Abstract
Simple Summary
Neurotoxicity is an on-target side effect of GD2-directed immunotherapy due to the expression of GD2 on healthy cells. Patients with high-risk neuroblastoma who receive treatment with anti-GD2 immunotherapy, therefore, require close observation and supportive management to improve treatment tolerance and avoid the persistence of neurological symptoms. This study reports on the incidence, clinical course and management of patients who experienced neurotoxicity due to treatment with the anti-GD2 antibody dinutuximab beta, given with or without interleukin-2, in two clinical trials. The majority of severe neurotoxic events were observed in patients treated with dinutuximab beta combined with interleukin-2, with most patients recovering following supportive management. Given the increased risk of neurotoxic events and the lack of clinical benefit reported for the combination treatment in clinical trials, adding interleukin-2 to dinutuximab beta therapy is not recommended. The clinical experiences described here may aid clinicians in managing neurotoxicity associated with dinutuximab beta more effectively.
Abstract
Neurotoxicity is an off-tumour, on-target side effect of GD2-directed immunotherapy with monoclonal antibodies. Here, we report the frequency, management and outcome of patients enrolled in two prospective clinical trials who experienced severe neurotoxicity during immunotherapy with the anti-GD2 antibody dinutuximab beta (DB) administered as short-term infusion (HR-NBL1/SIOPEN study, randomisation R2, EudraCT 2006-001489-17) or as long-term infusion (HR-NBL1/SIOPEN study, randomisation R4, EudraCT 2006-001489-17 and LTI/SIOPEN study, EudraCT 2009-018077-31), either alone or with subcutaneous interleukin-2 (scIL-2). The total number of patients included in this analysis was 1102. Overall, 44/1102 patients (4.0%) experienced Grade 3/4 neurotoxicities (HR-NBL1 R2, 21/406; HR-NBL1 R4, 8/408; LTI study, 15/288), including 27 patients with severe neurotoxicities (2.5%). Events occurred predominantly in patients receiving combined treatment with DB and scIL-2. Neurotoxicity was treated using dexamethasone, prednisolone, intravenous immunoglobulins and, in two patients, plasmapheresis, which was highly effective. While neurological recovery was observed in 16 of 21 patients with severe neurotoxicities, 5/1102 (0.45%) patients experienced persistent and severe neurological deficits. In conclusion, severe neurotoxicity is most commonly observed in patients receiving DB with scIL-2. Considering the lack of clinical benefit for IL-2 in clinical trials so far, the administration of IL-2 alongside DB is not recommended.
Keywords: neuroblastoma, anti-GD2 antibody, dinutuximab beta, neurotoxicity
1. Introduction
The promising results of clinical trials with anti-GD2 monoclonal antibodies in patients with high-risk neuroblastoma led to the introduction of immunotherapy as the standard of care in the maintenance phase of first-line treatment settings [1,2,3,4,5,6]. Immunotherapy with anti-GD2 antibodies is administered either as monotherapy or in combination with cytokines, following induction chemotherapy and consolidation therapy with high-dose chemotherapy and autologous stem cell rescue [1,3]. In the ANBL0032 study of the Children’s Oncology Group (COG), treatment with dinutuximab, a human/mouse chimeric anti-GD2 antibody produced in SP2/0 cells (ch14.18) [7], resulted in improved survival in patients with high-risk neuroblastoma when given in combination with granulocyte-macrophage colony-stimulating factor (GM-CSF) and intravenous (i.v.) interleukin-2 (IL-2) in alternating cycles [3]. In addition, two clinical trials conducted by the International Society of Paediatric Oncology European Neuroblastoma Group (SIOPEN) also demonstrated a benefit for patients with high-risk neuroblastoma treated with dinutuximab beta (DB), a similar anti-GD2 antibody produced in Chinese hamster ovary (CHO) cells (ch14.18/CHO) [1,2,8,9,10]. Short-term (8 h for 5 days) or long-term infusion (LTI; continuous infusion over 10 days) of DB with or without subcutaneous IL-2 (scIL-2) improved survival in the first-line maintenance setting (HR-NBL1/SIOPEN study) [1,2,8] as well as in patients with relapsed and refractory neuroblastoma (LTI/SIOPEN study) [9,10]. Although effective, the therapy is commonly associated with significant side effects, such as neuropathic pain, capillary leak syndrome and allergic reactions. Most of the side effects can be managed using appropriate prophylaxis and adequate supportive treatment during immunotherapy [11].
Neurotoxicity has also been reported in association with anti-GD2 immunotherapy [1,8,11]. While it is a relatively uncommon side effect, it specifically relates to the target antigen GD2 [1,6,8,11]. Although GD2 is a well-recognised tumour-associated antigen, its expression is not restricted to neuroectodermal tumours such as neuroblastoma, but can also be found on healthy tissues, in particular those of neuronal origin [7,12]. The expression of GD2 in the central nervous system (CNS) and the peripheral nervous system (PNS) results in on-target/off-tumour side effects when GD2-directed immunotherapies are administered [6,13,14]. One well known off-tumour side effect of anti-GD2 antibody therapy is the induction of neuropathic pain [11]. In animal models, which approximate the pain associated with anti-GD2 antibodies in humans in terms of timing and quality, anti-GD2-specific binding to Aδ and C pain fibers results in decreased mechanical stimulus thresholds [15]. Therefore, clinical use of anti-GD2 antibody therapy requires intensive co-administration of analgesic drugs, including i.v. morphine, to make this treatment tolerable [11].
On-target/off-tumour side effects associated with the expression of GD2 in the CNS may be more severe than in the PNS. In particular, the occurrence of transverse myelitis associated with paraplegia has been described in patients receiving anti-GD2 antibody therapy [11,16]. Given that anti-GD2 antibody therapy for high-risk neuroblastoma has been developed in combination with cytokines, in particular IL-2 [1,3], it is important to note that uncommon, but severe, neurotoxicity has also been reported for IL-2 therapy alone, including coma, convulsions and paralysis (≥1/1000 to <1/100 patients) [17]. Other side effects include changes in mental status, ataxia, and blindness, with evidence of demyelination as a radiological finding [17,18,19]. There are also reports of permanent neurological defects. More common side effects associated with IL-2 therapy are dizziness, paresthesia and somnolence, followed by neuropathy and lethargy [17].
Here, we report the incidence, clinical course and management of severe neurotoxicity observed in patients with high-risk neuroblastoma treated with DB with and without scIL-2 in two prospective SIOPEN trials (LTI study and HR-NBL1 study Randomisation 2 and 4 (R2 and R4)).
2. Materials and Methods
2.1. Patients
The occurrence and management of neurotoxicity was assessed in patients with high-risk neuroblastoma enrolled in two prospective SIOPEN studies: HR-NBL1/SIOPEN [1,8] and LTI/SIOPEN [10]. This analysis includes all 814 patients of the R2 and R4 phases of the HR-NBL1/SIOPEN study and all 288 patients enrolled in the LTI/SIOPEN study.
2.2. Study Designs
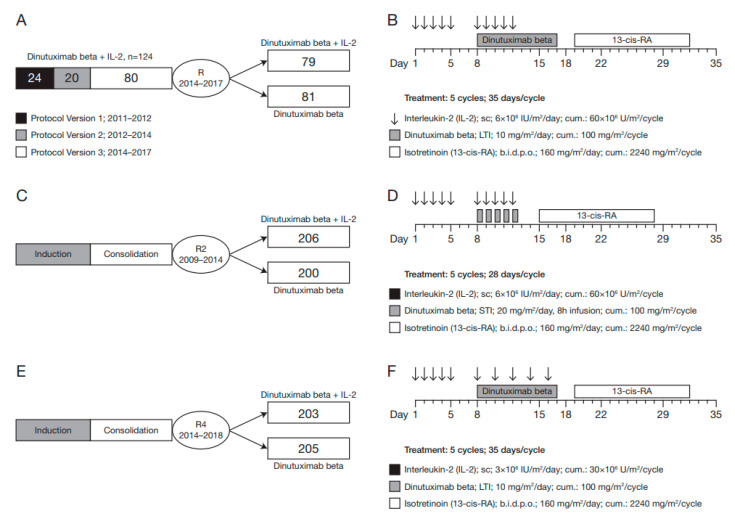
The LTI/SIOPEN study (EudraCT 2009-018077-31) is a prospective Phase II trial in patients with relapsed and refractory high-risk neuroblastoma (Figure 1A,B) [10]. The trial started as a single-arm study, where patients were treated with DB in combination with scIL-2, and was later amended to a randomised study, where patients received DB with or without scIL-2 [10]. The treatment regimen in the LTI/SIOPEN study included scIL-2 given once a day for five days (Days 1–5, 6 × 106 IU/m2/day), followed by combined administration of scIL-2 once a day (Days 8–12, 6 × 106 IU/m2/day) and a 10-day continuous i.v. infusion of DB (Days 8–17 10 mg/m2/day) [10]. Oral isotretinoin was given for 2 weeks following DB infusion (Days 19–32, 160 mg/m2/day) [10]. Patients received up to five 35-day treatment cycles [10]. In the randomised phase of the trial, the treatment schedule for DB, scIL-2 and isotretinoin remained unchanged but was tested against a treatment arm without scIL-2 [10].
Figure 1.
Schematic overview of study designs and treatment schedules in the LTI/SIOPEN study and the HR-NBL1/SIOPEN study. (A,B) Study design and treatment schedule of the LTI study. The study was initiated as a single-arm study of DB combined with scIL-2 in patients with relapsed/refractory high-risk neuroblastoma and amended in 2014 to include a randomizat design. Patients received either DB alone or DB combined with scIL-2. DB was administered as LTI (10 mg/m2 continuous infusion over 10 days; total dose 100 mg/m2). (C,D) Study design and treatment schedule of HR-NBL1-R2. Newly diagnosed patients with high-risk neuroblastoma were randomizat in the maintenance treatment phase to receive either DB alone or DB combined with scIL-2. DB was administered as STI (20 mg/m2/day on 5 consecutive days, 8 h infusions; total dose 100 mg/m2). (E,F) Study design and treatment schedule of HR-NBL1-R4. The study was amended to evaluate LTI of DB and a dose-reduced relaxed schedule of scIL-2. B.i.d.p.o., twice-daily oral administration; DB, dinutuximab beta; LTI, long-term infusion; R, randomization; RA, retinoic acid; scIL-2, subcutaneous interleukin-2; STI, short-term infusion.
The HR-NBL-1/SIOPEN study (EudraCT 2006-001489-17) is a prospective Phase III trial in patients with newly diagnosed high-risk neuroblastoma and includes four completed randomisations (R1–4), which are described elsewhere [1,8,20,21,22]. In the randomisation phase 2 (R2, Figure 1C,D), patients received DB as short-term infusion (STI) consisting of 20 mg/m2/day given as 8 h infusion on 5 consecutive days (Days 8–12) either with or without scIL-2 (Days 1–5 and Days 8–12; 6 × 106 IU/m2/day) [1]. Oral isotretinoin was given for 2 weeks (Days 15–28; 160 mg/m2/day). All patients received up to five 35-day cycles [1]. In the randomisation phase 4 (R4, Figure 1E,F), the infusion schedule of DB was changed to 10 days continuous infusion (Days 8–17; 10 mg/m2/day), with the same cumulative dosage of 100 mg/m2 per cycle as the STI [8]. The total dose of scIL-2 was reduced to 30 × 106 IU/m2/cycle, which was administered in a relaxed schedule during the combination treatment with DB (Days 1−5, 8, 10, 12, 14; 16; 3 × 106 IU/m2/day) [8]. The cumulative dosage of isotretinoin remained the same, but it was given on Days 19–32 of the treatment cycle [8].
Both trials stopped recruitment, and results of HR-NBL1-R2 were reported by Ladenstein et al. [1] The LTI study and the R4 phase of HR-NBL1 are currently in the follow-up phase awaiting data maturity to report activity and efficacy endpoints, with early results communicated in 2019 [8,10].
2.3. Assessments
All data on Grade 3/4 adverse events classified as neurotoxicity according to the Common Terminology Criteria for Adverse Events (CTCAE) were collected from both trials and analysed. A subgroup of patients who experienced severe CNS neurotoxicity underwent more detailed analysis. Criteria for severe CNS neurotoxicity are shown in Table 1. For the purpose of this report, we focused on severe CNS neurotoxicity with substantial and prolonged neurological deficits observed among patients with Grade 3/4 toxicity and causal relationship to DB. Severe DB-related CNS neurotoxicity was defined by the occurrence of clinical symptoms of the CNS without any other detectable reason. If radiological imaging was performed, results had to be consistent with the clinical findings, including the presence of CNS inflammation and/or demyelination (Table 1). Patients with severe pain and ophthalmoplegia occurring without any additional neurological sensory or motor dysfunction were not included as severe CNS neurotoxicity in this report.
Table 1.
Criteria of severe CNS neurotoxicity.
| Clinical Symptoms of CNS Dysfunction | Radiological Signs |
|---|---|
|
|
| |
| |
| |
| |
|
CNS, central nervous system.
3. Results
Overall, 44 of the 1102 patients (4.0%) included in the two SIOPEN trials experienced Grade 3/4 neurotoxicities, with 27/1102 (2.45%) patients fulfilling the criteria of severe CNS neurotoxicity (Table 1).
The majority of patients recovered, namely, 33 of 38 (86.8%), including 16 patients presenting with severe CNS neurotoxicity; data were not available for six patients. Only 5/1102 (0.45%) patients presented with persistent and severe neurological deficits. The distribution and outcomes of all patients with Grade 3/4 neurotoxicity are shown in Table 2 and the clinical characteristics and management of all patients with severe CNS neurotoxicity are shown in Table 3. Case descriptions and therapeutic interventions are provided in the Supplemental Materials.
Table 2.
Distribution and outcome of patients with severe neurotoxicity in the LTI and HR-NBL1 (R2 and R4) study.
| Patients, n | LTI Study | HR-NBL1 (R2) | HR-NBL1 (R4) | Total |
|---|---|---|---|---|
| Enrolled | 288 | 406 | 408 | 1102 |
| Gr 3/4 neurotoxicity * | 15 | 21 | 8 | 44 |
| with DB | 0 | 4 | 5 | 9 |
| with DB + scIL-2 | 15 | 17 | 3 | 35 |
| Severe CNS neurotoxicity # | 10 | 9 | 8 | 27 |
| with DB | 0 | 1 | 5 | 6 |
| with DB + scIL-2 | 10 | 8 | 3 | 19 |
| Recovery | 10 | 17 | 6 | 33 † |
| Persistent severe neurotoxicity | 3 | 2 | 0 | 5 |
* All neurotoxicity events reported according to CTCAE. # Severe neurotoxicity according to the definition outlined in Table 1; † Data were missing for 6 patients with severe CNS neurotoxicity. DB, dinutuximab beta; CNS, central nervous system; CTCAE, Common Terminology Criteria for Adverse Events; Gr, grade; R, randomisation; scIL-2, subcutaneous interleukin-2.
Table 3.
Clinical characteristics and management of severe CNS neurotoxicity in the LTI study and the HR-NBL1 study.
| Pt | Study | Schedule | Time of Onset |
Symptoms | MRI Findings | CSF | HSV/VZV | DB Level (Serum) * | DB Level (CSF) | Treatment | Rechallenge with DB |
Symptom Resolution |
MRI Follow-Up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | LTI | DB LTI + scIL-2 |
C1/D13 |
|
Cytotoxic oedema in brain stem |
ND | No | 2.5 µg/mL | ND |
|
No | No (minimal improvement) |
NA |
| 2 | LTI | DB LTI + scIL-2 |
C1/D14 |
|
Myelitis (thoracic to lumbar) |
|
Yes (thoracic shingles 6 wks before therapy) |
4.3 µg/mL | ND |
|
No | No | Partial resolution after 8 months |
| 3 | LTI | DB LTI + scIL-2 |
C1/D15 |
|
Myelitis (thoracic) |
|
Yes (skull skin shingles after the first round of scIL-2 during C1/D1–D5) |
5.8 µg/mL | ND |
|
No | Yes | Complete resolution |
| 4 | LTI | DB LTI + IL-2 |
C3/D17 |
|
Myelitis (thoracic) |
|
Yes (HSV 3 wks before DB) |
14.8 µg/mL | 0.02 µg/mL |
|
No | Yes | Complete resolution |
| 5 | LTI | DB LTI + scIL-2 |
C1/D15 |
|
Demyelinating neuropathy of dorsal roots |
|
No | 4.6 µg/mL | <0.01 µg/mL |
|
Yes (symptoms reoccurred after 4 h at 50% dose) |
Yes | NA |
| 6 | LTI | DB LTI + scIL-2 |
C1/D9 |
|
Myelitis | ND | No | ND | <0.01 µg/mL |
|
No | No (persistent neurogenic bladder) |
NA |
| 7 | LTI | DB LTI + scIL-2 |
C1/D9 |
|
ND | ND | ND | ND | ND | No treatment | No | Yes | NA |
| 8 | LTI | DB LIT + scIL-2 |
C2/D11 |
|
ND | ND | ND | 2.0 µg/mL | ND | No treatment | No | Yes | NA |
| 9 | LTI | DB LTI + scIL-2 |
ND |
|
ND | ND | ND | 14.9 µg/mL | ND | ND | ND | ND | ND |
| 10 | LTI | DB LTI + scIL-2 |
ND |
|
ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 11 | HR-NBL1R2 | DB STI + scIL-2 |
C4/D10 |
|
Encephalomyelitis | ND | ND | ND | ND |
|
No | No | NA |
| 12 | HR-NBL1R2 | DB STI | C2/D7 |
|
PRES | ND | ND | ND | ND |
|
Yes (no recurrence) |
Yes | Complete resolution |
| 13 | HR-NBL1R2 | DB STI + scIL-2 |
C2/D3 |
|
ND | ND | ND | ND | ND |
|
ND | Yes | NA |
| 14 | HR-NBL1R2 | DB STI + scIL-2 |
C1/D5 |
|
ND | ND | ND | ND | ND |
|
Yes (no recurrence) |
Yes | NA |
| 15 | HR-NBL1R2 | DB STI + scIL-2 |
C1/ND |
|
ND | ND | ND | ND | ND | ND | Yes (only DB, no scIL-2; no recurrence) | Yes | NA |
| 16 | HR-NBL1R2 | DB STI + scIL-2 |
C1/D9 |
|
ND | ND | ND | ND | ND | ND | ND | ND | NA |
| 17 | HR-NBL1R2 | DB STI + scIL-2 |
C1/D9 |
|
ND | ND | ND | ND | ND | No treatment | ND | Yes (child needs glasses) |
NA |
| 18 | HR-NBL1R2 | DB STI + scIL-2 |
C1/D15 |
|
PRES | ND | ND | ND | ND | ND | ND | ND | NA |
| 19 | HR-NBL1R2 | DB STI + scIL-2 |
ND |
|
ND | ND | ND | ND | ND | ND | ND | No | ND |
| 20 | HR-NBL1R4 | DB LTI + scIL-2 |
C1/D10 |
|
Encephalitis | Normal | ND | ND | ND |
|
No | Yes | Residual focal lesions |
| 21 | HR-NBL1R4 | DB LTI | C3/D13 |
|
Encephalitis |
|
No (blood and CSF) |
ND | ND |
|
No | Yes | ND |
| 22 | HR-NBL1R4 | DB LTI | C3/D17 |
|
Discreet infiltration of the acoustic- facial package |
Normal | ND | ND | ND |
|
Yes (no recurrence) |
Yes | ND |
| 23 | HR-NBL1R4 | DB LTI | C4/ND |
|
Mucosal thickening of left mastoid cell |
ND | ND | ND | ND |
|
ND | ND | ND |
| 24 | HR-NBL1R4 | DB LTI | C1/D22 |
|
ND | ND | ND | ND | ND |
|
Yes (symptoms reoccurred after 90 min of infusion) |
Yes | NA |
| 25 | HR-NBL1R4 | DB LTI | C1/D11 |
|
Sensory axonal neuropathy |
|
ND | ND | ND |
|
ND | ND | ND |
| 26 | HR-NBL1R4 | DB LTI + scIL-2 |
C1/D15 |
|
Normal | ND | ND | ND | ND | ND | Yes (no recurrence) |
Yes | ND |
| 27 | HR-NBL1R4 | DB LTI + scIL-2 |
C1/D27 |
|
ND | ND | ND | ND | ND | ND | ND | Yes | ND |
* The expected maximum serum concentration of DB at the end of infusion was 12.56 ± 0.68 µg/mL, as previously reported for patients in the LTI study [23]. CNS, central nervous system; C, cycle; CSF, cerebrospinal fluid; D, day; DB: dinutuximab beta; EEG, electroencephalogram; glc, glucose; h, hours; HSV, herpes simplex virus; IVIG, intravenous immunoglobulins; LTI, long-term infusion; mins, minutes; MRI, magnetic resonance spectroscopy; NA, not available; ND, not determined; PCR, polymerase chain reaction; PRES, posterior reversible encephalopathy syndrome; pt, patient; scIL-2, subcutaneous IL-2; STI short-term infusion; VZV, varicella zoster virus, wks, weeks.
The most common severe neurological side effects observed were paresis or hypotonia (n = 10), neurogenic bladder (n = 7), seizures (n = 6), ataxia and/or gait disturbances (n = 4) and cranial nerve palsy (n = 3). Patients also reported pain, sensory loss, somnolence, mood and behavioural changes, and visual disturbances, including one patient with blindness. In 12 patients, neurotoxicity presented with more than one symptom. The most common single symptoms were seizures and cranial nerve disturbances, all of which resolved without sequelae. No patient with severe neurotoxicity had meningeal or CNS involvement from their neuroblastoma.
3.1. LTI/SIOPEN Study
In the LTI/SIOPEN study, 15 out of 288 patients (5.2%) reported Grade 3/4 neurotoxicity (Table 2), including 10 patients with severe CNS neurotoxicity (3.5% of all LTI patients), 3 of whom experienced persistent neurological deficits (1.0% of all LTI patients). All events in this study occurred in patients treated with DB plus scIL-2. No neurotoxic events were observed in the first week of scIL-2 (Days 1–5 of the cycle) or during therapy with DB alone. There was no correlation between the disease status at study entry and the incidence of severe neurotoxicity (patients with neurotoxiciy: 60% not in complete response (non-CR) vs. 40% in complete response (CR), patients without neurotoxicity: 60% non-CR vs. 40% CR; p = 10,000; Fishers exact test). The serum levels of DB determined at the occurrence of symptoms did not exceed expected values of 12.56 ± 0.68 µg/mL [23]: mean concentration of 7.0 µg/mL ± 1.9 µg/mL (range 2.0–14.9 µg/mL) (Table 3). In two cases where samples were available, proinflammatory cytokines interleukin-6 (IL-6) and interferon-ɣ (IFN-ɣ) in peripheral blood were increased. Therapy with DB was stopped in all patients with severe neurotoxicity and six patients received immunosuppressive treatment.
Cerebrospinal fluid (CSF) was analysed in 5/10 patients with severe neurotoxicity. There were no relevant findings in routine parameters of CSF except for a distinct increase in the protein level (albumin) in four patients (0.55–1.8 g/L), which might be explained by a treatment-associated disturbance of the blood–brain barrier. The leukocyte number was only slightly increased (from 2 to 26 cells/µL). The concentration of DB in the CSF was determined in three patients, with levels of ≤0.02 µg/mL detected. Viral examinations, including polymerase chain reaction (PCR) for varicella zoster virus (VZV), were negative in all evaluated cases. Interestingly, 3/10 patients had VZV or herpes simplex virus (HSV) infection during or shortly before the DB therapy.
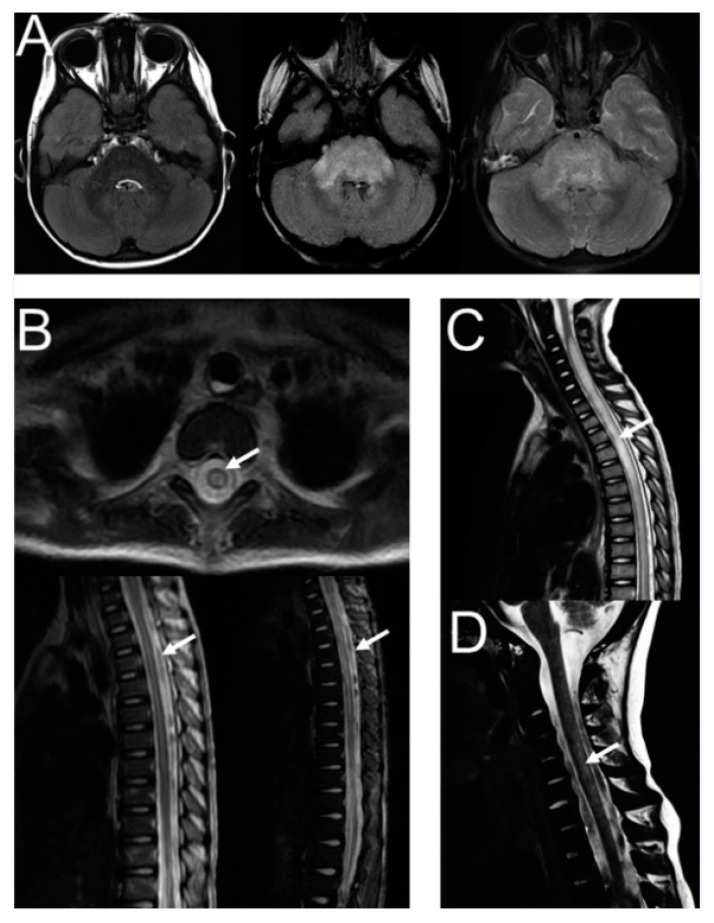
In 10 patients with available magnetic resonance imaging (MRI) scans, radiological review revealed inflammatory CNS lesions (Figure 2). One of these patients was diagnosed with cytotoxic brain stem oedema (Figure 2A) and five patients demonstrated demyelination, including four with transverse myelitis (Figure 2B–D) and 1 with demyelinating neuropathy involving the dorsal roots of the cauda equina. MRI scans were performed in a timely manner (within days) after the onset of clinical symptoms. More details on these cases are provided in the Supplementary Materials.
Figure 2.
Imaging results of patients with severe CNS neurotoxicities during immunotherapy with DB and scIL-2. (A) Patient 1. MRI of brain showing inflammation and oedema of mesencephalon, pons and medulla oblongata. (B) Patient 3. Frontal and sagittal MRI of spinal cord, with hyperintensive zone in STIR- and T2-weighted images. (C,D) Patient 2 and 4, respectively. Sagittal MRI of spinal cord, with hyperintensive zone in T2-weighted images. Arrows indicate areas of inflammatory response. DB, dinutuximab beta; CNS, central nervous system; MRI, magnetic resonance imaging; scIL-2, subcutaneous interleukin-2; STIR, short-TI inversion recovery.
3.2. HR-NBL1/SIOPEN Study (R2 and R4)
In the R2 phase of the HR-NBL1/SIOPEN study (n = 406), 21 patients (5.2%) experienced Grade 3/4 neurotoxicity (14 central and 7 peripheral events), including 9 patients with severe CNS neurotoxicity (Table 2 and Table 3).
Of the 14 patients with central neurotoxicities, 3 patients were treated with DB alone and 11 were treated with DB in combination with scIL-2. Initially, only eight patients were classified as severe CNS neurotoxicity; two presented with posterior reversible encephalopathy (PRES), four with seizures, one with mood disturbances followed by motor weakness, photophobia and taste impairment; and one with toxic demyelinating encephalopathy (paresis and coma) who did not fully recover. All severe neurotoxicities occurred in the group of patients treated with DB and scIL-2, except for one patient who experienced PRES while treated with DB alone.
Of the seven patients with Grade 3/4 peripheral neurotoxicity (paraesthesia and deficits in motor function), six were in the group of patients treated with DB plus scIL-2 and one in the group treated with DB alone. One patient treated with the combination therapy was classified as peripheral neuropathy, despite the occurrence of tetraparesis. The patient also experienced urinary retention and mydriasis. Based on the clinical symptoms, it is likely that this patient had transverse myelitis, and was not assigned to the correct neurotoxicity category. The patient did not recover, and was finally classified as having severe CNS neurotoxicity. Overall, 9 of 406 (2.2%) patients in the R2 phase of the HR-NBL1 study presented with severe CNS neurotoxicity, with 2 patients experiencing persistent neurological deficits (0.5% of all R2-HR-NBL1 patients).
In the R4 phase of the HR-NBL1 study (n = 408), eight (2%) patients presented with Grade 3/4 neurotoxicities (four central and four peripheral events), with five reported in the DB only group and three in the DB plus scIL-2 group. In two patients, CNS inflammatory lesions were identified on radiological imaging, with one patient demonstrating normal CSF results. All eight patients recovered without sequelae. HR-NBL1 study patients (R2 and R4 combined) who were not in complete remission (non-CR) before immunotherapy showed a trend towards a higher incidence of severe neurotoxicity (patients with severe neurotoxicity: 86% non-CR vs. 15% CR, patients without neurotoxicity: 55% non-CR vs. 45% CR; p = 0.0628; Fishers exact test).
In summary, we observed 44 out of 1102 patients with Grade 3/4 neurotoxicities: 17/814 patients (2%) in the front-line setting and 27 of 288 patients in the relapse/refractory setting (9.4%). The majority of Grade 3/4 neurotoxicities (35/44; 79.5%) occurred in patients treated with DB plus scIL-2. Recovery was observed in 33/38 patients (86.8%), which was achieved with different, non-standardised interventions, including the use of dexamethasone, prednisolone, i.v. immunoglobulins (IVIGs) and plasmapheresis. A total of 12 patients received immunomodulatory treatment combinations of IVIGs and/or steroids, and in 2 patients, who did not respond to this treatment, plasmapheresis resulted in the complete resolution of symptoms (Supplementary Materials). However, 5/44 patients (11.4%) demonstrated persisting severe neurological deficits.
4. Discussion
Here, we report the occurrence and management of severe neurotoxicity experienced by patients with high-risk neuroblastoma who received treatment with DB with or without scIL-2 in the front-line or relapse/refractory setting within two prospective clinical trials.
Neurotoxicity is a side effect of anti-GD2 antibodies occurring irrespective of their origin (mouse, chimeric, humanised). Neuropathic pain, confusion and sensorimotor neuropathy caused by demyelination have been described in a Phase I study investigating the murine monoclonal anti-GD2 antibody 14G2a in patients with metastatic melanoma [24]. Similarly, a Phase I study with the human/mouse chimeric anti-GD2 antibody ch14.18 reported optic nerve atrophy in two patients with neuroblastoma who also received radiotherapy [25]. In the prospective COG trial in children with high-risk neuroblastoma, patients randomised to dinutuximab (ch14.18 produced in SP2/0 cells [7]) in combination with i.v. IL-2 and GM-CSF, CNS toxicity was reported in 6 of 137 patients (4.4%) and manifested as encephalopathy, confusion, psychosis and seizure [3]. Neurotoxicity was also reported in the preceding Phase I study of dinutuximab in combination with GM-CSF in children with neuroblastoma [26]. In addition, treatment with the murine anti-GD2 antibody 3F8 was reported to be associated with neurotoxicity, with 5 of 215 patients (2.3%) developing PRES [27]. There was no case of transverse myelitis in this study, which might be explained by the absence of concomitant IL-2 therapy.
Transverse myelitis has, however, been reported in three patients treated with dinutuximab therapy [16]. Clinical symptoms were similar to those observed in our analysis and included weakness of bilateral lower extremities, urinary retention and progression to paraplegia [16]. Two of these patients were treated with concomitant IL-2 and GM-CSF, while one patient received dinutuximab combined with chemotherapy [16]. All symptoms resolved after immunomodulatory treatment with steroids and/or IVIG and/or plasmapheresis [16]. As all patients experienced neurotoxicity during consecutive courses of dinutuximab, the authors suggested that the previous exposure to the drug induced an anti-idiotypic immune response with crossreactivity against neuronal tissue [16]. However, in our analysis, neurotoxicity occurred in the first treatment cycle and most of these patients did not receive previous anti-GD2 immunotherapy, suggesting that other mechanisms may be involved.
The pathogenesis of the observed neurotoxicity is not entirely clear. This “on-target, off-tumour” side effect is not observed with human mouse chimeric monoclonal antibodies that target other antigens, such as CD20 (e.g., rituximab) or tumour necrosis factor-alpha (e.g., infliximab). Thus, neurotoxicity is associated with antibodies binding to GD2 followed by the induction of an inflammatory response against GD2-expressing neuronal tissue [28]. Since anti-GD2 antibodies are often used in combination with cytokines, the neurotoxic inflammation may be further enhanced, in particular in the presence of IL-2 [1,3,11]. As mentioned earlier, severe neurotoxicity is a reported side effect of IL-2 monotherapy including neuropathy, coma, convulsions, paralysis and leukoencephalopathy [17]. However, in both trials analysed in this report, neurotoxicity was not observed during the first five days of the monotherapy period with scIL-2, except for one patient who had an event during the first five days of Cycle 2, which might be due to carry-over exposure to DB from the previous cycle. Therefore, the anti-GD2 antibody appears to be the major factor for this neurotoxicity to occur. The frequency of severe neurotoxicity is very low in patients treated with DB alone, which indicates that IL-2 may be largely responsible for increasing the frequency and amplitude of this side effect, potentially by enhancing the inflammation triggered by the antibody effector function. The observation of neurotoxicity as a side effect of treatment with anti-GD2 antibodies is not new and appears to be a specific effect of anti-glycolipid antibodies [29,30]. Various antibodies directed against more than 20 different glycolipids have been associated with acute and chronic neuropathy syndromes [30].
Our analysis revealed a substantial number of patients with severe CNS neurotoxicities who had a reactivation/infection with the herpes viruses VZV and HSV (3/10 patients in the LTI study). However, it is not clear if there is a causal relationship. The herpes viruses family is known to exhibit neurotropism; VZV in particular is known to cause myelitis, even without involvement of the skin (zoster sine herpete) [31,32]. Myelitis might also be the only presentation of VZV infection, and examination of CSF in such cases may show normal results [32]. However, not all patients with HSV or VZV infection may present with neurotoxicities. In our analysis, there were 2 reported patients with active infections who received DB combined with scIL-2 and did not exhibit any neurotoxic side effects. Considering the poor prognosis for patients with high-risk neuroblastoma, the diagnosis of HSV or VZV infection should not exclude patients from receiving DB. The infection should be treated first until complete resolution, and acyclovir prophylaxis should be given during treatment with DB.
Since the neurotoxic effect of DB treatment is not observed with other chimeric human mouse monoclonal antibodies, it is likely to be associated with the GD2 specificity of the antibody. Moreover, the observation that removing DB by plasmapheresis led to a reduction in symptoms in two patients, and that the attempt of therapy reintroduction caused recurrence of symptoms in two patients, suggests that there is a causal relationship between DB and neurotoxicity. However, it is surprising that we did not find DB in the CSF of these three patients with neurotoxicity, suggesting that these events might also be due to indirect bystander effects of the immunological activation. In addition, the serum concentrations of DB were also not unusually high compared to the expected maximum concentration of 12.56 ± 0.68 µg/mL [23], indicating that high DB levels are unlikely to be responsible for the severe events observed. As mentioned earlier, IL-2 may be the primary driver of severe neurotoxicity in these patients.
In patients with neurotoxicity reported in our analysis, temporary immunosuppression with steroids (prednisolone and dexamethasone) or IVIG in standard doses was initiated immediately after the occurrence of symptoms, resulting in recovery in the majority of patients. In two patients who did not improve after immunosuppression, plasmapheresis was effective. One of these cases has recently been reported [33]. Although increased proinflammatory cytokine levels were found in some cases, other immunosuppressive drugs such as anti-IL-6 monoclonal antibodies were not administered due to lack of experience with these. As the recurrence of neurological symptoms has been described in three out of seven patients after the attempt of drug rechallenge, DB therapy should be discontinued permanently after the occurrence of severe neurotoxicity.
Based on our experience, we suggest the following management in case of neurological symptoms during immunotherapy (Table 4): Once symptoms occur, it is crucial to start immunosuppression with dexamethasone or prednisolone and intravenous immunoglobulin immediately. In parallel, other causes for neurological symptoms need to be ruled out, including infection and disease progression. Therefore, immediate imaging studies and CSF analysis are important steps. Until an infectious cause is excluded, antimicrobial and antiviral treatment is advisable. Taking into consideration a possible influence of HSV/VZV infection/reactivation in the pathogenesis of myelitis, even in the absence of evident skin lesions, viral diagnostics by PCR for VZV and HSV should be performed using blood and CSF. Acyclovir treatment should be considered at least until PCR results come back negative, even if no evidence of VZV/HSV is found in laboratory examinations. Furthermore, special care should be taken when DB therapy is initiated in patients who had active VZV or HSV infections shortly before planned treatment. Based on the cases reported here, it is not possible to recommend a clear time frame between infection and the start of therapy, but a complete resolution of active HSV/VZV infection should be achieved before starting DB. In addition, early intervention is recommended to reduce the severity and duration of neurological symptoms and prevent persistent disability. Follow-up examinations are recommended in patients with persistent symptoms and/or initial MRI findings upon the onset of neurotoxicity every 4–6 weeks to determine the course and make a decision about the escalation or de-escalation of therapy.
Table 4.
Management recommendations for suspected neurotoxicity during immunotherapy with DB.
|
|
|
|
|
|
|
DB, dinutuximab beta; cMRI, cardiac magnetic resonance imaging; CSF, cerebrospinal fluid; EEG, electroencephalogram; HSV, herpes simplex virus; IVIG, intravenous immunoglobulins; MRI, magnetic resonance imaging; PCR, polymerase chain reaction; VZV, varicella zoster virus.
5. Conclusions
Severe neurotoxic events observed in the two SIOPEN trials occurred more frequently in patients receiving DB combined with scIL-2 than in those receiving DB monotherapy. Although supportive management resulted in recovery in the majority of patients, a very small number of patients with severe CNS neurotoxicities demonstrated persistent neurological deficits, which are likely due to the co-administration of scIL-2. Since scIL-2 has not shown any clinical benefit when added to DB therapy [2,3,4], the findings reported here provide further evidence that scIL-2 should be omitted from the treatment regimen. When using DB therapy, it is crucial to strictly follow the patients to diagnose symptoms of neurotoxicity early, stop DB treatment and start immunosuppression. In patients who experience severe neurotoxicity, DB treatment should be discontinued permanently.
Acknowledgments
Special thanks go to all clinical investigator teams of SIOPEN centers as well as patients and their families.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cancers14081919/s1, Individual case descriptions and therapeutic interventions.
Author Contributions
Conceptualization: A.W. and H.N.L. Data curation: U.P., I.P., S.F. and A.W. Formal analysis: A.W., H.N.L., U.P. and I.P. Collection, compilation and validation of clinical data: A.W., H.N.L., C.M., A.G., J.G, V.P., D.V.-C., K.Z. and R.L. Methodology: H.N.L. and A.W. Supervision: H.N.L. and R.L. Validation: H.N.L. and R.L. Writing—original draft preparation: A.W. Writing—review and editing: A.W., H.N.L., V.P., J.G., D.V.-C. and R.L. All authors have read and agreed to the published version of the manuscript.
Funding
The St. Anna Kinderkrebsforschung e.V (Vienna, Austria) acted as academic sponsor of both studies. The HR-NBL study received funding from the European Commission 5th Framework Grant (SIOPEN-R-NET EC grant number QLRI-CT-2002-01768). The investigational medical product (IMP) dinutuximab beta (ch14.18/CHO) was first recloned and produced by Polymun Scientific (Vienna, Austria), commissioned by the St. Anna Kinderkrebsforschung e.V on behalf of respective European charities and institutions after a Europe-wide fund-raising campaign. During the course of studies, industry partners Apeiron Biologics (Vienna, Austria) and EUSA Pharma Ltd. (Hemel Hempstead, United Kingdom) took over the antibody production and provided IMP free of charge to both studies. The project was in part carried out within TEMICARE (German-Polish pediatric oncology center integrated by telemedicine in the Euroregion Pomerania (INT113)) funded by the European Union. Editorial assistance was provided by Katrin Male from mXm Medical Communications and the article-processing charge and editorial support were funded by EUSA Pharma. The content of the article represents the views of the authors and has not been influenced by third-party sponsorship.
Institutional Review Board Statement
The studies were conducted according to the guidelines of the Declaration of Helsinki, and approved by the lead ethical board of the Medical University in Vienna according to national practice. The Approval Number was: 693/2010.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the academic sponsor.
Conflicts of Interest
The Polymun Scientific, the charities, Apeiron Biologics and EUSA Pharma did not have a role in the study design or analysis. A.W., R.L. and H.L. report consultant fees and travel grants from EUSA Pharma outside the submitted work. C.M. declares that she has no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. A.G. and J.G. declare that they have previously received consultancy fees for advisory boards and speaker fees from EUSA. D.V.C. has worked with EUSA Pharma on various projects, including advisory boards, the financial support of which was used by D.V.C.’s institution to support the clinical research of the department. D.V.C. has no personal conflict of interest and has not received any payment from any pharmaceutical company. V.P. received speaker honoraria from EUSA Pharma. K.Z, U.P., I.P. and S.F. declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ladenstein R., Pötschger U., Valteau-Couanet D., Luksch R., Castel V., Yaniv I., Laureys G., Brock P., Michon J.M., Owens C., et al. Interleukin 2 with anti-GD2 antibody ch14.18/CHO (dinutuximab beta) in patients with high-risk neuroblastoma (HR-NBL1/SIOPEN): A multicentre, randomised, phase 3 trial. Lancet Oncol. 2018;19:1617–1629. doi: 10.1016/S1470-2045(18)30578-3. [DOI] [PubMed] [Google Scholar]
- 2.Ladenstein R., Pötschger U., Valteau-Couanet D., Luksch R., Castel V., Ash S., Laureys G., Brock P., Michon J.M., Owens C., et al. Investigation of the Role of Dinutuximab Beta-Based Immunotherapy in the SIOPEN High-Risk Neuroblastoma 1 Trial (HR-NBL1) Cancers. 2020;12:309. doi: 10.3390/cancers12020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu A.L., Gilman A.L., Ozkaynak M.F., London W.B., Kreissman S.G., Chen H.X., Smith M., Anderson B., Villablanca J.G., Matthay K.K., et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N. Engl. J. Med. 2010;363:1324–1334. doi: 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu A.L., Gilman A.L., Ozkaynak M.F., Naranjo A., Diccianni M.B., Gan J., Hank J.A., Batova A., London W.B., Tenney S.C., et al. Long-Term Follow-up of a Phase III Study of ch14.18 (Dinutuximab) + Cytokine Immunotherapy in Children with High-Risk Neuroblastoma: COG Study ANBL0032. Clin. Cancer Res. 2021;27:2179–2189. doi: 10.1158/1078-0432.CCR-20-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.UNITUXIN (dinutuximab) Prescribing Information. [(accessed on 17 February 2022)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125516s000lbl.pdf.
- 6.Qarziba (Dinutuximab Beta) Summary of Product Characteristics. [(accessed on 17 February 2022)]. Available online: https://www.ema.europa.eu/en/documents/product-information/qarziba-epar-product-information_en-0.pdf.
- 7.Sait S., Modak S. Anti-GD2 immunotherapy for neuroblastoma. Expert Rev. Anticancer Ther. 2017;17:889–904. doi: 10.1080/14737140.2017.1364995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ladenstein R.L., Poetschger U., Valteau-Couanet D., Gray J., Luksch R., Balwierz W., Castel V., Ash S., Popovic M.B., Laureys G., et al. Randomization of dose-reduced subcutaneous interleukin-2 (scIL2) in maintenance immunotherapy (IT) with anti-GD2 antibody dinutuximab beta (DB) long-term infusion (LTI) in front–line high-risk neuroblastoma patients: Early results from the HR-NBL1/SIOPEN trial. J. Clin. Oncol. 2019;37((Suppl. S15)):10013. [Google Scholar]
- 9.Mueller I., Ehlert K., Endres S., Pill L., Siebert N., Kietz S., Brock P., Garaventa A., Valteau-Couanet D., Janzek E., et al. Tolerability, response and outcome of high-risk neuroblastoma patients treated with long-term infusion of anti-GD(2) antibody ch14.18/CHO. MAbs. 2018;10:55–61. doi: 10.1080/19420862.2017.1402997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lode H.N., Valteau-Couanet D., Gray J., Luksch R., Wieczorek A., Castel V., Ash S., Laureys G., Papadakis V., Owens C., et al. Randomized use of anti-GD2 antibody dinutuximab beta (DB) long-term infusion with and without subcutaneous interleukin-2 (scIL-2) in high-risk neuroblastoma patients with relapsed and refractory disease: Results from the SIOPEN LTI-trial. J. Clin. Oncol. 2019;37((Suppl. S15)):10014. doi: 10.1200/JCO.2019.37.15_suppl.10014. [DOI] [Google Scholar]
- 11.Barone G., Barry A., Bautista F., Brichard B., Defachelles A.S., Herd F., Manzitti C., Reinhardt D., Rubio P.M., Wieczorek A., et al. Managing Adverse Events Associated with Dinutuximab Beta Treatment in Patients with High-Risk Neuroblastoma: Practical Guidance. Pediatr. Drugs. 2021;23:537–548. doi: 10.1007/s40272-021-00469-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doronin I.I., Vishnyakova P.A., Kholodenko I.V., Ponomarev E.D., Ryazantsev D.Y., Molotkovskaya I.M., Kholodenko R.V. Ganglioside GD2 in reception and transduction of cell death signal in tumor cells. BMC Cancer. 2014;14:295. doi: 10.1186/1471-2407-14-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marconi S., De Toni L., Lovato L., Tedeschi E., Gaetti L., Acler M., Bonetti B. Expression of gangliosides on glial and neuronal cells in normal and pathological adult human brain. J. Neuroimmunol. 2005;170:115–121. doi: 10.1016/j.jneuroim.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Svennerholm L., Boström K., Fredman P., Jungbjer B., Lekman A., Månsson J.E., Rynmark B.M. Gangliosides and allied glycosphingolipids in human peripheral nerve and spinal cord. Biochim. Biophys. Acta. 1994;1214:115–123. doi: 10.1016/0005-2760(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 15.Xiao W.H., Yu A.L., Sorkin L.S. Electrophysiological characteristics of primary afferent fibers after systemic administration of anti-GD2 ganglioside antibody. Pain. 1997;69:145–151. doi: 10.1016/S0304-3959(96)03280-0. [DOI] [PubMed] [Google Scholar]
- 16.Ding Y.Y., Panzer J., Maris J.M., Castañeda A., Gomez-Chiari M., Mora J. Transverse myelitis as an unexpected complication following treatment with dinutuximab in pediatric patients with high-risk neuroblastoma: A case series. Pediatr. Blood Cancer. 2018;65:e26732. doi: 10.1002/pbc.26732. [DOI] [PubMed] [Google Scholar]
- 17.Proleukin (Interleukin-2) Summary of Product Characteristics. [(accessed on 17 February 2022)]. Available online: https://www.medicines.org.uk/emc/medicine/19322#gref.
- 18.Dutcher J.P., Schwartzentruber D.J., Kaufman H.L., Agarwala S.S., Tarhini A.A., Lowder J.N., Atkins M.B. High dose interleukin-2 (Aldesleukin)—expert consensus on best management practices-2014. J. Immunother. Cancer. 2014;2:26. doi: 10.1186/s40425-014-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karp B.I., Yang J.C., Khorsand M., Wood R., Merigan T.C. Multiple cerebral lesions complicating therapy with interleukin-2. Neurology. 1996;47:417–424. doi: 10.1212/WNL.47.2.417. [DOI] [PubMed] [Google Scholar]
- 20.Ladenstein R., Pötschger U., Pearson A.D.J., Brock P., Luksch R., Castel V., Yaniv I., Papadakis V., Laureys G., Malis J., et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): An international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18:500–514. doi: 10.1016/S1470-2045(17)30070-0. [DOI] [PubMed] [Google Scholar]
- 21.Ladenstein R., Valteau-Couanet D., Brock P., Yaniv I., Castel V., Laureys G., Malis J., Papadakis V., Lacerda A., Ruud E., et al. Randomized Trial of prophylactic granulocyte colony-stimulating factor during rapid COJEC induction in pediatric patients with high-risk neuroblastoma: The European HR-NBL1/SIOPEN study. J. Clin. Oncol. 2010;28:3516–3524. doi: 10.1200/JCO.2009.27.3524. [DOI] [PubMed] [Google Scholar]
- 22.Garaventa A., Poetschger U., Valteau-Couanet D., Castel V., Elliott M., Ash S., Chan G.C.F., Laureys G., Popovic M.B., Vettenranta K., et al. The randomised induction for high-risk neuroblastoma comparing COJEC and N5-MSKCC regimens: Early results from the HR-NBL1.5/SIOPEN trial. J. Clin. Oncol. 2018;36:10507. doi: 10.1200/JCO.2018.36.15_suppl.10507. [DOI] [Google Scholar]
- 23.Siebert N., Eger C., Seidel D., Jüttner M., Zumpe M., Wegner D., Kietz S., Ehler K., Veal G.J., Siegmund W., et al. Pharmacokinetics and pharmacodynamics of ch14.18/CHO in relapsed/refractory high-risk neuroblastoma patients treated by long-term infusion in combination with IL-2. MAbs. 2016;8:604–616. doi: 10.1080/19420862.2015.1130196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleh M.N., Khazaeli M.B., Wheeler R.H., Dropcho E., Liu T., Urist M., Miller D.M., Lawson S., Dixon P., Russell C.H., et al. Phase I trial of the murine monoclonal anti-GD2 antibody 14G2a in metastatic melanoma. Cancer Res. 1992;52:4342–4347. [PubMed] [Google Scholar]
- 25.Handgretinger R., Anderson K., Lang P., Dopfer R., Klingebiel T., Schrappe M., Reuland P., Gillies S.D., Reisfeld R.A., Neithammer D. A phase I study of human/mouse chimeric antiganglioside GD2 antibody ch14.18 in patients with neuroblastoma. Eur. J. Cancer. 1995;31:261–267. doi: 10.1016/0959-8049(94)00413-Y. [DOI] [PubMed] [Google Scholar]
- 26.Ozkaynak M.F., Sondel P.M., Krailo M.D., Gan J., Javorsky B., Reisfeld R.A., Matthay K.K., Reaman G.H., Seeger R.C. Phase I study of chimeric human/murine anti-ganglioside G(D2) monoclonal antibody (ch14.18) with granulocyte-macrophage colony-stimulating factor in children with neuroblastoma immediately after hematopoietic stem-cell transplantation: A Children’s Cancer Group Study. J. Clin. Oncol. 2000;18:4077–4085. doi: 10.1200/JCO.2000.18.24.4077. [DOI] [PubMed] [Google Scholar]
- 27.Kushner B.H., Modak S., Basu E.M., Roberts S.S., Kramer K., Cheung N.K. Posterior reversible encephalopathy syndrome in neuroblastoma patients receiving anti-GD2 3F8 monoclonal antibody. Cancer. 2013;119:2789–2795. doi: 10.1002/cncr.28137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ceylan K., Jahns L.J., Lode B.N., Ehlert K., Kietz S., Troschke-Meurer S., Siebert N., Lode H.N. Inflammatory response and treatment tolerance of long-term infusion of the anti-GD(2) antibody ch14.18/CHO in combination with interleukin-2 in patients with high-risk neuroblastoma. Pediatr. Blood Cancer. 2018;65:e26967. doi: 10.1002/pbc.26967. [DOI] [PubMed] [Google Scholar]
- 29.Yuki N., Yamada M., Tagawa Y., Takahashi H., Handa S. Pathogenesis of the neurotoxicity caused by anti-GD2 antibody therapy. J. Neurol. Sci. 1997;149:127–130. doi: 10.1016/S0022-510X(97)05390-2. [DOI] [PubMed] [Google Scholar]
- 30.Willison H.J., Yuki N. Peripheral neuropathies and anti-glycolipid antibodies. Brain. 2002;125:2591–2625. doi: 10.1093/brain/awf272. [DOI] [PubMed] [Google Scholar]
- 31.Gilden D., Mahalingam R., Nagel M.A., Pugazhenthi S., Cohrs R.J. Review: The neurobiology of varicella zoster virus infection. Neuropathol. Appl. Neurobiol. 2011;37:441–463. doi: 10.1111/j.1365-2990.2011.01167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Broucker T., Mailles A., Chabrier S., Morand P., Stahl J.P. Acute varicella zoster encephalitis without evidence of primary vasculopathy in a case-series of 20 patients. Clin. Microbiol. Infect. 2012;18:808–819. doi: 10.1111/j.1469-0691.2011.03705.x. [DOI] [PubMed] [Google Scholar]
- 33.Papadakis V., Kelaidi C., Zisaki K., Antoniadi K., Pitsoulakis G., Polychronopoulou S. Dinutuximab beta-related severe neurotoxicity: Resolution with the use of plasmapheresis. Pediatr. Blood Cancer. 2021;69:e29465. doi: 10.1002/pbc.29465. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the academic sponsor.