Abstract
Aims
Bacopa floribunda (BF), an African traditional plant and its species have been widely used as brain tonic for memory enhancement. It has also been reported to help relieve anxiety and some psychological disorders. This study aimed to investigate the mechanisms of action of BF on Amyloid beta (Aβ) 1–42 peptides induced cognitive deficit in male Wistar rats.
Main methods
A total of 48 healthy male wistar rats were used for this study. Some groups were pre-treated with 200 mg/kg of BF extracts before a single bilateral injection of Aβ 1–42 while some were post-treated with BF for 21 days after Aβ1-42 exposure. Cognitive performance was evaluated using Y-Maze and Novel Object recognition tests. After treatments, hippocampal homogenates were assayed for the levels of Acetylcholinesterase, Na–K/ATPase activities, glutamate and Aβ1-42 concentrations among others.
Key findings
It was observed that Aβ1-42 caused cognitive impairment and BF extracts especially the ethanol extract was able to significantly (p < 0.05) reverse almost all the perturbations including lipid imbalance caused by Aβ1-42 assault mainly at the post-treatment level.
Significance
Administration of ethanol and aqueous extracts of BF mitigated the hazardous effect of Aβ1-42 observed in the blood plasma and hippocampal homogenates. In this context, we conclude that BF is an efficient cognitive enhancer that can help alleviate some symptoms associated with Alzheimer’s disease.
Keywords: Bacopa floribunda, Glutamate excitotoxicity, Na–K/ATPase, Acetylcholinesterase, Cognitive enhancer
Graphical abstract
Bacopa floribunda, Glutamate excitotoxicity, Na–K/ATPase, Acetylcholinesterase, Cognitive enhancer.
1. Introduction
Alzheimer's disease (AD) is an irreversible, dynamic brain issue that gradually annihilates memory and thinking aptitudes, and in the long run the capacity to do the least difficult tasks [1]. It is a degenerative disease and the commonest type of dementia in which structural and chemical brain disintegration is related to a steady loss of numerous parts of reasoning and conduct [2]. Around, 5.7 million Americans of all ages are living with Alzheimer's dementia [3] and, approximately 200,000 people under age 65 have a younger onset of AD. Presenilin (PSEN1) mutation, a risk factor for early onset of AD has been traced to Africa (specifically West Africans who were imported as captives to Columbia in the 16th century) [4]. Formally, records show that deaths from Alzheimer's disease have risen sharply (aprox 123%) between 2000 and 2015 while demise from the main cause of death (heart disease) diminished by 11 percent [3]. The two primary histopathological signs of AD (extracellular amyloid plaques and intracellular neurofibrillary tangles) are found in endangered brain regions like the hippocampus and cortex. The amyloid plaques (A.K.A senile plaques) are made of amyloid-beta peptides (Aβ), a large portion of which are 38–43 amino acids long [5, 6]. Aβ is a pro-inflammatory and extremely harmful material that results in neuroinflammation in the brain tissue [7]. A developing body of scientific reports alongside clinical proof supports the possibility that raised Aβ levels can be linked with impairment of cognition and can cause pathological events which result in cognitive defects as seen in AD [8, 9].
Blood-based biomarkers represent a less invasive and conceivably less expensive method that aids AD detection compared with cerebrospinal fluid and some neuroimaging biomarkers [10]. An expanding body of evidence similarly recommended a relationship between AD and possibly modifiable processes, including dyslipidemia and inflammation. Researchers have noticed, high serum cholesterol levels as been associated with increased risk of AD [11, 12], and this suggests that phospholipids may assume a significant role in modulating AD-associated pathogenesis.
In spite of the endorsed medications such as Tacrine, Rivastigmine, and Donepezil among others for the treatment of AD, inhibition of the disease progression remains unresolved because these drugs have short half-lives and horrible unfavorable impacts such as hepatotoxicity and gastrointestinal disorders [13, 14]. However, a therapeutic intervention with the possibility to cure AD has been recommended to be mechanistic disease-modifying therapies (DMT), beta-amyloid cleaving enzyme (BACE) inhibitors, passive immunotherapy e.t.c. that can slow, halt and reverse the neurodegenerative process [15, 16]. The species of Bacopa floribunda (R.Br.) Wettst (family: Scrophulariaceae) have been accounted for in Ayurvedic and traditional medicines for various disturbances, such as anxiety, intellect, and poor memory [17]. Their leaves are utilized in traditional and Ayurvedic medicine as a brain tonic for enhancing memory and forestalling a series of psychological disorders [18,19]. Therefore, this study was designed to determine the effect of Amyloid beta (Aβ) 1–42 on an acute reactant phase inflammatory biomarker (C-Reactive protein), some plasma lipid profiles together with some hippocampal AD biomarkers and to evaluate and compare the potency of ethanol and aqueous extract of Bacopa floribunda (BF) on Alzheimer’s disease model.
2. Materials and method
2.1. Animal care and management
Young adult, male wistar rats (n = 48; 170–220g) were obtained from the animal house section, University of Ilorin, Ilorin. Ethical approval was obtained from the University of Ilorin ethical review committee (UERC/ASN/2018/1470). The rats were fed standard rats’ pellets and water ad libitum and were maintained at standard laboratory conditions. The research was conducted in accordance with the internationally accepted regulation for laboratory animal use and care as found in US guidelines (NIH publication) revised in 1985.
2.2. Plant materials
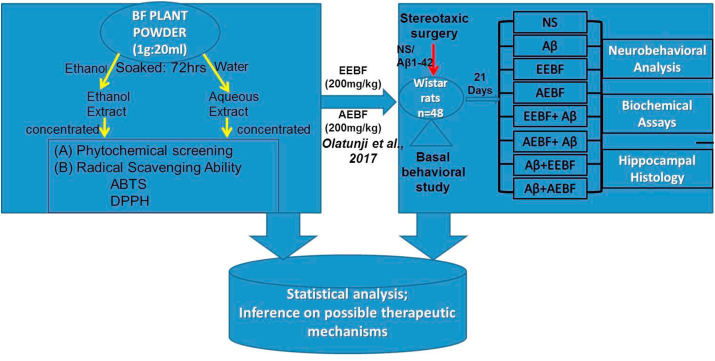
BF known as oniyemiye in Yoruba language was used for this study. Fresh leaves of BF were purchased from Oja Oba market, Ado-Ekiti, Nigeria. The plant was authenticated in the University herbarium, Ado-Ekiti, and a voucher specimen with the number UHAE2020059 and identification number UHAE 0182 was deposited there. Fresh leaves of B. floribunda were air-dried and pulverized in an electric blender, ethanol and aqueous extracts were then prepared separately as highlighted in Figure 1.
Figure 1.
Flowchart of BF extraction, Invito assays and animal grouping for invivo experiments
2.3. Main reagents and drugs
Amyloid beta (Aβ1-42) was obtained from Gen script, USA. Before injection, the Aβ1-42 peptide was dissolved in a physiological saline solution at a concentration of 1mg per 250 μL and incubated at 37 °C for 72 h to induce aggregation [20].
2.4. Plant extract analysis (in-vitro)
The total phenol content was determined according to the method of Singleton et al. [21], and the absorbance was measured at 765 nm in the UV-Visible spectrophotometer. The total phenol content was subsequently calculated as gallic acid equivalent (GAE). The total flavonoid content was determined using a slightly modified method reported by Meda et al., [22]. The absorbance of the reaction mixture was measured at 415nm in the UV-Visible spectrophotometer and total flavonoid content was calculated using quercetin (QA) as standard. ABTS (2,2-Azino-bis(3-Ethylbenzthiazoline-6-Sulphonic Acid)) radical scavenging assay was quantified using the method designed by Re et al., [23] and the Trolox equivalent antioxidant capacity (TEAC) was calculated. DPPH (1,1-Diphenyl-2 Picryhydrazyl) radical scavenging capacity was determined using the method of Gyamfi et al. [24], the reaction mixture was incubated in the dark for 30 min and the color intensity was measured at 516 nm. While alkaloid content determination was done by employing the method described by Harbone [25], determination of total saponin content was done by slightly modified methods of Davis [26].
The LD50 for these extracts was calculated to be 3,536 mg/kg body weight using Lorke’s [27] method as described in Eq. (1).
| LD50 = √ (maximal dose for all survival × dose for all death) | Equation (1) |
| LD50 = √ (2500 ₓ 5000) = 3,536 mg/kg body weight |
2.5. Animal grouping and treatment
Forty-eight rats ranging from 170-220g were randomly divided into eight groups of six rats each. Grouping was done as highlighted in Figure 1.
2.6. Surgery and induction of Alzheimer’s disease
The animal model of Alzheimer’s disease was achieved through a single bilateral intra-cerebroventricular (ICV) injection of the previously obtained Aβ 1–42 aggregates. Paxinos and Watson atlas was used to get the appropriate coordinate and the following stereotaxic coordinates: anteroposterior:-0.8mm from bregma, medial/lateral:+/-1.4mm and dorsal/ventral:-4.0mm were employed to inject substances into the lateral ventricles through a stainless steel cannula using a Hamilton microsyringe. Aβ 1–42 (4 μg/μl/site) was injected into experimental rats while sham rats were injected with the same volume of normal saline (1μl/site). After surgery, the rats were housed individually and had access to food and water freely. Antibiotic cream was applied daily and the rats were allowed 7days to recover from surgery. The general condition of the animals including the body weight, food and water intake was monitored daily after surgery.
2.7. Behavioural study
The animals were subjected to the Y-maze test and Novel object recognition test (NOR) for spatial memory on days 19 and 20 of administration respectively. The behavioral tests were carried out in a proper sound-controlled and highly illuminated behavioral analysis room.
Short-term memory was assessed by spontaneous alternation behavior in the Y-maze task using a standard rats’ Y-maze. The procedure was carried out as described by Hritcu et al. [28], and the extent of cognitive deficit was assessed using NOR as reviewed by Grayson et al., [29].
2.8. Animal sacrifice, excision of brain tissues and sample preparation for biochemical and histological procedures
Twenty-four hours after the completion of treatment, rats were sacrificed by cervical dislocation and blood was collected through cardiac puncture. Brain tissues were excised using appropriate forceps. For biochemical assays, the cerebrum was sagitally divided into two at the corpus callosum and the hippocampus isolated on ice surface. The hippocampi tissues were immediately homogenized (10% w/v) in 100mM phosphate buffer, pH 7.4, containing 0.2mM KCl and some whole brain tissues were fixed in 10% formal saline for the histological procedure.
2.8.1. Biochemical assays
Malondialdehyde (MDA) was measured spectrophotometrically at 532nm by the method described by Colado et al., [30] using 1, 1, 3, 3-tetra ethoxy propane as standard. Glutathione peroxidase (GPx) was measured by the method described by Sinet et al. [31], using t-butyl-HPx as substrate. Na+, K+-ATPase activity was determined by the method described by Wyse et al., [32]. Released inorganic phosphate (Pi) was measured by the method of Chan et al., [33]. The specific activity of the enzyme was expressed as μmol Pi released per mg of protein per hour (μmol Pi/mgprot./hr). AChE activity was determined according to Ellman et al. [34], with some modifications. Glutamate and Aβ1-42 were assayed using a glutamate colorimetric assay kit from bio vision and rat Aβ42 ELISA kit from Fine test with a catalog no: ERO 77. The manufacturers’ procedures for assays were strictly followed.
2.8.2. Histological procedure
The hippocampus specimens were fixed in formal saline. Blocks were made and 5μm thick of paraffin sections were obtained from the tissues using a Leica rotary microtome (Bright B5143 Huntington, England). Sections were stained using hematoxylin and eosin stain, this was examined under the microscope with photomicrographs taken with a Leica DM750 microscope, interfaced with Leica ICC 50 camera.
2.9. Statistical analysis
The results obtained were expressed as mean ± SEM. The data were analyzed using One-way ANOVA followed by Tukey’s Multiple Comparison Tests using Graph pad 5.03 (GraphPad Software Inc., CA, U.S.A). The results were considered significant when p < 0.05.
3. Results
3.1. Percentage yield of Bacopa floribunda extracts
The mean yield of Bacopa floribunda extracts after two extraction runs was 48.3g for ethanol extract and 29.6g for an aqueous extract which represented a mean percentage yield of 24.2 and 14.8 respectively based on the dry weight of the leaves (Table 1). The results indicate that the ethanol extract had more yield when compared to the aqueous extract.
Table 1.
Percentage yield of Bacopa floribunda extract.
| Experimental Run | Weight of BF powder (g) | Weight of AEBF obtained (g) | % yield | Weight of EEBF obtained (g) | % yield |
|---|---|---|---|---|---|
| 1 | 200 | 12.8 | 6.4 | 25.5 | 12.8 |
| 2 | 200 | 16.8 | 8.4 | 22.8 | 11.4 |
| Total | 400 | 29.6 | 14.8 | 48.3 | 24.2 |
3.2. Phytochemical screening of BF extracts
Table 2 shows the values of different Phytochemicals present in the aqueous and ethanol extracts of Bf and it revealed the presence of Phenols, flavonoids, Saponin and alkaloids. It was observed that the aqueous extract had significantly higher phenolic content in comparison with its ethanol counterpart however, the ethanol extract had more Alkaloids and Saponins contents.
Table 2.
Phytochemical screening of Ethanol and Aqueous extracts of Bacopa floribunda. ∗∗ significantly (p<0.05) higher in comparison with the other extract.
| Phytochemicals | Ethanol extract | Aqueous extract |
|---|---|---|
| Total Phenols | 2.98 ± 0.07 | 4.13 ± 0.08∗∗ |
| Flavonoids | 2.98 ± 0.07 | 3.42 ± 0.14 |
| Saponins | 22.63 ± 1.43∗∗ | 10.6 ± 0.85 |
| Alkaloids | 48.13 ± 0.83∗∗ | 32.15 ± 1.06 |
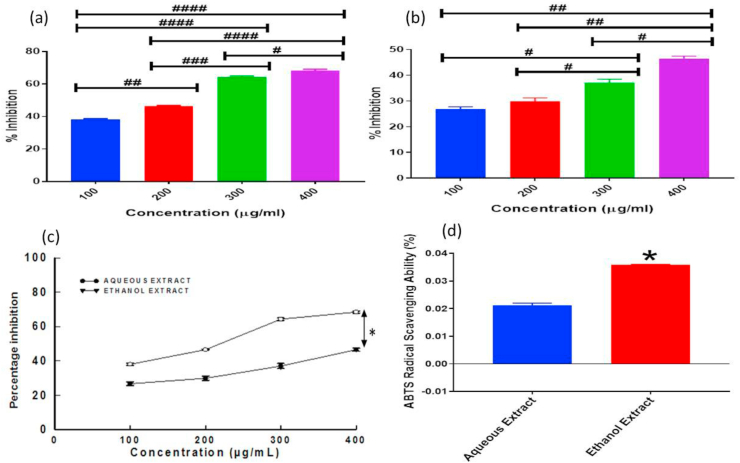
3.3. Ethanol extract possesses more in-vitro antioxidant activity
The results of the measurement of DPPH inhibition percentages determined by various concentrations of ethanol and aqueous Bf extracts are shown in Figure 2a and b respectively. It was observed that the higher the concentration of the extracts, the higher the DPPH free radical scavenging ability, Moreover, the ethanol extract showed significantly (p < 0.05) higher DPPH scavenging ability in comparison with the aqueous extract as shown in Figure 2c. Fig ure 2d revealed that the ethanol extract exhibited a significantly (p < 0.05) higher level of ABTS radical scavenging capability in comparison with the aqueous extract.
Figure 2.
Showing the different invitro antioxidant assays at various concentrations at a significant level of p < 0.05; (a) DPPH Radical Scavenging Ability of Ethanol Extract {# (p = 0.0213), ##(p = 0.0014), ### (p = 0.0001), #### (p < 0.0001)}, (b) DPPH Radical Scavenging Ability of Aqueous Extract {# (p < 0.05), ##(p < 0.005)}, (c) Comparison of DPPH Radical Scavenging Ability of both Aqueous and Ethanol Extract {∗ (p < 0.05) observed at all concentrations} and (d) ABTS Radical Scavenging Ability of both Aqueous and Ethanol Extract {∗ (p < 0.05)}.
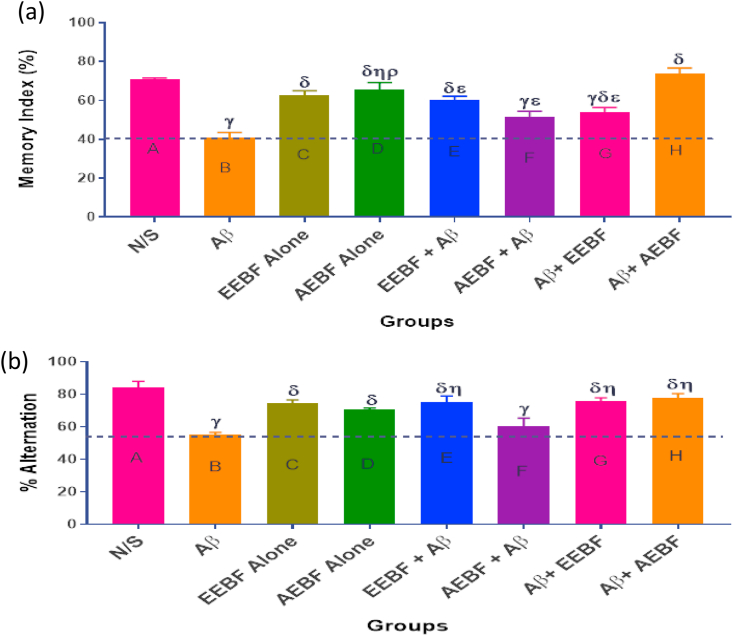
3.4. Effect of treatment on Novel Object recognition (non-spatial memory test) and Y-maze exploratory activity (spatial memory test)
A significantly (p < 0.05) increased cognitive ability was observed in animals in group A (N/S) when compared with those in groups B (Aβ), G (Aβ+EEBF) and F (AEBF + Aβ). Also a significant (p < 0.05) decrease in cognition was observed in animals in group B (Aβ) when compared with every other group except F (AEBF + Aβ) as represented in Figure 3a, this same observation was seen in Figure 3b during the exploratory activity of each group of rats in Y-Maze apparatus.
Figure 3.
Effect of treatment on (a) Memory index as recorded during the NOR test. (b) Spontaneous alternation of rats. The following symbols signify the levels of significance at p < 0.05 across groups, using ANOVA and Tukey’s posthoc test, γ = significantly different from N/S, δ = significantly different from Aβ, η = significantly different from AEBF + Aβ, ε = significantly different from Aβ+AEBF.
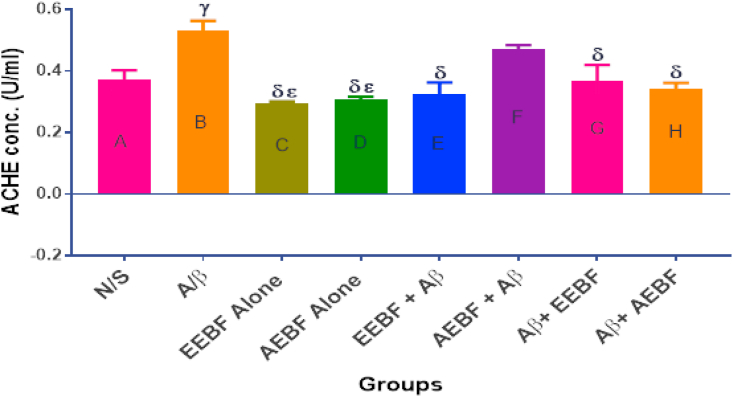
3.5. Effect of treatment on acetylcholinesterase concentration in rats’ hippocampus
Figure 4 shows the concentration of AChE in the hippocampus after the different treatment regimens. AChE concentration was observed to increase significantly (p < 0.05) in the Aβ group in comparison with all other groups except the group pre-treated with the aqueous extract (ABBF + Aβ).
Figure 4.
Effect of treatments on hippocampal acetylcholinesterase level. The following symbols signify the levels of significance at p < 0.05 across groups, using ANOVA and Tukey’s posthoc test, γ = significantly different from N/S, δ = significantly different from A/β, ε = significantly different from AEBF + Aβ.
3.6. Effect of treatment on lipid peroxidation and antioxidant enzyme in rats’ hippocampus and blood plasma
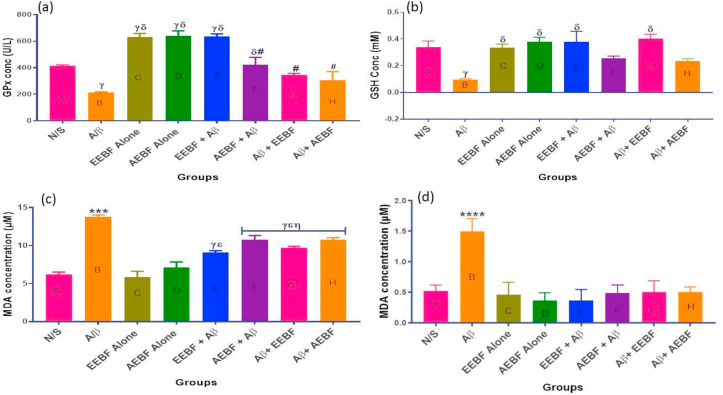
The result of treatment on antioxidants and lipid peroxidation is shown in Figure 5. It was observed in this study that the extract given was able to increase the level of Glutathione peroxidase (GPx), above the control level especially in the AEBF, EEBF and EEBF + Aβ groups as observed in Figure 5a. Reduced glutathione (GSH) was equally observed (Figure 5b) to decrease significantly (p < 0.05) in the untreated group (Aβ) in comparison with every other group except the aqueous extract treated groups. Likewise, the product of lipid peroxidation (MDA) was observed to increase significantly in the hippocampal homogenate and blood plasma of rats in the untreated group (Aβ) in comparison with every other group as seen in Figure 5c and d.
Figure 5.
Showing the levels of some antioxidant markers and product of lipid peroxidation in the hippocampus and blood; (a) Hippocampal Glutathione peroxidase (GPx) level, (b) Reduced glutathione (GSH) level in the blood, (c) Hippocampal Malondialdehyde (MDA) concentration and (d) MDA conc. in the blood. The following symbols signify the levels of significance at p < 0.05 across groups, using ANOVA and Tukey’s posthoc test, # = significantly different from groups C, D and E, ∗∗∗ = significantly different from all groups; γ = sig diff from N/S, δ = sig diff from Aβ, ε = sig diff from EEBF Alone, η = sig diff from AEBF Alone, and ρ = significantly different from EEBF + Aβ.
3.7. Effect of various treatment interventions on plasma C-Reactive protein (CRP)
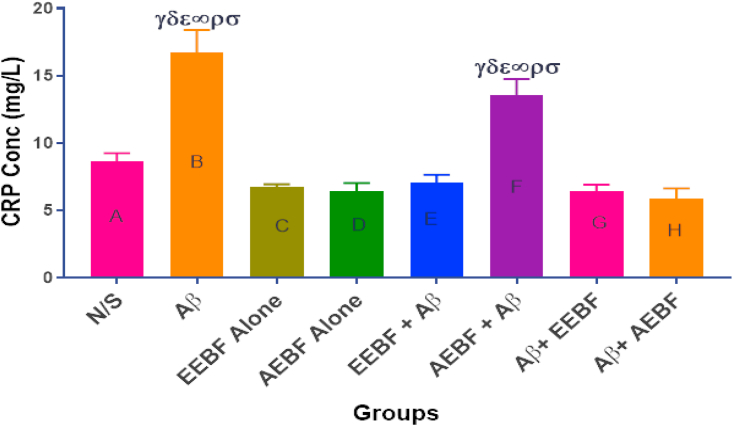
CRP concentration increased in the untreated group (Aβ) and the group pre-treated with an aqueous extract of Bf (AEBF + Aβ) when compared with all other groups as presented in Figure 6.
Figure 6.
C-Reactive Protein (CRP) concentration as measured in the blood after the different treatment regimen. The following symbols depict a level of significance at p < 0.05 across groups; γ = sig diff from N/S, δ = sig diff from EEBF Alone, ε = sig diff from AEBF Alone, ∞ = sig diff from EEBF + Aβ, ρ = sig diff from Aβ+EEBF and σ = sig diff from Aβ+AEBF.
3.8. Effects of treatments on hippocampal Na+/K + -ATPase concentration and glutamate level
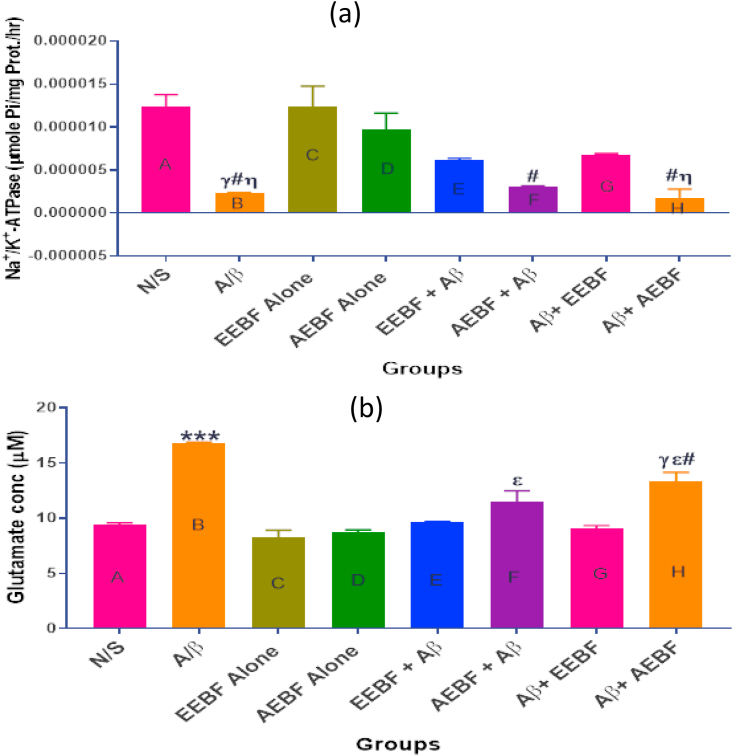
Results observed in Figure 7a showed a decreased hippocampal level of Na+/K + -ATPase in the untreated group (Aβ) as compared with the control groups. However, the figure also shows that the ethanol extract (both the pre-treatment and post-treatment models) was able to slightly restore the Na+/K + ATPase concentration towards normal though it was not significantly (p > 0.05) different either from the N/S or Aβ groups. Figure 7b represents the hippocampal concentration of glutamate. The group that received only Aβ had a significantly (p < 0.05) increased level of glutamate (glutamate excitotoxicity) in comparison to every other group. On the other hand, results showed that the ethanol extracts (pre and post-treatment) prevented glutamate excitotoxicity to a great extent.
Figure 7.
Effect of treatment on (a) hippocampal Na+/K+-ATPase (b) hippocampal glutamate concentration of rats in each group after different treatment interventions. The following symbols signify the levels of significance at p < 0.05 across groups, using ANOVA and Tukey’s posthoc test, γ = significantly different from N/S, ε = significantly different from EEBF alone, η = significantly different from AEBF alone, # = significantly different from groups D (AEBF alone), E (EEBF + Aβ) and F (AEBF + Aβ).
3.9. Effects of treatments on plasma lipid profiles
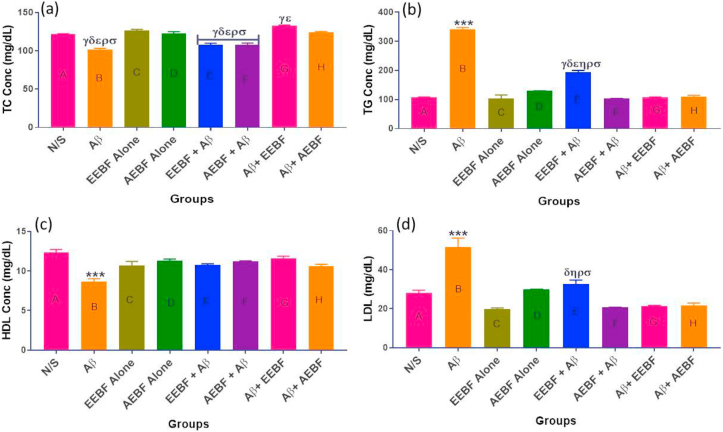
Total cholesterol (TC), Triglyceride (TG), High-Density Lipoprotein (HDL) and Low-Density Lipoprotein (LDL) concentrations are represented in Figure 8a, b, c, and d respectively. It was observed that the concentration of total cholesterol which consists of the good cholesterol (HDL) was significantly high in the control (N/S), the extract control (EEBF and AEBF alone) and post-treated (Aβ+EEBF and Aβ+AEBF) groups in comparison with the untreated group (Aβ). Likewise, the untreated group presented with a significantly (p < 0.05) high level of LDL and triglyceride with a significantly low level of HDL (p < 0.05) in comparison with all other groups.
Figure 8.
Showing the levels of blood lipid profiles in mg/dL; (a) Total cholesterol (TC), (b) Triglyceride (TG), (c) High Density Lipoprotein (HDL) and (d) Low Density Lipoprotein (LDL). The following symbols signify the levels of significance at p < 0.05 across groups, using ANOVA and Tukey’s posthoc test, ∗∗∗ = significantly different from all groups; γ = sig diff from N/S, δ = sig diff from EEBF Alone, ε = sig diff from AEBF Alone, η = sig diff from AEBF + Aβ, ρ = sig diff from Aβ+EEBF and σ = sig diff from Aβ+AEBF.
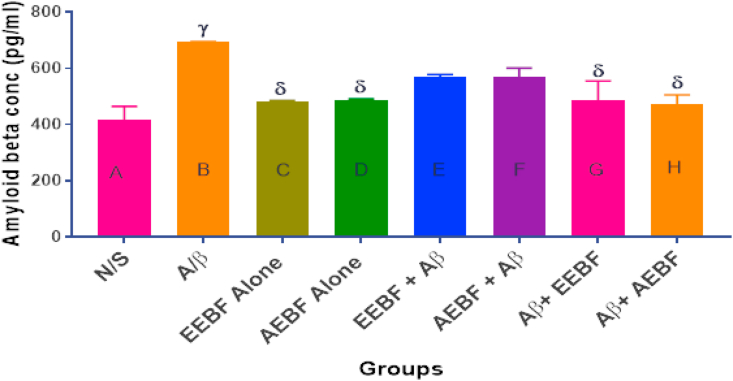
3.10. Amyloid-beta concentration in the whole brain after treatment
In Figure 9, a significant (p < 0.05) increase in Aβ1-42 concentration was observed in the brain of rats in group B (Aβ) when compared with the vehicle control group (N/S), those in groups C and D (EEBF and AEBF alone), G and H (post-treatment models). However, the pre-treatment models had no observed significant (p > 0.05) change in comparison with all other groups.
Figure 9.
Effect of treatments on hippocampal Aβ level. The following symbols signify the levels of significance at p < 0.05 across groups, using ANOVA and Tukey’s posthoc test, γ = significantly different from N/S, δ = significantly different from A/β.
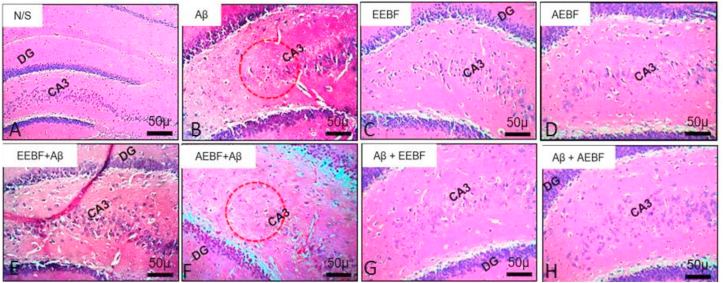
3.11. Effects of treatments on hippocampal architecture using H&E stains
Figure 10 shows the dentate gyrus of the hippocampus which is the site of neurogenesis and it is responsible for learning, memory and mood. The N/S, EEBF and AEBF groups showed the presence of the granule cells in the granular cell layer which was smaller in the untreated group (Aβ) and the presence of some pyramidal neurons in the molecular layer. Aβ (untreated group) revealed the presence of deposits and large vacuoles in the molecular layer of the hippocampus suggesting cell death. The post-treated models (Aβ+EEBF and Aβ+AEBF) revealed the presence of granule cells in the granular layer similar to the control animals, however; they had scanty pyramidal cells in the molecular layer than the control with few vacuolations. Likewise, the pre-treated models presented with the presence of vacuoles in the granular cell layer and molecular layer suggesting cell death, though not as much as that of the untreated group. Ethanol extract mitigated the deleterious effect of Aβ on the hippocampus than the aqueous extract.
Figure 10.
Effect of treatments on the dentate gyrus and CA3 of the hippocampus of experimental animals. This revealed the presence of granule cells in the granular layer and some pyramidal neurons in the molecular layer in the vehicle and extract control groups. These were not prominent in the untreated group as the cells became degenerated while the degeneration was prevented in the post treated models, it was mildly preserved in the pre-treated model.
4. Discussions and conclusion
Oxidative stress occurs as a result of an imbalance between Reactive Oxygen species and the antioxidant defense systems and this forms the basis of various diseases. The ethanol extract showed a better antioxidant activity at all concentrations with a significantly higher DPPH scavenging ability and ABTS scavenging ability and this implies that the ethanol extract has a higher free radical scavenging ability, hence, can proffer a redox-functioned proton ion for unstable free radicals thereby could play a vital role in stabilizing detrimental free radicals within the human body [35]. ICV injection of Aβ1–42 significantly decreased the levels of glutathione peroxidase (GPx) and reduced glutathione (GSH) and it invariably increased malondialdehyde (MDA) level in the blood plasma and hippocampus homogenates of experimental rats. This shows a connection between an increase in oxidative stress and AD due partly to enhanced production of oxygen species and loss of different antioxidant enzymes [36, 37]. However, BF extracts, (especially the ethanol extract) were able to increase the level of GPx and GSH reversing the influence of Aβ42.
Impairment in cognitive performance of rat model of Aβ1–42 was observed in this study. Accordingly, a few investigations have revealed that aggregation of Aβ causes synaptic dysfunction and neurodegeneration particularly in the hippocampal neurons and working memory [38, 39] and a decrease in spontaneous alternations percentage within the Y-maze task [40, 41]. Cognitive decline and impairment of short-term memory caused by Aβ42 were significantly reversed by BF extracts suggesting that our extract can enhance cognitive ability. Acetylcholinesterase (ACHE) is the target employed by the cholinesterase inhibitors for addressing cholinergic deficits in AD [42]. The reduction of AChE in explicit regions of the brain prompts difficulty in learning and memory [43] as equally observed in this study but the administered extracts decreased the level and consequently alleviated the effect of AChE, thereby enhancing cognitive functions.
C-reactive protein (CRP) has been documented to take an integral part in the systemic response to inflammation and it is usually measured as a biomarker of acute inflammation [44] because of its immediate increase in response to inflammation and infection. Studies have observed neuronal expression of CRP in AD post-mortem brains [45, 46] and chronic activation of immune response was reported to be involved in the etiology, advancement and prognosis of several neurodegenerative diseases such as AD and PD and this leads to cognitive dysfunction [47]. A significantly increased serum CRP concentration in the untreated group signifies an increase in systemic inflammation in response to Aβ1-42 ICV injection. However, BF extract especially, the ethanol extract showed a better ameliorative effect by suppressing inflammation and invariably CRP production.
Decreased Na/K-ATPase activity has been implicated as one of the early markers of AD [48, 49, 50]. Na/K-ATPase plays a key role in maintaining ion balance and resting potential and hence is important for the regulation of neuronal excitability, and synaptic transmission. Many literary reviews have associated a decrease in the activity of Na/K-ATPase to precede the course of neurodegeneration rather than being its outcome [49, 50, 51, 52]. Glutamate is the primary excitatory neurotransmitter in the central nervous system of mammals [53, 54]. It plays a major role in the physiology of the central nervous system because it controls numerous activities such as memory, learning, cognitive, emotional, endocrine and other visceral functions. Glutamate excitotoxicity is caused by excessive release of glutamate from presynaptic nerve terminals and astrocytes into the extracellular space and this may occur as a result of either over-stimulation of both ionotropic and metabotropic glutamate receptors promoting the excessive release, decreased uptake, or modification of receptor functions [55] and this is common in some neurodegenerative disorders such as AD, Huntington's disease, Parkinson's disease e.t.c [56]. Glutamate excitotoxicity and Na+/K + -ATPase are linked because the glial glutamate carriers (GluTs) that mediate glutamate uptake depend solely on the Na+/K + -ATPase electrochemical gradient. Therefore, reduced Na+/K + -ATPase activity in this study could be responsible for decreased uptake of glutamate from the synaptic cleft which might have been accountable for the observed glutamate excitotoxicity under the influence of Aβ42. However, the ethanol extract of BF was able to mitigate glutamate excitotoxicity through the suppression of Na+/K + -ATPase activity.
The role of lipids in the diagnosis and advancement of AD remains unclear because blood lipid profiles have recently been vaguely reported as biomarkers of AD. Though it is believed that the presence of Aβ in the brain is the key trigger of pathological processes observed in AD, the relationship between AD and dyslipidemia has not been well established. Therefore, this study was designed to explore if a relationship exists between Aβ-induced AD and cholesterol dysfunction. Higher total cholesterol was not directly associated with AD dementia in this study. However, an increase in low-density lipoprotein cholesterol (LDL-c) and triglyceride correlated positively with dementia while a decrease in high-density lipoprotein cholesterol (HDL-c) was observed with AD progression. In spite of conflicting documented works of literature on the relationship between hypercholesterolemia and AD dementia, it has been reported that a higher level of LDL-c is usually associated with earlier AD onset [57] through the induction of cortical amyloid deposition [58]. However, Borroni et al. [59], reported a correlation between higher total cholesterol and faster cognitive decline in AD patients using cholinesterase inhibitors. In line with observed dyslipidemia caused by Aβ42 peptides, extracts from Bacopa floribunda showed great therapeutic effect through the reversal of Aβ1-42-induced dyslipidemia, especially at the post-treated level.
Likewise, one of the metabolic features in neurodegenerative diseases pathology, including Alzheimer’s disease is an alteration in lipid composition of cells of the central nervous system. Hence, lipotoxicity has been linked to predisposing tissues to cellular damage, leading to inhibition of ATP synthesis [60] and invariably ATP depletion. Therefore, the observed depression of hippocampal Na+/K+-ATPase activity in the untreated group (Aβ) in this study is traceable to lipotoxicity and accompanied by increased amyloid-beta concentration. However, the BF extract (especially the ethanol extract) suppressed the accumulation of lipids, preventing lipotoxicity and elevated Aβ concentration.
To further test the potency of our extract, we measured endogenous Aβ 1–42 concentration after the whole treatment and the post-treated models demonstrated promising therapeutic effect against Aβ accumulation. Photomicrographs obtained from H&E stains supported the biochemical observations in this study because the dentate gyrus was spared from the toxic effect of Aβ under the influence of BF extract, especially, the ethanol extract. Thus, our results revealed that BF extracts demonstrated a robust protective action against Aβ1-42-induced damage and it is, therefore, considered to play a significant role in relieving some symptoms associated with AD dementia. Finally, the effects of BF observed in this study could have been mediated by the summation of activities of several phytochemicals within the plant (synergism or additive actions) or by a single component of the different phytochemicals.
5. Conclusion
Conclusively, Bacopa floribunda is an efficient cognitive enhancer that can help alleviate the symptoms of Alzheimer’s disease, hence, can be considered an alternative supply medicine for relieving memory impairment. Though, the ethanol extract had a better ameliorative effect than the aqueous extract. The phytochemical analysis revealed more saponins and alkaloids in ethanol extract as compared to the aqueous extract, hence the observed differences. Therefore, the various components of this extract responsible for its mechanisms of action should be further examined.
5.1. Limitation of the study
Renal and liver function tests were not included in the design of this study; therefore, the effect of these extracts could not be elucidated in the present study.
Declarations
Author contribution statement
Oyeleke, Mosunmola Busayo:Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Oni, Heritage Tolulope:Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Arokoyo, Oluwatamilore Lois:Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Owoyele, Bamidele Victor:Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.NIA . 2016. Alzheimer’s and Related Dementias Education and Referral (ADEAR) Center.www.nia.nih.gov/alzheimers [Google Scholar]
- 2.Adewusi E.A., Moodley N., Steenkamp V. Medicinal plants with cholinesterase inhibitory activity: a Review. Afr. J. Biotechnol. 2010;9(49):8257–8276. [Google Scholar]
- 3.Alzheimer's Association Alzheimer's disease facts and figures. Alzheimer's Dementia. 2018;14(5):701. [Google Scholar]
- 4.Aguilar L.R., Acosta-Uribe J., Giraldo M.M., Moreno S., Baena A., Alzate D., Cuastumal R., Aguillón D., Madrigal L., Saldarriaga A., et al. Genetic origin of a large family with a novel PSEN1 mutation (Ile416Thr) Alzheimer's Dementia. 2019;15(5):709–719. doi: 10.1016/j.jalz.2018.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crews L., Masliah E. Molecular mechanisms of neurodegeneration in Alzheimer’s disease. Hum. Mol. Genet. 2010;19:R12–R20. doi: 10.1093/hmg/ddq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selkoe D.J. Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat. Cell Biol. 2004;6(11):1054–1061. doi: 10.1038/ncb1104-1054. [DOI] [PubMed] [Google Scholar]
- 7.Tuppo E.E., Arias H.R. The role of inflammation in Alzheimer’s disease. Int. J. Biochem. Cell Biol. 2005;37(2):289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Bharadwaj P.R., Dubey A.K., Masters C.L., Martins R.N., Macreadie I.G. Aβ aggregation and possible implications in Alzheimer’s disease pathogenesis. J. Cell Mol. Med. 2009;13:412–421. doi: 10.1111/j.1582-4934.2009.00609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigue K.M., Kennedy K.M., Park D.C. Beta-amyloid deposition and the aging brain. Neuropsychol. Rev. 2009;19:436–450. doi: 10.1007/s11065-009-9118-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi L., Baird Alison L., Sarah Westwood, Hye A., Dobson R., Thambisetty M., Lovestone S. A decade of blood biomarkers for Alzheimer’s disease research: an evolving field, improving study designs, and the challenge of replication. J. Alzheim. Dis. 2018;62(3):1181–1198. doi: 10.3233/JAD-170531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shepardson N.E., Shankar G.M., Selkoe D.J. Cholesterol level and statin use in Alzheimer disease, I: review of epidemiological and preclinical studies. Arch. Neurol. 2011;(68):1239–1244. doi: 10.1001/archneurol.2011.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsuzaki T., Sasaki K., Hata J., Hirakawa Y., Fujimi K., Ninomiya T., Suzuki S.O., Kanba S., Kiyohara Y., Iwaki T. Association of Alzheimer disease pathology with abnormal lipid metabolism: the Hisayama Study. Neurology. 2011;77:1068–1075. doi: 10.1212/WNL.0b013e31822e145d. [DOI] [PubMed] [Google Scholar]
- 13.Sung S.H., Kang S.Y., Lee K.Y., Park M.J., Kim J.H., Park J.H., Kim Y.C., Kim J., Kim Y.C. (+)-Alpha-viniferin, a stilbene trimer from Caragana chamlague, inhibits acetylcholinesterase. Biol. Pharm. Bull. 2002;25:125–127. doi: 10.1248/bpb.25.125. [DOI] [PubMed] [Google Scholar]
- 14.Allain H., Bentué-Ferrer D., Tribut O., Gauthier S., Michel B.F., Rochelle C.D.L. Alzheimer’s disease: the pharmacological pathway. Fundam. Clin. Pharmacol. 2003;17(4):419–428. doi: 10.1046/j.1472-8206.2003.00153.x. [DOI] [PubMed] [Google Scholar]
- 15.Cummings J., Lee G., Mortsdorf T., Ritter A., Zhong K. Alzheimer’s disease drug development pipeline: 2017. Alzheimer’s & Dementia. Translational Research & Clinical Interventions. 2017;3:367–384. doi: 10.1016/j.trci.2017.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nazem A., Mansoori G. Nanotechnology solutions for Alzheimer’s disease: advances in research tools, diagnostic methods and therapeutic agents. J. Alzheim. Dis. 2008;13:199–223. doi: 10.3233/jad-2008-13210. [DOI] [PubMed] [Google Scholar]
- 17.Cyril-Olutayo C.M., Oladele A.T., Elufioye T.O. Ethnobotanical survey of plants used as memory enhancer and antiaging in Ondo state, Nigeria. Int. J. Pharm. 2012;2(1):26–32. [Google Scholar]
- 18.Elufioye T.O., Oladele A.T., Cyril-Olutayo C.M., Agbedahunsi J.M., Adesanya S.A. Ethnomedicinal study and screening of plants used for memory enhancement and antiaging in Sagamu, Nigeria. Eur. J. Med. Plants. 2012;2(3):262–275. [Google Scholar]
- 19.Olatunji B.P., Fasola T.R., Onasanwo S.A., Akinyemi A.J., Adeniyi P.A., Ishola A.O. Neuronal alterations and antioxidant status of lipopolysaccharide induced neuronal damage in mice: efficacy of three medicinal plants. J. Appl. Pharmaceut. Sci. 2017;7(12):156–162. [Google Scholar]
- 20.Diaz A., Escobedo C., Treviño S., Chávez R., Lopez-Lopez G., Moran C., Guevara J., Venegas B., Muñoz-Arenas G. Metabolic syndrome exacerbates the recognition memory impairment and oxidative-inflammatory response in rats with an intrahippocampal injection of amyloid beta 1–42. Oxid. Med. Cell. Longev. 2018 doi: 10.1155/2018/1358057. 1358057. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–ciocalteau’s reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 22.Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. J. Food Chem. 2005;91(3):571–577. [Google Scholar]
- 23.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorisation assay. J. Free Radic. Biol. Med. 1999;26(9):1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 24.Gyamfi M.A., Yonamine M., Aniya Y. Free-radical scavenging action of medicinal herbs from Ghana: thonningiasanguinea on experimentally-induced liver injuries. J. Gener. Pharmacol. 1999;32(6):661–667. doi: 10.1016/s0306-3623(98)00238-9. [DOI] [PubMed] [Google Scholar]
- 25.Harborne J.B. second ed. Chapman & Hall; London: 1984. Phytochemical Methods - a Guide to Modern Techniques of Plant Analysis; pp. 4–16. [Google Scholar]
- 26.Davis J. Food Safety Watch; 2006. Glycoalkaloids.http://www.foodsafetywatch [Google Scholar]
- 27.Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983;54(4):275–287. doi: 10.1007/BF01234480. [DOI] [PubMed] [Google Scholar]
- 28.Hritcu L., Cioanca O., Hancianu M. Effects of lavender oil inhalation on improving scopolamine induced spatial memory impairment in laboratory rats. Phytomedicine. 2012;19(6):529–534. doi: 10.1016/j.phymed.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Grayson B., Leger M., Piercy C., Adamson L., Harte M., Neill J.C. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav. Brain Res. 2015;285:176–193. doi: 10.1016/j.bbr.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 30.Colado M.I., O'Shea E., Granados R., Murray T.K., Green A.R. In vivo evidence for free radical involvement in the degeneration of rat brain 5-HT following administration of MDMA (‘ecstasy') and p-chloroamphet amine but not the degeneration following fenfluramine. Br. J. Pharmacol. 1997;121:889–900. doi: 10.1038/sj.bjp.0701213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sinet P.M., Michelson A.M., Bazin A., Lejeune J., Jeerome H. Increase in glutathi18.One peroxidase activity in erythrocytes from trisomy 21 subjects. Biochem. Biophys. Res. Commun. 1975;67:910–915. doi: 10.1016/0006-291x(75)90763-9. [DOI] [PubMed] [Google Scholar]
- 32.Wyse A.T.S., Streck E.L., Worm P., Wajner A., Ritter F., Netto C.A. Preconditioning prevents the inhibition of Na+, K+-ATPase activity after brain ischemia. Neurochem. Res. 2000;25:971–975. doi: 10.1023/a:1007504525301. [DOI] [PubMed] [Google Scholar]
- 33.Chan K., Delfer D., Junger K.D. A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal. Biochem. 1986;57:375–380. doi: 10.1016/0003-2697(86)90640-8. [DOI] [PubMed] [Google Scholar]
- 34.Ellman G.L., Courtney K.D., Andres V., Jr., Feather-Sotne R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 35.Postua P.A., Sadiki F.Z., Idrissi M.E., Cioanca O., Trifan A., Hancianu M., Hritcu L. Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1-42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmacother. 2019;112:108673. doi: 10.1016/j.biopha.2019.108673. [DOI] [PubMed] [Google Scholar]
- 36.Gella A., Durany N. Oxidative stress in Alzheimer disease. Cell Adhes. Migrat. 2009;3:88–93. doi: 10.4161/cam.3.1.7402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y., Zhao B. Oxidative stress and the pathogenesis of Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2013;2013:10. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y., Wang R., Dong Y., Tucker D., Zhao N., Ahmed M.E., Zhu L., Liu T.C., Cohen R.M., Zhang Q. Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol. Aging. 2017;49:165–182. doi: 10.1016/j.neurobiolaging.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai H.Y., Wang Z.J., Hölscher C., Yuan L., Zhang J., Sun P., Li J., Yang W., Wu M.N., Qi J.S. Lixisenatide attenuates the detrimental effects of amyloid b protein on spatial working memory and hippocampal neurons in rats. Behav. Brain Res. 2017;318:28–35. doi: 10.1016/j.bbr.2016.10.033. [DOI] [PubMed] [Google Scholar]
- 40.Hritcu L., Noumedem J.A., Cioanca O., Hancianu M., Kuete V., Mihasan M. Methanolic extract of Piper nigrum fruits improves memory impairment by decreasing brain oxidative stress in amyloid beta(1–42) rat model of Alzheimer’s Disease. Cell. Mol. Neurobiol. 2014;34:437–449. doi: 10.1007/s10571-014-0028-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cioanca O., Hritcu L., Mihasan M., Hancianu M. Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid beta (1–42) rat model of Alzheimer’s disease. Physiol. Behav. 2013;120:193–202. doi: 10.1016/j.physbeh.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 42.Hlila M.B., Mosbah H., Mssada K., Jannet H.B., Aouni M., Selmi B. Acetylcholinesterase inhibitory and antioxidant properties of roots extracts from the Tunisian Scabiosa arenaria Forssk. Ind. Crop. Prod. 2015;67:62–69. [Google Scholar]
- 43.Kar S., Slowikowski S.P., Westaway D., Mount H.T. Interactions between beta-amyloid and central cholinergic neurons:implications for Alzheimer’s disease. J. Psychiatry Neurosci. 2004;29:427–441. [PMC free article] [PubMed] [Google Scholar]
- 44.Black S., Kushner I., Samols D. C-reactive protein. J. Biol. Chem. 2004;279:48487–48490. doi: 10.1074/jbc.R400025200. [DOI] [PubMed] [Google Scholar]
- 45.Yasojima K., Schwab C., McGeer E.G., McGeer P.L. Human neurons generate C-reactive protein and amyloid P: upregulation in Alzheimer's disease. Brain Res. 2000;887:80–89. doi: 10.1016/s0006-8993(00)02970-x. [DOI] [PubMed] [Google Scholar]
- 46.Mancinella A., Mancinella M., Carpinteri G., Bellomo A., Fossati C., Gianturco V., Jori A., Ettorre E., Troisi V., Marigliano V. Is there a relationship between high C-reactive protein (CRP) levels and dementia? Arch. Gerentol. Geriatr. 2009;49:185–194. doi: 10.1016/j.archger.2009.09.028. [DOI] [PubMed] [Google Scholar]
- 47.Amor S., Peferoen L.A.N., Vogel D.Y.S., Breur M., van der Valk P., Baker D., van Noort J.M. Inflammation in neurodegenerative diseases - an update. Immunology. 2014;142:151–166. doi: 10.1111/imm.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kairane C., Roots K., Uusma T., Bogdanovic N., Karelson E., Kõks S., Zilmer M. Regulation of the frontocortical sodium pump by Na+ in Alzheimer’s disease: difference from the age-matched control but similarity to the rat model. FEBS Lett. 2002;531:241–244. doi: 10.1016/s0014-5793(02)03510-x. [DOI] [PubMed] [Google Scholar]
- 49.Dickey C.A., Gordon M.N., Wilcock D.M., Herber D.L., Freeman M.J., Morgan D. Dysregulation of Na+/K+ ATPase by amyloid in APP+PS1 transgenic mice. BMC Neurosci. 2005;2:1–7. doi: 10.1186/1471-2202-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vitvitsky V.M., Garg S.K., Keep R.F., Albin R.L., Banerjee R. Na+ and K+ ion imbalances in Alzheimer’s disease. Biochim. Biophys. Acta. 2012;1822:1671–1681. doi: 10.1016/j.bbadis.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gu Q.B., Zhao J.X., Feib J., Sshwarz W. Modulation of Na-K pumping and neurotransmitter uptake by b-amyloid. Neuroscience. 2004;126:61–67. doi: 10.1016/j.neuroscience.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Pákáski M., Kálmán J. Interactions between the amyloid and cholinergic mechanisms in Alzheimer’s disease. Neurochem. Int. 2008;53:103–111. doi: 10.1016/j.neuint.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Fairman W.A., Amara S.G. Functional diversity of excitatory amino acid transporters: ion channel and transport modes. Am. J. Physiol. 1999;277(4):F481–F486. doi: 10.1152/ajprenal.1999.277.4.F481. [DOI] [PubMed] [Google Scholar]
- 54.Ezza H.S.A., Khadrawyb Y.A. Glutamate excitotoxicity and neurodegeneration. J. Mol. Genet. Med. 2014;8:141. [Google Scholar]
- 55.Gagliardi R.J. Neuroprotection, excitotoxicity and NMDA antagonists. Arq. Neuro. Psiquiatr. 2000;58:583–588. doi: 10.1590/s0004-282x2000000300030. [DOI] [PubMed] [Google Scholar]
- 56.Mattson M. Excitotoxic and excitoprotective mechanisms: abundant targets for the prevention and treatment of neurodegenerative disorders. Neuromolec. Med. 2003;3:65–94. doi: 10.1385/NMM:3:2:65. [DOI] [PubMed] [Google Scholar]
- 57.De Oliveira F.F., Bertolucci P.H.F., Chen E.S., Smith M.C. Risk factors for age at onset of dementia due to Alzheimer’s disease in a sample of patients with low mean schooling from São Paulo, Brazil. Int. J. Geriatr. Psychiatr. 2014;29:1033–1039. doi: 10.1002/gps.4094. [DOI] [PubMed] [Google Scholar]
- 58.Rodríguez E., Mateo I., Infante J., Llorca J., Berciano J., Combarros O. Cholesteryl ester transfer protein (CETP) polymorphism modifies the Alzheimer’s disease risk associated with APOE 4 allele. J. Neurol. 2006;253:181–185. doi: 10.1007/s00415-005-0945-2. [DOI] [PubMed] [Google Scholar]
- 59.Borroni B., Pettenati C., Bordonali T., Akkawi N., Di Luca M., Padovani A. Serum cholesterol levels modulate long-term efficacy of cholinesterase inhibitors in Alzheimer disease. Neurosci. Lett. 2003;343:213–215. doi: 10.1016/s0304-3940(03)00336-7. [DOI] [PubMed] [Google Scholar]
- 60.Abdul-Ghani M.A., Muller F.L., Liu Y., Chavez A.O., Balas B., Zuo P., Chang Z., Tripathy D., Jani R., Molina-Carrion M., Monroy A., Folli F., Van Remmen H., DeFronzo R.A. Deleterious action of FA metabolites on ATP synthesis: possible link between lipotoxicity, mitochondrial dysfunction and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2008;295(3):E678–E685. doi: 10.1152/ajpendo.90287.2008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.