Abstract
To determine the efficacy of trovafloxacin as a possible treatment for intra-abdominal abscesses, we have developed an anaerobic time-kill technique using different inocula to study the in vitro killing of Bacteroides fragilis in pure culture or in mixed culture with either Escherichia coli or a vancomycin-resistant strain of Enterococcus faecium (VREF). With inocula of 5 × 105 CFU/ml and trovafloxacin concentrations of ≤2 μg/ml, a maximum observed effect (Emax) of ≥6.1 (log10 CFU/ml) was attained with all pure and mixed cultures within 24 h. With inocula of 108 CFU/ml, a similar Emax and a similar concentration to produce 50% of Emax (EC50) for B. fragilis were found in both pure cultures and mixed cultures with E. coli. However, to produce a similar killing of B. fragilis in the mixed cultures with VREF, a 14-fold increase in the concentration of trovafloxacin was required. A vancomycin-susceptible strain of E. faecium and a trovafloxacin-resistant strain of E. coli were also found to confer a similar “protective” effect on B. fragilis against the activity of trovafloxacin. Using inocula of 109 CFU/ml, the activity of trovafloxacin was retained for E. coli and B. fragilis and was negligible against VREF. We conclude that this is a useful technique to study the anaerobic killing of mixed cultures in vitro and may be of value in predicting the killing of mixed infections in vivo. The importance of using mixed cultures and not pure cultures is clearly shown by the difference in the killing of B. fragilis in the mixed cultures tested. Trovafloxacin will probably be ineffective in the treatment of infections involving large numbers of enterococci. However, due to its ability to retain activity against large cultures of B. fragilis and E. coli, trovafloxacin could be beneficial in the treatment of intra-abdominal abscesses.
Several factors must be taken into consideration when selecting an antibiotic for the treatment of intra-abdominal abscesses. Firstly, the presence of mixed infections of anaerobic and aerobic bacteria with different susceptibilities requires the use of either dual therapy, e.g., a penicillin plus an aminoglycoside, or broad-spectrum antibiotics, such as carbapenems or cephalosporins (29). Secondly, the large numbers of bacteria in a stationary phase of growth, as well as the pH and anaerobic environment of the abscesses (4), could all affect the activity of an antibiotic and consequently influence the choice of treatment.
Bacteroides fragilis and Escherichia coli are the most frequently isolated organisms from intra-abdominal abscesses although other anaerobes and facultative bacteria, including enterococcal strains, have been isolated in about 10 to 20% of cases (10, 29). Recently, we have developed a subcutaneous abscess model in the mouse by using a mixed infection of B. fragilis, E. coli, and autoclaved cecal contents (ACC) (I. C. Gyssens, L. E. T. Stearne, S. L. C. E. Buijk, J. W. Mouton, I. A. J. M. Bakker-Woudenberg, and H. A. Verbrugh, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-37, 1998). Treatment of these abscesses with high doses of imipenem or ceftizoxime was disappointing despite the fact that both strains were susceptible to both antibiotics, and, from kinetic studies, the concentrations of the drugs were shown to be above MIC levels in serum and abscesses for the entire dosing interval. The inoculum effect shown with both these antibiotics (26; Gyssens et al., 38th ICAAC) as well as the reduced activity of β-lactams against stationary cultures (30, 32) could be a possible explanation for the reduced efficacy in vivo.
The quinolones have had a limited use in anaerobic mixed infections due to their marginal activity or inactivity against anaerobes (27) or gram-positive strains (8). Trovafloxacin is a broad-spectrum fluoroquinolone that is effective not only against gram-negative organisms but also has an increased activity against gram-positive and anaerobic strains (12). In one study, trovafloxacin has been shown to inhibit 99.3% of isolates from intra-abdominal infections (6). In addition, the activity of trovafloxacin is virtually unaffected by changes in pH, inoculum size, or anaerobic conditions (1, 8, 24) and is also active against some cultures of nondividing cells (21). These properties indicate that trovafloxacin could be a possible choice of treatment for intra-abdominal abscesses while the long half-life (3) and postantibiotic effect (25) would facilitate its possible use in once-daily dosing regimens.
To date, the in vitro activity of trovafloxacin has been reported in MIC and minimum bactericidal concentration (MBC) (12, 13) or time-kill (28, 31) studies using single cultures. However, the killing of bacteria in pure culture may not necessarily be identical to the killing in mixed culture (15, 22). We have developed a mixed culture anaerobic time-kill technique for a comparative study of the in vitro activity of trovafloxacin against B. fragilis in pure or mixed culture with either E. coli or a vancomycin-resistant strain of Enterococcus faecium (VREF). Since abscesses contain large numbers of bacteria in a stationary phase of growth, we have used, in addition to standard inocula (5 × 105 CFU/ml), large numbers of actively growing (108 CFU/ml) or static (109 CFU/ml) cultures to simulate in vivo conditions. In addition, due to the elevated (protein) binding capacity reported for trovafloxacin (3), we have investigated the effect of intestinal contents (ACC) on the in vitro activity of trovafloxacin.
(This study was presented in part at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, abstr. 2333, p. 289, 1999.)
MATERIALS AND METHODS
Materials.
Wilkens Chalgren (WC) broth, WC agar, Eosin Methylene Blue (EM) agar, Diagnostic Sensitivity Test (DST) agar, and AnaeroGen anaerobic sachets and jars were all supplied by Unipath Ltd. (Haarlem, The Netherlands). Columbia blood agar plates came from Becton Dickinson B.V. (Woerden, The Netherlands). Gentamicin was obtained from Centrafarm Services B.V. (Etten-Leur, The Netherlands) and vancomycin was from Eli Lilly Nederland B.V. (Nieuwegein, The Netherlands). Trovafloxacin (CP-99, 219-27, lot 25381-086-02) was supplied by Pfizer Inc. (Groton, Conn.). The supplier of 5-ml polypropylene tubes was Greiner B.V. (Alphen a/d Rijn, The Netherlands).
Bacterial strains.
E. coli ATCC 25922, B. fragilis ATCC 23745, and E. faecium BM4147, a vancomycin-resistant clinical isolate, were used throughout the experiments. For comparative studies, B. fragilis ATCC 25285, E. faecium SH17, a vancomycin-susceptible clinical isolate, and E. coli 29020, a trovafloxacin-resistant clinical isolate, were also employed. Overnight cultures were obtained by inoculating 30-ml volumes of WC broth with 0.1 ml of standardized frozen bacterial suspensions and incubating aerobically (E. coli, VREF, and E. faecium) or anaerobically (B. fragilis) at 37°C for 18 h.
ACC.
ACC were obtained as previously described (19). Briefly, the cecal contents were removed from approximately 100 Swiss mice (Broekman Instituut B.V., Someren, The Netherlands), diluted 1:4 with WC broth, homogenized, and filtered twice through surgical gauze. The suspension was autoclaved in 5-ml volumes at 121°C for 2 h and stored at −80°C. Batches were standardized by measuring the dry weight (Edwards Super Modulyo freeze dryer) of a 1-ml sample of the autoclaved contents.
MIC and MBC determinations.
MICs were determined by the standard broth microdilution method using inocula of 105 or 108 CFU/ml (23). All tests were performed in duplicate and repeated on a separate day using freshly prepared trovafloxacin mixtures and inocula. Microtiter plates were incubated at 37°C aerobically for 20 h (E. coli, VREF, and E. faecium) or anaerobically for 48 h (B. fragilis). In experiments with the higher inocula (which were visually turbid), the MIC was defined as the lowest concentration of trovafloxacin that prevented a ≥0.1 increase in optical density at 600 nm (Biokinetics reader EL 340; Bio-tek Instruments) compared with that of controls incubated at 4°C. MBCs were determined by plating 100-μl samples from all wells showing no bacterial growth after incubation onto blood agar and then incubating at 37°C for 24 h (E. coli, VREF, and E. faecium) or 48 h (B. fragilis), and the MBC was defined as the lowest concentration producing ≥99.9% killing of the initial inoculum. Suspensions with the higher inocula (108 CFU/ml) were first diluted 1:100 in phosphate-buffered saline (PBS) before determining the MBCs. To determine the effect of ACC on the MBCs, tests were repeated using inocula containing 4 mg (dry weight) of ACC/ml (final concentration).
Mixed-culture anaerobic time-kill technique.
Twofold-increasing trovafloxacin concentrations (0.015 to 64 μg/ml) were used with final inocula of 5 × 105, 1 × 108, or 1 × 109 CFU/ml for each test strain. Concentrated solutions of trovafloxacin were diluted in prewarmed WC broth to yield two times the final required antibiotic concentrations, from which four 2-ml volumes were placed in polypropylene tubes with loose caps. Control samples contained 2 ml of WC broth. Inocula were prepared by diluting 18-h cultures of B. fragilis ATCC 23745 and either E. coli ATCC 25922 or VREF in prewarmed WC broth. Tubes containing broth with or without antibiotic were carefully inoculated with 1 ml of E. coli (or VREF) culture followed by 1 ml of B. fragilis culture. Cultures were placed in four separate anaerobic jars and incubated at 37°C on a shaker for 2, 4, 6, or 24 h. To prevent carryover of any antibiotic, 1 ml of each test suspension was washed by centrifuging at 13,000 × g for 2 min, removing 0.9 ml of the supernatant and resuspending the pellet in 0.9 ml of PBS. The entire procedure was repeated, and bacterial counts were performed on the resulting suspensions by making duplicate serial 10-fold dilutions of cultures in PBS and plating 20 μl of appropriate dilutions onto EM agar (E. coli), blood agar (VREF), or WC agar containing 100 mg of gentamicin/liter (B. fragilis). Plates were incubated at 37°C aerobically for 24 h (EM or blood agar) or anaerobically for 48 h (WC agar). Experiments were also performed using pure cultures of B. fragilis ATCC 23745. Counts were expressed as log10 CFU/milliliter, and the lower threshold limit was 2.5 log10 CFU/ml. Experiments with the different inocula were performed on different days. To determine daily variation, individual experiments were repeated using a more limited number of trovafloxacin concentrations.
For comparison, the technique was repeated with pure and mixed cultures (108 CFU/ml) of other bacterial strains, namely B. fragilis ATCC 25285, E. faecium SH17, and E. coli 29020, using 2 μg of trovafloxacin/ml, and after 24 h of incubation, bacterial counts were determined (when in mixed culture with E. faecium, B. fragilis counts were determined on WC agar containing 6.25 mg of vancomycin/liter). The experiment was performed three times on all pure and mixed cultures on separate days by using freshly prepared trovafloxacin and inocula. Control cultures without trovafloxacin were included with each experiment, from which the reduction in bacterial counts of each culture could be determined. In addition, the concentration of residual trovafloxacin present in the supernatants of the enterococcal mixed cultures after incubation was also measured using an agar diffusion bioassay on DST agar with a Bacillus subtilis isolate as the test organism. Standards were prepared in WC broth.
To determine the effect of intestinal contents on the activity of trovafloxacin, the time-kill technique was performed using concentrations of 0.25, 1, or 4 μg/ml and inocula of E. coli ATCC 25922-B. fragilis ATCC 23745 mixed cultures (108 CFU/ml) with or without 4 mg (dry weight) of ACC/ml (final concentration). To exclude the possibility that the ACC could affect the removal of trovafloxacin during the washing procedure outlined above by “trapping” the antibiotic in the pellet, a suspension containing 4 μg of trovafloxacin/ml and 4 mg of ACC/ml was incubated at 37°C for 24 h and centrifuged, and the pellet was washed twice in PBS. The trovafloxacin concentration in the pellet was measured in the bioassay previously described. Approximately 0.35 μg of trovafloxacin/ml was recovered from the pellet. However, in the time-kill study, this concentration would be further diluted 1:50 by plating out onto agar plates and would therefore have little influence on the outcome of the bacterial counts.
The degree of ACC binding to trovafloxacin was assessed by incubating 0.5 to 16 μg of trovafloxacin/ml in WC broth with or without 4 mg (dry weight) of ACC/ml for 24 h at 37°C. After incubation, the trovafloxacin concentrations in the suspensions were determined in the bioassay. The binding capacity of ACC was determined by measuring the percent loss of activity of trovafloxacin at the different concentrations.
Statistical analysis.
The effect of trovafloxacin on the bacterial counts of the different pure and mixed cultures (E) was expressed as the difference, after 6 h of incubation, between the log10 CFU/milliliter in the absence and in the presence of trovafloxacin as described by the following equation: E = (Emax × Cs)/(EC50s + Cs), where Emax is the maximum observed effect, C is the concentration, EC50 is the concentration at which 50% of the maximum effect is reached, and s is a parameter for the steepness of the concentration-effect relationship. EC50 and s were calculated for each pure and mixed culture by using nonlinear least-squares regression techniques (GraphPad Prism version 3.0 for Windows; GraphPad Software, San Diego, Calif.). The 95% confidence intervals were used to determine significant differences between cultures.
Comparison of the mean reduction in bacterial counts of the pure cultures of B. fragilis, VREF, E. faecium, or E. coli 29020 with the corresponding mixed cultures was analyzed using the Tukey-Kramer multiple comparison test. A P value of <0.05 was considered significant.
RESULTS
MIC and MBC determinations.
The MICs and MBCs of trovafloxacin against B. fragilis ATCC 23745, B. fragilis ATCC 25285, E. coli ATCC 25922, VREF, and E. faecium using different inocula are shown in Table 1. MICs and MBCs of trovafloxacin increased for all strains when the inoculum was increased from 105 to 108 CFU/ml. However, the >64-fold increase against VREF and E. faecium was much greater than the 4- to 8-fold increase for E. coli and the two B. fragilis strains. Trovafloxacin had MICs of 16 and >64 μg/ml for trovafloxacin-resistant E. coli 29020 inocula of 105 and 108 CFU/ml, respectively. The bactericidal activity of trovafloxacin was decreased in the presence of ACC as indicated by the 2- to 16-fold increase in the MBCs of all strains.
TABLE 1.
In vitro activity of trovafloxacin against B. fragilis, E. coli, VREF, and E. faecium using different inocula with and without ACC
| Inoculum (CFU/ml) | ACC (mg/ml) | Trovafloxacin activity (μg/ml) against:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
B. fragilis ATCC 23745
|
B. fragilis ATCC 25285
|
E. coli ATCC 25922
|
VREF BM4147
|
E. faecium SH17
|
|||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | ||
| 105 | 0.25 | 0.25 | 0.125 | 0.125 | 0.06 | 0.06 | 1 | 1 | 1 | 1 | |
| 108 | 2 | 2 | 1 | 1 | 0.25 | 0.25 | >64 | >64 | >64 | >64 | |
| 105 | 4 | —a | 2 | — | 1 | — | 1 | — | 8 | — | 8 |
| 108 | 4 | — | 4 | — | 2 | — | 4 | — | >64 | — | >64 |
a—, MIC could not be read due to turbidity caused by ACC.
Time-kill studies.
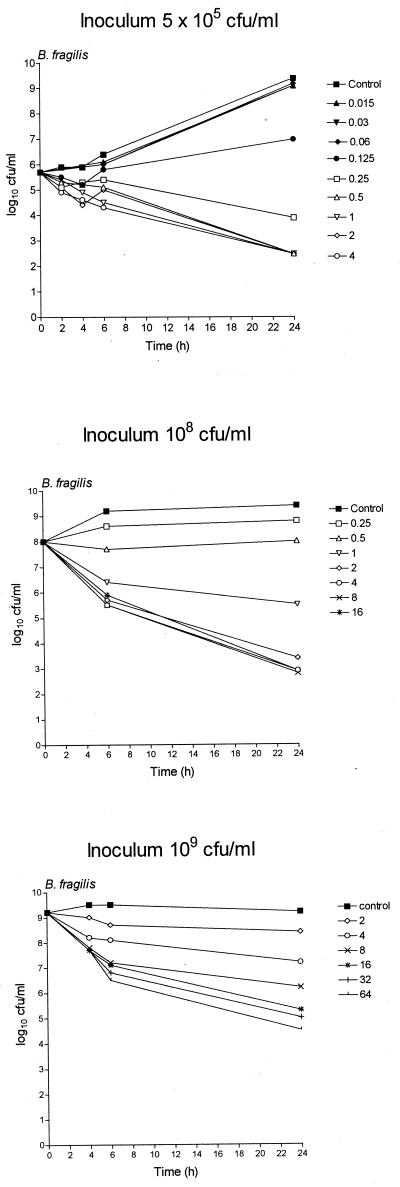
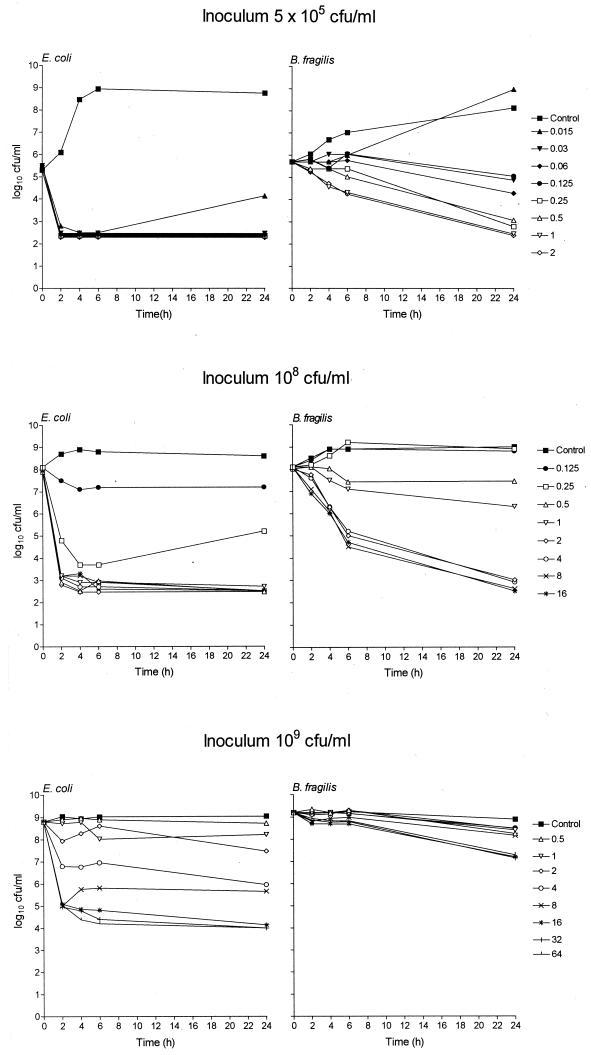
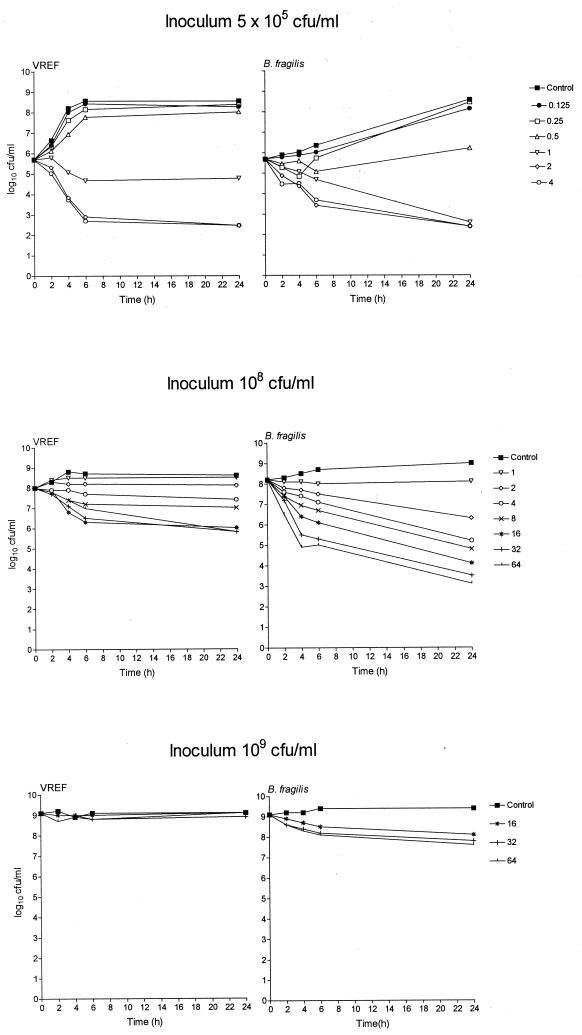
Fig. 1, 2, and 3 show the in vitro activity of trovafloxacin against B. fragilis ATCC 23745 in pure culture (Fig. 1) or in mixed culture with either E. coli ATCC 25922 (Fig. 2) or VREF (Fig. 3) using different inocula in the time-kill technique. Trovafloxacin produced a biphasic dose response whereby the bacterial killing was concentration dependent up to an optimum bactericidal concentration that resulted in a maximum log10 CFU/milliliter reduction in bacterial counts compared to counts of controls (Emax). The technique was reproducible with the mean difference in bacterial counts (log10 CFU/milliliter) between duplicate experiments having a standard deviation (SD) of <0.6. Trovafloxacin was effective in killing all standard inocula (5 × 105 CFU/ml) within 24 h at concentrations of 0.5 μg/ml (B. fragilis pure culture), 1 μg/ml (B. fragilis-E. coli mixed culture), and 2 μg/ml (B. fragilis-VREF mixed culture), resulting in an Emax of ≥6.1 for all strains. Similar Emaxs were attained with B. fragilis pure cultures and B. fragilis-E. coli mixed cultures when the inoculum was increased to 108 CFU/ml although a 2- to 4-fold increase in the trovafloxacin concentration (2 μg/ml) was required compared to the concentration required for the standard inocula. When the inoculum was further increased to 109 CFU/ml, trovafloxacin activity was retained against B. fragilis in pure culture. However, when in mixed culture with E. coli, the killing of B. fragilis was reduced. Trovafloxacin activity was retained against E. coli in the same mixed culture.
FIG. 1.
Time-kill studies of trovafloxacin against pure cultures of B. fragilis ATCC 23745 using inocula of 5 × 105, 1 × 108, or 1 × 109 CFU/ml. All stated trovafloxacin concentrations are in micrograms/milliliter.
FIG. 2.
Time-kill studies of trovafloxacin against mixed cultures of B. fragilis ATCC 23745 and E. coli ATCC 25922 using inocula of 5 × 105, 1 × 108, or 1 × 109 CFU/ml. All stated trovafloxacin concentrations are in micrograms/milliliter.
FIG. 3.
Time-kill studies of trovafloxacin against mixed cultures of B. fragilis ATCC 23745 and VREF BM4147 using inocula of 5 × 105, 1 × 108, or 1 × 109 CFU/ml. All stated trovafloxacin concentrations are in micrograms/milliliter.
The presence of large and static inocula had a more pronounced effect on the activity of trovafloxacin against mixed cultures of B. fragilis-VREF. To investigate these discrepancies further, the Emax and the EC50 were calculated from the bacterial counts measured after 6 h of incubation with trovafloxacin. Table 2 presents the Emax and the EC50 for B. fragilis ATCC 23745 in pure culture or in mixed culture with E. coli ATCC 25922 or VREF. There was little difference in the Emax for B. fragilis between the pure culture and the two mixed cultures with inocula of 5 × 105 and 1 × 108 CFU/ml. In addition, using the same inocula, a similar EC50 was found for B. fragilis in the pure cultures and the mixed cultures with E. coli. However, when in mixed culture with VREF, there was a 2-fold (inoculum, 5 × 105 CFU/ml) and a 14-fold (inoculum, 108 CFU/ml; P < 0.05) increase in the EC50 for B. fragilis compared to those of the pure cultures. With the higher inoculum, this difference in EC50 was significantly different (P < 0.05) from those of both the pure culture and the mixed culture with E. coli. Increasing the inoculum from 5 × 105 to 1 × 108 CFU/ml resulted in a significant 12-fold increase in EC50 for B. fragilis in mixed culture with VREF. With inocula of 109 CFU/ml, the EC50 for the mixed cultures could not be accurately determined due to inadequate killing of B. fragilis. However, compared to the other pure cultures, a significant increase in the EC50 of B. fragilis in pure culture was detected.
TABLE 2.
Activity of trovafloxacin against B. fragilis ATCC 23745 in pure culture or in mixed culture with either E. coli ATCC 25922 or VREF BM4147 determined by using the time-kill techniquea
| Inoculum (CFU/ml) | Culture | Emax (log10 CFU/ml ± SE) | EC50 (μg/ml ± SE) |
|---|---|---|---|
| 5 × 105 | B. fragilis | 2.0 ± 0.26 | 0.32 ± 1.50 |
| B. fragilis + E. coli | 3.0 ± 0.37 | 0.38 ± 1.35 | |
| B. fragilis + VREF | 3.0 ± 0.60 | 0.77 ± 1.48 | |
| 108 | B. fragilis | 3.6 ± 0.16 | 0.66 ± 1.20 |
| B. fragilis + E. coli | 4.0 ± 0.20 | 0.80 ± 1.18 | |
| B. fragilis + VREF | 4.0 ± 0.33 | 9.53 ± 1.27* | |
| 109 | B. fragilis | 3.2 ± 0.19 | 3.65 ± 1.19** |
| B. fragilis + E. coli | 0.5 ± 0.06 | ND | |
| B. fragilis + VREF | ND | ND |
Activities were determined after 6 h. ND, not determined, inadequate killing, or too few points to accurately calculate Emax and EC50. *, significantly different (P < 0.05) compared to the pure culture (108 CFU/ml), the mixed culture with E. coli (108 CFU/ml), and the mixed culture with VREF (5 × 105 CFU/ml). **, significantly different (P < 0.05) compared to other pure cultures.
The size of the inoculum also had a significant effect on the killing of VREF in the mixed culture with B. fragilis. Increasing the inoculum from 5 × 105 to 1 × 108 CFU/ml reduced the Emax from 5.8 ± 1.1 (best-fit value ± standard error) to 2.1 ± 0.24 log10 CFU/ml and increased the EC50 from 0.86 ± 1.03 to 4.63 ± 1.36 μg/ml, respectively (results not shown). In contrast, an Emax of >5 log10 CFU/ml for E. coli was found in all of the mixed cultures with B. fragilis (results not shown). The EC50 for E. coli in the 109-CFU/ml mixed culture was 2.2 ± 2.2 μg/ml. (The EC50 for E. coli in the 5 × 105 and 1 × 108 CFU/ml cultures could not be accurately determined.)
These results suggested that VREF conferred a “protective” effect on B. fragilis against the activity of trovafloxacin. To determine whether this phenomenon was also found with other strains, experiments were repeated using inocula of 108 CFU/ml of pure and mixed cultures of B. fragilis ATCC 23745, B. fragilis ATCC 25285, VREF, E. faecium, and trovafloxacin-resistant strain E. coli 29020. A trovafloxacin concentration of 2 μg/ml was used, and the bacterial counts were determined after 24 h (Table 3). (This concentration was chosen because it was effective in the killing of B. fragilis in both pure cultures and mixed cultures with E. coli.) The reduction in bacterial counts of both B. fragilis strains by trovafloxacin was approximately 3 log10 CFU/ml lower when these strains were grown in mixed culture with either VREF, E. faecium, or E. coli 29020 compared to that found with the pure cultures. This difference between the pure and mixed cultures was significant (P < 0.01). There was no significant difference in the reduction of B. fragilis bacterial counts between the different mixed cultures. The killing of VREF, E. faecium, and E. coli 29020 in all pure and mixed cultures was negligible. The residual concentrations of trovafloxacin in the supernatants of the enterococcal mixed cultures after 24 h incubation at 37°C were also measured in an agar diffusion bioassay. A bacteria-free control had a trovafloxacin concentration of 1.92 μg/ml compared to an initial measured concentration of 2.2 μg/ml. The trovafloxacin concentrations measured in all culture supernatants ranged from 1.4 to 2.16 μg/ml (73 to 113% [median, 94%] of the concentration found in the control).
TABLE 3.
Reduction in bacterial counts of pure or mixed cultures of B. fragilis, VREF, E. faecium, or E. coli strainsa
| Culture | Avg reduction(s) in bacterial counts (log10 CFU/ml ± SD) compared to those of controls b |
|---|---|
| B. fragilis ATCC 23745 | 5.3 ± 1.0 |
| B. fragilis ATCC 25285 | 6.6 ± 0.3 |
| VREF BM4147 | 0.9 ± 0.2 |
| E. faecium SH17 | 1.0 ± 0.3 |
| E. coli 29020 | 0.1 ± 0.1 |
| B. fragilis ATCC 23745 + VREF BM4147 | 2.4 ± 0.2c/0.8 ± 0.5 |
| B. fragilis ATCC 23745 + E. faecium SH17 | 3.0 ± 0.4d/1.0 ± 0.3 |
| B. fragilis ATCC 23745 + E. coli 29020 | 2.2 ± 0.3c/0.1 ± 0.1 |
| B. fragilis ATCC 25285 + VREF BM4147 | 3.4 ± 0.3c/0.9 ± 0.5 |
| B. fragilis ATCC 25285 + E. faecium SH17 | 3.9 ± 0.3c/1.0 ± 0.2 |
| B. fragilis ATCC 25285 + E. coli 29020 | 4.0 ± 0.2c/0.1 ± 0.1 |
Counts were taken after a 24-h incubation with 2 μg of trovafloxacin/ml and were determined by the time-kill technique.
Reductions are averages of three experiments using inocula of 108 CFU/ml.
Significantly different from counts obtained with pure cultures of B. fragilis (P < 0.001).
Significantly different from counts obtained with pure cultures of B. fragilis (P < 0.01).
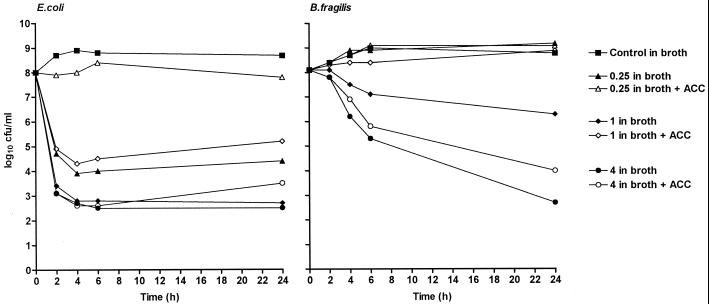
The effect of ACC on the activity of trovafloxacin was also investigated by using the time-kill technique. Figure 4 shows the killing of B. fragilis ATCC 23745 and E. coli ATCC 25922 mixed cultures by trovafloxacin with or without 4 mg of ACC/ml. The intestinal contents had a detrimental influence on the killing of both strains by trovafloxacin at concentrations of 0.25 and 1 μg/ml. However, this negative effect was minimal (B. fragilis) or not found (E. coli) with a trovafloxacin concentration of 4 μg/ml. This concentration dependent observation can be explained by the binding of ACC to trovafloxacin. At a concentration of 0.5 μg/ml, there was a >90% loss of antibacterial activity of trovafloxacin in the presence of ACC. However, at 2 and 16 μg/ml, the loss of activity was reduced to 62 and 46%, respectively. These results suggest that there is a saturation point in the binding of ACC to trovafloxacin.
FIG. 4.
Effect of ACC on the in vitro activity of trovafloxacin against mixed cultures of B. fragilis ATCC 23745 and E. coli ATCC 25922 in the time-kill study. Trovafloxacin concentrations (micrograms/milliliter) were inoculated with 108 CFU of either strain/ml with or without 4 mg (dry weight) of ACC/ml.
DISCUSSION
The aim of the present study was to investigate the in vitro activity of trovafloxacin, under anaerobic conditions, against mixed cultures of B. fragilis-E. coli or B. fragilis-VREF to gain insight into the factors that could influence its efficacy in the treatment of intra-abdominal abscesses. Since abscesses contain large numbers of bacteria in a stationary phase (Gyssens et al., 38th ICAAC), we studied the killing of large numbers of actively growing (108 CFU/ml) and static (109 CFU/ml) cultures to simulate conditions found in vivo.
The time-kill technique is a standard method used to follow the kinetics of bacterial killing in vitro (9). Time-kill studies under anaerobic conditions have been described by Spangler et al. (28); however, in contrast to their procedure, we have developed an anaerobic time-kill technique that does not require an anaerobic chamber. Although cultures were prepared under normal laboratory conditions, an anaerobic environment was obtained within 30 min of incubation by using Anaerogen sachets (17) and, as shown by control cultures in Fig. 1, 2, and 3, standard bacterial growth curves were obtained throughout the 24-h incubation period. The rate and extent of bacterial killing by trovafloxacin was concentration dependent up to an optimum bactericidal concentration, with further concentration increases producing similar bactericidal activity. This finding was reported also by Morrissey (21).
The MICs of trovafloxacin have been reported as being marginally affected by an increase in inoculum size (1, 8, 24). We have found a moderate effect of the inoculum on the killing of both B. fragilis strains and E. coli as indicated by the increase in MICs and MBCs when the inoculum was increased from 105 to 108 CFU/ml. However, these strains remained below the susceptible breakpoint for trovafloxacin of ≤2 μg/ml (12). In contrast, a large inoculum effect was found with both VREF and E. faecium in the present study and is similar to that reported by Hayden et al. (14) on the activity of levofloxacin against other clinically isolated enterococci.
In the present study, the EC50 was superior to the Emax in differentiating between the activities of trovafloxacin in the pure and mixed cultures. Previous studies also found the EC50 to be a valuable parameter in the comparison of the in vitro activities of different antibiotics against Staphylococcus aureus strains (16). The killing of B. fragilis ATCC 23745 in mixed culture with E. coli was similar to the killing of the anaerobe in pure culture by the time-kill technique with inocula of 5 × 105 and 1 × 108 CFU/ml. However, when in mixed culture with VREF, there was a 2-fold (inoculum, 5 × 105 CFU/ml) and a 14-fold (inoculum, 108 CFU/ml) increase in the trovafloxacin concentration required to obtain the same killing of B. fragilis as in the pure cultures. The effect of the inoculum on the killing of B. fragilis was also dependent on the other bacterial strain present in the mixed culture. Increasing the inoculum from 5 × 105 to 1 × 108 CFU/ml resulted in only a 2-fold increase in EC50 when the anaerobe was in mixed culture with E. coli but a 12-fold increase in the VREF mixed culture. This negative influence of VREF on the activity of trovafloxacin was also found with a vancomycin-susceptible strain of E. faecium in mixed culture with different B. fragilis strains. Nagy and Földes (22) have reported a similar protection of B. fragilis against the activity of metronidazole by Enterococcus faecalis. The authors observed that the antibiotic was inactivated by a cell extract of the enterococcus strain but not by culture supernatants. However, the inactivation of trovafloxacin by VREF or E. faecium was not found in the present study since approximately 94% of trovafloxacin activity was recovered from culture supernatants after a 24-h incubation compared to the activity of a bacteria-free control. Another possible explanation could be the production of an antagonistic substance by the enterococcal strains (5, 15) that could interfere with the activity of trovafloxacin. We have found, however, that culture supernatants from VREF and E. faecium failed to confer protection on B. fragilis ATCC 23745 (results not shown). The emergence and selection of trovafloxacin-resistant mutants (2, 5) with possible transfer of this resistance to B. fragilis in the mixed cultures (7, 11, 18, 20) could also account for this protection although further investigations would be required to confirm this hypothesis. It was interesting to observe that this phenomenon was not restricted to enterococcal mixed cultures but could be detected also in mixed cultures with a trovafloxacin-resistant E. coli strain.
These results suggest that, since the killing of VREF, E. faecium, or E. coli 29020 in the mixed cultures was minimal, the increased number of viable organisms was an important factor in the reduced activity of trovafloxacin against B. fragilis. However, in mixed cultures with B. fragilis and E. coli ATCC 25922 (inoculum, 109 CFU/ml), the activity of trovafloxacin against the anaerobe was also reduced despite the fact that there was an effective killing of the E. coli strain. However, the approximately 2 log10 CFU/ml larger number of viable E. coli cells remaining in these mixed cultures compared to the 108 CFU/ml cultures may also be a significant factor. The findings of the time-kill studies show, therefore, that the activity of trovafloxacin against large numbers of B. fragilis in mixed culture with different bacterial strains could be compromised when the bacterial numbers of the coexisting strain persist. Furthermore, the enterococcal strains, which under standard laboratory tests would be regarded as trovafloxacin susceptible, become totally resistant (inoculum, 109 CFU/ml) or require a significant increase in trovafloxacin concentrations to produce an effective killing of these strains (inoculum, 108 CFU/ml). For these reasons, trovafloxacin would appear to have little use in the treatment of infections involving large numbers of enterococci.
Previous studies have described different bactericidal mechanisms of action of trovafloxacin (21). Mechanism A is the basic quinolone mechanism and requires bacteria to be undergoing multiplication as well as protein or RNA synthesis while mechanism B does not require these processes to be present. In the same study, trovafloxacin was found to possess bactericidal mechanism B against E. coli KL16 and mechanism A against E. faecalis ATCC 19433. Similarly, by employing inocula that remained stable throughout the 24-h incubation period, we have detected differences in the killing by trovafloxacin against the different bacterial strains. Bactericidal activity was retained against E. coli ATCC 25922 and B. fragilis (in pure culture), indicating that active bacterial cell division was not a requirement for trovafloxacin with these strains. On the other hand, active bacterial multiplication appears to be of more importance for the bactericidal activity of trovafloxacin against VREF, as demonstrated by the negligible killing of the strain in the 109-CFU/ml cultures.
The protein binding capacity for trovafloxacin has been reported to be 70% in humans (3). ACC is composed of various constituents, including proteins, fibrous material, and bacteria, and since intra-abdominal abscesses comprise a certain amount of fecal material, we wished to determine the binding capacity of this complex mixture and investigate its effect on the activity of trovafloxacin. We have found that there is a saturation point in the binding of ACC to trovafloxacin, which was confirmed by the in vitro activity studies. In the MIC and MBC tests, a concentration of ≥1 μg/ml was required to kill ≥99.9% of all strains, while in the time-kill studies, ACC had a detrimental effect on the activity of trovafloxacin only when the antibiotic concentrations were <4 μg/ml. These results suggest that, providing that sufficiently high doses are employed, the presence of fecal materials in intra-abdominal abscesses should have little effect on the in vivo activity of trovafloxacin.
B. fragilis and E. coli are the most frequently isolated organisms from intra-abdominal abscesses (4, 29) and the effective in vitro killing of mixed cultures of these strains outlined in the present experiments have been confirmed by us in vivo using a mixed-infection subcutaneous abscess mouse model (I. C. Gyssens, L. E. T. Stearne, J. W. Mouton, W. H. Goessens, and H. A. Verbrugh, 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 800, 1999). Daily dosing of high concentrations of trovafloxacin resulted in not only a significant reduction in B. fragilis ATCC 23745 and E. coli ATCC 25922 bacterial counts but also a significant reduction in abscess weight and inflammation. In a similar mixed-infection mouse model with B. fragilis ATCC 23745 and VREF BM4147, the killing of VREF was ineffective while the killing of B. fragilis was significantly lower than killing in the mouse model with E. coli. These results corroborate the in vitro results described here.
In conclusion, the time-kill technique is a useful procedure to study the anaerobic killing of mixed cultures in vitro and may be of value in predicting the killing of mixed bacteria in vivo. It is important that the bacterial numbers and the mixed cultures studied in vitro reflect those found in vivo when determining the efficacy of an antibiotic as a treatment for mixed infections, as shown by the difference in the killing of B. fragilis in the mixed cultures tested. Due to its ability to retain activity against large cultures of B. fragilis and E. coli, trovafloxacin could be beneficial in the treatment of intra-abdominal abscesses.
ACKNOWLEDGMENTS
This study was financially supported by Pfizer B. V., Capelle aan de IJssel, The Netherlands.
H. Mattie is gratefully acknowledged for his valuable comments on the manuscript.
REFERENCES
- 1.Aldridge K E, Ashcraft D, Bowman K A. Comparative in vitro activities of trovafloxacin (CP 99, 219) and other antimicrobials against clinically significant anaerobes. Antimicrob Agents Chemother. 1997;41:484–487. doi: 10.1128/aac.41.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Lorian V, editor. Antibiotics in laboratory medicine. 3rd ed. Baltimore, Md: Williams & Wilkens Co.; 1991. pp. 53–105. [Google Scholar]
- 3.Bightly K E, Gootz T D. The chemistry and biological profile of trovafloxacin. J Antimicrob Chemother. 1997;39(Suppl. B):1–14. doi: 10.1093/jac/39.suppl_2.1. [DOI] [PubMed] [Google Scholar]
- 4.Brook I. In vitro susceptibility vs in vivo efficacy of various antimicrobial agents against the Bacteroides fragilis group. Rev Infect Dis. 1991;13:1170–1180. doi: 10.1093/clinids/13.6.1170. [DOI] [PubMed] [Google Scholar]
- 5.Brook I. Inoculum effect. Rev Infect Dis. 1989;11:361–368. doi: 10.1093/clinids/11.3.361. [DOI] [PubMed] [Google Scholar]
- 6.Citron D M, Appleman M D. Comparative in vitro activities of trovafloxacin (CP-99, 219) against 221 aerobic and 217 anaerobic bacteria isolated from patients with intra-abdominal infections. Antimicrob Agents Chemother. 1997;41:2312–2316. doi: 10.1128/aac.41.10.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clewell D B. Plasmids, drug resistance and gene transfer in the genus Streptococcus. Microbiol Rev. 1981;45:409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coque T M, Singh K V, Murray B E. Comparative in-vitro activity of the new fluoroquinolone trovafloxacin (CP-99, 219) against Gram-positive cocci. J Antimicrob Chemother. 1996;37:1011–1016. doi: 10.1093/jac/37.5.1011. [DOI] [PubMed] [Google Scholar]
- 9.Craig W A, Ebert S C. Killing and regrowth of bacteria in vitro: a review. Scand J Infect Dis. 1991;74(Suppl.):63–70. [PubMed] [Google Scholar]
- 10.Dunn D L, Simmons R L. The role of anaerobic bacteria in intraabdominal infections. Rev Infect Dis. 1984;6(Suppl. 1):S139–S146. doi: 10.1093/clinids/6.supplement_1.s139. [DOI] [PubMed] [Google Scholar]
- 11.El Amin N, Jalal S, Wretlind B. Alterations in GyrA and ParC associated with fluoroquinolone resistance in Enterococcus faecium. Antimicrob Agents Chemother. 1999;43:947–949. doi: 10.1128/aac.43.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garey K W, Amsden G W. Trovafloxacin: an overview. Pharmacotherapy. 1999;19:21–34. doi: 10.1592/phco.19.1.21.30507. [DOI] [PubMed] [Google Scholar]
- 13.Haria M, Lamb H M. Trovafloxacin. Drugs. 1997;54:435–445. doi: 10.2165/00003495-199754030-00006. [DOI] [PubMed] [Google Scholar]
- 14.Hayden M K, Matushek M G, Trenholme G M. Comparison of the in vitro activity of levofloxacin and other antimicrobial agents against vancomycin-susceptible and vancomycin-resistant Enterococcus species. Diagn Microbiol Infect Dis. 1995;22:349–352. doi: 10.1016/0732-8893(95)00144-1. [DOI] [PubMed] [Google Scholar]
- 15.Heilman F. Ampicillin and ampicillin-sulbactam dilution tests with mixed cultures of Bacteroides fragilis, Escherichia coli and Enterococcus. Infection. 1993;21:187–191. doi: 10.1007/BF01710545. [DOI] [PubMed] [Google Scholar]
- 16.Hoogeterp J J, Mattie H, Krul A M, Terporten P, van Furth R. Comparison of in vivo and in vitro activities of antibiotics with different modes of action against a tolerant and a non-tolerant Staphylococcus aureus strain. Scand J Infect Dis. 1989;21:95–101. doi: 10.3109/00365548909035686. [DOI] [PubMed] [Google Scholar]
- 17.Imhof A, Heinzer I. Continuous monitoring of oxygen concentrations in several systems for cultivation of anaerobic bacteria. J Clin Microbiol. 1996;34:1646–1648. doi: 10.1128/jcm.34.7.1646-1648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob A E, Hobbs S J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974;117:360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joiner K A, Onderdonk A B, Gelfand J A, Bartlett J G, Gorbach S L. A quantitative model for subcutaneous abscess formation in mice. Br J Exp Pathol. 1980;61:97–107. [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez-Martinez L, Pascual A, Jacoby G A. Quinolone resistance from a transferable plasmid. Lancet. 1998;351:797–799. doi: 10.1016/S0140-6736(97)07322-4. [DOI] [PubMed] [Google Scholar]
- 21.Morrissey I. Bactericidal activity of trovafloxacin (CP-99, 219) J Antimicrob Chemother. 1996;38:1061–1066. doi: 10.1093/jac/38.6.1061. [DOI] [PubMed] [Google Scholar]
- 22.Nagy E, Foldes J. Inactivation of metronidazole by Enterococcus faecalis. J Antimicrob Chemother. 1991;27:63–70. doi: 10.1093/jac/27.1.63. [DOI] [PubMed] [Google Scholar]
- 23.National Committee for Clinical Laboratory Standards. Methods for antimicrobial susceptibility testing of anaerobic bacteria. 2nd ed. 9. Approved standard. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 24.Neu H C, Chin N. In vitro activity of the new fluoroquinolone CP-99,219. Antimicrob Agents Chemother. 1994;38:2615–2622. doi: 10.1128/aac.38.11.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pankuch G A, Jacobs M R, Appelbaum P C. Postantibiotic effect of trovafloxacin against gram-positive and gram-negative organisms. Antimicrob Agents Chemother. 1998;42:1503–1505. doi: 10.1128/aac.42.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soriano F, Edwards R, Greenwood D. Effect of inoculum size on bacteriolytic activity of cefminox and four other β-lactam antibiotics against Escherichia coli. Antimicrob Agents Chemother. 1992;36:223–226. doi: 10.1128/aac.36.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spangler S K, Jacobs M R, Appelbaum P C. Activity of CP 99,219 compared with those of ciprofloxacin, grepafloxacin, metronidazole, cefoxitin, piperacillin and piperacillin-tazobactam against 489 anaerobes. Antimicrob Agents Chemother. 1994;38:2471–2476. doi: 10.1128/aac.38.10.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spangler S K, Jacobs M R, Appelbaum P C. Time-kill study of the activity of trovafloxacin compared with ciprofloxacin, sparfloxacin, metronidazole, cefoxitin, piperacillin and piperacillin/tazobactam against six anaerobes. J Antimicrob Chemother. 1997;39(Suppl. B):23–27. doi: 10.1093/jac/39.suppl_2.23. [DOI] [PubMed] [Google Scholar]
- 29.Tally F P. Factors affecting the choice of antibiotics in mixed infections. J Antimicrob Chemother. 1988;22(Suppl. A):87–100. doi: 10.1093/jac/22.supplement_a.87. [DOI] [PubMed] [Google Scholar]
- 30.Tuomanen E, Cozens R, Tosch W, Zak O, Tomasz A. The rate of killing of Escherichia coli by B-lactam antibiotics is strictly proportional to the rate of bacterial growth. J Gen Microbiol. 1986;132:1297–1304. doi: 10.1099/00221287-132-5-1297. [DOI] [PubMed] [Google Scholar]
- 31.Visalli M A, Jacobs M R, Appelbaum P C. Activity of CP 99,219 (trovafloxacin) compared with ciprofloxacin, sparfloxacin, clinafloxacin, lomefloxacin and cefuroxime against ten penicillin-susceptible and penicillin-resistant pneumococci by time-kill methodology. J Antimicrob Chemother. 1996;37:77–84. doi: 10.1093/jac/37.1.77. [DOI] [PubMed] [Google Scholar]
- 32.Widmer A F, Wiestner A, Frei R, Zimmerli W. Killing of non-growing and adherent Escherichia coli determines drug efficacy in device-related infections. Antimicrob Agents Chemother. 1991;35:741–746. doi: 10.1128/aac.35.4.741. [DOI] [PMC free article] [PubMed] [Google Scholar]