Abstract
Azo dyes have become a staple in various industries, as colors play an important role in consumer choices. However, these dyes pose various health and environmental risks. Although different wastewater treatments are available, the search for more eco-friendly options persists. Bioremediation utilizing microorganisms has been of great interest to researchers and industries, as the transition toward greener solutions has become more in demand through the years. This review tackles the health and environmental repercussions of azo dyes and its metabolites, available biological approaches to eliminate such dyes from the environment with a focus on the use of different microorganisms, enzymes that are involved in the degradation of azo dyes, and recent trends that could be applied for the treatment of azo dyes.
Keywords: azo dye degradation, decolorization, bioremediation, immobilization, p-phenylenediamine, xenobiotics
1. Introduction
Colors play an important role to different industries and in consumer choices. It also contributes to the aesthetic quality of a product, which drives consumers to purchase and therefore contributes to economic growth. The different colors and hues that we see are often derived from the dyes that were used. Natural dyes are the safer and more eco-friendly option compared to synthetic dyes [1]. They also pose other advantages such as having antimicrobial properties and offering protection from UV light [2,3,4,5].
Although natural dyes are the safer and the more environmentally friendly option to use, they are quite costly, more tedious to apply, and more difficult to procure. Tyrian purple, for example, is a natural dye from the mucus of Murex sp. snails that retails for about €2000 per gram [6,7]. Therefore, the use of natural dyes has been deemed to be impractical for many commercial applications. Moreover, natural sources of dyes usually contain only about 2% of actual coloring material, which means that uneconomic amounts of raw material might be needed to obtain the desired shades and hues. This drives up its cost and is thus undesirable for mass production use. It is nearly impossible to reproduce the same shade from batch to batch and fastness properties are rather poor, which make it difficult to apply on textiles [1].
As an alternative, synthetic dyes such as azo dyes have become the primary coloring agent in industries such as the food, cosmetics, and textile industries as trace amounts of dyeing material already produce an intense color [8,9,10]. Azo dyes take account for most of the synthetic dyes used as they are easy to synthesize, thus cost-efficient to produce, and generate a variety of colors as there are about 10,000 different azo dyes available [10,11,12]. Since azo dyes are synthesized chemically, careful downstream treatments are needed to ensure safety in its usage and disposal [13]. It is important to consider that only 10% of the dye is transferred into the material permanently, and the majority goes into wastewater as effluents [13]. At the same time, trace amounts of dye can lead to severe environmental and health hazards as some azo dyes are toxic, carcinogenic, and mutagenic [14,15]. The presence of these synthetic dyes can also hamper various biological activities [16]. Therefore, it is important to implement tight regulations for their treatment and disposal.
Several physical and chemical procedures are available for the downstream treatment and waste handling of azo dye containing effluents [17,18,19]. Quite often, these approaches are met with skepticism, as these have major downsides such as the ecologically questionable waste disposal for filters and charcoals, generation of toxic intermediates, production of sludge, and the high costs of equipment [19,20]. Thus, finding a more environmentally friendly approach is essential. Here, biological methods can be a more promising solution. This review tackles the treatment methods available for azo dyes especially on the biological context (primarily bacteria) with emphasis on the approach of whole-cell biocatalysis and/or enzymatic degradation. Physical parameters that affect dye decolorization and degradation, such as pH and temperature, are not within the scope of this review.
2. Impacts of Azo Dyes on Human Health and the Environment
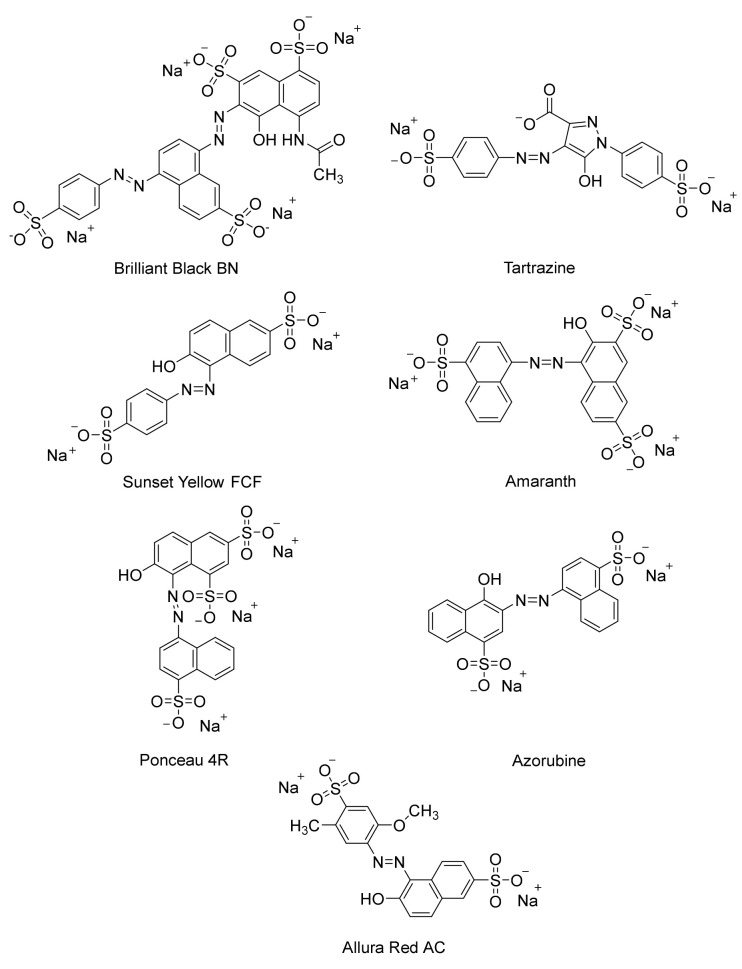
Azo dyes are characterized by the presence of an azo bond (-N = N-) between two or more aromatic rings [21,22]. The versatility of azo dyes renders them very appealing for various industries such as food and textiles. However, the xenobiotic nature of these dyes calls for proper evaluation of their harmful effects. In the food industry, the use of azo dyes should be critically assessed as they are often used as colorants for sweets and desserts with children as target consumers [23]. Although there are regulations on which dyes may be used or not, these are different by country, which adds to the difficulty of standardizing protocols for the usage of dyes [23]. There are thousands of azo dyes used in various industries. Some of the better-known azo dyes used in the food industry are Brilliant Black BN, Tartrazine, Sunset Yellow FCF, Amaranth, Azorubine, Ponceau 4R, and Allura Red AC [23] (Figure 1).
Figure 1.
Commonly used azo dyes in the food industry.
The toxicity of some of these azo dyes can be attributed to the reduction of the azo bond, which produces aromatic amines [24,25,26]. When ingested orally, the dye reaches the gastrointestinal tract, and the intestinal microflora or mammalian azoreductases cleave the azo bond [25,27,28]. The aromatic amines, which are often the final product of azo dye reduction, are subsequently hydroxylated or acetylated, and this adds to the mutagenicity and carcinogenicity of these compounds. The intake of azo dyes can also increase the risk of human bladder cancer, splenic sarcomas, hepatocarcinomas, and nuclear anomalies [25]. They can also cause allergies, dermatitis, and even DNA damage that results in the formation of malignant tumors [29,30]. Methyl Yellow, which is now banned in different countries, was formerly used for dyeing butter, and it was found to cause liver cancer in rats after two to three months of exposure [31,32]. Another example is the dye Amaranth, which was shown to induce DNA damage in the colon epithelium of mice [33,34,35], while Brilliant Black BN has shown genotoxicity in human lymphocytes based on in vitro experiments [36]. Sunset Yellow FCF can cause DNA damage and has shown to have toxic effects on the reproductive and neurobehavioral system of tested rodents and chick embryos [37,38]. Tartrazine was shown to bind to albumin, induce neurotoxicity, impair mental functions, and promote various reactions such as angioedema, nasal congestion, itchy skin, and hives [23]. In the cosmetic and textile industries, 4-aminobenzene or Aniline Yellow, a dye used for printers and as a precursor of other dyes, has been shown to cause high hepatocarcinogenicity and induce tumors to rats [39]. Sudan III, used for coloring non-polar substances such as acrylic emulsions, was also reported as a carcinogen [40].
Aside from health issues, another significant problem of these azo dyes is their presence in the environment. Trace amounts of azo dyes in watercourses can cause aesthetic pollution. It also leads to different chain reactions. Poor sunlight penetration into the water decreases the photosynthetic activity from waterborne organisms, leading to a reduction in dissolved O2 concentration, and it can as a consequence cause acute effects to the aquatic community [26]. The dissolved O2 concentration primarily restricts the growth and development of plants. These dyes can also affect germination rates and even inhibit the elongation of shoots and root seedlings [22,26]. As plants have a significant role in ecology such as serving as a habitat for organisms, providing organic matters that contribute to soil fertility, and keeping the soil from erosion, all these services are threatened as dye-containing effluents, and other xenobiotics continue to be discharged to the environment [22]. Moreover, the presence of these pollutants negatively affects the chlorophyll content of plants, as they trigger the production of chlorophyllase and abscissic acid, both of which can lead to chlorophyll degradation [26,41]. Aquatic organisms such as fishes are affected by the water quality of their environment. The presence of azo dyes and other toxic compounds impair the growth of several fishes—showing reduced growth and affecting the muscles, gills, liver, and intestines [26]. The edible freshwater fingerling, Catla catla, has shown histopathological alterations in gills such as hyperplasia and degenerated central axis when treated with Reactive Red 120 [42]. It was also shown that the exposure of embryo larval fathead minnows, Pimephales promelas, to the azo dyes Disperse Yellow 7 and Sudan Red G decreased the survival of larval fish with LC50 values of 25.4 µg/L and 16.7 µg/L, respectively [43]. These examples strikingly show how hazardous azo dyes can be to all areas of nature if not properly treated.
3. Impact of Azo Dye Metabolites
As mentioned earlier, not all azo dyes are harmful. However, dyes that were not found to be harmful still pose a threat, since oxidative and reductive metabolism could lead to the formation of toxic aromatic amines. Different studies have identified compounds derived from azo dye metabolism such as benzidine, p-phenylenediamine, aniline, and toluene, which show carcinogenic and mutagenic properties [24,25,27,31,39].
Benzidine is a building block of azo dyes such as Congo Red, Direct Black 38, and Direct Red 39. Earlier studies have shown that it can induce tumor cells on different body parts such as the gastrointestinal tract, pancreas, liver, and gallbladder [39]. Benzidine and its congeners, such as 3′3-dimethylbenzidine and 3′3′-dichlorobenzidine, were shown to induce carcinoma and tumors. 3′,3′,-5,-5′ Tetramethylbenzidine was the only congener of benzidine that was not found to be carcinogenic [39].
Meanwhile, p-phenylenediamine is another compound that is used as a henna substitute, for the manufacturing of certain polymers in different industries, as a developing agent for films, and as a major component for hair dyes. Initial investigations on p-phenylenediamine showed contrasting results. On the one hand, it was shown to be carcinogenic via an Ames test. On the other hand, it was shown that it did not pose any carcinogenic potential to F344 rats regardless of the sex and the exposure time [44]. It was also shown to not affect pregnant rats, and a multigenerational reproduction study also showed no negative effects [44,45]. However, it was discovered that p-phenylenediamine only becomes mutagenic after oxidation [46]. This was corroborated when p-phenylenediamine, in the presence of microsomal fraction and after H2O2 oxidation, became mutagenic to a Salmonella typhimurium tester strain TA1538 [47]. This poses a problem as most permanent hair dyes need to be oxidized upon usage to exert their dyeing properties. It was shown that upon absorption, p-phenylenediamine can lead to the formation of tumors in the liver, kidney, urinary bladder, and thyroid gland of rats [48,49]. It was also found that it can increase the expression of p53 proteins, thereby suggesting an increase in apoptosis and affecting cell viability [49]. A correlation was also observed on the usage of hair dyes and Non-Hodgkin lymphoma and cancer [50].
Other monocyclic aromatic amines such as p-nitroaniline, o-toluidine (2-methylaniline), 2-nitro-p-phenylenediamine, and o-phenylenediamine were also shown to have carcinogenic and mutagenic properties [39]. These compounds are all highly relevant toxins, as occupational exposure to aromatic amines can explain 25% of bladder cancer cases [51].
4. Physical and Chemical Treatment of Dyes
As azo dyes pose different risks and hazards to health and the environment, it is necessary to find ways to treat these dyes. Various physical and chemical methods have been explored [18,29,52]. Physical methods include techniques such as adsorption and filtration [8]. Adsorption uses materials such as activated carbon that can accumulate compounds to be removed from wastewater at their surface [53,54,55]. Although activated carbon is effective and has been the primary option for this kind of treatment, it is not often used due to its high cost. Alternatives have been explored, such as peat, banana peels, clay, corn cob, maize, and wheat straw [55,56,57,58]. However, there are some drawbacks due to the problematic waste disposal of these cheaper alternatives. Another frequently used physical method is filtration. It often involves the use of membranes to remove suspended solids and other unwanted materials from water [19]. Although effective, it likewise has some drawbacks such as similarly high costs for investment and of materials, deterioration of the membrane or membrane fouling, production of potentially toxic sludge, and again problematic waste disposal [19,22].
Chemical treatment involves the use of different chemicals or techniques such as coagulation–flocculation, Fenton’s reagent, ozone, and electrochemical methods [22]. Coagulation–flocculation followed by sedimentation are processes used in conventional wastewater treatment facilities [59,60]. Coagulation involves the use of coagulants to neutralize suspended solids (often with an opposite charge to the coagulant). Once neutralized, the suspended solids can collide and form microflocs. During flocculation, these microflocs can form macroflocs and sediment, upon which they can be removed from water using gravity. Furthermore, the Fenton reaction allows the generation of hydroxyl radicals using Fenton reagent (H2O2 and Fe2+ ions), which can destroy toxic pollutants in wastewater [61]. Although the overall process is cheap, it can lead to a high sludge production as secondary waste. Ozonation uses reactive ozone (O3) to oxidize and disassemble preferentially the chromophores of dyes, but its unstable nature makes it undesirable for wastewater treatment [62].
These physical and chemical treatments have been used traditionally for wastewater treatment. However, as mentioned earlier, these approaches can pose several drawbacks. Furthermore, dyes may be resistant or recalcitrant to these available treatments [63]. Therefore, it is necessary to find safer, more eco-friendly ways that can handle these synthetic dyes, especially azo dyes.
5. Biological Treatment of Dyes
Microorganisms are ubiquitous. Their ability to withstand harsh conditions and to thrive even in the most polluted environment make them a melting pot for the study of interesting enzymes and metabolites that can be harnessed to potentially solve environmental problems. The use of microorganisms as an alternative to physicochemical treatments is called bioremediation [64,65]. Bioremediation has sparked serious interest in the past decades, and microorganisms have been well-documented to destroy polyaromatic hydrocarbons [66,67,68], to transform heavy metals to less harmful or immobilized forms [69,70], to degrade pesticides [71,72], and to reduce or mineralize azo dyes [73,74].
For dye degradation, the use of microorganisms has several advantages. In a comparably cost-efficient manner, the dyes are decomposed to a vast degree whilst less water is required and less sludge produced. The mechanisms of how microorganisms can decolorize (i.e., reduce azo bonds to aromatic amines) and degrade (i.e., break down azo dyes into small molecules leading to H2O, CO2, and mineral by-products) azo dyes have been a subject of interest in different studies [75,76,77,78,79,80]. Microbiological dye removal or even degradation can be achieved by means of various ways such as via adsorption, via the production of enzymes that can attack the dyes, and even via the combination of both [81,82]. Adsorption occurs through ion exchange as the microbial cell wall has hydroxy and carboxy groups that can serve as binding sites for the dyes to adhere to [83,84]. Adsorption can be achieved by either live cells or dead cell biomass [85,86]. It has several disadvantages: azo dyes cannot be transformed into non-toxic forms, thus making waste disposal similarly problematic as in classical physical methods. As opposed to adsorption, the degradation of azo dyes has been of significant interest as ideally, the dyes can be completely degraded by the microbial enzymes. Microorganisms capable of decolorizing and degrading dyes include filamentous fungi [87,88], yeasts [89,90], algae [91,92], and bacteria [93,94,95].
5.1. Biological Treatment of Dyes Using Filamentous Fungi
Filamentous fungi are versatile microorganisms that can produce intracellular and extracellular enzymes able to degrade a variety of xenobiotics. They can be isolated everywhere such as in soil and even from waste organic materials. The mechanisms on how filamentous fungi decolorize and degrade azo dyes rely upon the combined activities of the biosorption process and the extracellular enzymes produced [80,96,97]. Moreover, investigations on filamentous fungi as a biosorbent for dye-containing effluents have been performed on both living and dead biomass setups.
The dead biomass of fungi has been of significant interest, as it was shown to take up higher concentration of dyes than the living biomass. The use of dead cells often relies on physicochemical interactions such as adsorption, deposition, and ion exchange to serve as a biosorbent for the treatment of dyes. In this process, dye molecules adhere to the fungal mycelia via ion exchange [80]. The heteropolysaccharides present in the cell wall of fungi such as chitin, chitosan, glucan, lipids, and phospholipids serve as binding sites with functional groups such as carboxy, hydroxy, and phosphoryl groups that help facilitate the biosorption process [80]. It was shown that dead fungal cells of Aspergillus niger can take up the dyes Basic Blue 9, Acid Blue 29, and Reactive Brilliant Red [96,98,99]. The integrity of the cell wall also plays an important role, as disrupted cells showed less efficiency in adsorbing dye solutions after 24 h than intact ones [99,100]. Filamentous fungi also serve as a powerhouse source for different enzymes that can convert various dyes. Some of the known fungal enzymes involved in dye degradation are laccases, lignin peroxidases, and manganese peroxidases (Table 1).
Table 1.
List of fungal cultures from various decolorization studies and involved enzymes.
| Enzyme Class Involved | Culture | Dyes | % Decolorization | References |
|---|---|---|---|---|
| Laccases | Marasmius scorodonius | Congo Red | 90% | [101] |
| Malachite Green | 82% | |||
| Crystal Violet | 69% | |||
| Methylene Green | 63% | |||
| Reactive Orange 16 | 48% | |||
| (+ 1-hydroxybenzotriazole) | ||||
| Remazol Brilliant Blue R | 61% | |||
| (+ 1-hydroxybenzotriazole) | ||||
| Myceliopthora thermophila | Acid Blue 74 | 15.20% | [97] | |
| Acid Blue 25 | 53.30% | |||
| Acid Green 27 | 67% | |||
| Reactive Blue 19 | 31.20% | |||
| Direct Red 28 | 9.60% | |||
| Trametes versicolor | Acid Blue 74 | 88.40% | [97] | |
| Acid Blue 25 | 66.00% | |||
| Acid Green 27 | 76.00% | |||
| Reactive Blue 19 | 64.50% | |||
| Direct Red 28 | 11.90% | |||
| Aspergillus ochraceus NCIM 1146 | Reactive Navy Blue HER | 90.00% | [102] | |
| Reactive Golden Yellow HER | 90.00% | |||
| Methyl Orange | ||||
| 56.00% | ||||
| Lignin peroxidases | Phanerochaete chrysosporium (Crude lignin peroxidases with 2 mM veratryl alcohol) | Bromophenol Blue | 93% | [103] |
| Congo Red | 54% | |||
| Methylene Blue | ~85% | |||
| Methyl Green | ~85% | |||
| Methyl Orange | ~85% | |||
| Remazol Brilliant Blue R | ~70% | |||
| Toluidine Blue | 80% | |||
| Poly R-478 | 46% | |||
| Poly S-119 | 80% | |||
| Poly T-128 | 48% | |||
| Ganoderma lucidum IBL-05 (with 4 mM veratryl alcohol) | Sandal-fix Red C4BLN | 66% | [104] | |
| Sandal-fix Turq Blue GWF | 59% | |||
| Sandal-fix Foron Blue E2BLN | 52% | |||
| Sandal-fix Black CKF | 40% | |||
| Sandal-fix Golden Yellow CRL | 48% | |||
| Bjerkandera adusta CX-9 | Acid Blue 158 | ~40% | [105] | |
| Cibacet Brilliant Blue BG | 25% | |||
| Poly R-478 | ~30% | |||
| Methyl Green | 75% | |||
| Indigo Carmine | 50% | |||
| Remazol Brilliant Blue R | ~90% | |||
| Remazol Brilliant Violet 5R | <20% | |||
| Manganese peroxidase | Bjerkandera adusta CX-9 | Acid Blue 158 | 91% | [105] |
| Cibacet Brilliant Blue BG | 70% | |||
| Poly R-478 | 80% | |||
| Methyl Green | <20% | |||
| Indigo Carmine | ~45% | |||
| Remazol Brilliant Blue R | ~40% | |||
| Remazol Brilliant Violet 5R | 70% | |||
| Cerrena unicolor BBP6 | Congo Red | 54% | [106] | |
| Methyl Orange | 78% | |||
| Remazol Brilliant Blue R | 81% | |||
| Bromophenol Blue | 62% | |||
| Crystal Violet | 81% | |||
| Azure Blue (+gallic acid) | 63% |
Several studies have compared these two approaches for fungal biomass application for dye treatments. It was shown that living and dead cells were equally effective for dye color removal [107]. Meanwhile, a wide screening across different kinds of microorganisms showed that dead forms had better decolorization rates for Reactive Black 5 and Reactive Blue 19 [108]. A study of Przytas et al., compared the efficiency of immobilized fungi, namely Pleurotus ostreatus BWPH, Gleophyllym odoratum DCa, and Polyporus picipes—in living and in autoclaved form, and corroborated previous findings where it was shown that the decolorization rates of the used dyes were higher in dead fungal biomass [109].
Both approaches for fungal biomass use have advantages and disadvantages. As mentioned earlier, living cells can have a variety of different mechanisms for dye decolorization and degradation. However, this entails optimizing operating conditions such as pH, moisture, temperature, nutrient supply, and culture maintenance, as all can affect the ability of fungi to secrete enzymes. On the other hand, dead biomass seems to be an effective biosorbent, but just like any (physical) adsorbent, the question of waste disposal will always remain. This is especially true for the then dye-enriched adsorbent. The fungal biomass probably degrades fast, and the previously adsorbed dye persists at the disposal site and might be exposed to weathering.
5.2. Biological Treatment of Dyes Using Yeasts
Yeasts are not widely used in dye decolorization and have not been as extensively studied as bacteria and filamentous fungi. However, yeasts hold potential in this field especially because they present a biotechnological advantage as they are fast-growers and can also thrive in harsh conditions. Yeasts typically remove dyes by the process of biosorption. The dyes adhere to cell peripheries and eventually enter into the cell based on interactions made by the functional groups present on the cell surface through electrostatic interactions, ion exchange, or ion chelation. In a screening by Yu and Wen (2005), Reactive Brilliant Red K-2BP was removed through biosorption by Saccharomyces cerevisae, Saccharomyces uvarum, Saccharomyces lipolytica, and Turolopsis candida [110].
Dye-degrading enzymes have not yet been well-studied on yeasts. However, it is possible that yeasts also produce putative dye-degrading enzymes similar to filamentous fungi. Some putative oxidative enzymes such as ligninolytic enzymes were found from Pyricularia oryzae [111], while some lignin peroxidase activities were detected from the cells of Saccharomyces cerevisiae after the decolorization of Methyl Red [112].
5.3. Biological Treatment of Dyes Using Algae
Algae are photosynthetic organisms widely distributed in different aquatic habitats. Just like the microorganisms described above, they also exhibit dye degradation capabilities. The algal cell wall contains many functional groups such as carboxy, carbonyl, hydroxy, phosphoryl, and amide groups that play important roles in dye decolorization [113]. Some algae are also able to assimilate the dye chromophores. They possess enzymes that can transform the dyes to H2O and CO2, leading to the production of algal biomass [75].
Microalgae isolates, namely Chlorella vulgaris, Anabaena oryzae, and Wollea sacata, showed efficient decolorization and degradation of different dyes from various dye classes, including the azo dye Orange G [114]. It was also shown that species from Chlorella and Oscillatoria degrade azo dyes to aromatic amines and further degrade these aromatic amines to simpler organic compounds [115]. Thus, algal biomass can be exploited for the potential treatment of azo dyes, especially as they thrive predominantly on aquatic environments, where textile dye effluents go.
5.4. Biological Treatment of Dyes Using Bacteria
Bacteria have been the subject of different studies since they offer several advantages in a biotechnological perspective such as being fast growers, having a plethora of degradative enzymes, and being able to degrade a wide range of dyes. Compared with all discussed microorganism groups, bacterial decolorization has been of considerable interest [22,75]. Although filamentous fungi are effective and potent agents for dye degradation, as discussed above, bacteria are more preferred owing to their faster growth rate and easier handling. The mechanism of dye decolorization and degradation relies on the ability of bacteria to produce enzymes such as azoreductases that can cleave the azo bond (-N = N-) [22,75,116]. Moreover, the ability of bacteria to further reduce or decompose aromatic amines, either aerobically or anaerobically, allows them to have more versatility compared to other organisms [22]. Bacterial decolorization furthermore has more potential for wastewater treatment application, as bacteria are less problematic to handle than filamentous fungi. Some bacterial strains have shown a wide range of substrates that can be reduced. Some have even shown the potential to completely degrade azo dyes. They are also more environmentally friendly and produce less sludge [117,118,119,120,121].
Studies on bacterial decolorization range from pure cultures to mixed bacterial cultures (Table 2 and Table 3). The study of pure cultures in the context of dye degradation allows an in-depth understanding on the mechanism of bacteria toward its behavior against the dyes. It also allows the study of the metabolic and degradation pathways involved [22,122]. Meanwhile, mixed bacterial cultures allow the possibility to explore synergistic activities as well as functional redundancies that can be helpful on dye degradation and may thus exploit alternative ways to degrade dyes [123,124]. Pure cultures can be limited in this sense, as they may encode only for one single activity or pathway to attack azo compounds. In mixed bacterial cultures, it is possible to mix, match, and explore multiple possibilities that would lead to dye degradation. However, the downside is that it is important to identify which bacterial isolates can be mixed and matched to untap the best results.
Table 2.
List of single bacterial cultures used on different dye decolorization studies.
| Culture | Dyes | % Decolorization | References |
|---|---|---|---|
| (Time of Incubation) | |||
| Bacillus sp. AK1 | Metanil Yellow | 99% (24 h) | [125] |
| Sphingomonas paucimobilis | Methyl Red | 99.6% (10 h) | [126] |
| Proteus mirabilis | Red RBN | 95% (20 h) | [127] |
| Aeromonas hydrophila | Red RBN | 90% (8 days) | [128] |
| Brevibacterium sp. VN-15 | Reactive Yellow 107 | 98% (96 h) | [129] |
| Reactive Black 5 | 95% (144 h) | ||
| Reactive Red 198 | 97% (120 h) | ||
| Direct Blue 71 | 94% (168 h) | ||
| Acinetobacter calcoaceticus NCIM 2890 | Amaranth | 93% (48 h) | [93] |
| Methyl Red | 95% (24 h) | ||
| Amido Black 10 B | 87% (72 h) | ||
| Congo Red | 17% (72 h) | ||
| Bacillus firmus H4 | Novacron Red | 80–89% (24 h) | [130] |
| Bacillus filamentosus T13 | Novacron Red | 80–89% (24 h) | [130] |
| Bacillus subterraneus A36 | Novacron Red | 80–89% (24 h) | [130] |
| Micrococcus luteus 24M | Congo Red | 99% (11 days) | [131] |
| Pseudomonas sp. SUK1 | Red BLI | 99% (1 h) | [132] |
| Pseudomonas sp. SUK1 | Reactive Red 2 | >80% (48 h to 72 h) | [133] |
| Shewanella putrefaciens | Acid Red 88 | 100% (4 h) | [134] |
| Direct Red 81 | 100% (4 h) | ||
| Reactive Black 5 | 100% (6 h) | ||
| Disperse Orange 3 | 100% (8 h) | ||
| Kocuria indica DP-K7 | Methyl Red | 68% (160 h) | [135] |
| Arthrobacter bambusae DP-A9 | Methyl Red | 100% (24 h) | [136] |
| Brilliant Black | 100% (24 h) | ||
| Leifsonia shinshuensis DP-L11 | Methyl Red | 53% (24 h) | [136] |
| Brilliant Black | 85% (24 h) | ||
| Dermacoccus nishinomiyaensis DP-D10 | Methyl Red | 84% (24 h) | [136] |
| Brilliant Black | 100% (24 h) | ||
| Paraburkholderia sp. DP-P12 | Methyl Red | 58% (24 h) | [136] |
| Brilliant Black | 62.5% (24 h) | ||
| Rhodococcus sp. UCC 0008 | Methyl Red | 100% (72 h) | [137] |
| Rhodococcus sp. UCC 0016 | Methyl Red | 100% (24 h) | [137] |
| Staphylococcus sp. EY-3 | Congo Red | >96% (48 h) | [138] |
| Kocuria rosea MTCC 1532 | Methyl Orange | 100% (72 h) | [139] |
| Citrobacter sp. CK3 | Reactive Red 180 | 95% (36 h) | [140] |
| Bacillus sp. YZU1 | Reactive Black 5 | 95% (120 h) | [141] |
Table 3.
List of microbial consortia used in different dye decolorization studies.
| Culture | Dyes | % Decolorization (Time of Incubation) | References | |
|---|---|---|---|---|
| Bacterial consortium | Bacillus circulans BPB8 | Textile effluents with mixed azo dyes (Reactive Red, Reactive Brown, Reactive Black) and Cr(VI) | 82% (5 days) | [142] |
| Bacillus circulans HQB947 | ||||
| Bacillis subtilis | ||||
| Terribacillus gorriensis | ||||
| Fungal–bacterial consortium |
White Rot fungus 8-4* Pseudomonas |
Direct Fast Scarlet 4BS (Sole Carbon Source) |
100% (30 h) | [143] |
| Bacterial consortium | Pseudomonas sp. ARa | Reactive Red 195 (Maltose and Proteose Peptone) |
100% (14 h) | [144] |
| Bacillus sp. ARc | ||||
| Bacillus sp. ARd | ||||
| Ochrobactrum sp. ARf | ||||
| Bacterial consortium | Bacillus cereus BN-7 | Acid Red 88 | 100% (24 h) | [145] |
| Pseudomonas putida BN-4 | ||||
| Pseudomonas fluorescence BN-5 | ||||
| Stenotrophomonas acidaminiphila BN-3 | ||||
| Fungal–bacterial consortium |
Brevibacillus laterosporus
Galactomyces geotrichum |
Reactive Red 198 | 92% (18 h) | [146] |
| Fungal–bacterial consortium |
Aspergillus ochraceous NCIM-1146 | Rubine GFL | 95% (30 h) | [147] |
| Pseudomonas sp. SUK1 | Textile effluent | 98% (35 h) | ||
| Bacterial consortium | Bacillus sp. AK1 | Ponceau 4R | 100% (18 h) | [148] |
| Lysinibacillus sp. AK2 | ||||
| Kerstersia sp. VKY1 | ||||
| Bacterial consortium | Paenibacillus polymyxa | Reactive Violet 5R | 100% (36 h) | [149] |
| Micrococcus luteus | ||||
| Micrococcus sp. | ||||
| Bacterial consortium | Enterobacter dissolvens AGYP1 | Acid Maroon V | 93% (20 h) | [150] |
| Pseudomonas aeruginosa AGYP2 | ||||
| Bacterial consortium | Bacillus odyssey SUK3 | Red HE3B | 97% (24 h) | [151] |
| Morganella morganii SUK5 | ||||
| Proteus sp. SUK7 | ||||
| Bacterial consortium | Providencia sp. SDS | Red HE3B | 100% (1 h) | [152] |
| Pseudomonas aeruginosa BCH | ||||
| Bacterial consortium | Proteus vulgaris NCIM-2027 (PV) | Scarlet Red Dye Mixture (Scarlet R, Navy Blue HER, Red HE7B, Green HE4BD, Orange HE2R, Navy Blue G, Red HE3B, Navy Blue HE2R, Golden Yellow 24D, Brilliant Blue G, Direct Brown MR, Direct Blue GLL) |
100% (3 h) | [153] |
|
Micrococcus glutamicus NCIM-2168 (MG) |
88% (72 h) | |||
| Bacterial consortium | Bacillus subtilis WGI3 | Direct Red 23 | 70% (48 h) | [154] |
| Bacillus subtilis WGI4 | Direct Yellow 12 | 84% (48 h) | ||
| Bacillus cereus WGI9 | Direct Blue 15 | 66% (48 h) | ||
| Dye Mixture | 75% (48 h) |
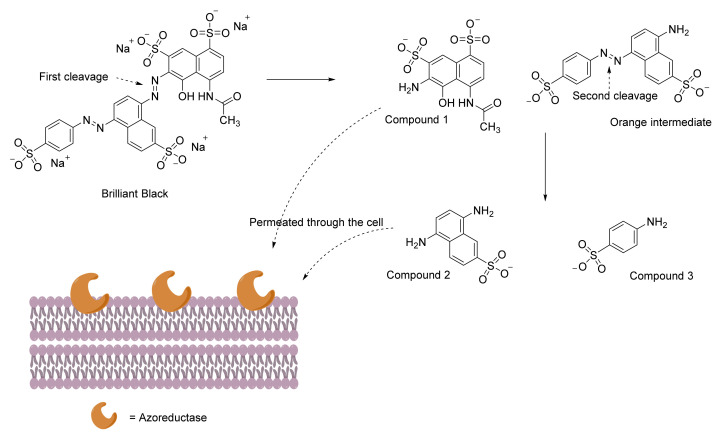
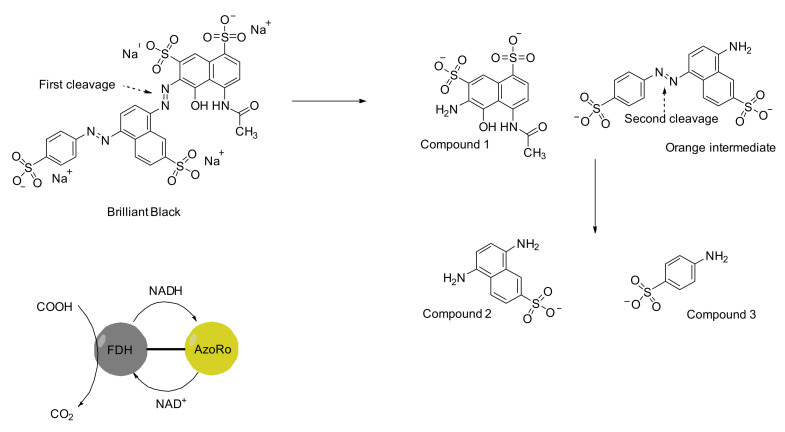
As the number of studies on dye decolorization and degradation increase, it is also important to investigate the mechanism of how azo dyes are being attacked by these bacteria. Brilliant Black, despite having two azo bridges in its structure, is not reduced simultaneously by Dermacoccus abyssi MT1.1T (Figure 2). Most azoreductases of the organism are known to be localized at the cell membrane. It is discussed that the azo bridge between the two naphthalene rings is cleaved first, producing a naphthol-based compound (compound 1) and an orange intermediate, 8-amino-5-((4-sulfonatophenyl)diazenyl) naphthalene-2-sulfonic acid. The orange intermediate, which still bears an azo bridge, is attacked by the azoreductase again and produces the second naphthol-based compound (compound 2) plus sulfanilic acid (compound 3). Compounds 1 and 2 could not be detected, and therefore, it was proposed that they were translocated into the cell membrane [155].
Figure 2.
Proposed degradation pathway for Brilliant Black by Dermacoccus abyssi MT1.1T while the degradation compounds or intermediates were reported earlier [155].
6. Enzymatic Degradation of Azo Dyes
There are several enzymes that have been found to reduce and degrade dyes. Some of the best-known enzymes are manganese peroxidases [156,157,158], lignin peroxidases [103,111,159], laccases [111,160], dye peroxidases [161,162], and azoreductases [163,164,165,166], all of which will be introduced in this section.
Manganese peroxidase is an oxidative enzyme that can destroy phenolic compounds and other xenobiotics with the oxidation of two Mn(II) to Mn(III) [167,168,169]. The Mn(III) compounds are active oxidants, which are typically stabilized by chelating organic acids such as oxalic acid [170]. Manganese peroxidases can break down lignin but can as well decolorize azo dyes and phthalocyanine. It was furthermore demonstrated to degrade the highly recalcitrant polymeric dye, Poly R-478 [171]. Although manganese peroxidase was first discovered in the white rot fungi Phanaerochaete chrysosporium, the enzyme was also reported to be responsible for the decolorization of the azo dye Ranocid Fast Blue and the anthraquinone dye Procion Brilliant Blue-H-GR by the bacterium Serratia marcescens [172].
Lignin peroxidases are also oxidative enzymes that can degrade lignin, polychlorinated biphenyls, and synthetic dyes [173]. Lignin peroxidases degrade dyes through the oxidation of the phenolic group at the carbon bearing the azo bond to produce a radical group [111]. The water attacks this phenolic carbon and then produces phenyldiazene, which can subsequently be oxidized by a one-electron reaction generating N2 [111]. Like manganese peroxidases, this enzyme is often produced by fungal systems. However, there are also bacteria that were reported to have lignin peroxidase activity, such as Bacillus sp. strain VUS and Acinetobacter calcoaceticus NCIM 2890 [93,174]. Pseudomonas desmolyticum NCIM 2112 was also reported to degrade Direct Blue 6 with the involvement of lignin peroxidases, laccases, and tyrosinases [175].
Dye-decolorizing peroxidases (DyP) are heme-peroxidases that were found to have different sequences, structures, and features compared to the classic plant and mammalian peroxidases [176]. These enzymes were discovered to attack azo dyes and the anthraquinone skeleton and hence earned the name dye-decolorizing peroxidases [177]. They contain the highly conserved GXXDG motif in their primary sequences and in addition a conserved Asp, a distal Arg, and a proximal His, which are important for stability, heme-binding, and biocatalysis [162,178,179]. DyPs can be classified into four types (A–D), where types A to C are widespread in bacteria and type D is of fungal origin [180,181].
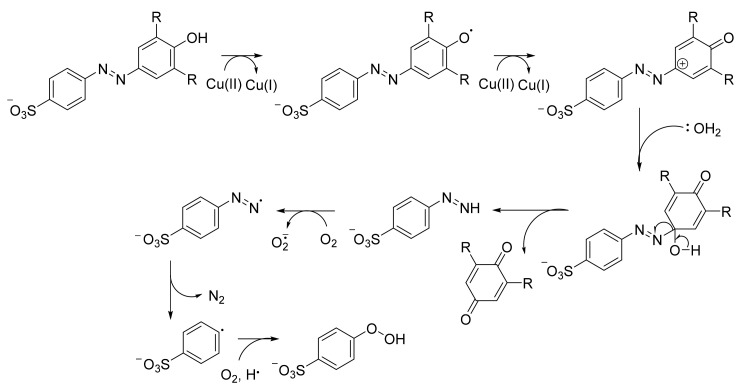
Laccases, on the other hand, are copper-containing enzymes that can oxidize a wide range of aromatic and inorganic substances [182]. The four Cu2+ ions in their active site play an important role in the oxidation of their substrate by taking four electrons from the compound, while the four Cu2+ are reduced to Cu+ [183] (Figure 3). The reduced laccase transfers the electrons to dioxygen and thereby produces water as it returns to its resting state [183] (Figure 3). Meanwhile, the oxidized substrate automatically decomposes into simple products as it has become an active cation radical [183] (Figure 3). Although some oxidized substrates can revert to the original state, ABTS or 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) can be used as a redox mediator for dye decolorization and degradation [184]. Most laccases have also been discovered from fungi such as Pichia pastoris and Trametes versicolor [185,186,187]. However, a small number of reports has shown that bacteria can exhibit phenol oxidase activity on azo dyes, as in the case of Pseudomonas desmolyticum NCIM 2112 [175].
Figure 3.
Proposed degradation pathway of azo dyes by laccases. Such activities were proposed for the ascomycete Pyricularia oryzae [111].
Although most of the enzymes mentioned are oxidative enzymes, reductive enzymes such as azoreductases are also involved in dye decolorization and degradation. In fact, azoreductases have been a subject of interest for most azo dye decolorization and degradation studies [17,19,22,75,121], thus being worthy of some more in-depth discussion.
7. An Insight to Azoreductases
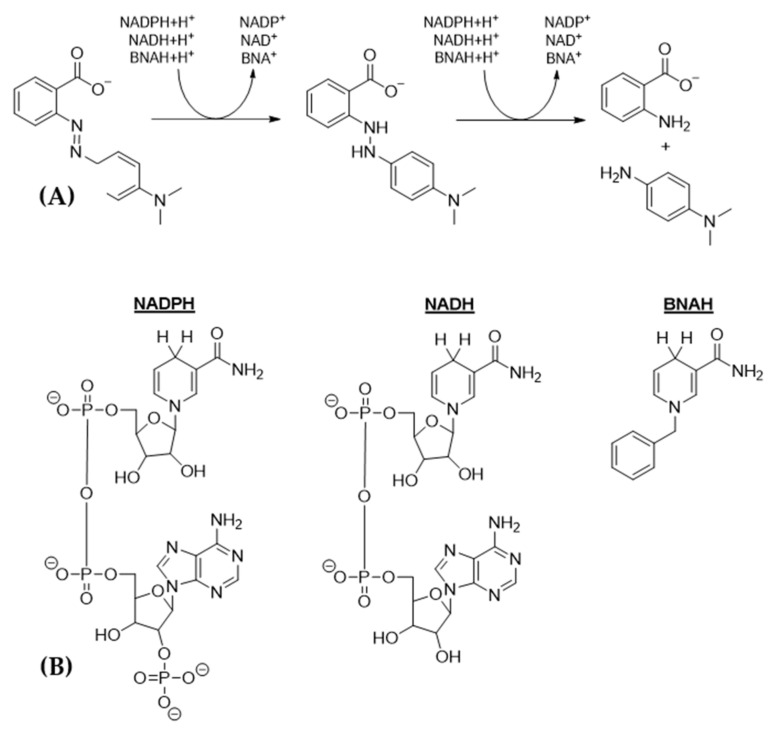
Azoreductases are enzymes that cleave azo bonds (-N = N-) present on azo dyes and therefore lead to the formation of colorless aromatic amines [116,122,153]. Most azoreductases operate via a ping-pong bi–bi mechanism (Figure 4). Earlier classifications of azoreductases were based on their prosthetic group. Azorecuctases can depend on flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD), or they can also be flavin-free [188,189,190,191]. Moreover, azoreductases can be further classified based on their preferred co-substrate—NADH, NADPH, or both. However, a recent study by Suzuki (2019) summarized that azoreductases can also be categorized based on sequences [121].
Figure 4.
(A) Reduction of the model azo dye substrate, Methyl Red, by an arbitrary azoreductase. Most azoreductases use NADPH or NADH as an electron donor to proceed with the reaction. However, some azoreductases can also use an NAD(P)H mimic, such as BNAH, as an electron donor. (B) Structure of the electron donors that can be used by azoreductases for the reduction of azo dyes.
Accounting for the sequence-based classification, clade I azoreductases have the NADPH-binding motif, GXGXXG, which was first observed on AZR from Bacillus sp. OY1-2 [164]. The azoreductases that belong to the members of this clade preferentially use NADPH and display about 52–100% sequence identities to each other [121]. Some of the known azoreductases from this group are from Geobacillus stearothermophilus, Rhodobacter sphaeroides, Bacillus subtilis ATCC 6633, and YhdA from Bacillus subtilis [117,192,193,194].
Meanwhile, clade II azoreductases do not contain the GXGXXG motif. The primary azoreductase sequences from this clade share about 30–80% identities and prefer NADH over NADPH. Some of the known members are the azoreductases from the human intestinal microflora such as Enterococcus faecalis and Enterococcus faecium [188,195]. Another member is the YvaB, which was found together with YhdA from Bacillus subtilis but lacks the conserved binding motif and in comparison, YvaB and YhdA share only about 26% sequence similarity [121].
Clade III members have about 201 amino acids and share about 25–71% sequence identities [121]. Like clade II members, they lack the NADPH-binding motif, and most have been known as flavoproteins. Some of the known members are azoreductases from Shewanella oneidensis, Rhodococcus opacus, Halomonas elongata, and Pseudomonas putida [116,190,196,197,198]. Almost all members of this clade accept both NADH and NADPH. One member can even accept the NADH mimic, 1-benzyl-1,4-dihydronicotinamide (BNAH), as an alternative electron donor. It was shown that AzoRo from Rhodococcus opacus 1CP can accept BNAH, which can allow the fast turnover of Methyl Red at pH 7 [198]. Most likely, more members of this enzyme group can accept alternative electron donors, which remains to be demonstrated.
As for clade IV azoreductases, the members do not have the GXGXXG motif but an alternative motif, GXXGXXG, at the N-terminus. Some of the known members were discovered to be flavin-free, such as the azoreductase from Xenophilus azovorans [199], Klebsiella oxytoca [200], and Kocuria indica -DP-K7 [135]. Due to the lack of flavin, the ping-pong bi–bi mechanism does not seem applicable for the azoreductases from this group. It has been stipulated that they catalyze the azo reduction via the formation of a ternary complex where the enzyme binds to NADPH and the substrate transiently [201,202].
Through various studies, it was shown that that several microorganisms possess azoreductases. However, these azoreductases behave differently, as there are no degradation patterns that can be derived even if the enzymes belong to the same clade [121]. The question remains if the azo dye consumptions are just a side activity, as the real physiological role of azoreductases is still to be unraveled as more studies on quinones and azoreductases are completed.
Moreover, azoreductases have shown significant versatility in reactivity toward other substrates such as nitroaromatics [121]. Azoreductases have also been a subject of protein engineering. One of the bottlenecks for applying azoreductases is their need of NAD(P)H as an electron donor. NAD(P)H is quite costly and renders the application of azoreductases impractical. However, recent studies have shown that azoreductases can be combined with formate dehydrogenases, which are enzymes that oxidize formate (a cheaper substrate) and donate it to NAD+ (Figure 5). Although the fusion protein exhibited only partial degradation of Brilliant Black, this still shows that these enzymes have a potential not only for azo dye degradation but also for other interesting substrates and applications [121].
Figure 5.
Reduction of Brilliant Black by a fusion protein comprised of the formate dehydrogenase (FDH) from Candida boidinii and the azoreductase (AzoRo) from Rhodococcus opacus 1CP according to an earlier reported observation of respective degradation compounds or intermediates [203].
8. Mediators and Varying Energy Sources for a More Efficient Dye Degradation
Azo dye degradation can be improved by the addition of different carbon sources, nitrogen sources, and redox mediators. Some microorganisms can use dyes as a sole carbon source. The combination of a white rot fungus and Pseudomonas sp. as a co-culture showed 100% decolorization of Direct Fast Scarlet 4BS without the addition of any carbon source [143]. This was also shown on various actinobacterial isolates and for a Parabukholderia sp., where the named organisms were enriched beforehand and isolated by using solely Methyl Red as a carbon source [136]. This was also the case for the bacterium similar to Hydrogenophaga palleronii, which was shown to grow on 4-carboxy-4′-sulfoazobenzene [204]. The bacterium was known to degrade sulfanilate and was pre-adapted to the sulfonated azo compound [204]. It was also demonstrated that the microbial community comprised of different bacterial classes can partially degrade azo dyes in the absence of an external carbon source [205], while the bacterial consortium comprised of Pseudomonas aeruginosa strain MM01, Enterobacter sp. strain MM05, and Serratia marcescens strain MM06 could use Reactive Red 120 as a sole carbon source [206]
The addition of carbon sources can also increase the rate of dye decolorization and degradation. This was shown in a study of Saranraj et al., (2018), wherein the decolorization rate of different azo dyes (Reactive Orange 16, Reactive Black B, and Reactive Yellow MR) increased for various isolates, namely Bacillus odyssey, Bacillus thuringiensis, Bacillus subtilis, Bacillus cereus, Alcaligenes sp., and Nocardiopsis alba, when sucrose was added [207]. The addition of 1% glucose also improved the degradation of Brilliant Black BN for different isolates [136].
The use of redox mediators can furthermore enhance dye decolorization. Sun et al., (2013) showed that the usage of redox mediators such as anthraquinone-2,6-disulfonate (AQDS), riboflavin, and humic acid increased the decolorization of Congo Red by 394%, 450%, and 258%, respectively [208]. Halomonas sp. GYW showed a more efficient decolorization of Acid Red B with the addition of 1,5-dichloroanthraquinone, 1,8-dichloroanthraquinone, anthraquinone, and 1,4,5,8-tetrachloroanthraquinone more than 1.5-fold [209]. Quinones promote electron transfer in different chemical and microbiological reactions [19]. Therefore, the addition of quinones enhances the electron transfer from the electron donor to the electron acceptor, which is often the azo dye. This faster transfer usually leads to an enhanced color removal rate [19,210].
9. Prospects on Azo Dye Degradation
Modern solutions to mitigate the repercussions of wastes and pollution are continuously being explored. Azo dyes, which are often used in food, textile, cosmetics, and pharmaceutical industries, pose threats and risks to health and environment. As greener solutions are being considered for the treatment of azo dyes as discussed above, the techniques that can be applied for azo dye degradation also evolve over time. It is important to look through the advancements achieved for azo dye degradation listed below.
9.1. Immobilization
Immobilization is a technique of confining enzymes or whole cells into a matrix or onto supports [211]. It is used in different industries especially for the ones that switched to greener solutions. Immobilization allows enzymes or whole cells to be reused and makes them more stable in, e.g., different temperatures or pH. This makes industrial processes more cost-effective and robust. Several supports are available that can range from natural polymers such as alginate, chitin, and sepharose, via synthetic polymers such as Amberlite resins, and to inorganic materials such as zeolites, celites, and silica [211]. There are different ways to facilitate immobilization such as through adsorption, encapsulation, covalent binding, and cross-linking [211].
In the field of azo dye degradation, several studies have already shown the possibility of immobilizing enzymes or whole cells. The study of Chen et al., (2003) exhibited the possibility of immobilizing a microbial consortium with phosphorylated polyvinyl alcohol: immobilized cell beads showed about 75% dye decolorization even with 500 mg/L concentration of Red RBN after 12 h [212]. Another microbial consortium immobilized to polyvinyl alcohol also yielded better decolorization of Direct Fast Scarlet 4BS and could be reused for more than 30 cycles without affecting the dye degradation activity [143]. Although immobilization can be detrimental to the activity of cells or enzymes, the immobilization of Lysinibacillus sp. KPB6 in calcium alginate achieved about 98% degradation of Reactive Blue-250 after 48 h as opposed to free cells, which achieved about 95% degradation within 72 h duration [213]. Meanwhile, the enzyme AzoRo, an azoreductase from Rhodococcus opacus 1CP, was immobilized to mesoporous silica which showed significant improvement on its stability, exhibiting activity even on incubation at pH 4 for 60 h and showing better storability [197]. The immobilized laccase from Cyathus bulleri showed about 90–95% decolorization of simulated dye effluents for up to 20 cycles [214]. Another immobilized laccase of Weissella viridescens LB37 on magnetic chitosan nanoparticles showed increased relative activity, which was 2-fold compared to the free enzyme counterpart, and it presented a high removal capacity for Direct Blue 15, Evans Blue, Reactive Black 5, and Acid Red 37 [215].
9.2. Bioreactors
Alongside immobilization, specialized bioreactors are often employed in different industrial processes. Bioreactors are vessels or tanks designed to hold free/immobilized whole cells or enzymes for the transformation of substrates to products. The bacterial consortium comprising Sphingomonas paucimobilis, Bacillus sp. and an unidentified filamentous bacterium was placed in a continuous stirred bed reactor and continuously fed with textile wastewater where the predicted decolorization rate was at 86% [216]. The bacterium Enterobacter aerogenes ES014 was also investigated and tested in a batch reactor wherein after treatment, the water quality of the wastewater improved [217]. The possibility of constructing bioreactors using alkalophilic and thermophilic bacterial consortia was also investigated where the color removal efficiency was about 85 to 94% after 192 h [218]. The setup also exhibited a reduction of nitrites and nitrates and that the dye mixture became non-toxic after treatment [217]. In an airlift bioreactor, it was also shown that Bjerkandera adusta OBR105 exhibited more than 90% decolorization for Acid Red 114, Acid Blue 62, Acid Black 172, and Reactive Blue 4 after 10–15 h [219]. Another demonstration of the efficiency of dye decolorization in bioreactors was the immobilization of a laccase from Ganoderma sp. KU-Alk4 in copper–alginate beads. This immobilized biocatalyst was also applied in an airlift bioreactor for dye removal. Results showed an enhanced stability of the catalyst toward different temperatures, even maintaining its normal activity at 55 °C, and it displayed a decolorization of Indigo Carmine after 14 runs without supplementation [220]. Meanwhile, a rotating disk reactor was also investigated for the treatment of water containing Direct Red-80 and Mordant Blue-9 using immobilized cells of Phanerochaete chrysosporium, and it showed more than 90% decolorization efficiencies for individual dyes after 24 h [221]. This highlights the applicability of these bioreactors for wastewater treatment.
9.3. Microbial Fuel Cells
Another emerging technology at present is the application of microbial fuel cells (MFC) for different processes. MFCs take advantage of the ability of microorganisms to convert chemical energy (usually from organic matters) to electricity. It has been shown from different studies that electricity can be generated via wastewater treatment with the simultaneous oxidation of different compounds. This technology has been adapted for azo dye decolorization studies. In the study of Liu et al., the possibility of using azo dyes as cathode oxidants was investigated in a cell designed to accept electrons from the respiration of Klebsiella pneumoniae L17 in the anode [222]. It was also shown that different dyes can affect the performance of MFCs, as Methyl Orange generated better results than Orange I and Orange II [222]. Industrial wastes can also be used to feed MFCs, as, e.g., brewery waste was used and presented the possibility of reducing Direct Red 80 (200 mg/L) as confirmed by Fourier transform infrared spectroscopy (FT-IR) [223]. In addition, microbial communities that attached to the anode of the setup revealed the presence of proteobacteria, betaproteobacteria, and Desulfovibrio [223]. Aside from azo dye decolorization, the removal of sulfides was also observed and coupled with a maximum power output of about 23.5 mW/m in a single chamber air cathode MFC setup [224]. The use of glucose as a substrate to generate electricity and to degrade Acid Navy Blue R was also demonstrated [225]. Different concentrations of dyes were tested, and 200 ppm of dye attained 10.36% Coulombic efficiency and 2236 mW/m2 of power density [225].
10. Conclusions
Azo dyes have become important in different industries, especially because color plays a huge role in consumer choices. However, with the increased usage of azo dyes, several health and environmental problems have emerged, which are caused by some of these azo dyes and its metabolites. As modern societies are striving toward greener solutions, bioremediation should be taken advantage of. Microorganisms have shown versatile performance not only in the biomedical field but also in the realms of environmental application. Microorganisms have tremendous potential still to be explored, and there are numerous enzymes from various microorganisms that should be further studied. The ability of these microorganisms to accept a broad spectrum of xenobiotics must also be also looked upon. Modern technology has displayed tremendous progress over the past decades. The field of molecular biology and biochemistry has flourished, and the -omics approach is now also being used in different applications in various industries. The combination of these developments and the area of bioremediation, especially in the field of dye degradation, is still an exciting venture to research. Ultimately, these microorganisms can pave the way for a hazard-free conversion of azo dyes and other xenobiotics.
Author Contributions
Conceptualization, A.C.R.N. and D.T.; investigation, A.C.R.N.; writing—original draft preparation, A.C.R.N.; writing—review and editing, A.C.R.N. and D.T.; visualization, A.C.R.N.; supervision, D.T.; project administration, D.T.; funding acquisition, D.T. All authors have read and agreed to the published version of the manuscript.
Funding
A.C.R.N. was supported by a pre-doctoral scholarship from Katholischer Akademischer Ausländer Dienst (KAAD). This project is supported by the Federal Ministry for Economic Affairs and Climate Action (BMWK) on the basis of a decision by the German Bundestag, grant number KK5161102AD1. The Microbial Biotechnology Team was supported by the DFG Research Training Group GRK 2341 “Microbial Substrate Conversion (MiCon).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Saxena S., Raja A.S.M. Roadmap to Sustainable Textiles and Clothing. Springer; Singapore: 2014. Natural dyes: Sources, chemistry, application and sustainability issues; pp. 37–80. [Google Scholar]
- 2.Datta S., Uddin M.A., Afreen K.S., Akter S., Bandyopadhyay A. Assessment of antimicrobial effectiveness of natural dyed fabrics. Bangladesh J. Sci. Ind. Res. 2013;48:179–184. doi: 10.3329/bjsir.v48i3.17327. [DOI] [Google Scholar]
- 3.Sarkar A.K. An evaluation of UV protection imparted by cotton fabrics dyed with natural colorants. BMC Dermatol. 2004;4:1–8. doi: 10.1186/1471-5945-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chattopadhyay S.N., Pan N.C., Roy A.K., Saxena S., Khan A. Development of natural dyed jute fabric with improved colour yield and UV protection characteristics. J. Text. Inst. 2013;104:808–818. [Google Scholar]
- 5.Prabhu K.H., Teli M.D. Eco-dyeing using Tamarindus indica L. seed coat tannin as a natural mordant for textiles with antibacterial activity. J. Saudi Chem. Soc. 2014;18:864–872. doi: 10.1016/j.jscs.2011.10.014. [DOI] [Google Scholar]
- 6.Cooksey C. Tyrian purple: The first four thousand years. Sci. Prog. 2013;96:171–186. doi: 10.3184/003685013X13680345111425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tyrian Purple, Genuine. [(accessed on 9 February 2022)]. Available online: https://www.kremer-pigmente.com/en/shop/pigments/36010-tyrian-purple-genuine.html.
- 8.Popli S., Patel U. Destruction of azo dyes by anaerobic–aerobic sequential biological treatment: A review. Int. J. Environ. Sci. Technol. 2015;12:405–420. doi: 10.1007/s13762-014-0499-x. [DOI] [Google Scholar]
- 9.Singh P.K., Singh R.L. Bio-removal of azo dyes: A review. Int. J. Appl. Sci. Biotechnol. 2017;5:108–126. doi: 10.3126/ijasbt.v5i2.16881. [DOI] [Google Scholar]
- 10.Zollinger H. Azo dyes and pigments. Colour Chem.-Synth. Prop. Appl. Org. Dyes Pigment. 1987;1st edition:92–100. [Google Scholar]
- 11.Padamavathy S. Aerobic decolorization of reactive azo dyes in presence of various cosubstrates. Chem. Biochem. Eng. Q. 2003;17:147–152. [Google Scholar]
- 12.Sandhya S., Padmavathy S., Swaminathan K., Subrahmanyam Y.V., Kaul S.N. Microaerophilic–aerobic sequential batch reactor for treatment of azo dyes containing simulated wastewater. Process Biochem. 2005;40:885–890. doi: 10.1016/j.procbio.2004.02.015. [DOI] [Google Scholar]
- 13.Sarkar S., Banerjee A., Halder U., Biswas R., Bandopadhyay R. Degradation of synthetic azo dyes of textile industry: A sustainable approach using microbial enzymes. Water Conserv. Sci. Eng. 2017;2:121–131. doi: 10.1007/s41101-017-0031-5. [DOI] [Google Scholar]
- 14.Fernandes F.H., Bustos-Obregon E., Salvadori D.M.F. Disperse Red 1 (textile dye) induces cytotoxic and genotoxic effects in mouse germ cells. Reprod. Toxicol. 2015;53:75–81. doi: 10.1016/j.reprotox.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Ayed L., Bakir K., Mansour H.B., Hammami S., Cheref A., Bakhrouf A. In vitro mutagenicity, NMR metabolite characterization of azo and triphenylmethanes dyes by adherents bacteria and the role of the “cna” adhesion gene in activated sludge. Microb. Pathog. 2017;103:29–39. doi: 10.1016/j.micpath.2016.12.016. [DOI] [PubMed] [Google Scholar]
- 16.De Vasconcelos D., Maria G., Mulinari J., Ulson de Souza A.A., De Oliveira D., De Andrade C.J. Biodegradation of azo dye-containing wastewater by activated sludge: A critical review. World J. Microbiol. Biotechnol. 2021;37:101. doi: 10.1007/s11274-021-03067-6. [DOI] [PubMed] [Google Scholar]
- 17.Banat I.M., Nigam P., Singh D., Marchant R. Microbial decolorization of textile-dyecontaining effluents: A review. Bioresour. Technol. 1996;58:217–227. doi: 10.1016/S0960-8524(96)00113-7. [DOI] [Google Scholar]
- 18.Forgacs E., Cserháti T., Oros G. Removal of synthetic dyes from wastewaters: A review. Environ. Int. 2004;30:953–971. doi: 10.1016/j.envint.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Dos Santos A.B., Cervantes F.J., Van Lier J.B. Review paper on current technologies for decolourisation of textile wastewaters: Perspectives for anaerobic biotechnology. Bioresour. Technol. 2007;98:2369–2385. doi: 10.1016/j.biortech.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Stolz A. Basic and applied aspects in the microbial degradation of azo dyes. Appl. Microbiol. Biotechnol. 2001;56:69–80. doi: 10.1007/s002530100686. [DOI] [PubMed] [Google Scholar]
- 21.Pandey A., Singh P., Iyengar L. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007;59:73–84. doi: 10.1016/j.ibiod.2006.08.006. [DOI] [Google Scholar]
- 22.Saratale R.G., Saratale G.D., Chang J.S., Govindwar S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011;42:138–157. doi: 10.1016/j.jtice.2010.06.006. [DOI] [Google Scholar]
- 23.Mota I.G.C., Neves R.A.M.D., Nascimento S.S.D.C., Maciel B.L.L., Morais A.H.D.A., Passos T.S. Artificial dyes: Health risks and the need for revision of international regulations. Food Rev. Int. 2021;27:1–16. doi: 10.1080/87559129.2021.1934694. [DOI] [Google Scholar]
- 24.Chung K.T. The significance of azoreduction in the mutagenesis and carcinogenesis of azo dyes. Mutat. Res. Rev. Genet. Toxicol. 1983;114:269–281. doi: 10.1016/0165-1110(83)90035-0. [DOI] [PubMed] [Google Scholar]
- 25.Chung K.T., Stevens S.E., Cerniglia C.E. The reduction of azo dyes by the intestinal microflora. Crit. Rev. Microbiol. 1992;18:175–190. doi: 10.3109/10408419209114557. [DOI] [PubMed] [Google Scholar]
- 26.Puvaneswari N., Muthukrishnan J., Gunasekaran P. Toxicity assessment and microbial degradation of azo dyes. Indian J. Exp. Biol. 2006;44:618–626. [PubMed] [Google Scholar]
- 27.Chung K.T., Cerniglia C.E. Mutagenicity of azo dyes: Structure-activity relationships. Mutat. Res. Rev. Genet. Toxicol. 1992;277:201–220. doi: 10.1016/0165-1110(92)90044-A. [DOI] [PubMed] [Google Scholar]
- 28.Chen H. Recent advances in azo dye degrading enzyme research. Curr. Protein Pept. Sci. 2006;7:101–111. doi: 10.2174/138920306776359786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carmen Z., Daniela S. Textile Organic Dyes-Characteristics, Polluting Effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview. Volume 3. IntechOpen; Rijeka, Croatia: 2012. pp. 55–86. [Google Scholar]
- 30.Khan S., Malik A. Environmental Deterioration and Human Health. Springer; Dordrecht, The Netherlands: 2014. Environmental and health effects of textile industry wastewater; pp. 55–71. [Google Scholar]
- 31.Chung K.T., Fulk G.E., Andrews A.W. Mutagenicity testing of some commonly used dyes. Appl. Environ. Microbiol. 1981;42:641–648. doi: 10.1128/aem.42.4.641-648.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishra V., Mishra M., Chaudhari B.P., Khanna R., Das M. Argemone oil and butter yellow induced toxicity in hepatic and extra hepatic tissues. Bioenergetics. 2014;3:111. [Google Scholar]
- 33.Tsuda S., Murakami M., Matsusaka N., Kano K., Taniguchi K., Sasaki Y.F. DNA damage induced by red food dyes orally administered to pregnant and male mice. Toxicol. Sci. 2001;61:92–99. doi: 10.1093/toxsci/61.1.92. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki Y.F., Kawaguchi S., Kamaya A., Ohshita M., Kabasawa K., Iwama K., Taniguchi K., Tsuda S. The comet assay with 8 mouse organs: Results with 39 currently used food additives. Mutat. Res. /Genet. Toxicol. Environ. Mutagenesis. 2002;519:103–119. doi: 10.1016/S1383-5718(02)00128-6. [DOI] [PubMed] [Google Scholar]
- 35.Shimada C., Kano K., Sasaki Y.F., Sato I., Tsuda S. Differential colon DNA damage induced by azo food additives between rats and mice. J. Toxicol. Sci. 2010;35:547–554. doi: 10.2131/jts.35.547. [DOI] [PubMed] [Google Scholar]
- 36.Macioszek V.K., Kononowicz A.K. The evaluation of the genotoxicity of two commonly used food colors: Quinoline Yellow (E 104) and Brilliant Black BN (E 151) Cell. Mol. Biol. Lett. 2004;9:107–122. [PubMed] [Google Scholar]
- 37.Ali M.Y., Hassan G.M., Hassan A.M.S., Mohamed Z.A., Ramadan M.F. In vivo genotoxicity assessment of sunset yellow and sodium benzoate in female rats. Drug Chem. Toxicol. 2020;43:504–513. doi: 10.1080/01480545.2018.1510416. [DOI] [PubMed] [Google Scholar]
- 38.El-Borm H.T., Badawy G.M., Hassab El-Nabi S., El-Sherif W.A., Atallah M.N. Toxicity of sunset yellow FCF and tartrazine dyes on DNA and cell cycle of liver and kidneys of the chick embryo: The alleviative effects of curcumin. Egypt. J. Zool. 2020;74:43–55. doi: 10.21608/ejz.2020.42218.1040. [DOI] [Google Scholar]
- 39.Chung K.T. Azo dyes and human health: A review. J. Environ. Sci. Health Part C. 2016;34:233–261. doi: 10.1080/10590501.2016.1236602. [DOI] [PubMed] [Google Scholar]
- 40.Zanoni T.B., Lizier T.M., Das Dores Assis M., Zanoni M.V.B., De Oliveira D.P. CYP-450 isoenzymes catalyze the generation of hazardous aromatic amines after reaction with the azo dye Sudan III. Food Chem. Toxicol. 2013;57:217–226. doi: 10.1016/j.fct.2013.03.035. [DOI] [PubMed] [Google Scholar]
- 41.Gadallah M.A.A. Phytotoxic effects of industrial and sewage waste waters on growth, chlorophyll content, transpiration rate and relative water content of potted sunflower plants. Water Air Soil Pollut. 1996;89:33–47. doi: 10.1007/BF00300420. [DOI] [Google Scholar]
- 42.Jagruti B. Evaluation of azo dye toxicity using some haematological and histopathological alterations in fish Catla catla. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2015;9:415–418. [Google Scholar]
- 43.Parrott J.L., Bartlett A.J., Balakrishnan V.K. Chronic toxicity of azo and anthracenedione dyes to embryo-larval fathead minnow. Environ. Pollut. 2016;210:40–47. doi: 10.1016/j.envpol.2015.11.037. [DOI] [PubMed] [Google Scholar]
- 44.Katsumi I., Yuichi I., Osamu N., Keisuke N., Nobuyuki I. Carcinogenicity and toxicity tests on p-phenylenediamine in F344 rats. Toxicol. Lett. 1983;16:259–269. doi: 10.1016/0378-4274(83)90186-8. [DOI] [PubMed] [Google Scholar]
- 45.Burnett C., Loehr R., Corbett J. Dominant lethal mutagenicity study on hair dyes. J. Toxicol. Environ. Health Part A Curr. Issues. 1977;2:657–662. doi: 10.1080/15287397709529467. [DOI] [PubMed] [Google Scholar]
- 46.Lin G.H., Solodar W.E. Structure—Activity relationship studies on the mutagenicity of some azo dyes in the Salmonella/microsome assay. Mutagenesis. 1988;3:311–315. doi: 10.1093/mutage/3.4.311. [DOI] [PubMed] [Google Scholar]
- 47.Watanabe M., Ishidate M., Jr., Nohmi T. Sensitive method for the detection of mutagenic nitroarenes and aromatic amines: New derivatives of Salmonella typhimurium tester strains possessing elevated O-acetyltransferase levels. Mutat. Res. Environ. Mutagenesis Relat. Subj. 1990;234:337–348. doi: 10.1016/0165-1161(90)90044-O. [DOI] [PubMed] [Google Scholar]
- 48.Sontag J.M. Carcinogenicity of substituted-benzenediamines (phenylenediamines) in rats and mice. J. Natl. Cancer Inst. 1981;66:591–602. [PubMed] [Google Scholar]
- 49.Chen S.C., Chen C.H., Chern C.L., Hsu L.S., Huang Y.C., Chung K.T., Chye S.M. P-phenylenediamine induces p53-mediated apoptosis in Mardin–Darby canine kidney cells. Toxicol. Vitr. 2006;20:801–807. doi: 10.1016/j.tiv.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Rollison D.E., Helzlsouer K.J., Pinney S.M. Personal hair dye use and cancer: A systematic literature review and evaluation of exposure assessment in studies published since 1992. J. Toxicol. Environ. Health Part B. 2006;9:413–439. doi: 10.1080/10937400600681455. [DOI] [PubMed] [Google Scholar]
- 51.Vineis P., Pirastu R. Aromatic amines and cancer. Cancer Causes Control. 1997;8:346–355. doi: 10.1023/A:1018453104303. [DOI] [PubMed] [Google Scholar]
- 52.Vandevivere P.C., Bianchi R., Verstraete W. Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1998;72:289–302. doi: 10.1002/(SICI)1097-4660(199808)72:4<289::AID-JCTB905>3.0.CO;2-#. [DOI] [Google Scholar]
- 53.Amin N.K. Removal of reactive dye from aqueous solutions by adsorption onto activated carbons prepared from sugarcane bagasse pith. Desalination. 2008;223:152–161. doi: 10.1016/j.desal.2007.01.203. [DOI] [Google Scholar]
- 54.Faria P.C.C., Órfão J.J.M., Figueiredo J.L., Pereira M.F.R. Adsorption of aromatic compounds from the biodegradation of azo dyes on activated carbon. Appl. Surf. Sci. 2008;254:3497–3503. doi: 10.1016/j.apsusc.2007.11.043. [DOI] [Google Scholar]
- 55.Velmurugan P., Rathinakumar V., Dhinakaran G. Dye removal from aqueous solution using low cost adsorbent. Int. J. Environ. Sci. 2011;1:1492–1503. [Google Scholar]
- 56.Das S., Singh S., Garg S. Agri-residual waste, wheat bran as a biosorbent for mitigation of dye pollution in industrial wastewaters. J. Basic Microbiology. 2022;62:465–479. doi: 10.1002/jobm.202100502. [DOI] [PubMed] [Google Scholar]
- 57.Paredes-Quevedo L.C., González-Caicedo C., Torres-Luna J.A., Carriazo J.G. Removal of a textile azo-dye (Basic Red 46) in water by efficient adsorption on a natural clay. Water Air Soil Pollut. 2021;232:4. doi: 10.1007/s11270-020-04968-2. [DOI] [Google Scholar]
- 58.Wang F., Li L., Iqbal J., Yang Z., Du Y. Preparation of magnetic chitosan corn straw biochar and its application in adsorption of amaranth dye in aqueous solution. Int. J. Biol. Macromolecules. 2022;199:234–242. doi: 10.1016/j.ijbiomac.2021.12.195. [DOI] [PubMed] [Google Scholar]
- 59.Golob V., Vinder A., Simonič M. Efficiency of the coagulation/flocculation method for the treatment of dyebath effluents. Dye. Pigment. 2005;67:93–97. doi: 10.1016/j.dyepig.2004.11.003. [DOI] [Google Scholar]
- 60.Sonal S., Mishra B.K. Water Pollution and Management Practices. Springer; Singapore: 2021. Role of coagulation/flocculation technology for the treatment of dye wastewater: Trend and future aspects; pp. 303–331. [Google Scholar]
- 61.Azami M., Bahram M., Nouri S., Naseri A. Central composite design for the optimization of removal of the azo dye, methyl orange, from wastewater using fenton reaction. J. Serb. Chem. Soc. 2012;77:235–246. doi: 10.2298/JSC110315165A. [DOI] [Google Scholar]
- 62.Tizaoui C., Grima N. Kinetics of the ozone oxidation of Reactive Orange 16 azo-dye in aqueous solution. Chem. Eng. J. 2011;173:463–473. doi: 10.1016/j.cej.2011.08.014. [DOI] [Google Scholar]
- 63.Garg S.K., Tripathi M. Microbial strategies for discoloration and detoxification of azo dyes from textile effluents. Res. J. Microbiol. 2017;12:1–19. doi: 10.3923/jm.2017.1.19. [DOI] [Google Scholar]
- 64.Vidali M. Bioremediation. An overview. Pure Appl. Chem. 2001;73:1163–1172. doi: 10.1351/pac200173071163. [DOI] [Google Scholar]
- 65.Dangi A.K., Sharma B., Hill R.T., Shukla P. Bioremediation through microbes: Systems biology and metabolic engineering approach. Crit. Rev. Biotechnol. 2019;39:79–98. doi: 10.1080/07388551.2018.1500997. [DOI] [PubMed] [Google Scholar]
- 66.Mrozik A., Piotrowska-Seget Z., Labuzek S. Bacterial degradation and bioremediation of polycyclic aromatic hydrocarbons. Pol. J. Environ. Stud. 2003;12:15–25. [Google Scholar]
- 67.Chaudhary P., Sahay H., Sharma R., Pandey A.K., Singh S.B., Saxena A.K., Nain L. Identification and analysis of polyaromatic hydrocarbons (PAHs)—Biodegrading bacterial strains from refinery soil of India. Environ. Monit. Assess. 2015;187:391. doi: 10.1007/s10661-015-4617-0. [DOI] [PubMed] [Google Scholar]
- 68.Nanca C.L., Neri K.D., Ngo A.C.R., Bennett R.M., Dedeles G.R. Degradation of polycyclic aromatic hydrocarbons by moderately halophilic bacteria from luzon salt beds. J. Health Pollut. 2018;8:180915. doi: 10.5696/2156-9614-8.19.180915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bennett R.M., Cordero P.R.F., Bautista G.S., Dedeles G.R. Reduction of hexavalent chromium using fungi and bacteria isolated from contaminated soil and water samples. Chem. Ecol. 2013;29:320–328. doi: 10.1080/02757540.2013.770478. [DOI] [Google Scholar]
- 70.Kang C.H., Kwon Y.J., So J.S. Bioremediation of heavy metals by using bacterial mixtures. Ecol. Eng. 2016;89:64–69. doi: 10.1016/j.ecoleng.2016.01.023. [DOI] [Google Scholar]
- 71.Paul D., Pandey G., Meier C., Roelof van der Meer J., Jain R.K. Bacterial community structure of a pesticide-contaminated site and assessment of changes induced in community structure during bioremediation. FEMS Microbiol. Ecol. 2006;57:116–127. doi: 10.1111/j.1574-6941.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- 72.Góngora-Echeverría V.R., García-Escalante R., Rojas-Herrera R., Giácoman-Vallejos G., Ponce-Caballero C. Pesticide bioremediation in liquid media using a microbial consortium and bacteria-pure strains isolated from a biomixture used in agricultural areas. Ecotoxicol. Environ. Saf. 2020;200:110734. doi: 10.1016/j.ecoenv.2020.110734. [DOI] [PubMed] [Google Scholar]
- 73.Barsing P., Tiwari A., Joshi T., Garg S. Application of a novel bacterial consortium for mineralization of sulphonated aromatic amines. Bioresour. Technol. 2011;102:765–771. doi: 10.1016/j.biortech.2010.08.098. [DOI] [PubMed] [Google Scholar]
- 74.Dissanayake M., Liyanage N., Herath C., Rathnayake S., Fernando E.Y. Mineralization of persistent azo dye pollutants by a microaerophilic tropical lake sediment mixed bacterial consortium. Environ. Adv. 2021;3:100038. doi: 10.1016/j.envadv.2021.100038. [DOI] [Google Scholar]
- 75.Khan R., Bhawana P., Fulekar M.H. Microbial decolorization and degradation of synthetic dyes: A review. Rev. Environ. Sci. Bio/Technol. 2013;12:75–97. doi: 10.1007/s11157-012-9287-6. [DOI] [Google Scholar]
- 76.Yesilada O., Birhanli E., Geckil H. Mycoremediation and Environmental Sustainability. Springer; Cham, Switzerland: 2018. Bioremediation and decolorization of textile dyes by white rot fungi and laccase enzymes; pp. 121–153. [Google Scholar]
- 77.Akansha K., Chakraborty D., Sachan S.G. Decolorization and degradation of methyl orange by Bacillus stratosphericus SCA1007. Biocatal. Agric. Biotechnol. 2019;18:101044. doi: 10.1016/j.bcab.2019.101044. [DOI] [Google Scholar]
- 78.Barathi S., Aruljothi K.N., Karthik C., Padikasan I.A. Optimization for enhanced ecofriendly decolorization and detoxification of Reactive Blue160 textile dye by Bacillus subtilis. Biotechnol. Rep. 2020;28:e00522. doi: 10.1016/j.btre.2020.e00522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang C., Luo H., Cheng W., Jiang K., Lu L., Ling L. Decolorization characteristics and mechanism of methyl orange dye by using Stenotrophomonas acidaminiphila EFS1. Int. J. Environ. Sci. Technol. 2022:1–10. doi: 10.1007/s13762-021-03846-6. [DOI] [Google Scholar]
- 80.Dhir B. Dye Biodegradation, Mechanisms and Techniques. Springer; Singapore: 2022. Degradation of dyes using filamentous fungi; pp. 51–66. [Google Scholar]
- 81.Solís M., Solís A., Pérez H.I., Manjarrez N., Flores M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012;47:1723–1748. doi: 10.1016/j.procbio.2012.08.014. [DOI] [Google Scholar]
- 82.Sen S.K., Raut S., Bandyopadhyay P., Raut S. Fungal decolouration and degradation of azo dyes: A review. Fungal Biol. Rev. 2016;30:112–133. doi: 10.1016/j.fbr.2016.06.003. [DOI] [Google Scholar]
- 83.Kyzas G.Z., Fu J., Matis K.A. The change from past to future for adsorbent materials in treatment of dyeing wastewaters. Materials. 2013;6:5131–5158. doi: 10.3390/ma6115131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fomina M., Gadd G.M. Biosorption: Current perspectives on concept, definition and application. Bioresour. Technol. 2014;160:3–14. doi: 10.1016/j.biortech.2013.12.102. [DOI] [PubMed] [Google Scholar]
- 85.Du L.N., Wang B., Li G., Wang S., Crowley D.E., Zhao Y.H. Biosorption of the metal-complex dye Acid Black 172 by live and heat-treated biomass of Pseudomonas sp. strain DY1: Kinetics and sorption mechanisms. J. Hazard. Mater. 2012;205:47–54. doi: 10.1016/j.jhazmat.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 86.Hernández-Zamora M., Cristiani-Urbina E., Martínez-Jerónimo F., Perales-Vela H.V., Ponce-Noyola T., Montes-Horcasitas M.D.C., Cañizares-Villanueva R.O. Bioremoval of the azo dye Congo Red by the microalga Chlorella vulgaris. Environ. Sci. Pollut. Res. 2015;22:10811–10823. doi: 10.1007/s11356-015-4277-1. [DOI] [PubMed] [Google Scholar]
- 87.Singh S., Pakshirajan K. Enzyme activities and decolourization of single and mixed azo dyes by the white-rot fungus Phanerochaete chrysosporium. Int. Biodeterior. Biodegrad. 2010;64:146–150. doi: 10.1016/j.ibiod.2009.11.003. [DOI] [Google Scholar]
- 88.Bhattacharya S., Das A. Mycoremediation of Congo red dye by filamentous fungi. Braz. J. Microbiol. 2011;42:1526–1536. doi: 10.1590/S1517-83822011000400040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dias A.A., Lucas M.S., Sampaio A., Peres J.A., Bezerra R.M. Biodegradation of Azo Dyes. Springer; Berlin/Heidelberg, Germany: 2010. Decolorization of azo dyes by yeasts; pp. 183–193. [Google Scholar]
- 90.Ngo A.C.R., Devanadera M.K.P., Dedeles G.R. Decolorization of selected synthetic textile dyes by yeasts from leaves and fruit peels. J. Health Pollut. 2016;6:42–55. doi: 10.5696/2156-9614-6-10.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shetty K., Krishnakumar G. Algal and cyanobacterial biomass as potential dye biodecolorizing material: A review. Biotechnol. Lett. 2020;42:2467–2488. doi: 10.1007/s10529-020-03005-w. [DOI] [PubMed] [Google Scholar]
- 92.El-Sheekh M.M., El-Shanshoury A.R., Abou-El-Souod G.W., Gharieb D.Y., El Shafay S.M. Decolorization of dyestuffs by some species of green algae and cyanobacteria and its consortium. Int. J. Environ. Sci. Technol. 2021;18:3895–3906. doi: 10.1007/s13762-020-03108-x. [DOI] [Google Scholar]
- 93.Ghodake G., Jadhav U., Tamboli D., Kagalkar A., Govindwar S. Decolorization of textile dyes and degradation of mono-azo dye amaranth by Acinetobacter calcoaceticus NCIM 2890. Indian J. Microbiol. 2011;51:501–508. doi: 10.1007/s12088-011-0131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shah M.P., Patel K.A., Nair S.S., Darji A.M. Microbial decolourization of methyl orange dye by Pseudomonas spp. OA Biotechnology. 2013;2:10. doi: 10.13172/2052-0069-2-1-497. [DOI] [Google Scholar]
- 95.Haque M.M., Haque M.A., Mosharaf M.K., Marcus P.K. Decolorization, degradation and detoxification of carcinogenic sulfonated azo dye methyl orange by newly developed biofilm consortia. Saudi J. Biol. Sci. 2021;28:793–804. doi: 10.1016/j.sjbs.2020.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fu Y., Viraraghavan T. Removal of Congo Red from an aqueous solution by fungus Aspergillus niger. Adv. Environ. Res. 2002;7:239–247. doi: 10.1016/S1093-0191(01)00123-X. [DOI] [Google Scholar]
- 97.Claus H., Faber G., König H.J.A.M. Redox-mediated decolorization of synthetic dyes by fungal laccases. Appl. Microbiol. Biotechnol. 2002;59:672–678. doi: 10.1007/s00253-002-1047-z. [DOI] [PubMed] [Google Scholar]
- 98.Fu Y., Viraraghavan T. Removal of a dye from an aqueous solution by the fungus Aspergillus niger. Water Qual. Res. J. 2000;35:95–112. doi: 10.2166/wqrj.2000.006. [DOI] [Google Scholar]
- 99.Gallagher K.A., Healy M.G., Allen S.J. Studies in Environmental Science. Volume 66. Elsevier; Amsterdam, The Netherlands: 1997. Biosorption of synthetic dye and metal ions from aqueous effluents using fungal biomass; pp. 27–50. [Google Scholar]
- 100.Brahimi-Horn M.C., Lim K.K., Liang S.L., Mou D.G. Binding of textile azo dyes by Myrothecium verrucaria. J. Ind. Microbiol. Biotechnol. 1992;10:31–36. [Google Scholar]
- 101.Jeon S.J., Lim S.J. Purification and characterization of the laccase involved in dye decolorization by the white-rot fungus Marasmius scorodonius. J. Microbiol. Biotechnol. 2017;27:1120–1127. doi: 10.4014/jmb.1701.01004. [DOI] [PubMed] [Google Scholar]
- 102.Telke A.A., Kadam A.A., Jagtap S.S., Jadhav J.P., Govindwar S.P. Biochemical characterization and potential for textile dye degradation of blue laccase from Aspergillus ochraceus NCIM-1146. Biotechnol. Bioprocess Eng. 2010;15:696–703. doi: 10.1007/s12257-009-3126-9. [DOI] [Google Scholar]
- 103.Ollikka P., Alhonmäki K., Leppänen V.M., Glumoff T., Raijola T., Suominen I. Decolorization of azo, triphenyl methane, heterocyclic, and polymeric dyes by lignin peroxidase isoenzymes from Phanerochaete chrysosporium. Appl. Environ. Microbiol. 1993;59:4010–4016. doi: 10.1128/aem.59.12.4010-4016.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shaheen R., Asgher M., Hussain F., Bhatti H.N. Immobilized lignin peroxidase from Ganoderma lucidum IBL-05 with improved dye decolorization and cytotoxicity reduction properties. Int. J. Biol. Macromol. 2017;103:57–64. doi: 10.1016/j.ijbiomac.2017.04.040. [DOI] [PubMed] [Google Scholar]
- 105.Bouacem K., Rekik H., Jaouadi N.Z., Zenati B., Kourdali S., El Hattab M., Badis A., Annane R., Bejar S., Hacene H., et al. Purification and characterization of two novel peroxidases from the dye-decolorizing fungus Bjerkandera adusta strain CX-9. Int. J. Biol. Macromol. 2018;106:636–646. doi: 10.1016/j.ijbiomac.2017.08.061. [DOI] [PubMed] [Google Scholar]
- 106.Zhang H., Zhang J., Zhang X., Geng A. Purification and characterization of a novel manganese peroxidase from white-rot fungus Cerrena unicolor BBP6 and its application in dye decolorization and denim bleaching. Process Biochem. 2018;66:222–229. doi: 10.1016/j.procbio.2017.12.011. [DOI] [Google Scholar]
- 107.Mou D.G., Lim K.K., Shen H.P. Microbial agents for decolorization of dye wastewater. Biotechnol. Adv. 1991;9:613–622. doi: 10.1016/0734-9750(91)90734-D. [DOI] [PubMed] [Google Scholar]
- 108.Polman K., Breckenridge C.R. Biomass-mediated binding and recovery of textile dyes from waste effluents. Text. Chem. Colorist. 1996;28:31–35. [Google Scholar]
- 109.Przystaś W., Zabłocka-Godlewska E., Grabińska-Sota E. Efficiency of decolorization of different dyes using fungal biomass immobilized on different solid supports. Braz. J. Microbiol. 2018;49:285–295. doi: 10.1016/j.bjm.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yu Z., Wen X. Screening and identification of yeasts for decolorizing synthetic dyes in industrial wastewater. Int. Biodeterior. Biodegrad. 2005;56:109–114. doi: 10.1016/j.ibiod.2005.05.006. [DOI] [Google Scholar]
- 111.Chivukula M., Renganathan V. Phenolic azo dye oxidation by laccase from Pyricularia oryzae. Appl. Environ. Microbiol. 1995;61:4374–4377. doi: 10.1128/aem.61.12.4374-4377.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jadhav J.P., Parshetti G.K., Kalme S.D., Govindwar S.P. Decolourization of azo dye methyl red by Saccharomyces cerevisiae MTCC 463. Chemosphere. 2007;68:394–400. doi: 10.1016/j.chemosphere.2006.12.087. [DOI] [PubMed] [Google Scholar]
- 113.Mahalakshmi S., Lakshmi D., Menaga U. Biodegradation of different concentration of dye (Congo red dye) by using green and blue green algae. Int. J. Environ. Res. 2015;9:735–744. [Google Scholar]
- 114.Abd Ellatif S., El-Sheekh M.M., Senousy H.H. Role of microalgal ligninolytic enzymes in industrial dye decolorization. Int. J. Phytoremediation. 2021;23:41–52. doi: 10.1080/15226514.2020.1789842. [DOI] [PubMed] [Google Scholar]
- 115.Acuner E., Dilek F.B. Treatment of tectilon yellow 2G by Chlorella vulgaris. Process Biochem. 2004;39:623–631. doi: 10.1016/S0032-9592(03)00138-9. [DOI] [Google Scholar]
- 116.Qi J., Schlömann M., Tischler D. Biochemical characterization of an azoreductase from Rhodococcus opacus 1CP possessing methyl red degradation ability. J. Mol. Catal. B Enzym. 2016;130:9–17. doi: 10.1016/j.molcatb.2016.04.012. [DOI] [Google Scholar]
- 117.Deller S., Sollner S., Trenker-El-Toukhy R., Jelesarov I., Gübitz G.M., Macheroux P. Characterization of a thermostable NADPH: FMN oxidoreductase from the mesophilic bacterium Bacillus subtilis. Biochemistry. 2006;45:7083–7091. doi: 10.1021/bi052478r. [DOI] [PubMed] [Google Scholar]
- 118.Ngo A.C.R., Qi J., Juric C., Bento I., Tischler D. Identification of molecular basis that underlie enzymatic specificity of AzoRo from Rhodococcus opacus 1CP: A potential NADH: Quinone oxidoreductase. Arch. Biochem. Biophys. 2022;717:109123. doi: 10.1016/j.abb.2022.109123. [DOI] [PubMed] [Google Scholar]
- 119.Rafii F., Cerniglia C.E. Reduction of azo dyes and nitroaromatic compounds by bacterial enzymes from the human intestinal tract. Environ. Health Perspect. 1995;103((Suppl. S5)):17–19. doi: 10.1289/ehp.95103s417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ryan A. Azoreductases in drug metabolism. Br. J. Pharmacol. 2017;174:2161–2173. doi: 10.1111/bph.13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suzuki H. Remarkable diversification of bacterial azoreductases: Primary sequences, structures, substrates, physiological roles, and biotechnological applications. Appl. Microbiol. Biotechnol. 2019;103:3965–3978. doi: 10.1007/s00253-019-09775-2. [DOI] [PubMed] [Google Scholar]
- 122.Misal S.A., Gawai K.R. Azoreductase: A key player of xenobiotic metabolism. Bioresour. Bioprocess. 2018;5:17. doi: 10.1186/s40643-018-0206-8. [DOI] [Google Scholar]
- 123.Shanmugam B.K., Mahadevan S. Metabolism and biotransformation of azo dye by bacterial consortium studied in a bioreaction calorimeter. Bioresour. Technol. 2015;196:500–508. doi: 10.1016/j.biortech.2015.07.108. [DOI] [PubMed] [Google Scholar]
- 124.Shanmugam B.K., Easwaran S.N., Lakra R., Deepa P.R., Mahadevan S. Metabolic pathway and role of individual species in the bacterial consortium for biodegradation of azo dye: A biocalorimetric investigation. Chemosphere. 2017;188:81–89. doi: 10.1016/j.chemosphere.2017.08.138. [DOI] [PubMed] [Google Scholar]
- 125.Anjaneya O., Souche S.Y., Santoshkumar M., Karegoudar T.B. Decolorization of sulfonated azo dye Metanil Yellow by newly isolated bacterial strains: Bacillus sp. strain AK1 and Lysinibacillus sp. strain AK2. J. Hazard. Mater. 2011;190:351–358. doi: 10.1016/j.jhazmat.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 126.Ayed L., Mahdhi A., Cheref A., Bakhrouf A. Decolorization and degradation of azo dye Methyl Red by an isolated Sphingomonas paucimobilis: Biotoxicity and metabolites characterization. Desalination. 2011;274:272–277. doi: 10.1016/j.desal.2011.02.024. [DOI] [Google Scholar]
- 127.Chen K.C., Huang W.T., Wu J.Y., Houng J.Y. Microbial decolorization of azo dyes by Proteus mirabilis. J. Ind. Microbiol. Biotechnol. 1999;23:686–690. doi: 10.1038/sj.jim.2900689. [DOI] [PubMed] [Google Scholar]
- 128.Chen K.C., Wu J.Y., Liou D.J., Hwang S.C.J. Decolorization of the textile dyes by newly isolated bacterial strains. J. Biotechnol. 2003;101:57–68. doi: 10.1016/S0168-1656(02)00303-6. [DOI] [PubMed] [Google Scholar]
- 129.Franciscon E., Grossman M.J., Paschoal J.A.R., Reyes F.G.R., Durrant L.R. Decolorization and biodegradation of reactive sulfonated azo dyes by a newly isolated Brevibacterium sp. strain VN-15. SpringerPlus. 2012;1:37. doi: 10.1186/2193-1801-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guembri M., Neifar M., Saidi M., Ferjani R., Chouchane H., Mosbah A., Cherif A., Saidi N., Ouzari H.I. Decolorization of textile azo dye Novacron Red using bacterial monoculture and consortium: Response surface methodology optimization. Water Environ. Res. 2021;93:1346–1360. doi: 10.1002/wer.1521. [DOI] [PubMed] [Google Scholar]
- 131.Ito T., Shimada Y., Suto T. Potential use of bacteria collected from human hands for textile dye decolorization. Water Resour. Ind. 2018;20:46–53. doi: 10.1016/j.wri.2018.09.001. [DOI] [Google Scholar]
- 132.Kalyani D.C., Patil P.S., Jadhav J.P., Govindwar S.P. Biodegradation of reactive textile dye Red BLI by an isolated bacterium Pseudomonas sp. SUK1. Bioresour. Technol. 2008;99:4635–4641. doi: 10.1016/j.biortech.2007.06.058. [DOI] [PubMed] [Google Scholar]
- 133.Kalyani D.C., Telke A.A., Dhanve R.S., Jadhav J.P. Ecofriendly biodegradation and detoxification of Reactive Red 2 textile dye by newly isolated Pseudomonas sp. SUK1. J. Hazard. Mater. 2009;163:735–742. doi: 10.1016/j.jhazmat.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 134.Khalid A., Arshad M., Crowley D.E. Accelerated decolorization of structurally different azo dyes by newly isolated bacterial strains. Appl. Microbiol. Biotechnol. 2008;78:361–369. doi: 10.1007/s00253-007-1302-4. [DOI] [PubMed] [Google Scholar]
- 135.Kumaran S., Ngo A.C.R., Schultes F.P.J., Tischler D. Draft genome sequence of Kocuria indica DP-K7, a methyl red degrading actinobacterium. 3 Biotech. 2020;10:175. doi: 10.1007/s13205-020-2136-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kumaran S., Ngo A.C.R., Schultes F., Saravanan V.S., Tischler D. In vitro and in silico analysis of Brilliant Black degradation by Actinobacteria and a Paraburkholderia sp. Genomics. 2022;114:110266. doi: 10.1016/j.ygeno.2022.01.003. [DOI] [PubMed] [Google Scholar]
- 137.Maniyam M.N., Ibrahim A.L., Cass A.E. Decolourization and biodegradation of azo dye methyl red by Rhodococcus strain UCC 0016. Environ. Technol. 2020;41:71–85. doi: 10.1080/09593330.2018.1491634. [DOI] [PubMed] [Google Scholar]
- 138.Park E.H., Jang M.S., Cha I.H., Choi Y.L., Cho Y.S., Kim C.H., Lee Y.C. Decolorization of a sulfonated azo dye, Congo Red, by Staphylococcus sp. EY-3. J. Microbiol. Biotechnol. 2005;15:221–225. [Google Scholar]
- 139.Parshetti G.K., Telke A.A., Kalyani D.C., Govindwar S.P. Decolorization and detoxification of sulfonated azo dye methyl orange by Kocuria rosea MTCC 1532. J. Hazard. Mater. 2010;176:503–509. doi: 10.1016/j.jhazmat.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 140.Wang H., Su J.Q., Zheng X.W., Tian Y., Xiong X.J., Zheng T.L. Bacterial decolorization and degradation of the reactive dye Reactive Red 180 by Citrobacter sp. CK3. Int. Biodeterior. Biodegrad. 2009;63:395–399. doi: 10.1016/j.ibiod.2008.11.006. [DOI] [Google Scholar]
- 141.Wang Z.W., Liang J.S., Liang Y. Decolorization of Reactive Black 5 by a newly isolated bacterium Bacillus sp. YZU1. Int. Biodeterior. Biodegrad. 2013;76:41–48. doi: 10.1016/j.ibiod.2012.06.023. [DOI] [Google Scholar]
- 142.Biju L.M., Pooshana V., Kumar P.S., Gayathri K.V., Ansar S., Govindaraju S. Treatment of textile wastewater containing mixed toxic azo dye and chromium (VI) BY haloalkaliphilic bacterial consortium. Chemosphere. 2022;287:132280. doi: 10.1016/j.chemosphere.2021.132280. [DOI] [PubMed] [Google Scholar]
- 143.Fang H., Wenrong H., Yuezhong L. Investigation of isolation and immobilization of a microbial consortium for decoloring of azo dye 4BS. Water Res. 2004;38:3596–3604. doi: 10.1016/j.watres.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 144.Khan Z., Jain K., Soni A., Madamwar D. Microaerophilic degradation of sulphonated azo dye–Reactive Red 195 by bacterial consortium AR1 through co-metabolism. Int. Biodeterior. Biodegrad. 2014;94:167–175. doi: 10.1016/j.ibiod.2014.07.002. [DOI] [Google Scholar]
- 145.Khehra M.S., Saini H.S., Sharma D.K., Chadha B.S., Chimni S.S. Comparative studies on potential of consortium and constituent pure bacterial isolates to decolorize azo dyes. Water Res. 2005;39:5135–5141. doi: 10.1016/j.watres.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 146.Kurade M.B., Waghmode T.R., Jadhav M.U., Jeon B.H., Govindwar S.P. Bacterial–yeast consortium as an effective biocatalyst for biodegradation of sulphonated azo dye Reactive Red 198. RSC Adv. 2015;5:23046–23056. doi: 10.1039/C4RA15834B. [DOI] [Google Scholar]
- 147.Lade H.S., Waghmode T.R., Kadam A.A., Govindwar S.P. Enhanced biodegradation and detoxification of disperse azo dye Rubine GFL and textile industry effluent by defined fungal-bacterial consortium. Int. Biodeterior. Biodegrad. 2012;72:94–107. doi: 10.1016/j.ibiod.2012.06.001. [DOI] [Google Scholar]
- 148.Masarbo R.S., Niranjana S.R., Monisha T.R., Nayak A.S., Karegoudar T.B. Efficient decolorization and detoxification of sulphonated azo dye Ponceau 4R by using single and mixed bacterial consortia. Biocatal. Biotransformation. 2019;37:367–376. doi: 10.1080/10242422.2019.1568414. [DOI] [Google Scholar]
- 149.Moosvi S., Kher X., Madamwar D. Isolation, characterization and decolorization of textile dyes by a mixed bacterial consortium JW-2. Dye. Pigment. 2007;74:723–729. doi: 10.1016/j.dyepig.2006.05.005. [DOI] [Google Scholar]
- 150.Yogesh P., Chitra M., Akshaya G. Assessment of biological decolorization and degradation of sulfonated di-azo dye Acid Maroon V by isolated bacterial consortium EDPA. Int. Biodeterior. Biodegrad. 2012;75:187–193. [Google Scholar]
- 151.Patil P.S., Phugare S.S., Jadhav S.B., Jadhav J.P. Communal action of microbial cultures for Red HE3B degradation. J. Hazard. Mater. 2010;181:263–270. doi: 10.1016/j.jhazmat.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 152.Phugare S.S., Kalyani D.C., Surwase S.N., Jadhav J.P. Ecofriendly degradation, decolorization and detoxification of textile effluent by a developed bacterial consortium. Ecotoxicol. Environ. Saf. 2011;74:1288–1296. doi: 10.1016/j.ecoenv.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 153.Saratale R.G., Saratale G.D., Kalyani D.C., Chang J.S., Govindwar S.P. Enhanced decolorization and biodegradation of textile azo dye Scarlet R by using developed microbial consortium-GR. Bioresour. Technol. 2009;100:2493–2500. doi: 10.1016/j.biortech.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 154.Thiruppathi K., Rangasamy K., Ramasamy M., Muthu D. Evaluation of textile dye degrading potential of ligninolytic bacterial consortia. Environ. Chall. 2021;4:100078. doi: 10.1016/j.envc.2021.100078. [DOI] [Google Scholar]
- 155.Lang W., Sirisansaneeyakul S., Martins L.O., Ngiwsara L., Sakairi N., Pathom-Aree W., Okuyama M., Mori H., Kimura A. Biodecolorization of a food azo dye by the deep sea Dermacoccus abyssi MT1. 1T strain from the Mariana Trench. J. Environ. Manag. 2014;132:155–164. doi: 10.1016/j.jenvman.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 156.Harazono K., Watanabe Y., Nakamura K. Decolorization of azo dye by the white-rot basidiomycete Phanerochaete sordida and by its manganese peroxidase. J. Biosci. Bioeng. 2003;95:455–459. doi: 10.1016/S1389-1723(03)80044-0. [DOI] [PubMed] [Google Scholar]
- 157.Urek R.O., Pazarlioglu N.K. Enhanced production of manganese peroxidase by Phanerochaete chrysosporium. Braz. Arch. Biol. Technol. 2007;50:913–920. doi: 10.1590/S1516-89132007000700001. [DOI] [Google Scholar]
- 158.Ali S.S., Al-Tohamy R., Sun J. Performance of Meyerozyma caribbica as a novel manganese peroxidase-producing yeast inhabiting wood-feeding termite gut symbionts for azo dye decolorization and detoxification. Sci. Total Environ. 2022;806:150665. doi: 10.1016/j.scitotenv.2021.150665. [DOI] [PubMed] [Google Scholar]
- 159.Ghodake G.S., Kalme S.D., Jadhav J.P., Govindwar S.P. Purification and partial characterization of lignin peroxidase from Acinetobacter calcoaceticus NCIM 2890 and its application in decolorization of textile dyes. Appl. Biochem. Biotechnol. 2009;152:6–14. doi: 10.1007/s12010-008-8258-4. [DOI] [PubMed] [Google Scholar]
- 160.Hao J., Song F., Huang F., Yang C., Zhang Z., Zheng Y., Tian X. Production of laccase by a newly isolated deuteromycete fungus Pestalotiopsis sp. and its decolorization of azo dye. J. Ind. Microbiol. Biotechnol. 2007;34:233. doi: 10.1007/s10295-006-0191-3. [DOI] [PubMed] [Google Scholar]
- 161.Santos A., Mendes S., Brissos V., Martins L.O. New dye-decolorizing peroxidases from Bacillus subtilis and Pseudomonas putida MET94: Towards biotechnological applications. Appl. Microbiol. Biotechnol. 2014;98:2053–2065. doi: 10.1007/s00253-013-5041-4. [DOI] [PubMed] [Google Scholar]
- 162.Ngo A.C.R., Conrad C., Gómez Baraibar Á., Matura A., Van Pee K.H., Tischler D. Characterization of two hydrogen peroxide resistant peroxidases from Rhodococcus opacus 1CP. Appl. Sci. 2021;11:7941. doi: 10.3390/app11177941. [DOI] [Google Scholar]
- 163.Hu T.L. Degradation of azo dye RP2B by Pseudomonas luteola. Water Sci. Technol. 1998;38:299–306. doi: 10.2166/wst.1998.0650. [DOI] [Google Scholar]
- 164.Suzuki Y., Yoda T., Ruhul A., Sugiura W. Molecular cloning and characterization of the gene coding for azoreductase from Bacillus sp. OY1-2 isolated from soil. J. Biol. Chem. 2001;276:9059–9065. doi: 10.1074/jbc.M008083200. [DOI] [PubMed] [Google Scholar]
- 165.Moutaouakkil A., Zeroual Y., Dzayri F.Z., Talbi M., Lee K., Blaghen M. Purification and partial characterization of azoreductase from Enterobacter agglomerans. Arch. Biochem. Biophys. 2003;413:139–146. doi: 10.1016/S0003-9861(03)00096-1. [DOI] [PubMed] [Google Scholar]
- 166.Leelakriangsak M., Borisut S. Characterization of the decolorizing activity of azo dyes by Bacillus subtilis azoreductase AzoR1. Songklanakarin J. Sci. Technol. 2012;34:509–516. [Google Scholar]
- 167.Gold M.H., Youngs H.L., Gelpke M.D.S. Manganese peroxidase. In: Sigel A., Sigel H., editors. Metal Ions in Biological Systems. Marcel Dekker Inc.; New York, NY, USA: 2000. pp. 559–586. [PubMed] [Google Scholar]
- 168.Hofrichter M. lignin conversion by manganese peroxidase (MnP) Enzym. Microb. Technol. 2002;30:454–466. doi: 10.1016/S0141-0229(01)00528-2. [DOI] [Google Scholar]
- 169.Husain Q. Peroxidase mediated decolorization and remediation of wastewater containing industrial dyes: A review. Rev. Environ. Sci. Bio/Technol. 2010;9:117–140. doi: 10.1007/s11157-009-9184-9. [DOI] [Google Scholar]
- 170.Heinfling A., Martinez M.J., Martínez A.T., Bergbauer M., Szewzyk U. Transformation of industrial dyes by manganese peroxidases from Bjerkandera adusta and Pleurotus eryngii in a manganese-independent reaction. Appl. Environ. Microbiol. 1998;64:2788–2793. doi: 10.1128/AEM.64.8.2788-2793.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Moreira M.T., Palma C., Mielgo I., Feijoo G., Lema J.M. In vitro degradation of a polymeric dye (Poly R-478) by manganese peroxidase. Biotechnol. Bioeng. 2001;75:362–368. doi: 10.1002/bit.10052. [DOI] [PubMed] [Google Scholar]
- 172.Verma P., Madamwar D. Decolourization of synthetic dyes by a newly isolated strain of Serratia marcescens. World J. Microbiol. Biotechnol. 2003;19:615–618. doi: 10.1023/A:1025115801331. [DOI] [Google Scholar]
- 173.Novotný Č., Svobodová K., Erbanová P., Cajthaml T., Kasinath A., Lang E., Šašek V. Ligninolytic fungi in bioremediation: Extracellular enzyme production and degradation rate. Soil Biol. Biochem. 2004;36:1545–1551. doi: 10.1016/j.soilbio.2004.07.019. [DOI] [Google Scholar]
- 174.Dawkar V.V., Jadhav U.U., Ghodake G.S., Govindwar S.P. Effect of inducers on the decolorization and biodegradation of textile azo dye Navy blue 2GL by Bacillus sp. VUS. Biodegradation. 2009;20:777–787. doi: 10.1007/s10532-009-9266-y. [DOI] [PubMed] [Google Scholar]
- 175.Kalme S.D., Parshetti G.K., Jadhav S.U., Govindwar S.P. Biodegradation of benzidine based dye Direct Blue-6 by Pseudomonas desmolyticum NCIM 2112. Bioresour. Technol. 2007;98:1405–1410. doi: 10.1016/j.biortech.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 176.Sugano Y., Muramatsu R., Ichiyanagi A., Sato T., Shoda M. DyP, a unique dye-decolorizing peroxidase, represents a novel heme peroxidase family: ASP171 replaces the distal histidine of classical peroxidases. J. Biol. Chem. 2007;282:36652–36658. doi: 10.1074/jbc.M706996200. [DOI] [PubMed] [Google Scholar]
- 177.Sugano Y., Yoshida T. DyP-type peroxidases: Recent advances and perspectives. Int. J. Mol. Sci. 2021;22:5556. doi: 10.3390/ijms22115556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hofrichter M., Ullrich R., Pecyna M.J., Liers C., Lundell T. New and classic families of secreted fungal heme peroxidases. Appl. Microbiol. Biotechnol. 2010;87:871–897. doi: 10.1007/s00253-010-2633-0. [DOI] [PubMed] [Google Scholar]
- 179.Krahe N.K., Berger R.G., Ersoy F. A DyP-type peroxidase of Pleurotus sapidus with alkene cleaving activity. Molecules. 2020;25:1536. doi: 10.3390/molecules25071536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Colpa D.I., Fraaije M.W., Van Bloois E. DyP-type peroxidases: A promising and versatile class of enzymes. J. Ind. Microbiol. Biotechnol. 2014;41:1–7. doi: 10.1007/s10295-013-1371-6. [DOI] [PubMed] [Google Scholar]
- 181.Singh R., Eltis L.D. The multihued palette of dye-decolorizing peroxidases. Arch. Biochem. Biophys. 2015;574:56–65. doi: 10.1016/j.abb.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 182.Madhavi V., Lele S.S. Laccase: Properties and applications. BioResources. 2009;4:1694–1717. [Google Scholar]
- 183.Wong Y., Yu J. Laccase-catalyzed decolorization of synthetic dyes. Water Res. 1999;33:3512–3520. doi: 10.1016/S0043-1354(99)00066-4. [DOI] [Google Scholar]
- 184.Hou H., Zhou J., Wang J., Du C., Yan B. Enhancement of laccase production by Pleurotus ostreatus and its use for the decolorization of anthraquinone dye. Process Biochem. 2004;39:1415–1419. doi: 10.1016/S0032-9592(03)00267-X. [DOI] [Google Scholar]
- 185.Li J.F., Hong Y.Z., Xiao Y.Z., Xu Y.H., Fang W. High production of laccase B from Trametes sp. in Pichia pastoris. World J. Microbiol. Biotechnol. 2007;23:741–745. doi: 10.1007/s11274-006-9286-2. [DOI] [Google Scholar]
- 186.Lu L., Zhao M., Liang S.C., Zhao L.Y., Li D.B., Zhang B.B. Production and synthetic dyes decolourization capacity of a recombinant laccase from Pichia pastoris. J. Appl. Microbiol. 2009;107:1149–1156. doi: 10.1111/j.1365-2672.2009.04291.x. [DOI] [PubMed] [Google Scholar]
- 187.Zheng M., Chi Y., Yi H., Shao S. Decolorization of Alizarin Red and other synthetic dyes by a recombinant laccase from Pichia pastoris. Biotechnol. Lett. 2014;36:39–45. doi: 10.1007/s10529-013-1323-2. [DOI] [PubMed] [Google Scholar]
- 188.Chen H., Wang R.F., Cerniglia C.E. Molecular cloning, overexpression, purification, and characterization of an aerobic FMN-dependent azoreductase from Enterococcus faecalis. Protein Expr. Purif. 2004;34:302–310. doi: 10.1016/j.pep.2003.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bürger S., Stolz A. Characterisation of the flavin-free oxygen-tolerant azoreductase from Xenophilus azovorans KF46F in comparison to flavin-containing azoreductases. Appl. Microbiol. Biotechnol. 2010;87:2067–2076. doi: 10.1007/s00253-010-2669-1. [DOI] [PubMed] [Google Scholar]
- 190.Mendes S., Pereira L., Batista C., Martins L.O. Molecular determinants of azo reduction activity in the strain Pseudomonas putida MET94. Appl. Microbiol. Biotechnol. 2011;92:393–405. doi: 10.1007/s00253-011-3366-4. [DOI] [PubMed] [Google Scholar]
- 191.Ooi T., Ogata D., Matsumoto K.I., Nakamura G., Yu J., Yao M., Kitamura M., Taguchi S. Flavin-binding of azoreductase: Direct evidences for dual-binding property of apo-azoreductase with FMN and FAD. J. Mol. Catal. B Enzym. 2012;74:204–208. doi: 10.1016/j.molcatb.2011.10.006. [DOI] [Google Scholar]
- 192.Matsumoto K.I., Mukai Y., Ogata D., Shozui F., Nduko J.M., Taguchi S., Ooi T. Characterization of thermostable FMN-dependent NADH azoreductase from the moderate thermophile Geobacillus stearothermophilus. Appl. Microbiol. Biotechnol. 2010;86:1431–1438. doi: 10.1007/s00253-009-2351-7. [DOI] [PubMed] [Google Scholar]
- 193.Bin Y., Jiti Z., Jing W., Cuihong D., Hongman H., Zhiyong S., Yongming B. Expression and characteristics of the gene encoding azoreductase from Rhodobacter sphaeroides AS1. 1737. FEMS Microbiol. Lett. 2004;236:129–136. doi: 10.1111/j.1574-6968.2004.tb09638.x. [DOI] [PubMed] [Google Scholar]
- 194.Sugiura W., Yoda T., Matsuba T., Tanaka Y., Suzuki Y. Expression and characterization of the genes encoding azoreductases from Bacillus subtilis and Geobacillus stearothermophilus. Biosci. Biotechnol. Biochem. 2006;70:1655–1665. doi: 10.1271/bbb.60014. [DOI] [PubMed] [Google Scholar]
- 195.Macwana S.R., Punj S., Cooper J., Schwenk E., John G.H. Identification and isolation of an azoreductase from Enterococcus faecium. Curr. Issues Mol. Biol. 2009;12:43–48. [PubMed] [Google Scholar]
- 196.Mugerfeld I., Law B.A., Wickham G.S., Thompson D.K. A putative azoreductase gene is involved in the Shewanella oneidensis response to heavy metal stress. Appl. Microbiol. Biotechnol. 2009;82:1131–1141. doi: 10.1007/s00253-009-1911-1. [DOI] [PubMed] [Google Scholar]
- 197.Qi J., Paul C.E., Hollmann F., Tischler D. Changing the electron donor improves azoreductase dye degrading activity at neutral pH. Enzym. Microb. Technol. 2017;100:17–19. doi: 10.1016/j.enzmictec.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 198.Eslami M., Amoozegar M.A., Asad S. Isolation, cloning and characterization of an azoreductase from the halophilic bacterium Halomonas elongata. Int. J. Biol. Macromol. 2016;85:111–116. doi: 10.1016/j.ijbiomac.2015.12.065. [DOI] [PubMed] [Google Scholar]
- 199.Blümel S., Busse H.J., Stolz A., Kämpfer P. Xenophilus azovorans gen. nov., sp. nov., a soil bacterium that is able to degrade azo dyes of the Orange II type. Int. J. Syst. Evol. Microbiol. 2001;51:1831–1837. doi: 10.1099/00207713-51-5-1831. [DOI] [PubMed] [Google Scholar]
- 200.Hua J.Q., Yu L. Cloning and characterization of a Flavin-free oxygen-insensitive azoreductase from Klebsiella oxytoca GS-4-08. Biotechnol. Lett. 2019;41:371–378. doi: 10.1007/s10529-019-02647-9. [DOI] [PubMed] [Google Scholar]
- 201.Kim M.H., Kim Y., Park H.J., Lee J.S., Kwak S.N., Jung W.H., Lee S.G., Kim D., Lee Y.C., Oh T.K. Structural insight into bioremediation of triphenylmethane dyes by Citrobacter sp. triphenylmethane reductase. J. Biol. Chem. 2008;283:31981–31990. doi: 10.1074/jbc.M804092200. [DOI] [PubMed] [Google Scholar]
- 202.Chen H., Feng J., Kweon O., Xu H., Cerniglia C.E. Identification and molecular characterization of a novel flavin-free NADPH preferred azoreductase encoded by azoB in Pigmentiphaga kullae K24. BMC Biochem. 2010;11:13. doi: 10.1186/1471-2091-11-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ngo A.C.R., Josef Schultes F.P., Maier A., Hadewig S.N.H., Tischler D. Improving biocatalytic properties of an azoreductase via the N-terminal fusion of formate dehydrogenase. ChemBioChem. 2022;23:e202100643. doi: 10.1002/cbic.202100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.BluȠmel S., Contzen M., Lutz M., Stolz A., Knackmuss H.J. Isolation of a bacterial strain with the ability to utilize the sulfonated azo compound 4-carboxy-4′-sulfoazobenzene as the sole source of carbon and energy. Appl. Environ. Microbiol. 1998;64:2315–2317. doi: 10.1128/AEM.64.6.2315-2317.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tan L., Ning S., Xia H., Sun J. Aerobic decolorization and mineralization of azo dyes by a microbial community in the absence of an external carbon source. Int. Biodeterior. Biodegrad. 2013;85:210–216. doi: 10.1016/j.ibiod.2013.02.018. [DOI] [Google Scholar]
- 206.Manogaran M., Yasid N.A., Othman A.R., Gunasekaran B., Halmi M.I.E., Shukor M.Y.A. Biodecolourisation of Reactive Red 120 as a sole carbon source by a bacterial consortium—Toxicity assessment and statistical optimisation. Int. J. Environ. Res. Public Health. 2021;18:2424. doi: 10.3390/ijerph18052424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Saranraj P., Sivasakthi S., Jayaprakash A. Studies on the effect of carbon and nitrogen sources for the decolourization of reactive textile dyes by bacterial isolates. World Appl. Sci. J. 2018;36:767–773. [Google Scholar]
- 208.Sun J., Li W., Li Y., Hu Y., Zhang Y. Redox mediator enhanced simultaneous decolorization of azo dye and bioelectricity generation in air-cathode microbial fuel cell. Bioresour. Technol. 2013;142:407–414. doi: 10.1016/j.biortech.2013.05.039. [DOI] [PubMed] [Google Scholar]
- 209.Guo J., Liu H., Qu J., Lian J., Zhao L., Jefferson W., Yang J. The structure activity relationship of non-dissolved redox mediators during azo dye bio-decolorization processes. Bioresour. Technol. 2012;112:350–354. doi: 10.1016/j.biortech.2012.02.106. [DOI] [PubMed] [Google Scholar]
- 210.Walker R., Ryan A.J. Some molecular parameters influencing rate of reduction of azo compounds by intestinal microflora. Xenobiotica. 1971;1:483–486. doi: 10.3109/00498257109041513. [DOI] [PubMed] [Google Scholar]
- 211.Datta S., Christena L.R., Rajaram Y.R.S. Enzyme immobilization: An overview on techniques and support materials. 3 Biotech. 2013;3:1–9. doi: 10.1007/s13205-012-0071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Chen K.C., Wu J.Y., Huang C.C., Liang Y.M., Hwang S.C.J. Decolorization of azo dye using PVA-immobilized microorganisms. J. Biotechnol. 2003;101:241–252. doi: 10.1016/S0168-1656(02)00362-0. [DOI] [PubMed] [Google Scholar]
- 213.Pandey K., Saha P., Rao K.B. A study on the utility of immobilized cells of indigenous bacteria for biodegradation of reactive azo dyes. Prep. Biochem. Biotechnol. 2020;50:317–329. doi: 10.1080/10826068.2019.1692219. [DOI] [PubMed] [Google Scholar]
- 214.Chhabra M., Mishra S., Sreekrishnan T.R. Mediator-assisted decolorization and detoxification of textile dyes/dye mixture by Cyathus bulleri laccase. Appl. Biochem. Biotechnol. 2008;151:587–598. doi: 10.1007/s12010-008-8234-z. [DOI] [PubMed] [Google Scholar]
- 215.Nadaroglu H., Mosber G., Gungor A.A., Adıguzel G., Adiguzel A. Biodegradation of some azo dyes from wastewater with laccase from Weissella viridescens LB37 immobilized on magnetic chitosan nanoparticles. J. Water Process Eng. 2019;31:100866. doi: 10.1016/j.jwpe.2019.100866. [DOI] [Google Scholar]
- 216.Ayed L., Achour S., Bakhrouf A. Application of the mixture design to decolourise effluent textile wastewater using continuous stirred bed reactor. Water Sa. 2011;37:21–26. doi: 10.4314/wsa.v37i1.64102. [DOI] [Google Scholar]
- 217.Al-Ansari M.M., Li Z., Masood A., Rajaselvam J. Decolourization of azo dye using a batch bioreactor by an indigenous bacterium Enterobacter aerogenes ES014 from the waste water dye effluent and toxicity analysis. Environ. Res. 2022;205:112189. doi: 10.1016/j.envres.2021.112189. [DOI] [PubMed] [Google Scholar]
- 218.Iqbal A., Ali N., Shang Z.H., Malik N.H., Rehman M.M.U., Sajjad W., Rehman M.L.U., Khan S. Decolorization and toxicity evaluation of simulated textile effluent via natural microbial consortia in attached growth reactors. Environ. Technol. Innov. 2022;26:102284. doi: 10.1016/j.eti.2022.102284. [DOI] [Google Scholar]
- 219.Sodaneath H., Lee J.I., Yang S.O., Jung H., Ryu H.W., Cho K.S. Decolorization of textile dyes in an air-lift bioreactor inoculated with Bjerkandera adusta OBR105. J. Environ. Sci. Health Part A. 2017;52:1099–1111. doi: 10.1080/10934529.2017.1340753. [DOI] [PubMed] [Google Scholar]
- 220.Teerapatsakul C., Parra R., Keshavarz T., Chitradon L. Repeated batch for dye degradation in an airlift bioreactor by laccase entrapped in copper alginate. Int. Biodeterior. Biodegrad. 2017;120:52–57. doi: 10.1016/j.ibiod.2017.02.001. [DOI] [Google Scholar]
- 221.Pakshirajan K., Singh S. Decolorization of synthetic wastewater containing azo dyes in a batch-operated rotating biological contactor reactor with the immobilized fungus Phanerochaete chrysosporium. Ind. Eng. Chem. Res. 2010;49:7484–7487. doi: 10.1021/ie1007079. [DOI] [Google Scholar]
- 222.Liu L., Li F.B., Feng C.H., Li X.Z. Microbial fuel cell with an azo-dye-feeding cathode. Appl. Microbiol. Biotechnol. 2009;85:175–183. doi: 10.1007/s00253-009-2147-9. [DOI] [PubMed] [Google Scholar]
- 223.Miran W., Nawaz M., Kadam A., Shin S., Heo J., Jang J., Lee D.S. Microbial community structure in a dual chamber microbial fuel cell fed with brewery waste for azo dye degradation and electricity generation. Environ. Sci. Pollut. Res. 2015;22:13477–13485. doi: 10.1007/s11356-015-4582-8. [DOI] [PubMed] [Google Scholar]
- 224.Dai Q., Zhang S., Liu H., Huang J., Li L. Sulfide-mediated azo dye degradation and microbial community analysis in a single-chamber air cathode microbial fuel cell. Bioelectrochemistry. 2020;131:107349. doi: 10.1016/j.bioelechem.2019.107349. [DOI] [PubMed] [Google Scholar]
- 225.Khan M.D., Abdulateif H., Ismail I.M., Sabir S., Khan M.Z. Bioelectricity generation and bioremediation of an azo-dye in a microbial fuel cell coupled activated sludge process. PLoS ONE. 2015;10:e0138448. doi: 10.1371/journal.pone.0138448. [DOI] [PMC free article] [PubMed] [Google Scholar]