Abstract
Patients still die from Streptococcus pneumoniae pneumonia after initiation of antibiotic therapy, when tissues are sterile and the pneumonia is clearing. There is growing evidence that overwhelming inflammation resulting from toxin release contributes to tissue injury, shock, and death. Monitoring host response may help us understand the consequences of antibiotic therapy for the inflammatory processes that occur in bacterial pneumonia. HMR 3004 is a ketolide that displays excellent in vitro activity against S. pneumoniae. In the present experiment, we investigated the chronology of inflammatory events that occur during pneumococcal pneumonia in mice treated with HMR 3004. Infection of mice with 107 CFU of living S. pneumoniae resulted in 100% mortality within 5 days. HMR 3004 given at 12.5 mg/kg of body weight/dose twice daily from 48 h postinfection achieved complete bacterial clearance from lungs and blood within 36 h and ensured survival of mice. Recruitment of neutrophils and monocytes from blood to lungs was significantly reduced, and nitric oxide release was totally prevented. Interleukin-6 secretion in lungs and blood became rapidly undetectable after initiation of therapy. Histological examination of lung tissue showed protection of interstitium against edema. By controlling bacterial invasion, HMR 3004 led to rapid and profound modifications of the host response in lungs, which may protect mice from deleterious inflammatory reactions.
Streptococcal pneumonia is the most common cause of community-acquired bacterial pneumonia. The worldwide increase in the resistance of Streptococcus pneumoniae to penicillin G and other antimicrobials has dramatically complicated the management of pneumococcal infections (2, 16, 17, 25). HMR 3004 belongs to the ketolide family, which represents a class of 14-membered ring macrolide agents that are characterized by a keto group at position 3 of the macrolactone ring, which replaces the l-cladinose moiety of other members of the macrolide group. Studies with HMR 3004 have indicated that ketolides offer a potential alternative for treating penicillin- and erythromycin-resistant S. pneumoniae strains (1, 7, 12, 20, 21). HMR 3004 activity was tested against erythromycin-resistant pneumococci in murine pneumonia models: the bactericidal activity of the drug in the lungs was associated with good pulmonary diffusion and a slightly prolonged half-life compared to those of typical 14-membered ring macrolides (P. Rajagopalan-Levasseur, E. Vallée, C. Agouridas, J. F. Chantot, and J. J. Pocidalo, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F173, 1995).
Pulmonary phagocytic cell recruitment and inflammatory mediator release also play a pivotal role in the effective killing of respiratory pathogens. However, there is increasing evidence indicating that during acute bacterial pneumonia the combination of bacterial virulence factors and excessive inflammatory reactions from the host together contribute to induce severe lung injury, shock, and death (reviewed in references 3 and 4). Only a few investigators have evaluated the chronology and magnitude of inflammatory events that accompany bacterial clearance during antibiotic therapy. In this study, we investigated the antibacterial activity of HMR 3004 in a murine model of pneumococcal pneumonia and the concomitant evolution of the inflammatory response, including phagocytic cell recruitment and release of cytokines and nitric oxide (NO) in blood, bronchoalveolar lavage fluid (BALF), and lung tissue. Correlations of these factors with histopathology and outcome of pneumonia were made.
MATERIALS AND METHODS
Bacteria.
A penicillin-susceptible clinical strain of S. pneumoniae serotype 3 was used for all experiments. HMR 3004 had a MIC at which 90% of isolates were inhibited of 0.015 mg/liter for this strain.
Animals.
Male CD1 Swiss mice (25 to 28 g) were obtained from Charles River (Québec, Canada). Animals had free access to food and water and were exposed to alternate standardized light/dark periods of 14 and 10 h/day.
Pneumococcal pneumonia model.
To prepare the inoculum, an overnight culture of bacteria was initially grown in brain heart infusion agar and was frozen in 1-ml aliquots at −80°C. For each experiment, a 1-ml volume was thawed and used to seed fresh brain heart infusion agar, and then the mixture was incubated overnight at 37°C in a 5% CO2 atmosphere. The cultures were centrifuged, washed, and resuspended in phosphate-buffered saline (PBS) to obtain the appropriate concentration for inoculation of animals. Mice were infected as previously described (3), with minor modifications. Briefly, lightly anesthetized mice received an inoculum of 107 log-phase CFU of bacteria in 50 μl of PBS, which was applied at the tip of the nose and involuntarily inhaled. To facilitate migration of the inoculum to the alveoli, mice were held in a vertical position for 2 min. Control mice received intranasal PBS.
Determination of HMR 3004 dosage.
The HMR 3004 dosage was chosen to approximate peak serum drug concentrations of 2 μg/ml, which were measured in human serum after administration of a standard dosage of HMR 3647 (B. Lenfant, E. Sultan, C. Wable, M. H. Pascual, and B. H. Meyer, Abstr. 38th Intersci. Conf. Antimicrob. Agents Chemother., abstr. A49, 1998). Since maximum levels of HMR 3004 in plasma in mice were about 4.5 μg/ml after an oral dose of 50 mg/kg of body weight, we assumed that a total daily dose of 25 mg/kg was suitable for the experiments (18).
Survival rate study.
Determination of the efficacy of HMR 3004 against pneumococcal pneumonia was first established in survival rate studies. Groups of 12 mice were infected as described above. Treatments with HMR 3004 at 12.5 mg/kg/dose (by gavage) were initiated either 24, 48, or 72 h postinfection (p.i.) and were maintained every 12 h for up to 5 days or until death of the animals (total daily administration of 25 mg/kg). Control mice received sterile saline. Survival rate was recorded every 24 h until day 5 p.i.
Influence of HMR 3004 on the pathophysiology of pneumococcal pneumonia: experimental protocol.
Based on the observation that 25 mg/kg/day was very effective in protecting against mortality, and considering that in clinical practice therapy most likely is initiated once pneumonia is well established, we undertook a series of pathogenesis studies with therapy initiated 48 h p.i. at the dosage of 25 mg/kg/day. Four groups of mice received either bacteria alone, HMR 3004 alone, bacteria plus HMR 3004, or the appropriate control diluent. Twelve animals in each group were sacrificed at 0, 6, 12, 24, 30, and 36 h after initiation of treatment, which corresponds to the 48- to 84-h period p.i. that is critical for death or survival in untreated versus HMR 3004-treated mice. Blood, BALF, and lung tissue were sampled to determine the cellular response and to quantify inflammatory mediators. Histopathology was also done on tissue sections. Bacterial counts were determined in lung tissue, and bacteremia was also verified.
Development of infection.
Lungs and heart were taken together and weighed before and after blood removal with 20 ml of sterile saline infused through the right ventricle until the effluent was clear. Lungs were then homogenized with a Potter homogenizer at a ratio of 1 g/10 ml of 50 mM potassium phosphate buffer (pH 6.5). Bacteria were quantified in this homogenate by plating 10-fold dilutions on blood sheep agar. S. pneumoniae cells were counted and results were expressed as CFU per gram of tissue. Dissemination of bacteria to the bloodstream was followed by sampling blood from the retro-orbital sinus of the left eye with a heparinized capillary tube, followed by direct plating on blood agar. Bacteremia was reported as the percentage of positive hemocultures after an incubation period of 12 h at 37°C in 5% CO2.
Hematological parameters.
Blood was collected in heparinized microtainer tubes and leukocytes were analyzed with a Coulter counter. Differentiation of cell populations was obtained after counting 100 leukocytes on a smear stained with Wright reagent.
Inflammatory cells.
Leukocyte recruitment to alveoli was determined in the BALF. Briefly, animals were killed by cervical dislocation, the trachea was exposed and intubated with a catheter, and then repeated 1-ml injections of PBS were made until a total of 3 ml of BALF was recovered. BALF was centrifuged at 3,400 × g for 10 min, and supernatant was frozen at −80°C until analysis of inflammatory mediators. Cells in the pellet were resuspended in PBS for quantification of leukocytes with a hemacytometer, and cell populations were enumerated from Diff-Quick (Baxter, Pointe-Claire, Québec, Canada)-stained cytospin preparations.
Neutrophil (polymorphonuclear cell [PMN]) infiltration in lung tissue was quantified through the measurement of myeloperoxidase (MPO), as previously described (3). Briefly, blood-free lung homogenates were sonicated and centrifuged at 3,000 × g for 30 min at 4°C. MPO was evaluated by adding 150 μl of the supernatant to 825 μl of phosphate buffer, 75 μl of o-dianisidine at 1.25 mg/ml in distilled water, and 75 μl of hydrogen peroxide at 0.05%. The enzymatic reaction was stopped after 15 min by adding 75 μl of sodium azide (1%), and absorbance was read at 450 nm against a standard curve made with commercial MPO (Sigma, Oakville, Ontario, Canada).
Inflammatory mediators.
Interleukin-6 (IL-6) levels were detected in the supernatant of BALF, in the supernatant of lung homogenates, and in the serum of control and infected animals, treated or not treated with HMR 3004. Lung homogenates were prepared in potassium phosphate buffer containing aprotinin (20 U) and 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) (0.2%). They were centrifuged at 3,000 × g in a microcentrifuge for 30 min at 4°C, and resulting supernatants were subjected to a sandwich enzyme-linked immunosorbent assay to quantify IL-6 (KM-IL-6 kit; Endogen, Cambridge, Mass.). The release of NO was also evaluated through the measurement of its oxidized nitrite and nitrate metabolites, according to the colorimetric method of Griess, as described previously (10).
Histopathology.
At each time point, the left lung from one animal per group was fixed in formaldehyde, embedded in paraffin, and processed for light microscopy as previously described (3). Tissue sections were stained with hematoxylin and eosin.
Statistical analysis.
Statistical differences between groups were analyzed by using the Mann-Whitney U test for nonparametric data and Fisher's protected least significant difference test for normally distributed data. A P value of <0.05 was considered significant. All data are presented as means ± standard errors of the means (SEM).
RESULTS
Therapeutic efficacy of HMR 3004 against mortality in experimental pneumococcal pneumonia.
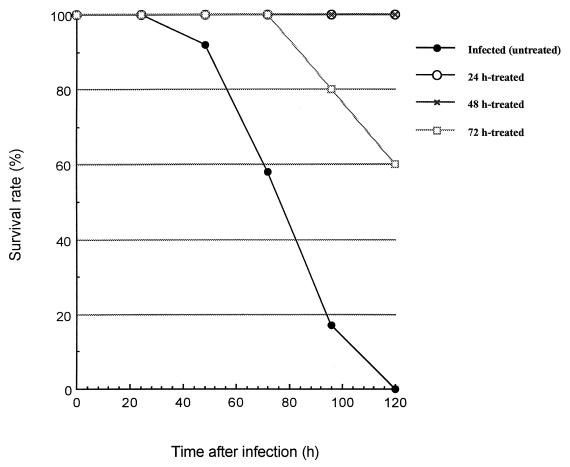
Inoculation of mice with 107 CFU of S. pneumoniae resulted in 100% mortality in untreated animals within 5 days p.i. (Fig. 1). HMR 3004 at 25 mg/kg/day initiated 24, 48, or 72 h p.i. was associated with survival rates of 100% (P < 0.000001), 100% (P < 0.000001), and 60% (P < 0.02), respectively, over the same period.
FIG. 1.
Number of survivors in different groups of untreated and HMR 3004-treated infected mice. HMR 3004 was delivered by gavage at a dose of 25 mg/kg (12.5 mg/kg/day given twice a day), started 24, 48, or 72 h after intranasal inoculation of animals with 107 S. pneumoniae bacteria. Twelve mice per group were infected to determine survival rate.
Impact of HMR 3004 on the pathophysiology of pneumonia. (i) Mouse infection model.
All infected animals presented with tachypnea and piloerection, observable 4 h after infection. Untreated mice developed a gradually pronounced hypodynamic state (lifeless behavior, prostration) thereafter, which was associated with a gradual loss in body weight from 24 h p.i. until death. By contrast, average body weight, which had fallen from 28.0 g at the time of infection to 22.1 g at 48 h p.i. (time when therapy with HMR 3004 was initiated), started to increase again thereafter in infected treated animals and reached 23.0, 24.8, and 25.5 g at 24, 48, and 72 h, respectively, after initiation of therapy.
(ii) Evaluation of bacterial parameters: bacterial clearance from lungs and bacteremia.
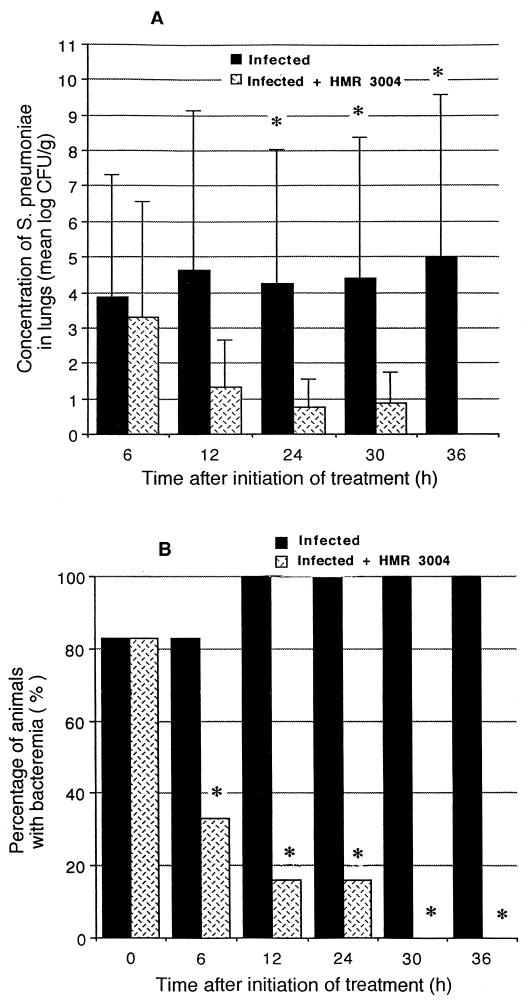
Bacterial counts in lung tissue had reached 104 CFU/g when antibiotic treatment was initiated. They remained high in untreated animals over the subsequent 36 h (Fig. 2A). By comparison, the tissue burden of S. pneumoniae significantly decreased after initiation of treatment with HMR 3004, and lungs were sterile over a 36-h period. Eighty-three percent of animals were bacteremic by the time therapy was initiated (Fig. 2B). A significant reduction (P < 0.05) gradually occurred in infected treated mice from 6 to 30 h after initiation of therapy; all treated animals had sterile blood by 30 h.
FIG. 2.
Quantitative lung tissue cultures (A) and percentage of animals developing bacteremia (B) in experimental pneumococcal pneumonia. Tissue burden of S. pneumoniae was rapidly reduced in mice treated with HMR 3004 at a dose of 25 mg/kg/day, started 48 h after infection (n = 6 at each time point). ∗, P < 0.05, treated versus untreated mice.
(iii) Evaluation of cellular parameters. (a) Inflammatory cells in blood.
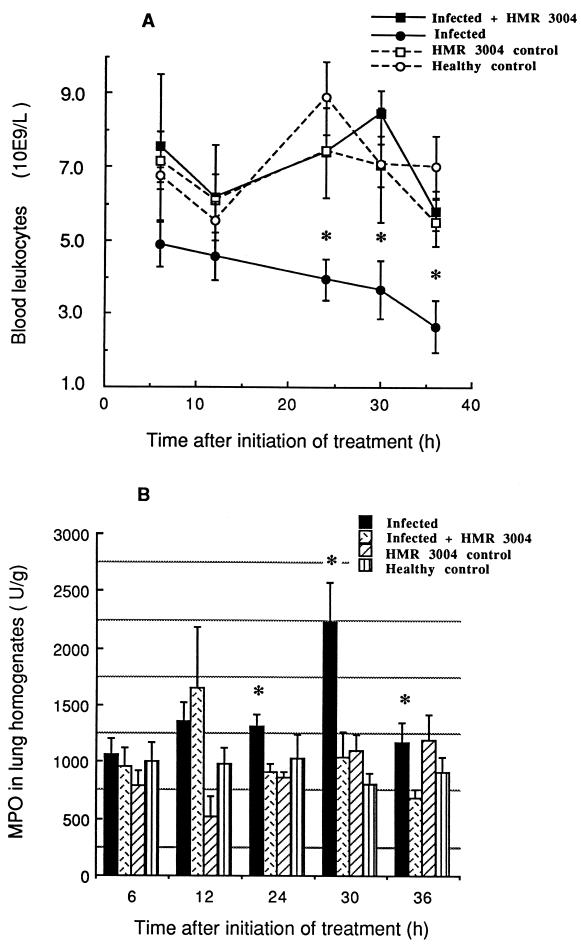
There were no significant differences in mean blood leukocyte counts between uninfected controls and infected mice treated with HMR 3004 (Fig. 3A). By contrast, untreated infected mice underwent progressive leukopenia. Comparisons between treated and untreated infected animals in terms of leukocyte counts (109 cells per liter) reached statistical significance 24 h (7.40 ± 1.23 versus 3.94 ± 0.55, P = 0.03), 30 h (8.46 ± 0.60 versus 3.66 ± 0.81, P = 0.006), and 36 h (5.82 ± 0.530 versus 2.65 ± 0.71, P = 0.008) after initiation of therapy.
FIG. 3.
Mean (±SEM) blood leukocyte counts (A) and MPO levels (B) in lung homogenates of infected and healthy mice, treated or not with HMR 3004 at 25 mg/kg/day. Significant leukopenia was seen in the untreated infected mice from 24 to 36 h after initiation of treatment (which was started 48 h p.i.) compared to the healthy controls or the HMR 3004-treated infected mice. Recruitment of PMNs to the lung (MPO levels) was greatly reduced by HMR 3004. ∗, P < 0.05.
(b) Neutrophils in lung tissue (MPO activity).
Infection resulted in high MPO levels in lung tissue in this experiment (Fig. 3B). HMR 3004 prevented this increase. Significant differences were seen between treated and untreated infected mice 24 h (P = 0.01), 30 h (P = 0.02), and 36 h (P = 0.02) after initiation of therapy.
(c) Inflammatory cells in BALF.
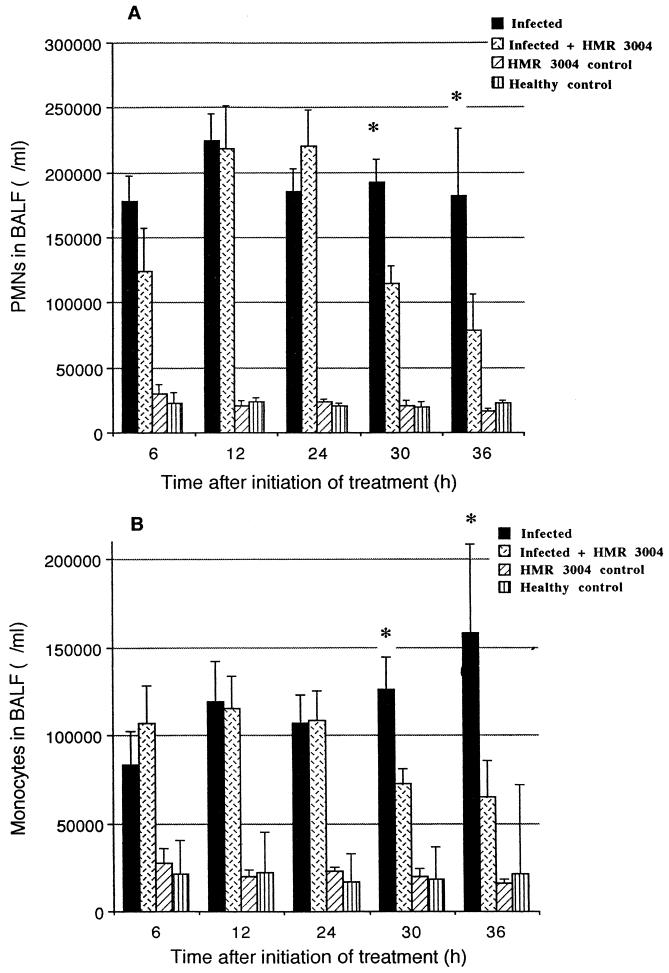
Compared to healthy controls, all infected mice exhibited high steady PMN counts in BALF at every time point of the experiment (Fig. 4A). A significant decrease in PMN recruitment occurred from 30 h after initiation of therapy, which corresponds to a gradual cure from bacterial invasion. As for the monocyte/macrophage recruitment in alveoli (BALF), a gradual increase was noted in untreated infected mice (Fig. 4B). A significant reduction in these cell counts was observed at 30 h (P = 0.02) and 36 h (P = 0.04) after initiation of treatment.
FIG. 4.
Mean (±SEM) neutrophil (A) and monocyte (B) counts in BALF of infected and healthy mice, treated or not with HMR 3004 at 25 mg/kg/day. Significant decreases in both cell populations were seen by 30 h after initiation of therapy, which was started 48 h p.i. ∗, P < 0.05.
(iv) Evaluation of inflammatory parameters. (a) Protection from lung edema during HMR 3004 therapy.
Edema was followed as a criterion to evaluate host inflammatory reactions. Right-lung weights in infected mice were significantly higher than in control or infected treated mice at 24 h (186 ± 15 mg in infected mice versus 125 ± 11 mg in infected treated mice, P = 0.012) and 36 h (174 ± 11 mg versus 131 ± 7 mg, P = 0.016) after initiation of treatment.
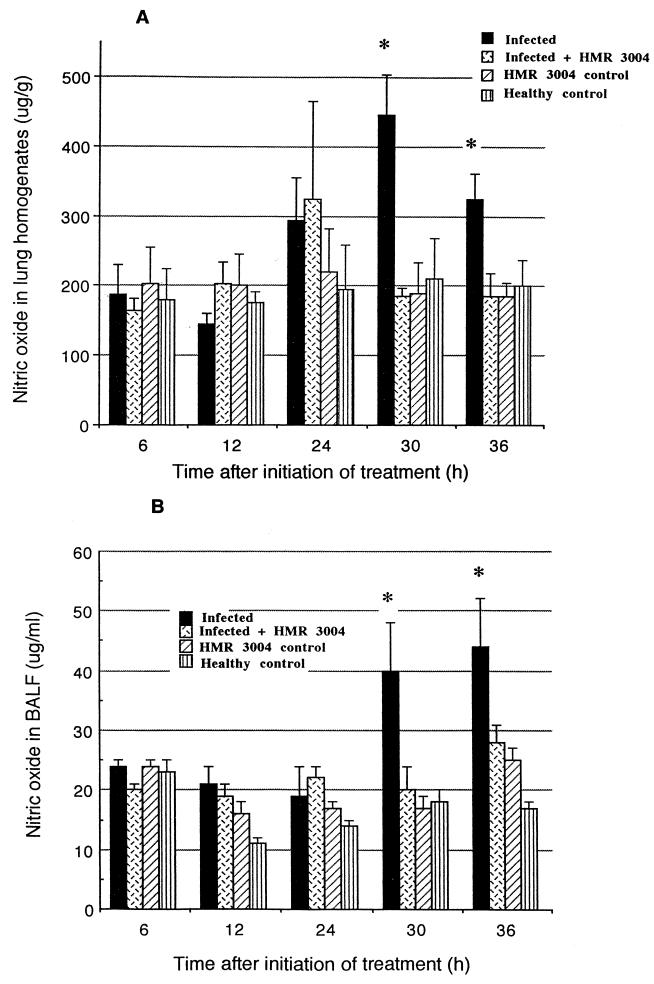
(b) Pulmonary NO release.
High NO secretion was observed in lung tissue (Fig. 5A) and BALF (Fig. 5B) of infected mice over the indicated 30- to 36-h period (which corresponds to 78 to 84 h p.i.). Statistical significance reached levels of P = 0.002 and P = 0.04 in lung tissue at the respective times and P = 0.05 at both times in BALF. HMR 3004 totally abrogated the effect of infection, as levels similar to the control uninfected group were observed in infected treated animals.
FIG. 5.
Mean (±SEM) NO levels in lung homogenates (A) and BALF (B) of infected and healthy mice, treated or not with HMR 3004 at 25 mg/kg/day. A significant prevention in NO release was noted in infected treated mice in comparison to untreated infected mice. Therapy was initiated 48 h p.i. ∗, P < 0.05.
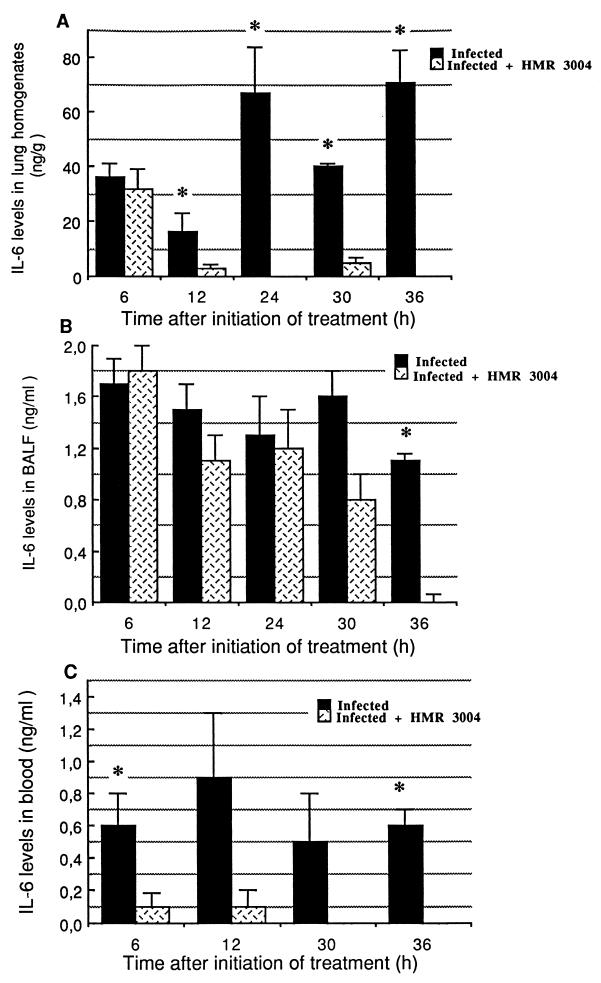
(c) Profile of IL-6 release in BALF, lung homogenates, and blood.
IL-6 was detected in BALF, lung homogenates, and blood. While IL-6 remained below the limit of detection in uninfected animals, infection with pneumococci stimulated IL-6 secretion in lung tissue (Fig. 6A), BALF (Fig. 6B), and blood (Fig. 6C). Strong inhibition of IL-6 in blood and lung tissue was noted in HMR 3004-treated animals as early as 6 h (P = 0.03) and 12 h (P = 0.001), respectively. IL-6 was detected over a longer period in BALF of treated animals, but significantly lower levels were also noted at 36 h (P = 0.0003).
FIG. 6.
Mean (±SEM) IL-6 levels in lung homogenates (A), BALF (B), and serum (C) of infected and healthy mice, treated or not with HMR 3004 at 25 mg/kg/day. Significant reduction in IL-6 was rapidly observed in blood and lung tissue after initiation of therapy, which was started 48 h p.i. IL-6 remained undetectable in uninfected animals. ∗, P < 0.05.
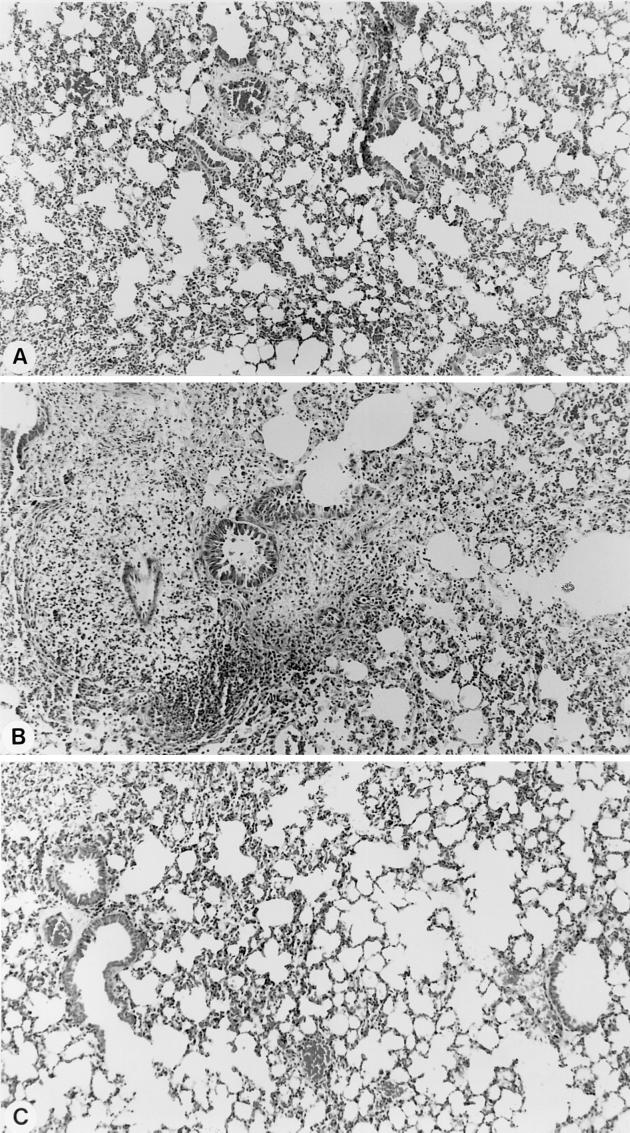
(v) Histopathology.
Figure 7 shows the protection afforded by HMR 3004 on tissue integrity in infected mice. While infection contributed to swelling of tissue interstitium and edema by 78 h p.i. (Fig. 7B), HMR 3004 administered from 48 h p.i. resulted, within 30 h after initiation of therapy (corresponding to 78 h p.i.), in almost complete protection from inflammation (Fig. 7A); tissues showed little edema and looked quite the same as tissues from healthy controls (Fig. 7C).
FIG. 7.
Histology of lung tissue in infected mice treated with HMR 3004 (A), untreated infected mice (B), or healthy control mice (C). Pictures were taken 30 h after initiation of therapy, which corresponds to 78 h p.i. Diffuse edema with swelling of interstitium was noted in untreated infected animals, while HMR 3004-treated mice recovered very fast and had tissue profiles similar to those of healthy controls.
DISCUSSION
While many bacterium-induced pneumonia rodent models are being used to evaluate antibiotic pharmacokinetics and efficacy, few investigators have evaluated the impact of antibiotic therapy on bacterial clearance, host response, and tissue integrity at the same time. Studying inflammation is particularly important in view of the fact that patients still die from pneumonia and bacteremia despite effective antibiotic therapy, and overwhelming inflammation most likely contributes to pulmonary dysfunction, multiple organ failure, and shock (reviewed in references 3 and 4). Our previous observations (3, 4) actually allowed us to identify five pathogenesis steps that characterize fatal pneumococcal pneumonia in mice. In the present experiment, we investigated the pathogenesis of pneumonia in animals treated with HMR 3004 at a dose that ensured 100% survival despite infection with a lethal inoculum. Our goal was to monitor simultaneously (in blood, lung tissue, and BALF of treated and untreated mice) various microbiological and inflammatory factors, to better correlate them with the histopathology and outcome of pneumonia. In this context, we followed the impact of HMR 3004 on bacterial kinetics in lungs and blood, on phagocyte infiltration into the lungs, and on the chronology of activation and inhibition of IL-6 and NO, as well as on pulmonary edema and survival of the animals. We showed that bacterial clearance by HMR 3004 at a dose of 25 mg/kg/day was associated with a concomitant reduction in inflammation, rapid recovery from tissue injury, and complete protection from death. Such extensive data on the influence of a ketolide on the pathogenesis of pneumococcal pneumonia have not been reported elsewhere.
In our model, HMR 3004 showed very effective antibacterial activity, as bacteria were cleared from both lungs and blood within 36 h after initiation of treatment, which was started 48 h p.i., a time when untreated mice are very sick and start dying. By the time lungs and blood were sterile, animals had received only three doses of HMR 3004 (at 12.5 mg/kg/dose every 12 h). Our investigation also showed that HMR 3004 administration was closely associated with a marked decrease in pulmonary inflammation that was noticeable early after initiation of therapy but was further evidenced over a short 36-h period. The large decrease in bacterial counts was associated with a profound and rapid modification in leukocyte trafficking. In fact, PMN recruitment to the lungs rapidly fell in HMR 3004-treated animals, as demonstrated by the significant decrease in MPO levels in tissues, PMN counts in BALF, and limited leukocytosis in blood. By contrast, untreated infected animals experienced sustained leukopenia until death, resulting from continuous recruitment of leukocytes from the bloodstream to lung compartments. Also, HMR 3004 prevented the late monocyte recruitment to the lungs which we have shown to precede mortality in murine pneumococcal pneumonia (3). Rapid normalization of leukocyte trafficking under antimicrobial therapy may thus represent a critical issue for a beneficial outcome of pneumonia, inasmuch as these cells secrete oxidative intermediates and proteolytic enzymes that would exert detrimental effects on structural cells once bacteria have been cleared by the drug (22).
As infection and inflammation progressed in untreated mice, lung morphological changes consisted mainly of gradual edema and tissue “hepatization” (having a liver-like appearance), with a consequent increase in lung weight. By contrast, regression of pulmonary inflammation in HMR 3004-treated infected mice prevented this increase and thus facilitated health recovery.
As for the inflammatory mediators, NO is now recognized as a critically important participant in the host response to infection (8). Strong evidence indicates that excessive NO production has deleterious effects during infectious states: NO is involved in the vascular collapse that is the hallmark of septic shock and a major contributor to mortality (26). NO formation at the late stages of inflammatory processes is detrimental for tissue integrity and organ function, mostly through the formation of toxic peroxynitrites (11, 14). We previously demonstrated concomitant NO release, monocyte recruitment, tissue injury, and death in pneumococcal pneumonia in mice (3). In the present study, we showed that HMR 3004 prevents NO release, which might contribute to protection of cell membranes from further damage.
IL-6 is consistently detected in the circulation of patients with bacterial infections (5). Pulmonary IL-6 secretion has been observed in the lungs of patients with pneumonia as well as in murine models of pneumococcal pneumonia (3, 5, 15). It plays a major role in the induction of the cytokine network in lungs and the acute-phase protein response, so that IL-6 gene-deficient mice show an impaired defense against pneumococcal pneumonia (23). On the other hand, IL-6 is also considered an anti-inflammatory cytokine, since it has the ability to inhibit proinflammatory cytokine and chemokine release in various inflammatory conditions (27). This cytokine was considered as a marker for the severity of bacterial challenge (13). According to Puren et al. (19), IL-6 rather reflects the severity of stress, whether of an infectious or noninfectious origin. Overall, IL-6 represents a relevant marker for the evolution of a host response, and this was further confirmed in our study, as shown by the early drop in tissue levels of IL-6 following HMR 3004 administration.
It has previously been demonstrated that HMR 3004 can modify the host immune response. This drug strongly accumulates within phagocytes and can modulate cell functions (24); using an experimental model of pulmonary inflammation with inhaled heat-killed S. pneumoniae, we previously observed that HMR 3004 down-regulated proinflammatory cytokines and NO production (6). However, the present study did not allow us to draw any conclusion about a direct immunomodulating effect of HMR 3004 that is independent from its antibacterial efficacy. Only a comparative study with an antibiotic devoid of anti-inflammatory activity, such as amoxicillin, could provide information about an intrinsic immunomodulating action of HMR 3004 that may be of useful clinical importance.
Although proinflammatory cytokine production in the lungs is useful to protect a host against colonization and infection of the lower respiratory tract (9, 13), antibiotic therapy in clinical practice is usually started once infection has been established and deleterious inflammation prevails. Our study suggests that, following effective antimicrobial therapy with HMR 3004 at clinically relevant doses, very potent regulatory mechanisms of inflammation are modified in ways that favorably affect disease outcome. Our present data show that effective control of bacterial growth and inflammation (either directly or indirectly) hopefully will improve therapy of threatening infections. For each new antibiotic investigated, not only in vitro MICs and in vivo pharmacokinetic data but also immunological determinations should be provided.
ACKNOWLEDGMENT
This work was supported by a grant from Hoechst Marion Roussel, Romainville, France.
REFERENCES
- 1.Agouridas C, Bonnefoy A, Chantot J F. Antibacterial activity of RU 64004 (HMR 3004), a novel ketolide derivative active against respiratory pathogens. Antimicrob Agents Chemother. 1997;41:2149–2158. doi: 10.1128/aac.41.10.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartlett J, Breiman R, Mandell L, File T. Community-acquired pneumonia in adults: guidelines for management. Clin Infect Dis. 1998;26:811–838. doi: 10.1086/513953. [DOI] [PubMed] [Google Scholar]
- 3.Bergeron Y, Ouellet N, Deslauriers A M, Simard M, Olivier M, Bergeron M G. Cytokine kinetics and other host factors in response to pneumococcal pulmonary infection in mice. Infect Immun. 1998;66:912–922. doi: 10.1128/iai.66.3.912-922.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergeron Y, Bergeron M G. Why does pneumococcus kill? Can J Infect Dis. 1999;10(Suppl.):49C–60C. [Google Scholar]
- 5.Dehoux M S, Boutten A, Ostinelli J, Seta N, Dombret M C, Crestani B, Deschenes M, Trouillet J L, Aubier M. Compartmentalized cytokine production within the human lung in unilateral pneumonia. Am J Respir Crit Care Med. 1994;150:710–716. doi: 10.1164/ajrccm.150.3.8087341. [DOI] [PubMed] [Google Scholar]
- 6.Duong M, Simard M, Bergeron Y, Ouellet N, Côté-Richer M, Bergeron M G. Immunomodulating effects of HMR 3004 on pulmonary inflammation caused by heat-killed Streptococcus pneumoniae in mice. Antimicrob Agents Chemother. 1998;42:3309–3312. doi: 10.1128/aac.42.12.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ednie L M, Spangler S K, Jacobs M R, Appelbaum P C. Susceptibilities of 228 penicillin- and erythromycin-susceptible and -resistant pneumococci to RU 64004, a new ketolide, compared with susceptibilities to 16 other agents. Antimicrob Agents Chemother. 1997;41:1033–1036. doi: 10.1128/aac.41.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fang F C. NO contest: nitric oxide plays complex roles in infection. ASM News. 1997;63:668–673. [Google Scholar]
- 9.Fox-Dewhurst R, Alberts M K, Kajikawa O, Caldwell E, Johnson II M C, Skerrett S J, Goodman R B, Ruzinski J T, Wong V A, Chi E Y, Martin T R. Pulmonary and systemic inflammatory responses in rabbits with gram-negative pneumonia. Am J Respir Crit Care Med. 1997;155:2030–2040. doi: 10.1164/ajrccm.155.6.9196112. [DOI] [PubMed] [Google Scholar]
- 10.Green L C, Tannenbaum S R, Goldman P. Nitrate synthesis in the germfree and conventional rat. Science. 1981;212:56–58. doi: 10.1126/science.6451927. [DOI] [PubMed] [Google Scholar]
- 11.Gross S S, Wolin M S. Nitric oxide: pathophysiological mechanisms. Annu Rev Physiol. 1995;57:737–769. doi: 10.1146/annurev.ph.57.030195.003513. [DOI] [PubMed] [Google Scholar]
- 12.Jamjian C, Biedenbach D, Jones R. In vitro evaluation of a novel ketolide antimicrobial agent, RU-64004. Antimicrob Agents Chemother. 1997;41:454–459. doi: 10.1128/aac.41.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lukacs N W, Ward P A. Inflammatory mediators, cytokines and adhesion molecules in pulmonary inflammation and injury. Adv Immunol. 1996;62:257–291. doi: 10.1016/s0065-2776(08)60432-0. [DOI] [PubMed] [Google Scholar]
- 14.Lyons C R. The role of nitric oxide in inflammation. Adv Immunol. 1995;60:323–355. doi: 10.1016/s0065-2776(08)60589-1. [DOI] [PubMed] [Google Scholar]
- 15.Moussa K, Michie H J, Cree I A, McCafferty A C, Winter J H, Dhillon D P, Stephens S, Brown R A. Phagocyte function and cytokine production in community-acquired pneumonia. Thorax. 1994;49:107–111. doi: 10.1136/thx.49.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nava J M, Bella F, Garau J, Lite J, Morera M, Marti C, Fontanals D, Font B, Pineda V, Uriz S, Deulofeu F, Calderon A, Duran P, Grau M, Agudo A. Predictive factors for invasive disease due to penicillin-resistant Streptococcus pneumoniae: a population-based study. J Infect Dis. 1994;19:884–890. doi: 10.1093/clinids/19.5.884. [DOI] [PubMed] [Google Scholar]
- 17.Pallares R, Lineares J, Vadillo M, Cabellos C, Manresa F, Viladrich P F, Martin R, Gudiol F. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 18.Piper K E, Rouse M S, Steckelberg J M, Wilson W R, Patel R. Ketolide treatment of Haemophilus influenzae experimental pneumonia. Antimicrob Agents Chemother. 1999;43:708–710. doi: 10.1128/aac.43.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puren A J, Feldman C, Savage N, Becker P J, Smith C. Patterns of cytokine expression in community-acquired pneumonia. Chest. 1995;107:1342–1349. doi: 10.1378/chest.107.5.1342. [DOI] [PubMed] [Google Scholar]
- 20.Reinert R R, Bryskier A, Lutticken R. In vitro activities of the new ketolide antibiotics HMR 3004 and HMR 3647 against Streptococcus pneumoniae in Germany. Antimicrob Agents Chemother. 1998;42:1509–1511. doi: 10.1128/aac.42.6.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schülin T, Wennersten C B, Moëllering R C, Jr, Eliopoulos G M. In vitro activity of RU 64004, a new ketolide antibiotic, against gram-positive bacteria. Antimicrob Agents Chemother. 1997;41:1196–1202. doi: 10.1128/aac.41.5.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sibille Y, Reynolds H Y. Macrophage and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis. 1990;141:471–501. doi: 10.1164/ajrccm/141.2.471. [DOI] [PubMed] [Google Scholar]
- 23.Van der Poll T, Keogh C V, Guirao X, Buurman W A, Kopf M, Lowry S F. Interleukin-6 gene-deficient mice show impaired defense against pneumococcal pneumonia. J Infect Dis. 1997;176:439–444. doi: 10.1086/514062. [DOI] [PubMed] [Google Scholar]
- 24.Vazifeh D, Bryskier A, Labro M T. Effect of proinflammatory cytokines on the interplay between roxithromycin, HMR 3647, or HMR 3004 and human polymorphonuclear neutrophils. Antimicrob Agents Chemother. 2000;44:511–521. doi: 10.1128/aac.44.3.511-521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watanakunakorn C, Bailey T. Adult bacteremic pneumococcal pneumonia in a community hospital, 1992–1996. Arch Intern Med. 1997;157:1965–1971. [PubMed] [Google Scholar]
- 26.Wright C E, Ress D, Moncada S. Protective and pathological roles of nitric oxide in endotoxin shock. Cardiovasc Res. 1992;26:48–57. doi: 10.1093/cvr/26.1.48. [DOI] [PubMed] [Google Scholar]
- 27.Xing Z, Gauldie J, Cox G, Baumann H, Jordana M, Lei X, Achong M K. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory response. J Clin Investig. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]