Abstract
Women with adverse pregnancy outcomes (APOs) later experience excess hypertension and cardiovascular disease, but how the events are linked is unknown. Examination of the placenta may provide clues to vascular impairments after delivery. Maternal vascular malperfusion lesions (MVM) were abstracted from clinical reports, validated, and characterized using clinical guidelines and severity score. A total of 492 women (170 with MVM, 322 without MVM) participated in a study visit 8–10 years after delivery to assess blood pressure (BP), cardiometabolic factors and sublingual microvascular features using sidestream dark field imaging. Covariates included age, race, APOs (preeclampsia, small for gestational age, preterm birth), and health behaviors. Women with vs. without MVM had a distinct sublingual microvascular profile comprised of a) lower microvascular density [−410 micrometers/mm2, p=0.015), b) higher red blood cell filling as a marker of perfusion (2 percent, p=0.004) and c) smaller perfused boundary region (−0.07 μm, p=0.025) as a measure of glycocalyx integrity, adjusted for covariates including APOs. Women with MVM also had higher adjusted diastolic BP (+2.6 mmHg, p=0.021), total and LDL-cholesterol (+11.2 mg/dl, p=0.016; +8.7 mg/dl, p=0.031). MVM associations with subsequent cardiovascular measures did not vary by type of APO, except among women with preterm births where BP was higher only among those with MVM. Results were similar when evaluated as MVM severity. A decade after delivery, women with placental vascular lesions had an adverse cardiovascular profile comprised of microvascular rarefaction, higher BP and more atherogenic lipids. Placental histopathology may reveal a woman’s early trajectory toward subsequent vascular disease.
Keywords: pregnancy, placenta, blood pressure, microvascular rarefaction, lipids
Women with a history of adverse pregnancy outcomes, such as preeclampsia and delivery of preterm or small-for-gestational age infants, are at significantly increased risk of hypertension and cardiovascular disease later in life.1 Although there is evidence of cardiac and vascular mechanisms linking preeclampsia to short- and long-term cardiovascular abnormalities including cardiac remodeling, inflammation, oxidative stress and endothelial dysfunction,2–4 we considered that the placental vasculature may provide clues regarding the pathophysiology that connects adverse pregnancy outcomes to later maternal health. Indeed, a shared finding in a portion of preeclampsia and other adverse pregnancy outcomes is the failure of maternal decidual adaptation, with subsequent inability to perfuse the placenta sufficiently for the support of fetal growth and development.5 This decidual vasculopathy includes insufficient remodeling of the spiral arteries into highly dilated conduits feeding the placenta and related spiral artery pathologies. Decidual vasculopathy and concomitant hypoxia/reperfusion lesions in the placenta are collectively termed maternal vascular malperfusion (MVM).5
While causes of MVM are not known, one possibility is a maternal predisposition to microvascular dysfunction, which is unmasked by the pregnancy and apparent in the placenta. Thus, pregnancy acts like a stress test, similar to a treadmill test for occult heart disease. Regardless of its causes, MVM can result in a poorly perfused placenta, angiogenic imbalance, systemic vasoconstriction and circulating placenta-derived microparticles which themselves may induce or exacerbate maternal systemic vessel injury, perhaps via oxidative stress damage.6–10 Thus, this initial damage may extend both spatially outside the placenta and temporally beyond the pregnancy event. For example, MVM is a risk factor for adverse pregnancy outcomes in subsequent pregnancies, even in women without prior complications.11 Most research has focused on MVM as a marker of pregnancy outcomes and fetal health. Only recently has the placenta emerged as an accelerated life course model of maternal health, supported by emerging evidence that MVM may be a novel risk marker for vascular dysfunction later in life.12–16 This may extend to microvascular impairments, as preeclampsia predicts capillary rarefaction in the finger nail 17 and skin,18–20 reduced venular diameters in conjunctiva,17 increased microvascular reactivity in skin blood flow,21, 22 impaired coronary microvascular function,23 and sublingual small vessel impairments.11, 20, 24–26 This is relevant, as microvascular disease is a more common feature of CVD in women than in men.27, 28
We tested the hypothesis that women with placenta MVM would have an adverse blood pressure, cardiometabolic, and microvascular profile 8 to 10 years after pregnancy. We also considered that this profile may be detected in women with and without adverse pregnancy outcomes, including preeclampsia, preterm birth, and delivery of small-for-gestational age infants which are all conditions known to be related to MVM.
Methods:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
We enrolled women in 2016–2019 with deliveries in 2008–2009 identified from the Steve N. Caritis Magee Obstetric Maternal and Infant (MOMI) database, a clinical registry of women with detailed pregnancy data abstracted from medical records. About 45% of deliveries (n=4,048) had a placental pathology evaluation at the time of delivery, and women with these births were the source population for the study.29 Of the 3,947 women who were eligible (alive, non-pregnant and without chronic hypertension or diabetes prior to the index pregnancy), 1070 declined to participate, 2379 were unable to be contacted, and 498 enrolled. Participation was monitored to reflect the prevalence of MVM in our source population. Enrolled women were, on average, slightly older, more likely to be of African American race/ethnicity and less likely to smoke compared to those who refused or were unable to be contacted. There were no differences in these groups according to rates of adverse pregnancy outcomes (Table S1). Enrolled women attended a cardiovascular screening visit and comprised the study population. The study was approved by the University of Pittsburgh IRB and women provided written informed consent.
Delivery characteristics were abstracted from hospital records including gestational age (based mainly on prenatal ultrasounds), maternal age at delivery, race/ethnicity, pre-pregnancy BMI and smoking status. Preeclampsia was adjudicated via chart review as de novo blood pressure elevations after 20 weeks gestation accompanied by evidence of end organ impairment such as proteinuria.30 Preterm delivery was defined as delivery before 37 completed weeks gestation. Small-for-gestational age (SGA) was defined as birthweight for gestational age lower than the 10th percentile based on race-specific nomograms.31
Presence of placental lesions was abstracted from clinical pathology reports and categorized as MVM using definitions in place at the time of our study. MVM was defined as presence of any of these lesions; we also considered severity by using a weighted score from most to least severe or absent (range, 9–0; vasculopathy>infarct>advanced villous maturation>perivillous or intervillous fibrin deposition) and summed. Diagnostic criteria32–35 and severity scoring for these features are summarized in Table S2 and presented in Figure 1. Since the launch of our study the Amsterdam consensus statement has added low placental weight to this diagnosis, but our analysis revealed this feature alone is unrelated to maternal cardiometabolic health after delivery so we did not include it.36 37 For births delivered 2008–2009 two perinatal pathologists prepared all evaluations following a standardized protocol using a uniform reporting approach and identical diagnostic criteria. Placental slides for enrolled women were re-evaluated (n=459 were available to be retrieved) by study pathologist (WTP) who was blinded to all clinical information except gestational age. There was substantial agreement between the blinded review and the clinical report of MVM (kappa=0.78) and our analysis utilized the clinical pathology report of MVM as this allowed all enrolled women to be included and our blinded review demonstrated these clinical pathology reports were reliable.
Figure 1.

Representative placental lesions. (A) Normal villous morphology for 31 weeks gestation. (B) Advanced villous maturation. Chorionic villi are markedly smaller than normal 31-week villi, and syncytial knots are much more frequent. (C) Distal villous hypoplasia. Long, narrow villi with limited branching are apparent. Cross sections reveal tiny round villi, while the villi on longitudinal sections are long and thin with minimal branching. (D) Villous infarction. Nearly the entirety of the lower left quadrant of this image is occupied by an old villous infarction. The devitalized villi have lost their nuclear basophilia, and the ghost outlines of the infarcted villi have collapsed together. (E) Decidual vasculopathy with fibrinoid necrosis. The smooth muscle wall of this spiral artery has been replaced by dense, waxy, darkly eosinophilic fibrinoid material. (F) Decidual vasculopathy with mural hypertrophy. The smooth muscle wall of this spiral artery from the extraplacental membranes shows marked thickening, with multiple additional layers of smooth muscle and significant narrowing of the vascular lumen.
Blood pressure was measured during the study visit following a standardized research protocol. After five minutes of rest, trained research staff took three measurements separated by one minute using a validated automated device (Microlife A6 PC / BP 3GUI-8X) and appropriately sized cuff based on measured arm circumference. The average systolic and diastolic blood pressure were calculated. BMI was calculated using measured height (via a stadiometer) and weight (Tanita scale TBF-300A) obtained at the study visit after removing shoes, socks and excessive clothing. Cardiometabolic markers were quantified in fasting serum at the UPMC Core laboratory following standardized protocols. Briefly, Beckman Coulter AU 5800 system procedures were utilized to measure high sensitivity CRP (run precision of <5% CV, and total precision <10% CV), Glucose, HDL, LDL, and Triglycerides (all CV <3%). Hemoglobin A1C was quantified with The Tosoh Automated Glycohemoglobin Analyzer HLC-723G8 (<2% CV) and insulin was measured using the Siemens immulite (IMMULITE® 2000) method (total CV 4.1%−7.3%).
Sublingual microvascular functional density, perfusion, and endothelial glycocalyx barrier integrity (depth) were imaged (Figure 2) using a handheld sidestream dark field video microscope (MicroVision; Wallingford PA) and GlycoCheck software (Microvascular Health Solutions Inc., Salt Lake City, Utah) to obtain and analyze video clips of red blood cell (RBC) flow.38, 39 Briefly, perfused vessels in the 5–25 μm diameter range were identified in 10 μm segments. Functional microvascular density was determined by the total length of vascular segments perfused with RBCs per area of tissue visualized, expressed as total length in micrometers per mm2 of area of tissue (μm/mm2).38 Red blood cell (RBC) filling was calculated as the percentage of time individual vascular segments were occupied by RBCs, reported as the average across all detected microvascular segments, and vessel diameter was estimated as the median RBC column width determined by the software. The glycocalyx on the luminal surface of endothelial cells is comprised of a negatively charged carbohydrate-rich layer, which contributes to both vascular homeostasis as well as vascular function and signaling.40 The perfused boundary region was the portion of the glycocalyx permeable to RBC, with increases reflective of impaired glycocalyx barrier integrity.41–43
Figure 2.
Sublingual microvasculature using sidestream dark field imaging (SDF). (A) Image from the SDF camera. The black contrast reveals the RBC-perfused lumen of the vessels. (B) Identification of sublingual vessels (red), in 10 um segments passing quality control (green lines) or invalid segments (yellow lines). Total valid segment length/total area examined is vessel functional density. Red blood cell filling percentage is calculated as the percentage of time individual vascular segments were occupied by RBCs (perfusion). The vessel diameter is estimated as the median RBC column width. The glycocalyx at the interface of the endothelial wall and RBCs helps to regulate vascular function. The perfused boundary region represents the portion of the glycocalyx permeable to RBCs. An increase in perfused boundary region indicates reduced glycocalyx integrity. In the sublingual vascular bed, dysfunction can be reductions in density, diameter, perfusion, and/or glycocalyx integrity.
Years of education completed, current smoking, and medications used for blood pressure control were self-reported at the study visit 8–10 years after pregnancy. Women also reported sedentary time via a single item from the Global Physical Activity Questionnaire44, 45 and moderate/vigorous activity using the Paffenberger Physical Activity Questionnaire.46, 47 Sodium intake was assessed using the validated Sodium Screener developed by Block(©NutritionQuest 2011).48
Analysis:
Descriptive statistics, such as means and standard deviations for continuous variables and counts and percentages for discrete variables, were first used to describe the distributions of variables in the study population. The two-sample t-test was used to compare continuous variables between women with MWM and those without MWM. For continuous variables whose distribution was skewed, the median and inter-quartile range was used to describe their distribution and the Wilcoxon rank-sum test was used to compare their distribution between women with and without MWM. The Pearson’s chi-square test was used to compare discrete variables between groups. Multiple linear regression models were used to assess whether the cardiovascular markers differed between women with and without MWM after adjusting for race, age, follow-up time, pre-pregnancy obesity, sodium, preeclampsia, small for gestational age birth, preterm delivery, and anti-hypertensive medication use. Models were replicated using MVM severity as a continuous predictor. Potential interactions between MWM and APOs were assessed by likelihood ratio tests that compared a full model with the interaction terms and the sub-model without the corresponding interaction terms. Any comparison or test with p-value less than 0.05 was regarded as statistically significant.
Results:
There were 170 women enrolled with prior MVM in the placenta, and 322 women with no MVM. Those with MVM tended to be heavier prior to pregnancy (BMI 27.6 [SD 7.1] vs. 26.6 [6.4], p=0.09) and, as expected, the MVM-affected pregnancy was more likely to have resulted in an adverse pregnancy outcome compared to women without MVM (p<0.001; Table 1). For this study, women with MVM placentas were more likely to be evaluated a year sooner post-delivery than women without MVM (8.5 [0.7] years vs. 9.5 [0.8], p<0.001), and they reported a lower average sodium intake (3077 [1259] mg/day vs. 3310 [1186], p=0.044). There were no significant differences in other assessed lifestyle factors (daily sedentary time or physical activity below recommendations) or subsequent medical or pregnancy conditions (parity, adverse outcomes in other pregnancies, blood pressure medication use or type II diabetes).
Table 1.
Maternal characteristics at pregnancy and at follow up 8–10 years later according to presence of maternal vascular malperfusion lesions in the placenta during pregnancy ()
| No MVM | MVM | P-Value | |
|---|---|---|---|
| Maternal characteristics | (n=322) | (n=170) | |
|
| |||
| Pregnancy | |||
| Age, years | 28.3 (6.1) | 28.8 (6.0) | 0.395 |
| Black race/ethnicity (n (%)) | 114 (35.4) | 63 (37.1) | 0.244 |
| Pre-pregnancy BMI, kg/m2 | 26.6 (6.4) | 27.6 (7.1) | 0.094 |
| Smoking during pregnancy (n (%)) | 58 (18.0) | 32 (18.8) | 0.825 |
| Nulliparous (n (%)) | 184 (57.1) | 92 (54.1) | 0.52 |
| Adverse pregnancy outcome (n (%)) | 110 (34.2) | 94 (55.3) | < 0.001 |
| Preterm birth < 37 weeks’ (n (%)) | 59 (18.3) | 47 (27.7) | 0.017 |
| Preeclampsia (n (%)) | 36 (11.2) | 39 (22.9) | 0.001 |
| Small-for-gestational-age (n (%)) | 31 (9.6) | 41 (24.1) | < 0.001 |
| Gestational hypertension (n (%)) | 22 (6.8) | 14 (8.2) | 0.570 |
| Gestational diabetes (n (%)) | 27 (8.4) | 12 (7.1) | 0.605 |
| Placental Lesion (n (%)) | |||
| Decidual vasculopathy | 0 (0.0) | 33 (19.4) | |
| Infarct | 0 (0.0) | 41 (24.1) | |
| Advanced villous maturation | 0 (0.0) | 38 (22.4) | |
| Fibrin (intervillous or perivillous) | 0 (0.0) | 58 (34.1) | |
| None | 322 (100.0) | 0 (0.0) | |
| Follow-up 8–10 years after pregnancy | |||
| Follow-up, years | 9.5 (0.8) | 8.5 (0.7) | < 0.001 |
| High school education or less (n (%)) | 75 (23.3) | 42 (24.9) | 0.714 |
| BMI, kg/m*m | 29.9 (7.8) | 30.6 (7.7) | 0.330 |
| Waist circumference, inches | 38 (7.1) | 38.4 (6.3) | 0.574 |
| Blood pressure, mmHg | 116/75 | 118/78 | 0.027 |
| Blood pressure medication (n (%)) | 30 (9.4) | 14 (8.2) | 0.682 |
| hsCRP, mg/dL (median, IQR)* | 0.2 (0.08, 0.52) | 0.18 (0.08, 0.42) | 0.599 |
| Glucose, mg/dL* | 88 (83, 95) | 89 (82, 98) | 0.398 |
| LDL, mg/dL | 101 (32) | 110 (37.2) | 0.010 |
| VLDL, mg/dL* | 16 (11, 23) | 15 (11, 23) | 0.590 |
| Cholesterol, mg/dL | 174 (39) | 184 (44) | 0.025 |
| Triglyceride, mg/dL* | 79 (57, 117) | 77 (55, 117) | 0.613 |
| HDL, mg/dL | 55.1 (14.4) | 54.7 (14.1) | 0.755 |
| Insulin, uIU/mL* | 10.1 (20) | 11 (23.1) | 0.441 |
| HOMA-IR* | 1.3 (0.6, 2.7) | 1.2 (0.4, 2.5) | 0.668 |
| Sodium intake, mg/day | 3310 (1186) | 3077 (1259) | 0.044 |
| Physical Activity below recommendations (n (%)) | 281 (87.6) | 147 (86.5) | 0.147 |
| Sedentary time, hours/day | 6.1 (3.5) | 6 (3.8) | 0.886 |
| Type II diabetes | 17 (5.5) | 13 (8.1) | 0.275 |
| Parity | 2.7 (1.6) | 2.7 (1.5) | 0.851 |
| Preterm birth < 37 weeks’ in other births (n (%)) | 58 (18.0) | 24 (14.1) | 0.270 |
| Preeclampsia in other births (n (%)) | 26 (8.1) | 13 (7.7) | 0.867 |
| Small-for-gestational-age in in other births (n (%)) | 50 (15.5) | 27 (15.9) | 0.918 |
| Microvascular features | |||
| Vessel density, μm /mm2* | 3930 (3110, 4690) | 3610 (2920, 4850) | 0.326 |
| Vessel diameter, μm* | 9.0 (8.3, 9.6) | 8.7 (8.2, 9.4) | 0.087 |
| Red blood cell filling (perfusion), percent* | 71 (0.68, 0.74) | 73 (0.69, 0.76) | 0.006 |
| Perfused boundary region (glycocalyx integrity), μm* | 2.07 (1.94, 2.23) | 2.03 (1.83, 2.20) | 0.018 |
MVM, Maternal vascular malperfusion lesions; Missing values: pre-pregnancy BMI, n=10; Education, n=2; BMI or waist circumference, n=8; blood draw, n=19; microvascular features, n=78;
Wilcoxon rank sum p-value; data reported as mean +/−SD and median (IQR) for normally and non-normally distributed continuous data, respectively, and n (%) for categorical data
Evaluated 8 to 10 years after delivery, measurements of sublingual microvascular features were notably different between the groups. Compared to women with no MVM, the 5–25 μm diameter microvessels of women with MVM had a smaller perfused boundary region (2.03 μm [IQR 1.83, 2.20] vs. 2.07 [1.94, 2.23], p=0.018) accompanied by a greater percentage of time that vessels were perfused with red blood cells (RBC filling (73 percent [0.69, 0.76] vs. 71 percent [0.68, 0.74], p=0.006). They also tended, on average, to have smaller diameter microvessels and lower vessel density (functional rarefaction) compared to women without MVM history although these unadjusted differences were not statistically significant (estimated mean vessel diameter 8.7 μm [8.2, 9.4] vs. 9.0 [8.3, 9.6], p=0.087; vessel density 3610 [IQR 2920, 4850] micrometers/mm2 vs. 3930 [3110, 4690], p=0.326]). After adjustment for confounders (race, age, follow-up time, pre-pregnancy obesity, sodium, preeclampsia, SGA birth, preterm delivery, and anti-hypertensive medication use), women with MVM had significantly lower vessel density (−410 number/mm2, [CI −740, −80] p=0.015), along with higher RBC filling percent (p=0.004) and smaller perfused boundary region (p=0.031).
There were also cardiometabolic differences 8 to 10 years after pregnancy in women with a history of MVM. Those with MVM had higher diastolic BP (77.7 [10.7] mmHg vs. 75.3 [10.9], p=0.018) compared to those without MVM. This diastolic BP difference according to MVM history persisted after accounting for covariates (Table 2A). Both total and LDL-cholesterol were higher in women with MVM (184 vs. 174 mg/dL for total cholesterol, p=0.025; 110 vs. 101 mg/dL for LDL, p=0.010) and these differences persisted after adjustment for confounders (p=0.016 and 0.031, respectively). MVM was unrelated to other metabolic markers (BMI, waist circumference, HDL-C, triglycerides, insulin or glucose). Results were similar when analyzed as a MVM severity score, where the most severe lesions were weighted more heavily (mean severity score for women with MVM was 2.8 [SD 1.9]). MVM severity was correlated with more adverse microvascular features (r=−0.14 for estimated mean vessel diameter and r=0.14 for RBC filling percent), LDL-cholesterol (r=0.11), diastolic BP (r=0.17), and systolic BP (r=0.15; p<0.05 for all measures). Similarly, after accounting for confounders, systolic and diastolic BP, microvessel diameter and RBC filling were more adverse as MVM severity increased (Table 2B).
Table 2A.
Blood pressure, microvascular features and cholesterol according to presence of placental maternal vascular malperfusion (MVM) lesions
| Difference + Malperfusion | Adjusted Difference* + Malperfusion | |||||
|---|---|---|---|---|---|---|
| Maternal characteristic | Difference | CI | p-value | Difference | CI | p-value |
|
| ||||||
| Systolic blood pressure, mmHg | 1.92 | (0.22, 3.63) | 0.027 | 2.52 | (−0.44, 5.48) | 0.095 |
| Diastolic blood pressure, mmHg | 2.40 | (1.17, 3.63) | <.001 | 2.56 | (0.39, 4.74) | 0.021 |
|
| ||||||
| - | - | - | - | - | - | - |
|
| ||||||
| Vessel density, micrometers/mm2* | −210 | (−470, 50) | 0.117 | −410 | (−740, −80) | 0.015 |
| Vessel diameter, μm* | −0.34 | (−0.57, −0.12) | 0.003 | −0.24 | (−0.52, 0.04) | 0.091 |
| Red blood cell filling, percent* | 0.02 | (0.01, 0.01) | <.001 | 0.02 | (0.01, 0.03) | 0.004 |
| Perfused boundary region, μm* | −0.07 | (−0.12, −0.02) | 0.004 | −0.07 | (−0.13, −0.01) | 0.025 |
|
| ||||||
|
| ||||||
| Total cholesterol, mg/dl | 9.5 | 1.8, 17.3 | 0.016 | 11.2 | (2.1, 20.4) | 0.016 |
| LDL cholesterol, mg/dl | 9.2 | 2.7, 15.6 | 0.005 | 8.7 | (0.9, 16.4) | 0.031 |
Table 2B.
Blood pressure, microvascular features and cholesterol according to severity of MVM (range 0–9)
| per 1 unit increase in MVM severity | ||||
|---|---|---|---|---|
| unadjusted | p-value | adjusted* | p-value | |
|
| ||||
| Systolic blood pressure, mmHg | 1.23 | 0.001 | 1.05 | 0.010 |
| Diastolic blood pressure, mmHg | 1.08 | 0.0001 | 0.89 | 0.003 |
|
| ||||
| Vessel density, micrometers/mm2* | −30 | 0.423 | −60 | 0.211 |
| Vessel diameter, μm* | −0.10 | 0.003 | −0.08 | 0.039 |
| Red blood cell filling, percent* | 0.50% | 0.001 | 0.30% | 0.085 |
| Perfused boundary region, μm* | −0.013 | 0.071 | −0.010 | 0.267 |
|
| ||||
| Total cholesterol, mg/dl | 2.26 | 0.014 | 1.57 | 0.148 |
| LDL cholesterol, mg/dl | 2.04 | 0.065 | 1.66 | 0.194 |
Adjusted for race, age, follow-up time, pre-pregnancy obesity, sodium, preeclampsia, small for gestational age birth, preterm delivery, anti-hypertensive medication use
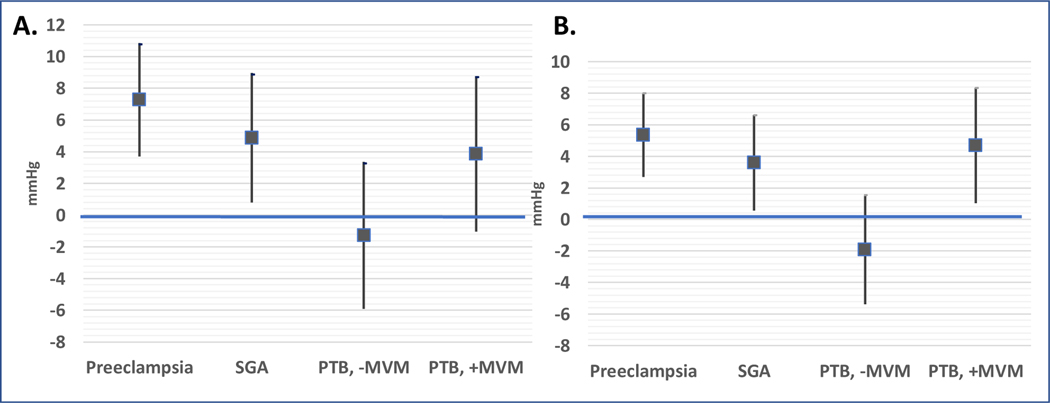
We then examined whether these MVM associations varied by occurrence of each adverse pregnancy outcome. The associations between MVM lesions, microvascular features and cholesterol were detected regardless of adverse pregnancy outcomes (all p-interaction >0.30). In contrast, the associations between MVM lesions and blood pressure were more complex. While higher diastolic blood pressure was detected in women with preeclampsia or SGA regardless of MVM (p-interaction=0.507 and 0.528, respectively), diastolic BP was higher in women with preterm birth only when accompanied by placental evidence of MVM (Preterm+MVM +4.7 mmHg [95% CI 1.02, 8.34], Preterm-MVM −1.9 mmHg [−5.4, 1.5], p-interaction, 0.019; Figure 3). Results were similar but not as precise for SBP (p-interaction=0.106). When evaluated according to strata of APO with and without MVM (Table S3), findings were consistent such that blood pressure abnormalities were more strongly associated with APO occurrence while microvascular and atherogenic lipid findings were associated with MVM occurrence.
Figure 3.
Differences in systolic BP (mmHg, panel A) and diastolic BP (panel B) 8–10 years after pregnancies complicated by preeclampsia, small for gestational age (SGA), preterm birth (PTB) without (−) or with (+) placental maternal vascular malperfusion lesions (MVM); women with no adverse pregnancy outcomes are the referent (horizontal line). Interaction of preeclampsia and SGA with MVM was not significant (P>0.3) and therefore these groups are not stratified by presence or absence of MVM. In contrast, there was evidence of effect modification by MVM status in women with prior PTB (p for interaction=0.02) so this group is stratified by presence of MVM.
Discussion
We present a novel approach to evaluating maternal cardiovascular risk a decade after pregnancy by focusing on occurrence of maternal vascular malperfusion lesions in the placenta as a marker of later life vascular susceptibility. These lesions comprise a group of related placental abnormalities that are thought to derive from early maldevelopment of the spiral arteries—the small arteries that supply the maternal blood to the placenta. While outside of pregnancy atherosis is a feature of large vessels (coronary arteries, aorta, etc), the spiral arteries (50–100 μm) are among the larger vessels of the uterine microvasculature. Early in gestation, deep migration of fetal extravillous trophoblasts into the maternal decidua and inner myometrium leads to the remodeling of these vessels, with dissolution of their smooth muscle walls and replacement of the walls by extravillous trophoblast-derived fibrinoid. Remodeling converts the otherwise narrow, tightly coiled vessels into looser funnel-shaped structures that have lost the capacity to respond to maternal vascular signals. When remodeling is incomplete or fails to occur, the spiral arteries retain their muscular walls and vascular tone. The resulting blood flow into the placenta retains its arterial character, with pulsatile, high velocity flow that mixes poorly and potentially damages the placental chorionic villi. The specific lesions of incomplete or failed remodeling are termed decidual vasculopathy and include incomplete remodeling of basal plate arteries, fibrinoid necrosis and/or atherosis of spiral artery walls, and mural hypertrophy of the spiral artery branches in the extraplacental membranes. Decidual vasculopathy may then lead to occlusion of spiral arteries with infarction of the overlying placenta or to hypoxic/ischemic damage to the placental parenchyma, including advanced (or accelerated) villous maturation, an alteration in villous growth in response to poor fetal perfusion, or increased fibrin deposition, a response to localized necrosis of the syncytiotrophoblast covering the chorionic villi.
Despite reasonable agreement about which individual lesions fall within the diagnostic category of MVM, there is little agreement on which features or how many individual lesions are necessary for a diagnosis of MVM. In fact, even the Amsterdam criteria, the most recent reassessment and recategorization of placental pathology features, only described the features of MVM and pointedly did not attempt to grade or stage this diagnosis. Given the lack of direction in this area, our study employed a two-pronged approach. Our primary approach was to follow the current clinical guidelines that any single MVM lesion was sufficient for a diagnosis of MVM. The risk with using this low-threshold, minimal criterion for MVM, however, is that contributing lesions likely do not represent equal probabilities for the development or severity of MVM, and less impactful criteria may dilute the significance of the more representative criteria. To mitigate this risk, we explored a severity index that assigned each placenta a score based on the types and numbers of individual lesions. Importantly, significant differences in three vascular domains examined (microvasculature, cholesterol and blood pressure) were detected using a minimal, dichotomous criteria for MVM as well as a severity score. These findings are consistent with our hypothesis that MVM may be manifestations of an underlying maternal vascular phenotype relevant to later vascular health. Supporting this interpretation is the lack of association between MVM and components of metabolic syndrome (waist circumference, HDL-C, glucose, triglycerides), pointing to a vascular rather than metabolic abnormality. We also note that the inclusion of low placental weight, in addition to the direct assessment of spiral artery-related abnormalities, did not improve the precision of associations with later life maternal vascular impairments. It is possible that low placental weight is the composite result of these impairments and that small placentas without these MVM findings are physiologically (vs. pathologically) small. It is also possible that low placental weight may be more reflective of fetal rather than maternal sequelae or is a less precise marker of maternal vascular malperfusion. These possibilities warrant further study.
Our results present several notable findings. Aligned with our hypothesis that women with MVM would have an adverse vascular profile, most robust were the changes in the sublingual microvasculature as assessed by sidestream dark field imaging. Using only the minimal criteria for MVM, women with MVM placentas had lower vessel functional (perfused) density, higher RBC filling percent and a lower perfused boundary region. Effectively, these women had functional rarefaction of their microvasculature with increased flow in this smaller number of perfused vessels. When these same variables were then evaluated in the context of MVM severity, those women with worse MVM (higher severity score) had even narrower vessel diameters. The other microvascular parameters also trended to severe values (further decreased vessel density and increased red blood cell filling percentage). Importantly, these variations in the microvasculature were independent of adverse pregnancy outcomes, suggesting that the findings may represent an intrinsic propensity to an altered microvasculature in those women with MVM.
Microvascular impairments have been implicated in the occurrence of MVM in the placenta due potentially to underlying hemodynamic aberrations. For example, low plasma volume, a well-established feature of preeclampsia, may signal cardiovascular reserve capacity and reduced reserve may contribute to and be a consequence of microvascular damage.12, 49 Reduced functional capillary density 5–10 years after a hypertensive pregnancy has been reported using capillaroscopy.50 To our knowledge, however, our study may be a first to assess microvascular features 8 to 10 years after pregnancy associated with specific placental pathological features. Interestingly, not all changes were in the hypothesized direction. Of note, loss of glycocalyx integrity quantified as deeper red blood cell penetration (larger perfused boundary region) has been associated with renal disease,51 lacunar stroke,52 and coronary heart disease,38, 53 and our prediction was that MVM cases would have larger perfused boundary regions. In contrast, our findings were opposite, such that women with MVM had a smaller perfused boundary region accompanied by a higher RBC filling percentage, lower vessel density and, on average, smaller diameter microvessels. We can only speculate about these unexpected findings. One possibility is that they reflect a maternal microvasculature that may be less able to respond to stressors, such as pregnancy, and later life adaptations required for healthy aging, but this warrants future study.54 Aligned with this possibility, our group has reported that women with preeclampsia and MVM have lower cognition scores and blunted cerebrovascular reactivity in relevant brain regions after pregnancy compared to non-preeclampsia cases or preeclampsia cases without MVM.55
Consistent with our hypothesis that MVM will identify susceptibility to vascular impairments that may be distinct from the clinical outcomes of preeclampsia, preterm birth and SGA, our findings indicate that diastolic blood pressure, total and LDL-cholesterol, and small vessel sublingual derangements appear to be related to MVM occurrence that is not entirely explained by these adverse pregnancy outcomes. Although the magnitude of these vascular differences assessed 8–10 years after pregnancy were modest, they were detected in young adult women at a mean age of 38 years and thus may be early markers of a high-risk profile warranting further study. It is noteworthy that large vessel (blood pressure) and small vessel (sublingual) sequelae of placental impairments presented differently. While diastolic blood pressure was higher in women with preeclampsia or SGA regardless of placental features, only the subset of women with preterm births accompanied by vascular placental lesions had higher blood pressure. In contrast, women with maternal vascular malperfusion lesions had a distinct sublingual microvascular phenotype regardless of an accompanying clinical presentation of preeclampsia, small for gestational age or preterm birth. Taken together, our findings raise the possibility that vascular impairments revealed in the placenta as well as the maternal and fetal responses which are manifest as clinical adverse outcomes may accrue in both the large and small vessels after delivery. For example, MVM was not associated with hypertension after delivery in our data, but whether progression to hypertension may converge with microvascular features detected in women following MVM and contribute to overt CVD warrants additional study. The evidence that traditional cardiometabolic risk factors, including hypertension, do not fully explain the link between adverse pregnancy outcomes and CVD supports this possibility.56
Several lines of evidence identify the placenta as a marker of pre-existing susceptibility to vascular impairment as well as a contributor to vessel injury remote from the uterus and from the pregnancy event. First, our group and others have reported that Black race,57, 58 obesity59 and neighborhood deprivation60 all are associated with higher risk of MVM and thus pre-conception susceptibility is likely. There is also accumulating evidence that a poorly perfused placenta leads to oxidative stress and placentally-derived materials that are shed into the maternal circulation that may damage the endothelium systemically.8 How or if these injuries persist post-partum are unknown, but our data suggests they are detectable 8–10 years after delivery. While many prior studies have investigated individual adverse pregnancy outcomes, we opted instead to study what we hypothesized would be a shared vascular pregnancy phenotype, a malperfused placenta.61 This approach makes comparisons challenging, but there is evidence that seven months after delivery, women with preeclampsia with accompanying decidual vasculopathy (the most severe, chronic and likely underlying precursor lesion to other MVM features) had higher diastolic BP, lower left ventricular stroke volume and higher total peripheral vascular resistance compared to women with preeclampsia but no decidual vasculopathy.12 A large, prospective, community based U.S. cohort has also reported that mural hyperplasia, a feature of decidual vasculopathy characterized by thickening of the smooth muscle wall of the spiral arteries, is associated with risk of hypertension a decade after delivery when accompanied by modest blood pressure elevations in pregnancy.62 Consistent with our findings, we have reported in a separate cohort that women with normotensive preterm births accompanied by placenta vascular malperfusion have a more atherogenic profile a decade after delivery compared to those with preterm births without placental malperfusion.14 Other smaller studies that evaluated mothers soon after delivery have reported higher LDL-C and triglycerides in women with atherosis, a subtype of dedicual vasculopathy.63, 64 Our data reporting modest blood pressure elevations and a more atherogenic lipid profile following MVM that was independent of adverse pregnancy outcomes are consistent with these findings and extend them to women assessed a decade after delivery.
Strengths of our study included a comprehensive approach to evaluating the maternal vasculature a decade after pregnancy that included novel (microvascular) and traditional measures. This is important because CVD has sex-specific features. Women present with more coronary microvascular disease and heart failure with preserved ejection fraction compared to men.65, 66 Blood pressure increases twice as fast from age 20 to 40 in women vs. men, and these patterns predict CVD outcomes more commonly in women compared to men, so identifying the sex-specific contributors is essential.67 Adverse pregnancy outcomes such as preeclampsia and delivery of preterm or SGA infants, which themselves are heterogenous outcomes, are associated with excess CVD risk but when added to risk prediction models do not appear to improve precision.68 These findings suggest that subtypes and novel precursors to established risk factors, such as those with evidence of placental vascular impairments, may be informative.
Potential limitations of our study include the requirement that a woman have had a delivery from which the placenta was sent for examination. Since only 45% of placentas were evaluated during this time, this may have biased the findings towards the null as our non-MVM group was not completely normal. Future studies of non-selected evaluations are warranted to expand and replicate our findings. Another potential limitation is that no distinction was made among the different MVM lesions for inclusion into the study. Subsequent studies focused more tightly on specific lesions such as decidual vasculopathy or advanced villous maturation are still needed to more precisely delineate MVM risks. The use of placental pathology reports to identify study subjects is also a potential limitation as diagnosis of placental MVM lesions is known to have high interobserver variability. To mitigate this, the placental pathology slides from all available cases were re-reviewed and we demonstrate good concordance between clinical and blinded research review. Although we considered occurrence of adverse outcomes in other pregnancies, placental evaluations for these were not available to be included. Future studies are needed to examine if recurrent MVM in subsequent pregnancies may signal a more severe post-delivery maternal vascular phenotype.
Perspectives:
Adverse pregnancy outcomes are established harbingers of excess hypertension and cardiovascular disease in women.1 For each of these adverse outcomes (preeclampsia, preterm birth and a small for gestational age infant) a subset of the placentas will show evidence of MVM on pathologic examination. Studies such as ours are beginning to evaluate the relations between these adverse pregnancy outcomes, an aberrant placental vascular phenotype and subsequent cardiovascular risk in women. Our study, which evaluated women 8–10 years after delivery, has demonstrated that MVM lesions are indeed associated with significantly worse vascular parameters, including rarefaction of the microvasculature, elevated total and LDL-cholesterol, and increased diastolic blood pressure. These findings suggest that an underlying vascular phenotype may connect these different clinical entities, and they may help guide future studies attempting to define the molecular and genetic aspects of this vascular phenotype.
Supplementary Material
Novelty and Significance:
What is new:
The subset of women with evidence of maternal vascular malperfusion (MVM) in the placenta have an impaired vascular profile a decade after pregnancy, including rarefaction of the microvasculature, elevated total and LDL-cholesterol, and increased diastolic blood pressure
These findings were independent of adverse pregnancy outcomes (APO) including preeclampsia, small for gestational age or preterm infant, although the group with both APO and MVM may be the most severely affected.
We detected differences in maternal sequelae this using both a minimal criterion and a novel composite severity score
What is relevant:
Adverse pregnancy outcomes are heterogenous and all are associated with higher cardiovascular risk after delivery. The placenta may identify an underlying vascular phenotype connecting pregnancy to later life vascular sequelae.
Rather than rely upon a clinical diagnosis of pregnancy outcome, the placenta may provide tissue-specific clues to microvascular and atherogenic etiologies relevant to pregnancy health and later maternal vascular susceptibility.
Summary:
Women with adverse pregnancy outcomes (APOs) later experience excess hypertension and cardiovascular disease, but how the events are linked is still unknown. Examination of the placenta may provide clues to vascular impairments after delivery. Our study demonstrates that a decade after delivery, women with placental vascular lesions had an adverse cardiovascular profile comprised of microvascular rarefaction, higher diastolic blood pressure and more atherogenic lipids. Placental histopathology may provide etiologic insight into a woman’s early trajectory toward subsequent vascular disease.
Acknowledgements:
We gratefully acknowledge the study participants and study staff at the Magee-Womens Research Institute.
Sources of Funding: This work was funded by the American Heart Association Go Red for Women Strategic Focused Research Network (16SFRN28930000, 16SFRN27810001, and 16SFRN28340000).
Footnotes
Disclosures: The authors have no financial disclosures or conflicts to report.
References
- 1.Parikh NI, Gonzalez JM, Anderson CAM, et al. Adverse Pregnancy Outcomes and Cardiovascular Disease Risk: Unique Opportunities for Cardiovascular Disease Prevention in Women: A Scientific Statement From the American Heart Association. Circulation. 2021;143(18):e902–e916. doi:doi: 10.1161/CIR.0000000000000961 [DOI] [PubMed] [Google Scholar]
- 2.Kräker K, O’Driscoll JM, Schütte T, et al. Statins Reverse Postpartum Cardiovascular Dysfunction in a Rat Model of Preeclampsia. Hypertension. 2020;75(1):202–210. doi:doi: 10.1161/HYPERTENSIONAHA.119.13219 [DOI] [PubMed] [Google Scholar]
- 3.Ormesher L, Higson S, Luckie M, et al. Postnatal Enalapril to Improve Cardiovascular Function Following Preterm Preeclampsia (PICk-UP):: A Randomized Double-Blind Placebo-Controlled Feasibility Trial. Hypertension. Dec 2020;76(6):1828–1837. doi: 10.1161/hypertensionaha.120.15875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller EC. Preeclampsia and Cerebrovascular Disease. Hypertension. Jul 2019;74(1):5–13. doi: 10.1161/hypertensionaha.118.11513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parks WT. Placental hypoxia: The lesions of maternal malperfusion. Seminars in Perinatology. 2// 2015;39(1):9–19. doi: 10.1053/j.semperi.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 6.Das S, Halushka MK. Extracellular vesicle microRNA transfer in cardiovascular disease. Cardiovascular Pathology. 2015/07/01/ 2015;24(4):199–206. doi: 10.1016/j.carpath.2015.04.007 [DOI] [PubMed] [Google Scholar]
- 7.González-Quintero VH, Smarkusky LP, Jiménez JJ, et al. Elevated plasma endothelial microparticles: Preeclampsia versus gestational hypertension. American Journal of Obstetrics and Gynecology. 2004/10/01/ 2004;191(4):1418–1424. doi: 10.1016/j.ajog.2004.06.044 [DOI] [PubMed] [Google Scholar]
- 8.Hecht JL, Zsengeller ZK, Spiel M, Karumanchi SA, Rosen S. Revisiting decidual vasculopathy. Placenta. Jun 2016;42:37–43. doi: 10.1016/j.placenta.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 9.Rana S, Burke SD, Karumanchi SA. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am J Obstet Gynecol. Oct 20 2020;doi: 10.1016/j.ajog.2020.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weissgerber TL, Milic NM, Milin-Lazovic JS, Garovic VD. Impaired Flow-Mediated Dilation Before, During, and After Preeclampsia: A Systematic Review and Meta-Analysis. Hypertension. Feb 2016;67(2):415–23. doi: 10.1161/hypertensionaha.115.06554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauspurg A, Redman EK, Assibey-Mensah V, et al. Placental findings in non-hypertensive term pregnancies and association with future adverse pregnancy outcomes: a cohort study. Placenta. Dec 15 2018;74:14–19. doi: 10.1016/j.placenta.2018.12.008 [DOI] [PubMed] [Google Scholar]
- 12.Stevens DU, Al-Nasiry S, Fajta MM, et al. Cardiovascular and thrombogenic risk of decidual vasculopathy in preeclampsia. American Journal of Obstetrics and Gynecology. 6// 2014;210(6):545.e1–545.e6. doi: 10.1016/j.ajog.2013.12.029 [DOI] [PubMed] [Google Scholar]
- 13.Stevens DU, Smits MP, Bulten J, Spaanderman ME, van Vugt JM, Al-Nasiry S. Prevalence of hypertensive disorders in women after preeclamptic pregnancy associated with decidual vasculopathy. Hypertens Pregnancy. 2015;34(3):332–41. doi: 10.3109/10641955.2015.1034803 [DOI] [PubMed] [Google Scholar]
- 14.Catov JM, Muldoon MF, Reis SE, et al. Preterm birth with placental evidence of malperfusion is associated with cardiovascular risk factors after pregnancy: a prospective cohort study. BJOG. Jul 2018;125(8):1009–1017. doi: 10.1111/1471-0528.15040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension. Dec 2010;56(6):1026–34. doi: 10.1161/HYPERTENSIONAHA.110.157743 [DOI] [PubMed] [Google Scholar]
- 16.Staff AC, Dechend R, Redman CWG. Review: Preeclampsia, acute atherosis of the spiral arteries and future cardiovascular disease: Two new hypotheses. Placenta. 2013;34:S73–S78. doi:DOI 10.1016/j.placenta.2012.11.022 [DOI] [PubMed] [Google Scholar]
- 17.Houben AJ, de Leeuw PW, Peeters LL. Configuration of the microcirculation in pre-eclampsia: possible role of the venular system. J Hypertens. Aug 2007;25(8):1665–70. doi: 10.1097/HJH.0b013e3281900e0e00004872-200708000-00021 [pii] [DOI] [PubMed] [Google Scholar]
- 18.Cornette J, Herzog E, Dubekot JJ, Tibboel D, Buijs EA, Steegers EA. Microcirculation analysed by sidestream dark field imaging (SDF) technique in women with severe pre-eclampsia. Reprod Sci. 2011;18(4):S-197. [Google Scholar]
- 19.Hasan KM, Manyonda IT, Ng FS, Singer DR, Antonios TF. Skin capillary density changes in normal pregnancy and pre-eclampsia. J Hypertens. Dec 2002;20(12):2439–43. doi: 10.1097/01.hjh.0000042883.24999.6e [DOI] [PubMed] [Google Scholar]
- 20.Gandley RE, Bregand-White J, Brands J, et al. 253. Sublingual microvascular density and glycocalyx barrier dynamics, during and after normal and preeclamptic pregnancy. Pregnancy Hypertension. 2018/10/01/ 2018;13:S111. doi: 10.1016/j.preghy.2018.08.328 [DOI] [Google Scholar]
- 21.Beinder E, Schlembach D. Skin flux during reactive hyperemia and local hyperthermia in patients with preeclampsia. Obstetrics and gynecology. Aug 2001;98(2):313–8. doi:S0029-7844(01)01456-9 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Vollebregt KC, Boer K, Mathura KR, de Graaff JC, Ubbink DT, Ince C. Impaired vascular function in women with pre-eclampsia observed with orthogonal polarisation spectral imaging. BJOG. Nov 2001;108(11):1148–53. [DOI] [PubMed] [Google Scholar]
- 23.Ciftci FC, Caliskan M, Ciftci O, et al. Impaired coronary microvascular function and increased intima-media thickness in preeclampsia. Journal of the American Society of Hypertension : JASH. Nov 2014;8(11):820–6. doi: 10.1016/j.jash.2014.08.012 [DOI] [PubMed] [Google Scholar]
- 24.Weissgerber TL, Garcia-Valencia O, Milic NM, et al. Early Onset Preeclampsia Is Associated With Glycocalyx Degradation and Reduced Microvascular Perfusion. J Am Heart Assoc. Feb 19 2019;8(4):e010647. doi: 10.1161/JAHA.118.010647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brands J, Hauspurg A, Bregand-White J, et al. 209. The microvascular endothelial glycocalyx: impaired barrier function in preeclampsia with small gestational age neonates. Pregnancy Hypertension. 2018/10/01/ 2018;13:S99. doi: 10.1016/j.preghy.2018.08.294 [DOI] [Google Scholar]
- 26.Brands J, Jeyabalan A, Hauspurg A, McGonigal SC, Gandley RE, Hubel CA. 210. Reduced barrier function of the microvascular endothelial glycocalyx in women with a history of preeclampsia, one year after delivery. Pregnancy Hypertension. 2018/10/01/ 2018;13:S99–S100. doi: 10.1016/j.preghy.2018.08.295 [DOI] [Google Scholar]
- 27.Bairey Merz CN. Testing for Coronary Microvascular Dysfunction. Jama. Nov 18 2019;doi: 10.1001/jama.2019.16625 [DOI] [PubMed] [Google Scholar]
- 28.Pepine CJ, Ferdinand KC, Shaw LJ, et al. Emergence of Nonobstructive Coronary Artery Disease: A Woman’s Problem and Need for Change in Definition on Angiography. J Am Coll Cardiol. Oct 27 2015;66(17):1918–33. doi: 10.1016/j.jacc.2015.08.876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Catov JM, Peng Y, Scifres CM, Parks WT. Placental pathology measures: Can they be rapidly and reliably integrated into large-scale perinatal studies? Placenta. Jun 2015;36(6):687–92. doi: 10.1016/j.placenta.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 30.ACOG Practice Bulletin No. 202: Gestational Hypertension and Preeclampsia. Obstet Gynecol. Jan 2019;133(1):e1–e25. doi: 10.1097/aog.0000000000003018 [DOI] [PubMed] [Google Scholar]
- 31.Ding G, Tian Y, Zhang Y, Pang Y, Zhang JS, Zhang J. Application of a global reference for fetal-weight and birthweight percentiles in predicting infant mortality. BJOG. Dec 2013;120(13):1613–21. doi: 10.1111/1471-0528.12381 [DOI] [PubMed] [Google Scholar]
- 32.Katzman PJ. Chronic inflammatory lesions of the placenta. Semin Perinatol. Feb 2015;39(1):20–6. doi: 10.1053/j.semperi.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 33.Redline R, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic Infection Syndrome: Nosology and Reproducibility of Placental Reaction Patterns. Pediatric an Developmental Pathology. 2003;6:435–448. [DOI] [PubMed] [Google Scholar]
- 34.Redline RW, Ariel I, Baergen RN, et al. Fetal vascular obstructive lesions: nosology and reproducibility of placental reaction patterns. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. Sep-Oct 2004;7(5):443–52. doi: 10.1007/s10024-004-2020-x [DOI] [PubMed] [Google Scholar]
- 35.Redline RW, Boyd T, Campbell V, et al. Maternal vascular underperfusion: nosology and reproducibility of placental reaction patterns. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society. May-Jun 2004;7(3):237–49. doi: 10.1007/s10024-003-8083-2 [DOI] [PubMed] [Google Scholar]
- 36.Gandley RE, Brands J, Hubel C, Hauspurg A, Parks WT, Catov Janet M. Maternal Placental Vascular Malperfusion Lesions Associated with Increased Cardiometabolic Risk and Reduced Microvascular Density in Women a Decade after Delivery: Which Placental Features Matter?. Circulation. 2020;142(Suppl 3) [Google Scholar]
- 37.Khong TY, Mooney EE, Ariel I, et al. Sampling and Definitions of Placental Lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. Jul 2016;140(7):698–713. doi: 10.5858/arpa.2015-0225-CC [DOI] [PubMed] [Google Scholar]
- 38.Brands J, Hubel CA, Althouse A, Reis SE, Pacella JJ. Noninvasive sublingual microvascular imaging reveals sex-specific reduction in glycocalyx barrier properties in patients with coronary artery disease. Physiological reports. Jan 2020;8(2):e14351. doi: 10.14814/phy2.14351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dane MJ, Khairoun M, Lee DH, et al. Association of kidney function with changes in the endothelial surface layer. Clinical journal of the American Society of Nephrology : CJASN. Apr 2014;9(4):698–704. doi: 10.2215/cjn.08160813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Potje SR, Paula TD, Paulo M, Bendhack LM. The Role of Glycocalyx and Caveolae in Vascular Homeostasis and Diseases. Front Physiol. 2020;11:620840. doi: 10.3389/fphys.2020.620840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Broekhuizen LN, Lemkes BA, Mooij HL, et al. Effect of sulodexide on endothelial glycocalyx and vascular permeability in patients with type 2 diabetes mellitus. Diabetologia. Dec 2010;53(12):2646–55. doi: 10.1007/s00125-010-1910-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nieuwdorp M, Meuwese MC, Mooij HL, et al. Measuring endothelial glycocalyx dimensions in humans: a potential novel tool to monitor vascular vulnerability. Journal of applied physiology (Bethesda, Md : 1985). Mar 2008;104(3):845–52. doi: 10.1152/japplphysiol.00440.2007 [DOI] [PubMed] [Google Scholar]
- 43.Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince C. Sidestream Dark Field (SDF) imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Opt Express. Nov 12 2007;15(23):15101–14. doi: 10.1364/oe.15.015101 [DOI] [PubMed] [Google Scholar]
- 44.Bull FC, Maslin TS, Armstrong T. Global physical activity questionnaire (GPAQ): nine country reliability and validity study. Journal of physical activity & health. Nov 2009;6(6):790–804. doi: 10.1123/jpah.6.6.790 [DOI] [PubMed] [Google Scholar]
- 45.Spehar SM, Gibbs BB, Muldoon M, Catov JM. Association of sedentary time with blood pressure in women of reproductive age. Prev Med Rep. Dec 2020;20:101219. doi: 10.1016/j.pmedr.2020.101219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.LaPorte RE, Black-Sandler R, Cauley JA, Link M, Bayles C, Marks B. The assessment of physical activity in older women: analysis of the interrelationship and reliability of activity monitoring, activity surveys, and caloric intake. J Gerontol. Jul 1983;38(4):394–7. [DOI] [PubMed] [Google Scholar]
- 47.Ainsworth BE, Leon AS, Richardson MT, Jacobs DR, Paffenbarger RS, Jr. Accuracy of the College Alumnus Physical Activity Questionnaire. J Clin Epidemiol. Dec 1993;46(12):1403–11. [DOI] [PubMed] [Google Scholar]
- 48.Tangney CC, Rasmussen HC, Rusch J, et al. Validation of a Sodium Screener in Two Samples. The FASEB Journal. 2016/04/01 2016;30(1_supplement):293.6–293.6. doi: 10.1096/fasebj.30.1_supplement.293.6 [DOI] [Google Scholar]
- 49.Scholten RR, Sep S, Peeters L, Hopman MTE, Lotgering FK, Spaanderman MEA. Prepregnancy Low-Plasma Volume and Predisposition to Preeclampsia and Fetal Growth Restriction. Obstetrics & Gynecology. 2011;117(5) [DOI] [PubMed] [Google Scholar]
- 50.Boardman H, Lamata P, Lazdam M, et al. Variations in Cardiovascular Structure, Function, and Geometry in Midlife Associated With a History of Hypertensive Pregnancy. Hypertension. 2020;75(6):1542–1550. doi:doi: 10.1161/HYPERTENSIONAHA.119.14530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H. Damage of the Endothelial Glycocalyx in Dialysis Patients. Journal of the American Society of Nephrology. 2012;23(11):1900–1908. doi: 10.1681/asn.2011121181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martens RJ, Vink H, van Oostenbrugge RJ, Staals J. Sublingual microvascular glycocalyx dimensions in lacunar stroke patients. Cerebrovasc Dis. 2013;35(5):451–4. doi: 10.1159/000348854 [DOI] [PubMed] [Google Scholar]
- 53.Gorshkov AY, Klimushina MV, Boytsov SA, Kots AY, Gumanova NG. Increase in perfused boundary region of endothelial glycocalyx is associated with higher prevalence of ischemic heart disease and lesions of microcirculation and vascular wall. Microcirculation (New York, NY : 1994). May 2018;25(4):e12454. doi: 10.1111/micc.12454 [DOI] [PubMed] [Google Scholar]
- 54.Lee DH, Dane MJ, van den Berg BM, et al. Deeper penetration of erythrocytes into the endothelial glycocalyx is associated with impaired microvascular perfusion. PLoS One. 2014;9(5):e96477. doi: 10.1371/journal.pone.0096477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shaaban B, Rosano C, Cohen AD, et al. Cognition and cerebrovascular reactivity in midlife women with history of preeclampsia and placental evidence of maternal vascular malperfusion. Frontiers in Aging Neuroscience. 2021;(in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang YX, Arvizu M, Rich-Edwards JW, et al. Hypertensive Disorders of Pregnancy and Subsequent Risk of Premature Mortality. J Am Coll Cardiol. Mar 16 2021;77(10):1302–1312. doi: 10.1016/j.jacc.2021.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Assibey-Mensah V, Parks WT, Gernand AD, Catov JM. Race and risk of maternal vascular malperfusion lesions in the placenta. Placenta. Sep 2018;69:102–108. doi: 10.1016/j.placenta.2018.07.017 [DOI] [PubMed] [Google Scholar]
- 58.Matoba N, Mestan KK, Collins JW. Understanding Racial Disparities of Preterm Birth Through the Placenta. Clinical Therapeutics. 2021/02/01/ 2021;43(2):287–296. doi: 10.1016/j.clinthera.2020.12.013 [DOI] [PubMed] [Google Scholar]
- 59.Avagliano L, Monari F, Po G, et al. The Burden of Placental Histopathology in Stillbirths Associated With Maternal Obesity. American journal of clinical pathology. Jul 7 2020;154(2):225–235. doi: 10.1093/ajcp/aqaa035 [DOI] [PubMed] [Google Scholar]
- 60.Assibey-Mensah V, Mendez DD, Carey K, Parks W, Catov J. Neighborhood Deprivation and Decidual Vasculopathy presented at: Society for Reproductive Investigation 6th Annual Scientific Meeting; March 12-16, 2019 2019; [Google Scholar]
- 61.Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. Jun 2009;30(6):473–82. doi:S0143-4004(09)00066-6 [pii] 10.1016/j.placenta.2009.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holzman CB, Senagore P, Xu J, et al. Maternal risk of hypertension 7–15 years after pregnancy: clues from the placenta. Bjog. Sep 15 2020;doi: 10.1111/1471-0528.16498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Moe K, Alnaes-Katjavivi P, Storvold GL, et al. Classical Cardiovascular Risk Markers in Pregnancy and Associations to Uteroplacental Acute Atherosis. Hypertension. Sep 2018;72(3):695–702. doi: 10.1161/hypertensionaha.118.10964 [DOI] [PubMed] [Google Scholar]
- 64.Veerbeek JH, Brouwers L, Koster MP, et al. Spiral artery remodeling and maternal cardiovascular risk: the spiral artery remodeling (SPAR) study. J Hypertens. Aug 2016;34(8):1570–7. doi: 10.1097/hjh.0000000000000964 [DOI] [PubMed] [Google Scholar]
- 65.Dean J, Cruz SD, Mehta PK, Merz CNB. Coronary microvascular dysfunction: sex-specific risk, diagnosis, and therapy. Nature Reviews Cardiology. 2015/07/01 2015;12(7):406–414. doi: 10.1038/nrcardio.2015.72 [DOI] [PubMed] [Google Scholar]
- 66.Beale AL, Meyer P, Marwick TH, Lam CSP, Kaye DM. Sex Differences in Cardiovascular Pathophysiology. Circulation. 2018;138(2):198–205. doi:doi: 10.1161/CIRCULATIONAHA.118.034271 [DOI] [PubMed] [Google Scholar]
- 67.Ji H, Kim A, Ebinger JE, et al. Sex Differences in Blood Pressure Trajectories Over the Life Course. JAMA cardiology. Jan 15 2020;5(3):19–26. doi: 10.1001/jamacardio.2019.5306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Markovitz AR, Stuart JJ, Horn J, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J. Apr 7 2019;40(14):1113–1120. doi: 10.1093/eurheartj/ehy863 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.