Abstract
A unique series of simple “unnatural” nucleosides has been discovered to inhibit hepatitis B virus (HBV) replication. Through structure-activity analysis it was found that the 3′-OH group of the β-l-2′-deoxyribose of the β-l-2′-deoxynucleoside confers specific antihepadnavirus activity. The unsubstituted nucleosides β-l-2′-deoxycytidine, β-l-thymidine, and β-l-2′-deoxyadenosine had the most potent, selective, and specific antiviral activity against HBV replication. Human DNA polymerases (α, β, and γ) and mitochondrial function were not affected. In the woodchuck model of chronic HBV infection, viral load was reduced by as much as 108 genome equivalents/ml of serum and there was no drug-related toxicity. In addition, the decline in woodchuck hepatitis virus surface antigen paralleled the decrease in viral load. These investigational drugs, used alone or in combination, are expected to offer new therapeutic options for patients with chronic HBV infection.
Infection with hepatitis B virus (HBV) is a major world health problem, affecting 5% of the population. More than 2 billion people have been infected with the virus, and 350 million of them are chronic carriers at risk of death from cirrhosis and liver cancer (49).
Several strategies have been evaluated for the treatment of chronic HBV infection with the goal of eliminating persistent viral replication and preventing progression to chronic active hepatitis and liver failure. Currently, the only approved treatment options are alpha interferon (IFN) and lamivudine (β-l-2′,3′-dideoxy-3′-thiacytidine [3TC]). Unfortunately, the rate of response to IFN is low, and drug-associated side effects are significant (24, 55). Individuals who are immunosuppressed (e.g., transplant recipients or those coinfected with the human immunodeficiency virus [HIV]) rarely respond to IFN therapy (13). Lamivudine is a well-known example of the class of β-l-nucleoside analogs that has recently drawn attention as antiviral and anticancer agents (52). As with IFN, however, a complete antiviral response, as assessed by HBe seroconversion, is seen in only a minority of patients after 1 year of treatment (27). In addition, cessation of lamivudine therapy or development of viral resistance may lead to a marked rebound in viral replication which can be life threatening (hepatitis flare) in HIV-HBV-coinfected patients (2, 30). Lamivudine resistance is now recognized in 16 to 32% of HBV-infected patients after 1 year of treatment and in as many as 58% after 2 to 3 years (14, 27, 30).
Since the Food and Drug Administration approved lamivudine for the treatment of HIV infection in the United States in 1996 and for HBV in 1998, intensive studies on “unnatural” l-nucleosides as agents against HIV, HBV, and herpesviruses (including Epstein-Barr virus [EBV]) and as anticancer agents have been conducted (23). Now, through an extensive structure-activity analysis, we have found that the 3′-OH group of the β-l-2′-deoxyribose of the β-l-2′-deoxynucleoside series confers unique specificity for anti-HBV activity. In this chemical series, β-l-2′-deoxycytidine (l-dC), β-l-thymidine (l-dT), and β-l-2′-deoxyadenosine (l-dA) had the most potent, selective, and specific activity against HBV replication.
MATERIALS AND METHODS
Chemicals.
l-dC, l-dT, and l-dA were synthesized as previously described (D. Dukhan, C. Pierra, M. Bryant, J.-P. Sommadossi, J.-L. Imbach, and G. Gosselin, Abstr. 13th Int. Conf. Antiviral Res., abstr. 89, 2000; C. Pierra, D. Dukhan, M. Bryant, J.-P. Sommadossi, J.-L. Imbach and G. Gosselin, Abstr. 13th Int. Conf. Antivir. Res., abstr. 90, 2000). The 5′-triphosphates of l-dT, l-dC, and l-dA were chemically synthesized and characterized using previously described methods (16). Radiolabeled nucleotides were obtained from Moravek Biochemicals, Inc. (Brea, Calif.). All other chemicals were of the highest grade available.
Extracellular HBV DNA analysis.
Inhibition of HBV replication in vitro was assessed using the 2.2.15 cell line as previously described (26). Briefly, cells were cultured at a density of 2 × 105 cells per ml in 24-well plates in Dulbecco minimal essential medium supplemented with 4% dialyzed fetal bovine serum and 0.5 mM l-glutamine for 9 days in the presence or absence of drug, with medium changes every 3 days. The antiviral activities of l-dC, l-dT, and l-dA were determined as the reduction of extracellular virus-associated DNA. HBV DNA isolated from virions was analyzed by Southern blot analysis. Inhibition of viral DNA replication was assessed by comparison of HBV DNA from drug-treated and untreated cultures using hybridization to a 32P-labeled HBV-specific probe followed by autoradiography and densitometry (i.e., detection and quantitation).
HIV antiviral and cytotoxicity assays in PBM cells.
Anti-HIV-1 activity of the compounds was determined in human peripheral blood mononuclear (PBM) cells as previously described (41). Cells were infected with HIV-1LAI at a multiplicity of infection of 0.01. Virus obtained from cell culture supernatant was quantitated on day 6 after infection using a reverse transcriptase assay and (rA)n · (dT)12–18 as a template primer. The toxicity of the compounds was assessed in human PBM cells as previously described (41). The antiviral 50% effective concentration (EC50) and 50% cytotoxic concentration were determined from the concentration-response curve using the median effect method (1).
Virion-associated WHV DNA polymerase assay.
For the woodchuck hepatitis virus (WHV) DNA polymerase assay, virus particles were concentrated from sera of woodchucks chronically infected with WHV. Briefly, virus-containing serum was ultracentrifuged at 55,000 × g using a 30% sucrose gradient supplemented with 1% bovine serum albumin and 1 μM EDTA for 12 h at 4oC. The viral pellet was suspended in 400 μl of Tris-HCl buffer (pH 7.6) containing 10% Nonidet P-40 and 15 mM β-mercaptoethanol. Reaction mixtures in a final volume of 50 μl contained 80 mM Tris-HCl (pH 7.6); 20 mM MgCl2; 60 mM NH4Cl; 100 μM concentrations of dGTP, dATP, dCTP, and TTP with substitution of 0.75 μM [3H]TTP, [3H]dCTP, or [3H]dATP; and various concentrations of the β-l-2′-deoxynucleoside 5′-triphosphate derivative (e.g., l-TTP was assayed in the presence of 0.75 μM [3H]TTP and 100 μM concentrations each of dGTP, dATP, and dCTP). The reaction was started by adding the disrupted virus particles. The mixture was incubated for 2 h at 37oC, and aliquots were spotted onto Whatman DE81 filters. The filters were washed three times with 125 mM Na2HPO4 buffer (pH 7.0) and rinsed once with water and once with ethanol. The filters were dried and counted in 4 ml of scintillation liquid (Econo-safe liquid; Research Products International [RPI], Mount Prospect, Ill.) using a Beckman LS TA 5000 scintillation counter.
Drug cytotoxicity studies in human bone marrow progenitor cells.
Human bone marrow cells were collected by aspiration from the posterior iliac crest of normal healthy donors, and the mononuclear cell population was isolated by Ficoll-Hypaque gradient centrifugation (44). Assays of granulocyte-macrophage CFU (GM-CFU) and erythroid burst-forming units (E-BFU) were performed using a bilayer soft-agar–methylcellulose method described previously (44). After incubation for 14 days in the presence or absence of drug, GM-CFU and E-BFU were counted with an inverted microscope.
Human DNA polymerase assays.
Human DNA polymerases α and γ were a generous gift from William Parker (Southern Research Institute, Birmingham, Ala.) and human DNA polymerase β was purchased from Molecular Biology Resources, Inc. (Milwaukee, Wis.). Reaction mixtures for DNA polymerase α contained 50 mM Tris-HCl (pH 8.0); 10 mM MgCl2; 1.0 mg of bovine serum albumin/ml; 1.0 mM dithiothreitol; 10 μg of activated calf thymus DNA; 50 μM concentrations of dGTP, dATP, dCTP, and TTP with substitution of 0.75 μM [3H]dCTP, [3H]TTP, or [3H]dATP; and various concentrations of the β-l-2′-deoxynucleoside 5′-triphosphate derivative (e.g., l-dCTP was assayed in the presence of 0.75 μM [3H]dCTP and 100 μM concentrations each of dGTP, dATP, and TTP). The DNA polymerase β assay was carried out in the same buffer as for DNA polymerase α except that 60 mM Tris-HCl at pH 8.7, 100 mM KCl, and 15% (vol/vol) glycerol were used. Reactions for the DNA polymerase γ assay were carried out in the buffer used for DNA polymerase α except that 100 mM KCl was added. Reactions were initiated by the addition of 1.5 U of DNA polymerase α, β, or γ. All reaction mixtures were incubated for 60 min at 37oC, and 40-μl aliquots were spotted onto Whatman DE81 filters. The filters were washed and counted as described above.
Mitochondrial and cytotoxicity studies in HepG2 cells.
To measure lactic acid production in drug-treated cultures, HepG2 cells (2.5 × 104 cells/ml) were plated in 12-well culture plates and treated with 10 μM l-dC, l-dT, or l-dA as previously described (8). Briefly, cells were cultured in minimal essential medium with nonessential amino acids supplemented with 10% heat-inactivated dialyzed fetal bovine serum and 1% sodium pyruvate. Cells were cultured for 14 days with medium changes every 4 days. Lactic acid content in the culture media was measured using a lactic acid assay kit (Boehringer Mannheim Corp., Mannheim, Germany). Cell number was determined with a hemocytometer. The effect of the β-l-2′-deoxynucleosides on mitochondrial DNA (mtDNA) content in HepG2 cells was determined essentially as described previously (8). Briefly, after 14 days of incubation in the presence or absence of drug with medium changes every 4 days, cells were lysed, and the DNA was immobilized on nylon membranes. The DNA slot blots were probed with a specific mitochondrial probe to quantitate mtDNA levels. A β-actin probe was used to standardize total DNA loading. For morphological evaluation, HepG2 cells (2.5 × 104 cells/ml) were plated in 35- by 10-mm culture dishes and incubated in the absence or presence of 10 μM l-dA, l-dT, or l-dC for 14 days with medium changes every 4 days. For electron microscopic examination (Hitachi 7000), the cells were fixed, postfixed, dehydrated, embedded, and sectioned as previously described (8). The effect of the β-l-2′-deoxynucleosides on cell growth relative to that of untreated cells was determined by the neutral red dye technique as described previously (39).
Woodchucks.
Chronically infected 16- to 18-month-old male and female woodchucks were stratified into comparable groups of three animals each (four animals in the control group) based on sex, weight, levels of WHV DNA in serum, and γ-glutamyl transpeptidase levels were measured 30 days prior to the start of the study. Each drug was dissolved in water (30 mg/ml), and woodchucks in the treatment group received a daily oral dose of 10 mg of drug/kg of body weight in approximately 5 ml of liquid woodchuck control diet (Dyets, Inc., Bethlehem, Pa.) by dose syringe. The control group received an equivalent volume of water administered with liquid woodchuck control diet. The woodchucks were anesthetized (50 mg of ketamine/kg and 5 mg of xylazine/kg), and body weights were determined and serum samples were taken at the indicated time points.
Viral load determinations.
Serum WHV DNA levels were measured by a dot blot hybridization technique using a full-length (3.2-kb) gel-purified WHV DNA probe as previously described (47). Amounts of viral DNA were determined by reference to a known amount of cloned viral DNA. Serum samples with WHV DNA levels below the limit of detection using the dot blot procedure were reanalyzed by PCR as previously described (34). The WHV core region, nucleotides 2041 to 2411, was amplified with the forward primer 5′-CATTGTTCACCTCACCATACTGCAC-3′ and the reverse primer 5′-GATTGAGACCTTCGTCTGCGAG-3′. The amplified product was bound to nitrocellulose membranes using a 96-well dot blot manifold and quantitated using a genomic WHV probe. A reference serum sample containing 7 × 1010 WHV genomes was used to calibrate the PCRs and to quantitate the amount of WHV DNA present in the woodchuck serum samples. The limit of detection of the WHV DNA detection procedure was approximately 300 WHV genome equivalents/ml of woodchuck serum.
RESULTS AND DISCUSSION
The β-l-2′-deoxynucleoside series is specific for HBV.
The structure-activity relationships (SARs) established among the l-dC, l-dT, and l-dA series are presented in Table 1. Substitution of a halogen atom at the 5 position (R1) in the pyrimidine ring of l-dC, without modification of the deoxyribose sugar (e.g., β-l-2′-deoxy-5-fluorocytidine [l-5-FdC] and β-l-2′-deoxy-5-chlorocytidine [l-5-CldC]), decreased the potency against HBV but did not affect the specificity for HBV. In contrast, analogs of l-dC which lacked the 3′-OH group (R3) on the deoxyribose sugar (e.g., β-l-2′,3′-dideoxycytidine [l-ddC], 3TC, and β-l-2′,3′-didehydro-2′,3′-dideoxycytidine [l-d4C]) lost antiviral specificity for HBV and showed activity against HIV. Similarly, replacement of the 3′-OH group with a 3′-fluoro- moiety (e.g., β-l-2′,3′-dideoxy-3′-fluorocytidine [l-3′-FddC]) eliminated the antiviral specificity, although antiviral potency against HBV and HIV was retained.
TABLE 1.
SARs of l-dC, l-dT, and l-dA analogs
| Compound | R1 | R2 | R3 | X | EC50 (μM)a
|
Structure | |
|---|---|---|---|---|---|---|---|
| Anti-HBV in 2.2.15 cells | Anti-HIV in PBM cells | ||||||
| l-dC | H | H | OH | CH | 0.24 ± 0.08 | >200 | 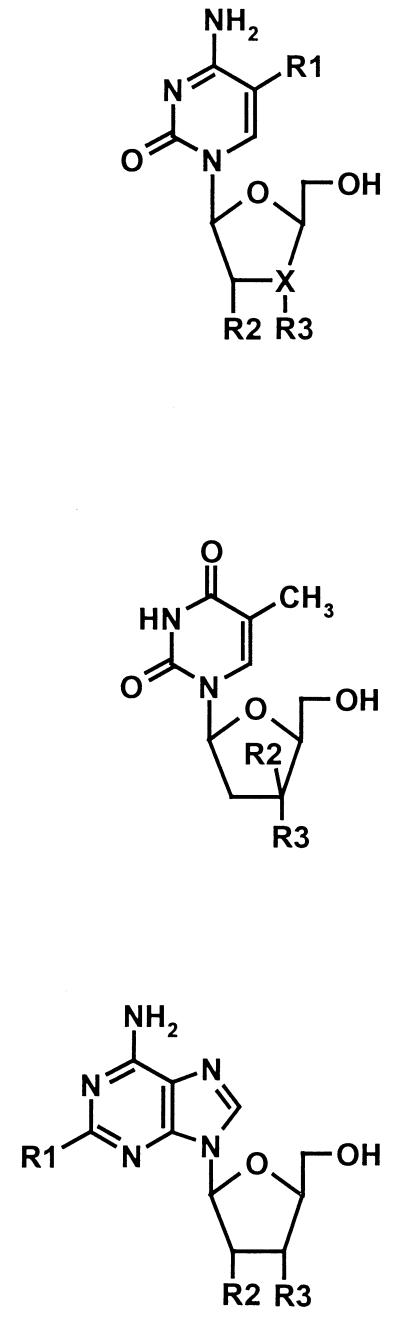 |
| l-5-FdC | F | H | OH | CH | 5 | >100 | |
| l-5-CldC | Cl | H | OH | CH | 10 | >100 | |
| l-ddC | H | H | H | CH | 0.1 | 0.26 | |
| 3TC | H | H | —b | S | 0.05 ± 0.01 | 0.002 | |
| l-3′-azido-5-FddC | F | H | N3 | CH | 0.11 ± 0.09 | 0.05 | |
| l-3′-FddC | H | H | F | CH | 0.5 | 82 | |
| FTC | F | H | — | S | 0.04 | 0.008 | |
| l-5-ClddC | Cl | H | H | CH | 10 | >100 | |
| l-d4C | H | — | — | CH | <0.1 | 1.0 | |
| l-d4FC | F | — | — | CH | <0.1 | 0.034 | |
| l-3′-F-5-FddC | F | — | F | CH | 4 | >100 | |
| l-5-FddC | F | — | — | CH | 0.10 ± 0.05 | 0.021 | |
| l-dT | H | OH | 0.19 ± 0.09 | >200 | |||
| l-ddT | H | H | >10 | >100 | |||
| l-3′-FddT | H | F | >10 | >100 | |||
| l-3′-azido-ddT | H | N3 | >10 | >100 | |||
| l-3′-amino-ddT | H | NH2 | >10 | >10 | |||
| l-d4T | — | — | >10 | >100 | |||
| l-xylo-dT | OH | H | >10 | >10 | |||
| l-dA | H | H | OH | 0.10–1.9 | >10 | ||
| l-2-CldA | Cl | H | OH | >10 | >10 | ||
| l-ddA | H | H | H | 5 | >10 | ||
| l-d4A | H | — | — | 0.80 ± 0.10 | 0.38 | ||
| l-3′-azido-ddA | H | H | N3 | 5 | >10 | ||
| l-3′-amino-ddA | H | H | NH2 | >10 | >10 | ||
| l-3′-fluoro-ddA | H | H | F | >10 | >100 | ||
| l-ddAMP-bis(terbutyl-SATE) | H | H | H | 0.08 ± 0.03 | 0.002 | ||
| l-3′-azido-d4A | H | — | N3 | >10 | >100 | ||
EC50s were determined as described in Materials and Methods; values with a “greater than” symbol are the highest concentrations at which the compounds were tested. Values are means of at least three independent experiments. Anti-HIV data for l-ddC, 3TC, FTC, l-5-FddC, and l-d4FC are from references 22, 40, and 43. l-d4T, l-ddA, and l-d4A data are from references 3 and 21.
—, no substituent.
In addition, substitutions at the 5 position (R1) of the pyrimidine base of l-ddC lacking the 3′-OH group (e.g., β-l-2′,3′-dideoxy-5-fluorocytidine [l-5-FddC], β-l-2′,3′-dideoxy-5-chlorocytidine [l-5-ClddC], β-l-2′,3′-dideoxy-3′-thia-5-fluorocytidine [FTC], β-l-2′,3′-didehydro-2′,3′-dideoxy-5-fluorocytidine [l-d4FC], β-l-2′,3′-dideoxy-3′-fluoro-5-fluorocytidine [l-3′-F-5-FddC], and β-l-2′,3′-dideoxy-3′-azido-5-fluorocytidine [l-3′-azido-5-FddC]) further affected the antiviral potency of these analogs against HBV as well as HIV. These studies suggest that the 3′-OH of the β-l-2′-deoxyribose of l-dC plays a crucial role in inhibiting virus replication, possibly by specific interaction with the HBV DNA polymerase.
The SARs for the l-dT and l-dA series (Table 1) were similar to those observed for the l-dC series. The specific anti-HBV activity of l-dT and l-dA was lost upon removal or substitution of the 3′-OH group (R3). β-l-2′-Deoxy-xylo-thymidine (l-xylo-dT), which is identical to l-dT except that the 3′-OH group is in the opposite orientation (R2), also lost anti-HBV activity, further emphasizing the importance of the 3′-OH group in the interaction with the HBV DNA polymerase. An l-dT analog with a fluorine substitution at the 2′ up position (β-l-2′-deoxy-2′-fluoro-5-methyl-arabinofuranosyl uracil [l-FMAU]) has been reported to have activity against both HBV and EBV (6). Thus, it is possible that modification of the 2′ position in addition to the 3′ position of l-dT may also change antiviral specificity for HBV.
Substitution at the 2 position (R1) on the purine base of l-dA (e.g., β-l-2′-deoxy-2-chloroadenosine [l-2-CldA]) had a negative effect on anti-HBV activity. The analogs of l-dA lacking the 3′-OH group with or without further modification of the deoxyribose sugar lost specificity and were not as potent against HBV. The marginal antiviral activity of β-l-2′,3′-dideoxyadenosine (l-ddA), despite its potent inhibitory activity against both HIV reverse transcriptase and WHV DNA polymerase (Placidi et al., unpublished data), can be explained by the low intracellular concentrations of the phosphorylated form due to rapid and extensive catabolism (36). This conclusion is also supported by recent studies that demonstrated potent antiviral activity of an l-ddA 5′-monophosphate prodrug (β-l-2′,3′-ddAMP-terbutyl-S-acyl-2-thioethyl [l-ddAMP-bis(terbutyl-SATE)]). The prodrug form decreases the intracellular catabolism of the parent molecule (L. Placidi et al., Proc. 2nd Int. Conf. Ther. Vir. Hepatitis, abstr. A22, 1998 [Antivir. Ther. 3, Suppl. 3]) and releases the 5′-monophosphate derivative inside the cell. When used in this pronucleotide form, l-ddA was active against both HIV and HBV, further supporting the importance of the 3′-OH group for antiviral specificity. As in the l-dC and l-dT series, unmodified β-l-2′-deoxyadenosine most potently and specifically inhibited HBV replication.
To further assess their antiviral specificities, l-dC, l-dT, and l-dA were screened against 15 different RNA and DNA viruses. The β-l-2′-deoxynucleosides inhibited hepadnavirus replication as previously defined by the SAR but had no activity against HIV-1, herpes simplex virus types 1 and 2, varicella-zoster virus, EBV, human cytomegalovirus, adenovirus type 1, influenza A and B viruses, measles virus, parainfluenzavirus type 3, rhinovirus type 5, or respiratory syncytial virus type A at concentrations as high as 200 μM. Potent antiviral activity against WHV, determined using an in vivo model of chronic HBV infection, is described below. Thus, the unmodified β-l-2′-deoxynucleosides l-dC, l-dT, and l-dA are uniquely specific for the hepadnaviruses HBV, duck HBV (DHBV), and WHV.
Selectivity of β-l-2′-deoxynucleosides.
Since long-term treatment is expected for chronic HBV infection, drug selectivity is a critical issue. Toxic side effects have been a major problem, limiting the clinical use of some nucleoside analogs (17, 25, 53, 54). The 5′-triphosphates of l-dC, l-dT, and l-dA did not inhibit human DNA polymerases α, β, and γ at concentrations up to 100 μM. Semizarov and coworkers also reported that the 5′-triphosphates of l-dC and l-dT were not substrates for human DNA polymerases (42). l-dC, l-dT, and l-dA had no cytotoxic effect on the human hepatoma cell line 2.2.15 (50% cytotoxic concentration > 1,000 μM), primary human PBM cells, human foreskin fibroblasts, or other cell types of mammalian and avian origin. In addition, studies by Verri et al. demonstrated that l-dC was not cytotoxic toward lymphoblastoid T cells (51). Human bone marrow stem cells in primary culture have been shown to be a good predictor of potential nucleoside analog-induced hematotoxicity in patients (15, 45). GM-CFU and E-BFU precursors exposed to l-dC, l-dT, and l-dA in clonogenic assays at concentrations up to 10 μM were not affected. These results suggest that l-dC, l-dT, and l-dA are highly selective and that their phosphorylated forms will be nontoxic in vivo.
l-dC, l-dT, and l-dA were efficiently metabolized (activated) to their respective 5′-triphosphate derivatives in HepG2 cells and human hepatocytes in primary culture (Placidi et al., 3rd Int. Conf. Ther. Vir. Hepatitis, abstr. A122, 1999 [Antivir. Ther. 4, Suppl. 4]). Earlier studies reported limited intracellular activation of l-dT (18, 46). Together with the potent in vitro antiviral activity, these data suggest that like other nucleoside analogs, the intracellular phosphorylated form was responsible for inhibition of the viral polymerase. Furthermore, the 5′-triphosphates of l-dC, l-dT, and l-dA each inhibited WHV DNA polymerase with a 50% inhibitory concentration of 0.24 to 1.82 μM. In addition, exposure of HepG2 cells to l-dC led to a second 5′-triphosphate derivative, i.e., β-l-2′-dUTP (l-dUTP) which also inhibited WHV DNA polymerase, with a 50% inhibitory concentration of 5.26 μM (Faraj et al. and Placidi et al., 3rd Int. Conf. Ther. Vir. Hepatitis, abstr. A119 and A122, 1999 [Antivir. Ther. 4, Suppl. 4]). Similar to β-l-cytidine analogs (4, 19, 31, 51), l-dC was not a substrate for cytosolic cytidine deaminase, which suggested that the 5′-monophosphate metabolite of l-dC may be susceptible to deamination through deoxycytidylate deaminase. The inhibition of HBV replication by these β-l-2′-deoxynucleosides and inhibition of hepadnaviral polymerase by their corresponding 5′-triphosphates suggested that, like most nucleoside analogs, l-dC, l-dT, and l-dA may act by inhibiting the reverse transcription of HBV pregenomic RNA. Demonstration that l-deoxynucleoside triphosphate analogs inhibit HBV reverse transcriptase and/or DNA polymerase activity does not preclude other mechanisms of action. Inhibition of other important activities of the polymerase (which include RNase H activity, priming of reverse transcription, and coordination of intracellular virion assembly) and the possibility of internal incorporation of l-deoxynucleoside monophosphates into viral DNA as a mechanism of inhibition are currently under investigation.
β-l-2′-Deoxynucleosides have no effect on mitochondrial function or morphology.
Nucleoside analogs used in AIDS therapy, such as zidovudine (β-d-3′-azido-3′-deoxythymidine), stavudine (β-d-2′,3′-didehydro-2′,3′-dideoxythymidine [d4T]), didanosine (β-d-2′,3′-dideoxyinosine [ddI]), and zalcitabine (β-d-2′,3′-dideoxycytidine [ddC]), have shown clinically limiting delayed toxicities such as peripheral neuropathy, myopathy, and pancreatitis (17, 25, 53, 54). This nucleoside analog-related cellular toxicity has been attributed to decreased mtDNA content and altered mitochondrial function, leading to increased lactic acid production (5, 9–12, 28, 35). Concomitant morphological changes in mitochondria (e.g., loss of cristae, matrix dissolution and swelling, and lipid droplet formation) can be observed with ultrastructural analysis using transmission electron microscopy (10, 29, 35). In HepG2 cells incubated with 10 μM fialuridine (FIAU; 1,2′-deoxy-2′-fluoro-1-β-d-arabinofuranosly-5-iodo-uracil), a substantial increase in lactic acid production was observed (data not shown). Electron micrographs of these cells showed the presence of enlarged mitochondria with morphological changes consistent with mitochondrial dysfunction. Lamivudine (10 μM) did not affect mitochondrial structure or function. Under similar conditions, exposure of HepG2 cells to 10 μM l-dC, l-dT, or l-dA for 14 days had no effect on lactic acid production, mtDNA content, or morphology (data not shown).
In vivo antiviral activity and toxicity.
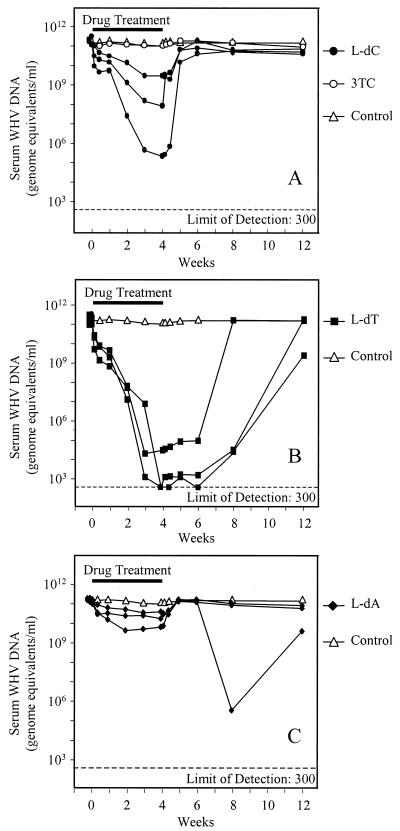
The woodchuck model of chronic HBV infection has proven to be a positive predictor of the antiviral activity and safety of antiviral drug candidates for the treatment of human chronic HBV infection (47, 48). l-dC, l-dT, and l-dA were given orally to woodchucks once daily at 10 mg/kg/day. The levels of WHV DNA in serum during 4 weeks of drug treatment and 8 weeks of posttreatment follow-up were determined by DNA dot blot hybridization (detection limit, approximately 107 genome equivalents/ml of serum) and by quantitative PCR (detection limit, 300 genome equivalents/ml of serum). The WHV DNA replication was significantly inhibited within the first few days of treatment and this inhibition was maintained throughout the treatment period. Notably, serum WHV DNA levels (HBV viremia) decreased in the l-dT-treated animals by as much as 8 logs (Fig. 1). Following drug withdrawal, viral rebound reached near-pretreatment levels between week 4 and week 8. In the l-dC-treated animals, serum WHV DNA levels decreased by up to 6 logs by the third week of therapy. Viral rebound was detected within the first week posttreatment. Animals receiving l-dA showed a decrease in serum WHV DNA levels of approximately 1.5 logs within the first week of treatment, and this decrease was also followed by viral rebound. In addition to the determination of viral load, WHV surface antigen (WHsAg), which is assumed to represent the level of intercellular gene expression, was measured using the method of Cote et al. (7). In general, the serologic profiles paralleled the decrease in viral load and continued to fall for several weeks after drug removal.
FIG. 1.
Woodchuck hepatitis virus levels in serum in animals at 4 weeks of treatment with l-dC (A), l-dT (B), or l-dA (C) and 8 weeks posttreatment. Data are presented for individual animals administered 10 mg/kg/day orally (n = 3) and untreated control animals (n = 4).
The cytidine analog lamivudine (10 mg/kg/day), used for comparison to the l-dC treatment group, reduced the number of HBV genome equivalents per milliliter in serum by 0.5 log. This weak effect is consistent with previous studies using similar doses of lamivudine (20). Much higher doses (40 to 200 mg/kg) are required to produce significant antiviral activity in this model (32). The low activity of lamivudine in the woodchuck model has been explained in part by the low rate of conversion of lamivudine and other cytidine analogs to their active 5′-triphosphate forms in woodchuck liver compared to that in human liver. In addition, the oral bioavailability of lamivudine in woodchucks was reported to be 18 to 54%, whereas the oral bioavailability observed in humans was 82% (37, 50).
The woodchuck model was also valuable for the preclinical toxicological evaluation of nucleoside analogs. This model identified the delayed severe hepatocellular toxicity induced by FIAU in humans not seen in preclinical evaluation in rats, dogs, or monkeys (38, 47). The FIAU-induced toxicity observed in woodchucks, including significant weight loss, wasting, and hepatocellular damage seen upon liver biopsy, was identified beginning 6 to 8 weeks from onset of treatment and was similar to that observed in the treated HBV-infected patients (33, 47). Using this model we found in additional studies that the unmodified β-l-2′-deoxynucleosides l-dC, l-dT, and l-dA were well tolerated and caused no drug-related toxicity through 12 weeks of treatment and 4 weeks of follow-up (data not shown).
In summary, this is the first report of β-l-2′-deoxynucleosides with potent, selective, and specific activity against HBV replication. This series of drug candidates has in common the presence of a hydroxyl group in the 3′ position that determines specific activity against hepadnavirus. In the woodchuck model of chronic HBV infection, oral administration of these β-l-2′-deoxynucleosides reduced serum viral load by as much as 108 genome equivalents/ml without toxicity. These β-l-2′-deoxynucleosides are highly attractive clinical development candidates for the treatment of chronic HBV infection.
REFERENCES
- 1.Belen'kii S M, Schinazi R S. Multiple drug effect analysis with confidence interval. Antivir Res. 1994;25:1–11. doi: 10.1016/0166-3542(94)90089-2. [DOI] [PubMed] [Google Scholar]
- 2.Bessesen M, Ives D, Condreay L, Lawrence S, Sherman K E. Chronic active hepatitis B exacerbations in human immunodeficiency virus-infected patients following development of resistance to or withdrawal of lamivudine. Clin Infect Dis. 1999;28:1032–1035. doi: 10.1086/514750. [DOI] [PubMed] [Google Scholar]
- 3.Bolon P J, Wang P, Chu C, Gosselin G, Boudou V, Pierra C, Mathe C, Imbach J L, Faraj A, Alaoui A, Sommadossi J P, Pai S B, Zhu Y L, Lin J S, Cheng Y C, Schinazi R F. Anti-human immunodeficiency and anti-hepatitis B virus activities of beta-l-2′,3′-dideoxy purine nucleosides. Bioorg Med Chem Lett. 1996;6:1657–1662. [Google Scholar]
- 4.Chang C N, Doong S L, Zhou J H, Beach J W, Jeong L S, Chu C K, Tsai C H, Cheng Y C, Liotta D, Schinazi R. Deoxycytidine deaminase-resistant stereoisomer is the active form of (+/−)-2′,3′-dideoxy-3′-thiacytidine in the inhibition of hepatitis B virus replication. J Biol Chem. 1992;267:13938–13942. [PubMed] [Google Scholar]
- 5.Chen C H, Cheng Y C. Delayed cytotoxicity and selective loss of mitochondrial DNA in cells treated with the anti-human immunodeficiency virus compound 2′,3′-dideoxycytidine. J Biol Chem. 1989;264:11934–11937. [PubMed] [Google Scholar]
- 6.Chu C K, Ma T, Shanmuganathan K, Wang C, Xiang Y, Pai S B, Yao G Q, Sommadossi J P, Cheng Y C. Use of 2′-fluoro-5-methyl-beta-l-arabinofuranosyluracil as a novel antiviral agent for hepatitis B virus and Epstein-Barr virus. Antimicrob Agents Chemother. 1995;39:979–981. doi: 10.1128/aac.39.4.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cote R J, Roneker C, Cass K, Schodel F, Peterson D, Tennant B, De Noronha F, Gerin J. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol. 1993;6:161–169. doi: 10.1089/vim.1993.6.161. [DOI] [PubMed] [Google Scholar]
- 8.Cui L, Faraj A, Sommadossi J P. Effect of 1-(2-deoxy-2-fluoro-beta-d-arabinofuranosyl)-5-ethyluracil on mitochondrial functions in HepG2 cells. Antivir Res. 1999;43:201–207. doi: 10.1016/s0166-3542(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 9.Cui L, Locatelli L, Xie M Y, Sommadossi J P. Mitochondrial DNA effect of nucleoside analogs on neurite regeneration and mitochondrial DNA synthesis in PC-12 cells. J Pharmacol Exp Ther. 1997;280:1228–1234. [PubMed] [Google Scholar]
- 10.Cui L, Schinazi R F, Gosselin G, Imbach J-L, Chu C K, Rando R F, Revankar G R, Sommadossi J-P. Effect of β-enantiomeric and racemic nucleoside analogues on mitochondrial functions in HepG2 cells. Biochem Pharmacol. 1996;52:1577–1584. doi: 10.1016/s0006-2952(96)00562-x. [DOI] [PubMed] [Google Scholar]
- 11.Cui L, Yoon S, Schinazi R F, Sommadossi J P. Cellular and molecular events leading to mitochondrial toxicity of 1-(2-deoxy-2-fluoro-1-β-d-arabinofuranosyl)-5-iodouracil in human liver cells. J Clin Investig. 1995;95:555–563. doi: 10.1172/JCI117698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dalakas C M, Illa I, Pezeshkpour G H, Laukaitis J P, Cohen B, Griffin J L. Mitochondrial myopathy caused by long-term zidovudine therapy. N Engl J Med. 1990;322:1098–1105. doi: 10.1056/NEJM199004193221602. [DOI] [PubMed] [Google Scholar]
- 13.Davis G L. Interferon treatment of viral hepatitis in immunocompromised patients. Semin Liver Dis. 1989;9:267–272. doi: 10.1055/s-2008-1040522. [DOI] [PubMed] [Google Scholar]
- 14.Dienstag J L, Schiff E R, Wright T L, Perrillo R P, Hann H L, Goodman Z, Crowther L, Condreay L D, Woessner M, Rubin M, Brown N A for the U.S. Lamivudine Investigator Group. Lamivudine as initial treatment for chronic hepatitis B in the United States. N Engl J Med. 1999;341:1256–1263. doi: 10.1056/NEJM199910213411702. [DOI] [PubMed] [Google Scholar]
- 15.Faraj A, Fowler D A, Bridges E G, Sommadossi J-P. Effects of 2′,3′-dideoxynucleosides on proliferation and differentiation of human pluripotent progenitors in liquid culture and their effects on mitochondrial DNA synthesis. Antimicrob Agents Chemother. 1994;38:924–930. doi: 10.1128/aac.38.5.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Faraj J, Agrofolio L A, Wakefield J K, McPherson S, Morrow C D, Gosselin G, Mathe C, Imbach J-L, Schinazi R F, Sommadossi J-P. Inhibition of human immunodeficiency virus type 1 reverse transcriptase by the 5′-triphosphate beta enantiomers of cytidine analogs. Antimicrob Agents Chemother. 1994;38:2300–2305. doi: 10.1128/aac.38.10.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faulds D, Brogden R N. Didanosine. A review of its antiviral activity, pharmacokinetic properties and therapeutic potential in human immunodeficiency virus infection. Drugs. 1992;44:94–116. doi: 10.2165/00003495-199244010-00008. [DOI] [PubMed] [Google Scholar]
- 18.Focher F, Maga G, Bendiscioli A, Capobianco M, Colonna F, Garbesi A, Spadari S. Stereospecificity of human DNA polymerases alpha, beta, gamma, delta and epsilon, HIV-reverse transcriptase, HSV-1 DNA polymerase, calf thymus terminal transferase and Escherichia coli DNA polymerase I in recognizing d- and l-thymidine 5′-triphosphate as substrate. Nucleic Acids Res. 1995;23:2840–2847. doi: 10.1093/nar/23.15.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Furman P A, Davis M, Liotta D C, Paff M, Frick L W, Nelson D J, Dornsife R E, Wurster J A, Wilson L J, Fyfe J A, et al. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (−) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2686–2692. doi: 10.1128/aac.36.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Genovesi E V, Lamb L, Medina I, Taylor D, Seifer M, Innaimo S, Colonno R J, Standring D N, Clark J M. Efficacy of the carbocyclic 2′-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob Agents Chemother. 1998;42:3209–3217. doi: 10.1128/aac.42.12.3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gosselin G, Boudou V, Griffon J F, Pavia G, Pierra C, Imbach J L, Aubertin A M, Schinazi R F, Faraj A, Sommadossi J P. New unnatural l-nucleoside enantiomers: from their stereoselective synthesis to their biological activities. Nucleosides Nucleotides. 1997;16:1389–1398. [Google Scholar]
- 22.Gosselin G, Mathe C, Bergogne M C, Aubertin A M, Kirn A, Schinazi R F, Sommadossi J P, Imbach J L. Enantiomeric 2′,3′-dideoxycytidine derivatives are potent human immunodeficiency virus inhibitors in cell culture. C R Acad Sci. 1994;317:85–89. [PubMed] [Google Scholar]
- 23.Graciet J C G, Schinazi R F. From d- to l-nucleoside analogs as antiviral agents. Adv Antivir Drug Design. 1999;3:1–68. [Google Scholar]
- 24.Hoofnagle J H, di Bisceglie A M. The treatment of chronic viral hepatitis. N Engl J Med. 1997;336:347–356. doi: 10.1056/NEJM199701303360507. [DOI] [PubMed] [Google Scholar]
- 25.Hurst M, Noble S. Stavudine: an update of its use in the treatment of HIV infection. Drugs. 1999;58:919–949. doi: 10.2165/00003495-199958050-00012. [DOI] [PubMed] [Google Scholar]
- 26.Korba B E, Gerin J L. Use of a standardized cell culture assay to assess activities of nucleoside analogs against hepatitis B virus replication. Antivir Res. 1992;19:55–70. doi: 10.1016/0166-3542(92)90056-b. [DOI] [PubMed] [Google Scholar]
- 27.Lai C L, Chien R N, Leung N W, Chang T T, Guan R, Tai D I, Ng K Y, Wu P C, Dent J C, Barber J, Stephenson S L, Gray D F. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 28.Lewis W, Gonzalez B, Chomyn A, Papoian T. Zidovudine induces molecular, biochemical, and ultrastructural changes in rat skeletal muscle mitochondria. J Clin Investig. 1992;89:1354–1360. doi: 10.1172/JCI115722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis W, Levine E S, Griniuviene B, Tankersly K O, Colacino J M, Sommadossi J P, Watanabe K A, Perrino F W. Fialuridine and its metabolites inhibit DNA polymerase gamma at sites of multiple adjacent analog incorporation, decrease mtDNA abundance, and cause mitochondrial structural defects in cultured hepatoblasts. Proc Natl Acad Sci USA. 1996;93:3592–3597. doi: 10.1073/pnas.93.8.3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liaw Y F, Chien R N, Yeh C T, Tsai S L, Chu C M. Acute exacerbation and hepatitis B virus clearance after emergence of YMDD motif mutation during lamivudine therapy. Hepatology. 1999;30:567–572. doi: 10.1002/hep.510300221. [DOI] [PubMed] [Google Scholar]
- 31.Martin L T, Faraj A, Schinazi R F, Gosselin G, Mathe C, Imbach J L, Sommadossi J P. Effect of stereoisomerism on the cellular pharmacology of beta- enantiomers of cytidine analogs in Hep-G2 cells. Biochem Pharmacol. 1997;53:75–87. doi: 10.1016/s0006-2952(96)00653-3. [DOI] [PubMed] [Google Scholar]
- 32.Mason W S, Cullen J, Moraleda G, Saputelli J, Aldrich C E, Miller D S, Tennant B, Frick L, Averett D, Condreay L D, Jilbert A R. Lamivudine therapy of WHV-infected woodchucks. Virology. 1998;245:18–32. doi: 10.1006/viro.1998.9150. [DOI] [PubMed] [Google Scholar]
- 33.McKenzie R, Fried M W, Sallie R, Conjeevaram H, Di Bisceglie A M, Park Y, Savarese B, Kleiner D, Tsokos M, Luciano C, Pruett T, Stotka J L, Straus S E, Hoofnagle J H. Hepatic failure and lactic acidosis due to fialuridine (FIAU), an investigational nucleoside analogue for chronic hepatitis B. N Engl J Med. 1995;333:1099–1105. doi: 10.1056/NEJM199510263331702. [DOI] [PubMed] [Google Scholar]
- 34.Morrey J D, Korba B E, Sidwell R W. Transgenic mice as a chemotherapeutic model for hepatitis B virus infection. In: Schinazi R F, Sommadossi J P, Thomas H C, editors. Therapies for viral hepatitis. London, United Kingdom: International Medical Press; 1998. pp. 161–170. [PubMed] [Google Scholar]
- 35.Pan-Zhou X R, Cui L, Zhou X-J, Sommadossi J-P, Darley-Usmar V M. Differential effects of antiretroviral nucleoside analogs on mitochondrial function in HepG2 cells. Antimicrob Agents Chemother. 2000;44:496–503. doi: 10.1128/aac.44.3.496-503.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Placidi L, Cretton-Scott E, Gosselin G, Pierra C, Schinazi R F, Imbach J L, el Kouni M H, Sommadossi J P. Intracellular metabolism of β-l-2′,3′-dideoxyadenosine: relevance to its limited antiviral activity. Antimicrob Agents Chemother. 2000;44:853–858. doi: 10.1128/aac.44.4.853-858.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rajagopalan P, Boudinot F D, Chu C K, Tennant B C, Baldwin B H, Schinazi R F. Pharmacokinetics of (−)-2′-3′-dideoxy-3′-thiacytidine in woodchucks. Antimicrob Agents Chemother. 1996;40:642–645. doi: 10.1128/aac.40.3.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Richardson F C, Engelhardt J A, Bowsher R R. Fialuridine accumulates in DNA of dogs, monkeys, and rats following long-term oral administration. Proc Natl Acad Sci USA. 1994;91:12003–12007. doi: 10.1073/pnas.91.25.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schinazi R F, Gosselin G, Faraj A, Korba B E, Liotta D C, Chu C K, Mathe C, Imbach J L, Sommadossi J P. Pure nucleoside enantiomers of beta-2′,3′-dideoxycytidine analogs are selective inhibitors of hepatitis B virus in vitro. Antimicrob Agents Chemother. 1994;38:2172–2174. doi: 10.1128/aac.38.9.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schinazi R F, McMillan A, Cannon D, Mathis R, Lloyd R M, Peck A, Sommadossi J P, St. Clair M, Wilson J, Furman P A, et al. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992;36:2423–2431. doi: 10.1128/aac.36.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schinazi R F, Sommadossi J-P, Saalmann V, Cannon D L, Xie M-Y, Hart G C, Smith G A, Hahn E F. Activities of 3′-azido-3′-deoxythymidine nucleotide dimers in primary lymphocytes infected with human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1990;34:1061–1067. doi: 10.1128/aac.34.6.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Semizarov D G, Arzumanov A A, Dyatkina N B, Meyer A, Vichier-Guerre S, Gosselin G, Rayner B, Imbach J L, Krayevsky A A. Stereoisomers of deoxynucleoside 5′-triphosphates as substrates for template-dependent and -independent DNA polymerases. J Biol Chem. 1997;272:9556–9560. doi: 10.1074/jbc.272.14.9556. [DOI] [PubMed] [Google Scholar]
- 43.Shi J, McAtee J J, Schlueter Wirtz S, Tharnish P, Juodawlkis A, Liotta D C, Schinazi R F. Synthesis and biological evaluation of 2′,3′-didehydro-2′,3′- dideoxy-5-fluorocytidine (D4FC) analogues: discovery of carbocyclic nucleoside triphosphates with potent inhibitory activity against HIV-1 reverse transcriptase. J Med Chem. 1999;42:859–867. doi: 10.1021/jm980510s. [DOI] [PubMed] [Google Scholar]
- 44.Sommadossi J-P, Carlisle R, Schinazi R F, Zhou Z. Uridine reverses the toxicity of 3′-azido-3′-deoxythymidine in normal human granulocyte-macrophage progenitor cells in vitro without impairment of antiretroviral activity. Antimicrob Agents Chemother. 1988;32:997–1001. doi: 10.1128/aac.32.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sommadossi J-P, Carlisle R, Zhou Z. Cellular pharmacology of 3′-azido-3′-deoxythymidine with evidence of incorporation into DNA of human bone marrow cells. Mol Pharmacol. 1989;36:9–14. [PubMed] [Google Scholar]
- 46.Spadari S, Maga G, Focher F, Ciarrocchi G, Manservigi R, Arcamone F, Capobianco M, Carcuro A, Colonna F, Iotti S, et al. l-Thymidine is phosphorylated by herpes simplex virus type 1 thymidine kinase and inhibits viral growth. J Med Chem. 1992;35:4214–4220. doi: 10.1021/jm00100a029. [DOI] [PubMed] [Google Scholar]
- 47.Tennant B C, Baldwin B H, Graham L A, Ascenzi M A, Hornbuckle W E, Rowland P H, Tochkov I A, Yeager A E, Erb H N, Colacino J M, Lopez C, Engelhardt J A, Bowsher R R, Richardson F C, Lewis W, Cote P J, Korba B E, Gerin J L. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology. 1998;128:179–191. doi: 10.1002/hep.510280124. [DOI] [PubMed] [Google Scholar]
- 48.Tennant B C, Peek S F, Tochkov I A, Baldwin B H, Hornbuckle W E, Korba B E, Cote P J, Gerin J L. The woodchuck in preclinical assessment of therapy for hepatitis B virus infection. In: Schinazi R F, Sommadossi J-P, Thomas H C, editors. Therapies for viral hepatitis. London, United Kingdom: International Medical Press; 1998. pp. 171–176. [Google Scholar]
- 49.UNAIDS. Report on the global HIV/AIDS epidemic. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS; 1998. [Google Scholar]
- 50.van Leeuwen R, Lange J M, Hussey E K, Donn K H, Hall S T, Harker A J, Jonker P, Danner S A. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in patients with HIV infection: a phase I study. AIDS. 1992;6:1471–1475. doi: 10.1097/00002030-199212000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Verri A, Focher F, Priori G, Gosselin G, Imbach J L, Capobianco M, Garbesi A, Spadari S. Lack of enantiospecificity of human 2′-deoxycytidine kinase: relevance for the activation of beta-l-deoxycytidine analogs as antineoplastic and antiviral agents. Mol Pharmacol. 1997;51:132–138. doi: 10.1124/mol.51.1.132. [DOI] [PubMed] [Google Scholar]
- 52.Wang P, Hong J H, Cooperwood J S, Chu C K. Recent advances in l-nucleosides: chemistry and biology. Antivir Res. 1998;40:19–44. doi: 10.1016/s0166-3542(98)00041-2. [DOI] [PubMed] [Google Scholar]
- 53.Whittington R, Brogden R N. Zalcitabine. A review of its pharmacology and clinical potential in acquired immunodeficiency syndrome (AIDS) Drugs. 1992;44:656–683. doi: 10.2165/00003495-199244040-00009. [DOI] [PubMed] [Google Scholar]
- 54.Wilde I M, Langtry H D. Zidovudine. An update of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy. Drugs. 1993;46:515–578. doi: 10.2165/00003495-199346030-00010. [DOI] [PubMed] [Google Scholar]
- 55.Wong D K, Cheung A M, O'Rourke K, Naylor C D, Detsky A S, Heathcote J. Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Ann Intern Med. 1993;119:312–323. doi: 10.7326/0003-4819-119-4-199308150-00011. [DOI] [PubMed] [Google Scholar]