Abstract
Women from racial and ethnic minority groups face a disproportionate burden of cervical and breast cancers in the United States. The Coronavirus Disease 2019 (COVID-19) pandemic might exacerbate these disparities as supply and demand for screening services are reduced. The National Breast and Cervical Cancer Early Detection Program (NBCCEDP) provides cancer screening services to women with low income and inadequate health insurance. We examined COVID-19's impact on NBCCEDP screening services during January-June 2020. We found the total number of NBCCEDP-funded breast and cervical cancer screening tests declined by 87% and 84%, respectively, during April 2020 compared with the previous 5-year averages for that month. The extent of declines varied by geography, race/ethnicity, and rurality. In April 2020, screening test volume declined most severely in Health and Human Services Region 2 - New York (96% for breast, 95% for cervical cancer screening) compared to the previous 5-year averages. The greatest declines were among American Indian/Alaskan Native women for breast cancer screening (98%) and Asian Pacific Islander women for cervical cancer screening (92%). Test volume began to recover in May and, by June 2020, NBCCEDP breast and cervical cancer screening test volume was 39% and 40% below the 5-year average for that month, respectively. However, breast cancer screening remained over 50% below the 5-year average among women in rural areas. NBCCEDP programs reported assisting health care providers resume screening.
Keywords: COVID-19, Cervical cancer screening, Breast cancer screening, Public health, Medically underserved
1. Introduction
Racial and ethnic minority women face a disproportionate burden of cervical and breast cancers. In the United States (US), Black women and Hispanic women have the highest rates of cervical cancer incidence at 8.3 and 8.9 per 100,000 women respectively, compared with 7.3 per 100,000 among White women (USCS, 2020). Black women and Hispanic women also have the highest rates of cervical cancer deaths. For breast cancer, the incidence rate is highest and similar among White women (125.8) and Black women (121.3). However, Black women have the highest rate of deaths due to breast cancer at 26.9 compared with White women at 19.4 per 100,000 women (USCS, 2020). Additionally, Black and Hispanic women are more likely than White women to be diagnosed with triple negative breast cancer which is especially aggressive and associated with poor prognosis (Scott et al., 2019).
There is extensive evidence demonstrating the effectiveness of cervical and breast cancer screening in reducing cancer-related mortality. The US Preventive Services Task Force (USPSTF) recommends women at average risk aged 2129 years receive cervical cytology alone every three years and that women aged 30–65 years receive primary high-risk human papillomavirus (HPV) testing every five years, both cervical cytology and high-risk HPV testing (co-testing) every five years, or cervical cytology alone every three years (USPSTF, 2018). For breast cancer screening, the USPSTF recommends that average risk women, aged 50–74 years receive screening mammograms biennially and that women age 40–49 should make an individual decision on when to start screening mammography based on their personal values including potential harms and benefits (USPSTF, 2016). Screening uptake for both cervical and breast cancer are lower among women with low incomes and living in rural areas (Doescher and Jackson, 2009; Henley et al., 2017). These women often face many barriers to screening which include lack of health insurance, decreased likelihood of having a usual source of primary care and access to health care, distrust of medical system, lack of transportation, need for childcare, and no access to language translation services (Fiscella et al., 2011).
The National Breast and Cervical Cancer Early Detection Program (NBCCEDP) was established to reduce disparities in morbidity and mortality due to breast and cervical cancer by providing cancer screening and diagnostic services to women with low income and inadequate health insurance. The NBCCEDP was authorized by the Breast and Cervical Cancer Mortality Prevention Act of 1990 (Public Law 101-354, 42 U.S.C. §300 k) and is administered by the U.S. Centers for Disease Control and Prevention (CDC) to fund cooperative agreements with states, tribes and territories (Wong and Miller, 2019). CDC currently funds all 50 state health departments or their bona fide agents, the District of Columbia, and 13 tribal and 6 territorial organizations (i.e., NBCCEDP awardees) to implement the NBCCEDP. Each NBCCEDP awardee has established a screening delivery system including existing clinics and providers within their jurisdiction that are contracted by awardees to deliver breast and cervical screening and diagnostic services according to USPSTF guidelines. Awardees also conduct activities (e.g., outreach) to identify program-eligible women in communities and link them to clinics for screening services. The Breast and Cervical Cancer Prevention and Treatment Act of 2000 (Public Law 106-354, 42 U.S.C. §1396) allows states the option to offer cancer treatment to women diagnosed with cancer or precancer through the NBCCEDP through the Medicaid program. Currently, all but two states, Arkansas and Maryland, extend this coverage (U.S. Government Accountability Office, 2020).
With the advent of the Coronavirus Disease 2019 (COVID-19) pandemic, there is concern that the racial and ethnic disparities seen in breast and cervical cancer morbidity and mortality could be exacerbated, based on declines in supply of or demand for cancer care. One report, authored by Murray and Kleinrock for IQVIA Institute for Human Data Science (Murray and Kleinrock, 2020), looking at U.S. insurance claims found greater than 80% drop in weekly mammography and Pap test claims in early April 2020. These authors also report a modeling study on this impact over a 3-month period (ending June 5, 2020) which estimated an almost 70% drop in both breast and cervical cancer screening that would result in 38,500 women having a delay in cancer diagnosis. Delays in cancer screening, diagnosis, and treatment due to COVID-19 could lead to worse health outcomes. This is especially concerning given that serious outcomes of both cancer and COVID-19 disproportionately affect Hispanic persons, Black persons, and American Indians/Alaskan Natives (AI/AN) based on issues including structural racism (Stokes et al., 2020). Studying the impact of COVID-19 on cancer screening among populations experiencing health inequities is critical to determine the effects of COVID-19 on widening disparities in cancer outcomes.
The purpose of this paper is to examine the impact of COVID-19 on NBCCEDP screening services during the early months of the pandemic, January through June 2020, in the United States. Specifically, we address two questions:
-
1.
What is the impact of COVID-19 on the number of breast and cervical cancer screening tests in the NBCCEDP?
-
2.
How has COVID-19 affected the availability of screening services and NBCCEDP awardees' capacity to support partner clinics?
2. Methods
We used NBCCEDP administrative and program data reported to CDC by awardees to address the two evaluation questions. These include minimum data elements (MDEs) collected on women served through the NBCCEDP, annual awardee survey data, and annual awardee budgets.
2.1. Minimum data elements (MDEs)
All awardees collect and report a standardized record on each woman served through the NBCCEDP (Yancy et al., 2014). The MDEs include patient demographics; screening date, test performed, and result; final diagnosis and related date; and treatment initiation, if indicated. An MDE record reflects a screening cycle, therefore, multiple tests can be included in a single record if more than one test is performed. Awardees submit a de-identified, cumulative MDE data file semi-annually, in April and October, that coincides with the 12-month NBCCEDP program year (PY), funded from July 1 through June 30. Data submitted in April include MDE records through the prior December 31; the October submission includes records through the prior June 30. Diagnostic data typically lag screening data by one reporting period given the time needed for women to schedule and receive diagnostic tests. Following each MDE data submission, reports are produced to monitor awardee performance, provide feedback on data quality and performance, and inform technical assistance delivered by CDC. The MDEs have Office of Management and Budget (OMB, #0920-0571, exp. 03.31.2022). This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.1
For this study, we assessed the number of breast cancer screening tests (mammograms) and cervical cancer screening tests (Pap tests and/or HPV tests) conducted during January-June for years 2015, 2016, 2017, 2018, 2019, and 2020. We excluded diagnostic data due to the data lag noted above. Sixty-five of 70 awardees were included in the analysis. Five were excluded either due to being newly funded awardees in the NBCCEDP, therefore lacking sufficient data for all six years of the study period, or for data quality issues. Data for January–June 2020, representing the time period when COVID-19 emerged in the U.S., were compared with data for the 5-year average for the same months for 2015–2019. Data were stratified based on the 10 Health and Human Services (HHS) public health regions (Fig. 1 ), race/ethnicity, and rurality. The HHS regions are named based on the physical location of the HHS regional office (e.g., Boston for Region 1). We assigned each NBCCEDP tribal program to an HHS region based on the location of the tribe or tribal organization's NBCCEDP program headquarters. Rurality was determined using the residence county of the NBCCEDP client based on the U.S. Department of Agriculture Urban Rural Continuum Codes. Each client was assigned to one of three categories: metropolitan (metro), urban, or rural. Statistical tests for the difference of monthly counts between the 5-year average and 2020 was calculated using a Poisson regression model with a covariate to indicate the year 2020. A likelihood ratio test was performed to test the null hypothesis that the parameter for the 2020 indicator variable is zero, and a two-sided p-value was calculated for this test. All analyses were performed using Proc GENMOD with SAS 9.4 (TS1M5) (SAS Institute Inc., Cary, North Carolina).
Fig. 1.
NBCCEDP awardees by HHS region.
2.2. NBCCEDP annual awardee survey
CDC administers a web-based, annual survey of all NBCCEDP awardees to collect information about program management, technical assistance needs, participating provider sites where screening services are delivered, non-CDC resources supporting the program, and other aspects of program implementation. Data are self-reported and collected following the end of each PY. Survey data are used for reporting to CDC and other government agencies and to inform CDC technical assistance delivery to awardees. The survey has been approved by OMB (#0920-1046, exp. 11.30.2021). This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.2 In fall 2020, CDC received OMB approval for seven additional survey questions addressing awardee's ability to provide screening services due to interruptions caused by COVID-19 (Appendix 1). These questions addressed the effects of COVID-19 on awardee staffing (i.e., deployment of staff to work on COVID-19, staff furloughs) and on participating provider clinics (i.e., clinic closures, suspension of screening services). Information on the number of staff positions expressed as full-time equivalents (FTEs) was collected, because individual staff can be supported by more than one funding source and work on other programs or projects in addition to the NBCCEDP. Respondents could enter information on up to 10 staff that were deployed. A final, open-ended question allowed awardee respondents to describe any other effects of COVID-19 on their programs.
A link to the web-based, annual survey for PY3 (July 2019–June 2020) was emailed to the 70 Awardee Program Directors on August 24, 2020. The survey closed on October 2, 2020. Because CDC received OMB approval for the seven additional COVID-19 related questions in October, these questions were sent to the same respondents and fielded separately during November 13–December 11, 2020. CDC contacted awardees to verify responses that fell outside expected ranges. For example, we contacted all awardee program directors that reported having deployed staff for the COVID-19 response in their jurisdictions for more than 16 weeks during January–June 2020. We conducted descriptive analysis of quantitative survey responses using SAS 9.4 (TS1M5) (SAS Institute Inc., Cary, North Carolina) and thematic analysis of qualitative data. A total of 68 of 70 awardees completed the standard annual survey, and 68 of 70 completed the seven-question COVID-19 survey. All four non-responders were tribal programs that were especially impacted by the pandemic.
2.3. Annual awardee budgets
Each February, awardees submit a workplan and proposed budget for the next PY. We analyzed PY3 (July 2019–June 2020) staffing using awardee budgets approved by CDC. While awardees may deviate slightly from approved budgets, changes greater than 25% of funding must be approved by CDC. Any approved changes were included for this analysis to provide the most up-to-date calculation on the number of staff. Using Microsoft Excel, we abstracted data on each staff person by job title. The total number of staff for each awardee was then calculated.
3. Results
3.1. Client data
A total of 630,264 breast and 594,566 cervical cancer screening tests were conducted during the study time period (January–June 2015–2020). A total of 487,645 unique women received breast cancer screening services and 353,398 unique women received cervical cancer screening services over the 6 years (2015–2020). Among those receiving breast cancer screening services, the distribution by mutually exclusive racial/ethnic group was: 47.5% Hispanic, 28.1% White, 14.9% Black, 4.3% Asian Pacific Islander (API), 3.5% AI/AN, 0.4% multi-racial, and 1.2% unknown. These women resided largely in metro areas (80.5%), followed by urban (14.9%), rural (2.1%) and unknown (2.5%). For women who received cervical cancer screening services, the distribution by ethnic or racial group was 52.1% Hispanic, 26.4% White, 10.7% Black, 4.4% API, 4.7% AI/AN, 0.6% multi-racial, and 1.1% unknown. Nearly 80% lived in metro areas (79.7%) followed by urban (15.3%), rural (1.9%), and unknown (3.1%). For both breast and cervical cancer screening, a slightly greater percentage of clients were Hispanic women in 2020 when compared with the percentage for the time period 2015–2019. Finally, a slightly greater percentage of women receiving screening tests in 2020 were from metro areas when compared with those in the previous 5-year period (data not shown).
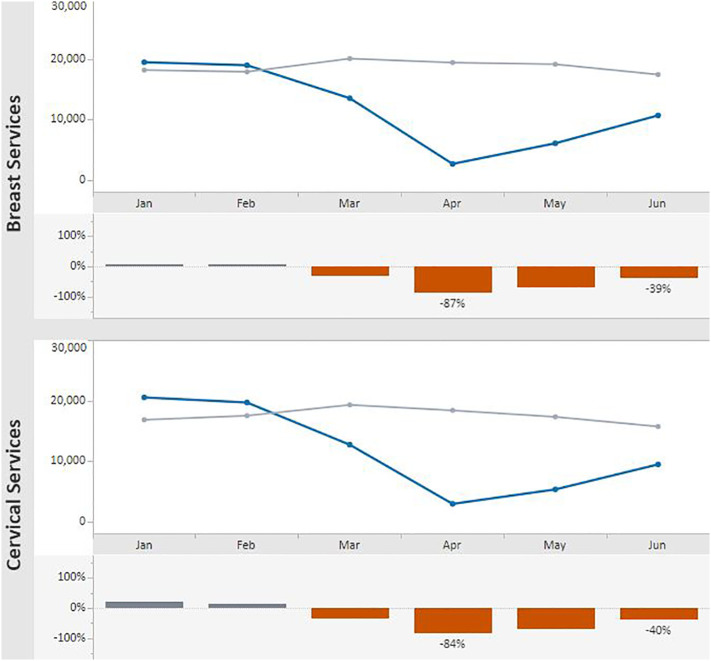
Overall, the volume of screening tests in 2020 was well below that for the previous 5-year averages for the months March–June. A sharp decline in the number of breast and cervical cancer screening tests was observed in March–April 2020 compared with the previous 5-year averages for the same months (Fig. 2 ). Screening test volumes for both breast and cervical cancer were lowest in April 2020. In that month, breast cancer screening tests declined 87% from the previous 5-year average of 19,366 to 2607; cervical cancer screening tests declined 84% from the 5-year average of 18,347 to 2880. Test volume began to recover in May, and by June 2020, breast tests were 39% below the 5-year average (10,626 vs. 17,385.4). Similarly, in June 2020, cervical cancer screening tests represented a 40% decline from the 5-year average (9413 vs. 15,681). Likelihood ratio tests for the differences in the number of tests for the months of March compared with June were all statistically significant with P < 0.001.
Fig. 2.
Monthly NBCCEDP Breast and Cervical Cancer Screening Tests for January–June 2020* Compared with the 5-Year Average in 2015—2019.
Footnote: Blue line represents screening tests conducted in 2020; grey line represents average screening tests conducted over the 5-year period, 2015–2019. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
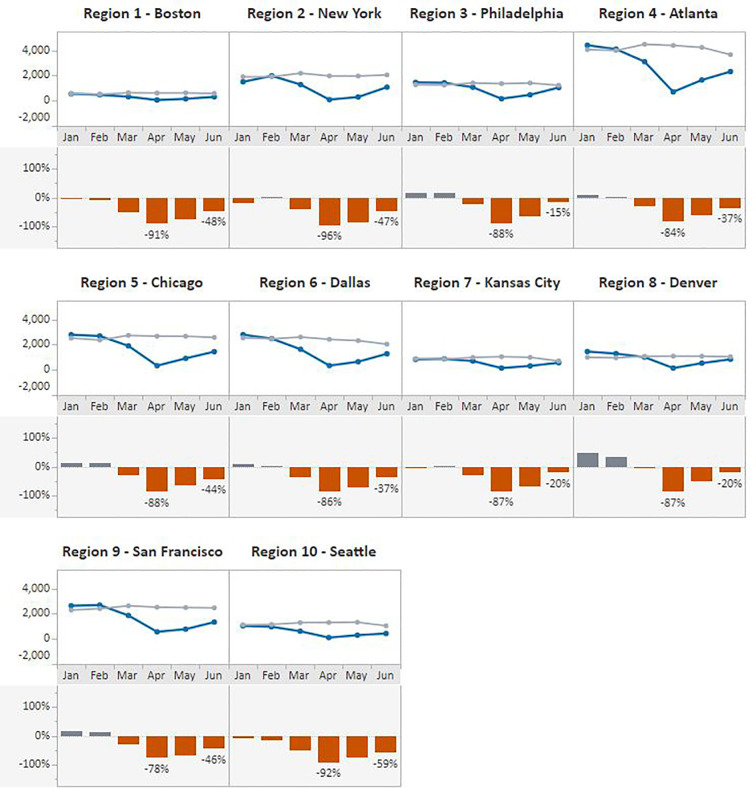
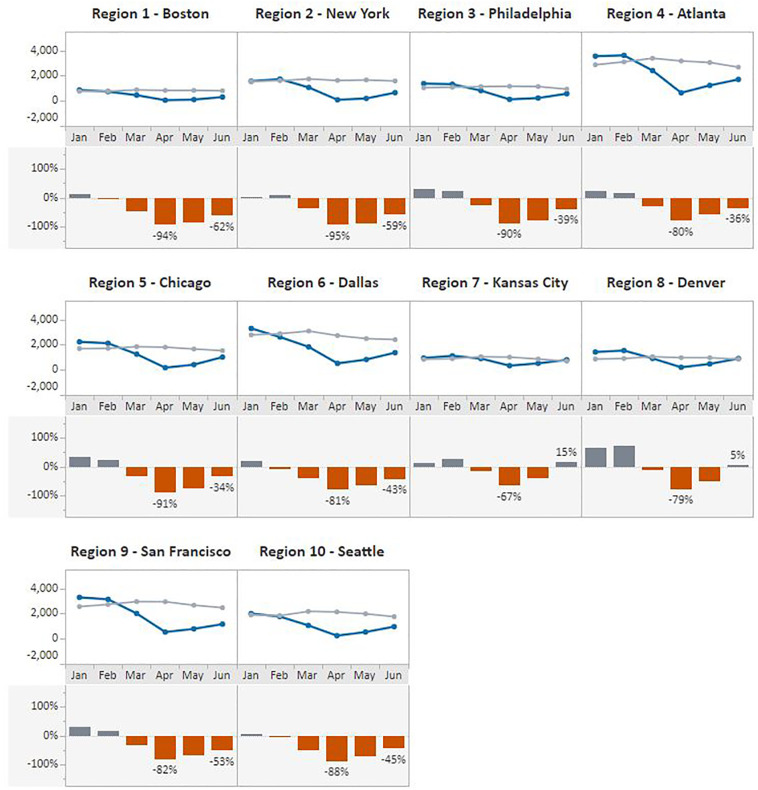
The same pattern was observed when data were stratified by HHS regions (Fig. 3, Fig. 4 ). In April 2020, Region 2 – New York, experienced the greatest declines in both in breast (96%) and cervical (95%) cancer screening test volume compared with the previous 5-year average. In contrast, regions with the lowest declines for April included Region 9 - San Francisco at 78% for breast cancer and Region 7 - Kansas City at 67% for cervical cancer screening test volume. Volumes increased in May and June across all HHS regions, with the greatest gains made in the Kansas City region where, in June, the number of cervical cancer screening tests surpassed the 5-year average by 15% and in Region 3 – Philadelphia where breast cancer screening tests recovered to 15% below the average.
Fig. 3.
Monthly NBCCEDP Breast Cancer Screening Tests for January–June 2020 Compared with the 5-Year Average in 2015–2019, by HHS Region.
Footnote: Blue line represents screening tests conducted in 2020; grey line represents average screening tests conducted over the 5-year period, 2015–2019. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Monthly NBCCEDP Cervical Cancer Screening Tests for January–June 2020 Compared with the 5-Year Average in 2015–2019, by HHS Region.
Footnote: Blue line represents screening tests conducted in 2020; grey line represents average screening tests conducted over the 5-year period, 2015–2019. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
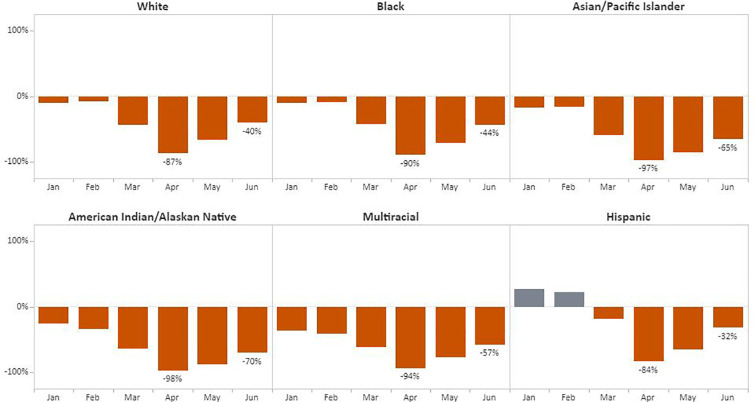
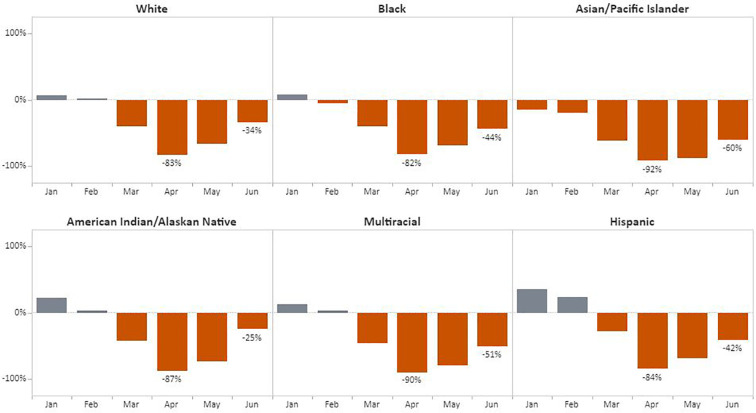
Regarding race/ethnicity, the greatest declines in the number of breast cancer screening tests was during April among American Indian/Alaskan Native (AI/AN) women (98%) followed by Asian/Pacific Islander (API) women (97%), with Hispanic women experiencing a decline of 84% from the 5-year average (Fig. 5 ). Women with unknown racial/ethnic group accounted for 1.1% (6894) of breast and 1.1% (6337) of cervical cancer screening tests. The most significant recovery was for Hispanic women; by June, screening tests had recovered to 68% of baseline. For cervical cancer, the number of tests declined from the 5-year average by 92% for API women and 90% for women identifying as multi-racial (Fig. 6 ). By June, screening among AI/AN women returned to 75% of previous cervical cancer screening test levels, the strongest rebound among the racial/ethnic groups.
Fig. 5.
Monthly NBCCEDP Breast Cancer Screening Tests for January–June 2020 Compared with the 5-Year Average in 2015–2019, by Racial/Ethnic Group.
Fig. 6.
Monthly NBCCEDP Cervical Cancer Screening Tests for January–June 2020 Compared with the 5-Year Average in 2015–2019, by Racial/Ethnic Group.
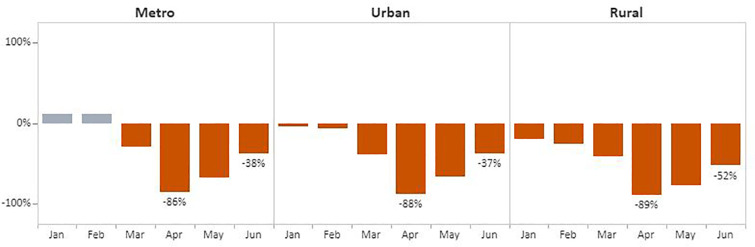
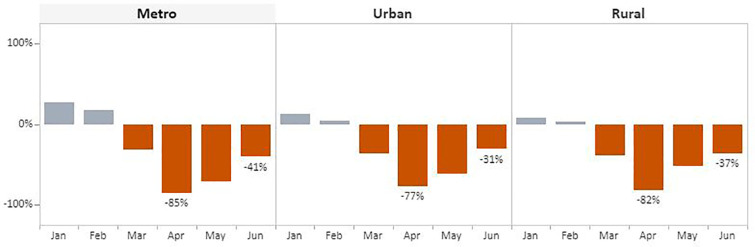
The overall pattern also held for breast and cervical cancer screening test volume when data were stratified by rurality (Fig. 7, Fig. 8 ). In April, the number of screening tests for breast cancer declined in metro (86%), urban (88%), and rural (89%) areas when compared with the respective 5-year averages. The decline was slightly less for cervical cancer screening tests during this month compared with the 5-year average, with reductions of 85% and 82% for metro and rural areas, respectively, and 77% for urban areas. Volume began trending upward in May and June across all three categories. Rural areas recovered the least with breast cancer test volume remaining 52% below the 5-year average. Comparatively, metro and urban areas experienced far greater improvement, with breast cancer screening tests at 38% and 37% below the 5-year average, respectively. Improvements were slightly greater for cervical cancer screening tests in rural and urban areas: volume was 37% and 31% below the 5-year average, respectively. In metro areas, tests remained 41% below the 5-years average.
Fig. 7.
Monthly NBCCEDP Breast Cancer Screening Tests in January–June 2020 Compared with the 5-Year Average in 2015–2019, by Rurality.
Fig. 8.
Monthly NBCCEDP Cervical Cancer Screening Tests in January–June 2020 Compared with the 5-Year Average in 2015–2019, by Rurality.
3.2. Survey
Among the 68 awardees responding to the survey, 44 (65%) reported having deployed NBCCEDP-funded staff to assist with the COVID-19 response in their area. Among those 44 awardees, 191 staff (39%) were deployed for the COVID-19 response among the total 489 staff persons funded in-full or in-part by the NBCCEDP. The number of staff deployed per awardee ranged from 1 to 10 with an average of 4.3 persons per awardee. (Note that we limited the number of staff that could be listed to 10 in the survey). A total of 81.5 FTEs (43%) were deployed among the 191 staff. By HHS region, the percent of NBCCEDP-funded staff deployed ranged from 23% in Region 2 – New York to 59% in Region 8 – Denver (Table 1 ). The average length of time deployed ranged from 6.9 weeks in Region 9 – San Francisco to 13.2 in Region 5 - Chicago. Only four awardees reported furloughing NBCCEDP-funded staff during the time period January to June 2020 due to COVID-19, all from different HHS regions. The duration of the furloughs ranged from 6 to 17 weeks.
Table 1.
Awardees with NBCCEDP-funded staff⁎ deployed to assist on the COVID-19 response, January–June 2020 (N = 68), by HHS region.
| HHS region | Awardees with staff deployed | Total number of people budgeted | Total number of staff deployed | % of number of staff deployed | Total FTE time staff deployed | Average of weeks of staff deployed |
|---|---|---|---|---|---|---|
| 1 | Yes (N = 4) | 31 | 16 | 52% | 6.2 | 12.3⁎⁎ |
| No (N = 2) | ||||||
| 2 | Yes (N = 1) | 44 | 10 | 23% | 5.1 | 11.2 |
| No (N = 2) | ||||||
| 3 | Yes (N = 3) | 24 | 6 | 25% | 4.2 | 12.2 |
| No (N = 3) | ||||||
| 4 | Yes (N = 6) | 86 | 32 | 37% | 10.5 | 8.0 |
| No (N = 2) | ||||||
| 5 | Yes (N = 5) | 61 | 23 | 38% | 10.3 | 13.2 |
| No (N = 2) | ||||||
| 6 | Yes (N = 3) | 38 | 16 | 42% | 7.5 | 12.4 |
| No (N = 4) | ||||||
| 7 | Yes (N = 3) | 46 | 17 | 37% | 3.9 | 7.8 |
| No (N = 1) | ||||||
| 8 | Yes (N = 6) | 49 | 29 | 59% | 13.7 | 11.3 |
| No (N = 1) | ||||||
| 9 | Yes (N = 6) | 54 | 18 | 33% | 7.4 | 6.9 |
| No (N = 5) | ||||||
| 10 | Yes (N = 7) | 56 | 24 | 43% | 12.7 | 11.3⁎⁎ |
| No (N = 2) |
NBCCEDP program staff are those working for the awardee, not staff working at screening delivery sites.
Two awardees in HHS region 1 and one awardee in HHS region 10 were not included in the calculation due to unknown weeks for all staff deployed for the grantee; one awardee in HHS region 8 and one awardee in HHS Region 10 were unable to complete the COVID-19 survey.
During the study period, 63 awardees partnered with a total of 13,085 provider sites to deliver breast and cervical screening services; three awardees who reported ‘unknown’ for the number of provider sties were excluded from the analysis. The reported number of provider sites excludes sites providing specialty services such as imaging and diagnostic tests. Based on the COVID-19 survey, 43 (63%) of respondents reported that “some” provider sites closed for business due to COVID-19 compared with 25 (37%) that reported “none” had closed (Table 2 ). Thirty-seven (54%) of respondents reported that “some” provider sites temporarily suspended or reduced breast and/or cervical cancer screening services in contrast to 31 (46%) that reported “none” (Table 2). All three awardees in Region 2 – New York reported that “some” provider sites had closed and that “some” had suspended or reduced breast and cervical cancer screening services.
Table 2.
Number of NBCCEDP Awardees Reporting ‘Some’ Provider Site Closures and ‘Some’ Provider Sites where Screening Services were Suspended, January–June 2020.
| HHS region |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Closed | 3 (50%) | 3 (100%) | 5 (83%) | 6 (75%) | 5 (71%) | 5 (71%) | 2 (50%) | 6 (86%) | 5 (45%) | 3 (33%) |
| Suspended | 2 (33%) | 3 (100%) | 2 (33%) | 4 (50%) | 4 (57%) | 4 (57%) | 2 (50%) | 4 (57%) | 6 (55%) | 6 (67%) |
Footnote: Survey options included “none”, “some”, “all” and “do not know”. No respondent answered “all” or “do not know”. Therefore, the only other response was “none”.
Forty-five (66%) awardees reported assisting provider sites to restart routine clinical care. Of those 45, 19 awardees described their efforts to give provider sites information about preventing COVID-19 transmission. One awardee noted, “[We] discussed CDC's recommended COVID-19 precautions (promote wearing masks, practice social distance, etc.)” and another wrote, “We provided educational webinars on COVID-19 and resuming cancer screening, also provided monthly technical assistance calls.” Eighteen awardees described giving operational support to providers. For example, one wrote, “The program collaborated with health provider clinics to have patients enroll [in NBCCEDP] on-site at the clinic while providing technical assistance on enrollment forms and eligibility.” Another mentioned, “[We provided] technical assistance on the enrollment protocol [enrolling NBCCEDP clients] and maintaining a screening schedule, and we provided small media and other resources on COVID-19.” Other awardees discussed conducting outreach to clients to reassure them of safety guidelines that were followed by clinics and encouraging them to be screened. One awardee mentioned their efforts to reach women who may have been recently unemployed due to the pandemic, “[We used] language for Facebook, Twitter, Instagram messages to reach newly unemployed/uninsured women to inform them of the [NBCCEDP] program.”
Finally, 60 (88%) awardees reported that their NBCCEDP was affected and challenged by COVID-19 during PY3 2020 in ways other than deployments and furloughs. Twenty-nine awardees described program activities that could no longer be provided. One respondent wrote, “[We were] unable to do community outreach or continue to build relationships with community partners, limiting the number of events we could attend.” Another reported, “Staff from most of our coordinating agencies, mostly county health departments, had to focus on COVID-19 and were unable to carry out most of their NBCCEDP responsibilities.” And one mentioned, “Some contracted community health workers were furloughed or shifted duties as a response to COVID-19. This resulted in a decrease of outreach, enrollment, and screening activity.” Twenty-four respondents wrote about the challenges they faced when their partner provider sites either reduced or eliminated screening services for some time period. One awardee wrote, “[Our] governor stopped non-emergency health care visits from March until May to help conserve personal protective equipment, stopping most cancer screening.” Another U.S. territory awardee said, “The only clinic that provides all primary care services such as clinical breast exams, Pap tests, and HPV tests was activated as a Tier 2 clinic to test and treat COVID-19 patients which affected program screening.” Many (n = 16) identified challenges in remote working such as training partners virtually. Respondents also identified challenges such as clients' reluctance to go to clinics, even when clinic operations were restored, for fear of contracting COVID-19 and noted reductions in their own staff due to childcare needs at home.
4. Discussion
The NBCCEDP provides a unique opportunity to examine the impact of COVID-19 on breast and cervical cancer screening among women with lower incomes who are underserved and represent diverse racial and ethnic groups. Results of this study show a significant decline in screening tests beginning in March 2020 for both cancers with decreases evident across geography, ethnic and racial group, and rurality. This decline in screening coincided with the escalation of COVID-19 cases and response activities across the United States, reduced screening access, and public health's shift in priorities to respond with public education, testing, and contact tracing. Our findings are consistent with a study of insured populations that found decreases in mammograms and Pap tests of 87% and 83%, respectively, based on an analysis of medical claims data in April 2020 compared with February (Murray and Kleinrock, 2020). Similarly, a study of 1.5 million women insured through Kaiser Permanente Southern California found a precipitous drop in screening during the COVID-19 statewide stay-at-home order in place during March 19–June 12, 2020. Cervical cytology screening rates per 100 person-months decreased by 78% among women aged 21–29 years and high-risk human papillomavirus test screening rates per 100 person-months decreased 82% among women aged 30–65 years (Miller et al., 2021).
Overall, Region 2 – New York, the area hardest hit early in the pandemic, experienced the greatest declines in screening test volume, with drops that were 9 and 11 percentage points greater than the NBCCEDP average for breast and cervical cancer screening, respectively. This is not surprising given the effect of COVID-19 on the region in spring 2020. All 3 NBCCEDP awardees in Region 2 reported having clinics closed and cancer screening suspended during the same time period. Breast cancer screening tests were impacted particularly among AI/AN women in the NBCCEDP where the drop in volume was 11 percentage points greater than for White women. AI/AN populations have been especially affected by COVID-19. A recent study in 14 states found that COVID-19 associated deaths among AI/AN persons were nearly twice that of non-Hispanic White persons (Arrazola et al., 2020). AI/AN populations experience social inequities, including access to quality health care, and other challenges that may contribute to these disparities. In our study, API women were also significantly impacted in terms of breast and cervical cancer screening with declines well above the NBCCEDP average. Factors that might have contributed to screening declines in the NBCCEDP during this time period, include screening site closures and the temporary suspension of breast and cervical cancer screening services. Requirements or encouragement to stay at home and women's fear of contracting COVID-19 also likely deterred them from seeking health care services, including cancer screening (Hacker and Briss, 2021).
Although it is promising that screening test volume trended upward toward the 5-year average in May and June, significant discrepancies remained in nearly all regions. In particular, breast cancer screening volume in rural areas remained over 50% below the 5-year average in June. Assistance provided to screening sites by NBCCEDP awardees might have contributed to the increases that were observed in May and June; however, many NBCCEDP programs were short-staffed for some amount of time due to staff deployments to assist with the COVID-19 response, and in some cases, the necessity to stay at home given school and childcare closures. The capacity of NBCCEDP programs to maintain program activities was likely diminished during the study period given the deployment of staff and, for some staff, childcare responsibilities. Staffing limitations might have contributed to decreased screening at their partner clinics as awardees may have had less time to conduct outreach and other activities such as patient navigation that facilitate screening. Regardless of such challenges, however, a large number of awardees reported flexibility and creative efforts to reach women and support clinics resumption of clinical care, including screening, during the COVID-19 pandemic.
The declines in screening within the NBCCEDP and elsewhere might unfortunately result in delayed diagnosis and the identification of later stage disease with worse outcomes for women. A recent modeling study from the United Kingdom estimated an increase of 7.9 to 9.6% in breast cancer deaths up to five years after diagnosis compared with pre-pandemic levels due to delays in women receiving diagnostic tests (Maringe et al., 2020). In the U.S., the effects of COVID-19 on screening and treatment have been estimated to result in 10,000 additional deaths due to breast and colorectal cancers (Sharpless, 2020). Prolonged delays in screening due to COVID-19 will lead to more delays in cancer diagnosed in the general population and women of color will likely experience further disparities (Carethers et al., 2020). A previous study showed that women in the NBCCEDP were diagnosed with later stage breast cancer than non-enrolled women (Wu et al., 2015). Based on that study and the results of the one presented here where some racial minorities experienced greater declines in screening volume, such disparities might be further exacerbated by COVID-19. Future study is needed to examine the effects of COVID-19 on breast and cervical cancer outcomes, including assessing outcomes by race/ethnicity.
Cancer screening along with other preventive health care services are important to safely maintain, even during this pandemic. The NBCCEDP can play an important role in encouraging women with low incomes to resume cancer screening if it can be provided in a safe environment where COVID-19 transmission is minimized. Delays in testing should be especially minimized for women experiencing symptoms concerning for breast or cervical cancer and those at otherwise high risk (American Society for Colposcopy and Cervical Pathology, 2020). Efforts to curtail women missing a full screening cycle, which would delay screening by years, are of utmost importance. By providing education about the importance of routine screening and addressing women's concerns about COVID-19 transmission, NBCCEDP awardees can work to minimize increases in cancer disparities. Women served through the NBCCEDP might also need help with transportation, childcare, and other services that have also been affected by the pandemic. Patient navigators funded through NBCCEDP can help women overcome these barriers. Importantly, for women with low incomes who have lost their jobs and employer-provided health insurance due to the pandemic, the NBCCEDP offers resources for screening and diagnostic services, if needed. The NBCCEDP, through activities aimed at facilitating clinical access for women in the community, may also help ensure these women receive vaccination for COVID-19 when vaccine availability is more widespread. Awardees can also assist their clinic provider partners by facilitating the availability of assistance from public health agencies that can provide them with COVID-19 educational materials and other support.
This study provides a first look at the impact of COVID-19 on the NBCCEDP, including screening volume, awardee staffing, and program activities. As additional data become available, more analysis would be needed to assess screening volume over the full 12 months of 2020 and the impact on diagnostic follow-up. The pandemic's effects ebbed and flowed over various parts of the country throughout 2020, and it likely contributed to more disruptions in health care based on geography than were observed in the first six months. The results of this study point to the importance of future research to examine the impact of observed declines in screening due to COVID-19 on cancer mortality.
Limitations in the study should be acknowledged. As noted, diagnostic data representing our study time period were not yet available, therefore an analysis of the effects of COVID-19 on timeliness of diagnostic follow-up and final diagnosis was not possible. Additionally, small numbers of women enrolled within certain racial and ethnic groups required that we combine some categories. Next, some awardee staff are funded by multiple sources with a portion of their FTE allocation supported by NBCCEDP funds. Consequently, we were unable to determine the precise percentage of NBCCEDP-funded FTEs that were deployed to assist on COVID-19 efforts. In addition, the changes in screening volume cannot be entirely attributed to COVID-19. Other factors likely influence variation in screening. Finally, we are unable to examine whether specific women who went unscreened during the peak months of the observed decline were subsequently screened in May and June when screening volume began to rebound.
5. Conclusion
The NBCCEDP provides critical cancer screening services to a population of women with low incomes from diverse racial/ethnic groups who would otherwise likely remain unscreened. The COVID-19 pandemic dramatically reduced cancer screening in the U.S in early spring 2020, including among NBCCEDP clients. Our data show, however, that among the women who are served by the NBCCEDP, screening recovered in a similar way to insured populations, and, overall, by June was roughly 60% of pre-COVID-19 levels. The declines in breast cancer screening test volume due to COVID-19 identified in this study may lead to later stage breast cancer diagnosis and mortality while declines in cervical cancer screening may result in increased cervical cancer incidence, later stage diagnoses, and mortality, furthering cancer disparities among this population. The capacity of NBCCEDP awardees' was hindered by the pandemic, affecting their ability to carry out program activities. However, NBCCEDP awardees reported assisting health care providers to resume screening. Future studies will examine the effect of the pandemic on screening during the second half of 2020, when surges of COVID-19 and their timing varied geographically.
Disclosure
The authors report no conflicts of interest.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
See e.g., 45C.F.R. part 46; 21C.F.R. part 56; 42 U.S.C. §241(d), 5 U.S.C. §552a, 44 U.S.C. §3501 et seq.
See e.g., 45C.F.R. part 46; 21C.F.R. part 56; 42 U.S.C. §241(d), 5 U.S.C. §552a, 44 U.S.C. §3501 et seq.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ypmed.2021.106559.
Appendix A. Supplementary data
NBCCEDP Annual Awardee Survey COVID-19 Questions.
References
- U.S. Preventive Services Task Force Screening for breast Cancer: US preventive services task force recommendation statement. Ann. Intern. Med. 2016;164:279–296. doi: 10.7326/M15-2886. [DOI] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force Screening for cervical Cancer: U.S. preventive services task force recommendation statement. JAMA. 2018;320(7):674–686. doi: 10.1001/jama.2018.10897. [DOI] [PubMed] [Google Scholar]
- U.S. Cancer Statistics Working Group. United States Cancer Statistics: 1999–2017. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; www.cdc.gov/cancer/dataviz, released in June 2020. Accessed December 20, 2020.
- U.S. Government Accountability Office . 2020. Federal programs provide screening and treatment for breast and cervical cancer. GAO-21-35.https://www.gao.gov/assets/720/710372.pdf [Google Scholar]
- American Society for Colposcopy and Cervical Pathology . 2020. The American Society for Colposcopy and Cervical Pathology prioritizes high grade abnormal results for diagnostic workup.https://journals.lww.com/jlgtd/Fulltext/2020/04000/2019_ASCCP_Risk_Based_Management_Consensus.3.aspx [Google Scholar]
- Arrazola J., Masiello M.M., Joshi S., et al. COVID-19 mortality among American Indian and Alaska Native persons — 14 States, January–June 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:1853–1856. doi: 10.15585/mmwr.mm6949a3externalicon. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carethers J.M., Sengupta R., Blakey R., Ribas A., D’Souza G. Cancer Prevention Research; 2020. Disparities in Cancer Prevention in the COVID-19 Era. Published Online First: September 17, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doescher M.P., Jackson J.E. Trends in cervical and breast cancer screening practices among women in rural and urban areas of the United States. J. Public Health Manag. Pract. 2009;15(3):200–209. doi: 10.1097/PHH.0b013e3181a117da. [DOI] [PubMed] [Google Scholar]
- Fiscella K., Humiston S., Hendren S., et al. Eliminating disparities in cancer screening and follow-up of abnormal results: what will it take? J. Health Care Poor Underserved. 2011 doi: 10.1353/hpu.2011.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker K., Briss P. An ounce of prevention is still worth a pound of cure, especially in the time of COVID-19. Prev Chronic Dis. 2021;18:200627. doi: 10.5888/pcd18.200627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henley J.S., Anderson R.N., Thomas C.C., Massetti G.M., Peaker B., Richardson L.C. Invasive cancer incidence, 2004–2013, and deaths, 2006–2015, in nonmetropolitan and metropolitan counties – United States. MMWR Surveill. Summ. 2017;66(14):1–14. doi: 10.15585/mmwr.ss6614a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020 doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.J., Xu L., Qin J., et al. 2020. Impact of COVID-19 on cervical cancer screening rates among women aged 21–65 years in a large integrated health care system — Southern California, January 1–September 30, 2019, and January 1–September 30, 2020. MMWR Morb. Mortal. Wkly Rep. 2021;70:109–113. doi: 10.15585/mmwr.mm7004a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A., Kleinrock M. IQVIA Institute for Human Data Science; 2020. Shifts in Healthcare Demand, Delivery and Care during the COVID-19 Era.https://www.iqvia.com/insights/the-iqvia-institute/covid-19/shifts-in-healthcare-demand-delivery-and-care-during-the-covid-19-era Accessed December 20, 2020. [Google Scholar]
- Scott L.C., Mobley L.R., Tzy-Mey K., Il’yasova D. Update on triple-negative breast cancer disparities for the United States: a population-based study from the United States Cancer Statistics database, 2010–2014. Cancer. 2019;125(19):2417–3412. doi: 10.1002/cncr.32207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless N.E. COVID-19 and cancer. Science. 2020;368(6497):1290. doi: 10.1126/science.abd3377. [DOI] [PubMed] [Google Scholar]
- Stokes E.K., Zambrano L.D., Anderson K.N., et al. Coronavirus disease 2019 case surveillance — United States, January 22–May 30, 2020. MMWR Morb. Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong F.L., Miller J.W. Center for Disease Control and Prevention’s National Breast and Cervical Cancer Early Detection Program: increasing access to screening. J. Women’s Health. 2019;28(4):427–431. doi: 10.1089/jwh.2019.7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Austin H., Eheman C.R., Miles Z., Miller J., Royalty J., Ryerson A.B. A comparative analysis of breast cancer stage between women enrolled in the National Breast and Cervical Cancer Early Detection Program and women not participating in the program. Cancer Causes Control. 2015 doi: 10.1007/s10552-015-0548-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy B., Royalty J., Marroulis S., Mattingly C., Benard V., DeGroff A. Using data to effectively manage a national screening program. Cancer. 2014;120(16):2575–2583. doi: 10.1002/cncr.28821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
NBCCEDP Annual Awardee Survey COVID-19 Questions.