density of tumor-infiltrating lymphocytes than nonresponders. Expression of chemokines known to enable trafficking of adaptive effector immune populations was significantly higher in responders than in nonresponders: CCL4 (mean [SE], 6.17 [0.12] vs 4.20 [0.11]; P < .001), CXCL9 (median, 7.44 [IQR, 7.09-7.79] vs 4.70 [IQR, 1.86-7.54]; P = .03), and CXCL10 (mean [SE], 6.81 [0.13] vs 4.51 [0.39]; P = .003) (Figure). The expression of these chemokines was previously shown to correlate with a T-cell–inflamed phenotype in PDAC.
Figure. Box Plots of 4 Chemokines of Interest in Responders vs Nonresponders.
CCL4, CXCL9, and CXCL10 are genes associated with a T-cell–inflamed phenotype in pancreatic adenocarcinoma. Patients 1 and 2 (complete response [CR]) and 8 and 9 (progressive disease [PD]) had adequate pretreatment tissue and were included in this analysis. All comparisons were done using an unpaired t test with the Welch correction except CXCL9, which was analyzed using the Mann-Whitney U test because of the distribution of the samples. Horizontal lines in the middle of the boxes indicate median values; outer horizontal lines, upper and lower bounds of the IQRs; error bars, upper and lower bounds of the range; and circles, sample replicates.
aP < .001.
bP = .10.
cP = .03.
dP = .003.
Discussion
This case series showed that ipilimumab/nivolumab therapy was associated with clinical benefit in a biomarker-selected group of patients with PDAC and PGVs in genes encoding for HRD, with pretreatment biopsy analysis supporting biological plausibility. Most patients had platinum and PARP inhibitor–refractory disease and would typically have an unfavorable prognosis but showed marked improvement with ipilimumab/nivolumab therapy. Of the 4 patients who achieved CR, 3 discontinued therapy after 2 years and all remained without evidence of disease. Limitations of the study were the small number of patients and few tumor specimens for analysis; however, differences were revealed in the pretreatment tumor characteristics between responders and nonresponders, including more tumor-infiltrating lymphocytes and a T-cell–inflamed signature on RNA expression analysis in responders.
Typically, PDAC is characterized by few or no infiltrating immune effector cells, low antigenicity, and multiple immunosuppressive factors in the tumor microenvironment. Treatment with ICIs has not shown meaningful benefit for PDAC except for microsatellite instability–high tumors. This study showed an association between germline HRD status and sensitivity to ICIs, advancing previous evidence of an association between BRCA1/2 variants in other tumors and immunotherapy response.
References
- 1.Pishvaian MJ, Bender RJ, Halverson D, et al. Molecular profiling of patients with pancreatic cancer: initial results from the Know Your Tumor initiative. Clin Cancer Res. 2018;24(20):5018-5027. doi: 10.1158/1078-0432.CCR-18-0531 [DOI] [PubMed] [Google Scholar]
- 2.Golan T, Hammel P, Reni M, et al. Maintenance olaparib for germline BRCA-mutated metastatic pancreatic cancer. N Engl J Med. 2019;381(4):317-327. doi: 10.1056/NEJMoa1903387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sokol ES, Pavlick D, Khiabanian H, et al. Pan-cancer analysis of BRCA1 and BRCA2 genomic alterations and their association with genomic instability as measured by genome-wide loss of heterozygosity. JCO Precis Oncol. 2020;4:442-465. doi: 10.1200/PO.19.00345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587-13598. doi: 10.18632/oncotarget.7277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Reilly EM, Oh DY, Dhani N, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5(10):1431-1438. doi: 10.1001/jamaoncol.2019.1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romero JM, Grünwald B, Jang GH, et al. A four-chemokine signature is associated with a T-cell–inflamed phenotype in primary and metastatic pancreatic cancer. Clin Cancer Res. 2020;26(8):1997-2010. doi: 10.1158/1078-0432.CCR-19-2803 [DOI] [PubMed] [Google Scholar]