Abstract
Atopic dermatitis is one of the most common chronic inflammatory skin conditions and is associated with sleep disturbances in 47% to 80% of children and 33% to 90% of adults. Herein, we review the literature on sleep disturbances experienced by patients with atopic dermatitis, as well as the mechanisms that may underlie this. We present subjective and objective methods for measuring sleep quantity and quality and discuss strategies for management. Unfortunately, the literature on this topic remains sparse, with most studies evaluating sleep as a secondary outcome using subjective measures. The development of portable, at-home methods for more objective measures offers new opportunities to better evaluate sleep disturbances in atopic dermatitis research studies and in clinical practice. Published by Elsevier Inc. on behalf of the American Academy of Allergy, Asthma & Immunology
Keywords: Atopic dermatitis, Sleep, Type 2 immunity, Insomnia, Pruritus
INTRODUCTION
Atopic dermatitis (AD) is one of the most common chronic inflammatory skin conditions and is commonly associated with sleep disturbances.1 Although approximately 10% to 41% of children2-4 and 7% to 48% of adults5-7 in the general population experience sleep disturbances, in patients with AD, this figure climbs to 47% to 80% in children8-17 and 33% to 90% in adults.8,17-19 In 2019, the Federal Drug Administration’s (FDA’s) Patient-Focused Drug Development Initiative found that sleep was 1 of the 3 most problematic symptoms for patients with AD and their families.20 Impaired sleep compromises function (at work and home), mood, and interpersonal relationships. Chronic sleep disturbance can increase the risk for cardiovascular,21 metabolic,22 and psychiatric diseases.23 However, objectively and efficiently quantifying AD sleep disturbance, understanding its pathophysiology, and identifying effective management strategies remain challenging.
“Sleep disturbance” is a broad term encompassing poor sleep, disrupted sleep, sleep loss, or a specific sleep disorder (eg, insomnia and obstructive sleep apnea). Methods to assess sleep disturbance vary widely. Single dichotomous (eg, “Do you have trouble sleeping?”) or Likert-type questions (eg, “On a scale of 1-5, what is the quality of your sleep?”) are used in epidemiologic studies, or when sleep is a secondary or tertiary outcome measure. When sleep is the primary focus, numerous validated, self-reported measures of sleep, sleep quality, and sleep disorders can be deployed. In addition, some attributes of sleep can be measured objectively in the laboratory or home environment. It is unclear what method(s) is most appropriate to assess sleep disturbances in patients with AD. In this review, we summarize studies on sleep disturbance in AD, discuss possible mechanisms, critique methods for measurement, outline management strategies, and recommend areas for further study.
SLEEP DISTURBANCES IN AD
Children
Most studies of sleep disturbance in AD have been conducted on children, reflecting that although prevalence ranges vary widely across studies, sleep disturbances are more common in children with AD than in adults.20 Children with AD report difficulty falling asleep,13,24 frequent and long nighttime awakenings,11,24-26 difficulty waking up,13,24 and excessive daytime sleepiness.13,24 This has been corroborated to some degree by polysomnography (PSG) studies, which, although not uniformly consistent, have shown prolonged sleep-onset latency (SOL) (time required to fall asleep),13,27 more wake time after sleep onset (WASO), sleep fragmentation,27-31 and lower sleep efficiency (SE) (proportion of time in bed spent asleep).28,29,32 Some studies indicate a reduction in total sleep duration,13,24 but most, including a large longitudinal study,33 found no significant differences in sleep duration compared with healthy controls.25,28,29,31,34 Similarly, some studies found that younger children,9,15 females,35 and those of lower socioeconomic status9 were more likely to have reduced sleep quality, whereas others found no such differences.15,36
Generally, as AD severity increases, the prevalence and severity of sleep disturbances also increase.12,37,38 During AD flares, sleep disturbances increased from 60% to 83%.9 Increased disease severity as measured by the SCORing Atopic Dermatitis (SCORAD) was associated with more bedtime resistance, nocturnal awakenings, and daytime sleepiness (all P < .0001).34 Increases in SCORAD were also associated with lower SE (r = −0.73), increased fragmentation (r = 0.70), longer WASO (r = 0.62), and increased movements during sleep (r = −0.70) (all P < .001).13
Sleep disturbances can significantly impact the quality of life of children with AD, their caregivers, and other family members.10,26,39 Disturbed sleep has been associated with reduced happiness,12 impaired performance on neurobehavioral tasks,40,41 hyperactivity/inattention,9,40,42,43 behavioral and emotional disturbances,9,14,41,43 and stunted growth.44 In addition, 60% to 65% of parents and 63% of siblings of children with AD reported disturbed sleep.12,14,38,45 In fact, 30% of parents coslept with their child because of AD symptoms,12 which exacerbated disturbances for both child and parent.46 During disease flares, 86% of parents had disturbed sleep, losing up to 2.6 hours of sleep per night.47,48 Parental sleep disturbances were maintained throughout childhood, with mothers of children with AD consistently being more likely to experience difficulty falling asleep (adjusted odds ratio, 1.36; 95% CI, 1.01-1.83) and subjectively insufficient sleep (adjusted odds ratio, 1.43; 95% CI, 1.24-1.66).49 These disturbances resulted in reduced parental happiness,12,46,50 interpersonal conflicts,14 and exhaustion.14,38,46
Adults
Studies on adults with AD are more limited. Adults reported difficulty falling asleep,17,51-54 early morning awakenings,51,52 and daytime fatigue.54,55 Although very few objective studies have been conducted, 2 actigraphy studies demonstrated lower SE,55 twice as many nocturnal awakenings,55 and twice as many movements56 as controls. Furthermore, increased disease severity was associated with reduced sleep quality,52,54,57,58 increased SOL,17,37,53,57 and reduced SE.59 As in children, total sleep duration was similar to that in healthy controls.55,60 Sleep disturbance was associated with more missed workdays, visits to the doctor, and poorer overall health (P < .0001).61
Overall, most large AD studies that assess sleep use self-reports or surveys that often assess a single item drawn from a mood or quality-of-life instrument, which do not measure the complexities of sleep.62 Not surprisingly, self-reports do not always corroborate more objective measures of sleep.55,56,59,63,64 Even studies using objective measures have found conflicting results and significant intraindividual and interindividual variation.56 Notably, almost all studies conducted thus far are cross-sectional. In a chronic, relapsing, and remitting disease such as AD, longitudinal studies would provide more information about how fluctuations in disease activity affect sleep. In addition, few objective studies have been conducted on AD caregivers, partners, or other family members. Large-scale trials using objective measures of sleep quality are needed to draw clearer conclusions.
PUTATIVE MECHANISMS OF SLEEP DISTURBANCES IN AD
We propose 3 mechanisms by which sleep disturbance and AD may be associated or even causally connected. First, as demonstrated in other chronic conditions,65 the mere stress of having AD can precipitate acute insomnia, which becomes chronic over time. Nocturnal scratching disrupts sleep and sets the stage for cognitive and behavioral factors that reinforce insomnia as a conditioned response. When persons with insomnia are awake in bed, they may ruminate and/or worry about stressors, their pruritus, falling back asleep, and/or daytime consequences of poor sleep. They may also engage in behaviors that are counterproductive (eg, remaining in bed while awake to maximize sleep opportunity, staying in bed past typical rise times, and taking naps), which reduce nocturnal sleep drive and disrupt circadian rhythm. Insomnia can persist even when the co-occurring condition has resolved.66 One study found that children with AD in remission (n = 14) still had more arousals from sleep per hour than healthy controls (n = 9) (24.1 ± 8.1 vs 15.4 ± 6.2; P < .001).31 This suggests that sleep disturbances may in part be mediated by learned behavioral patterns that persist even when the skin is clear.
Second, a commonly held belief is that pruritus disrupts sleep.62 Pruritus is a key driver of impaired quality of life and commonly worsens at night.15,17,20,26,59,62 It results in scratching, which can disturb sleep. In several studies, healthy controls displayed minimal scratching during sleep, whereas patients with AD spent up to 14.3% of their sleep time scratching.60,64,67 Scratching was associated with nocturnal awakenings, sleep fragmentation (r = 0.5; P < .001), and reduced SE (r = −0.54; P < .001).11,13,29,67,68 The “itch-scratch cycle” may lead to tissue damage and the release of inflammatory mediators/pruritogens, further exacerbating sleep disturbances.69 These include (1) eosinophil granule proteins and nerve growth factors and neuropeptides, which lead to sensory hypersensitivity70-74; (2) circadian variations in skin blood flow, transepidermal water loss, and cortisol levels, which can promote nocturnal pruritus75-77; and (3) cytokines overexpressed in AD lesions that may mediate pruritus, including IL-31,75,78-80 IL-4, IL-13, and thymic stromal lymphopoietin.80 However, studies have found conflicting results or only weak correlations with pruritus, and very few have investigated the direct impact of these biomarkers on itch or scratching behaviors, and more importantly, on sleep disturbances. One study of children with AD (n = 28) found that brain-derived neurotropic factor (r = 0.905; P < .001) and substance P (r = 0.925; P < .001) levels were associated with increased nocturnal wrist activities.81 However, only brain-derived neurotropic factor was associated with AD severity, and neither was associated with subjective assessments of sleep loss or pruritus. Another study found that IL-31 was associated with subjective sleep loss and disease severity, but not pruritus.82
A third hypothesis on sleep-AD mechanisms focuses on circadian variations in cytokines and melatonin production. Generally, cytokines such as IL-1β, IL-2, IL-6, TNF-α, and IFN-γ increase at night and promote sleep, whereas cytokines such as IL-4, IL-10, and IL-13 increase after waking and promote wakefulness.62,83-85 The altered expression of these cytokines in AD is thought to disrupt normal circadian patterns. Some studies found that high levels of IL-6, particularly in the morning, were associated with reduced sleep quality in patients with AD.32,59 However, a larger study found that a lower ratio of IFN-γ/IL-4 was associated with reduced SE, whereas levels of IL-1β, IL-6, and IL-10 had no effect on sleep.13 Melatonin, which regulates sleep and the circadian rhythm,86,87 can also have immunomodulatory, antioxidant, and cytoprotective functions.88 In 1 study, only 22% of patients with AD had a normal melatonin secretion pattern, with most lacking the normal nocturnal melatonin surge.89 A second study found that patients with AD with higher nocturnal melatonin had greater SE (r = 0.4; P = .004) and less fragmentation (r = −0.34; P = .004).13
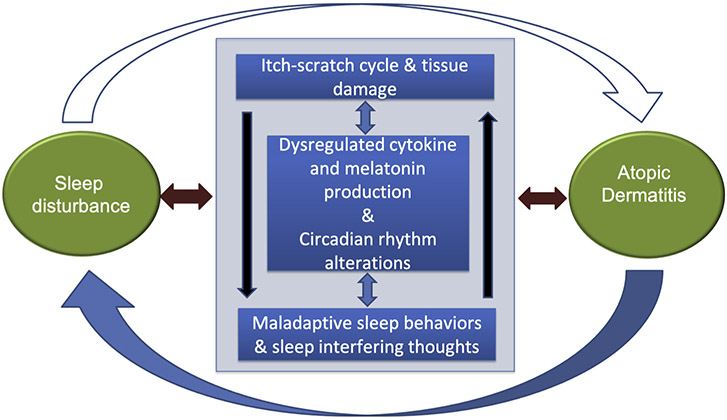
Unfortunately, the evidence for any of these pathways in AD is not strong. Although pruritus and scratching appear to play a role, they may not account for most of the sleep disturbances the patients experience.31,90 The molecular basis of sleep disturbance/homeostasis may involve a set of genes (termed “clock genes”)91,92 whose role in human sleep is being investigated, albeit not yet in patients with AD. Although far more studies are needed to investigate the nature of the sleep-AD relationship before we can draw any firm conclusions, Figure 1 is a simplified conceptual model.
FIGURE 1.
The large semicircular arrows reflect the macro-level bidirectional between sleep and AD. The large interior box reflects the putative mechanisms both driving the sleep-AD relationship and driven by current status of both sleep and AD. The individual putative mechanism boxes reflect evidence suggesting that each of these mechanisms may themselves have bidirectional relationships with each other.
METHODS FOR MEASURING AND IDENTIFYING SLEEP DISTURBANCES
There are multiple methods to evaluate sleep including PSG, portable sleep monitors, actigraphy, patient-reported outcomes (PROs), and sleep diaries.
Polysomnography
The “criterion” standard for objective measurement of sleep is PSG, an overnight test performed in a sleep laboratory that involves placement of more than 12 electrodes on the face/scalp, thorax, abdomen, and legs. PSG captures brain activity (electroencephalography), eye movements (electrooculography), muscle activity/activation (electromyography), and respiratory end points. The readouts are respiration metrics, limb movements, sleep stages (nonrapid eye movement stages N1, N2, and N3, and rapid eye movement), arousals from sleep, SOL, WASO, total sleep time, and SE. This is a data-rich, but time- and resource-intensive test that is not widely available and can be burdensome, uncomfortable, and costly for the patient.
Portable monitors
Because of these PSG challenges, portable monitors that can be used in the home were developed. There are 4 portable monitor classes, with type I being an ambulatory PSG and types II to IV having fewer end points than PSG.
Digital wearables
Actigraphy involves typically wrist-worn devices that use an internal accelerometer to measure gross movement. The accompanying software uses the data to estimate activity and sleep/wake periods. Actigraphy is noninvasive, less expensive than PSG and portable monitors, and can be used at home. It has been shown to correlate with PSG-measured end points such as SOL, WASO, total sleep time, SE, and arousals, but it has limited ability to assess sleep stages.
Another wearable is an electroencephalography headband, with sophisticated versions having 5 electrodes, an accelerometer, pulse oximeter, and an audio component. The resulting data have correlated with some PSG readouts but not others (SOL and WASO). However, similar to actigraphy devices, headbands can have battery life or software issues and are positionally sensitive (ie, can fall off during sleep). For these reasons, they may not yield consistent and useful data.
Nonwearables
These are devices placed near or under the sleeping person. Mattress-based sensors are one example, which use ballistocardiography to measure body motion generated by the ejection of blood during the cardiac cycle. They also track thoracic movements associated with respiration. Although mattress sensors correlate somewhat with validated objective measures, they are confounded by a number of factors: mattress thickness, mattress size, patient weight, body movements/location, and bed partners (human and animal).
PROs and sleep diaries
PROs and sleep diaries are commonly used to assess the impact of sleep disturbances on patients’ well-being. The daily sleep diary is a standard PRO with 6 to 10 items to be completed daily for 1 to 2 weeks, which provide weekly average values for SOL, WASO, total sleep time, and SE93 Although it is widely used to capture these measures, it typically does not correlate well with the same measures collected by PSG or actigraphy. Several variants of sleep diaries exist including a consensus sleep diary.93 For an overview of other commonly used sleep PROs, see Table I.
TABLE I.
Tools commonly used to measure sleep disturbances
| Method | Variables measured | Advantages | Limitations |
|---|---|---|---|
| Subjective measures | |||
| Pittsburgh Sleep Quality Index94 | A total of 19 items answered by patient and 5 items answered by partner or caregiver regarding sleep duration and quality in the past month | Identifies specific aspects of sleep that are impaired High internal consistency Tested in various disease states and countries Translated into many languages |
Limited to adults May primarily reflect sleep on workdays95 |
| Medical Outcomes Study Sleep Scale96,97 | A total of 12 items answered by patient to measure 6 sleep dimensions: initiation (time to fall asleep), quantity (hours of sleep each night), maintenance, respiratory problems, perceived adequacy, and somnolence | Tested in various disease states and countries Developed using a large number of patients served in primary care and specialty practice settings Covers multiple dimensions of sleep Translatable into multiple languages |
Limited to adults Does not have a cutoff score Responsiveness to change/sensitivity has not been established |
| Children’s Sleep Habits Questionnaire98 | A total of 35 items regarding bedtime behavior, nocturnal awakenings, and sleep disorders over the past week in children aged 4-10 y | High specificity and sensitivity in identifying sleep disturbances99 Incorporates questions regarding sleep disorders Validated in children with a wide variety of diseases Validated in longitudinal studies99 Available in multiple languages |
Limited to parental report, which may not be accurate Time frame limited to past week, which may not capture typical sleep habits in some circumstances |
| Brief Infant Sleep Questionnaire100 | A total of 13 items regarding initiation and maintenance of sleep in infants in the past week | Sensitive and specific tool to identify sleep problems99 Correlated with actigraphy100 Translated into several languages |
Proxy reporting of infant sleep by parents may be inaccurate |
| Epworth Sleepiness Scale101 | A total of 8 items regarding present-day daytime sleepiness | Correlated with PSG to some degree102 Easy to administer Useful in screening for sleep disorders101 |
Limited to daytime sleepiness, which may be affected by other factors Does not investigate specific disturbances during sleep May have limited validity between individuals103 |
| Insomnia Severity Index | A total of 7 items evaluating patient’s assessment of his or her insomnia and its impact on daily functioning in the past 2 wk104 | Sensitive, validated screening tool to identify patients with insomnia and assess treatment response105 Brief, easy to administer |
Use is limited to assessing insomnia, rather than specific parameters of sleep quality |
| Patient Reported Outcomes Information System106,107 | A total of 41 items related to sleep habits, sleep disturbances, and daytime sleep-related impairments in the past 7 d | Validated in adults with AD19 Used internationally108 Easy to administer |
Inconsistent results in adolescents108 |
| Sleep diaries93 | Variable (typically logs of daily sleep duration and disturbances) | Cost-effective May be more accurate than retrospective estimates of sleep quality over the past week/month109 |
May not accurately reflect objective measures109,110 Require daily completion Most are not formally validated106 |
| Children’s Dermatology Life Quality Index | A total of 10 items assessing skin symptoms and impacts on various aspects of quality of life in children aged 4-16 y in the past week | Dermatology-specific Easy to administer Translated into many languages High internal consistency111 Self-reported |
May not correlate well with other quality-of-life measures112 Only 1 item regarding sleep |
| Dermatitis Family Impact14 | A total of 10 items assessing impacts of child’s disease on caregiver’s daily activities and quality of life | AD-specific One of the few tools assessing caregiver sleep Easy to administer High internal consistency111 |
May be impacted by parental health and comorbidities Only 1 item assessing sleep |
| Infant’s Dermatitis Quality of Life Index10 | A 10-item questionnaire assessing infant daily activities and quality of life in children aged <4 y in the past week | AD-specific Easy to administer Shows test-retest reliability111 |
Weak reliability and content validity113 Only 2 items addressing sleep |
| Objective measures | |||
| PSG: criterion standard | Brain function, heart rate, eye movements, muscle movements, and respiratory parameters | Multiple biophysiological variables measured Can aid in diagnosing sleep disorders, including disordered breathing Can detect disturbances that are not associated with body movements |
Not done in the individual’s natural habitat May cause skin irritation in patients with AD May require monitoring for multiple nights to capture some disturbances Costly |
| Actigraphy | Limb acceleration (activity count in a certain amount of time) | Can be completed at home Less invasive than PSG Correlates well with PSG13 |
Limited to measuring movements (unable to accurately measure sleep fragmentation or respiratory parameters) Correlation with PSG may depend on population studied and make of actigraphy device Quiet wakefulness may be measured as sleep Value of readouts is dependent on the software algorithm used to analyze the data |
| Video monitoring/observation | Visualized movements or other disturbances during sleep | Allows for direct visualization of sleeping and waking114 Can be done at home Allows for differentiation between scratching behaviors and other movements64,115 |
May not capture brief nocturnal awakenings not associated with movements May not capture disordered breathing May not be as accurate in timings of events Rating and coding video is labor-intensive and can present rater variability |
| Biomotion sensors and mattress sensors | Changes in pressure, body movements, or respiratory movements detected by sensors placed in mattresses | Correlates fairly well with validated objective measures116-119 Can be used at home Does not require wearing a device |
Limited evidence Lack of movement may be detected as sleep May be confounded by mattress thickness and size, patient weight, and bed partners |
| Headband electroencephalograms | Stages of sleep detected through brain electrical patterns | Can be performed at home Agrees fairly well with other objective measures120-122 |
Limited evidence Does not always stay on throughout the night120 Was not able to accurately measure sleep- onset latency and WASO in some studies120 |
The instruments and PROs/diaries briefly reviewed above and in Table I provide a range of options to use in clinical practice and/or research trials. They vary in cost, logistical challenges, burden to investigator and patient, diagnostic utility, ideal patient population, and validity. In AD clinical trials in which sleep is often a secondary outcome measure, the inclusion of 1 or more of these measures will improve our understanding of sleep health in this population.
METHODS FOR MANAGEMENT OF SLEEP DISTURBANCES IN AD
Treating the AD
It is unclear whether AD treatments directed at inflammation and/or pruritus will also treat sleep disturbances. Therefore, we begin by reporting sleep improvements noted from systemic therapies that are in common use, as well as those in late-phase clinical development. The evidence on topical therapies is briefly outlined in Table II. Most studies have been conducted on adults.
TABLE II.
Treatment strategies to manage sleep disturbances in patients with AD
| Method | Advantages | Limitations | Quality of evidence in AD |
|---|---|---|---|
| Optimizing treatment of AS | |||
| Topical corticosteroids123-127 | Easy to use, with minimal side effects Well tolerated in adults and children |
Must be applied regularly (often daily) Chronic use may thin skin |
Nine RCTs with varied control groups and results Improvements in sleep assessed through VAS of sleep loss in nearly all studies |
| Topical calcineurin inhibitors (eg, tacrolimus and pimecrolimus)126,128 | Easy to use, with minimal side effects | Must be applied regularly (often daily) Can cause burning/stinging on application |
Most studies assessed sleep on a VAS Actigraphy used in 1 RCT on pimecrolimus, found no differences in sleep outcomes with treatment128 |
| Topical phosphodiesterase-4 inhibitors (eg, crisaborole) | Easy to use, with minimal side effects | Must be applied regularly (often daily) Typically not sufficient for severe cases Can cause burning/stinging on application |
Improved sleep of children and caregivers in phase 3 trials129 Data largely based on single item assessing sleep in quality-of-life questionnaires |
| Topical JAK inhibitors (eg, ruxolitinib) | Easy to use, with minimal side effects | No trials in children | Phase 3 trials showed improvements in patient-reported perceptions of sleep quality, sleep depth, and restoration associated with sleep (PROMIS Short Form-Sleep Disturbance (8b) questionnaire) Phase 2 trials showed improved pruritus and quality of life, but sleep was not specifically discussed130 |
| Systemic immunosuppressants used off-label for AD (eg, cyclosporine,131,132 methotrexate,133 | Lead to significant improvements in AD | Side effects and toxicities limit them to short-term use | RCTs131,132,134 and open-label study133 showing positive effect Limited evidence on children Results on sleep are based on VASs |
| Anti—type 2 immunity approaches (eg, dupilumab and IL—13 targeting therapies)135-142 | Lead to significant improvements in AD Well tolerated with minimal side effects Typically dosed once every few weeks |
Require injection Few trials in pediatric patients for most therapies |
Several RPCTs with large sample sizes showing positive effect Sleep outcomes based on VASs of sleep loss |
| Sleep aids | |||
| Melatonin143 | Minimal side effects, and little potential for addiction or withdrawal144 May improve disease severity143 |
Not recommended for patients with bronchial asthma, due to potential for exacerbating inflammation88 May worsen autoimmune diseases145 |
One RDBPCT (n = 73) with cross-over on children with AD Sleep outcomes measured using actigraphy and PSG Only improved SOL |
| First-generation antihistamines | Can reduce inflammatory effects of mast cells146 | May develop tolerance147 Anticholinergic side effects148 Excessive sedation may impede daytime performance |
No RCTs or high-level evidence on sleep quality in AD149 RCT on nocturnal itch/scratch showed similar efficacy to placebo150 |
| Benzodiazepines151 | Can also be effective for concurrent parasomnias145 | Side effect profile: behavioral problems, daytime sleepiness, muscle relaxation (especially problematic in asthma)152 Potential for addiction, tolerance, and withdrawal Rebound insomnia on discontinuation145 |
RDBPCT with small sample size (n = 10 adults) Reduced frequency but increased duration of scratching No RCT on children |
| Alpha-receptor agonists | Can also be effective for treating comorbid ADHD145 | Adrenergic side effects145 Potential for overdose given narrow therapeutic index145 |
Case report in pediatric patient with AD showing positive effect on reported sleep quality153 |
| Cognitive-behavioral therapy154 | Does not require medications Minimal side effects Addresses behavioral and psychological aspects of sleep disturbances |
Limited evidence in AD | Small uncontrolled study (n = 10) showing no effect154 |
| Biofeedback (eg, progressive muscle relaxation)155 | Does not require medications Minimal side effects Addresses behavioral and psychological aspects of sleep disturbances |
Limited evidence in AD | RCT with small sample size (n = 25) showing positive effect Sleep outcomes assessed using VAS evaluating sleep loss |
| Sleep hygiene (eg, blue light therapy, altering bedtime routines)156,157 | Does not require medications Minimal side effects Addresses behavioral aspects of sleep disturbances |
Limited evidence in AD | No RCTs on sleep quality in AD Sleep outcomes based on VASs or global assessment of “sleepiness”157 |
| Acupuncture | May address psychological aspects of sleep disturbances May relieve pruritus158 |
Limited evidence | Sham RCT (n = 30) showed positive effects on VAS of insomni159 A second RCT underway using EEG to evaluate sleep160 |
EEG, Electroencephalography; RCT, randomized controlled trial; RDBPCT, randomized, double-blind, placebo-controlled trial; RPCT, randomized placebo-controlled trial.
Systemic immunosuppressants such as cyclosporine, methotrexate, and azathioprine are not less commonly used to treat moderate to severe AD. However, few studies have explored their effects on sleep. Uncontrolled trials of cyclosporine (1 adult, 1 pediatric) showed approximately 50% improvement in patient-reported sleep loss using a visual analog scale (VAS) after 8 weeks of treatment.131,132 Similarly, small trials of azathioprine (n = 37)134 and methotrexate (n = 12)133 in adults demonstrated reductions in sleep disturbance VAS scores of 30% to 50% by week 12 for azathioprine (not statistically significant) and week 24 for methotrexate (P < .05).
More targeted therapies have limited data with regard to sleep end points. Dupilumab, a fully humanized mAb targeting IL-4Rα, has been shown to significantly reduce pruritus within days of treatment initiation.161 Dupilumab (300 mg biweekly) resulted in an improvement in SCORAD sleep loss scores by 3.3 points at 16 weeks, with 51% of subjects reporting absence of sleep disturbances.139 Other targeted therapies with data on sleep disturbances include the Janus kinase (JAK) inhibitors and antibodies against IL-31Rα. Trials of JAKi have demonstrated improvements in disease severity, inflammatory biomarkers, pruritus, and in some cases, sleep. In a phase 2 trial of baricitinib (2 mg/d; JAK1 & 2 selective), SCORAD sleep loss scores were significantly reduced (P < .01) after 16 weeks; phase 3 trials showed significant reductions in the Atopic Dermatitis Sleep Scale score (item no. 2) as early as 1 week into treatment (P < .05; 1, 2 and 4 mg/d).162,163 Phase 3 trials of abrocitinib (100 and 200 mg/d; JAK1 selective) showed significant reductions in pruritus numerical rating scale score compared with placebo by 2 weeks (P < .01), but sleep was not evaluated.164 IL-31 is a pruritogen that binds to IL-31Rα, expressed on sensory neurons and a wide range of other cell types.165 Phase 2 trials with nemolizumab (10, 30, and 90 mg subcutaneous every 4 weeks; anti—IL-31Rα) resulted in reduced pruritus and sleep disturbance VAS by approximately 60% at 4 weeks and 90% by 52 weeks.166,167 A small, 16-week, phase 3 trial (60 mg subcutaneous every 4 weeks) observed a greater percentage of patients achieving an Insomnia Severity Index score of 7 or less after treatment.168
Antibodies to IL-13 (eg, tralokinumab and lebrikizumab), thymic stromal lymphopoietin (eg, tezepelumab), and the H4 histamine receptor (ZPL-3893787) have also been investigated. IL-13 inhibitors, tezepelumab, and ZPL-3893787 improved some measures of pruritus; however, tezepelumab studies have not investigated sleep outcomes.142,169,170 ZPL-3893787 significantly improved SCORAD sleep scores compared with placebo by week 1 (P < .01), while lebrikizumab and tralokinumab both resulted in improved sleep scores at week 12 (P = .023) and week 16 (P < .01), respectively.140-142 Overall, nearly all studies on the effects of AD therapies on sleep have investigated sleep as a secondary outcome using PROs. Incorporating more objective measures may add significant depth to current findings.
Treating the sleep disturbance directly
The optimal management of sleep disturbance relies on accurate identification of the specific disturbance. Assuming that sleep disorders such as sleep apnea, restless legs syndrome, parasomnias, and a circadian rhythm disorder have been ruled out, the sleep disturbance that is most commonly associated with AD is insomnia.171 The Insomnia Severity Index is a PRO that can reliably screen for and quantify insomnia (see Table I).104,105 Clinical practice guidelines for the management of insomnia strongly recommend that the first-line management of adult insomnia be cognitive-behavioral therapy for insomnia (CBT-I).172-174
CBT-I is a multicomponent intervention with established efficacy and durability in adolescents and adults.175-178 This approach is effective in reducing not only insomnia but also mood and anxiety symptoms.179 Typically, it is delivered in 6 to 8 weekly psychotherapy sessions, although briefer formats have been shown to be effective.180,181 Detailed descriptions of CBT-I are available elsewhere, but its 3 core components are sleep restriction therapy, stimulus control therapy, and sleep-specific cognitive therapy, which are supplemented by sleep psychoeducation and sleep hygiene.171,182 Notably, sleep hygiene is a very small part of CBT-I and the least effective behavioral intervention.183 When feasible, the full complement of CBT-I components should be used, which can also be accomplished via digital platforms.184
No other nonpharmacologic treatment options for insomnia (eg, hypnosis, biofeedback, acupuncture, meditation, and relaxation) have been consistently effective, and none are as effective as CBT-I or sedative-hypnotic medications. The same can be said for over-the-counter formulations and supplements. Over-the-counter sedating antihistamines have limited data supporting their effectiveness for insomnia. Some studies support the use of diphenhydramine, but a recent task force recommended against its use for insomnia.185 Supplementation with melatonin is an established treatment for circadian rhythm disturbances186 such as delayed sleep-phase disorder.187 Evidence for its effectiveness in treating insomnia, however, is mixed and this, coupled with wide variations in dose and expedients in over-the-counter formulations, and concerns about long-term safety, should limit its use.144,188
Several options exist for the pharmacologic treatment of insomnia (in adults) if the first-line treatment (CBT-I) is ineffective or unavailable. These were reviewed in the 2017 clinical practice guideline from the American Academy of Sleep Medicine.185 None of the agents warranted a “strong” evidence rating, but many were rated “weak” (as opposed to not enough evidence to evaluate). Eight were recommended for the treatment of sleep-onset and/or sleep maintenance insomnia and were FDA-approved for insomnia. These include 2 benzodiazepines (triazolam and temazepam), 3 non-benzodiazepine receptor agonists (zolpidem, zaleplon, and eszopiclone), the orexin antagonist suvorexant, the melatonin agonist ramelteon, and low-dose (3-6mg) doxepin, the tricyclic antidepressant with antihistaminergic properties. Notably, 6 agents had enough evidence to recommend against their use: the herbal supplement valerian, the amino-acid supplement tryptophan, exogenous melatonin, the antihistamine diphenhydramine, the gamma aminobutyric acid-reuptake inhibitor tiagabine, and the serotonin antagonist reuptake inhibitor trazodone. None of these 6 agents has an FDA indication for insomnia; yet, trazodone is one of the most widely prescribed agents for insomnia despite ongoing safety concerns (eg, morning sedation, falls, and suicide attempts).189
It is striking that very few insomnia or “sleep disturbance” intervention trials have been conducted in patients with AD. Among these, a small (n = 73), well-designed, cross-over trial comparing melatonin to placebo in children with AD with sleep disturbance found modest improvements on SOL, but no other sleep outcomes.143 One randomized controlled trial evaluating the antihistamine chlorpheniramine found no difference from placebo in relieving nocturnal AD symptoms.150 A small trial (N = 10) of the benzodiazepine nitrazepam found that it reduced the frequency, but increased the duration, of scratching.151 A randomized controlled trial (n = 24) of progressive muscle relaxation found no difference on a single-item 0 to 10 scale for “loss of sleep” compared with controls,155 and 1 sham-controlled randomized controlled trial of acupuncture (n = 30) showed positive effects on a VAS of insomnia, although the participants in the sham condition had much higher baseline insomnia that was not controlled for in analyses.159 Remarkably, no trials of CBT-I or of medications with FDA indications for insomnia have been conducted in populations with AD.
CONCLUSIONS
Despite the fact that eczema has the highest disability-adjusted life-years of any skin condition worldwide, and more than 50% of children with AD report sleep disturbance as 1 of the most bothersome symptoms, we still know very little about this AD comorbidity.190 We have reviewed what is known about sleep disturbances in populations with AD, provided an overview of the strengths and weaknesses of tools (both wearables and surveys) used to characterize, and in some cases quantify, sleep and/or sleep metrics, summarized several theories about pathogenesis, and reviewed traditional sleep treatments and the potential benefits of newer inflammation-directed AD therapies. Unfortunately, this exercise has highlighted the stark reality that we have much more to learn. Future studies should focus on characterizing AD-associated sleep disturbance(s), understanding their pathogenesis, identifying at-risk populations with AD, defining the role that sleep disturbances play in other AD-associated comorbidities (anxiety, depression, attention-deficit disorders, social anxiety, etc), and effectively managing it.
Acknowledgments
F.B. was supported by the University of Rochester Clinical and Translational Science Institute award number TL1 TR000096 from the National Center for Advancing Translational Sciences of the National Institutes of Health. L.A.B. is supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health (award no. U19AI117673).
Abbreviations used
- AD
Atopic dermatitis
- CBT-I
Cognitive-behavioral therapy for insomnia
- FDA
Federal Drug Administration
- JAK
Janus kinase
- PSG
polysomnography
- PRO
Patient-reported outcome
- SCORAD
CORing Atopic Dermatitis
- SE
Sleep efficiency
- SOL
Sleep-onset latency
- VAS
Visual analog scale
- WASO
Wake time after sleep onset
Footnotes
Conflicts of interest: C. A. Northcott is an employee of Pfizer, Inc, and owns company stock. L. A. Beck is a consultant for Abbvie, AI/Benevolent, Allakos, Astra-Zeneca, Galderma, Incyte, LEO Pharma, Lilly, Naos Bioderma, Novartis, Pfizer, PrincipiaBio, Regeneron, Sanofi, UCB, and Vimalan; is or has been an investigator on Abbvie, LEO Pharma, Pfizer, and Regeneron atopic dermatitis trials; and owns stock in Moderna, Gilead, and Medtronic. W. R. Pigeon has consulted for Curaegis Technologies and is an employee of the US Department of Veterans Affairs (VA). F. Bawany has no relevant conflicts of interest. The content is solely the responsibility of the authors and does not necessarily represent the official views of the US government, the National Institutes of Health, or the VA.
REFERENCES
- 1.Weidinger S, Beck LA, Bieber T, Kabashima K, Irvine AD. Atopic dermatitis. Nat Rev Dis Primers 2018;4:1. [DOI] [PubMed] [Google Scholar]
- 2.Magee CA, Robinson L, Keane C. Sleep quality subtypes predict health-related quality of life in children. Sleep Med 2017;35:67–73. [DOI] [PubMed] [Google Scholar]
- 3.Johnson CM. Infant and toddler sleep: a telephone survey of parents in one community. J Dev Behav Pediatr 1991;12:108–14. [PubMed] [Google Scholar]
- 4.Nena E, Cassimos D, Kaditis A, Kourantzi M, Trakada G, Economou NT, et al. Predictors of sleep duration and sleep disturbance in children of a culturally diverse region in North-Eastern Greece. Front Pediatr 2020;8:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hinz A, Glaesmer H, Brahler E, Loffler M, Engel C, Enzenbach C, et al. Sleep quality in the general population: psychometric properties of the Pittsburgh Sleep Quality Index, derived from a German community sample of 9284 people. Sleep Med 2017;30:57–63. [DOI] [PubMed] [Google Scholar]
- 6.Doi Y, Minowa M, Okawa M, Uchiyama M. Prevalence of sleep disturbance and hypnotic medication use in relation to sociodemographic factors in the general Japanese adult population. J Epidemiol 2000;10:79–86. [DOI] [PubMed] [Google Scholar]
- 7.Dregan A, Armstrong D. Cross-country variation in sleep disturbance among working and older age groups: an analysis based on the European Social Survey. Int Psychogeriatr 2011;23:1413–20. [DOI] [PubMed] [Google Scholar]
- 8.Chang YS, Chiang BL. Sleep disorders and atopic dermatitis: a 2-way street? J Allergy Clin Immunol 2018;142:1033–40. [DOI] [PubMed] [Google Scholar]
- 9.Camfferman D, Kennedy JD, Gold M, Martin AJ, Winwood P, Lushington K. Eczema, sleep, and behavior in children. J Clin Sleep Med 2010;6:581–8. [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis-Jones MS, Finlay AY, Dykes PJ. The Infants’ Dermatitis Quality of Life Index. Br J Dermatol 2001;144:104–10. [DOI] [PubMed] [Google Scholar]
- 11.Bartlet LB, Westbroek R, White JE. Sleep patterns in children with atopic eczema. Acta Derm Venereol 1997;77:446–8. [DOI] [PubMed] [Google Scholar]
- 12.Chamlin SL, Mattson CL, Frieden IJ, Williams ML, Mancini AJ, Cella D, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med 2005;159:745–50. [DOI] [PubMed] [Google Scholar]
- 13.Chang YS, Chou YT, Lee JH, Lee PL, Dai YS, Sun C, et al. Atopic dermatitis, melatonin, and sleep disturbance. Pediatrics 2014;134:e397–405. [DOI] [PubMed] [Google Scholar]
- 14.Lawson V, Lewis-Jones MS, Finlay AY, Reid P, Owens RG. The family impact of childhood atopic dermatitis: the Dermatitis Family Impact Questionnaire. Br J Dermatol 1998;138:107–13. [DOI] [PubMed] [Google Scholar]
- 15.Hon KL, Leung TF, Wong KY, Chow CM, Chuh A, Ng PC. Does age or gender influence quality of life in children with atopic dermatitis? Clin Exp Dermatol 2008;33:705–9. [DOI] [PubMed] [Google Scholar]
- 16.Long CC, Funnell CM, Collard R, Finlay AY. What do members of the National Eczema Society really want? Clin Exp Dermatol 1993;18:516–22. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez-Perez J, Dauden-Tello E, Mora AM, Lara Surinyac N. Impact of atopic dermatitis on health-related quality of life in Spanish children and adults: the PSEDA study. Actas Dermosifiliogr 2013;104:44–52. [DOI] [PubMed] [Google Scholar]
- 18.Simpson EL, Bieber T, Eckert L, Wu R, Ardeleanu M, Graham NM, et al. Patient burden of moderate to severe atopic dermatitis (AD): insights from a phase 2b clinical trial of dupilumab in adults. J Am Acad Dermatol 2016;74: 491–8. [DOI] [PubMed] [Google Scholar]
- 19.Lei D, Yousaf M, Janmohamed SR, Vakharia PP, Chopra R, Chavda R, et al. Validation of four single-item patient-reported assessments of sleep in adult atopic dermatitis patients. Ann Allergy Asthma Immunol 2020;124:261–6. [DOI] [PubMed] [Google Scholar]
- 20.McCleary KK. The more than skin deep “Voice of the Patient” report; 2020. Available from: http://www.morethanskindeep-eczema.org/uploads/1/2/5/3/125377765/mtsd_report_-_digital_file.pdf. Accessed September 30, 2020. [Google Scholar]
- 21.Alibhai FJ, Tsimakouridze EV, Reitz CJ, Pyle WG, Martino TA. Consequences of circadian and sleep disturbances for the cardiovascular system. Can J Cardiol 2015;31:860–72. [DOI] [PubMed] [Google Scholar]
- 22.Whitaker KM, Lutsey PL, Ogilvie RP, Pankow JS, Bertoni A, Michos ED, et al. Associations between polysomnography and actigraphy-based sleep indices and glycemic control among those with and without type 2 diabetes: the Multi-Ethnic Study of Atherosclerosis. Sleep 2018;41:zsy172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Charrier A, Olliac B, Roubertoux P, Tordjman S. Clock genes and altered sleep-wake rhythms: their role in the development of psychiatric disorders. Int J Mol Sci 2017;18:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dahl RE, Bernhisel-Broadbent J, Scanlon-Holdford S, Sampson HA, Lupo M. Sleep disturbances in children with atopic dermatitis. Arch Pediatr Adolesc Med 1995;149:856–60. [DOI] [PubMed] [Google Scholar]
- 25.Dogan DG, Canaloglu SK, Kivilcim M, Kum YE, Topal E, Catal F. Sleep patterns of young children with newly diagnosed atopic dermatitis. Postepy Dermatol Alergol 2017;34:143–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricci G, Bendandi B, Bellini F, Patrizi A, Masi M. Atopic dermatitis: quality of life of young Italian children and their families and correlation with severity score. Pediatr Allergy Immunol 2007;18:245–9. [DOI] [PubMed] [Google Scholar]
- 27.Treister AD, Stefek H, Grimaldi D, Rupani N, Zee P, Yob J, et al. Sleep and limb movement characteristics of children with atopic dermatitis coincidentally undergoing clinical polysomnography. J Clin Sleep Med 2019;15:1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fishbein AB, Mueller K, Kruse L, Boor P, Sheldon S, Zee P, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: a case-control study. J Am Acad Dermatol 2018;78:336–41. [DOI] [PubMed] [Google Scholar]
- 29.Stores G, Burrows A, Crawford C. Physiological sleep disturbance in children with atopic dermatitis: a case control study. Pediatr Dermatol 1998;15:264–8. [DOI] [PubMed] [Google Scholar]
- 30.Monti JM, Vignale R, Monti D. Sleep and nighttime pruritus in children with atopic dermatitis. Sleep 1989;12:309–14. [DOI] [PubMed] [Google Scholar]
- 31.Reuveni H, Chapnick G, Tal A, Tarasiuk A. Sleep fragmentation in children with atopic dermatitis. Arch Pediatr Adolesc Med 1999;153:249–53. [DOI] [PubMed] [Google Scholar]
- 32.Hon KL, Leung TF, Ma KC, Li AM, Wong Y, Yin JA, et al. Resting energy expenditure, oxygen consumption and carbon dioxide production during sleep in children with atopic dermatitis. J Dermatolog Treat 2005;16:22–5. [DOI] [PubMed] [Google Scholar]
- 33.Ramirez FD, Chen S, Langan SM, Prather AA, McCulloch CE, Kidd SA, et al. Association of atopic dermatitis with sleep quality in children. JAMA Pediatr 2019;173:e190025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urrutia-Pereira M, Sole D, Rosario NA, Neto HJC, Acosta V, Almendarez CF, et al. Sleep-related disorders in Latin-American children with atopic dermatitis: a case control study. Allergol Immunopathol (Madr) 2017;45:276–82. [DOI] [PubMed] [Google Scholar]
- 35.Vlaski E, Stavric K, Isjanovska R, Seckova L, Kimovska M. Overweight hypothesis in asthma and eczema in young adolescents. Allergol Immunopathol (Madr) 2006;34:199–205. [DOI] [PubMed] [Google Scholar]
- 36.Al-Riyami BM, Al-Rawas OA, Al-Riyami AA, Jasim LG, Mohammed AJ. A relatively high prevalence and severity of asthma, allergic rhinitis and atopic eczema in schoolchildren in the Sultanate of Oman. Respirology 2003;8:69–76. [DOI] [PubMed] [Google Scholar]
- 37.Kong TS, Han TY, Lee JH, Son SJ. Correlation between severity of atopic dermatitis and sleep quality in children and adults. Ann Dermatol 2016;28: 321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capozza K, Gadd H, Kelley K, Russell S, Shi V, Schwartz A. Insights from caregivers on the impact of pediatric atopic dermatitis on families: “I’m tired, overwhelmed, and feel like I’m failing as a mother”. Dermatitis 2020;31: 223–7. [DOI] [PubMed] [Google Scholar]
- 39.Beattie PE, Lewis-Jones MS. An audit of the impact of a consultation with a paediatric dermatology team on quality of life in infants with atopic eczema and their families: further validation of the Infants’ Dermatitis Quality of Life Index and Dermatitis Family Impact score. Br J Dermatol 2006;155:1249–55. [DOI] [PubMed] [Google Scholar]
- 40.Touchette E, Petit D, Seguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep 2007;30:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sadeh A, Gruber R, Raviv A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev 2002;73:405–17. [DOI] [PubMed] [Google Scholar]
- 42.Romanos M, Gerlach M, Warnke A, Schmitt J. Association of attention-deficit/hyperactivity disorder and atopic eczema modified by sleep disturbance in a large population-based sample. J Epidemiol Community Health 2010;64: 269–73. [DOI] [PubMed] [Google Scholar]
- 43.Schmitt J, Chen CM, Apfelbacher C, Romanos M, Lehmann I, Herbarth O, et al. Infant eczema, infant sleeping problems, and mental health at 10 years of age: the prospective birth cohort study LISAplus. Allergy 2011;66: 404–11. [DOI] [PubMed] [Google Scholar]
- 44.Silverberg JI, Paller AS. Association between eczema and stature in 9 US population-based studies. JAMA Dermatol 2015;151:401–9. [DOI] [PubMed] [Google Scholar]
- 45.Ridolo E, Caffarelli C, Olivieri E, Montagni M, Incorvaia C, Baiardini I, et al. Quality of sleep in allergic children and their parents. Allergol Immunopathol (Madr) 2015;43:180–4. [DOI] [PubMed] [Google Scholar]
- 46.Angelhoff C, Askenteg H, Wikner U, Edell-Gustafsson U. “To cope with everyday life, I need to sleep”—a phenomenographic study exploring sleep loss in parents of children with atopic dermatitis. J Pediatr Nurs 2018;43: e59–65. [DOI] [PubMed] [Google Scholar]
- 47.Reid P, Lewis-Jones MS. Sleep difficulties and their management in preschoolers with atopic eczema. Clin Exp Dermatol 1995;20:38–41. [DOI] [PubMed] [Google Scholar]
- 48.Kemp AS. Atopic eczema: its social and financial costs. J Paediatr Child Health 1999;35:229–31. [DOI] [PubMed] [Google Scholar]
- 49.Ramirez FD, Chen S, Langan SM, Prather AA, McCulloch CE, Kidd SA, et al. Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol 2019;155:556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moore K, David TJ, Murray CS, Child F, Arkwright PD. Effect of childhood eczema and asthma on parental sleep and well-being: a prospective comparative study. Br J Dermatol 2006;154:514–8. [DOI] [PubMed] [Google Scholar]
- 51.Yu SH, Attarian H, Zee P, Silverberg JI. Burden of sleep and fatigue in US adults with atopic dermatitis. Dermatitis 2016;27:50–8. [DOI] [PubMed] [Google Scholar]
- 52.Mann C, Dreher M, Weess HG, Staubach P. Sleep disturbance in patients with urticaria and atopic dermatitis: an underestimated burden. Acta Derm Venereol 2020;100:adv00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaaz K, Szepietowski JC, Matusiak L. Influence of itch and pain on sleep quality in atopic dermatitis and psoriasis. Acta Derm Venereol 2019;99: 175–80. [DOI] [PubMed] [Google Scholar]
- 54.Li JC, Fishbein A, Singam V, Patel KR, Zee PC, Attarian H, et al. Sleep disturbance and sleep-related impairment in adults with atopic dermatitis: a cross-sectional study. Dermatitis 2018;29:270–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bender BG, Leung SB, Leung DY. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: an objective life quality measure. J Allergy Clin Immunol 2003;111:598–602. [DOI] [PubMed] [Google Scholar]
- 56.Bringhurst C, Waterston K, Schofield O, Benjamin K, Rees JL. Measurement of itch using actigraphy in pediatric and adult populations. J Am Acad Dermatol 2004;51:893–8. [DOI] [PubMed] [Google Scholar]
- 57.Yano C, Saeki H, Ishiji T, Ishiuji Y, Sato J, Tofuku Y, et al. Impact of disease severity on sleep quality in Japanese patients with atopic dermatitis. J Dermatol Sci 2013;72:195–7. [DOI] [PubMed] [Google Scholar]
- 58.de Bruin-Weller M, Gadkari A, Auziere S, Simpson EL, Puig L, Barbarot S, et al. The patient-reported disease burden in adults with atopic dermatitis: a cross-sectional study in Europe and Canada. J Eur Acad Dermatol Venereol 2020;34:1026–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bender BG, Ballard R, Canono B, Murphy JR, Leung DY. Disease severity, scratching, and sleep quality in patients with atopic dermatitis. J Am Acad Dermatol 2008;58:415–20. [DOI] [PubMed] [Google Scholar]
- 60.Noro Y, Omoto Y, Umeda K, Tanaka F, Shiratsuka Y, Yamada T, et al. Novel acoustic evaluation system for scratching behavior in itching dermatitis: rapid and accurate analysis for nocturnal scratching of atopic dermatitis patients. J Dermatol 2014;41:233–8. [DOI] [PubMed] [Google Scholar]
- 61.Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. J Invest Dermatol 2015;135:56–66. [DOI] [PubMed] [Google Scholar]
- 62.Chang YS, Chiang BL. Mechanism of sleep disturbance in children with atopic dermatitis and the role of the circadian rhythm and melatonin. Int J Mol Sci 2016; 17:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hon KL, Leung TF, Wong Y, Fok TF. Lesson from performing SCORADs in children with atopic dermatitis: subjective symptoms do not correlate well with disease extent or intensity. Int J Dermatol 2006;45:728–30. [DOI] [PubMed] [Google Scholar]
- 64.Benjamin K, Waterston K, Russell M, Schofield O, Diffey B, Rees JL. The development of an objective method for measuring scratch in children with atopic dermatitis suitable for clinical use. J Am Acad Dermatol 2004;50:33–40. [DOI] [PubMed] [Google Scholar]
- 65.Pigeon WR, Cribbet MR. The pathophysiology of insomnia: from models to molecules (and back). Curr Opin Pulm Med 2012;18:546–53. [DOI] [PubMed] [Google Scholar]
- 66.Carney CE, Segal ZV, Edinger JD, Krystal AD. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J Clin Psychiatry 2007;68: 254–60. [DOI] [PubMed] [Google Scholar]
- 67.Ebata T, Aizawa H, Kamide R, Niimura M. The characteristics of nocturnal scratching in adults with atopic dermatitis. Br J Dermatol 1999;141:82–6. [DOI] [PubMed] [Google Scholar]
- 68.Aoki T, Kushimoto H, Hishikawa Y, Savin JA. Nocturnal scratching and its relationship to the disturbed sleep of itchy subjects. Clin Exp Dermatol 1991; 16:268–72. [DOI] [PubMed] [Google Scholar]
- 69.Irwin MR. Sleep and inflammation: partners in sickness and in health. Nat Rev Immunol 2019;19:702–15. [DOI] [PubMed] [Google Scholar]
- 70.Ozawa M, Tsuchiyama K, Gomi R, Kurosaki F, Kawamoto Y, Aiba S. Neuroselective transcutaneous electrical stimulation reveals neuronal sensitization in atopic dermatitis. J Am Acad Dermatol 2009;60:609–14. [DOI] [PubMed] [Google Scholar]
- 71.Misery L. Atopic dermatitis and the nervous system. Clin Rev Allergy Immunol 2011;41:259–66. [DOI] [PubMed] [Google Scholar]
- 72.Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, et al. Eosinophils increase neuron branching in human and murine skin and in vitro. PLoS One 2011;6:e22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Takano N, Sakurai T, Ohashi Y, Kurachi M. Effects of high-affinity nerve growth factor receptor inhibitors on symptoms in the NC/Nga mouse atopic dermatitis model. Br J Dermatol 2007;156:241–6. [DOI] [PubMed] [Google Scholar]
- 74.Yamaguchi J, Aihara M, Kobayashi Y, Kambara T, Ikezawa Z. Quantitative analysis of nerve growth factor (NGF) in the atopic dermatitis and psoriasis horny layer and effect of treatment on NGF in atopic dermatitis. J Dermatol Sci 2009;53:48–54. [DOI] [PubMed] [Google Scholar]
- 75.Patel T, Ishiuji Y, Yosipovitch G. Nocturnal itch: why do we itch at night? Acta Derm Venereol 2007;87:295–8. [DOI] [PubMed] [Google Scholar]
- 76.Vaughn AR, Clark AK, Sivamani RK, Shi VY. Circadian rhythm in atopic dermatitis—pathophysiology and implications for chronotherapy. Pediatr Dermatol 2018;35:152–7. [DOI] [PubMed] [Google Scholar]
- 77.Yosipovitch G, Sackett-Lundeen L, Goon A, Yiong Huak C, Leok Goh C, Haus E. Circadian and ultradian (12 h) variations of skin blood flow and barrier function in non-irritated and irritated skin—effect of topical corticosteroids. J Invest Dermatol 2004;122:824–9. [DOI] [PubMed] [Google Scholar]
- 78.Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol 2006;117:418–25. [DOI] [PubMed] [Google Scholar]
- 79.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol 2006; 117:411–7. [DOI] [PubMed] [Google Scholar]
- 80.Trier AM, Kim BS. Cytokine modulation of atopic itch. Curr Opin Immunol 2018;54:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hon KL, Lam MC, Wong KY, Leung TF, Ng PC. Pathophysiology of nocturnal scratching in childhood atopic dermatitis: the role of brain-derived neurotrophic factor and substance P. Br J Dermatol 2007; 157: 922–5. [DOI] [PubMed] [Google Scholar]
- 82.Raap U, Weissmantel S, Gehring M, Eisenberg AM, Kapp A, Folster-Holst R. IL-31 significantly correlates with disease activity and Th2 cytokine levels in children with atopic dermatitis. Pediatr Allergy Immunol 2012;23:285–8. [DOI] [PubMed] [Google Scholar]
- 83.Geiger SS, Fagundes CT, Siegel RM. Chrono-immunology: progress and challenges in understanding links between the circadian and immune systems. Immunology 2015;146:349–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Opp MR. Cytokines and sleep. Sleep Med Rev 2005;9:355–64. [DOI] [PubMed] [Google Scholar]
- 85.Krueger JM, Obál FJ, Fang J, Kubota T, Taishi P. The role of cytokines in physiological sleep regulation. Ann N Y Acad Sci 2001;933:211–21. [DOI] [PubMed] [Google Scholar]
- 86.Arendt J, Skene DJ. Melatonin as a chronobiotic. Sleep Med Rev 2005;9: 25–39. [DOI] [PubMed] [Google Scholar]
- 87.Cagnacci A, Krauchi K, Wirz-Justice A, Volpe A. Homeostatic versus circadian effects of melatonin on core body temperature in humans. J Biol Rhythms 1997;12:509–17. [DOI] [PubMed] [Google Scholar]
- 88.Marseglia L, D’Angelo G, Manti S, Salpietro C, Arrigo T, Barberi I, et al. Melatonin and atopy: role in atopic dermatitis and asthma. Int J Mol Sci 2014; 15:13482–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schwarz W, Birau N, Hornstein OP, Heubeck B, Schonberger A, Meyer C, et al. Alterations of melatonin secretion in atopic eczema. Acta Derm Venereol 1988;68:224–9. [PubMed] [Google Scholar]
- 90.Hon KL, Lam MC, Leung TF, Kam WY, Lee KC, Li MC, et al. Nocturnal wrist movements are correlated with objective clinical scores and plasma chemokine levels in children with atopic dermatitis. Br J Dermatol 2006; 154: 629–35. [DOI] [PubMed] [Google Scholar]
- 91.Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci 2009;29:1820–9. [DOI] [PubMed] [Google Scholar]
- 92.Rosenwasser AM, Turek FW. Neurobiology of circadian rhythm regulation. Sleep Med Clin 2015;10:403–12. [DOI] [PubMed] [Google Scholar]
- 93.Carney CE, Buysse DJ, Ancoli-Israel S, Edinger JD, Krystal AD, Lichstein KL, et al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep 2012;35:287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Buysse DJ, Reynolds CF III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989;28:193–213. [DOI] [PubMed] [Google Scholar]
- 95.Pilz LK, Keller LK, Lenssen D, Roenneberg T. Time to rethink sleep quality: PSQI scores reflect sleep quality on workdays. Sleep 2018;41:zsy029. [DOI] [PubMed] [Google Scholar]
- 96.Smith MT, Wegener ST. Measures of sleep: The Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Care Res 2003;49: S184–96. [Google Scholar]
- 97.Kaaz K, Szepietowski JC, Matusiak Ł. Sleep quality among adult patients with chronic dermatoses. Postepy Dermatol Alergol 2019;36:659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep 2000;23:1043–51. [PubMed] [Google Scholar]
- 99.Lewandowski AS, Toliver-Sokol M, Palermo TM. Evidence-based review of subjective pediatric sleep measures. J Pediatr Psychol 2011;36:780–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sadeh A. A brief screening questionnaire for infant sleep problems: validation and findings for an Internet sample. Pediatrics 2004;113:e570–7. [DOI] [PubMed] [Google Scholar]
- 101.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 1991;14:540–5. [DOI] [PubMed] [Google Scholar]
- 102.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics 2004;114:768–75. [DOI] [PubMed] [Google Scholar]
- 103.Lee JL, Chung Y, Waters E, Vedam H. The Epworth sleepiness scale: reliably unreliable in a sleep clinic population. J Sleep Res 2020;29:e13019. [DOI] [PubMed] [Google Scholar]
- 104.Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2001;2: 297–307. [DOI] [PubMed] [Google Scholar]
- 105.Morin CM, Belleville G, Bélanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep 2011;34:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep 2010;33:781–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, et al. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med 2011;10:6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.van Kooten J, van Litsenburg RRL, Yoder WR, Kaspers GJL, Terwee CB. Validation of the PROMIS Sleep Disturbance and Sleep-Related Impairment item banks in Dutch adolescents. Qual Life Res 2018;27:1911–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arora T, Broglia E, Pushpakumar D, Lodhi T, Taheri S. An investigation into the strength of the association and agreement levels between subjective and objective sleep duration in adolescents. PLoS One 2013;8: e72406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Short MA, Gradisar M, Lack LC, Wright H, Carskadon MA. The discrepancy between actigraphic and sleep diary measures of sleep in adolescents. Sleep Med 2012;13:378–84. [DOI] [PubMed] [Google Scholar]
- 111.Vakharia PP, Cella D, Silverberg JI. Patient-reported outcomes and quality of life measures in atopic dermatitis. Clin Dermatol 2018;36:616–30. [DOI] [PubMed] [Google Scholar]
- 112.van Geel MJ, Maatkamp M, Oostveen AM, de Jong EM, Finlay AY, van de Kerkhof PC, et al. Comparison of the Dermatology Life Quality Index and the Children’s Dermatology Life Quality Index in assessment of quality of life in patients with psoriasis aged 16-17 years. Br J Dermatol 2016;174: 152–7. [DOI] [PubMed] [Google Scholar]
- 113.Heinl D, Prinsen CA, Drucker AM, Ofenloch R, Humphreys R, Sach T, et al. Measurement properties of quality of life measurement instruments for infants, children and adolescents with eczema: protocol for a systematic review. Syst Rev 2016;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kayyali HA, Weimer S, Frederick C, Martin C, Basa D, Juguilon JA, et al. Remotely attended home monitoring of sleep disorders. Telemed J E Health 2008;14:371–4. [DOI] [PubMed] [Google Scholar]
- 115.Ebata T, Aizawa H, Kamide R. An infrared video camera system to observe nocturnal scratching in atopic dermatitis patients. J Dermatol 1996;23:153–5. [DOI] [PubMed] [Google Scholar]
- 116.Tenhunen M, Elomaa E, Sistonen H, Rauhala E, Himanen SL. Emfit movement sensor in evaluating nocturnal breathing. Respir Physiol Neurobiol 2013;187:183–9. [DOI] [PubMed] [Google Scholar]
- 117.Merilahti J, Saarinen A, Parkka J, Antila K, Mattila E, Korhonen I. Long-term subjective and objective sleep analysis of total sleep time and sleep quality in real life settings. Conf Proc IEEE Eng Med Biol Soc 2007;2007:5202–5. [DOI] [PubMed] [Google Scholar]
- 118.Pallin M, O’Hare E, Zaffaroni A, Boyle P, Fagan C, Kent B, et al. Comparison of a novel non-contact biomotion sensor with wrist actigraphy in estimating sleep quality in patients with obstructive sleep apnoea. J Sleep Res 2014;23: 475–84. [DOI] [PubMed] [Google Scholar]
- 119.De Chazal P, Fox N, O’Hare E, Heneghan C, Zaffaroni A, Boyle P, et al. Sleep/wake measurement using a non-contact biomotion sensor. J Sleep Res 2011;20:356–66. [DOI] [PubMed] [Google Scholar]
- 120.Griessenberger H, Heib DP, Kunz AB, Hoedlmoser K, Schabus M. Assessment of a wireless headband for automatic sleep scoring. Sleep Breath 2013; 17:747–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhuo Z, Cuntai G, Ti Eu C, Juanhong Y, Ng AK, Haihong Z, et al. Automatic sleep onset detection using single EEG sensor. Conf Proc IEEE Eng Med Biol Soc 2014;2014:2265–8. [DOI] [PubMed] [Google Scholar]
- 122.Tonetti L, Cellini N, de Zambotti M, Fabbri M, Martoni M, Fabregas SE, et al. Polysomnographic validation of a wireless dry headband technology for sleep monitoring in healthy young adults. Physiol Behav 2013;118:185–8. [DOI] [PubMed] [Google Scholar]
- 123.Gonzalez-Lopez G, Ceballos-Rodriguez RM, Gonzalez-Lopez JJ, Feito Rodriguez M, Herranz-Pinto P. Efficacy and safety of wet wrap therapy for patients with atopic dermatitis: a systematic review and meta-analysis. Br J Dermatol 2017;177:688–95. [DOI] [PubMed] [Google Scholar]
- 124.Nicol NH, Boguniewicz M, Strand M, Klinnert MD. Wet wrap therapy in children with moderate to severe atopic dermatitis in a multidisciplinary treatment program. J Allergy Clin Immunol Pract 2014;2:400–6. [DOI] [PubMed] [Google Scholar]
- 125.Abeck D, Brockow K, Mempel M, Fesq H, Ring J. Behandlung des akut exazerbierten atopischen Ekzems mit fett-feuchten Verbanden und topischem Chlorhexidin. Der Hautarzt 1999;50:418–21. [DOI] [PubMed] [Google Scholar]
- 126.Doss N, Kamoun MR, Dubertret L, Cambazard F, Remitz A, Lahfa M, et al. Efficacy of tacrolimus 0.03% ointment as second-line treatment for children with moderate-to-severe atopic dermatitis: evidence from a randomized, double-blind non-inferiority trial vs. fluticasone 0.005% ointment. Pediatr Allergy Immunol 2010;21:321–9. [DOI] [PubMed] [Google Scholar]
- 127.Sugarman JL, Parish LC. Efficacy of a lipid-based barrier repair formulation in moderate-to-severe pediatric atopic dermatitis. J Drugs Dermatol 2009;8: 1106–11. [PubMed] [Google Scholar]
- 128.Leo HL, Bender BG, Leung SB, Tran ZV, Leung DY. Effect of pimecrolimus cream 1% on skin condition and sleep disturbance in children with atopic dermatitis. J Allergy Clin Immunol 2004;114:691–3. [DOI] [PubMed] [Google Scholar]
- 129.Simpson EL, Paller AS, Boguniewicz M, Eichenfield LF, Feldman SR, Silverberg JI, et al. Crisaborole ointment improves quality of life of patients with mild to moderate atopic dermatitis and their families. Dermatol Ther (Heidelb) 2018;8:605–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim BS, Sun K, Papp K, Venturanza M, Nasir A, Kuligowski ME. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, dose-ranging, vehicle- and active-controlled study. J Am Acad Dermatol 2020;82:1305–13. [DOI] [PubMed] [Google Scholar]
- 131.Harper JI, Ahmed I, Barclay G, Lacour M, Hoeger P, Cork MJ, et al. Cyclosporin for severe childhood atopic dermatitis: short course versus continuous therapy. Br J Dermatol 2000;142:52–8. [DOI] [PubMed] [Google Scholar]
- 132.Czech W, Brautigam M, Weidinger G, Schopf E. A body-weight-independent dosing regimen of cyclosporine microemulsion is effective in severe atopic dermatitis and improves the quality of life. J Am Acad Dermatol 2000;42: 653–9. [PubMed] [Google Scholar]
- 133.Weatherhead SC, Wahie S, Reynolds NJ, Meggitt SJ. An open-label, dose-ranging study of methotrexate for moderate-to-severe adult atopic eczema. Br J Dermatol 2007;156:346–51. [DOI] [PubMed] [Google Scholar]
- 134.Berth-Jones J, Takwale A, Tan E, Barclay G, Agarwal S, Ahmed I, et al. Azathioprine in severe adult atopic dermatitis: a double-blind, placebo-controlled, crossover trial. Br J Dermatol 2002;147:324–30. [DOI] [PubMed] [Google Scholar]
- 135.de Bruin-Weller M, Thaci D, Smith CH, Reich K, Cork MJ, Radin A, et al. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol 2018; 178: 1083–101. [DOI] [PubMed] [Google Scholar]
- 136.Tsianakas A, Luger TA, Radin A. Dupilumab treatment improves quality of life in adult patients with moderate-to-severe atopic dermatitis: results from a randomized, placebo-controlled clinical trial. Br J Dermatol 2018; 178:406–14. [DOI] [PubMed] [Google Scholar]
- 137.Simpson EL, Gadkari A, Worm M, Soong W, Blauvelt A, Eckert L, et al. Dupilumab therapy provides clinically meaningful improvement in patient-reported outcomes (PROs): a phase IIb, randomized, placebo-controlled, clinical trial in adult patients with moderate to severe atopic dermatitis (AD). J Am Acad Dermatol 2016;75:506–15. [DOI] [PubMed] [Google Scholar]
- 138.Tofte SJ, Papp K, Sadick N, Bohnert K, Simpson E, Thaci D, et al. Efficacy and safety of dupilumab for the treatment of moderate-to-severe atopic dermatitis in adults: a pooled analysis of two phase 2 clinical trials. J Am Assoc Nurse Pract 2018;30:529–41. [DOI] [PubMed] [Google Scholar]
- 139.Cork MJ, Eckert L, Simpson EL, Armstrong A, Barbarot S, Puig L, et al. Dupilumab improves patient-reported symptoms of atopic dermatitis, symptoms of anxiety and depression, and health-related quality of life in moderate-to-severe atopic dermatitis: analysis of pooled data from the randomized trials SOLO 1 and SOLO 2. J Dermatolog Treat 2020;31: 606–14. [DOI] [PubMed] [Google Scholar]
- 140.Simpson EL, Flohr C, Eichenfield LF, Bieber T, Sofen H, Taïeb A, et al. Efficacy and safety of lebrikizumab (an anti-IL-13 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by topical corticosteroids: a randomized, placebo-controlled phase II trial (TREBLE). J Am Acad Dermatol 2018;78:863–871.e11. [DOI] [PubMed] [Google Scholar]
- 141.Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour JP, et al. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2). Br J Dermatol 2021;184:437–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Werfel T, Layton G, Yeadon M, Whitlock L, Osterloh I, Jimenez P, et al. Efficacy and safety of the histamine H4 receptor antagonist ZPL-3893787 in patients with atopic dermatitis. J Allergy Clin Immunol 2019; 143: 1830–1837. e4. [DOI] [PubMed] [Google Scholar]
- 143.Chang YS, Lin MH, Lee JH, Lee PL, Dai YS, Chu KH, et al. Melatonin supplementation for children with atopic dermatitis and sleep disturbance: a randomized clinical trial. JAMA Pediatr 2016;170:35–42. [DOI] [PubMed] [Google Scholar]
- 144.Buscemi N, Vandermeer B, Hooton N, Pandya R, Tjosvold L, Hartling L, et al. Efficacy and safety of exogenous melatonin for secondary sleep disorders and sleep disorders accompanying sleep restriction: meta-analysis. BMJ 2006;332: 385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Owens JA, Babcock D, Blumer J, Chervin R, Ferber R, Goetting M, et al. The use of pharmacotherapy in the treatment of pediatric insomnia in primary care: rational approaches. A consensus meeting summary. J Clin Sleep Med 2005;1: 49–59. [PubMed] [Google Scholar]
- 146.Kelsay K Management of sleep disturbance associated with atopic dermatitis. J Allergy Clin Immunol 2006;118:198–201. [DOI] [PubMed] [Google Scholar]
- 147.Richardson GS, Roehrs TA, Rosenthal L, Koshorek G, Roth T. Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol 2002; 22:511–5. [DOI] [PubMed] [Google Scholar]
- 148.Patel D, Levoska M, Shwayder T. Managing sleep disturbances in children with atopic dermatitis. Pediatr Dermatol 2018;35:428–33. [DOI] [PubMed] [Google Scholar]
- 149.He A, Feldman SR, Fleischer AB Jr. An assessment of the use of antihistamines in the management of atopic dermatitis. J Am Acad Dermatol 2018;79: 92–6. [DOI] [PubMed] [Google Scholar]
- 150.Munday J, Bloomfield R, Goldman M, Robey H, Kitowska GJ, Gwiezdziski Z, et al. Chlorpheniramine is no more effective than placebo in relieving the symptoms of childhood atopic dermatitis with a nocturnal itching and scratching component. Dermatology 2002;205:40–5. [DOI] [PubMed] [Google Scholar]
- 151.Ebata T, Izumi H, Aizawa H, Kamide R, Niimura M. Effects of nitrazepam on nocturnal scratching in adults with atopic dermatitis: a double-blind placebo-controlled crossover study. Br J Dermatol 1998;138:631–4. [DOI] [PubMed] [Google Scholar]
- 152.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ 2000; 162: 225–33. [PMC free article] [PubMed] [Google Scholar]
- 153.Genois A, Haig M, Des Roches A, Sirard A, Le May S, McCuaig CC. Case report of atopic dermatitis with refractory pruritus markedly improved with the novel use of clonidine and trimeprazine. Pediatr Dermatol 2014;31:76–9. [DOI] [PubMed] [Google Scholar]
- 154.Hedman-Lagerlof E, Bergman A, Lindefors N, Bradley M. Exposure-based cognitive behavior therapy for atopic dermatitis: an open trial. Cogn Behav Ther 2019;48:300–10. [DOI] [PubMed] [Google Scholar]
- 155.Bae BG, Oh SH, Park CO, Noh S, Noh JY, Kim KR, et al. Progressive muscle relaxation therapy for atopic dermatitis: objective assessment of efficacy. Acta Derm Venereol 2012;92:57–61. [DOI] [PubMed] [Google Scholar]
- 156.LeBovidge JS, Elverson W, Timmons KG, Hawryluk EB, Rea C, Lee M, et al. Multidisciplinary interventions in the management of atopic dermatitis. J Allergy Clin Immunol 2016;138:325–34. [DOI] [PubMed] [Google Scholar]
- 157.Becker D, Langer E, Seemann M, Seemann G, Fell I, Saloga J, et al. Clinical efficacy of blue light full body irradiation as treatment option for severe atopic dermatitis. PLoS One 2011;6:e20566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tan HY, Lenon GB, Zhang AL, Xue CC. Efficacy of acupuncture in the management of atopic dermatitis: a systematic review. Clin Exp Dermatol 2015;40:711–5. quiz 5-6. [DOI] [PubMed] [Google Scholar]
- 159.Kang S, Kim YK, Yeom M, Lee H, Jang H, Park HJ, et al. Acupuncture improves symptoms in patients with mild-to-moderate atopic dermatitis: a randomized, sham-controlled preliminary trial. Complement Ther Med 2018; 41:90–8. [DOI] [PubMed] [Google Scholar]
- 160.Park JG, Park HJ, Chae Y, Kim YK, Lee H, Kim K. Acupuncture treatment for symptom management in atopic dermatitis: a study protocol for a randomized, participant- and assessor-blind, sham-controlled trial. Evid Based Complement Alternat Med 2019;2019:1907578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Simpson EL, Bieber T, Guttman-Yassky E, Beck LA, Blauvelt A, Cork MJ, et al. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med 2016;375:2335–48. [DOI] [PubMed] [Google Scholar]
- 162.Guttman-Yassky E, Silverberg JI, Nemoto O, Forman SB, Wilke A, Prescilla R, et al. Baricitinib in adult patients with moderate-to-severe atopic dermatitis: a phase 2 parallel, double-blinded, randomized placebo-controlled multiple-dose study. J Am Acad Dermatol 2019;80:913–921.e9. [DOI] [PubMed] [Google Scholar]
- 163.Simpson EL, Lacour JP, Spelman L, Galimberti R, Eichenfield LF, Bissonnette R, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol 2020;183: 242–55. [DOI] [PubMed] [Google Scholar]
- 164.Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol 2020; 156: 863–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Oetjen LK, Mack MR, Feng J, Whelan TM, Niu H, Guo CJ, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell 2017;171:217–228.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med 2017;376:826–35. [DOI] [PubMed] [Google Scholar]
- 167.Kabashima K, Furue M, Hanifin JM, Pulka G, Wollenberg A, Galus R, et al. Nemolizumab in patients with moderate-to-severe atopic dermatitis: randomized, phase II, long-term extension study. J Allergy Clin Immunol 2018; 142: 1121–1130.e7. [DOI] [PubMed] [Google Scholar]
- 168.Kabashima K, Matsumura T, Komazaki H, Kawashima M. Trial of nemolizumab and topical agents for atopic dermatitis with pruritus. N Engl J Med 2020;383:141–50. [DOI] [PubMed] [Google Scholar]
- 169.Wollenberg A, Howell MD, Guttman-Yassky E, Silverberg JI, Kell C, Ranade K, et al. Treatment of atopic dermatitis with tralokinumab, an anti-IL-13 mAb. J Allergy Clin Immunol 2019;143:135–41. [DOI] [PubMed] [Google Scholar]
- 170.Simpson EL, Parnes JR, She D, Crouch S, Rees W, Mo M, et al. Tezepelumab, an anti—thymic stromal lymphopoietin monoclonal antibody, in the treatment of moderate to severe atopic dermatitis: a randomized phase 2a clinical trial. J Am Acad Dermatol 2019;80:1013–21. [DOI] [PubMed] [Google Scholar]
- 171.Pigeon WR. Treatment of adult insomnia with cognitive-behavioral therapy. J Clin Psychol 2010;66:1148–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med 2016;165:125–33. [DOI] [PubMed] [Google Scholar]
- 173.Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res 2017;26:675–700. [DOI] [PubMed] [Google Scholar]
- 174.Mysliwiec V, Martin JL, Ulmer CS, Chowdhuri S, Brock MS, Spevak C, et al. The Management of Chronic Insomnia Disorder and Obstructive Sleep Apnea: Synopsis of the 2019 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guidelines. Ann Intern Med 2020; 172: 325–36. [DOI] [PubMed] [Google Scholar]
- 175.Smith MT, Perlis ML, Park A, Smith MS, Pennington J, Giles DE, et al. Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry 2002;159:5–11. [DOI] [PubMed] [Google Scholar]
- 176.Owens JA. Pediatric insomnia. Sleep Med Clin 2006;1:423–35. [Google Scholar]
- 177.Morgenthaler TI, Owens J, Alessi C, Boehlecke B, Brown TM, Coleman J Jr, et al. Practice parameters for behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep 2006;29:1277–81. [PubMed] [Google Scholar]
- 178.Blake MJ, Sheeber LB, Youssef GJ, Raniti MB, Allen NB. Systematic review and meta-analysis of adolescent cognitive-behavioral sleep interentions. Clin Child Fam Psychol Rev 2017;20:227–49. [DOI] [PubMed] [Google Scholar]
- 179.Okajima I, Inoue Y. Efficacy of cognitive behavioral therapy for comorbid insomnia: a meta-analysis. Sleep Biol Rhythms 2018;16:21–35. [Google Scholar]
- 180.Edinger JD, Wohlgemuth WK, Radtke RA, Coffman CJ, Carney CE. Dose-response effects of cognitive-behavioral insomnia therapy: a randomized clinical trial. Sleep 2007;30:203–12. [DOI] [PubMed] [Google Scholar]
- 181.Pigeon WR, Funderburk JS, Cross W, Bishop TM, Crean HF. Brief CBT for insomnia delivered in primary care to patients endorsing suicidal ideation: a proof-of-concept randomized clinical trial. Transl Behav Med 2019;9: 1169–77. [DOI] [PubMed] [Google Scholar]
- 182.Perlis ML, Jungquist C, Smith MT, Posner D. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. New York: Springer; 2006. [Google Scholar]
- 183.Sidani S, Epstein DR, Fox M, Collins L. Comparing the effects of single- and multiple-component therapies for insomnia on sleep outcomes. Worldviews Evid Based Nurs 2019;16:195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zachariae R, Lyby MS, Ritterband LM, O’Toole MS. Efficacy of internet-delivered cognitive-behavioral therapy for insomnia—a systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev 2016;30: 1–10. [DOI] [PubMed] [Google Scholar]
- 185.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN, Heald JL. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2017;13:307–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Auger RR, Burgess HJ, Emens JS, Deriy LV, Thomas SM, Sharkey KM. Clinical practice guideline for the treatment of intrinsic circadian rhythm sleep-wake disorders: advanced sleep-wake phase disorder (ASWPD), delayed sleep-wake phase disorder (DSWPD), non-24-hour sleep-wake rhythm disorder (N24SWD), and irregular sleep-wake rhythm disorder (ISWRD). An Update for 2015: An American Academy of Sleep Medicine Clinical Practice Guideline. J Clin Sleep Med 2015;11:1199–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep 2010;33:1605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Andersen LP, Gogenur I, Rosenberg J, Reiter RJ. The safety of melatonin in humans. Clin Drug Investig 2016;36:169–75. [DOI] [PubMed] [Google Scholar]
- 189.Lavigne JE, Hur K, Kane C, Au A, Bishop TM, Pigeon WR. Prescription medications for the treatment of insomnia and risk of suicide attempt: a comparative safety study. J Gen Intern Med 2019;34:1554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Karimkhani C, Dellavalle RP, Coffeng LE, Flohr C, Hay RJ, Langan SM, et al. Global skin disease morbidity and mortality: an update from the Global Burden of Disease Study 2013. JAMA Dermatol 2017; 153:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]