Over the past decade, advances in sequencing and genomics technology have transformed our understanding of the genome. Unexpectantly, these studies revealed that non-coding RNAs (ncRNAs) account for the vast majority of the transcribed genome1, which has spurred intense interest in determining the functions of these molecules. ncRNAs are a diverse family of transcripts that can be classified by size with RNAs <200 nucleotides designated as small non-coding RNAs, including microRNAs (miRNAs) and small interfering RNAs (siRNAs), and those >200 nucleotides termed long non-coding RNAs (lncRNAs). miRNAs function as post-transcriptional repressors of gene expression that directly bind to target messenger RNAs to inhibit translation or induce transcript degradation. miRNAs play essential roles in cardiovascular physiology and pathophysiology and clinical trials using promising miRNA targets in heart failure have recently been started2. Far less is currently known about lncRNAs, which are a more heterogenous class of RNAs that participate in diverse cellular processes including transcriptional regulation, nuclear domain organization, and direct regulation of proteins or RNA molecules. lncRNAs have recently emerged as additional key players in cardiovascular health and disease and have gained attention both as potential therapeutic targets and biomarkers for a broad range of diseases3.
During cardiac development and in response to physiological stimuli or pathological stress, the heart undergoes hypertrophic growth, which is characterized by an increase in cardiomyocyte size4. Several lncRNAs have been identified that are directly involved in cardiac hypertrophy and are differentially regulated in response to pathological stress including cardiac hypertrophy-associated transcript (Chast)5 and cardiac hypertrophy-associated epigenetic regulator (Chaer)6, which are both up-regulated in mice in response to transverse aortic constriction (TAC), and myosin heavy-chain-associated RNA transcript (Mhrt)7, which is down-regulated following TAC. Additional cardiac lncRNAs are dynamically regulated during the compensatory (hypertrophic) and decompensatory (heart failure) phases of TAC-induced pressure-overload including H198 and non-coding repressor of NFAT (NRON)9. These studies have shown that lncRNAs can serve as therapeutic targets, evidenced by the cardioprotection observed in mouse models of TAC in response to targeted knockdown or deletion of specific lncRNAs (Chast5, Chaer6, NRON9) or via overexpression (Mhrt7, H198).
Whereas a clear role for lncRNAs has been established in pathological cardiac hypertrophy, much less is currently known about their contributions to physiological growth processes, which involve distinct signaling mechanisms4. Exercise-induced physiological hypertrophy provides significant protective effects in heart injury and heart failure and is associated with cardiomyogenesis10, however the role of lncRNAs in these processes remains largely unexamined. To date, cardiac physiological hypertrophy-associated regulator (CPhar)11 is the only lncRNA that has been demonstrated to be required for exercise-induced physiological cardiac hypertrophy in mice based on a forced swim training model.
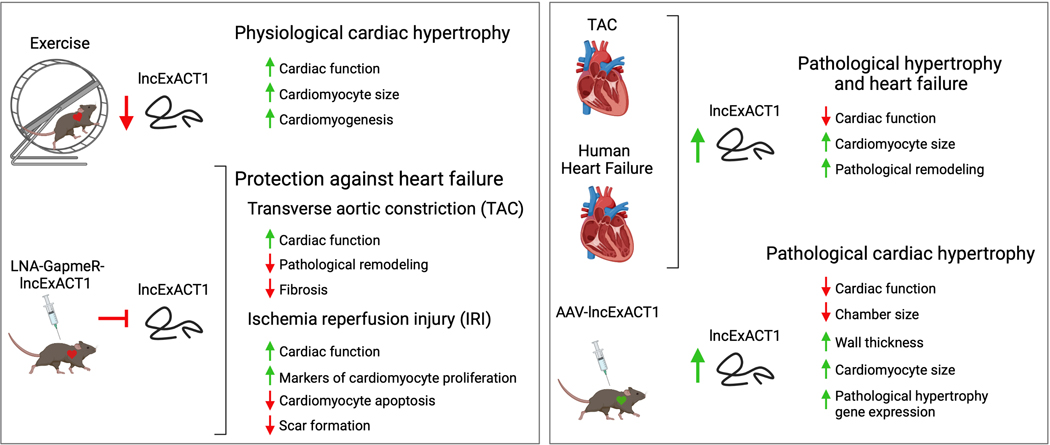
In this issue of Circulation, Li et al12 set out to comprehensively identify lncRNAs that are expressed differentially in physiological cardiac hypertrophy versus pathological hypertrophy and heart failure. RNA-sequencing was performed on hearts from sedentary mice or mice subjected to voluntary wheel-running for 8-weeks and compared to mice that underwent TAC surgery for 2-weeks (hypertrophic response) or 8-weeks (decompensated heart failure). This analysis pipeline identified 25 lncRNAs that were dynamically regulated by exercise, collectively termed long non-coding Exercise Associated Transcripts (lncExACTs), and of these molecules, a subset of 6 were also changed in the TAC disease model in the opposite direction compared to exercise. lncExACT1 was selected for further study because it was the only identified lncRNA that was downregulated in response to exercise and upregulated in response to TAC (Figure), a pattern that was also observed in human heart failure samples, suggesting that a knockdown approach may have therapeutic benefit. Importantly, despite a relatively high degree of sequence conservation of lncExACT1 across mammalian species, it was rigorously shown to lack protein-coding capacity.
Figure.
The role of lncExACT1 in physiological and pathological cardiac hypertrophy.
Left. LncRNA screening identified lncExACT1 downregulation during exercise (wheel-running) of mice that was associated with physiological cardiac hypertrophy. Mimicking exercise-induced lncExACT1 downregulation by systemic injection of antisense locked nucleic acid (LNA)-GapmeR-lncExACT1 protected against the development of heart failure in 2 disease models. Right. lncExACT1 expression was upregulated in hearts of mice in response to thoracic aortic constriction (TAC) and in human heart failure patients with non-ischemic cardiomyopathy. Adeno-associated virus (AAV)-mediated overexpression of lncExACT1 in mouse hearts induced cardiac dysfunction and pathological cardiac hypertrophy. (Figure created with BioRender.com)
Li et al12 performed a series of comprehensive studies showing that cardiomyocyte-specific overexpression of lncExACT1 is sufficient to induce pathological hypertrophy and heart failure in mice, whereas antisense locked nucleic acid (LNA) GapmeR-mediated silencing of lncExACT1 was cardioprotective in response to TAC and ischemia/reperfusion injury (IRI) and induced a phenotype consistent with physiological growth with preserved cardiac function and protection against fibrosis (Figure). Whereas both overexpression and inhibition of lncExACT1 stimulated cardiac hypertrophy, a distinct profile of pathological hypertrophy was seen in response to lncExACT1 overexpression versus that of physiological growth driven by lncExACT1 inhibition. Notably, lncExACT1 overexpression induced a gene expression profile characteristic of pathological remodeling including robust reactivation of the fetal gene program driven in part through calcineurin signaling, a well-established driver of pathological remodeling4, culminating in cardiac dysfunction and heart failure. In sharp contrast, inhibition of lncExACT1 in vivo resulted in improved cardiac function at baseline and after pathological stress, which was accompanied by an increase in markers of cardiomyocyte proliferation at baseline as well as after TAC and IRI. These findings are consistent with cardiomyogenesis and were further supported by in vitro data showing an increase in cardiomyocyte number after lncExACT1 inhibition. Whereas the data presented fall short of conclusively proving new cardiomyocytes are generated in vivo and integrated into the myocardium, they are highly supportive of a proliferative phenotype.
Detailed mechanistic studies revealed that lncExACT1 works in several distinct ways to exert its effects, and these modes of action highlight several unique features of lncRNAs. Notably, the molecular functions of lncRNAs can change depending on their subcellular localization. lncExACT1 is present both in cardiomyocyte nuclei and in the cytoplasm, with ~2.5-fold enrichment in the nucleus. In the cytoplasm, lncExACT1 binds directly to and inhibits the cardiac-expressed miRNA miR-222, which the authors previously showed was necessary but not sufficient for physiological cardiac growth and exercise-induced cardiomyogenesis13. In the nucleus, where lncExACT1 is more abundant, lncExACT1 binds to the promoter region of its neighboring gene, dachsous cadherin-related 2 (DCHS2), and positively regulates its transcription. Nuclear lncRNAs often regulate the expression of nearby genes and are classified as those that modify chromatin structure or gene expression locally “in cis” versus those that leave the site of transcription and perform their functions distally “in trans”, however such distinct mechanisms are not always clear-cut. Whereas lncExACT1 appears to function locally in geographical terms by binding to the promoter region of the neighboring DCHS2 gene, exogenous expression of the lncExACT1 transcript can mediate this effect, indicating its regulatory function is more consistent with that of a trans-acting lncRNA. DCHS2 is positively regulated by lncExACT1, with lncExACT1 overexpression in mice resulting in DCHS2 upregulation and lncExACT1 inhibition correlating with DCHS2 downregulation. These parallel expression patterns are also consistent in human heart failure patients in which both lncExACT1 and DCHS2 are upregulated. Importantly, the authors provide evidence that DCHS2 is both necessary and sufficient for lncExACT1’s effects on cardiomyocyte growth, where DCHS2 knockdown prevented the pathological hypertrophy seen with lncExACT1 overexpression, and DCHS2 overexpression blocked the physiological hypertrophy seen with lncExACT1 knockdown. Thus, DCHS2 is proposed to be a downstream effector of lncExACT1 activity.
DCHS2 has been shown in other systems to be involved in regulating cell proliferation through Hippo/Yap1 signaling14, which is a highly conserved regulatory pathway of cell size and proliferation in many organ systems including the heart. Therefore, this was probed as a potential contributing mechanism downstream of lncExACT1 activity. Consistent with this logic, both exercise and the inhibition of lncExACT1 or DCHS2 increased the nuclear fraction of Yap1, which is pro-proliferative, resulting in the expression of downstream Yap1 targets. DCHS2 knockdown also reduced cytoplasmic expression of phosphorylated MST1/2, which is a core regulatory protein that works upstream of Yap1 in the Hippo pathway. These data collectively implicate lncExACT1 and DCHS2 as novel regulators of cardiac Yap1, which likely contributes to the observed phenotypes of exercise-induced heart growth and cardiomyogenesis. However, it is important to note that prior studies have shown that postnatal activation of cardiac Yap1 does not increase cardiomyocyte size15, which suggests that Yap1 activation is not sufficient to induce physiological growth. Therefore, it is likely that the effects observed by Li et al12 on cardiac hypertrophy are mediated by Yap1-independent pathways downstream of lncExACT1 and DCHS2, such as via miR-222 and/or calcineurin as discussed above.
Importantly, the authors provide new evidence that lncExACT1 can serve as a novel heart failure therapeutic target via an inhibition strategy12. Silencing of RNA molecules can be readily achieved using antisense oligonucleotides (ASO) or RNA interference (RNAi), and such therapies have already been applied in clinical trials2, 3. GapmeRs, which are the class of ASOs used to target lncExACT1 in this study, are highly effective in vivo because they can enter both the cytoplasm and nucleus, thereby enabling the efficient targeting of both cytoplasmic and nuclear RNA molecules (such as lncExACT1). Whereas the inhibition of several lncRNAs that are upregulated in response to pressure overload is sufficient to attenuate pathological remodeling and dysfunction in experimental mouse models (i.e. Chast5, Chaer6, NRON9), the silencing of lncExACT1 is the first known example to concomitantly promote cardiomyogenesis and physiological hypertrophy, indicating it is a therapeutic target that warrants further detailed investigation. Future studies should aim to assess efficacy in additional heart failure models and large animal models as well as to study potential off-target effects and toxicity.
Acknowledgments:
C.A.M. is supported by grants from the National Institutes of Health (HL-141630 and HL-160569) and Cincinnati Children’s Research Foundation (Trustee Award).
T.T. is supported by the Deutsche Forschungsgemeinschaft (SFB/Transregio (TRR267); SFB1470 and KFO311).
Footnotes
Conflict of interest disclosures:
Dr. Thum has filed and licensed patents in the field of cardiovascular ncRNAs. Dr. Thum is founder and shareholder of Cardior Pharmaceuticals GmbH.
References:
- 1.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, et al. Landscape of transcription in human cells. Nature. 2012;489:101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taubel J, Hauke W, Rump S, Viereck J, Batkai S, Poetzsch J, Rode L, Weigt H, Genschel C, Lorch U, et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur Heart J. 2021;42:178–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beermann J, Piccoli MT, Viereck J and Thum T. Non-coding RNAs in Development and Disease: Background, Mechanisms, and Therapeutic Approaches. Physiol Rev. 2016;96:1297–325. [DOI] [PubMed] [Google Scholar]
- 4.Heineke J and Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7:589–600. [DOI] [PubMed] [Google Scholar]
- 5.Viereck J, Kumarswamy R, Foinquinos A, Xiao K, Avramopoulos P, Kunz M, Dittrich M, Maetzig T, Zimmer K, Remke J, et al. Long noncoding RNA Chast promotes cardiac remodeling. Sci Transl Med. 2016;8:326ra22. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Zhang XJ, Ji YX, Zhang P, Deng KQ, Gong J, Ren S, Wang X, Chen I, Wang H, et al. The long noncoding RNA Chaer defines an epigenetic checkpoint in cardiac hypertrophy. Nat Med. 2016;22:1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han P, Li W, Lin CH, Yang J, Shang C, Nuernberg ST, Jin KK, Xu W, Lin CY, Lin CJ, et al. A long noncoding RNA protects the heart from pathological hypertrophy. Nature. 2014;514:102–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viereck J, Buhrke A, Foinquinos A, Chatterjee S, Kleeberger JA, Xiao K, Janssen-Peters H, Batkai S, Ramanujam D, Kraft T, et al. Targeting muscle-enriched long non-coding RNA H19 reverses pathological cardiac hypertrophy. Eur Heart J. 2020;41:3462–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoepfner J, Leonardy J, Lu D, Schmidt K, Hunkler HJ, Biss S, Foinquinos A, Xiao K, Regalla K, Ramanujam D, et al. The long non-coding RNA NRON promotes the development of cardiac hypertrophy in the murine heart. Mol Ther. 2021; 10.1016/j.ymthe.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vujic A, Lerchenmuller C, Wu TD, Guillermier C, Rabolli CP, Gonzalez E, Senyo SE, Liu X, Guerquin-Kern JL, Steinhauser ML, et al. Exercise induces new cardiomyocyte generation in the adult mammalian heart. Nat Commun. 2018;9:1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gao R, Wang L, Bei Y, Wu X, Wang J, Zhou Q, Tao L, Das S, Li X and Xiao J. Long Noncoding RNA Cardiac Physiological Hypertrophy-Associated Regulator Induces Cardiac Physiological Hypertrophy and Promotes Functional Recovery After Myocardial Ischemia-Reperfusion Injury. Circulation. 2021;144:303–317. [DOI] [PubMed] [Google Scholar]
- 12.Li H, Trager LE, Liu X, Hastings MH, Xiao C, Guerra J, To0 S, Li G, Yeri A, Rodosthenous R, et al. lncExACT1 and DCHS2 Regulate Physiological and Pathological Cardiac Hypertrophy. Circulation. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Xiao J, Zhu H, Wei X, Platt C, Damilano F, Xiao C, Bezzerides V, Bostrom P, Che L, et al. miR-222 is necessary for exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell Metab. 2015;21:584–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bagherie-Lachidan M, Reginensi A, Pan Q, Zaveri HP, Scott DA, Blencowe BJ, Helmbacher F and McNeill H. Stromal Fat4 acts non-autonomously with Dchs1/2 to restrict the nephron progenitor pool. Development. 2015;142:2564–73. [DOI] [PubMed] [Google Scholar]
- 15.von Gise A, Lin Z, Schlegelmilch K, Honor LB, Pan GM, Buck JN, Ma Q, Ishiwata T, Zhou B, Camargo FD, et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc Natl Acad Sci U S A. 2012;109:2394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]