Abstract
Conventional antiretroviral therapy involves administration of standard fixed doses to adults and adolescents. This approach ignores interindividual variability in pharmacokinetics and results in substantial differences in systemic concentrations among patients. Thus, variability in systemic concentrations contributes to variability in response to therapy. This study was designed to evaluate the feasibility and safety of a regimen of zidovudine, lamivudine, and indinavir designed to achieve select target concentrations versus standard dose therapy. Twenty-four antiretroviral-naïve subjects completed the 24-week study; 13 received standard therapy, and 11 received concentration-controlled therapy. There were no differences in baseline characteristics. Oral clearance for all three drugs was not different between weeks 2 and 28; average ratios of week 2 oral clearance to week 28 oral clearance were 0.95, 1.09, and 1.06 for zidovudine, lamivudine, and indinavir, respectively, with 95% confidence intervals including 1. The selected target concentrations were average steady-state concentrations of 0.19 mg/liter for zidovudine and 0.44 mg/liter for lamivudine and a trough concentration of 0.15 mg/liter for indinavir; mean concentrations achieved at week 28 in the concentration-controlled arm were 0.20, 0.54, and 0.19 mg/liter, respectively. Concentration-controlled therapy significantly reduced interpatient variability in zidovudine concentrations and significantly increased indinavir concentrations. There was no difference in adverse drug effects or adherence. This investigation has provided a pharmacologic basis for concentration-controlled therapy by demonstrating that it is feasible and has a safety profile no different from that of standard therapy. Additional studies to evaluate the virologic effect of the concentration-controlled approach to antiretroviral therapy are warranted.
Combination therapy with three or more highly active antiretroviral agents is advocated for the treatment of human immunodeficiency virus (HIV) infection (b; Panel on Clinical Practices for Treatment of HIV Infection [http://www.hivatis.org/trtgdlns.html]. Recommended first-line regimens include the use of two nucleoside reverse transcriptase inhibitors with either one or two protease inhibitors, or with a nonnucleoside reverse transcriptase inhibitor. Among these combination regimens, zidovudine, lamivudine, and indinavir have demonstrated the most effective and most durable response to date (15–17, 20). Unfortunately, not all patients adequately respond to highly active antiretroviral therapy. This heterogeneity has been attributed to pharmacologic, virologic, immunologic, and behavioral differences among patients. Conventional antiretroviral therapy involves the administration of standard fixed doses to adults and adolescents. This approach ignores interindividual variability in pharmacokinetic processes and results in substantial differences in systemic concentrations among patients. It is becoming increasingly evident that pharmacodynamic relationships exist between antiretroviral drug concentrations and response for all classes of antiretrovirals (1, 4, 5, 11, 14, 18, 19, 22, 23, 31, 33; E. P. Acosta et al., 7th Conf. Retrovir. Opportun. Infect., abstr. 455, 2000; A. S. Joshi et al., 39th Int. Conf. Antimicrob. Agents Chemother., abstr. I-1201, 1999; D. Slain et al., 38th Int. Conf. Antimicrob. Agents Chemother., abstr. A-74, 1998). Therefore, heterogeneity in the response to antiretroviral therapy may arise from variability in systemic antiretroviral concentrations. Employing dosing regimens to achieve a target systemic concentration may improve clinical outcome by reducing variability in the pharmacologic contribution to therapeutic success. The specific aims of this study were, first, to determine whether a novel dose adjustment strategy we developed to achieve and maintain selected concentrations of zidovudine, lamivudine, and indinavir in plasma was feasible and, second, to evaluate the safety of the concentration-controlled approach versus the standard dose regimen.
MATERIALS AND METHODS
Human subjects and study design.
This investigation was approved by the Human Subjects Committee of the University of Minnesota and was conducted at the Outpatient Clinic of the General Clinical Research Center at the University of Minnesota. Subjects were informed about the study and gave written consent prior to participation. Antiretroviral-naïve, HIV-infected persons (age, 18 to 60 years) with plasma HIV RNA levels of ≥5,000 copies/ml and CD4 T-lymphocyte counts of ≥100 cells/μl were eligible for participation. Exclusion criteria included active opportunistic infection that would require interruption of antiretroviral therapy and known history of nonadherence with medications or scheduled physician and clinic visits. After enrollment, individuals missing scheduled clinic visits and not rescheduling within 1 week or in <85% adherence with their assigned regimen as assessed by medication counts or interview were discontinued from the study.
This study was a randomized, open-label study of standard dose therapy compared with concentration-controlled therapy. The initial phase of the study was 6 months; long-term follow-up will be presented separately. All participants were initially treated with lamivudine (150 mg twice daily) and indinavir (800 mg every 8 h) for the first 2 weeks. Zidovudine was started at a dose of 100 mg twice daily for the first week and then increased to 200 mg twice daily for the second week to minimize gastrointestinal side effects. At week 2, patients were randomized to either standard therapy—consisting of zidovudine (300 mg twice daily), lamivudine (150 mg twice daily), and indinavir (800 mg every 8 h)—or concentration-controlled therapy. Randomization was performed using a permuted block approach with assignments contained in sealed, opaque envelopes sequentially numbered. Patients randomized to standard therapy received separate zidovudine and lamivudine tablets for the 6-month study period. Study participants randomized to concentration-controlled therapy received an individualized regimen developed to maintain targeted antiretroviral drug concentrations in plasma. An average steady-state concentration in plasma (Css) of 0.19 mg/liter was selected for zidovudine. This concentration was based on our previous experience with concentration-controlled zidovudine monotherapy (12). The target Css selected for lamivudine was 0.44 mg/liter. This target was selected because it is the Css that persons receiving 150 mg of lamivudine would have if they were perfectly adherent and had average values for lamivudine bioavailability and total body clearance (30). This was the same conceptual approach originally used to define the target concentration for zidovudine. The Css was chosen for both drugs based upon in vitro data showing that the amount of intracellular triphosphate formed is related to the extracellular concentration of the parent drug (21). A trough concentration (Cmin) of 0.15 mg/liter was selected for indinavir based on two considerations. First, the concentration of indinavir necessary to inhibit 95% of HIV replication in vitro ranges from 0.015 to 0.061 mg/liter for wild-type HIV isolates. Plasma protein binding of indinavir is approximately 56%; therefore, a concentration in plasma above 0.110 mg/liter in vivo would theoretically be necessary to achieve unbound concentrations sufficient to inhibit 95% of wild-type virus. Second, an exploratory study of indinavir concentrations and effect in a cohort of 23 persons receiving nucleoside therapy plus indinavir found that the median indinavir Cmin in patients with undetectable HIV RNA was 0.147 mg/liter, whereas it was 0.037 mg/liter (P = 0.007) in those with detectable HIV RNA (1). Taken together, these considerations led us to choose 0.15 mg/liter as the target Cmin for indinavir.
Pharmacokinetic and adherence evaluations.
Blood samples for plasma zidovudine, lamivudine, and indinavir concentrations were obtained from all study participants at weeks 2 and 28 at the following times: predose and 0.5, 1, 2, 3, 4, 5, 6, 7, and 8 h postdose. All subjects received the standard dose of zidovudine, lamivudine, and indinavir for the week 2 pharmacokinetic studies. At the week 28 pharmacokinetic study, standard therapy recipients received their doses of zidovudine and lamivudine as a single tablet (Combivir; Glaxo Wellcome) twice daily. Concentration-controlled recipients received their individualized doses for the week 28 visit. Patients were not allowed to eat 1 h before or 2 h after ingestion of their medications since food has been shown to affect absorption of these drugs (3, 7, 24, 26, 32, 35). Blood samples were also obtained between 2 and 5 h following drug administration at weeks 4, 8, 12, 16, 20, and 24. This time frame was chosen to avoid the absorption phase and obtain postabsorption concentrations within an optimal window, as assessed by d-optimality criteria, as previously described for zidovudine (27).
Zidovudine and lamivudine were quantitated by a validated simultaneous high-performance liquid chromatography procedure. The mobile phase consisted of 8.5% acetonitrile and 91.5% 50 mM phosphate buffer containing 50 mM triethylamine at pH 7. The chromatographic separation was performed on a Waters Spherisorb, 4.6- by 250-mm reversed-phase octyl column with a 5-μm particle diameter. Detection was achieved by a SpectraFocus forward-scanning UV detector at 266 nm with Chrom Perfect software used for data capture and quantification. A GBC model LC1650 autoinjector and model LC1150 pump were used. A 200-μl sample volume was spiked with 25 μl of 50 μM β-hydroxyethyl-theophylline as the internal standard and applied to an Empore C18 SPE cartridge. The final elution of 300 μl of 100% methanol was dried under nitrogen and reconstituted in the mobile phase and 50 μl was injected. Standards for both zidovudine and lamivudine ranged from 25 to 2,500 ng/ml. The within-day coefficient of variation (CV) ranged from 5.3 to 1.5% for zidovudine and 9.3 to 4.8% for lamivudine. Accuracy ranged from 98 to 105.5% for zidovudine and 97.2 to 102.7% for lamivudine. Quality controls were used at 75,300, and 1,500 ng/ml. These within-day CVs ranged from 5.3 to 1.5% for zidovudine and 14.8 to 6.7% for lamivudine. Accuracy ranged from 97.2 to 102.5% for zidovudine and 92.6 to 99.2% for lamivudine. Analysis of variance (ANOVA) calculations were also performed on the quality controls using triplicate determinations on five separate days to determine the total assay variation expected between days for a single replicate. The CVs ranged from 1.0 to 3.8%, with an overall accuracy of 99.2%, for zidovudine and from 5.5 to 9.4%, with an overall accuracy of 93.7%, for lamivudine. Indinavir concentrations in plasma were quantitated by high-performance liquid chromatography (13). The lower limit of quantitation was 0.02 mg/liter, with a CV of <10% at all concentrations. Interday and intraday CVs were less than 7 and 5%, respectively, for each of the three quality control samples.
Pharmacokinetic parameters for zidovudine, lamivudine, and indinavir were calculated for all subjects at week 2 and week 28. A one-compartment model with first-order absorption, an absorption lag phase, and first-order elimination was fit to the concentration-time data using maximum a posteriori probability-Bayesian estimation (ADAPT II, version 4.0) (9). A proportional variance model was used to describe the error associated with the concentration-time data. The nominal values and the variances of the population parameters used in the Bayesian estimator were taken from published work with zidovudine and indinavir and literature sources for lamivudine (1, 2, 30). Model construction was guided by Akaike's information criterion and visual inspection of actual versus fitted concentrations (34). For only those patients randomized to concentration-controlled therapy, these parameters were used to calculate initial individualized doses, which were implemented at study week 4. Pharmacokinetic parameters were reevaluated every 4 weeks, incorporating the most-recent plasma concentration information, and further dose adjustments were made for the concentration-controlled recipients if necessary. The zidovudine dose (in milligrams/day) was calculated according to the formula dose = (CL/F)(0.19 mg/liter)(24 h), where CL/F is the individual patient's estimate of oral clearance in liters per hour and 0.19 mg/liter is the targeted concentration. Calculated doses were rounded to the nearest 100 mg, and an attempt was made to keep administration to three times a day (e.g., if a patient required 800 mg/day, the daily regimen would be 300 mg in the morning, 200 mg in the afternoon, and 300 mg in the evening). Twice-daily dosing of zidovudine was not used in the concentration-controlled regimens. Commercially available 100-mg zidovudine capsules (Glaxo Wellcome) were used. The daily dose of lamivudine (in milligrams/day) was calculated in a similar fashion: dose = (CL/F)(0.44 mg/liter)(24 h). Doses were adjusted to the nearest 75 mg; lamivudine dosing intervals were either twice or three times daily. Commercially available 150-mg lamivudine tablets (Glaxo Wellcome) were used.
Dose adjustment for indinavir was based on the following rearrangement of an equation for steady-state Cmin:
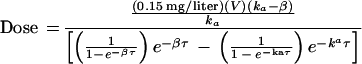 |
where V is the apparent volume of distribution in liters, ka is the oral absorption rate constant, β is the elimination rate constant, and τ is the dosing interval. Both 200- and 400-mg capsules (Merck and Co., Inc.) were available for dose adjustments. Dosing intervals were restricted to every 8 or every 6 h for patient convenience. At no point were patients' doses below the recommended daily doses of zidovudine (600 mg/day), lamivudine (300 mg/day), and indinavir (2,400 mg/day).
Medications were provided to the study participants at each visit in quantities sufficient to last until the next visit (i.e., 15- or 30-day supply). Thus, a total of eight visits per patient encompassing weeks 0 to 2, 2 to 4, 4 to 8, 8 to 12, 12 to 16, 16 to 20, 20 to 24, and 24 to 28 were possible. Study participants were requested to return their unused medications at each clinic visit. To be eligible for evaluation of adherence, a study participant must have returned their medication for at least 4 of the 8 visits. Adherence for each visit was calculated as the ratio of the number of dosage units taken, as determined by medication count adjusted for time lapsed between visits, to the number expected. Additional adherence assessments included a formal interview with the patient and inspection of plasma drug concentrations at each visit.
Laboratory evaluations.
A clinical assessment and measurement of hematologic parameters and clinical chemistries were performed with every clinic visit. Urinalysis and cholesterol and triglyceride analyses were performed every 3 to 4 months. Adverse reactions were graded and managed using the approach developed by the AIDS Clinical Trials Group (10). CD4 lymphocytes and plasma HIV RNA (Roche Amplicor Ultrasensitive Assay) were measured at baseline and every 4 weeks during the study.
Statistical analyses.
The sample size for this study was based on two considerations: first, the ability to detect a >40% difference in the variance of Css for zidovudine between the standard and concentration-controlled regimen, and second, the ability to detect a difference in Cmin of 0.09 mg/liter for indinavir. The effect size for indinavir was selected because it is the difference in indinavir Cmin found in an earlier study of patients with undetectable plasma HIV RNA levels compared with those that had HIV RNA detectable in plasma (1). For both of these considerations, a sample size of 24 patients was sufficient at an α of 0.05 and 80% power.
Baseline patient characteristics were evaluated with the Mann-Whitney U test. Comparisons of pharmacokinetic parameters between treatment groups and between weeks 2 and 28 were analyzed with repeated-measures ANOVA. Variances were compared by using the F test. The proportions of subjects achieving the target concentration in both groups were compared by using Fisher's exact test. A concentration between −10% of and more than the defined target was considered acceptable. Safety parameters were compared between groups by using the χ2 test. Assessment of adherence data was done by ANOVA. Excel 98 (Microsoft Inc., Redmond, Wash.) was used to maintain the patient database. Statview 5.0.1 (SAS Institute, Inc., Cary, N.C.) was used for statistical analyses. For all statistical analyses, a P value of <0.05 was considered significant.
RESULTS
Human subjects.
Thirty-two antiretroviral-naïve persons with HIV consented to participate in this study; all were determined to be eligible based upon laboratory criteria and were subsequently enrolled in the study. No potential participant was excluded from participation for a known history of nonadherence with medications or scheduled physician and clinic visits. Eight patients did not complete the 28-week study period. One subject moved out of state. Two participants at study weeks 4 and 8, respectively, were lost to follow-up. One participant randomized to concentration-controlled therapy withdrew from the study at week 8 because of an unwillingness to meet protocol requirements. Four individuals withdrew prior to week 28 for medical reasons or because of drug toxicity: two people (one each randomized to concentration-controlled and standard therapy) withdrew for gastrointestinal intolerance, one person (standard therapy) withdrew after the development of peripheral neuropathy at study week 4, and one person (standard therapy) was discontinued after the development of a brain lesion and anemia at week 12. Of the remaining 24 subjects, 13 were randomized to standard therapy and 11 were randomized to concentration-controlled therapy. There were no differences in baseline characteristics between the two treatment groups (Table 1).
TABLE 1.
Baseline characteristics
| Characteristic | Therapy group
|
|
|---|---|---|
| Standard | Concentration controlled | |
| No. of participants | 13 | 11 |
| Male sex (n) | 10 | 10 |
| Age (range) (yr) | 39 (28–59) | 38 (29–47) |
| Race or ethnic group (n) | ||
| White, non-Hispanic | 8 | 7 |
| African American | 5 | 2 |
| Hispanic | 0 | 1 |
| Asian Pacific or other | 0 | 1 |
| CD4 count (cells/μl) (mean ± SD) | 315 ± 182 | 300 ± 186 |
| HIV RNA (log10 copies/ml) (mean ± SD) | 4.56 ± 0.47 | 4.71 ± 0.65 |
Pharmacokinetic evaluations.
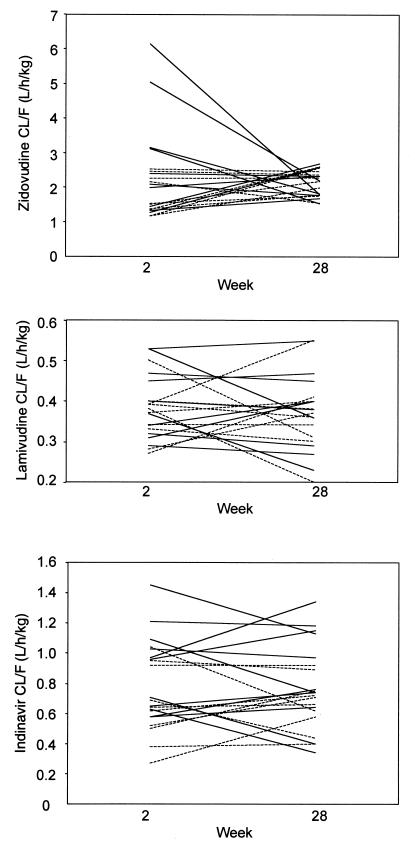
The week 2 and 28 pharmacokinetic parameters for zidovudine, lamivudine, and indinavir for both arms of the study are presented in Table 2. There was no difference in CL/F for zidovudine, lamivudine, and indinavir between week 2 and week 28 or between standard and concentration-controlled therapy recipients. Figure 1 presents CL/F values for all 24 patients at week 2 and week 28. The average ratios of week 2 to week 28 CL/F (and 95% confidence interval) for the following medications were as indicated: zidovudine, 0.95 (0.78 to 1.12); lamivudine, 1.09 (0.96 to 1.22); and indinavir, 1.06 (0.9 to 1.22).
TABLE 2.
Pharmacokinetic parameters of zidovudine, lamivudine, and indinavir at weeks 2 and 28
| Drug and parameter | Mean ± SD for indicated subjects at:
|
|||||
|---|---|---|---|---|---|---|
| Wk 2a
|
Wk 28b
|
|||||
| Stdf(n = 13) | CCg(n = 11) | All (n = 24) | Std (n = 13) | CC (n = 11) | All (n = 24) | |
| Zidovudine | ||||||
| CL/F (liters/h/kg) | 2.52 ± 1.52 | 1.78 ± 0.52 | 2.18 ± 1.21 | 2.07 ± 0.41 | 2.15 ± 0.37 | 2.11 ± 0.38 |
| V/F (liters/kg) | 2.31 ± 0.96 | 2.04 ± 0.55 | 2.21 ± 0.83 | 2.79 ± 0.42 | 2.41 ± 0.87 | 2.61 ± 0.68 |
| t1/2 (h) | 0.72 ± 0.29 | 0.71 ± 0.17 | 0.71 ± 0.24 | 0.96 ± 0.16c | 0.74 ± 0.20 | 0.86 ± 0.21d |
| Css (mg/liter) | 0.19 ± 0.08 | 0.20 ± 0.06 | 0.20 ± 0.07 | 0.20 ± 0.06 | 0.20 ± 0.03e | 0.19 ± 0.05 |
| Lamivudine | ||||||
| CL/F (liters/h/kg) | 0.38 ± 0.09 | 0.37 ± 0.06 | 0.38 ± 0.08 | 0.38 ± 0.10 | 0.37 ± 0.09 | 0.38 ± 0.09 |
| V/F (liters/kg) | 1.47 ± 0.54 | 1.36 ± 0.35 | 1.42 ± 0.46 | 1.33 ± 0.33 | 1.38 ± 0.33 | 1.35 ± 0.32 |
| t1/2 (h) | 2.65 ± 0.61 | 2.53 ± 0.29 | 2.59 ± 0.49 | 2.46 ± 0.50 | 2.60 ± 0.40 | 2.53 ± 0.45 |
| Css (mg/liter) | 0.51 ± 0.11 | 0.51 ± 0.11 | 0.50 ± 0.09 | 0.52 ± 0.13 | 0.53 ± 0.12 | 0.53 ± 0.12 |
| Indinavir | ||||||
| CL/F (liters/h/kg) | 0.89 ± 0.26 | 0.64 ± 0.24 | 0.78 ± 0.28 | 0.85 ± 0.33 | 0.67 ± 0.24 | 0.77 ± 0.27 |
| V/F (liters/kg) | 1.23 ± 0.44c | 0.93 ± 0.35 | 1.09 ± 0.42 | 1.29 ± 0.45 | 1.02 ± 0.22 | 1.16 ± 0.38 |
| t1/2 (h) | 0.97 ± 0.23 | 1.05 ± 0.26 | 1.01 ± 0.24 | 1.09 ± 0.16 | 1.08 ± 0.13 | 1.08 ± 0.14 |
| Cmin (mg/liter) | 0.09 ± 0.05 | 0.14 ± 0.13 | 0.11 ± 0.10 | 0.10 ± 0.07c | 0.19 ± 0.10 | 0.14 ± 0.09 |
Week 2 results are pharmacokinetic parameters obtained with all subjects receiving the same dose and prior to any dose adjustments in those randomized to concentration-controlled therapy.
Week 28 results are pharmacokinetic parameters obtained with standard therapy subjects receiving the standard doses of zidovudine, lamivudine, and indinavir and the concentration-controlled subjects receiving the regimen designed to achieve the target concentrations which was implemented at week 4.
P < 0.05, standard therapy versus concentration-controlled therapy (repeated-measures ANOVA)
P < 0.05, week 2 versus week 28 (repeated-measures ANOVA).
P < 0.05, variance of standard therapy versus concentration-controlled therapy (F test).
Std, standard dose therapy.
CC, concentration-controlled therapy.
FIG. 1.
Estimated CL/F at weeks 2 and 28 for zidovudine (top), lamivudine (middle), and indinavir (bottom). Solid lines represent standard therapy patients (n = 13); dashed lines represent concentration-controlled patients (n = 11).
Dose adjustments for zidovudine, lamivudine, or indinavir were necessary for 10 out of 11 concentration-controlled patients. All initial dose adjustments were based on the pharmacokinetic study conducted at week 2 and were implemented at week 4. Nine subjects required a change in their dose of indinavir. These adjustments were a change from the standard dose of 800 mg every 8 h to 600 mg every 6 h (n = 2), 800 mg every 6 h (n = 4), or 1,000 mg every 8 h (n = 3). During the course of the 28-week study only one subject required a subsequent change in indinavir dose based upon pharmacokinetic data, and this was from 1,000 mg every 8 h to 800 mg every 6 h. Zidovudine doses were changed in five patients: one patient's dose was increased to 900 mg/day, one patient's dose was increased to 700 mg/day, and three patients' doses were changed to 800 mg/day. Only one patient had a subsequent change in zidovudine dose, which was a reduction from 800 to 600 mg/day due to nausea and vomiting. Only two patients required a change in their dose of lamivudine, both of which were an increase from 150 mg twice daily to 150 mg thrice daily.
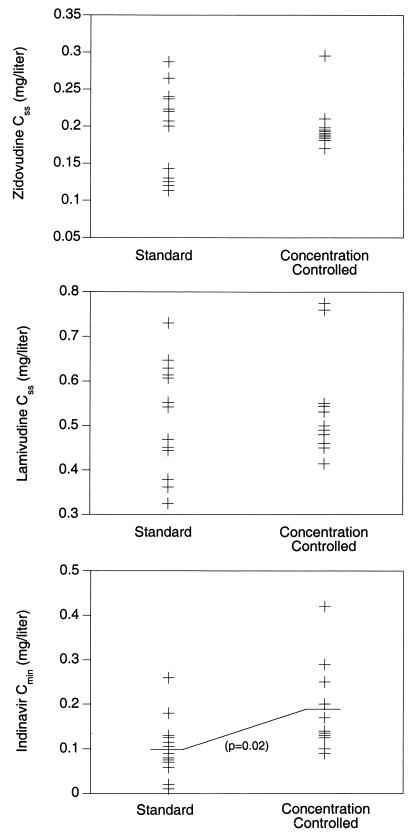
Figure 2 shows the measured Css for zidovudine at week 28 in the standard and concentration-controlled recipients. There was no difference in Css values between the treatment arms; however, variability in Css was significantly less in the concentration-controlled recipients (P = 0.001). All concentration-controlled recipients achieved the target zidovudine Css at week 28, whereas 8 of 13 (62%) of standard therapy recipients achieved the target (P = 0.04). There was no difference in lamivudine Css at week 28 between the two groups. Three patients in the standard arm had Css values below the desired target, compared with none in the concentration-controlled group.
FIG. 2.
Target concentrations at week 28. Css values for zidovudine and lamivudine and Cmin values for indinavir obtained at week 28 in patients receiving standard (n = 13) or concentration-controlled (n = 11) therapy. The variability in Css for zidovudine was significantly reduced with the concentration-controlled regimen compared with the standard dose regimen. Indinavir Cmin values were significantly higher in the recipients of concentration-controlled therapy than in those receiving standard dose therapy.
Figure 2 also presents indinavir trough concentrations measured at week 28 in the standard therapy and concentration-controlled recipients. The mean Cmin with standard therapy was 0.10 mg/liter, which was significantly less than the mean Cmin of 0.19 mg/liter achieved in concentration-controlled recipients (P = 0.02). At week 28, 9 of 11 (82%) concentration-controlled recipients had mean Cmin values at or above the target, compared with 3 of 13 (23%) standard dose recipients (P = 0.01).
Adherence evaluations.
Among the 24 study participants, 18 (75%) were eligible for adherence evaluation. These 18 subjects returned study medication for 87.5% (126 of 144) of the clinic visits. The overall mean adherence values were 96% for zidovudine, 97% for lamivudine, and 96% for indinavir. Standard therapy recipients (n = 10) had mean adherence values of 96, 95, and 94% for zidovudine, lamivudine, and indinavir, respectively. The mean adherence values for concentration-controlled patients were 96% for zidovudine and 98% for both lamivudine and indinavir. There was no difference in adherence between the two groups. No particular time period during the 28-week study was associated with more or less adherence. No subject was discontinued from the study for poor adherence.
Safety.
Overall, there was no significant difference in the number of adverse events between the standard and concentration-controlled treatment arms. Asymptomatic hyperbilirubinemia was the most frequent objective side effect noted. Grade I or II elevations in total bilirubin occurred in nine standard therapy recipients and seven concentration-controlled recipients. Two patients who received concentration-controlled therapy developed grade III hyperbilirubinemia; one patient was on 800 mg of indinavir every 8 h at the time, and the other was on 800 mg every 6 h. Three concentration-controlled recipients had grade I or II elevations in liver enzymes; a fourth patient had grade IV elevations but also had hepatitis C coinfection at the time.
Crystalluria was present in two patients, one from each group, during the study. The crystals were not specifically identified for origin but were believed to be indinavir related. Neither patient reported flank pain, dysuria, or any other symptoms related to nephrolithiasis. Flank pain was reported at weeks 12 and 16 in two patients randomized to standard therapy; one patient also had dysuria. One patient randomized to concentration-controlled therapy developed nephrolithiasis at week 28, requiring hospital admission. This patient was receiving an indinavir dose of 800 mg every 8 h.
Hematologic toxicities were mostly mild to moderate, with the exception of the one previously described case. Four patients receiving concentration-controlled therapy and three patients receiving standard therapy experienced a grade I decrease in absolute neutrophil count; one standard patient each developed a grade II and a grade IV drop. None of the 24 patients that completed 24 weeks of therapy required discontinuation or modification of therapy or supportive medications (i.e., transfusion or erythropoietin) for hematologic toxicities. Six patients (three in each arm) had nausea and fatigue during the initial part of therapy despite the zidovudine titration scheme. The majority of patients reported feeling better after 4 weeks of therapy. Nausea was not associated with the use of higher zidovudine doses.
Other side effects that were possibly related to study medications included partial hair loss (n = 3), hypercholesterolemia (n = 2), hyperglycemia (n = 1), and hypertension (n = 1). None of the patients developed fat redistribution syndrome or excessive weight gain or loss.
DISCUSSION
We have shown that the administration of zidovudine, lamivudine, and indinavir in a regimen designed to achieve a specific target concentration was feasible and had a safety and tolerance profile not different from the standard dose regimen. The concentration targets selected were average Css of 0.19 and 0.44 mg/liter for zidovudine and lamivudine, respectively, and a Cmin of 0.15 mg/liter for indinavir. The actual mean values at week 28 in the concentration-controlled recipients were 0.20 mg/liter for zidovudine, 0.54 mg/liter for lamivudine, and 0.19 mg/liter for indinavir. A significantly higher proportion of subjects receiving concentration-controlled compared with standard dose therapy achieved these targets for zidovudine and indinavir.
The 24 subjects who participated in this study and were randomized to receive either standard dose or concentration-controlled therapy were well balanced with respect to baseline characteristics, including CL/F (determined at week 2) for zidovudine, lamivudine, and indinavir. Thus, differences in concentrations accomplished with the use of a concentration-controlled regimen did not arise because of baseline differences in pharmacokinetic behavior. A necessary element for the successful application of a concentration-controlled strategy is for intrapatient pharmacokinetic variability to be less than interpatient variability. Figure 1 shows individual patient CL/F values at weeks 2 and 28 and illustrates a general consistency within patients over the 28-week study duration. There was no difference in CL/F for zidovudine, lamivudine, and indinavir between weeks 2 and 28. The mean week 2- to 28-week ratios of CL/F for all three drugs were between 0.95 and 1.09, with 95% confidence intervals encompassing 1. These data provide clear evidence that intrapatient variability over a 6-month period was low and did not result in statistically significant differences in CL/F.
The target concentrations selected for zidovudine and lamivudine were the average Css expected in a patient perfectly adherent with the standard dose regimen of 600 mg/day for zidovudine and 300 mg/day for lamivudine and who had the population average values for bioavailability and total body clearance. Thus, the concentration-controlled strategies for zidovudine and lamivudine were designed not to produce average Css values that were different from those with the standard dose but rather to reduce interpatient variability in Css, as we have previously shown in a study of zidovudine monotherapy (11). Use of the concentration-controlled regimen for lamivudine did not result in a significant reduction in interpatient variability in Css. This is not surprising, as only two persons in the concentration-controlled arm required a lamivudine dose adjustment to achieve the desired concentration. For zidovudine, however, interpatient variability in Css was significantly reduced by 50% with the concentration-controlled regimen. This magnitude of reduction is consistent with that found in an earlier concentration-controlled study with zidovudine monotherapy. The range of zidovudine doses used in the present study, 600 to 900 mg/day (average, 690 mg/day), to achieve a target Css of 0.19 mg/liter is less than the range needed in a previous study (with doses up to 1,200 mg/day) to reach the same target (11). The discrepancy between these studies may in part be due to a drug interaction between zidovudine and indinavir. Indinavir has been shown to increase zidovudine area under the curve by 17 to 36% (25).
The target concentration strategy for indinavir was designed to achieve a Cmin of ≥0.15 mg/liter. The average Cmin with the concentration-controlled approach was 0.19 mg/liter, which was significantly greater than the 0.10 mg/liter produced with the standard dose. Indinavir doses necessary for concentration-controlled regimens ranged from 2,400 to 3,200 mg/day; dosing intervals of every 8 h and every 6 h were employed. Even with these efforts, 2 of the 11 concentration-controlled recipients could not be dosed to attain the desired target concentration, as we chose to not exceed an indinavir dose of 3,200 mg/day for safety considerations. Such patients may represent rapid metabolizers of indinavir, and perhaps other drugs that are substrates for similar metabolic pathways, and may explain why some antiretroviral-naïve patients fail highly active antiretroviral therapy at standard doses despite good adherence. Attempts to prospectively identify these patients may prove useful. The erythromycin breath test has been suggested as a tool for this purpose, but a prospective study failed to demonstrate any utility (Slain et al., 38th ICAAC).
As assessed by counts of returned medications, overall adherence in this study was high for all three drugs. Furthermore, adherence was equally good between the concentration-controlled and standard therapy recipients. We were concerned that the more frequent dosing and greater capsule or tablet burden necessary to implement the concentration-controlled regimens would affect adherence adversely. However, we found no evidence to support this concern. This observation is consistent with a study of adherence that found no difference in patient adherence to a regimen of protease inhibitors given twice daily compared with thrice daily (29). These data indicate that factors distinct from the complexity of the regimen play an important role in adherence to a medication regimen; a patient's understanding of disease and drug therapy, motivation, and relationship with his or her health care provider are likely possibilities. The participants in this study may have been biased towards a more adherent population, as an exclusion criterion was a known history of nonadherence with medications or scheduled physician and clinic visits. While no potential participant was excluded based upon this criterion, we do not know if some individuals were not referred by their physician for potential participation in this study because of this criterion. Thus, the overall high degree of medication adherence achieved in both the concentration-controlled and standard therapy recipients may not extrapolate to a larger population of HIV-infected persons receiving antiretroviral therapy.
Overall, zidovudine, lamivudine, and indinavir were safe and well tolerated by the participants during this 28-week study. The adverse reactions of primary concern with this regimen were anemia, neutropenia, and nephrolithiasis. Grade III or IV adverse events occurred in 4 of the 24 patients (17%); there was no difference in events between the concentration-controlled and standard therapy recipients. This is consistent with the finding in our previous study of concentration-controlled zidovudine therapy where, despite an overall higher dose, systemic concentrations, and intracellular zidovudine triphosphate concentrations, there were no differences in the rates of anemia and neutropenia between standard dose and concentration-controlled therapy (11). This 17% rate is consistent also with the 26% incidence of grade III or IV adverse reactions in the AIDS Clinical Trials Group study (study 320) of zidovudine, lamivudine, and indinavir (17). We found no difference in the incidence of urologic complaints in standard dose recipients compared with concentration-controlled recipients. Frank nephrolithiasis developed in one patient during the course of treatment, and two others reported flank pain. All three patients had no prior history of renal disease. The concentration-controlled patient who developed the kidney stone had been receiving indinavir (800 mg every 8 h) throughout the course of study. It has been suggested that higher plasma concentrations of indinavir are associated with a higher incidence of urologic complaints, including nephrolithiasis (9). However, the basis for a higher dose in a patient receiving concentration-controlled therapy is to adjust for a higher-than-average clearance of the drug. Therefore, these patients may not have the same risk of dose- or concentration-related nephrolithiasis as would a person with average clearance receiving an increased dose. Nevertheless, antiretroviral therapy is a long-term undertaking, and a more prolonged comparative assessment of the safety and tolerance of concentration-controlled versus standard dose therapy would be important.
Irrefutable progress in the pharmacotherapy of HIV infection has been made (28). Improving the use of currently available antiretroviral agents is as important as the rational development of promising new compounds in order to advance therapeutics further. This investigation has provided a pharmacologic basis for concentration-controlled combination antiretroviral therapy by demonstrating that it is feasible and that it has a short-term safety profile comparable with the standard dose regimen. Studies to learn whether concentration-controlled therapy provides a virologic advantage over the conventional approach of administering the same dose of antiretroviral agents to all adults now appear warranted.
ACKNOWLEDGMENTS
This work was supported by grants RO1-AI33835-07 from the National Institute of Allergy and Infectious Diseases and MO1-RR00400 from the National Institute of Health, Center for Research Resources General Clinical Research Centers Program.
We thank Cynthia Gross for comments on study design and data analysis; Rory P. Remmel for his guidance with development of the zidovudine, lamivudine, and indinavir assays; Dennis Weller, Lane Bushman, Shao Mei Han, and Sagar Kawle for their technical assistance; Henry H. Balfour, Jr., Alejo Erice, and Carolyn Beatty of the Clinical Virology Laboratory for their assistance; the laboratory of J. Brooks Jackson at Johns Hopkins University Medical School for measurement of HIV RNA in plasma; the staff of the General Clinical Research Center for their outstanding patient care; Glaxo Wellcome for their donation of zidovudine, lamivudine, and Combivir; Merck and Co., Inc., for their donation of indinavir; and the patients for their involvement in this study.
REFERENCES
- 1.Acosta E P, Henry K, Baken L, Page L M, Fletcher C V. Indinavir concentrations and antiviral effect. Pharmacotherapy. 1999;19:708–712. doi: 10.1592/phco.19.9.708.31544. [DOI] [PubMed] [Google Scholar]
- 2.Acosta E P, Henry K, Page L M, Erice A, Balfour H H, Fletcher C V. Pharmacokinetics and safety of concentration-controlled oral zidovudine therapy. Pharmacotherapy. 1997;17:424–430. [PubMed] [Google Scholar]
- 3.Angel J, Hussey E, Hall S, Donn K, Morris D, McCormack J, Montaner J, Ruedy J. Pharmacokinetics of 3TC (GR109714X) administered with and without food to HIV-infected patients. Drug Investig. 1993;6:70–74. [Google Scholar]
- 4.Beltangady M, Knupp C, Gustafson N, Barbhaiya R, Dolin R, Seidlin M, Cooley T, Rozencweig M. Relation between plasma concentrations of didanosine and markers of antiviral efficacy in adults with AIDS or AIDS-related complex. Clin Infect Dis. 1993;16:26–31. doi: 10.1093/clinids/16.supplement_1.s26. [DOI] [PubMed] [Google Scholar]
- 5.Burger D M, Hoetelmans R M, Hugen P W, Mulder J W, Meenhorst P L, Koopmans P P, Brinkman K, Keuter M, Dolmans W, Hekster Y A. Low plasma concentrations of indinavir are related to virological treatment failure in HIV-1 infected patients on indinavir-containing triple therapy. Antivir Ther. 1998;3:215–220. [PubMed] [Google Scholar]
- 6.Carpenter C C, Cooper D A, Fischl M A, Gatell J M, Gazzard B G, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schechter M, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA panel. JAMA. 2000;283:381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- 7.Carver P L, Fleisher D, Zhou S Y, Kaul D, Kazanjian P, Li C. Meal composition effects on the oral bioavailability of indinavir in HIV-infected patients. Pharm Res. 1999;16:718–724. doi: 10.1023/a:1018880726035. [DOI] [PubMed] [Google Scholar]
- 8.d'Argenio D, Schumitzky A, Wolf W. Simulation of linear compartment models with application to nuclear medicine kinetics. Comput Methods Programs Biomed. 1988;27:47–54. doi: 10.1016/0169-2607(88)90102-2. [DOI] [PubMed] [Google Scholar]
- 9.Dieleman J P, Gyssens I C, van der Ende M E, de Marie S, Burger D M. Urological complaints in relation to indinavir plasma concentrations in HIV-infected patients. AIDS. 1999;13:473–478. doi: 10.1097/00002030-199903110-00005. [DOI] [PubMed] [Google Scholar]
- 10.Division of AIDS. Division of AIDS table for grading severity of adult adverse experiences. Rockville, Md: Division of AIDS, NIH; 1996. [Google Scholar]
- 11.Fletcher C V, Acosta E P, Henry K, Page L M, Gross C R, Kawle S P, Remmel R P, Erice A, Balfour H H., Jr Concentration-controlled zidovudine therapy. Clin Pharmacol Ther. 1998;64:331–338. doi: 10.1016/S0009-9236(98)90182-5. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher C V, Balfour H H., Jr Variability in zidovudine serum concentrations. Pharmacotherapy. 1996;16:1154–1158. [PubMed] [Google Scholar]
- 13.Fletcher C V, Brundage R C, Remmel R P, Page L M, Weller D, Calles N R, Simon C, Kline M W. Pharmacologic characteristics of indinavir, didanosine, and stavudine in human immunodeficiency virus-infected children receiving combination therapy. Antimicrob Agents Chemother. 2000;44:1029–1034. doi: 10.1128/aac.44.4.1029-1034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gieschke R, Fotteler B R, Buss N, Steimer J-L. Relationships between exposure to saquinavir monotherapy and antiviral response in HIV-positive patients. Clin Pharmacokinet. 1999;37:75–86. doi: 10.2165/00003088-199937010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Gulick R M, Mellors J W, Havlir D, Eron J, Gonzalez C, McMahon D, Jonas L, Meibohm A, Holder D, Schleif W A, Condra J H, Emini E A, Isaacs R, Chodakewitz J A, Richman D D. Simultaneous vs sequential initiation of therapy with indinavir, zidovudine, and lamivudine for HIV-1 infection. JAMA. 1998;280:35–41. doi: 10.1001/jama.280.1.35. [DOI] [PubMed] [Google Scholar]
- 16.Gulick R M, Mellors J W, Havlir D, Eron J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, Emini E A, Chodakewitz J A. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 17.Hammer S M, Squires K E, Hughes M D, Grimes J M, Demeter L M, Currier J S, Eron J J, Feinberg J E, Balfour H H, Jr, Deyton L R, Chodakewitz J A, Fischl M A. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 counts of 200 per cubic millimeter or less. N Engl J Med. 1997;337:725–733. doi: 10.1056/NEJM199709113371101. [DOI] [PubMed] [Google Scholar]
- 18.Harris M, Durakovic C, Rae S, Raboud J, Fransen S, Shillington A, Conway B, Montaner J. A pilot study of nevirapine, indinavir, and lamivudine among patients with advanced human immunodeficiency virus disease who have had failure of combination nucleoside therapy. J Infect Dis. 1998;177:1514–1520. doi: 10.1086/515317. [DOI] [PubMed] [Google Scholar]
- 19.Havlir D, Cheeseman S H, McLaughlin M, Murphy R, Erice A, Spector S A, Greenough T C, Sullivan J L, Hall D, Myers M, Lamson M, Richman D D. High-dose nevirapine: safety, pharmacokinetics, and antiviral effect in patients with human immunodeficiency virus infection. J Infect Dis. 1995;171:537–545. doi: 10.1093/infdis/171.3.537. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch M, Steigbigel R, Staszewski S, Mellors J, Scerpella E, Hirschel B, Lange J, Squires K, Rawlins S, Meibohm A, Leavitt R. A randomized, controlled trial of indinavir, zidovudine, and lamivudine in adults with advanced human immunodeficiency virus type 1 infection and prior antiretroviral therapy. J Infect Dis. 1999;180:659–665. doi: 10.1086/314948. [DOI] [PubMed] [Google Scholar]
- 21.Ho H-T, Hitchcock M J M. Cellular pharmacology of 2′,3′-dideoxy-2′,3′-didehydrothymidine, a nucleoside analog active against human immunodeficiency virus. Antimicrob Agents Chemother. 1989;33:844–849. doi: 10.1128/aac.33.6.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilby J M, Hopkins S, Venetta T M, DiMassimo B, Cloud G A, Lee J Y, Alldredge L, Hunter E, Lambert D, Bolognesi D, Matthews T, Johnson M R, Nowak M A, Shaw G M, Saag M S. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 23.Lorenzi P, Yerly S, Abderrakim K, Fathi M, Rutschmann O T, van Overbeck J, Leduc D, Perrin L, Hirschel B. Toxicity, efficacy, plasma drug concentrations and protease mutations in patients with advanced HIV infection treated with ritonavir plus saquinavir. AIDS. 1997;11:F95–F99. doi: 10.1097/00002030-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Lotterer E, Ruhnke M, Trautmann M, Beyer R, Bauer F. Decreased and variable systemic availability of zidovudine in patients with AIDS if administered with a meal. Eur J Clin Pharmacol. 1991;40:305–308. doi: 10.1007/BF00315215. [DOI] [PubMed] [Google Scholar]
- 25.Malaty L I, Kuper J J. Drug interactions of HIV protease inhibitors. Drug Safety. 1999;20:147–169. doi: 10.2165/00002018-199920020-00005. [DOI] [PubMed] [Google Scholar]
- 26.Moore K H, Shaw S, Laurent A L, Lloyd P, Duncan B, Morris D M, O'Mara M J, Pakes G E. Lamivudine/zidovudine as a combined formulation: bioequivalence compared with lamivudine and zidovudine administered concurrently and the effect of food on absorption. J Clin Pharmacol. 1999;39:593–605. doi: 10.1177/00912709922008209. [DOI] [PubMed] [Google Scholar]
- 27.Noormohamed S E, Henry W K, Rhame F S, Balfour H H, Jr, Fletcher C V. Strategies for control of zidovudine concentrations in serum. Antimicrob Agents Chemother. 1995;39:2792–2797. doi: 10.1128/aac.39.12.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palella F J, Jr, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 29.Paterson D L, Swindells S, Mohr J, Brester M, Vergis E N, Squier C, Wagener M M, Singh N. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 30.Pluda J, Cooley T, Montaner J, Shay L, Reinhalter N, Warthan S. A phase I/II study of 2′-deoxy-3′-thiacytidine (lamivudine) in patients with advanced human immunodeficiency virus infection. J Infect Dis. 1995;171:1438–1447. doi: 10.1093/infdis/171.6.1438. [DOI] [PubMed] [Google Scholar]
- 31.Schapiro J M, Winters M A, Stewart F, Efron B, Norris J, Kozal M J, Merigan T C. The effect of high-dose saquinavir on viral load and CD4+ T-cell counts in HIV-infected patients. Ann Intern Med. 1996;124:1039–1050. doi: 10.7326/0003-4819-124-12-199606150-00003. [DOI] [PubMed] [Google Scholar]
- 32.Unadkat J, Collier A, Crosby S, Cummings D, Opheim K, Corey L. Pharmacokinetics of oral zidovudine (azidothymidine) in patients with AIDS when administered with and without a high-fat meal. AIDS. 1990;4:229–232. doi: 10.1097/00002030-199003000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Vanhove G F, Gries J-M, Verotta D, Sheiner L B, Coombs R, Collier A C, Blaschke T F. Exposure-response relationships for saquinavir, zidovudine, and zalcitabine in combination therapy. Antimicrob Agents Chemother. 1997;41:2433–2438. doi: 10.1128/aac.41.11.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion in the evaluation of linear pharmacokinetic equations. J Pharmacokinet Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- 35.Yeh K C, Deutsch P J, Haddix H, Hesney M, Hoagland V, Ju W D, Justice S J, Osborne B, Sterrett A T, Stone J A, Woolf E, Waldman S. Single-dose pharmacokinetics of indinavir and the effect of food. Antimicrob Agents Chemother. 1998;42:332–338. doi: 10.1128/aac.42.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]