Abstract
The current revolution of digital health technology and machine learning offers enormous potential to improve patient care. Nevertheless, it is essential to recognize that dermatology requires an approach different from those of other specialties. For many dermatological conditions, there is a lack of standardized methodology for quantitatively tracking disease progression and treatment response (clinimetrics). Furthermore, dermatological diseases impact patients in complex ways, some of which can be measured only through patient reports (psychometrics). New tools using digital health technology (e.g., smartphone applications, wearable devices) can aid in capturing both clinimetric and psychometric variables over time. With these data, machine learning can inform efforts to improve health care by, for example, the identification of high-risk patient groups, optimization of treatment strategies, and prediction of disease outcomes. We use the term personalized, data-driven dermatology to refer to the use of comprehensive data to inform individual patient care and improve patient outcomes. In this paper, we provide a framework that includes data from multiple sources, leverages digital health technology, and uses machine learning. Although this framework is applicable broadly to dermatological conditions, we use the example of a serious inflammatory skin condition, chronic cutaneous graft-versus-host disease, to illustrate personalized, data-driven dermatology.
Abbreviations: AAD, American Academy of Dermatology; cGVHD, chronic graft-versus-host disease; NIH, National Institutes of Health; PRO, patient-reported outcome
Introduction
The growing amounts of healthcare data combined with advances in computational and analytical approaches have yielded real-world improvements in patient outcomes (Abuabara et al., 2018; Ginsburg and Phillips, 2018). Although risk prediction and prognostic models have been developed in the past using static, cross-sectional data, fields such as cardiology and oncology have more recently adopted data sources and methodology that incorporate longitudinal electronic health data. In fact, longitudinal data more accurately capture fluctuations in physiological factors over time. For instance, variables such as systolic and diastolic blood pressure, blood glucose level, total cholesterol, triglyceride level, and body mass index, which are often measured and recorded into electronic medical records, can be incorporated into predictive algorithms (Rajkomar et al., 2019; Yu et al., 2018; Yuan et al., 2021; Zhao et al., 2019). However, to capitalize on the advances in healthcare technology and analytics, dermatology faces unique challenges. Most skin conditions cannot be followed by traditional vital signs or laboratory values. In addition, the severity of most skin diseases is not easily assessed or communicated with typical medical record documentation. Furthermore, the lack of a standardized methodology for monitoring dermatological conditions makes it challenging to quantitatively track disease progression and treatment response. In addition, a dermatological disease often raises concerns regarding the patient’s overall health, which requires communication and comanagement across different subspecialists (Chamlin and Chren, 2010; Chen et al., 2002; Chren, 2020, 2012, 2005; Chren et al., 2001, 1996).
In this paper, we propose a framework for personalized, data-driven dermatology, which we define as the use of comprehensive data (including both clinimetrics and psychometrics) to inform individual patient care. With machine learning combined with digital health technology to capture novel measurements of skin conditions, there is enormous potential to improve patient outcomes by optimizing care at the individual patient level.
Measurements in dermatology
Accurate assessment of the impact of dermatological diseases on a patient’s health state requires the collection of both clinimetric (clinical) and psychometric (patient-reported) data (Chren, 2020, 2005; Chren et al., 1996). Clinimetric data can include both objective information (e.g., laboratory values, vital signs, clinical images, biopsy results) and subjective clinician/provider assessment (e.g., clinically assessed disease severity). However, because dermatological conditions often impact patients not only physically but also psychosocially, clinimetrics alone are often inadequate to capture the degree of impact the disease has on the patient’s overall life. For instance, measures such as Skindex, Dermatology Life Quality Index, Patient-Oriented Eczema Measure, and Psoriasis Symptom Inventory have been developed to assess psychometric data in a standardized manner. Through these measures, the patients’ perspectives are captured systematically through domains such as health-related QOL, somatic symptoms (e.g., pain, itch), function (e.g., activities of daily living, movement), and emotional/social (e.g., anxiety, depression). Given that patient-reported assessment of disease severity may differ from clinician/provider assessment, using patient reports (psychometrics) in conjunction with traditionally gathered information from the history, physical examination, and clinical data (clinimetrics) can provide a more complete picture and allow for individualized care to address the patients’ needs (Barbieri and Gelfand, 2021; Kirby, 2022).
The use of digital health technology (e.g., smartphone applications, sensors, wearables) can increase the feasibility of capturing these data sources. When machine learning approaches are applied to these data, it may be possible to improve our understanding of patients’ health states and inform our strategies to optimize their care (Lee et al., 2021; Wongvibulsin et al., 2020, 2019). In particular, digital health technology that captures patient-generated data can help engage patients in their health care and better inform clinicians of the disease trajectory in the context of the patient’s everyday life. These tools offer the opportunity to gather a rich source of real-time information on symptoms and medication tracking, exposure/environmental/behavioral data, QOL, and patient-reported outcomes (PROs). In fact, PROs have already been successfully implemented in dermatological clinical trials (Copley-Merriman et al., 2017). Efforts for personalized, data-driven dermatology can build on these approaches by facilitating the ease of patient engagement and integration into clinical records through digital tools to capture PROs in real-time. Incorporation of PROs is particularly important given the literature supporting that changes in PROs may reflect changes most important to patients and may be more sensitive in capturing these differences than physician-assessed measures (Cohen et al., 2004; Strand et al., 1999). The increasing recognition of the importance of PROs is reflected in the Society of Thoracic Surgery Adult Cardiac Surgery Database, one of the most comprehensive clinical data registries developed after approximately three decades of iterations to refine the database. Previously, the Society of Thoracic Surgery measured surgical outcomes only with standard clinical metrics. However, they acknowledged in their 2018 Update on Outcomes and Quality that these objective measures may not always reflect what is important to patients. As a result, they established a Patient Reported Outcomes Task Force and adopted the Patient Reported Outcomes Measurement Information System with the goal of collecting PROs data in a format that allows for standardized measurement of patient-reported symptoms and health outcomes in a longitudinal manner through advances in health information technology. In addition, the Society of Thoracic Surgery established the Informatics Task Force as a central computer science and informatics resource for their national database and for investigation of novel methodology for linking longitudinal database records (Badhwar et al., 2018; Bowdish et al., 2020; D’Agostino et al., 2018; O’Brien et al., 2018; PROMIS, 2022). Numerous opportunities exist for the field of dermatology to benefit from these examples of capturing patients’ journeys through longitudinal information of both clinimetric and psychometric data. For instance, innovative, multifaceted longitudinal data have already been recognized as valuable and necessary sources of information toward the goals of early diagnosis and preemptive therapy in chronic graft-versus-host disease (cGVHD; further details are provided in the section “The potential of personalized, data-driven dermatology in the care of patients with cGVHD”) (Kitko et al., 2021; Pavletic et al., 2021; Pidala et al., 2021; Shakshouk et al., 2021).
Overall, integrating data from multiple sources, such as biological, clinical, PROs, smartphone, and wearable sensor data, is essential to fully capture patients’ dermatological conditions over time. With accurate representation of dermatological diseases captured through digital health technology, machine learning can assist in the optimization of treatment strategies, prediction of disease flares, and management plans. This framework can also benefit clinical trials and drug development in dermatology.
Personalized, data-driven dermatology
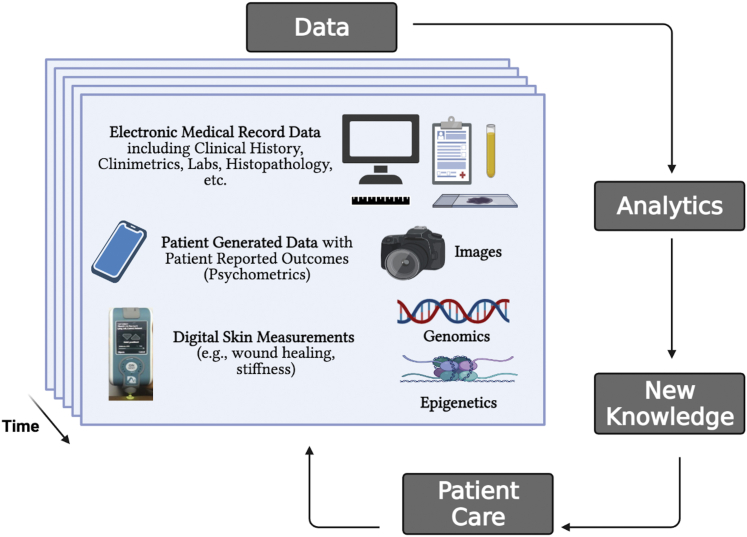
As shown in Figure 1, the key components of our framework include (i) data captured over time, (ii) analytics to generate new insights on disease course and treatment response, and (iii) the application of this knowledge to patient care. Moreover, in the process of patient care, additional data are collected, enabling the cycle of learning to continue (i.e., learning healthcare systems) (Olsen et al., 2007; Wongvibulsin and Zeger, 2020). Importantly, data in the framework are composed of not only traditionally gathered clinical data but also data generated by patients and through digital technology. For example, relevant data sources might include biospecimens with genetic and epigenetic information; the electronic medical record (e.g., clinical history, clinimetrics, laboratory results, histopathology); clinical images; digital skin measurements; and patient-generated data, including PROs.
Figure 1.
Framework for personalized, data-driven dermatology. The key components of the framework include longitudinal data, analytics, and new knowledge that can be applied to patient care to improve patient outcomes. Note that the data in the framework are composed of not only traditionally gathered clinical data but also data generated by patients (e.g., tracking their symptoms and self-reported outcomes as well as physiological measurements ranging from home blood pressure and glucose levels to step count and sleep duration/quality) and through digital technology such as ones enabling skin measurements of stiffness and wound healing. Labs, laboratory measurements.
The potential of personalized, data-driven dermatology in the care of patients with cGVHD
This framework of multiple data sources, digital health technology, and machine learning is applicable broadly to the care of most dermatological conditions. In this paper, we use the specific example of cGVHD to provide a concrete example. cGVHD is a rich model to convey this framework for several reasons. First, cGVHD is a complex, serious disease with high variability in clinical presentations and treatment responses that are difficult to predict. Furthermore, there is already widespread clinician familiarity with the Lee Symptom Scale, an extensively validated PRO measure (Lee et al., 2002), which can serve as an example for further approaches in dermatological disease quantification. Moreover, the lack of readily accessible centers for the care of patients with cGVHD heightens the need for digital approaches for remote monitoring as well as patient education and engagement (Kitko et al., 2021). Owing to the complexity of cGVHD management, the multidisciplinary nature of cGVHD care can benefit from quantitative measurements of disease trajectories to facilitate effective communication and comanagement of patients’ conditions (Jacobsohn et al., 2012). With the recent publication of the National Institutes of Health (NIH) Consensus Development Project on Criteria for Clinical Trials in cGVHD (Pavletic et al., 2021), this is an opportune time to introduce the framework for personalized, data-driven dermatology in the context of cGVHD (Figure 2).
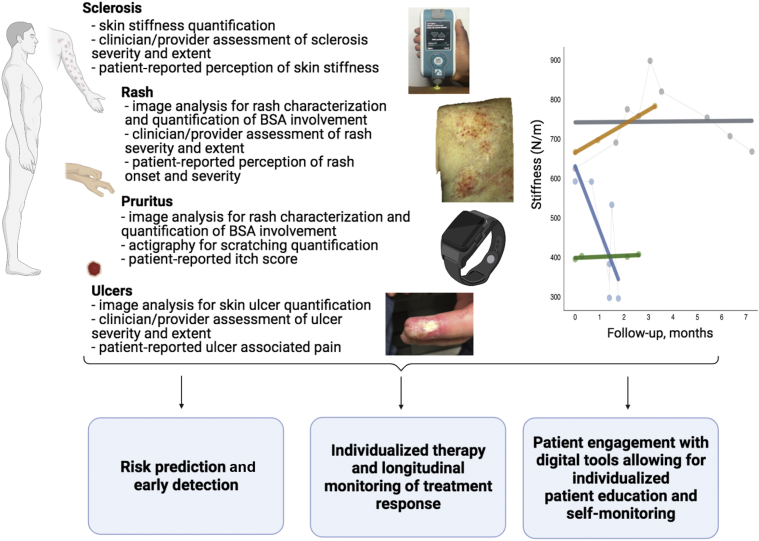
Figure 2.
The potential of personalized, data-driven dermatology in the care of patients with cGVHD. This framework can allow for the quantification of the severity and extent of GVHD through both clinimetrics and psychometrics. An example of data that can be collected includes measurements of skin stiffness as shown in the plot, where dynamic stiffness measurements are shown over time where each color corresponds to a different patient’s trajectory (gray: clinically stable, gold: disease progression, blue: clinical improvement, green: no skin involvement; modified from Baker et al., 2021b). These data can be collected longitudinally to enable tools in three main categories: (i) risk prediction and early detection, (ii) individualized therapy and longitudinal monitoring of treatment response, and (iii) patient engagement with digital tools allowing for individualized patient education and self-monitoring. Although the figure in this paper focuses on illustrating the digital measurements related to the skin, the same framework can be considered for a much broader set of digital measurements. For example, including physiological measurements such as home blood pressure and glucose levels to step count and sleep duration/quality can help to inform skin disease biology and management as well as underscore the relationship of skin diseases with other systemic conditions. Images: MyotonPRO Device (Baker et al., 2021b); image capturing erythematous lesion severity of GVHD (Tkaczyk et al., 2018; reprinted with permission from Elsevier). BSA, body surface area; cGVHD, chronic graft-versus-host disease; GVHD, graft-versus-host disease.
cGVHD has been extensively investigated, particularly with regard to the pulmonary aspect of cGVHD, which is the leading cause of mortality (Curtis et al., 2014; Palmer et al., 2014). In fact, research focused on the pulmonary symptoms of cGVHD provides an important foundation for potential advances in quantitative approaches in the dermatological aspects of cGVHD. For instance, the NIH symptom-based lung score has been shown to be statistically associated with nonrelapse mortality and overall survival (Palmer et al., 2016). More recently, a study using machine learning identified distinct cGVHD phenotypes. Although there is potential for this work to translate to the clinical setting, additional research for validation in prospective, independent cohorts will be necessary to determine the ability of the algorithm to uncover clinically relevant clusters for patients with cGVHD for applications in risk stratification (Gandelman et al., 2019). In addition, research with the eGVHD application has shown the accuracy and usability of the digital tool to support healthcare professionals in assessing the severity of graft-versus-host disease (Schoemans et al., 2018a, 2018b, 2016). These developments in cGVHD research overall set the stage for advances in the management of the dermatological aspects of cGVHD through quantitative, data-driven approaches.
As shown in Figure 2, our framework focuses on quantification of the severity and extent of cGVHD through both clinimetrics and psychometrics. For instance, sclerosis, rash, pruritus, and ulcers can all be characterized by both clinical measurements and patient reports. Furthermore, skin stiffness is a clinical manifestation that has traditionally been difficult to monitor accurately. However, with the introduction of the MyotonPRO Device (Myoton, Tallinn, Estonia), skin stiffness can now be quantified and tracked dynamically (Baker et al., 2021b). With skin biomechanical parameters measured using the MyotonPRO, recent investigation using logistic regression and machine learning models found that the frequency and relaxation time offered the highest diagnostic yield for distinguishing patients with sclerotic cGVHD from post-hematopoietic cell transplantation controls. Although the study of most complex diseases utilizes a data-driven approach, tools at the point of care are not currently available to provide an indication of which are the most important variables that correlate with the outcome of interest for the patient being treated. With further research using this framework of personalized, data-driven dermatology, it may be possible to identify differences in the key variables at varying times in the disease course of cGVHD. For instance, early in the disease course, PROs and subclinical fibrosis assessed by measurements of skin stiffness may be most predictive, whereas during the treatment phase, early predictors of adverse events from therapy may be more predictive than skin stiffness. These developments could inform future quantitative biomechanical studies of sclerotic cGVHD as well as other sclerosing diseases (Baker et al., 2021a).
Current advances and next steps
As computational healthcare technology and methodology advance, there is enormous potential to gather, integrate, and translate data collected to improve health outcomes (Deo, 2015; Rajkomar et al., 2019; Sidey-Gibbons and Sidey-Gibbons, 2019; Topol, 2019, 2016). For instance, improved computational speed and algorithms have enabled image analysis that can offer diagnostic support as well as lesion tracking over time (Esteva et al., 2021). In addition, a recent publication focused specifically on the development of a robust and scalable algorithm for intensive longitudinal data from electronic health records and personal wearable devices (German et al., 2021).
The American Academy of Dermatology (AAD) has also set data collection as a top priority. Although AAD’s DataDerm was initially established with the goals of facilitating quality demonstration, its goals now also include (i) standardization of data within and across institutions, (ii) integration of diverse data sources (including PROs and mobile applications to minimize the burden of data entry), (iii) aggregation of skin disease registries, (iv) acceleration of population-based and health systems research, and (v) incorporation of data at scale from large health systems (Park et al., 2018). Efforts such as DataDerm are an important step toward the integration of information not only across a single provider but also across multiple providers and institutions to allow for the acceleration of research and clinical impact. Furthermore, it will also be equally important to inform dermatologists of these new tools and encourage their contribution to these data sources. It is essential that individuals from diverse backgrounds are involved in the process of development of these technological advances to help ensure an inclusive approach that represents diverse skin types and helps reduce rather than further widen healthcare disparities (Adamson and Smith, 2018). Lack of inclusion of skin of color in initial machine learning algorithm developments as well as barriers to digital health access in underserved populations are important considerations as the field of medicine increasingly adopts a digital infrastructure (Lyles et al., 2021). Although the framework we propose includes data more broadly, encompassing both digital and inherently nondigital data (e.g., clinical assessments or patient reports), an important caveat is the consideration of digital health equity to ensure that digital inputs that are based on wearables, smartphones, or other technologies are not skewed toward patients from resource-rich settings while leaving others behind. As the COVID-19 pandemic has highlighted, there are long-standing disparities in health and health care, particularly related to racial, ethnic, and socioeconomic status. Given that medicine is at a transition point of a digital health transformation, it is essential that proactive planning is undertaken to develop effective solutions to ensure that the digital healthcare revolution improves health equity rather than further widens healthcare disparities. The path forward in this area will require multilevel efforts, ranging from the individual to community to policy levels, as described in the digital health equity mapped to the socioecological framework (Lyles et al., 2021).
As digital health technology and machine learning become increasingly integrated into the field of medicine as a whole, incorporation of fundamentals of these topics in medical education can serve as a starting point to ensure the familiarity of clinicians with these emerging tools for enhancing health care (Kolachalama and Garg, 2018). In addition, as recently discussed (Li et al., 2022), recruitment, training, development, and retention of leaders in investigative dermatology will be essential for the field as a whole and in particular to accelerate the application of data-driven approaches to dermatology to advance personalized care and improve outcomes. As real-world experience accumulates with personalized, data-driven dermatology over time, continuing to refine this framework on the basis of results regarding items such as patient outcomes and clinician satisfaction will allow the field to further embrace and benefit from the infrastructure of continuous learning, as shown in Figure 3.
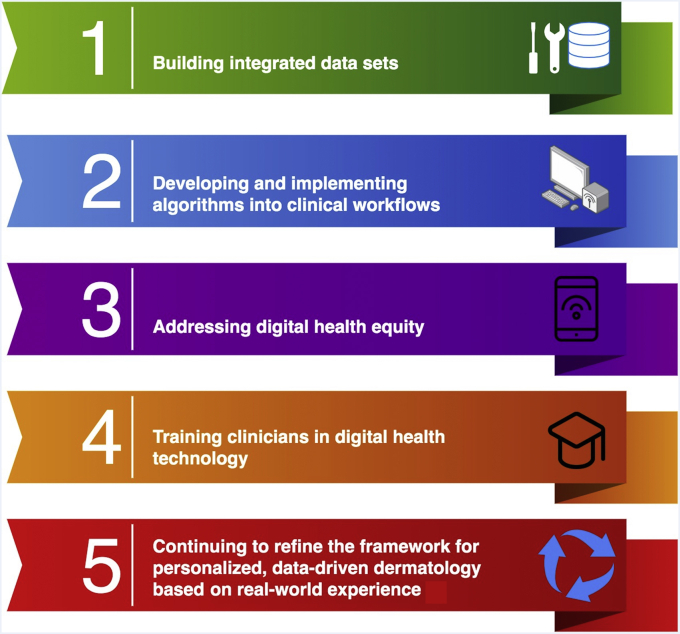
Figure 3.
Key next steps. This figure outlines the key next steps toward achieving personalized, data-driven dermatology.
Conclusion and outlook
Advances in computational and analytical approaches combined with the increasing amounts of healthcare data offer enormous potential for precision medicine and learning healthcare systems. Nevertheless, it is essential to recognize that approaches to the care of patients in dermatology require a different strategy from that of other specialties because the clinical severity and course of skin diseases can be challenging to measure, and skin diseases can impact patients in ways that may not be captured through traditional vital signs or laboratory measurements. Instead, the accurate measurement of dermatological diseases includes both clinimetrics (clinical data) and psychometrics (patient-reported data). Moreover, the collection and integration of these diverse data sources can be facilitated through the use of digital health technology. When combined with machine learning, these skin measurements may increase our understanding of the patient’s health state and help optimize multidisciplinary management strategies to improve patient outcomes. As illustrated with cGVHD, this framework provides the opportunity to enhance patient care in numerous areas, ranging from risk prediction and early detection to individualized treatment strategies and patient engagement tools. Overall, personalized, data-driven dermatology has the potential to provide a more comprehensive understanding of skin conditions at the individual level and improve patient outcomes.
ORCIDs
Shannon Wongvibulsin: http://orcid.org/0000-0002-1390-7440
Tracy M. Frech: http://orcid.org/0000-0002-5472-3840
Mary-Margaret Chren: http://orcid.org/0000-0001-8009-7014
Eric R. Tkaczyk: http://orcid.org/0000-0002-2850-4740
Author Contributions
Conceptualization: SW, ERT, MMC; Investigation: SW, TMF, ERT, MMC; Supervision: ERT, MMC; Writing - Original Draft Preparation: SW; Writing - Review and Editing: SW, TMF, ERT, MMC
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflict of Interest
The authors state no conflict of interest.
Acknowledgments
SW’s efforts were supported by the National Institutes of Health (NIH) F30HL142131 and 5T32GM007309 grants. TMF is supported by NIH K23AR067889. MMC is supported by NIH R01AR073001. ERT’s effort was supported by Career Development Award number IK2 CX001785 from the United States Department of Veterans Affairs Clinical Science R&D Service.
accepted XXX; corrected proof published online XXX
Footnotes
Cite this article as: JID Innovations 2022;X:100105
References
- Abuabara K., Asgari M.M., Chen S.C., Dellavalle R.P., Kalia S., Secrest A.M., et al. How data can deliver for dermatology. J Am Acad Dermatol. 2018;79:400–402. doi: 10.1016/j.jaad.2018.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamson A.S., Smith A. Machine learning and health care disparities in dermatology. JAMA Dermatol. 2018;154:1247–1248. doi: 10.1001/jamadermatol.2018.2348. [DOI] [PubMed] [Google Scholar]
- Badhwar V., Rankin J.S., Thourani V.H., D’Agostino R.S., Habib R.H., Shahian D.M., et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 update on research: outcomes analysis, quality improvement, and patient safety. Ann Thorac Surg. 2018;106:8–13. doi: 10.1016/j.athoracsur.2018.04.052. [DOI] [PubMed] [Google Scholar]
- Baker L.X., Chen F., Cronin A., Chen H., Vain A., Jagasia M., et al. Optimal biomechanical parameters for measuring sclerotic chronic graft-versus-host disease. JID Innov. 2021;1:100037. doi: 10.1016/j.xjidi.2021.100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker L.X., Chen F., Ssempijja Y., Byrne M., Kim T.K., Vain A., et al. Longitudinal tracking of skin dynamic stiffness to quantify evolution of sclerosis in chronic graft-versus-host disease. Bone Marrow Transplant. 2021;56:989–991. doi: 10.1038/s41409-020-01158-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbieri J.S., Gelfand J.M. Patient-reported outcome measures as complementary information to clinician-reported outcome measures in patients with psoriasis. JAMA Dermatol. 2021;157:1236–1237. doi: 10.1001/jamadermatol.2021.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdish M.E., D’Agostino R.S., Thourani V.H., Desai N., Shahian D.M., Fernandez F.G., et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2020 update on outcomes and research. Ann Thorac Surg. 2020;109:1646–1655. doi: 10.1016/j.athoracsur.2020.03.003. [DOI] [PubMed] [Google Scholar]
- Chamlin S.L., Chren M.M. Quality-of-life outcomes and measurement in childhood atopic dermatitis. Immunol Allergy Clin North Am. 2010;30:281–288. doi: 10.1016/j.iac.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S.C., Yeung J., Chren M.M. Scalpdex: a quality-of-life instrument for scalp dermatitis. Arch Dermatol. 2002;138:803–807. doi: 10.1001/archderm.138.6.803. [DOI] [PubMed] [Google Scholar]
- Chren M.M. Measurement of vital signs for skin diseases. J Invest Dermatol. 2005;125:viii–ix. doi: 10.1111/j.0022-202X.2005.23796.x. [DOI] [PubMed] [Google Scholar]
- Chren M.M. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatol Clin. 2012;30:231–236. doi: 10.1016/j.det.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chren M.M. Challenges in understanding, scoring, and comparing patients’ reports. JAMA Dermatol. 2020;156:369–370. doi: 10.1001/jamadermatol.2019.4658. [DOI] [PubMed] [Google Scholar]
- Chren M.M., Lasek R.J., Quinn L.M., Mostow E.N., Zyzanski S.J. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. J Invest Dermatol. 1996;107:707–713. doi: 10.1111/1523-1747.ep12365600. [DOI] [PubMed] [Google Scholar]
- Chren M.M., Lasek R.J., Sahay A.P., Sands L.P. Measurement properties of Skindex-16: a brief quality-of-life measure for patients with skin diseases. J Cutan Med Surg. 2001;5:105–110. doi: 10.1007/BF02737863. [DOI] [PubMed] [Google Scholar]
- Cohen S.B., Strand V., Aguilar D., Ofman J.J. Patient- versus physician-reported outcomes in rheumatoid arthritis patients treated with recombinant interleukin-1 receptor antagonist (anakinra) therapy. Rheumatology (Oxford) 2004;43:704–711. doi: 10.1093/rheumatology/keh152. [DOI] [PubMed] [Google Scholar]
- Copley-Merriman C., Zelt S., Clark M., Gnanasakthy A. Impact of measuring patient-reported outcomes in dermatology drug development. Patient. 2017;10:203–213. doi: 10.1007/s40271-016-0196-6. [DOI] [PubMed] [Google Scholar]
- Curtis L.M., Grkovic L., Mitchell S.A., Steinberg S.M., Cowen E.W., Datiles M.B., et al. NIH response criteria measures are associated with important parameters of disease severity in patients with chronic GVHD. Bone Marrow Transplant. 2014;49:1513–1520. doi: 10.1038/bmt.2014.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino R.S., Jacobs J.P., Badhwar V., Fernandez F.G., Paone G., Wormuth D.W., et al. The Society of Thoracic Surgeons Adult Cardiac Surgery Database: 2018 update on outcomes and quality. Ann Thorac Surg. 2018;105:15–23. doi: 10.1016/j.athoracsur.2017.10.035. [DOI] [PubMed] [Google Scholar]
- Deo R.C. Machine learning in medicine. Circulation. 2015;132:1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteva A., Chou K., Yeung S., Naik N., Madani A., Mottaghi A., et al. Deep learning-enabled medical computer vision. NPJ Digit Med. 2021;4:5. doi: 10.1038/s41746-020-00376-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandelman J.S., Byrne M.T., Mistry A.M., Polikowsky H.G., Diggins K.E., Chen H., et al. Machine learning reveals chronic graft-versus-host disease phenotypes and stratifies survival after stem cell transplant for hematologic malignancies. Haematologica. 2019;104:189–196. doi: 10.3324/haematol.2018.193441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German C.A., Sinsheimer J.S., Zhou J., Zhou H. WiSER: robust and scalable estimation and inference of within-subject variances from intensive longitudinal data [e-pub ahead of print] Biometrics. 2021 doi: 10.1111/biom.13506. (accessed 21 February 2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg G.S., Phillips K.A. Precision medicine: from science to value. Health Aff (Millwood) 2018;37:694–701. doi: 10.1377/hlthaff.2017.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsohn D.A., Kurland B.F., Pidala J., Inamoto Y., Chai X., Palmer J.M., et al. Correlation between NIH composite skin score, patient-reported skin score, and outcome: results from the Chronic GVHD Consortium. Blood. 2012;120:2545–2574. doi: 10.1182/blood-2012-04-424135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby J.S. Patient-reported outcomes in dermatology. JAMA Dermatol. 2022;158:97–98. doi: 10.1001/jamadermatol.2021.1559. [DOI] [PubMed] [Google Scholar]
- Kitko C.L., Pidala J., Schoemans H.M., Lawitschka A., Flowers M.E., Cowen E.W., et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IIa. The 2020 clinical implementation and early diagnosis working group report. Transplant Cell Ther. 2021;27:545–557. doi: 10.1016/j.jtct.2021.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolachalama V.B., Garg P.S. Machine learning and medical education. NPJ Digit Med. 2018;1:54. doi: 10.1038/s41746-018-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.Y., Maloney N.J., Cheng K., Bach D.Q. Machine learning for precision dermatology: advances, opportunities, and outlook. J Am Acad Dermatol. 2021;84:1458–1459. doi: 10.1016/j.jaad.2020.06.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.K., Cook E.F., Soiffer R., Antin J.H. Development and validation of a scale to measure symptoms of chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2002;8:444–452. doi: 10.1053/bbmt.2002.v8.pm12234170. [DOI] [PubMed] [Google Scholar]
- Li S., Yancey K.B., Cruz P.D., Le L.Q. Training physician‒scientists for careers in investigative dermatology. JID Innov. 2022;2:100061. doi: 10.1016/j.xjidi.2021.100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyles C.R., Wachter R.M., Sarkar U. Focusing on digital health equity. JAMA. 2021;326:1795–1796. doi: 10.1001/jama.2021.18459. [DOI] [PubMed] [Google Scholar]
- O’Brien S.M., Feng L., He X., Xian Y., Jacobs J.P., Badhwar V., et al. The Society of Thoracic Surgeons 2018 adult cardiac surgery risk models: part 2-statistical methods and results. Ann Thorac Surg. 2018;105:1419–1428. doi: 10.1016/j.athoracsur.2018.03.003. [DOI] [PubMed] [Google Scholar]
- Olsen L.A., Aisner D., McGinnis J.M., editors. The learning healthcare system. National Academies Press; Washington, DC: 2007. [PubMed] [Google Scholar]
- Palmer J., Chai X., Pidala J., Inamoto Y., Martin P.J., Storer B., et al. Predictors of survival, nonrelapse mortality, and failure-free survival in patients treated for chronic graft-versus-host disease. Blood. 2016;127:160–166. doi: 10.1182/blood-2015-08-662874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer J., Williams K., Inamoto Y., Chai X., Martin P.J., Tomas L.S., et al. Pulmonary symptoms measured by the National Institutes of Health lung score predict overall survival, nonrelapse mortality, and patient-reported outcomes in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2014;20:337–344. doi: 10.1016/j.bbmt.2013.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A.J., Ko J.M., Swerlick R.A. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643–644. doi: 10.1016/j.jaad.2017.08.053. [DOI] [PubMed] [Google Scholar]
- Pavletic S.Z., Martin P.J., Schultz K.R., Lee S.J. The future of chronic graft-versus-host disease: introduction to the 2020 National Institutes of Health consensus development project reports. Transplant Cell Ther. 2021;27:448–451. doi: 10.1016/j.jtct.2021.02.034. [DOI] [PubMed] [Google Scholar]
- Pidala J., Kitko C., Lee S.J., Carpenter P., Cuvelier G.D.E., Holtan S., et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: IIb. The 2020 Preemptive Therapy Working Group report. Transplant Cell Ther. 2021;27:632–641. doi: 10.1016/j.jtct.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PROMIS, https://www.promishealth.org/; 2022 (accessed 18 February 2022).
- Rajkomar A., Dean J., Kohane I. Machine learning in medicine. N Engl J Med. 2019;380:1347–1358. doi: 10.1056/NEJMra1814259. [DOI] [PubMed] [Google Scholar]
- Schoemans H., Goris K., Durm R.V., Vanhoof J., Wolff D., Greinix H., et al. Development, preliminary usability and accuracy testing of the EBMT ‘eGVHD App’ to support GvHD assessment according to NIH criteria—a proof of concept. Bone Marrow Transplant. 2016;51:1062–1065. doi: 10.1038/bmt.2016.26. [DOI] [PubMed] [Google Scholar]
- Schoemans H.M., Goris K., Van Durm R., Fieuws S., De Geest S., Pavletic S.Z., et al. The eGVHD App has the potential to improve the accuracy of graft-versus-host disease assessment: a multicenter randomized controlled trial. Haematologica. 2018;103:1698–1707. doi: 10.3324/haematol.2018.190777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoemans H.M., Goris K., Van Durm R., Vanbrabant K., De Geest S., Maertens J., et al. Accuracy and usability of the eGVHD app in assessing the severity of graft-versus-host disease at the 2017 EBMT annual congress. Bone Marrow Transplant. 2018;53:490–494. doi: 10.1038/s41409-017-0017-0. [DOI] [PubMed] [Google Scholar]
- Shakshouk H., Tkaczyk E.R., Cowen E.W., El-Azhary R.A., Hashmi S.K., Kenderian S.J., et al. Methods to assess disease activity and severity in cutaneous chronic graft-versus-host disease: a critical literature review. Transplant Cell Ther. 2021;27:738–746. doi: 10.1016/j.jtct.2021.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidey-Gibbons J.A.M., Sidey-Gibbons C.J. Machine learning in medicine: a practical introduction. BMC Med Res Methodol. 2019;19:64. doi: 10.1186/s12874-019-0681-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand V., Tugwell P., Bombardier C., Maetzel A., Crawford B., Dorrier C., et al. Function and health-related quality of life: results from a randomized controlled trial of leflunomide versus methotrexate or placebo in patients with active rheumatoid arthritis. Leflunomide Rheumatoid Arthritis Investigators Group. Arthritis Rheum. 1999;42:1870–1878. doi: 10.1002/1529-0131(199909)42:9<1870::AID-ANR11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Tkaczyk E.R., Chen F., Wang J., Gandelman J.S., Saknite I., Dellalana L.E., et al. Overcoming human disagreement assessing erythematous lesion severity on 3D photos of chronic graft-versus-host disease. Bone Marrow Transplant. 2018;53:1356–1358. doi: 10.1038/s41409-018-0211-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topol E. Digital medicine: empowering both patients and clinicians. Lancet. 2016;388:740–741. doi: 10.1016/S0140-6736(16)31355-1. [DOI] [PubMed] [Google Scholar]
- Topol E.J. High-performance medicine: the convergence of human and artificial intelligence. Nat Med. 2019;25:44–56. doi: 10.1038/s41591-018-0300-7. [DOI] [PubMed] [Google Scholar]
- Wongvibulsin S., Ho B.K.T., Kwatra S.G. Embracing machine learning and digital health technology for precision dermatology. J Dermatolog Treat. 2020;31:494–495. doi: 10.1080/09546634.2019.1623373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongvibulsin S., Martin S.S., Saria S., Zeger S.L., Murphy S.A. An individualized, data-driven digital approach for precision behavior change. Am J Lifestyle Med. 2019;14:289–293. doi: 10.1177/1559827619843489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wongvibulsin S., Zeger S.L. Enabling individualised health in learning healthcare systems. BMJ Evid Based Med. 2020;25:125–129. doi: 10.1136/bmjebm-2019-111190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K.H., Beam A.L., Kohane I.S. Artificial intelligence in healthcare. Nat Biomed Eng. 2018;2:719–731. doi: 10.1038/s41551-018-0305-z. [DOI] [PubMed] [Google Scholar]
- Yuan Q., Cai T., Hong C., Du M., Johnson B.E., Lanuti M., et al. Performance of a machine learning algorithm using electronic health record data to identify and estimate survival in a longitudinal cohort of patients with lung cancer. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Feng Q.P., Wu P., Lupu R.A., Wilke R.A., Wells Q.S., et al. Learning from longitudinal data in electronic health record and genetic data to improve cardiovascular event prediction. Sci Rep. 2019;9:717. doi: 10.1038/s41598-018-36745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]