Abstract
Lectins are defined as carbohydrate-binding proteins/glycoproteins of none immune origin, they are ubiquitous in nature, exist from bacteria to human cells. And due to their carbohydrate-binding recognition capacity, they have been a useful biological tool for the purification of glycoproteins and their subsequent characterization. Some plant lectins have also been revealed to own antinociceptive, antiulcer, and anti-inflammatory properties, where these features, in many instances, depending on the lectin carbohydrate-binding site. Coronavirus disease of 2019 (COVID-19) is a respiratory disease that struck the entire world leaving millions of people dead and more infected. Although COVID-19 vaccines have been made available, and quite a large number of world populations have already been immunized, the viral infection rates remained in acceleration, which continues to provoke major concern about the vaccines' efficacy. The belief in the ineffectiveness of the vaccine has been attributed in part to the recurrent mutations that occur in the epitope determinant fragments of the virus. Coronavirus envelope surface is extensively glycosylated being covered by more than sixty N-linked oligomannose, composite, and hybrid glycans with a core of Man3GlcNAc2Asn. In addition some O–linked glycans are also detected. Of these glyco-chains, many have also been exposed to several mutations, and a few remained conserved. Therefore, numerous plant lectins with a specificity directed towards these viral envelope sugars have been found to interact preferentially with them and are suggested to be scrutinized as a possible future tool to combat coronaviruses including severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) through blocking the viral attachment to the host cells. In this review, we will discuss the possible applications of plant lectins as anti-coronaviruses including SARS-CoV-2, antinociceptive, anti-inflammatory, and antiulcer agents with the proposed mechanism of their actions.
Keywords: Plant lectins, Anti-coronavirus, SARS-CoV-2, Antiulcer, Antinociceptive, Anti-inflammatory
1. Introduction
Cell surface glycoconjugates coat play a crucial role in several biological paths, such as cell–cell adhesion, inflammatory relocation, host-pathogen interactions, immune response initiation, and cancer metastasis ((Colgan et al., 1995, Gorelik et al., 2001, Hevey, 2019, Nardy et al., 2016), and references therein). These processes are collectively mediated through carbohydrate-binding proteins that span the surface of the opposing cells (Brandley and Schnaar 1986).
Since ancient times, and to date plant parts such as leaves, barks, seeds, and roots have been used by human communities in the prevention and treatment of diseases, and for healthcare (Jones et al. 2018). In Africa and Asia over 90% of folk medicine protocols are comprised of plants, and the majority of these remedies are used by villagers or nomads who are often delocalized, and away from governmental medical facilities access. These plant products have been routinely used for treatments of various ailments such as infectious diseases caused by bacteria, fungi, and viruses, as well as non-communicable diseases including heart disease, cancer, diabetes, chronic lung conditions, etc. (Sofowora et al. 2013). It is generally thought that the presently in-use analgesia-inducing medications such as opioids and Non-steroidal anti-inflammatory drugs (NSAIDs) are not suitable for many patients due to their side effects and low efficiency (Ahmadiani et al. 1998). Consequently, the search for new drugs becomes necessary.
The fortuitous discovery made by the German doctoral student Peter Hermann Stillmark in 1888 that the castor bean (Ricinus communis) extract can agglutinate erythrocytes, a mechanism confirmed later to occur through erythrocytes’ surface glycoconjugates, had signified the milestone for our existing understanding of plant carbohydrate-binding protein in particular, and its animal/microbial counterparts in general (Sharon and Lis 2004). These glycan interacting proteins were then termed lectin, “Legere” in Latin which means to choose or pick up. In subsequent years research has confirmed the presence of such proteins not merely in plant and animal cells but also in bacteria, viruses, yeast, and parasites where they could facilitate the attachment processes of these microbes to the glycoproteins and glycolipids, and permeate the host cell surface (Sharon and Lis 1997). By their feature or capacity of being able to interact with glycans, these proteins have been defined as any multivalent protein/glycoprotein that possesses at least a single non-catalytic domain that could interact reversibly with sugars or carbohydrates and thereby causes agglutination of the cells (Goldstein et al. 1980). Owing to their being furnished with such unique carbohydrate-binding site(s) lectins are assigned to perform many biological functions such as endocytosis, act as intracellular transport vehicles for glycoproteins, and regulate the protein content in the blood ((Dias et al. 2015) and the references cited therein). Nevertheless, animal lectin was discovered before plant lectin in 1872 but they were not recognized as glycan-binding protein (Kilpatrick 2002). Although animal and plant lectins share no apparent primary structure resemblances, they share the aptitude to interact and recognize specific glycan receptors emphasizing the role of these proteins in molecular recognition (Reyes-Montaño and Vega-Castro 2018). Animal and microbial lectins are found in comparatively lesser amounts as compared to the plant lectins, the latter being detected almost in every part of the plant; seeds, leaves, bark, stem, flower, roots, etc (Mishra et al. 2019). Although not all plants contain lectins, however, when present they may account for up to 10–12% of the total seeds soluble protein (Roopashree et al., 2006, Sathe and Deshpande, 2003). The abundance of plant lectin (Spilatro et al. 1996), their ease to isolate (Mishra et al. 2019), and the accelerated advancement in preparation of affinity chromatographies that facilitated the purification of plant lectin in a single or two steps (Freeze, 1995, O'Connor et al., 2017), have assisted in performing deep studies to solve their structures’ ambiguity, possible biological effects, and clinical applications. While many plant lectins are sharing primary and secondary structures, they exhibit different biological effects which are probably ascribed to their varying glycan recognition specificities. Moreover, scientific proofs that present some plant lectins with antinociceptive, anti-inflammatory, antioxidant, and gastroprotective properties are also accumulating. While others are recognized to inhibit many microbes like viruses, parasites, nematodes, and bacteria (Breitenbach Barroso Coelho et al., 2018, Gaofu et al., 2008, Lusvarghi and Bewley, 2016, Pinto et al., 2019, Vanderlei et al., 2010). Coronavirus is the main causative of COVID-19 the acute respiratory syndrome which originates from Wuhan China and caused an outbreak worldwide leaving million dead and infected (Hu et al. 2021). Many vaccines have been manufactured and approved worldwide to curb the disease’s rapid mortality and morbidity rates. However, due to the accelerated frequencies of the virus spike protein mutations, the efficacy of these vaccines becomes questionable (Baraniuk, 2021, Hayawi et al., 2021, Khan et al., 2021). However, the fact that the various N-linked glycosylation points of coronavirus-2 protein, which play a major role in the viral virulence are largely conserved, opened widely the window for the conceivable use of carbohydrate-binding agents such as lectins for targeting this virus’ glycans, and thereby interfering with its initial binding stage to the host cell surface receptors (Ahmed et al., 2022, Barre et al., 2021, Martinez et al., 2021). In this review, we aim to discuss, in-depth, the potential applications of plant lectins to combat coronavirus diseases as well as their uses as, antiulcer, anti-inflammatory, and antinociceptive agents.
2. The spike protein (S) glycosylation
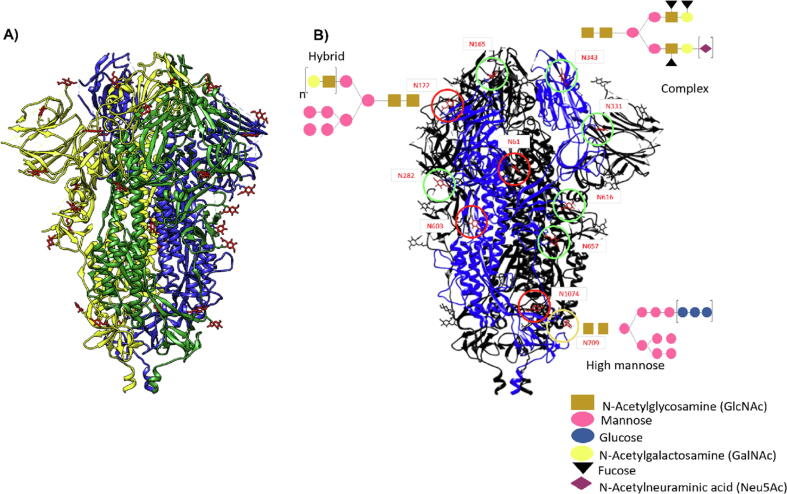
The coronavirus comprises four main structural proteins: spike (S), membrane (M), envelope (E), and nucleocapsid (N) proteins. Spike protein (S) is the main structural protein antigen of SARS-CoV-2, which mediates the fusion of the coronaviruses into susceptible human cells through their interactions with the angiotensin-converting enzyme- 2 (ACE-2) receptor (Hsieh et al. 2005). However, unlike other SARS-CoV-2 viral proteins, it is accountable for triggering the host immune response, and the antibodies directed towards the S protein can bring about protective immunity against subsequent infections (Boechat et al. 2021). The S protein (180–200 kDa) is a homotrimer of two subunits S1 and S2, linked through a membrane-embedded serine 2 protease. S1 contains the receptor-binding site, while S 2 is devoted to the viral fusion of the host cells. Structural investigations of the S protein by cryo-EM and mass spectrometry revealed that S protein to be extensively glycosylated with as many as 66 N-glycosylation points (22 per monomer), covering the surface of the protein and assisting partly in mediating the virulence of the pathogen, and at the same time shielding the susceptible viral receptor-binding domain (RBD) against the neutralizing human antibodies (Huang et al., 2021, Shajahan et al., 2021) Fig. 1. Of distinct interest, the glycosylation points as N165 and N234 (mannose-rich glycans) are located at the vicinity of the ACE-2 RBD, they have been revealed by all-atom molecular dynamic simulation (MD) to play a role in the ACE2-S protein interaction. Point mutation of N165A and N234A, which resulted in glycosylation depletion at these sites, reduced effectively the receptor-glycan interaction, though didn’t utterly nullify it. These results underlined the possible importance of these glycosylation points in suitably orienting the conformation of the receptor-binding domain of the virus ((Zhao et al. 2021) and references cited therein). Through using different expression vectors, several workers have reported recurrent mutations at N-glycans (Shajahan et al. 2021). Nonetheless, 19 glycosylation sites were proven to remain conserved. O-glycosylation has also been shown to occur, however, to a limited extent (Hayawi et al. 2021). Expression of SARS-CoV-2 genome on human cell line HEK-293 offered varied complex glycosylation patterns, habitually of the mannose-rich type glycan.
Fig. 1.
Structural presentation of SARS-CoV-2 spike protein (S) (PDB ID: 6VXX). A) The homotrimer structure of the S-protein showing the N-glycans (Presented in red). B) The monomer of the S-protein A chain is highlighted in blue, of the 22 N-glycans, 11 were presented in 3 different types (hybrid, complex, and high mannose glycans), O-glycans are not shown. The 3D structures were visualized and edited using UCSF-Chimera 1.8v software.
2.1. The use of lectins to target SARS-CoV spike glycoprotein
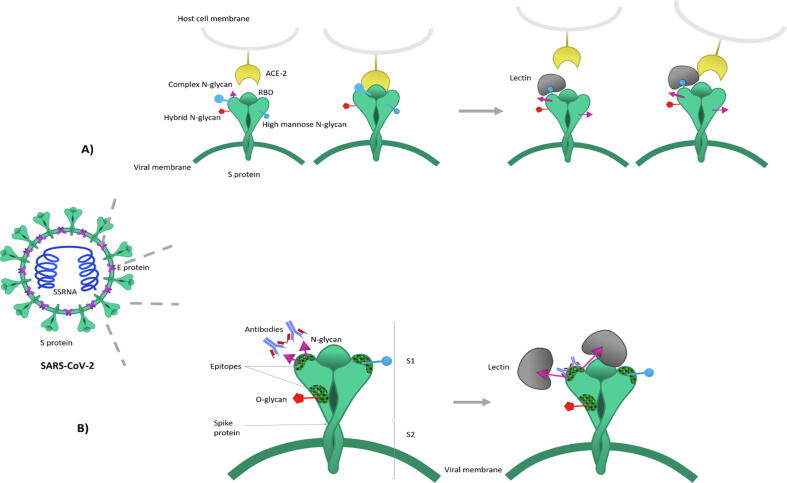
In the search for appropriate and effective treatment for SARS-CoV-2 infection, drug repurposing has already been underway. Inclusion of the antimalarial drugs such as chloroquine and hydroxychloroquine in COVID-19 treatment protocol has made a considerable debate before they were withdrawn after proven futile (Altulea et al., 2021, Ferner and Aronson, 2020). Anticancer drugs have also been proposed to be promising against viral replication however, higher toxicity described with the administration of such drugs is criticized (El Bairi et al. 2020). As stated the heavily glycosylated SARS-CoV-2 S protein surface made it a tempting choice for glycan-binding proteins (lectins) especially those of plausible interaction with these glycans. It is largely believed that they will likely hinder the attachment of the virus to the host cells by inducing a conformational change that would favour uncovering the epitope recognition site of the virus, thereby neutralizing the virulence effect by the triggered immune response (Fig. 2). At the vicinity to the RBD of the spike protein, the presence of two glycosylation sequences N165 and N234 which mainly comprised of Man3 GlcNAc core could represent an excellent target for plant lectins with complex-type biantennary oligo-mannosyl saccharides. Almost four decades ago, Greig and Bouillant had revealed extensive binding of Concanavalin A (ConA); the lectin from the Canavalia ensiformis to encephalomyelitis virus, a Coronavirus. Removal of the viral surface glycans by snake venom phospholipase A abolished ConA-virus interactions, emphasizing the importance of the sugar chains in the viral attachment process (Greig and Bouillant 1977).
Fig. 2.
Lectins assumed mechanism of action against SARS-CoV-2. A) ACE-2 recognition of by the viral S-protein and interference of plant lectin. B) Action of plant lectin on exposing of the virus epitope determinant site. The Virus N-glycan shield the virus epitope determinant and prevent the antibodies recognition. Upon lectin binding, conformational changes occur and lead to exposing the epitope site.
On the other hand, Urtica dioica agglutinin (UDA); the lectin purified from the Nettle (Urtica dioica L.), is a small 8.7 kDa protein with a specificity directed towards N, N′, N″- tri-acetylchitotriose (polymer of acetylated acetylglucosamines). This peptide lectin besides its sugar-binding site also had a hydrophobic binding region adjacent to its carbohydrate-binding site (Shibuya et al. 1986). It inhibits the SARS-CoV replication cycle by interfering with the viral attachment to the host cell probably through binding to spike protein N-acetylglucosamine (GlcNAc) units (Kumaki et al., 2011, Saul et al., 2000). Investigating the effect of the mannose-binding lectin griffithsin against MERS-CoV infection revealed that griffithsin even though displaying no apparent cytotoxicity, offered a strong inhibitory effect on MERS-CoV infection through binding to the mannose-rich viral spike protein (Millet et al. 2016). The same lectin, in several other publications, was demonstrated to exhibit a broad antiviral spectrum. HIV-1 N-linked glycosylation sites on the gp-120 were affirmed to be recognized by griffithsin, the binding of lectin would lead to a change in the structure of gp-120, which would result in exposing the virus CD4 binding site (Alexandre et al., 2011, Fischer et al., 2019). Similar results were obtained for banana (Musa acuminata) lectin which could inhibit HIV-1 by recognizing the mannose-rich gp-120 viral outer layer glycoprotein in a range of picomole quantities, hence interfering with the viral adherence to the human cell (Swanson et al. 2010). Testing a collection of 33 plant lectins with different specificities indicated that the strongest antiviral activity was principally confined among lectins with mannose-binding specificity (Keyaerts et al. 2007). Using Vero B4 cells, the Wheat Germ Agglutinin (WGA) purified from Triticum vulgaris exhibited antiviral activity not only against the initially emerged SARS-CoV-2, but also its recent major two variants Alpha and Beta were found effective with IC50 of < 10 ng/mL at both the pre-incubation period with the virus or during the viral infection. Interestingly, this lectin had a narrow specificity that was merely directed towards coronaviruses as it had no effect with other non-coronaviruses causing respiratory tract distresses (Auth et al. 2021). In a very recent publication, Barre and his colleagues studied the viral envelope shielding glycans of several viruses like Ebola, herpes simplex, human cytomegalovirus, human immunodeficiency, influenza, chikungunya, Lassa, MERS-CoV, SARS-CoV, SARS-CoV-2, and Zika, which exhibited high coat glycans heterogeneities. Based on that they have suggested some homodimeric mannose-specific legume lectins with a high affinity for α1,6-fucosylated Man3 3GlcNAc2 core. They have concluded that lectins from Pisum sativum, Lens culinaris, Lathyrus ochrus, Canavalia ensiformis, Pterocarpus angolensis, and Vicia faba could be classified to offer the best SARS-CoV-2 spike envelope binding capacity (Barre et al., 2021, Garcia-Pino et al., 2007). However, since these lectins merely bind to the mannose-rich glycan receptors on the viral envelope surface and don’t interfere with the inside viral genome, they did not consider them as coronavirus replication inhibitors, but from a future perspective point of view, could be used in preventing the viral attachment to the host cells and therefore block the initial stages in the virulence processes. Table 1 shows different plant/algal lectins with reported antiviral activity including the recent publications concerning SARS-CoV-2.
Table 1.
Plant /algal lectins with antiviral activities.
| Plant/algae Latin name | Family | Plant part | subunit / Mr | Specificity | Virus inhibited | References |
|---|---|---|---|---|---|---|
| Grateloupia chiangii | Halymeniaceae | Whole | Monomer/25 kDa | Mannose | Influenza virus, HIV type 1, and herpes | (Hwang et al. 2020) |
| Pandanus amaryllifolius Roxb. | Pandanaceae | Leaf | Monomer/8kDa | Mannose | Herpes simplex virus type-1, influenza virus, N1H1 | (Ooi et al. 2004) |
| Phaseolus vulgaris L. | Fabaceae | Seed | Homodimer/30 kDa | Complex | HIV-1 | (Sharma et al. 2009) |
| Capparis spinosa L. | Capparaceae | Seed | Homodimer/ 31 kDa | Raffinose, lactose, rhamnose and galactose |
HIV-1 | (Lam et al. 2009) |
| Lablab purpureus (L.) Sweet | Fabaceae | Seed | Pentamer (varying associations) /67 kDa | glucose/mannose | Influenza, SARS-CoV-2 | (Liu et al., 2020, Mo et al., 1999) |
| Polygonatum odoratum (Mill.) Druce | Asparagales | Rhizome | Homotetramer/12 kDa | mannose | Herpes Simplex Virus | (Yang et al. 2011) |
| Clematis montana Buch.-Ham. ex DC. | Ranunculaceae | Stem | Homodimer/12 kDa | Complex of mannose units glycans | HIV, HIV-1, HIV-2, Influenza A H1N1, Influenza A H3N2, Influenza B, Para-influenza-3 and virus reovirus-1 | (Peng et al. 2009) |
|
Myrianthus holstii Engl. |
Urticaceae | Stem, Root | Monomer/9kDa | N-acetylglucosamine | HIV-1RF | (Charan et al. 2000) |
| Musa acuminata Colla * | Musaceae | Fruit | Homodimer/13 kDa | MERS-CoV, SARS-CoV-2 including variants Alpha and Beta | (David et al., 2022, Koshte et al., 1990) | |
| Lens culinaris Medik. | Fabaceae | Seed | Heterodimer/17 & 4 kDa | oligomannose-type glycans and GlcNAc | SARS-COV-2 variants | (Chan et al., 2015, Wang et al., 2021) |
| Triticum aestivum L. | Pooideae | Seed | Monomer/23 kDa | N-acetyl-D-glucosamine | SARS-CoV-2 including variants Alpha and Beta | (Auth et al., 2021, LeVine et al., 1972) |
| Maackia amurensis Rupr. | Fabaceae | Seed | Heterodimer/32 & 37 kDa | Sialic acid | SARS-CoV-2 | (Sheehan et al., 2020, Van Damme et al., 1997) |
| Urtica dioica | Urticaceae | Rhizomes | Monomer /8.5 KDa | N-acetylglucosamine | HIV-1 | (Gordts et al. 2015) |
| Nicotiana tabacum | Solanaceae | Leaf | Monomer/19 KDa | N-acetylglucosamine | HIV-1 | (Gordts et al. 2015) |
| Galanthus nivalis | Amaryllidaceae | Bulb | Tetramer (13 KDa/monomer) | Mannose-specififc | HIV-1, HIV-2 | (Balzarini et al. 2004) |
| Hippeastrum hybrid | Amaryllidaceae | Bulb | Homomtetramer /14 KDa/monomer | Mannose-specific | HIV-1, HIV-2 | (Balzarini et al. 2004) |
| Tamaridus insica | Fabaceae | seed | Monomer /34 kDa | N-acetyl glucosamine | Chikungunya virus | (Kaur et al. 2019) |
Genetically engineered lectin with preserved antiviral activity however with a reduced mitogenic capacity (Swanson et al. 2010).
3. Plant lectins as antinocieptive and anti-inflammatory agaents
Apart from the booming development that has arisen in recent years accompanied by studious progress in pharmaceutical biotechnology and the development of drugs manufacturing, the need for effective and powerful analgesics is in continuous demand (Khan et al. 2020). A great number of currently prescribed drugs were initially extracted from medicinal plants like morphine, thebaine, and the recently isolated and commercialized serratiopeptidase (Bhagat et al., 2013, Jehan et al., 2017). Local communities from different countries, especially in Africa and Asia are customed to practice the use of several plants parts for pain relief and reducing inflammation. It was estimated that 65% and 90% of Indian and Sudanese populations depend on traditional medicine for their healthcare, respectively (Karar and Kuhnert, 2017, Prashantkumar and Vidyasagar, 2008). Clove (Syzygium aromaticum) buds are routinely used by natives to relieve toothache, treating experimental animals with clove aqueous extract is reported to significantly increase the latency period upon thermal stimuli (hotplate test), confirming the analgesics property of the plant (Kamkar Asl et al. 2013). The aromatic herb peppermint leaves are used on daily basis to soothe an upset stomach (Uritu et al. 2018). Examining the anti-inflammatory and antinociceptive properties of mint oil extracted from three species Mentha piperita L. var. pallescens, Mentha spicata L. subsp. Crispata, and Mentha suaveolens Ehrh resulted in a significant pain reduction (Mogosan et al. 2017). While many plant crude aqueous extracts have been tested for their antinociceptive properties and gave a positive significant outcome, very few publications were devoted to isolating the active ingredient responsible for such effect. To evaluate lectin anti-nociceptive properties in mice or rat models, variable methods such as abdominal writhing, formalin and the hotplate tests are largely accepted. Whereas the anti-inflammatory reactions are often assessed by challenging animals with carrageenan, dextran or serotonin to induce paw oedema. Neutrophils and leukocytes' migration to the peritoneal cavity is followed to confirm the lectin anti-inflammatory action (Nunes et al. 2009). An affinity-purified galactose-specific lectin isolated from the leaves of Bauhinia monandra exhibited antinociceptive and anti-inflammatory in a dose-dependent manner when mice were challenged with 1% carrageenan-induced inflammation and 0.8% acetic acid-induced abdominal writhing. At a concentration of 60 mg lectin/kg mice, there was a 60% inflammation reduction. Whereas 71.3% agony reduction was recorded in the case of acetic acid pain induction. The authors concluded their results by attributing the routine use of this plant in traditional medicine as an anti-inflammatory and analgesic agent to the presence of lectin in this plant (Campos et al. 2016). A heterodimer lectin-like protein with an ambiguous sugar specificity purified from the seeds of Clitoria fairchildiana displayed no apparent toxicity to human RBCs, owned an anti-inflammatory activity of 64% attenuation in the mice paws oedema caused by administration of carrageenan. Moreover, the lectin was successful in inhibiting neutrophils migration. This lectin had also shown a 72% diminution in the mice's abdominal writhing when the pain is induced by acetic acid and therefore is considered to possess both anti-inflammatory and antinociceptive characteristics (Leite et al. 2012). Several algal lectins were also characterized by their analgesic and sometimes anti-inflammatory properties such as Caulerpa cupressoides lectin, this protein could decrease the effect of the writhing induced by acetic acid to up to 86%, however, it was not being able to produce significant antinociceptive effects in the hot plate experiment, indicating the sole involvement of the peripheral rather than central acting mechanism (Vanderlei et al. 2010). The antinociceptive properties of lectins have been, in many instances, attributed to the probable inhibition of inflammation producing molecules such as bradykinin, prostaglandins, substance P, and some cytokines, such as IL-1β and TNFα which will lead to activation of chemosensitive nociceptors and hence induction of pain (Vanderlei et al. 2010). Similar observations were also noticed with the marine red algae Pterocladiella capillacea lectin (Silva et al 2010). The writhing effect induced by acetic acid was significantly reduced when Amansia multifida Lamouroux lectin was administered along with the glycoprotein avidin at 1 mg/kg. The authors ascribed the occurred attenuation in the antinociceptive property to the blockage of the lectin’s active site by the inhibitory glycoprotein avidin, therefore concluding the involvement of the lectin sugar-binding site in the obtained analgesic effect (Neves et al. 2007). To examine the involvement of the opioid system, the analgesic morphine was used, and to block the thermal stimuli, the morphine antagonist naloxone was administered which resulted in complete reserve of the morphine effect indicating the involvement of the opioid receptor in the pain sensation (Neves et al. 2007). The red seaweed Bryothamnion triquetrum lectin was found potent at 10 mg/kg in inhibiting paw oedema induced by both carrageenan and dextran. This anti-inflammatory response was linked by inhibiting the neutrophil migration towards the peritoneal fluid (Fontenelle et al. 2018). A mannose-specific lectin isolated from the seeds of Andira anthelmia expressed a potent antinociceptive property; it inhibited mice writhings caused by parenteral administration of 0.7% acetic acid by about 68% in as low as 10 µg/kg dose, this effect was neutralized in presence of mannose (Nascimento et al. 2016). To wrap up, many legume lectins discussed in this review were proven to attenuate the inflammatory response including the lectin isolated from Canavalia boliviana seeds in a reaction mediated by the lectin binding site (Figueiredo et al. 2009), however, astonishingly, Canavalia virosa seed lectin which was purified by affinity interaction to Sephadex®-G50 (polymer of glucose units), was rather found to trigger inflammation when injected subcutaneously into mice paw. The noticed paw oedema produced by lectin administration was dramatically reverted when the lectin was initially incubated with its haptenic sugar glucose, indicating the role of sugar affinity site in the observed inflammatory reaction (Osterne et al. 2017). Another interesting work on Lonchocarpus araripensis seed lectin published by a Brazilian group, where the N-acetyl-D-glucosamine (GlcNAc) specific lectin demonstrated antinociceptive effect through a unique mechanism involving the nitric oxide pathway, additionally the same protein was also characterized by GlcNAc specific anti-inflammatory property, inhibiting the neutrophil migration to the intraperitoneal cavity of the experimental animal. The administration of the anti-steroidal drug niflumic acid, instead of initiating an analgesic effect, aggravates the pain sensation. Since this drug is used as a calcium-activated chloride channel blocker, the involvement of the calcium channel, in this case, as a first-ever report, is apparent (Assreuy et al., 2020, de Freitas Pires et al., 2019). Similarly, the pretreatment of the animal with Cannabinoid receptor 1 (CB-1) and receptor 2 (CB-2) antagonist AM251 and AM630 respectively overturned the antinociceptive effect of Lonchocarpus araripensis seed lectin, emphasizing the contribution of endocannabinoid receptor (Amorim et al. 2021). Table 2 presents to date literature compilation of plant lectins with both antinociceptive and anti-inflammatory effects. Mechanistically, plant lectins can exert their anti-inflammatory response by interfering with the pro-inflammatory cascade in either of two different ways. They can inhibit vascular inflammation mediated through competitive binding of lectin with the glycosylation molecules found at the surface of the white blood cells, thus preventing them from interacting with selectin molecules and inducing cell transmigration to the site of inflammation. Or by inhibiting the cytokines and chemokines production related to white blood cells migration (Alencar et al., 1999, Jandú et al., 2017).
Table 2.
Plant lectins with reported anti-inflammatory and antinociceptive activities.
| Source | Plant/algae Latin name | Family | Plant part | Subunit / Mr | Sugar specificity | Activity |
References |
|---|---|---|---|---|---|---|---|
| Andira anthelmia | Fabaceae | seed | Heterotrimer 20, 17, 15, 13 kDa |
Mannose | Anti-inflammatory | (Do Nascimento et al. 2021) | |
| Bauhinia monandra | Fabaceae | leaves | Monomer 33 kDa |
Galactose | Anti-inflammatory and antinociceptive | (Coelho and da Silva, 2000, Campos et al., 2016) | |
| PLant Lectins | Dioclea guianensis Benth. | Fabaceae | Seed | Two isolectins of 47 and 100 kDa, Heterotridimers 12,18 & 30 kDa |
Mannose/glucose | Anti-inflammatory | (Assreuy et al., 1997, Vasconcelos et al., 1991) |
|
Dioclea grandiflora Benth. |
Fabaceae | Seed | Three isolectins 25–26 kDa 13–14 kDa 8–9 kDa |
Mannose/glucose | Anti-inflammatory | (Assreuy et al., 1997, Moreira et al., 1983) | |
|
Dioclea violacea Benth. |
Fabaceae | Seed | Heterotrimer 11.7, 15.8 and 29.5 kDa |
Mannose/glucose | Anti-inflammatory & Antinociceptive |
(Assreuy et al., 1997, Holanda et al., 2009, Renato et al., 1996) | |
|
Dioclea virgata (Rich.) Amshoff |
Fabaceae | Seed | Heterotrimer 30.9, 16.2 & 12 kDa |
Mannose/glucose | Anti-inflammatory | (Assreuy et al., 1997, Márcio et al., 1996) | |
| Clitoria fairchildiana | Fabaceae | Seeds | Heterodimer 100, 116 kDa | Unspecific to known sugars and glyconjugates | Anti-inflammatory /antinociceptive |
(Leite et al. 2012) | |
| Cratylia floribunda Benth. | Fabaceae | Seed | Heterotrimers 29–30 kDa 16–18 kDa 12–13 kDa |
Mannose/glucose | Anti-inflammatory | (Assreuy et al., 1997, Oliveira et al., 1991) | |
|
Canna limbata |
Cannaceae | Seed | Homodimer of 21 kDa | N-acetylglucosamine | Anti-inflammatory Antinociceptive |
(Araújo et al. 2013) | |
| Parkia biglobosa (Jacq.) G.Don | Fabaceae | Seed | Homodimer 46 kDa | Mannose/glucose | Anti-inflammatory Antinociceptive |
(Silva et al. 2013) | |
| Parkia playcephala | Fabaceae | seed | Monomer 48 kDa | Mannose/glucose | Antinociceptive | (De Oliveira Leite et al. 2020) | |
| Praecitrullus fistulosus | Cucurbitaceae | Phloem exudates | Semi-Purified | ND | Anti-inflammatory | (Madhu and sharada 2019) | |
|
Crataeva tapia L. |
Capparaceae | Bark | Heterodimer 21 and 40 kDa |
Mannose/glucose | Anti-inflammatory Antinociceptive |
(Araújo et al., 2011, Araújo et al., 2012) | |
| Lonchocarpus campestris Mart. ex Benth. | Fabaceae | Seed | Two Isolectins 10 & 25 kDa |
Mannose | Anti-inflammatory Antinociceptive |
(De Freitas Pires et al. 2019) | |
| Machaerium acutifolium Vogel | Fabaceae | Seed | Heterotrimer 29, 13, & 8 kDa | Mannose/ N-acetyl- glucosamine | Antinociceptive | (Santos et al. 2019) | |
| Microgranna vacciniifolia | Polypodiaceae | Rhizome | Monomer 54 kDa | Glucose | Anti-inflammatory Antinociceptive |
(Cavalcante da Silva et al. 2021) | |
| Mucuna pruriens | Fabaceae | Seeds | Monomer 60 kDa |
Complex specificity | Anti-inflammatory Antinociceptive |
(Lacerda et al. 2015) | |
| Tetracarpidium conophorum | Euphorbiaceae | seeds | Heterodimer 17 and 34 kDa |
Lactose/galactose | Anti-inflammatory Anti-inflammatory and Antinociceptive |
(Oladokun et al. , 2019) | |
| Schinus terebinthifolia | Anacardiaceae | Leaf | Monomer 12.4–14 kDa kDa | Chitin | Anti-inflammatory and Antinociceptive | (Gomes et al., 2013, de Souza Feitosa Lima et al., 2019, Ramos et al., 2020) | |
| Luetzelburgia auriculata | Fabacaea | seeds | Homotetramer 123.5 kDa | N-acetyl-D-galactosamine | Anti-inflammatory | (Oliveira et al., 2002, Alencar et al., 2010) | |
| Synadenium carinatum | Euphorbiaceae | Latex | Heterodimer 28 and 30 kDa |
Galactose | Anti-inflammatory | (Rogerio et al. 2007) | |
| Algal Lectins | Bryothamnion triquetrum | Alsidieae | Whole | Monomer 9 kDa |
Mucin | Anti-inflammatory | (Fontenelle et al. 2018) |
| Solieria filiformis | Solieriaceae | Whole | Monomer 28 kDa | Complex glycan | Anti-inflammatory Antinociceptive |
(Abreu et al. 2016) | |
| Caulerpa cupressoides | Caulerpaceae | Whole | Homodimer 23 kDa | Lactose | Anti-inflammatory Antinociceptive |
(Vanderlei et al. 2010) | |
| Hypnea cervicornis | Gigartinaceae | Whole | Heterodimer 9.1, 9.9 kDa | Complex glycan | Anti-inflammatory Antinociceptive |
(Bitencourt Fda et al. 2008) |
ND: No information available.
4. Plant lectins as antiulcer
An ulcer is defined as a pain that originates from damage that occurs in the internal coating of the stomach or small intestine, when the problem is associated with stomach mucosal lesions it is termed gastric ulcer which is caused by acid secretion or pepsin (Sverdén et al. 2019). In Nigeria, natives routinely use Carica papaya seeds flour for the treatment of peptic ulcers, whereas in Sudan Acacia Senegal and Aerva javanica are known for their healing effect of peptic ulcers (Karar and Kuhnert 2017). In the experimental models, many gastric-lesion inducers have been employed such as ethanol, Indomethacin and Aspirin. A homotetrameric galactose-binding lectin purified from Artocarpus incise conventionally named frutalin was successful in providing considerable protection against both ethanol and indomethacin gastric injury in mice, however in a dose-unrelated manner where a lectin at a concentration as low as 500 μg/kg was able to produce potent protection. Yet, the pre-treatment with the α2- receptor antagonist Yohimbine didn’t revert or attenuate frutalin protection against ethanol lesions which indicated no involvement of α2-receptor in the caused action of the lectin. Simultaneously administration of glibenclamide, a K+ATP channel inhibitor, resulted in a partial however significant reduction of the action frutalin, demonstrating the influence of K+ATP channel in shielding the stomach lining against the external mucosal attacker (De Vasconcellos Abdon et al. 2012). A rabbit erythrocytes specific seeds lectin purified from the Brazilian plant Mucuna pruriens (L.) DC (MpLec), was also examined for its protective effect against ethanol-induced gastro-damage in mice. The lectin was able to provide a significant reduction in stomach lesions in comparison to control, however, pre-treatment with Yohimbine abolished the MpLec protective effect, which emphasize the role of α2 adrenoceptors in the attained defensive mechanism (Pinto et al. 2019). Another interesting highly stable GlcNAc specific seeds lectin, which agglutinates only the human A-blood group was capable of reducing the ethanol damage by up to 63%. The lectin was administered at three different doses 10, 100 and 1000 µg/kg, even though all of them lead to a significant outcome, major protection was offered by the 1000 µg/kg dose.
5. Conclusion
The information on the great potency of plant lectins on microorganisms such as bacteria, viruses, parasites, and fungi as well as their reported action as antioxidants, antinociceptive, antitumors and antiulcer have been accumulating. And due to their peculiar sugar recognition site that compliments in minute details with the acknowledged tumor cellular glycan changes, clinical trials on their possible application as a drug shuttle for cancer treatment are underway (Wijetunge et al., 2018, Wode et al., 2020). Some of their unfavourable characteristics such as large molecular weight which will likely induce immunogenicity and toxicity may limit the widespread use of these interesting proteins in drug applications. Therefore the suggestion of the implementation of small molecular weight lectins is promising and may pave the way to overcome the immunogenic hurdle. While there is still much to be investigated and disclosed about the in-vivo and in-vitro triggered biological effects of plant lectins, the currently ongoing research on genetically engineered lectins with reduced undesired activity without resulting in major structural alterations may retain hope in future applications of plant lectin in drug synthesis and applications. Additionally, our laboratory's ongoing research activities on many lectins from tropical medicinal plants with extreme thermal and chemical stability hold significant probabilities of thrilling discoveries shortly.
Funding
This work was not funded.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Emadeldin Konozy, Email: ehkonozy@act.gov.sd.
Makarim Osman, Email: makarim84@gmail.com.
Amina Dirar, Email: aminadirar2007@gmail.com.
References
- Abreu T., Ribeiro N., Chaves H., Jorge R., Bezerra M., Monteiro H., Vasconcelos I., Mota É., Benevides N. Antinociceptive and Anti-inflammatory Activities of the Lectin from Marine Red Alga Solieria filiformis. Planta Med. 2016;82(07):596–605. doi: 10.1055/s-0042-101762. [DOI] [PubMed] [Google Scholar]
- Ahmadiani A., Fereidoni M., Semnanian S., Kamalinejad M., Saremi S. Antinociceptive and anti-inflammatory effects of Sambucus ebulus rhizome extract in rats. J. Ethnopharmacol. 1998;61(3):229–235. doi: 10.1016/s0378-8741(98)00043-9. [DOI] [PubMed] [Google Scholar]
- Ahmed M.N., Jahan R., Nissapatorn V., Wilairatana P., Rahmatullah M. Plant lectins as prospective antiviral biomolecules in the search for COVID-19 eradication strategies. Biomed. Pharmacother. 2022;146:112507. doi: 10.1016/j.biopha.2021.112507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandre K.B., Gray E.S., Pantophlet R., Moore P.L., McMahon J.B., Chakauya E., O'Keefe B.R., Chikwamba R., Morris L. Binding of the mannose-specific lectin, griffithsin, to HIV-1 gp120 exposes the CD4-binding site. J Virol. 2011;85(17):9039–9050. doi: 10.1128/JVI.02675-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alencar N.M.N., Oliveira R.S.B., Figueiredo J.G., Cavalcante I.J.M., Matos M.P.V., Cunha F.Q., Nunes J.V.S., Bomfim L.R., Ramos M.V. An anti-inflammatory lectin from Luetzelburgia auriculata seeds inhibits adhesion and rolling of leukocytes and modulates histamine and PGE2 action in acute inflammation models. Inflamm Res. 2010;59(4):245–254. doi: 10.1007/s00011-009-0092-9. [DOI] [PubMed] [Google Scholar]
- Alencar N.M.N., Teixeira E.H., Assreuy A.M.S., Cavada B.S., Flores C.A., Ribeiro R.A. Leguminous lectins as tools for studying the role of sugar residues in leukocyte recruitment. Mediators Inflamm. 1999;8(2):107–113. doi: 10.1080/09629359990603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altulea D., Maassen S., Baranov M.V., van den Bogaart G., Wu J. What makes (hydroxy) chloroquine ineffective against COVID-19: insights from cell biology. J. Mol. Cell Biol. 2021;13(3):175–184. doi: 10.1093/jmcb/mjab016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim R.M.F., Oliveira M.V.d., Cavada B.S., Nascimento K.S., Assreuy A.M.S., Pires A.d.F. The Antinociceptive Effect of Lonchocarpus Araripensis Lectin is Mediated by Endocannabinoid Receptors. IJSCIA. 2021;2(4) doi: 10.51542/ijscia.v2i4.21. [DOI] [Google Scholar]
- Araújo R.M.S., Vaz A.F.M., Aguiar J.S., Coelho L.C.B.B., Paiva P.M.G., Melo A.M.M., Silva T.G., Correia M.T.S. Lectin from Crataeva tapia bark exerts antitumor, anti-inflammtory and analgesic activities. Nat Prod Bioprospect g. 2011;1(2):97–100. [Google Scholar]
- Araújo R.M.S.d., Ferreira R.d.S., Napoleão T.H., Carneiro-da-Cunha M.D.G., Coelho L.C.B.B., Correia M.T.D.S., Oliva M.L.V., Paiva P.M.G. Crataeva tapia bark lectin is an affinity adsorbent and insecticidal agent. Plant Sci. 2012;183:20–26. doi: 10.1016/j.plantsci.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Araújo T.S., Teixeira C.S., Falcão M.A.P., Junior V.R.P., Santiago M.Q., Benevides R.G., Delatorre P., Martins J.L., Alexandre-Moreira M.S., Cavada B.S., Campesatto E.A., Rocha B.A.M. Anti-inflammatory and Antinociceptive Activity of Chitin-binding Lectin from Canna limbata Seeds. Appl Biochem Biotechnol. 2013;171(8):1944–1955. doi: 10.1007/s12010-013-0470-1. [DOI] [PubMed] [Google Scholar]
- Assreuy A.M.S., Shibuya M.D., Martins G.J., De Souza M.L.P., Cavada B.S., Moreira R.A., Oliveira J.T.A., Ribeiro R.A., Flores C.A. Anti-inflammatory effect of glucose-mannose binding lectins isolated from Brazilian beans. Mediators Inflamm. 1997;6(3):201–210. doi: 10.1080/09629359791695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assreuy A.M.S., Amorim R.M.F., Martins S.L., de Queiroz Martins M.G., Cajazeiras J.B., da Silva M.T.L., Pires A.F., Nascimento K.S., Cavada B.S., Mota M.R.L. Antinociceptive effect of Lonchocarpus araripensis lectin: activation of L-arginine/NO/cGMP/K(+)ATP signaling pathway. Inflammopharmacology. 2020;28(6):1623–1631. doi: 10.1007/s10787-020-00729-z. [DOI] [PubMed] [Google Scholar]
- Auth J., Fröba M., Große M., Rauch P., Ruetalo N., Schindler M., Morokutti-Kurz M., Graf P., Dolischka A., Prieschl-Grassauer E., Setz C., Schubert U. Lectin from Triticum vulgaris (WGA) Inhibits Infection with SARS-CoV-2 and Its Variants of Concern Alpha and Beta. International Int. J. Mol. Sci. 2021;22(19):10205. doi: 10.3390/ijms221910205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzarini J., Hatse S., Vermeire K., Princen K., Aquaro S., Perno C.-F., De Clercq E., Egberink H., Vanden Mooter G., Peumans W., Van Damme E., Schols D. Mannose-specific plant lectins from the Amaryllidaceae family qualify as efficient microbicides for prevention of human immunodeficiency virus infection. Antimicrob Agents Chemother. 2004;48(10):3858–3870. doi: 10.1128/AAC.48.10.3858-3870.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraniuk C. Covid-19: How effective are vaccines against the delta variant? BMJ (Online) 2021;374 doi: 10.1136/bmj.n1960. [DOI] [PubMed] [Google Scholar]
- Barre A., Van Damme E.J.M., Simplicien M., Le Poder S., Klonjkowski B., Benoist H., Peyrade D., Rougé P. Man-Specific Lectins from Plants, Fungi, Algae and Cyanobacteria, as Potential Blockers for SARS-CoV, MERS-CoV and SARS-CoV-2 (COVID-19) Coronaviruses: Biomedical Perspectives. Cells. 2021;10(7):1619. doi: 10.3390/cells10071619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagat S., Agarwal M., Roy V. Serratiopeptidase: A systematic review of the existing evidence. Int. J. Surg. 2013;11(3):209–217. doi: 10.1016/j.ijsu.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Bitencourt F.d.S., Figueiredo J.G., Mota M.R.L., Bezerra C.C.R., Silvestre P.P., Vale M.R., Nascimento K.S., Sampaio A.H., Nagano C.S., Saker-Sampaio S., Farias W.R.L., Cavada B.S., Assreuy A.M.S., de Alencar N.M.N. Antinociceptive and anti-inflammatory effects of a mucin-binding agglutinin isolated from the red marine alga Hypnea cervicornis. Naunyn Schmiedebergs Arch. Pharmacol. 2008;377(2):139–148. doi: 10.1007/s00210-008-0262-2. [DOI] [PubMed] [Google Scholar]
- Boechat J.L., Chora I., Morais A., Delgado L. The immune response to SARS-CoV-2 and COVID-19 immunopathology – Current perspectives. Pulmonology. 2021;27(5):423–437. doi: 10.1016/j.pulmoe.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandley B.K., Schnaar R.L. Cell-surface carbohydrates in cell recognition and response. J Leukoc Biol. 1986;40(1):97–111. doi: 10.1002/jlb.40.1.97. [DOI] [PubMed] [Google Scholar]
- Breitenbach Barroso Coelho L.C., Marcelino dos Santos Silva P., Felix de Oliveira W., de Moura M.C., Viana Pontual E., Soares Gomes F., Guedes Paiva P.M., Napoleão T.H., dos Santos Correia M.T. Lectins as antimicrobial agents. J. Appl. Microbiol. 2018;125(5):1238–1252. doi: 10.1111/jam.14055. [DOI] [PubMed] [Google Scholar]
- Campos J.K.L., Araújo C.S.F., Araújo T.F.S., Santos A.F.S., Teixeira J.A., Lima V.L.M., Coelho L.C.B.B. Anti-inflammatory and antinociceptive activities of Bauhinia monandra leaf lectin. Biochimie Open. 2016;2:62–68. doi: 10.1016/j.biopen.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcante da Silva G., Macário de Oliveira A., Soares de Freitas A.F., Paiva P.M.G., Napoleão T.H. Antinociceptive and Anti-Inflammatory Effects of Saline Extract and Lectin-Rich Fraction from Microgramma vacciniifolia Rhizome in Mice. Chem Biodivers. 2021;18(6) doi: 10.1002/cbdv.202100125. [DOI] [PubMed] [Google Scholar]
- Chan Y.S., Yu H., Xia L., Ng T.B. Lectin from green speckled lentil seeds (Lens culinaris) triggered apoptosis in nasopharyngeal carcinoma cell lines. Chin Med. 2015;10(1) doi: 10.1186/s13020-015-0057-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charan R.D., Munro M.H.G., O'Keefe B.R., Sowder R.C., McKee T.C., Currens M.J., Pannell L.K., Boyd M.R. Isolation and Characterization of Myrianthus holstii Lectin, a Potent HIV-1 Inhibitory Protein from the Plant Myrianthus holstii. J. Nat. Prod. 2000;63(8):1170–1174. doi: 10.1021/np000039h. [DOI] [PubMed] [Google Scholar]
- Coelho L.C., da Silva M.B. Simple method to purify milligram quantities of the galactose-specific lectin from the leaves of Bauhinia monandra. Phytochem Anal. 2000;11(5):295–300. doi: 10.1002/1099-1565(200009/10)11:5<295::AID-PCA517>3.0.CO;2-S. [DOI] [Google Scholar]
- Colgan S.P., Parkos C.A., McGuirk D., Brady H.R., Papayianni A.A., Frendl G., Madara J.L. Receptors involved in carbohydrate binding modulate intestinal epithelial-neutrophil interactions. J. Biol. Chem. 1995;270(18):10531–10539. doi: 10.1074/jbc.270.18.10531. [DOI] [PubMed] [Google Scholar]
- David M, et al. (2022) A molecularly engineered, broad-spectrum anti-coronavirus lectin inhibits SARS-CoV-2 and MERS-CoV infection in vivo. Research Square, https://doi.org/10.21203/rs.3.rs-516695/v1 doi:10.21203/rs.3.rs-516695/v1. [DOI] [PMC free article] [PubMed]
- de Freitas Pires A., Bezerra M.M., Amorim R.M.F., do Nascimento F.L.F., Marinho M.M., Moura R.M., Silva M.T.L., Correia J.L.A., Cavada B.S., Assreuy A.M.S., Nascimento K.S. Lectin purified from Lonchocarpus campestris seeds inhibits inflammatory nociception. Int. J. Biol. Macromol. 2019;125:53–60. doi: 10.1016/j.ijbiomac.2018.11.233. [DOI] [PubMed] [Google Scholar]
- de Oliveira Leite G., Santos S.A.A.R., Bezerra F.M.D.H., Sena e Silva F.E., de Castro Ribeiro A.D., Roma R.R., Silva R.R.S., Santos M.H.C., Santos A.L.E., Teixeira C.S., Campos A.R. Is the orofacial antinociceptive effect of lectins intrinsically related to their specificity to monosaccharides? Int. J. Biol. Macromol. 2020;161:1079–1085. doi: 10.1016/j.ijbiomac.2020.06.132. [DOI] [PubMed] [Google Scholar]
- de Souza Feitosa Lima I.M., Zagmignan A., Santos D.M., Maia H.S., dos Santos Silva L., da Silva Cutrim B., Vieira S.L., Bezerra Filho C.M., de Sousa E.M., Napoleão T.H., Krogfelt K.A., Løbner-Olesen A., Paiva P.M.G., Nascimento da Silva L.C. Schinus terebinthifolia leaf lectin (SteLL) has anti-infective action and modulates the response of Staphylococcus aureus-infected macrophages. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-54616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vasconcellos Abdon A.P., Coelho de Souza G., Noronha Coelho de Souza L., Prado Vasconcelos R., Araújo Castro C., Moreira Guedes M., Pereira Lima Júnior R.C., de Azevedo Moreira R., de Oliveira Monteiro-Moreira A.C., Rolim Campos A. Gastroprotective potential of frutalin, a d-galactose binding lectin, against ethanol-induced gastric lesions. Fitoterapia. 2012;83(3):604–608. doi: 10.1016/j.fitote.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Dias R., Machado L., Migliolo L., Franco O. Insights into animal and plant lectins with antimicrobial activities. Molecules. 2015;20(1):519–541. doi: 10.3390/molecules20010519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do Nascimento FLF, et al (2021). The Anti-Inflammatory Effect of Andira Anthelmia Lectin In Rats Involves Inhibition of The Prostanoid Pathway, TNF-α And Lectin Domain. ResearchSquare. DOI: 10.21203/rs.3.rs-718940/v1. [DOI] [PubMed]
- El Bairi K., Trapani D., Petrillo A., Le Page C., Zbakh H., Daniele B., Belbaraka R., Curigliano G., Afqir S. Repurposing anticancer drugs for the management of COVID-19. Eur. J. Cancer. 2020;141:40–61. doi: 10.1016/j.ejca.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferner R.E., Aronson J.K. Chloroquine and hydroxychloroquine in covid-19. BMJ. 2020;369 doi: 10.1136/bmj.m1432. [DOI] [PubMed] [Google Scholar]
- Figueiredo J.G., da Silveira Bitencourt F., Beserra I.G., Teixeira C.S., Luz P.B., Bezerra E.H.S., Mota M.R.L., Assreuy A.M.S., de Queiroz Cunha F., Cavada B.S., de Alencar N.M.N. Antinociceptive activity and toxicology of the lectin from Canavalia boliviana seeds in mice. Naunyn Schmiedebergs Arch. Pharmacol. 2009;380(5):407–414. doi: 10.1007/s00210-009-0448-2. [DOI] [PubMed] [Google Scholar]
- Fischer K., Nguyen K., LiWang P.J. Griffithsin Retains Anti-HIV-1 Potency with Changes in gp120 Glycosylation and Complements Broadly Neutralizing Antibodies PGT121 and PGT126. Antimicrob Agents Chemother. 2019;64(1) doi: 10.1128/AAC.01084-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontenelle T.P.C., Lima G.C., Mesquita J.X., Lopes J.L.d.S., de Brito T.V., Vieira Júnior F.D.C., Sales A.B., Aragão K.S., Souza M.H.L.P., Barbosa A.L.D.R., Freitas A.L.P. Lectin obtained from the red seaweed Bryothamnion triquetrum: Secondary structure and anti-inflammatory activity in mice. Int. J. Biol. Macromol. 2018;112:1122–1130. doi: 10.1016/j.ijbiomac.2018.02.058. [DOI] [PubMed] [Google Scholar]
- Freeze H.H. Lectin Affinity Chromatography. Current Protocols in Protein Science. 1995;00(1) doi: 10.1002/0471140864.ps0901s00. [DOI] [PubMed] [Google Scholar]
- Gaofu Q.i., Shiqing M., Fayin Z., Zhiniu Y.u., Xiuyun Z. In vitro assessment of plant lectins with anti-pinwood nematode activity. J. Appl. Microbiol. 2008;98(1):40–45. doi: 10.1016/j.jip.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Garcia-Pino A., Buts L., Wyns L., Imberty A., Loris R. How a plant lectin recognizes high mannose oligosaccharides. Plant Physiol. 2007;144(4):1733–1741. doi: 10.1104/pp.107.100867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I.J., et al. What should be called a lectin? Nature. 1980;285(5760):66. [Google Scholar]
- Gomes F.S., Procópio T.F., Napoleão T.H., Coelho L.C.B.B., Paiva P.M.G. Antimicrobial lectin from S Chinus terebinthifolius leaf. J. Appl. Microbiol. 2013;114(3):672–679. doi: 10.1111/jam.12086. [DOI] [PubMed] [Google Scholar]
- Gordts S.C., Renders M., Férir G., Huskens D., Van Damme E.J.M., Peumans W., Balzarini J., Schols D. NICTABA and UDA, two GlcNAc-binding lectins with unique antiviral activity profiles. J. Antimicrob. Chemother. 2015;70(6):1674–1685. doi: 10.1093/jac/dkv034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelik E., et al. On the role of cell surface carbohydrates and their binding proteins (lectins) in tumor metastasis. Cancer metastasis rev. 2001;20(3–4):245–277. doi: 10.1023/a:1015535427597. [DOI] [PubMed] [Google Scholar]
- Greig A.S., Bouillant A.M. Binding effects of concanavalin A on a coronavirus. Can J. Comp. Med. 1977;41(1):122–126. [PMC free article] [PubMed] [Google Scholar]
- Hayawi K., Shahriar S., Serhani M.A., Alashwal H., Masud M.M. Vaccine versus Variants (3Vs): Are the COVID-19 Vaccines Effective against the Variants? A Systematic Review. VBSABP. 2021;9(11):1305. doi: 10.3390/vaccines9111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevey R. Strategies for the Development of Glycomimetic Drug Candidates. Pharmaceuticals (Basel) 2019;12(2):55. doi: 10.3390/ph12020055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holanda F.R., et al. Antinociceptive activity of lectins from Diocleinae seeds on acetic acid-induced writhing test in mice. Protein Pept. Lett. 2009;16(9):1088–1092. doi: 10.2174/092986609789055304. [DOI] [PubMed] [Google Scholar]
- Hsieh P.-K., Chang S.C., Huang C.-C., Lee T.-T., Hsiao C.-W., Kou Y.-H., Chen I.-Y., Chang C.-K., Huang T.-H., Chang M.-F. Assembly of severe acute respiratory syndrome coronavirus RNA packaging signal into virus-like particles is nucleocapsid dependent. J. Virol. 2005;79(22):13848–13855. doi: 10.1128/JVI.79.22.13848-13855.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B., Guo H., Zhou P., Shi Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021;19(3):141–154. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H.-C., Lai Y.-J., Liao C.-C., Yang W.-F., Huang K.-B., Lee I.-J., Chou W.-C., Wang S.-H., Wang L.-H., Hsu J.-M., Sun C.-P., Kuo C.-T., Wang J., Hsiao T.-C., Yang P.-J., Lee T.-A., Huang W., Li F.-A., Shen C.-Y., Lin Y.-L., Tao M.-H., Li C.-W. Targeting conserved N-glycosylation blocks SARS-CoV-2 variant infection in vitro. EBioMedicine. 2021;74:103712. doi: 10.1016/j.ebiom.2021.103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang H.-J., Han J.-W., Jeon H., Cho K., Kim J.-H., Lee D.-S., Han J.W. Characterization of a Novel Mannose-Binding Lectin with Antiviral Activities from Red Alga Grateloupia chiangii. Biomolecules. 2020;10(2):333. doi: 10.3390/biom10020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jandú, Jannyson JB, et al. Targeting the immune system with plant lectins to combat microbial infections. Front. Pharmacol. 8 (2017): 671. doi: 10.3389/fphar.2017.00671. [DOI] [PMC free article] [PubMed]
- Jehan ARN, et al (2017) Analgesic Potential of Extracts and Derived Natural Products from Medicinal Plants, Pain Relief In: Maldonado C (ed) Analgesics to Alternative Therapies vol DOI: 10.5772/intechopen.68631. Available from: https://www.intechopen.com/chapters/54987. IntechOpen.
- Jones M.B., Kansiime F., Saunders M.J. The potential use of papyrus (Cyperus papyrus L.) wetlands as a source of biomass energy for sub-Saharan Africa. GCB Bioenergy. 2018;10(1):4–11. [Google Scholar]
- Kamkar Asl M., et al. Analgesic effect of the aqueous and ethanolic extracts of clove. Avicenna J. Phytomedicine. 2013;3(2):186–192. [PMC free article] [PubMed] [Google Scholar]
- Karar MGE& Kuhnert N (2017) Herbal Drugs from Sudan: Traditional Uses and Phytoconstituents. Pharmacogn Rev 11(22):83-103 doi:10.4103/phrev.phrev_15_15, [DOI] [PMC free article] [PubMed]
- Kaur R., et al. Glycan-dependent chikungunya viral infection divulged by antiviral activity of NAG specific chi-like lectin. Virol. J. 2019;526:91–98. doi: 10.1016/j.virol.2018.10.009. [DOI] [PubMed] [Google Scholar]
- Keyaerts E., Vijgen L., Pannecouque C., Van Damme E., Peumans W., Egberink H., Balzarini J., Van Ranst M. Plant lectins are potent inhibitors of coronaviruses by interfering with two targets in the viral replication cycle. Antiviral Res. 2007;75(3):179–187. doi: 10.1016/j.antiviral.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A., Khan T., Ali S., Aftab S., Wang Y., Qiankun W., Khan M., Suleman M., Ali S., Heng W., Ali S.S., Wei D.-Q., Mohammad A. SARS-CoV-2 new variants: Characteristic features and impact on the efficacy of different vaccines. Biomed. Pharmacother. 2021;143:112176. doi: 10.1016/j.biopha.2021.112176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan H., Pervaiz A., Intagliata S., Das N., Nagulapalli Venkata K.C., Atanasov A.G., Najda A., Nabavi S.M., Wang D., Pittalà V., Bishayee A. The analgesic potential of glycosides derived from medicinal plants. Daru : DARU J. Pharm. Sci. 2020;28(1):387–401. doi: 10.1007/s40199-019-00319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. Animal lectins: a historical introduction and overview. B (BBA) 2002;1572(2-3):187–197. doi: 10.1016/s0304-4165(02)00308-2. [DOI] [PubMed] [Google Scholar]
- Koshte V.L., van Dijk W., van der Stelt M.E., Aalberse R.C. Isolation and characterization of BanLec-I, a mannoside-binding lectin from Musa paradisiac (banana) Biochem. 1990;272(3):721–726. doi: 10.1042/bj2720721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaki Y., Wandersee M.K., Smith A.J., Zhou Y., Simmons G., Nelson N.M., Bailey K.W., Vest Z.G., Li J.-K., Chan P.-S., Smee D.F., Barnard D.L. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin Urtica dioica agglutinin. Antiviral Res. 2011;90(1):22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacerda R.R., et al. Lectin isolated from Brazilian seeds of velvet bean (Mucuna pruriens (L) DC.) presents analgesic, anti-inflammatory and antihemolytic action. J. Med. Plant Res. 2015;9(8):231–242. [Google Scholar]
- Lam S., Han Q., Ng T. Isolation and characterization of a lectin with potentially exploitable activities from caper (Capparis spinosa) seeds. Biosci. Rep. 2009;29(5):293–299. doi: 10.1042/BSR20080110. [DOI] [PubMed] [Google Scholar]
- Leite J.F.M., Assreuy A.M.S., Mota M.R.L., Bringel P.H.d.S.F., e Lacerda R.R., Gomes V.d.M., Cajazeiras J.B., do Nascimento K.S., Pessôa H.d.L.F., Gadelha C.A.d.A., Delatorre P., Cavada B.S., Santi-Gadelha T. Antinociceptive and anti-inflammatory effects of a lectin-like substance from Clitoria fairchildiana R Howard seeds. Molecules. 2012;17(3):3277–3290. doi: 10.3390/molecules17033277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeVine D., Kaplan M.J., Greenaway P.J. The purification and characterization of wheat-germ agglutinin. Biochem. 1972;129(4):847–856. doi: 10.1042/bj1290847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.-M., Shahed-Al-Mahmud M.d., Chen X., Chen T.-H., Liao K.-S., Lo J.M., Wu Y.-M., Ho M.-C., Wu C.-Y., Wong C.-H., Jan J.-T., Ma C. A Carbohydrate-Binding Protein from the Edible Lablab Beans Effectively Blocks the Infections of Influenza Viruses and SARS-CoV-2. Cell Rep. 2020;32(6):108016. doi: 10.1016/j.celrep.2020.108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusvarghi S., Bewley C.A. Griffithsin: An Antiviral Lectin with Outstanding Therapeutic Potential. Viruses. 2016;8(10) doi: 10.3390/v8100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhu CS & sharada AC. (2019) Anti-inflammatory activity of partially purified lectin from Praecitrullus Fistulosus Phloem Exudates. Asian J Pharm Clin Res. 12(1):91-4. DOI: https://doi.org/10.22159/ajpcr.2019.v12i1.28670.
- Márcio VR, et al (1996) Purification and Partial Characterization of a Lectin From Dioclea virgata Benth Seeds. Fascículos:Revista Brasileira de Fisiologia Vegetal 8 (37-42).
- Martinez D., Amaral D., Markovitz D., Pinto L. The Use of Lectins as Tools to Combat SARS-CoV-2. Curr. Pharm. Des. 2021;27(41):4212–4222. doi: 10.2174/1381612827666210830094743. [DOI] [PubMed] [Google Scholar]
- Millet J.K., Séron K., Labitt R.N., Danneels A., Palmer K.E., Whittaker G.R., Dubuisson J., Belouzard S. Middle East respiratory syndrome coronavirus infection is inhibited by griffithsin. Antiviral Res. 2016;133:1–8. doi: 10.1016/j.antiviral.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A., Behura A., Mawatwal S., Kumar A., Naik L., Mohanty S.S., Manna D., Dokania P., Mishra A., Patra S.K., Dhiman R. Structure-function and application of plant lectins in disease biology and immunity. Food Chem Toxicol. 2019;134:110827. doi: 10.1016/j.fct.2019.110827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mo H., Meah Y., Moore J.G., Goldstein I.J. Purification and characterization of Dolichos lablab lectin. Glycobiol. 1999;9(2):173–179. doi: 10.1093/glycob/9.2.173. [DOI] [PubMed] [Google Scholar]
- Mogosan C., Vostinaru O., Oprean R., Heghes C., Filip L., Balica G., Moldovan R. A Comparative Analysis of the Chemical Composition, Anti-Inflammatory, and Antinociceptive Effects of the Essential Oils from Three Species of Mentha Cultivated in Romania. Molecules. 2017;22(2):263. doi: 10.3390/molecules22020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira R.A., Barros A.C.H., Stewart J.C., Pusztai A. Isolation and characterization of a lectin from the seeds of Dioclea grandiflora (Mart.) Planta. 1983;158(1):63–69. doi: 10.1007/BF00395404. [DOI] [PubMed] [Google Scholar]
- Nardy A.F.F.R., Freire-de-Lima L., Freire-de-Lima C.G., Morrot A. The Sweet Side of Immune Evasion: Role of Glycans in the Mechanisms of Cancer Progression. Front. Oncol. 2016;6 doi: 10.3389/fonc.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento K.S., Nascimento F.L.F.d., Silva M.T.L., Nobre C.B., Moreira C.G., Brizeno L.A.C., da Ponte E.L., Assreuy A.M.S., Cavada B.S. Purification of a thermostable antinociceptive lectin isolated from Andira anthelmia. J. Mol. Recognit. 2016;29(6):248–252. doi: 10.1002/jmr.2523. [DOI] [PubMed] [Google Scholar]
- Neves S.A., Freitas A.L.P., Souza B.W.S., Rocha M.L.A., Correia M.V.O., Sampaio D.A., Viana G.S.B. Antinociceptive properties in mice of a lectin isolated from the marine alga Amansia multifida Lamouroux Braz. J. Med. Biol. 2007;40(1):127–134. doi: 10.1590/s0100-879x2007000100016. [DOI] [PubMed] [Google Scholar]
- Nunes B.S., Rensonnet N.S., Dal-Secco D., Vieira S.M., Cavada B.S., Teixeira E.H., Moura T.R., Teixeira C.S., Clemente-Napimoga J.T., Cunha F.Q., Napimoga M.H. Lectin extracted from Canavalia grandiflora seeds presents potential anti-inflammatory and analgesic effects. Naunyn-Schmiedeb. Arch. Pharmacol. 2009;379(6):609–616. doi: 10.1007/s00210-009-0397-9. [DOI] [PubMed] [Google Scholar]
- O'Connor B.F., et al. Lectin Affinity Chromatography (LAC) Methods mol. biol. 2017;1485:411–420. doi: 10.1007/978-1-4939-6412-3_23. [DOI] [PubMed] [Google Scholar]
- Oladokun, et al. Anti-nociceptive and anti-inflammatory activities of Tetracarpidium conophorum seed lectin. Sci. Afr. 2019;3 [Google Scholar]
- Oliveira J.T.A., Melo V.M.M., Câmara M.F.L., Vasconcelos I.M., Beltramini L.M., Machado O.L.T., Gomes V.M., Pereira S.P., Fernandes C.F., Nunes E.P., Capistrano G.G.G., Monteiro-Moreira A.C.O. Purification and physicochemical characterization of a cotyledonary lectin from Luetzelburgia auriculata. Phytochem. 2002;61(3):301–310. doi: 10.1016/s0031-9422(02)00239-x. [DOI] [PubMed] [Google Scholar]
- Oliveira J.T.A.D., et al. Isolation and partial characterization of a lectin from Cratylia floribunda mart. seeds. Rev Bras Bot. 1991;14(1):61–66. [Google Scholar]
- Ooi L.S.M., Sun S.S.M., Ooi V.E.C. Purification and characterization of a new antiviral protein from the leaves of Pandanus amaryllifolius (Pandanaceae) Int. J. Biochem. Cell Biol. 2004;36(8):1440–1446. doi: 10.1016/j.biocel.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Osterne V.J.S., Silva-Filho J.C., Santiago M.Q., Pinto-Junior V.R., Almeida A.C., Barreto A.A.G.C., Wolin I.A.V., Nascimento A.P.M., Amorim R.M.F., Rocha B.A.M., Delatorre P., Nagano C.S., Leal R.B., Assreuy A.M.S., Nascimento K.S., Cavada B.S. Structural characterization of a lectin from Canavalia virosa seeds with inflammatory and cytotoxic activities. Int. J. Biol. Macromol. 2017;94:271–282. doi: 10.1016/j.ijbiomac.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Peng H., Lv H., Wang Y., Liu Y.-H., Li C.-Y., Meng L., Chen F., Bao J.-k. Clematis montana lectin, a novel mannose-binding lectin from traditional Chinese medicine with antiviral and apoptosis-inducing activities. Peptides. 2009;30(10):1805–1815. doi: 10.1016/j.peptides.2009.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto I.R., Chaves H.V., Vasconcelos A.S., de Sousa F.C.F., Santi-Gadelha T., de Lacerda J.T.J.G., Ribeiro K.A., Freitas R.S., Maciel L.M., Filho S.M.P., Viana A.F.S.C., de Almeida Gadelha C.A., Filho G.C., de Paulo Teixeira Pinto V., Pereira K.M.A., Rodrigues e Silva A.A., Bezerra M.M. Antiulcer and Antioxidant Activity of a Lectin from Mucuna pruriens Seeds on Ethanol- induced Gastropathy: Involvement of Alpha-2 Adrenoceptors and Prostaglandins. Curr. Pharm. Des. 2019;25(12):1430–1439. doi: 10.2174/1381612825666190524081433. [DOI] [PubMed] [Google Scholar]
- Prashantkumar P, & Vidyasagar GM (2008) Traditional knowledge on medicinal plants used for the treatment of skin diseases in Bidar district, Karnataka Council of Scientific & Industrial Research (CRIS). vol http://hdl.handle.net/123456789/1588. (CRIS), India, p 273-276.
- Ramos D.d.B.M., Araújo M.T.d.M.F., Araújo T.C.d.L., Silva Y.A., dos Santos A.C.L.A., e Silva M.G., Paiva P.M.G., Mendes R.L., Napoleão T.H. Antinociceptive activity of Schinus terebinthifolia leaf lectin (SteLL) in sarcoma 180-bearing mice. J. Ethnopharmacol. 2020;259:112952. doi: 10.1016/j.jep.2020.112952. [DOI] [PubMed] [Google Scholar]
- Renato D.A.M., et al. isolation and partial characterization of a lectin from seeds of Dioclea violacea. Braz. J. Plant Physiol. 1996;8(1):23–29. [Google Scholar]
- Reyes-Montaño E.A., Vega-Castro N. Plant Lectins with Insecticidal and Insectistatic Activities, Insecticides Agriculture and Toxicology, Ghousia Begum. IntechOpen. vol. 2018 doi: 10.5772/intechopen.74962. [DOI] [Google Scholar]
- Rogerio A.P., Cardoso C.R., Fontanari C., Souza M.A., Afonso-Cardoso S.R., Silva É.VG., Koyama N.S., Basei F.L., Soares E.G., Calixto J.B., Stowell S.R., Dias-Baruffi M., Faccioli L.H. Anti-asthmatic potential of a D-galactose-binding lectin from Synadenium carinatum latex. Glycobiol. 2007;17(8):795–804. doi: 10.1093/glycob/cwm053. [DOI] [PubMed] [Google Scholar]
- Roopashree S., Singh S., Gowda L., Rao A.G.A. Dual-function protein in plant defence: seed lectin from Dolichos biflorus (horse gram) exhibits lipoxygenase activity. Biochem. J. 2006;395(3):629–639. doi: 10.1042/BJ20051889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos A.L.E., Leite G.O., Carneiro R.F., Roma R.R., Santos V.F., Santos M.H.C., Pereira R.O., Silva R.C., Nagano C.S., Sampaio A.H., Rocha B.A.M., Delatorre P., Campos A.R., Teixeira C.S. Purification and biophysical characterization of a mannose/N-acetyl-d-glucosamine-specific lectin from Machaerium acutifolium and its effect on inhibition of orofacial pain via TRPV1 receptor. Arch. Biochem. Biophys. 2019;664:149–156. doi: 10.1016/j.abb.2019.02.009. [DOI] [PubMed] [Google Scholar]
- Sathe S.K., Deshpande S.S. In: Encyclopedia of Food Sciences and Nutrition. Second Edition. Caballero B., editor. Academic Press; Oxford: 2003. BEANS; pp. 403–412. [Google Scholar]
- Saul F.A., Rovira P., Boulot G., Van Damme E.JM., Peumans W.J., Truffa-Bachi P., Bentley G.A. Crystal structure of Urtica dioica agglutinin, a superantigen presented by MHC molecules of class I and class II. Structure. 2000;8(6):593–603. doi: 10.1016/s0969-2126(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Shajahan A., Pepi L.E., Rouhani D.S., Heiss C., Azadi P. Glycosylation of SARS-CoV-2: structural and functional insights. Anal. Bioanal. Chem. 2021;413(29):7179–7193. doi: 10.1007/s00216-021-03499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A., Ng T.B., Wong J.H., Lin P. Purification and Characterization of a Lectin from Phaseolus vulgaris cv (Anasazi Beans) Biomed. 2009;2009:1–9. doi: 10.1155/2009/929568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Microbial lectins and their glycoprotein receptors. New Compr. Biochem. 1997;29:475–506. doi: 10.1016/S0167-7306(08)60626-2. [DOI] [Google Scholar]
- Sharon N., Lis H. History of lectins: from hemagglutinins to biological recognition molecules. Glycobiol. 2004;14(11):53R–62R. doi: 10.1093/glycob/cwh122. [DOI] [PubMed] [Google Scholar]
- Sheehan SA, et al. (2020) Evidence that Maackia amurensis seed lectin (MASL) exerts pleiotropic actions on oral squamous cells to inhibit SARS-CoV-2 infection and COVID-19 disease progression. Res Sq:rs.3.rs-93851 doi:10.21203/rs.3.rs-93851/v1. [DOI] [PMC free article] [PubMed]
- Shibuya N., Goldstein I.J., Shafer J.A., Peumans W.J., Broekaert W.F. Carbohydrate binding properties of the stinging nettle (Urtica dioica) rhizome lectin. Arch. Biochem. Biophys. 1986;249(1):215–224. doi: 10.1016/0003-9861(86)90577-1. [DOI] [PubMed] [Google Scholar]
- Silva H.C., Bari A.U., Rocha B.A.M., Nascimento K.S., Ponte E.L., Pires A.F., Delatorre P., Teixeira E.H., Debray H., Assreuy A.M.S., Nagano C.S., Cavada B.S. Purification and primary structure of a mannose/glucose-binding lectin from Parkia biglobosa Jacq. seeds with antinociceptive and anti-inflammatory properties. J. Mol. Recognit. 2013;26(10):470–478. doi: 10.1002/jmr.2289. [DOI] [PubMed] [Google Scholar]
- Silva L.M.C.M., Lima V., Holanda M.L., Pinheiro P.G., Rodrigues J.A.G., Lima M.E.P., Benevides N.M.B. Antinociceptive and anti-inflammatory activities of lectin from marine red alga Pterocladiella capillacea. Biol. Pharm. Bull. 2010;33(5):830–835. doi: 10.1248/bpb.33.830. [DOI] [PubMed] [Google Scholar]
- Sofowora A., et al. The role and place of medicinal plants in the strategies for disease prevention. African J. Traditional, J. Altern. Complement Med. 2013;10(5):210–229. doi: 10.4314/ajtcam.v10i5.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spilatro S.R., Cochran G.R., Walker R.E., Cablish K.L., Bittner C.C. Characterization of a new lectin of soybean vegetative tissues. Plant Physiol. 1996;110(3):825–834. doi: 10.1104/pp.110.3.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sverdén E, et al (2019) Peptic ulcer disease. BMJ 367:l5495 doi:10.1136/bmj.l5495 %J BMJ. [DOI] [PubMed]
- Swanson M.D., Winter H.C., Goldstein I.J., Markovitz D.M. A Lectin Isolated from Bananas Is a Potent Inhibitor of HIV Replication*. Int. J. Biol. Chem. 2010;285(12):8646–8655. doi: 10.1074/jbc.M109.034926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uritu C.M., Mihai C.T., Stanciu G.-D., Dodi G., Alexa-Stratulat T., Luca A., Leon-Constantin M.-M., Stefanescu R., Bild V., Melnic S., Tamba B.I. Medicinal Plants of the Family Lamiaceae in Pain Therapy: A Review. Pain Res. Manag. 2018;2018:1–44. doi: 10.1155/2018/7801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme E.J.M., et al. Isolation, characterization and molecular cloning of the bark lectins from Maackia amurensis. Glycoconj. J. 1997;14(4):449–456. doi: 10.1023/A:1018595300863. [DOI] [PubMed] [Google Scholar]
- Vanderlei E.S.O., Patoilo K.K.N.R., Lima N.A., Lima A.P.S., Rodrigues J.A.G., Silva L.M.C.M., Lima M.E.P., Lima V., Benevides N.M.B. Antinociceptive and anti-inflammatory activities of lectin from the marine green alga Caulerpa cupressoides. Int. Immunopharmacol. 2010;10(9):1113–1118. doi: 10.1016/j.intimp.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Vasconcelos I.M., Cavada B.S., Moreira R.DE.A., Oliveira J.T.A.DE. Purification and partial characterization of a lectin from the seeds of Dioclea guianensis. J. Food Biochem. 1991;15(2):137–154. [Google Scholar]
- Wang W., Li Q., Wu J., Hu Y.u., Wu G., Yu C., Xu K., Liu X., Wang Q., Huang W., Wang L., Wang Y. Lentil lectin derived from Lens culinaris exhibit broad antiviral activities against SARS-CoV-2 variants. Emerg. Microbes Infect. 2021;10(1):1519–1529. doi: 10.1080/22221751.2021.1957720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijetunge S.S., Wen J., Yeh C.-K., Sun Y. Lectin-Conjugated Liposomes as Biocompatible, Bioadhesive Drug Carriers for the Management of Oral Ulcerative Lesions. ACS Appl. 2018;1(5):1487–1495. doi: 10.1021/acsabm.8b00425. [DOI] [PubMed] [Google Scholar]
- Wode K., Hök Nordberg J., Kienle G.S., Elander N.O., Bernhardson B.-M., Sunde B., Sharp L., Henriksson R., Fransson P. Efficacy of mistletoe extract as a complement to standard treatment in advanced pancreatic cancer: study protocol for a multicentre, parallel group, double-blind, randomised, placebo-controlled clinical trial (MISTRAL) Trials. 2020;21(1) doi: 10.1186/s13063-020-04581-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Xu H.-L., Zhang Z.-T., Liu J.-J., Li W.-W., Ming H., Bao J.-k. Characterization, molecular cloning, and in silico analysis of a novel mannose-binding lectin from Polygonatum odoratum (Mill.) with anti-HSV-II and apoptosis-inducing activities. Phytomedicine. 2011;18(8-9):748–755. doi: 10.1016/j.phymed.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Zhao X., et al. Glycans of SARS-CoV-2 Spike Protein in Virus Infection and Antibody Production. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmob.2021.629873. [DOI] [PMC free article] [PubMed] [Google Scholar]