Abstract
Moxifloxacin activity against Mycobacterium avium complex (MAC) was evaluated in vitro against 25 strains. The MIC was determined to range from 0.125 to 2.0 μg/ml. In addition, U937 macrophage monolayers infected with MAC strain 101 (serovar 1) were treated with moxifloxacin (0.25 to 8 μg/ml) daily, and the number of intracellular bacteria was quantitated after 4 days. Moxifloxacin showed inhibitory activity at 0.5 μg/ml and higher. To assess the activity of moxifloxacin containing regimens in vivo, we infected C57BL bg+/bg+ mice with 3 × 107 MAC strain 101 bacteria intravenously. One week later treatment was begun with the following: (i) moxifloxacin (50 mg/kg/day or 100 mg/kg/day), ethambutol (100 mg/kg/day), or a combination of moxifloxacin and ethambutol; or (ii) moxifloxacin (100 mg/kg/day), azithromycin (200 mg/kg/day), or rifabutin (40 mg/kg/day) as oral monotherapy; or (iii) all permutations of two-drug therapy or all three drugs in combination. All groups contained at least 14 animals, and the control group received the drug vehicle. After 4 weeks, quantitative blood cultures were obtained and the number of bacteria in liver and spleen was quantitated. Moxifloxacin, ethambutol, and azithromycin were active as single agents in liver, spleen, and blood. Rifabutin showed inhibitory activity only in the blood. Two-drug combinations containing azithromycin were no more active than azithromycin alone. Similarly, the three-drug combination was not more active than azithromycin alone in the spleen. Rifabutin did not add to the activity of any other single agent or two-drug combination. Moxifloxacin at both concentrations in combination with ethambutol was significantly more active than each drug alone.
Infection caused by organisms of the Mycobacterium avium complex (MAC) is common in AIDS patients with fewer than 50 CD4+ T cells per cm3 of blood (11, 12). In this population, M. avium has been associated with bacteremia and disseminated disease (11, 12). M. avium is a well-recognized cause of chronic lung infection in some immunocompetent patients (7, 14).
One of the characteristics of M. avium is its resistance to many antimicrobials, including the most common conventional antituberculosis agents (10). New macrolides such as azithromycin and clarithromycin are active in humans but resistance to clarithromycin and consequently to azithromycin has been reported after a short course of monotherapy (6). In addition, once AIDS patients undergoing either prophylactic or therapeutic treatment develop infection with a macrolide-resistant strain, the management of their infection becomes a challenge for the clinicians because of the lack of alternative effective regimens.
Moxifloxacin is a new 8-methoxyquinolone with a structure similar to that of BAY y 3118 (the difference between them is that moxifloxacin has a methoxy instead of Cl− at the 8 position), a compound that has been shown to be active against M. avium in an experimental model of infection (2). It has broad-spectrum activity against gram-positive, gram-negative, and anaerobic bacteria (8). Another attractive property of this compound is that it has a half-life that allows once-a-day dosing in humans (24).
A few studies have demonstrated the activity of moxifloxacin against Mycobacterium tuberculosis in vitro and in vivo (15, 19). However, M. tuberculosis, in contrast to M. avium, is susceptible in vitro to a number of quinolone agents. In our experience, no quinolone except BAY y 3118 has shown significant activity against M. avium in the beige mouse model (2).
In this study we evaluated the activity of moxifloxacin alone and in combination with azithromycin, rifabutin, or ethambutol.
MATERIALS AND METHODS
Mycobacteria.
The M. avium strains used in this study (100 to 105, 107 to 109, 110, 111, 113, 116, 117, 128, 500 to 508, 511 to 513, JJL, and JWL) were isolated from the blood of AIDS patients with disseminated MAC disease (each strain was isolated from a different patient). Each isolate was identified as M. avium using a commercially available DNA probe (Gen-Probe, Inc., San Diego, Calif.). MAC 101 Clari-R is a clarithromycin-resistant strain isolated from mice (5). MAC strains 510 to 513, JJL, and JWL are clarithromycin-resistant strains isolated from patients. MAC 101 is a virulent strain in the mouse test system that causes reproducible levels of infection and mortality in beige mice (1). MAC organisms were cultured in Middlebrook 7H10 medium (Difco Laboratories, Detroit, Mich.) supplemented with oleic acid, albumin, dextrose, and catalase (OADC; Difco) for 10 days at 37°C. Only transparent colony types were used in the studies. For the macrophage assays and mice studies, colonies were harvested and suspended in Hank's buffered salt solution (HBSS) to concentrations of 4 × 108 and 3 × 108 CFU/ml, respectively, by comparison with a McFarland no. 1 turbidity standard; samples were then plated onto 7H10 agar to confirm the concentration of the inoculum.
Prior to the infection of macrophages, the suspension was vortex agitated for 2 min and passed through a 23-gauge needle five times to disperse clumps. Microscopic observation confirmed the dispersion of the inoculum.
Antimicrobials.
Azithromycin was a gift from Pfizer (Groton, N.J.). Rifabutin was provided by Pharmacia and Upjohn (Kalamazoo, Mich.), and moxifloxacin was provided by Bayer Corporation (West Haven, Conn.). Ethambutol was purchased from Sigma Chemical Co. (St. Louis, Mo.).
In vitro susceptibility testing.
MICs were determined using a radiometric broth macrodilution method and the T100 method of data analysis and by the broth microdilution method (13). The inoculum for susceptibility testing was prepared by placing 5 to 10 colonies from a 7H11 agar plate into 7H9 broth and was tested directly or frozen at −70°C. The inoculum was adjusted to approximately 5 × 104 CFU/ml by comparison with a McFarland no. 1 turbidity standard. Isolates that clumped and could not be easily dispersed were shaken with glass beads. Controls included the inoculum undiluted without drug added (no drug control), the inoculum diluted to a 1 to 100 ratio (99% control), and the inoculum diluted to a 1 to 1,000 ratio (99.9% control). In addition, one vial was inoculated with a suspension of mycobacteria, which were boiled for 5 min prior to the inoculation in order to monitor the non-growth-related release of carbon dioxide in the BACTEC system. The period of observation and end points were determined by daily monitoring of the control and test cultures, but a period of 7 days was sufficient for most isolates. MAC strain 101 was tested against amikacin to control for overall performance.
Macrophage test system.
The source of macrophages was the human monocyte cell line U937 cultured in RPMI 1640 medium (pH 7.2) (Gibco, Chicago, Ill.) supplemented with 5% fetal bovine serum (Sigma Chemical Co.) and 2 mM l-glutamine. The assays were performed as previously described (2). Briefly, cells were grown to a density of 5 × 108 cells per ml and then centrifuged, washed, and resuspended in supplemented RPMI 1640 medium. The concentration of cells was adjusted to 106 cells per ml, and 1 ml of the cell suspension was added to each well of a 24-well tissue culture plate (Costar, Cambridge, Mass.). Monolayers were treated with 1 μg of phorbol myristate acetate per ml for 24 h to stimulate maturation of the monocytes. The monolayers were infected with MAC strain 101 at the ratio of 10 bacteria to 1 cell as described previously (2). Following the establishment of the baseline level of infection, the treatment with moxifloxacin (ranging from 0.25 to 8 μg/ml) was initiated daily for 4 days. Inhibitory activity was considered when the number of CFU/ml in the treated group was smaller than the number of CFU/ml in the untreated control at the same time point but greater than the number of CFU/ml in monolayers before treatment was initiated (time 0). Bactericidal activity was considered when treatment decreased the number of CFU/ml below the bacterial number prior to treatment (time 0).
Animal test system.
The potential therapeutic efficacy of moxifloxacin was evaluated by using the beige mouse test system as previously described (M. A. Bertram, C. B. Inderlied, S. Yadegar, P. Kolonoski, J. K. Yamada, and L. S. Young, Letter, J. Infect. Dis. 154:194–195, 1986). This system employs 8- to 10-week-old female C57BL/6 bg+/bg+ mice (Jackson Laboratories, Bar Harbor, Maine). Briefly, each mouse was infected through the tail vein with 3 × 107 CFU in 100 μl of MAC strain 101; after 7 days, treatment was initiated with antibiotic (moxifloxacin, 50 or 100 mg/kg/day; rifabutin, 40 mg/kg/day; ethambutol, 100 mg/kg/day; azithromycin, 200 mg/kg/day) daily for 4 weeks. The concentration of moxifloxacin used was chosen based on previous studies (15, 19) and information about the pharmacokinetics of the drug in mice (22, 23). The drugs were administered by gavage. Mice were harvested 48 h after the end of therapy to prevent a carryover effect of the drug. An evaluation of the results by plating different dilutions did not suggest an effect from drug present in the lysate. A control group of mice was infected but received a drug vehicle in place of those antibiotics. An additional group of mice was sacrificed 7 days after infection in order to establish the level of infection in the liver, spleen, and blood before initiation of therapy. A total of 14 or more mice were used for each of the control and experimental groups. At the termination of therapy the livers and spleens of control and treated mice were aseptically removed, weighed, and then homogenized in 5 ml of 7H9 broth (Difco) with a tissue homogenizer. The blood was collected and inoculated in BACTEC bottles as previously described (2). The tissue suspensions were then serially diluted in 7H9 broth and plated onto 7H11 agar plates supplemented with oleic acid, albumin, dextrose, and catalase for the quantitation of viable bacteria. The numbers of bacteria per milliliter of blood both before and after treatment were compared. The results represent the difference in CFU/ml.
Statistical analysis.
The differences between results for untreated control and experimental groups in macrophage experiments at identical time points were determined by the Mann-Whitney nonparametric test. The statistical significance of the differences between the number of organisms recovered from deep organs of mice was evaluated by analysis of variance. Differences between the results for experimental groups and control groups, as well as among experimental groups, are represented as means ± standard deviations. The difference was considered statistically significant if P values were <0.05.
RESULTS
Activity of moxifloxacin in vitro.
The inhibitory concentration of moxifloxacin against 25 macrolide-susceptible strains of M. avium ranged from 0.125 to 2 μg/ml (mode MIC equals 0.5 μg/ml), while the MIC against 5 macrolide-resistant strains of M. avium ranged from 0.25 to 2 μg/ml.
Effect of moxifloxacin against intracellular M. avium in macrophages.
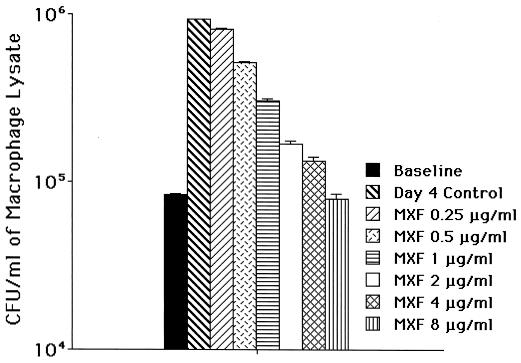
As shown in Fig. 1, after 4 days of treatment 0.5 μg of moxifloxacin/ml and greater concentrations were associated with significant inhibition of the intracellular bacterial growth. Nonetheless, in this model, moxifloxacin up to 8 μg/ml/day had only bacteriostatic activity.
FIG. 1.
Activity of moxifloxacin against the MAC 101 strain using the macrophage system, as described in Materials and Methods. Infected U937 macrophages were treated with moxifloxacin (at different concentrations) for 4 days. The experiment was repeated six times. The data represent the mean ± the standard deviation. Concentrations of ≥0.5 μg/ml to ≤1.0 μg/ml had a P value of 0.04 compared with the untreated control. For treatment with 2 μg/ml the P value was 0.03, and for the differences between 4 and 8 μg/ml and the control the P value was 0.01. Both 4 and 8 μg/ml of moxifloxacin were bactericidal in this system.
Efficacy of moxifloxacin and the combination of moxifloxacin and ethambutol in vivo.
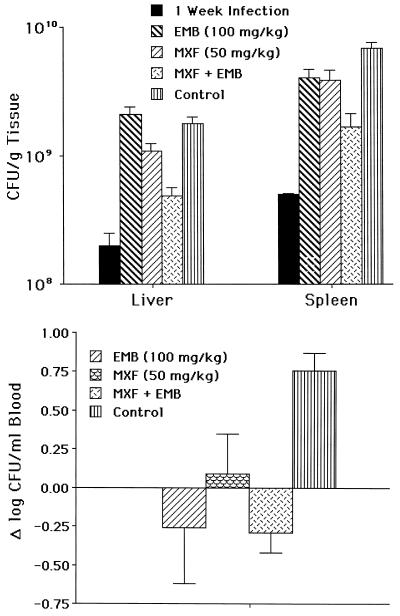
The efficacy of moxifloxacin against M. avium in vivo was first examined at the concentration of 50 mg/kg. At 50 mg/kg/day, moxifloxacin was inhibitory for the growth of bacteria in both spleen and liver. The association of moxifloxacin (50 mg/kg/day) with ethambutol (100 mg/kg/day) resulted in a significant increase in the antimicrobial effect compared to either drug alone (Fig. 2), although it was bacteriostatic as well.
FIG. 2.
(A) Activity of moxifloxacin (MXF) alone at 50 mg/kg in liver and spleen or in combination with ethambutol (EMB) at 100 mg/kg. Fourteen mice were used per group per time point. P was <0.05 when moxifloxacin at 50 mg/kg was compared to the control at the same time point in both liver and spleen. P was <0.05 when moxifloxacin plus ethambutol in both liver and spleen was compared with moxifloxacin or ethambutol alone. The combination was bacteriostatic. (B) Blood. Moxifloxacin activity was statistically better than the control (P < 0.05) but not superior to ethambutol.
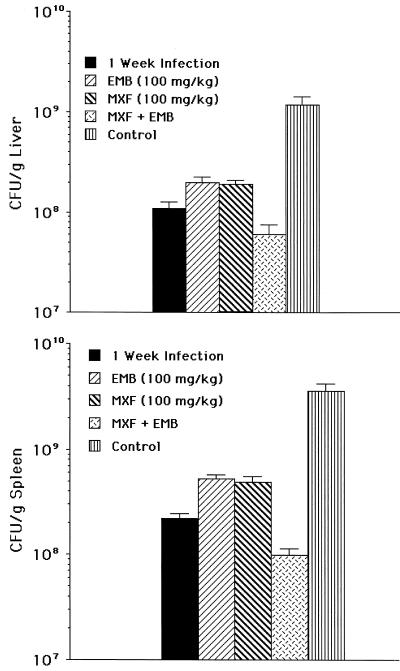
We then investigated whether moxifloxacin given at 100 mg/kg/day had more potent anti-M. avium activity than at 50 mg/kg/day. As shown in Fig. 3, moxifloxacin at 100 mg/kg/day was significantly more effective than at 50 mg/kg/day (P < 0.05). Treatment of infected mice resulted in a bacteriostatic effect, i.e., 90 to 95% inhibition of mycobacterial growth in liver and spleen compared to the untreated control at the same time point (bacteriostatic activity).
FIG. 3.
Effect of treatment of disseminated M. avium infection with moxifloxacin (MXF) (100 mg/kg) alone or in combination with ethambutol (EMB). (A) Liver. (B) Spleen. Moxifloxacin at 100 mg/kg, compared to the control at the same time point, was bacteriostatic (P < 0.05). The P value was <0.05 for the comparison between the combination moxifloxacin-ethambutol and the control at the same time point and for the comparison between the combination moxifloxacin-ethambutol and either moxifloxacin or ethambutol alone. The effect was bactericidal (P < 0.05 compared with the 1-week control).
In addition, the association of moxifloxacin (100 mg/kg/day) with ethambutol (100 mg/kg/day) had a modest bactericidal effect in both spleen and liver (Fig. 3) and was significantly more effective in inhibiting M. avium growth in the liver and spleen than treatment with either drug by itself. Of note was the observation that ethambutol had more pronounced activity in the second set of experiments in the first set of experiments. The difference in efficacy between the activity of ethambutol seen in Fig. 1 and Fig. 2 can possibly be explained by the smaller number of mice (n, 14) used in the experiment shown in Fig. 1 compared to 30 mice per time point per group in the experiments shown in Fig. 2.
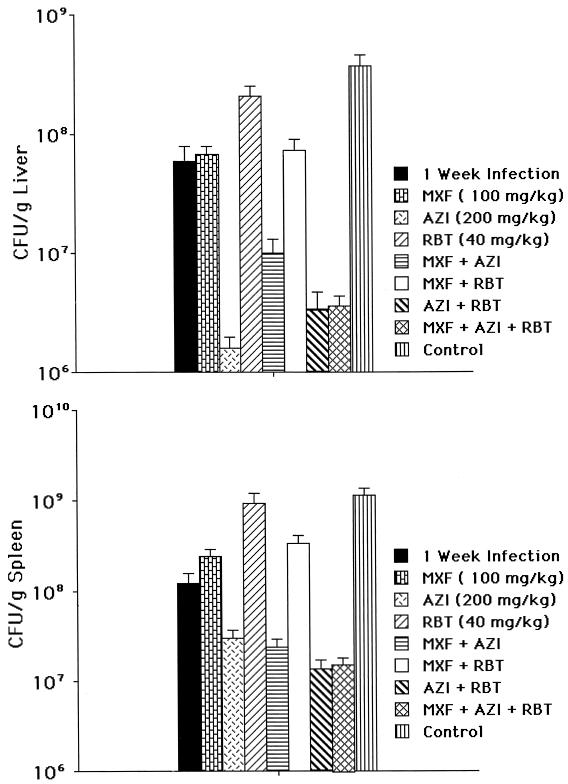
Effect of therapy with moxifloxacin combined with azithromycin and rifabutin.
Because azithromycin and rifabutin are used clinically for therapy for disseminated M. avium in AIDS patients, we sought to investigate the effect of moxifloxacin in combination with either azithromycin or rifabutin as well as the efficacy of the three drugs together. Moxifloxacin was administered at a dose of 100 mg/kg, azithromycin at 200 mg/kg, and rifabutin at 40 mg/kg. The result shown in Fig. 4A and B is that treatment with either moxifloxacin or azithromycin alone resulted in a significant decrease in the bacterial load in spleen and liver compared with the control. A combination of azithromycin and moxifloxacin was not superior to azithromycin alone. Similarly, the combination of moxifloxacin and rifabutin was not more efficacious than moxifloxacin alone. The combination of three drugs was not more effective than azithromycin, although it may improve the ability to combat resistance.
FIG. 4.
Effect of therapy for disseminated M. avium infection with moxifloxacin (MXF) alone and in combination with rifabutin (RBT) and/or azithromycin (AZI). (A) Liver. (B) Spleen. Thirty mice were used per group and per time point. P was <0.05 for all of the following comparisons: between moxifloxacin or azithromycin and the control; between the combination moxifloxacin-azithromycin and moxifloxacin and between the combination azithromycin-rifabutin and rifabutin alone; between the combinations moxifloxacin-azithromycin, moxifloxacin-rifabutin, azithromycin-rifabutin, and moxifloxacin-azithromycin-rifabutin and the untreated control at the same time point; between the combination azithromycin-moxifloxacin and moxifloxacin alone or azithromycin alone; between the combination azithromycin-rifabutin and rifabutin alone or azithromycin alone; and between the combination moxifloxacin-rifabutin and rifabutin alone or moxifloxacin alone. In the liver, the combination moxifloxacin-azithromycin was significantly (P < 0.05) less active than azithromycin alone. The combination of the three drugs was not more active than azithromycin alone (P > 0.05 for all comparisons), nor were the combinations azithromycin-moxifloxacin and azithromycin-rifabutin.
DISCUSSION
Moxifloxacin is active against M. avium in vitro, in cultured macrophages, and in the beige mouse test system. Not surprisingly, moxifloxacin was less active against M. avium than M. tuberculosis (15, 19), as has been demonstrated with every quinolone evaluated against both mycobacteria thus far (2, 16–18). As a single agent, moxifloxacin was bacteriostatic in liver, spleen, and blood. Moxifloxacin is superior to BAY y 3118, the first quinolone shown to have significant anti-M. avium activity in beige mice (2). Thus, the substitution of a halogen at the C-8 position by a methoxy group not only abrogates the phototoxicity associated with BAY y 3118 administration but also increases its anti-M. avium activity. Additionally, moxifloxacin concentrates intracellularly, achieving greater levels than ofloxacin and levofloxacin and an intracellular concentration similar to that of sparfloxacin (20).
Extrapolation of animal experimental data to humans is challenging. The doses of moxifloxacin employed in our study are larger than those used in human therapeutic trials thus far for respiratory infections, but rodents “turn over” drugs much faster than humans and the calculated correction factor of 8- to 10-fold is often used in projecting a human dose on successful rodent studies (9). Nonetheless, larger doses of moxifloxacin have been used to treat patients with respiratory disease.
Despite moxifloxacin's anti-M. avium activity, it did not enhance the antimycobacterial effect of azithromycin. The combination with ethambutol, however, was significantly more effective than either drug alone. In contrast, combining moxifloxacin with rifabutin did not improve the anti-M. avium activity. These results suggest that moxifloxacin and ethambutol should be further evaluated as a nonmacrolide regimen, since the combination was bactericidal against M. avium.
Moxifloxacin, in a manner similar to BAY y 3118, was bacteriostatic in vitro for M. avium (data not shown). The observation that moxifloxacin in combination with ethambutol was bactericidal in vivo is an interesting finding in which two compounds that are bacteriostatic by themselves become bactericidal when administered together. One possible explanation that has been suggested in the past is that ethambutol would affect the mycobacterial cell wall, increasing the permeability as a consequence and thus facilitating the uptake of a second drug, in this case moxifloxacin (4). This suggested effect of ethambutol has not been seen in our experience with M. avium in beige mice (12, 21), and the current finding represents the first time that an antimicrobial has its activity significantly increased when combined with ethambutol (Fig. 3).
Although M. avium as a significant opportunistic pathogen in AIDS patients has declined in importance as a result of improved antiretroviral therapy, some cases due to macrolide-resistant organisms still pose a major therapeutic challenge. We continue to seek novel molecular entities with in vivo activity against the bacterium. Moxifloxacin and mefloquine (3) are two of the more promising agents that do not share cross-resistance with macrolides.
ACKNOWLEDGMENTS
This work was supported in part by a grant from Bayer Corporation and in part by contract no. NO1-A1-25140 from the National Institute of Allergy and Infectious Diseases.
We thank Karen Allen for preparing the manuscript.
REFERENCES
- 1.Bermudez L E, Inderlied C B, Kolonoski P, Petrofsky M, Young L S. Clarithromycin, dapsone, and a combination of both used to treat or prevent disseminated Mycobacterium avium infection in beige mice. Antimicrob Agents Chemother. 1994;38:2717–2721. doi: 10.1128/aac.38.12.2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bermudez L E, Inderlied C B, Kolonoski P, Wu M, Barbara-Burnham L, Young L S. Activities of BAY Y 3118, levofloxacin, and ofloxacin alone or in combination with ethambutol against Mycobacterium avium complex in vitro, in human macrophages, and in beige mice. Antimicrob Agents Chemother. 1996;40:546–551. doi: 10.1128/aac.40.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bermudez L E, Kolonoski P, Wu M, Aralar P A, Inderlied C B, Young L S. Mefloquine is active in vitro and in vivo against Mycobacterium avium complex. Antimicrob Agents Chemother. 1999;43:1870–1874. doi: 10.1128/aac.43.8.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bermudez L E, Kolonoski P, Young L S, Inderlied C B. Activity of KRM 1648 alone or in combination with ethambutol or clarithromycin against Mycobacterium avium in beige mouse model of disseminated infection. Antimicrob Agents Chemother. 1994;38:1844–1848. doi: 10.1128/aac.38.8.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bermudez L E, Petrofsky M, Kolonoski P, Young L S. Emergence of Mycobacterium avium populations resistant to macrolides during experimental chemotherapy. Antimicrob Agents Chemother. 1998;42:180–183. doi: 10.1128/aac.42.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaisson R E, Benson C A, Dube M P, Heifets L B, Korvick J A, Elkin S, Smith T, Craft J C, Sattler F R. Clarithromycin therapy for bacteremic Mycobacterium avium complex disease. A randomized, double-blind, dose-ranging study in patients with AIDS. AIDS Clinical Trials Group Protocol 157 Study Team. Ann Intern Med. 1994;121:905–911. doi: 10.7326/0003-4819-121-12-199412150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Contreras M A, Cheung O T, Sanders D E, Goldstein R S. Pulmonary infection with nontuberculous mycobacteria. Am Rev Respir Dis. 1988;137:149–152. doi: 10.1164/ajrccm/137.1.149. [DOI] [PubMed] [Google Scholar]
- 8.Fass R J. In vitro activity of Bay 12-8039, a new 8-methoxyquinolone. Antimicrob Agents Chemother. 1997;41:1818–1824. doi: 10.1128/aac.41.8.1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freireich E J, Gehan E A, Rall D P, Schmidt L H, Skipper H E. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey and man. Cancer Chemother Rep. 1996;50:219–244. [PubMed] [Google Scholar]
- 10.Hawkins C C, Gold J W, Whimbey E, Kiehn T E, Brannon P, Cammarata R, Brown A E, Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986;105:184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- 11.Horsburgh C R., Jr Mycobacterium avium complex infection in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:1332–1338. doi: 10.1056/NEJM199105093241906. [DOI] [PubMed] [Google Scholar]
- 12.Inderlied C B, Kemper C A, Bermudez L E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993;6:266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inderlied C B, Young L S, Yamada J K. Determination of in vitro susceptibility of Mycobacterium avium complex isolates to antimycobacterial agents by various methods. Antimicrob Agents Chemother. 1987;31:1697–1702. doi: 10.1128/aac.31.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iseman M D, Buschman D L, Ackerson L M. Pectus excavatum and scoliosis. Thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis. 1991;144:914–916. doi: 10.1164/ajrccm/144.4.914. [DOI] [PubMed] [Google Scholar]
- 15.Ji B, Lounis N, Maslo C, Truffot-Pernot C, Bonnafous P, Grosset J. In vitro and in vivo activities of moxifloxacin and clinafloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1998;42:2066–2069. doi: 10.1128/aac.42.8.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ji B, Lounis N, Truffot-Pernot C, Grosset J. Effectiveness of various antimicrobial agents against Mycobacterium avium complex in the beige mouse model. Antimicrob Agents Chemother. 1994;38:2521–2529. doi: 10.1128/aac.38.11.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji B, Lounis N, Truffot-Pernot C, Grosset J. In vitro and in vivo activities of levofloxacin against Mycobacterium tuberculosis. Antimicrob Agents Chemother. 1995;39:1341–1344. doi: 10.1128/aac.39.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji B, Truffot-Pernot C, Grosset J. In vitro and in vivo activities of sparfloxacin (AT-4140) against Mycobacterium tuberculosis. Tubercle. 1991;72:181–186. doi: 10.1016/0041-3879(91)90004-c. [DOI] [PubMed] [Google Scholar]
- 19.Miyazaki E, Miyazaki M, Chen J M, Chaisson R E, Bishai W R. Moxifloxacin (BAY12–8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob Agents Chemother. 1999;43:85–89. doi: 10.1128/aac.43.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascual A, Garcia I, Ballesta S, Perea E J. Uptake and intracellular activity of moxifloxacin in human neutrophils and tissue-cultured epithelial cells. Antimicrob Agents Chemother. 1999;43:12–15. doi: 10.1128/aac.43.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rastogi N, Labrousse V, Bryskier A. Intracellular activities of roxithromycin used alone and in association with other drugs against Mycobacterium avium complex in human macrophages. Antimicrob Agents Chemother. 1995;39:976–978. doi: 10.1128/aac.39.4.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siefert H M, Domdey-Bette A, Henninger K, Hucke F, Kohlsdorfer C, Stass H H. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: a comparison in humans and other mammalian species. J Antimicrob Chemother. 1999;43(Suppl. B):69–76. doi: 10.1093/jac/43.suppl_2.69. [DOI] [PubMed] [Google Scholar]
- 23.Siefert H M, Kohlsdorfer C, Steinke W, Witt A. Pharmacokinetics of the 8-methoxyquinolone, moxifloxacin: tissue distribution in male rats. J Antimicrob Chemother. 1999;43(Suppl. B):61–67. doi: 10.1093/jac/43.suppl_2.61. [DOI] [PubMed] [Google Scholar]
- 24.Stass H, Dalhoff A, Kubitza D, Schuhly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]