Abstract
BACKGROUND AND PURPOSE:
Herpes zoster (HZ) is associated with increased risk of stroke, and zoster vaccine live (ZVL, Zostavax) reduces the risk of HZ. No study has examined the association between ZVL (Zostavax) and risk of stroke. Present study examined association between receipt of ZVL (Zostavax) and risk of stroke among older US population.
METHODS:
Our study included 1 603 406 US Medicare fee-for-service beneficiaries aged ≥66 years without a history of stroke and who received ZVL (Zostavax) during 2008 to 2014, and 1 603 406 propensity score-matched unvaccinated beneficiaries followed through to December 31, 2017. We used Cox proportional hazard models to examine association between ZVL (Zostavax) and composite fatal or nonfatal incident stroke outcomes.
RESULTS:
During a median of 5.1 years follow-up (interquartile range, 3.9–6.7), we documented 64 635 stroke events, including 43 954 acute ischemic strokes and 6727 hemorrhagic strokes, among vaccinated beneficiaries during 8 755 331 person-years. The corresponding numbers among unvaccinated beneficiaries were 73 023, 50 476, and 7276, respectively, during 8 517 322 person-years. Incidence comparing vaccinated to unvaccinated beneficiaries were 7.38 versus 8.57 per 1000 person-years for all stroke, 5.00 versus 5.90 for acute ischemic stroke, and 0.76 versus 0.84 for hemorrhagic stroke (P<0.001 for all difference). Adjusted hazard ratios comparing vaccinated to unvaccinated beneficiaries were 0.84 (95% CI, 0.83–0.85), 0.83 (0.82–0.84), and 0.88 (0.85–0.91) for all stroke, acute ischemic stroke, and hemorrhagic stroke, respectively. The association between ZVL (Zostavax) and risk of stroke appeared to be stronger among younger beneficiaries, beneficiaries who did not take antihypertensive or statin medications and who had fewer comorbid conditions (P<0.05 for interaction) but largely consistent across sex, low-income status, and racial groups.
CONCLUSIONS:
Among Medicare fee-for-service beneficiaries, receipt of ZVL (Zostavax) was associated with lower incidence of stroke. Our findings may encourage people to get vaccinated against HZ to reduce HZ and HZ-associated stroke risk.
Keywords: association, herpes zoster, Medicare, population, vaccines
GRAPHIC ABSTRACT:
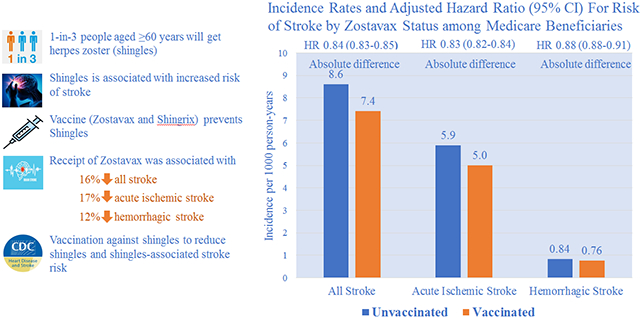
Herpes zoster (HZ), also known as shingles, is a painful rash caused by reactivation of latent varicella zoster virus (VZV) infection generally acquired at young ages.1-3 Approximately one million HZ cases, more than half among individuals aged 60 years or older, occur annually in the United States,2,3 and 1 in 3 people who had VZV infection develop HZ during their lifetime (https://www.cdc.gov/shingles/about/overview.html). The Advisory Committee on Immunization Practices recommended routine zoster vaccine live (ZVL; ZOSTAVAX, a 1-dose HZ live-attenuated vaccine) for preventing HZ among all persons aged 60 years or older during 2006,4 and in 2017, Advisory Committee on Immunization Practices recommended a new HZ vaccine (Shingrix, a 2-dose, adjuvant, recombinant HZ vaccine) for people aged 50 years or older.5 The coverage of ZVL increased significantly since 2006, and about one-third of adults had received ZVL as of 2016.5,6
Many studies suggested that the risk of stroke increases significantly following the HZ.7-15 Other studies confirmed the effectiveness of ZVL on prevention of HZ among people recommended for the vaccine,16-20 immunocompromised patients, and among those with end-stage renal disease.21,22 However, no prior study examined the effect of ZVL on risk of stroke. The present study examined this association among adults aged 66 years or older who enrolled in the Medicare fee-for-service (FFS) program during 2008 to 2017 in the United States.
METHODS
The Medicare data used in this study cannot be shared by authors because of the data usage agreement, but the investigators can access to these data by application to Centers for Medicare and Medicaid Services.
Study Cohort
Identifying FFS Beneficiaries With ZVL
We used Medicare’s enrollment databases to generate this population-based cohort. First, we identified all Medicare FFS beneficiaries aged 65 years or older with at least 12 months continuous enrollment in Medicare Part A (hospitalization) and B (office-based care) and eligible for Part D (≥1-month prescription drug coverage) from 2007 to 2014. Second, we identified FFS beneficiaries who had the recoded ZVL that occurred from 2007 to 2014, including multiple ZVL administrations (22 240 [1.4%] beneficiaries had >1 ZVL administrated and the date of first ZVL was chosen). National Drug Code from the Medicare Part D data and Current Procedural Terminology code from inpatient and outpatient claims data were used to identify the status of ZVL (Table I in the Data Supplement)8,22 We used a 12-month or longer review period to identify the first ZVL (look-back period where no ZVL codes were billed), and the length of review time varied by the years of Medicare enrollment. For example, we used 12 months for beneficiaries 66 years of age (Medicare eligible at age 65 years) and 24 months for beneficiaries aged 67 years, and January 1, 2006, was the earliest date of look-back since ZVL was recommended in 2006. Therefore, this cohort consisted of FFS beneficiaries aged 66 years or older who had their first ZVL during 2008 to 2014. Among vaccinated FFS beneficiaries, we excluded (1) those who were in institutional long-term care; (2) those with a history of stroke or transient ischemic attack on the basis of the Chronic Conditions Warehouse definition used by the Centers for Medicare and Medicaid Services (www.ccw-data.org/web/guest/condition-categories) or the hospitalizations recorded in Medicare Provider Analysis and Review that occurred before the index date of ZVL; (3) those who had a history of HZ before the index date of ZVL (Table II in the Data Supplement for International Classification of Diseases, Ninth Revision [ICD-9-CM] or ICD-10-CM codes for HZ); and (4) those with history of immunocompromised conditions because ZVL was not recommended for those beneficiaries (Table III in the Data Supplement).
Selecting FFS Unvaccinated Beneficiaries
Among FFS unvaccinated beneficiaries, we followed the similar selection criteria as for vaccinated beneficiaries. However, before matching vaccinated to unvaccinated beneficiaries, there was no index date for unvaccinated beneficiaries (date of first ZVL was index date for vaccinated beneficiaries), and we could not completely exclude the beneficiaries with a history of stroke/transient ischemic attack, HZ, or immunocompromised conditions. Therefore, we adopted the 2-step exclusion process among unvaccinated beneficiaries. First, we excluded all unvaccinated beneficiaries who had a history of stroke/transient ischemic attack, HZ, or immunocompromised conditions before the start of the baseline cohort year. For example, we excluded the unvaccinated beneficiaries who entered the cohort in 2008, who were aged 66 years or older, and who had these exclusion conditions recorded before January 1, 2008. The main purpose of the first-step exclusion is to reduce the size of the matching pool and improve the matching process. Second, for each matched beneficiary, we assigned the vaccinated beneficiary’s index date of ZVL as the unvaccinated beneficiary’s index date, then we excluded the unvaccinated beneficiaries who had a stroke/transient ischemic attack, HZ, or immunocompromised conditions before the assigned index date and excluded the corresponding vaccinated beneficiaries. Figure I in the Data Supplement shows the flowchart of Medicare FFS beneficiaries’ selection and matching process, and the final cohort included 1 603 406 vaccinated beneficiaries from 2008 to 2014 and the same number of matched unvaccinated beneficiaries.
Propensity Score Matching
Propensity score matching is used to select controls who were similar to cases to minimize treatment selection bias when estimating the treatment effects in nonrandomized studies.23 We derived the propensity score by using multivariate logistic regression that exactly matched on age, sex, race, and year of cohort entry, and included socioeconomic conditions, health care utilization characteristics, frailty characteristics, medications use, Centers for Medicare and Medicaid Services Hierarchical Condition Category score, and coexisting health conditions (Table IV in the Data Supplement for the list of matching variables). We excluded FFS beneficiaries with missing values for the matching variables before matching. We used a greedy nearest neighbor one-to-one matching on the logit of propensity score with caliper of width 0.2 in SAS Proc PSMATCH procedure (release 9.4; SAS Institute, Inc, Cary, NC). Balance of the covariates was assessed by the standardized difference where differences <0.10 were considered negligible.24
Outcomes
The primary outcome was the composite fatal/nonfatal incident stroke during follow-up. We used Medicare Provider Analysis and Review files to identify the hospitalizations with all stroke, acute ischemic stroke (AIS), and hemorrhagic stroke. The Medicare Provider Analysis and Review files contained inpatient hospital and skilled nursing facility stay records for all Medicare beneficiaries, and we used the primary diagnosis codes ICD-9-CM for 2008 to 2015 and ICD-10-CM for the last quarter of 2015 to 2017 to identify the beneficiaries with stroke (Table V in the Data Supplement). The secondary outcome was the incident HZ. Incident HZ cases were identified by the first HZ diagnosis that occurred during follow-up in the inpatient or outpatient visit claims data. Follow-up of beneficiaries continued until incident stroke (fatal or nonfatal), and the status of fatal stroke death was determined by the National Death Index for primary outcomes or first occurrence of HZ for the secondary outcome, with censoring at the time of death other than stroke death or through end of follow-up period (December 31, 2017).
Assessment of Confounding and Selection Bias
FFS vaccinated beneficiaries may differ from unvaccinated beneficiaries in their risk profile for stroke or HZ. To assess the possible effect of confounding and selection bias, we used 13 acute symptomatic conditions as the negative control outcomes that ZVL should not protect against these conditions (Table VI in the Data Supplement).17,19 A negative control outcome is one that shares the same potential sources of bias with the primary outcome (stroke) but cannot plausibly be related to the treatment of interest (ZVL vaccine).25,26 We calculated the incidence rates and adjusted hazard ratios (HRs) for each negative control outcome comparing vaccinated to unvaccinated beneficiaries. We expect that these HRs should be distributed around 1.0, and a significant departure from 1.0 may indicate the effect of confounding or selection bias.26
Statistical Analysis
We calculated the mean and percentages of the selected covariates by ZVL status. We used Cox proportional hazards regression to estimate HRs and 95% CIs for the composite all stroke, AIS, and hemorrhagic stroke events associated with ZVL. We presented propensity score-adjusted HRs and fully adjusted HRs that included all matching variables accounting for the residual confounding. The proportional hazards assumption of the Cox models was evaluated with Schoenfeld residuals, which revealed no important departures from proportionality in hazards. The Cox models were stratified by year of receiving ZVL.
We conducted several sensitivity analyses. First, we conducted stratified analyses of ZVL and risk of stroke by age (66–74, 75–84, and ≥85 years), sex, race or ethnicity (non-Hispanic White, non-Hispanic Black, Hispanic, and other), low-income subsidy status (yes/no), antihypertensive use (yes/no), statins use (yes/no), and Charlson Comorbidity Index (CCI; 0, 1, 2, 3, and ≥4), and we tested for interactions of ZVL status with these covariates by including interactions terms in analysis and presented adjusted P value by using the Holm method for multiple comparison.27,28 Second, we examined the association between ZVL and risk of HZ by age, sex, and race or ethnicity. Third, we examined the association between ZVL and 13 negative outcomes. Data were analyzed by using SAS software (version 9.4). All tests were 2-sided, and P<0.05 was considered significant.
RESULTS
Table 1 is information for participants. Among 1 603 406 Medicare FFS vaccinated beneficiaries and the same number of unvaccinated FFS beneficiaries, the mean age was 73.4 years (95% CI, 73.4–73.5), about 2 in 3 beneficiaries were aged younger than 75 years and female. Nearly 90% of beneficiaries were non-Hispanic White; over 70% were taking antihypertensive medications; more than half were on statins; and >60% of beneficiaries had a CCI of ≥1. The vaccinated FFS beneficiaries differed from the original cohort, but the vaccinated and matched unvaccinated beneficiaries were well balanced after propensity score matching by the selected characteristics with the standardized differences <0.03 (Table 1 and Table VII in the Data Supplement).
Table 1.
Characteristics of Medicare Fee-for-Service Beneficiaries Aged 66 Years or Older by ZVL Status, Medicare 2008–2017 Matched Cohort
| Characteristics | ZVL status | ||||
|---|---|---|---|---|---|
| No. of vaccinated beneficiaries |
Vaccinated mean/% (95% CI) |
No. of unvaccinated beneficiaries |
Unvaccinated mean/% (95% CI) |
Standardized differences/%* |
|
| All | 1 603 406 | 1 603 406 | |||
| Age, mean | 73.4 (73.4–73.5) | 73.4 (73.4–73.5) | 0.000 | ||
| Age group (%) | |||||
| 66–74 y | 1 028 245 | 64.1 (64.0–64.3) | 1 028 245 | 64.1 (64.0–64.3) | 0.000 |
| 75–84 y | 478 584 | 29.8 (29.8–29.9) | 478 584 | 29.8 (29.8–29.9) | 0.000 |
| ≥85 y | 96 577 | 6.02 (5.99–6.06) | 96 577 | 6.02 (5.99–6.06) | 0.000 |
| Sex (%) | |||||
| Men | 597 294 | 37.3 (37.2–37.3) | 597 294 | 37.3 (37.2–37.3) | |
| Women | 1 006 112 | 62.7 (62.6–62.9) | 1 006 112 | 62.7 (62.6–62.9) | 0.000 |
| Race/ethnicity (%) | |||||
| Non-Hispanic White | 1 438 651 | 89.7 (89.6–89.9) | 1 438 651 | 89.7 (89.6–89.9) | 0.000 |
| Non-Hispanic Black | 36 681 | 2.29 (2.26–2.31) | 36 681 | 2.29 (2.26–2.31) | 0.000 |
| Hispanic | 44 898 | 2.8 (2.77–2.83) | 44 898 | 2.8 (2.77–2.83) | 0.000 |
| Other | 83 176 | 5.19 (5.15–5.22) | 83 176 | 5.19 (5.15–5.22) | 0.000 |
| Low-income subsidy (%) | |||||
| Yes | 207 346 | 12.9 (12.9–13.0) | 210 903 | 13.2 (13.1–13.2) | |
| No | 1 396 060 | 87.1 (86.9–87.2) | 1 392 503 | 86.8 (86.7–87.0) | 0.007 |
| Antihypertensive use status (%) | |||||
| Yes | 1 138 334 | 71.0 (70.9–71.1) | 1 140 006 | 71.1 (71.0–71.2) | |
| No | 465 072 | 29.0 (28.9–29.1) | 463 400 | 28.9 (28.8–29.0) | 0.002 |
| Statin use status (%) | |||||
| Yes | 865 128 | 54.0 (53.8–54.1) | 861 495 | 53.7 (53.6–53.8) | |
| No | 738 278 | 46.0 (45.9–46.1) | 741 911 | 46.3 (46.2–46.4) | 0.002 |
| Charlson Comorbidity Index (%) | |||||
| 0 | 600 806 | 37.5 (37.4–37.6) | 606 806 | 37.8 (37.8–37.9) | 0.008 |
| 1 | 405 528 | 25.3 (25.2–25.4) | 413 712 | 25.8 (25.7–25.9) | 0.012 |
| 2 | 257 887 | 16.1 (16.0–16.1) | 257 532 | 16.1 (16.0–16.1) | 0.001 |
| 3 | 168 556 | 10.5 (10.5–10.6) | 167 496 | 10.4 (10.4–10.5) | 0.002 |
| 4+ | 170 629 | 10.6 (10.6–10.7) | 157 781 | 9.84 (9.79–9.89) | 0.026 |
ZVL indicates zoster vaccine live.
Standardized differences were the differences in means or proportions between vaccinated and unvaccinated beneficiaries divided by standard errors where differences <0.10 were considered negligible.
During a median of 5.1 years follow-up (interquartile range, 3.9–6.7), we documented 64 635 stroke events, including 43 954 AIS and 6727 hemorrhagic strokes, among vaccinated beneficiaries during 8 755 331 person-years. The corresponding numbers for unvaccinated beneficiaries were 73 023, 50 476, and 7276 during 8 517 322 person-years. Incidence rates of stroke per 1000 person-years among vaccinated and unvaccinated beneficiaries were 7.38 (95% CI, 7.38–7.38) versus 8.57 (95% CI, 8.57–8.58) for all stroke, 5.00 (95% CI, 5.90–5.00) versus 5.90 (95% CI, 5.90–5.91) for AIS, and 0.76 (95% CI, 0.76–0.76) versus 0.84 (95% CI, 0.84–0.84) for hemorrhagic stroke (P<0.001 for difference). Adjusted HRs comparing vaccinated to unvaccinated beneficiaries were 0.84 (95% CI, 0.83–0.85), 0.83 (95% CI, 0.82–0.84), and 0.88 (95% CI, 0.85–0.91) for all stroke, AIS, and hemorrhagic stroke, respectively (Table 2). The adjusted HRs decreased significantly with increased age and increased CCI for all stroke and AIS (adjusted P<0.005 for interaction). The association between ZVL and all stroke and AIS appeared to be stronger among beneficiaries who did not take antihypertensive medications or statins (adjusted P<0.05 for interaction; Figure [A and B] and Table VIII in the Data Supplement). The pattern of association between ZVL and hemorrhagic stroke was largely consistent across subpopulations (adjusted P>0.05; Figure [C] and Table VIII in the Data Supplement). The adjusted HRs for the 13 negative outcomes ranged from 0.82 for hip fracture to 1.15 for hemorrhoids (mean=1.03; SD=0.098). Only hip fracture had comparable HR to the association between ZVL and risk of stroke (Table IX and Figure II in the Data Supplement).
Table 2.
Incidence Rates and Adjusted HR (95% CI) For Risk of Stroke by ZVL Status, Medicare 2008–2017 Matched Cohort
| Characteristics | ZVL status | P value* | |
|---|---|---|---|
| Unvaccinated beneficiaries |
Vaccinated beneficiaries |
||
| All stroke | |||
| Events (person-years)† | 73 023 (8 517 322) | 64 635 (8 755 331) | |
| Incidence/per 1000 person-years (95% CI) | 8.57 (8.57–8.58) | 7.38 (7.38–7.38) | |
| HR (95% CI) | 1.0 | 0.86 (0.85–0.87) | <0.0001 |
| Fully adjusted HR (95% CI)‡ | 1.0 | 0.84 (0.83–0.85) | <0.0001 |
| Acute ischemic stroke | |||
| Events (person-years)† | 50 476 (8 548 663) | 43 954 (8 786 492) | |
| Incidence/per 1000 person-years (95% CI) | 5.90 (5.89–5.91) | 5.00 (5.00–5.00) | |
| HR (95% CI) | 1.0 | 0.84 (0.83–0.86) | <0.0001 |
| Fully adjusted HR (95% CI)‡ | 1.0 | 0.83 (0.82–0.84) | <0.0001 |
| Hemorrhagic stroke | |||
| Events (person-years)† | 7276 (8 660 334) | 6727 (8 887 693) | |
| Incidence/per 1000 person-years (95% CI) | 0.84 (0.84–0.84) | 0.76 (0.76–0.76) | |
| HR (95% CI) | 1.0 | 0.90 (0.87–0.93) | <0.0001 |
| Fully adjusted HR (95% CI)‡ | 1.0 | 0.88 (0.85–0.91) | <0.0001 |
HR indicates hazard ratio; and ZVL, zoster vaccine live.
P value for difference in risk of stroke between beneficiaries who received ZVL and matched controls; all tests were 2-tailed.
Stroke events included both fatal and nonfatal incident events.
In addition to propensity score matching, adjusted for all matching variables to control for potential residual confounding.
Figure. Adjusted hazard ratio (95% CI) for risk of stroke, acute ischemic stroke, and hemorrhagic stroke by selected covariates, Medicare 2008–2017 matched cohort.
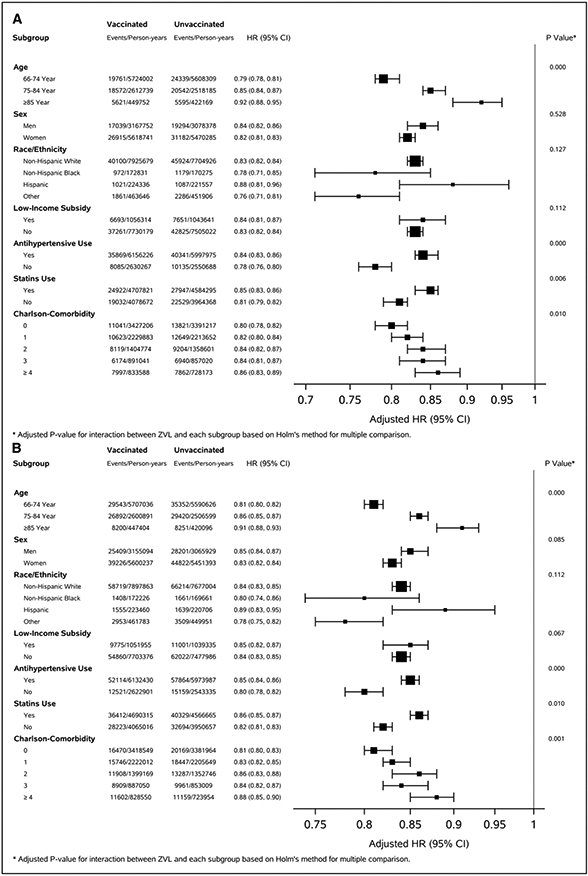
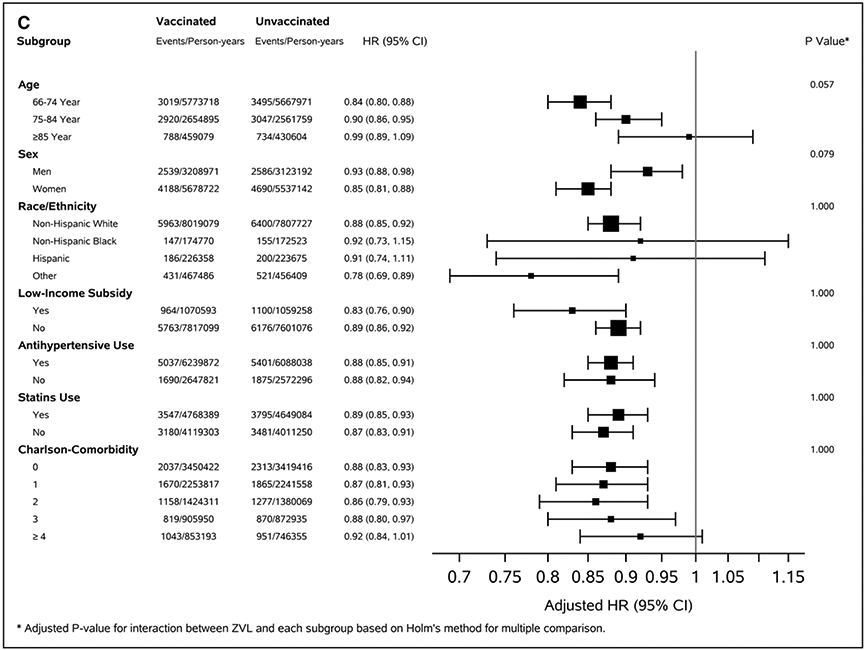
A, All stroke. B, Acute ischemic stroke. C, Hemorrhagic stroke.
The incidence of HZ per 1000 person-years among unvaccinated and vaccinated beneficiaries was 11.1 (95% CI, 11.1–11.1) and 8.51 (95% CI, 8.50–8.51) with an adjusted HRs of 0.76 (95% CI, 0.75–0.77; Table 3). The adjusted HRs decreased significantly with increased age but were similar between men and women.
Table 3.
Incidence Rates and Adjusted Hazards Ratios (95% CI) for Risk of Herpes Zoster Associated With ZVL, Medicare 2008–2017 Matched Cohort
| Characteristics | ZVL status | P value* | |
|---|---|---|---|
| Unvaccinated beneficiaries |
Vaccinated beneficiaries |
||
| Total | |||
| HZ events (person-years)† | 93 046 (8 373 925) | 73 692 (8 663 578) | |
| Incidence/per 1000 person-years (95% CI) | 11.1 (11.1–11.1) | 8.51 (8.50–8.51) | |
| HR (95% CI) | 1.0 | 0.76 (0.76–0.77) | <0.0001 |
| Fully adjusted HR (95% CI)‡ | 1.0 | 0.76 (0.75–0.77) | <0.0001 |
| Age group | |||
| 66–74 y | |||
| HZ events (person-years)† | 61 137 (5 681 945) | 46 648 (5 841 556) | |
| Incidence/per 1000 person-years (95% CI) | 10.8 (10.8–10.8) | 7.99 (7.98–7.99) | |
| HR (95% CI) | 1.0 | 0.74 (0.73–0.75) | <0.0001 |
| Fully adjusted HR (95% CI)‡ | 1.0 | 0.74 (0.73–0.75) | <0.0001 |
| 75–84 y | |||
| HZ events (person-years)† | 27 550 (2 326 499) | 23 157 (2 432 484) | |
| Incidence/per 1,000 person-years (95% CI) | 11.8 (11.8–11.8) | 9.52 (9.52–9.52) | |
| HR (95% CI) | 1.0 | 0.80 (0.79–0.82) | <0.0001 |
| Fully adjusted HR (95% CI)‡ | 1.0 | 0.80 (0.78–0.81) | <0.0001 |
| ≥85 y | |||
| HZ events (person-years)† | 4359 (365 481) | 3887 (389 538) | |
| Incidence/per 1,000 person-years (95% CI) | 11.9 (11.9–11.9) | 9.98 (9.97–9.99) | |
| HR (95% CI) | 1.0 | 0.84 (0.80–0.87) | <0.0001 |
| Fully adjusted HR (95% CI)‡ | 1.0 | 0.82 (0.79–0.86) | <0.0001 |
| Male | |||
| HZ events (person-years)† | 28 506 (3 039 104) | 22 132 (3 144 280) | |
| Incidence/per 1000 person-years (95% CI) | 9.38 (9.38–9.38) | 7.04 (7.04–7.04) | |
| HR (95% CI) | 1.0 | 0.75 (0.74–0.76) | <0.0001 |
| Fully adjusted HR (95% CI)‡ | 1.0 | 0.75 (0.73–0.76) | <0.0001 |
| Female | |||
| HZ events (person-years)† | 64 540 (5 334 821) | 51 560 (5 519 299) | |
| Incidence/per 1000 person-years (95% CI) | 12.1 (12.1–12.1) | 9.34 (9.34–9.34) | |
| HR (95% CI) | 1.0 | 0.77 (0.76–0.78) | <0.0001 |
| Fully adjusted HR (95% CI)‡ | 1.0 | 0.77 (0.76–0.78) | <0.0001 |
HR indicates hazard ratio; HZ, herpes zoster; and ZVL, zoster vaccine live.
P value for difference in risk of HZ between beneficiaries who received ZVL and matched controls; all tests were 2-tailed.
First HZ event during follow-up.
In addition to propensity score matching, adjusted for ail matching variables to control for potential residual confounding.
DISCUSSION
Among this population-based cohort of Medicare FFS beneficiaries aged 66 years or older, receipt of ZVL was associated with reduced risk of stroke during a median of 5-year follow-up. Compared with unvaccinated beneficiaries, vaccinated beneficiaries had about 16%, 17%, and 12% lower risk for all, AIS, and hemorrhagic stroke, respectively. ZVL-associated reduction in risk of all stroke and AIS decreased by increased age and increased CCI and appeared to be stronger among FFS beneficiaries who did not take antihypertensive medications or statins.
Findings from many observational studies suggested that the risk of stroke increased significantly following HZ, especially within the first few months of HZ onset.7-11,15,29,30 In 2006, Advisory Committee on Immunization Practices recommended routine ZVL vaccine for persons aged 60 years or older to prevent HZ.4 Although the effectiveness of ZVL in prevention of HZ in real-world populations was lower than the findings from the randomized control trials,16 many studies confirmed its effectiveness among populations and showed its reduced effectiveness with increased age and time since the vaccination.17,18,20,31 Our results of ZVL effectiveness were comparable with the findings of the studies using similar data sources.17,18,31 To the best of our knowledge, our study is the first to seek to determine whether receipt of ZVL may be associated with reduced risk of stroke. The biological mechanisms of a potential causal association between ZVL and reduced risk of stroke are not clear. Studies suggested that the HZ viral ability to replicate in cerebral arteries where the infection spreads along the nerve fibers to the blood vessels could cause subsequent inflammation leading to pathological vascular remodeling and increased risk of stroke.32,33 The receipt of ZVL reduces the risk of having HZ; therefore, ZVL-associated reduction in stroke risk may be, at least partly, a result of reduced risk of HZ among vaccinated beneficiaries. Our results showed that the ZVL-associated reduction in stroke risk decreased significantly with increased age and was consistent with the findings of decreased effectiveness of ZVL against HZ with increased age (https://www.merck.com/product/usa/pi_circulars/z/zostavax/zostavax_pi2.pdf). Studies suggested that VZV, same virus as HZ and primary infection causes varicella (chickenpox) usually among children, is associated with increased risk of stroke among children.34-36 Compared with adults, children are less likely to have underlying immune deficiency or risk factors associated with increased risk for stroke and the VZV associated risk for stroke may suggest a potential causal association between varicella-zoster infection (either VZV or HZ) and stroke.36 In addition, HZ may also increase blood pressure because of pain or stress associated with HZ.10 High blood pressure is the leading risk factor for stroke.37 Reduction of HZ-associated blood pressure increase by ZVL protection against HZ may also help to reduce the risk of stroke. Further study is needed to clarify the association between ZVL and reduced risk of stroke.
The ZVL-associated reduction in risk of stroke appeared to decrease with increased number of CCI. Age was significantly correlated with number of CCI (eg, the median age of those with a CCI of 0 was 71 years and increased to 74 years among those with a CCI of 4 or higher), and age was associated with reduced effectiveness of ZVL against HZ,17,18 which may partly explain the reduced protection of ZVL for risk of stroke among beneficiaries with increased comorbid conditions. The association between ZVL and risk of all stroke and AIS appeared to be stronger among FFS beneficiaries who did not take antihypertensive medications or statins. Medication treatments for high blood pressure and dyslipidemia reduce the risk of stroke.38 However, at least one study reported that individuals with hypertension who were taking antihypertensive medications had higher risk of stroke compared with the individuals with untreated hypertension or individuals without hypertension among the population.39 Hypertensive medication treatment may be a marker reflecting greater severity of hypertension and a cluster of other cardiovascular risk factors, such as diabetes, dyslipidemia, or atrial fibrillation, which leads to a higher risk of stroke.39-43 FFS beneficiaries who were taking antihypertensive medications or statins may have a higher baseline risk of stroke, and this higher risk could attenuate the association between ZVL and risk of stroke.
The association between ZVL and hemorrhagic stroke appeared to be weaker compared with AIS (adjusted HRs 0.83 [95% CI, 0.82–0.84] for AIS versus 0.88 [95% CI, 0.85–0.91] for hemorrhagic stroke, P<0.05). The association between ZVL and hemorrhagic stroke was largely consistent across age, sex, race or ethnicity, and other groups. Few studies examined the association between HZ and hemorrhagic stroke, with inconsistent findings: some suggested significant association,12 whereas others found insignificant association.7,15,44 Limited sample size, number of studies, and different study designs may contribute to inconsistent findings. Our study had a reasonable number of hemorrhagic stroke events (≈14 000 events) and found a weaker but significant association between ZVL and hemorrhagic stroke. High blood pressure is the most important risk factor for hemorrhagic stroke. If ZVL reduces the HZ-associated high blood pressure caused by the pain or stress associated with HZ,10 then this may partly explain the association between ZVL and hemorrhagic stroke. However, if taking antihypertensive medications or statins suggests a higher baseline risk of stroke, attenuating the association between ZVL and AIS, then we would expect to see a similar pattern for hemorrhagic stroke. Yet there was no difference in adjusted HRs between the beneficiaries who took and did not take antihypertensive medications or statins. The reasons for these findings are unclear, and further studies are needed to clarify these issues.
Our study included a large, population-based cohort of Medicare FFS beneficiaries aged 66 years or older. We used the propensity score matching to identify the comparable controls with a comprehensive list of matching variables to account for confounding and selection bias. The primary limitation of our study is the selection of the comparable unvaccinated beneficiaries. Although we included a comprehensive list of matching variables, we cannot rule out the effect of unobserved confounding. However, the results of 13 negative control outcomes suggested that confounding and selection bias may not play a major role in our findings; (only hip fracture had a comparable HR to HRs of ZVL and risk of stroke). Second, ZVL was recommended for adults aged 60 years or older, and there was no ZVL information before age 65 years in Medicare data. Our study may misclassify the beneficiaries who had ZVL before age 65 years. Among adults aged 60 to 64 years, 20.1% reported ZVL vaccination in 2014,45 and the coverage was significantly lower during the earlier years.46 Third, our study was conducted when only ZVL information was available in Medicare. Advisory Committee on Immunization Practices recommended Shingrix for people aged 50 years or older in 2017.5 The effectiveness of Shingrix at preventing HZ is >90%, and future studies are needed to examine the association between Shingrix and risk of stroke. Fourth, stroke diagnosis was based on ICD-9/10-CM codes from Medicare claims data and subject to misclassification. Fifth, our study included Medicare FFS beneficiaries, and the findings may not apply to the beneficiaries who enrolled in Medicare Advantage plans.
CONCLUSIONS
Among this population-based cohort of Medicare FFS beneficiaries aged 66 years or older, receipt of ZVL vaccine (Zostavax) was associated with reduced risk of stroke. Although we cannot rule out the possible effect of unaccounted stroke risk factors, our findings may encourage people to get vaccinated against HZ to prevent HZ and HZ-associated stroke risk.
Supplementary Material
Acknowledgments
We thank Linda Schieb, Division for Heart Disease and Stroke Prevention, Centers for Disease Control and Prevention, for providing information on US family income by ZIP code of residences.
Nonstandard Abbreviations and Acronyms
- AIS
acute ischemic stroke
- CCI
Charlson Comorbidity Index
- FFS
fee-for-service
- HR
hazard ratio
- HZ
herpes zoster
- ICD
International Classification of Diseases
- VZV
varicella zoster virus
- ZVL
zoster vaccine live
Footnotes
Disclosures
None.
REFERENCES
- 1.Schmader K Herpes zoster. Ann Intern Med. 2018;169:897. doi: 10.7326/L18-0558 [DOI] [PubMed] [Google Scholar]
- 2.Hales CM, Harpaz R, Joesoef MR, Bialek SR. Examination of links between herpes zoster incidence and childhood varicella vaccination. Ann Intern Med. 2013;159:739–745. doi: 10.7326/0003-4819-159-11-201312030-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sampathkumar P, Drage LA, Martin DP. Herpes zoster (shingles) and postherpetic neuralgia. Mayo Clin Proc. 2009;84:274–280. doi: 10.1016/S0025-6196(11)61146-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harpaz R, Ortega-Sanchez IR, Seward JF; Advisory Committee on Immunization Practices (ACIP) Centers for Disease Control and Prevention (CDC). Prevention of herpes zoster: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2008;57(RR-5):1; quiz CE2–30; quiz CE2. [PubMed] [Google Scholar]
- 5.Dooling KL, Guo A, Patel M, Lee GM, Moore K, Belongia EA, Harpaz R. Recommendations of the advisory committee on immunization practices for use of herpes zoster vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103–108. doi: 10.15585/mmwr.mm6703a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harpaz R, Leung JW. The epidemiology of herpes zoster in the United States during the era of varicella and herpes zoster vaccines: changing patterns among older adults. Clin Infect Dis. 2019;69:341–344. doi: 10.1093/cid/ciy953 [DOI] [PubMed] [Google Scholar]
- 7.Lin HC, Chien CW, Ho JD. Herpes zoster ophthalmicus and the risk of stroke: a population-based follow-up study. Neurology. 2010;74:792–797 doi: 10.1212/WNL.0b013e3181d31e5c [DOI] [PubMed] [Google Scholar]
- 8.Minassian C, Thomas SL, Smeeth L, Douglas I, Brauer R, Langan SM. Acute cardiovascular events after herpes zoster: a self-controlled case series analysis in vaccinated and unvaccinated older residents of the United States. PLoS Med. 2015;12:e1001919. doi: 10.1371/journal.pmed.1001919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calabrese LH, Xie F, Yun H, Winthrop KL, Baddley JW, Calabrese C, Curtis JR. Herpes zoster and the risk of stroke in patients with autoimmune diseases. Arthritis Rheumatol. 2017;69:439–446. doi: 10.1002/art.39855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self-controlled case-series study. Clin Infect Dis. 2014;58:1497–1503. doi: 10.1093/cid/ciu098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sreenivasan N, Basit S, Wohlfahrt J, Pasternak B, Munch TN, Nielsen LP, Melbye M. The short- and long-term risk of stroke after herpes zoster - a nationwide population-based cohort study. PLoS One. 2013;8:e69156. doi: 10.1371/journal.pone.0069156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schink T, Behr S, Thöne K, Bricout H, Garbe E. Risk of stroke after herpes zoster - evidence from a German self-controlled case-series study. PLoS One. 2016;11:e0166554. doi: 10.1371/journal.pone.0166554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Luo G, Huang Y, Yu Q, Wang L, Li K. Risk of stroke/transient ischemic attack or myocardial infarction with herpes zoster: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2017;26:1807–1816. doi: 10.1016/j.jstrokecerebrovasdis.2017.04.013 [DOI] [PubMed] [Google Scholar]
- 14.Kim MC, Yun SC, Lee HB, Lee PH, Lee SW, Choi SH, Kim YS, Woo JH, Kim SH, Kwon SU. Herpes zoster increases the risk of stroke and myocardial infarction. J Am Coll Cardiol. 2017;70:295–296. doi: 10.1016/j.jacc.2017.05.015 [DOI] [PubMed] [Google Scholar]
- 15.Wu PH, Chuang YS, Lin YT. Does herpes zoster increase the risk of stroke and myocardial infarction? A comprehensive review. J Clin Med. 2019;8:547 doi: 10.3390/jcm8040547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ansaldi F, Trucchi C, Alicino C, Paganino C, Orsi A, Icardi G. Real-world effectiveness and safety of a live-attenuated herpes zoster vaccine: a comprehensive review. Adv Ther. 2016;33:1094–1104. doi: 10.1007/s12325-016-0355-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izurieta HS, Wernecke M, Kelman J, Wong S, Forshee R, Pratt D, Lu Y, Sun Q, Jankosky C, Krause P, et al. Effectiveness and duration of protection provided by the live-attenuated herpes zoster vaccine in the medicare population ages 65 years and older. Clin Infect Dis. 2017;64:785–793. doi: 10.1093/cid/ciw854 [DOI] [PubMed] [Google Scholar]
- 18.Tricco AC, Zarin W, Cardoso R, Veroniki AA, Khan PA, Nincic V, Ghassemi M, Warren R, Sharpe JP, Page AV, et al. Efficacy, effectiveness, and safety of herpes zoster vaccines in adults aged 50 and older: systematic review and network meta-analysis. BMJ. 2018;363:k4029. doi: 10.1136/bmj.k4029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160–166. doi: 10.1001/jama.2010.1983 [DOI] [PubMed] [Google Scholar]
- 20.Tseng HF, Sy LS. Use of real-world evidence to evaluate the effectiveness of herpes zoster vaccine. J Infect Dis. 2018;218(suppl_2):S63–S67 doi: 10.1093/infdis/jiy263 [DOI] [PubMed] [Google Scholar]
- 21.Tseng HF, Luo Y, Shi J, Sy LS, Tartof SY, Sim JJ, Hechter RC, Jacobsen SJ. Effectiveness of herpes zoster vaccine in patients 60 years and older with end-stage renal disease. Clin Infect Dis. 2016;62:462–467. doi: 10.1093/cid/civ930 [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Xie F, Delzell E, Chen L, Winthrop KL, Lewis JD, Saag KG, Baddley JW, Curtis JR. Association between vaccination for herpes zoster and risk of herpes zoster infection among older patients with selected immune-mediated diseases. JAMA. 2012;308:43–49. doi: 10.1001/jama.2012.7304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. doi: 10.1002/sim.5984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107 doi: 10.1002/sim.3697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arnold BF, Ercumen A. Negative control outcomes: a tool to detect bias in randomized trials. JAMA. 2016;316:2597–2598. doi: 10.1001/jama.2016.17700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipsitch M, Tchetgen Tchetgen E, Cohen T. Negative controls: a tool for detecting confounding and bias in observational studies. Epidemiology. 2010;21:383–388. doi: 10.1097/EDE.0b013e3181d61eeb [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86:726–728. doi: 10.2105/ajph.86.5.726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holm SA. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 29.Breuer J, Pacou M, Gautier A, Brown MM. Herpes zoster as a risk factor for stroke and TIA: a retrospective cohort study in the UK. Neurology. 2014;83:e27–e33. doi: 10.1212/WNL.0000000000000584 [DOI] [PubMed] [Google Scholar]
- 30.Erskine N, Tran H, Levin L, Ulbricht C, Fingeroth J, Kiefe C, Goldberg RJ, Singh S. A systematic review and meta-analysis on herpes zoster and the risk of cardiac and cerebrovascular events. PLoS One. 2017;12:e0181565. doi: 10.1371/journal.pone.0181565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng HF, Harpaz R, Luo Y, Hales CM, Sy LS, Tartof SY, Bialek S, Hechter RC, Jacobsen SJ. Declining effectiveness of herpes zoster vaccine in adults Aged ≥60 years. J Infect Dis. 2016;213:1872–1875. doi: 10.1093/infdis/jiw047 [DOI] [PubMed] [Google Scholar]
- 32.Gilden D, Nagel MA, Mahalingam R, Mueller NH, Brazeau EA, Pugazhenthi S, Cohrs RJ. Clinical and molecular aspects of varicella zoster virus infection. Future Neurol. 2009;4:103–117. doi: 10.2217/14796708.4.1.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagel MA, Gilden D. The relationship between herpes zoster and stroke. Curr Neurol Neurosci Rep. 2015; 15:16. doi: 10.1007/s11910-015-0534-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amlie-Lefond C, Gilden D. Varicella zoster virus: a common cause of stroke in children and adults. J Stroke Cerebrovasc Dis. 2016;25:1561–1569. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Askalan R, Laughiin S, Mayank S, Chan A, MacGregor D, Andrew M, Curtis R, Meaney B, deVeber G. Chickenpox and stroke in childhood: a study of frequency and causation. Stroke. 2001;32:1257–1262. doi: 10.1161/01.str.32.6.1257 [DOI] [PubMed] [Google Scholar]
- 36.Grose C. Biological plausibility of a link between arterial ischemic stroke and infection with varicella-zoster virus or herpes simplex virus. Circulation. 2016;133:695–697. doi: 10.1161/CIRCULATIONAHA.116.021459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weinstein SJ, Vogt TM, Gerrior SA. Healthy Eating Index scores are associated with blood nutrient concentrations in the third National Health And Nutrition Examination Survey. J Am Diet Assoc. 2004;104:576–584. doi: 10.1016/j.jada.2004.01.005 [DOI] [PubMed] [Google Scholar]
- 38.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asayama K, Ohkubo T, Yoshida S, Suzuki K, Metoki H, Harada A, Murakami Y, Ohashi Y, Ueshima H, Imai Y; Japan Arteriosclerosis Longitudinal Study (JALS) Group. Stroke risk and antihypertensive drug treatment in the general population: the Japan arteriosclerosis longitudinal study. J Hypertens. 2009;27:357–364. doi: 10.1097/HJH.0b013e32831967ca [DOI] [PubMed] [Google Scholar]
- 40.Almgren T, Persson B, Wilhelmsen L, Rosengren A, Andersson OK. Stroke and coronary heart disease in treated hypertension – a prospective cohort study over three decades. J Intern Med 2005;257:496–502. doi: 10.1111/j.1365-2796.2005.01497.x [DOI] [PubMed] [Google Scholar]
- 41.Benetos A, Petrovic M, Strandberg T. Hypertension management in older and frail older patients. Circ Res. 2019;124:1045–1060. doi: 10.1161/CIRCRESAHA.118.313236 [DOI] [PubMed] [Google Scholar]
- 42.Kang MG, Kim SW, Yoon SJ, Choi JY, Kim KI, Kim CH. Association between frailty and hypertension prevalence, treatment, and control in the elderly Korean population. Sci Rep. 2017;7:7542. doi: 10.1038/s41598-017-07449-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Verdecchia P, Angeli F, Reboldi G, Hypertension and atrial fibrillation: doubts and certainties from basic and clinical studies. Circ Res. 2018;122:352–368. doi: 10.1161/CIRCRESAHA.117.311402 [DOI] [PubMed] [Google Scholar]
- 44.Lian Y, Zhu Y, Tang F, Yang B, Duan R. Herpes zoster and the risk of ischemic and hemorrhagic stroke: a systematic review and meta-analysis. PLoS One. 2017;12:e0171182. doi: 10.1371/journal.pone.0171182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams WW, Lu PJ, O'Halloran A, Kim DK, Grohskopf LA, Pilishvili T, Skoff TH, Nelson NP, Harpaz R, Markowitz LE, et al. ; Centers for Disease Control and Prevention (CDC). Surveillance of vaccination coverage among adult populations - United States, 2014. MMWR Surveill Summ. 2016;65:1–36. doi: 10.15585/mmwr.ss6501a1 [DOI] [PubMed] [Google Scholar]
- 46.Centers for Disease Control and Prevention (CDC). Adult vaccination coverage--United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:66–72. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


