Key Points
Question
Do physicians accurately detect treatment-associated toxic effect symptoms in patients with breast cancer?
Findings
In this cohort study of 13 725 patients with breast cancer who received treatment with radiotherapy after undergoing lumpectomy, when patient reports were compared with matched physician reports for 9868 patients, more than half of those patients experiencing substantial acute toxic effect symptoms during radiotherapy had symptoms that were underrecognized by physicians during their treatment course (2933 of 5510). Factors independently significantly associated with underrecognition were younger age, Black or Other race and ethnicity (including American Indian/Alaska Native, Arab/Middle Eastern, and Asian), conventional fractionation, male physician sex, and 2-field radiotherapy.
Meaning
The findings of this study suggest that physicians may systematically miss substantial symptoms in certain patients, particularly patients who are young or Black individuals or those of Other race and ethnicity; improving symptom detection may be a targetable mechanism to reduce disparities in experiences and outcomes.
Abstract
Importance
Understanding whether physicians accurately detect symptoms in patients with breast cancer is important because recognition of symptoms facilitates supportive care, and clinical trials often rely on physician assessments using Common Toxicity Criteria for Adverse Events (CTCAE).
Objective
To compare the patient-reported outcomes (PROs) of patients with breast cancer who received radiotherapy from January 1, 2012, to March 31, 2020, with physicians’ CTCAE assessments to assess underrecognition of symptoms.
Design, Setting, and Participants
This cohort study included a total of 29 practices enrolled in the Michigan Radiation Oncology Quality Consortium quality initiative. Of 13 725 patients with breast cancer who received treatment with radiotherapy after undergoing lumpectomy, 9941 patients (72.4%) completed at least 1 PRO questionnaire during treatment with radiotherapy and were evaluated for the study. Of these, 9868 patients (99.3%) were matched to physician CTCAE assessments that were completed within 3 days of the PRO questionnaires.
Exposures
Patient and physician ratings of 4 symptoms (pain, pruritus, edema, and fatigue) were compared.
Main Outcomes and Measures
We used multilevel multivariable logistic regression to evaluate factors associated with symptom underrecognition, hypothesizing that it would be more common in racial and ethnic minority groups.
Results
Of 9941 patients, all were female, 1655 (16.6%) were Black, 7925 (79.7%) were White, and 361 (3.6%) had Other race and ethnicity (including American Indian/Alaska Native, Arab/Middle Eastern, and Asian), either as self-reported or as indicated in the electronic medical record. A total of 1595 (16.0%) were younger than 50 years, 2874 (28.9%) were age 50 to 59 years, 3353 (33.7%) were age 60 to 69 years, and 2119 (21.3%) were 70 years or older. Underrecognition of symptoms existed in 2094 of 6781 (30.9%) observations of patient-reported moderate/severe pain, 748 of 2039 observations (36.7%) of patient-reported frequent pruritus, 2309 of 4492 observations (51.4%) of patient-reported frequent edema, and 390 of 2079 observations (18.8%) of patient-reported substantial fatigue. Underrecognition of at least 1 symptom occurred at least once for 2933 of 5510 (53.2%) of those who reported at least 1 substantial symptom. Factors independently associated with underrecognition were younger age (younger than 50 years compared with 60-69 years: odds ratio [OR], 1.35; 95% CI, 1.14-1.59; P < .001; age 50-59 years compared with 60-69 years: OR, 1.19; 95% CI, 1.03-1.37; P = .02), race (Black individuals compared with White individuals: OR, 1.56; 95% CI 1.30-1.88; P < .001; individuals with Other race or ethnicity compared with White individuals: OR, 1.52; 95% CI, 1.12-2.07; P = .01), conventional fractionation (OR, 1.26; 95% CI, 1.10-1.45; P = .002), male physician sex (OR, 1.54; 95% CI, 1.20-1.99; P = .002), and 2-field radiotherapy (without a supraclavicular field) (OR, 0.80; 95% CI, 0.67-0.97; P = .02).
Conclusions and Relevance
The results of this cohort study suggest that PRO collection may be essential for trials because relying on the CTCAE to detect adverse events may miss important symptoms. Moreover, since physicians in this study systematically missed substantial symptoms in certain patients, including younger patients and Black individuals or those of Other race and ethnicity, improving symptom detection may be a targetable mechanism to reduce disparities.
This cohort study examines patient-reported outcomes of patients with breast cancer who received radiotherapy and their physicians’ Common Toxicity Criteria for Adverse Events assessments to assess underrecognition of symptoms.
Introduction
Toxic effects of cancer therapy can impair patients’ quality of life and may sometimes limit the ability to deliver treatments optimally to ensure disease control. Understanding whether physicians are adequately detecting when patients are experiencing substantial toxic effects is important because recognition of symptoms is a necessary precondition to delivering adequate supportive care.1,2
In the context of clinical trials and data registries, recognition of symptoms is also important because it affects the broader understanding of patient experiences with various treatments. The generalizable knowledge derived from trials and registries affects the treatment decisions made by other clinicians and patients in the future, so the accuracy of information collected in those settings merits scrutiny. Several prior studies have raised questions about whether physician ratings of toxic effects accurately capture patient experiences.3,4,5,6
Recently, we reported finding differences between White individuals and individuals in racial and ethnic minority groups regarding the short-term toxic effects of treatment with radiotherapy for breast cancer, with Black patients more likely to experience pain during adjuvant therapy in a large multicenter prospective cohort in the US.7 Because this data set includes information from physician and patient reports, we more recently initiated an analysis to evaluate whether treating physicians were recognizing symptoms when patients were reporting substantial toxic effects and whether this varied by patient or clinician characteristics. We hypothesized that physicians’ underrecognition of toxic effects would be particularly pronounced among patients from racial and ethnic minority groups, and we report our findings in this article.
Methods
Patients with breast cancer who received treatment with radiotherapy after undergoing lumpectomy at 29 practices were enrolled in a quality initiative, the Michigan Radiation Oncology Quality Consortium (MROQC). The MROQC received institutional review board approval from the University of Michigan Medical School as a quality improvement initiative with an informed consent waiver because the primary purpose of data collection was for quality improvement rather than research, and its structure has been detailed elsewhere.8,9 In brief, detailed information was collected through this initiative for all patients receiving adjuvant whole breast radiotherapy for nonmetastatic unilateral breast cancer in participating practices. Weekly during treatment, physicians submitted toxic effect evaluations using standard forms that contained detailed relevant measures from the Common Toxicity Criteria for Adverse Events (CTCAE), version 4.0. Patients are also asked to participate in voluntary weekly patient-reported outcomes (PROs) assessments during treatment.
Of 13 725 female patients who completed radiotherapy between January 1, 2012, and March 31, 2020, 9941 (72.4%) completed at least 1 PRO questionnaire during radiotherapy. When physician assessments were available within 3 days of patient-reported evaluation, patient and physician ratings of 4 symptoms were compared.
Patients reported breast pain via an approved modification of the Brief Pain Inventory,10 asking for ratings in the last 24 hours of pain at its worst, least, average, and “right now.” Substantial pain was defined as moderate or severe pain reported by patients. Physicians were deemed to underrecognize pain when patients reported moderate pain (score, 4-6) but physicians graded pain as 0 (absent) on the CTCAE, or when patients reported severe pain (score, 7-10) but physicians’ CTCAE grade was 1 or less.
Patients reported bother from pruritus and edema of the breast using modified scaled measures adapted from the Skindex instrument.11 To evaluate bother from pruritus, patients were asked, “During the past week, how often have you been bothered by itching of the skin of the treated breast?” To evaluate bother from edema, patients were asked, “During the past week, how often have you been bothered by swelling of the treated breast?” Response options were never, rarely, sometimes, often, or all of the time. Physicians were deemed to underrecognize pruritus and edema if they graded these as absent (grade 0) when patients reported substantial symptoms (defined as bother often or all of the time from itching or swelling, respectively).
Finally, patients reported fatigue as described previously,9 using a single item asked on the end-of-treatment questionnaire: “How often did you feel significant fatigue?” Response options were always, most of the time, sometimes, rarely, or never during the past 4 weeks. Physicians were deemed to underrecognize fatigue if they graded fatigue as absent (grade 0) when patients reported having substantial symptoms (defined as significant fatigue most of the time or always).
In our analyses, after describing the association between physician and patient pain scores and calculating the Spearman rank correlation, we reported the patterns of underrecognition for each substantial symptom. We then described the proportion of patients who experienced substantial symptoms for whom underrecognition of at least 1 of these 4 symptoms occurred at least once during the treatment course. We used multilevel multivariable logistic regression modeling to evaluate factors that were associated with a patient experiencing this underrecognition and test the hypothesis that it would be a more common experience for racial and ethnic minority groups.
We include as fixed effects in the model the following covariates: age group (<50, 50-59, 60-69, and ≥70 years), body mass index (calculated as weight in kilograms divided by height in meters squared) category (underweight [<18.5], normal [18.5-<25], overweight [25 to <30], obesity I [30 to <35], obesity II [35 to <40], and obesity III [≥40]), racial and ethnic group (Black, White, or Other [(including American Indian/Alaska Native, Arab/Middle Eastern, and Asian]), use of a supraclavicular nodal field (yes/no), radiation dose fractionation (conventional [1.8-2 Gy]/hypofractionation [>2-2.8 Gy]), use of a lumpectomy bed boost (yes/no), whether the treating site was an academic facility (teaching resident/fellows: yes/no), and the sex of the treating radiation oncologist (female/male). As random effects, patients were clustered by treating radiation oncologist, and patients and radiation oncologists were clustered by treating facility. Race and ethnicity were based on self-report for the 5006 of 5510 (91% of the modeled population) who provided this information directly via surveys, and the remainder were based on information in the electronic medical record. Black race included patients who indicated Black or African American, and Other included all other racial identities (within which the largest subcategory was Asian, followed by Arab/Middle Eastern, followed by American Indian/Alaska Native; these groups were combined as each did not, individually, contain enough patients to permit meaningful or generalizable results).
We used a 3-level multilevel model for describing the association of these variables, with underrecognition of short-term toxic effects by the physician when substantial symptoms were reported by the patient, at the bivariate level (single fixed-effects explanatory variable plus the clustering effects) and for the multivariable model (including all fixed-effects explanatory variables plus clustering effects simultaneously). All statistical analyses were conducted using SAS, version 9.4(SAS Institute). P values at or less than .05 were considered significant except in the presence of multiple testing, when significant P values were compared with .05/the number of tests (Bonferroni correction).
Results
eTable 1 in Supplement 1 provides the characteristics of the 9941 patients who completed at least 1 PRO questionnaire; it provides a comparison of those who experienced at least 1 form of substantial toxic effects with those who did not, similar to a previously reported analysis.7 Of the patients, 1655 (16.6%) were Black, 7925 (79.7%) were White, and 361 (3.6%) had Other race. Nearly half (4469 [45%]) were younger than 60 years. In this sample, 3434 of 9940 patients (34.5%) who had at least 1 patient-reported pain assessment reported substantial breast pain. Of 9923 who received at least 1 patient-reported assessment of bother from pruritus, 3039 (30.6%) reported frequent bother from pruritus. Of 9906 who had at least 1 patient-reported assessment of bother from edema, 2363 (23.9%) reported frequent bother from edema. Finally, of 8860 who responded regarding fatigue, 2209 (24.9%) reported substantial fatigue.
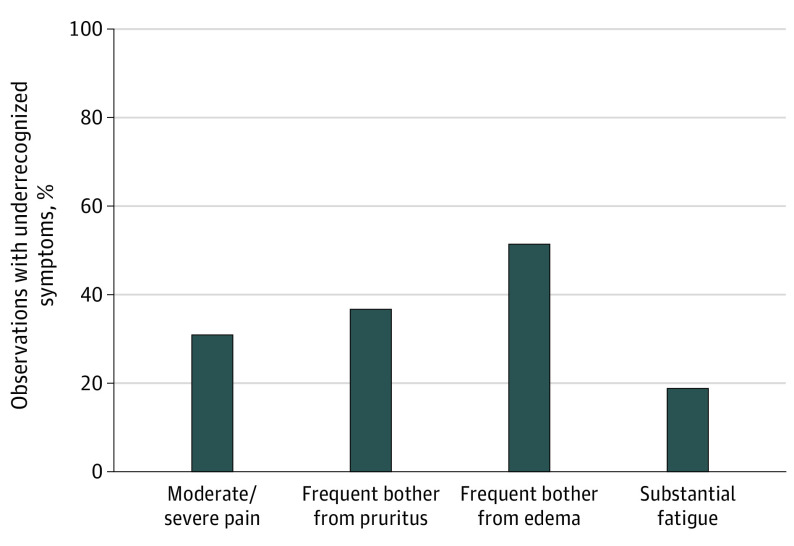
We could evaluate underrecognition in 9868 patients, with 37 593 independent paired observations of patient and physician reports. Of these, 35 797 observations (95.2%) occurred on the exact same date, and 1796 (4.8%) occurred within 3 days. Detailed findings from an analysis of concordance between physician and patient reports of pain is provided in eTable 2 in Supplement 1. This demonstrated a strong association between patient and physician ratings, with a Spearman rank correlation between physician CTCAE and patient score groups of 0.58 (P < .001). Nevertheless, as shown in Figure 1, underrecognition existed in 2094 of 6781 observations (30.9%) of patient-reported moderate or severe pain, 748 of 2039 (36.7%) of patient-reported frequent bother from pruritus, 2309 of 4492 (51.4%) of patient-reported frequent bother from edema, and 390 of 2079 (18.8%) of patient-reported substantial fatigue.
Figure 1. Percentage of Observations With Underrecognized Symptoms.
The Table presents the characteristics of the 5510 unique patients who reported experiencing at least 1 substantial symptom during treatment with radiotherapy who constitute the sample in which evaluation of underrecognition of substantial symptoms is possible. Underrecognition of at least 1 of the 4 symptoms occurred at least once during the patient’s treatment course for 2933 of 5510 patients (53.2%) who reported at least 1 substantial symptom during treatment with radiotherapy.
Table. Characteristics of the 5510 Patients Who Experienced at Least 1 Substantial Symptom During Treatment With Breast Radiotherapy.
| Variable/level | All (n = 5510), No. (column %) | No. (column %) [row %] | |
|---|---|---|---|
| Underrecognition occurred at least once when substantial symptom reported (n = 2951) | No underrecognition (n = 2559) | ||
| Age group, y | |||
| <50 | 1115 (20.2) | 656 (22.2) [58.8] | 459 (17.9) [41.2] |
| 50-59 | 1799 (32.7) | 988 (33.5) [54.9] | 811 (31.7) [45.1] |
| 60-69 | 1704 (30.9) | 854 (28.9) [50.1] | 850 (33.2) [49.9] |
| ≥70 | 892 (16.2) | 453 (15.4) [50.8] | 439 (17.2) [49.2] |
| BMI category | |||
| Underweight (<18.5) | 95 (1.7) | 44 (1.5) [46.3] | 51 (2.0) [53.7] |
| Normal (18.5 to <25) | 1144 (20.8) | 592 (20.1) [51.7] | 552 (21.6) [48.3] |
| Overweight (25 to <30) | 1574 (28.6) | 829 (28.1) [52.7] | 745 (29.1) [47.3] |
| Obesity | |||
| I (30 to <35) | 1276 (23.2) | 691 (23.4) [54.2] | 585 (22.9) [45.8] |
| II (35 to <40) | 749 (13.6) | 414 (14.0) [55.3] | 335 (13.1) [44.7] |
| III (≥40) | 672 (12.2) | 381 (12.9) [56.7] | 291 (11.4) [43.3] |
| Racial and ethnic groupa | |||
| Black | 1066 (19.3) | 715 (24.2) [67.1] | 351 (13.7) [32.9] |
| White | 4240 (77.0) | 2108 (71.4) [49.7] | 2132 (83.3) [50.3] |
| Other | 204 (3.7) | 128 (4.3) [62.7] | 76 (3.0) [37.3] |
| Supraclavicular field used | |||
| No | 4774 (86.6) | 2570 (87.1) [53.8] | 2204 (86.1) [46.2] |
| Yes | 736 (13.4) | 381 (12.9) [51.8] | 355 (13.9) [48.2] |
| Fractionation | |||
| Conventional | 2975 (54.0) | 1643 (55.7) [55.2] | 1332 (52.1) [44.8] |
| Hypofractionated | 2535 (46.0) | 1308 (44.3) [51.6] | 1227 (48.0) [48.4] |
| Boost used | |||
| No | 680 (12.3) | 349 (11.8) [51.3] | 331 (12.9) [48.7] |
| Yes | 4830 (87.7) | 2602 (88.2) [53.9] | 2228 (87.1) [46.1] |
| Academic treatment facility | |||
| No | 3486 (63.3) | 1782 (60.4) [51.1] | 1704 (66.6) [48.9] |
| Yes | 2024 (36.7) | 1169 (39.6) [57.8] | 855 (33.4) [42.2] |
| Sex of treating clinician | |||
| Female | 1939 (35.2) | 1001 (33.9) [51.6] | 938 (36.7) [48.4] |
| Male | 3571 (64.8) | 1950 (66.1) [54.6] | 1621 (63.3) [45.4] |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Racial and ethnic identities are based on self-report for 91% who completed surveys asking their race and ethnicity and from electronic medical records for the remainder. Black includes patients who indicated “Black” or “African American”; Other includes all other racial and ethnic identities (in which the largest subgroup was Asian, followed by Arab/Middle Eastern, followed by American Indian/Alaska Native).
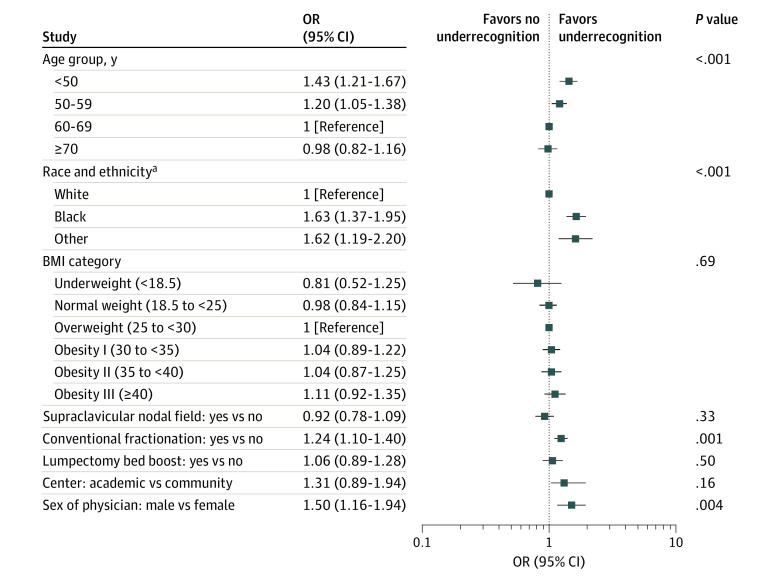
Results of the bivariate analyses are presented in Figure 2. Factors significantly associated with underrecognition, comparing P values with a multiple testing corrected significance value of .00625, were patient age (Age <50 years vs 60-69: odds ratio [OR], 1.43; 95% CI, 1.21-1.67; P < .001), race and ethnicity (Black vs White individuals: OR, 1.63; 95% CI, 1.37-1.95; P < .001; individuals of Other race and ethnicity vs White individuals: OR, 1.62; 95% CI, 1.19-2.20; P < .001), dose-fractionation (conventional fractionation vs hypofractionation: OR, 1.24; 95% CI, 1.10-1.40; P = .001), and attending physician sex (male vs female physicians: OR, 1.50; 95% CI, 1.16-1.94; P = .004).
Figure 2. Bivariate Associations for the Fixed-Effects Explanatory Variables.
P = .00625 considered significant because of multiple testing. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared).
aRacial and ethnic identities are based on self-report for 91% who completed surveys asking their race and ethnicity and from electronic medical records for the remainder. Black includes patients who indicated “Black” or “African American”; Other includes all other racial and ethnic identities (in which the largest subgroup was Asian, followed by Arab/Middle Eastern, followed by American Indian/Alaska Native).
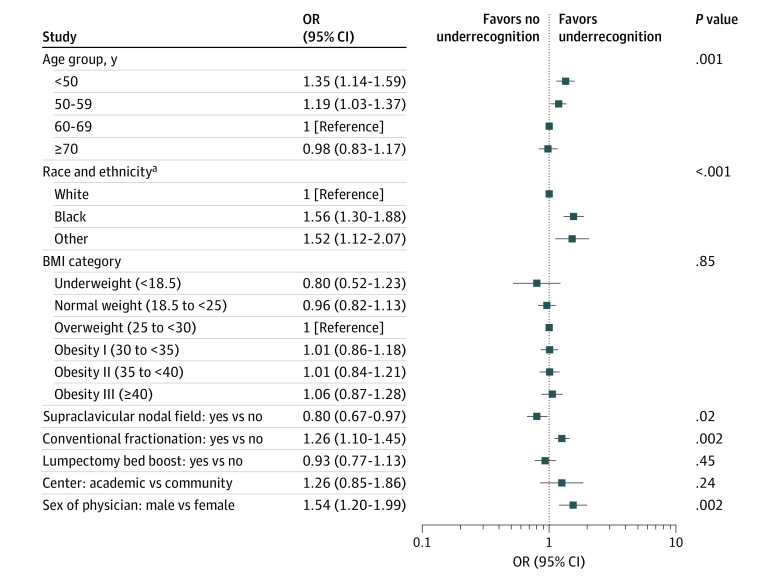
On multivariable modeling, as shown in Figure 3, the factors independently associated with underrecognition were younger age (younger than 50 years compared with 60-69 years: odds ratio [OR], 1.35; 95% CI, 1.14-1.59; P < .001; age 50-59 years compared with 60-69 years: OR, 1.19; 95% CI, 1.03-1.37; P = .02), race (Black individuals compared with White individuals: OR, 1.56; 95% CI 1.30-1.88; P < .001; individuals with Other race compared with White individuals: OR, 1.52; 95% CI, 1.12-2.07; P = .01), conventional fractionation OR, 1.26; 95% CI, 1.10-1.45; P = .002), and male physician sex (OR, 1.54; 95% CI, 1.20-1.99; P = .002). In addition, on multivariable analysis, receiving a supraclavicular field was independently associated with lower odds of underrecognition (OR, 0.80; 95% CI, 0.67-0.97; P = .02). Calculation of the intraclass correlation coefficients demonstrated that the clustering of patients within physicians and facilities explained only 6.84% of the overall variance in underrecognition, of which 4.33% was because of physician-level clustering and 2.51% because of facility-level clustering.
Figure 3. Multiple Variable Associations for the Fixed-Effects Explanatory Variables.
P < .05 considered significant. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared).
aRacial and ethnic identities are based on self-report for 91% who completed surveys asking their race and ethnicity and from electronic medical records for the remainder. Black includes patients who indicated “Black” or “African American”; Other includes all other racial and ethnic identities (in which the largest subgroup was Asian, followed by Arab/Middle Eastern, followed by American Indian/Alaska Native).
Discussion
In this cohort study using a large prospective observational data set, meaningful differences in patient and physician reports of short-term toxic effects existed. More than half of all patients who experienced a substantial short-term toxic effect symptom based on their own report did not have timely recognition of that symptom by their physician. Particularly worrisome is the finding that underrecognition of substantial symptoms was most common in patients of racial and ethnic minority groups, even after controlling for other differences in patient and treatment characteristics and the clustering of patients within treating physicians and facilities.
Several other studies have suggested that physicians may underrecognize symptoms that trouble their patients and that patient-reported outcomes measures are more likely to reveal serious toxic effects than clinician reports.4,5,6 In the trial NRG 1203,3 clinicians were found to have substantially underreported symptomatic gastrointestinal adverse events compared with patients themselves, with important implications for the primary outcome of the research. However, others have shown higher levels of agreement between physician-reported and patient-reported outcomes.12 The current study focused on the clinically meaningful question of whether there was timely recognition of substantial symptoms, with disappointing findings.
Prior studies have documented that patients of racial and ethnic minority groups experience higher rates of short-term toxic effects.7 Although some of this difference might be associated with biological differences in genes associated with inflammation, wound repair, and fibrotic response or other effects of radiation,13,14 racial and ethnic disparities in experiences likely also reflect differences in social factors, including resources and behaviors.15 Black patients are less likely to receive hypofractionated radiation therapy,16 an approach less likely to be followed by short-term toxic effects,9 but the association between race and ethnicity and toxic effects in the current study existed even after controlling for differences in fractionation. Given evidence that clinicians may be less sensitive to pain experienced by Black patients and less likely to prescribe them pain medication,17,18,19,20,21,22,23,24,25,26 and also given that skin reactions to radiation may be less easily observed in patients with darker skin pigmentation and physicians may be less familiar with the ways radiation dermatitis manifests in patients with darker skin tones,27 we hypothesized that underrecognition of symptoms would be more likely for individuals of racial and ethnic minority groups. This study’s findings confirmed that hypothesis.
Higher underrecognition of short-term toxic effects among individuals of racial and ethnic minority groups may reflect differences in the resources available to clinicians who serve primarily racial and ethnic minority groups. However, these findings persisted even after a multivariable analysis that adjusted for clustering of patients within clinicians and facilities. Moreover, the intraclass correlation coefficients suggested that most of the variance in underrecognition was associated with individual patient-level factors, including patient race and ethnicity, rather than differences at the level of the clinicians or facilities where they receive care.
Differences in clinician recognition of symptoms by patient race and ethnicity are not necessarily the result of differences in the skills or behavior of these physicians. Racism has understandably led to distrust of the health care system by many in racial and ethnic minority groups.28,29,30,31,32,33,34,35,36 Those who have repeatedly faced undertreatment of pain25,37,38,39,40,41 might reasonably assume that health care clinicians are unlikely to help and be less likely to mention symptoms in a different context. Those who are socioeconomically underserved may be less likely to have a family member who can take time off to accompany them on their visits, potentially changing the likelihood that concerns are brought to the physician’s attention. Thus, even if all of the physicians in this study treated patients they encountered in an unbiased way, differences in recognition of symptoms could nevertheless emerge.
That said, changes in clinician behaviors may still be essential to increase the trustworthiness of the health care system. The current study identifies a remediable deficit: a gap in communication about symptoms. Because this deficit is more pronounced in patients of racial and ethnic minority groups, this is not only a target for general quality improvement but also mitigation of disparities in oncology care. Promoting the engagement of nursing and other support staff and optimizing communication within interdisciplinary teams regarding symptom identification and supportive care delivery is a promising way forward.42
In addition to underrecognition being more common for patients of racial and ethnic minority groups, underrecognition was more common in younger patients, those who did not receive a supraclavicular field, and those who received conventional fractionation. Perhaps physicians may be most vigilant in asking about and recognizing short-term toxic effects when encountering patients they perceive to be at highest risk for toxic effects. Underrecognition was also more common in those seen by male physicians. Prior research has shown that female physicians may have different communication styles and spend more time in direct patient care per visit.43 Further research is necessary to identify what physician behaviors optimize the accurate communication of information about the toxic effects of patients.
Limitations
The limitations of this study include its situation within a single state within the US; given the social construction of race and ethnicity, differences may vary in other parts of the US and the world. Other limitations include the difficulty of using observational data of this sort to understand root causes of observed differences. We lacked sufficient data on factors like socioeconomic status that might explain some of the associations observed. Finally, there are many ways that agreement between patient and physician reports can be assessed. We attempted to focus on a meaningful form of underrecognition for clinical care delivery: timely recognition of severe symptoms at least once during the patient’s treatment course. We deliberately limited our analyses to this prespecified measure to minimize erroneous inferences associated with multiplicity of testing. Additionally, the analyses of clinician and facility characteristics was limited to sex and teaching institution status, and factors were not able to be assessed for the subgroups included in Other race and ethnicity. Additional research to collect other clinician characteristics, such as clinician race and ethnicity, and to investigate any association of patient-clinician racial and ethnic concordance, might also be a valuable endeavor in the future.
Conclusions
In this cohort study, PRO collection appeared essential for trials because relying on the CTCAE to detect adverse events may miss important symptoms. Moreover, since in this study physicians seemed to systematically miss substantial symptoms in certain patients, including those who were younger or of racial and ethnic minority groups, improving symptom detection may be a targetable mechanism to reduce disparities in radiotherapy experiences and outcomes. Promising efforts to facilitate routine PRO collection during and after treatment via electronic platforms are already underway.44 For now, all physicians should inquire directly about potential toxic effects in detail with all patients to avoid missing symptoms they might help to mitigate.
eTable 1. Characteristics of the 9,941 Patients Who Report At Least One Patient-Reported Outcomes Survey during the period on treatment to end of treatment assessment
eTable 2. Detailed Evaluation of Concordance Between Physician and Patient Ratings of Pain for 37,248 Paired Observations of Pain
Nonauthor collaborators
References
- 1.Henson LA, Maddocks M, Evans C, Davidson M, Hicks S, Higginson IJ. Palliative care and the management of common distressing symptoms in advanced cancer: pain, breathlessness, nausea and vomiting, and fatigue. J Clin Oncol. 2020;38(9):905-914. doi: 10.1200/JCO.19.00470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hui D, Hoge G, Bruera E. Models of supportive care in oncology. Curr Opin Oncol. 2021;33(4):259-266. doi: 10.1097/CCO.0000000000000733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yeung AR, Pugh SL, Klopp AH, et al. Improvement in patient-reported outcomes with intensity-modulated radiotherapy (RT) compared with standard RT: a report from the NRG Oncology RTOG 1203 study. J Clin Oncol. 2020;38(15):1685-1692. doi: 10.1200/JCO.19.02381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xiao C, Polomano R, Bruner DW. Comparison between patient-reported and clinician-observed symptoms in oncology. Cancer Nurs. 2013;36(6):E1-E16. doi: 10.1097/NCC.0b013e318269040f [DOI] [PubMed] [Google Scholar]
- 5.Gilbert A, Ziegler L, Martland M, et al. Systematic review of radiation therapy toxicity reporting in randomized controlled trials of rectal cancer: a comparison of patient-reported outcomes and clinician toxicity reporting. Int J Radiat Oncol Biol Phys. 2015;92(3):555-567. doi: 10.1016/j.ijrobp.2015.02.021 [DOI] [PubMed] [Google Scholar]
- 6.Basch E, Dueck AC, Rogak LJ, et al. Feasibility assessment of patient reporting of symptomatic adverse events in multicenter cancer clinical trials. JAMA Oncol. 2017;3(8):1043-1050. doi: 10.1001/jamaoncol.2016.6749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jagsi R, Griffith KA, Vicini F, et al. ; Michigan Radiation Oncology Quality Consortium . Toward improving patients’ experiences of acute toxicity from breast radiotherapy: insights from the analysis of patient-reported outcomes in a large multicenter cohort. J Clin Oncol. 2020;38(34):4019-4029. doi: 10.1200/JCO.20.01703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran JM, Feng M, Benedetti LA, et al. ; Michigan Radiation Oncology Quality Consortium . Development of a model web-based system to support a statewide quality consortium in radiation oncology. Pract Radiat Oncol. 2017;7(3):e205-e213. doi: 10.1016/j.prro.2016.10.002 [DOI] [PubMed] [Google Scholar]
- 9.Jagsi R, Griffith KA, Boike TP, et al. Differences in the acute toxic effects of breast radiotherapy by fractionation schedule: comparative analysis of physician-assessed and patient-reported outcomes in a large multicenter cohort. JAMA Oncol. 2015;1(7):918-930. doi: 10.1001/jamaoncol.2015.2590 [DOI] [PubMed] [Google Scholar]
- 10.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23(2):129-138. [PubMed] [Google Scholar]
- 11.Chren M-M, Lasek RJ, Sahay AP, Sands LP. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5(2):105-110. doi: 10.1177/120347540100500202 [DOI] [PubMed] [Google Scholar]
- 12.Lam E, Yee C, Wong G, et al. A systematic review and meta-analysis of clinician-reported versus patient-reported outcomes of radiation dermatitis. Breast. 2020;50:125-134. doi: 10.1016/j.breast.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alam A, Mukhopadhyay ND, Ning Y, et al. A preliminary study on racial differences in HMOX1, NFE2L2, and TGFβ1 gene polymorphisms and radiation-induced late normal tissue toxicity. Int J Radiat Oncol Bio Physics. 2015;93(2):436-443. doi: 10.1016/j.ijrobp.2015.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grossberg AJ, Lei X, Xu T, et al. Association of transforming growth factor β polymorphism C-509T with radiation-induced fibrosis among patients with early-stage breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(12):1751-1757. doi: 10.1001/jamaoncol.2018.2583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd R, Lindo E, Weeks L, McLemore M. On racism: a new standard for publishing on racial health inequities. Accessed July 30, 2021. https://www.healthaffairs.org/do/10.1377/hblog20200630.939347/full/
- 16.Laucis AM, Jagsi R, Griffith KA, et al. The role of facility variation on racial disparities in use of hypofractionated whole breast radiation therapy. Int J Radiat Oncol Bio Physics. 2020;107(5):949-958. doi: 10.1016/j.ijrobp.2020.04.035 [DOI] [PubMed] [Google Scholar]
- 17.Anderson KO, Green CR, Payne R. Racial and ethnic disparities in pain: causes and consequences of unequal care. J Pain. 2009;10(12):1187-1204. doi: 10.1016/j.jpain.2009.10.002 [DOI] [PubMed] [Google Scholar]
- 18.Bonham VL. Race, ethnicity, and pain treatment: striving to understand the causes and solutions to the disparities in pain treatment. J Law Med Ethics. 2001;29(1):52-68. doi: 10.1111/j.1748-720X.2001.tb00039.x [DOI] [PubMed] [Google Scholar]
- 19.Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med. 2006;9(6):1454-1473. doi: 10.1089/jpm.2006.9.1454 [DOI] [PubMed] [Google Scholar]
- 20.Cleeland CS, Gonin R, Baez L, Loehrer P, Pandya KJ. Pain and treatment of pain in minority patients with cancer: the Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med. 1997;127(9):813-816. doi: 10.7326/0003-4819-127-9-199711010-00006 [DOI] [PubMed] [Google Scholar]
- 21.Freeman HP, Payne R. Racial injustice in health care. N Engl J Med. 2000;342(14):1045-1047. doi: 10.1056/NEJM200004063421411 [DOI] [PubMed] [Google Scholar]
- 22.Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4(3):277-294. doi: 10.1046/j.1526-4637.2003.03034.x [DOI] [PubMed] [Google Scholar]
- 23.Shavers VL, Bakos A, Sheppard VB. Race, ethnicity, and pain among the U.S. adult population. J Health Care Poor Underserved. 2010;21(1):177-220. doi: 10.1353/hpu.0.0255 [DOI] [PubMed] [Google Scholar]
- 24.Smedley BD, Stith AY. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. National Academies Press; 2003. [PubMed] [Google Scholar]
- 25.Todd KH, Deaton C, D’Adamo AP, Goe L. Ethnicity and analgesic practice. Ann Emerg Med. 2000;35(1):11-16. doi: 10.1016/S0196-0644(00)70099-0 [DOI] [PubMed] [Google Scholar]
- 26.Hoffman KM, Trawalter S, Axt JR, Oliver MN. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113(16):4296-4301. doi: 10.1073/pnas.1516047113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shumway DA, Kapadia N, Walker EM, et al. Development of an illustrated scale for acute radiation dermatitis in breast cancer patients. Pract Radiat Oncol. 2021;11(3):168-176. doi: 10.1016/j.prro.2020.09.002 [DOI] [PubMed] [Google Scholar]
- 28.Kaiser K, Rauscher GH, Jacobs EA, Strenski TA, Ferrans CE, Warnecke RB. The import of trust in regular providers to trust in cancer physicians among white, African American, and Hispanic breast cancer patients. J Gen Intern Med. 2011;26(1):51-57. doi: 10.1007/s11606-010-1489-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doescher MP, Saver BG, Franks P, Fiscella K. Racial and ethnic disparities in perceptions of physician style and trust. Arch Fam Med. 2000;9(10):1156-1163. doi: 10.1001/archfami.9.10.1156 [DOI] [PubMed] [Google Scholar]
- 30.Halbert CH, Armstrong K, Gandy OH Jr, Shaker L. Racial differences in trust in health care providers. Arch Intern Med. 2006;166(8):896-901. doi: 10.1001/archinte.166.8.896 [DOI] [PubMed] [Google Scholar]
- 31.Dawson MA, Giger JN, Powell-Young Y, Brannon CB. Why African-Americans are hesitant to take the newly proposed COVID-19 vaccines: Tuskegee revisited. J Natl Black Nurses Assoc. 2020;31(2):vi-viii. [PubMed] [Google Scholar]
- 32.Wolinetz CD, Collins FS. Recognition of research participants’ need for autonomy: remembering the legacy of Henrietta Lacks. JAMA. 2020;324(11):1027-1028. doi: 10.1001/jama.2020.15936 [DOI] [PubMed] [Google Scholar]
- 33.Khabele D, Holcomb K, Connors NK, Bradley L. A perspective on James Marion Sims, MD, and antiblack racism in obstetrics and gynecology. J Minim Invasive Gynecol. 2021;28(2):153-155. doi: 10.1016/j.jmig.2020.10.027 [DOI] [PubMed] [Google Scholar]
- 34.Bajaj SS, Stanford FC. Beyond Tuskegee—vaccine distrust and everyday racism. N Engl J Med. 2021;384(5):e12. doi: 10.1056/NEJMpv2035827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devakumar D, Shannon G, Bhopal SS, Abubakar I. Racism and discrimination in COVID-19 responses. Lancet. 2020;395(10231):1194. doi: 10.1016/S0140-6736(20)30792-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nong P, Raj M, Creary M, Kardia SLR, Platt JE. Patient-reported experiences of discrimination in the US health care system. JAMA Netw Open. 2020;3(12):e2029650. doi: 10.1001/jamanetworkopen.2020.29650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pletcher MJ, Kertesz SG, Kohn MA, Gonzales R. Trends in opioid prescribing by race/ethnicity for patients seeking care in US emergency departments. JAMA. 2008;299(1):70-78. doi: 10.1001/jama.2007.64 [DOI] [PubMed] [Google Scholar]
- 38.Shah AA, Zogg CK, Zafar SN, et al. Analgesic access for acute abdominal pain in the emergency department among racial/ethnic minority patients: a nationwide examination. Med Care. 2015;53(12):1000-1009. doi: 10.1097/MLR.0000000000000444 [DOI] [PubMed] [Google Scholar]
- 39.Lee HH, Lewis CW, McKinney CM. Disparities in emergency department pain treatment for toothache. JDR Clin Trans Res. 2016;1(3):226-233. doi: 10.1177/2380084416655745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goyal MK, Kuppermann N, Cleary SD, Teach SJ, Chamberlain JM. Racial disparities in pain management of children with appendicitis in emergency departments. JAMA Pediatr. 2015;169(11):996-1002. doi: 10.1001/jamapediatrics.2015.1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goyal MK, Johnson TJ, Chamberlain JM, et al. ; Pediatric Emergency Care Applied Research Network . Racial and ethnic differences in emergency department pain management of children with fractures. Pediatrics. 2020;145(5):e20193370. doi: 10.1542/peds.2019-3370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kozul C, Stafford L, Little R, et al. Breast cancer survivor symptoms: a comparison of physicians’ consultation records and nurse-led survivorship care plans. Clin J Oncol Nurs. 2020;24(3):E34-E42. doi: 10.1188/20.CJON.E34-E42 [DOI] [PubMed] [Google Scholar]
- 43.Ganguli I, Sheridan B, Gray J, Chernew M, Rosenthal MB, Neprash H. Physician work hours and the gender pay gap—evidence from primary care. N Engl J Med. 2020;383(14):1349-1357. doi: 10.1056/NEJMsa2013804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lapen K, Sabol C, Tin AL, et al. Development and pilot implementation of a remote monitoring system for acute toxicity using electronic patient-reported outcomes for patients undergoing radiation therapy for breast cancer. Int J Radiat Oncol Biol Phys. 2021;111(4):979-991. doi: 10.1016/j.ijrobp.2021.07.1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Characteristics of the 9,941 Patients Who Report At Least One Patient-Reported Outcomes Survey during the period on treatment to end of treatment assessment
eTable 2. Detailed Evaluation of Concordance Between Physician and Patient Ratings of Pain for 37,248 Paired Observations of Pain
Nonauthor collaborators