Abstract
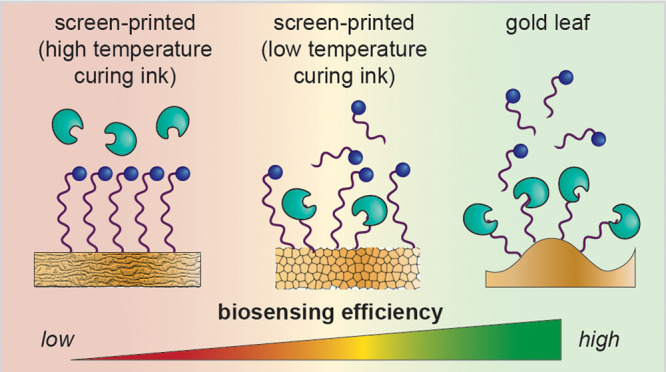
Electrochemical biosensors are promising technologies for detection and monitoring in low-resource settings due to their potential for easy use and low-cost instrumentation. Disposable gold screen-printed electrodes (SPEs) are popular substrates for these biosensors, but necessary dopants in the ink used for their production can interfere with biosensor function and contribute to the heterogeneity of these electrodes. We recently reported an alternative disposable gold electrode made from gold leaf generated using low-cost, equipment-free fabrication. We have directly compared the surface topology, biorecognition element deposition, and functional performance of three disposable gold electrodes: our gold leaf electrodes and two commercial SPEs. Our leaf electrodes significantly outperformed the SPEs for reproducible and effective biosensing in a DNase I assay and are nearly an order of magnitude less expensive than the SPEs. Therefore, these electrodes are promising for further development as point-of-care diagnostics, especially in low-resource settings.
Keywords: gold leaf, biosensor, diagnostic, electrochemistry, affordable
Electrochemical biosensors are promising technologies for detection and monitoring in low-resource settings (LRS) due to their ease of use and low-cost instrumentation.1,2 However, these devices have had limited commercial applications beyond the glucometer in large part because of challenges with electrode fabrication and modification.3 Such sensors have been developed to detect biomolecules ranging from DNA to antibodies.4 For effective detection with these systems, biorecognition elements must be immobilized on the electrode surface. Thus, gold electrodes are often used due to the ease of formation and strength of gold–thiol bonds.5−7 Traditionally, gold electrodes are fabricated in cleanrooms using methods such as photolithography or shadow mask lithography, which generate high-quality crystalline surfaces, allowing for stable alkanethiol monolayer formation.8 However, this type of fabrication is costly, inefficient, and laborious, limiting its use to well-funded, specialized laboratories and making it inaccessible to many.9
Recently, disposable gold screen-printed electrodes (SPEs)10 have risen in prominence as affordable alternatives to traditionally fabricated electrodes. SPEs are generated by applying a gold ink to a substrate through a patterned mask. To effectively print with this method, gold inks are doped with other materials,11,12 but unfortunately, these can interfere with biosensor function and contribute to the heterogeneity and irreproducibility found with these electrodes. To circumvent these issues and eliminate the need for dopants, we recently reported a protocol to fabricate disposable gold electrodes using 24 karat gold leaf.13 These electrodes were partly inspired by a preceding study that similarly applied gold leaf to generate working electrodes without the need for cleanroom fabrication.14 In our system, the gold leaf is applied directly to an adhesive, and the resulting electrode is a pure gold surface devoid of contaminants. These electrodes are made using low-cost, equipment-free fabrication and maintain attributes of high-quality crystalline surfaces, making them favorable for applications that necessitate detection in LRS.
Despite the promise of gold leaf electrodes, their efficacy for biosensing as compared to commercial SPEs has never been directly evaluated. Here, we provide the first study that directly compares key parameters, including the gold surface topology, biorecognition element deposition, and functional performance, of three disposable gold electrodes: our in-house fabricated leaf electrodes as well as two commercial SPEs. To determine the impact of surface quality and topology on biosensor performance, redox-labeled DNA was immobilized on electrodes as reporters to detect DNase I endonuclease activity; our findings indicate that electrode morphology and functionalization are essential for the detection of biological activity. Further, we found that the gold leaf electrodes outperform both types of the screen-printed gold electrodes evaluated, demonstrating higher sensitivity and stability. This work sheds light on the key factors that must be considered for effective electrode development for biosensing, including electroactive surface area and the local domain size. Additionally, the potential of gold leaf electrodes is evident from this work, and we anticipate this platform to be widely implementable for diagnostics in LRS.
Results
Electrode Surface Characterization
We assessed the quality of electrode surfaces based on their morphology and attributes, where high-quality surfaces exhibit characteristics of crystalline gold and contain smooth regions that allow for the formation of stable, homogeneous self-assembled monolayers. Three types of electrode surfaces were assessed in these studies: the leaf electrodes fabricated in-house and two commonly used gold SPEs that differ in their ink compositions. The high-temperature curing ink electrodes will be referred to as “AT” electrodes, as defined by their manufacturer, and the low-temperature ones will be referred to as “BT” electrodes.15 The three types of electrodes have vastly different surface morphologies, as indicated by atomic force microscopy and scanning electron microscopy (Figure 1). The leaf electrodes contain undulating features. They appear smooth on the nanometer scale but contain micrometer-scale hills and valleys. The AT and BT electrodes are rough and heterogeneous on both the nanometer and micrometer scales.
Figure 1.
Microscopy-based electrode characterization. (a) Dropsens 220AT electrodes. (b) Dropsens 220BT electrodes. (c) Leaf electrodes. Atomic force microscopy images of the electrodes (ii). Scanning Electron Microscopy images of the electrodes with varying magnifications (iii,iv). The leaf electrodes exhibit nanoscale smoothness and micron-scale undulating features.
To assess the crystallinity of the electrodes, cyclic voltammetry in sulfuric acid was performed. The potential window for each type of electrode was determined to prevent oxygen evolution at high potentials. Because the three types of electrodes contain different pseudoreference and working electrode materials, this evolution occurs at slightly different measured potentials, and the surfaces remain stable across different potential windows.16 Though the potential windows used differ slightly, they all enabled evaluation of the gold oxidation peak characteristics.17,18 The occurrence of three oxidative peaks is characteristic of crystalline gold19 (Figure 2a). Whereas the BT and leaf electrodes show characteristics of crystalline gold, the AT electrodes do not. Based on these results, the leaf electrodes exhibit characteristics of crystalline gold with homogeneous, smooth features, whereas the AT ones do not exhibit characteristics of crystalline gold and have heterogeneous, rough features.
Figure 2.
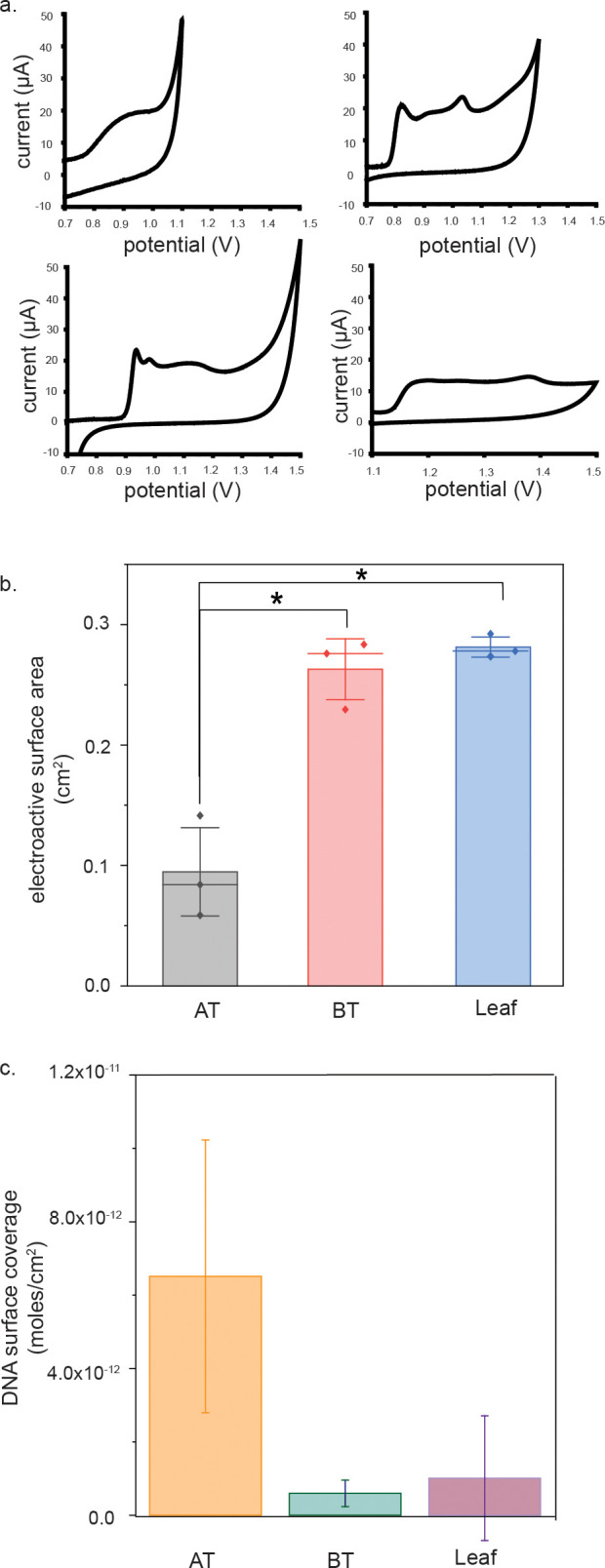
Electroactive surface area determination. (a) Oxidative peaks of cyclic voltammograms of bare electrodes in 0.5 M H2SO4 for AT (top left), BT (top right), leaf (bottom left), and rod electrodes (bottom right). BT, leaf, and rod electrodes show characteristics of crystalline gold. (b) AT electrodes have the smallest electroactive surface area. (c) Oligonucleotides are most densely packed on the AT electrodes.
Self-Assembled Monolayer Formation
Electrodes were functionalized with methylene blue (MB)-labeled DNA and assessed for surface density on the different electrodes.20 The average DNA surface coverage on the AT electrodes (6.51 × 10–12 mol/cm2) is greater than that on the BT (5.96 × 10–13 mol/cm2) or leaf electrodes (1.01 × 10–12 mol/cm2) (Figure 2c). We attribute the differences in density to differences in the amount of gold available for binding for the same concentration of thiolated DNA incubated with surfaces. From our calculations of the electroactive surface area of the three types of electrodes, AT electrodes have the smallest electroactive surface area, whereas the BT and leaf electrodes have the largest electroactive surface area (Figure 2b), as is indicated by differences in the area under the reductive peaks in cyclic voltammograms of 0.5 M H2SO4, which is proportional to the electroactive surface area of gold on the electrode surface.21 The difference in electroactive area between the AT and other electrodes was statistically significant (p < 0.05, according to a t-test). Surface coverages for self-assembled DNA monolayers on gold vary significantly based on the assembly conditions and initial concentration of thiolated DNA used. Typical concentrations range from 30 to 50 pmol/cm2,22,23 which is nearly an order of magnitude larger than that of our most densely formed monolayer on the AT electrodes. Due to the fact that we are using low-density monolayers, the finding that the AT electrodes have a smaller electroactive surface area is consistent with our findings that the DNA surface coverage on the AT electrodes is denser than that on the leaf or BT electrodes, despite the electrodes having the same geometrical surface area. The fact that the AT electrodes have the smallest electroactive surface area could be attributed to the presence of impurities in the AT electrodes, which has been previously reported.11
Biosensor Performance
Oligonucleotide-functionalized gold electrodes were used to detect DNase I activity (Figure 3a). In the presence of the enzyme, the MB-modified oligonucleotides are cleaved, removing the MB from the surface, resulting in a signal decrease (Figure 3b). Thus, if DNase I is active, a decrease in the electrochemical signal is expected due to the loss of the MB. Prior to DNase I treatment, MB signals were on the order of microamperes, whereas successful detection of DNase I resulted in MB signals on the order of nanoamperes.
Figure 3.
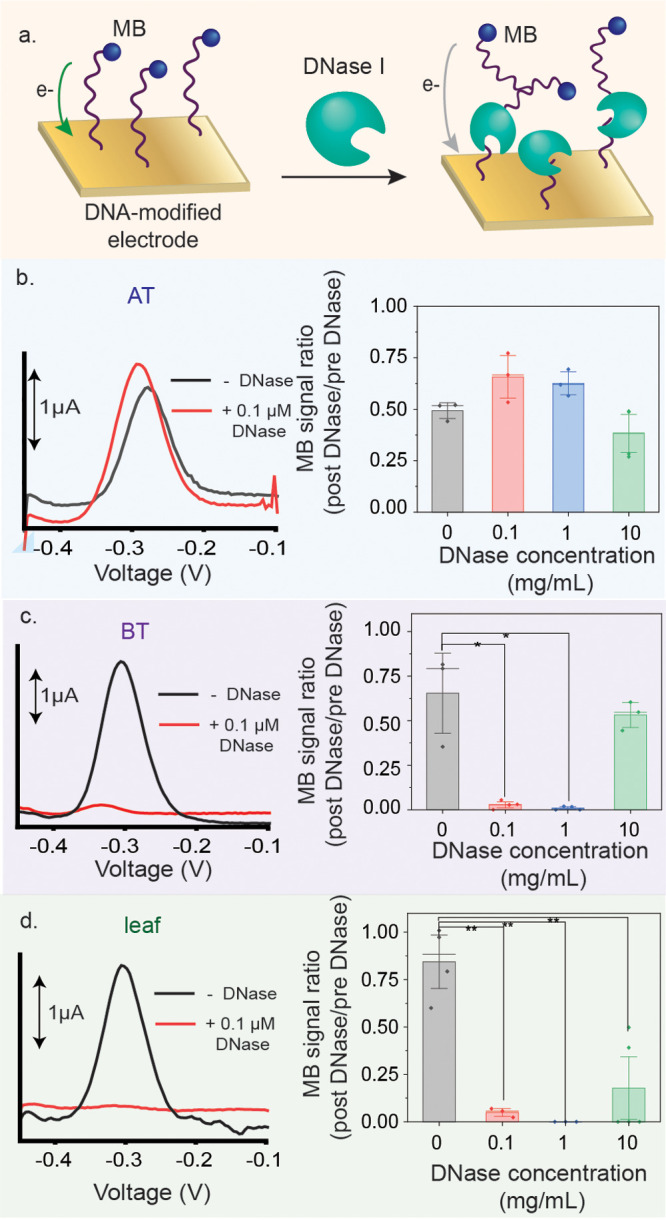
(a) Addition of DNase I cleaves MB-tagged oligonucleotides on the surface of the electrode, resulting in a decrease in signal from the MB. Representative square wave voltammograms measured before and after addition of 0.1 μM DNase onto the electrodes. (b–d) Signal decrease from MB following DNase I addition to the different electrodes. Detection of 0.1 and 1 mg/mL of DNase I is statistically significant with the BT electrodes with a p < 0.05 and is statistically significant with the leaf electrodes with a p < 0.01. The AT electrodes did not allow for detection of DNase I activity.
The AT electrodes were not able to sense DNase I activity at all. With BT electrodes, detection of 0.1 and 1 mg/mL DNase was statistically significant with p < 0.05 according to a t-test. Detection of 10 mg/mL DNase was not achieved, likely due to steric hindrance effects, meaning that DNase I was unable to cleave the immobilized probes. Using the leaf electrodes, detection of 0.1, 1, and 10 mg/mL of DNase was statistically significant with p < 0.01 according to a t-test (Figure 3d). These results mean that the DNase I was successfully cleaving MB-tagged oligonucleotides on the electrode surface.
Discussion
A high-quality gold surface is crystalline gold, which is atomically flat and smooth, allowing for the formation of highly ordered self-assembled monolayers on the surface.24 Unlike the AT electrodes, the BT and leaf electrodes both showed characteristics of crystalline gold, suggesting that the electrodes contain local regions of highly ordered self-assembled monolayers on atomically smooth gold, despite nanometer- and micrometer-level surface roughness (Figure 1). The AT electrodes do not contain any crystalline characteristics (Figure 2a), suggesting that the surface of the AT electrodes does not allow for the formation of highly ordered, high-quality monolayers, but instead that the monolayers may contain defects that allow for nonspecific adsorption of the MB-tagged DNA to the electrode. The nonspecific adsorption of biomolecules (namely, single-stranded DNA) to an imperfect self-assembled monolayer is well-established.25−28 This interaction would inhibit biosensor performance by decreasing solvent access to the biorecognition elements.26,29 This may also explain why the MB signal ratio for some of the AT electrodes upon treatment is greater than one (Figure 3b).
In addition to the quality of the gold surface, the increased electroactive surface area of the BT and leaf electrodes appears to improve their biosensor performance. As the electroactive surface area increases, the density of oligonucleotides on the electrodes decreases, making each oligo more accessible to solvent and therefore to cleavage by DNase I.30 Although the BT and leaf electrodes appear to have similar oligonucleotide densities, the leaf electrodes had higher sensitivity for DNase I activity. A factor that may contribute to this biosensor performance is that there is a smaller decrease in current for the negative controls using the leaf electrodes as compared to the BT electrodes. This result suggests that the monolayers on the leaf electrodes may be more stable than those on the BT electrodes.
Not only do the leaf electrodes outperform the AT and BT electrodes, the leaf electrodes are nearly an order of magnitude less expensive than the AT or BT electrodes and do not require any specialized equipment to fabricate. Therefore, they are well-positioned to be widely adopted for point-of-care diagnostics in LRS.
We have completed the first study to compare the gold leaf electrodes to commercially available electrodes; we demonstrate that gold leaf electrodes are superior to the AT or BT electrodes for biosensor fabrication because they detect DNase I activity with sensitivity better than that of either the AT or the BT electrodes. These differences in biosensor performance can be attributed to the quality and surface area of the electrode, providing an important design roadmap for future electrochemical technologies.
Acknowledgments
We would like to acknowledge the MIT J-WAFS program and the MIT Research Support Committee for supporting this work. Support for this research was also provided by a core center grant P30-ES002109 from the National Institute of Environmental Health Sciences, National Institutes of Health.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmeasuresciau.1c00042.
Methods and materials (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Zamani M.; Furst A. L.; Klapperich C. M. Strategies for Engineering Affordable Technologies for Point-of-Care Diagnostics of Infectious Diseases. Acc. Chem. Res. 2021, 54, 3772–3779. 10.1021/acs.accounts.1c00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataide V. N.; Mendes L. F.; Gama L. I. L. M.; de Araujo W. R.; Paixão T. R. L. C. Electrochemical Paper-Based Analytical Devices: Ten Years of Development. Anal. Methods 2020, 12 (8), 1030–1054. 10.1039/C9AY02350J. [DOI] [Google Scholar]
- Cho I. H.; Kim D. H.; Park S. Electrochemical Biosensors: Perspective on Functional Nanomaterials for on-Site Analysis. Biomater. Res. 2020, 24 (1), 1–12. 10.1186/s40824-019-0181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y.; Xu J.; Liu J.; Wang X.; Chen B. Disease-Related Detection with Electrochemical Biosensors: A Review. Sensors 2017, 17 (10), 2375. 10.3390/s17102375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhaus F. V.; Frense D.; Beckmann D. Immobilization Techniques for Aptamers on Gold Electrodes for the Electrochemical Detection of Proteins: A Review. Biosensors 2020, 10 (5), 45. 10.3390/bios10050045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q.-Z.; Lin M.-W.; Hsu W.-E.; Lin C.-T. Review—Advancements of Nanoscale Structures and Materials in Impedimetric Biosensing Technologies. ECS J. Solid State Sci. Technol. 2020, 9 (11), 115027. 10.1149/2162-8777/abbcb3. [DOI] [Google Scholar]
- Dong S.; Li J. Self-Assembled Monolayers of Thiols on Gold Electrodes for Bioelectrochemistry and Biosensors. Bioelectrochem. Bioenerg. 1997, 42 (1), 7–13. 10.1016/S0302-4598(96)05172-0. [DOI] [Google Scholar]
- Lee M. S.; Hong S. C.; Kim D. Fabrication of Patterned Gold Electrodes with Spin-Coated-and-Fired Au(1 1 1) Film by the Soft Lithography. Appl. Surf. Sci. 2006, 252 (14), 5019–5025. 10.1016/j.apsusc.2005.07.019. [DOI] [Google Scholar]
- Sui Y.; Zorman C. A. Review—Inkjet Printing of Metal Structures for ElectrocSui, Y., & Zorman, C. A. (2020). Review—Inkjet Printing of Metal Structures for Electrochemical Sensor Applications. Journal of The Electrochemical Society, 167(3), 037571. Https://Doi.Org/10.1149/194. J. Electrochem. Soc. 2020, 167 (3), 037571 10.1149/1945-7111/ab721f. [DOI] [Google Scholar]
- Yamanaka K.; Vestergaard M. C.; Tamiya E. Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application. Sensors 2016, 16, 1761. 10.3390/s16101761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernalte E.; Marín-Sánchez C.; Pinilla-Gil E.; Brett C. M. A. Characterisation of Screen-Printed Gold and Gold Nanoparticle-Modified Carbon Sensors by Electrochemical Impedance Spectroscopy. J. Electroanal. Chem. 2013, 709, 70–76. 10.1016/j.jelechem.2013.09.007. [DOI] [Google Scholar]
- Bernalte E.; Sánchez C. M.; Gil E. P. Determination of Mercury in Ambient Water Samples by Anodic Stripping Voltammetry on Screen-Printed Gold Electrodes. Anal. Chim. Acta 2011, 689 (1), 60–64. 10.1016/j.aca.2011.01.042. [DOI] [PubMed] [Google Scholar]
- Zamani M.; Robson J. M.; Fan A.; Bono M. S.; Furst A. L.; Klapperich C. M. Electrochemical Strategy for Low-Cost Viral Detection. ACS Cent. Sci. 2021, 7 (6), 963–972. 10.1021/acscentsci.1c00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M. S. F.; Ameku W. A.; Gutz I. G. R.; Paixão T. R. L. C. Gold Leaf: From Gilding to the Fabrication of Disposable, Wearable and Low-Cost Electrodes. Talanta 2018, 179, 507–511. 10.1016/j.talanta.2017.11.021. [DOI] [PubMed] [Google Scholar]
- Metrohm DropSens; https://www.dropsens.com/ (accessed 2021-11-09).
- García-Cerdán J. G.; Furst A. L.; McDonald K. L.; Schünemann D.; Francis M. B.; Niyogi K. K. A Thylakoid Membrane-Bound and Redox-Active Rubredoxin (RBD1) Functions in de Novo Assembly and Repair of Photosystem II. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (33), 16631–16640. 10.1073/pnas.1903314116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyabharathi C.; Hasse U.; Ahrens P.; Scholz F. Oxygen Electroreduction on Polycrystalline Gold Electrodes and on Gold Nanoparticle-Modified Glassy Carbon Electrodes. J. Solid State Electrochem. 2014, 18 (12), 3299–3306. 10.1007/s10008-014-2657-y. [DOI] [Google Scholar]
- Sukeri A.; Saravia L. P. H.; Bertotti M. A Facile Electrochemical Approach to Fabricate a Nanoporous Gold Film Electrode and Its Electrocatalytic Activity towards Dissolved Oxygen Reduction. Phys. Chem. Chem. Phys. 2015, 17 (43), 28510–28514. 10.1039/C5CP05220C. [DOI] [PubMed] [Google Scholar]
- Elnagar M. M.; Hermann J. M.; Jacob T.; Kibler L. A. Electrochimica Acta An Affordable Option to Au Single Crystals through Cathodic Corrosion of a Wire: Fabrication, Electrochemical Behavior, and Applications in Electrocatalysis and Spectroscopy. Electrochim. Acta 2021, 372, 137867. 10.1016/j.electacta.2021.137867. [DOI] [Google Scholar]
- Bard A. J.; Faulkner L. R.. Electrochemical Methods: Fundamentals and Applications, 2nd ed.; John Wiley & Sons, Inc.: New York, 2001; Vol. 677. [Google Scholar]
- Burke L. D.; Nugent P. F. The Electrochemistry of Gold: I. The Redox Behaviour of the Metal in Aqueous Media. Gold Bull. 1997, 30 (2), 43–53. 10.1007/BF03214756. [DOI] [Google Scholar]
- Furst A. L.; Hill M. G.; Barton J. K. DNA-Modified Electrodes Fabricated Using Copper-Free Click Chemistry for Enhanced Protein Detection. Langmuir 2013, 29 (52), 16141–16149. 10.1021/la403262v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauphin-Ducharme P.; Arroyo-Currás N.; Plaxco K. W. High-Precision Electrochemical Measurements of the Guanine-, Mismatch-, and Length-Dependence of Electron Transfer from Electrode-Bound DNA Are Consistent with a Contact-Mediated Mechanism. J. Am. Chem. Soc. 2019, 141 (3), 1304–1311. 10.1021/jacs.8b11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki M.; Sato T.; Tanaka T.; Takao K. Rapid Self-Assembly of Alkanethiol Monolayers on Sputter-Grown Au(111). Langmuir 2000, 16 (4), 1719–1728. 10.1021/la990310z. [DOI] [Google Scholar]
- Lichtenberg J. Y.; Ling Y.; Kim S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19 (11), 2488. 10.3390/s19112488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methods of reducing non-specific adsorption in microfluidic biosensors - IOPscience.
- Azzaroni O.; Salvarezza R. C. Chemisorbed Self-Assembled Monolayers. Supramol. Chem. 2012, 10.1002/9780470661345.smc147. [DOI] [Google Scholar]
- Nano A.; Furst A. L.; Hill M. G.; Barton J. K. DNA Electrochemistry: Charge-Transport Pathways through DNA Films on Gold. J. Am. Chem. Soc. 2021, 143, 11631. 10.1021/jacs.1c04713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamdad C. A DNA Self-Assembled Monolayer for the Specific Attachment of Unmodified Double- or Single-Stranded DNA. Biophys. J. 1998, 75 (4), 1997–2003. 10.1016/S0006-3495(98)77641-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biagiotti V.; Porchetta A.; Desiderati S.; Plaxco K. W.; Palleschi G.; Ricci F. Probe Accessibility Effects on the Performance of Electrochemical Biosensors Employing DNA Monolayers. Anal. Bioanal. Chem. 2012, 402 (1), 413–421. 10.1007/s00216-011-5361-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



